Abstract
Immune checkpoint inhibitor therapy has revolutionized the treatment of advanced malignancies in recent years. Numerous reports have detailed the myriad of possible adverse inflammatory effects of immune checkpoint therapies, including within the cardiovascular system. However, these reports have been largely limited to myocarditis. The critical role of inflammation and adaptive immunity in atherosclerosis has been well characterized in preclinical studies, and several emerging clinical studies indicate a potential role of immune checkpoint targeting therapies in the development and exacerbation of atherosclerosis. In this review, we provide an overview of the role of T-cell immunity in atherogenesis and describe the molecular effects and clinical associations of both approved and investigational immune checkpoint therapy on atherosclerosis. We also highlight the role of cholesterol metabolism in oncogenesis and discuss the implications of these associations on future treatment and monitoring of atherosclerotic cardiovascular disease in the oncologic population receiving immune checkpoint therapy.
Keywords: Inflammation, Immunology, Cardiovascular Disease, Cardio-Oncology, Atherosclerosis
Condensed Abstract
In the last decade, immune checkpoint inhibitors have been revolutionary in the field of oncology and has become the standard of care in numerous advanced malignancies. As T cell immunity plays an integral role in atherogenesis through promotion of inflammation, preclinical studies and recently emerging clinical studies indicate a role of immune checkpoint inhibitors in the development of atherosclerosis. This review provides a comprehensive analysis of the molecular effects and clinical associations between immune checkpoint altering therapy and atherosclerotic cardiovascular disease (ASCVD) risk, and identifies important considerations to the surveillance and treatment of atherosclerosis in patients receiving immune checkpoint therapy.
Introduction: Emergence of Immune Checkpoint Inhibitors
Modern day immunotherapy originates from the cancer immunosurveillance hypothesis, which states that immune cells are responsible for the surveillance and elimination of nascent transformed cells in host tissues(1). Enhanced appreciation for the ability for tumor cells to evade immune surveillance (2) galvanized efforts to develop therapies restoring the host immune system’s antineoplastic defenses. The culmination of these efforts led to the development of immune checkpoint inhibitors (ICIs) that restore the host T cell immune response against cancer cells. The development of ICIs in the last decade has rapidly revolutionized cancer therapy, with applications of ICIs targeting CTLA-4 and PD-1/PDL-1 pathways in cancers including melanoma, non-small cell lung cancers (NSCLC), and urothelial carcinomas(3-5).
When ICIs were being approved, it was anticipated that diffuse leveraging of the immune system would lead to off-tumor adverse events, which occur in up to 70% of patients and may affect any organ system, but are typically easily managed. Initial reports detailing the potential cardiovascular effects of ICIs focused principally on the uncommon, but highly morbid, occurrence of myocarditis, mediated by direct T cell infiltration of the myocardium expressing PD-L1(6). More recently, the potential for ICIs to affect the cardiovascular system beyond myocarditis has been reported. Specifically, preclinical evidence supports that treatment with ICIs can theoretically promote the development and acceleration of atherosclerosis, and recent emerging clinical evidence suggests an increase in atherosclerotic-related cardiovascular events in patients receiving ICI therapy and a possible connection between ICIs and increased atherosclerotic cardiovascular disease (ASCVD) risk (7).
The role of inflammation in atherosclerosis has been well established, with the CANTOS, LoDoCo, and COLCOT trials demonstrating that targeting immune responses can improve clinical cardiovascular outcomes(8-10). These studies showed that targeting innate immunity, such as inhibition of IL-1β via canakinumab and neutrophil function via colchicine, may mitigate the progression of ASCVD. As adaptive immunity via T cell activation has also been shown to play a crucial role in the development and progression of atherosclerosis, ICIs may have important implications on atherosclerotic disease (11). This review aims to describe the role of T-cell mediated immunity in atherogenesis, the molecular implications of various pathways of immune checkpoint alteration in atherosclerosis, the current clinical associations between treatment with ICIs and atherosclerosis, and potential treatment avenues.
Overview of T Cell Mediated Immunity and Key Immune Checkpoint Pathways
1. Overview of T Cell Mediated Immunity
Tumor associated antigens are recognized and phagocytosed by antigen presenting cells (APCs) such as dendritic cells and macrophages. These antigens are presented via major histocompatibility complex (MHC) molecules on the surface of APCs, which interact with T cell receptors (TCRs) and serve as the primary stimulatory signal leading to the intracellular cascade that activates naïve CD4+ and CD8+ T cells. However, full T cell activation is dependent on the presence of co-stimulatory signals involving the binding of CD28 on T cell surfaces to B7-1 (CD80) or B7-2 (CD86) on APCs (Figure 1A).
Figure 1. T Cell Activation and Effect of Immune Checkpoints.
A. T Cell Activation. Antigen presenting cells (APCs) present antigens via major histocompatibility complex (MHC) molecules that bind to T cell receptors (TCR) on naïve CD4+ and CD8+ T cells. T cell activation requires the binding of costimulatory molecules B7.1(CD80) or B7.2(CD86) on APCs to CD28. Naïve CD8+ T cells are activated into cytotoxic T cells that secrete perforins and granzymes, leading to apoptotic cascades in target cells. The presence of particular cytokines and growth factors in the microenvironment determine naïve CD4+ T cell differentiation.
B. Immune Alterations in Cancer and Effect of Immune Checkpoint Inhibitors. Prolonged inflammation in cancer leads to T cell exhaustion that promotes recruitment of Treg cells and increased expression of cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 receptor (PD-1) on T cells. Programmed cell death protein 1 receptor ligand (PD-L1) on tumor cells binds to PD-1 to decrease the production of inflammatory cytokines. Immune checkpoint inhibitors enhance tumor cell killing by targeting PD-1, PD-L1 and CTLA-4 to restore T cell activation and inflammatory cytokine production. Created with BioRender.
Subsequently, the cytokine composition of the surrounding environment determine the fate of T cell differentiation into various subclasses, including CD8+ cells into cytotoxic T cells, and CD4+ T cells into helper T cells (Th1, Th2 , and Th17) and regulatory T cells (Treg) (Figure 1A). Cytotoxic T cells secrete cytotoxins and promote apoptosis of its target cells. Of the CD4+ T cell derivatives, Th1 promotes cell-based immunity by activating macrophages and cytotoxic T cells, while Th2 cells promote antibody-mediated immunity and the recruitment of eosinophils. Th17 cells assist with extracellular pathogen clearance at mucosal surfaces and promote antibody production (12). Treg cells promote peripheral tolerance by secreting inhibitory cytokines, promoting cytolysis, and suppressing the activation, proliferation and cytokine production of CD4+ and CD8+ T cells (13).
2. T Cell Anergy and Co-Inhibitory Signaling
Several mechanisms exist to prevent immune overactivation and promote self-tolerance. Activation of T cells requires binding of a second co-stimulatory signal with stimulatory checkpoint molecules such as CD28 to B7-1/B7-2, without which T cells would remain anergic. Another class of immune checkpoint molecules induce co-inhibitory signals, including cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and the programmed cell death protein −1 (PD-1) receptor.
CTLA-4 is an analog similar to CD28 that is constitutively expressed on Treg cell surfaces and upregulated on other T cell classes after initial activation. It exerts inhibitory effects in the early phases of T cell activation within lymphoid tissues by directly competing with CD28 to bind to B7-1 and B7-2 ligands on APCs with higher affinity. Its binding launches a signaling cascade preventing TCR signal transduction (14). Later studies showed a signaling-independent mechanism of inhibition via activation of trans-endocytosis that removes B7 ligands from the surfaces of APCs (15). CTLA-4 expression has been demonstrated in T cells of atherosclerotic plaque (16), and CTLA-4 inhibition has been linked to multiorgan lymphocyte infiltration, including the heart, leading to ICI fulminant myocarditis (17).
