Abstract
Objective
To develop an efficient, clinical-grade, freezing protocol toward experimental clinical cryopreservation of testicular tissues in prepubertal boys suffering from cancer.
Design
Experimental cryopreservation of testicular tissue.
Setting
University Medical Center.
Patient(s)
Adult patients undergoing orchiectomy for various tumors and prepubertal boys scheduled for gonadotoxic treatment.
Intervention(s)
None.
Main Outcome Measure(s)
Histopathological analysis of tissue architecture, structural integrity, and cellular morphology was performed for control and frozen-thawed cryopreserved tissues.
The number of seminiferous tubules per testicular section was calculated. The survival of spermatogonial stem cells (SSCs) and Sertoli cells of the control and frozen-thawed cryopreserved tissues was analyzed by immunofluorescence staining.
Result(s)
Uncontrolled Slow Freezing, Controlled slow freezing, and vitrification similarly preserved the integrity of the adult testicular tissues and the survival of SSCs and Sertoli cells. Controlled slow freezing of prepubertal testicular tissues effectively preserved their architecture, the number of tubules, SSCs, and Sertoli cells. In addition, we observed SSC loss after chemotherapy in prepubertal boys, reemphasizing the importance of fertility preservation before gonadotoxic treatment.
Conclusion(s)
Future fertility restoration for male survivors of pediatric cancers depends on the development of an optimal prepubertal testicular tissue cryopreservation method. Our findings demonstrate the effectiveness of controlled slow freezing for cryopreservation of human prepubertal testicular tissues and may contribute to more effective banking of these tissues and potential fertility restoration.
Clinical Trial Registration Number
NIH research clinical trials number: NCT02529826.
Key Words: Fertility preservation, prepubertal boys, human testicular cryopreservation, controlled slow freezing, male infertility
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-21-00046
As the effectiveness of cancer treatments has improved, many children diagnosed with cancer can enjoy a longer life free of the disease (1, 2). To date, it is estimated that one in 250 young adults is a cancer survivor (3). Although oncological treatments are highly effective, long-term survivors of pediatric cancers often suffer from fertility problems due to gonadotoxic therapies (4, 5). Whereas pubertal male patients are encouraged to cryopreserve sperm, this is not possible for prepubertal boys in whom spermatogenesis, the process of differentiation of spermatogonial stem cells (SSCs) to mature sperm, has not yet begun (2). Two different approaches may be considered for fertility preservation in prepubertal boys before gonadotoxic treatments, cryopreservation of isolated SSCs obtained after enzymatic digestion of the testicular tissue and cryopreservation of testicular tissue fragments that preserve both the SSCs and their supporting microenvironment (6). Testicular tissue cryopreservation is advantageous to SSC freezing because it could potentially enable the use of either tissue or SSCs for fertility restoration. Although this method is not yet feasible for use in humans (6, 7), it was successfully applied to restore fertility in several animal models (8, 9). Recently, autologous transplantation of frozen-thawed prepubertal rhesus testicular tissue was reported to produce mature sperms that were used for in vitro fertilization (IVF) to produce offspring, suggesting that this approach may be applicable in the future for fertility restoration in humans (10).
At present, a standardized protocol for freezing human testicular tissue has not been established, and the optimal cryopreservation method is still unknown (11). Several groups have developed protocols for cryopreservation of adult testicular tissues. These protocols were predominantly based on controlled slow freezing (CSF) and uncontrolled slow freezing (USF) methods (12, 13, 14). The various publications on CSF differed in the composition and concentrations of the cryoprotective agents (dimethyl sulfoxide [DMSO], ethylene glycol, sucrose, and human serum albumin [HSA]) (6, 13, 15). Only a single study compared the efficacy of cryopreservation by CSF and USF with open pulled-straw vitrification (13).
The potential of the various cryopreservation methods to restore fertility in pediatric cancer survivors has not been shown. Studies evaluating the efficacy of prepubertal tissue cryopreservation are scarce (12, 16, 17, 18, 19, 20, 21). Nevertheless, currently, medical and academic centers offer experimental slow freezing testicular tissue cryopreservation to prepubertal cancer-affected boys in anticipation that these tissues would be used in the future to restore fertility (11, 15, 22, 23)
The objective of the present study was to develop an efficient clinical-grade freezing protocol toward experimental clinical cryopreservation of testicular tissues in prepubertal boys suffering from cancer. First, we compared the effectiveness of cryopreservation of adult human testicular tissues by three methods: USF, CSF, and vitrification. Then, we studied the efficacy of CSF in cryopreservation of prepubertal human testicular tissues from prepubertal patients with cancer.
Materials and methods
Tissue Collection and Processing
Adult testicular tissues were obtained from men undergoing orchiectomy for various tumors. A wedge biopsy was performed by a urologist after obtaining written informed consent. The testicular tissue was immediately transferred in cold Hank's buffered salt solution (HBSS) (SH30588.01, Hyclone, Fisher Scientific, Pittsburgh, PA) on ice to the laboratory. It was washed with cold HBSS and manually dissected into small fragments (approximately 4 × 4 × 4 mm3) using a scalpel. One fragment was fixed in 4% paraformaldehyde (PFA) and served as a nonfrozen control. The remaining fragments were cryopreserved using three freezing protocols: USF, CSF, and vitrification (adapted from Baert et al. [13]). Prepubertal testicular tissue cryopreservation was offered as an experimental procedure to young prepubertal boys before gonadotoxic treatment, or after an earlier treatment/relapse of the disease when further gonadotoxic treatment was planned. After obtaining written informed consent from the parents, a pediatric urologist performed a wedge biopsy from one testis under anesthesia. The biopsied tissues were immediately transported to the laboratory in cold HBSS on ice, washed, and manually dissected using a scalpel into fragments of approximately 3 × 3 × 3 mm3, and CSF cryopreservation was performed. Some of the cryopreserved tissues were retained for research, and the remaining tissues were stored at the Hadassah Fertility Cryobank.
