Abstract
Background:
The NSABP B-36 compared 4 cycles of doxorubicin and cyclophosphamide (AC) with 6 cycles of 5-fluoururacil, epirubicin, cyclophosphamide (FEC-100) in node-negative early-stage breast cancer. A sub-study within B-36, focusing on symptoms, quality of life (QOL), menstrual history (MH), and cardiac function (CF) was conducted.
Patients and methods:
Patients completed the QOL questionnaire at baseline, during treatment, and every 6 months through 36 months. FACT-B Trial Outcome Index (TOI), symptom severity, and SF-36 Vitality and Physical Functioning (PF) scales scores were compared between the two groups using a mixed model for repeated measures analysis. MH was collected at baseline and subsequently assessed if menstrual bleeding occurred within 12 months prior to randomization. Post-chemotherapy amenorrhea outcome was examined at 18 months and was defined as lack of menses in the preceding year. Logistic regression was used to test for association of amenorrhea and treatment. CF assessment was done at baseline and 12 months. Correlation analysis was used to address associations between changes in baseline- and 12-month PF and concurrent CF changes measured by LVEF.
Results:
FEC-100 patients had statistically significantly lower TOI scores during chemotherapy (P=0.02) and at 6 months (P<0.001); lower Vitality score at 6 months (P<0.01), and lower PF score during the first year than AC patients. There were no statistically significant QOL score differences between the two groups beyond 12 months. No significant differences in symptom severity between the two groups were observed. Rates of amenorrhea were significantly different between FEC-100 and AC (67.4% vs. 59.1%, P<0.001). There was no association between changes in LVEF and PF (P=0.38).
Conclusions:
Statistically significant QOL differences between the two groups favored AC; however, the magnitude was small and unlikely to be clinically meaningful. There was a clinical and statistically significant difference in risk for amenorrhea, favoring AC.
Keywords: Quality of life, amenorrhea, cardiac function, early-stage breast cancer, adjuvant chemotherapy
Introduction
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-36 trial was designed to evaluate whether standard adjuvant therapy with 4 cycles of doxorubicin and cyclophosphamide (AC) for node-negative early stage breast cancer could be improved upon by using a more intensive 6-cycle anthracycline therapeutic regimen (5-fluoururacil, epirubicin, and cyclophosphamide: FEC-100), which was widely used in Europe and Canada. In the early years of the 21st century, when this trial was designed, genomic tests were not yet available to identify patients with node-negative breast cancer, who were at low risk of recurrence1 and/or who could benefit from chemotherapy.2 All women with invasive breast cancers >1 centimeter in size were considered appropriate for adjuvant chemotherapy.3 Contemporary use of adjuvant chemotherapy in this patient population is limited to patients with high recurrence risk or unfavorable biological features; however, understanding the impact of these two treatment regimens on quality of life (QOL), symptoms, and other post-treatment survivorship concerns was an important consideration at the time the B-36 trial was launched.
We designed a behavioral and health outcomes study within the B-36 clinical trial, focusing on symptoms and quality of life. In addition, we hypothesized that women in the AC arm would have a lower likelihood of amenorrhea than those in the FEC-100 arm, and we planned to explore whether or not post-treatment amenorrhea would have an effect on survival outcomes. Finally, we were interested in the impact of the two different anthracycline regimens and their cumulative doses on cardiac function as measured by MUGA scan and self-report measures in a sub-study of patients who had baseline and one-year assessments. In this report, we describe the results of our findings from the behavioral and health outcomes study.
Methods
Patient-reported outcomes
QOL and symptoms were measured by a survey battery with the following measures: Functional Assessment of Cancer Therapy (FACT-B),4 a treatment-specific symptom checklist, Vitality and Physical Functioning (PF) scales from the short form health survey (SF-36) instrument.5 All of the questionnaires are well established and have been previously used in breast cancer clinical trials.6,7 A 23-item FACT-B trial outcome index (TOI), the primary outcome of the QOL sub-study, ranges from 0 to 92, with a higher score indicating better QOL, and a 5-point difference being clinically meaningful.8 Symptoms were assessed using 32 items from the Breast Cancer Prevention Trial symptom checklist9–11 and other NSABP symptom checklist toxicity questions. Patients rated each symptom during the past 7 days with a bother rating of 0=“not at all” to 4=“very much.” Total symptom severity scores ranged from 0 to 128, with higher being worse.
