Abstract
DNA damaging agents have been a cornerstone of cancer therapy for nearly a century. The discovery of many of these chemicals, particularly the alkylating agents, are deeply entwined with the development of poisonous materials originally intended for use in warfare. Over the last decades, their anti-proliferative effects have focused on the specific mechanisms by which they damage DNA, and the factors involved in the repair of such damage. Due to the variety of aberrant adducts created even for the simplest alkylating agents, numerous pathways of repair are engaged as a defense against this damage. More recent work has underscored the role of RNA damage in the cellular response to these agents, although the understanding of their role in relation to established DNA repair pathways is still in its infancy. In this review, we discuss the chemistry of alkylating agents, the numerous ways in which they damage nucleic acids, as well as the specific DNA and RNA repair pathways which are engaged to counter their effects.
History of alkylating agents
Over a century ago during World War I, attempts at creating poisonous chemicals for the purpose of warfare birthed the introduction of numerous hazardous chemicals, including alkylating agents. One of the most potent of these was the “mustard gas”, di-(−2-chloroethyl) sulphide or “sulfur mustard” (Figure 1A). The designation was a misnomer; it was not a gas, but a liquid at room temperature, and its mustard smell was due to associated impurities and not the parent compound. As a highly potent vesicant, its effects on the surface of the skin and mucous membranes were devastating. This quality deemed it potentially advantageous on the battlefield relative to chlorine or other agents, which had an impact only upon inhalation (Brookes, 1990). Therefore, gas masks alone provided insufficient protection against mustard gas. Beyond this, the effect of such agents in killing rapidly dividing blood cells was recognized initially in 1919 during examination and treatment of exposed soldiers (Brookes, 1990). Due to these anti-proliferative effects, the possibility of more peaceful uses of mustard gas as an anti-cancer agent were explored, using animal models as well as early clinical trials in humans during the 1930s (Adair and Bagg, 1931). Despite some initial positive results, the very narrow therapeutic window of mustard gas and major challenges with its practical use limited widespread clinical application.
Figure 1. Examples of important alkylating agents.
(A) Sulfur mustards and nitrogen mustards are bifunctional alkylating agents originally developed for chemical warfare. Nitrogen mustards are now clinically important anticancer agents. (B) Temozolomide, dacarbazide and streptocotocin are clinically used methylating agents that undergo activation to form a methyl diazonium ion to react with DNA. (C) Cyclophosphamide, carmustine, bulsulfan and mitomycin C are examples of bifunctional alkylating agents used in antitumor therapy that form DNA interstrand crosslinks. (D) NKK (4-(methylnitrosamino-1-(3-pyridyl)-1-butanone) is a tobacco-specific nitrosamine that can form two alkylation products with DNA. N-nitroso-glycine can be formed from the amino acid glycine to produce O6-carboxymethyl-G, a lesion linked to colorectal cancer development. The alkyl groups transferred to the DNA are shown in red for each molecule.
The years during and following World War II saw a surge in both the development of new alkylating agents, as well as further research into their effects on biological systems. In fact, the first example of chemical mutagenesis by Auerbach and Robson used mustard gas (Auerbach and Robson, 1946). Using Drosophila melanogaster as a model system, they demonstrated that treatment of adult male flies with sublethal doses of the agent caused a marked increase in sex-linked lethal mutations. The similarity of the biological effects of alkylating agents and X-rays was also recognized, resulting in the coining of the term “radiomimetic” (Brookes, 1990). Research on similar nitrogen-based alkylating compounds (hence the term “nitrogen mustard”; Figure 1A) for chemical warfare confirmed their reactivity with cellular macromolecules, potentially explaining their cytotoxic effects. This culminated in the first clinical trial using alkylators as a cancer chemotherapeutic, which was reported in 1946 (Gilman and Philips, 1946). The development of additional, more complex compounds of various classes for therapeutic purposes continued over the next decades, with many of them remaining as frontline agents for specific cancers and as immunosuppressive agents (Figure 1B–1C). In general, these agents can be categorized into monofunctional agents that cause simple methylation lesions in DNA (Figure 1B) and bifunctional agents with the ability to covalently link two strands of DNA, forming DNA interstrand crosslinks (ICLs; Figure 1C).The cellular target of these agents was initially unclear, yet the possibility that they impact chromosomes was suggested even prior to the discovery of the structure of DNA (Biesele et al., 1950). Soon thereafter, definitive evidence arose for the reactivity of alkylating agents with nucleic acids, although the precise nature of the chemical modification(s) they induce remained a matter of intense debate for some time (reviewed in (Brookes, 1990)).
Nucleic acids as alkylation damage targets
Besides the synthetic agents above, a vast number of endogenous and environmental alkylating agents exist that can similarly modify nucleic acids. For example, high concentrations of the cellular methyl donor, S-adenosylmethionine (SAM) may be capable of nonenzymatically methylating DNA (Rydberg and Lindahl, 1982). Other endogenous alkylating agents can be generated from the nitrosation of amino acids or bile acids, which have been shown to produce O-alkyl adducts (Busby et al., 1985; Garcia-Santos Mdel, Calle, and Casado, 2001), or from lipid peroxidation, which target DNA bases to form genotoxic exocyclic etheno(ε)-adducts (Gros et al., 2004). Exogenous alkylating agents include environmental chemicals such as halogenated hydrocarbons (Ballschmiter, 2003), nitrosamines from food products, and tobacco smoke (Hecht, 1999; Song, Wu, and Guan, 2015) (Figure 1D). As alluded to above, alkylating agents are one of the most widely used systemic therapies for cancer treatment. Detailed information about the types and functions of alkylating agents for cancer chemotherapy has been extensively reviewed elsewhere (Fu, Calvo, and Samson, 2012).
Following the treatment with monofunctional methylating agents, the most abundant lesion is 7-methylguanine (7meG), which comprises more than 70% of the total proportion of all DNA alkylation lesions (Beranek, 1990). Genome-wide mapping of alkylated lesions in yeast treated with methyl methansulfonate (MMS) has confirmed that the vast majority of the lesions are 7meG (~80%) and 3-methyladenine (3meA; ~10%) (Figure 2) (Mao et al., 2017). While 7meG itself is not cytotoxic or mutagenic, it can undergo spontaneous depurination and generate apurinic (AP) sites, which are toxic and mutagenic. 7meG is furthermore prone to ring-opening, leading to the formation Fapy-7meG, which blocks E. coli DNA polymerase in vitro (Tudek, 2003). The other relatively less common alkylated lesions are 1-methyladenine (1meA), 3-methylcytosine (3meC) and O6-methylguanine (O6meG) (Beranek, 1990). Besides 7meG, these minor lesions are considered more toxic since they inhibit DNA polymerases because the methyl group blocks formation of base pairing (Larson et al., 1985). Though O6meG by itself does not cause stalling of DNA polymerases, it readily causes mispairing with T rather than C, resulting in G:C to A:T transition mutations (Warren, Forsberg, and Beese, 2006). In the context of an O6meG-T mispair, this lesion is recognized by the mismatch repair (MMR) machinery, which targets the non-alkylated strand for removal (York and Modrich, 2006). If the O6meG remains, replication may yet again produce O6meG:T, initiating a futile cycle that leads to DNA strand breaks. This is believed to be the biochemical mechanism by which O6meG induces cell death (York and Modrich, 2006; Kat et al., 1993). Hence, the loss of MMR factors, or silencing of their expression, is one way to suppress O6meG cytotoxicity. These DNA alkylated lesions and their corresponding cytotoxic and mutagenic effects are summarized in Figure 2. We should note that these same lesions may also occur in RNA, although their effects on cellular fitness remains unclear (Yan and Zaher, 2019).
