Abstract
Objectives:
To determine which surgical factors are associated with quality-of-life (QOL) outcomes in oral cavity cancer survivors after free flap reconstruction of the oral cavity.
Patients and Methods:
A cross-sectional study was conducted from a multidisciplinary head and neck cancer (HNC) survivorship clinic. Oral cavity cancer survivors with at least 6-months of postoperative follow-up from ablation and free flap reconstruction were included. Primary outcome measures were validated patient-reported outcome measures (PROMs) including the Eating Assessment Tool-10 (EAT-10) measure of swallowing-specific QOL, University of Washington Quality of Life (UW-QOL) physical and social-emotional subscale scores and feeding tube dependence.
Results:
Extent of tongue resection was associated with EAT-10 and the UW-QOL Physical subscale scores. Patients with oral tongue defects reported worse scores than with composite defects in the EAT-10 and UW-QOL physical domain (p = 0.0004, 0.0025, respectively). This association no longer applies when controlling for differences in extent of tongue resection. Patients with anterior composite resections reported worse EAT-10 scores than lateral resections (p = 0.024). This association no longer applies when controlling for extent of tongue resection (p = 0.46). Gastric tube dependence demonstrates similar trends to PROMs.
Conclusion:
Extent of tongue resection was strongly associated with poor QOL outcomes after free tissue reconstruction of the oral cavity and mediates the associations between other defect characteristics and QOL. These findings demonstrate the need for emphasis on expected oral tongue defects when counseling patients and highlight the need to address QOL in a multidisciplinary fashion post-operatively.
Keywords: Head and neck cancer, Survivorship, Oral cavity, Free flap, Quality-of-life
Introduction
A recent focus on head and neck cancer (HNC) survivorship has been prompted in part by limited understanding of the long-term toxicities that affect quality-of-life (QOL) after treatment. Within the reconstructed oral cavity, there are conflicting data as to how defect and reconstructive characteristics influence QOL outcomes. While primary closure and local reconstruction are options for smaller defects, more advanced malignancies require free flap reconstruction. The use of free tissue transfer has widely expanded the reconstructive possibilities in HNC patients. There is a substantial body of literature describing functional outcomes after HNC treatment. However, the factors that influence functional outcomes in the unique population of patients who undergo free flap reconstruction are not fully understood. With complex extirpative defects involving multiple oral cavity subsites, such patients are at risk for significant treatment sequelae [1-3]. Advanced disease stage often necessitates larger ablative defects requiring free tissue transfer, and increases the likelihood of adjuvant radiation and possibly chemotherapy, which have been shown to worsen QOL [3,4].
Treatment toxicities span multiple domains including swallowing, chewing, speech, taste, salivation, pain, neck and shoulder disability, appearance, mental health, and finances. Prior studies have shown significant toxicity in all these domains [2,3,5-8]. Most QOL metrics tend to improve over time, except for swallowing and neck disability which improve initially and then may worsen steadily several years later in patients who undergo adjuvant treatment [3,5,9]. In order to better understand treatment toxicities and QOL outcomes, numerous patient-reported outcome measures (PROMs) have been developed or adopted from other fields and validated in HNC patients [5,10-17]. The PROMs provide important insight into patient experience in domains that are otherwise difficult to study in a systematic manner.
As we refine microsurgical techniques for oral cavity reconstruction, it has become more important to understand the factors that influence QOL outcomes after treatment [18,19]. Oral cavity cancer has variable involvement of the oral tongue, mandible, and extension into the oropharynx. These factors affect extirpative and reconstructive needs and may influence post-treatment QOL in oral cavity cancer survivors. Conflicting data exist as to how reconstructive modality and defect characteristics affect QOL [1,2,4,9,20-24]. This study aims to determine which surgical factors are associated with QOL outcomes in oral cavity cancer survivors after free flap reconstruction of the oral cavity.
Patients and methods
This study was conducted under a research registry approved by the University of Pittsburgh Human Research Protection Office Institutional Review Board (PRO1303037), which allows for collation and stratification of clinical data. PROMs were administered to all patients presenting to the UPMC HNC Survivorship clinic as part of their clinical evaluation per our standard of care. In addition to patient history and examination, review of PROMs helps dictate each survivor’s need for speech-language pathology, physical therapy, dental, nutrition, audiology and mental health services.