Uncontrolled Slow Freezing
Several testicular tissue fragments were placed into a prelabeled (preprinted with Brady label) cryovial tube and filled with 1 ml cold buffer, composed of Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 medium (DMEM/F12- 21331020, Invitrogen, Thermo Fisher Scientific, Waltham, MA), containing 1.5 M DMSO (CryoSure DMSO solution, USP grade, WAK-DMSO-10, WAK-Chemie Medical GmbH, Steinbach, Germany), 0.15 M sucrose (S-9378, Sigma-Aldrich, St. Louis, MO), and 10 mg/mL human serum albumin (HSA-9988 FUJIFILM, Irvine Scientific, Santa Ana, CA). After equilibration for 15 minutes on ice, the cryovial tube was placed in an isopropyl alcohol container (Mr. Frosty Freezing Container- 5100-0001, Nalgene, Thermo Fisher Scientific) and put in a -80 °C freezer overnight. The next day, the cryovial tubes were transferred to liquid nitrogen for long-term storage. The frozen samples were thawed at 37 °C in a water bath for 2 minutes, then washed twice in prewarmed (37 °C) DMEM/F12 containing 10 mg/mL HSA.
Controlled Slow Freezing
Several testicular tissue fragments were placed in a cryovial tube filled with 1 ml of cold sterile HBSS, containing 1.5 M DMSO, 0.1 M sucrose, and 10 mg/mL HSA. After equilibration for 30 minutes on ice, the cryovial tubes were placed in a programmable freezer (Kryo 360-1.7, Planer Limited, Sunbury-on-Thames, UK). Cooling was performed at 1 °C/min with a holding period at 0 °C for 5 minutes, followed by cooling at 0.5 °C/min until -8 °C. When the temperature reached -8 °C, the program was put on hold for 10 minutes to allow manual seeding. Cooling was resumed at a rate of 0.5 °C/min until -40 °C, put on hold for 10 minutes, and continued cooling to -70 °C at 7 °C/min. Cryovial tubes were then transferred to liquid nitrogen for long-term storage. The frozen samples were thawed at 37 °C in a water bath for 2 minutes and washed twice in cold HBSS on ice for 5 minutes.
Vitrification
Several testicular tissue fragments were incubated at room temperature for 10 minutes in vitrification solution I composed of Dulbecco's modified Eagle medium (DMEM)/F12 containing 1.05 M DMSO, 1.35 M ethylene glycol (433810010, Acros Organics, Thermo Fisher). This was followed by incubation at room temperature for 5 minutes in vitrification solution II composed of DMEM/F12 containing 2.1 M DMSO, 2.7 M ethylene glycol, and 20 mg/mL HAS. Cryovial tubes (2 ml, Nalgene cryogenic vials) were filled with liquid nitrogen, and using a floater, partially immersed in a thermos filled with liquid nitrogen. Open pulled vitrification straws (CryoTip cat 40709, Irvine Scientific, CA) were manually cut at their widest diameter, removing the fine tip and the metal cover sleeve, leaving approximately 3 cm of the wide part of the straw able to fit the cryovial tubes. Using a 1 ml syringe and an adaptor, 2–3 testicular tissue fragments were carefully aspirated into the wide tip of the straws under a binocular microscope. The nonsealed vitrification straws were immediately submerged into the liquid nitrogen-filled cryovial tubes. When vitrification was completed, the straws with the cryopreserved tissues were transferred to new liquid nitrogen-empty cryovial tubes that were capped and transferred to liquid nitrogen for long-term storage. The frozen straws were thawed by immersing them in a prewarmed (37 °C) thawing solution composed of DMEM/F12 containing 1.5 M Sucrose and 20 mg/mL HSA. They were further incubated at 37 °C for 2 minutes in a solution composed of DMEM/F12 containing 20 mg/mL HSA.
Histological Analysis
The frozen-thawed and the control testicular tissue fragments were fixed in 4% PFA, embedded in paraffin, and serially sectioned into 6-μm sections. Every other section was mounted on slides (2–3 sections per slide, and 20 slides for each fragment). Four slides (slides 3, 8, 13, and 18) containing 8–12 sections from various parts of the testicular fragments were stained with hematoxylin and eosin (H&E). Histological analysis to evaluate cellular morphology, tissue architecture, and structural integrity was performed by a certified pathologist. The number of tubules per 1,500 × 1,000 μm2 was calculated using light microscopy at ×100 magnifications for adult donors from 5 to 11 random images and ×200 magnification for prepubertal patients from 4 to 10 random images. The number of images depended on the amount of donated testicular tissue that was available.
