Abstract
Background
Late in the nineteenth century, it was theorized that a circulating product produced by the parathyroid glands could negatively impact skeletal homeostasis. A century later, intermittent administration of that protein, namely parathyroid hormone (PTH), was approved by the FDA and EMA as the first anabolic agent to treat osteoporosis. Yet, several unanswered but important questions remain about the skeletal actions of PTH.
Scope of review
Current research efforts have focused on improving the efficacy of PTH treatment by designing structural analogs and identifying other targets (e.g., the PTH or the calcium sensing receptor). A unique but only recently described aspect of PTH action is its regulation of cellular bioenergetics and metabolism, namely in bone and adipose tissue but also in other tissues. The current review aims to provide a brief background on PTH's previously described actions on bone and highlights how PTH regulates osteoblast bioenergetics, contributing to greater bone formation. It will also shed light on how PTH could alter metabolic homeostasis through its actions in other cells and tissues, thereby impacting the skeleton in a cell non-autonomous manner.
Major conclusions
PTH administration enhances bone formation by targeting the osteoblast through transcriptional changes in several pathways; the most prominent is via adenyl cyclase and PKA. PTH and its related protein, PTHrP, also induce glycolysis and fatty acid oxidation in bone cells and drive lipolysis and thermogenic programming in adipocytes; the latter may indirectly but positively influence skeletal metabolism. While much work remains, alterations in cellular metabolism may also provide a novel mechanism related to PTH's temporal actions. Thus, the bioenergetic impact of PTH can be considered another of the myriad anabolic effects of PTH on the skeleton. Just as importantly from a translational perspective, the non-skeletal metabolic effects may lead to a better understanding of whole-body homeostasis along with new and improved therapies to treat musculoskeletal conditions.
Keywords: Bone, Bioenergetics, Anabolic, Osteoblasts, Adipocytes, Fat, Mitochondria
1. Introduction
Unraveling the relationship between parathyroid hormone (PTH), also called parathormone or parathyrin, and bone has been a complex and evolving story. While it is complicated, it has proven to be incredibly rewarding from a translational perspective. Approved in 2001, PTH (1–34), or teriparatide, was the first FDA-approved osteoanabolic agent to treat severe osteoporosis [1]. Previously, the relationship between PTH and the skeleton was observational and loosely connected since Virchow and Erdeheim, both anatomical pathologists, had described enlarged parathyroid glands in patients with bone disease as early as the late nineteenth century. However, after trial and error in the early twentieth century, it was established that patients with hyperparathyroidism suffer from severe bone disease resulting in multiple fractures, and hypoparathyroidism is associated with a milder bone phenotype [2]. Despite this fact, and somewhat paradoxical, Selye reported in 1932 that PTH could also stimulate osteogenesis; remarkably, this finding went underappreciated for years [3]. It was not until the late 1990s that this “rediscovery” was re-proven; it is now widely accepted that intermittent administration of PTH stimulates bone formation greater than bone resorption in what is referred to as an “anabolic window.” That window, approximately 6 months in duration, ultimately leads to anabolic action of bone that results in an increase in bone mineral density (BMD) and a reduction in osteoporotic fractures, making it an ideal therapeutic agent to treat osteoporosis [4]. Conversely, during chronic elevation of PTH, as in hyperparathyroidism, this osteoanabolic action is counterbalanced by elevated catabolic function or bone loss [4,5,6]. As the field of bone biology continues to mature, bone cell metabolic programming has become a provocative area of research. As such, PTH-induced increases in osteoblast “workload,” or bone formation, must also include some regulation of cellular metabolism to fuel those cells. The current review will cover the impact of PTH on regulating systemic and cellular metabolism and how these actions influence skeletal homeostasis. While some of these details remain speculative, a strong connection exists between PTH and metabolic processes; this connection will be highlighted throughout this review.
To fully appreciate these complex mechanisms, it is important to establish the primary function of PTH, which in essence is the regulation of calcium homeostasis. PTH is exclusively produced and secreted by chief cells located in the parathyroid gland [7]. Conversely, PTH- related peptide (PTHrP) is synthesized and expressed by various tissues including skin, blood vessels, growth plate chondrocytes, bone, smooth muscle, and in neuronal tissues to act in a paracrine fashion [8]. While PTH and PTHrP only share 16% homology in their overall sequence, significant homology is clustered within their N-terminal; therefore, both peptides can serve as ligands for the same receptor, PTH1R [8]. As its main physiological actions, circulating PTH regulates extracellular calcium homeostasis such that under physiological states, blood calcium is maintained at 2.0 mM and experiences no more than a 20% variation [2,7,9]. Half of the calcium in circulation is bound to blood proteins including albumin; it is the unbound extracellular ionic calcium (Ca2+) concentrations (∼1.2 mM) that must be precisely controlled to maintain potential difference in excitable cell membranes. That said, PTH regulates calcium homeostasis via multiple mechanisms mainly targeting tissues with high expression of PTH1R such as the bone and kidney. The skeleton serves as an excellent and never-ending reservoir for stored calcium, which can be liberated via activation of the PTH1R on bone-forming osteoblasts to signal the release of receptor-activator of NF-κB ligand (RANKL) to drive osteoclastogenesis and bone resorption. This allows acute and chronic maintenance of serum calcium within a very tight range.
The skeleton, as a dynamic tissue, undergoes continuous remodeling involving bone resorbing osteoclasts, bone-forming osteoblasts, and matrix-embedded mechano-sensing osteocytes. Bone formation by the osteoblast begins with the secretion of a predominantly type 1 collagen matrix called osteoid that essentially lays the template for mineralization to occur. It is this mineral of hydroxyapatite crystals [Ca10(PO4)6(OH)2] that provides much of the structural integrity to the bone and perhaps inadvertently also creates the reservoir for stored calcium. In this capacity, when extracellular calcium levels are low, the calcium-sensing receptor (CaSR) promotes PTH release into the circulation [10,11]. PTH then acts to stimulate bone resorption via osteoblast release of RANKL, which drives osteoclastogenesis [12,13]. It remains controversial whether mammalian osteoclasts express PTH1R in a species-specific manner [14,15]. This raises the possibility there is a direct PTH action on osteoclasts to enhance bone resorption. Nevertheless, this indirect mechanism is fundamental to understanding the skeletal actions of PTH, which in this context could be considered a “super-remodeler” because it stimulates new bone formation but is coupled to an increase in bone resorption. PTH directly stimulates osteoblasts and bone formation [16]; if sustained chronically, formation plateaus and is followed by an increase in bone resorption due to enhanced RANKL [13]. Conversely, intermittent administration of PTH capitalizes on that short “anabolic” window when bone formation exceeds that of bone resorption, leading to a net gain of bone. Finally, given the relationship between PTH and bone remodeling, it remains unclear from a teleological standpoint why PTH stimulates bone formation if in fact skeletal resorption and calcium release are the ultimate goal. Some researchers have speculated that the initial response of PTH to trigger osteoblastogenesis is to protect the skeleton from an acute calcemic effect. It is also possible that PTH signals to the osteoblast first to “prime” the bone with newly synthesized osteoid capable of providing said calcium following resorption. While the process remains unclear, this is an intriguing concept for further investigation.
