Abstract
Background
For decades, the dopamine D2 receptor (D2R) has been known as the main target of antipsychotic medications, but the mechanism for antipsychotic effects beyond this pharmacological target remains unclear. Disrupted-in-schizophrenia 1 (DISC1) is a gene implicated in the etiology of schizophrenia, and we have found elevated levels of the D2R-DISC1 complex in the postmortem brain tissue of patients with schizophrenia.
Methods
We used coimmunoprecipitation to measure D2R-DISC1 complex levels in peripheral blood samples from patients with schizophrenia and unaffected controls in 3 cohorts (including males and females) from different hospitals. We also used label-free mass spectrometry to conduct proteomic analysis of these samples.
Results
Levels of the D2R-DISC1 complex were elevated in the peripheral blood samples of patients with schizophrenia from 3 independent cohorts, and were normalized with antipsychotic treatment. Proteomic analysis of the blood samples from patients with high D2R-DISC1 complex levels that were normalized with antipsychotic treatment revealed a number of altered proteins and pathways associated with D2R, DISC1 and the D2R-DISC1 complex. We identified additional proteins and pathways that were associated with antipsychotic treatment in schizophrenia, and that may also be novel targets for schizophrenia treatment.
Limitations
Sample sizes were relatively small, but were sufficient to detect associations between D2R-DISC1 levels, schizophrenia and treatment response. The relevance of leukocyte changes to the symptoms of schizophrenia is unknown. The coimmunoprecipitation lanes included several nonspecific bands.
Conclusion
Levels of the D2R-DISC1 complex were elevated in patients with schizophrenia and reduced with antipsychotic treatment. This finding reinforces the independent role of each protein in schizophrenia. Our results enhanced our understanding of the molecular pathways involved in schizophrenia and in antipsychotic medications, and identified novel potential molecular targets for treating schizophrenia.
Introduction
Schizophrenia is a chronic mental illness characterized by episodes of psychotic symptoms, with persistent cognitive and social impairments that emerge in early adulthood. Dopamine D2 receptors (D2Rs) are the main target of antipsychotic medications,1,2 but some antipsychotics also block serotonin 2A receptors.3 Unfortunately, current antipsychotics are ineffective in many patients, and even with good symptom control, functional outcomes remain poor.4,5 Current antipsychotic drugs also cause serious adverse effects, including extra-pyramidal symptoms, tardive dyskinesia, sexual dysfunction, weight gain and diabetes.6 A better understanding of the mechanisms of antipsychotic action is critical for developing more effective antipsychotics that have fewer adverse effects.
D2Rs are G-protein-coupled receptors that also activate non-G-protein pathways7 such as β-arrestin 2, phosphatase 2A, Akt and glycogen synthase kinase 3 (GSK3).8 D2Rs are regulated by kinase-mediated desensitization, endocytosis and endosomal trafficking that is initiated by G-protein-coupled receptor kinase phosphorylation. In turn, this leads to the formation of a complex with β-arrestin, adaptor protein 2 and clathrin.9 D2R function is also regulated by various interacting proteins that modulate its signalling, trafficking and stability.10,11 These D2R-associated protein complexes are potential targets for new antipsychotic treatments.
Disrupted in Schizophrenia 1 (DISC1) is a susceptibility gene for schizophrenia and other psychiatric disorders;12–19 it acts as a scaffold protein, interacting with many important signalling molecules, including GSK3.20–26 As a result, the role of DISC1 in mental illness involves a complex network of interacting proteins and pathways that have various functions depending on developmental stage, signalling pathway and brain region.27–30 The Disc1-L100P (334T/C) mutant mouse has characteristics consistent with schizophrenia: enlarged lateral ventricles, abnormal cortical lamination, abnormal prepulse inhibition, disrupted latent inhibition and decreased social interaction.31,32 These behavioural alterations are ameliorated by antipsychotic medications.
We reported previously that D2Rs form a protein complex with DISC1 that facilitates D2R-mediated GSK3 signalling.33 Levels of the D2R-DISC1 complex are increased in conjunction with decreased GSK3α/β (Ser21/9) phosphorylation in post-mortem brain tissue from patients with schizophrenia. Levels of the D2R-DISC1 complex are also higher in Disc1-L100P mice than in wild-type control mice, and those increased levels are normalized with haloperidol, a classical antipsychotic drug. Agonist activation of D2R with quinpirole also increased D2R-DISC1 complex levels in vitro. Disrupting the D2R-DISC1 complex with an interfering peptide successfully reversed schizophrenia-relevant behaviours in Disc1-L100P mice.
The in vivo effect of antipsychotic medication on the D2R-DISC1 complex remains unclear, because our previous work analyzed postmortem brain tissue from patients with schizophrenia who had all received antipsychotic medication.34 It is also unclear whether the D2R-DISC1 complex is detectable in peripheral blood, and whether blood would also show increased D2R-DISC1 levels in patients with schizophrenia. Both D2R and DISC1 proteins are expressed in human peripheral blood leukocytes,35,36 so it is probable that the D2-DISC1 complex is also present in these cells.
Analysis of postmortem human tissue is also affected by many confounds, such as the agonal state, prescribed medications, substance use and delay in tissue collection, which causes variable degrees of degradation that can affect molecular analyses.34,37 There is also a variable interval between the last available clinical assessment and the time of death, and the generic problem of retrospective diagnostic determinations that hamper the accuracy of associations between molecular analyses, histological analyses and psychological variables.38 A crucial question is whether antipsychotic medication normalizes alterations in the brains of patients with schizophrenia. Answering this question experimentally is difficult, because it would require brain tissue from patients with schizophrenia who have not been treated with antipsychotic medication. Untreated patients typically do not receive psychiatric care, and thus it is rare to have a clear diagnosis of schizophrenia in postmortem analyses.
Several experimental designs can partially compensate for this lack of brain tissue from antipsychotic-naive patients with schizophrenia. The first is the dose–response strategy, comparing patients with more or less cumulative exposure to antipsychotic medication.39 An example of this approach revealed that patients treated with higher amounts of antipsychotic medication had less abnormal protein expression, suggesting that antipsychotics normalized protein levels. The second strategy is to identify protein alterations in postmortem human brain tissue from (treated) patients with schizophrenia, and then to treat animals with antipsychotic medications to determine whether the identified proteins are normalized. Examples of this approach have identified reduced expression of the oxytocin receptor gene in schizophrenia that was not increased by antipsychotic medication in animal models,40 and altered glutamate transporter levels in schizophrenia that were partially changed with clozapine treatment in rats.41 The third strategy is what we have chosen in this paper: to examine peripheral tissues in patients with schizophrenia before and after treatment with antipsychotic medication. Other groups have also taken this approach.42 Each of the above strategies has shortcomings, but these are unavoidable because it is so difficult to obtain sufficient brain tissue samples from patients with schizophrenia who have not received antipsychotic medication.
The current study had 2 objectives, one very specific and the other quite broad. The first was to extend our previous data from postmortem brain tissue by analyzing peripheral blood samples from living participants with schizophrenia. This would allow us to determine whether elevated levels of the D2R-DISC1 complex in the brain are also reflected in the blood. More interesting was the question of whether antipsychotic treatment normalized (lowered) levels of the D2R-DISC1 protein complex in patients with schizophrenia. The second, more general, objective was to conduct a proteomic screen of peripheral leukocytes in participants with high levels of D2R-DISC complex that were normalized with anti-psychotic treatment. In this screen, we sought to identify additional proteins and signalling pathways related to D2R and DISC1 that are involved in antipsychotic treatment response.
