Abstract
Background
Adenosine is a purinergic signaling molecule with a wide range of physiological functions including anti- and pronociceptive properties. Adenosine receptors are expressed in the trigeminovascular system, and adenosine receptor antagonist, caffeine, relieves migraine headache. We performed a systematic review of the literature of preclinical data addressing the role of adenosine in migraine pathophysiology.
Methods
PubMed and EMBASE were searched for pre-clinical studies on the role of adenosine in migraine pathophysiology on September 5th, 2021.
Results
A total of 2510 studies were screened by title and abstract. Of these, thirteen pre-clinical studies evaluating adenosine, adenosine A1, A2A and A3 receptors were included.
These studies showed that adenosine signaling pathway is involved in controlling vascular tone. Furthermore, electrical stimulation of the trigeminal ganglion modulates the expression of adenosine A1 and A2A receptors in the trigeminal ganglion and trigeminal nucleus caudalis implicating adenosine signaling pathway in pain transmission.
Conclusion
Preclinical studies showed that adenosine has a dual effect on vasodilation and trigeminal pain pathway due to different receptor activation, suggesting a possible role of adenosine in migraine pathophysiology. Studies investigating pharmacological characteristics of subtypes of adenosine receptors are needed to further elucidate their role as a potential target for migraine treatment.
Keywords: Headache, Adenosine receptor, Pre-clinical, Pain
Introduction
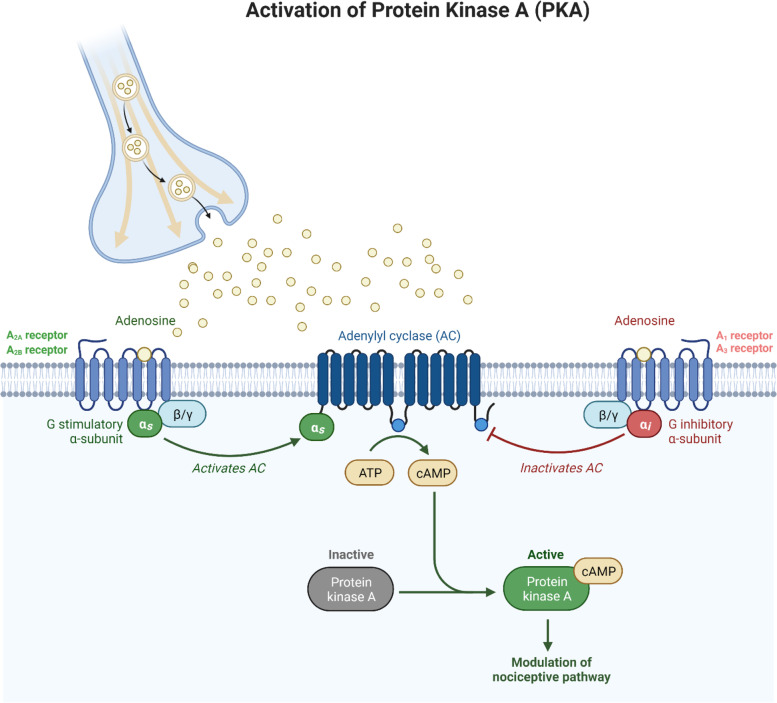
Adenosine, a vasoactive amine produced by the hydrolysis of adenosine monophosphate (AMP) or S-adenosylhomocysteine (SAH) [43], is involved in numerous physiological processes such as metabolism, inflammation, respiration and pain [39]. Adenosine binds to four G-protein coupled receptors (GPCR), A1, A2A, A2B and A3, with a unique profile of tissue distribution, signaling pathways and function (Table 1 & Fig. 1) [20, 21]. Adenosine receptors activate the mitogen-activated protein kinase (MAPK), leading to survival, cell growth, and differentiation [20], and modulate the activity of adenylate cyclase [11, 20], the enzyme that regulates intracellular concentration of cyclic adenosine monophosphate (cAMP) [38]. Adenosine A1 and A3 receptors are coupled to a Gαi subunit that downregulates cAMP by inhibiting adenylate cyclase [20, 38], while A2A and A2B receptors are coupled to a Gαs subunit which stimulates adenylate cyclase to upregulate cAMP [20, 38]. A1 and A3 receptors are considered to have anti-nociceptive effects, whereas, activation of A2A and A2B receptors induces nociception [11]. Adenosine A1 receptors are expressed at the trigeminovascular system (TVS), including the trigeminal ganglion (TG) and trigeminal nucleus caudalis (TNC) [6, 32], which is considered to be the anatomical and physiological substrate of migraine pain [2]. Stimulation of this receptor causes inhibition of the TVS by reducing neuronal firing from the trigeminal nucleus and decreasing the release of calcitonin gene-related peptide (CGRP) [6, 13]. A2A and A2B receptors are located in vascular smooth muscle cells [20, 38] and in pre- and postsynaptic nerve terminals [38], and stimulation of these receptors causes dural vasodilation [16], leading to stimulation of the TVS. Collectively, adenosine signaling pathways are complex and might be involved in headache and migraine pathophysiology.
Table 1.
Adenosine receptors and their functions
| Receptor | Subunit | Signaling pathway |
|---|---|---|
| Adenosine A1 receptor | Gαi-subunit | Inhibits adenylate cyclase, decreases cAMP formation [20, 38], leading to activation of KATP channels [4, 26, 35, 39] and inactivation of BKCa channels [28] |
| Gβγ subunits | Stimulates PLC and increases IP3 [20, 38] | |
| Adenosine A2A receptor | Gαs-subunit | Activates adenylate cyclase and increases cAMP formation [20, 38], leading to activation of KATP channels [27] |
| Pertussis toxin insensitive Gα15 and Gα16 proteins | Activates PLC and upregulates IP3 [20] | |
| Adenosine A2B receptor | Gαs-subunit | Stimulates adenylate cyclase, increases cAMP formation [38], leading to activation of KATP channels [27] |
| Gq subunit | Activates PLC and upregulates IP3 [38] | |
| Adenosine A3 receptor | Gαi -subunit | Inhibits adenylate cyclase and decreases cAMP [11, 20] |
| Gβγ subunits | Increases the activity of PLC and PLD [38] | |
| All | Modulates MAPK [20] |
cAMP cyclic adenosine monophosphate, IP3 inositol 1,4,5-triphosphate, MAPK mitogen-activated protein kinase, PLC phospholipase C, PLD phospholipase D
Fig. 1.