2. Old dog: PTH's classically described mechanisms on bone tissue
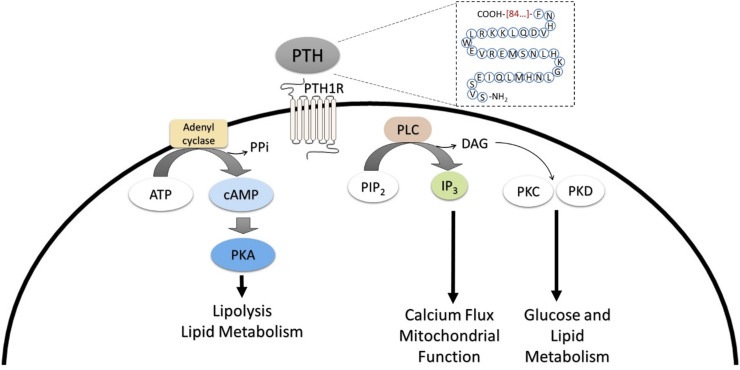
Multiple mechanisms have been proposed for intermittent PTH's anabolic actions on bone. These involve different signaling pathways and various targets. Like other G-protein-coupled receptors (GPCRs), signaling cascades downstream of PTH1R most notably include cyclic adenosine monophosphate (cAMP) or adenylyl cyclase and phospholipase C (PLC) [16]. The predominant physiological pathway involves the stimulation of cAMP, leading to the phosphorylation and activation of protein kinase A (PKA); this can in-turn regulate a multitude of cellular processes. Additionally, PTH1R activation can lead to PLC's cleavage of phosphatidylinositol bisphosphate (PIP2) to yield inositol triphosphate (IP3) and diacylglycerol (DAG). Both can increase intracellular calcium and activate protein kinase C (PKC), respectively, in osteoblasts [17,18]. Interestingly, activation of the PLC pathway has been demonstrated to only occur when an agonist is administered at a high (micromolar) concentration, whereas cAMP is activated at sub-nanomolar concentrations (i.e., in the range of physiological PTH) [19,20]. This is particularly noteworthy as it may represent a way by which the cell preferentially activates the PLC pathway via high, local concentrations of PTHrP, as is observed in the growth plate [21]. While these pathways have been extensively studied relative to osteoblast cellular function, as both are critical regulators of intracellular metabolism, they are often overlooked when examining PTH-PTH1R signaling (Figure 1).
Figure 1.
Parathyroid hormone (PTH)'s Primary Signaling Pathways in Osteoblastic Cells. PTH is a polypeptide containing 84 amino acids (AA). The peptide fragment essential for signaling (1–34 AA) are demonstrated in blue circles. PTH binds to PTH receptor (PTH1R), a G-protein coupled receptor, and activates adenylyl cyclase and phospholipase C (PLC). Adenyl cyclase activation then converts adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP), which now acts as a secondary messenger to activate protein kinase A (PKA). PKA can directly interact with proteins on the lipid droplet membrane to trigger the breakdown of triglycerides to free fatty acids, or lipolysis. In addition to the cAMP/PKA pathway, PTH-PTH1R signaling triggers PLC hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol triphosphate (IP3). IP3 is now capable of signaling within the endoplasmic reticulum to release calcium stores, thereby altering the intracellular calcium flux that is critical for regulating mitochondrial function. Upon IP3 formation, diacylglycerol (DAG) is also formed and can act as secondary messengers, regulating protein kinase C (PKC) and protein kinase D (PKD), both of which are important for glucose and lipid metabolism. Although both pathways have been demonstrated in osteoblastic cells, it is likely that these pathways are also stimulated within other cell types which express PTH1R.
Cells of the osteoblast lineage contain machinery for both cAMP/PKA and PLC pathways. PTH signaling profoundly impacts these cells by targeting genes/proteins important for bone formation and have been previously described to include ephrin B2, insulin-like growth factor (IGF-1), fibroblast growth factor (FGF-2), salt inducible kinase (SIK2), Wnt/β-catenin, and matrix metalloproteinase (MMP-13) [22,23,24,25,26,27,28,29,30]. Signaling through these pathways in cells of the osteoblast lineage results in the activation of bone lining cells, increased mineralized matrix deposition, and suppressed apoptosis [17,18]. For example, PTH can activate bone lining cells and lead to an increase in the number of osteoblasts on the bone surface, as well as delay the osteoblast-to-bone lining cell transition that can occur late in remodeling [31]. In addition to increased osteoblast numbers, PTH also promotes enhanced matrix deposition such that more bone is formed per osteoblast [32]. Fundamental to these cellular processes is the ability of bone forming osteoblasts to acquire and utilize substrates for the production of cellular energy or adenosine triphosphate (ATP). In this regard, the anabolic actions of PTH require the coordination and generation of ATP in osteoblasts to effectively enhance bone formation while also activating bone lining cells.
3. New tricks: PTH's role in metabolism
3.1. Bone
There is substantial evidence that the secretion of matrix proteins and mineralization vesicles by the osteoblast that results in bone formation is an energy demanding process [33,34,35]. ATP is required for supporting such processes, which are described as cellular “bioenergetics” and include a series of sequential reactions. These PTH-induced changes in osteoblast activity are further supported by Denton and McCormack's parallel activation mode whereby increases in energy demand produce an increase in bioenergetic capacity [36,37]. Take, for example, collagen synthesis and secretion; these have been shown to rely heavily on cellular ATP:ADP ratios [33,34,35]. If one considers further that it takes ∼199 ATP to translate a protein composed of 50 amino acids and that type 1 collagen is 1,465 amino acids, this results in 5,855 ATP molecules required to make 1 chain of collagen. And since collagen exists in a tight triple helical structure or fibril, the entire process demands 17,565 mol of ATP. Moreover, to synthesize additional proteins in the bone matrix (e.g., osteocalcin, bone sialoprotein, and osteopontin) along with the coordination of proper secretion of mineralization vesicles, collective “bone formation” would require an additional amount of ATP, underscoring the importance of osteoblast bioenergetic status in relation to anabolic treatments.
The bioenergetic capacity of osteoblasts is generally considered within the framework of two mechanisms, glycolysis and oxidative phosphorylation via mitochondrial respiration. These processes rely on glucose, exclusively for the former, along with glucose and fatty acids for the latter. It was initially established that PTH modulates intracellular metabolism by increasing aerobic glycolysis in osteoblastic MC3T3-E1 cells [38]. That work focused on the PTH-IGF1-mTORC2 axis by which glucose was metabolized via aerobic glycolysis when treated for 48 h [38]. However, due to the dynamic nature of osteoblast differentiation during bone remodeling, there are likely to be temporally related changes in bioenergetic substrates. Our laboratories demonstrated that oxidative phosphorylation predominates early in stromal cell differentiation, whereas glycolysis is the major metabolic pathway later in osteoblast differentiation [39]. While the mechanism regulating this switch remains unclear, it could be a function of oxygen availability. In this capacity, oxidative phosphorylation relies on oxygen, and these stromal/undifferentiated osteoblasts are presumed to be mostly positioned close to a blood supply, while mature osteoblasts are expected to experience local hypoxia close to and within the bone niche [40]. It should be noted that although it is true that mature osteoblasts demonstrate an increase in glycolysis, oxidative phosphorylation is still active and believed to contribute to the ATP pool [39].
