Abstract
The human urinary microbiome, also termed urobiome, has been overlooked due to the clinical dogma of sterile urine, as reported by routine culture. However, evolving sensitive tools such as expanded quantitative urine culture, 16S ribosomal RNA gene sequencing, and next-generation sequencing have discovered a vast number of microorganisms present in urine, even in healthy individuals. Microbiome dysbiosis and its links to disease is a heavily explored area in several microbial niches. Presently, urobiome dysbiosis and its correlation to urinary system-related diseases is at its infancy but rapidly emerging, as it provides potential therapeutic insights. This review outlines the changes in the human urinary microbiome concerning globally prevalent diseases affecting kidney function, such as chronic kidney disease (CKD), diabetes mellitus (DM), hypertension (HT), and urinary tract infection (UTI). Alterations to urine microbial diversity, including differences in the abundance and species richness of particular microbial genera, notably Lactobacillus, Prevotella, Streptococcus, Staphylococcus, Klebsiella, Enterococcus, between diseased and healthy samples are discussed utilising studies to date. Subsequent research needs to move beyond correlation to understand the roles of the urinary microbiota in diseases, thereby clarifying whether urinary dysbiosis has causal contributions that may provide important insight for diagnostics, pathophysiology, and therapy in renal pathologies.
Keywords: Dysbiosis, Human urinary microbiome, Urinary system-related diseases
Introduction
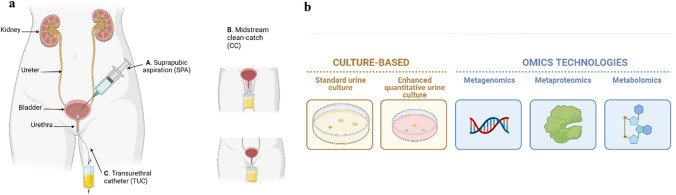
The human body is loaded with approximately 38 trillion commensal and pathogenic microorganisms, similar in proportion to all the human cells put together [1]. These microorganisms in and on the body are called microbiota and range from bacteria and eukaryotic viruses to protozoa and fungi. They are found in several physical locations like the gastrointestinal tract, respiratory tract, nasal tract, urogenital tract, skin and play crucial roles to sustain human health [2–5]. The Human Microbiome Project (HMP) is an initiative by the United States National Institutes of Health (NIH) to uncover the microbial composition of the human body and their roles in health and disease, such as the existence of a characteristic microbiome associated with particular health status [6]. In 2008, the initial phase of this project (HMP1) characterised microbial communities in 300 healthy participants at five significant sites: nasal passageway, oral cavity, skin, gastrointestinal tract, and urogenital tract. Various research has been done on these more common microbial niches [7–11], but it wasn’t until much recently the urinary microbiome; specifically, the bladder microbiome was studied because of the clinical dogma that urine (which represents the bladder microbiome) of healthy asymptomatic individuals is sterile until the urethra, as shown by routine culture [12, 13]. However, emerging research utilising more sensitive techniques such as enhanced quantitative urine culture and 16S ribosomal RNA (16S rRNA) gene sequencing identifies extensive microorganisms in urine, even in the bladder [13–17]. The urinary microbiota comprises microorganisms residing in the bladder. Still, it may be contaminated with microorganisms in the lower urinary tract or urogenital tract based on the sampling method used to obtain urine (Fig. 1) [18].
Fig. 1.
a. Three urine collection methods that are currently used to sample urine for urinary microbiome analyses. A. Suprapubic aspiration (SPA) utilizes a syringe placed perpendicular to the skin to directly sample urine from the bladder, B. Midstream clean-catch (CC) method involves thorough sanitation of the genital area to aseptically collect the mid-portion of urine flow into a sterile urine cup, C. Transurethral catheter (TUC) samples urine from the bladder via the urethra. SPA suprapubic aspiration, CC midstream clean-catch, TUC transurethral catheter. b Techniques that are employed to study taxonomy and/or functional profile of the urinary microbiome. Culture-based & OMICs, [metagenomics (16S rRNA gene sequencing and whole-genome sequencing), metaproteomics and metabolomics]
A surprising outcome of HMP1 was that even among healthy individuals, there were differences in the microbial diversity in niches, including the gut, skin, and vagina, potentially due to differences in environment, diet, and medication [19]. Therefore, relying on the composition of the human microbiome of healthy individuals as a definition for a “healthy status” is problematic. Another fascinating discovery from other HMP human cohort studies that examined subjects with diseases in the gastrointestinal tract, oral cavity, or urogenital tract was that differences existed in the microbiomes between these diseased participants and healthy controls [20]. These differences were based on the proportion of particular microorganisms and microbial metabolism properties rather than the total microbial composition. This fact led researchers to look beyond microbiome composition to understand the role of the human microbiome in health and disease. The concept of “dysbiosis,” a change in abundance/ gain or loss of microbes in a community, leading to an “imbalance,” has gained a lot of attention due to its potential link to disease [21–24]. Dysbiosis of the microbiome manifests with one or more of the following characteristics: An increase in the proportion of pathogenic microorganisms, a decrease in the numbers of commensal microorganisms, and a reduction in microbial diversity [22]. The onset of dysbiosis is governed by environmental and host-related factors ranging from diet, infection, inflammation, antibiotics use, and genetics [22, 24, 25]. Association between dysbiosis and various diseases, including inflammatory, autoimmune and neurodegenerative diseases, have been established, but the question remains whether dysbiosis is a cause or consequence of disease [25].
The urinary system consists of two kidneys, two ureters, a bladder, and a urethra, and collectively works to eliminate waste products present in the blood. Diseases that may hamper this process contributes to poor renal health as measured by a low estimated glomerular filtration rate and increased urinary albumin [26, 27]. Chronic kidney disease (CKD) is a highly prevalent maladaptive condition of the kidneys [26, 28], with diabetes mellitus (DM) and hypertension (HT) being the leading causes of it, hence complications of either DM or HT can pose a threat to the healthy functioning of the urinary system [27, 29]. CKD also weakens the immune system and puts patients at risk of infections like urinary tract infection (UTI), further exacerbating the urinary system’s functioning if not treated at the onset [30]. The global disease burden of CKD, DM, HT and UTI is high, presenting as serious public health problems [26–29, 31].
Additionally, antimicrobial resistance limits antibiotic treatment options for UTI or UTI comorbid outcomes from these diseases, warranting alternative treatment options [32–36]. Dysbiosis of the urinary microbiome and its association to diseases implicating the urinary system is currently an emerging field [12, 37–41]. It offers potential insights on diagnostics, pathophysiology and microbiome-based treatment for urinary pathologies [42]. Given the significant burden of such diseases and the need for more therapeutic options to alleviate kidney infection-related morbidities, urinary microbiome dysbiosis and microbiome-based treatment options may be worthy of investigating.
This review focuses on human urinary microbiome dysbiosis concerning CKD, DM, HT, and UTI, all significant global public health concerns affecting the urinary system. It presents the current links between urobiome dysbiosis and disease and highlights lapses and how this area may contribute to therapy. The keywords “chronic kidney disease” OR “diabetes” OR “hypertension” OR “urinary tract infection” AND “urinary microbiome” were searched (Google Scholar and PubMed), and articles in the past 20 years were chosen for this review.