Adenosine signaling pathway. Adenosine binds to G-protein coupled receptors (GPCR) resulting in either activation (adenosine A2A and A2B receptors) or inactivation (adenosine A1 and A3 receptors) of adenylyl cyclase (AC). Activation of AC increases the formation of cyclic adenosine monophosphate (cAMP) which binds to protein kinase A (PKA). Active PKA then phosphorylates and thereby modulates cellular responses. ATP: adenosine triphosphate
Here, we systematically review preclinical studies on the involvement of adenosine in trigeminal pain pathway, to make the case that adenosine signaling pathways may play a role in migraine and discuss adenosine receptors as potential target for future treatment of migraine (Table 2).
Table 2.
Molecules presented here that target adenosine receptors. None are approved for treatment
| Name | Target | Clinical use |
|---|---|---|
| Adenosine receptor agonists | ||
| GR79236 | Highly potent and selective adenosine A1 receptor agonist [12] | Ischemic heart disease [3, 25], sleep apnea [5], modulation of lipolysis and insulin sensitivity [18] |
| GR79236X | Selective adenosine A1 receptor agonist [6] | Ischemic heart disease [44] and dental pain [41] |
| GR190178 | Low efficacy (partial) adenosine A1 receptor agonist [13] | Cluster headache [13] |
| CGS21680 | Selective adenosine A2A receptor agonist [6] | Huntington’s disease [7], spinal cord injury [36], bone regeneration [33] and overactive bladder [47] |
| 2-CI-IB-MECA | Selective adenosine A3 receptor agonist [6] | Atrial function [49], damage of the optic nerve and white matter ischemic damage [14] |
| Adenosine receptor antagonists | ||
| Caffeine | Non-selective adenosine A1 and A2A antagonist [34] | Treatment of headache, pain, apnea in premature children and neurodegenerative diseases [10] |
| DPCPX | Selective adenosine A1 receptor antagonist [6, 13] | Depression [42], cancer [8, 30, 50] and neuroprotection [37] |
| SCH58261 | Potent and highly selective A2A receptor antagonist [15] | Spinal cord injury [36], epilepsy [29] and preeclampsia [40] |
| JNJ-41942914, JNJ-39928122, JNJ-40529749, JNJ-40064440, and JNJ-41501798 | A2A receptor antagonists [16] | None |
Method
Data source
We conducted two searches on PubMed and Embase on September 5th, 2021. Firstly, we searched “(“adenosine”[MeSH Terms] OR “adenosine”[All Fields] OR “adenosin”[All Fields] OR “adenosine s”[All Fields] OR “adenosines”[All Fields]) AND (“migrain”[All Fields] OR “migraine disorders”[MeSH Terms] OR (“migraine”[All Fields] AND “disorders”[All Fields]) OR “migraine disorders”[All Fields] OR “migraine”[All Fields] OR “migraines”[All Fields] OR “migraine s”[All Fields] OR “migraineous”[All Fields] OR “migrainers”[All Fields] OR “migrainous”[All Fields])”. Secondly, we searched for “(“adenosine”[MeSH Terms] OR “adenosine”[All Fields] OR “adenosin”[All Fields] OR “adenosine s”[All Fields] OR “adenosines”[All Fields]) AND (“headache”[MeSH Terms] OR “headache”[All Fields] OR “headaches”[All Fields] OR “headache s”[All Fields])”. Both searches were restricted to English language.
Selection criteria and study inclusion
Studies were restricted to pre-clinical or clinical studies that investigated adenosine, adenosine agonist, adenosine antagonist, adenosine deaminase, adenosine deaminase inhibitor and adenosine reuptake inhibitors in headache and migraine pathophysiology. We excluded reviews, meta-analysis, conference proceedings and case reports.
Two investigators (J.T. and L.K.) screened all studies by title and abstract, followed by full text screening to confirm eligibility. References of the included studies were screened to find studies that were missed by the search. For each included study, both investigators (J.T. and L.K.) extracted hypothesis or purpose of the study, method, sample size, main outcome, conclusion, and limitations. Any disagreements were resolved through discussion by the two investigators (J.T. and L.K.). If the conflict remained, a third investigator (M.M.K) made the final decision.
Results
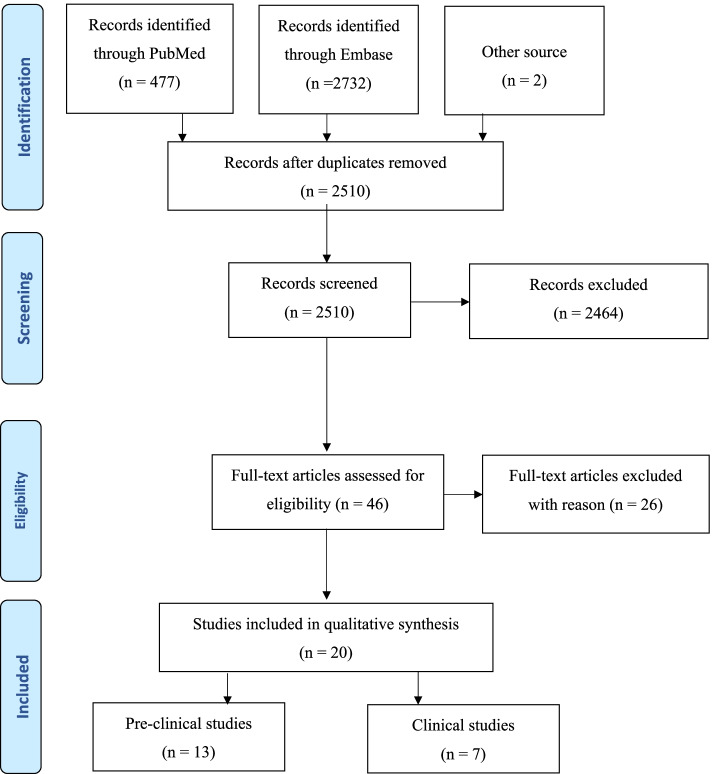
The database search identified 3209 citations of which 701 were duplicates (Fig. 2). An additional two studies were included through a manual search of identified primary articles. A total of 2510 studies were screened by title and abstract and 44 were full text screened. Of these, 20 studies were included – 13 preclinical (Table 3) and 7 clinical studies. Data for clinical studies has been published recently [45].
Fig. 2.
Flow chart of search strategy
Table 3.