Design
We performed a retrospective cross-sectional study of PROMs in HNC survivors seen in a multidisciplinary HNC survivorship clinic between December 2016 and September 2020 after undergoing oral cavity free flap reconstruction. Inclusion criteria were: 1) age 18 years or older; 2) prior oral cavity resection and free flap reconstruction. Survivors were excluded in cases of distant metastases, active locoregional disease, missing surgical or treatment details (such as those treated in an outside hospital), or if they had<6 months of post-operative survivorship follow-up. Patients who experienced post-operative flap failure and revision with primary closure or locoregional flap were also excluded. In addition to PROMs, we collected demographic and clinical data from the electronic medical record including: sex, marital status, race, age at the time of surgery, histology, staging (American Joint Committee on Cancer), treatment modalities, time since treatment completion, whether free flap reconstruction was used for primary disease, recurrence or second primary, free flap donor site. Defect characteristics were also collected including the presence of a composite or isolated oral tongue defect, oropharyngeal component, and the extent of tongue resection. For composite resection defects, anterior or lateral site was defined by inclusion of the mandibular symphysis in mandibulectomy.
Patient-reported outcomes
A single survey containing numerous PROMs is completed by survivors during each survivorship clinic visit as part of the standard clinical evaluation. Validated PROMs are used to collect data on primary outcomes of swallowing function, neck disability, physical and social-emotional QOL, depression and anxiety. PROM surveys included the Eating Assessment Tool-10 (EAT-10), the Functional Oral Intake Scale (FOIS), University of Washington Quality of Life (UW-QOL) physical and social-emotional domains, the Neck Disability Index (NDI), the Patient Health Questionnaire (PHQ-2) depression screen, and the General Anxiety Disorder questionnaire (GAD-2) screen.
Given the numerous available PROMs and potential overlap of content between measures, a Spearman correlation coefficient matrix was used to select a single PROM from highly correlated pairs of PROMs for subsequent analysis (see Supplemental Materials). This served to limit the number of endpoints and prevent analysis of redundant measures. The EAT-10 and UW-QOL physical and social-emotional domains segregated into distinct clusters and were selected, while the NDI, PHQ-2, GAD-2 were highly correlated with selected PROMs and were excluded from further analysis. Gastric tube dependence was described as an objective correlate to PROMs.
Swallowing function was measured using the EAT-10, a 10-item subjective dysphagia questionnaire that has been validated in HNC patients [11]. A score of 2 or less is normal. Higher scores indicate a higher dysphagia burden (maximum score of 40). Physical and social-emotional quality of life were measured using the 16-item University of Washington Quality of Life (UW-QOL version 4) which has been validated as a QOL instrument in HNC patients [10]. Higher scores indicate better QOL and the score range is from 0 to 100, with a minimal clinically important difference (MCID) of 7 points [25]. The UW-QOL physical subscale score is based on the domains of chewing, swallowing, speech, taste, salivation, and appearance. The social-emotional subscale includes domains of anxiety, mood, pain, activity, recreation.
Statistics
Differences among patient groups were tested with the Wilcoxon test for two groups and the Kruskal-Wallis test for three or more groups. Standard univariate and multivariate regression analysis were used to categorize effects of surgical and clinical variables and demographic variables upon PROMS. Individual tests of association among surgical and clinical factors with PROMs were first individually assessed before regression model building. Final models were checked for distribution of the residuals and non-linearity; interaction between pairs of independent variables was examined and ruled out. The categorical variable, gastric tube dependence, was tested with Fisher’s exact test.
Results
Clinical and surgical patient characteristics
Patient clinical and surgical characteristics are summarized in Table 1. A total of 80 patients (47 men, 33 women) with a median age of 60 years at the time of surgery met inclusion criteria (interquartile range [IQR] = 53–68). Survivors were seen at a median of 36 months from surgery (IQR = 16–85 months, range = 6 months to 17 years). Most included patients had advanced disease. Of the patients who underwent fibula free flap reconstruction, 3 patients underwent simultaneous anterolateral thigh for reconstruction of an external soft tissue defect.
Table 1.
Patient details.
| Patient details | N (%) |
|---|---|
| Age at Surgery | |
| Median | 60 |
| IQR | 53–68 |
| Range | 29 – 84 |
| Histology | |
| SCC | 75 (94%) |
| Adenoid Cystic | 4 (5%) |
| Ameloblastoma | 1 (1%) |
| AJCC Stage (1) | |
| I | 11 (14%) |
| II | 10 (13%) |
| III | 13 (17%) |
| IVa | 41 (54%) |
| IVb | 1 (1%) |
| Additional Treatment | |
| None | 17 (21%) |
| Radiation | 30 (38%) |
| Chemoradiation | 33 (41%) |
| Treatment for recurrence | 14 (18%) |
| Treatment for second primary | 15 (19%) |
| Free Flap | |
| ALT | 18 (22%) |
| Fibula | 36 (45%) |
| Radial Forearm | 18 (22%) |
| Other | 8 (10%) |
| Second Simultaneous Flap | 3 (4%) |
| Composite Resection | 56 (70%) |
| Oral Tongue Resection | 24 (30%) |
| Composite Site | |
| Lateral | 38 (68%) |
| Anterior | 18 (32%) |
| Oropharyngeal Component | 28 (35%) |
| Extent of Tongue Resection | |
| None | 29 (36%) |
| < 1/3 | 14 (18%) |
| 1/3 – 1/2 | 29 (36%) |
| > 1/2 | 8 (10%) |
| Mandibulectomy | |
| Segmental | 45 of 56 (80%) |
| Marginal | 11 of 56 (20%) |
Four patients were missing AJCC Stage.