The aim of these experiments was to link the main treatment target for schizophrenia (D2R) with a gene and protein (DISC1) that, when mutated, is known to cause schizophrenia.43 The dopamine hypothesis for schizophrenia has a compelling symmetry: excess dopamine signalling causes psychosis, which in turn can be treated with D2R antagonists.44 Although evidence of abnormal or excessive dopamine neurotransmission in schizophrenia certainly exists,45,46 it is also clear that schizophrenia does not primarily arise from genetic mutations in dopamine-system genes.47 Our goal was to investigate whether a direct protein–protein interaction between D2R and DISC1 is increased in schizophrenia and decreased by antipsychotic medications, as well as to provide a direct molecular connection between a cause of psychosis and the target of antipsychotic medications.
Methods
Participant recruitment and clinical assessment
All participants provided written informed consent, and their capacity to provide consent was assessed by a trained research assistant. We obtained informed consent after we had explained the nature and possible consequences of the studies. All control participants were recruited through advertisements.
Shanghai Mental Health Centre
The study protocol was approved by the institutional review board at Shanghai Mental Health Centre (SMHC 2013–10).
Patients with schizophrenia were recruited via advertisements from the outpatient department of Shanghai Mental Health Centre. Inclusion criteria were as follows: ages 16 to 45 years; a diagnosis of schizophrenia or schizophreniform disorder based on the Structured Clinical Interview for DSM IV-TR; medication-naive at baseline; and no diagnosis of substance abuse, mood disorder, head injury or seizure. Healthy controls were medication-free, and we excluded those with Axis I disorders using the Structured Clinical Interview for DSM IV-TR.
Schizophrenia symptoms were quantified at baseline and after 2 months of treatment with the Chinese version of the Positive and Negative Syndrome Scale (PANSS; first cohort),48 or the Brief Psychiatric Rating Scale (second cohort).49 Patients were treated with second-generation anti-psychotics (Appendix 1, Tables S1 and S2, available at www.jpn.ca/lookup/doi/10.1503/jpn.210145/tab-related-content).
Beijing AnDing Hospital
The study protocol was approved by the ethics review committee of the Beijing AnDing Hospital (protocol 2014–68–201886XG-5).
Patients with schizophrenia were recruited via referral from health care professionals. Inclusion criteria were as follows: aged 18 to 65 years; a diagnosis of schizophrenia confirmed by the Mini-International Neuropsychiatric Interview;50 and no diagnoses of substance abuse, mood disorder, head injury, seizure or adverse drug reactions. Patients with schizophrenia were not medication-naive at baseline. Control participants met the same criteria but were free of any psychiatric diagnosis (DSM-IV).
Schizophrenia symptoms were quantified at the time of admission using the PANSS.48
Isolation of leukocytes from peripheral blood samples
Peripheral blood samples were collected in EDTA whole blood tubes (BD Biosciences), and leukocytes were isolated using Ficoll-Paque PLUS (GE Healthcare) according to the manufacturer’s instructions. More details can be found in Appendix 1.
Coimmunoprecipitation and Western blot
Fasting blood cell samples were collected in EDTA whole blood tubes, and were centrifuged and divided into aliquots for storage at −80°C. Coimmunoprecipitation and Western blot analyses were performed as previously described.51,52
Shanghai samples underwent total protein extraction with the Hemoglobin Depletion and Protein Enrichment kit (Biotech Support Group). Beijing samples underwent total protein extraction with lysis buffer. More details can be found in Appendix 1.
Mass spectrometry analysis
Total protein (120 μg) extracted from samples in the first Shanghai cohort underwent mass spectrometry analysis at the National Centre for Protein Science Shanghai and Beijing Qinglian Biotech Co., Ltd. After protein precipitation and digestion, samples were analyzed by liquid chromatography tandem mass spectrometry. After we obtained the raw data, we conducted protein identification and quantification, followed by functional analysis. See Appendix 1 for detailed experimental procedures, mass spectra analysis, quality control and assessment, and proteomic data processing.
Statistical analysis
We analyzed coimmunoprecipitation and Western blot data using 1-way analysis of variance (ANOVA), followed by a Tukey post hoc test in Prism 6 (GraphPad). We also performed linear regression and correlation analysis of coimmunoprecipitation data with clinical assessment scores using Prism 6. Outliers were excluded using a Grubbs test if the data were normally distributed.
Results
D2R-DISC1 complex levels in patients with schizophrenia
Shanghai Mental Health Centre
We confirmed that both D2R and DISC1 protein were expressed in peripheral blood leukocytes from control participants (Appendix 1, Figure S1A).
We used coimmunoprecipitation to measure D2R-DISC1 complex levels, with a fixed amount of D2R antibodies incubated with equal amounts of protein extracted from each blood sample. The DISC1 protein was coimmunoprecipitated together with D2R by D2R antibodies. We then used DISC1 antibodies to visualize the coimmunoprecipitated protein on Western blots, permitting quantification of the D2R-DISC1 complex with densitometric analysis of individual bands on each blot. We found higher levels of the D2R-DISC1 complex in peripheral blood samples from patients with schizophrenia (n = 34 for each group; F2,99 = 2.447, p < 0.05; Figure 1A to C).
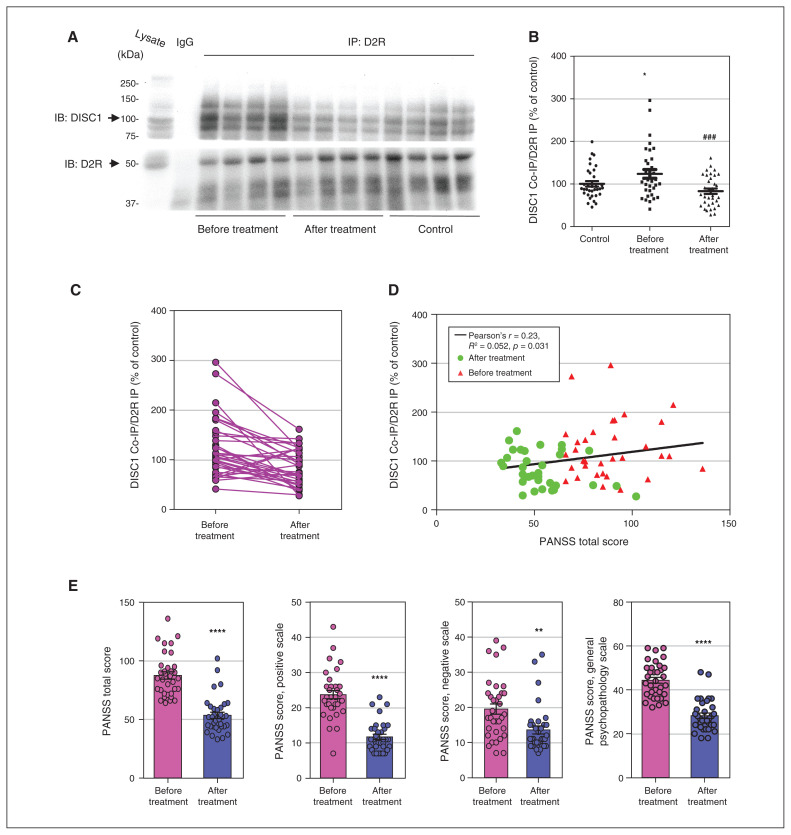
Figure 1.
Antipsychotic medications normalized elevated D2R-DISC1 complex levels in peripheral blood samples from patients with schizophrenia (Shanghai first cohort). (A) Coimmunoprecipitation shows that antipsychotic medications reduced D2R-DISC1 complex levels in periphal blood samples from patients with schizophrenia. (B) Densitometric analysis of DISC1 coimmunoprecipitated by D2R from peripheral blood samples of patients with schizophrenia before and after antipsychotic treatment, and of unaffected controls. *p < 0.05 versus controls. ###p < 0.001 versus patients with schizophrenia before treatment. One-way analysis of variance followed by a Tukey post hoc test (n = 34 patients with schizophrenia before and after treatment; n = 34 unaffected controls). (C) Graph displaying D2R-DISC1 complex levels before and after treatment. (D) PANSS total score was positively correlated with D2R-DISC1 complex levels in patients with schizophrenia before and after antipsychotic treatment (n = 34 patients with schizophrenia). (E) PANSS scores for patients with schizophrenia before and after treatment (n = 34 patients with schizophrenia); t test, **p < 0.01, ****p < 0.001 versus before treatment. Co-IP = coimmunoprecipitation; D2R = dopamine 2 receptor; DISC1 = disrupted in schizophrenia 1; IB = immunoblotting; IgG = immunoglobulin G; IP = immunoprecipitation; PANSS = Positive and Negative Syndrome Scale.
