ABSTRACT
Background
Trauma- and stress-related disorders, such as post-traumatic stress disorder (PTSD), are more common in females than in males. Sex hormones affect learning and emotional memory formation and may be associated with the development of PTSD. Most previous studies have indexed these hormones in isolation. Objectives: To investigate associations of sex hormones and cortisol during memory consolidation on the development of intrusive memories. Methods: We employed an experimental trauma film paradigm in 61 healthy women and indexed salivary testosterone, progesterone, estradiol, and cortisol on day one and day two post experimental trauma exposure and their effects on intrusion frequency, distress, and vividness. Intrusive trauma memories were indexed by means of a diary in which participants documented intrusion frequency, distress, and vividness. Results and conclusion: Participants reported an average of 5.3 intrusions over the course of seven days (SD = 4.6, range 0-26). Progesterone, and estradiol indexed on day one predicted intrusion frequency, with higher progesterone and lower estradiol predicting more intrusive memories (p-values AUC progesterone 0.01 and estradiol 0.02). There was no evidence for associations between hormone concentration indices on day two and intrusion outcomes. Further research on the roles of gonadal and adrenal hormones in trauma memory formation is needed to advance our efforts to understand their influence on PTSD development.
KEYWORDS: Intrusive emotional memories, memory consolidation, post-traumatic stress disorder, sex hormones, cortisol
Abstract
Antecedentes: Los trastornos relacionados con el trauma y el estrés, como el trastorno de estrés postraumático (TEPT), son más comunes en mujeres que en hombres. Las hormonas sexuales afectan el aprendizaje y la formación de la memoria emocional y pueden estar asociadas con el desarrollo del TEPT. La mayoría de los estudios previos han indexado estas hormonas de forma aislada.
Objetivos: Investigar las asociaciones de hormonas sexuales y cortisol durante la consolidación de la memoria en el desarrollo de recuerdos intrusivos.
Métodos: Empleamos un paradigma de trauma experimental de película en 61 mujeres sanas e indexamos la testosterona salival, la progesterona, el estradiol y el cortisol en el día 1 y el día 2 después de la exposición al trauma experimental y sus efectos sobre la frecuencia de intrusión, la angustia y la vividez. Los recuerdos traumáticos intrusivos se indexaron por medio de un diario en el que los participantes documentaron la frecuencia, la angustia y la vividez de la intrusión.
Resultados y conclusión: Los participantes informaron un promedio de 5,3 intrusiones en el transcurso de 7 días (SD = 4,6, rango 0-26). La progesterona y el estradiol indexados en el día 1 predijeron la frecuencia de intrusión, con progesterona más alta y estradiol más bajo prediciendo más recuerdos intrusivos (valores de p de AUC progesterona 0.01 y estradiol 0.02). No hubo evidencia de asociaciones entre los índices de concentración de hormonas en el día 2 y los resultados de la intrusión. Se necesita más investigación sobre los roles de las hormonas gonadales y suprarrenales en la formación de recuerdos traumáticos para avanzar en nuestros esfuerzos por comprender su influencia en el desarrollo del TEPT.
PALABRAS CLAVE: Memoria emocional, recuerdos intrusivos, consolidación, Trastorno de estrés postraumático, hormonas sexuales, cortisol
HIGHLIGHTS: • We examined effects of sex hormones and cortisol post-experimental trauma on intrusive memories.• Progesterone and estradiol indexed on day one were associated with intrusion frequency.• No significant association between hormones on day two and intrusive memory outcomes emerged
Abstract
背景: 创伤和应激相关疾病, 如创伤后应激障碍 (PTSD), 在女性中比在男性中更常见。性激素影响学习和情绪记忆的形成, 并可能与 PTSD发展有关。以前大多数研究都孤立地索引了这些激素。
目的: 研究记忆巩固过程中性激素和皮质醇与闯入性记忆发展的关系。
方法: 我们在 61 名健康女性中使用了实验性创伤影片范例, 并在实验性创伤暴露后第 1 天和第 2 天指数化唾液睾酮、孕酮、雌二醇和皮质醇及其对闯入频率、痛苦和生动性的影响。闯入性创伤记忆通过日记的方式进行指数化, 参与者在日记中记录了闯入频率、痛苦和生动性。
结果和结论: 参与者报告在 7 天内平均发生 5.3 次闯入 (标准差 4.6, 范围 0-26)。第 1 天索引的孕酮和雌二醇预测闯入频率, 较高的孕酮和较低的雌二醇预测更多的闯入性记忆 (p 值 AUC 孕酮 0.01 和雌二醇 0.02)。没有证据表明第 2 天的激素浓度指数与闯入结果之间存在关联。需要进一步研究性腺和肾上腺激素在创伤记忆形成中的作用, 以推动我们努力了解其对 PTSD 发展的影响。
关键词: 情绪记忆, 闯入性记忆, 巩固, 创伤后应激障碍, 性激素, 皮质醇
1. Introduction
A proportion of individuals exposed to a traumatic experience develops post-traumatic stress disorder (PTSD) (Kearns et al., 2012; Wittchen, 2006). PTSD is twice as likely to occur in female trauma survivors compared to their male counterparts (Breslau and Anthony, 2007; Seligowski et al., 2020a; Tolin and Foa, 2006). Several factors may account for this difference, including psychological and sociocultural aspects (Breslau and Anthony, 2007), or sex differences in stress- and trauma-related memory consolidation processes (Felmingham et al., 2012; Hsu et al., 2018). Effects of estradiol and progesterone as well as adrenal hormones, which have been associated with stress-related and emotional learning, have all been suggested as robust PTSD predictors (Seligowski et al., 2020b). However, clinical studies have produced inconsistent findings in relating gonadal hormones, which fluctuate around the menstrual cycle, with PTSD. Many of these studies have focused on intrusive memories, the core characteristic of the disorder. Here we expand on previous and partly inconsistent results (Christiansen and Berke, 2020; Garcia et al., 2018; Josephs et al., 2017; Ney et al., 2019), and investigate associations of several sex hormones and cortisol with intrusive memory formation during memory consolidation in an experimental trauma paradigm, hence accounting for concurrent adrenal and gonadal hormonal influences.
A key mechanism thought to underlie PTSD is enhanced consolidation of an often fragmented and emotionally charged memory of the trauma due to incomplete memory processing (Brewin, 2011; Marks et al., 2018; McGaugh, 2015). Initially fragile memories consolidate gradually and convert from a labile short-term into a long-term permanent state, which might facilitate endogenous processes to adjust the strength of an experience (Kearns et al., 2012; McGaugh, 2000). This is exemplified in synaptic and cellular representations (Dudai et al., 2015). In this process of recurrent reactivation, taking place during wakefulness and sleep, information is distributed to additional brain locations and integrated into existing knowledge (Dudai et al., 2015). According to contemporary memory consolidation accounts, the time period during which memories are consolidated can range from seconds to months or years (Brewin, 2018; Dudai et al., 2015; McGaugh, 2000, 2015). Arousal and hormones, including cortisol and other adrenal hormones, influence brain processes and memory consolidation (McGaugh, 2015). However, existing studies have mainly analyzed the effects of sex hormones and cortisol, during encoding and consolidation in the early aftermath of trauma, such as minutes or hours post-exposure (Chou et al., 2014; Christiansen and Berke, 2020; Jiang et al., 2019; Soni et al., 2013). The process of memory consolidation in the later phase and renewed consolidation following initial memory processing, is relatively less researched although potential effects could have important practical implications. In this study, we focus on both, the early consolidation phase on the day of experimental trauma exposure (day one) as well as on the following day (day two). The latter phase of memory consolidation has far often been neglected. Any considerable effects that might be identified on day two could be exploited for prevention and intervention science, such as in an emergency room setting (Kearns et al., 2012; Marks et al., 2018), where the majority of patients are admitted within 24 h post-trauma (Rothbaum et al., 2012).