The PD-1 receptor is present on activated T cells and exerts an inhibitory effect via binding its two ligands PD-L1 (CD274) and PD-L2 (CD273) in peripheral tissues. While PD-L2 is present mostly on macrophages and dendritic cells, PD-L1 is expressed in hematopoietic cells and tissue cells in various organs, including in cardiomyocytes, vascular endothelial cells, and leukocytes within atherosclerotic plaque (18-20). The binding of PD-1 to PD-L1 or PD-L2 leads activation of the Akt pathway that decreases the production of inflammatory cytokines and cell survival proteins (21). Disruption in the PD-1/PD-L1 pathway in PD-1 knockout mice led to rapid onset autoimmune mediated dilated cardiomyopathy with diffuse IgG deposition, and is a potential mechanism of cardiotoxicity in ICI induced myocarditis (19).
3. Immune Checkpoint Alterations in Cancer and the Advent of ICIs
In the tumor microenvironment, tumor cells promote recruitment and production of CTLA-4 expressing Treg cells via TGF-β secretion. (22). In addition, prolonged activation of T cells in the cancer immune response leads to exhaustion, where T cells upregulate inhibitory molecules including PD-1 and CTLA-4 to limit their own proliferative potential (23) (Figure 1B). The surrounding tissue hypoxia promotes an increase in PD-L1 expression in various tumors (18).
Targeted ICIs utilize the altered oncologic expression of immune checkpoints to augment the host immune response, beginning with the approval of the CTLA-4 checkpoint inhibitor ipilimumab (Yervoy) for use in metastatic melanoma in 2011 (4). Shortly after came approval for PD-1 inhibitors pembrolizumab (Keytruda) and nivolumab (Opdivo) (3, 5) (Figure 1B). Since their initial FDA approval, the indications for ICIs have expanded rapidly to include NSCLCs, non-Hodgkin’s lymphoma, urothelial carcinoma, colorectal cancers, and more. Several investigational therapies targeting other immune checkpoints are in various stages of development (24). The pathways utilized in anti-CTLA-4 and PD-1/PD-L1 inhibitor therapies as well as those of investigational immune checkpoint agents have been implicated in atherogenesis (Central Illustration, Table 1).
Central Illustration: Summary of Immune Checkpoint Alterations and their Effects on Atherogenesis.
CTLA-4 and PD-1/PDL-1 blockade are well-studied examples of co-inhibitory molecule blockade that worsen atherosclerosis. CD40/CD40L agonism and GITR agonism are examples of co-stimulatory molecule agonism that worsen atherosclerosis. CD-47 blockade restores efferocytosis and may be atheroprotective. APC indicates antigen presenting cell; MHC, major histocompatibility complex, TCR, T cell receptor, CTLA4, cytotoxic T-lymphocyte associated protein 4; PD-1, Programmed cell death protein 1 receptor; PD-L1, Programmed cell death protein 1 receptor ligand; SIRP-α, signal-regulatory protein alpha; CD, cluster of differentiation; GITR, glucocorticoid-induced tumor necrosis factor family-related protein; GITRL, glucocorticoid-induced tumor necrosis factor family-related protein ligand; Treg, regulatory T cell; VCAM, vascular cell adhesion molecule; and MMP, matrix metalloproteinase. Created with BioRender.
Table 1:
Summary of Immune Checkpoint Pathway Alterations and Effects on Cancer Therapy and ASCVD
| Immune Checkpoint Class |
Immune Checkpoint Target |
Effect on Cancer Therapy† | Effect on ASCVD† | Example Therapies |
|---|---|---|---|---|
| Co-Inhibitory Signal Blockade | CTLA-4 | Restores T cell activation and enhances cancer cell destruction (3, 24) | -Increases atherosclerotic lesion size (63-65) -Progression to advanced phenotype (65) -Decreases collagen content (65) |
Ipilimumab (4) Tremelimumab (151)* Zalifrelimab (AGEN1884)* (152) |
| PD-1 and PD-L1 | -Increases atherosclerotic lesion size (67-69) -Enhances TNF-α secretion (67, 68) -Enhances lesion helper T cell, cytotoxic T cell and macrophage activation (67-69) |
Anti-PD-1: nivolumab
(5), pembrolizumab (3), cemiplimab (153), spartalizumab* (154), camrelixumab* (154),
tislelizumab* (154) Anti- PD-L1: atezolizumab (155), avelumab (156), durvalumab (157), cosebelimab*(154),sugemalimab*(154), CX-072*(154) |
||
| TIM-3* | -Increases atherosclerotic lesion size (105) -Increases lesion macrophage content -Decreases lesion Treg content -Enhances TNF-α and IFN-γ secretion in combination with anti-PD-1 in human cells (106) |
Cobolimab* (24) BMS-986258*(158), MBG453* (158), TSR-022*(158), Sym023*(158), BGB-A425* (158) | ||
| LAG-3* | -Effect unknown, a marker positively correlated with ASCVD in a human observational study (109) | Relatlimab*, Eftilagimod alpha*, Tebotelimab*, Favezelimab*, LAG525*, REGN 3767* (159) | ||
| Co-Stimulatory Signal Agonism | GITR* | -Enhances macrophage activity (90) -Promotes CD4+ and CD8+ T cell activation (89, 90) -Decreases immunosuppressive ability of Treg (89) |
-Promotes macrophage secretion of atherogenic
cytokines (94, 95) -Increases MMP production (95) -Enhances plaque leukocyte recruitment via ICAM-1 (95) -GITR inhibition decreased plaque size and increased plaque stability (94) |
AMG228*, MEDI-1873*, BMS-986156* (160) |
| CD40 and CD40L* | -Enhances antigen presentation by APCs (74) -Promotes B cell proliferation and antibody production (74) -Promotes tumor cell apoptosis (75) |
-Increases secretion of IL-1β, IL-6,
TNF-α, IFN-γ (73) -Endothelial cell and smooth muscle cell activation in human cells (78) -Increases leukocyte recruitment by expression of VCAM-1 and P-selectin (77) -Increases MMP production (77) -Promotes foam cell production (77) Inhibition of CD40L: -increased plaque stability (82-85) - inconsistently decreased plaque size (82, 85) vs no change (83, 84) -reduced Th1 polarization and IFN-γ secretion (87) -reduced atherothrombosis (87) |
Selicrelumab* (161), dazetuzumab* (162), CP-870893*(162) , JNJ-107*(162), APX005M (162)* | |
| CD27 and CD70* | -Enhanced T cell expansion and survival (96) -Enhanced memory T cell production (96) -Polarization toward IFN-γ producing effector T cells (96) |
-decreased lesion size (98, 99) -enhanced macrophage efferocytosis (98) -enhanced oxLDL clearing (98) -promotion of Treg survival (98, 99) |
Anti-CD27: Varlilumab*, AMG-172* Anti-CD70: Cusatuzumab*, BMS-936561* (163) |
|
| ICOS and ICOSL* | -Promotion of cytotoxic T cells (100) -Enhancing effector T cell function (100) -Promotion of Treg activity (100) |
Inhibition of ICOS: - increased atherosclerotic plaque size (101, 102) -reduced atherosclerotic Treg population (102) -increased IFN-γ secretion (101) -decreased IL-10 secretion (101) |
Anti- ICOS
Agonists: Feladilimab*,vopratelimab (100) Anti- ICOS Antagonists: MEDI-570*, KY1044 *(100) |
|
| “Don’t eat me” Blockade | CD47* | -Blockade of interaction with SIRP-α
(111) -Enhances phagocytosis of apoptotic cells and debris (111) -Reduces inflammation (111) |
-Reduces atherosclerotic lesion size and
inflammation (112, 113) -Decreases macrophage response to IL-1β and IFN-γ (113) -Improves clearance of VSMCs (114) |
Magrolimab* (115), SRF231*(164), AO-176*(164), CC-90002* (164) |
denotes investigational therapies.
