Abstract
Introduction: Several studies identified a link between gastroesophageal reflux disease (GERD) and obstructive sleep apnea (OSA). GERD is a condition in which acid reflux from the stomach to the esophagus causes troublesome symptoms. On the other hand, OSA is defined as a sleep-related breathing disorder in which airflow significantly decreases or ceases due to upper airway obstruction, leading to arousal from sleep. OSA was found to be associated with GERD. In this study, we aim to study the characteristics and concurrent risk factors associated with GERD and OSA in a large population-based study.
Methods: Patients with the diagnosis of GERD were extracted from the National Inpatient Database (NIS) for the years 2016 to 2019. Patients' age, gender, race, and hospital information, including region and bed size, were extracted and considered as baseline characteristics. The comorbidities included are hypertension (HTN), atrial fibrillation (AFib), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), pulmonary hypertension (PHTN), obesity, and smoking. Patients younger than 18 years old were excluded from this study.
Results: Out of 22,677,620 patients with the diagnosis of GERD, 12.21% had a concurrent diagnosis of OSA (compared to 4.79% in patients without GERD, p-value <0.001). The mean age of patients with GERD and OSA was 64.47 years vs 65.42 years in patients without OSA (p-value <0.001). The GERD and OSA group had almost identical gender distribution compared to the GERD only group, as it was predominantly female patients. The white and black races were slightly more prevalent in the GERD and OSA group compared to the GERD only group. Regarding comorbidities, the prevalence of obesity was more clear in the GERD and OSA group. It was noted that the group of patients who carry a diagnosis of GERD and OSA have more prevalence of diabetes (DM), hypertension (HTN), obesity, atrial fibrillation (Afib), congestive heart failure (CHF), and pulmonary hypertension (PHTN). Patients with GERD and OSA were 21% less likely to be older than 65 years rather than younger (95% CI: 0.79-0.8, p-value <0.001), 35% less likely to be females (95% CI: 0.65-0.65, p-value <0.001), and 22% less likely to be non-white (95% CI: 0.77-0.8, p-value <0.001). Obesity was found to be the strongest association with this population, followed by PHTN, CHF, DM, HTN, Afib, and lastly smoking.
Conclusion: Patients with GERD and OSA were found more likely to be female, white, living in the southern part of the United States, obese, diabetes mellitus type 2, and being active smokers.
Keywords: obstructive sleep apnea, gerd pathophysiology, prevalance, prevalence of gerd, osa
Introduction
Several studies identified a link between gastroesophageal reflux disease (GERD) and obstructive sleep apnea (OSA) [1,2]. GERD is a condition in which acid reflux from the stomach to the esophagus causes troublesome symptoms (typically including heartburn or regurgitation) and/or esophageal complications. Globally, GERD affects 8% to 33% of people, affecting both genders and all age groups [2]. It is mainly diagnosed clinically based on reported symptoms, but sometimes specific examinations such as impedance-pH monitoring and gastroscopy can support the diagnosis [3]. Known complications of GERD include erosive esophagitis, Barrett's esophagus, esophageal stricture, and asthma [4,5]. On the other hand, OSA is defined as a sleep-related breathing disorder in which airflow significantly decreases or ceases due to upper airway obstruction, mainly in the oropharynx, leading to arousal from sleep. As a result, partial pressure of oxygen in arterial blood (PaO2) is decreased, while partial pressure of carbon dioxide (PaCO2) is increased. This leads to complications such as hypoxic pulmonary vasoconstriction leading to pulmonary hypertension and cor pulmonale along with secondary hypertension in the setting of increased sympathetic activity [6].