Menstrual history (MH) sub-study
MH status was self-reported for all women at study entry by assessment of surgical menopause and menstrual bleeding pattern in the previous 12 months. Pre- or perimenopausal women, defined as those with menstrual bleeding within 12 months prior to random assignment and not having had a hysterectomy and/or bilateral oophorectomy, were included in the MH sub-study. Follow-up questionnaires tracked changes in MH and possible surgical menopause only for these patients.
MH and QOL questionnaires were administered at baseline, during chemotherapy (cycle 4, day 1), and every 6 months up to 36 months after randomization. A missing-data form was completed by the institutional staff when a questionnaire was not completed for a given assessment.
Cardiac functioning (CF) sub-study
The first 450 patients enrolled in B-36 were included in the CF sub-study. CF assessment was done at baseline and 12 months after study enrollment with echocardiogram or MUGA scan (investigator discretion), at institutional clinical facilities. Baseline was clinically required for study entry and the 12-month assessment was paid for by the research study. Follow-up assessment was scheduled at the same facility if possible and with the same assessment method for the two time points.
Patients who experienced a breast cancer recurrence or second primary cancer were not expected to continue the MH and QOL assessments or have the 12-month echocardiogram or MUGA.
Statistical methods
Demographic and medical characteristics of patients participating in the QOL, MH, and/or CF sub-studies were compared between the two treatment groups and to the characteristics of B-36 patients not in these sub-studies. Questionnaire compliance was evaluated using a mixed model for repeated measures logistic regression.
TOI, symptom severity, and Vitality and PF scores were compared between the two treatment groups using a mixed model for repeated measures with adjustment for the baseline scores, type of surgery, and hormone receptor status, examining the first 12 months and the later time points in the separate models with the primary analysis focusing on the pattern of patient-reported outcomes during the first year after randomization. We assessed potential interactions between the covariates for the models evaluating the first year after randomization.
Post-chemotherapy amenorrhea was defined as the lack of menstrual periods during the 12 months preceding the 18-month follow-up evaluation and was chosen as the primary outcome for the MH sub-study. Logistic regression, adjusted for age, was used to test for association of amenorrhea status and treatment. A log-rank test was used to assess the association of post-chemotherapy amenorrhea with survival outcomes (DFS and OS). These analyses were conditional on a patient’s participation in the MH sub-study and on her DFS for at least 18 months following randomization.
The primary focus of the CF sub-study was assessing whether changes in PF from baseline to the 12-month evaluation were related to concurrent changes in cardiac functioning, as measured by LVEF. Correlation analysis, adjusted for age at study entry, was used to address this relationship. Partial correlation coefficient was reported. The association between treatment group and changes in LVEF was also explored by means of linear regression.
All analyses used 2-sided tests at 0.05 significance level.
The protocol was approved by institutional IRBs. Written informed consent was required of all participants.
RESULTS
Patient characteristics
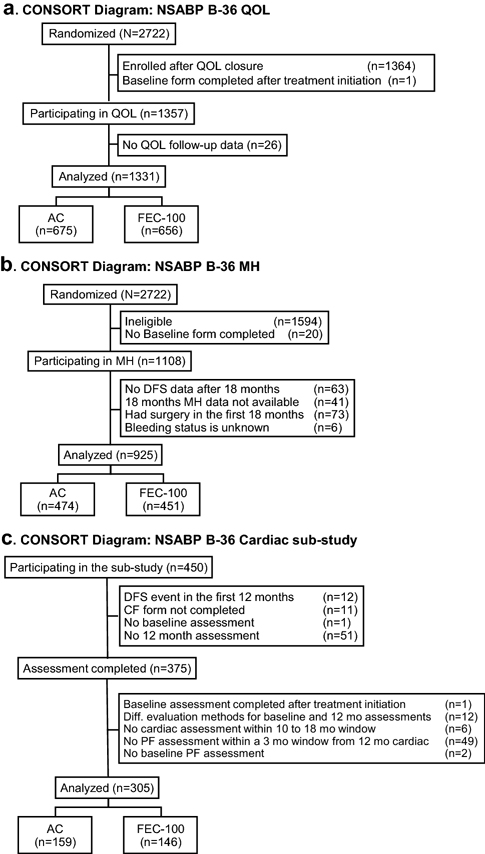
Between May 2004 and July 2008, 2,722 patients were randomly assigned to either AC (n=1,361) or FEC-100 (n=1,361). QOL sub-study accrual was closed on August 11, 2006 with 1,358 patients enrolled and completing the baseline form. There were 1,331 patients (AC:675, FEC-100:656) who had at least one follow-up QOL form and were included in the analyses (Figure 1a). QOL sub-study patient characteristics are presented in Table 1. Lumpectomy was the primary surgery for 68% of patients with 95% receiving adjuvant radiotherapy. Nine percent of mastectomy patients received radiotherapy. Women who participated in the QOL sub-study were slightly younger than the rest of the B-36 patients. The distribution of baseline QOL measures were similar between the two treatment groups.