Immunohistochemistry Analysis
The frozen-thawed and control testicular tissues were fixed in 4% PFA, embedded in paraffin, and sectioned. Six-micron sections were dewaxed and rehydrated. Antigen retrieval was performed in 20 mM citrate buffer pH 6 (Invitrogen, Thermofisher Scientific). Nonspecific binding was blocked in CAS Block buffer (Zymed Laboratories, San Francisco, CA) for 30 minutes at room temperature, and blocking of endogenous peroxidase was performed with H2O2 (3%) (Merck, Darmstadt, Germany). Sections were then incubated overnight at 4 °C in a humidified chamber with the specific primary anti-human antibodies against Oct4 (mouse anti-Oct4; sc-5279, 1:100, Santa Cruz Biotechnology), MAGE-A4 (mouse anti-human monoclonal antibody purified from hybridoma 57B, kindly provided by Giulio Spagnoli, MD, University of Basel, Switzerland; 1:50), and vimentin (mouse monoclonal antibody clone V9, M0725, 1:50, Dako, Heverlee, Belgium). Visualization was performed using EnVision+System-horseradish peroxidase labeled polymer goat anti-mouse (Dako). DAB Chromogen (Invitrogen, Thermofisher Scientific) was used as a substrate for the horseradish peroxidase reaction. Sections were counterstained using H&E. For the negative controls, the primary antibody was omitted. The number of MAGE-A4, Oct4, and vimentin positively stained cells per tubule section was calculated from 3 to 5 random images (×100 or ×200 magnifications). For each marker, 4–20 tubule sections were analyzed. Detailed IHC analysis of stem and Sertoli cells particularly from donor 3, was performed because a large quantity of testicular tissue, which was cryopreserved using the three methods (UCSF, CSF, and vitrification), was available from this donor.
Statistical Analysis
To test the difference between three or more independent groups (control and cryopreservation methods), the Kruskal-Wallis nonparametric test and the post hoc pairwise nonparametric Mann-Whitney U test with the Bonferroni correction of the significance level was applied. Repeated measures analysis using generalized estimating equations was used to evaluate the effect of the freezing methods, taking into account the dependence between the measurements taken from the same donor. All tests used were two-tailed, and P≤.05 was considered statistically significant. Because of the variety in SCC numbers related to the age and condition of the prepubertal patients (i.e., before or after gonadotoxic treatment), each prepubertal sample was analyzed separately.
Ethical Approval
All the experiments in this study were approved by the institutional review board of Hadassah Hebrew University Medical Center (IRB no: 0266-10-HMO and 0530-14-HMO).
Results
Cryopreservation of Adult Human Testicular Tissues by CSF, USF, and Vitrification
Four adult patients were enrolled in the study. After an open testicular biopsy, the testicular tissues were cryopreserved using three protocols: USF, CSF, and vitrification (Supplemental Table 1, available online). The slow-freeze protocols were adapted from Baert et al. (13), using cryopreservation solutions containing DMSO, sucrose, and HSA. The vitrification protocol was based on Baert et al. (24) using vitrification solutions containing DMSO, ethylene glycol, and HSA. We optimized the three protocols for clinical applications with the use of clinical-grade reagents when available. After storage in liquid nitrogen, the cryopreserved testicular tissues were thawed, fixed, embedded in paraffin, and sectioned. Testicular sections were analyzed after both H&E as well as immunostaining to evaluate their structural integrity, cellular morphology, and the survival of SSCs, spermatogonia, and components of the testicular niche.
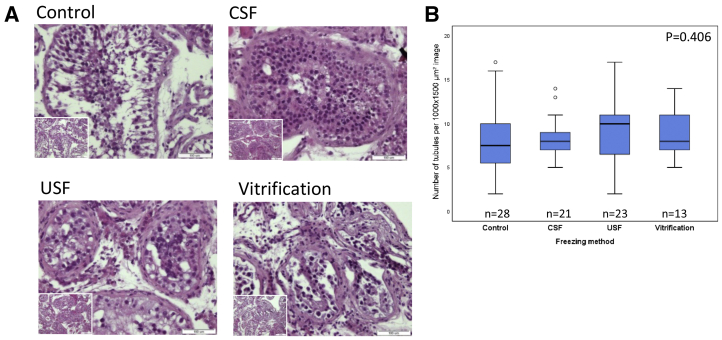
Histopathological analysis of the adult control and frozen-thawed cryopreserved tissues revealed typical seminiferous tubule architecture. Sertoli cells were located near the basement membrane. Consistent with active spermatogenesis, spermatogonia, differentiating spermatids, and mature spermatozoa were observed. Tissue architecture, structural integrity, and cellular morphology were similar when comparing the control and cryopreserved samples after CSF, USF, and vitrification (Fig. 1A). Quantitation of the number of intact seminiferous tubules in the control group and three cryopreservation groups showed that the average numbers of tubules per 1,500 × 1,000 μm2 were 7.96 ± 4.09, 8.52 ± 2.18, 8.91 ± 3.52, and 9.15 ± 2.85, in the control, CSF, USF, and vitrification groups, respectively. Statistical analysis showed no significant differences between the control and three cryopreservation groups (P=.406) (Fig. 1B).
Figure 1.
Histological analysis of control and cryopreserved adult human testicular tissues. Representative hematoxylin-eosin stained sections of control and frozen-thawed testicular tissues from donor 3 cryopreserved by controlled slow freezing (CSF), uncontrolled slow freezing (USF), and vitrification. Bar = 100 μm; Bar for insets = 200 μm (A). Boxplots displaying the distribution of intact tubules numbers (per 1,000 × 1,500 μm, ×100 magnification) in control, CSF, USF, and vitrification cryopreserved testicular tissues from donors 3, 4, and 2, respectively (Supplemental Table 1). The line in the center of each box represents the median. The top and bottom parts of each box represent 75% and 25% percentiles, respectively. The lines extending from each box show the maximum and minimum values of the data. P value was determined by the Kruskal-Wallis H test (nonpaired) (B). n = number of analyzed tubule sections.
