Abstract
Herpes virus infections is not uncommon in solid organ transplantation patients. We report 3 cases with primary Herpes simplex virus type-1 (HSV1) infection with acute liver failure (ALF). This is a rare and potentially fatal entity that could be a donor-derived infection. Although the initial clinical presentation is non-specific, it should be considered as a differential diagnosis in HSV-negative serology patients with liver failure and empirical treatment must be started in combination with a drastic reduction of immunosuppression. A strategy of HSV prophylaxis for pre-transplant HSV seronegative patients must be stablished in order to reduce the risk of clinical disease.
Keywords: Herpes simplex virus hepatitis, HSV-1, Transplantation, Donor derived transmitted disease, SOT
Highlights
-
•
HSV hepatitis is a rare and fatal entity that can be a donor-derived infection.
-
•
It should be suspected in highly immunosuppressed transplant patients who develop acute liver failure.
-
•
The treatment delay is associated with higher fatality.
-
•
In transplant patients with negative HSV serology, prophylaxis is recommended if they are not with prophylaxis for CMV.
Introduction
Classically, solid organ transplant (SOT) recipients have a higher risk of opportunistic infections due to their state of immunosuppression, especially in the first 6 months’ post-transplantation. The prevalence of type 1 herpes simplex virus seropositivity is high in the general population and reaches up to 75% in SOT, of which 8.9% develop a symptomatic infection [1], [2]. Most cases are mucocutaneous infections, however, in a lower percentage they develop systemic infections. The acute liver failure (ALF) due to primary or reactivated infection is an extremely rare and potentially fatal entity [3]. We report 3 solid-organ transplantation patients who developed ALF due to HSV-1 primary infection in the immediate post-transplant period.
Clinical cases and review of previously reported cases
First case
A 66-year-old female underwent liver transplantation (LT) for a hepatitis C virus (HCV)related end-stage liver disease. Before LT, patient was admitted due to a stenotrophomonas maltophilia bacteriemia, which was treated and solved with intravenous trimethoprim/sulfamethoxazole and tigecycline. Basiliximab, mycophenolate, and corticosteroids were indicated as induction therapy. On the fifth day after surgery, patient required splenic artery embolization due to splenic artery steal syndrome. Cyclosporine was administered at the 6th day post transplantation, but it had to be switched to everolimus due to neurologic side effects attributed to the calcineurin inhibitor. Donor was tested positive for cytomegalovirus (CMV) IgG, toxoplasma, hepatitis B virus (HBV) anticore IgG, strongyloid and negative for HIV, HBV surface antigen and HCV IgG. Recipient serologies were positive for CMV IgG and negative for other viruses including HSV. Prophylaxis with lamivudine, ivermectin, clotrimoxazole, and nystatin was administered, but valganciclovir prophylaxis was not indicated.
Fourteen days after LT, the patient presented right leg cellulitis associated with an elevation of C-reactive protein (15.5 mg/dl) and empirical antibiotic treatment with meropenem and vancomycin was started. Four days later, she developed vesicular lesions spreaded over the right leg, abdomen and mouth. Given the suspicion of a herpetic lesion, the patient was treated with parenteral acyclovir. However, she further presented fever, and altered liver function tests with aspartate aminotransferase (AST) 1700 IU/L, alanin aminotransferase (ALT) 900 IU/L, alkaline phosphatase (AP) 840 IU/L, gamma glutamyltranspeptidase (GGT) 740 IU/L, total bilirubin 2.3 mg/dL, INR 1.23 and thrombocytopenia 21^109 cells/L. The abdominal ultrasound ruled out vascular or bile duct obstruction. Patient was tested negative for hepatotropic viruses and HHV-6, but serum PCR for herpes virus 2 resulted positive. The transjugular liver biopsy showed abundant foci of coagulative necrosis and scarce inflammatory reaction compatible with herpetic hepatitis, and the immunohistochemistry confirmed the presence of HSV1 and HSV2. The cutaneous vesicles biopsy and Tzanck test showed epithelial cells with cytopathic changes compatible with herpetic infection. As the patient was diagnosed with disseminated herpetic infection, acyclovir dose was increased, foscarnet and immunoglobulins were added to treatment.