Figure 2. Alkylated sites on DNA bases and the corresponding cellular effects as well as the responsive repair proteins for the lesions.
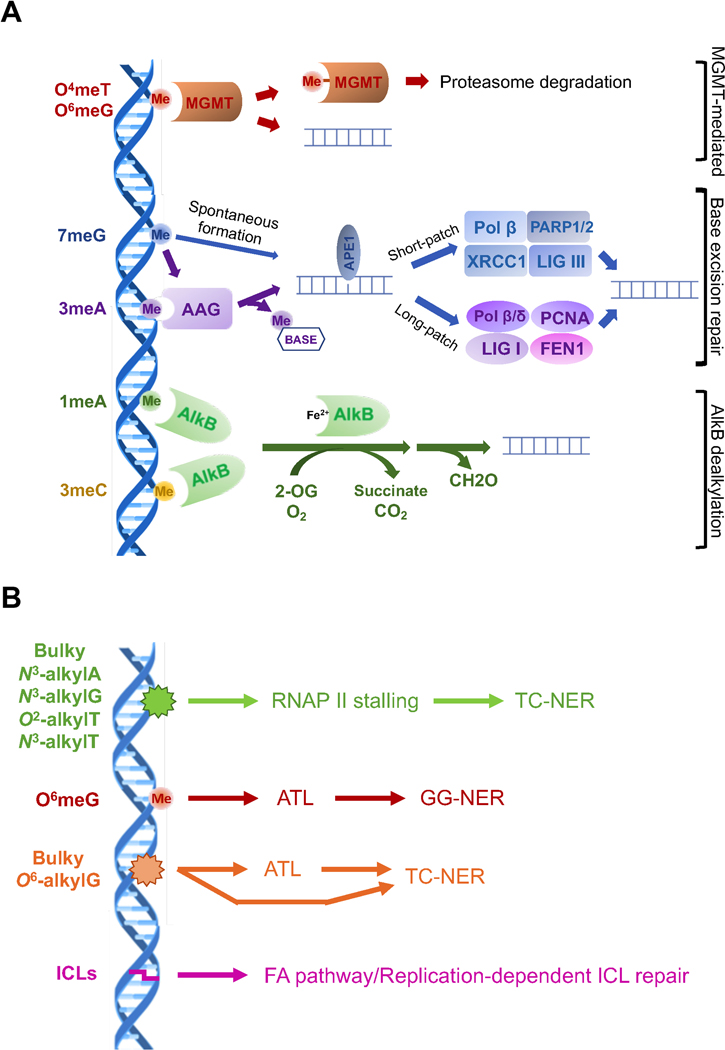
The different nitrogen or oxygen positions on DNA bases targeted by alkylating agents are shown in different colors and the repair proteins responsive to the lesions are shown with the same colors of alkylated lesions with outlines. The full-colored rectangles indicate the different toxic effects of alkylated lesions. MGMT, O6-methylguanine-DNA methyltransferase; AAG, alkyladenine glycosylase; APE1, apurinic/apyrimidinic endonuclease. AlkB, Fe(ΙΙ)/α-ketoglutarate-dependent dioxygenase; Fapy-G, 5-N-methyl-2,6-diamino-4-hydroxyformamidopyrimidine; Alkyl-PTE, alkylated phosphotriester.
Mechanisms of DNA alkylation repair
In response to the various types of DNA alkylation adducts, cells have an arsenal of pathways for repairing these lesions, each of which will be discussed individually (Figure 3A–B). These distinct pathways of repair are necessary due to the vast array of different lesions created, even in response to simple methylation damage.
Figure 3. The repair pathways for different DNA alkylated lesions.
A. Three major pathways for the simple methylated lesions with the proteins and molecules involved in the pathways. LIG, DNA ligase; FEN1, Flap endonuclease; PCNA, proliferating cell nuclear antigen; PARP, Poly(ADP-ribose)-polymerase. B. NER-mediated repair pathways for the bulky alkylated lesions and the repair mechanisms responsive to the interstrand crosslinks (ICLs). ALT, alkyltransferase-like protein.
Methylguanine methyltransferase (MGMT)-mediated repair
MGMT (also known as alkyl guanine transferase - AGT), is a highly conserved protein, having originally been described in E. coli as part of the adaptive response to DNA damage (Teo et al., 1986). It encodes a suicide protein – and not a bona fide methyltransferase enzyme – as it reverts O-linked lesions from DNA by covalently transferring the methyl group onto a cysteine residue in the active site (Teo et al., 1986). While the major lesion that is considered to be reverted by this pathway is O6meG, other relatively minor adducts, such as O4meT and the methyl groups on the phosphate group of the DNA backbone, can also be reversed by MGMT, albeit at a lower efficiency (Samson et al., 1997; Sassanfar et al., 1991; Wu, Wang, and Wang, 2018) (Figure 3A). In eukaryotes, alkylated (and hence inactivated) MGMT is ubiquitinated and targeted for proteosomal degradation (Xu-Welliver and Pegg, 2002). Consistent with its non-enzymatic function in repairing DNA, tumors with higher levels of MGMT expression are more resistant to exogenous alkylating agents (Kitange et al., 2009). Indeed, the clinical efficacy of alkylating chemotherapeutics in various cancers, particularly glioblastoma, is known to be dependent on MGMT protein levels (Sarkaria et al., 2008). As a result, the determination of MGMT gene promoter methylation status, which serves as a correlate to MGMT expression, was one of the earliest proven markers for personalized chemotherapy for glioblastoma, which is commonly treated with the methylating agent temozolomide (Hegi et al., 2005).
Base excision repair (BER)
The most common lesions in DNA generated by alkylating agents, 7meG and 3meA, are repaired through BER (Krokan and Bjoras, 2013). In this pathway, alkyladenine glycosylase (AAG; also known as its HUGO name MPG, N-methylpurine-DNA glycosylase) recognizes these adducts and cleaves the glycosidic bond in the DNA, leaving an abasic site (also known as an apurinic (AP) site. Alkyladenine DNA glycosylases were originally discovered in bacteria. E. coli has two such enzymes, Tag and AlkA. Tag is consitutively expressed and narrowly focused on 3meA, while AlkA is the functional homolog of AAG. Intriguingly, the AlkA gene is part of an operon that is induced by alkylated MGMT (also called Ada in bacteria) and also includes AlkB (Evensen and Seeberg, 1982; Karran, Hjelmgren, and Lindahl, 1982; Lindahl et al., 1988). Subsequent cloning of these genes, as well as biochemical and structural studies, revealed that both AlkA and AAG are enzymes with relatively broad substrate specificity and recognize a variety of purine bases, including 3meA, 7meG, hypoxanthine and ethenoadenine (Wyatt et al., 1999). The abasic site formed by the glycosylase reaction is recognized by AP endonuclease (APE1) to cleave the phosphodiester bond 5’ to the lesion. DNA polymerase β (Polβ) has a dual role in BER in processing of the remaining deoxyribose phosphate to create a single nucleotide gap and fill in the one nucleotide gap (Matsumoto and Kim, 1995; Sobol et al., 1996) followed by ligation of the nick by XRCC1/DNA ligase III in short patch BER (Kubota et al., 1996). Alternatively, in long patch BER, a longer stretch of nucleotides is inserted in strand-displacement synthesis by Polβ or DNA polymerase δ (Polδ), creating a single-stranded flap that is cleaved by flap endonuclease (FEN1) prior to ligation (Frosina et al., 1996). The coordination between initiation of alkylation repair by AAG and subsequent processing by the BER machinery is critical, as overexpression of AAG causes increased mutagenesis, likely due to accumulation of BER intermediates, such as abasic sites (Glassner et al., 1998).