The differentiation of mesenchymal progenitors into mature osteoblasts, as noted, is a dynamic process not only in respect to transcriptional profiling but also regarding substrate utilization. Hence, demands on the osteoblast require not only access to substrate but the need for varying amounts of ATP molecules necessary for a particular stage of activity. In this capacity, although osteoblasts rely partly on aerobic glycolysis to generate ATP [39,41,42], fatty acids yield more energy per molecule than glucose when catabolized. To this point, in addition to increased glucose utilization demonstrated by the Long group [38], the authors also noted that PTH increased mitochondrial oxidative phosphorylation from an undetermined, non-glucose substrate source. Although scarce data exists relative to PTH and fatty acid oxidation, it has been confirmed that fatty acids are an important substrate source for normal bone formation and during anabolic stimulation of WNT-LRP5 signaling [43,44]. This is a critical point for further exploration because osteoblasts have been shown to utilize both intracellular lipid droplets and exogenous fatty acids as sources for energy generation [44,45]. Finally, PTH can also increase osteoblast amino acid uptake, namely proline and glutamine, for enhanced collagen synthesis [46,47,48]. It is conceivable these amino acids are also capable of altering osteoblast bioenergetics, as glutamine has previously been shown to support the TCA cycle via α-ketoglutarate production [49] and proline coordinates reactions of the electron transport chain [50,51]. Many of these mechanistic studies have been performed in vitro using cell lines, bone marrow stromal cell-derived osteoblasts, and/or calvaria osteoblasts; caution should be exercised because these systems introduce artificial environmental factors. Nonetheless, the timing and sequence of fuel utilization after PTH exposure are physiologically relevant as osteoblasts strive to increase their workload within a restrained and hypoxic environment.
In addition to studies on substrate utilization, given the pathways signaled downstream of PTH1R in osteoblasts, additional mechanisms must be involved in modulating osteoblast bioenergetics to facilitate PTH-induced bone formation and mineralization. For example, PTH treatment has been shown to alter mitochondrial membrane potential in vitro [52]. This may occur through PTH-mediated changes in mitochondrial calcium (Ca2+m) flux. As previously described, PTH binding to PTH1R results in signaling cascades involving the PLC pathway in osteoblasts [16]. Downstream targets, such as IP3, act as a ligand for IP3 receptors (IP3R) located on the ER, and this causes the release of Ca2+, which enter the mitochondria. Once inside the mitochondria, Ca2+ activation of oxidative phosphorylation occurs at multiple levels including (1) production of TCA cycle intermediates pyruvate dehydrogenase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase; (2) stimulation of the ATP synthase; (3) stimulation of α-glycerophosphate dehydrogenase; and (4) stimulation of adenine nucleotide translocase (ANT) [53]. The potential relevance of the activation of this pathway is further supported by previous reports that PTH-PTH1R-PLC is required for proper skeletal homeostasis, specifically under “stressed” physiological conditions [54]. In this regard, transgenic mice expressing PTH1R modified to signal via adenyl cyclase normally, but attenuated PLC (DESL mice) fed a low calcium diet exhibited reduced trabecular bone volume fraction as well as impaired osteoblastogenesis [54].
In addition to the PLC pathway, the dominant pathway signaled via PTH-PTH1R activation, cAMP-PKA, has also been noted to modulate metabolism [55]. Therefore, it is highly likely that activation of both pathways in osteoblasts is contributing to the sum of cellular bioenergetics that support PTH-induced bone formation. Certainly, there is a cell-autonomous role by which PTH impacts osteoblast bioenergetics. However, three caveats should be noted. First, as emphasized, energy utilization by the osteoblast is temporally dependent, hence in vitro data that support energy utilization by various substrates, e.g., glucose, fatty acids, or glutamate, may miss critical switches in fuel utilization that occur in vivo. Second, in that regard, the relative impact of PTH on the pentose phosphate pathway for obligatory nucleotide synthesis withing osteoblasts has not been investigated. Third, it is possible that whole-body metabolic changes could also be contributing to secondary cell non-autonomous changes in the osteoblast, resulting in enhanced skeletal remodeling. Notwithstanding, more studies are needed to fully understand the impact of PTH on energy metabolism in osteoblasts, particularly in relation to acute versus chronic exposure, reactive oxygen species (ROS) generation, mitochondrial biogenesis, and mitophagy. This is particularly relevant with the emerging data that PTH uncouples mitochondrial respiration in adipocytes [56].
3.2. Adipose tissue
The relationship between the skeleton and adipose tissue has been appreciated for some time through clinical studies, suggesting a protective effect of greater body weight and adipose depots on fracture risk [57,58,59]. However, visceral adiposity in obese individuals is inflammatory and through adipokine and cytokine release can stimulate bone resorption and thus cause bone loss. Nevertheless, the unique capacity of adipose tissue to store excess energy in the form of fatty acids provides a relatively straightforward mechanism for bone cells to enhance their fuel utilization during periods of bone growth, remodeling, and regeneration.
PTH interacts with adipose tissue in a unique way. An early study reported that PTH administration increased glycerol release, a secreted byproduct of lipolysis, 3–5 fold in rat epididymal adipocytes [60]. Norman Bell and colleagues [61,62] demonstrated that PTH treatment of human subcutaneous fat depots led to glycerol release in vitro and showed peak plasma fatty acids following an ∼30–60 min injection of PTH [61]. Plasma-free fatty acids remained elevated compared to baseline following 150 min of PTH, but no additional timepoints were collected [61]. More recently, Larsson et al. provided mechanistic insights into this phenomenon, demonstrating that both PTH and PTHrP were able to stimulate cytoplasmic lipolysis via cAMP-PKA activation of hormone sensitive lipase (HSL) [63]. In vivo data about PTH regulation of fat mass are somewhat less convincing. There is one cross-sectional study of young, nonobese, Caucasian women that demonstrated a significant association between fasting serum PTH and fat mass that was independent of serum calcium [64]. Evidence has also emerged that bone quality is adversely impacted by the magnitude of adipose tissue [65,66]. Yet, there is no evidence that sustained PTH secretion (i.e., primary or secondary hyperparathyroidism) or intermittent administration of PTH or PTHrP reduce fat mass or body weight. Notably, while no overt alterations in fat mass have been demonstrated during these scenarios, activation of lipolysis does not necessarily result in a reduction in fat mass [67]. Moreover, there is relatively low expression of PTH1R on peripheral white adipose depots, thus masking any such overt clinical observation. Taken together, these data support the tenet that under certain experimental circumstances (i.e., various conditions and/or diseases), or ex vivo, the PTH1R on adipocytes could be activated by PTH and drive lipolysis. Reconciling those experimental data with the reports that osteoblasts can use fatty acids to enhance collagen synthesis [42,43,44,68] suggests that PTH may impact the skeleton through non-cell autonomous mechanisms involving white adipose tissue.