Antipsychotic treatment for 12 weeks significantly lowered D2R-DISC1 complex levels (n = 34; F2,99 = 2.447, p < 0.001; Figure 1A to C). These results were consistent with our previous findings in cellular and animal model systems.33 D2R-DISC1 complex levels were correlated with PANSS total scores (Pearson r = 0.23, R2 = 0.052, p = 0.031; Figure 1D). Antipsychotic treatment was effective in reducing schizophrenia symptoms (n = 34; Figure 1E). Details of anti-psychotic treatments are provided in Appendix 1, Table S1. Demographic information for patients and controls (first cohort) is shown in Appendix 1, Table S2.
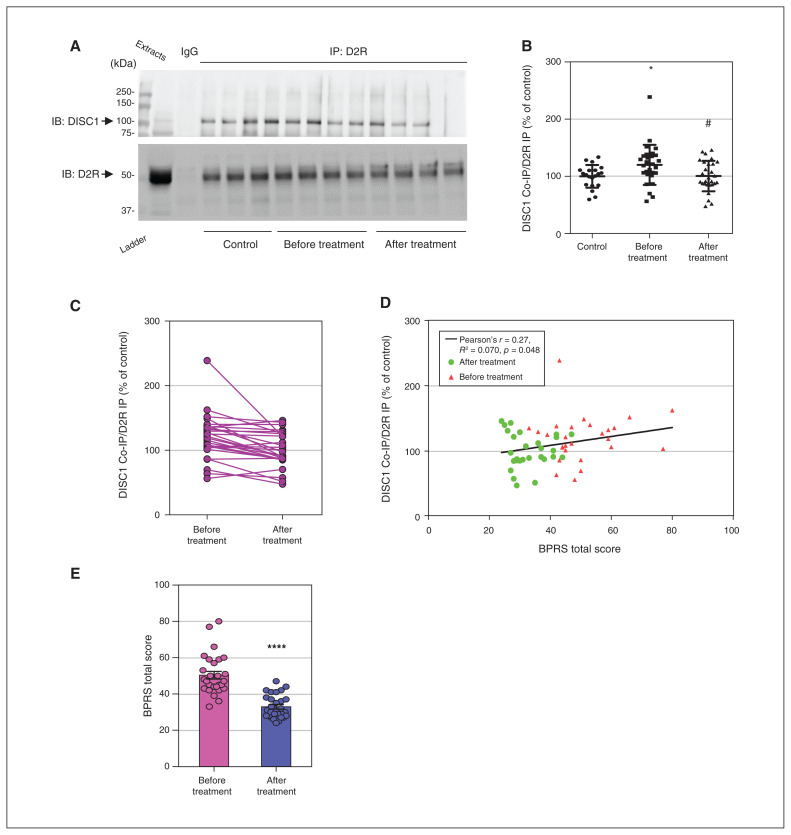
To strengthen our results by replication, we repeated the same experiments in a second cohort of Shanghai patients treated for 8 weeks, and found similar results (n = 20 controls, n = 28 patients; F2,73 = 1.435, p < 0.05; Figure 2A to C). Higher D2R-DISC1 complex levels were correlated with a higher total score on the Brief Psychiatric Rating Scale (Pearson r = 0.27, R2 = 0.070, p = 0.048; Figure 2D). As in the first cohort, antipsychotic treatment was effective in reducing schizophrenia symptoms (Figure 2E). Details of antipsychotic treatments are provided in Appendix 1, Table S3. Demographic information for patients and controls (second cohort) is shown in Appendix 1, Table S4.
Figure 2.
Antipsychotic medications normalized elevated D2R-DISC1 complex levels in peripheral blood samples from patients with schizophrenia (Shanghai second cohort). (A) Coimmunoprecipitation shows that antipsychotic medications reduced D2R-DISC1 complex levels in peripheral blood samples from patients with schizophrenia. (B) Densitometric analysis of DISC1 coimmunoprecipitated by D2R from peripheral blood samples of patients with schizophrenia before and after antipsychotic treatment, and of unaffected controls. *p < 0.05 versus controls, #p < 0.05 versus patients with schizophrenia before treatment. One-way analysis of variance followed by a Tukey post hoc test (n = 28 patients with schizophrenia before and after treatment; n = 20 unaffected controls). (C) Graph displaying D2R-DISC1 complex levels before and after treatment. (D) BPRS total score was positively correlated with D2R-DISC1 complex levels in patients with schizophrenia before and after anti-psychotic treatment (n = 28 participants with schizophrenia). (E) BPRS total scores for patients with schizophrenia before and after treatment; t test, ****p < 0.001 compared to before treatment. BPRS = Brief Psychiatric Rating Scale; Co-IP = coimmunoprecipitation; D2R = dopamine 2 receptor; DISC1 = disrupted in schizophrenia 1; IB = immunoblotting; IgG = immunoglobulin G; IP = immunoprecipitation.
Beijing AnDing Hospital
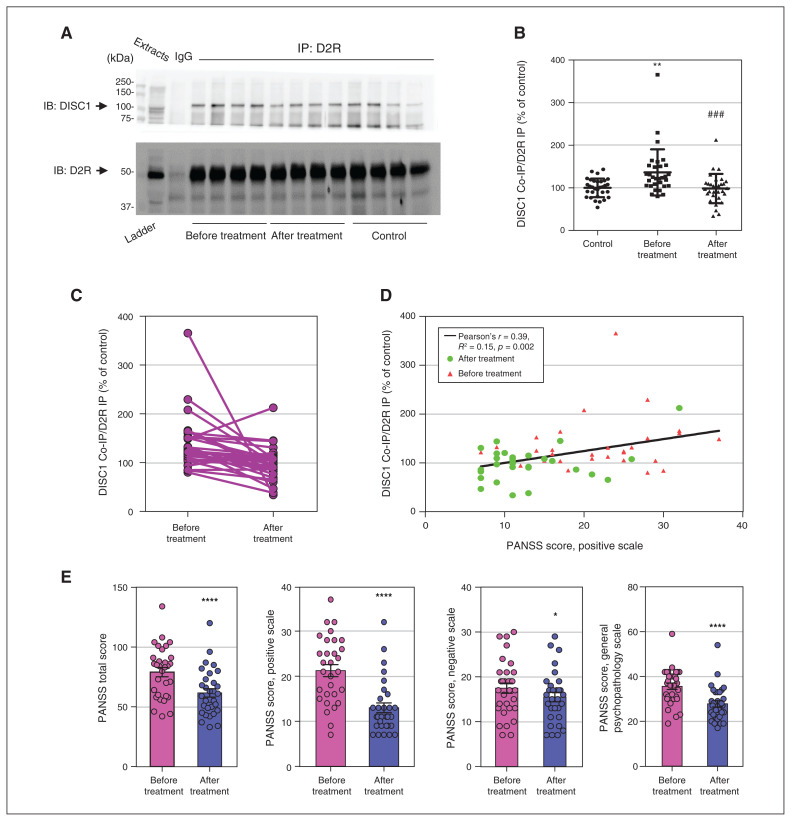
To further replicate the findings above, we examined another cohort of participants treated for 4 weeks at Beijing AnDing Hospital. Our results were consistent with those for the Shanghai cohorts, showing that 4 weeks of antipsychotic treatment decreased D2R-DISC1 complex levels compared to pretreatment levels (n = 12 controls, n = 31 patients; F2,90 = 1.757, p < 0.001; Figure 3A to C). Post-treatment D2R-DISC1 levels in patients with schizophrenia were comparable to those of unaffected controls. Higher D2R-DISC1 complex levels were correlated with positive symptoms (Pearson r = 0.39, R2 = 0.15, p = 0.002; Figure 3D). The mean scores on the PANSS positive symptom subscale were 21.26 ± 1.33 before treatment and 12.81 ± 0.95 after treatment (Figure 3E). Details of antipsychotic treatments are provided in Appendix 1, Table S5. Demographic information for patients and controls is shown in Appendix 1, Table S6.