The Healthy Microbiome
As previously mentioned, there is vast interpersonal diversity in the microbiome of healthy individuals; thus, any attempts to identify a so-called “healthy microbiome” in each site may be challenging [43]. As a result, researchers moved on to an alternate concept: a “healthy functional core” to define a healthy microbiome, which corresponds to a microbiome capable of metabolic and molecular functions needed for the healthy life of these microbes: expresses housekeeping genes correctly, has resilience against external and internal changes (e.g., medication and age), and can hold a mutually beneficial relationship with the host [44]. The idea is that although the composition of microbes may vary from healthy person to person, a healthy microbiome has a healthy functional profile that supports its survivability. Dysbiosis likely happens when the external or internal perturbations are more potent than the resilience capabilities of the microbiome [22].
The Healthy Urinary Microbiome
Interestingly, similar to the healthy lungs, the bladder was considered sterile and free from bacteria not long ago. These myths were debunked, and their associated microbiomes are thought to play essential roles in urinary and respiratory health, respectively [13, 45, 46]. Colonisation in urine by microorganisms seems counterintuitive as its low pH of about 6 and high urea concentration makes it inhabitable to many bacteria [47]. Host factors have been suggested to play a role in the colonisation of these resident microorganisms, such as the expression of receptors for the adherence of bacteria to the uroepithelium; however, this requires further scientific analysis [42]. The source of these colonising microorganisms in the bladder microbiome is hypothesised to be genital [48]. The resident gut microbiota is implicated as the source of colonising uropathogens in urinary tract infections [48, 49].
Healthy urine microbiota includes a range of bacterial genera, predominantly, Lactobacillus, Corynebacterium, Staphylococcus, Streptococcus, Veillonella, Prevotella with sex-specific differences: Lactobacillus found mainly in healthy women, and Corynebacterium or Streptococcus found mainly in healthy men [12, 50]. Healthy females tend to have a more diverse composition of bacterial genera than males [51]. Catheterized microbiomes, including urethral samples, have a higher abundance of Staphylococcus, Neisseria, and Veillonella, while midstream voided urine samples have Streptococcus, Lactobacillus, and Gardnerella [50] predominantly. The healthy lung microbiome consists mainly of Streptococcus, Prevotella, Veillonella, Neisseria, and Fusobacterium [52]. Fusobacterium has also been detected in the urinary microbiome in abundance, but in bladder cancer patients, not in the healthy urobiome [53].
It is also noted that the “core'' healthy urinary microbiome exists in an age group-specific manner, where a change in the abundance of particular genera and new genera are seen with age: urobiome diversity decreases with age, and the genera Jonquetella, Parvimonas, Proteiniphilum, and Saccharofermentans are shown to have age-specific occurrences in those over 70 years [51, 54].
Antibiotic Use and Urinary Microbiome Dysbiosis
Microbiome dysbiosis has been correlated with the occurrence of various diseases, but what is the onset of it? Antibiotics are likely to contribute to microbial dysbiosis, as they can affect microbial abundance [42]. The impact of antibiotics use on gut microbiome dysbiosis has been explored extensively [55] but not so much regarding urinary microbiome dysbiosis. The influence of antibiotics on the resident microorganisms occupying the urinary tract has been studied in older adults [56]. It was found that the microbiota before and after antibiotic therapy was different, with Escherichia coli being the most abundant species and Lactobacillus being the most reduced genera after antimicrobial drug use. A similar finding was obtained in a very recent study that monitored the urinary microbiota of a patient given oral Cephalexin over seven days, leading to the depletion of commensal Lactobacillus sp. and recurrent cystitis [57]. These studies suggest that antibiotics may contribute to urinary microbiome dysbiosis. Therefore, these therapies must be carefully controlled to deplete uropathogens but not commensal microorganisms associated with healthy states. This control is also necessary to minimise antimicrobial resistance when treating urological diseases, especially with broad-spectrum antibiotics [58].
Urinary Microbiome Analysis
Three primary urine collection methods are employed to collect urine from individuals to study the urinary microbiome (Fig. 1) [59]. Suprapubic aspiration (SPA) is excellent for explicitly sampling urine directly from the bladder without contamination from local microbiota [18]. Nonetheless, it is very invasive, involving inserting a needle at the suprapubic area directly above the bladder [17]. The midstream clean-catch (CC) urine technique is a commonly used non-invasive method to obtain urine samples by avoiding the initial and final portions of urine flow to reduce skin and urethral contamination [50]. The urine travels the entire lower urinary tract (from the ureters, bladder, and out of the urethra). It may risk skin, perineum, and vagina contamination if these regions are not sterilised with sterile wipes before [60]. The use of a transurethral catheter (TUC) involves inserting a catheter into the bladder through the urethra [18]. Although this method is better at targeting the urinary microbiome than with CC, it is invasive and may perturb the urethral microbiota [60]. Taking measures to minimise contamination effects, such as ensuring participants sanitise their periurethral regions sufficiently when providing a mid-stream urine sample and taking urethral swabs with TUC samples to assess their level of contamination [18].
Currently, the urine microbiome is commonly studied using various culture, molecular, proteomics-based, and bioinformatics techniques such as conventional culture, enhanced quantitative urine culture, followed by metagenomic sequencing, and metaproteomics [12, 14, 50, 59]. Metagenomic amplicon-based 16S rRNA gene sequencing is commonly used to identify the urinary microbiome owing to their conserved primers and hypervariable regions (V1–V9), providing species-specific identification [15–17, 59]. Nevertheless, it does not provide functional aspects of the bacteria as it does not sequence all the genes. Therefore, shotgun metagenomic sequencing, metaproteomics, and, more recently, metabolomics are used to understand urinary microbial functional properties, which may be crucial to deciphering host-microbiome interactions in disease [12, 18, 60, 88]. Metatranscriptomics has not been used for urinary microbiome analyses till-date, to our knowledge. Apart from the identification of microbiota, studies also use species richness estimates such as Chao1 and ACE indices to assess the number of different species, and diversity indices such as Shannon and Simpson indices, for the total number of species and the relative abundance of each species, in the urobiome [37, 61]. The presence of viable but nonculturable (VBNC) bacteria in urine makes it difficult to culture all urine microbiota using routine microbiological media [62]. The use of sensitive molecular techniques, such as 16S rRNA gene sequencing and enhanced quantitative urine culture that makes use of several culture media and incubation conditions, allows a wide variety of genitourinary bacteria to be identified, which may otherwise not be detected by standard urine culture alone [14, 37]. The main limitation of culture-based techniques as opposed to gene sequencing is that they are insufficient to identify the urine microbiome completely [51]. However, they benefit from verifying microbial viability, which is not as straightforward with sequencing experiments.
Urinary Microbiome and Disease
The term dysbiosis was first coined in the early twentieth century with the human gut microbiota [63]. This field has since quickly emerged into an active area of research in other microbiome locations [54]. Recently, studies have tried to correlate kidney-related diseases and comorbidities to dysbiosis of the urine microbiota. Following intestinal dysbiosis, these studies suggest changes in diversity and abundance of microorganisms in the urine microbiome associated with diseases, including CKD, DM, HT, hyperlipidemia (HL), and UTI [12, 37, 40, 64]. The following sections will briefly explore the specific changes to the urinary microbiome in CKD, DM, HT, and UTI patients compared to their healthy counterparts and characterise diseased urobiomes (Table 1).
Table 1.