denotes findings of preclinical/animal studies unless otherwise specified. AGEN indicates Agenus; AMG, Amgen; AO, Arch Oncology; APX, Apexigen; APCs, antigen presenting cells; ASCVD, atherosclerotic cardiovascular disease; BGB-A, BeiGene; BMS, Bristol Myers Squibb; CC, Celgene; CD, cluster of differentiation; CTLA-4, cytotoxic T-lymphocyte associated protein; CX, CytomX; GITR, glucocorticoid-induced tumor necrosis factor receptor-related protein; ICAM-1, intracellular adhesion molecule 1; IFN-γ, interferon gamma; IL, interleukin; JNJ, Johnson & Johnson; KY, Kymab; LAG-3, lymphocyte-activation gene 3; MMP, matrix metalloproteinase; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; REGN, Regeneron Pharmaceuticals; SIRP-α, signal regulatory protein alpha; SRF, Surface Oncology; Sym, Symphogen; TIM-3, T cell immunoglobulin and mucin-domain-containing-3; TNF-α, tumor necrosis factor alpha; Treg, regulatory T cell; VCAM-1, vascular cell adhesion molecule 1; and VSMCs, vascular smooth muscle cells.
Role of Immune Cells in Atherosclerosis and Effects of Current Immune Checkpoint Inhibitors
1. Overview of Atherogenesis
The pathophysiology of atherosclerosis begins with damaged endothelial cell expression of cellular adhesion molecules that attract monocytes to enter the subendothelium, where they transform into macrophages that are polarized into subtypes depending on their microenvironment. The classically activated pro-inflammatory phenotype is promoted by exposure to free fatty acids, oxidized lipids, and factors such as IFN-γ whereas the alternatively activated anti-inflammatory phenotype is promoted by factors such as IL-4, IL-13, and IL-10. Pro-inflammatory macrophages predominate in early atherosclerotic plaque and are responsible for the accumulation of intracellular lipids, formation of foam cells, and secretion proinflammatory cytokines such as IL-1β and IL-6 (25) (Figure 2). Anti-inflammatory macrophages promote collagen formation and effective lipid clearance, and are associated with atherosclerosis regression (26). Necrotic core formation is the hallmark of chronic inflammation in atherosclerosis, and is rich in lipids and cellular debris as a result of foam cell and vascular smooth muscle cell (VSMC) apoptosis and necrosis (27).
Figure 2. Atherogenesis and Effects of T Cell Activation.
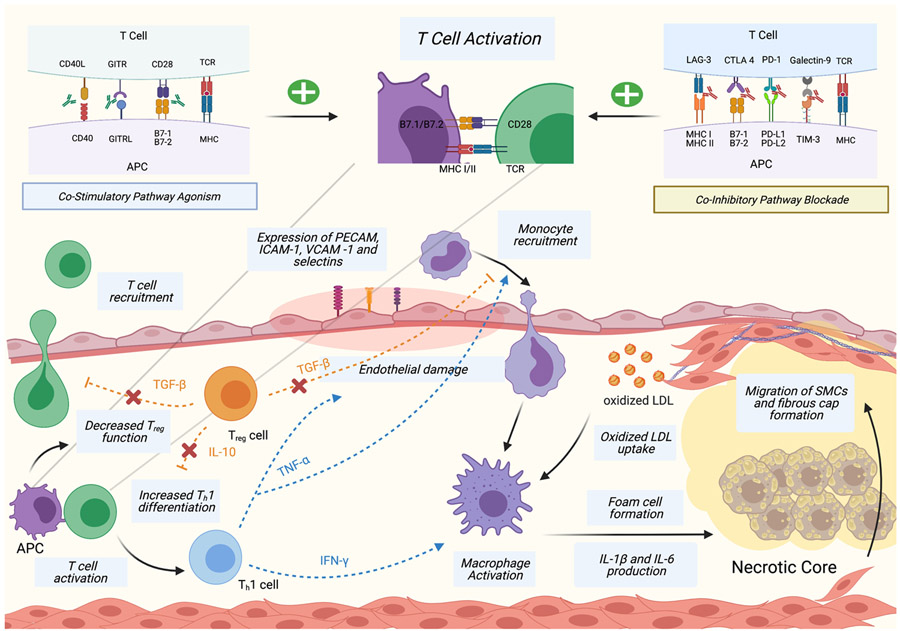
Damaged endothelial cells express platelet endothelial cell adhesion molecules (PECAM), intracellular adhesion molecules (ICAM), vascular cell adhesion protein (VCAM) and selectins that recruit monocytes to the subendothelium. Monocytes are activated into macrophages that consume oxidized low density lipoproteins (ox-LDL) and produce inflammatory cytokines, leading to foam cell and necrotic core formation. T cells are recruited to the subendothelium and activated by APCs. Activation of T helper 1 (Th1) cells leads to tumor necrosis factor alpha (TNF-α) secretion that promotes monocyte recruitment and endothelial damage, and interferon gamma (IFN-γ) secretion that promotes macrophage activation. T cell activation decreases the function of regulatory T cells (Treg) that normally decreases T cell and monocyte recruitment via transforming growth factor-beta (TGF-β) secretion and reduces Th1 differentiation via interleukin-10 (IL-10) secretion. T cell activation is enhanced by immune checkpoint altering antibodies. Created with BioRender.
T cell activation is also integral to atherogenesis. During early atherosclerosis, APCs present atheroma-related antigens to naïve T cells in lymphoid tissues, leading to T cell migration towards the plaque (28). The initial recruitment of T cells is nonspecific, and they undergo further selective activation and clonal expansion into atherogenic T cell subtypes (28, 29). Atheroma-related T cell antigens have been difficult to identify, but include oxidized LDL (oxLDL) particles, heat shock proteins, and Apolipoprotein B (30). OxLDL are abundant in atheromatous plaque, and serves as an antigen triggering T cell autoimmune response that leads to inflammation and macrophage activation via IFN-γ secretion (31). CD8+ cytotoxic T cells, Th1 cells, Th2 cells, Th17 cells, and Treg cells have all been identified in atherosclerotic plaques (30).
2. Role of Differentiated T cells in Atherogenesis
Th1 cells are the predominant CD4+ T cell present in plaques (32) and the most directly associated with atherogenesis due to their production of inflammatory cytokines, including IFN-γ and TNF-α (33, 34) (Figure 2). The production of IFN-γ enhances recruitment of macrophages and T cells and promotes macrophage polarization, cytokine secretion and foam cell formation (35). IFN-γ is also thought to inhibit vascular smooth muscle cell proliferation, thereby contributing to decreased plaque stability (36). The atherogenic role of IFN-γ is most clearly demonstrated in a study where mice with dysfunctional IFN-γ receptors developed smaller and more phenotypically stable atheromas compared to controls (37). TNF-α promotes atherosclerosis through leukocyte recruitment, inflammatory cytokine production, and promotion of endothelial cell damage and oxidative stress (38). TNF-α deficient mice have been shown to exhibit smaller plaque lesions (33), and the presence of TNF-α is associated with increased lesion necrosis and more advanced plaque progression in mice (39). The inhibition of Th1 differentiation in mice is also atheroprotective and reduces the amount of IFN-γ detected in plaques (40).