Glucocorticoids influence human cognitive function and memory formation, which has an effect on the development of PTSD (Brewin, 2011; Bryant et al., 2011; de Quervain et al., 2009; McEwen and Sapolsky, 1995; Melmed et al., 2016). Memory consolidation is enhanced by cortisol, in rats and humans by influencing directly, or via the release of norepinephrine in the basolateral amygdala, brain regions involved in memory consolidation, such as the hippocampus, neocortex and caudate nucleus (McGaugh, 2015). Directly after a trauma film in men and women, high levels of cortisol have been associated with more intrusive memories (Cheung et al., 2015). At encoding, there is evidence for a positive correlation between cortisol levels and memory recall in women and intrusion frequency in women and men (Andreano et al., 2008; Chou et al., 2014; van Ast et al., 2014). Patients with PTSD from both sexes compared to controls, as well as participants following experimental trauma who report previous trauma exposure, all show lower salivary cortisol levels (Pan et al., 2018). Participants of a trauma film study who have recently experienced more traumatic events, had lower salivary cortisol after trauma film, which led to more vivid intrusions (Chou et al., 2014).
There is an intricate interplay between sex hormones and cortisol in stress regulation and emotional memory consolidation (Zorawski et al., 2006). Adrenal stress-related hormones, such as glucocorticoids, suppress gonadotropin-releasing hormone (GnRH). As a consequence, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and the sex hormones estradiol, progesterone, and testosterone often increase (Melmed et al., 2016). During the luteal phase, progesterone levels rise. Women in this phase have higher levels of bioavailable free salivary cortisol compared to women in the follicular phase or to men (Kirschbaum et al., 1999; Shibui et al., 2000). Heightened estradiol induces a higher norepinephrine and stress hormone release from the hypothalamic pituitary adrenal (HPA) axis in anterior frontal, insula, and cingulate cortices, brain regions that are, among other functions, responsible for arousal (Goldstein et al., 2005). Lower estradiol and progesterone levels lead to a decrease in serotonin and allopregnanolone, which in turn results in less effective stress regulation (Li and Graham, 2017). Previous studies have mostly investigated adrenal and gonadal hormone effects in isolation (Seligowski et al., 2020a; Seligowski et al., 2020b).
In terms of gonadal hormones, testosterone has been associated with PTSD development, anxiolytic and antidepressant effects, neuronal plasticity, and anxiety symptom reduction during exposure therapy (Christiansen and Berke, 2020; Davis and Wahlin-Jacobsen, 2015; Hutschemaekers et al., 2020; Josephs et al., 2017; McHenry et al., 2014; Pitman et al., 2012a). In men, reduced testosterone and cortisol under stress was associated with a higher risk of developing PTSD (Josephs et al., 2017). Lower pre-deployment testosterone levels also predicted later development of PTSD symptoms (Reijnen et al., 2015). Higher testosterone levels, together with heightened estradiol and lower cortisol levels, demonstrated beneficial effects and were associated with fewer PTSD symptoms in both sexes (Pitman et al., 2012). In rodents, testosterone impaired memory consolidation and influenced extinction memory consolidation (Harooni et al., 2008; Maeng et al., 2017). The impacts of testosterone on emotional memory consolidation, and intrusive memories in particular, have rarely been analyzed in humans, and results remain inconclusive to date (Davis and Wahlin-Jacobsen, 2015; Hutschemaekers et al., 2020). To the best of our knowledge, no studies have examined testosterone and intrusive memories in women.
Estradiol and progesterone have been proposed as key factors influencing intrusive emotional memories in humans (Cover et al., 2014; Li and Graham, 2017; Miedl et al., 2018; Ney et al., 2019; Seligowski et al., 2020a). High levels of estradiol have been associated with an increased consolidation of fear extinction memory, while low estradiol levels have been correlated with more and stronger intrusive memories at encoding and consolidation (Cover et al., 2014; Soni et al., 2013; Wegerer et al., 2014). In addition, high levels of progesterone at encoding, have been associated with more intrusion frequency, and emotional memories (Ferree et al., 2011; Soni et al., 2013). During early consolidation, high levels of progesterone have caused an increased consolidation of emotional memories and amygdala activity (Sundström Poromaa and Gingnell, 2014). For emotionally arousing stimuli in women, high progesterone levels have shown enhanced memory consolidation (Ertman et al., 2011; Maeng and Milad, 2015). However, other studies revealed no association between progesterone and intrusive memories or fear extinction memory during encoding and consolidation (Cover et al., 2014; Wegerer et al., 2014).
Overall, there are few studies investigating effects of testosterone and memory consolidation in women. Findings are mixed regarding the impacts of progesterone and estradiol levels during memory consolidation. Moreover, there is evidence that cortisol interacts with these hormones during memory consolidation (Zorawski et al., 2006). Most studies have investigated the influence of hormone concentrations during encoding or early consolidation without investigating their influence in later consolidation phases. Therefore, the present study aimed to investigate effects of testosterone, estradiol, progesterone, and cortisol during early, as well as later memory consolidation, i.e. on day one and two post-experimental trauma on intrusive memory characteristics. We hypothesized that higher concentrations of cortisol and progesterone and lower concentrations of estradiol and testosterone predict greater intrusion frequency, distress, and vividness.
2. Methods
2.1. Study design and procedure
Participants were recruited through online advertisement, mailing lists, and billboards, and they were accepted for inclusion following a telephone screening. On the first day of the study, participants visited the laboratory and, after providing informed consent, completed an independent task for evaluating attention control as well as standardized questionnaires. Participants were asked if they have seen the film scene before and then exposed to a traumatizing 12-minute film sequence, that depicts strong interpersonal violence. Such experimental procedure has been referred to as an analog trauma-film paradigm (Holmes and Bourne, 2008; Holmes et al., 2004). The film viewing took place during a time window extending from 11:00 AM to 3:00 PM to control for circadian fluctuations in hormones and other relevant processes of, for example, attention and cognitive functioning. Participants could stop the experiment at any time and access the support of an experienced psychotherapist. After viewing the film sequence, the participants were instructed on how to fill in the intrusion diary, received an explanation of the hormone self-measurements, and scheduled their next appointment. Salivary hormone measurements were obtained from participants immediately and 20 minutes post- film and at 6:00 PM of the day of laboratory trauma exposure (day one), and at 8:00 AM, 12:00 PM, and 6:00 PM on day two (via self-selection). From day one to day seven, participants used an intrusion diary to document their trauma-film-related intrusive memories during this post experimental analogue trauma period in regard to the type, content, frequency, associated vividness, and distress. In a second session, which was held after one week, the participants received their payment of 50 CHF or course credit points. Moreover, the results were discussed, and intrusions were coded. The local ethics board approved this study, which was reported in alignment with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (von Elm et al., 2014).
2.2. Participants
The research sample of this observational study consisted of 61 healthy women. All of the participants provided informed consent. To meet the inclusion criteria, participants had to be female, between the ages of 18 and 40, fluent in the German language, and have a regular menstrual cycle of between 24 and 32 days. Exclusion criteria were the use of psychotropic medication or hormonal contraception, depressive or anxious psychopathology, pregnancy, hormonal disorders such as polycystic ovary syndrome, endometriosis, or a prior experience of interpersonal violence, experienced either by themselves or by a significantly close other. Participants were included following a telephone screening, where we indexed pregnancy and medication use (yes to any of these lead to study exclusion), a screening question for depression, i.e. whether participants experienced a period of time where they felt depressed or down most of the time (yes lead to study exclusion), suicidality, i.e. whether they experienced suicidal ideation or had attempted suicide during the last four weeks (yes lead to study exclusion), psychosis, as well as interpersonal violence, i.e. were they or anyone in their close surroundings ever victim of interpersonal violence (yes to any of these questions lead to study exclusion). Demographic variables, such as age, marital status, employment, education, and nationality, were collected via questionnaire items. Participants also provided information about their consumption of nicotine, cannabis, and alcohol as well as their use of medication. Prior experience of interpersonal violence was identified through the German abbreviated version of the Post-Traumatic Stress Diagnostic Scale by Foa, Cashman, Jaycox, and Perry (Griesel et al., 2006) and the Childhood Trauma Questionnaire (CTQ) by Bernstein and Fink (Klinitzke et al., 2012). Depressive or anxious psychopathology was assessed with the German versions of the Beck Depression Inventory II (BDI-II) by Hautzinger (Hautzinger et al., 2000) and the Beck Anxiety Inventory (BAI) by Beck and Steer (Margraf and Ehlers, 2007).