Summary of literature on urinary microbiome dysbiosis in CKD, DM, HT, and UTI. CKD chronic kidney disease, DM diabetes mellitus, UTI urinary tract infection, HT hypertension
| Disease and reference group/s (if relevant) (n) | Age (years mean ± standard deviation) | Method of sample collection | Study techniques | Main findings | Reference |
|---|---|---|---|---|---|
| Stage 3–5 non-dialysis dependent CKDa: males (36), females (41) | 71.5 ± 7.9 | CCb | 16S rRNA sequencing (V4 region, Illumina), diversity measures: inverse Simpson, Chao, and Shannon indices | Most abundant bacterial genera or family: Corynebacterium, Staphylococcus, Streptococcus, Lactobacillus, Gardnerella, Prevotella, Escherichia Shigella, and Enterobacteriaceae | [37] |
|
Females with type 2 DMc (25), DM + HTd (24), DM + HLe (7), DM + HT + HL (11) |
DM only: 56.28 ± 13.91 DM + HT: 70.42 ± 9.00 DM + HL: 54.43 ± 10.66 DM + HT + HLP: 69.81 ± 9.64 |
Modified midstream urine collection | 16S rRNA sequencing (V3–V4 regions, Illumina), diversity measures: number of reads, OTUsf, Chao1, ACE, Shannon and Simpson indices | Number of bacterial genera and most abundant genera: DM: 320, Lactobacillus, Prevotella, Acinetobacter. DM + HT: 303, Prevotella, Streptococcus, Bacteroides. DM + HL: 236, Lactobacillus, Prevotella, Halomonas. DM + HT + HL: 225, Prevotella, Lactobacillus, Bacillus | [40] |
| Females with type 2 DM (70) and female controls (70) | all: 26–35, 36–45, 46–55, 56–65, 66–75, 76 and above | Modified midstream urine collection | 16S rRNA sequencing (V3–V4 regions, Illumina), diversity measures: number of reads, OTUs, Chao1, ACE, Shannon and Simpson indices |
Bacterial genera with different relative abundances between the type 2 DM cohort and controls: Prevotella*, Lactobacillus*, Shuttleworthia*, Acinetobacter, Bacteroides, Halmonas, Blautia, Faecalibacterium, Corynebacterium, Klebsiella, Pseudomonas |
[67] |
| Females with type 2 DM with detectable and undetectable urine IL-8 g (70) and female controls (70) | all: 26–85 | Modified midstream urine collection | 16S rRNA sequencing (V3–V4 regions, Illumina), ELISAh, diversity measures: OTUs, Chao1, Shannon, and Simpson indices |
11 bacterial genera were more abundant in the type 2 DM with detectable IL-8 cohort than the type 2 DM with undetectable IL-8 cohort: Shuttleworthia, Mobiluncus, Peptoniphilus, Corynebacterium, Thermus, Gemella, Enterococcus, Acinetobacter, Akkermansia, Aquaspirillum, and Geobacillus |
[66] |
| Females with type 2 DM (32) and female controls (26) |
DM: 56.97 ± 8.01 controls: 57.62 ± 9.24 |
CC | Standard culture, 16S rRNA sequencing (V3–V4 regions, Illumina), diversity measures: Observed Species, Chao1, ACE, Shannon and Simpson indices | Bacterial genera that were over-represented in the type 2 DM cohort: Escherichia-shigella, Klebsiella, Aerococcus, Delftia, Enterococcus, Alistipes, Stenotrophomonas, Micrococcus, Deinococcus, Rubellimicrobium | [61] |
| Kidney stone disease with normotension, prehypertension and HT (50) and controls (12) |
Kidney stone + normotension: 47.33 ± 14.95, prehypertension: 54.09 ± 13.03, HT: 54.74 ± 12.36, controls: 58.91 ± 18.97 |
BUA with a cystoscopei and TUCj | Expanded quantitative urine culture, 16S rRNA sequencing (V3–V4 regions, Illumina), diversity measures: Observed Species, Chao1, Shannon, Simpson indices |
Bacterial genera that were significantly different between the kidney stone cohorts and controls: Comamonas, Enterococcus, Bifidobacterium, Lactobacillus |
[39] |
| Females with DOSk (pelvic floor surgery) positive urine culture (13), postoperative UTIl (4) and DOS negative urine culture with postoperative no-UTI/ negative (37) |
DOS positive urine culture: 67, postoperative UTI: 60, negative: 56 |
TUC | Urine culture, 16S rRNA sequencing (Life Technologies, RDP classifier), ELISA, protease assay |
Lactobacillus was abundant in all three cohorts Most abundant bacterial genera in postoperative UTI cohort versus postoperative no-UTI (negative) cohort: Dyella, Fulvimonas, Klebsiella, and Lactobacillus |
[77] |
| Catheter-associated UTI: males (8), females (2) | 70.9 | TUC | Urine culture, 16S rRNA sequencing (V4 region, Illumina), diversity measures: observed OTUs, and Shannon index | Study subjects that developed catheter-associated UTI had a low diverse urinary microbiome | [75] |
| Females with UTI-like symptoms (75) and females without UTI-like symptoms (75) | all: 62.3 ± 14.9 | TUC | Standard culture, modified standard culture, expanded quantitative urine culture, diversity measure: species accumulation curves and Shannon index | Bacterial species that had substantially higher average CFU/ml in the UTI-cohort than no-UTI cohort: Escherichia coli, Klebsiella pneumoniae, Streptococcus agalactiae, Aerococcus urinae, Enterococcus faecalis, Staphylococcus aureus, Streptococcus anginosus | [14] |
| Females with urogynaecology surgery (pelvic organ prolapse and/or urinary incontinence) (104) | 57 | TUC | 16S rRNA sequencing (V4 region, Illumina), diversity measures: Chao 1, ACE, Shannon, and Simpson indices |
Postoperative UTI risk was associated with an abundance of diverse pathogens in the preoperative bladder microbiome: Enterobacteriaceae, Pseudomonas, Staphylococcus, the species Lactobacillus delbrueckii, Actinotignum schaalii, Anaerococcus obesiensis, Corynebacterium tuberculostearicum, Streptococcus anginosus, Aerococcus christensenii and Anaerococcus murdochii Increased Lactobacillus iners was protective against postoperative UTI risk |
[76] |
| UTI: males (149), females (234) | 56 | CC | Urinalysis, urine culture, 16S rRNA sequencing (broad range archaeal primers, mcrA gene, Technelysium) | The archaeal methanogen Methanobrevibacter smithii was present in 54% of the patients diagnosed with UTI | [64] |
| Cystitis: males (12), females (16) | 66 | CC, TUC | Standard culture, 16S rRNA sequencing (V3–V4 regions, Illumina), diversity measures: observed OTUs |
15 distinct phyla were detected in all cystitis patients. The most abundant phyla: Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria |
[57] |
aChronic kidney disease
bMidstream clean-catch method
cDiabetes mellitus
dHypertension
eHyperlipidemia
fOperational taxonomic units
gInterleukin-8
hEnzyme-linked immunosorbent assay
iBladder urine aspirate
jTransurethral catheter
kDay of Surgery
lUrinary tract infection
*most abundant genera in type 2 DM
Chronic Kidney Disease
There are limited research governing associations between the urobiome and CKD, thus identifying any correlations between them proves to be challenging. Emerging research is necessary to explore this area to gain a reliable understanding of the urobiome in chronic kidney pathologies. At the time of writing this review, only the work done by Kramer et al. is relevant to assessing the CKD urobiome in humans [37]. Their work used midstream urine samples of adults, covering stages 3 to 5 non-dialysis dependent chronic kidney disease. A majority of the specimens had particular genera that were more abundant than others: Corynebacterium, Staphylococcus, Streptococcus, Lactobacillus, Gardnerella, Prevotella, Escherichia Shigella, and Enterobacteriaceae. There were also high levels of diversity in the samples, where participants with higher estimated glomerular filtration rates and CKD at stage 3 had more diverse urobiomes. More recently, bladder microbiome dysbiosis has been demonstrated in cats with CKD, where Escherichia Shigella was the dominant species [65]. As CKD is a risk factor for infections, close attention must be paid to the abundance of uropathogens in the CKD urobiome.