According to the Wisconsin cohort study, the prevalence of OSA in people aged 30 to 60 years ranges from 9% to 24% in men and 4% to 9% in women [7]. Usually, the main complaints are chronic fatigue, nocturia, poor concentration, and depressed mood [8]. As the reported symptoms and physical exam can be nonspecific or not indicative, several tools were developed to identify patients with a potential diagnosis of OSA, such as Epworth Sleepiness Scale (ESS) and Stop-Bang Questionnaires. The diagnosis is mainly confirmed by polysomnography (PSG) [9]. Cardiovascular diseases, stroke, and diabetes risk were found to be increased with OSA [10]. OSA and GERD have a strong relationship. Approximately 40%-60% of people with OSA also suffer from GERD, which can be extremely difficult to treat and result in extremely disruptive sleep patterns [11,12]. In this study, we aim to study the characteristics and concurrent risk factors associated with GERD and OSA in a large population-based study.
Materials and methods
Data source
This is a retrospective cohort study of patients who were admitted to hospitals in the United States between the years 2016 and 2019. The data were extracted from the Healthcare Cost and Utilization Project National Inpatient Sample (NIS) database. The NIS is sponsored by the Agency for Healthcare Research Quality (AHRQ) and is considered the largest publicly available inpatient healthcare database in the United States. The database includes data from at least 46 states and covers more than 97% of the US population [13]. A 20% probability sample was collected and subsequently weighted to ensure that the selected population was nationally representative. Each admission in the database was assigned one principal diagnosis, up to 40 secondary diagnoses, and 25 procedures. These variables are defined via the International Classification of Disease, 10th Revision, and Clinical Modification (ICD-10-CM) codes.
Study variables
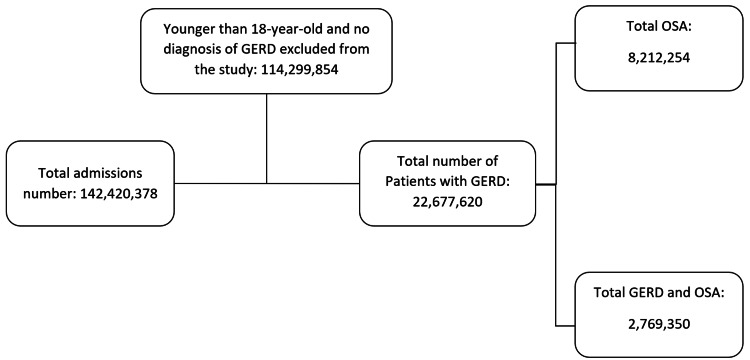
Using ICD-10-CM codes, we were able to identify patients who carry diagnoses of OSA and GERD. Patients' age (in years), gender, race (white, black, Hispanic, others), and hospital information (region and bed size) were collected and considered as baseline characteristics. The comorbidities included are hypertension (HTN), atrial fibrillation (AFib), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), pulmonary hypertension (PHTN), obesity, and smoking. Patients younger than 18 years old were excluded from this study. Patients with a body mass index (BMI) of more than 24.9 were considered obese. A flow chart of the study population selection process is demonstrated in Figure 1.
Figure 1. Flow diagram of patients selection.
GERD, gastroesophageal reflux disease; OSA, obstructive sleep apnea.
Statistical analysis
The statistical analysis was done using STATA software, version 17.0 (StataCorp., College Station, TX, USA). The characteristics of patients with OSA alone and those who had both OSA and GERD were described using descriptive statistics. In this study, multivariate logistic regression analyses were performed to determine factors associated with in-hospital mortality. Variables that were not statistically significant (p-value <0.1) on univariate analysis were excluded from the multivariate analysis. The odds ratio at 95% CI was used to describe the association between the study and outcome variables. Statistical significance was defined as a two-tailed p-value of <0.05.