2.3. Trauma film
In a low-stimulus and darkened laboratory room, participants viewed alone a 12-minute film sequence from the R-rated French movie Irréversible by Gaspar Noé (Noé, 2002) on a laptop monitor that had a diagonal of 17 in. with a resolution of 1280 × 1024 pixels. The film sequence depicted scenes of strong interpersonal violence against a woman, including injury and humiliation. In a prior study that examined a number of films, the results indicated significant emergence of intrusive memories following the viewing of this film (Weidmann et al., 2009). In the present study, participants were instructed to always look at the screen. They were asked to watch the scene as if they were witness to the situation. Eye tracking was not used.
2.4. Hormone salivary assessment
In this study, hormone measurements of cortisol and testosterone as well as estradiol and progesterone were obtained. Salivary assessments measured free biologically active hormones (Dabbs and de La Rue, 1991; Melmed et al., 2016). Salivary hormones were assessed at the day of and one day after the laboratory trauma. On the first day of the study in the laboratory, participants received instructions for the collection of the saliva samples. The first sample was taken while still at the laboratory, and they took two further saliva samples under supervision in the laboratory. Participants were instructed to refrain from drinking, eating, or smoking for at least 30 min before collecting the saliva sample. They collected saliva samples with Salivette® collection devices (Sarstedt, Sevelen/Switzerland), salicaps (SC), recorded the collection using a form and kept them in their fridge at home. A reminder and instruction for obtaining saliva samples was included on the front page of the intrusive memory diary. Participants brought the saliva samples to the second session, where they were frozen and send to the independent laboratory at the University of Dresden in Germany (DSL, Sinsheim, Germany). This laboratory centrifuged all samples and assayed hormone levels by using commercially available radioimmunoassay kits that were adopted for the analysis of salivary samples. The sensitivity of the cortisol assay was 0.11 nmol/l of the progesterone assay is 2.6 pg/ml of the estradiol assay is 0.3 pg/ml, and of the testosterone assay is 1.8 pg/ml (DSL, Sinsheim, Germany).
2.5. Intrusive memory diary
The intrusion diary was based on prior studies (Holmes and Bourne, 2008; Holmes et al., 2004) and included a definition of the nature of intrusive memories as well as instructions for how to use and fill in the intrusion diary. The paper-pencil diaries were filled in daily, with the request to enter intrusions as soon as possible after their occurrence. Participants recorded information about the memory content, distress, and vividness as indexed on a scale from one (‘not at all’) to ten (‘extremely’), and they noted whether the intrusion occurred spontaneously. Starting from day one, the participants filled in the diary for seven days. The data for this study only included memories that had been coded as intrusive. The experimenter also checked through the diary together with participants when they returned to the laboratory to resolve potential inconsistencies. Two independent raters, both trained psychology graduate students, categorized each memory as intrusive or non-intrusive based on the content descriptions and participants’ scores relating to vividness and spontaneous occurrence.
2.6. Outcomes
Intrusion frequency post-film was the primary outcome, distress, and vividness of intrusive memories the secondary outcome. Measures were obtained from the diary completed by participants from day one to seven post-film.
2.7. Statistical methods
All analyses were conducted with the software R (R, version 3.6.3, 29.02.2020) (RStudioTeam, 2016). Descriptive statistics included minimum and maximum, mean, and standard deviation (SD) for approximately normal continuous variables, median and interquartile range (IQR) for skewed continuous variables and frequency and percentage for categorical variables. Interrater reliability was assessed using Cohen's Kappa. Participants with missing values were excluded from the respective analysis; as a consequence, the data reflect only those participants with complete observations. We assumed that the mechanism generating the missingness was missing completely at random (MCAR).
A negative binomial regression model (McCullagh and Nelder, 1999; Venables and Ripley, 2010) was applied to model the primary outcome (i.e. intrusion frequency), with the independent variables being the log of the area under the curve (AUC) of the hormone concentrations of testosterone, cortisol, progesterone, and estradiol. In addition, linear regression modeling (Field, 2012; Hastie and Chambers, 1992) was used to quantify the association with distress and vividness of intrusions from the same hormone concentrations of testosterone, cortisol, progesterone, and estradiol, if participants reported intrusions. No adjustments were made for multiple testing. The independent variables were the hormone concentrations of testosterone, cortisol, progesterone, and estradiol that were measured on day one and two. In order to utilize the total diurnal hormonal output and exposure for the participants and ensure the same calculation method for each hormone, for the early consolidation phase we calculated the AUC based on the concentration of the saliva samples SC2 immediately after the film, SC3 20 minutes after the film and SC4 at 6:00 PM at the day of experimental analogue trauma (Antypa et al., 2018; Pruessner et al., 2003). For day two, the day after trauma-film, we calculated the AUC based on the concentration of SC5 at 8:00 AM, SC6 at 12:00 PM, and SC7 at 6:00 PM one day after experimental analogue trauma. To enhance homoscedasticity and the linear relation of the independent variables to the dependent variable, hormone data were log transformed after the calculation of the AUC.
Tobacco use was controlled for as a confounding variable. As tobacco may influence cortisol and testosterone, the menstrual cycle, other neuroendocrinological factors and is a risk factor for the development of PTSD, tobacco use was included as a confounder in the models (Hawkins and Cougle, 2013; Kudielka and Kirschbaum, 2003; Pitman et al., 2012; van der Velden et al., 2008; Zhao et al., 2016). We defined it as a binary variable with two categories: tobacco use and no tobacco use. Model results including additional confounders alcohol and cannabis use can be found in the Supplement (Tables 8 and 9).
2.8. Sample size considerations
There was no a priori sample size calculation for this study. However, we conducted a sensitivity analysis for the sample size at hand (Perugini et al., 2018) using the R package RSPS, a significance level α of 5%, and an anticipated power of 80%. Two scenarios for 1.3-fold and 1.5-fold change in number of intrusions as outcome in a negative binomial model were evaluated regarding power for varying sample sizes for the regression analysis. The resulting figure is shown in the supplementary material (see Figure 2). For a power of 80% and an expected medium effect size of 1.5-fold increase, a sample size of at least 35 subjects would be sufficient to detect this effect with 80% power, which our study fulfilled.
3. Results
3.1. Descriptive and sample characteristics
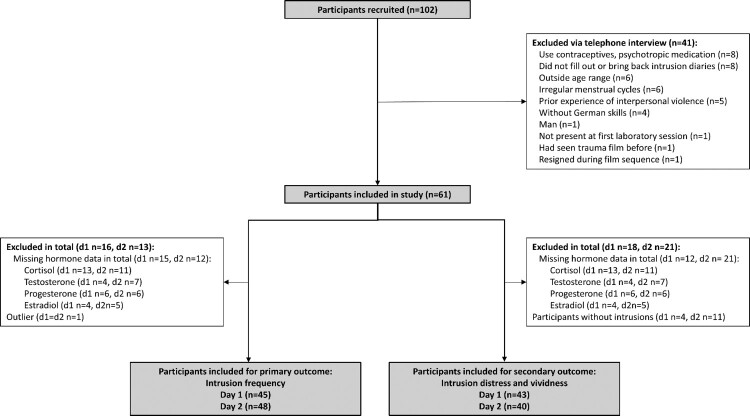
Our sample consisted of 61 healthy women. Following inclusion, eight participants had to be excluded from the study due to noncompletion of the intrusion diary, one for not being present at the first laboratory session, one had seen the distressing film clip before, and one resigned during the film sequence, see Figure 1. Mean age of the resulting 61 participants was 25.1 years (SD = 5.3, range 18–40), see Table 1. Depression and anxiety symptoms were low (mean BDI = 4.5; SD = 4.2, mean BAI = 6.5; SD = 5.4). Participants experienced three traumatic events on average (mean = 3.2; SD = 2.0), with a mean childhood trauma score of 32.5 (SD = 7.7). One participant reported interpersonal violence in the questionnaires later but had not reported this in the initial screening. This participant remained in the study and her exclusion did not significantly change the results of the three regression models. With respect to self-reported menstrual phases, 41 participants were in their follicular phase, while 20 were in their luteal cycle phase. Hormone salivary measurements did not fully reflect the self-evaluated menstrual phases, see Supplements Figure 4. Hormone concentrations were used for regression analysis. Both independent raters who categorized each memory as intrusive or non-intrusive had an interrater reliability of Cohen's Kappa Ƙ = 1.0 (95% CI from 1.0 to 1.0).