Regarding defect characteristics, 24 (30%) patients underwent oral tongue resection, while 56 (70%) patients underwent composite resection with mandibulectomy, of which 18 contained an anterior component. Of all composite defects, 18 (32%) were anterior, 38 (68%) were lateral resections. Among patients with composite resections, 29 (52%) underwent no tongue resection, 14 (25%) underwent resection of<1/3 of the oral tongue, 11 (20%) underwent resection of 1/3 to ½ of the oral tongue, and 2 (4%) underwent resection of more than ½ of the oral tongue. Among patients with oral tongue resections, none underwent resection of<1/3 of the oral tongue, 18 (75%) underwent resection of 1/3 to ½ of the oral tongue and 6 (25%) underwent resection of more than ½ of the oral tongue. Of 24 patients with oral tongue resections, 12 (50%) were gastric tube dependent. Of these, 6 (25%) were totally gastric tube dependent. Of 56 patients with composite resections, 7 (12.5%) were gastric tube dependent. Of these, 4 (7%) were totally gastric tube dependent.
Clinical factors and PROMs
Scores and associations between clinical factors and PROMs are listed in Table 2. Sex was the only demographic or clinical factor associated with PROM scores (Table 2). Men reported better QOL on the UW-QOL Physical domain and Social-emotional domain than women patients (p = 0.0076; p = 0.003). Patients who underwent adjuvant treatment had worse scores on the UW-QOL Physical domain (p = 0.04), compared to patients not undergoing adjuvant therapy, but no difference on the UW-QOL social-emotional domain (p = 0.18). PROMS were examined for influence of elapsed time between surgery and the clinic visit and no time-dependent patterns were observed.
Table 2.
Associations between clinical details and PROMs.
| PROM | Clinical detail | Median Score (IQR) |
P value |
|---|---|---|---|
| UW-QOL | Male | 71 (61 – 82) | p = 0.0076 |
| Physical | Female | 59 (45 – 69) | |
| Surgery alone | 72 (63 – 82) | p = 0.0401 | |
| Surgery + RT | 61 (45 – 85) | ||
| Surgery + CRT | 63 (48 – 71) | ||
| Stage I | 71 (46 – 88) | p = 0.9005 | |
| Stage II | 68 (64 – 74) | ||
| Stage III | 61 (57 – 72) | ||
| Stage IV | 67 (47 – 78) | ||
| Primary disease | 67 (52 – 78) | p = 0.835 | |
| Recurrence or 2nd | 65 (54 – 73) | ||
| primary | |||
| UW-QOL Social | Male | 82 (70 – 91) | p = 0.003 |
| Female | 64 (61 – 82) | ||
| Surgery alone | 87 (65 – 92) | p = 0.18 | |
| Surgery + RT | 80 (64 – 88) | ||
| Surgery + CRT | 70 (62 – 82) | ||
| Stage I | 78 (66 – 96) | p = 0.8498 | |
| Stage II | 79 (64 – 82) | ||
| Stage III | 73 (61 – 87) | ||
| Stage IV | 72 (64 – 87) | ||
| Primary disease | 75 (63 – 87) | p = 0.8665 | |
| Recurrence or 2nd | 72 (63 – 92) | ||
| primary | |||
| EAT-10 | Male | 7 (2 – 18) | p = 0.0706 |
| Female | 16 (4 – 26) | ||
| Surgery alone | 6 (2 – 10) | p = 0.2837 | |
| Surgery + RT | 11 (2 – 27) | ||
| Surgery + CRT | 12 (4–23 | ||
| Stage I | 6 (2 – 22) | p = 0.3947 | |
| Stage II | 4 (2 – 21) | ||
| Stage III | 12 (0 – 27 | ||
| Stage IV | 10 (4 – 26) | ||
| Primary disease | 10 (2 – 21) | p = 0.3394 | |
| Recurrence or 2nd | 10 (4–25 | ||
| primary |
Defect characteristics and PROMs in all patients