Summary of preclinical studies
| Author | Purpose of the study | Study population (n) | Method | Main outcome(s) | Conclusion |
|---|---|---|---|---|---|
| Arulmani et al. [1] | To investigate the effects of GR79236 on capsaicin-induced carotid hemodynamic changes and plasma CGRP release | Pigs (15) | Capsaicin infusion after treatment with adenosine A1 receptor agonist, GR79236, or vehicle (saline) | GR79236 attenuated capsaicin-induced carotid hemodynamic changes dose-dependently, but not CGRP release | GR79236 might have potential as migraine treatment because of its vasoconstrictive effects rather than its inhibition of trigeminal CGRP release |
| Carruthers et al. [6] | To investigate the mechanisms of CGRP release and its potential modulation by adenosine agonists | Rats (n = NR) |
1) Immunocytochemistry and western blotting 2) In vitro application of forskolin and adenosine A1 receptor agonist (GR79236X), adenosine A1 receptor antagonist (DPCPX), adenosine A2A agonist (CGS21680) or A3 receptor agonist (2-CI-IB-MECA) to trigeminal ganglion neuron cultures. CGRP was measured using ELISA |
1) Adenosine A1 receptor was expressed in the trigeminal ganglion 2) GR79236X inhibited forskolin stimulated CGRP release 3) DPCPX abolished inhibition of CGRP release mediated by GR79236X 4) CGS21680 and 2-CI-IB-MECA did not have effect on forskolin-induced CGRP secretion |
A1 adenosine receptors on CGRP-positive neurons can inhibit cAMP induced CGRP release from trigeminal neurons. Adenosine receptors has significant potential for development as therapeutic targets for pain |
| Faraci et al. [9] | To investigate the vascular responses of the dura mater to adenosine | Dogs (n = 9)a | Intravenous infusion of 5 µM/kg/min adenosine while measuring blood flow with labelled, radioactive microspheres |
Adenosine infusion showed 1) aortic pressure decreased 2) vascular resistance in the dura decreased 3) blood flow to the dura increased 4) no effect on cerebral blood flow |
Adenosine did not affect cerebral blood flow but increased dural blood flow Vasomotor responses of the dural circulation might contribute to some forms of vascular headache |
| Goadsby et al. [13] | To investigate the role of adenosine A1 receptors in an animal model of nociceptive activation of the trigeminovascular system | Cats (n = 14) |
Blood samples were taken at: baseline, after electrical stimulation of superior sagittal sinus (SSS), after electrical stimulation and GR79236 or GR190178. A single dose of DPCPX was administered after highest dose of GR79236 |
1) GR79236 and GR190178 had a dose-dependent inhibitory effect on SSS stimulation-induced trigeminovascular activation in trigeminal nucleus caudalis (TNC) 2) GR79236 and GR190178 inhibited SSS stimulation-induced CGRP release in cranial circulation 3) DPCPX reversed the inhibitory effect of GR79236 on the trigeminal nucleus |
Activation of adenosine A1 receptor causes neuronal inhibition without concurrent vasoconstriction, proposing a novel avenue for treating migraine and cluster headache |
| Haanes et al. [15] | To investigate the vasomotor effects of purinergic receptor in the middle meningeal artery (MMA), using functional myograph with natural and designed agonists | Rats (n = NR) |
1) In vitro application of adenosine, caffeine and a A2A receptor antagonist, SCH58261 2) mRNA expression was measured using PCR |
1) Adenosine caused vasodilation in pre-contracted MMA segments 2) Caffeine blocked adenosine-induced vasodilation and caused adenosine to elicit a contraction 3) SCH58261 partly mimic the effect of caffeine 4) All purinergic receptor mRNAs were present in the trigeminal ganglion at same base pair size as for MMA |
Purinergic receptors might partly regulated blood flow through the MMA. Adenosine mainly binds to A2A receptors, the strongest expressed adenosine receptor, to cause relaxation of MMAs. The relaxation is inhibited by SCH58261. Similar response is seen when adding physiological caffein concentrations (50 µM). This gives a putative molecular explanation for benefit and use of coffee/caffeine as a MMA vasoconstrictor potentially related to sensation of cranial pain |
| Haanes et al. [16] | To investigate the effects of five novel adenosine A2A receptor antagonists on the vasodilation of the middle meningeal artery produced by an adenosine A2 receptor agonist (CGS21680) or endogenous CGRP | Rats (n = 57) |
1) Intravenous adenosine and caffeine 2) Periarterial electrical stimulation or intravenous CGS21680 followed by either i.v. bolus injections of JNJ-41942914, JNJ-39928122, JNJ-40529749, JNJ-40064440 and JNJ-41501798 Diameter was captured through closed cranial window |
1) Adenosine caused dural arterial dilation and decreased blood pressure, which was inhibited by pre-treatment with caffeine 2) Caffeine increased blood pressure 3) JNJ antagonists did not affect electrical stimulated neurogenic vasodilation 4) CGS21680 caused dural arterial dilation and decrease in blood pressure 5) All JNJ-adenosine A2A receptor antagonists blocked CGS21680-induced dural vasodilation with a more potent respond with A2A over A1 selectivity |
Selective A2A receptor antagonists may offer a novel approach to antimigraine therapy that still needs to be determined |
| Hardebo et al. [17] | To investigate whether adenosine and closely related adenine compounds (AMP, cyclic AMP, ADP, and ATP) may cause a sufficiently high degree of vasodilatation in vitro to account for a possible involvement in initiating the vasodilatory phase of a migraine attack | Cats (n = 24) & Humans (n = 3) |
In vitro application of adenosine, cAMP, ADP, and ATP to arteries (MCA, lingual and external maxillary artery), followed by measurement of tension and dilatory response |
All adenine compounds caused 1) dilation of pial arteries before and after application of prostaglandin F2a (PGF2a) 2) did not affect the extracranial arteries in both cats and humans 3) a less pronounced dilation of pial arteries when extracellular K+ concentration was increased |
Adenine compounds might initiate the dilatory phase in an attack or reactive hyperemia in intracranial circulation since a marked dilated of intracranial dilation was caused by these compounds |
| Honey et al. [19] | To investigate the effect of a selective adenosine A1 receptor agonist (GR79236) on neurogenic dural blood vessel dilation in anaesthetized rats | Rats (n = NR) | Electrically or CGRP evoked dural vasodilatation after treatment with an adenosine A1 receptor agonist, GR79236, or both GR79236 and an adenosine A1 receptor antagonist (DPCPX) through a cranial window |