Treg cells’ atheroprotective role has been well studied, and their effects are primarily mediated through secretion of TGF-β and IL-10 (Figure 2). TGF-β inhibits recruitment and activation of T-cells and macrophages, and increases plaque stability by promoting VSMC proliferation (41). Mice with defective TGF-β receptors have larger atherosclerotic lesions with increased T cell and macrophage composition, increased IFN-γ expression, and more vulnerable plaque phenotype (42). IL-10 reduces Th1 differentiation and prevents the recruitment and cytokine secretion of T cells and macrophages (43). A study with IL-10 deficient mice demonstrated increased susceptibility to atherosclerosis, and higher T cell infiltration and IFN-γ expression compared to controls (44). In addition, Treg secretion of IL-10 promotes the transformation of macrophages from the pro-inflammatory to the anti-inflammatory phenotype, potentiating its atheroprotective effect (45). Ait-Oufella et al. demonstrated that deficiency in Treg was associated with increased plaque size and more advanced plaque phenotype in mice, and that subsequent co-transference of Treg reduced inflammatory cell infiltration and plaque size (46). In addition, the number of Treg cells was inversely correlated with plaque vulnerability in human carotid arteries (47).
The roles of the remaining T cell subtypes in atherosclerosis are less well defined. Th2 cells oppose the production of IFN-γ, which suggested a potentially atheroprotective role. This was further supported as deficiency in IL-5, one of the primary Th2 secreted cytokines, was shown to accelerate atherosclerosis development in mice (48, 49). However, the deletion of another Th2 cytokine, IL-4, has been shown to decrease plaque lesion size in mice (50). Thus, the role of Th2 cells in atherogenesis remains unclear.
Th17 cells have received considerable attention in atherosclerosis in recent years, though its definitive role is still controversial. A study analyzing human coronary atherosclerotic plaques demonstrated that IL-17, the principal cytokine released by Th17, worked synergistically with IFN-γ to promote inflammation by increasing secretion of IL-6 (51). However, cytokine secretion of Th17 cells relies on environmental context, and subsets secrete IL-17 in conjunction with the anti-inflammatory IL-10, making the role of Th17 cells in atherosclerosis difficult to define (52). Several studies have indicated an atherogenic role to IL-17,(53) while others suggest an atheroprotective role and promotion of plaque stability via Type I collagen production (54, 55). Despite these inconclusive findings, the ratio between Th17 and Treg cells have been implicated in atherosclerosis progression, with increased Th17 and decreased Treg levels observed in patients with coronary atherosclerosis. Recent data suggests that inhibition of CD69, a key molecule expressed on early T lymphocytes regulating T cell differentiation, leads to elevated Th17/Treg ratios and exacerbation of atherosclerosis in mice (56). Indeed, the delicate balance between Th17 and Treg cells has important implications on autoimmune conditions and off-target adverse events related to ICI therapy, including myocarditis (57, 58).
Despite CD8+ T cells comprising about 50% of lymphocytes in atherosclerotic lesions (59), fewer studies have addressed the role of CD8+ cells. Cytotoxic CD8+ T cells have been shown to promote atherosclerosis in mouse models through IFN-γ secretion (60) and necrotic core formation by perforin and granzyme B-mediated apoptosis of macrophages (61). However, another mouse model suggest an atheroprotective role by promoting cytolysis of APCs (62).
Immune Checkpoint Therapies and Atherosclerosis
1. B7-1/B7-2 and CD28/CTLA-4 Pathway
The immunoregulatory effects of CTLA-4 and B7-1/B7-2 binding suggests an atheroprotective role for this pathway. The use of abatacept, a synthetic analog of CTLA-4, prevented CD4+ cell activation and reduced atherosclerosis development in murine femoral arteries by 78%, whereas the administration of CTLA-4 blocking antibodies increased atherosclerotic lesion sizes (63). Similarly, mice with elevated homocysteine levels had larger atherosclerotic plaque sizes associated with decreased membrane expression of CTLA-4, and pretreatment of these mice with abatacept ameliorated plaque development with reduced IFN-γ and IL-2 production and decreased macrophage content (64). Matsumoto et al. demonstrated that transgenic mice with constitutive CTLA-4 overexpression exhibited a significant reduction in atherosclerotic lesion size at the aortic root (16). In this study, CTLA-4 overexpression appeared to reduce atherosclerosis by decreasing plaque inflammation, as evidenced by a 38% decrease in macrophage accumulation and 42% decrease in CD4+ T cell infiltration, and downregulation of T cell proliferative capacity and proinflammatory cytokine production (16).
Modeling the role of ICIs in atherosclerosis, Poels et al. evaluated the role of antibody mediated CTLA-4 inhibition on atherogenesis. They demonstrated a two-fold increase in the size of atherosclerotic lesions in mice treated with CTLA-inhibiting antibodies, which was primarily mediated by a transition to an activated T cell profile without significant alterations in the macrophage inflammatory profile. CTLA-4 inhibition was also associated with plaque progression to more advanced phenotypes, with decreased collagen content and increased intimal thickening and necrotic core areas (65).
2. PD-1 and PD-L1/PD-L2 Pathway
PD-1 has been shown to suppress Th1 cytokine production and promote the development of Treg cells, suggesting a potentially atheroprotective role for this pathway (66). In hyperlipidemic mice, both PD-L1/PD-L2 deficiency and PD-1 receptor inhibition have been associated with increased atherosclerotic lesion size, increased plaque T cell activation , and enhanced TNF-α secretion (67, 68) . In contrast to CTLA-4 inhibition, PD-1 inhibition also exhibited increased lesion macrophage content, and enhanced the cytotoxicity of lesion CD8+ T cells (68). PD-1 deficiency has been shown to activate both CD4+ T cells and regulatory T cells, but the net effect was still exacerbated atherosclerotic lesion growth and increased plaque T cell infiltration (69).
In humans, several studies have observed decreased expression of PD-1 or its ligands in patients with coronary artery disease (CAD) and acute coronary syndrome (ACS), suggesting its protective role in atherogenesis and progression to advanced plaque phenotype (70, 71). At baseline, human atherosclerotic plaque T cells express high levels of PD-1 as a marker of T cell exhaustion in chronic inflammation (72). Thus, the delicate co-existence of PD-1 expressing T cells with activated T-cells within human plaques raises concern that PD-1 inhibition would lead to exacerbation of atherosclerosis.
Investigational Immune Checkpoint Targets and Atherosclerosis
1. CD40-CD40L Pathway
CD40 is a T cell costimulatory molecule and a member of the TNF receptor family that is present on APCs as well as non-immune cells such as endothelial cells, VSMCs, and platelets (73). Its classic ligand, CD40L, is expressed on activated CD4+ T cells. The binding of CD40 to CD40L promotes B cell proliferation and improves dendritic cell antigen presentation and T cell activation (74). CD40/CD40L ligation leads to recruitment of TNF receptor associated factors (TRAFs), that lead to downstream increases in atherogenic cytokines including IL-1β, IL-6, TNF-α and IFN-γ, which enhances the activities of macrophages (73).
Activation of the CD40/CD40L pathway has been shown to enhance antitumor immune response and promote tumor cell apoptosis and has been explored in cancer immunotherapy(75). Several ongoing Phase I and II clinical trials are evaluating the effect of anti-CD40 agonist antibodies, such as elicrelumab and dacetuzumab in various hematologic and solid tumor malignancies (76).
Several studies have explored role of the CD40/CD40L pathway in atherosclerosis. CD40L ligation to CD40 promotes thrombosis due to its activating effects on endothelial cells and platelets and its promotion of tissue factor production(77, 78). Endothelial cells exposed to CD40L exhibit increased expression of adhesion molecules such as VCAM-1 and P-selectin that enhances leukocyte recruitment, a key process in atherosclerosis initiation (79). CD40L has also been shown to promote foam cell formation in atherosclerotic plaques (80). Bruemmer et al. observed that in human iliac arteries, the expression of CD40 increased with atherosclerotic stage, and the largest increases occurred in the early stages of atherosclerosis (81). In later stages of atherosclerosis, the CD40/CD40L pathway stimulation increased secretion of matrix-degrading metalloproteinases that increase plaque rupture vulnerability (78).