Figure 1.
Flow chart participants.
Table 1.
Descriptive Statistics (n = 61).
| N | % | ||
|---|---|---|---|
| Marital status | Single | 56 | 91.8 |
| Married | 4 | 6.6 | |
| Divorced | 1 | 1.6 | |
| Education | Baccalaureate | 34 | 55.7 |
| University degree | 20 | 32.8 | |
| Secondary school | 3 | 4.9 | |
| Elementary School | 2 | 3.3 | |
| Others | 2 | 3.3 | |
| Employment | Studying | 46 | 75.4 |
| Employed | 8 | 13.1 | |
| Unemployed | 5 | 8.2 | |
| In school | 2 | 3.3 | |
| Nationality | Swiss | 37 | 60.7 |
| German | 10 | 16.4 | |
| Turkish | 2 | 3.3 | |
| French | 1 | 1.6 | |
| Italian | 1 | 1.6 | |
| Others | 10 | 16.4 | |
| Tobacco use | Yes | 19 | 31.1 |
| No | 42 | 68.9 | |
| Cannabis use | Yes | 9 | 14.8 |
| No | 52 | 85.2 | |
| Alcohol use | Yes | 49 | 80.3 |
| No | 12 | 19.7 | |
| Medical use | Yes | 47 | 77.0 |
| No | 6 | 9.8 | |
| Without information | 8 | 13.1 | |
| Menstrual cyclea | Early follicular cycle phase | 24 | 39.3 |
| Late follicular cycle phase | 17 | 27.9 | |
| Luteal cycle phase | 20 | 32.8 | |
| M | SD | ||
| Age | 25.1 | 5.3 | |
| Beck Depression index (BDI)b | 4.5 | 4.2 | |
| Beck Anxiety index (BAI)c | 6.4 | 5.4 | |
| Childhood Trauma Questionnaire (CTQ)d | 32.5 | 7.7 | |
| Emotional abuse | 7.0 | 3.0 | |
| Physical abuse | 5.5 | 1.2 | |
| Sexual abuse | 5.3 | 1.2 | |
| Emotional neglect | 8.6 | 3.7 | |
| Physical neglect | 6.1 | 1.7 | |
| Life Event Scale (LE)e | 3.2 | 2.0 | |
Note. Descriptive statistics of demographic and clinical sample characteristics.
aSelf-reported menstrual phase.
bBDI range 0-63, moderate depression > 19.
cBAI range 0-63, moderate anxiety > 15.
dCTQ range 5–25 for each subscale –, assessment of moderate traumatic childhood experiences: emotional (>12), physical (>9), and sexual (>7) abuse, as well as emotional (>14) and physical neglect (>9).
eLE range 0–16 critical traumatic life events, based on DSM IV PTSD criteria.
3.2. Cortisol, testosterone, estradiol, and progesterone
The mean AUC of cortisol post-experimental analogue trauma exposure was 72.8 (day 1, SD = 29.3) and 90.1 (day 2, SD = 42.9). For testosterone, the mean AUC was 210.6 (day 1, SD = 161.2) and 314.8 (day 2, SD = 422.0). The mean AUC for progesterone after experimental analogue trauma was 2860.7 (day 1, SD = 7580.3) and 925.9 (day 2, SD = 772.1). For estradiol, the mean AUC was 46.5 (day 1, SD = 25.1) and 44.0 (day 2, SD = 27.4; see Table 2). Due to missing hormone data 15 (day 1) and 12 (day 2) participants had to be excluded from the regression analysis (see Figure 1 flow chart participants). For information purposes, Spearman correlation coefficients between hormone concentrations, as well as hormone concentrations and intrusions of day one, can be found in the Supplement Table 5.
Table 2.
Descriptive statistics of AUC hormone levels (day 1 and 2) and intrusive memories (day 1–7 and 2–7).
| Day 1 | Day 2 | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Cortisol, nmol/l | 72.8 | 29.3 | 90.1 | 42.9 |
| Testosterone, pg/ml | 210.6 | 161.2 | 314.8 | 422.0 |
| Progesterone, pg/mla | 2860.7 | 7580.3 | 925.9 | 772.1 |
| Estradiol, pg/ml |
46.5 |
25.1 |
44.0 |
27.4 |
| Day 1–7 | Day 2–7 | |||
| Mean | SD | Mean | SD | |
| Intrusion frequency | 5.3 | 4.6 | 3.7 | 3.9 |
| Median | IQR | Median | IQR | |
| Intrusion distressb | 4.0 | 2.8, 6.4 | 3.2 | 2.1, 6.3 |
| Intrusion vividnessb | 5.5 | 4.0, 6.8 | 4.5 | 3.0, 6.4 |
Note:
Median progesterone levels did not differ significantly for day 1 and day 2; mean differences were driven by single individuals.
Intrusion distress and vividness are indexed on a scale from 1 ‘not at all’ to 10 ‘extremely’.
3.3. Intrusion frequency, distress, and vividness
There were individual differences in intrusion frequency and participants reported an average of 5.3 intrusions (day 1-7, SD = 4.6, range 0-26) and 3.7 intrusions (day 2-7, SD = 3.9; range 0–22), see Supplement Figure 3. 4 participants (day 1-7, 7%) and 11 participants (day 2-7, 18%) reported no intrusions. Average intrusion distress revealed low to moderate distress, with a median of 4.0 (day 1-7, IQR = 2.8-6.4) and 3.2 (day 2-7, IQR = 2.1–6.3). Vividness ratings conveyed moderate overall vividness (day 1–7 Mdn = 5.5, IQR = 4.0–6.8; day 2–7 Mdn = 4.5, IQR = 3.0–6.4).
3.4. Effects of hormones on intrusive memories
Hormone data were missing for 15 participants (day 1) and 12 participants (day 2), one participant was identified and excluded as an outlier based on the residual and Cooks’ distance. For the analysis of intrusion distress and vividness, four participants (day 1-7) and eleven participants (day 2-7) were excluded, as they had not experienced intrusions. Since there was an overlap between missing hormone data and no experience of intrusions, 45 (day 1) and 48 (day 2) participants were included in the analysis of the primary outcome, and 43 (day 1) and 40 (day 2) in the analysis of the secondary outcome (see Figure 1).
For hormone indices obtained on day one, results of the negative binomial model regressing the intrusion count between day one and seven on log-transformed AUC values for cortisol, testosterone, progesterone, and estradiol directly after trauma film are reported in Table 3. There was evidence of an association between progesterone and estradiol concentrations and intrusion frequency (estimates of log AUC of progesterone 0.21 (95% CI from 0.02 to 0.41, p = 0.01) of log AUC of estradiol −0.71 (95% CI from −1.39 to −0.06, p = 0.02)). There was no evidence for an association between cortisol, testosterone, and intrusion frequency, (estimates of log AUC of cortisol −0.02 (95% CI from −0.61 to 0.57, p = 0.95), and of log AUC of testosterone −0.10 (95% CI from −0.42 to 0.22, p = 0.53) with intrusion frequency). A linear model regressing intrusion distress and vividness on the same hormone concentrations provided no evidence for an association between hormone concentrations and these characteristics (estimates of log AUC of cortisol 0.16 (95% CI from −1.91 to 2.23, p = 0.88), of log AUC of testosterone 0.70 (95% CI from −0.60 to 2.00, p = 0.28), of log AUC of progesterone 0.09 (95% CI from −0.55 to 0.73, p = 0.78), and of log AUC of estradiol −0.25 (95% CI from −2.48 to 1.97, p = 0.82) with intrusion distress, respectively, and estimates of log AUC of cortisol 0.54 (95% CI from –1.24 to 2.33, p = 0.54), of log AUC of testosterone 0.17 (95% CI from −0.96 to 1.29, p = 0.77), of log AUC of progesterone −0.02 (95% CI from −0.57 to 0.53, p = 0.94), and of log AUC of estradiol −0.19 (95% CI from −2.11 to 1.72, p = 0.84) with intrusion vividness; see Table 3). There was evidence for an association between the confounder tobacco use and intrusion frequency as well as vividness (estimates of tobacco use with intrusion frequency −0.37 (95% CI from −0.82 to 0.08, p = 0.1) and estimates of tobacco use with intrusion vividness 1.14 (95% CI from –0.15 to 2.43, p = 0.08); see Table 3) and little evidence of tobacco use with intrusion distress (estimates of tobacco use with intrusion frequency 1.16 (95% CI from −0.34 to 2.66, p = 0.13; see Table 3)).