Diabetes Mellitus
Although much research has been exploring links between DM (type 1 and 2) and the gut microbiome, there is a limited body of knowledge analysing the urinary microbiome regarding DM. The urobiome of DM patients has been studied more than that of CKD patients. A significant study that utilised urine samples from women with type 2 DM only and comorbidities of HT and HL is currently the only study that assessed whether these comorbidities might alter the urinary microbiome in DM patients [40]. DM patients with different comorbidities had differences in the predominant bacterial genera present in their urine: for the DM cohort, it was Lactobacillus, Prevotella, and Acinetobacter. For the DM and HL cohort, it was Lactobacillus, Prevotella, and Halomonas. For DM and HT, it was Streptococcus, Prevotella, and Bacteroides. This suggests that specific changes in the urine microbiome may be associated with disease and the kind of comorbidities. Interestingly, some cohorts had completely absent species in other cohorts: Deinococcus aquatilis was found in the DM-only cohort but was not found in the DM and HT cohort. Such disease-specific microbiome species have also been found in the lung microbiome, where lung cancer patients showed bacterial species such as Corynebacterium tuberculostearicum and Keratinibaculum paraultunense, not in bronchiectasis patients [45].
The study by Ling et al. used 16S rRNA gene sequencing to assess urinary microbiota in female type 2 DM [66]. They went a step further to check for links between dysbiosis of urinary microbiota and proinflammatory chemokine interleukin-8 levels (IL-8) for the first time. They showed IL-8 level-dependent differences in the abundance of specific urinary microbes, shedding light on possible interactions between the urobiome and inflammation, which is significant as type 2 DM has been established as an inflammatory disease [66]. This uncovers possibilities for urinary microbiome-based therapy in type 2 DM.
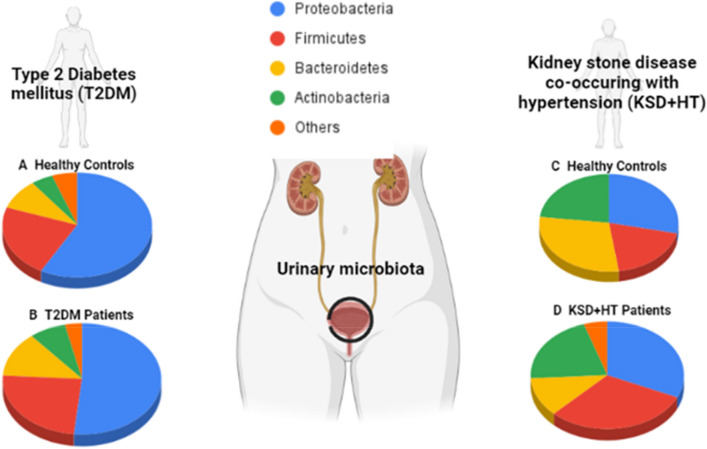
Chen et al. recruited female type 2 DM patients and healthy controls to find urinary dysbiosis linked to diabetes: there were higher abundances in the pathogens Escherichia-Shigella, Klebsiella, and Enterococcus in type 2 DM patients compared to controls [61]. Another study that investigated links between urinary microbiota and type 2 DM found reduced bacterial diversity and richness in Chinese type 2 DM patients compared to healthy controls, associated with decreased carbohydrate and amino acid metabolism [67]. These findings suggest that therapy focused on altering urinary microbiome dysbiosis may modulate metabolism in type 2 DM patients, a hypothesis that needs to be assessed in future studies. Additionally, the phyla Actinobacteria was more abundant in type 2 DM patients than healthy controls (Fig. 2) and served as a biomarker to differentiate between them. A lapse in research in all these studies is an imbalance of gender. Most DM participants were female and not male, highlighting the need for more studies with male DM participants so that sex hormone influence on urine microbiota can be discerned.
Fig. 2.
Composition of bacterial phyla in the urinary microbiome present in type 2 diabetes mellitus (T2DM) patients [67], and kidney stone disease co-occurring with hypertension (KSD + HT) patients [39], as compared to their healthy counterparts. A. Relative abundance of phyla present in matched healthy controls and B. T2DM patients [67]. C. Relative abundance of phyla present in healthy controls with neither kidney stones nor hypertension and D. KSD + HT patients [39]. T2DM type 2 diabetes mellitus, KSD + HT kidney stone disease co-occurring with hypertension
Hypertension
Similarly, with CKD and DM, there has been relatively little research exploring the urobiome of HT patients, although there is extensive work that links the gut microbiome to HT. Apart from the work of Liu et al. on DM and comorbidities of HT and HL [40], there is one other research characterising the urinary microbiome of HT patients with kidney stone disease [39]. There isn’t any literature that explores the urobiome of patients suffering only from HT (without co-occurring diseases).
Of the work currently done, Liu et al. found that patients with kidney stone disease and HT had a higher abundance of the phyla Firmicutes associated with the genus Lactobacillus than healthy controls (Fig. 2) [39]. Whether this abundance of commensal Lactobacillus is part of a host inflammatory response or related to disease pathology is worth investigating. Interestingly, the urobiome profile changes based on the extent of hypertension (normotension, prehypertension, and hypertension) in kidney stone disease patients, indicating microbiome-based tools for monitoring kidney stone disease progression and treatment response in those complicated with hypertension.
Urinary Tract Infection
There are recent studies that observed the urinary microbiome and its influence on UTI. Additionally, antimicrobial resistance has been implicated in bacteria in UTI, signifying the necessity to study the urobiome of UTI to inform and assess antibiotic use [57, 68–70].
The study titled “Microbial metagenome of urinary tract infection” employed 16S ribosomal DNA (16S rDNA) and metagenome sequencing of the microbiome in 121 midstream clean-catch samples [71]. They found that the two clusters that showed infectiousness of the urinary tract had Proteobacteria as the most abundant phylum. Additionally, uropathogens like Escherichia, Klebsiella, Pseudomonas, Enterobacter, and Citrobacter and less known genera in infection like Acidovorax, Rhodanobacter, Oligella were uncovered. These findings may indicate urobiome dysbiosis in UTI, owing to a microbial imbalance with an abundant presence of uropathogens. It is interesting to understand the roles of the other genera present in UTI, which are usually less common in infection [71], as they may provide additional insight into UTI pathogenicity. This presence of several uropathogens uncovers the possibility for polymicrobial/ microbe-microbe interactions and how this influences disease, which has been reviewed [72].
Recently, potential bacterial microbiome dysbiosis in lower male urinary tract symptoms (LUTS) was studied [73]. Streptococcus and Enterococcus spp. were abundantly represented in the LUTS cohort in comparison to the controls. Additionally, recurrent UTI is a cause for concern, and this has been explored concerning the urinary microbiome in a study by Burnett et al. [74]. Forty-three women with recurrent UTIs were included in this study, and their urine was obtained via catheterisation and voiding. These samples underwent standard and expanded quantitative urine culture. Culture results were associated with five clinical profile clusters for the subjects based on patient-reported symptoms like frequency, urgency, pain, cloudiness. Interestingly, one clinical profile group that used vaginal estrogen showed a significantly higher proportion of Lactobacillus, which is commonly associated with a healthy status, suggesting that perhaps for recurrent diseases, restoring commensal microbiota alone is insufficient for complete recovery [74]. Nevertheless, this study had small numbers of subjects in each clinical profile, thus the findings need additional data for validation.