Results
Demographic and clinical characteristics
Out of 22,677,620 patients carrying the diagnosis of GERD, it was found that 12.21% were diagnosed with concurrent OSA (compared to 4.79% in patients without GERD, p-value <0.001) (Figure 1). The mean age of patients with GERD and concurrent OSA was slightly less than that of patients with GERD only (64.47 years vs 65.42 years, p-value <0.001). The GERD and OSA group had almost identical gender distribution compared to the GERD only group, as it was predominantly female patients. The white and black races were slightly more prevalent in the GERD and OSA group compared to the GERD only group. In terms of hospital information, the GERD and OSA group was more likely to be in a large hospital in the midwest region compared to the GERD only group, but the southern region remained the most prevalent region in both groups (Table 1). Regarding comorbidities, the prevalence of obesity was more clear in the GERD and OSA group. It was noted that the group of patients who carry a diagnosis of GERD and OSA have more prevalence of DM, HTN, obesity, Afib, CHF, and PHTN.
Table 1. Breakdown of characteristics and their statistical significance.
GERD, gastroesophageal reflux disease; OSA, obstructive sleep apnea; DM, diabetes; HTN, hypertension; Afib, atrial fibrillation; CHF, congestive heart failure; PHTN, pulmonary hypertension.
| Characteristics | GERD only (22,677,620) | OSA and GERD (2,769,350) | P-value |
| Age (years) | 65.42 | 64.47 | <0.0001 |
| Gender (%) | <0.0001 | ||
| Males | 40.38 | 49.37 | |
| Females | 59.62 | 50.63 | |
| Race (%) | <0.0001 | ||
| White | 75.91 | 78.27 | |
| Black | 12.5 | 13.65 | |
| Hispanic | 7.21 | 5.3 | |
| Others | 4.38 | 2.78 | |
| Hospital region (%) | |||
| Northeast | 19.18 | 16.82 | <0.0001 |
| Midwest | 24.06 | 31.17 | |
| South | 41.45 | 37.09 | |
| West | 15.32 | 14.91 | |
| Hospital bed size (%) | <0.0001 | ||
| Small | 20.93 | 19.85 | |
| Medium | 28.91 | 27.66 | |
| Large | 50.16 | 52.48 | |
| Comorbidities (%) | <0.0001 | ||
| Obesity | 16.59 | 45.25 | |
| Smoke | 41.8 | 43.87 | |
| DM | 30.37 | 47.62 | |
| HTN | 31 | 22.42 | |
| Afib | 16.61 | 23 | |
| CHF | 19.85 | 32.33 | |
| PHTN | 0.04 | 0.1 |
Potential predictors of concurrent OSA diagnosis in GERD patients
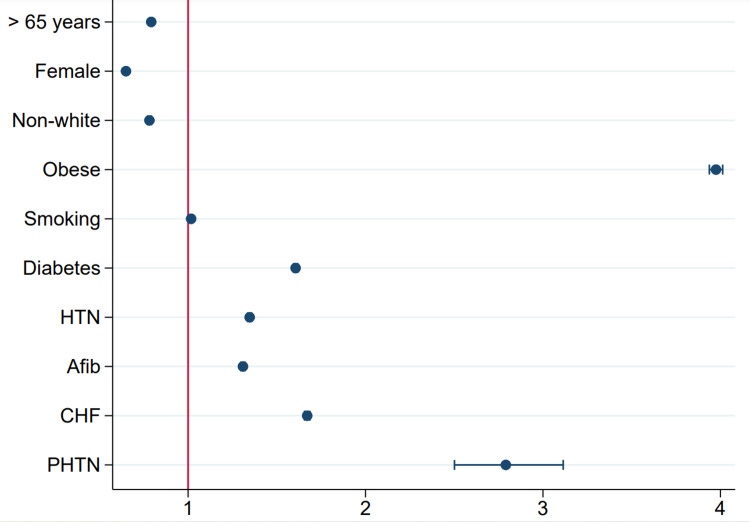
These patients were 21% less likely to be older than 65 years rather than younger (95% CI: 0.79-0.8, p-value <0.001), 35% less likely to be females (95% CI: 0.65-0.65, p-value <0.001), and 22% less likely to be non-white (95% CI: 0.77-0.8, p-value <0.001). Obesity was found to be the strongest association with this population, followed by PHTN, CHF, DM, HTN, Afib, and lastly smoking (Table 2). A plot demonstrating predictors of OSA in the GERD population is shown in Figure 2.