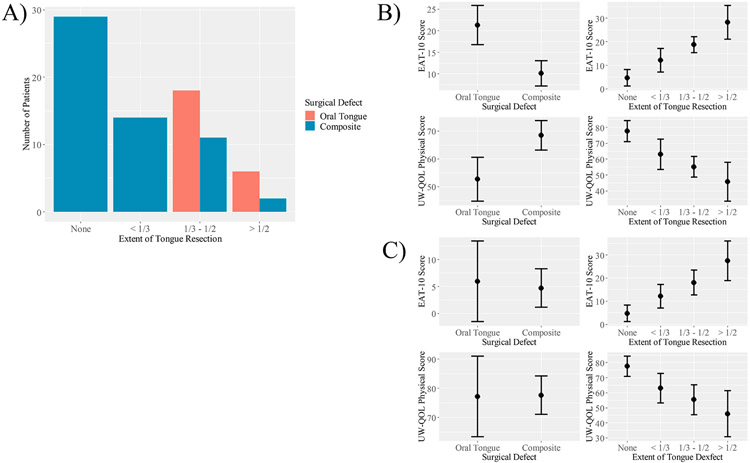
Scores and associations between defect characteristics and PROMs are listed in Table 3. Among all patients (composite and oral tongue defects), several defect characteristics were associated with PROM scores. Greater extent of tongue resection was associated with substantially worse EAT-10 (p < 0.0001) and UW-QOL Physical (p < 0.0001), but not UW-QOL Social-emotional (p = 0.2433) scores. Oral tongue defects were associated with worse scores on the EAT-10 (p = 0.0004) and UW-QOL Physical scale (p = 0.0025) than composite defects. Patients with isolated oral tongue defects underwent more extensive oral tongue resection than patients with composite resection defects. All patients in the oral tongue group underwent hemi- or subtotal glossectomy, while greater than half of composite resections lacked an oral tongue component (Figure 1). When controlling for the extent of tongue resection on EAT-10 and UW-QOL Physical score with a bivariate regression model, composite and oral tongue resections are no longer different (p = 0.71 for EAT-10; p = 0.94 for UW-QOL Physical; Figure 1). In contrast, extent of tongue resection retains a strong association with PROM scores when controlling for oral tongue or composite site (p < 0.001 for EAT-10 and UW-QOL Physical scores; Figure 1). This indicates that the association between composite or oral tongue resection and EAT-10 and UW-QOL Physical score is conditional on the extent of tongue resection.
Table 3.
Associations between defect characteristics and PROMs.
| PROM | Defect Characteristic | Median Score (IQR) |
P value | |
|---|---|---|---|---|
| UW-QOL | Oropharyngeal component | Yes | 72 (53 – 78) | p = 0.2723 |
| Physical | No | 63 (52 – 73) | ||
| Resection type | Oral tongue | 48 (36 – 70) | p = 0.0025 | |
| Composite | 68 (59 – 81) | |||
| Extent of tongue resection | None | 78 (64 – 92) | p < 0.0001 | |
| < 1/3 | 68 (57 – 76) | |||
| 1/3–1/2 | 61 (42 – 68) | |||
| > 1/2 | 40 (32 – 53) | |||
| UW-QOL | Oropharyngeal component | Yes | 83 (63 – 91) | p = 0.39 |
| Social | No | 70 (63 – 87) | ||
| Resection type | Oral tongue | 73 (62–83 | p = 0.3738 | |
| Composite | 77 (63 – 89) | |||
| Extent of tongue resection | None | 78 (65 – 88) | p = 0.2433 | |
| < 1/3 | 69 (62 – 81) | |||
| 1/3–1/2 | 82 (66 – 91) | |||
| > 1/2 | 66 (60 – 73) | |||
| EAT-10 | Oropharyngeal component | Yes | 4 (2 – 22) | p = 0.3850 |
| No | 10 (4 – 24) | |||
| Resection type | Oral tongue | 25 (10 – 32 | p = 0.0004 | |
| Composite | 10 (2 – 18) | |||
| Extent of tongue resection | None | 2 (1 – 7) | p < 0.0001 | |
| < 1/3 | 8 (4 – 16) | |||
| 1/3–1/2 | 19 (7 – 31) | |||
| > 1/2 | 28 (24 – 33) |
Figure 1.
The association between resection type and PROMs is conditional on the extent of tongue resection. A) Bar graph demonstrating differences in the extent of tongue resected between the composite and oral tongue groups. B) Univariate models demonstrating an association between PROMs and resection type or extent of tongue resection. C) Bivariate models demonstrating that the association between resection type and PROMs is conditionalon the extent of tongue resection.