In addition to evidence that PTH-PTH1R signaling regulates lipolysis in white adipocytes, emerging data suggest that PTH1R-signaling pathways can alter the white adipocyte profile to that of a brown, thermogenic adipocyte. Seminal work by the Spiegelman laboratory, using a conditional deletion of the PTH1R with an adipoCre mouse, first demonstrated that the cancer-related cachexia mediated by high levels of PTHrP was related to its action on thermogenic adipocytes [69]. Both PTH and tumor-derived PTHrP were shown to convert (i.e., ‘beige’) white adipocyte tissue (WAT) into a thermogenic depot [69,70]. Mechanistic studies showed that PTH, due to production from neoplastic cells or in chronic renal failure, induces a molecular thermogenic program that enhances uncoupled mitochondrial activity and beige adipocyte markers in human subcutaneous white adipose precursor cells [56]. Clinical support for that premise came from one study in patients with hyperparathyroidism, where some adipocytes expressed higher levels of beige markers compared to those with normal PTH levels [70]. Moreover, a recent translational study further expanded on these alterations by demonstrating that ice-water swimmers and non-shivering thermogenesis protocols resulted in increased serum PTH and thyroid stimulating hormone (TSH), accompanied by a whole-body metabolic preference for lipids and increased BAT volume [71]. This interaction also highlights the complex endocrine actions associated with changes in circulating PTH, to include other endocrine factors. As such, TSH, along with growth hormones, (GH) and insulin-like growth factor-1 (IGF-1) increase lipolysis and alter lipid metabolism [72,73,74,75] and also elicit an anabolic response on the skeleton [76,77,78]. Thus, in addition to PTH-stimulated lipolysis, these lines of evidence support the ability of PTH to brown or “beige” white adipocytes by reprogramming. The precise mechanism for that adipocyte “conversion” has not been fully elucidated, although preliminary studies from our lab and others suggest that Zinc finger proteins (Zfps), 423, 467, and 521, the latter two which are regulated by PTH and PTHrP, may play important roles in metabolic reprogramming of adipocyte [79,80,81,82]. These studies establish the premise PTH has a distinct impact on lipid metabolism, namely in adipocytes, which can pose a multitude of effects on addition tissues.
Finally, our laboratories have been particularly interested in how PTH modulates metabolism in a unique adipose depot, bone marrow adipocyte tissue (BMAT). The bone marrow compartment provides a microenvironment in which communication occurs between white blood cells, red blood cells, platelets, and immune cells, in addition to classic bone cells (osteoblasts, osteoclasts, and osteocytes) that can both directly and indirectly impact skeletal homeostasis [83]. Bone marrow adipocytes (BMAdipo) are found interspersed throughout the marrow compartment in the axial and appendicular skeleton. Our understanding of BMAT has advanced significantly in the past decade, although many questions remain relative to their lineage and function. Unlike peripheral adipocytes or WAT progenitors, which are primarily derived from mesenchymal stem cells (MSC) through vascular infiltration [84,85], the definitive lineage of BMAdipo remains controversial [86]. Recent work by Ling Qin et al. at the University of Pennsylvania described a marrow adipocyte like progenitor, or MALP, that expresses the same genes found in BMAdipo but without the lipid droplet [87]. These cells are distinct from peripheral white adipocyte progenitors and carry adiponectin as well as the leptin receptor and other classic white adipocyte markers [87]. Importantly, these progenitors also express RANKL and PTH1R and may be the source of the critical factor that drives bone resorption during enhanced marrow adipogenesis [88]. The defining morphological feature of BMAdipo, as well as other peripheral adipocytes, is their ability to store lipids as a large, unilocular lipid droplet. However, unlike other fat cells, BMAdipo store lipids even during states of profound nutritional deficiency, such as anorexia nervosa or calorie restriction [65].
BMAdipo function is arguably even less well understood as is the fate of individual terminally differentiated marrow fat cells. BMAdipo can regulate blood cell recruitment in the marrow [89]. After marrow injury (e.g., radiation, chemotherapy, and mechanical ablation), these cells could serve as a “place holder” for hematopoietic stem cells so that during reconstitution, a ready source of fuel can be easily accessed. While the impact of BMAdipo on bone is complex, evidence generally indicates that an inverse relationship exists between BMAT and bone mass [65]. Clinical scenarios of compromised bone health including anorexia nervosa, aging and gonadal hormone deficiency, glucocorticoid treatment, alcoholism, and unloading/weightlessness are all associated with profound increases in BMAT [65]. Presumably, due to their intimate relationship with osteoblast progenitor cells, BMAdipo express PTH1R, as do MALPS, and thereby this relationship holds true relative to PTH—that is, the bone anabolic effect of intermittent PTH is also accompanied by a decrease in BMAT [90]. Relative to this observation, we showed [91] that intermittent PTH treatment initiated prior to BMAT expansion in a calorie-restricted model of anorexia nervosa decreased the BMAdipo number. Interestingly, intermittent PTH treatment subsequent to BMAT expansion not only decreased BMAdipo size by activation of lipolysis but also resulted in a greater bone anabolic response compared to control (∼2–3 fold) [91]. Given the intimate proximity of BMAdipo to osteoblasts and osteoclasts along with their precursors, it is conceivable that BMAT can directly influence bone mass by providing a fuel source during states of stress or accelerated bone formation. For example, we have preliminary data that in the treatment of hypoparathyroidism with PTH, adipocyte number and size are reduced, and this is temporally related to an increase in bone formation. As such, this is an area of active exploration because it could hold clues related to PTH-dosing and efficacy for the management of osteoporosis, as well as leading to novel therapeutic targets. For example, it remains plausible that osteoporotic patients unresponsive to anabolic treatment with PTH might demonstrate low BMAT and/or attenuated lipolytic signaling. If the osteoanabolic effect of PTH requires fatty acids, it stands to reason that PTH treatment coordinated with exogenous substrates could enhance bone formation. While this remains under investigation, it does provide a glimpse into how such mechanisms would prove clinically beneficial.
3.3. Other tissues
While it was the goal of the current review to highlight PTH's ability to exert an osteoanabolic impact on bone by its potential regulation of osteoblast and adipocyte metabolism, it is recognized that PTH administration can influence other tissues. For example, due to the kidney's regulation of calcium and vitamin D, along with the high expression of PTH1R in renal tubules, this is another important target tissue of PTH. In the kidney, PTH promotes calcium reabsorption and increases 1,25-dihydroxycholecalciferol while decreasing phosphate reabsorption. Given PTH's pharmacokinetics and short half-life [92,93], it was expected that intermittent PTH for the treatment of osteoporosis has limited effect on this tissue. However, it is conceivable that intermittent PTH could exert an acute response in kidney metabolism. The first line of evidence includes patients with chronic kidney disease (CKD) who often have secondary hyperparathyroidism. These individuals also develop hypercholesterolemia that includes elevated low-density lipoprotein cholesterol (LDL-C) and reduced high-density lipoprotein cholesterol (HDL-C), as well increased serum triglycerides and fatty acids [94]. Interestingly, fatty acids are the main energy substrate source in the kidney with uptake mediated by CD36, followed by mitochondrial β-oxidation for the generation of ATP. Similar to the previously described mechanism involving intracellular calcium flux via PLC pathway, PTH has been shown to alter TCA cycle intermediates and calcium flux on mitochondria from rat kidneys [95]. Therefore, given the high expression of PTH1R, along with the fatty acid and lipid demand of the kidney, it is plausible that acute, intermittent injection of PTH alters renal cell metabolism and fatty acid metabolism, although more data are needed.
No evidence supports PTH's ability to alter energy metabolism in both skeletal and cardiac muscle. Somewhat counterintuitive to the increased energy production presented to explain PTH's osteoanabolic effect on osteoblasts using the parallel activation model theory, rats treated with PTH for four days demonstrated reduced mitochondrial oxygen consumption, increased ROS, and lower energy production in skeletal and cardiac muscle [96,97]. These alterations were attributed to enhanced entry and accumulation of calcium [96,97]. Relative muscle dysfunction and wasting is observed in some patients with long-standing secondary and tertiary hyperparathyroidism, although cause and effect have not been established. As previously noted, however, muscle wasting, or cachexia, described in CKD and cancer (Lewis lung carcinoma) were also shown to be related to ligand activation of PTH-PTH1R signaling and beiging in adipocytes [70]. These data bring multiple mechanisms into perspective and further underscore the complex, systemic impact PTH signaling has on multiple tissues while implicating altered metabolic processes.