1) GR79236 dose-dependently inhibited electrically induced neurogenic vasodilation 2) DPCPX reversed the inhibitory effect of GR79236 on electrically evoked vasodilation 3) GR79236 did not inhibit CGRP induced vasodilation |
It is possible that A1 agonists might be clinically effective in migraine because of an inhibitory effect both in the brain and periphery. This mechanism might offer a novel approach to migraine therapy |
| Jenkins et al. [22] | To investigate the receptors and mechanisms involved in prostanoid-induced CGRP release in cultured rat trigeminal neurons | Rats (n = NR) | In vitro application of adenosine deaminase to trigeminal neuronal culture | Adenosine deaminase did not alter baseline CGRP level nor CGRP release evoked by 1 µM PGE2 | Not reported regarding adenosine |
| Lindquist et al. [31] | To investigate whether metabolic status could modulate adenosine accumulation in brain slices exposed to spreading depolarization (SD), and compare SD-associated adenosine release in vivo, under healthy, hypoglycemic, and ischemic conditions | Mice (n = NR) | Coronal slices were prepared at 250 μm, 350 μm, and 450 μm thicknesses. Adenosine measurements were done with amperometric recordings in brain slices in vivo |
1) SD caused adenosine accumulation in vivo 2) Adenosine signals triggered by SD could reliably report underlying metabolic status in brain slices |
Adenosine or adenosine derivates might be useful as biomarkers of SD incidence in different clinical conditions |
| Lu et al. [32] | To investigate whether CGRP, A2AR and A1R are involved in migraine pain information transmission in the electrical stimulation of the trigeminal ganglion (ESTG) in migraine rat model and exploring the mechanisms of Tianshi capsule (TSC) as migraine treatment | Male rats (n = 40) | ESTG for 30 min. in one group, sham-operation without ESTG in another group and Tianshu capsule (TSC) followed by electrical stimulation of TG in the last group. The TNC and ipsilateral TG were removed for western blot analysis or RT-qPCR to evaluate CGRP, A1R and A2AR expression |
1) Electrical stimulation increased CGRP and A2AR expression, and decreased A1R expression in the TNC and ipsilateral TG compared with the blank groups and sham-operated groups 2) Treatment with TSC caused: - decreased CGRP and A2AR expression, - increased A1R expression in the TNC and ipsilateral TG compared to ESTG group |
CGRP, A1R and A2AR mediates pain transmission and regulates the process during migraine. TSC regulates the expression of the three proteins |
| Wei et al. [46] | To investigate the possibility that nitric oxide donors, nitroglycerin and/or sodium nitroprusside activate trigeminovascular fibers by promoting neuropeptide release and vasodilation within the pial vasculature. Additionally, it was examined whether LY83583, a drug that lowers cyclic GMP, blocks the relaxation mediated by the topical application of the released neuropeptide CGRP or by sodium nitroprusside | Cats (n = 10)a |
1) Application of adenosine before and after the CGRP antagonist (CGRP (8–37)) 2) Application of adenosine before and after application of guanylate cyclase inhibitor (LY83583) Cranial window over the parietal cortex was used to observe arteries |
1) CGRP-induced dilation was completely blocked by the CGRP antagonist, but the adenosine-induced vasodilation was not affected by the CGRP antagonist 2) Adenosine-induced vasodilation was not affected by guanylate cyclase inhibitor |
Not reported regarding adenosine |
| Yegutkin et al. [48] | To investigate pro-nociceptive effects of adenine nucleotides in control and in migraine-like conditions modeled with the neuropeptide CGRP | Male rats (n = NR) |
1) Bioluminescent and fluorometric techniques to measure purine levels in trigeminal ganglion cells, before and after pre-treatment with CGRP 2) Electro-physiological recordings of nociceptive spikes in trigeminal nerves in meningeal tissues |
1) Basal ATP and ADP levels in trigeminal cultures were maintained at very low level, meanwhile basal adenosine and AMP levels were almost one-two orders higher. CGRP pretreatment led to decreased adenosine levels by ∼ 50% in trigeminal cultures, but no changes in CGRP-treated meninges 2) Adenosine could not activate nociceptive firing in meningeal nerves |
Data are consistent with the purinergic hypothesis of migraine and proposes new targets against trigeminal pain |
A1R A1 receptor, A2AR A2A receptor, ADP adenosine 5’-diphosphate, AMP adenosine monophosphate, ATP adenosine triphosphate, cAMP cyclic adenosine 3’-5’-cyclic monophosphate, CGRP calcitonin gen-related peptide, ESTG electrical stimulation of the trigeminal ganglion, MMA meningeal media artery, mRNA messenger ribonucleic acid, NR. not reported, PCR polymerase chain reaction, PGE2 prostaglandin E2, PGF2a prostaglandin F2a, SD spreading depressing, SSS superior sagittal sinus, TG trigeminal ganglion, TNC trigeminal nucleus caudalis, TSC Tianshu capsule, anumber of subjects exposed to adenosine related substances
Narrative summaries
Arulmani et al. [1]. In pigs, intravenous infusion of adenosine A1 receptor agonist, GR79236, was compared to vehicle prior to capsaicin infusion. Total carotid blood flow, conductance, and plasma CGRP concentrations in jugular vein were assessed at baseline and after the infusions. GR79236 dose-dependently attenuated the capsaicin-induced carotid hemodynamic changes but not the CGRP release, compared to vehicle infusion.
Carruthers et al. [6]. In rats, adenosine agonists and antagonist (A1 receptor agonist GR79236X, adenosine A1 receptor antagonist, DPCPX, adenosine A2A agonist, CGS21680, and A3 receptor agonist, 2-CI-IB-MECA) were applied to cultured trigeminal neurons in combination with forskolin or vehicle to induce release CGRP. Immunocytochemical studies and Western analysis assessed whether these pharmacological agents could modulate the forskolin induced CGRP release. GR79236X concentration-dependently inhibited forskolin-stimulated CGRP release, while DPCPX abolished GR79236X´s effect. CGS21680 and 2-CI-IB-MECA were unable to attenuate forskolin-induced CGRP secretion.
Faraci et al. [9]. Intravenous infusion of adenosine was administered to anesthetized dogs. Blood flow was measured with labelled, radioactive microspheres. Adenosine decreased aortic pressure along with blood flow and vascular resistance in the dura. Adenosine infusion did not alter cerebral blood flow.