AlkB family-mediated oxidative dealkylation
Like several DNA repair enzymes, the α-ketoglutarate-dependent dioxygenase AlkB (AlkB) is highly conserved in viruses, bacteria, and mammals, although they appear to be absent in certain yeasts (Fedeles et al., 2015; Shen et al., 2014). AlkB was originally discovered in bacteria, where it is located in the same operon as AlkA and Ada (Lindahl et al., 1988; Kataoka and Sekiguchi, 1985). The function of AlkB remained mysterious for a long time and a first clue to its function came from the observation that AlkB is important for the repair of alkylation damage in the context of single stranded DNA (Dinglay et al., 2000). The enzymatic activity of AlkB was suggested when bioinformatic analysis classified the enzyme as an iron and α-ketoglutarate dependent dioxygenase (Aravind and Koonin, 2001). Two groups independently demonstrated that AlkB can oxidize and remove methyl groups from DNA as formaldehyde (Falnes, Johansen, and Seeberg, 2002; Trewick et al., 2002). AlkB enzymes have been demonstrated to dealkylate many N-linked lesions in DNA, particularly those located on the base pairing interface (Li et al., 2013). Notably, many of these proteins, including those from viruses, E. coli AlkB, and human ALKBH3, can also repair RNA (Aas et al., 2003; van den Born et al., 2008). To date, total nine AlkB members have been identified in the human genome, but only ALKBH2 and ALKBH3 have demonstrated activity on 1meA and 3meC in DNA, while most of the other AlkB proteins have roles in RNA epitranscriptomics. ALKBH2 prefers double-stranded DNA as a substrate, and ALKBH3 prefers single-stranded DNA or RNA (Aas et al., 2003). ALKBH1 has been suggested to demethylate 3meC in single-stranded DNA and RNA in vitro, but evidence of this function in vivo is lacking (Westbye et al., 2008). In spite of the preference for single-stranded DNA, ALKBH3 has shown to be capable of repairing alkylated lesions in double-stranded DNA by forming a complex with ASCC3, which can open the duplex using its has helicase activity to unwind DNA, exposing the single-stranded substrate needed for demethylation (Dango et al., 2011). Bacterial AlkB has been shown to repair a number of bulkier N-linked lesions in vitro, although it preference is to repair these lesions in a single-stranded DNA context (Li et al., 2013).
The role of NER in repairing alkylated lesions
In contrast to the narrow substrate specificity of the above pathways acting on methylated bases, nucleotide excision repair (NER) is a pathway that has evolved to clear the genome of structurally diverse lesions, such as those formed by UV irradiation and various environmental agents. NER occurs in two main subpathways, global genome NER (GG-NER), for the repair lesions anywhere in the genome, and transcription-coupled NER (TC-NER), for the accelerated repair of lesions in the transcribed strands of active genes (Marteijn et al., 2014; Scharer, 2013). GG-NER is initiated by the XPC-RAD23B and UV-DDB proteins, with a preference for lesions that thermodynamically destabilize the DNA duplex. TC-NER is initiated by an RNA polymerase that has stalled at a lesion. While the two pathways differ in the initial damage recognition step, both utilize the transcription and repair factor TFIIH to verify the presence of a lesion and to assemble the full NER pre-incision complex that excises the damage as part of an oligonucleotide. Based on this mechanism, simple alkylated bases are not obvious candidates for NER substrates, but early studies suggested that O6-meG can be excised by the GG-NER machinery in vitro, albeit with low efficiency (Huang et al., 1994). An important role for NER in the repair of O6-meG was not substantiated in subsequent studies, although the repair of O6-alkylG lesions with larger substituents was shown to be influenced by NER (Du et al., 2019). Similarly, there may be a minor contribution to the repair of 7meG or 3meA adducts by NER (Plosky et al., 2002; Smith and Engelward, 2000), while there is more substantial evidence for the repair of larger 7-alkylG and 3-alkylA adducts by NER (Grant, Bessho, and Reardon, 1998; Sitaram et al., 1997).
The recognition of alkyl lesions by GG-NER is unlikely to be efficient for alkyl lesions that do not destabilize the DNA duplex. By contrast, several alkyl lesion can block the translocation of RNA polymerase II and trigger TC-NER (Malvezzi et al., 2017; Xu et al., 2017) (Figure 3B). The stalling of RNA polymerase II is also dependent on the size and structure of a lesion, although the parameters are distinct from those for GG-NER. 3meA or O4-EtT provide only a minor obstacle for translocation, while bulkier N3-alkyl-A adducts or O2-Et-T and N3-Et-T provide a potent block. Interestingly, simple alkyl lesions such as 7meG or 3meA still have the potential to trigger TC-NER if they are transformed to abasic sites, either by spontaneous depurination of these positively charged alkylated bases or by the activities of DNA glycosylases. Abasic sites have been shown to arrest RNA polymerase II and trigger TC-NER. Consistent with these observations, N-ethyl purine lesions have been shown to be repair by TC-NER (Sitaram et al., 1997), while for example O6-meG mainly induces transcriptional mutagenesis by miscoding during transcription (Burns et al., 2010).
An intriguing variant of alkylation repair exists, at least in bacteria and yeast, to facilitate NER of bulky O6-alkylG adducts. These organisms have alkyltransferase-like (ATL) genes, close homologs of AGT that lack the active-site cysteine (Margison et al., 2007; Mazon et al., 2009). Instead, ATL proteins have binding pockets that can bind O6-alkylG adducts of increased size and induce distortion in the DNA upon binding lesions (Tubbs et al., 2009). In S. pombe, ATL has been shown to bind smaller O6-alkylG lesion with moderate affinity and upon dissociation, shuttle them into the GG-NER pathway, possibly fulfilling a role akin to UV-DDB for these lesions (Latypov et al., 2012) (Figure 3B). By contrast, ATL has a lower dissociation constant on bulky O6-alkylG lesions and such complexes are instead associated with TC-NER or a block of replication. Whether such a mechanism is also operating in higher eukaryotes is currently unknown, as an obvious ATL ortholog has not been found in metazoans.
Multiple pathways cooperate to repair ICLs formed by alkylating agents.
Bifunctional alkylating agents, including the nitrogen mustards and choloroethyl nitrosoureas (CENUs), have the potential to form DNA interstrand crosslinks (ICLs), which are clinically the most important lesions formed by these agents (Figure 1) (Brookes and Lawley, 1960; Kohn, Spears, and Doty, 1966). The covalent linkage between two strands of DNA makes ICLs extraordinarily cytotoxic and therefore useful in cancer therapy. Their structures necessitate complex repair pathways that combine the activities of many proteins, and those involved in direct repair, BER and NER have all been shown to contribute to ICL repair (De Silva et al., 2000; Nojima et al., 2005; Semlow et al., 2016; Wang et al., 2001). However, it is thought that the main pathway for the repair of ICLs is the Fanconi Anemia (FA) pathway, which occurs during replication. This is initiated by stalling of the replication fork, activation of the Fanconi anemia signaling pathway, unhooking of the ICL to separate the two strands, translesion synthesis to restore one of the two strands and homologous recombination to restore the replication fork. While ICL repair has been extensively reviewed elsewhere (Kottemann and Smogorzewska, 2013; Roy and Scharer, 2016; Zhang and Walter, 2014), it is worth noting that classical alkylation repair pathways also counteract the effects of ICLs. For example, the repair of CENU-adducts may be mediated by MGMT/AGT before they can form ICLs (Margison and Santibanez-Koref, 2002). More recent work has uncovered a role of specific DNA glycosylases in the repair of certain ICLs (Semlow et al., 2016). The regulatory mechanisms that govern which of these pathways are deployed for ICL repair remains largely unknown.