4. Conclusions
Targeting metabolic pathways in bone cells is an approach that could be used clinically to reduce osteoporotic related fractures. In fact, anti-resorptive drugs such as the bisphosphonates (i.e., first-generation etidronate and clodronate, second-generation alendronate, zoledronate, and residronate) are effective treatments for osteoporosis and work in part to inhibit osteoclast metabolism (ATP and cholesterol, respectively). We provided preliminary evidence that teriparatide (PTH 1–34) and abaloparatide (PTHrP), two agents that form the cornerstones of anabolic therapy for osteoporosis, are likely to impact the bioenergetics of both bone and adipose tissue. However, more work is needed to understand the exact metabolomic signatures of these hormones and the role that bone marrow adipose tissues play in fueling skeletal and hematopoietic responses.
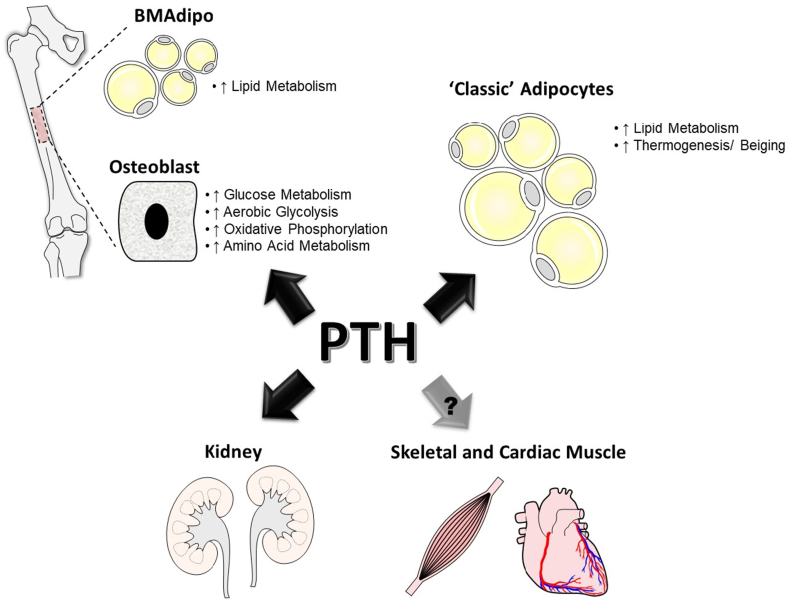
In conclusion, this review highlighted the current knowledge related to PTH's ability to alter osteoblast bioenergetics and systemic metabolic processes by targeting other tissues and cell types, including adipocyte populations, kidneys, and skeletal muscle (Figure 2). There are tantalizing clues as to the molecular basis for the modulation of skeletal and non-skeletal cellular metabolism by PTH. The potential to translate these findings into new and improved therapies combating a host of musculoskeletal conditions remains high.
Figure 2.
Parathyroid hormone (PTH)'s Actions on Metabolic Process in Various Tissues. PTH has been shown to modulate cellular metabolic processes within the bone, as in osteoblasts and bone marrow adipocytes (BMAdipo), and within classic adipocyte populations, including subcutaneous and epididymal fat. Additionally, as a primary target of PTH, the kidney is expected to experience some of the effects as well. To a lesser extent, data does exist describing alterations occurring in skeletal and cardiac muscles following PTH treatment.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants K01AR072123 (ERR) and R01AR073774 (CJR) and the National Institute on Aging Grant R01AG069795 (ERR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest
None declared.
Contributor Information
Elizabeth Rendina-Ruedy, Email: elizabeth.rendina-ruedy@vumc.org.
Clifford J. Rosen, Email: Rosenc@mmc.org.
References
- 1.Ishtiaq S., Fogelman I., Hampson G. Treatment of post-menopausal osteoporosis: beyond bisphosphonates. Journal of Endocrinological Investigation. 2015;38(1):13–29. doi: 10.1007/s40618-014-0152-z. [DOI] [PubMed] [Google Scholar]
- 2.Potts J.T., Jr. A short history of parathyroid hormone, its biological role, and pathophysiology of hormone excess. Journal of Clinical Densitometry. 2013;16(1):4–7. doi: 10.1016/j.jocd.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Selye H. ON the stimulation OF new bone-formation with parathyroid extract and irradiated ergostero. Endocrinology. 1932;16(5):547–558. [Google Scholar]
- 4.Neer R.M., Arnaud C.D., Zanchetta J.R., Prince R., Gaich G.A., Reginster J.Y., et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. New England Journal of Medicine. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 5.Jilka R.L., O'Brien C.A., Bartell S.M., Weinstein R.S., Manolagas S.C. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. Journal of Bone and Mineral Research. 2010;25(11):2427–2437. doi: 10.1002/jbmr.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dempster D.W., Cosman F., Kurland E.S., Zhou H., Nieves J., Woelfert L., et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. Journal of Bone and Mineral Research. 2001;16(10):1846–1853. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 7.Goltzman D. Physiology of parathyroid hormone. Endocrinology and Metabolism Clinics of North America. 2018;47(4):743–758. doi: 10.1016/j.ecl.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Sutkeviciute I., Clark L.J., White A.D., Gardella T.J., Vilardaga J.P. PTH/PTHrP receptor signaling, allostery, and structures. Trends in Endocrinology and Metabolism. 2019;30(11):860–874. doi: 10.1016/j.tem.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilezikian J.P., Brandi M.L., Cusano N.E., Mannstadt M., Rejnmark L., Rizzoli R., et al. Management of hypoparathyroidism: present and future. Journal of Clinical Endocrinology & Metabolism. 2016;101(6):2313–2324. doi: 10.1210/jc.2015-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz-Soto G., Rocher A., Garcia-Rodriguez C., Nunez L., Villalobos C. The calcium-sensing receptor in health and disease. Int Rev Cell Mol Biol. 2016;327:321–369. doi: 10.1016/bs.ircmb.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Goltzman D., Mannstadt M., Marcocci C. Physiology of the calcium-parathyroid hormone-vitamin D Axis. Frontiers of Hormone Research. 2018;50:1–13. doi: 10.1159/000486060. [DOI] [PubMed] [Google Scholar]
- 12.McSheehy P.M., Chambers T.J. Osteoblastic cells mediate osteoclastic responsiveness to parathyroid hormone. Endocrinology. 1986;118(2):824–828. doi: 10.1210/endo-118-2-824. [DOI] [PubMed] [Google Scholar]
- 13.Shiotani A., Takami M., Itoh K., Shibasaki Y., Sasaki T. Regulation of osteoclast differentiation and function by receptor activator of NFkB ligand and osteoprotegerin. The Anatomical Record. 2002;268(2):137–146. doi: 10.1002/ar.10121. [DOI] [PubMed] [Google Scholar]
- 14.Langub M.C., Monier-Faugere M.C., Qi Q., Geng Z., Koszewski N.J., Malluche H.H. Parathyroid hormone/parathyroid hormone-related peptide type 1 receptor in human bone. Journal of Bone and Mineral Research. 2001;16(3):448–456. doi: 10.1359/jbmr.2001.16.3.448. [DOI] [PubMed] [Google Scholar]
- 15.Dempster D.W., Hughes-Begos C.E., Plavetic-Chee K., Brandao-Burch A., Cosman F., Nieves J., et al. Normal human osteoclasts formed from peripheral blood monocytes express PTH type 1 receptors and are stimulated by PTH in the absence of osteoblasts. Journal of Cellular Biochemistry. 2005;95(1):139–148. doi: 10.1002/jcb.20388. [DOI] [PubMed] [Google Scholar]
- 16.Datta N.S., Abou-Samra A.B. PTH and PTHrP signaling in osteoblasts. Cellular Signalling. 2009;21(8):1245–1254. doi: 10.1016/j.cellsig.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Civitelli R., Reid I.R., Westbrook S., Avioli L.V., Hruska K.A. PTH elevates inositol polyphosphates and diacylglycerol in a rat osteoblast-like cell line. American Journal of Physiology. 1988;255(5 Pt 1):E660–E667. doi: 10.1152/ajpendo.1988.255.5.E660. [DOI] [PubMed] [Google Scholar]
- 18.Reid I.R., Civitelli R., Halstead L.R., Avioli L.V., Hruska K.A. Parathyroid hormone acutely elevates intracellular calcium in osteoblastlike cells. American Journal of Physiology. 1987;253(1 Pt 1):E45–E51. doi: 10.1152/ajpendo.1987.253.1.E45. [DOI] [PubMed] [Google Scholar]