Figure 3.
Antipsychotic medications normalized elevated D2R-DISC1 complex levels in peripheral blood samples from patients with schizophrenia (Beijing cohort). (A) Coimmunoprecipitation shows that antipsychotic medications reduced D2R-DISC1 complex levels in peripheral blood samples from patients with schizophrenia. (B) Densitometric analysis of DISC1 coimmunoprecipitated by D2R from peripheral blood samples of patients with schizophrenia before and after antipsychotic treatment, and of unaffected controls. **p < 0.01 versus controls. ###p < 0.001 versus patients with schizophrenia before treatment. One-way analysis of variance followed by a Tukey post hoc test (n = 31 patients with schizophrenia before and after treatment; n = 12 unaffected controls). (C) Graph displaying D2R-DISC1 complex levels before and after treatment. (D) PANSS positive scale score was positively correlated with D2R-DISC1 complex levels in patients with schizophrenia before and after antipsychotic treatment (n = 31 patients with schizophrenia). (E) PANSS scores for patients with schizophrenia before and after treatment (n = 31 patients with schizophrenia before and after treatment; n = 12 unaffected controls); t test, *p < 0.05, ****p < 0.001 compared to before treatment. Co-IP = coimmunoprecipitation; D2R = dopamine 2 receptor; DISC1 = disrupted in schizophrenia 1; IB = immunoblotting; IgG = immunoglobulin G; IP = immunoprecipitation; PANSS = Positive and Negative Syndrome Scale.
Summary
In the Western blots used to generate the data above, equal amounts of protein from each sample were incubated with anti-D2R antibody, and the precipitated proteins were immunoblotted with either DISC1 or D2R antibody. Each blot included 4 samples from each group, and results for each sample are presented as the percentage of the mean of 4 control samples on the same blot. These data from multiple cohorts confirmed that D2R-DISC1 complex levels were elevated in schizophrenia and normalized with antipsychotic treatment, in conjunction with symptom improvement.
Proteomic analysis of peripheral blood samples of patients with schizophrenia from Shanghai Mental Health Centre
To search for additional proteins associated with antipsychotic treatment response in schizophrenia — especially those proteins associated with D2R and DISC1 — we performed proteomic analysis of blood samples from 6 patients with schizophrenia before and after 12 weeks of antipsychotic treatment. We also analyzed blood samples from 6 unaffected controls. The 12 participants in this analysis were a subset of the first cohort of 34 patients recruited in Shanghai. The 6 samples from patients with schizophrenia were chosen based on the fact that they showed significant changes in D2R-DISC1 complex levels after treatment (Appendix 1, Figure S1B; n = 6 for each group; F2,15 = 9.699, p < 0.01). As before, antipsychotic treatment was effective in reducing symptoms of schizophrenia (Appendix 1, Figure S1C). Demographic information is shown in Appendix 1, Table S7. We conducted all analyses using the same mass spectrometer with internal standards. Our goal was to identify proteins that were present at significantly higher or lower levels in patients with schizophrenia, and that were normalized with antipsychotic treatment; we paid special attention to proteins known to be associated with D2R and DISC1.19
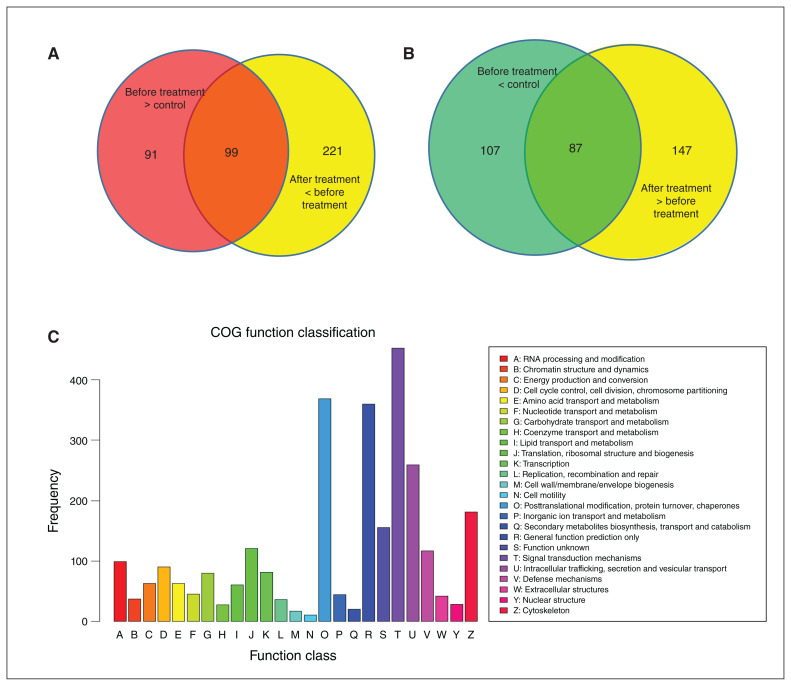
Quality control assessment of data from the label-free mass spectra analysis showed that 20 886 of 28 953 peptides had 0 missed cleavage, indicating appropriate reduction, alkylation and digestion (Appendix 1, Figure S2). The label-free mass spectra analysis identified 2964 proteins from all samples tested. Compared to controls, 194 proteins had significantly lower levels and 190 proteins had significantly higher levels in patients with schizophrenia before treatment (baseline; Appendix 1, Figure S3A). After 12 weeks of anti-psychotic treatment, 320 proteins had significantly lower levels than before treatment, and 234 proteins had significantly higher levels. Also after 12 weeks of treatment, levels of 285 proteins were significantly lower and 224 proteins were significantly higher than in patients with schizophrenia compared to controls. Among these proteins, 99 were significantly higher in patients before treatment and decreased significantly with treatment (Figure 4A). Similarly, 87 proteins were lower in patients before treatment and increased after treatment (Figure 4B). These 186 proteins (99 + 87) are of interest because they were altered in patients with schizophrenia and normalized with antipsychotic treatment.
Figure 4.
Identification, expression and COG analysis of proteins detected in mass spectrometry of blood samples from patients with schizophrenia before and after treatment, as well as unaffected controls. (A) Number of proteins with significantly higher levels in patients with schizophrenia versus controls, and with significantly lower levels in patients with schizophrenia after treatment versus before treatment. (B) Number of proteins with significantly lower levels in patients with schizophrenia versus controls, and with significantly higher levels in patients with schizophrenia after treatment versus before treatment. (C) COG analysis of all the proteins identified in mass spectrometry analysis; 2964 proteins were grouped into 25 COG function classifications. COG = Clusters of Orthologous Groups.