Table 2. Potential predictors of concurrent OSA diagnosis in GERD patients.
OSA, obstructive sleep apnea; GERD, gastroesophageal reflux disease; DM, diabetes; HTN, hypertension; Afib, atrial fibrillation; CHF, congestive heart failure; PHTN, pulmonary hypertension.
| Variable | Adjusted OR (95% CI) | P-value |
| Age | <0.001 | |
| 18-65 years | Reference | |
| >65 years | 0.79 (0.79-0.8) | |
| Gender | <0.001 | |
| Male | Reference | |
| Female | 0.65 (0.65-0.65) | |
| Race | <0.001 | |
| White | Reference | |
| Non-white | 0.78 (0.77-0.8) | |
| Comorbidities | <0.001 | |
| Obesity | 3.98 (3.94-4.01) | |
| Smoking | 1.02 (1.01 -1.03) | |
| DM | 1.6 (1.59-1.62) | |
| HTN | 1.35 (1.34-1.36) | |
| Afib | 1.31 (1.3-1.32) | |
| CHF | 1.67 (1.66-1.69) | |
| PHTN | 2.79 (2.5-3.11) |
Figure 2. Plot demonstrating predictors of OSA in the GERD population.
GERD, gastroesophageal reflux disease; OSA, obstructive sleep apnea; HTN, hypertension; Afib, atrial fibrillation; CHF, congestive heart failure; PHTN, pulmonary hypertension.
Discussion
This study attempts to further elucidate the relationship between GERD and OSA. Prior studies over the past decade have demonstrated the growing evidence of GERD complications and the importance of employing a multidisciplinary approach to target the most recognized cardiovascular, pulmonary, laryngeal, and dental complications associated with this common medical condition [14]. Only more recently, a potential relationship between GERD and OSA has been studied [15]. One proposed mechanism is that the greater respiratory effort and the cough associated with respiratory conditions increase the pressure gradient across the lower esophageal sphincter (LES), which in turn increases the pressure and promotes the opening of the LES, concomitantly increasing abdominal pressure and ultimately increasing the risk of gastric reflux by facilitating the retrograde movement of gastric contents [16]. Other explanations include hyperinflation interfering with the normal diaphragmatic contribution to the LES, the relaxing effect of bronchodilator medication on the LES function, and the high prevalence of hiatal hernia in these patients [17-19]. Finally, both OSA and GERD share many of the same risk factors. While the prevalence of GERD and OSA is high in the general population, there is currently no explanation of any potential causal relationship between these two conditions.
In our study, the prevalence of OSA in patients with GERD is more than that in patients without GERD. Moreover, some factors were associated with a higher chance of having OSA in patients with GERD such as female gender, the white race, living in the southern part of the United States, obesity, DM type 2, and active smoking. Obesity correlates well with the risk of OSA [20]. A number of mechanisms may explain the association of excess body mass with OSA, including increased upper airway collapsibility and impaired neuromuscular control [21]. In one study, it was suggested that an increase in weight by 10% could lead to a six-fold increase in OSA risk [22]. Obesity has also been found to be associated with transient relaxations of the lower esophageal sphincter (TRLES). TRLES is primarily triggered by gastric distension, especially in the fundus, and is considered to be one of the main mechanisms for GERD development [23].
Smoking was associated with an increase in nasal airflow resistance, which is hypothesized to be a contributing factor for OSA development [24,25]. Compared to those who had never smoked or who had smoked in the past, current smokers were three times as likely to have OSA [26]. On the other hand, smoking tobacco was found to reduce the LES resting pressure, which can eventually lead to GERD [27]. It is possible that OSA and DM type 2 may share a bidirectional relationship. This is because diabetic neuropathy can alter central control of respiration and upper airway nerves, resulting in OSA [28]. There are several mechanisms contributing to GERD in DM, including motor and LES abnormalities, hormonal changes, extra-abdominal pressure, obesity, and decreased parotid gland secretion, which reduces the clearance of esophageal acid [29].