- 19.Cheloha R.W., Gellman S.H., Vilardaga J.P., Gardella T.J. PTH receptor-1 signalling-mechanistic insights and therapeutic prospects. Nature Reviews Endocrinology. 2015;11(12):712–724. doi: 10.1038/nrendo.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo J., Iida-Klein A., Huang X., Abou-Samra A.B., Segre G.V., Bringhurst F.R. Parathyroid hormone (PTH)/PTH-related peptide receptor density modulates activation of phospholipase C and phosphate transport by PTH in LLC-PK1 cells. Endocrinology. 1995;136(9):3884–3891. doi: 10.1210/endo.136.9.7649096. [DOI] [PubMed] [Google Scholar]
- 21.Xu T., Yang K., You H., Chen A., Wang J., Xu K., et al. Regulation of PTHrP expression by cyclic mechanical strain in postnatal growth plate chondrocytes. Bone. 2013;56(2):304–311. doi: 10.1016/j.bone.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Allan E.H., Hausler K.D., Wei T., Gooi J.H., Quinn J.M., Crimeen-Irwin B., et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. Journal of Bone and Mineral Research. 2008;23(8):1170–1181. doi: 10.1359/jbmr.080324. [DOI] [PubMed] [Google Scholar]
- 23.Qiu T., Crane J.L., Xie L., Xian L., Xie H., Cao X. IGF-I induced phosphorylation of PTH receptor enhances osteoblast to osteocyte transition. Bone Res. 2018;6:5. doi: 10.1038/s41413-017-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabbieti M.G., Agas D., Xiao L., Marchetti L., Coffin J.D., Doetschman T., et al. Endogenous FGF-2 is critically important in PTH anabolic effects on bone. Journal of Cellular Physiology. 2009;219(1):143–151. doi: 10.1002/jcp.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao L., Fei Y., Hurley M.M. FGF2 crosstalk with Wnt signaling in mediating the anabolic action of PTH on bone formation. BoneKEy Reports. 2018;9:136–144. doi: 10.1016/j.bonr.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wein M.N., Liang Y., Goransson O., Sundberg T.B., Wang J., Williams E.A., et al. SIKs control osteocyte responses to parathyroid hormone. Nature Communications. 2016;7:13176. doi: 10.1038/ncomms13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S., Yang L., He S., Yang J., Liu D., Bao Q., et al. Preactivation of beta-catenin in osteoblasts improves the osteoanabolic effect of PTH in type 1 diabetic mice. Journal of Cellular Physiology. 2020;235(2):1480–1493. doi: 10.1002/jcp.29068. [DOI] [PubMed] [Google Scholar]
- 28.Tian Y., Xu Y., Fu Q., He M. Parathyroid hormone regulates osteoblast differentiation in a Wnt/beta-catenin-dependent manner. Molecular and Cellular Biochemistry. 2011;355(1–2):211–216. doi: 10.1007/s11010-011-0856-8. [DOI] [PubMed] [Google Scholar]
- 29.Mohanakrishnan V., Balasubramanian A., Mahalingam G., Partridge N.C., Ramachandran I., Selvamurugan N. Parathyroid hormone-induced down-regulation of miR-532-5p for matrix metalloproteinase-13 expression in rat osteoblasts. Journal of Cellular Biochemistry. 2018;119(7):6181–6193. doi: 10.1002/jcb.26827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyman J.S., Lynch C.C., Perrien D.S., Thiolloy S., O'Quinn E.C., Patil C.A., et al. Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. Journal of Bone and Mineral Research. 2011;26(6):1252–1260. doi: 10.1002/jbmr.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leaffer D., Sweeney M., Kellerman L.A., Avnur Z., Krstenansky J.L., Vickery B.H., et al. Modulation of osteogenic cell ultrastructure by RS-23581, an analog of human parathyroid hormone (PTH)-related peptide-(1-34), and bovine PTH-(1-34) Endocrinology. 1995;136(8):3624–3631. doi: 10.1210/endo.136.8.7628402. [DOI] [PubMed] [Google Scholar]
- 32.Lee D.J., Southgate R.D., Farhat Y.M., Loiselle A.E., Hammert W.C., Awad H.A., et al. Parathyroid hormone 1-34 enhances extracellular matrix deposition and organization during flexor tendon repair. Journal of Orthopaedic Research. 2015;33(1):17–24. doi: 10.1002/jor.22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruse N.J., Bornstein P. The metabolic requirements for transcellular movement and secretion of collagen. Journal of Biological Chemistry. 1975;250(13):4841–4847. [PubMed] [Google Scholar]
- 34.Gonzales S., Wang C., Levene H., Cheung H.S., Huang C.C. ATP promotes extracellular matrix biosynthesis of intervertebral disc cells. Cell and Tissue Research. 2015;359(2):635–642. doi: 10.1007/s00441-014-2042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanotelli M.R., Goldblatt Z.E., Miller J.P., Bordeleau F., Li J., VanderBurgh J.A., et al. Regulation of ATP utilization during metastatic cell migration by collagen architecture. Molecular Biology of the Cell. 2018;29(1):1–9. doi: 10.1091/mbc.E17-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCormack J.G., Denton R.M. Role of calcium ions in the regulation of intramitochondrial metabolism. Properties of the Ca2+-sensitive dehydrogenases within intact uncoupled mitochondria from the white and brown adipose tissue of the rat. Biochemical Journal. 1980;190(1):95–105. doi: 10.1042/bj1900095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormack J.G., Halestrap A.P., Denton R.M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiological Reviews. 1990;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 38.Esen E., Lee S.Y., Wice B.M., Long F. PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. Journal of Bone and Mineral Research. 2015;30(11):1959–1968. doi: 10.1002/jbmr.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misra B.B., Jayapalan S., Richards A.K., Helderman R.C.M., Rendina-Ruedy E. Untargeted metabolomics in primary murine bone marrow stromal cells reveals distinct profile throughout osteoblast differentiation. Metabolomics. 2021;17(10):86. doi: 10.1007/s11306-021-01829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S.Y., Abel E.D., Long F. Glucose metabolism induced by Bmp signaling is essential for murine skeletal development. Nature Communications. 2018;9(1):4831. doi: 10.1038/s41467-018-07316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guntur A.R., Le P.T., Farber C.R., Rosen C.J. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology. 2014;155(5):1589–1595. doi: 10.1210/en.2013-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tencerova M., Rendina-Ruedy E., Neess D., Faergeman N., Figeac F., Ali D., et al. Metabolic programming determines the lineage-differentiation fate of murine bone marrow stromal progenitor cells. Bone Res. 2019;7:35. doi: 10.1038/s41413-019-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frey J.L., Li Z., Ellis J.M., Zhang Q., Farber C.R., Aja S., et al. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Molecular and Cellular Biology. 2015;35(11):1979–1991. doi: 10.1128/MCB.01343-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S.P., Li Z., Zoch M.L., Frey J.L., Bowman C.E., Kushwaha P., et al. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight. 2017;2(16) doi: 10.1172/jci.insight.92704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rendina-Ruedy E., Guntur A.R., Rosen C.J. Intracellular lipid droplets support osteoblast function. Adipocyte. 2017;6(3):250–258. doi: 10.1080/21623945.2017.1356505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phang J.M., Downing S.J. Amino acid transport in bone: stimulation by cyclic AMP. American Journal of Physiology. 1973;224(1):191–196. doi: 10.1152/ajplegacy.1973.224.1.191. [DOI] [PubMed] [Google Scholar]