Regulation of alkylation damage repair
As with other pathways of DNA repair, it is important that these various alkylation reversal mechanisms are regulated in order to maximize efficient repair while being minimally disruptive to other DNA template dependent pathways such as replication and transcription. It is well established that certain DNA repair pathways are regulated throughout the cell cycle, particularly for the machinery participating in DNA double-stranded break repair (Panier and Durocher, 2013). However, the potential mechanisms of cell cycle regulation of alkylation reversal are still largely unexplored. Some of these alkylation repair pathways are coupled to replication, particularly MGMT and ALKBH2. Recent evidence has suggested that MGMT possesses a PCNA-interacting protein (PIP) motif which may serve to recruit it to the replication machinery (Mostofa et al., 2018). This same study suggested that MGMT is more tightly recruited to PCNA upon alkylation challenge, although the mechanism of this was not further explored. In the same vein, although the expression of ALKBH2 is not significantly altered throughout the cell cycle, it forms a complex with PCNA during S phase, using its eponymous APIM (ALKBH2 PCNA interacting motif) domain (Gilljam et al., 2009). It is interesting to note that the loss of a specific translesion polymerase, Pol κ, significantly increases the rate of mutagenesis in response to MMS damage, suggesting that canonical alkylation reversal pathways of alkylation repair are not sufficient to counter these lesions prior to replication (Volkova et al., 2020). What regulatory mechanisms may exist that govern these interactions with the replication machinery during S-phase, or how they are recruited to damaged DNA outside S-phase, remains unknown.
Although BER is generally considered as a G1 phase repair pathway (Dianov and Hubscher, 2013), a number of BER genes including AAG are upregulated in the during the S-phase of the cell cycle (Bouziane et al., 2000; Mjelle et al., 2015). The interaction between AAG, APE1 and PCNA in vitro, and the enrichment of AAG as well as other BER proteins on nascent DNA further implies that the repair of alkylated lesions could occur during DNA replication (Xia et al., 2005). Notably, the modest enrichment of BER factors with the replisome suggests BER may function in concert with replication or in a post-replicative manner (Bj Ras et al., 2017). AAG deficient cells show a delay of cell-cycle progression through S-phase after MMS treatment, suggesting that BER activity is important for maintaining the genome either prior to or during replication (Bj Ras et al., 2017). Given that cells need to minimize the occurrence and lifetime of single-stranded break intermediates created by BER during DNA replication to avoid formation of double-stranded breaks upon replication, a mechanism must exist to prevent AP site processing during S-phase. It is possible that these are masked by AAG or the newly discovered HMCES protein, or bypassed via translesion synthesis (Mehta et al., 2020).
We previously reported that the ASCC complex is recruited to euchromatic nuclear speckle bodies during alkylation damage induction (Brickner et al., 2017). The recruitment of ASCC to these foci appears to be restricted to G1 phase. Given that ALKBH2 and ALKBH3 have distinct substrate specificities and potential differences in cell cycle regulation, we speculate that there may be a handoff between the ALKBH3-ASCC pathway to ALKBH2-PCNA as cells transition from G1 to S-phase. Further investigation is still needed to elucidate how the cell cycle coordinates or switches between these repair pathways in response to alkylation damage. The ASCC complex has also been shown to interact with RNAP II (Boeing et al., 2016). As with the interaction of AAG with the Elongator complex (Montaldo et al., 2019), it is possible that both AAG-mediated BER and direct dealkylation repair may be coupled to sites of active transcription.
Potential integration of DNA and RNA alkylation repair: An example of the ASCC complex
Alkylating agents generate damage lesions in both DNA and RNA (Drablos et al., 2004). In fact, likely due to the single-stranded nature of RNA and its greater abundance, RNA is more readily alkylated in vivo relative to DNA. Consistent with this concept, MMS treatment induces at least an order of magnitude more alkylation of RNA relative to DNA in E. coli (Vagbo et al., 2013). Some major types of alkylation such as N1-methyladenine (m1A), N3-methylcytosine (m3C) and N7-methylguanine (m7G) are also common physiological RNA modifications in the cell (Thapar et al., 2019; Xiong, Li, and Yi, 2018). The overlap between these marks and damage lesions suggest the cellular response to alkylation may produce effects related to the physiological functions of these methylation marks. Several studies have shown that human ALKBH1 and ALKBH3 have demethylation activity on m1A and m3C in RNA (Li et al., 2016; Liu et al., 2016; Ma et al., 2019; Ueda et al., 2017). Indeed, such RNA repair may help to maintain mRNA and tRNA functionality (Ougland et al., 2004), although the biological effect of AlkB-mediated RNA dealkylation on cellular functions remains poorly understood.
In addition to the physiological roles of methylated RNA, the specific effects of aberrant RNA methylation caused by alkylating agents are just beginning to be understood. We previously reported that the recruitment of ASCC complex to RNA-rich nuclear speckles is crucial for repairing DNA alkylation damage. This recruitment is mediated by the ASCC2 subunit, via a ubiquitin recognition domain (CUE) recognizing K63-polyubiquitination chain generated by the RNF113A E3 ligase (Brickner et al., 2017). Intriguingly, recent studies have also shown that ASCC complex functions in the cytosol by disassembling collided ribosomes as part of the ribosome quality control pathway (Hashimoto et al., 2020; Juszkiewicz et al., 2020). Analogous to RNF113A mediated ubiquitination, ribosome disassembly requires ZNF598-mediated ubiquitination of the 40S ribosomal subunit (Juszkiewicz et al., 2020). Given that RNF113A is associated with the spliceosome (Hegele et al., 2012; Shostak et al., 2020) and that ASCC is recruited to active spliceosomal components present in nuclear speckles (Brickner et al., 2017), we speculate that this may serve as a general mechanism for the function of the ASCC complex, both in the cytosol and in the nucleus (Figure 4). Similar to the activation of ZNF598 upon ribosome collision, RNF113A may be activated upon “stalling” of the active spliceosome when it encounters an alkylated lesion in pre-mRNA, thereby generating ubiquitin chains to recruit the ASCC complex to the damaged region. This recruitment may be important for resuming RNA splicing. While the outcome of ASCC recruitment to the spliceosome is unclear, one possible outcome is to disassemble spliceosome components via ATP-dependent helicase activity of ASCC3, in turn potentially recycling the spliceosome components as well as increasing the accessibility for potentially degrading the damaged nascent RNA. The other possibility is to directly demethylate these alkylated residues via recruitment of ALKBH3, which physically interacts with ASCC3 (Dango et al., 2011), thereby resuming RNA splicing (Figure 4).
Figure 4. A purposed common role of ASCC complex in the disassembly of stalled ribosomes and spliceosomes.
In the cytosol (left panel), a damaged mRNA causes ribosome stalling. This activates ZNF598 to ubiquitinate the 40S ribosomal subunit, which is required for the recruitment of the ASCC complex for splitting of the stalled ribosome. In the nucleus (right panel), the ASCC complex may bind to active spliceosomes that are “stalled” due to the damage induced by methylation or alkylation. ASCC may similarly disassemble the spliceosome or directly repair the damage in the nascent RNA by ALKBH3-mediated demethylation.