Figure 1, CONSORT Diagram: NSABP B-36.
A. Quality-of-life (QOL) sub-study
B. Menstrual History (MH) sub-study
C. Cardiac sub-study
AC, doxorubicin and cyclophosphamide; FEC-100, 5-fluorouracil, epirubicin and cyclophosphamide.
Table 1.
Baseline patient and tumor characteristics of women enrolled in NSABP B-36
| Characteristic | QOL study population |
Other B-36 patients (n=1,391) n (%) | P*†value | |||
|---|---|---|---|---|---|---|
| AC (n=675) n (%) | FEC-100 (n=656) n (%) | P* value | Total (n=1,331) n (%) | |||
| Age (years) | ||||||
| ≤ 49 | 280 (41.5) | 284 (43.3) | 0.50 | 564 (42.4) | 534 (38.4) | 0.03 |
| ≥ 50 | 395 (58.5) | 372 (56.7) | 767 (57.6) | 857 (61.6) | ||
| Race | ||||||
| White | 555 (82.2) | 565 (86.1) | 0.049 | 1120 (84.1) | 1177 (84.6) | 0.84 |
| Black/African American | 75 (11.1) | 52 (7.9) | 127 (9.5) | 130 (9.3) | ||
| Other | 26 (3.9) | 30 (4.6) | 56 (4.2) | 61 (4.4) | ||
| Unknown | 19 (2.8) | 9 (1.4) | 28 (2.1) | 23 (1.7) | ||
| Ethnicity | ||||||
| Hispanic or Latina | 40 (5.9) | 40 (6.1) | 0.91 | 80 (6.0) | 100 (7.2) | 0.34 |
| Not Hispanic or Latina | 590 (87.4) | 576 (87.8) | 1166 (87.6) | 1213 (87.2) | ||
| Unknown | 45 (6.7) | 40 (6.1) | 85 (6.4) | 78 (5.6) | ||
| Hormone receptor status | ||||||
| ER− and PgR− | 227 (33.6) | 227 (34.6) | 0.71 | 454 (34.1) | 488 (35.1) | 0.59 |
| ER+ and/or PgR+ | 448 (66.4) | 429 (65.4) | 877 (65.9) | 903 (64.9) | ||
| Surgery type | ||||||
| Lumpectomy | 457 (67.7) | 453 (69.1) | 0.60 | 910 (68.4) | 933 (67.1) | 0.47 |
| Mastectomy | 218 (32.3) | 203 (30.9) | 421 (31.6) | 458 (32.9) | ||
| Tumor grade | ||||||
| Low | 82 (12.1) | 61 (9.3) | 0.23 | 143 (10.7) | 143 (10.3) | 0.86 |
| Intermediate | 246 (36.4) | 263 (40.1) | 509 (38.2) | 518 (37.2) | ||
| High | 343 (50.8) | 330 (50.3) | 673 (50.6) | 722 (51.9) | ||
| Unknown | 4 (0.6) | 2 (0.3) | 6 (0.5) | 8 (0.6) | ||
|
| ||||||
| Size of primary tumor | ||||||
| ≤ 2 cm | 414 (61.3) | 404 (61.6) | 0.49 | 818 (61.5) | 827 (59.5) | 0.38 |
| 2.1 – 5 cm | 253 (37.5) | 239 (36.4) | 492 (37.0) | 537 (38.6) | ||
| > 5 cm | 8 (1.2) | 13 (2.0) | 21 (1.6) | 25 (1.8) | ||
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.1) | ||
| Mean (STD) | Mean (STD) | |||||
| FACT-B TOI | 69.48 (12.15) | 69.73 (11.76) | 0.71 | |||
| SF-36 Vitality subscale | 63.13 (20.11) | 63.20 (19.78) | 0.95 | |||
| Symptoms score | 13.84 (10.97) | 14.11 (11.60) | 0.66 | |||
| SF-36 PF-10 subscale | 76.43 (23.08) | 78.53 (21.51) | 0.09 | |||
P value is based on the chi-square test for categorical variables, t-test for continuous measures.
To compare patients who participated in the QOL sub-study to other B-36 patients.