Defect characteristics and PROMs in composite resection patients
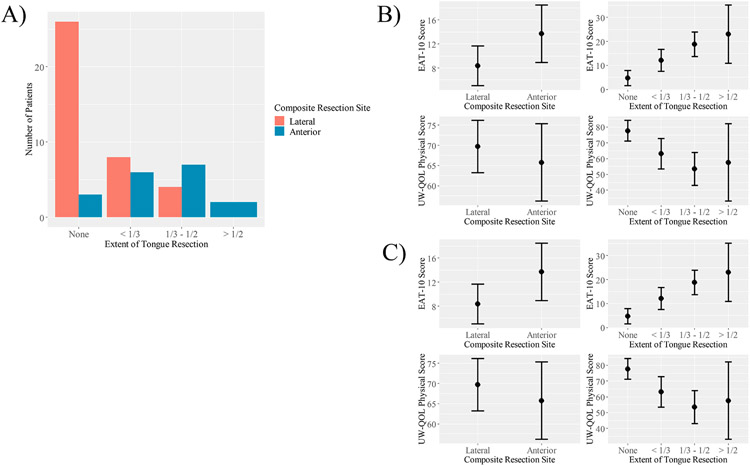
In the 56 patients who underwent composite resection with mandibulectomy, segmental and marginal mandibulectomy were not associated with differences in the UW-QOL Physical domain (p = 0.78), Social-emotional domain (p = 0.57), or EAT-10 (p = 0.82). Patients undergoing anterior composite resections reported worse EAT-10 scores than patients undergoing lateral composite resection (p = 0.024; Figure 2). UW-QOL physical and social-emotional scores were not different between the two groups. Patients with anterior composite defects underwent more extensive oral tongue resection than patients with lateral defects (Figure 2). Half of patients with anterior composite resections underwent hemi- or subtotal glossectomy, whereas only 4 of 38 (10.5%) patients with lateral resections underwent hemi- or subtotal glossectomy. When controlling for the extent of tongue resection on EAT-10 and UW-QOL Physical score with a bivariate regression model, anterior defects are no longer associated with worse EAT-10 scores than lateral composite defects (p = 0.46) and are associated with better UW-QOL Physical scores (p = 0.44; Figure 2). The extent of tongue resection retains a strong association with PROM scores when controlling for composite resection site (p < 0.001 for EAT-10 and UW-QOL Physical scores; Figure 2). This indicates that the association between composite resection site and EAT-10 and UW-QOL Physical scores is conditional on the extent of tongue resection. Patients with anterior composite resections and less extensive tongue defects did not have worse QOL outcomes than patients with lateral resections and similar tongue defects.
Figure 2.
The association between anterior defects and PROMs is conditional on the extent of tongue resection in patients who underwent composite resections. A) Bar graph demonstrating differences in the extent of tongue resected between patients with anterior and lateral composite defects. B) Univariate models demonstrating an association between PROMs and composite site or extent of tongue resection. C) Bivariate model demonstrating that the association between composite resection site and PROMs is conditional on the extent of tongue resection.
Defect characteristics and gastric tube dependence
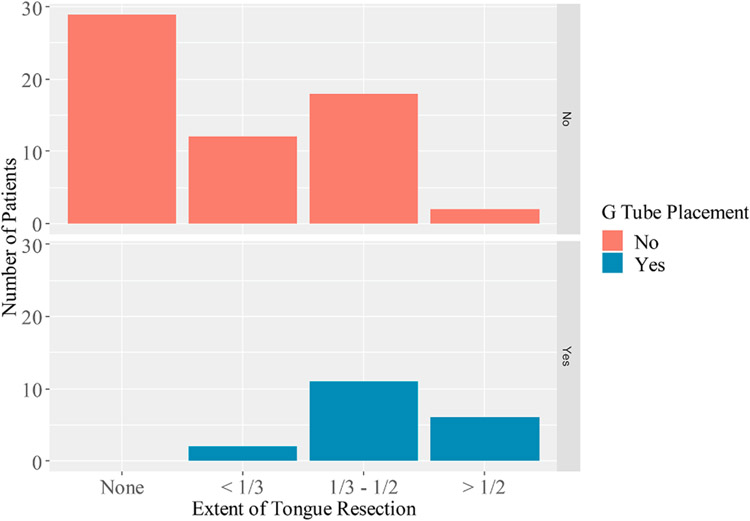
Gastric tube dependence was examined as an objective correlate to PROMs (Figure 3). Like differences identified in EAT-10 and UW-QOL scores, patients with more extensive tongue resection were more likely to be gastric tube dependent (Figure 3). Of 19 cases of gastric tube dependence, all had an oral tongue defect component (p = 0.0001).
Figure 3.
Bar graph demonstrating that gastric tube dependence demonstrates a similar trend to PROMs. The proportion of patients with gastric tube dependence increases with extent of tongue resection.