Why such an intricate set of pathways would evolve to signal damage to RNA that converges on the ASCC complex is not entirely clear. Since ALKBH3 repairs DNA as well as RNA, it is possible that recruitment of this complex serves to signal damage within a general region of the nucleus, with the capacity to repair either type of nucleic acid. Yet the main function may be to rescue ribonucleoprotein complexes stalled on damaged RNA, with DNA repair being a secondary benefit. It is also interesting to note that ALKBH3 prefers m3C in RNA over m1A (Aas et al., 2003), since m3C marks physiological R-loop formation in the nucleolus triggered by the METTL8 methyltransferase (Zhang et al., 2020). The fact that alkylating agents also induce R-loops in cells suggest that such R-loop formation may be caused at least in part by RNA methylation (Lockhart et al., 2019). Since the deregulation of RNA splicing is known to induce genome instability partly due to R-loop formation (Chakraborty, Huang, and Hiom, 2018; Niehrs and Luke, 2020), it is possible that the function of the ASCC complex evolved for the purposes of R-loop resolution, whether pathological or physiological, with spliceosomal components serving as the upstream sensor.
Conclusion and Perspectives
Although individual alkylation repair pathways and regulatory mechanisms of each pathway are well-understood, the coordination of these individual repair pathways with each other needs to be further investigated. In addition, compared to DNA alkylation repair, the consequences of aberrant RNA alkylation, whether this be repair or degradation, remains largely unexplored. Several crucial questions remain to be solved: What is the evolutionary pressure to evolve such pathways, particularly for RNA repair? In addition to the ASCC-ALKBH3 pathway, what other connections exist between RNA and DNA alkylation damage repair? Notably, YTH domain-containing proteins that bind to methylated RNA as epitranscriptomic “readers” were recently reported to bind to m1A in RNA (Dai et al., 2018; Seo and Kleiner, 2020). It would be interesting to determine whether such YTH domain proteins function to mark regions of alkylated RNA for repair by the AlkB or other alkylation reversal mechanism. Exploring these questions will provide us with a better understandings of nucleic acid biology, cellular damage responses, along with potential implications for cancer chemotherapy.
Acknowledgments
Declaration of Interest
The authors have no conflicts of interest to declear. NM and ODS are members of the Structural Cell Biology of DNA Repair program project (NIH P01 CA092584). Work in the NM laboratory is supported the by the NIH (R01 CA193318 and R01 CA227001), the American Cancer Society research scholar program (RSG-18–156-01-DMC), and the Alvin J. Siteman Cancer Center Investment Program, which is supported by the Foundation for Barnes-Jewish Hospital Cancer Frontier Fund and the National Cancer Institute, Cancer Support Grant P30 CA091842. Work in the ODS laboratory is supported by the Korean Institute for Basic Science (IBS-R022-A1) and the NIH (R01 CA218315).
References
- 1.Brookes P. (1990).The early history of the biological alkylating agents, 1918–1968. Mutat Res, 233, 3–14 [DOI] [PubMed] [Google Scholar]
- 2.Adair FE, Bagg HJ. (1931).Experimental and Clinical Studies on the Treatment of Cancer by Dichlorethylsulphide (Mustard Gas. Ann Surg, 93, 190–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auerbach C, Robson JM. (1946).Chemical production of mutations. Nature, 157, 302. [DOI] [PubMed] [Google Scholar]
- 4.Gilman A, Philips FS. (1946).The Biological Actions and Therapeutic Applications of the B-Chloroethyl Amines and Sulfides. Science, 103, 409–36 [DOI] [PubMed] [Google Scholar]
- 5.Biesele JJ, Philips FS, Thiersch JB, Burchenal JH, Buckley SM, Stock CC, Loveless A, Ross WC. (1950).Chromosome alteration and tumour inhibition by nitrogen mustards; the hypothesis of crosslinking alkylation. Nature, 166, 1112–4 [DOI] [PubMed] [Google Scholar]
- 6.Rydberg B, Lindahl T. (1982).Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-L-methionine is a potentially mutagenic reaction. The EMBO journal, 1, 211–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busby WF Jr., Shuker DE, Charnley G, Newberne PM, Tannenbaum SR, Wogan GN. (1985).Carcinogenicity in rats of the nitrosated bile acid conjugates N-nitrosoglycocholic acid and N-nitrosotaurocholic acid. Cancer Res, 45, 1367–71 [PubMed] [Google Scholar]
- 8.Garcia-Santos Mdel P, Calle E, Casado J. (2001).Amino acid nitrosation products as alkylating agents. J Am Chem Soc, 123, 7506–10 [DOI] [PubMed] [Google Scholar]
- 9.Gros L, Maksimenko AV, Privezentzev CV, Laval J, Saparbaev MK. (2004).Hijacking of the human alkyl-N-purine-DNA glycosylase by 3,N4-ethenocytosine, a lipid peroxidation-induced DNA adduct. J Biol Chem, 279, 17723–30 [DOI] [PubMed] [Google Scholar]
- 10.Ballschmiter K. (2003).Pattern and sources of naturally produced organohalogens in the marine environment: biogenic formation of organohalogens. Chemosphere, 52, 313–24 [DOI] [PubMed] [Google Scholar]
- 11.Hecht SS. (1999).DNA adduct formation from tobacco-specific N-nitrosamines. Mutat Res, 424, 127–42 [DOI] [PubMed] [Google Scholar]
- 12.Song P, Wu L, Guan W. (2015).Dietary Nitrates, Nitrites, and Nitrosamines Intake and the Risk of Gastric Cancer: A Meta-Analysis. Nutrients, 7, 9872–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu D, Calvo JA, Samson LD. (2012).Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat Rev Cancer, 12, 104–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beranek DT. (1990).Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat Res, 231, 11–30 [DOI] [PubMed] [Google Scholar]
- 15.Mao P, Brown AJ, Malc EP, Mieczkowski PA, Smerdon MJ, Roberts SA, Wyrick JJ. (2017).Genome-wide maps of alkylation damage, repair, and mutagenesis in yeast reveal mechanisms of mutational heterogeneity. Genome Res, 27, 1674–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tudek B. (2003).Imidazole ring-opened DNA purines and their biological significance. J Biochem Mol Biol, 36, 12–9 [DOI] [PubMed] [Google Scholar]
- 17.Larson K, Sahm J, Shenkar R, Strauss B. (1985).Methylation-induced blocks to in vitro DNA replication. Mutat Res, 150, 77–84 [DOI] [PubMed] [Google Scholar]
- 18.Warren JJ, Forsberg LJ, Beese LS. (2006).The structural basis for the mutagenicity of O(6)-methyl-guanine lesions. Proc Natl Acad Sci U S A, 103, 19701–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.York SJ, Modrich P. (2006).Mismatch repair-dependent iterative excision at irreparable O6-methylguanine lesions in human nuclear extracts. J Biol Chem, 281, 22674–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kat A, Thilly WG, Fang WH, Longley MJ, Li GM, Modrich P. (1993).An alkylation-tolerant, mutator human cell line is deficient in strand-specific mismatch repair. Proc Natl Acad Sci U S A, 90, 6424–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan LL, Zaher HS. (2019).How do cells cope with RNA damage and its consequences? J Biol Chem, 294, 15158–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teo I, Sedgwick B, Kilpatrick MW, McCarthy TV, Lindahl T. (1986).The intracellular signal for induction of resistance to alkylating agents in E. coli. Cell, 45, 315–24 [DOI] [PubMed] [Google Scholar]