Compliance with data collection
The QOL form submission rates were high, ranging from 92% during chemotherapy to 86% at the 24, 30, and 36-month time-points with no difference between the treatment groups. Slightly better compliance was observed in white (P value=0.01), older (≥50, P value=0.03) patients, who had lumpectomy as their primary surgery (P value=0.02). These results are consistent with other reports from our group.12
QOL outcomes
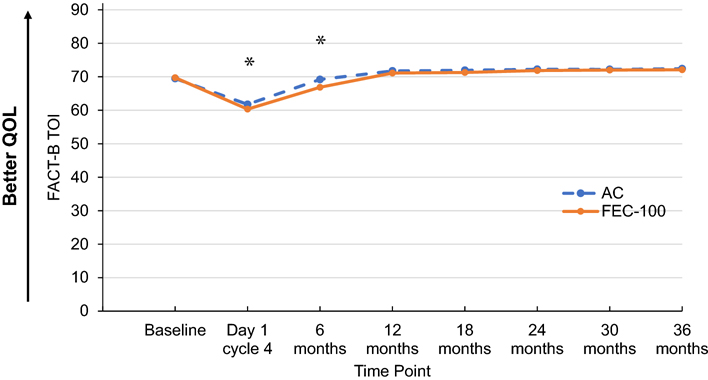
Patients receiving FEC-100 had statistically significantly lower TOI score than did patients receiving AC during chemotherapy (day 1 of cycle 4) and at 6 months, with no difference at 12 months after randomization (time-by-treatment interaction P value=0.03). There were no statistically significant differences in TOI scores between the two treatment groups beyond 12 months (P value=0.34) (Figure 2).
Figure 2. FACT-B Trial Outcome Index (TOI) by treatment group and time point.
Adjusted least-square mean scores for all timepoints after baseline are obtained from mixed model for repeated measures analysis of the score.
AC, doxorubicin and cyclophosphamide; FEC-100, 5-fluorouracil, epirubicin and cyclophosphamide.
Footnote: *statistically significant differences observed: Day 1: FEC-100:60.34; AC:61.76, difference= −1.42, 95%CI=(−2.63,−0.22), P value=0.02; 6 months: FEC-100:66.86; AC:69.21; difference= −2.35, 95%CI=(−3.57,−1.13), P value<0.001.
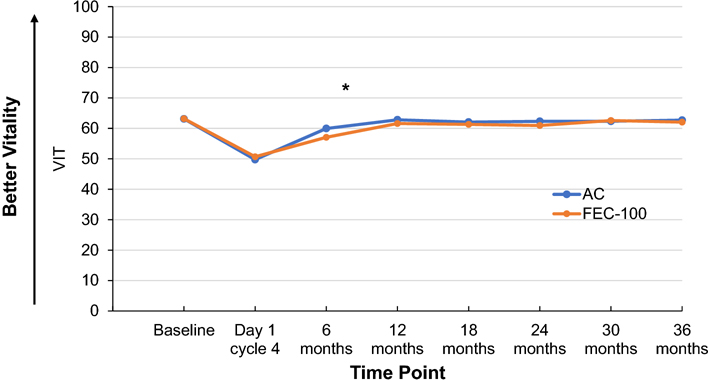
Patients receiving FEC-100 had a statistically significantly lower Vitality score than did patients receiving AC at 6 months with no difference during chemotherapy and at 12 months after randomization (time-by-treatment interaction P value=0.004). There were no statistically significant differences in Vitality scores between the two treatment groups beyond 12 months (P value=0.31) (Figure 3).
Figure 3. SF-36 Vitality scale score by treatment group and time point.
Adjusted least-square mean scores for all timepoints after baseline are obtained from mixed model for repeated measures analysis of the score.
AC, doxorubicin and cyclophosphamide; FEC-100, 5-fluorouracil, epirubicin and cyclophosphamide Footnote: *statistically significant differences observed: 6 months: FEC-100:57.06; AC:59.97, difference=−2.91, 95%CI=(− 4.89,−0.93), P value=0.004.
On average, patients receiving FEC-100 had slightly more bothersome symptoms than did patients receiving AC during the first year after treatment initiation and beyond 12 months, though neither of the differences were statistically significant (P values=0.13 and 0.57) (Supplemental Figure S1).
Among women who were not bothered by a particular symptom at baseline or bothered “a little bit,” the most commonly reported symptoms at levels “somewhat” or higher three years after randomization were hot flashes (38.6%), joint pains (38.5%), weight gain (32.0%), general aches and pains (32.0%), muscle stiffness (30.0%), and forgetfulness (29.1%). See supplemental figures to show the pattern of severity scores over time. (Supplemental Figures S2–S7).