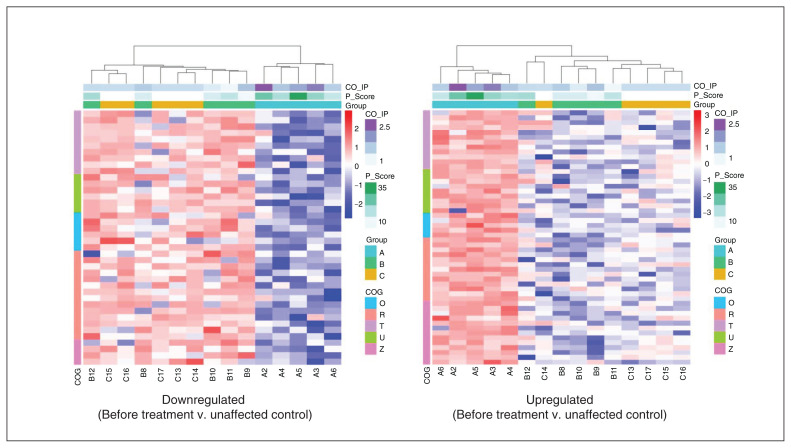
To predict and classify the functions of the identified proteins, we searched in the Clusters of Orthologous Groups (COG) database. Overall, we grouped proteins into 25 COG function classifications (Figure 4C). The top 5 were as follows: signal transduction mechanisms (T: 453, 15.86 %); posttranslational modification, protein turnover and chaperones (O: 369, 12.92 %); general function prediction only (R: 360, 12.60 %); intracellular trafficking, secretion and vesicular transport (U: 259, 9.07 %); and cytoskeleton (Z: 181, 6.34 %). Hierarchical clustering analysis showed that the proteins with significantly higher or lower levels in patients before treatment (and normalized after treatment) were associated with a reduction in score on the PANSS positive scale, as well as a decrease in D2R-DISC1 complex levels (coimmunoprecipitation; Figure 5). One sample with outlier data from each group was excluded, because its heatmap was obviously different from the other samples in the same group.
Figure 5.
Hierarchical clustering analysis of differentially expressed proteins of 5 function groups in COG analysis (Figure 4). One sample with outlier data was excluded from each group. The left panel shows analysis of proteins that had lower levels in patients with schizophrenia before treatment versus controls. The right panel shows proteins with higher levels in patients with schizophrenia before treatment versus controls. Differential expression thresholds were a fold change > 1.2 and p < 0.05. Each row indicates a protein, and each column represents a sample. The proteomic profile was associated with clinical assessment of positive symptoms (PANSS positive scale score; P_Score) and D2R-DISC1 complex levels (CO_IP). The heatmap depicts the relative abundance of proteins. Biological functions related to these proteins are denoted on the left (for a key, see Figure 4). Group A: patients with schizophrenia before treatment; group B: patients with schizophrenia after treatment; group C: unaffected controls. COG = Clusters of Orthologous Groups; D2R = dopamine 2 receptor; DISC1 = disrupted in schizophrenia 1; PANSS = Positive and Negative Syndrome Scale.
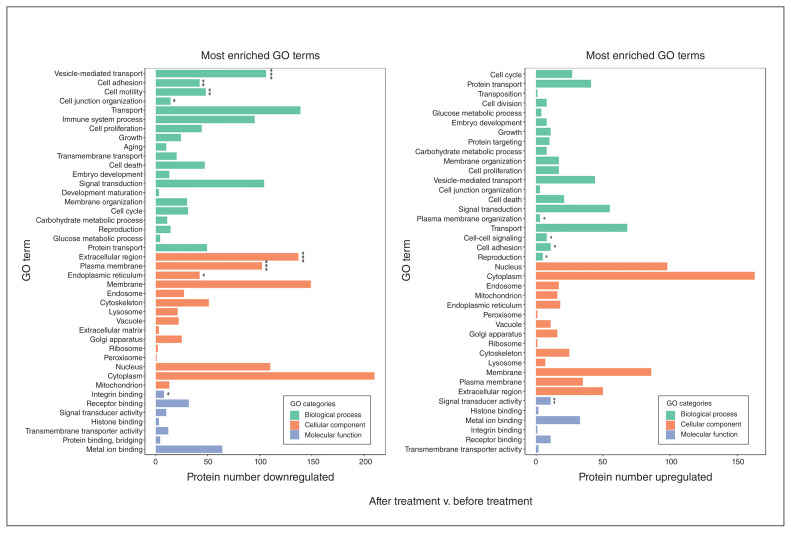
We also conducted Gene Ontology (GO) term enrichment analysis to group proteins with similar functions and associations. These differentially expressed proteins were enriched into 8286 GO terms for biological processes, cellular components and molecular functions. In the proteins that were decreased with treatment, we identified the top 40 terms for 20 biological processes, 13 cellular components and 7 molecular functions. Of these 40 GO terms, transport, vesicle-mediated transport and signal transduction were the 3 most enriched. GO terms from the cellular component showed many proteins involved in cytoplasm, membrane and extracellular regions. Enrichment in the molecular function group was for proteins involved in metal ion binding and receptor binding. In the proteins increased by treatment, we identified the top 40 GO terms for 20 biological processes, 14 cellular components and 6 molecular functions. In these 40 terms, many proteins were involved in biological processes, including transport, signal transduction and vesicle-mediated transport, similar to the proteins decreased by treatment, above. Cytoplasm, nucleus and membrane proteins were the top 3 terms for the cellular component. GO terms for molecular functions were metal ion binding, receptor binding and signal transduction, again similar to proteins decreased by treatment (Figure 6).
Figure 6.
Analysis of the differential expression of GO-enriched proteins in patients with schizophrenia after treatment versus before treatment. Left: GO enrichment of decreased proteins in patients with schizophrenia after treatment versus before treatment. Right: GO enrichment of increased proteins in patients with schizophrenia after treatment versus before treatment. The graph shows the GO term for each row and the number of differentially expressed proteins on the x axis. The green bars represent biological processes, the orange bars represent cellular components and the blue bars represent molecular functions. *p < 0.05, **p < 0.01, ***p < 0.001. GO = Gene Ontology.
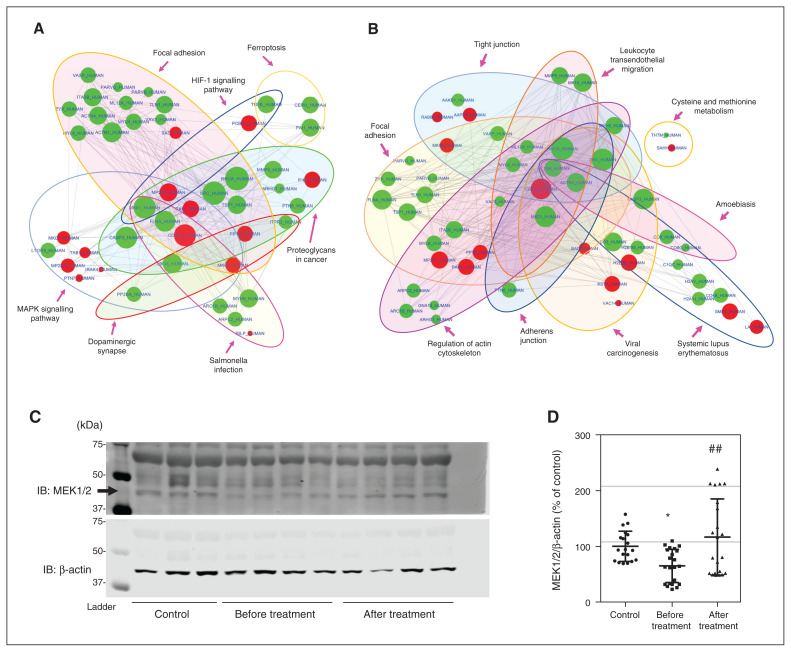
Finally, we used the KEGG (Kyoto Encyclopedia of Genes and Genomes) database53 to group differentially expressed proteins into pathways. We mapped all proteins to the reference pathway in the KEGG database; in total, they were involved in 312 pathways (Appendix 1, Figure S3B). Based on our previous studies of the D2R-DISC1 complex and its associated downstream pathways, as well as studies on the D2R-mediated signalling pathway, we focused on mitogen-activated protein kinase (MAPK), cyclic adenosine monophosphate (cAMP), the PI3K-Akt signalling pathway and the dopamine synapse. We prepared diagrams of protein–protein interaction networks by uploading to the STRING54 database the proteins involved in these pathways that were differentially expressed in patients after treatment versus before treatment. As shown in Figure 7A, multiple proteins in these pathways were altered with antipsychotic treatment, further supporting conclusions of previous studies from our laboratory and others that these pathways are involved in the pathophysiology of schizophrenia.8,33,55–61
Figure 7.