Discussion
In this cross-sectional study, we describe clinical and surgical factors that are associated with post-treatment QOL outcomes in patients who underwent free flap reconstruction of the oral cavity. There have been conflicting data as to how reconstructive modality influences long-term QOL. Several studies have described an association with worsened QOL and the use of free flap reconstruction [1,20]. However, it is thought that this difference may be due to lack of controlling for likely confounders such as stage, defect characteristics, and the need for adjuvant therapy [2]. This is supported by other studies demonstrating superior or equivalent QOL outcomes with free flap reconstruction compared to other reconstructive techniques in patients with similar defect characteristics [21,22]. For this reason, we examined only patients who underwent free flap reconstruction. Only 3 patients underwent reconstruction with two simultaneous free-flaps, but other studies have demonstrated that this population is at risk of poor QOL outcomes [26]. Our sample consisted of patients with mostly locally advanced disease, with a high proportion of patients that underwent adjuvant treatment and are thus at risk for poor QOL outcomes [1,3].
Most notably, EAT-10 and UW-QOL physical domain scores were strongly associated with extent of oral tongue resection. This was true among all patients with oral cavity cancer and among patients undergoing composite resection of the mandible. Patients with isolated oral tongue defects reported lower QOL than those with composite defects. This difference appears to be mediated by less extensive oral tongue involvement in composite resections compared to oral tongue defects (Figure 1). All patients in the oral tongue group underwent hemi- or subtotal glossectomy, while greater than half of composite resections lacked an oral tongue component. This data may seem to conflict with prior reports describing better outcomes in isolated tongue defects [9]. This may be due to baseline differences in the disease stage and treatment. Yan YB et al. examined all patients with primary tongue malignancies, including small primary lesions that did not require free flap reconstruction [9]. Other studies have shown that patients with limited tongue resections can expect near-baseline QOL in the long-term [27]. In contrast, our study compares patients with locally advanced disease requiring free flap reconstruction. This difference in results underscores the importance of studying outcomes in the unique population of HNC survivors who have undergone free flap reconstruction. In this setting, it appears that having a primary malignancy of the tongue portends worse QOL outcomes. This is in line with other studies that have demonstrated that extensive extirpative defects portend worse outcomes in most QOL domains [28,29]. Poor QOL outcomes in this group demonstrate that replacing dynamic tongue with static soft tissue is insufficient to maintain QOL. As an objective correlate, gastric tube dependence demonstrates similar trends to PROMs. Gastric tube dependence was far more likely with more extensive oral tongue resection.
Like prior reports, anterior composite resection was associated with worse swallowing-specific QOL scores than lateral resections [23]. Physical QOL (measured by the UW-QOL Physical domain) was similar in the two groups. However, when controlling for differences in the extent of tongue resected, EAT-10 scores were similar and UW-QOL Physical scores were better in the anterior composite resection group (Figure 2). This suggests that the extent of tongue resected mediates the worse QOL outcomes associated with anterior composite resections. These data have significant implications for patient counseling and setting QOL expectations, especially in the less common settings of lateral composite resections with a large oral tongue component (where patients may have a worse QOL outcome than expected) and anterior composite resections with a small oral tongue component (where patients may have better QOL outcomes than expected).
Our data demonstrate that segmental and marginal mandibulectomy do not portend different QOL outcomes in the setting of free flap reconstruction. Some reports describe conflicting data regarding the extent of mandibular resection. Becker et al. described worse QOL outcomes in patients undergoing segmental mandibulectomy in comparison to marginal resections [20]. There are numerous potential confounders however, and patients with marginal resections tended to have less locally advanced tumors [20]. As with our data, other studies have largely failed to find an association between extent of mandible resection and QOL [4,23].
Limitations
The subjective nature of PROMs is one limitation of this study. Because of the subjectivity, PROMs are influenced by individual patient differences that are difficult to measure. However, they provide an important insight into the patient experience and are critical to consider in providing patient-centered oncologic care.
While our survivorship database was built with prospectively collected data, this analysis is retrospective. Because of the cross-sectional study design, PROMs were measured at variable times from completion of treatment. To account for differences in QOL based on time from treatment, any patients with PROMs collected at<6 months post-treatment were excluded. There was no association between time from treatment and PROMs suggesting that QOL neither improved nor declined with longer time to complete the PROM surveys. However, data were cross-sectional and no within-patient comparisons were calculated, which will be necessary to validate the lack of effect of elapsed time. Other longitudinal analyses have shown that QOL tends to stabilize after 6 months [30].
Regarding the selection of PROMs for data analysis, it would seem ideal to include all available PROMs and even perform individual item-level analyse. This had to be balanced with the risk of having too many endpoints for a sample size of 80 patients, thus increasing false positive results and diluting the statistical validity of our results. Another reason to avoid analysis of many PROMs at once is that several measures have significant redundancy in content. Further research is needed to identify the most pertinent PROMs for each domain with the goal of standardizing how QOL is assessed in HNC survivors. We are unable to make subscale-specific conclusions about chewing, speech, taste, salivation, appearance, anxiety, mood, pain, activity, and recreation. However, these subscales are represented by the more global domains of physical and social-emotional QOL, from which we are able to draw conclusions.