Price et al. used enhanced quantitative urine culture to identify uropathogens in female urogynaecology patients with UTI-like symptoms and no-UTI-like symptoms [14]. It was found that the no-UTI cohort had more diverse and richer species than the UTI cohort, which is in line with the findings of Horwitz et al., who also demonstrated a lower urobiome diversity in those with UTI [75]. The uropathogens Escherichia coli, Klebsiella pneumoniae, Enterococcus faecalis, among others, were more abundant in the UTI cohort [14].
Apart from bacterial dysbiosis in the urinary microbiota, the archaeal role in UTI has also been studied [64]. It was shown that the archaeal methanogen Methanobrevibacter smithii might be a constituent of the urinary microbiome. It was co-cultured every single time with enterobacteria, including Escherichia coli, Klebsiella pneumoniae, Enterobacter sp. 19/34 patients diagnosed with UTI had M. smithii present in their urine samples. This may suggest a potential mutually beneficial relationship between M. smithii and enterobacteria, which may contribute to UTI in some patients, as enterobacteria are known uropathogens in this regard. Further research exploring the contribution of M. smithii to UTI, such as its influence on enterobacteria dysbiosis and pathogenesis in UTI, is necessary.
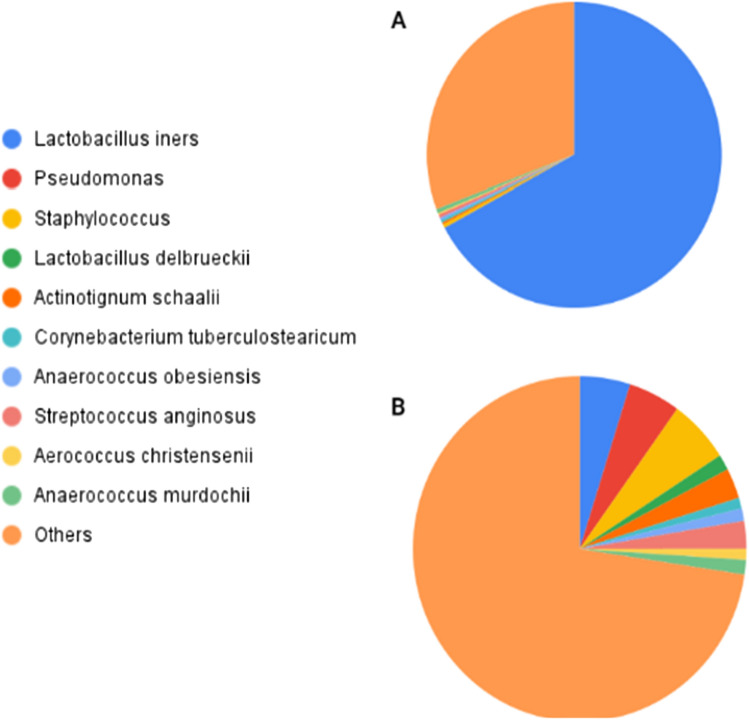
Postoperative UTI is a common complication that may occur after urogynecology surgery. Studies have assessed microbiome-based markers and host antimicrobial peptide profiles to identify patients at risk for postoperative UTI [76, 77]. These studies found that the urinary microbiota composition present on the day of surgery was associated with postoperative UTI risk, where an increased presence of Lactobacillus iners (Fig. 3) and urinary HBD1 may reduce the risk of postoperative UTI after surgery for pelvic floor disorders such as pelvic organ prolapse [76, 77].
Fig. 3.
Comparison between two clusters of pre-operative bladder microbiomes associated with differential post-operative urinary tract infection (UTI) statuses [76]. Pre-operative bladder microbiomes were divided into two clusters: A. Less Dispersed Cluster (LDC) has an abundance of Lactobacillus iners and all women in this cluster did not develop post-operative UTI [76]. B. More Dispersed Cluster (MDC) has more diversity in uropathogens and included more women who developed post-operative UTIs later on [76]. UTI urinary tract infection, LDC less dispersed cluster, MDC more dispersed cluster
Cystitis is the most common form of UTI, which may be complicated by antimicrobial resistance. The very recent work done by Ceprnja et al., investigated changes in the urinary microbiome of cystitis patients and its dynamics when prescribed with antimicrobial therapy [57]. 16S rRNA gene sequencing data of 28 patients suspected of cystitis was used to infer models about urine microbial interactions and dynamics: Actinobacteria and Bacilli demonstrated protective roles against pathogens, such as bacterial cystitis indicating Gammaproteobacteria, which was the class pathogen associated with a majority of cases in this study. Notably, a single female patient’s microbiota was monitored during the entire 7-day period of oral Cephalexin therapy, and it was found that the national guidelines on antimicrobial treatment duration for UTI used should be altered, as the 7-day treatment led to the depletion of commensal Lactobacillus sp. contributing to Candida and recurring cystitis [57]. The study found that two days of therapy was sufficient to reduce the relative abundance of the uropathogen in question from 94% to 1.04%. The need for more studies with larger cohorts that monitor urobiome dynamics during antimicrobial therapy may provide essential insights for the rational use of antibiotics.
Future Perspectives: Host-Urobiome Interactions, Biomarkers, Microbiome and Metabolome-Based Therapy
The question remains, is dysbiosis a cause or result of disease? Current research has established links between urinary microbiome dysbiosis and urinary system diseases, paving the way for additional work that needs to assess whether there are disease-specific causal attributes of imbalanced urobiomes, thereby identifying targets for therapy. This is an essential future perspective as merely having information about microbial community changes does not specify whether those changes are functionally detrimental to the host, secondary outcomes from diseases or individual host-related factors [22, 25]. It is therefore suggested that future research focuses not only on correlating urinary microbiome changes to diseases but also on interactions between the host and microbiome, such as the impact of imbalanced urobiomes to host functions (e.g., the immune system, glomerular filtration), and on host factors that influence the microbiome. For instance, Rudick et al. utilised asymptomatic bacteriuria Escherichia coli (ASB E. coli) to treat UTI and suggested that ASB E. coli exhibits its anti-infective effects by improving the host immune response to uropathogens thereby reducing their abundance [79]. This highlights the need also to consider host factors such as immunity that may affect the microbiota balance [25]. Similarly, it is necessary to investigate whether urinary dysbiosis has measurable, maladaptive functional implications to the host [22]. Studying the microbiome in the host context provides insights that are paramount to understanding how urinary dysbiosis contributes to diagnostics, aetiology and discovery of therapeutic targets.
Biomarkers can serve as valuable diagnostic tools, helping to identify the extent of disease (high-risk versus low-risk patients) and explain pathophysiology [77, 80, 81]. Biomarker identification based on microbiota signatures for diseased urinary microbiomes is an important direction for future studies. Currently, urine biomarkers have been explored with community-acquired pneumonia, where they were used in predictive models to identify two cytokines, thirteen microbial taxa, and metabolites that can be used to differentiate between bacterial and viral pneumonia [80]. A recent review identifies urine microbial extracellular vesicles as novel biomarkers for allergic diseases [82]. Extracellular vesicles are involved in host and microbiome interactions; therefore, it is interesting to study whether urinary microbial-derived extracellular vesicles in disease provide insights into urinary system-related pathologies [83]. Future studies can also explore urinary microbial signatures, metabolites, among others, as potential biomarkers for diseases implicating the urinary system such as CKD, HT, DM and UTIs.