Goadsby et al. [13]. Intravenous infusion of adenosine A1 receptor agonists were administered in anesthetized dogs, following electrical stimulation of the superior sagittal sinus (SSS). Jugular vein blood samples were taken at baseline, immediately after the SSS stimulation and following the A1 agonist infusion, for detection of CGRP levels. Both A1 receptor agonists, GR79236 and GR190178, inhibited SSS-induced activation in TNC and CGRP release in cranial circulation, in a dose-dependent manner. Moreover, adenosine A1 receptor antagonist, DPCPX, was able to reverse GR79236's inhibitory effect on TNC activation.
Haanes et al. [15]. Adenosine was applied to pre-contracted middle meningeal artery (MMA) segments isolated from rats. RT-PCR was used to characterize the expression of purinergic receptor and myography to access the vascular effects. Notably, all purinergic receptor mRNAs were detected in the trigeminal ganglion and MMA. Adenosine caused dilation of MMA, which was reversed by SCH58261 (A2A receptor antagonist) and caffeine (adenosine receptor antagonist).
Haanes et al. [16]. Adenosine and caffeine were administered intravenously to six rats. Adenosine resulted in dural vasodilation and decrease in blood pressure. However, pre-treatment with caffeine inhibited adenosine´s effect. Caffeine caused an increase in blood pressure and a non-significant dilation of dural arteries. Secondly, intravenous infusions of different adenosine A2A receptor antagonists (JNJ compounds) were given following intravenous administration of adenosine A2A receptor agonist, CGS21680, or periarterial electrical stimulation (mode of CGRP-release), in rats. The closed cranial window was used to evaluate the antagonists´ effect on the CGS21680 and CGRP -induced dural dilation. CGS21680 caused vasodilation and decrease in arterial blood pressure. All A2A receptor antagonists blocked CGS21680-induced dural vasodilation with a more potent respond with A2A over A1 selectivity, while they did not affect electrical stimulated neurogenic vasodilation.
Hardebo et al. [17]. Adenosine, cAMP, ADP and ATP were applied in segments of middle cerebral artery and extracranial arteries of feline and humans. The dissected vessels were pre-constricted by prostaglandin F2a (PGF2a) or 5-hydroxytryptamine (5-HT). The tension was measured with force displacement transducers and recorded on a Grass polygraph. All adenine compounds dilated feline pial arteries, however the dilatory response was less pronounced when extracellular K+ concentration increased. Adenine compounds did not influence the diameter of human and feline extracranial arteries.
Honey et al. [19]. In rats, intravenous infusion of adenosine A1-receptor agonist, GR79236, was compared to saline infusion in models of neurogenic dural vasodilation. Vasodilation was induced by either electrical stimulation of perivascular trigeminal nerves or intravenous CGRP. Cranial window was used to evaluate the vascular responses. GR79236 inhibited electrically induced neurogenic vasodilation in a dose-dependent manner but had no effect on vasodilation caused by CGRP. Selective A1 receptor antagonist, DPCPX, inhibited the effect of GR79236 on electrically evoked vasodilation, compared to vehicle.
Jenkins et al. [22]. Adenosine deaminase was applied in cultured rat trigeminal neurons. Application of prostaglandin E2 (PGE2) led to CGRP release from the cultured cells. Adenosine deaminase did not alter baseline or PGE2-evoked CGRP levels.
Lindquist et al. [31]. Accumulation of adenosine following spreading depolarization (SD) was investigated in brain slices of mice and in vivo. Amperometric recordings from adenosine-sensitive enzyme-linked electrochemical were made in brain slices and applied in vivo. SD generated transient adenosine accumulation in vivo which could reliably report underlying metabolic status in brain slices.
Lu et al. [32]. Εlectrical stimulation of the trigeminal ganglion (ESTG) or sham operation was performed in rats to investigate its effect on CGRP, adenosine A1 receptor and adenosine A2A receptor expression. RT-qPCR and Western analysis was used for detection and quantification of the proteins. In the trigeminal nucleus caudalis (TNC) and ipsilateral trigeminal ganglion (TG), CGRP and A2A expression increased following ESTG, while A1 decreased. Interestingly, pretreatment with hinese medicine Tianshu capsule (TSC) decreased CGRP and A2A expression and increased A1 receptor expression.
Wei et al. [46]. In cats, the effect of topical application of adenosine and adenosine diphosphate was investigated before and following application of CGRP receptor antagonist, CGRP8-37. Cranial window was used to evaluate the vascular responses. CGRP8-37 was not able to reverse vasodilating effect of adenosine and adenosine diphosphate. Moreover, guanylate cyclase inhibitor, LY83583, had no effect on adenosine-induced vasodilation.
Yegutkin et al. [48]. The effects of adenine nucleotides were assessed in meninges of rats and cultured trigeminal cells following application of CGRP or placebo. Bioluminescent and fluorometric techniques were used to measure purine levels in trigeminal ganglion cells, and electrophysiology to record the nociceptive spikes in the meningeal trigeminal nerves, before and after pre-treatment with CGRP. CGRP decreased adenosine levels in cultured cells but not in the meninges, while adenosine was not able to activate nociceptive firing in the meningeal nerves. Moreover, basal levels of adenosine and AMP where higher compared to ATP and ADP in trigeminal cells.
Discussion
The main findings of the present systematic review are that adenosine receptors modulate pain transmission through the TVS. While A1 receptor has an inhibitory effect, stimulation of A2A receptor causes vasodilation and activation of trigeminal pain pathway.
In rats, electrical stimulation of the TG decreased adenosine A1 receptor expression and increased adenosine A2A receptor expression in ipsilateral TG and TNC [32]. The former is suggested to be involved in migraine attack initiation, while upregulation of adenosine A1 receptors or activation of this receptor might block migraine attacks [32]. In support, one study found that treatment with adenosine A1 receptor agonists, GR79236 and GR190178, inhibited TVS activation after electrical stimulation of the superior sagittal sinus in cats [13]. Upregulation of both adenosine A2A and CGRP receptors following electrical stimulation implies that when combined, the two receptors activate trigeminal pain transmission and cause migraine [32]. Overall, these findings implicate both adenosine A1 and A2A receptors in pain regulation and transmission during migraine [13, 32].
Three studies showed that adenosine caused prominent dilation of pre-contracted middle meningeal artery, dural and pial arteries in vitro [15–17]. Another study showed that adenosine caused dural vasodilation in dogs [9]. Pretreatment with caffeine or adenosine A2A receptor antagonist, SCH58261, was able to block adenosine-induced dilation in vitro [15, 16], suggesting that adenosine-induced vasodilation might be mainly dependent on adenosine A2A receptor [15].