Several preclinical studies have explored the inhibition of CD40/CD40L in reducing atherosclerosis. Blockade of CD40L either via antibodies or knock out models resulted in decreased leukocyte composition in the atherosclerotic plaque and phenotypic evidence of increased plaque stability (82-85). However, CD40L blockade was inconsistent in achieving plaque size reduction, where studies by Bavendiek et al. and Mach et al. observed reduced plaque size (82, 85), while two studies from Lutgens et al. did not (83, 84). In addition, CD40L inhibitory antibodies has been shown to increase the risk of thromboembolic events via platelet activation, limiting its systemic use in atherosclerosis treatment (86). Recent data from Lacy et al. demonstrated differing roles for CD40L depletion on atherosclerosis depending on cell source, with T cell CD40L inhibition leading to reduced Th1 polarization and IFN-γ secretion, whereas platelet-CD40L inhibition led to reduced atherothrombosis (87). These data hold promise for the development of cell-specific CD40L inhibition in the treatment of atherosclerosis.
With further research, CD40L/CD40 blockade may be pursued as a treatment for atherosclerosis. Conversely, with CD40/CD40L agonists being studied in clinical trials for cancer immunotherapy, their possible implications on cardiovascular disease must be carefully considered.
2. GITR-GITRL Pathway
Similar to CD40, glucocorticoid-induced TNF receptor-related protein (GITR) is a T cell costimulatory molecule present on T and NK cells and binds to its ligand, GITRL, expressed on APCs and endothelial cells (88). GITR-GITRL signaling promotes macrophage activity, increases CD8+ T cell cytotoxicity, and enhances effector T cell activation by increasing IFN-γ and IL-2 secretion (89, 90). Simultaneously, Treg cells experience a decrease in immunosuppressive effect downstream of GITR-GITRL interaction (89). The efficacy of tumor inhibition by GITR agonists was directly correlated with intratumor CD4+ and CD8+ levels, suggesting its therapeutic potential in NSCLC, renal cell carcinoma, and melanoma (91). Several phase I clinical trials have shown limited therapeutic response as monotherapy, but possible synergistic activity in combination with anti-PD-1 agents (92).
Macrophage and effector T cell activation and Treg suppression by the GITR/GITRL pathway suggests a proatherogenic role. GITR+ macrophages, T cells, and endothelial cells have been identified within atherosclerotic plaques (93, 94). Moreover, Shami et al. demonstrated increased GITR expression in carotid artery plaques in patients with symptomatic cerebrovascular disease, which correlated with increased plaque vulnerability (94). Kim et al. showed that GITR agonist antibodies promoted macrophage production of pro-atherogenic cytokines, plaque-destabilizing metalloproteinases, and intracellular cell adhesion molecule 1 (ICAM-1) (95). Conversely, GITR deficiency has been associated with decreased plaque size and increased plaque stability in mice (94). Thus, GITR agonism may be beneficial in combination with other ICIs such as PD-1 in cancer immunotherapy, but may worsen atherosclerosis, though studies of the direct effect of GITR agonistic monoclonal antibody on atherosclerosis are needed.
3. Other Costimulatory Pathways: CD27/CD70 and ICOS
Agonism of several other co-stimulatory pathways are being explored as immunotherapy. CD27 is a transmembrane glycoprotein that is part of the TNF superfamily that binds to CD70 (also known as CD27L) expressed on activated T cells. Its agonism enhances T cell expansion and promotion of memory T cells, with a predilection toward IFN-γ production (96). It has gained interest as a target for immunotherapy, with ongoing phase I/II trials for anti-CD27 agonists such as varlilumab (97). With its promotion of IFN-γ production, initial studies postulated that CD27/CD70 agonism would worsen atherogenesis. Surprisingly, chronic inflammation from CD70 overexpression was atheroprotective, as the CD27/CD70 pathway has shown important roles in macrophage efferocytosis, oxLDL clearing, and promotion of atheroma Treg survival (98, 99).
ICOS, or inducible costimulator, is a protein involved in CD4+ and CD8+ differentiation, survival and proliferation, promoting activation of cytotoxic T cells, enhancing effector T cell production of IL-4, IL-5, IL-10 and TNF-α, as well as promoting Treg activity (100). Given its prominent role in the survival of both pro-tumor and anti-tumor T cells, both ICOS agonists and antagonists are in early phase cancer immunotherapy trials (100). In preclinical studies, ICOS inhibition was associated with aggravated atherosclerosis via a reduced Treg population, as well as enhanced IFN-γ production and diminished IL-10 production (101, 102). Thus, contrary to CD40 and GITR, further study of CD27/CD70 and ICOS agonism may one day prove beneficial in atherosclerosis.
4. Investigational Co-Inhibitory Molecules: TIM-3 and LAG-3
TIM-3 is a negative regulatory immune checkpoint present on effector T cells, Treg cells, and dendritic cells. It binds to four separate ligands, most notably to galectin-9 that triggers Th1 and CD8+ T cell death (103, 104). Ongoing clinical trials are studying anti-TIM-3 antibodies such as cobolimab both as monotherapy or in combination with anti-PD-1 inhibitors(24).
Several preclinical studies have suggested that TIM-3 is a negative regulator of atherosclerosis. Foks et al. found that TIM-3 expression was increased in mice fed with atherogenic diet, and that TIM-3 antibody blockade was associated with larger atherosclerotic plaque size, increased lesion macrophage content, and decreased lesion Treg cells (105). In atherosclerotic lesions, CD8+ T cells exhibits co-expression of PD-1 and TIM-3, and inhibiting both immune checkpoints was associated with increase TNF-α and IFN-γ and decrease in IL-10 and IL-4) when compared to singular PD-1 or TIM-3 blockade (106). Thus, the development of anti-TIM-3 checkpoint inhibitors pose theoretical risks of exacerbating atherosclerosis.
LAG-3 is a cell surface protein analogous to CD4 present on T cells and dendritic cells that binds MHC class II molecules and serves as an inhibitory signal. It directly competes with TCR and CD4 binding to MHC class II, suppresses T cell expansion and promotes CD4+ T cell differentiation into Treg (107). LAG-3 targeting ICIs, such as relatlimab, are being investigated in Phase II and III clinical trials (108). One observational study of the Multiethnic Study of Atherosclerosis (MESA) cohort demonstrated that patients with CAD had higher LAG-3 levels, and LAG-3 was a significant coronary artery disease risk predictor (109). Despite this positive correlation, no causal relationship between LAG-3 and atherosclerosis has been established. Preclinical studies are needed to evaluate whether increased LAG-3 expression is a contributory or compensatory mechanism in atherosclerosis.
5. CD47-SIRPα Pathway
In addition to T cell mediated immune checkpoint inhibitors, novel classes of immune check therapies being explored include the macrophage-mediated immune checkpoint CD47. CD47 is an immunoglobulin-like molecule that binds to signal regulatory protein alpha (SIRPα) and impairs phagocytosis. In areas with high rates of apoptosis and cell turnover, such as within tumors and atherosclerotic necrotic cores, effective clearance of apoptotic cellular debris helps prevent further inflammatory response (110). This process, known as “efferocytosis” refers to programmed cell removal by which macrophages detect cell surface markers that signal phagocytosis, collectively termed “eat me” signals (111). By contrast, cells may express markers that impair phagocytosis, termed “don’t eat me” signals, such as CD47.
Kojima et al. demonstrated an upregulation of CD47 in both murine and human atherosclerotic plaque, particularly in the necrotic core (112). Treatment of atherosclerotic models with CD47 inhibitory antibodies markedly reduced atherosclerosis by restoring efferocytosis, evidenced by the enhanced removal of diseased vascular smooth muscles and macrophages in-vivo (112, 113). In addition, CD47 inhibition was shown to downregulate genes implicated in macrophage response to IL-1 and IFN-γ, leading to significant reduction in atherosclerotic inflammation in PET/CT imaging of mouse models (113). CD47 has also been suggested to reduce the ability of macrophages to remove opsonized targets, including the removal of opsonized clonal smooth muscle cells thought to give rise to the majority of cells in atherosclerotic plaques (114).