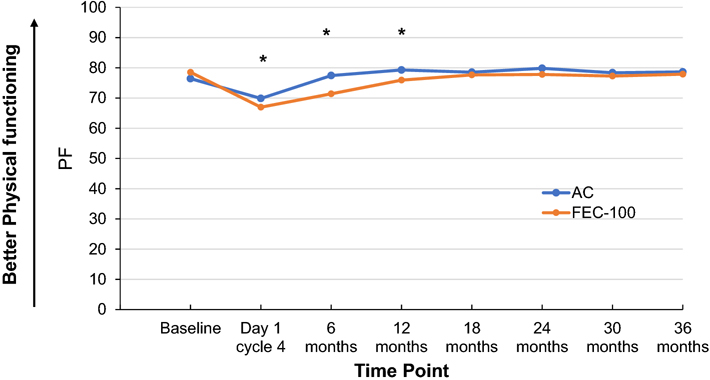
Patients receiving FEC-100 had statistically significantly lower PF scores than did patients receiving AC during the first year after randomization. The magnitude of the difference varied by time (time-by-treatment interaction P value=0.03). There were no statistically significant differences in PF scores between the two treatment groups beyond 12 months (P value=0.08) (Figure 4).
Figure 4. SF-36 Physical functioning score by treatment group and time point.
Adjusted least-square mean scores for all timepoints after baseline are obtained from mixed model for repeated measures analysis of the score.
AC, doxorubicin and cyclophosphamide; FEC-100, 5-fluorouracil, epirubicin and cyclophosphamide.
Footnote: *statistically significant differences observed: Day 1: FEC-100:66.97; AC:69.86, difference=−2.90, 95%CI=(−5.15, −0.65), P value=0.01; 6 months: FEC-100:71.40; AC:77.44, difference=−6.04, 95%CI=(−8.32, −3.77), P value<0.001; and 12 months: FEC-100:75.94; AC:79.29, difference=−3.35, 95%CI=(−5.64, −1.06), P value=0.004.
As expected, patients had worse QOL (lower scores for TOI, Vitality, and PF) and higher symptom burden while on treatment compared to baseline. Although patients recovered with time in terms of QOL, the symptoms scores remained elevated after randomization, not returning to baseline levels. Patients who reported poor baseline scores in terms of QOL or symptoms recovered the most by 12 months compared to the on-treatment assessment (time-by-corresponding baseline score interaction P value<0.05 for all considered endpoints). The difference between patients who received lumpectomy versus mastectomy was more pronounced for women who reported poor baseline TOI or PF scores, with mastectomy patients doing better (surgery-by-corresponding baseline score interaction P value<0.05 for TOI and PF). Mastectomy patients recovered the most by 12 months compared to the on-treatment assessment in terms of Vitality (time-by-surgery interaction P value=0.003). Symptom recovery was different based on patient’s receptor status with receptor-negative patients recovering the most by 12 months compared to the on-treatment assessment (time by receptor status interaction P value=0.003).
MH outcomes
There were 1,108 B-36 patients eligible for the MH sub-study, based on their reporting that they had menstrual bleeding within 12 months prior to randomization and had not received a hysterectomy and/or bilateral oophorectomy (Figure 1b). Among them, 1,004 were DFS-event-free and had MH data available at 18 months. Patients who had had a hysterectomy and/or bilateral oophorectomy sometime between randomization and the 18-month assessment were excluded from the analysis (n=73). In addition, for six patients, menstrual bleeding status within 12 months preceding the 18-month evaluation was unknown. Therefore, the analysis was based on 925 patients (AC:474, FEC-100:451). Patient and tumor characteristics of women included in the MH sub-study are presented in Supplemental Table S1. Rates of post-chemotherapy amenorrhea were statistically significantly different between the two treatment groups (FEC-100:67.4%, AC:59.1%, OR=2.0, 95%CI=1.4,2.8; P value<0.001 age-adjusted). Among 661 hormone receptor-positive patients, 25 did not take any hormonal therapy, 575 reported taking tamoxifen, and 59 reported taking other hormonal therapy (primarily an aromatase inhibitor) at some point during the first 18 months after randomization. The status was unknown for two patients. Among receptor-positive patients who did not take any hormonal therapy and receptor-positive patients who reported taking tamoxifen, the rates of amenorrhea were slightly higher than among receptor-negative patients (60% and 63% vs. 58%), however the difference among these three groups was not statistically significant (overall P value=0.47, adjusted for treatment and age). Among women who reported taking hormonal therapy other than tamoxifen, 90% were considered amenorrheic. In the exploratory analysis of the effect of chemotherapy on frequency of amenorrhea, an indication for possible interaction (P value=0.08) between chemotherapy and age was observed (Supplemental Figure S8). There were no statistically significant differences in DFS (HR=1.08, 95%CI=0.75,1.54, P value=0.70) and OS (HR=1.19, 95%CI=0.65,2.17, P value=0.58) for patients who developed post-chemotherapy amenorrhea compared to those who did not.