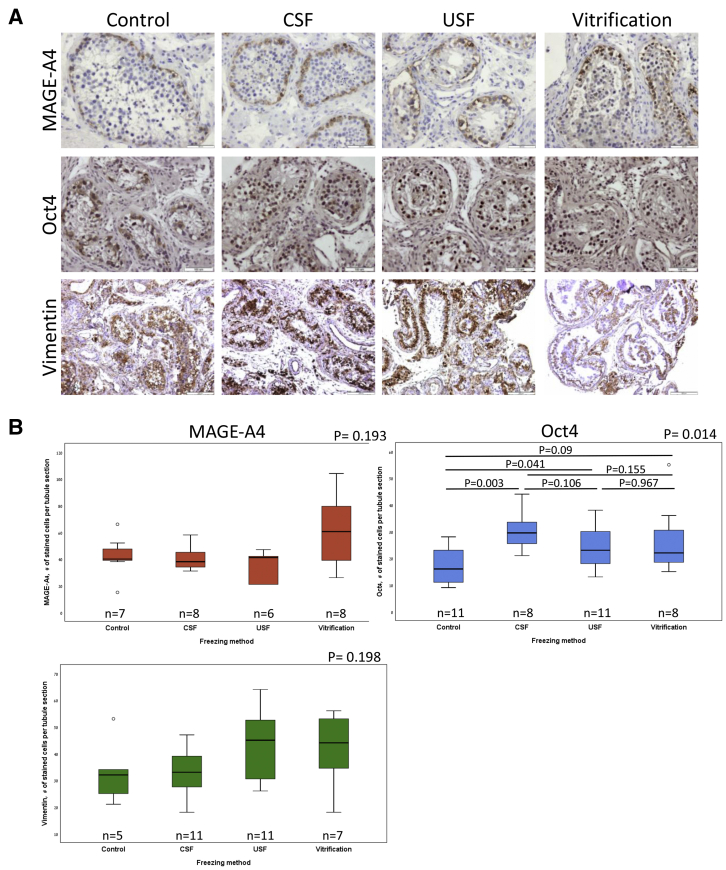
Spermatogonial stem cells are present in both prepubertal and adult testes (25). These stem cells maintain the balance between self-renewal and differentiation into spermatogonial progenitors, which will give rise to mature spermatozoa (26). Preservation of SSCs in the cryopreserved tissue is therefore crucial for the future implementation of fertility restoration. We analyzed the survival of SSCs in the postthawed tissues using two molecular markers: MAGE-A4, a cell-surface antigen expressed by human SSCs and spermatogonia (25, 27), and Oct4, a key pluripotency transcription factor (28), which was shown to be required for SSC proliferation (29). Immunohistochemical analysis of the control and frozen-thawed testicular tissues from donor 3 showed positive staining for MAGE-A4 and Oct4 in tubule sections of the control and three cryopreserved samples. MAGE-A4 and Oct4-expressing cells were located primarily adjacent to the basement membrane of the seminiferous tubules, indicating that these cells are SSCs and/or progenitor spermatogonia cells (Fig. 2A). Analysis of the number of MAGE-A4-positive cells per tubule section from donor 3 showed comparable numbers of stained cells in the control and cryopreserved tissues (42 ± 15.44, 40.38 ± 9.13, 35.5 ± 11.47, 61 ± 26.59 in the control, CSF, USF, and vitrification groups, respectively; P=.193). Quantitation of Oct4 staining showed 17.26 ± 6.96, 30.25 ± 7.29, 24.09 ± 7.84, 26.5 ± 13.14 stained cells in the control, CSF, USF, and vitrification groups, respectively. Statistical analysis showed a statistically significant difference between groups (P=.014). However, multiple comparisons between the different groups by post hoc pairwise Mann-Whitney U test (nonpaired) with Bonferroni corrections showed significantly higher numbers of Oct4 stained cells in the CSF group, compared with the control group (P=.003), but comparable numbers of Oct4 stained cells in all three cryopreserved samples (Fig. 2B).
Figure 2.
Immunohistochemical analysis of control and cryopreserved adult human testicular tissues from donor 3. The control and thawed testicular tissues sections are cryopreserved by uncontrolled slow freezing (USF), controlled slow freezing (CSF), and vitrification, stained for MAGE-A4, Oct4, and vimentin. Bar = 100 μm (A). Boxplots displaying the distribution of positively stained cell numbers per tubule section in control and cryopreserved testicular tissues. P values were determined by the Kruskal-Wallis H test (nonpaired). For Oct4, P values in multiple comparisons were determined by post hoc pairwise Mann-Whitney U test (nonpaired) with Bonferroni corrections (B). n = number of analyzed tubule sections.
Sertoli cells are one of the major components of the testicular niche and play a vital role in spermatogenesis (30). Positively stained cells for vimentin, a marker of Sertoli cells, were observed in the control and cryopreserved samples (Fig. 2A, lower panel). Analysis of the number of vimentin-positive cells per tubule section from donor 3 showed comparable numbers of stained cells in the control and cryopreserved tissues (33 ± 12.35, 32.36 ± 9.36, 43.09 ± 13.3, 41.86 ± 14.14 in the control, CSF, USF, and vitrification groups, respectively (P=.198) (Fig. 2B).
Taken together, the histological and IHC analyses demonstrated that all three cryopreservation methods similarly preserved the structural integrity and cellular morphology of the testicular tissue, as well as the survival of both SSCs and Sertoli cells.