Strengths and limitations
Several advantages of our study include the use of NIS, the largest publicly available inpatient database, which makes the results more representative since the sample size is large; it adjusts all outcomes to the most common baseline characteristics of both patients and hospitals to minimize confounding factors as much as possible, and it analyzes multiple demographics for patients with GERD and OSA. In our study, we encountered some limitations. First, it is a retrospective study, which makes it susceptible to nonrandomization. Second, the NIS database includes an administrative database, which means that administrative codes were used to identify GERD, OSA, and other diagnoses, leading to possible misclassifications, undercodings, or overcodings. The misclassification will, however, be seen as an error rather than a bias since it is likely to occur equally across all arms. Errors do not change the nature of the relationship between two variables; rather, statistical significance between them becomes more difficult to establish.
Conclusions
Our study demonstrated a close relationship between GERD and OSA and the characteristics and risk factors that would predispose someone with the diagnosis of GERD to also have OSA. Patients with GERD who carry the highest risk factors being female, white, living in the southern part of the United States, obese, diabetes mellitus type 2, and being an active smoker would likely benefit most from being screened to have a concomitant diagnosis of OSA. As GERD tends to be more symptomatic and more likely to be reported by patients compared to OSA, and given previous literature on the relationship between OSA and GERD that was reinforced by our paper, we propose that OSA screening might be warranted in patients with an underlying diagnosis of GERD, as it is associated with long-term significant morbidity and mortality. Nonetheless, more studies are needed on that content before any definitive conclusion can be made.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Gastro-oesophageal reflux symptoms are related to the presence and severity of obstructive sleep apnoea. Shepherd KL, James AL, Musk AW, Hunter ML, Hillman DR, Eastwood PR. J Sleep Res. 2011;20:241–249. doi: 10.1111/j.1365-2869.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- 2.Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. El-Serag HB, Sweet S, Winchester CC, Dent J. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modern diagnosis of GERD: the Lyon Consensus. Gyawali CP, Kahrilas PJ, Savarino E, et al. Gut. 2018;67:1351–1362. doi: 10.1136/gutjnl-2017-314722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erosive esophagitis is a risk factor for Barrett's esophagus: a community-based endoscopic follow-up study. Ronkainen J, Talley NJ, Storskrubb T, et al. Am J Gastroenterol. 2011;106:1946–1952. doi: 10.1038/ajg.2011.326. [DOI] [PubMed] [Google Scholar]
- 5.Sorting out the relationship between esophageal and pulmonary disease. Okwara NC, Chan WW. Gastroenterol Clin North Am. 2021;50:919–934. doi: 10.1016/j.gtc.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Obstructive sleep apnea in adults. Veasey SC, Rosen IM. N Engl J Med. 2019;380:1442–1449. doi: 10.1056/NEJMcp1816152. [DOI] [PubMed] [Google Scholar]
- 7.Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort Study. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2858234/ WMJ. 2009;108:246–249. [PMC free article] [PubMed] [Google Scholar]
- 8.Self-reported sleepiness and not the apnoea hypopnoea index is the best predictor of sleepiness-related accidents in obstructive sleep apnoea. Philip P, Bailly S, Benmerad M, et al. Sci Rep. 2020;10:16267. doi: 10.1038/s41598-020-72430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Parati G, Lombardi C, Hedner J, et al. Eur Respir J. 2013;41:523–538. doi: 10.1183/09031936.00226711. [DOI] [PubMed] [Google Scholar]