In recent years, CD47 inhibitor therapies have been used in clinical trials aimed at increasing tumor cell recognition and phagocytosis by macrophages. Magrolimab, the first-in-class anti-CD47 antibody, showed promising results in Phase 1B trials of relapsed and refractory non-Hodgkin’s lymphoma (115). It recently gained breakthrough therapy designation by the FDA, and ongoing trials are evaluating its efficacy in various hematologic and solid tumor malignancies. Interestingly, a small retrospective analysis on the non-Hodgkin’s lymphoma trial participants demonstrated a reduction of FDG uptake in the carotid arteries after 9 weeks of Magrolimab treatment, implying that CD47 inhibition reduces vascular inflammation (116). Thus, CD47 inhibition may be a shared pathway against both oncogenesis and atherogenesis.
Macrophage efferocytosis has also been shown to be influenced by Treg cell activity. Proto et al. showed that Treg cell expansion enhanced efferocytosis via expression of IL-13, which in turn increased IL-10 production in macrophages and led to apoptotic cell engulfment and clearance (117). Since existing PD-1 and CTLA-4 targeted ICI therapies have been suggested to suppress Treg activity, decreased efferocytosis may be another avenue by which other classes of immune checkpoint therapies may exacerbate atherosclerosis. However, extensive research is needed to substantiate this potential link.
Cholesterol Metabolism and T Cell Mediated Immunity in Cancer
Clinically, obesity and metabolic syndrome have been clearly linked with increased cancer risk, mediated by many shared risk factors (118). One shared link is energy metabolism, where alterations in lipid metabolism can shape the immune system’s response to tumor activity. Cholesterol metabolism is essential for cancer progression, including the formation of cellular membranes during rapid proliferation and in tumor migration and invasion (119).
The features of metabolic syndrome, including hypertriglyceridemia, hyperglycemia and hypercholesterolemia, promotes a chronic inflammatory state that paradoxically blocks physiologic immune function (120). Chronic inflammation and hypercholesterolemia promote an increase in the production of myeloid-derived suppressor cells, or MDSCs, through a process termed “emergency myelopoiesis” (121). MDSCs inhibit T cell functions through alterations in TCR receptors that impair downstream signaling, secretion of TGF-β, IL-10 and cytokines that decrease effector T cell function, and overexpression of PD-L1 (122). In addition, Ma et al. utilized an Apoe−/− mouse model traditionally used to study atherosclerosis to demonstrate negative regulatory effect of hypercholesterolemia on IL-9 levels, which impair CD8+ T cell differentiation and antitumor response (123). The enriched cholesterol content of the tumor microenvironment induces T cell exhaustion and the expression of immune checkpoints such as PD-1, TIM-3 and LAG-3 on CD8+ T cells (124).
Given the intricate relationship of hypercholesterolemia and repressed T cell function, it is anticipated that cholesterol levels modulate patient response to immunotherapy. Perrone et al. found that among patients receiving ICIs, baseline hypercholesterolemia was associated with improved overall survival rates (125). Other studies have established improved prognosis in obese patients treated with ICIs (126, 127). Thus it can be postulated that cancer patients with higher cholesterol levels may have worse T cell dysfunction by mechanisms described above, and subsequently exhibit a more pronounced response to the restoration of T cell function by immune checkpoint inhibition. However, these data are limited to date, and whether hypercholesterolemia is a causative factor in improved ICI response or simply a biomarker of chronic inflammation rendering patients more susceptible to immune restoration has yet to be established.
Cholesteryl esters are a storage form of cholesterol that are observed in increased numbers in the tumor microenvironment. One of the key enzymes in this process is ACAT1 (Acetyl-coenzyme A acetyltransferase), which promotes cholesterol esterification into the storage form and facilitates cholesterol transport in blood (128), has been studied in antitumor therapy and atherosclerosis. ACAT1 inhibition has been shown to decrease cancer cell migration and tumor progression in breast cancer and glioblastomas (129, 130). In the early stages of atherosclerosis, ACAT1 expression is increased in macrophages to enhance their ability to store free cholesterol, and the use of ACAT inhibitors had been shown to promote cell death (131). The ACAT1 inhibitor avasimibe has been studied in animals and humans with hyperlipidemia, however their effects on plasma cholesterol and atherosclerotic plaque size were inconsistent (132-134). Yang et al applied ACAT inhibition to cancer immunotherapy, and demonstrated that the addition of avasimibe to anti-PD-1 therapy was more effective in the treatment of melanoma in mice, with demonstrated enhancement in CD8+ T cell antitumor activity (135). Together, these findings imply an important relationship between hypercholesterolemia, a major driver of atherosclerosis, and tumor progression.
Clinical Implications of ICI Therapy on ASCVD
Recent clinical data have emerged to suggest an association between ICI use and accelerated atherosclerosis and atherosclerotic cardiovascular events. Initially, a case series of 11 patients suggested that PD-1 blockade may actually improve complicated plaque burden, as PD-1 blockade was associated with regression of atherosclerotic plaque in 3 patients (27%), no change in 7 (64%) and an increase in 1 (9%). However, this study was limited by a short CT scan follow up period and imaging protocols that were not intended for vascular imaging (136). Since, two case reports have linked PD-L1 and PD-1 inhibitors with rapid interval progression of CAD on left heart catheterization and fatal ACS in metastatic lung and giant bone cell cancer, respectively (137, 138).
Several single center studies have documented the incidence of ASCVD in ICI therapy. In a retrospective analysis of ICI clinical trials where PD-1 and PD-L1 inhibitors including nivolumab, pembrolizumab, atezolizumab, avelumab, and durvalumab were tested in patients with NSCLC, Hu et al. reported a 1% incidence of myocardial infarction and 2% incidence of stroke (139). In a single center registry by Oren et al. of 3,326 patients with any malignancy on ICI therapy including atezolizumab, avelumab, ipilimumab, nivolumab and pembrolizumab, there was a 7% incidence for both myocardial infarction and stroke within a 16 month period (140).
In the largest single center study to date, Drobni et al. compared 2842 patients on any ICIs (the majority of which being PD-1 inhibitors) with controls matched for age, cancer type and cardiovascular history on their risk of ASCVD related events over a 2 year follow up period. ICI use was associated with >4-fold greater risk for composite cardiovascular events (HR 4.7, [95% CI 3.5-6.2, p<0.001), >7 fold greater risk of myocardial infarction (HR 7.2 [95% CI 4.5-11.5, p<0.001], 3-fold greater risk of coronary revascularization (HR 3.3, [95% CI 2.0-5.5], p<0.001) and 4-fold greater risk of stroke (HR 4.6 [95% CI 2.9-7.2], p<0.001) (7). Small scale human imaging and histologic studies have attempted to substantiate the possibility that ICI therapy increases atheromatous inflammation and atherosclerosis. In Calabretta et al., PET scans from twenty melanoma patients treated with ICIs (80% PD-1 inhibitors, 5% CTLA-4 inhibitors and 15% combination) showed significant elevation in FDG uptake of all aortic segments about 6 months post-treatment, particularly in noncalcified and mildly calcified segments (p<0.001) (141). However, in a study by Poels et al., ten melanoma patients treated with pembrolizumab or combination nivolumab/ipilimumab showed no absolute change in overall vascular FDG-positive inflammation after 6 weeks.(142) These discrepant results may be due to small sample sizes and varying follow up periods. In a sub-study by Drobni et al., 40 melanoma patients were evaluated via CT at three different time points, and ICI treatment was shown to accelerate the rate of atherosclerotic plaque progression 3-fold (from 2.1%/yr pre-ICI to 6.7%/yr after ICI, p=0.02). In an autopsy study of 11 patients on ICI therapy, although the absolute number of T cells was unchanged, there was an increased ratio of CD3+ cells to CD68+ cells leading to a shift from macrophage- to lymphocyte-predominant inflammation (143). Future large-scale, long term imaging and histologic studies will be needed to further clarify the role of ICI on atheromatous plaque progression and immune response.