Cryopreservation of Prepubertal Human Testicular Tissues by CSF
We next proceeded to analyze the effect of cryopreservation on prepubertal human tissues using the CSF protocol. This methodology was chosen because its cooling process is machinery controlled and therefore may be less affected by variability between operators, and is therefore more suitable for an experimental clinical application. Though this protocol was used for the cryopreservation of adult human testicular tissues (13), its efficacy with prepubertal human tissues has not been reported.
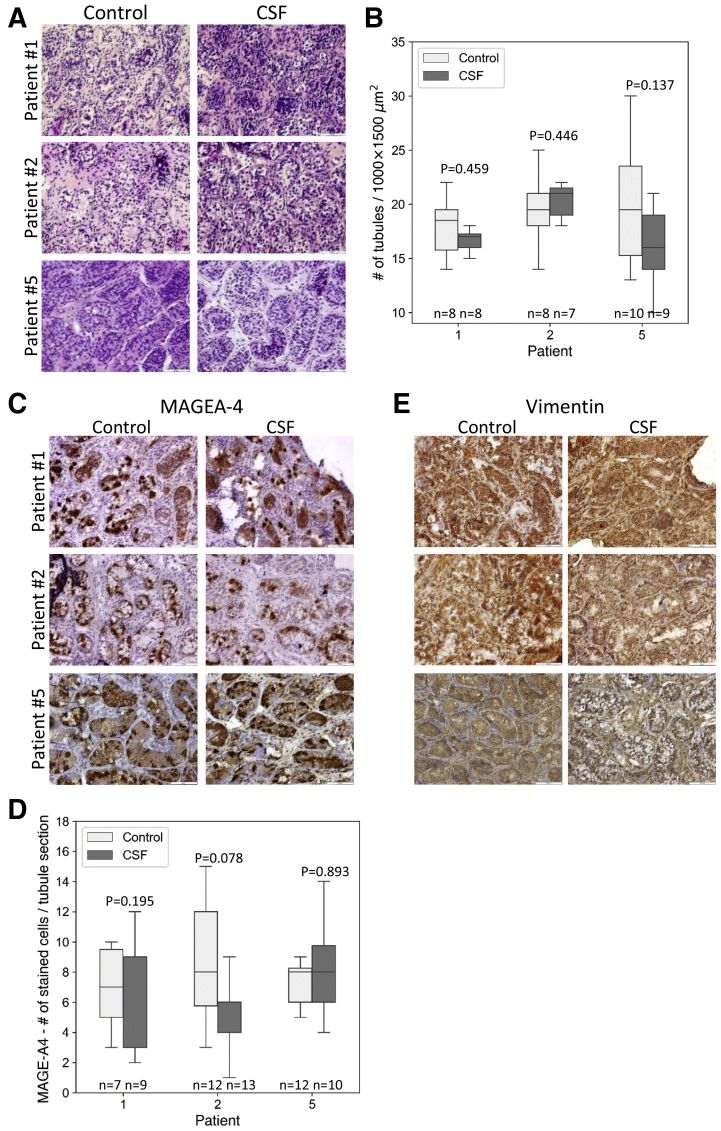
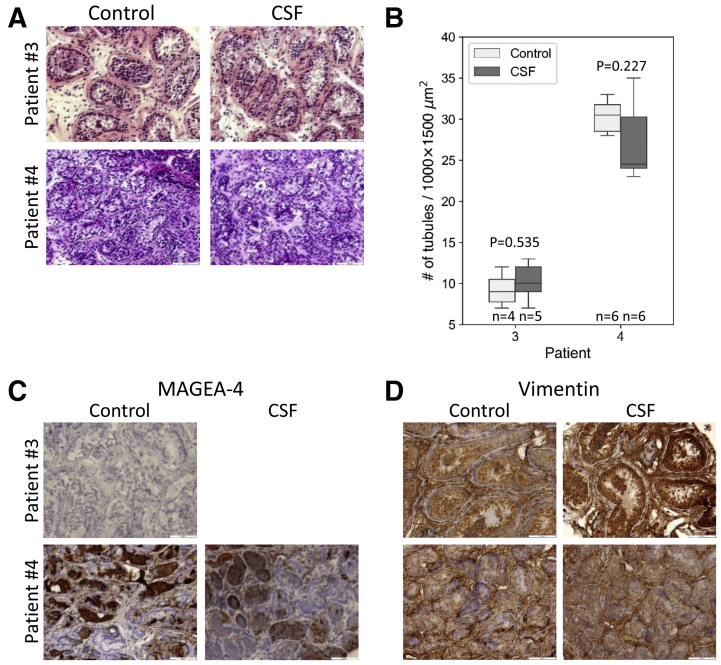
Five prepubertal patients with cancer were enrolled in the study. Two of them were previously treated with gonadotoxic therapies (Supplemental Table 2). After testicular biopsies and cryopreservation by CSF, we analyzed the postthawed tissues and compared them with control tissues. Hematoxylin and eosin staining of the testicular tissues from the three patients with cancer that did not receive previous treatments (patients 1, 2, and 5) demonstrated an intact architecture of the control and cryopreserved tissues, including the tubule structure and tubular niche. Spermatogonial stem cells/spermatogonia were observed in proximity to the basement membranes in all testicular tissues. As expected, mature spermatozoa were absent, indicating inactive spermatogenesis (Fig. 3A). Quantitative analysis of tubule numbers per 1,000 × 1,500 μm2 showed similar tubule numbers in the control and CSF-cryopreserved tissues (18 ± 2.8 in control vs. 17 ± 1.5 in CSF of patient 1, 19.25 ± 3.5 in control vs. 20.29 ± 1.7 in CSF of patient 2, and 20.2 ± 6.1 in control vs. 16 ± 3.5 in CSF of patient 5). Statistical analysis showed no significant differences between the control and CSF-cryopreserved groups (P values of .459, .446, and .137 for patients 1, 2, and 5, respectively) (Fig. 3B). Immunostaining for MAGE-A4 detected positively stained cells located mostly at the basement membranes, in both control and cryopreserved tissues, and the numbers of MAGE-A4 positive cells per tubule section were comparable in the control and cryopreserved samples (7 ± 2.8 in control vs. 5.33 ± 3.6 in CSF of patient 1, 8.5 ± 3.8 in control vs. 5.85 ± 2.8 in CSF of patient 2, and 8.25 ± 3.6 in control vs. 8.0 ± 3.2 in CSF of patient 5). Statistical analysis showed no significant differences between the control and CSF-cryopreserved groups (P values of .195, .078, and .893 for patients 1, 2, and 5, respectively) (Fig. 3C and D). Vimentin staining showed strong positive staining that could not be quantified in the control and cryopreserved samples (Fig. 3E). Analysis of the testicular tissues from the two patients with cancer with previous gonadotoxic treatments (patients 3 and 4) demonstrated preservation of intact architecture of the testicular niche after CSF, as well as similar numbers of tubules per 1,000 × 1,500 μm2 between control and CSF-cryopreserved tissues (9.25 ± 2.2 in control vs. 10.2 ± 2.4 in CSF of patient 3, and 30.33 ± 2.1 in control vs. 27.17 ± 5.0 in CSF of patient 