Two days later, she presented abdominal pain and hemodynamic instability which needed the administration of norepinephrine. The abdominal CT scan showed marked distension of small bowel and ascites. She was transferred to the intensive care unit (ICU) and antibiotic coverage was expanded. On the following days, a gradual impairment of the liver function was detected with serum bilirubin and INR up to 12 mg/dL and 2.11 respectively. In this setting, patient developed rapidly progressive encephalopathy requiring intubation and mechanical ventilation. Subsequent infection workup reported multiresistant pseudomonas aeruginosa in the ascitic fluid and bronchoalveolar lavage, Candida krusei in the urine culture and positive strongyloides stercolaris PCR in the stool. Patient progressed to multiorgan failure, andunfortunately, she passed away on the 34th day after LT.
Second case
A 69-year-old male diagnosed with alcohol related cirrhosis and hepatocarcinoma underwent LT. Induction therapy included methylprednisolone and tacrolimus. Both donor and recipient presented positivity for CMV IgG, so valganciclovir prophylaxis was not indicated. In addition, the recipient presented negative serology (IgG) for HSV. Three days after LT, the patient was diagnosed with influenza A and was treated with oseltamivir.
Thirteen days after LT, the patient started with fever abdominal pain and decreased peristalsis. Elevated C-reactive protein (16 mg/dl) and transaminases (AST 213 U/L, ALT 111 U/L) were also detected. The abdominal CT scan showed distension of the small boweland ascites, while no vascular or bile duct obstruction were observed. Patient was diagnosed with adynamic ileus, treatment with piperacillin/tazobactam and parenteral nutrition was started. Two days later, the patient’s clinical status deteriorated. Laboratory tests showed ALT 11.500 IU/L, AST 3.032 IU/L, total bilirubin 5.3 mg/dl, INR 2.5 and thrombocytopenia 18^109 cell/L. Serum HHV-6 PCR was positive, but negative for CMV.
A liver biopsy was performed, showing coagulative necrosis associated with the presence of neutrophils with karyorrhexis changes. In the context of this findings and the HHV-6 positive PCR, intravenous ganciclovir was started. As the clinical state of the patient impaired, he was transferred to the ICU, where vasoactive support and mechanical ventilation were needed. He developed impaired renal function which required hemodialysis and the liver function worsened with AST 20.000 IU/L, ALT 4.500 IU/L, total bilirubin 7 mg/dl, INR 5.9 and serum ammonia 148 mg/L. Subsequently, the biopsy immunohistochemistry showed positivity for HSV and negativity for HHV-6 and CMV. HSV-1 was identified in blood and cerebrospinal fluid by PCR and gancyvlovir treatment was switched to intravenous acyclovir. Patient finally died on the 24th day after LT from a refractory septic shock.
Third case
A 45-year-old female with unknown cause chronic kidney disease underwent a second deceased donor kidney transplant (KT), she presented high immunological risk with 99% pre-KT cPRA. She received induction thymoglobulin; tacrolimus, everolimus, and prednisone were used as maintenance therapy and trimethoprim-sulfamethoxazole prophylaxis. The donor and recipient had positive pre-transplant CMV IgG and the recipient was negative for HSV. Prophylactic treatment with valganciclovir was not started and close monitoring of CMV PCR was made. 24 h post-KT, she presented a urinary leak, requiring surgical repair. 10 days later, she had a urinary tract infection, and completed 7 days of Ertapenem. The allograft function was adequate, and the patient was discharged.
On the 21st day post-transplant, the patient started with fever (38 °C) and was admitted for further study and treatment. Laboratory tests showed ALT 5103 U/L, ALT 2564 U/L, LDH 6400 U/L, total bilirubin 1.70 mg/dl Leukopenia 1.580^109 cells/L,prothrombin time 60%, INR 1.3 and C-reactive protein 5.27 mg/dl. The liver ultrasound showed no signs of vascular or bile duct obstruction. The blood cultures were negative and Candida parapsilosis was isolated in urinary culture. After ruling out pharmacological causes and with the suspicion of viral hepatitis, empirical treatment with meropenem, teicoplanin, isavuconazole and ganciclovir was started. Viral tests resulted positive for HSV-1, Epstein Barr virus and HHV-6 serum PCR, and negative for hepatotropic viruses, CMV, and HIV. The transjugular liver biopsy showed extensive foci of confluent coagulative necrosis, and the immunohistochemistry was positive for HSV1 hepatitis. The patient was diagnosed with a secondary acute HSV1 hepatitis and antiviral coverage with acyclovir and foscarnet was started 3 days after the clinical onset. After the diagnosis, the donor serology was confirmed to be positive for this virus.