Role of Pharmacotherapy in Cardiovascular Risk Reduction in Patients Receiving ICIs
Growing clinical data signaling increased atherosclerosis in ICI therapy invite consideration of pharmacotherapies to reduce cardiovascular events in this patient population. In addition to lipid lowering, statins have been associated with plaque stabilization, endothelial dysfunction reversal, and inflammation reduction. How statins reduce inflammation are not fully understood, but may involve inhibition of beta-2 integrin leukocyte function antigen-1 (LFA-1), an adhesion molecule with a role in T cell activation (144). In Drobni et al. the markedly increased rate of aortic plaque progression associated with ICI therapy was attenuated by the use of statins, though no direct comparisons of cardiovascular events based on statin use were performed (7). PCSK9 inhibitors are an increasingly popular class of monoclonal antibodies that reduce serum LDL and atherosclerotic events in higher risk patients (145). In contrast to statins, there is much less known about potential anti-inflammatory effects of PCSK9 inhibitors.
The potential for cholesterol levels to modulate ICI response raises important considerations for the effects of these cholesterol lowering agents on ICI efficacy. Despite the correlation between hypercholesterolemia and improved outcomes of ICI therapy, statins and PCSK9 inhibitors have shown preliminary evidence of synergistic benefit when paired with ICI therapy independent of their cholesterol lowering effects. In particular, statins have been shown to inhibit protein prenylation leading to enhanced antigen presentation that may be synergistic with immunotherapy (146). Several clinical studies in patients with advanced NSCLC and malignant pleural mesothelioma treated with ICIs demonstrated that statins were associated with increased response rate, improved time to treatment failure, progression free and overall survival (147, 148). Liu et al. showed that proprotein convertase substilisin/kexin type 9 (PCSK9) inhibition with evolocumab synergized with anti-PD-1 therapy to suppress tumor growth by increasing the expression of MHC I proteins and enhancing lymphocyte proliferation into the tumor (149). These findings highlight the complex interplay between cholesterol metabolism and the immune system that requires further research, but demonstrate that statins and PCSK9 inhibitors have the potential to both enhance ICI efficacy and treat ICI related atherosclerosis. However, Drobni et al. recently demonstrated increased risk of myopathy in patients treated concurrently with statins and ICIs (150). Thus, further study is needed to confirm the safety of existing therapies and identify novel therapeutics in the treatment of ASCVD in the context of ICI use.
Conclusions
Numerous studies have demonstrated the role of various immune checkpoint pathways in atherogenesis, and emerging clinical studies have begun describing an association between ICI use and increased risk of ASCVD. Taken together, these reports indicate that the cardiovascular effects of immune checkpoint therapies extend beyond the rare incidences of myocarditis. As ASCVD is one of the most prominent causes of morbidity and mortality worldwide, if further clinical studies confirm this association, the risk of major adverse cardiovascular events in patients receiving ICIs must be carefully considered.
The current level evidence linking ICI therapy to atherogenesis and atherosclerotic events is not without its limitations. Preclinical studies thus far have not clearly delineated how immune checkpoint alteration will impact each stage of atherosclerosis. It has become increasingly recognized that the microenvironmental context of cell death and apoptosis is important in determining whether the net effect is atherogenic or atheroprotective (111). Thus, the effect of ICIs on atherosclerosis may prove to be more heterogeneous and evolve as clinical disease progresses. Careful mechanistic understanding of the effect of ICI at each stage of atherosclerosis is needed to determine the timing and need of interventions.
On a clinical level, the linkage between ICI use and atherosclerosis has only been suggested by smaller observational studies, and extensive further evidence with larger sample sizes and longer study duration are needed to confirm this association and estimate the rate of adverse cardiovascular events in this population. If future studies continue to demonstrate significant ASCVD burden in patients receiving ICIs, effort must be taken to identify atherosclerotic development and control reversible atherosclerosis risk factors. In this effort, integration of baseline and routine atherosclerotic imaging in these patients may help to quantify atherosclerotic burden.
Patients who develop ASCVD from ICI therapy represent a unique patient population that warrant our investigation of potential pharmacotherapeutic options. The role of statins, PCSK9 inhibitors and other pharmacotherapies should be explored in animal models, and randomized controlled trials are needed to prove their effectiveness and safety in this population.
Highlights.
Immune checkpoint therapy may increase atherosclerotic cardiovascular disease (ASCVD).
Cholesterol metabolism modulates the response to immune checkpoint therapies and may be a common target for treatment of both cancer and atherosclerosis.
Further research should define cardiovascular endpoints for patients receiving immune checkpoint therapies and standardize treatment and surveillance strategies.
Sources of Funding:
Dr. Stein-Merlob and Dr. Nayeri are supported by the National Institutes of Health Cardiovascular Scientist Training Program (grant T32HL007895). Dr. Sallam is supported by the AHA Transformational Project Award and the National Institutes of Health grant HL149766. Dr. Neilan is supported by a gift from A. Curt Greer and Pamela Kohlberg, and grants from the National Institutes of Health/National Heart, Lung, and Blood Institute grants R01HL130539, R01HL137562, K24HL150238.
Disclosures:
Dr. Neilan has been a consultant to and received fees from Amgen, H3-Biomedicine, and AbbVie outside of the current work. Dr. Neilan also reports consultant fees from Bristol Myers Squibb for a Scientific Advisory Board focused on myocarditis related to immune checkpoint inhibitors and grant funding from AstraZeneca on atherosclerosis with immune checkpoint inhibitors. Dr. Yang is supported by a grant from CSL Behring. The remaining authors have no disclosures.