- 47.Yee J.A. Effect of parathyroid hormone on amino acid transport by cultured neonatal mouse calvarial bone cells. Journal of Bone and Mineral Research. 1988;3(2):211–218. doi: 10.1002/jbmr.5650030214. [DOI] [PubMed] [Google Scholar]
- 48.Stegen S., Devignes C.S., Torrekens S., Van Looveren R., Carmeliet P., Carmeliet G. Glutamine metabolism in osteoprogenitors is required for bone mass accrual and PTH-induced bone anabolism in male mice. Journal of Bone and Mineral Research. 2021;36(3):604–616. doi: 10.1002/jbmr.4219. [DOI] [PubMed] [Google Scholar]
- 49.Karner C.M., Esen E., Okunade A.L., Patterson B.W., Long F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. Journal of Clinical Investigation. 2015;125(2):551–562. doi: 10.1172/JCI78470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanner J.J., Fendt S.M., Becker D.F. The proline cycle as a potential cancer therapy target. Biochemistry. 2018;57(25):3433–3444. doi: 10.1021/acs.biochem.8b00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hollinshead K.E.R., Munford H., Eales K.L., Bardella C., Li C., Escribano-Gonzalez C., et al. Oncogenic IDH1 mutations promote enhanced proline synthesis through PYCR1 to support the maintenance of mitochondrial redox homeostasis. Cell Rep. 2018;22(12):3107–3114. doi: 10.1016/j.celrep.2018.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Troyan M.B., Gilman V.R., Gay C.V. Mitochondrial membrane potential changes in osteoblasts treated with parathyroid hormone and estradiol. Experimental Cell Research. 1997;233(2):274–280. doi: 10.1006/excr.1997.3570. [DOI] [PubMed] [Google Scholar]
- 53.Boyman L., Karbowski M., Lederer W.J. Regulation of mitochondrial ATP production: Ca(2+) signaling and quality control. Trends in Molecular Medicine. 2020;26(1):21–39. doi: 10.1016/j.molmed.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo J., Liu M., Yang D., Bouxsein M.L., Thomas C.C., Schipani E., et al. Phospholipase C signaling via the parathyroid hormone (PTH)/PTH-related peptide receptor is essential for normal bone responses to PTH. Endocrinology. 2010;151(8):3502–3513. doi: 10.1210/en.2009-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.London E., Bloyd M., Stratakis C.A. PKA functions in metabolism and resistance to obesity: lessons from mouse and human studies. Journal of Endocrinology. 2020;246(3):R51–R64. doi: 10.1530/JOE-20-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hedesan O.C., Fenzl A., Digruber A., Spirk K., Baumgartner-Parzer S., Bilban M., et al. Parathyroid hormone induces a browning program in human white adipocytes. International Journal of Obesity. 2019;43(6):1319–1324. doi: 10.1038/s41366-018-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheon C.K. Association of obesity or overweight with bone health in childhood and adolescence: another health risk never to Be underestimated. Journal of Korean Medical Science. 2017;32(10):1561–1562. doi: 10.3346/jkms.2017.32.10.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lecka-Czernik B., Stechschulte L.A., Czernik P.J., Dowling A.R. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Molecular and Cellular Endocrinology. 2015;410:35–41. doi: 10.1016/j.mce.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Gerdhem P., Isaksson A., Akesson K., Obrant K.J. Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporosis International. 2005;16(12):1506–1512. doi: 10.1007/s00198-005-1877-5. [DOI] [PubMed] [Google Scholar]
- 60.Werner S., Low H. Stimulation of lipolysis and calcium accumulation by parathyroid hormone in rat adipose tissue in vitro after adrenalectomy and administration of high doses of cortisone acetate. Hormone and Metabolic Research. 1973;5(4):292–296. doi: 10.1055/s-0028-1093931. [DOI] [PubMed] [Google Scholar]
- 61.Sinha T.K., Thajchayapong P., Queener S.F., Allen D.O., Bell N.H. On the lipolytic action of parathyroid hormone in man. Metabolism. 1976;25(3):251–260. doi: 10.1016/0026-0495(76)90083-4. [DOI] [PubMed] [Google Scholar]
- 62.Taniguchi A., Kataoka K., Kono T., Oseko F., Okuda H., Nagata I., et al. Parathyroid hormone-induced lipolysis in human adipose tissue. The Journal of Lipid Research. 1987;28(5):490–494. [PubMed] [Google Scholar]
- 63.Larsson S., Jones H.A., Goransson O., Degerman E., Holm C. Parathyroid hormone induces adipocyte lipolysis via PKA-mediated phosphorylation of hormone-sensitive lipase. Cellular Signalling. 2016;28(3):204–213. doi: 10.1016/j.cellsig.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 64.Gunther C.W., Legowski P.A., Lyle R.M., Weaver C.M., McCabe L.D., McCabe G.P., et al. Parathyroid hormone is associated with decreased fat mass in young healthy women. International Journal of Obesity. 2006;30(1):94–99. doi: 10.1038/sj.ijo.0803066. [DOI] [PubMed] [Google Scholar]
- 65.Rendina-Ruedy E., Rosen C.J. Bone-fat interaction. Endocrinology and Metabolism Clinics of North America. 2017;46(1):41–50. doi: 10.1016/j.ecl.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rendina-Ruedy E., Rosen C.J. Lipids in the bone marrow: an evolving perspective. Cell Metabolism. 2020;31(2):219–231. doi: 10.1016/j.cmet.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Girousse A., Tavernier G., Valle C., Moro C., Mejhert N., Dinel A.L., et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol. 2013;11(2) doi: 10.1371/journal.pbio.1001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sinnott-Armstrong N., Sousa I.S., Laber S., Rendina-Ruedy E., Nitter Dankel S.E., Ferreira T., et al. A regulatory variant at 3q21.1 confers an increased pleiotropic risk for hyperglycemia and altered bone mineral density. Cell Metabolism. 2021 doi: 10.1016/j.cmet.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kir S., White J.P., Kleiner S., Kazak L., Cohen P., Baracos V.E., et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100–104. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kir S., Komaba H., Garcia A.P., Economopoulos K.P., Liu W., Lanske B., et al. PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metabolism. 2016;23(2):315–323. doi: 10.1016/j.cmet.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kovaničová Z., Kurdiová T., Baláž M., Štefanička P., Varga L., Kulterer O.C., et al. Cold exposure distinctively modulates parathyroid and thyroid hormones in cold-acclimatized and non-acclimatized humans. Endocrinology. 2020;161(7) doi: 10.1210/endocr/bqaa051. [DOI] [PubMed] [Google Scholar]