Despite the antiviral treatment, the liver function suffered a gradual impairment with AST 12983 IU/L, ALT 3739 IU/L, total bilirubin 2 mg/dL, INR 2.5 and patient evolved with encephalopathy, so she was transferred to the intensive care unit. As an LT was considered contraindicated in the setting of a disseminated herpetic infection, plasma exchanges were added to the supportive care. The patient presented progressive encephalopathy and required mechanical ventilation. She had Candida parapsilosis candidemia, developing rapid evolution of multiorgan failure and died on the 31st day after KT.
Previously Reported Cases
Four additional cases of acute hepatitis secondary to type 1 HSV primary infection in the immediate post-transplant phase were previously published. The main characteristics of these cases and those reported in the present work are summarized in (Table 1) [5], [6], [7], [8].
Table 1.
Case series.
| No | Ref. | Age | Gender | Type of transplant | Immunosuppression | Latency time (days) | HSV Donor serology (IgG) | HSV Receptor serology (IgG) | Diagnosis | CMV Prophylaxis | Graft dysfunction | Liver biopsy | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gabel H, et al. 1988 | 48 | M | Kidney | Ciclosporine, azathioprine and steroids | 21 days | Unknown | HSV- 1 Neg HSV-2 Neg |
HSV-1 PCR Positive | None | Failure | Unrealized | IV Aciclovir | Survived |
| 2 | Kunse S. Et al. 1991 | 26 | F | Liver | Cyclosporine and steroids | 18 days | HSV-1 Pos HSV-2 Pos |
HSV − 1 Neg HSV-2 Pos |
HSV-1 PCR Positive | ND | ND | ND | ND | Died |
| 3 | Al Midani et al. 2011 | 26 | F | Kidney | Basiliximab, tacrolimus, mycophenolate mofetil and steroids. | 14 days | HSV − 1 Pos | HSV − 1 Neg HSV − 2 Neg |
HSV-1 PCR > 10.4 log10 copies/ml | None | Failure | Unrealized | IV Aciclovir and IVIG | Survived |
| 4 | Côté D, et al. 2014 | 64 | M | Liver | Basiliximab,tacrolimus, azathioprine and steroids, | 10 days | HSV − 1 Pos | HSV-1 Neg HSV-2 Neg |
HSV-1 PCR Positive | None | Failure | Acute Hepatitis | IV Aciclovir | Died |
| 5 | Case 1 2014 | 69 | M | Liver | Tacrolimus and steroids | 13 days | Unknown | HSV Neg | HSV-1 PCR Positive | None | Failure | Acute Hepatitis | IV Aciclovir | Died |
| 6 | Case 3 2019 | 66 | F | Liver | Basiliximab, Ciclosporine and steorids | 14 days | Unknown | HSV Neg | HSV-1 and HVS-2 PCR Positive | None | Failure | Acute Hepatitis | IV Aciclovir, Foscarnet and IVIG | Died |
| 7 | Case 2 2021 | 45 | F | Kidney | Basiliximab Tacrolimus, everolimus and steroids | 12 days | HSV-1 Pos | HSV Neg | HSV-1 PCR Positive | None | Failure | Acute Hepatitis | IV Aciclovir Foscarnet IVIG and PE | Died |
ND No data; * * extra administration on suspicion of rejection; Pos Positive; Neg Negative; HIC Immunohistochemistry; HSV Herpes Simplex Virus IV: intravenous, PE: Plasma exchange
Discussion
Herpes virus infections is not uncommon in solid organ transplantation patients. It has been reported an incidence of herpes infection (HSV and herpes zoster virus) of 8% in the first month post-transplant, 10% in the first 6 months and 16% at 6 months’ post-transplant [4]. The prevalence of herpes simplex virus seropositivity in SOT is 75% with an incidence of primary infection of 6.7% at the first year [3], the majority of them are mild infections and less frequently severe systemic infections. HSV hepatitis is a rare and fatal entity that could be a donor-derived infection. The 7 reported cases, included ours, meet the criteria to be considered ‘probable’ donor-derived disease transmission according to the ad hoc Disease Transmission Advisory Committee (DTAC) [9] since the infection occurs after transplantation with seronegative recipients.
The mean age of patients was 49 (23−69) years old, the majority were women 4/7. Four patients received LT, the rest had kidney transplant. Immunosuppression varied significantly between cases due, in part, to the temporal changes in the indicated drugs among decades. In addition, immunosuppression therapy varied according to immunological risk and type of transplanted organs. It was observed that infection developed a few days after transplantation in a meantime of 14 (10−21) days, coinciding with the period of maximum immunosuppression caused by induction therapies. The relationship with immunosuppression has also been reported in two clinical cases where HSV-1 hepatitis was observed after 4 and 5 years after transplantation associated with an increase in immunosuppression (corticosteroid bolus or thymoglobulin) as therapy against graft rejection[10], [11].