- 23.Samson L, Han S, Marquis JC, Rasmussen LJ. (1997).Mammalian DNA repair methyltransferases shield O4MeT from nucleotide excision repair. Carcinogenesis, 18, 919–24 [DOI] [PubMed] [Google Scholar]
- 24.Sassanfar M, Dosanjh MK, Essigmann JM, Samson L. (1991).Relative efficiencies of the bacterial, yeast, and human DNA methyltransferases for the repair of O6-methylguanine and O4-methylthymine. Suggestive evidence for O4-methylthymine repair by eukaryotic methyltransferases. J Biol Chem, 266, 2767–71 [PubMed] [Google Scholar]
- 25.Wu J, Wang P, Wang Y. (2018).Cytotoxic and mutagenic properties of alkyl phosphotriester lesions in Escherichia coli cells. Nucleic Acids Res, 46, 4013–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu-Welliver M, Pegg AE. (2002).Degradation of the alkylated form of the DNA repair protein, O(6)-alkylguanine-DNA alkyltransferase. Carcinogenesis, 23, 823–30 [DOI] [PubMed] [Google Scholar]
- 27.Kitange GJ, Carlson BL, Schroeder MA, Grogan PT, Lamont JD, Decker PA, Wu W, James CD, Sarkaria JN. (2009).Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro-oncology, 11, 281–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkaria JN, Kitange GJ, James CD, Plummer R, Calvert H, Weller M, Wick W. (2008).Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res, 14, 2900–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. (2005).MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med, 352, 997–1003 [DOI] [PubMed] [Google Scholar]
- 30.Krokan HE, Bjoras M. (2013).Base excision repair. Cold Spring Harb Perspect Biol, 5, a012583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evensen G, Seeberg E. (1982).Adaptation to alkylation resistance involves the induction of a DNA glycosylase. Nature, 296, 773–5 [DOI] [PubMed] [Google Scholar]
- 32.Karran P, Hjelmgren T, Lindahl T. (1982).Induction of a DNA glycosylase for N-methylated purines is part of the adaptive response to alkylating agents. Nature, 296, 770–3 [DOI] [PubMed] [Google Scholar]
- 33.Lindahl T, Sedgwick B, Sekiguchi M, Nakabeppu Y. (1988).Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem, 57, 133–57 [DOI] [PubMed] [Google Scholar]
- 34.Wyatt MD, Allan JM, Lau AY, Ellenberger TE, Samson LD. (1999).3-methyladenine DNA glycosylases: structure, function, and biological importance. Bioessays, 21, 668–76 [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto Y, Kim K. (1995).Excision of deoxyribose phosphate residues by DNA polymerase beta during DNA repair. Science, 269, 699–702 [DOI] [PubMed] [Google Scholar]
- 36.Sobol RW, Horton JK, Kuhn R, Gu H, Singhal RK, Prasad R, Rajewsky K, Wilson SH. (1996).Requirement of mammalian DNA polymerase-beta in base-excision repair. Nature, 379, 183–6 [DOI] [PubMed] [Google Scholar]
- 37.Kubota Y, Nash RA, Klungland A, Schar P, Barnes DE, Lindahl T. (1996).Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein. EMBO J, 15, 6662–70 [PMC free article] [PubMed] [Google Scholar]
- 38.Frosina G, Fortini P, Rossi O, Carrozzino F, Raspaglio G, Cox LS, Lane DP, Abbondandolo A, Dogliotti E. (1996).Two pathways for base excision repair in mammalian cells. J Biol Chem, 271, 9573–8 [DOI] [PubMed] [Google Scholar]
- 39.Glassner BJ, Rasmussen LJ, Najarian MT, Posnick LM, Samson LD. (1998).Generation of a strong mutator phenotype in yeast by imbalanced base excision repair. Proc Natl Acad Sci U S A, 95, 9997–10002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fedeles BI, Singh V, Delaney JC, Li D, Essigmann JM. (2015).The AlkB Family of Fe(II)/alpha-Ketoglutarate-dependent Dioxygenases: Repairing Nucleic Acid Alkylation Damage and Beyond. J Biol Chem, 290, 20734–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen L, Song CX, He C, Zhang Y. (2014).Mechanism and function of oxidative reversal of DNA and RNA methylation. Annu Rev Biochem, 83, 585–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kataoka H, Sekiguchi M. (1985).Molecular cloning and characterization of the alkB gene of Escherichia coli. Mol Gen Genet, 198, 263–9 [DOI] [PubMed] [Google Scholar]
- 43.Dinglay S, Trewick SC, Lindahl T, Sedgwick B. (2000).Defective processing of methylated single-stranded DNA by E. coli AlkB mutants. Genes Dev, 14, 2097–105 [PMC free article] [PubMed] [Google Scholar]
- 44.Aravind L, Koonin EV. (2001).The DNA-repair protein AlkB, EGL-9, and leprecan define new families of 2-oxoglutarate- and iron-dependent dioxygenases. Genome Biol, 2, RESEARCH0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Falnes PO, Johansen RF, Seeberg E. (2002).AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature, 419, 178–82 [DOI] [PubMed] [Google Scholar]
- 46.Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. (2002).Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature, 419, 174–8 [DOI] [PubMed] [Google Scholar]
- 47.Li D, Fedeles BI, Shrivastav N, Delaney JC, Yang X, Wong C, Drennan CL, Essigmann JM. (2013).Removal of N-alkyl modifications from N(2)-alkylguanine and N(4)-alkylcytosine in DNA by the adaptive response protein AlkB. Chem Res Toxicol, 26, 1182–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aas PA, Otterlei M, Falnes PO, Vagbo CB, Skorpen F, Akbari M, Sundheim O, Bjoras M, Slupphaug G, Seeberg E, Krokan HE. (2003).Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature, 421, 859–63 [DOI] [PubMed] [Google Scholar]
- 49.van den Born E, Omelchenko MV, Bekkelund A, Leihne V, Koonin EV, Dolja VV, Falnes PO. (2008).Viral AlkB proteins repair RNA damage by oxidative demethylation. Nucleic Acids Res, 36, 5451–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westbye MP, Feyzi E, Aas PA, Vagbo CB, Talstad VA, Kavli B, Hagen L, Sundheim O, Akbari M, Liabakk NB, Slupphaug G, Otterlei M, Krokan HE. (2008).Human AlkB homolog 1 is a mitochondrial protein that demethylates 3-methylcytosine in DNA and RNA. The Journal of biological chemistry, 283, 25046–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dango S, Mosammaparast N, Sowa ME, Xiong LJ, Wu F, Park K, Rubin M, Gygi S, Harper JW, Shi Y. (2011).DNA unwinding by ASCC3 helicase is coupled to ALKBH3-dependent DNA alkylation repair and cancer cell proliferation. Mol Cell, 44, 373–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marteijn JA, Lans H, Vermeulen W, Hoeijmakers JH. (2014).Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol, 15, 465–81 [DOI] [PubMed] [Google Scholar]