Cardiac outcomes
Among the first 450 patients enrolled in B-36, 305 contributed data to the cardiac assessment analysis (Figure 1c, Supplemental Table S2). Average baseline LVEF was 64.8. There was a statistically significant decrease (p<0.001) in LVEF from baseline to 12 months (Table 2). The change from baseline to 12 months in terms of PF was not statistically significantly different (P value=0.14). There was no statistically significant difference between treatment groups in terms of LVEF changes (P value=0.96). Treatment effect on PF in the cardiac subgroup was similar to that observed for the whole QOL population, however the difference between the two treatment groups did not reach statistical significance (P value=0.25). There was no association between LVEF and patient-reported changes in PF between baseline and 12 months after randomization (partial Spearman correlation coefficient=−0.05, P value=0.38) (Supplemental Figure S9).
Table 2.
Relationship between treatment, physical, and cardiac functioning:* NSABP B-36
| Treatment | Change from baseline to 12 months |
|
|---|---|---|
| Physical Functioning | LVEF | |
| Overall | −1.74 (−4.03, 0.55) | −2.61 (−3.39,−1.83) |
| AC | −0.46 (−3.64, 2.71) | −2.61 (−3.70, −1.53) |
| FEC-100 | −3.18 (−6.49, 0.14) | −2.65 (−3.79, −1.52) |
| Difference FEC-100 vs. AC | −2.72 (−7.33, 1.89) | −0.04 (−1.61, 1.53) |
| P value | 0.25 | 0.96 |
Changes are model-based and adjusted for age and corresponding baseline measure; evaluated at average age of 51.8 and average baseline PF-10 score of 79.4, or average baseline LVEF score of 64.8.
Discussion
Although much has changed in the adjuvant treatment of early-stage breast cancer since the B-36 trial was initiated, we are unaware of any studies comparing these two regimens that have examined patient-reported outcomes, menstrual history, or cardiac outcomes in this setting. This is especially important, because the event-free survival for women with node-negative breast cancer is extremely good, and no statistically significant difference in OS was seen between the two treatment groups (Geyer, et al).13 At the time this study was conducted, however, there was uncertainty concerning the benefits of longer duration therapy, which does not appear to be beneficial, based on the outcomes of the B-36 trial.13
Based on the results of the B-36 trial,13 there can be no clinical justification for the longer and more costly FEC-100 regimen. This decision is further supported by the more prompt recovery of QOL, Vitality, symptoms, and PF among the AC-treated group, although by 12 months after randomization, both groups functioned at near baseline levels, with the exception of a greater delay in return of PF scores in the FEC-100 group. Certainly, by 2- and 3-years of follow-up, there are no treatment differences. It is also reassuring to note that the findings regarding recovery after AC treatment are similar to those reported in an earlier comparison of AC with CMF in the NSABP B-23 QOL study.7
Symptom severity summary scores nearly double during chemotherapy treatment, reflecting the acute treatment toxicities, but they do not return to baseline years after treatment ends. As can be seen with several of the selected symptoms (hot flashes, weight gain, joint pains and muscle aches, forgetfulness, see Supplemental Figures), women who had no symptoms prior to treatment are burdened with unremitting treatment-associated symptoms up to 36 months later. Endocrine therapy may also be contributing to this sustained elevation in symptoms.14 This type of information is important to share with patients during the survivorship period and demonstrates the need to provide symptom-focused post-treatment care, because these are the lasting effects of adjuvant chemotherapy, along with endocrine therapy, which often accompany this treatment.