4). Statistical analysis showed no significant differences between the control and CSF-cryopreserved groups (P values of .535 and .227 for patients 3 and 4, respectively) (Fig. 4A and 4B). Notably, both H&E and MAGE-A4 staining did not detect SSC/spermatogonia in tubular sections from patient 3, already in the control sample, indicating gonadotoxicity of previous treatments (platinum-based chemotherapy and radiation of the brain and the spine) (Fig. 4A and C). MAGE-A4 staining of the tubule sections from patient 4 showed staining in 82.3% ± 1.2% of the tubules in the control sample, and 65.7% ± 14.3% in the cryopreserved samples (P=.114), suggesting that previous treatments (chemotherapy without platinum [Adriamycin, Bleomycin, Vinblastin, and Dacarbazine]) were less gonadotoxic in this patient (Fig. 4C). In line with the preservation of the integrity of the testicular niche, vimentin-positive cells were similarly observed in the control and cryopreserved samples (Fig. 4D).
Figure 3.
Histological and immunohistochemical analysis of control and controlled slow freezing (CSF)-cryopreserved prepubertal human testicular tissues from patients with primary cancer. Hematoxylin-eosin stained sections of control and frozen-thawed testicular tissues cryopreserved by CSF from patients 1, 2, and 5. Bar = 100 μm (A). Boxplots displaying the distribution of intact tubules numbers (per 1,000 × 1,500 μm, ×200 magnification, in control and CSF-cryopreserved testicular tissues. P values were determined by the Mann-Whitney U test (nonpaired). (B). MAGE-A4 stained sections of control and CSF-cryopreserved testicular tissues. Bar = 100 μm (C). Boxplots displaying the distribution of MAGE-A4 positively stained cell numbers per tubule section, control, and CSF-cryopreserved testicular tissues (D) vimentin stained sections of control, and CSF-cryopreserved testicular tissues. Bar = 100 μm P values were determined by the Mann-Whitney U test (nonpaired).
Figure 4.
Histological and immunohistochemical analysis of control and controlled slow freezing (CSF)-cryopreserved prepubertal human testicular tissues from patients with recurrent cancer. Hematoxylin-eosin stained sections of control and frozen-thawed testicular tissues cryopreserved by CSF from patients 3 and 4. Bar = 100 μm (A). Boxplots displaying the distribution of intact tubules numbers (per 1,000 × 1,500 μm, ×200 magnification, in control and CSF-cryopreserved testicular tissues. P values were determined by the Mann-Whitney U test (nonpaired) (B). MAGE-A4. Because MAGE-4 positive cells were not demonstrated in fresh testicular tissues of patient 3 (control), immunohistochemical analysis post-thawing was not performed. (C) and vimentin (D) stained sections of control and frozen-thawed testicular tissues. Bar = 100 μm (C–D).
Taken together, the histological and IHC analyses showed that CSF preserved the structural integrity of the prepubertal testicular tissue, as well as the survival of both SSCs and Sertoli cells. They also emphasized the importance of cryopreservation of the prepubertal testicular tissue before gonadotoxic treatments.