- 53.Scharer OD. (2013).Nucleotide excision repair in eukaryotes. Cold Spring Harb Perspect Biol, 5, a012609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang JC, Hsu DS, Kazantsev A, Sancar A. (1994).Substrate spectrum of human excinuclease: repair of abasic sites, methylated bases, mismatches, and bulky adducts. Proc Natl Acad Sci U S A, 91, 12213–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du H, Wang P, Li L, Wang Y. (2019).Repair and translesion synthesis of O (6)-alkylguanine DNA lesions in human cells. J Biol Chem, 294, 11144–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plosky B, Samson L, Engelward BP, Gold B, Schlaen B, Millas T, Magnotti M, Schor J, Scicchitano DA. (2002).Base excision repair and nucleotide excision repair contribute to the removal of N-methylpurines from active genes. DNA Repair (Amst), 1, 683–96 [DOI] [PubMed] [Google Scholar]
- 57.Smith SA, Engelward BP. (2000).In vivo repair of methylation damage in Aag 3-methyladenine DNA glycosylase null mouse cells. Nucleic Acids Res, 28, 3294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant DF, Bessho T, Reardon JT. (1998).Nucleotide excision repair of melphalan monoadducts. Cancer Res, 58, 5196–200 [PubMed] [Google Scholar]
- 59.Sitaram A, Plitas G, Wang W, Scicchitano DA. (1997).Functional nucleotide excision repair is required for the preferential removal of N-ethylpurines from the transcribed strand of the dihydrofolate reductase gene of Chinese hamster ovary cells. Mol Cell Biol, 17, 564–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malvezzi S, Farnung L, Aloisi CMN, Angelov T, Cramer P, Sturla SJ. (2017).Mechanism of RNA polymerase II stalling by DNA alkylation. Proc Natl Acad Sci U S A, 114, 12172–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu L, Wang W, Wu J, Shin JH, Wang P, Unarta IC, Chong J, Wang Y, Wang D. (2017).Mechanism of DNA alkylation-induced transcriptional stalling, lesion bypass, and mutagenesis. Proc Natl Acad Sci U S A, 114, E7082–E91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burns JA, Dreij K, Cartularo L, Scicchitano DA. (2010).O6-methylguanine induces altered proteins at the level of transcription in human cells. Nucleic Acids Res, 38, 8178–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Margison GP, Butt A, Pearson SJ, Wharton S, Watson AJ, Marriott A, Caetano CM, Hollins JJ, Rukazenkova N, Begum G, Santibanez-Koref MF. (2007).Alkyltransferase-like proteins. DNA Repair (Amst), 6, 1222–8 [DOI] [PubMed] [Google Scholar]
- 64.Mazon G, Philippin G, Cadet J, Gasparutto D, Fuchs RP. (2009).The alkyltransferase-like ybaZ gene product enhances nucleotide excision repair of O(6)-alkylguanine adducts in E. coli. DNA Repair (Amst), 8, 697–703 [DOI] [PubMed] [Google Scholar]
- 65.Tubbs JL, Latypov V, Kanugula S, Butt A, Melikishvili M, Kraehenbuehl R, Fleck O, Marriott A, Watson AJ, Verbeek B, McGown G, Thorncroft M, Santibanez-Koref MF, Millington C, Arvai AS, Kroeger MD, Peterson LA, Williams DM, Fried MG, Margison GP, Pegg AE, Tainer JA. (2009).Flipping of alkylated DNA damage bridges base and nucleotide excision repair. Nature, 459, 808–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Latypov VF, Tubbs JL, Watson AJ, Marriott AS, McGown G, Thorncroft M, Wilkinson OJ, Senthong P, Butt A, Arvai AS, Millington CL, Povey AC, Williams DM, Santibanez-Koref MF, Tainer JA, Margison GP. (2012).Atl1 regulates choice between global genome and transcription-coupled repair of O(6)-alkylguanines. Mol Cell, 47, 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brookes P, Lawley PD. (1960).The reaction of mustard gas with nucleic acids in vitro and in vivo. Biochem J, 77, 478–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kohn KW, Spears CL, Doty P. (1966).Inter-strand crosslinking of DNA by nitrogen mustard. J Mol Biol, 19, 266–88 [DOI] [PubMed] [Google Scholar]
- 69.De Silva IU, McHugh PJ, Clingen PH, Hartley JA. (2000).Defining the roles of nucleotide excision repair and recombination in the repair of DNA interstrand cross-links in mammalian cells. Mol Cell Biol, 20, 7980–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nojima K, Hochegger H, Saberi A, Fukushima T, Kikuchi K, Yoshimura M, Orelli BJ, Bishop DK, Hirano S, Ohzeki M, Ishiai M, Yamamoto K, Takata M, Arakawa H, Buerstedde JM, Yamazoe M, Kawamoto T, Araki K, Takahashi JA, Hashimoto N, Takeda S, Sonoda E. (2005).Multiple repair pathways mediate tolerance to chemotherapeutic cross-linking agents in vertebrate cells. Cancer Res, 65, 11704–11 [DOI] [PubMed] [Google Scholar]
- 71.Semlow DR, Zhang J, Budzowska M, Drohat AC, Walter JC. (2016).Replication-Dependent Unhooking of DNA Interstrand Cross-Links by the NEIL3 Glycosylase. Cell, 167, 498–511 e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Peterson CA, Zheng H, Nairn RS, Legerski RJ, Li L. (2001).Involvement of nucleotide excision repair in a recombination-independent and error-prone pathway of DNA interstrand cross-link repair. Mol Cell Biol, 21, 713–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kottemann MC, Smogorzewska A. (2013).Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature, 493, 356–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roy U, Scharer OD. (2016).Involvement of translesion synthesis DNA polymerases in DNA interstrand crosslink repair. DNA Repair (Amst), 44, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, Walter JC. (2014).Mechanism and regulation of incisions during DNA interstrand cross-link repair. DNA Repair (Amst), 19, 135–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Margison GP, Santibanez-Koref MF. (2002).O6-alkylguanine-DNA alkyltransferase: role in carcinogenesis and chemotherapy. Bioessays, 24, 255–66 [DOI] [PubMed] [Google Scholar]
- 77.Panier S, Durocher D. (2013).Push back to respond better: regulatory inhibition of the DNA double-strand break response. Nat Rev Mol Cell Biol, 14, 661–72 [DOI] [PubMed] [Google Scholar]
- 78.Mostofa A, Punganuru SR, Madala HR, Srivenugopal KS. (2018).S-phase Specific Downregulation of Human O(6)-Methylguanine DNA Methyltransferase (MGMT) and its Serendipitous Interactions with PCNA and p21(cip1) Proteins in Glioma Cells. Neoplasia, 20, 305–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilljam KM, Feyzi E, Aas PA, Sousa MM, Muller R, Vagbo CB, Catterall TC, Liabakk NB, Slupphaug G, Drablos F, Krokan HE, Otterlei M. (2009).Identification of a novel, widespread, and functionally important PCNA-binding motif. The Journal of cell biology, 186, 645–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Volkova NV, Meier B, Gonzalez-Huici V, Bertolini S, Gonzalez S, Vohringer H, Abascal F, Martincorena I, Campbell PJ, Gartner A, Gerstung M. (2020).Mutational signatures are jointly shaped by DNA damage and repair. Nat Commun, 11, 2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dianov GL, Hubscher U. (2013).Mammalian base excision repair: the forgotten archangel. Nucleic Acids Res, 41, 3483–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bouziane M, Miao F, Bates SE, Somsouk L, Sang BC, Denissenko M, O’Connor TR. (2000).Promoter structure and cell cycle dependent expression of the human methylpurine-DNA glycosylase gene. Mutat Res, 461, 15–29 [DOI] [PubMed] [Google Scholar]