Understanding cause-effect relationships of urinary microbiome dysbiosis facilitate research on urinary microbiome-based therapy. The use of live biotherapeutic products (LBPs), such as using microorganisms as part of vaccines and probiotics, prebiotics and faecal microbiota transplantation, are explored to alleviate disease symptoms potentially by reconstituting the healthy, protective microbiome in various gastrointestinal and non-gastrointestinal diseases [24, 78, 79, 84–86]. Future studies can explore such microbiome-based therapies with diseases affecting the urinary system. Horwitz et al. demonstrated that bladder inoculation with benign E. coli HU2117 as an LBP did not prevent colonisation by uropathogens and the incidence of symptomatic UTI; however, a limitation of this study was the smaller sample size [75]. In contrast, in another study, ASB E. coli exerts anti-infective effects in UTI [79]. The need for future studies with sufficient samples that examine the use of LBPs in urological diseases is necessary for a consensus. A recent study by Aragón et al. also reviews the effectiveness of probiotics, prebiotics, and diet as ways to regulate an imbalanced microbiome [87]. The use of probiotics such as Lactobacillus casei in bladder cancer and Oxalobacter formigenes in kidney stone cases has shown promising disease management results. However, the latter example has had contradictory findings [87]. Future studies can clarify existing contradictions and explore probiotic use in other kidney-related diseases like CKD, DM, UTI, and HT. In addition to microbiome-based therapy, metabolite-based therapy is suggested to be a potential area for future translational research on urinary system diseases, given that dysbiosis of the urinary microbiome is also reflected in changes at the metabolite level [85, 88]. A combination of urine microbiome analyses and urine metabolomics would provide a reliable means to identify therapeutic targets and biomarkers [88].
Conclusion
The human urinary microbiome has implications for health and disease. Studying urobiome dysbiosis, such as changes to species richness and diversity, in kidney pathologies may provide new insights into disease pathogenesis and treatment interventions. As urobiome dysbiosis is a relatively understudied area, it is recommended that future studies continue to explore this field. This is necessary to form reliable and sound scientific conclusions. It is essential to look beyond solely correlating urinary microbiome dysbiosis with diseases and to investigate host-microbe interactions and potential microbial-derived biomarkers that may allow predictions to be made about disease diagnosis, mechanisms, and targets for therapy. Future studies can also explore ways of modifying urobiome dysbiosis and alleviating disease symptoms, such as introducing suitable LBPs to suppress uropathogens, utilising prebiotics, and diet modifications.
Acknowledgements
The authors received no funding.
Funding
None.
Availability of data and material
No data and material were used to support this study.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogunrinola GA, Oyewale JO, Oshamika OO, Olasehinde GI. The human microbiome and its impacts on health. Int J Microbiol. 2020;2020:8045646. doi: 10.1155/2020/8045646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäckhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, Versalovic J, Young V, Finlay BB. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12:611–622. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Mohajeri MH, Brummer RJ, Rastall RA, Weersma RK, Harmsen HJ, Faas M, Eggersdorfer M. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. 2018;57:1–4. doi: 10.1007/s00394-018-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimitri-Pinheiro S, Soares R, Barata P. The microbiome of the nose—friend or foe? Allergy Rhinol. 2020;11:2152656720911605. doi: 10.1177/2152656720911605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterolo. 2015;31:69–75. doi: 10.1097/mog.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahdavinia M, Keshavarzian A, Tobin MC, Landay AL, Schleimer RP. A comprehensive review of the nasal microbiome in chronic rhinosinusitis (CRS) Clin Exp Allergy. 2016;46:21–41. doi: 10.1111/cea.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma B, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and disease. Ann Rev Microbiol. 2012;66:371–389. doi: 10.1146/annurev-micro-092611-150157#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh MJ, Huang ST, Ljungberg I, Sprague BM, Lucas SK, Torralba M. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med. 2012;10:1–7. doi: 10.1186/1479-5876-10-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, Brubaker L, Gai X, Wolfe AJ, Schreckenberger PC. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52:871–876. doi: 10.1128/jcm.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price TK, Dune T, Hilt EE, Thomas-White KJ, Kliethermes S, Brincat C, Brubaker L, Wolfe AJ, Mueller ER, Schreckenberger PC. The clinical urine culture: enhanced techniques improve detection of clinically relevant microorganisms. J Clin Microbiol. 2016;54:1216–1222. doi: 10.1128/jcm.00044-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson DE, Van Der Pol B, Dong Q, Revanna KV, Fan B, Easwaran S, Sodergren E, Weinstock GM, Diao L, Fortenberry JD. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS ONE. 2010;5:e14116. doi: 10.1371/journal.pone.0014116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui H, Nederbragt AJ, Lagesen K, Jeansson SL, Jakobsen KS. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 2011;11:244. doi: 10.1186/1471-2180-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, FitzGerald M, Mueller ER, Schreckenberger P, Dong Q, Nelson DE, Brubaker L. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50:1376–1383. doi: 10.1128/jcm.05852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neugent ML, Hulyalkar NV, Nguyen VH, Zimmern PE, De Nisco NJ. Advances in understanding the human urinary microbiome and its potential role in urinary tract infection. MBio. 2020;11:e00218–e220. doi: 10.1128/mbio.00218-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NIH Human Microbiome Portfolio Analysis Team A review of 10 years of human microbiome research activities at the US National Institutes of Health, Fiscal Years 2007–2016. Microbiome. 2019;7:31. doi: 10.1186/s40168-019-0620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis CD. The gut microbiome and its role in obesity. Nutr Today. 2016;51:167–174. doi: 10.1097/nt.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 23.Sood U, Bajaj A, Kumar R, Khurana S, Kalia VC. Infection and microbiome: impact of tuberculosis on human gut microbiome of Indian cohort. Indian J Microbiol. 2018;58:123–125. doi: 10.1007/s12088-018-0706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rishi P, Thakur K, Vij S, Rishi L, Singh A, Kaur IP, Patel SK, Lee JK, Kalia VC. Diet, gut microbiota and COVID-19. Indian J Microbiol. 2020;60:1–10. doi: 10.1007/s12088-020-00908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiffany CR, Bäumler AJ. Dysbiosis: from fiction to function. Am J Physiol Gastrointest Liver Physiol. 2019;317:G602–G608. doi: 10.1152/ajpgi.00230.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cockwell P, Fisher LA. The global burden of chronic kidney disease. Lancet. 2020;395:662–664. doi: 10.1016/S0140-6736(19)32977-0. [DOI] [PubMed] [Google Scholar]
- 27.Girndt M. Diagnosis and treatment of chronic kidney disease. Internist (Berl) 2017;58:243–256. doi: 10.1007/s00108-017-0195-2. [DOI] [PubMed] [Google Scholar]