- 11.Gastroesophageal reflux in patients with morbid obesity: a role of obstructive sleep apnea syndrome? Sabaté JM, Jouët P, Merrouche M, et al. Obes Surg. 2008;18:1479–1484. doi: 10.1007/s11695-008-9508-9. [DOI] [PubMed] [Google Scholar]
- 12.Gastroesophageal reflux disease and sleep quality in a Chinese population. Chen MJ, Wu MS, Lin JT, et al. J Formos Med Assoc. 2009;108:53–60. doi: 10.1016/S0929-6646(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 13.HCUP National Inpatient Sample (NIS): Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. [ Mar; 2022 ];http://www.hcup-us.ahrq.gov/nisoverview.jsp 2012
- 14.The relationship between gastroesophageal reflux disease and obstructive sleep apnea. Demeter P, Pap A. J Gastroenterol. 2004;39:815–820. doi: 10.1007/s00535-004-1416-8. [DOI] [PubMed] [Google Scholar]
- 15.Gastroesophageal reflux disease: recent advances and its association with sleep. Oh JH. Ann N Y Acad Sci. 2016;1380:195–203. doi: 10.1111/nyas.13143. [DOI] [PubMed] [Google Scholar]
- 16.Extraesophageal manifestations of gastroesophageal reflux. Field SK, Field TS, Cowie RL. http://pubmed.ncbi.nlm.nih.gov/16493371/ Minerva Gastroenterol Dietol. 2001;47:137–150. [PubMed] [Google Scholar]
- 17.Pulmonary manifestations of gastroesophageal reflux disease. Gaude GS. Ann Thorac Med. 2009;4:115–123. doi: 10.4103/1817-1737.53347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The effects of nebulized albuterol on esophageal function in asthmatic patients. Lacy BE, Mathis C, DesBiens J, Liu MC. Dig Dis Sci. 2008;53:2627–2633. doi: 10.1007/s10620-007-0188-4. [DOI] [PubMed] [Google Scholar]
- 19.Poor sleep quality and obstructive sleep apnea in patients with GERD and Barrett's esophagus. Vela MF, Kramer JR, Richardson PA, Dodge R, El-Serag HB. Neurogastroenterol Motil. 2014;26:346–352. doi: 10.1111/nmo.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Increased prevalence of sleep-disordered breathing in adults. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. http://pubmed.ncbi.nlm.nih.gov/18250211/ Proc Am Thorac Soc. 2008;5:185–192. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longitudinal study of moderate weight change and sleep-disordered breathing. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 23.Increased frequency of transient lower esophageal sphincter relaxation induced by gastric distention in reflux patients with hiatal hernia. Kahrilas PJ, Shi G, Manka M, Joehl RJ. Gastroenterology. 2000;118:688–695. doi: 10.1016/s0016-5085(00)70138-7. [DOI] [PubMed] [Google Scholar]
- 24.Effect of heavy smoking on nasal resistance. Dessi P, Sambuc R, Moulin G, Ledoray V, Cannoni M. Acta Otolaryngol. 1994;114:305–310. doi: 10.3109/00016489409126061. [DOI] [PubMed] [Google Scholar]
- 25.Nasal obstruction as a risk factor for sleep-disordered breathing. Young T, Finn L, Kim H. J Allergy Clin Immunol. 1997;99:757–762. doi: 10.1016/s0091-6749(97)70124-6. [DOI] [PubMed] [Google Scholar]
- 26.Smoking as a risk factor for sleep-disordered breathing. Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Arch Intern Med. 1994;154:2219–2224. [PubMed] [Google Scholar]
- 27.Inhibitory effect of smoking on the lower esophageal sphincter. Dennish GW, Castell DO. N Engl J Med. 1971;284:1136–1137. doi: 10.1056/NEJM197105202842007. [DOI] [PubMed] [Google Scholar]
- 28.Obstructive sleep apnea and diabetes: a state of the art review. Reutrakul S, Mokhlesi B. Chest. 2017;152:1070–1086. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diabetes and the esophagus. Monreal-Robles R, Remes-Troche JM. Curr Treat Options Gastroenterol. 2017;15:475–489. doi: 10.1007/s11938-017-0153-z. [DOI] [PubMed] [Google Scholar]