Table 3.
Negative binomial model regressing intrusion frequency (day 1-7) (upper section) on cortisol, testosterone, and estradiol indexed on day 1 (n = 45). Linear model regressing mean intrusion distress (day 1-7) (middle section) and vividness (day 1-7) (lower section) on cortisol, testosterone, progesterone, and estradiol indexed on day 1 (n = 43).
| Estimate (SEa) | Lower CIb | Upper CIb | p value | |
|---|---|---|---|---|
| |
Intrusion frequencyc |
|
|
|
| Cortisol* | −0.02 (0.29) | −0.61 | 0.57 | 0.95 |
| Testosterone* | −0.10 (0.17) | −0.42 | 0.22 | 0.53 |
| Progesterone* | 0.21 (0.09) | 0.02 | 0.41 | 0.01 |
| Estradiol* | −0.71 (0.32) | −1.39 | −0.06 | 0.02 |
| Tobacco use | −0.37 (0.23) | −0.82 | 0.08 | 0.10 |
| |
Intrusion distress |
|
|
|
| Cortisol* | 0.16 (1.02) | −1.91 | 2.23 | 0.88 |
| Testosterone* | 0.70 (0.64) | −0.60 | 2.00 | 0.28 |
| Progesterone* | 0.09 (0.32) | −0.55 | 0.73 | 0.78 |
| Estradiol* | −0.25 (1.10) | −2.48 | 1.97 | 0.82 |
| Tobacco use | 1.16 (0.74) | −0.34 | 2.66 | 0.13 |
| |
Intrusion vividness |
|
|
|
| Cortisol* | 0.54 (0.88) | −1.24 | 2.33 | 0.54 |
| Testosterone* | 0.17 (0.55) | −0.96 | 1.29 | 0.77 |
| Progesterone* | −0.02 (0.27) | −0.57 | 0.53 | 0.94 |
| Estradiol* | −0.19 (0.95) | −2.11 | 1.72 | 0.84 |
| Tobacco use | 1.14 (0.64) | −0.15 | 2.43 | 0.08 |
Note:
* = log AUC value, day 1.
a Standard error.
b Two-sided 95% CI.
c Upper section for intrusion frequency prediction: Estimates depict the log of the expected count.
For hormone concentrations obtained on day two, no evidence for an association emerged for any of the hormones and intrusion frequency (estimates of log AUC of cortisol −0.01 (95% CI from −0.66 to 0.64, p = 0.99), of log AUC of testosterone −0.05 (95% CI from −0.41 to 0.31, p = 0.77), of log AUC of progesterone 0.22 (95% CI from −0.20 to 0.64, p = 0.25), and of log AUC of estradiol −0.08 (95% CI from −0.74 to 0.58, p = 0.81), Table 4). We also calculated univariate models for hormone influences on intrusion outcomes, see Table 6 and 7 in the Supplement. For intrusion distress and vividness no evidence for an association between hormone concentrations and intrusion distress or vividness emerged (estimates of log AUC of cortisol −0.87 (95% CI from −2.73 to 0.99, p = 0.35), of log AUC of testosterone 0.27 (95% CI from −0.73 to 1.26, p = 0.59), of log AUC of progesterone 0.09 (95% CI from −0.95 to 1.12, p = 0.87), and of log AUC of estradiol −0.77 (95% CI from −2.55 to 1.01, p = 0.39) with intrusion distress, respectively, and estimates of log AUC of cortisol 0.02 (95% CI from −1.70 to 1.73, p = 0.98), of log AUC of testosterone −0.07 (95% CI from −0.98 to 0.85, p = 0.88), of log AUC of progesterone 0.01 (95% CI from −0.95 to 0.96, p = 0.99), and of log AUC of estradiol −0.40 (95% CI from −2.04 to 1.24, p = 0.62) with intrusion vividness; see Table 4).
Table 4.
Negative binomial model regressing intrusion frequency (day 2-7) (upper section) on cortisol, testosterone, and estradiol indexed on day 2 (n = 48). Linear model regressing mean intrusion distress (day 2-7) (middle section) and vividness (day 2-7) (lower section) on cortisol, testosterone, progesterone, and estradiol indexed on day 2 (n = 40).
| Estimate (SEa) | Lower CIb | Upper CIb | p value | |
|---|---|---|---|---|
| |
Intrusion frequencyc |
|
|
|
| Cortisol* | −0.01 (0.29) | −0.66 | 0.64 | 0.99 |
| Testosterone* | −0.05 (0.17) | −0.41 | 0.31 | 0.77 |
| Progesterone* | 0.22 (0.19) | −0.20 | 0.64 | 0.25 |
| Estradiol* | −0.08 (0.32) | −0.74 | 0.58 | 0.81 |
| Tobacco use | −0.34 (0.31) | −0.95 | 0.27 | 0.28 |
| |
Intrusion distress |
|
|
|
| Cortisol* | −0.87 (0.92) | −2.73 | 0.99 | 0.35 |
| Testosterone* | 0.27 (0.49) | −0.73 | 1.26 | 0.59 |
| Progesterone* | 0.09 (0.51) | −0.95 | 1.12 | 0.87 |
| Estradiol* | −0.77 (0.88) | −2.55 | 1.01 | 0.39 |
| Tobacco use | 1.02 (0.85) | −0.71 | 2.75 | 0.24 |
| |
Intrusion vividness |
|
|
|
| Cortisol* | 0.02 (0.84) | −1.70 | 1.73 | 0.98 |
| Testosterone* | −0.07 (0.45) | −0.98 | 0.85 | 0.88 |
| Progesterone* | 0.01 (0.47) | −0.95 | 0.96 | 0.99 |
| Estradiol* | −0.40 (0.81) | −2.04 | 1.24 | 0.62 |
| Tobacco use | 0.54 (0.78) | −1.05 | 2.13 | 0.49 |
Note:
* = log AUC value, day 2.
a Standard error.
b Two-sided 95% CI.
c Upper section for intrusion frequency prediction: estimates depict the log of the expected count.
4. Discussion
We investigated gonadal and adrenal hormone influences during memory consolidation on the development of intrusive memories, which constitute a core PTSD symptom. To this end, we indexed hormones (i.e. progesterone, estradiol, testosterone, and cortisol) in an experimental analog trauma paradigm. Hormones were sampled on day one and day two post-experimental trauma during memory consolidation. Progesterone and estradiol fluctuated around the menstrual cycle. Participants reported intrusive emotional memories in a diary over the following seven days. In accordance with previous studies (Chou et al., 2014; Holmes and Bourne, 2008; Schultebraucks et al., 2019; Weidmann et al., 2009), the majority of participants (93%) reported intrusive memories during the seven-day period in which they maintained the diary, and 82% reported intrusions from day two until day seven. There were considerable between-person differences in intrusion frequency, and we investigated whether sex hormones and cortisol account for significant parts of this variance in intrusion development. In the early consolidation phase, there was evidence for an association between the salivary hormone concentrations of progesterone and estradiol levels, and intrusive memory frequency. One day after trauma film, in the later consolidation phase, there was no evidence of an association of any of the hormones that were indexed at day two with intrusion frequency, distress, or vividness.