Despite referral to HNC survivorship clinic being standard of care at our institution after oral cavity resection and free flap reconstruction, the potential for sampling bias is an additional limitation of this study. It is unknown whether patients who present to survivorship clinic are different from those who do not. Patients who attend may have more significant post-treatment toxicities thereby having greater need for survivorship clinic services than those who do not. It is also possible that patients with limited resources who are vulnerable to the significant financial toxicity associated with HNC are less likely to attend survivorship clinic. Among patients who present to survivorship clinic, there may also be variability in participation with treatment as recommended by speech-language pathology, dentistry, physical therapy, and mental health services. Our sample of almost exclusively white patients highlights a substantial disparity in patient access to survivorship services. Because most patients present to survivorship after the initiation or completion of treatment, pre-treatment comparisons cannot be made.
Conclusion
Taken together, our data suggest that the extent of oral tongue resection is crucial to consider when counseling patients on the impact that treatment will have on their QOL after free flap reconstruction of the oral cavity. Differences in the extent of tongue resection may mediate the association between anterior composite resection and worse QOL outcomes and may also mediate the worse QOL scores in the oral tongue resection group compared to the composite resection group. Future studies should examine whether the way we counsel patients on long-term QOL after treatment is concordant with their predicted outcomes based on expected defect characteristics.
Supplementary Material
Acknowledgements
There are no further acknowledgments to include.
Funding source
LJM was awarded NIH/NIDCD Research Training in Otolaryngology, Grant/Award Number: 5T32DC000066. This project utilized the UPMC Hillman Cancer Center Biostatistics Facility which is supported by the National Cancer Institute Cancer Center Support Grant: P30CA04790. The funding sources had no role in study design, data collection, analysis, interpretation, manuscript preparation or submission for publication.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.oraloncology.2021.105574.
References
- [1].Meier JK, Schuderer JG, Zeman F, et al. Health-related quality of life: a retrospective study on local vs. microvascular reconstruction in patients with oral cancer. BMC Oral Health. 2019;19(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chandu A, Smith AC, Rogers SN. Health-related Quality of Life in Oral Cancer: A Review. J Oral Maxillofac Surg. 2006;64(3):495–502. [DOI] [PubMed] [Google Scholar]
- [3].Nilsen MN, Mady LJ, Hodges J, Wasserman-Wincko T, Johnson JT. Burden of Treatment: Reported Outcomes in a Head and Neck Cancer Survivorship Clinic. Laryngoscope. 2019;129(12):E437–44. [DOI] [PubMed] [Google Scholar]
- [4].Rogers SN, Devine J, Lowe D, Shokar P, Brown JS, Vaugman ED. Longitudinal health-related quality of life after mandibular resection for oral cancer: a comparison between rim and segment. Head Neck. 2004;26(1):54–62. [DOI] [PubMed] [Google Scholar]
- [5].Nilsen MN, Lyu L, Belsky MA, et al. Impact of Neck Disability on Health-Related Quality of Life among Head and Neck Cancer Survivors. Otolaryngol Head Neck Surg. 2020;162(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cramer JD, Johnson JT, Nilsen ML. Pain in Head and Neck Cancer Survivors: Prevalence, Predictors, and Quality-of- Life Impact. Otolaryngol Head Neck Surg. 2018;159(5):853–8. [DOI] [PubMed] [Google Scholar]
- [7].Zhu J, Xiao Y, Liu F, Wang J, Yang W, Xie W. Measures of health-related quality of life and socio cultural aspects in young patients who after mandible primary reconstruction with free fibula flap. World J Surg Oncol. 2013;11:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mady LJ, Lyu L, Owoc MS, Peddada SD, Thomas TH, Sabik LM, et al. Understanding financial toxicity in head and neck cancer survivors. Oral Oncol. 2019;95:187–93. [DOI] [PubMed] [Google Scholar]
- [9].Yan YB, Meng L, Liu ZQ, Xu JB, Liu H, Shen J, et al. Quality of life in long-term oral cancer survivors: an 8-year prospective study in China. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(1):67–75. [DOI] [PubMed] [Google Scholar]