- 28.Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. doi: 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 29.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi: 10.2215/cjn.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankar M, Shashikala Narasimhappa MN. Urinary tract infection in chronic kidney disease population: a clinical observational study. Cureus. 2021;13:e12486. doi: 10.7759/cureus.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28:1–3. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Wang TZ, Kodiyanplakkal RP, Calfee DP. Antimicrobial resistance in nephrology. Nat Rev Nephrol. 2019;15:463–481. doi: 10.1038/s41581-019-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su G, Xu H, Riggi E, He Z, Lu L, Lindholm B, Marrone G, Wen Z, Liu X, Johnson DW, Carrero JJ. Association of kidney function with infections by multidrug-resistant organisms: an electronic medical record analysis. Sci Rep. 2018;8:13372. doi: 10.1038/s41598-018-31612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pietrucha-Dilanchian P, Hooton TM. Diagnosis, treatment, and prevention of urinary tract infection. Microbiol Spectr. 2016 doi: 10.1128/microbiolspec.uti-0021-2015. [DOI] [PubMed] [Google Scholar]
- 35.McLellan LK, Hunstad DA. Urinary tract infection: pathogenesis and outlook. Trends Mol Med. 2016;22:946–957. doi: 10.1016/j.molmed.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beerepoot MA, ter Riet G, Nys S, van der Wal WM, de Borgie CA, de Reijke TM, Prins JM, Koeijers J, Verbon A, Stobberingh E, Geerlings SE. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomised, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med. 2012;172:704–712. doi: 10.1001/archinternmed.2012.777. [DOI] [PubMed] [Google Scholar]
- 37.Kramer H, Kuffel G, Thomas-White K, Wolfe AJ, Vellanki K, Leehey DJ, Bansal VK, Brubaker L, Flanigan R, Koval J, Wadhwa A. Diversity of the midstream urine microbiome in adults with chronic kidney disease. Int Urol Nephrol. 2018;50:1123–1130. doi: 10.1007/s11255-018-1860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lecamwasam AR, Mohebbi M, Ekinci EI, Dwyer KM, Saffery R. Identification of potential biomarkers of chronic kidney disease in individuals with diabetes: protocol for a cross-sectional observational study. JMIR Res Protoc. 2020;9:e16277. doi: 10.2196/16277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu F, Zhang N, Jiang P, Zhai Q, Li C, Yu D, Wu Y, Zhang Y, Lv L, Xu X, Feng N. Characteristics of the urinary microbiome in kidney stone patients with hypertension. J Transl Med. 2020;18:130. doi: 10.1186/s12967-020-02282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu F, Ling Z, Xiao Y, Yang Q, Wang B, Zheng L, Jiang P, Li L, Wang W. Alterations of urinary microbiota in type 2 diabetes mellitus with hypertension and/or hyperlipidemia. Front Pysiol. 2017;8:126. doi: 10.3389/fphys.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Govender Y, Gabriel I, Minassian V, Fichorova R. The current evidence on the association between the urinary microbiome and urinary incontinence in women. Front Cell Infect Microbiol. 2019;9:133. doi: 10.3389/fcimb.2019.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract—a role beyond infection. Nat Rev Urol. 2015;12:81–90. doi: 10.1038/nrurol.2014.361. [DOI] [PubMed] [Google Scholar]
- 43.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shafquat A, Joice R, Simmons SL, Huttenhower C. Functional and phylogenetic assembly of microbial communities in the human microbiome. Trends Microbiol. 2014;22:261–266. doi: 10.1016/j.tim.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekanayake A, Madegedara D, Chandrasekharan V, Magana-Arachchi D. Respiratory bacterial microbiota and individual bacterial variability in lung cancer and bronchiectasis patients. Indian J Microbiol. 2020;60:196–205. doi: 10.1007/s12088-019-00850-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 2017;10:299–306. doi: 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang J. Microbiome in the urinary system—a review. AIMS Microbiol. 2017;3:143–154. doi: 10.3934/microbiol.2017.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meštrović T, Matijašić M, Perić M, Čipčić Paljetak H, Barešić A, Verbanac D. The role of gut, vaginal, and urinary microbiome in urinary tract infections: from bench to bedside. Diagnostics (Basel) 2020;11:7. doi: 10.3390/diagnostics11010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magruder M, Sholi AN, Gong C, Zhang L, Edusei E, Huang J, Albakry S, Satlin MJ, Westblade LF, Crawford C, Dadhania DM. Gut uropathogen abundance is a risk factor for development of bacteriuria and urinary tract infection. Nat Commun. 2019;10:1–9. doi: 10.1038/s41467-019-13467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pohl HG, Groah SL, Pérez-Losada M, Ljungberg I, Sprague BM, Chandal N, Caldovic L, Hsieh M. The urine microbiome of healthy men and women differs by urine collection method. Int Neurourol J. 2020;24:41–51. doi: 10.5213/inj.1938244.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis DA, Brown R, Williams J, White P, Jacobson SK, Marchesi J, Drake MJ. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front Cell Infect Microbiol. 2013;3:41. doi: 10.3389/fcimb.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sommariva M, Le Noci V, Bianchi F, Camelliti S, Balsari A, Tagliabue E, Sfondrini L. The lung microbiota: role in maintaining pulmonary immune homeostasis and its implications in cancer development and therapy. Cell Mol Life Sci. 2020;77:2739–2749. doi: 10.1007/s00018-020-03452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Popović VB, Šitum M, Chow CE, Chan LS, Roje B, Terzić J. The urinary microbiome associated with bladder cancer. Sci Rep. 2018;8:12157. doi: 10.1038/s41598-018-29054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wojciuk B, Salabura A, Grygorcewicz B, Kędzierska K, Ciechanowski K, Dołęgowska B. Urobiome: in sickness and in health. Microorganisms. 2019;7:548. doi: 10.3390/microorganisms7110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iizumi T, Battaglia T, Ruiz V, Perez GI. Gut microbiome and antibiotics. Arch Med Res. 2017;48:727–734. doi: 10.1016/j.arcmed.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Mulder M, Radjabzadeh D, Hassing RJ, Heeringa J, Uitterlinden AG, Kraaij R, Stricker BH, Verbon A. The effect of antimicrobial drug use on the composition of the genitourinary microbiota in an elderly population. BMC Microbiol. 2019;19:1–7. doi: 10.1186/s12866-018-1379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ceprnja M, Oros D, Melvan E, Svetlicic E, Skrlin J, Barisic K, Starcevic L, Zucko J, Starcevic A. Modeling of urinary microbiota associated with cystitis. Front Cell Infect Microbiol. 2021;11:643638. doi: 10.3389/fcimb.2021.643638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5:229–241. doi: 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cumpanas AA, Bratu OG, Bardan RT, Ferician OC, Cumpanas AD, Horhat FG, Licker M, Pricop C, Cretu OM. Urinary microbiota—are we ready for prime time? a literature review of study methods’ critical steps in avoiding contamination and minimising biased results. Diagnostics (Basel) 2020;10:343. doi: 10.3390/diagnostics10060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karstens L, Asquith M, Caruso V, Rosenbaum JT, Fair DA, Braun J, Gregory WT, Nardos R, McWeeney SK. Community profiling of the urinary microbiota: considerations for low-biomass samples. Nat Rev Urol. 2018;15:735–749. doi: 10.1038/s41585-018-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J, Zhao J, Cao Y, Zhang G, Chen Y, Zhong J, Huang W, Zeng J, Wu P. Relationship between alterations of urinary microbiota and cultured negative lower urinary tract symptoms in female type 2 diabetes patients. BMC Urol. 2019;19:78. doi: 10.1186/s12894-019-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rivers B, Steck TR. Viable but nonculturable uropathogenic bacteria are present in the mouse urinary tract following urinary tract infection and antibiotic therapy. Urol Res. 2001;29:60–66. doi: 10.1007/s002400000151. [DOI] [PubMed] [Google Scholar]