Adenosine A1 receptor agonist, GR79236, inhibited electrically-induced vasodilation and capsaicin-induced hemodynamic changes in carotid artery [1, 19]. Pretreatment with DPCPX prevented the inhibition following GR79236, indicating that its inhibitory or vasoconstricting effect is mediated through adenosine A1 receptor [19]. The same agonist, GR79236, inhibited CGRP release induced by adenylate cyclase activator, forskolin [6] without any effect on CGRP-induced vasodilation in rats [19]. These data indicate that GR79236 inhibits CGRP release via a pre-junctional inhibition, and that adenosine A1 receptors are present on CGRP-positive neurons [6, 13, 19]. Together with its vasoconstricting ability, it is suggested that GR79236 and adenosine A1 receptors hold anti-migraine potential [1, 6, 19].
In contrast to GR79236, adenosine A2A receptor agonist, CGS21680, had no effect on forskolin-induced CGRP release [6]. However, CGS21680 caused dural vasodilation that was blocked by adenosine A2A receptor antagonists (JNJ-compounds) [16]. The study showed that the lower the selectivity for A2A receptor over A1 receptors, the higher the potential to attenuate the CGS21680 induced vasodilation. It was suggested that blocking both A1 and A2A receptors might be necessary to completely attenuate dural vasodilation [16].
Of note, 2-CI-IB-MECA, an adenosine A3 receptor agonist, had no effect on forskolin-induced CGRP secretion in rats [6]. The involvement of adenosine A3 receptor in migraine has not been further investigated, however, adenosine A3 receptor agonist exhibited anti-nociceptive properties in models of chronic pain in rats and mice [24].
While CGRP antagonist, CGRP(8–37), and guanylate cyclase inhibitor, LY83583, inhibited CGRP and nitroglycerine induced vasodilation, both compounds did not alter adenosine-induced vasodilation [46]. This finding demonstrates that adenosine induced dilation is not dependent on activation of CGRP receptors or an increase in cyclic guanosine monophosphate. Another study showed that pretreatment of trigeminal ganglion cells with CGRP is followed by decreased adenosine levels compared to baseline [48]. It is suggested that the finding might be a part of migraine sensitization but due to other contradicting findings (i.e., no change in nociceptive firing), further investigation on adenosine’s mechanisms was recommended [48].
Collectively, studies showed adenosine receptors expression in the trigeminal pain pathway and indicated that adenosine-induced pronociceptive effect is mediated through A2A receptor activation, whilst A1 receptor mediates antinociception. A1 receptor agonist, GR79236, inhibits activation of the TVS and vasodilation, while A2A receptor antagonist, SCH58261, attenuated adenosine-induced vasodilation [13, 15, 19], designating adenosine A1 and A2A receptors as possible targets in the treatment of migraine.
Limitations and future perspective
The major limitations of the studies included, were differences in methodological approaches including designs, subjects, substances, and sampling sources. Additionally, concentrations and types of adenosine A1 receptor agonists applied, differed across the studies [6, 13, 19]. Different CGRP releasing mechanisms were applied throughout the studies, potentially affecting the potency of adenosine receptor agonists and antagonists in modulating the CGRP release [1, 6, 13, 22].
Human studies are needed to elucidate the headache inducing effect of adenosine in patients with migraine. A specific focus on adenosine A1 receptor agonists and A2A receptors antagonists would be of great interest because of their potentially opposite effects based on current knowledge. Several adenosine receptor agonists and antagonist are currently available for research purpose only, while only one adenosine receptor antagonist, istradefylline, is currently U.S. Food and Drug Administration (FDA) approved as treatment for Parkinson’s disease (JF and RA 2020). To our best knowledge, no studies have been conducted on the adenosine A2B receptor in migraine and adenosine A3 has only once been investigated in migraine [6]. This leaves a huge gap in our knowledge that needs to be explored in both clinical and pre-clinical setting.
Conclusion
Preclinical data demonstrated that adenosine caused vasodilation and modulated CGRP release. We suggest that the adenosine A1 receptor and adenosine A2A receptor could be potential targets for migraine treatment.
Acknowledgements
Not applicable.
Abbreviations
- A1R
A1 receptor
- A2AR
A2A receptor
- AC
Adenylyl cyclase
- AMP
Adenosine monophosphate
- ADP
Adenosine 5’-diphosphate
- ATP
Adenosine triphosphate
- cAMP
Cyclic adenosine monophosphate
- CGRP
Calcitonin gene related peptide
- DAG
Diacylglycerol
- ES
Electrical stimulation
- ESTG
Electrical stimulation of the trigeminal ganglion
- FDA
U.S. Food and Drug Administration
- GPCR
G-protein coupled receptors
- ICHD
International Classification of Headache Disorders
- IP3
Inositol 1,4,5-triphosphate
- MAPK
Mitogen-activated protein kinase
- MMA
Meningeal media artery
- MO
Migraine without aura
- mRNA
Messenger RNA
- NR
Not reported
- PKA
Protein kinase A
- PCR
Polymerase chain reaction
- PGE2
Prostaglandin E2
- PGF2a
Prostaglandin F2a
- PIP2
Phosphatidylinositol 4,5-biphosphate
- PLC
Phospholipase C
- PLD
Phospholipase D
- SAH
S-adenosylhomocysteine
- SD
Spreading depression
- TG
Trigeminal ganglion
- TNC
Trigeminal nucleus caudalis
- TSC
Tianshu capsule
- TVS
Trigeminovascular system
Authors’ contributions
JT did the search, screening of articles, data extraction and drafted first manuscript. LK did the screening of articles and data extraction. MMK and MA initiated, designed, supervised, and revised the paper. All authors reviewed and approved the final version.
Funding
M.A. was supported by the Lundbeck Foundation Professor Grant (R310-2018–3711).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JT, LK and MMK report no conflict of interest. MA has received consulting fees and honoraria for lectures/presentations from AbbVie, Allergan, Amgen, Eli Lily, Lundbeck, Novartis and Teva. MA has also received personal payments for participating on data Safety Monitoring Board or Advisory Board for AbbVie, Amgen, Eli Lily, Lundbeck and Novartis.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Janu Thuraiaiyah, Email: janu_thurai@hotmail.com.
Lili Kokoti, Email: lily.kokoti@gmail.com.
Mohammad Al-Mahdi Al-Karagholi, Email: mahdi.alkaragholi@gmail.com.
Messoud Ashina, Email: ashina@dadlnet.dk.