In the majority of cases the onset symptoms were nonspecific, they included fever and mild changes in liver profile. In less than 50% of cases were accompanied by skin lesions but these were non-characteristic of HSV infection[12], [13]. For example, in the second case the skin lesions were oriented as cellulitis, and antibiotic therapy was indicated leading to a delay in the diagnosis. Later, this patient presented established liver injury characterized by thrombocytopenia, coagulopathy, liver failure and septic shock. Similar outcomes were reported for the rest of the cases. Berrington et al. described the clinical characteristics in 29 patients with HSV viremia, observing that 54% of them presented with fever 72 h after the detection of HSV in peripheral blood, 46% had neurological symptoms such as confusion and clouding of consciousness, 31% had abdominal symptoms, 31% had respiratory failure with 23 requiring mechanical ventilation, and 38% had bacterial and fungal co-infections [14].
In the differential diagnosis of this disease we must include bacterial and viral infections that could cause acute hepatitis. In the setting of LT, especially at the first weeks after surgery, many entities could initially arise with abnormalities in the liver function tests, such as ischemia-reperfusion injury or vascular and biliary complications. However, the most common complication that must be suspected at this phase is rejection. The treatment comprises an increase in the inmmunosuppressive therapy. Hence, ruling out rejection should be of utmost importance as its treatment could be detrimental in the context of a herpetic infection [6].
Initial diagnosis was made through serum PCR in all cases[15], [16]. Zeidan et al. described one case of acute liver failure due to HSV-2 in a heart transplant recipient and conducted a literature review. They found 7 PCR-confirmed cases, of whom 5 and 2 had HSV-2 and HSV-1 infection, respectively [16]. However, in most of them, including our cases, diagnostic confirmation was performed with transjugular liver biopsy due to the risk of bleeding complications. Different studies define the histopathological findings of HSV-1 infection, which shows fragmented necrosis, low inflammatory infiltration and inadequate mesenchymal reaction [17]. However, radiological tests are not specific for the initial diagnosis of this disease[18].
The treatment of severe or disseminated HSV infections is parenteral acyclovir or foscarnet and cidofovir in the case of resistance [19]. Norvell et al. observed, in 137 patients with HSV hepatitis, that the treatment delay was associated with an increase in mortality with a mean 4.7 ± 0.48 versus 3.5 ± 0.27 days (p = 0.03) in those patients who died compared to those who survived [12]. In the first and second cases, ganciclovir was started on the 4th day due to CMV infection suspicion, and the specific HVS treatment was delayed until 5 and 8 days, respectively. In the third case, acyclovir and ganciclovir were started on the second day after symptoms onset and a mild improvement and stability of the liver profile was observed; however, the patient died due to multiple bacterial and fungal co-infections that triggered refractory septic shock. The mortality rate of disseminated HSV infections is high, Norvell et al. reported a 74% fatality rate [12]. Similar mortality rate was observed among the immunosuppressed patients described in Table 1.
There is controversy on monitoring of HVS infection in SOT recipients, and there is no clear recommendations on prophylaxis against HVS in recipients with negative HSV serology [20], [21]. There are different guidelines where it mentions the management of infections in transplant patients, such as Practice Guideline by The American Association for the Study of Liver Diseases (AASLD) published in 2012 [22], and the recent guidelines of the American Society of Transplantation (AST) Infectious Diseases Community of Practice in 2019 [19]. In AST Guidelines is reported that prophylaxis used for CMV is also effective against HSV [19]. Martin-Gandu et al. in a cohort of over 2781 SOT patients, observed that with the use of CMV antiviral prophylaxis, the incidence of HSV infections one year after transplantation was 3% in patients receiving antiviral prophylaxis versus 9.8% without prophylaxis [2]. In addition, in the subgroup of patients with positive donor serology for CMV, 2.9% of HSV infection was observed in patients who underwent prophylaxis compared to 10.6% in patients followed by the preventive approach [2]. This may explain the low incidence of post-transplant HVS infections in these patients. Furthermore, none of the previously reported cases received CMV prophylaxis [15], [16]. It cannot be ruled out that the primary HSV infection in this series is a donor-transmitted infection (in 4 cases, including one of ours, the HSV donor serology was positive).