In the early consolidation phase, there is evidence for an association between the salivary hormone concentrations of progesterone and estradiol, and intrusive memory frequency (see Table 3), as well as between the confounder tobacco use and intrusion frequency and vividness. Participants with higher progesterone and lower estradiol concentrations experienced more intrusive memories. Nonsmokers had more and more vivid intrusions. These findings are in line with previous studies investigating these hormones in the immediate aftermath (i.e. on the same day) of trauma, that have demonstrated significant influences of estrogen and progesterone on intrusive memories (Pitman et al., 2012) and estrogen and progesterone in PTSD (Garcia et al., 2018; Seligowski et al., 2020a). Higher progesterone levels could lead to more intrusions due to increased activity of the amygdala and increased consolidation of emotional memories (Sundstrom Poromaa and Gingnell, 2014). Additionally, lower estradiol levels might promote less activation of the prefrontal cortex, stronger emotional reaction, and memory formation (Cover et al., 2014; Wegerer et al., 2014). The confounder tobacco use might have influenced progesterone and estradiol levels, as well as dysregulated neural stress systems and enzymes involved in the regulation of dopamine and serotonin (van der Velden et al., 2008).
During memory consolidation, initially fragile memory traces are reorganized and integrated into long-term storage (McGaugh, 2000) in a process that can take up to years to fully develop (Diekelmann and Born, 2010). The later consolidation phase that was examined in this study as well (i.e. one day post-trauma) appears promising for early clinical interventions to prevent PTSD. Trauma survivors are often in contact with professional services during this time, and the majority of patients present to the emergency room within 24 hours post-trauma (Rothbaum et al., 2012). Identification of clinically relevant associations could support practical uses and implications for treatment and prevention in settings such as an emergency room, which can open up treatment opportunities. While significant sex hormone associations have often been reported—for example, between cortisol and testosterone (Josephs et al., 2017) or between Dehydroepiandrosteron (DHEA) and cortisol in recurrent depression (Mocking et al., 2015), we did not discover any evidence of associations between testosterone and cortisol directly after trauma film and between sex hormones or cortisol on day two and intrusions. It is possible that a significant effect was constrained to sub-groups that we have not identified. Interactions between endocrinological with physiological, psychological, and social factors might be complex, and while focusing on adrenal and gonadal hormonal influences, we did not model such complexity in our data.
While glucocorticoids, as well as gonadal hormones have both been associated with fear learning and emotional memory modulation, timing of their measurement has been key to demonstrate such associations and has recently been exploited for clinical application to augment extinction learning (Bentz et al., 2010; de Quervain et al., 2011; Milad et al., 2009; Soravia et al., 2014).
Our study is one of the first to explore the role of testosterone in women in an experimental analogue trauma context while accounting for adrenal and gonadal hormonal influences. Cortisol, testosterone in men, and estradiol follow circadian patterns, while no significant diurnal variation seems to exist for progesterone and evidence for diurnal testosterone variation in women has so far not been fully established (Al-Dujaili and Sharp, 2012; Melmed et al., 2016; Panico et al., 1990; Parikh et al., 2018; Strickler et al., 1981). To take advantage of this diurnal hormonal output and to match calculation methods across hormones, we calculated the AUC, but we found no evidence for an association. Despite our findings, testosterone could be a promising biological marker in women and warrants further investigation in larger studies.
Our study is not without limitations. First, participants reported a relatively low number of intrusions among a total of 48 participants for the primary outcome and 40 participants for the secondary outcome, although this finding approximates those of other studies that have employed the trauma film paradigm (Chou et al., 2014; Ferree et al., 2011; Miedl et al., 2018). This limitation could relate to the fact that our participants opted into the research while aware of the stressful nature of the film. Individuals who are more sensitive to such material and, possibly, more susceptible to the impact of trauma might have been unwilling to watch a potentially distressing film clip. Second, while we excluded women who had irregular menstrual cycles, used contraception or other medication, reported hormonal disorders, or with prior experience of interpersonal violence, we did not control for light exposure in the morning, sleep timing including the time of awakening, sleep restriction, exercise, or subjective stress early in the day, which are factors that may affect morning cortisol (Kudielka and Kirschbaum, 2003; Scheer and Buijs, 1999; Vgontzas et al., 2004; Williams et al., 2005). We did not measure the cortisol awakening response. Future studies are needed to investigate potential interactions and joint contributions of such processes. Complex interactions between sex hormones and sleep (Morssinkhof et al., 2020), in particular, warrant future investigations. Third, a higher number of salivary measurements throughout the day could have improved the validity of the AUC. Finally, even though we used a well-studied experimental setting with the trauma film paradigm, the design remains experimental and is not based on women who suffered from a real-life trauma.
Despite these limitations, this study presents important strengths. It is one of the first to examine several sex hormones on day one post-experimental analogue trauma in explaining intrusive memory formation. There is a need for future studies that feature larger samples and that use clinical samples after real-world trauma to better understand these complex relationships. Additional experiments, including animal studies, could provide insight into the exact mechanisms with which sex hormones shape long-term synaptic changes in memory that may explain their potential contribution to intrusive memory formation in PTSD.
Supplementary Material
Acknowledgments
We would like to thank Iva Jelezarova and Alexandra Fritschi for their help with the data collection.
Funding Statement
This work was supported by the Swiss National Science Foundation: [Grant number PZ00P1_126597; PZ00P1_150812; awarded to Birgit Kleim.
Data availability
The dataset used for the current analyses and R-Code supporting the findings of this study are available from the corresponding author [EK] upon request. The complete raw data file cannot be made accessible to the public due to local institutional review board regulations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Al-Dujaili, E., & Sharp, M. (2012). Female Salivary Testosterone: Measurement, Challenges and Applications. In S. M. Ostojic (Ed.), Steroids: From Physiology to Clinical Medicine InTech. 10.5772/53648 [DOI]
- Andreano, J. M., Arjomandi, H., & Cahill, L. (2008). Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology, 33, 874–882. [DOI] [PubMed] [Google Scholar]
- Antypa, D., Vuilleumier, P., & Rimmele, U. (2018). Suppressing cortisol at encoding reduces the emotional enhancement in subjective sense of recollection. Neurobiology of Learning and Memory, 155, 86–91. [DOI] [PubMed] [Google Scholar]
- Bentz, D., Michael, T., de Quervain, D. J., & Wilhelm, F. H. (2010). Enhancing exposure therapy for anxiety disorders with glucocorticoids: From basic mechanisms of emotional learning to clinical applications. Journal of Anxiety Disorders, 24. [DOI] [PubMed] [Google Scholar]