With regard to the MH component of this study, we found high rates of amenorrhea at 18 months, i.e., 12 months after the last chemotherapy. During this time, resumption of menses would indicate that the woman was not postmenopausal. The rate of 18 month amenorrhea was 8% higher in the FEC-100-treated patients, and as in our earlier MH study in the B-30 trial,6 we found a higher likelihood of amenorrhea at 18 months with use of endocrine therapy. A recent systematic review of factors associated with recovery of menses after chemotherapy15 noted that taxane therapy was not protective, and that endocrine therapy delayed recovery. Thus, for counseling younger patients who may want to consider a pregnancy after adjuvant chemotherapy, the shorter course of AC therapy may offer a greater chance of recovery of menstrual function. We could not document a significant correlation between decline in LVEF and PF and there was no evidence of statistically significant difference in LVEF decline from baseline to 12 months between the two treatment groups based on our cardiac sub-study.
There were a few other interesting findings that emerged from examination of interactions of medical covariates with the QOL and MH outcomes, specifically in relationship to the type of surgical treatment. Although TOI scores recovered after on-treatment nadir, among women who reported poor or average baseline TOI scores, mastectomy patients were doing better compared to lumpectomy patients. For Vitality, mastectomy patients recovered the most by 12 months compared to on-treatment assessments. We hypothesize that this may be related to the more extended treatment period in the lumpectomy patients who received post-chemotherapy radiotherapy. Receptor-negative patients recovered the most by 12 months compared to the on-treatment assessment in terms of symptoms, which may reflect the added burden of endocrine therapy in the hormone receptor-positive patients. We had hypothesized an interaction between age, amenorrhea, and treatment regimen, but only found support for a trend (p=.08), with women <45 years appearing to have higher rates of amenorrhea with FEC-100 than AC.
In conclusion, in this large sample of patients we detected statistically significant differences in QOL between the two treatment regimens, favoring AC; however, the magnitude of the differences was small and unlikely to be clinically meaningful. At the same time, difference in risk for amenorrhea, when comparing these two commonly used chemotherapy regimens, was clinically and statistically significant favoring AC over FEC-100. In this group of women with very favorable prognoses, using the more limited AC treatment regimen in clinical practice makes sense given the main survival outcomes from the B-36 trial,13 as well as the additionally favorable QOL outcomes. Unfortunately, we do not have the genomic characteristics of these tumors, which might have led to the overall avoidance of chemotherapy altogether. Finally, the detailed assessment of QOL, symptoms, amenorrhea, and cardiac function in this study of patients with early-stage breast cancer demonstrates the need for multi-disciplinary care in the years following treatment to manage ongoing and late effects of adjuvant chemotherapy. Although most patients do fare well, there will be some who need specialized follow-up care.
Supplementary Material
Acknowledgements
The authors would also like to acknowledge John Wilson, PhD (retired), who was the original Protocol Statistician for the study and was an invaluable part of the protocol team. The authors acknowledge the contributions of Barbara C. Good, PhD, Director of Scientific Publications, Christine I. Rudock, Publications and Graphics Specialist, and Wendy L. Rea, BA, Editorial Associate, all of whom are employees of NSABP. They were not compensated beyond their normal salaries for this work.
Disclosure of potential conflicts of interest
Charles E. Geyer, Jr. - Grants, non-financial support and other from Genentech/Roche, Daiichi/Sankyo, and AstraZeneca, during the conduct of the study, and personal fees from Exact Sciences and Athenex, outside the submitted work.
Johnathan Polikoff – Consultant, Natera.
Louis Provencher - Consulting or Advisory Role: Lilly, Pfizer, Roche, Novartis. Research Funding: Pfizer, Roche, Novartis, Merck, GlaxoSmithKline, Odonate Therapeutics
The remaining authors declare no conflicts of interest.
Funding
This work was supported by the National Institutes of Health grants U10-CA-180868, -189867, -180822, and -44066–26; and Pharmacia & Upjohn Company, a subsidiary of Pfizer, Inc.
Footnotes
ClinicalTrials.gov: NCT00087178; Date of Registration: 07/08/2004.
Availability of Data and Material (data transparency)
Individual participant data that underlie the results reported in this article, after deidentification, will generally be available within one year after publication and will be accessible through the NCTN Data Archive.
Compliance with Ethical Standards
Ethics Approval
The protocol was approved by institutional IRBs. All studies involving human participants are in accord with ethical standards of the institutional research board and the Helsinki declaration or comparable ethical standards.
Informed Consent to Participate
The study was approved by the local institutional review board. Written informed consent was required of all participants.
Code Availability (software application or custom code)
Analyses were performed using SAS standard procedures (v9.4; SAS Institute, Cary, NC
Consent for Publication
All authors have consented for publication.