References
- 1.Arulmani U, Heiligers JPC, Centurión D, et al. Lack of effect of the adenosine A1 receptor agonist, GR79236, on capsaicin-induced CGRP release in anaesthetized pigs. Cephalalgia. 2005 doi: 10.1111/j.1468-2982.2005.00967.x. [DOI] [PubMed] [Google Scholar]
- 2.Ashina M. Migraine. N Engl J Med. 2020;383:1866–1876. doi: 10.1056/NEJMra1915327. [DOI] [PubMed] [Google Scholar]
- 3.Baxter GF, Hale SL, Miki T, et al. Adenosine A1 agonist at reperfusion trial (AART): Results of a three-center, blinded, randomized, controlled experimental infarct study. Cardiovasc Drugs Ther. 2000;14:607–614. doi: 10.1023/A:1007850527878. [DOI] [PubMed] [Google Scholar]
- 4.Borea PA, Gessi S, Merighi S, et al. Pharmacology of Adenosine Receptors: The State of the Art. Physiol Rev. 2018;98:1591–1625. doi: 10.1152/physrev.00049.2017.-Adenosine. [DOI] [PubMed] [Google Scholar]
- 5.Carley DW, Hagan RM, Sheehan M, et al. Adenosine A1 receptor agonist GR79236 suppresses apnea during all sleep stages in the rat. Sleep. 1997;20:1093–1098. doi: 10.1093/sleep/20.12.1093. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers AM, Sellers LA, Jenkins DW, et al. Adenosine A1 receptor-mediated inhibition of protein kinase A-induced calcitonin gene-related peptide release from rat trigeminal neurons. Mol Pharmacol. 2001;59:1533–1541. doi: 10.1124/mol.59.6.1533. [DOI] [PubMed] [Google Scholar]
- 7.Chou SY, Lee YC, Chen HM, et al. CGS21680 attenuates symptoms of Huntington’s disease in a transgenic mouse model. J Neurochem. 2005;93:310–320. doi: 10.1111/j.1471-4159.2005.03029.x. [DOI] [PubMed] [Google Scholar]
- 8.Dastjerdi MN, Rarani MZ, Valiani A, Mahmoudieh M. The effect of adenosine A1 receptor agonist and antagonist on p53 and caspase 3, 8, and 9 expression and apoptosis rate in MCF-7 breast cancer cell line. Res Pharm Sci. 2016;11:303–310. doi: 10.4103/1735-5362.189301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faraci FM, Kadel KA, Heistad DD. Vascular responses of dura mater. Am J Physiol - Hear Circ Physiol. 1989;257:H157–61. doi: 10.1152/ajpheart.1989.257.1.h157. [DOI] [PubMed] [Google Scholar]
- 10.Faudone G, Arifi S, Merk D. The Medicinal Chemistry of Caffeine. J Med Chem. 2021;64:7156–7178. doi: 10.1021/ACS.JMEDCHEM.1C00261. [DOI] [PubMed] [Google Scholar]
- 11.Fried NT, Elliott MB, Oshinsky ML. The Role of Adenosine Signaling in Headache: A Review. Brain Sci. 2017;7:30. doi: 10.3390/brainsci7030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giffin NJ, Kowacs F, Libri V, et al. Effect of the adenosine A1 receptor agonist GR79236 on trigeminal nociception with blink reflex recordings in healthy human subjects. Cephalalgia. 2003;23:287–292. doi: 10.1046/j.1468-2982.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- 13.Goadsby PJ, Hoskin KL, Storer RJ, et al. Adenosine A1 receptor agonists inhibit trigeminovascular nociceptive transmission. Brain. 2002;125:1392–1401. doi: 10.1093/brain/awf141. [DOI] [PubMed] [Google Scholar]
- 14.González-Fernández E, Sánchez-Gómez MV, Pérez-Samartín A, et al. A3 Adenosine receptors mediate oligodendrocyte death and ischemic damage to optic nerve. Glia. 2014;62:199–216. doi: 10.1002/glia.22599. [DOI] [PubMed] [Google Scholar]
- 15.Haanes KA, Edvinsson L (2014) Expression and characterization of purinergic receptors in rat middle meningeal artery-potential role in migraine. PLoS One 9(9):e108782–e108782. 10.1371/journal.pone.0108782 [DOI] [PMC free article] [PubMed]
- 16.Haanes KA, Labastida-Ramírez A, Chan KY, et al. Characterization of the trigeminovascular actions of several adenosine A2A receptor antagonists in an in vivo rat model of migraine. J Headache Pain. 2018;19:1–10. doi: 10.1186/s10194-018-0867-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardebo JE, Edvinsson L. Adenine compounds: Cerebrovascular effects In Vitro with reference to their possible involvement in migraine. Stroke. 1979 doi: 10.1161/01.STR.10.1.58. [DOI] [PubMed] [Google Scholar]
- 18.Heseltine L, Webster JM, Taylor R. Adenosine effects upon insulin action on lipolysis and glucose transport in human adipocytes. Mol Cell Biochem. 1995;144:147–151. doi: 10.1007/BF00944394. [DOI] [PubMed] [Google Scholar]
- 19.Honey AC, Bland-Ward PA, Connor HE, et al. Study of an adenosine A1 receptor agonist on trigeminally evoked dural blood vessel dilation in the anaesthetized rat. Cephalalgia. 2002;22:260–264. doi: 10.1046/j.1468-2982.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson KA, Tosh DK, Jain S, Gao ZG. Historical and current adenosine receptor agonists in preclinical and clinical development. Front Cell Neurosci. 2019;13:1–17. doi: 10.3389/fncel.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins DW, Feniuk W, Humphrey PPA. Characterization of the prostanoid receptor types involved in mediating calcitonin gene-related peptide release from cultured rat trigeminal neurones. Br J Pharmacol. 2001;134:1296–1302. doi: 10.1038/sj.bjp.0704357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JF, Cunha RA. The belated US FDA approval of the adenosine A 2A receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signal. 2020;16:167–174. doi: 10.1007/S11302-020-09694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little JW, Ford A, Symons-Liguori AM, et al. Endogenous adenosine A3 receptor activation selectively alleviates persistent pain states. Brain. 2015;138:28–35. doi: 10.1093/BRAIN/AWU330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelion AD, Webb TP, Gardner MA, et al. Does a selective adenosine A1 receptor agonist protect against exercise induced ischaemia in patients with coronary artery disease? Heart. 2002;87:115–120. doi: 10.1136/heart.87.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirsch GE, Codina J, Birnbaumer L, Brown AM. Coupling of ATP-sensitive K+ channels to A1 receptors by G proteins in rat ventricular myocytes. 1990. [DOI] [PubMed] [Google Scholar]