Discussion
The objective of the present study was to develop an efficient clinical-grade protocol for cryopreservation of prepubertal human testicular tissues. Given the ethical restrictions and limited availability of human prepubertal testicular tissue, we first analyzed the efficiency of USF, CSF, and vitrification for cryopreservation of adult testicular tissues. Various slow freezing protocols for cryopreservation of adult (12, 13) and prepubertal (12, 16, 17, 18, 19) human testicular tissues were previously reported. The open pulled-straw vitrification method was reported as an alternative preservation technique of prepubertal human testicular tissues, with the potential to minimize freezing injuries caused by ice crystal formation (20, 21). The protocols we used were adapted from Baert et al. (13) and optimized for clinical use. Evaluation of the effects of both slow freezing and vitrification on adult testicular tissues showed that all three methods preserved the integrity and architecture of the testicular niche, including survival of SSCs and their supporting Sertoli cells. We observed a significantly higher number of Oct4-positive cells per tubule section in the CSF compared with the control group. The IHC staining of Oct4 after CSF was brighter than that of the controls, probably because of an obscure technical issue, and we assume that this was the reason for the higher quantification in this subgroup. The comparable quantification of MAGE-A4 expressing cells per tubule section between the various cryopreservation methods and control groups supports this assumption. Further assessment of the testicular tissue function after slow freezing vs. vitrification is necessary to determine the preferable cryopreservation method that may provide functional advantages for future fertility restoration in prepubertal boys undergoing gonadotoxic treatment.
We sought to further study the effect of CSF cryopreservation on prepubertal human testicular tissues because it might be less affected by the variability between operators and thus more suitable for an experimental clinical application. Of note, while this approach was previously used to cryopreserve adult human testicular tissues (13), its efficacy for the cryopreservation of prepubertal tissues was not evaluated. Our findings demonstrated that CSF was an efficient method for cryopreservation of prepubertal human testicular tissues. Supporting our results, a different CSF-based protocol is currently employed by several academic centers in the course of a clinical experimental testicular tissue cryopreservation trial for prepubertal cancer-affected boys (22). It should be noted that the function of CSF-cryopreserved prepubertal testicular tissues has not yet been reported in humans; however, it was recently demonstrated to preserve testicular function in nonhuman primates (10).
The present study included testicular tissue samples from two pediatric patients who previously received gonadotoxic treatments. The tubules of patient 3, who received chemotherapy with alkylating agents and radiation, did not contain SSC/spermatogonia, as shown by H&E and MAGE-A4 immunostaining. In patient 4, who received chemotherapy without alkylating agents, MAGE-A4 expression was observed in a proportion of the tubules, indicating a less severe effect on SSC/spermatogonia. These results are in line with previously reported data, demonstrating the toxicity of cancer treatments to the SSC and spermatogonia pool (4, 5, 31, 32, 33). In contrast, staining for vimentin showed a similar pattern in all control and cryopreserved samples from primary- and recurrent- cancer-affected boys, indicating that the testicular niche is less sensitive to gonadotoxic treatment. These results demonstrate the gonadotoxicity of the cancer treatments, especially to the SSCs, and emphasize the need to cryopreserve testicular tissues before gonadotoxic therapies, which may irreversibly affect the SSC pool, impeding future fertility.
A limitation of our study was the small number of samples. As fertility preservation for prepubertal boys will be practiced more commonly, additional studies with larger sample sizes may be performed. Additionally, the functionality of the thawed testicular tissue after CSF should be evaluated in future studies.
Conclusion
Our study presents a CSF-based clinical-grade protocol for cryopreservation of adult and prepubertal human testicular tissues. The presented protocol preserved the integrity and architecture of the testicular niche, including the survival of SSCs and their supporting Sertoli cells. Our results may contribute to more effective banking of prepubertal testicular tissues and potential fertility restoration in the future.
Acknowledgments
The authors thank the adult and prepubertal patients who participated in this study. We thank Sidney and Judy Swartz and the Gassner Fund for Medical Research for their generous donation that enabled the study.
Footnotes
D.K. and M.S. should be considered similar in author order.
D.K. has nothing to disclose. M.S. has nothing to disclose. M.G. has nothing to disclose. G.H. has nothing to disclose. T.M.D. has nothing to disclose. K.M. has nothing to disclose. A.R. has nothing to disclose. T.I. has nothing to disclose. B.R. has nothing to disclose.
Supplementary data
References
- 1.Stiller C.A., Desandes E., Danon S.E., Izarzugaza I., Ratiu A., Vassileva-Valerianova Z., et al. Cancer incidence and survival in European adolescents (1978-1997). Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2006–2018. doi: 10.1016/j.ejca.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Youlden D.R., Baade P.D., Valery P.C., Ward L.J., Green A.C., Aitken J.F. Differentials in survival for childhood cancer in Australia by remoteness of residence and area disadvantage. Cancer Epidemiol Biomarkers Prev. 2011;20:1649–1656. doi: 10.1158/1055-9965.EPI-11-0432. [DOI] [PubMed] [Google Scholar]
- 3.Stevens M., Frobisher C., Hawkins M., Jenney M., Lancashire E., Reulen R., et al. The British Childhood Cancer Survivor Study: objectives, methods, population structure, response rates and initial descriptive information. Pediatr Blood Cancer. 2008;50:1018–1025. doi: 10.1002/pbc.21335. [DOI] [PubMed] [Google Scholar]