PPI networks of D2R- and DISC1-interacting proteins detected in mass spectrometry, and expression levels of MEK1/2 in samples from the second cohort. (A) PPI network diagram showing D2R-interacting proteins with significantly different levels in patients with schizophrenia after treatment versus before treatment. The red nodes represent increased protein levels, and the green nodes represent decreased protein levels. (B) PPI network diagram showing DISC1-interacting proteins with significantly different levels in patients with schizophrenia after treatment versus before treatment. The red nodes represent increased protein levels and the green nodes represent decreased protein levels. (C and D) Representative Western blot images and densitometric analysis of MEK1/2 levels in unaffected controls and patients with schizophrenia before and after treatment (Shanghai second cohort). MEK1/2 levels were normalized to the control-group average on the same blot after normalization to β-actin levels. *p < 0.05 versus controls. ##p < 0.01 versus patients with schizophrenia before treatment. One-way analysis of variance followed by a Tukey post hoc test (n = 19 unaffected controls; n = 22 patients with schizophrenia before and after treatment). D2R = dopamine 2 receptor; DISC1 = disrupted in schizophrenia 1; IB = immunoblotting; ERK = extracellular signal–regulated kinase; HIF-1 = hypoxia-inducible factor 1; MAPK = mitogen-activated protein kinase; MEK1/2 = MAPK/ERK 1/2; PPI = protein–protein interaction.
For a more focused analysis, we specifically examined proteins known to be part of D2R- or DISC1-interacting pathways (Figures 7A and B). According to the BioGRID62 and IntAct63 databases, 98 proteins interact with D2R,64 and we detected 15 of those in our assay. Two of the 15 were significantly changed by treatment in our samples (Appendix 1, Figure S4A). These 2 proteins are specifically involved in 7 pathways, of which 6 were nominally significant (p < 0.05), as was the dopamine synapse pathway (Figure 7A). Similarly, we identified 547 proteins that directly or indirectly interacted with DISC1 from both databases and the literature.19,64–70 Of these, 134 were detected by mass spectrometry and 23 were significantly altered by anti-psychotic treatment (Appendix 1, Figure S4B). These 23 proteins are specifically involved in 15 pathways, of which 9 were nominally significant (Figure 7B). Furthermore, as shown in the volcano plots of proteins detected by mass spectrometry (Appendix 1, Figure S5), MAP2K1 (MEK1) was downregulated in patients with schizophrenia before treatment (compared to controls), and upregulated after antipsychotic treatment. To further validate these results, we confirmed expression levels of MEK1/2 from the above pathways in the Shanghai second cohort using Western blot analysis. As shown in Figures 7C and D, the alterations in MEK1/2 were consistent with mass spectrometry findings (n = 19 controls, n = 22 patients; F2,60 = 13.83, p < 0.01).
Discussion
In this study we confirmed and extended our previous findings showing that the D2R-DISC1 protein complex is elevated in patients with schizophrenia compared to unaffected controls.33 Our previous study examined postmortem brain tissue, in which almost all patients with schizophrenia had been treated with antipsychotic medications. In the current study, we analyzed peripheral blood leukocytes from 3 separate cohorts of patients and controls recruited at 2 different centres and compared samples from patients with schizophrenia before and after antipsychotic treatment. We confirmed that D2R-DISC1 complex levels were elevated in patients with schizophrenia compared to unaffected controls. We also found that antipsychotic treatment normalized the elevated levels, bringing them to levels similar to that of the controls. The 3 cohorts were treated for different periods of time before the second blood sample was drawn, but they yielded convergent results. In a proteomic screen, we identified a number of other proteins present at abnormal levels in patients with schizophrenia that were normalized with anti-psychotic treatment. Many of these proteins function in pathways known to be connected to dopamine signalling or the network of DISC1-interacting proteins, providing convergent evidence that the D2R-DISC1 protein complex plays a role in both schizophrenia and antipsychotic treatment response.
Our results suggest that D2R-DISC1 complex levels are related to schizophrenia symptoms and provide clinical evidence that the D2R-DISC1 complex is involved in anti-psychotic effects. Previously, we had shown in cellular and animal model systems that the D2R-DISC1 complex facilitates GSK3 signalling through decreased GSK3α/β (Ser21/9) phosphorylation, and that it inhibits agonist-induced D2R internalization. The human clinical observations in the current study provide additional insights into the mechanisms by which antipsychotic medications exert their therapeutic effects in schizophrenia. GSK3 is a well known downstream target of antipsychotic medication treatment,8 and our current findings reinforce the importance of this signalling pathway for antipsychotic effects. DISC1 also interacts with phosphodiesterase 4B (PDE4B), which metabolizes cAMP, an important second messenger of dopamine receptors. PDE4B has also been associated with schizophrenia,71 and rolipram, a drug targeting PDE4B, has antipsychotic-like effects in rodent models.72 One potential next step is to investigate how the D2R-DISC1 complex interacts with the DISC1-PDE4B complex to modulate psychotic symptoms and antipsychotic treatment.
In our proteomic analysis, we identified a number of D2R- and DISC1-related proteins that were altered in patients with schizophrenia and normalized with antipsychotic treatment. These data provide a starting point for further investigation of the molecular pathways through which antipsychotic medications exert their effects. For example, our results showed that MP2K1 (MEK1) was decreased in patients with schizophrenia before treatment and increased with treatment. MP2K1 protein directly inhibits GSK3 signalling,73 and the D2R-DISC1 complex activates GSK3.33 The functional categories of proteins that were altered in schizophrenia and normalized with antipsychotic medications were the same regardless of the direction of change (higher or lower in schizophrenia compared to controls).
Another significant implication of this work is that despite the heterogeneous genetic etiology of schizophrenia, common molecular changes were associated with treatment response. How central the dopamine system is to the etiology of schizophrenia has been an ongoing controversy. It is well accepted that targeting D2R is necessary for anti-psychotic medication to be effective, and there are numerous reports of alterations in the dopamine system in schizophrenia, most notably at the level of dopamine release.45,74 However, the extent to which alterations in the dopamine system are responsible for symptoms of the illness is still unclear. Our data provide support for the notion that the dopamine system, specifically the D2R-DISC1 complex, is involved in the origin of schizophrenia symptoms and in antipsychotic treatment effects. It is also notable that we saw broad improvements in all schizophrenia symptoms — not only in the positive symptom domain.
Limitations
A limitation of this study was that it was unclear how and if the D2R-DISC1 complex in peripheral blood leukocytes contributed to schizophrenia. Our previous analysis of this protein complex was in postmortem brain tissue from patients with schizophrenia and controls.33 It would be preferable to analyze brain tissue in participants with a psychiatric disorder such as schizophrenia, but postmortem tissue analyses do not permit within-subject comparisons to examine the effects of antipsychotic treatment. Postmortem analyses are necessarily cross-sectional and static, and a main aim of the current study was to determine whether the D2R-DISC1 complex varied dynamically in conjunction with the psychiatric symptoms of schizophrenia. Our proteomic analysis revealed that some leukocyte-related pathways were altered significantly by antipsychotic treatment, including proteins involved in systemic lupus erythematosus, complement and coagulation cascades, T cell receptor signalling, natural killer cell mediated cytotoxicity, platelet activation, and p53 signalling, which may be associated with adverse effects of antipsychotic treatment such as agranulocytosis. Another potential limitation of our study was a lack of information about substance use, which could have affected protein levels.