- 27.Kleppisch T, Nelson MT. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase (vasodilation/glibenclamide/hypoxia/P1 receptors) 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunduri S, Dick G, Nayeem M, Mustafa S. Adenosine A(1) receptor signaling inhibits BK channels through a PKCα-dependent mechanism in mouse aortic smooth muscle. Physiol Rep. 2013;1:e00037. doi: 10.1002/phy2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Kang R, Shi J, et al. Anticonvulsant Activity of B2, an Adenosine Analog, on Chemical Convulsant-Induced Seizures. PLoS ONE. 2013;8:67060. doi: 10.1371/journal.pone.0067060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Z, Yin P, Reierstad S, et al. Adenosine A1 receptor, a target and regulator of estrogen receptorα action, mediates the proliferative effects of estradiol in breast cancer. Oncogene. 2010;29:1114–1122. doi: 10.1038/onc.2009.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindquist BE, Shuttleworth CW. Spreading depolarization-induced adenosine accumulation reflects metabolic status in vitro and in vivo. J Cereb Blood Flow Metab. 2014;34:1779–1790. doi: 10.1038/jcbfm.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu W, Li B, Chen J, et al. Expression of calcitonin gene-related peptide, adenosine A2a receptor and adenosine A1 receptor in experiment rat migraine models. Biomed Reports. 2016;4:379–383. doi: 10.3892/br.2016.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mediero A, Wilder T, Perez-Aso M, Cronstein BN. Direct or indirect stimulation of adenosine A2Areceptors enhances bone regeneration as well as bone morphogenetic protein-2. FASEB J. 2015;29:1577–1590. doi: 10.1096/fj.14-265066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowaczewska M, Wiciński M, Kaźmierczak W. The Ambiguous Role of Caffeine in Migraine Headache: From Trigger to Treatment. Nutrients. 2020;12:1–16. doi: 10.3390/NU12082259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ocaña M, Baeyens JM. Role of ATP-sensitive K+ channels in antinociception induced by R-PIA, an adenosine A1 receptor agonist. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:57–62. doi: 10.1007/BF00180011. [DOI] [PubMed] [Google Scholar]
- 36.Paterniti I, Melani A, Cipriani S, et al. Selective adenosine A2Areceptor agonists and antagonists protect against spinal cord injury through peripheral and central effects. J Neuroinflammation. 2011;8:1–14. doi: 10.1186/1742-2094-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ragozzino D, Limatola C, Sobrero F, et al. Hippocampal Neurons Neuroprotection and Neuromodulation in CL1-Mediated 3 Required for CX Activity of Adenosine Receptors Type 1 Is. J Immunol Ref. 2021;180:7590–7596. doi: 10.4049/jimmunol.180.11.7590. [DOI] [PubMed] [Google Scholar]
- 38.Sachdeva S, Gupta M. Adenosine and its receptors as therapeutic targets: An overview. Saudi Pharm J. 2013;21:245–253. doi: 10.1016/j.jsps.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawynok J. Adenosine receptor targets for pain. Neuroscience. 2016;338:1–18. doi: 10.1016/j.neuroscience.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 40.Shen WT, Huang YJ, Zhang Q, et al. SCH58261, the antagonist of adenosine A2A receptor, alleviates cadmium-induced preeclampsia via sirtiun-1/hypoxia-inducible factor-1a pathway in rats. Eur Rev Med Pharmacol Sci. 2020;24:10941–10953. doi: 10.26355/eurrev_202011_23577. [DOI] [PubMed] [Google Scholar]
- 41.Sneyd RJ, Langton JA, Allan LG, et al. Multicentre evaluation of the adenosine agonist GR79236X in patients with dental pain after third molar extraction. Br J Anaesth. 2007;98:672–676. doi: 10.1093/bja/aem075. [DOI] [PubMed] [Google Scholar]
- 42.Szopa A, Poleszak E, Bogatko K, et al. DPCPX, a selective adenosine A1 receptor antagonist, enhances the antidepressant-like effects of imipramine, escitalopram, and reboxetine in mice behavioral tests. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:1361. doi: 10.1007/s00210-018-1551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabrizchi R, Bedi S. Pharmacology of adenosine receptors in the vasculature. Pharmacol Ther. 2001;91:133–147. doi: 10.1016/S0163-7258(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 44.Teoh LKK, Grant R, Hulf JA, et al. The effect of preconditioning (ischemic and pharmacological) on myocardial necrosis following coronary artery bypass graft surgery. Cardiovasc Res. 2002;53:175–180. doi: 10.1016/S0008-6363(01)00435-7. [DOI] [PubMed] [Google Scholar]
- 45.Thuraiaiyah J, Kokoti L, Al-Karagholi MA, Ashina M (2022) Involvement of adenosine signaling pathway in migraine pathophysiology: A systematic review of clinical studies. Cephalalgia. 10.1177/03331024221077665. [DOI] [PubMed]
- 46.Wei EP, Moskowitz MA, Boccalini P, Kontos HA. Calcitonin gene-related peptide mediates nitroglycerin and sodium nitroprusside-induced vasodilation in feline cerebral arterioles. Circ Res. 1992;70:1313–1319. doi: 10.1161/01.RES.70.6.1313. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Zhang H, Lu Q, et al. Suppression of adenosine A2a receptors alleviates bladder overactivity and hyperalgesia in cyclophosphamide-induced cystitis by inhibiting TRPV1. Biochem Pharmacol. 2021;183:114340. doi: 10.1016/j.bcp.2020.114340. [DOI] [PubMed] [Google Scholar]
- 48.Yegutkin GG, Guerrero-Toro C, Kilinc E, et al. Nucleotide homeostasis and purinergic nociceptive signaling in rat meninges in migraine-like conditions. Purinergic Signal. 2016;12:561–574. doi: 10.1007/s11302-016-9521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan K, Bai GY, Park WH, et al. Stimulation of ANP secretion by 2-Cl-IB-MECA through A3 receptor and CaMKII. Peptides. 2008;29:2216–2224. doi: 10.1016/j.peptides.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Y, Tong L, Chu X, et al. The Adenosine A1 Receptor Antagonist DPCPX Inhibits Tumor Progression via the ERK/JNK Pathway in Renal Cell Carcinoma. Cell Physiol Biochem. 2017;43:733–742. doi: 10.1159/000481557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.