The funders had no role in the design of the study; the collection, analysis, and/or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Previous, related work:
Ganz PA, Wilson JW, Bandos H, et al. Impact of treatment on quality of life (QOL) and menstrual history (MH) in the NSABP B-36: A randomized phase III trial comparing six cycles of 5-fluorouracil (5-FU), epirubicin, and cyclophosphamide (FEC) to four cycles of adriamycin and cyclophosphamide (AC) in patients (pts) with node-negative breast cancer. SABCS (San Antonio Breast Cancer Symposium) 2014;75(9 suppl). Published 5–1-15. Abstr P3–12-01.
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
REFERENCES
- 1.Paik S, Shak S, Tang, et al. : A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351:2817–2826. https://www.ncbi.nlm.nih.gov/pubmed/?term=15591335 [DOI] [PubMed] [Google Scholar]
- 2.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006; 24:3726–3734. https://www.ncbi.nlm.nih.gov/pubmed/?term=16720680 [DOI] [PubMed] [Google Scholar]
- 3.Early stage breast cancer. Consens Statement. 1990. Jun 18–21;8(6):1–19. Review. https://www.ncbi.nlm.nih.gov/pubmed/2247093 [PubMed] [Google Scholar]
- 4.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997; 15:974–986. https://www.ncbi.nlm.nih.gov/pubmed/?term=9060536 [DOI] [PubMed] [Google Scholar]
- 5.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992; 30:473–483. https://www.ncbi.nlm.nih.gov/pubmed/?term=1593914 [PubMed] [Google Scholar]
- 6.Ganz PA, Land SR, Geyer CE Jr, et al. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol. 2011; 29:1110–1116. https://www.ncbi.nlm.nih.gov/pubmed/?term=PMC3083866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Land SR, Kopec JA, Yothers G, Anderson S, et al. Health-related quality of life in axillary node-negative, estrogen receptor-negative breast cancer patients undergoing AC versus CMF chemotherapy: Findings from the National Surgical Adjuvant Breast and Bowel Project B-23. Breast Cancer Res Treat. 2004; 86:153–164. https://www.ncbi.nlm.nih.gov/pubmed/?term=15319567 [DOI] [PubMed] [Google Scholar]
- 8.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004; 57:898–910. https://www.ncbi.nlm.nih.gov/pubmed/?term=15504633 [DOI] [PubMed] [Google Scholar]
- 9.Ganz PA, Day R, Ware JE Jr, Redmond C, Fisher B. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995; 87(18):1372–1382. https://www.ncbi.nlm.nih.gov/pubmed/?term=7658498 [DOI] [PubMed] [Google Scholar]
- 10.Cella D, Land SR, Chang CH, et al. Symptom measurement in the Breast Cancer Prevention Trial (BCPT) (P-1): Psychometric properties of a new measure of symptoms for midlife women. Breast Cancer Res Treat. 2008; 109:515–526. https://www.ncbi.nlm.nih.gov/pubmed/?term=17851765 [DOI] [PubMed] [Google Scholar]
- 11.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005; 97:448–456. https://www.ncbi.nlm.nih.gov/pubmed/?term=15770009 [DOI] [PubMed] [Google Scholar]
- 12.Land SR, Ritter MW, Costantino JP, et al. Compliance with patient-reported outcomes in multicenter clinical trials: Methodologic and practical approaches. J Clin Oncol. 2007; 25:5113–5120. https://www.ncbi.nlm.nih.gov/pubmed/?term=17991930 [DOI] [PubMed] [Google Scholar]
- 13.Companion paper: Geyer, et al. Geyer CE Jr., Bandos H, Elledge RM, et al. Definitive Results of a Phase III Adjuvant Trial Comparing Six Cycles of FEC-100 to Four Cycles of AC in Women with Operable Node Negative Breast Cancer: The NSABP B-36 Trial (NRG Oncology). [DOI] [PMC free article] [PubMed]
- 14.Ganz PA, Cecchini RS, Julian TB, et al. : Patient-reported outcomes with anastrozole versus tamoxifen for postmenopausal patients with ductal carcinoma in situ treated with lumpectomy plus radiotherapy (NSABP B-35): A randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387(10021):857–865. https://pubmed.ncbi.nlm.nih.gov/26686960/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva C, Caramelo O, Almeida-Santos T, Ribeiro Rama AC. Factors associated with ovarian function recovery after chemotherapy for breast cancer: A systematic review and meta-analysis. Hum Reprod. 2016; 31:2737–2749. https://www.ncbi.nlm.nih.gov/pubmed/?term=27664208 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.