The coimmunoprecipitation lanes had several nonspecific bands, which could have occurred for a variety of reasons, including incorrectly titrated antibodies, excessive lysate loaded, impurities in the antibodies, degraded protein samples and the presence of different splice variants that share similar epitopes. However, the band we measured was of the predicted protein size, and all samples underwent the same experimental procedures, so these nonspecific bands should not have affected the main findings.
DISC1 is an important schizophrenia susceptibility gene because the drastic translocation mutation in the Scottish family in which DISC1 was first identified is almost certainly causal of mental illness in mutation carriers. However, as in most rare mutations with large effects, common mutations in the same gene in the general population do not have strong effects on schizophrenia risk.75–77 Nevertheless, DISC1 is of interest as an entry point into the biology of schizophrenia, and this is reinforced by the data presented here, showing that the D2R-DISC1 complex varies dynamically in conjunction with schizophrenia symptoms. This protein complex thus provides a direct molecular link between the origins of schizophrenia and antipsychotic treatment.
Conclusion
Our results provide support for the long-standing dopamine hypothesis of schizophrenia — not directly through the core elements of the dopamine system such as the receptors or dopamine synthesis machinery, but through DISC1, a protein that interacts directly with D2Rs. Although the overall explanation for how pharmacological D2R antagonists have antipsychotic effects remains elusive, the data presented here bring us one step further by identifying another molecular component in this process. With ongoing work, it may be possible to use this information to begin exploring other treatment targets for schizophrenia.
Supplementary Material
Acknowledgements
The authors thank the Mass Spectrometry System at the National Facility for Protein Science in Shanghai, Zhangjiang Lab, Shanghai Advanced Research Institute, Chinese Academy of Science, China for data collection and analysis. We thank Beijing Qinglian Biotech Co., Ltd for further analysis of the mass spectrometry data including protein identification and functional analysis.
Footnotes
Competing interests: A.H.C. Wong reports consulting fees and stock from FSD Pharma. No other competing interests declared.
Contributors: F. Liu supervised the overall project. A. Wong and F. Liu designed the study. J. Wang, L. Xu, A. Yuan, C. Li and T. Zhang recruited the patients and collected the blood samples and clinical data at Shanghai Mental Health Centre. J. Yang, F. Dong and J. Zhou recruited the patients and collected the blood samples and clinical data at Beijing AnDing Hospital. P. Su conducted the experiments and collected and analyzed the data from coimmunoprecipitation and Western blots in Beijing and Shanghai. P. Su also further analyzed the mass spectrometry data with helpful insights from A. Wong. J. Samsom helped with further analysis of the coimmunoprecipitation data. P. Su, A. Wong and F. Liu wrote the manuscript. J. Wang, J. Yang, L. Xu, A. Yuan., C. Li, T. Zhang, F. Dong, J. Zhou and J. Samsom reviewed the manuscript critically. All the authors approved the final version to be published and agree to be responsible for its content.
Funding: This study was supported by the National Key R&D Program of Ministry of Science and Technology of China (2016YFC1306800). A.H.C. Wong received support from the Miner’s Lamp Innovation Fund. F. Liu received support from the Canadian Institutes of Health Research (FRN#126075) and the Miner’s Lamp Innovation Fund. F. Liu holds the Tapscott Chair in Schizophrenia Studies.
References
- 1.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 1975;188:1217–9. [DOI] [PubMed] [Google Scholar]
- 2.Seeman P, Lee T, Chau-Wong M, et al. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 1976;261:717–9. [DOI] [PubMed] [Google Scholar]
- 3.Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ 2000;321:1371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry 2006;11:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of anti-psychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209–23. [DOI] [PubMed] [Google Scholar]
- 6.Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 2009;373:31–41. [DOI] [PubMed] [Google Scholar]
- 7.Missale C, Nash SR, Robinson SW, et al. Dopamine receptors: from structure to function. Physiol Rev 1998;78:189–225. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol 2009; 49:327–47. [DOI] [PubMed] [Google Scholar]
- 9.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol 2008;48:537–68. [DOI] [PubMed] [Google Scholar]
- 10.Fukunaga K, Shioda N. Novel dopamine D2 receptor signaling through proteins interacting with the third cytoplasmic loop. Mol Neurobiol 2012;45:144–52. [DOI] [PubMed] [Google Scholar]
- 11.Fuxe K, Borroto-Escuela DO, Marcellino D, et al. GPCR heteromers and their allosteric receptor-receptor interactions. Curr Med Chem 2012;19:356–63. [DOI] [PubMed] [Google Scholar]
- 12.Porteous DJ, Millar JK, Brandon NJ, et al. Drosoph Inf ServC1 at 10: connecting psychiatric genetics and neuroscience. Trends Mol Med 2011;17:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Clair D, Blackwood D, Muir W, et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet 1990;336:13–6. [DOI] [PubMed] [Google Scholar]
- 14.Hennah W, Varilo T, Kestila M, et al. Haplotype transmission analysis provides evidence of association for Drosoph Inf ServC1 to schizophrenia and suggests sex-dependent effects. Hum Mol Genet 2003;12:3151–9. [DOI] [PubMed] [Google Scholar]
- 15.Liu YL, Fann CS, Liu CM, et al. A single nucleotide polymorphism fine mapping study of chromosome 1q42.1 reveals the vulnerability genes for schizophrenia, GNPAT and Drosoph Inf ServC1: association with impairment of sustained attention. Biol Psychiatry 2006;60:554–62. [DOI] [PubMed] [Google Scholar]
- 16.Hamshere ML, Bennett P, Williams N, et al. Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to Drosoph Inf ServC1, and suggestive evidence at 22q11 and 19p13. Arch Gen Psychiatry 2005;62:1081–8. [DOI] [PubMed] [Google Scholar]
- 17.Hodgkinson CA, Goldman D, Jaeger J, et al. Disrupted in schizophrenia 1 (Drosoph Inf ServC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet 2004; 75:862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor MS, Devon RS, Millar JK, et al. Evolutionary constraints on the Disrupted in Schizophrenia locus. Genomics 2003;81:67–77. [DOI] [PubMed] [Google Scholar]
- 19.Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through Drosoph Inf ServC1. Nat Rev Neurosci 2011;12:707–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen S, Lang B, Nakamoto C, et al. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci 2008;28:10893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan X, Chang JH, Ge S, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 2007;130:1146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kvajo M, McKellar H, Arguello PA, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A 2008;105:7076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pletnikov MV, Ayhan Y, Nikolskaia O, et al. Inducible expression of mutant human Drosoph Inf ServC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry 2008;13:173–86. [DOI] [PubMed] [Google Scholar]
- 24.Mao Y, Ge X, Frank CL, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/ beta-catenin signaling. Cell 2009;136:1017–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niwa M, Kamiya A, Murai R, et al. Knockdown of Drosoph Inf ServC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron 2010;65:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee FH, Kaidanovich-Beilin O, Roder JC, et al. Genetic inactivation of GSK3alpha rescues spine deficits in Disc1-L100P mutant mice. Schizophr Res 2011;129:74–9. [DOI] [PubMed] [Google Scholar]
- 27.Singh KK, Ge X, Mao Y, et al. Dixdc1 is a critical regulator of Drosoph Inf ServC1 and embryonic cortical development. Neuron 2010;67:33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JY, Liu CY, Zhang F, et al. Interplay between Drosoph Inf ServC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell 2012;148:1051–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JY, Duan X, Liu CY, et al. Drosoph Inf ServC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 2009;63:761–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enomoto A, Asai N, Namba T, et al. Roles of disrupted-inschizophrenia 1–interacting protein girdin in postnatal development of the dentate gyrus. Neuron 2009;63:774–87. [DOI] [PubMed] [Google Scholar]