- 4.Green D.M., Kawashima T., Stovall M., Leisenring W., Sklar C.A., Mertens A.C., et al. Fertility of male survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2010;28:332–339. doi: 10.1200/JCO.2009.24.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meistrich M.L. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013;100:1180–1186. doi: 10.1016/j.fertnstert.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picton H.M., Wyns C., Anderson R.A., Goossens E., Jahnukainen K., Kliesch S., et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod. 2015;30:2463–2475. doi: 10.1093/humrep/dev190. [DOI] [PubMed] [Google Scholar]
- 7.Aslam I., Fishel S., Moore H., Dowell K., Thornton S. Fertility preservation of boys undergoing anti-cancer therapy: a review of the existing situation and prospects for the future: opinion. Hum Reprod. 2000;15:2154–2159. doi: 10.1093/humrep/15.10.2154. [DOI] [PubMed] [Google Scholar]
- 8.Shinohara T. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod. 2002;17:3039–3045. doi: 10.1093/humrep/17.12.3039. [DOI] [PubMed] [Google Scholar]
- 9.Snedaker A.K., Honaramooz A., Dobrinski I. A game of cat and mouse: xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J Androl. 2004;25:926–930. doi: 10.1002/j.1939-4640.2004.tb03163.x. [DOI] [PubMed] [Google Scholar]
- 10.Fayomi A.P., Peters K., Sukhwani M., Valli-Pulaski H., Shetty G., Meistrich M.L., et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science. 2019;363:1314–1319. doi: 10.1126/science.aav2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goossens E., Jahnukainen K., Mitchell R.T., van Pelt A.M.M., Pennings G., Rives N., et al. Fertility preservation in boys: recent developments and new insights. Hum Reprod Open. 2020;2020 doi: 10.1093/hropen/hoaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keros V., Rosenlund B., Hultenby K., Aghajanova L., Levkov L., Hovatta O. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod. 2005;20:1676–1687. doi: 10.1093/humrep/deh797. [DOI] [PubMed] [Google Scholar]
- 13.Baert Y., Van Saen D., Haentjens P., In’t Veld P., Tournaye H., Goossens E. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod. 2013:1816–1826. doi: 10.1093/humrep/det100. [DOI] [PubMed] [Google Scholar]
- 14.Moraveji S., Esfandiari F., Sharbatoghli M., Taleahmad S., Nikeghbalian S., Shahverdi A., et al. Optimizing methods for human testicular tissue cryopreservation and spermatogonial stem cell isolation. J Cell Biochem. 2019;120:613–621. doi: 10.1002/jcb.27419. [DOI] [PubMed] [Google Scholar]
- 15.Onofre J., Baert Y., Faes K., Goossens E. Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum Reprod Update. 2016;22:744–761. doi: 10.1093/humupd/dmw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyns C., Van Langendonckt A., Wese F.X., Donnez J., Curaba M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod. 2008;23:2402–2414. doi: 10.1093/humrep/den272. [DOI] [PubMed] [Google Scholar]
- 17.Keros V., Hultenby K., Borgström B., Fridström M., Jahnukainen K., Hovatta O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22:1384–1395. doi: 10.1093/humrep/del508. [DOI] [PubMed] [Google Scholar]
- 18.Kvist K., Thorup J., Byskov A.G., Høyer P.E., Møllgård K., Yding Andersen C. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Hum Reprod. 2006;21:484–491. doi: 10.1093/humrep/dei331. [DOI] [PubMed] [Google Scholar]
- 19.Wyns C., Curaba M., Martinez-Madrid B., Van Langendonckt A., François-Xavier W., Donnez J. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Hum Reprod. 2007;22:1603–1611. doi: 10.1093/humrep/dem062. [DOI] [PubMed] [Google Scholar]
- 20.Curaba M., Poels J., Van Langendonckt A., Donnez J., Wyns C. Can prepubertal human testicular tissue be cryopreserved by vitrification? Fertil Steril. 2011;95:2123.e9–2123.e12. doi: 10.1016/j.fertnstert.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Poels J., Van Langendonckt A., Many M.C., Wese F.X., Wyns C. Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod. 2013;28:578–589. doi: 10.1093/humrep/des455. [DOI] [PubMed] [Google Scholar]
- 22.Valli-Pulaski H., Peters K.A., Gassei K., Steimer S.R., Sukhwani M., Hermann B.P., et al. Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers. Hum Reprod. 2019;34:966–977. doi: 10.1093/humrep/dez043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braye A., Tournaye H., Goossens E. Setting up a cryopreservation programme for immature testicular tissue: lessons learned after more than 15 years of experience. Clin Med Insights Reprod Health. 2019;13 doi: 10.1177/1179558119886342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baert Y., Goossens E., Van Saen D., Ning L., In’T Veld P., Tournaye H. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertil Steril. 2012;97:1152–1157.e2. doi: 10.1016/j.fertnstert.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Fayomi A.P., Orwig K.E. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018;29:207–214. doi: 10.1016/j.scr.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tournaye H., Dohle G.R., Barratt C.L.R. Fertility preservation in men with cancer. Lancet. 2014;384:1295–1301. doi: 10.1016/S0140-6736(14)60495-5. [DOI] [PubMed] [Google Scholar]
- 27.He Z., Kokkinaki M., Jiang J., Dobrinski I., Dym M. Isolation, characterization, and culture of human spermatogonia1. Biol Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 29.Dann C.T., Alvarado A.L., Molyneux L.A., Denard B.S., Garbers D.L., Porteus M.H. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–2937. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- 30.Oatley J.M., Brinster R.L. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92:577–595. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson M.M. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol. 2010;116:1171–1183. doi: 10.1097/AOG.0b013e3181f87c4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kliesch S., Behre H.M., Jürgens H., Nieschlag E. Cryopreservation of semen from adolescent patients with malignancies. Med Pediatr Oncol. 1996;26:20–27. doi: 10.1002/(SICI)1096-911X(199601)26:1<20::AID-MPO3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Jahnukainen K., Mitchell R.T., Stukenborg J.B. Testicular function and fertility preservation after treatment for haematological cancer. Curr Opin Endocrinol Diabetes Obes. 2015;22:217–223. doi: 10.1097/MED.0000000000000156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.