- 83.Mjelle R, Hegre SA, Aas PA, Slupphaug G, Drablos F, Saetrom P, Krokan HE. (2015).Cell cycle regulation of human DNA repair and chromatin remodeling genes. DNA Repair (Amst), 30, 53–67 [DOI] [PubMed] [Google Scholar]
- 84.Xia L, Zheng L, Lee HW, Bates SE, Federico L, Shen B, O’Connor TR. (2005).Human 3-methyladenine-DNA glycosylase: effect of sequence context on excision, association with PCNA, and stimulation by AP endonuclease. J Mol Biol, 346, 1259–74 [DOI] [PubMed] [Google Scholar]
- 85.Bj Ras KO, Sousa MML, Sharma A, Fonseca DM, CK SG, Bj Ras M, Otterlei M. (2017).Monitoring of the spatial and temporal dynamics of BER/SSBR pathway proteins, including MYH, UNG2, MPG, NTH1 and NEIL1–3, during DNA replication. Nucleic Acids Res, 45, 8291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehta KPM, Lovejoy CA, Zhao R, Heintzman DR, Cortez D. (2020).HMCES Maintains Replication Fork Progression and Prevents Double-Strand Breaks in Response to APOBEC Deamination and Abasic Site Formation. Cell reports, 31, 107705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brickner JR, Soll JM, Lombardi PM, Vagbo CB, Mudge MC, Oyeniran C, Rabe R, Jackson J, Sullender ME, Blazosky E, Byrum AK, Zhao Y, Corbett MA, Gecz J, Field M, Vindigni A, Slupphaug G, Wolberger C, Mosammaparast N. (2017).A ubiquitin-dependent signalling axis specific for ALKBH-mediated DNA dealkylation repair. Nature, 551, 389–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boeing S, Williamson L, Encheva V, Gori I, Saunders RE, Instrell R, Aygun O, Rodriguez-Martinez M, Weems JC, Kelly GP, Conaway JW, Conaway RC, Stewart A, Howell M, Snijders AP, Svejstrup JQ. (2016).Multiomic Analysis of the UV-Induced DNA Damage Response. Cell reports [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Montaldo NP, Bordin DL, Brambilla A, Rosinger M, Fordyce Martin SL, Bjoras KO, Bradamante S, Aas PA, Furrer A, Olsen LC, Kunath N, Otterlei M, Saetrom P, Bjoras M, Samson LD, van Loon B. (2019).Alkyladenine DNA glycosylase associates with transcription elongation to coordinate DNA repair with gene expression. Nat Commun, 10, 5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drablos F, Feyzi E, Aas PA, Vaagbo CB, Kavli B, Bratlie MS, Pena-Diaz J, Otterlei M, Slupphaug G, Krokan HE. (2004).Alkylation damage in DNA and RNA--repair mechanisms and medical significance. DNA Repair (Amst), 3, 1389–407 [DOI] [PubMed] [Google Scholar]
- 91.Vagbo CB, Svaasand EK, Aas PA, Krokan HE. (2013).Methylation damage to RNA induced in vivo in Escherichia coli is repaired by endogenous AlkB as part of the adaptive response. DNA Repair (Amst), 12, 188–95 [DOI] [PubMed] [Google Scholar]
- 92.Thapar R, Bacolla A, Oyeniran C, Brickner JR, Chinnam NB, Mosammaparast N, Tainer JA. (2019).RNA Modifications: Reversal Mechanisms and Cancer. Biochemistry, 58, 312–29 [DOI] [PubMed] [Google Scholar]
- 93.Xiong X, Li X, Yi C. (2018).N(1)-methyladenosine methylome in messenger RNA and non-coding RNA. Curr Opin Chem Biol, 45, 179–86 [DOI] [PubMed] [Google Scholar]
- 94.Li X, Xiong X, Wang K, Wang L, Shu X, Ma S, Yi C. (2016).Transcriptome-wide mapping reveals reversible and dynamic N-methyladenosine methylome. Nat Chem Biol [DOI] [PubMed] [Google Scholar]
- 95.Liu F, Clark W, Luo G, Wang X, Fu Y, Wei J, Wang X, Hao Z, Dai Q, Zheng G, Ma H, Han D, Evans M, Klungland A, Pan T, He C. (2016).ALKBH1-Mediated tRNA Demethylation Regulates Translation. Cell, 167, 1897. [DOI] [PubMed] [Google Scholar]
- 96.Ma CJ, Ding JH, Ye TT, Yuan BF, Feng YQ. (2019).AlkB Homologue 1 Demethylates N(3)-Methylcytidine in mRNA of Mammals. ACS Chem Biol, 14, 1418–25 [DOI] [PubMed] [Google Scholar]
- 97.Ueda Y, Ooshio I, Fusamae Y, Kitae K, Kawaguchi M, Jingushi K, Hase H, Harada K, Hirata K, Tsujikawa K. (2017).AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Scientific reports, 7, 42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ougland R, Zhang CM, Liiv A, Johansen RF, Seeberg E, Hou YM, Remme J, Falnes PO. (2004).AlkB restores the biological function of mRNA and tRNA inactivated by chemical methylation. Mol Cell, 16, 107–16 [DOI] [PubMed] [Google Scholar]
- 99.Hashimoto S, Sugiyama T, Yamazaki R, Nobuta R, Inada T. (2020).Identification of a novel trigger complex that facilitates ribosome-associated quality control in mammalian cells. Scientific reports, 10, 3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Juszkiewicz S, Speldewinde SH, Wan L, Svejstrup JQ, Hegde RS. (2020).The ASC-1 Complex Disassembles Collided Ribosomes. Mol Cell, 79, 603–14 e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hegele A, Kamburov A, Grossmann A, Sourlis C, Wowro S, Weimann M, Will CL, Pena V, Luhrmann R, Stelzl U. (2012).Dynamic protein-protein interaction wiring of the human spliceosome. Mol Cell, 45, 567–80 [DOI] [PubMed] [Google Scholar]
- 102.Shostak K, Jiang Z, Charloteaux B, Mayer A, Habraken Y, Tharun L, Klein S, Xu X, Duong HQ, Vislovukh A, Close P, Florin A, Rambow F, Marine JC, Buttner R, Chariot A. (2020).The X-linked trichothiodystrophy-causing gene RNF113A links the spliceosome to cell survival upon DNA damage. Nat Commun, 11, 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang LH, Zhang XY, Hu T, Chen XY, Li JJ, Raida M, Sun N, Luo Y, Gao X. (2020).The SUMOylated METTL8 Induces R-loop and Tumorigenesis via m3C. iScience, 23, 100968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lockhart A, Pires VB, Bento F, Kellner V, Luke-Glaser S, Yakoub G, Ulrich HD, Luke B. (2019).RNase H1 and H2 Are Differentially Regulated to Process RNA-DNA Hybrids. Cell reports, 29, 2890–900 e5 [DOI] [PubMed] [Google Scholar]
- 105.Chakraborty P, Huang JTJ, Hiom K. (2018).DHX9 helicase promotes R-loop formation in cells with impaired RNA splicing. Nat Commun, 9, 4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Niehrs C, Luke B. (2020).Regulatory R-loops as facilitators of gene expression and genome stability. Nat Rev Mol Cell Biol, 21, 167–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dai X, Wang T, Gonzalez G, Wang Y. (2018).Identification of YTH Domain-Containing Proteins as the Readers for N1-Methyladenosine in RNA. Anal Chem, 90, 6380–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seo KW, Kleiner RE. (2020).YTHDF2 Recognition of N(1)-Methyladenosine (m(1)A)-Modified RNA Is Associated with Transcript Destabilization. ACS Chem Biol, 15, 132–39 [DOI] [PMC free article] [PubMed] [Google Scholar]