- 31.Clapcote SJ, Lipina TV, Millar JK, et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron 2007;54:387–402. [DOI] [PubMed] [Google Scholar]
- 32.Lee FH, Fadel MP, Preston-Maher K, et al. Disc1 point mutations in mice affect development of the cerebral cortex. J Neurosci 2011; 31:3197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su P, Li S, Chen S, et al. A dopamine D2 receptor-Drosoph Inf ServC1 protein complex may contribute to antipsychotic-like effects. Neuron 2014;84:1302–16. [DOI] [PubMed] [Google Scholar]
- 34.Torrey EF, Webster M, Knable M, et al. The Stanley Foundation brain collection and Neuropathology Consortium. Schizophr Res 2000;44:151–5. [DOI] [PubMed] [Google Scholar]
- 35.Trossbach SV, Fehsel K, Henning U, et al. Peripheral Drosoph Inf ServC1 protein levels as a trait marker for schizophrenia and modulating effects of nicotine. Behav Brain Res 2014;275:176–82. [DOI] [PubMed] [Google Scholar]
- 36.McKenna F, McLaughlin PJ, Lewis BJ, et al. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol 2002;132:34–40. [DOI] [PubMed] [Google Scholar]
- 37.Rossor M. Biological markers in mental disorders: post-mortem studies. J Psychiatr Res 1984;18:457–65. [DOI] [PubMed] [Google Scholar]
- 38.Webster MJ. Tissue preparation and banking. Prog Brain Res 2006; 158:3–14. [DOI] [PubMed] [Google Scholar]
- 39.Chan MK, Tsang TM, Harris LW, et al. Evidence for disease and antipsychotic medication effects in post-mortem brain from schizophrenia patients. Mol Psychiatry 2011;16:1189–202. [DOI] [PubMed] [Google Scholar]
- 40.Uhrig S, Hirth N, Broccoli L, et al. Reduced oxytocin receptor gene expression and binding sites in different brain regions in schizophrenia: a post-mortem study. Schizophr Res 2016;177:59–66. [DOI] [PubMed] [Google Scholar]
- 41.Wilmsdorff MV, Blaich C, Zink M, Treutlein J, et al. Gene expression of glutamate transporters SLC1A1, SLC1A3 and SLC1A6 in the cerebellar subregions of elderly schizophrenia patients and effects of antipsychotic treatment. World J Biol Psychiatry 2013;14:490–9. [DOI] [PubMed] [Google Scholar]
- 42.Abdolmaleky HM, Pajouhanfar S, Faghankhani M, et al. Anti-psychotic drugs attenuate aberrant DNA methylation of DTNBP1 (dysbindin) promoter in saliva and post-mortem brain of patients with schizophrenia and psychotic bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 2015;168:687–96. [DOI] [PubMed] [Google Scholar]
- 43.Porteous DJ, Millar JK, Brandon NJ, et al. Drosoph Inf ServC1 at 10: connecting psychiatric genetics and neuroscience. Trends Mol Med 2011;17:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong AH, Van Tol HH. Schizophrenia: from phenomenology to neurobiology. Neurosci Biobehav Rev 2003;27:269–306. [DOI] [PubMed] [Google Scholar]
- 45.Abi-Dargham A, Gil R, Krystal J, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry 1998;155:761–7. [DOI] [PubMed] [Google Scholar]
- 46.Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A 2000;97:8104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong AH, Buckle CE, Van Tol HH. Polymorphisms in dopamine receptors: what do they tell us? Eur J Pharmacol 2000;410:183–203. [DOI] [PubMed] [Google Scholar]
- 48.Kay SR, Flszbein A, Opfer LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261. [DOI] [PubMed] [Google Scholar]
- 49.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS). Psychol Rep 1962;10:799–812. [Google Scholar]
- 50.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22–33. [PubMed] [Google Scholar]
- 51.Pei L, Li S, Wang M, et al. Uncoupling the dopamine D1–D2 receptor complex exerts antidepressant-like effects. Nat Med 2010; 16:1393–5. [DOI] [PubMed] [Google Scholar]
- 52.Lee FJ, Pei L, Moszczynska A, et al. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J 2007;26:2127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogata H, Goto S, Sato K, et al. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 1999;27:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019;47:D607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molteni R, Calabrese F, Racagni G, et al. Antipsychotic drug actions on gene modulation and signaling mechanisms. Pharmacol Ther 2009;124:74–85. [DOI] [PubMed] [Google Scholar]
- 56.Brandon NJ. Uncovering the function of Disrupted in Schizophrenia 1 through interactions with the cAMP phosphodiesterase PDE4: contributions of the Houslay lab to molecular psychiatry. Cell Signal 2016;28:749–52. [DOI] [PubMed] [Google Scholar]
- 57.Weinstein JJ, Chohan MO, Slifstein M, et al. Pathway-specific dopamine abnormalities in schizophrenia. Biol Psychiatry 2017; 81:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Funk AJ, McCullumsmith RE, Haroutunian V, et al. Abnormal activity of the MAPK- and cAMP-associated signaling pathways in frontal cortical areas in postmortem brain in schizophrenia. Neuropsychopharm 2012;37:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kyosseva SV, Elbein AD, Griffin WS, et al. Mitogen-activated protein kinases in schizophrenia. Biol Psychiatry 1999;46:689–96. [DOI] [PubMed] [Google Scholar]
- 60.Matsuda S, Ikeda Y, Murakami M, et al. Roles of PI3K/AKT/GSK3 pathway involved in psychiatric illnesses. Diseases 2019;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry 2020;19:15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stark C, Breitkreutz BJ, Reguly T, et al. BioGRID: a general repository for interaction datasets. Nucleic Acids Res 2006;34:D535–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orchard S, Ammari M, Aranda B, et al. The MIntAct project — IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res 2014;42:D358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.The UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 2019;47:D506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camargo LM, Collura V, Rain JC, et al. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry 2007;12:74–86. [DOI] [PubMed] [Google Scholar]
- 66.Millar JK, Christie S, Porteous DJ. Yeast two-hybrid screens implicate Drosoph Inf ServC1 in brain development and function. Biochem Biophys Res Commun 2003;311:1019–25. [DOI] [PubMed] [Google Scholar]
- 67.Morris JA, Kandpal G, Ma L, et al. Drosoph Inf ServC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet 2003;12:1591–608. [DOI] [PubMed] [Google Scholar]
- 68.Ozeki Y, Tomoda T, Kleiderlein J, et al. Disrupted-in-schizophrenia-1 (Drosoph Inf ServC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci U S A 2003;100:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyoshi K, Asanuma M, Miyazaki I, et al. Drosoph Inf ServC1 localizes to the centrosome by binding to kendrin. Biochem Biophys Res Commun 2004;317:1195–9. [DOI] [PubMed] [Google Scholar]
- 70.Shinoda T, Taya S, Tsuboi D, et al. Drosoph Inf ServC1 regulates neurotrophin-induced axon elongation via interaction with Grb2. J Neurosci 2007;27:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Millar JK, Pickard BS, Mackie S, et al. Drosoph Inf ServC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 2005;310:1187–91. [DOI] [PubMed] [Google Scholar]
- 72.Siuciak JA, Chapin DS, McCarthy SA, et al. Antipsychotic profile of rolipram: efficacy in rats and reduced sensitivity in mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 2007;192:415–24. [DOI] [PubMed] [Google Scholar]
- 73.Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016;143:3050–60. [DOI] [PubMed] [Google Scholar]
- 74.Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A 1996;93:9235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet 2012;13:537–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sullivan PF. Questions about Drosoph Inf ServC1 as a genetic risk factor for schizophrenia. Mol Psychiatry 2013;18:1050–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Porteous DJ, Thomson PA, Millar JK, et al. Drosoph Inf ServC1 as a genetic risk factor for schizophrenia and related major mental illness: response to Sullivan. Mol Psychiatry 2014;19:141–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.