- Breslau, and Anthony . (2007). Gender differences in the sensitivity to posttraumatic stress disorder: An epidemiological study of urban young adults. Journal of Abnormal Psychology, 116. [DOI] [PubMed] [Google Scholar]
- Brewin, C. R. (2011). The nature and significance of memory disturbance in posttraumatic stress disorder. Annual Review of Clinical Psychology, 7, 203–227. [DOI] [PubMed] [Google Scholar]
- Brewin, C. R. (2018). Memory and forgetting. Current Psychiatry Reports, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, R. A., Felmingham, K. L., Silove, D., Creamer, M., O'Donnell, M., & McFarlane, A. C. (2011). The association between menstrual cycle and traumatic memories. Journal of Affective Disorders, 131, 398–401. [DOI] [PubMed] [Google Scholar]
- Cheung, J., Garber, B., & Bryant, R. A. (2015). The role of stress during memory reactivation on intrusive memories. Neurobiology of Learning and Memory, 123, 28–34. [DOI] [PubMed] [Google Scholar]
- Chou, C. Y., La Marca, R., Steptoe, A., & Brewin, C. R. (2014). Biological responses to trauma and the development of intrusive memories: An analog study with the trauma film paradigm. Biological Psychology, 103, 135–143. [DOI] [PubMed] [Google Scholar]
- Christiansen, D. M., & Berke, E. T. (2020). Gender- and Sex-based contributors to Sex differences in PTSD. Current Psychiatry Reports, 22, 19. [DOI] [PubMed] [Google Scholar]
- Cover, K. K., Maeng, L. Y., Lebron-Milad, K., & Milad, M. R. (2014). Mechanisms of estradiol in fear circuitry: Implications for sex differences in psychopathology. Translational Psychiatry, 4, e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs, J. M., Jr., and de La Rue D., 1991, Salivary testosterone measurements among women: Relative magnitude of circadian and menstrual cycles. Hormone Research, 35, 182–184. [DOI] [PubMed] [Google Scholar]
- Davis, S. R., & Wahlin-Jacobsen, S. (2015). Testosterone in women—the clinical significance. The Lancet Diabetes & Endocrinology, 3, 980–992. [DOI] [PubMed] [Google Scholar]
- de Quervain, D. J., Aerni, A., Schelling, G., & Roozendaal, B. (2009). Glucocorticoids and the regulation of memory in health and disease. Frontiers in Neuroendocrinology, 30, 358–370. [DOI] [PubMed] [Google Scholar]
- de Quervain, D. J., Bentz, D., Michael, T., Bolt, O. C., Wiederhold, B. K., Margraf, J., & Wilhelm, F. H. (2011). Glucocorticoids enhance extinction-based psychotherapy. Proceedings of the National Academy of Sciences of the United States of America, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann, S., & Born, J. (2010). The memory function of sleep. Nature Reviews Neuroscience, 11, 114–126. [DOI] [PubMed] [Google Scholar]
- Dudai, Y., Karni, A., & Born, J. (2015). The consolidation and transformation of memory. Neuron, 88, 20–32. [DOI] [PubMed] [Google Scholar]
- Ertman, N., Andreano, J. M., & Cahill, L. (2011). Progesterone at encoding predicts subsequent emotional memory. Learning & Memory, 18, 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham, K., Tran, T., Fong, W., & Bryant, R. (2012). Sex differences in emotional memory consolidation: The effect of stress-induced salivary alpha-amylase and cortisol. Biological Psychology, 89. [DOI] [PubMed] [Google Scholar]
- Ferree, N. K., Kamat, R., & Cahill, L. (2011). Influences of menstrual cycle position and sex hormone levels on spontaneous intrusive recollections following emotional stimuli. Consciousness and Cognition, 20, 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, A. (2012). Discovering Statistics Using R. Los Angeles, Los Angeles: Sage. [Google Scholar]
- Garcia, N. M., Walker, R. S., & Zoellner, L. A. (2018). Estrogen, progesterone, and the menstrual cycle: A systematic review of fear learning, intrusive memories, and PTSD. Clinical Psychology Review, 66, 80–96. [DOI] [PubMed] [Google Scholar]
- Goldstein, J. M., Jerram, M., Poldrack, R., Ahern, T., Kennedy, D. N., Seidman, L. J., & Makris, N. (2005). Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. Journal of Neuroscience, 25, 9309–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesel, D., Wessa, M., & Flor, H. (2006). Psychometric qualities of the German version of the posttraumatic diagnostic scale (PTDS). Psychological Assessment, 18, 262–268. [DOI] [PubMed] [Google Scholar]
- Harooni, H. E., Naghdi, N., Sepehri, H., & Rohani, A. H. (2008). Intra hippocampal injection of testosterone impaired acquisition, consolidation and retrieval of inhibitory avoidance learning and memory in adult male rats. Behavioural Brain Research, 188, 71–77. [DOI] [PubMed] [Google Scholar]
- Hastie, T., & Chambers, J. M. (1992). Statistical models in S. Boca Raton, FL: Chapman & Hall/CRC. [Google Scholar]
- Hautzinger, M., Keller, F., & Kühler, C. (2000). BDI II, Beck Depressionsinventar. Göttingen: Hogrefe. [Google Scholar]
- Hawkins, K. A., & Cougle, J. R. (2013). The effects of nicotine on intrusive memories in nonsmokers. Experimental and Clinical Psychopharmacology, 21, 434–442. [DOI] [PubMed] [Google Scholar]
- Holmes, E. A., & Bourne, C. (2008). Inducing and modulating intrusive emotional memories: A review of the trauma film paradigm. Acta Psychologica, 127, 553–566. [DOI] [PubMed] [Google Scholar]
- Holmes, E. A., Brewin, C. R., & Hennessy, R. G. (2004). Trauma films, information processing, and intrusive memory development. Journal of Experimental Psychology: General, 133, 3–22. [DOI] [PubMed] [Google Scholar]
- Hsu, C., Kleim, B., Nicholson, E., Zuj, D., Cushing, P., Gray, K., … Felmingham, K. (2018). Sex differences in intrusive memories following trauma. PloS ONE, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutschemaekers, M. H. M., de Kleine, R. A., Davis, M. L., Kampman, M., Smits, J. A. J., & Roelofs, K. (2020). Endogenous testosterone levels are predictive of symptom reduction with exposure therapy in social anxiety disorder. Psychoneuroendocrinology, 115, 104612. [DOI] [PubMed] [Google Scholar]
- Jiang, A., Tran, T. T., Madison, F. N., & Bakker, A. (2019). Acute stress-induced cortisol elevation during memory consolidation enhances pattern separation. Learning & Memory, 26, 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, R. A., Cobb, A. R., Lancaster, C. L., Lee, H. J., & Telch, M. J. (2017). Dual-hormone stress reactivity predicts downstream war-zone stress-evoked PTSD. Psychoneuroendocrinology, 78, 76–84. [DOI] [PubMed] [Google Scholar]
- Kearns, M. C., Ressler, K. J., Zatzick, D., & Rothbaum, B. O. (2012). Early interventions for PTSD: A review. Depression and Anxiety, 29, 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum, C., Kudielka, B. M., Gaab, J., Schommer, N. C., & Hellhammer, D. H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61, 154–162. [DOI] [PubMed] [Google Scholar]
- Klinitzke, G., Romppel, M., Hauser, W., Brahler, E., & Glaesmer, H. (2012). Die deutsche Version des Childhood Trauma Questionnaire (CTQ) – psychometrische Eigenschaften in einer bevölkerungsrepräsentativen Stichprobe. PPmP - Psychotherapie Psychosomatik Medizinische Psychologie, 62, 47–51. [DOI] [PubMed] [Google Scholar]
- Kudielka, B. M., & Kirschbaum, C. (2003). Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology, 28, 35–47. [DOI] [PubMed] [Google Scholar]
- Li, S. H., & Graham, B. M. (2017). Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. The Lancet. Psychiatry, 4. [DOI] [PubMed] [Google Scholar]
- Maeng, L., & Milad, M. (2015). Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Hormones and Behavior, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng, L. Y., Taha, M. B., Cover, K. K., Glynn, S. S., Murillo, M., Lebron-Milad, K., & Milad, M. R. (2017). Acute gonadotropin-releasing hormone agonist treatment enhances extinction memory in male rats. Psychoneuroendocrinology, 82, 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margraf, J., & Ehlers, A. (2007). Beck Angst Inventar. Deutschsprachige Adaption des Beck AnxietyInventory von A.T. Beck und R.A. Stern. Göttingen: Horgreve.