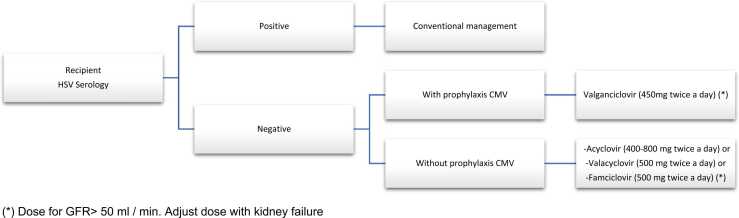
We propose this HSV prophylaxis algorithm for pre-transplant HSV seronegative patients (Fig. 1). The aim of the algorithm is to identify those patients who are seronegative for HSV and stratify the risk of transmission. Once identified the patients at higher risk, HVS specific prophylaxis is indicated in those patients who are not going to receive CMV prophylaxis during the first month. The donor serology is important for etiologic diagnosis, but it shouldn't totally influence the prophylaxis indication. From the theoretical point of view, patients with D- / R- HSV serology status could not receive prophylaxis, nevertheless HSV infection can occur from other causes [8], [23]. We propose that antiviral prophylaxis be performed in all negative HSV recipients regarding the donor serology in the immediate period after kidney transplantation and after rejection treatment. In seronegative HSV patients, short-term monitoring by serum PCR could be considered in order to know the efficacy of this prophylaxis.
Fig. 1.
HSV prophylaxis algorithm for pre-transplant HSV seronegative patients (*) Dose for GFR> 50 ml / min. Adjust dose with kidney failure.
In conclusion, herpes simplex virus (HSV) hepatitis is a rare and potentially fatal entity that could be a donor-derived infection. Although the initial clinical presentation is non-specific, it should be considered as a differential diagnosis in HSV-negative serology patients with liver failure and empirical treatment must be started in combination with a drastic reduction of immunosuppression. A strategy of HSV prophylaxis for pre-transplant HSV seronegative patients must be stablished in order to reduce the risk of clinical disease.
Concent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
C. Arana participated in data collection, analysis, and writing of the manuscript; F. Cofan contributed in the study design, conceptualization, data analysis, and review; P. Ruiz participated in the writing and review; E. Hermida, J. Fernández, J. Colmenero, X. Forns, L. Escude, D. Cucchiari, A. Moreno, M.Bodro, S. Herrera, C. Rodriguez, D. Paredes, F. Diekmann, participated in the critical review of the manuscript and approval of the final version.
Declaration of Competing Interest
The authors don’t have conflicts of interest, on the publication of this paper.
References
- 1.Looker K.J., Magaret A.S., May M.T., Turner K.M.E., Vickerman P., Gottlieb S.L., et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin-Gandul C., Stampf S., Héquet D., Mueller N.J., Cusini A., van Delden C., et al. Preventive strategies against cytomegalovirus and incidence of α-herpesvirus infections in solid organ transplant recipients: a nationwide cohort study. Am J Transpl. 2017;17:1813–1822. doi: 10.1111/ajt.14192. [DOI] [PubMed] [Google Scholar]
- 3.Riediger C., Sauer P., Matevossian E., Müller M.W., Büchler P., Friess H. Herpes simplex virus sepsis and acute liver failure. Clin Transpl. 2009;23(Suppl 21):37–41. doi: 10.1111/j.1399-0012.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 4.San Juan R., Aguado J.M., Lumbreras C., et al. Incidence, clinical characteristics and risk factors of late infection in solid organ transplant recipients: data from the RESITRA study group. Am J Transpl. 2007;7(4):964–971. doi: 10.1111/j.1600-6143.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- 5.Kusne S., Schwartz M., Breinig M.K., Dummer J.S., Lee R.E., Selby R., et al. Herpes simplex virus hepatitis after solid organ transplantation in adults. J Infect Dis. 1991;163:1001–1007. doi: 10.1093/infdis/163.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Côté-Daigneault J., Carrier F.M., Toledano K., Wartelle-Bladu C., Willems B. Herpes simplex hepatitis after liver transplantation: case report and literature review. Transpl Infect Dis. 2014;16:130–134. doi: 10.1111/tid.12178. [DOI] [PubMed] [Google Scholar]
- 7.Gäbel H., Flamholc L., Ahlfors K. Herpes simplex virus hepatitis in a renal transplant recipient: successful treatment with acyclovir. Scand J Infect Dis. 1988;20:435–438. doi: 10.3109/00365548809032482. [DOI] [PubMed] [Google Scholar]