- [10].Rogers SN, Gwanne S, Lowe D, Humphris G, Yueh B, Weymuller EA. The addition of mood and anxiety domains to the University of Washington quality of life scale. Head Neck. 2002;24(6):521–9. [DOI] [PubMed] [Google Scholar]
- [11].Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24. [DOI] [PubMed] [Google Scholar]
- [12].Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–92. [DOI] [PubMed] [Google Scholar]
- [13].Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–73. [DOI] [PubMed] [Google Scholar]
- [14].Ghiam MK, Mannion K, Dietrich MS, Stevens KL, Gilbert J, Murphy BA. Assessment of musculoskeletal impairment in head and neck cancer patients. Support Care Cancer. 2017;25(7):2085–92. [DOI] [PubMed] [Google Scholar]
- [15].Gane EM, O’Leary SP, Hatton AL, Panizza BJ, McPhail SM. Neck and upper limb dysfunction in patients following neck dissection: looking beyond the shoulder. Otolaryngol Head Neck Surg. 2017;157(4):631–40. [DOI] [PubMed] [Google Scholar]
- [16].Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7):409–15. [PubMed] [Google Scholar]
- [17].Hains F, Waalen J, Mior S. Psychometric properties of the neck disability index. J Manipulative Physiol Ther. 1998;21(2):75–80. [PubMed] [Google Scholar]
- [18].Cheraghlou S, Schettino A, Zogg CK, Judson BL. Changing prognosis of oral cancer: An analysis of survival and treatment between 1973 and 2014. Laryngoscope. 2018;128(12):2762–9. [DOI] [PubMed] [Google Scholar]
- [19].Chen S, Zhang Q, Guo Z, et al. Trends in clinical features and survival of oral cavity cancer: fifty years of experience with 3,362 consecutive cases from a single institution. Cancer Manag Res. 2018;10:4523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Becker ST, Menzebach M, Köchler T, Hetrampf K, Hans- Jürgen W, Wiltfang J. Quality of Life in Oral Cancer Patients-Effects of Mandible Resection and Socio-cultural Aspects. J Craniomaxillofac Surg. 2012;40(1):24–7. [DOI] [PubMed] [Google Scholar]
- [21].van Gemert J, Holtslag I, van der Bilt A, Merkx M, Koole R, Van Cann E. Health-related quality of life after segmental resection of the lateral mandible: Free fibula flap versus plate reconstruction. J Craniomaxillofac Surg. 2015;43(5):658–62. [DOI] [PubMed] [Google Scholar]
- [22].Shpitzer T, Gullane PJ, Neligan PC, et al. The free vascularized flap and the flap plate options: comparative results of reconstruction of lateral mandibular defects. Laryngoscope. 2000;110(12):2056–60. [DOI] [PubMed] [Google Scholar]
- [23].Warshavsky A, Fliss DM, Frenkel G, et al. Quality of life after mandibulectomy: the impact of the resected subsite. Int J Oral Maxillofac Surg. 2019;48(10):1273–8. [DOI] [PubMed] [Google Scholar]
- [24].Rogers SN, Lowe D, Brown JS, Vaughan ED. The University of Washington head and neck cancer measure as a predictor of outcome following primary surgery for oral cancer. Head Neck. 1999;21(5):394–401. [DOI] [PubMed] [Google Scholar]
- [25].Vartanian J, Carvalho A, Yueh B, et al. Long-term quality-of-life evaluation after head and neck cancer treatment in a developing country. Arch Otolaryngol Head Neck Surg. 2004;130:1209–13. [DOI] [PubMed] [Google Scholar]
- [26].Gao RW, Nuyen BA, Divi V, Sirjani D, Rosenthal EL. Outcomes in head and neck resections that require multiple-flap reconstructions: A systematic review. JAMA Otolaryngol Head Neck Surg. 2018;144(8):753–4. [DOI] [PubMed] [Google Scholar]
- [27].Ochoa E, Larson AR, Han M, Webb KL, Stanford-Moore GB, El-Sayed IH, et al. Patient-Reported Quality of Life After Resection With Primary Closure for Oral Tongue Carcinoma. Laryngoscope. 2021;131(2):312–8. [DOI] [PubMed] [Google Scholar]
- [28].Manrique OJ, Leland HA, Langevin CJ, Wong A, Carey JN, Ciudad P, et al. Optimizing Outcomes following Total and Subtotal Tongue Reconstruction: A Systematic Review of the Contemporary Literature. J Reconstr Microsurg. 2017;33(2):103–11. [DOI] [PubMed] [Google Scholar]
- [29].Rathod S, Livergant J, Klein J, Witterick I, Ringash J. A systematic review of quality of life in head and neck cancer treated with surgery with or without adjuvant treatment. Oral Oncol. 2015;51(10):888–900. [DOI] [PubMed] [Google Scholar]
- [30].Borggreven PA, Aaronson NK, Verdonck-de Leeuw IM, Muller MJ, Heiligers ML, Bree Rd, et al. Quality of life after surgical treatment for oral and oropharyngeal cancer: a prospective longitudinal assessment of patients reconstructed by a microvascular flap. Oral Oncol. 2007;43(10):1034–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.