- Marks, E. H., Franklin, A. R., & Zoellner, L. A. (2018). Can't get it out of my mind: A systematic review of predictors of intrusive memories of distressing events. Psychological Bulletin, 144, 584–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh, P., & Nelder, J. A. (1999). Generalized Linear Models, v. 37 Ed.2 Repr. 1999. Boca Raton, FL: Chapman & Hall/CRC. [Google Scholar]
- McEwen, B. S., & Sapolsky, R. M. (1995). Stress and cognitive function. Current Opinion in Neurobiology, 5, 205–216. [DOI] [PubMed] [Google Scholar]
- McGaugh, J. L. (2000). Memory–a century of consolidation. Science, 287, 248–251. [DOI] [PubMed] [Google Scholar]
- McGaugh, J. L. (2015). Consolidating memories. Annual Review of Psychology, 66, 1–24. [DOI] [PubMed] [Google Scholar]
- McHenry, J., Carrier, N., Hull, E., & Kabbaj, M. (2014). Sex differences in anxiety and depression: Role of testosterone. Frontiers in Neuroendocrinology, 35, 42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed, S., Polonsky, K. S., Larsen, P. R., & Kronenberg, H. (2016). Williams Textbook of Endocrinology: Textbook of Endocrinology. Philadelphia, PA: Elsevier. [Google Scholar]
- Miedl, S. F., Wegerer, M., Kerschbaum, H., Blechert, J., & Wilhelm, F. H. (2018). Neural activity during traumatic film viewing is linked to endogenous estradiol and hormonal contraception. Psychoneuroendocrinology, 87, 20–26. [DOI] [PubMed] [Google Scholar]
- Milad, M. R., Igoe, S. A., Lebron-Milad, K., & Novales, J. E. (2009). Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocking, R., Pellikaan, C., Lok, A., Assies, J., Ruhé, H., Koeter, M., … Schene, A. (2015). DHEAS and cortisol/DHEAS-ratio in recurrent depression: State, or trait predicting 10-year recurrence? Psychoneuroendocrinology, 59. [DOI] [PubMed] [Google Scholar]
- Morssinkhof, M. W. L., van Wylick, D. W., Priester-Vink, S., van der Werf, Y. D., den Heijer, M., van den Heuvel, O. A., & Broekman, B. F. P. (2020). Associations between sex hormones, sleep problems and depression: A systematic review. Neuroscience and Biobehavioral Reviews, 118. [DOI] [PubMed] [Google Scholar]
- Ney, L. J., Gogos, A., Ken Hsu, C. M., & Felmingham, K. L. (2019). An alternative theory for hormone effects on sex differences in PTSD: The role of heightened sex hormones during trauma. Psychoneuroendocrinology, 109, 104416. [DOI] [PubMed] [Google Scholar]
- Noé, G. (2002). Irréversible [Motion Picture]. France: StudioCanal. [Google Scholar]
- Pan, X., Wang, Z., Wu, X., Wen, S. W., & A, L. (2018). Salivary cortisol in post-traumatic stress disorder: A systematic review and meta-analysis. BMC Psychiatry, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panico, S., Pisani, P., Muti, P., Recchione, C., Cavalleri, A., Totis, A., & Berrino, F. (1990). Diurnal variation of testosterone and estradiol: A source of bias in comparative studies on breast cancer. Journal of Endocrinological Investigation, 13, 423–426. [DOI] [PubMed] [Google Scholar]
- Parikh, T. P., Stolze, B., Ozarda, Y., Jonklaas, J., Welsh, K., Masika, L., & Soldin, S. J. (2018). Diurnal variation of steroid hormones and their reference intervals using mass spectrometric analysis. Endocrine Connections, 7, 1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perugini, M., Gallucci, M., & Costantini, G. (2018). A Practical Primer to Power Analysis for Simple Experimental Designs. International Review of Social Psychology.
- Pitman, R. K., Rasmusson, A. M., Koenen, K. C., Shin, L. M., Orr, S. P., Gilbertson, M. W., & Liberzon, I. (2012). Biological studies of post-traumatic stress disorder: Nature reviews. Neuroscience, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916–931. [DOI] [PubMed] [Google Scholar]
- Reijnen, A., Geuze, E., & Vermetten, E. (2015). The effect of deployment to a combat zone on testosterone levels and the association with the development of posttraumatic stress symptoms: A longitudinal prospective Dutch military cohort study. Psychoneuroendocrinology, 51, 525–533. [DOI] [PubMed] [Google Scholar]
- Rothbaum, B. O., Kearns, M. C., Price, M., Malcoun, E., Davis, M., Ressler, K. J., … Houry, D. (2012). Early intervention may prevent the development of posttraumatic stress disorder: A randomized pilot civilian study with modified prolonged exposure. Biological Psychiatry, 72, 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudioTeam . (2016). RStudio: Integrated Development Environment for R. Boston, MA: RStudio, Inc. [Google Scholar]
- Scheer, F. A., & Buijs, R. M. (1999). Light affects morning salivary cortisol in humans. The Journal of Clinical Endocrinology & Metabolism, 84, 3395–3398. [DOI] [PubMed] [Google Scholar]
- Schultebraucks, K., Rombold-Bruehl, F., Wingenfeld, K., Hellmann-Regen, J., Otte, C., & Roepke, S. (2019). Heightened biological stress response during exposure to a trauma film predicts an increase in intrusive memories. Journal of Abnormal Psychology, 128, 645–657. [DOI] [PubMed] [Google Scholar]
- Seligowski, A., Hurly, J., Mellen, E., Ressler, K., & Ramikie, T. (2020a). Translational studies of estradiol and progesterone in fear and PTSD. European Journal of Psychotraumatology, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligowski, A. V., Harnett, N. G., Merker, J. B., & Ressler, K. J. (2020b). Nervous and endocrine system dysfunction in posttraumatic stress disorder: An overview and consideration of Sex as a biological variable. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibui, K., Uchiyama, M., Okawa, M., Kudo, Y., Kim, K., Liu, X., … Ishibashi, K. (2000). Diurnal fluctuation of sleep propensity and hormonal secretion across the menstrual cycle. Biological Psychiatry, 48, 1062–1068. [DOI] [PubMed] [Google Scholar]
- Soni, M., Curran, V. H., & Kamboj, S. K. (2013). Identification of a narrow post-ovulatory window of vulnerability to distressing involuntary memories in healthy women. Neurobiology of Learning and Memory, 104. [DOI] [PubMed] [Google Scholar]
- Soravia, L. M., Heinrichs, M., Winzeler, L., Fisler, M., Schmitt, W., Horn, H., … de Quervain, D. J. (2014). Glucocorticoids enhance in vivo exposure-based therapy of spider phobia. Depression and Anxiety, 31. [DOI] [PubMed] [Google Scholar]
- Strickler, R. C., Wiest, W. G., Borth, R., & Woolever, C. A. (1981). Daily and circadian variations in serum free testosterone levels are not clinically significant. American Journal of Obstetrics and Gynecology, 140. [DOI] [PubMed] [Google Scholar]
- Sundstrom Poromaa, I., & Gingnell, M. (2014). Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Frontiers in Neuroscience, 8, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin, D., & Foa, E. (2006). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132. [DOI] [PubMed] [Google Scholar]
- van Ast, V. A., Cornelisse, S., Meeter, M., & Kindt, M. (2014). Cortisol mediates the effects of stress on the contextual dependency of memories. Psychoneuroendocrinology, 41, 97–110. [DOI] [PubMed] [Google Scholar]
- van der Velden, P. G., Kleber, R. J., & Koenen, K. C. (2008). Smoking predicts posttraumatic stress symptoms among rescue workers: A prospective study of ambulance personnel involved in the enschede fireworks disaster. Drug and Alcohol Dependence, 94, 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables, W. N., & Ripley, B. D. (2010). Modern Applied Statistics with S. New York, NY: Springer Science + Business Media. [Google Scholar]
- Vgontzas, A. N., Zoumakis E., Bixler E. O., Lin H. M., Follett H., Kales A., and Chrousos G. P., 2004, Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines: The Journal of Clinical Endocrinology & Metabolism, v. 89, p. 2119-2126. [DOI] [PubMed] [Google Scholar]
- von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., Vandenbroucke, J. P., & Initiative, S. (2014).The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. International Journal of Surgery, 12. [Google Scholar]
- Wegerer, M., Kerschbaum, H., Blechert, J., & Wilhelm, F. H. (2014). Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiology of Learning and Memory, 116, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann, A., Conradi, A., Groger, K., Fehm, L., & Fydrich, T. (2009). Using stressful films to analyze risk factors for PTSD in analogue experimental studies – which film works best? Anxiety, Stress & Coping, 22, 549–569. [DOI] [PubMed] [Google Scholar]
- Williams, E., Magid, K., & Steptoe, A. (2005). The impact of time of waking and concurrent subjective stress on the cortisol response to awakening. Psychoneuroendocrinology, 30, 139–148. [DOI] [PubMed] [Google Scholar]
- Wittchen, H.-U. (2006). Klinische Psychologie und Psychotherapie. Heidelberg: Springer Medizin Verlag. [Google Scholar]
- Zhao, J., Leung, J. Y., Lin, S. L., & Schooling, C. M. (2016). Cigarette smoking and testosterone in men and women: A systematic review and meta-analysis of observational studies. Preventive Medicine, 85, 1–10. [DOI] [PubMed] [Google Scholar]
- Zorawski, M., Blanding, N. Q., Kuhn, C. M., & LaBar, K. S. (2006). Effects of Stress and sex on Acquisition and Consolidation of Human Fear Conditioning, 13. Cold Spring Harbor, NY: Learning & Memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used for the current analyses and R-Code supporting the findings of this study are available from the corresponding author [EK] upon request. The complete raw data file cannot be made accessible to the public due to local institutional review board regulations.