- 8.Al Midani A., Pinney J., Field N., Atkinson C., Haque T., Harber M. Fulminant hepatitis following primary herpes simplex virus infection. Saudi J Kidney Dis Transpl. 2011;22:107–111. [PubMed] [Google Scholar]
- 9.Ison M.G., Nalesnik M.A. An update on donor-derived disease transmission in organ transplantation. Am J Transpl. 2011;11:1123–1130. doi: 10.1111/j.1600-6143.2011.03493.x. [DOI] [PubMed] [Google Scholar]
- 10.Bissig K.-D., Zimmermann A., Bernasch D., Furrer H., Dufour J.-F. Herpes simplex virus hepatitis 4 years after liver transplantation. J Gastroenterol. 2003;38:1005–1008. doi: 10.1007/s00535-002-1186-0. [DOI] [PubMed] [Google Scholar]
- 11.Flohr T., Bonatti H., Frierson H., Harmon R.C., Berg C., Sifri C.D., et al. Herpes simplex virus hepatitis after renal transplantation. Transpl Infect Dis. 2008;10:377–378. doi: 10.1111/j.1399-3062.2008.00309.x. [DOI] [PubMed] [Google Scholar]
- 12.Norvell J.P., Blei A.T., Jovanovic B.D., Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428–1434. doi: 10.1002/lt.21250. [DOI] [PubMed] [Google Scholar]
- 13.Yiu D., Ballabio M., Fornoni G., Maggi U. Unusual oral presentation of HSV-1 lesions in an adult liver transplant recipient. BMJ Case Rep. 2019:12. doi: 10.1136/bcr-2018-227492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berrington W.R., Jerome K.R., Cook L., Wald A., Corey L., Casper C. Clinical correlates of herpes simplex virus viremia among hospitalized adults. Clin Infect Dis. 2009;49:1295–1301. doi: 10.1086/606053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pietrucha-Dilanchian P., Tanawuttiwat T., Abbo L., Regatieri A., Chaparro S., Ruiz P., et al. Fatal herpes simplex virus type 2 hepatitis in a heart transplant recipient: a case report and review of the literature. Transpl Infect Dis. 2013;15:E87–E96. doi: 10.1111/tid.12077. [DOI] [PubMed] [Google Scholar]
- 16.Zeidan J.H., Casingal V., Hippen B., Ahrens W., Lamm K., Gerber D.A., et al. Donor-derived herpes simplex virus hepatitis in a kidney transplant recipient and review of the literature. Transpl Infect Dis. 2021 doi: 10.1111/tid.13562. [DOI] [PubMed] [Google Scholar]
- 17.Jacques S.M., Qureshi F. Herpes simplex virus hepatitis in pregnancy: a clinicopathologic study of three cases. Hum Pathol. 1992;23:183–187. doi: 10.1016/0046-8177(92)90241-t. [DOI] [PubMed] [Google Scholar]
- 18.Montalbano M., Slapak-Green G.I., Neff G.W. Fulminant hepatic failure from herpes simplex virus: post liver transplantation acyclovir therapy and literature review. Transpl Proc. 2005;37:4393–4396. doi: 10.1016/j.transproceed.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Lee D.H., Zuckerman R.A., Infectious A.S.T. Diseases community of practice. herpes simplex virus infections in solid organ transplantation: guidelines from the american society of transplantation infectious diseases community of practice. Clin Transpl. 2019;33 doi: 10.1111/ctr.13526. [DOI] [PubMed] [Google Scholar]
- 20.Malinis M., Boucher H.W., Infectious A.S.T. Diseases community of practice. screening of donor and candidate prior to solid organ transplantation-guidelines from the american society of transplantation infectious diseases community of practice. Clin Transpl. 2019;33 doi: 10.1111/ctr.13548. [DOI] [PubMed] [Google Scholar]
- 21.Zuckerman R., Wald A. AST Infectious diseases community of practice. Herpes simplex virus infections in solid organ transplant recipients. Am J Transpl. 2009;9(Suppl 4):S104–S107. doi: 10.1111/j.1600-6143.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- 22.Lucey M.R., Terrault N., Ojo L., Hay J.E., Neuberger J., Blumberg E., Teperman L.W. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3–26. doi: 10.1002/lt.23566. [DOI] [PubMed] [Google Scholar]
- 23.Taylor R.J., Saul S.H., Dowling J.N., Hakala T.R., Peel R.L., Ho M. Primary disseminated herpes simplex infection with fulminant hepatitis following renal transplantation. Arch Intern Med. 1981;141:1519–1521. [PubMed] [Google Scholar]