- 63.Hooks KB, O’Malley MA. Dysbiosis and its discontents. MBio. 2017;8:e01492–e1517. doi: 10.1128/mbio.01492-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grine G, Lotte R, Chirio D, Chevalier A, Raoult D, Drancourt M, Ruimy R. Co-culture of Methanobrevibacter smithii with enterobacteria during urinary infection. EBioMedicine. 2019;43:333–337. doi: 10.1016/j.ebiom.2019.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim Y, Carrai M, Leung MH, Chin J, Li J, Lee PK, Beatty JA, Pfeiffer DU, Barrs VR. Dysbiosis of the urinary bladder microbiome in cats with chronic kidney disease. mSystems. 2021;6:e0051021. doi: 10.1128/msystems.00510-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ling Z, Liu F, Shao L, Cheng Y, Li L. Dysbiosis of the urinary microbiota associated with urine levels of proinflammatory chemokine interleukin-8 in female type 2 diabetic patients. Front Immunol. 2017;8:1032. doi: 10.3389/fimmu.2017.01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu F, Ling Z, Xiao Y, Lv L, Yang Q, Wang B, Lu H, Zheng L, Jiang P, Wang W, Li L. Dysbiosis of urinary microbiota is positively correlated with type 2 diabetes mellitus. Oncotarget. 2017;8:3798–3810. doi: 10.18632/oncotarget.14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nickel JC. Urinary tract infections and resistant bacteria: highlights of a symposium at the combined meeting of the 25th international congress of chemotherapy (icc) and the 17th European congress of clinical microbiology and infectious diseases (eccmid), March 31–April 3, 2007, Munich, Germany. Rev Urol. 2007;9:78–80. [PMC free article] [PubMed] [Google Scholar]
- 69.Thänert R, Reske KA, Hink T, Wallace MA, Wang B, Schwartz DJ, Seiler S, Cass C, Burnham CA, Dubberke ER, Kwon JH. Comparative genomics of antibiotic-resistant uropathogens implicates three routes for recurrence of urinary tract infections. mBbio. 2019;10:e01977–e2019. doi: 10.1128/mbio.01977-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naber KG, Kogan M, Wagenlehner FM, Siener R, Gessner A. How the microbiome is influenced by the therapy of urological diseases: standard versus alternative approaches. Clinical Phytoscience. 2017;3:1–4. doi: 10.1186/s40816-017-0045-8. [DOI] [Google Scholar]
- 71.Moustafa A, Li W, Singh H, Moncera KJ, Torralba MG, Yu Y, Manuel O, Biggs W, Venter JC, Nelson KE, Pieper R. Microbial metagenome of urinary tract infection. Sci Rep. 2018;8:4333. doi: 10.1038/s41598-018-22660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaston JR, Johnson AO, Bair KL, White AN, Armbruster CE (2021) Polymicrobial interactions in the urinary tract: is the enemy of my enemy my friend?.Infect and Immun IAI.00652–20. 10.1128/iai.00652-20 [DOI] [PubMed]
- 73.Thomas S, Dunn CD, Campbell LJ, Strand DW, Vezina CM, Bjorling DE, Penniston KL, Li L, Ricke WA, Goldberg TL. A multi-omic investigation of male lower urinary tract symptoms: potential role for JC virus. PLoS ONE. 2021;16:e0246266. doi: 10.1371/journal.pone.0246266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burnett LA, Hochstedler BR, Weldon K, Wolfe AJ, Brubaker L. Recurrent urinary tract infection: association of clinical profiles with urobiome composition in women. Neurourol Urodyn. 2021;40:1479–1489. doi: 10.1002/nau.24707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horwitz D, McCue T, Mapes AC, Ajami NJ, Petrosino JF, Ramig RF, Trautner BW. Decreased microbiota diversity associated with urinary tract infection in a trial of bacterial interference. J Infect. 2015;71:358–367. doi: 10.1016/j.jinf.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas-White KJ, Gao X, Lin H, Fok CS, Ghanayem K, Mueller ER, Dong Q, Brubaker L, Wolfe AJ. Urinary microbes and postoperative urinary tract infection risk in urogynecologic surgical patients. Int Urogynecol J. 2018;29:1797–1805. doi: 10.1007/s00192-018-3767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nienhouse V, Gao X, Dong Q, Nelson DE, Toh E, McKinley K, Schreckenberger P, Shibata N, Fok CS, Mueller ER, Brubaker L. Interplay between bladder microbiota and urinary antimicrobial peptides: mechanisms for human urinary tract infection risk and symptom severity. PLoS ONE. 2014;9:e114185. doi: 10.1371/journal.pone.0114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar R, Sood U, Gupta V, Singh M, Scaria J, Lal R. Recent advancements in the development of modern probiotics for restoring human gut microbiome dysbiosis. Indian J Microbiol. 2020;60:12–25. doi: 10.1007/s12088-019-00808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rudick CN, Taylor AK, Yaggie RE, Schaeffer AJ, Klumpp DJ. Asymptomatic bacteriuria Escherichia coli are live biotherapeutics for UTI. PLoS ONE. 2014;9:e109321. doi: 10.1371/journal.pone.0109321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pierre JF, Akbilgic O, Smallwood H, Cao X, Fitzpatrick EA, Pena S, Furmanek SP, Ramirez JA, Jonsson CB. Discovery and predictive modeling of urine microbiome, metabolite and cytokine biomarkers in hospitalised patients with community acquired pneumonia. Sci Rep. 2020;10:13418. doi: 10.1038/s41598-020-70461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bi H, Tian Y, Song C, Li J, Liu T, Chen Z, Chen C, Huang Y, Zhang Y. Urinary microbiota–a potential biomarker and therapeutic target for bladder cancer. J Med Microbiol. 2019;68:1471–1478. doi: 10.1099/jmm.0.001058. [DOI] [PubMed] [Google Scholar]
- 82.Choi Y, Park HS, Jee YK. Urine microbial extracellular vesicles can be potential and novel biomarkers for allergic diseases. Allergy Asthma Immunol Res. 2021;13:5–7. doi: 10.4168/aair.2021.13.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee HJ. Microbe-host communication by small RNAs in extracellular vesicles: vehicles for transkingdom RNA transportation. Int J Mol Sci. 2019;20:1487. doi: 10.3390/ijms20061487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta K, Stapleton AE, Hooton TM, Roberts PL, Fennell CL, Stamm WE. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonisation in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446–450. doi: 10.1086/515635. [DOI] [PubMed] [Google Scholar]
- 85.Wong AC, Levy M. New approaches to microbiome-based therapies. mSystems. 2019;4:e00122–e219. doi: 10.1128/msystems.00122-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bajic P, Wolfe AJ, Gupta GN. Old instillations and new implications for bladder cancer: the urinary microbiome and intravesical BCG. BJU Int. 2019;124:7–8. doi: 10.1111/bju.14683. [DOI] [PubMed] [Google Scholar]
- 87.Aragon IM, Herrera-Imbroda B, Queipo-Ortuño MI, Castillo E, Del Moral JS, Gomez-Millan J, Yucel G, Lara MF. The urinary tract microbiome in health and disease. Eur Urol Focus. 2018;4:128–138. doi: 10.1016/j.euf.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 88.Xu H, Tamrat NE, Gao J, Xu J, Zhou Y, Zhang S, Chen Z, Shao Y, Ding L, Shen B, Wei Z. Combined signature of the urinary microbiome and metabolome in patients with interstitial cystitis. Front Cell Infect Microbiol. 2021;11:711746. doi: 10.3389/fcimb.2021.711746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data and material were used to support this study.
Not applicable.