- 72.Gagnon A., Antunes T.T., Ly T., Pongsuwan P., Gavin C., Lochnan H.A., et al. Thyroid-stimulating hormone stimulates lipolysis in adipocytes in culture and raises serum free fatty acid levels in vivo. Metabolism. 2010;59(4):547–553. doi: 10.1016/j.metabol.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 73.Walczak K., Sieminska L. Obesity and thyroid Axis. International Journal of Environmental Research and Public Health. 2021;18(18) doi: 10.3390/ijerph18189434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergan-Roller H.E., Ickstadt A.T., Kittilson J.D., Sheridan M.A. Insulin and insulin-like growth factor-1 modulate the lipolytic action of growth hormone by altering signal pathway linkages. General and Comparative Endocrinology. 2017;248:40–48. doi: 10.1016/j.ygcen.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Kopchick J.J., Berryman D.E., Puri V., Lee K.Y., Jorgensen J.O.L. The effects of growth hormone on adipose tissue: old observations, new mechanisms. Nature Reviews Endocrinology. 2020;16(3):135–146. doi: 10.1038/s41574-019-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tritos N.A., Klibanski A. Effects of growth hormone on bone. Prog Mol Biol Transl Sci. 2016;138:193–211. doi: 10.1016/bs.pmbts.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 77.Zhang M., Xuan S., Bouxsein M.L., von Stechow D., Akeno N., Faugere M.C., et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. Journal of Biological Chemistry. 2002;277(46):44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 78.Lademann F., Tsourdi E., Hofbauer L.C., Rauner M. Thyroid hormone actions and bone remodeling - the role of the Wnt signaling pathway. Experimental and Clinical Endocrinology & Diabetes. 2020;128(6–07):450–454. doi: 10.1055/a-1088-1215. [DOI] [PubMed] [Google Scholar]
- 79.Longo M., Raciti G.A., Zatterale F., Parrillo L., Desiderio A., Spinelli R., et al. Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity. Diabetologia. 2018;61(2):369–380. doi: 10.1007/s00125-017-4471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quach J.M., Walker E.C., Allan E., Solano M., Yokoyama A., Kato S., et al. Zinc finger protein 467 is a novel regulator of osteoblast and adipocyte commitment. Journal of Biological Chemistry. 2011;286(6):4186–4198. doi: 10.1074/jbc.M110.178251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le P.T., Liu H., Alabdulaaly L., Vegting Y., Calle I.L., Gori F., et al. The role of Zfp467 in mediating the pro-osteogenic and anti-adipogenic effects on bone and bone marrow niche. Bone. 2021;144:115832. doi: 10.1016/j.bone.2020.115832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang S., Akerblad P., Kiviranta R., Gupta R.K., Kajimura S., Griffin M.J., et al. Regulation of early adipose commitment by Zfp521. PLoS Biol. 2012;10(11) doi: 10.1371/journal.pbio.1001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sebo Z.L., Rendina-Ruedy E., Ables G.P., Lindskog D.M., Rodeheffer M.S., Fazeli P.K., et al. Bone marrow adiposity: basic and clinical implications. Endocrine Reviews. 2019;40(5):1187–1206. doi: 10.1210/er.2018-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berry R., Rodeheffer M.S. Characterization of the adipocyte cellular lineage in vivo. Nature Cell Biology. 2013;15(3):302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanchez-Gurmaches J., Guertin D.A. Adipocyte lineages: tracing back the origins of fat. Biochimica et Biophysica Acta 1842. 2014;(3):340–351. doi: 10.1016/j.bbadis.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horowitz M.C., Berry R., Holtrup B., Sebo Z., Nelson T., Fretz J.A., et al. Bone marrow adipocytes, Adipocyte. 2017;6(3):193–204. doi: 10.1080/21623945.2017.1367881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhong L., Yao L., Tower R.J., Wei Y., Miao Z., Park J., et al. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. Elife. 2020;9 doi: 10.7554/eLife.54695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu W., Zhong L., Yao L., Wei Y., Gui T., Li Z., et al. Bone marrow adipogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. Journal of Clinical Investigation. 2021;131(2) doi: 10.1172/JCI140214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cuminetti V., Arranz L. Bone marrow adipocytes: the enigmatic components of the hematopoietic stem cell niche. Journal of Clinical Medicine. 2019;8(5) doi: 10.3390/jcm8050707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fan Y., Hanai J.I., Le P.T., Bi R., Maridas D., DeMambro V., et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metabolism. 2017;25(3):661–672. doi: 10.1016/j.cmet.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maridas D.E., Rendina-Ruedy E., Helderman R.C., DeMambro V.E., Brooks D., Guntur A.R., et al. Progenitor recruitment and adipogenic lipolysis contribute to the anabolic actions of parathyroid hormone on the skeleton. The FASEB Journal. 2019;33(2):2885–2898. doi: 10.1096/fj.201800948RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Satterwhite J., Heathman M., Miller P.D., Marín F., Glass E.V., Dobnig H. Pharmacokinetics of teriparatide (rhPTH[1-34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcified Tissue International. 2010;87(6):485–492. doi: 10.1007/s00223-010-9424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vall H., Parmar M. StatPearls Publishing LLC.; Treasure Island (FL): 2021. Teriparatide, StatPearls, StatPearls publishing copyright © 2021. [Google Scholar]
- 94.Lamprea-Montealegre J.A., Sharrett A.R., Matsushita K., Selvin E., Szklo M., Astor B.C. Chronic kidney disease, lipids and apolipoproteins, and coronary heart disease: the ARIC study. Atherosclerosis. 2014;234(1):42–46. doi: 10.1016/j.atherosclerosis.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 95.Rasmussen H., Shirasu H., Ogata E., Hawker C. Parathyroid hormone and mitochondrial metabolism. Specificity, sensitivity, and physiological correlates. Journal of Biological Chemistry. 1967;242(20):4669–4677. [PubMed] [Google Scholar]
- 96.Baczynski R., Massry S.G., Magott M., el-Belbessi S., Kohan R., Brautbar N. Effect of parathyroid hormone on energy metabolism of skeletal muscle. Kidney Int. 1985;28(5):722–727. doi: 10.1038/ki.1985.190. [DOI] [PubMed] [Google Scholar]
- 97.Baczynski R., Massry S.G., Kohan R., Magott M., Saglikes Y., Brautbar N. Effect of parathyroid hormone on myocardial energy metabolism in the rat. Kidney Int. 1985;27(5):718–725. doi: 10.1038/ki.1985.71. [DOI] [PubMed] [Google Scholar]