Nonstandard Abbreviations
- ASCVD
Atherosclerotic cardiovascular disease
- CANTOS
Canakinumab Anti-Inflammatory Thrombosis Outcomes Study
- COLCOT
Colchicine Cardiovascular Outcomes Trial
- CTLA-4
Cytotoxic T-lymphocyte associated protein 4
- ICI
Immune checkpoint inhibitor
- IFN-γ
Interferon gamma
- LAG-3
Lymphocyte-activation gene 3
- LoDoCo
Low-Dose Colchicine
- PD-1
Programmed cell death protein 1
- PD-L
Programmed cell death ligand
References
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Shreiber RD. Cancer Immunoediting: From Surveillance to Escape. Cancer Immunother. Immune Suppr. Tumor Growth Second Ed. 2013;3:85–99. [Google Scholar]
- 2.Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 2018;18:139–147. Available at: 10.1038/nrc.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–1492. Available at: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 4.Hodi SF, O’Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc 2020;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drobni ZD, Alvi RM, Taron J, et al. Association between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020:2299–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 9.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol 2013;61:404–410. [DOI] [PubMed] [Google Scholar]
- 10.Tardif J-C, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 11.Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ. Res 2019;124:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and Type 17 Helper T Cells. N. Engl. J. Med 2009;361:888–898. [DOI] [PubMed] [Google Scholar]
- 13.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat. Rev. Immunol 2008;8:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qureshi OS, Zheng Y, Nakamura K, et al. UKPMC Funders Group cell extrinsic function of CTLA-4. Science (80-. ). 2011;332:600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto T, Sasaki N, Yamashita T, et al. Overexpression of cytotoxic T-lymphocyte-associated antigen-4 prevents atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol 2016;36:1141–1151. [DOI] [PubMed] [Google Scholar]
- 17.Brunner-Weinzierl MC, Rudd CE. CTLA-4 and PD-1 control of T-cell motility and migration: Implications for tumor immunotherapy. Front. Immunol 2018;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018;48:434–452. Available at: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science (80-. ). 2001;291:319–322. [DOI] [PubMed] [Google Scholar]
- 20.Tajiri K, Ieda M. Cardiac Complications in Immune Checkpoint Inhibition Therapy. Front. Cardiovasc. Med 2019;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim ES, Kim JE, Patel MA, Mangraviti A, Ruzevick J, Lim M. Immune Checkpoint Modulators: An Emerging Antiglioma Armamentarium. J. Immunol. Res 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou W Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol 2006;6:295–307. [DOI] [PubMed] [Google Scholar]
- 23.Baitsch L, Baumgaertner P, Devêvre E, et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest 2011;121:2350–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019;18:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leitinger N, Schulman IG. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler. Thromb. Vasc. Biol 2013;33:1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett TJ. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol 2020;40:20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ. Res 2014;114:1852–1866. [DOI] [PubMed] [Google Scholar]
- 28.Robertson AKL, Hansson GK. T cells in atherogenesis: For better or for worse? Arterioscler. Thromb. Vasc. Biol 2006;26:2421–2432. [DOI] [PubMed] [Google Scholar]
- 29.Paulsson G, Zhou X, Törnquist E, Hansson GK. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol 2000;20:10–17. [DOI] [PubMed] [Google Scholar]
- 30.Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol 2020;17:387–401. Available at: 10.1038/s41569-020-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. U. S. A 1995;92:3893–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frostegård J, Ulfgren AK, Nyberg P, et al. Cytokine expression in advanced human atherosclerotic plaques: Dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 1999;145:33–43. [DOI] [PubMed] [Google Scholar]
- 33.Brånén L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-α reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol 2004;24:2137–2142. [DOI] [PubMed] [Google Scholar]
- 34.Rocha VZ, Folco EJ, Sukhova G, et al. Interferon-γ, a Th1 cytokine, regulates fat inflammation: A role for adaptive immunity in obesity. Circ. Res 2008;103:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gotsman I, Lichtman AH. Targeting interferon-γ to treat atherosclerosis. Circ. Res 2007;101:333–334. [DOI] [PubMed] [Google Scholar]
- 36.Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol 1991;11:1223–1230. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Pablo AM, Jiang XC, Wang N, Tall AR, Schindler C. IFN-γ, potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest 1997;99:2752–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat. Rev. Cardiol 2009;6:410–417. Available at: 10.1038/nrcardio.2009.57. [DOI] [PubMed] [Google Scholar]
- 39.Boesten LSM, Zadelaar ASM, Van Nieuwkoop A, et al. Tumor necrosis factor-α promotes atherosclerotic lesion progression in APOE*3-leiden transgenic mice. Cardiovasc. Res 2005;66:179–185. [DOI] [PubMed] [Google Scholar]
- 40.Laurat E, Poirier B, Tupin E, et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 2001;104:197–202. [DOI] [PubMed] [Google Scholar]
- 41.Grainger DJ. Transforming Growth Factor β and Atherosclerosis: So Far, So Good for the Protective Cytokine Hypothesis. Arterioscler. Thromb. Vasc. Biol 2004;24:399–404. [DOI] [PubMed] [Google Scholar]
- 42.Robertson AKL, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J. Clin. Invest 2003;112:1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou H xiao, Guo B bing, Liu Q, et al. Regulatory T cells as a new therapeutic target for atherosclerosis. Acta Pharmacol. Sin 2018;39:1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mallat Z, Besnard S, Duriez M, et al. Protective role of interleukin-10 in atherosclerosis. Circ. Res 1999;85. [DOI] [PubMed] [Google Scholar]
- 45.Lin J, Li M, Wang Z, He S, Xuming M, Li D. The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation. J. Lipid Res 2010;51:1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med 2006;12:178–180. [DOI] [PubMed] [Google Scholar]
- 47.Dietel B, Cicha I, Voskens CJ, Verhoeven E, Achenbach S, Garlichs CD. Decreased numbers of regulatory T cells are associated with human atherosclerotic lesion vulnerability and inversely correlate with infiltrated mature dendritic cells. Atherosclerosis 2013;230:92–99. Available at: 10.1016/j.atherosclerosis.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Binder CJ, Hartvigsen K, Chang MK, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest 2004;114:427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ketelhuth DFJ, Hansson GK. Adaptive Response of T and B Cells in Atherosclerosis. Circ. Res 2016;118:668–678. [DOI] [PubMed] [Google Scholar]
- 50.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler. Thromb. Vasc. Biol 2002;22:456–461. [DOI] [PubMed] [Google Scholar]
- 51.Eid RE, Rao DA, Zhou J, et al. Interleukin-17 and interferon-γ Are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 2009;119:1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taleb S, Tedgui A, Mallat Z. IL-17 and Th17 cells in atherosclerosis: Subtle and contextual roles. Arterioscler. Thromb. Vasc. Biol 2015;35:258–264. [DOI] [PubMed] [Google Scholar]
- 53.Smith E, Prasad KMR, Butcher M, et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 2010;121:1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gisterå A, Robertson AKL, Andersson J, et al. Transforming growth factor-β signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci. Transl. Med 2013;5:18–23. [DOI] [PubMed] [Google Scholar]
- 55.Danzaki K, Matsui Y, Ikesue M, et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol 2012;32:273–280. [DOI] [PubMed] [Google Scholar]
- 56.Tsilingiri K, De La Fuente H, Relaño M, et al. Oxidized Low-Density Lipoprotein Receptor in Lymphocytes Prevents Atherosclerosis and Predicts Subclinical Disease. Circulation 2019;139:243–255. [DOI] [PubMed] [Google Scholar]
- 57.Knochelmann HM, Dwyer CJ, Bailey SR, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol 2018;15:458–469. Available at: 10.1038/s41423-018-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rikhi R, Karnuta J, Hussain M, et al. Immune Checkpoint Inhibitors Mediated Lymphocytic and Giant Cell Myocarditis: Uncovering Etiological Mechanisms. Front. Cardiovasc. Med 2021;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gewaltig J, Kummer M, Koella C, Cathomas G, Biedermann BC. Requirements for CD8 T-cell migration into the human arterial wall. Hum. Pathol 2008;39:1756–1762. Available at: 10.1016/j.humpath.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 60.Kolbus D, Ramos OH, Berg KE, et al. CD8+T cell activation predominate early immune responses to hypercholesterolemia in Apoe−/−mice. BMC Immunol. 2010;11:58. Available at: http://www.biomedcentral.com/1471-2172/11/58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kyaw T, Winship A, Tay C, et al. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in ApoE-deficient mice. Circulation 2013;127:1028–1039. [DOI] [PubMed] [Google Scholar]
- 62.Chyu KY, Zhao X, Dimayuga PC, et al. CD8 + T cells mediate the athero-protective effect of immunization with an ApoB-100 peptide. PLoS One 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ewing MM, Karper JC, Abdul S, et al. T-cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int. J. Cardiol 2013;168:1965–1974. Available at: 10.1016/j.ijcard.2012.12.085. [DOI] [PubMed] [Google Scholar]
- 64.Ma K, Lv S, Liu B, et al. CTLA4-IgG ameliorates homocysteine-accelerated atherosclerosis by inhibiting T-cell overactivation in apoE−/− mice. Cardiovasc. Res 2013;97:349–359. [DOI] [PubMed] [Google Scholar]
- 65.Poels K, van Leent MMT, Reiche ME, et al. Antibody-Mediated Inhibition of CTLA4 Aggravates Atherosclerotic Plaque Inflammation and Progression in Hyperlipidemic Mice. Cells 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol 2001;2:261–268. [DOI] [PubMed] [Google Scholar]
- 67.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J. Clin. Invest 2007;117:2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bu DX, Tarrio M, Maganto-Garcia E, et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler. Thromb. Vasc. Biol 2011;31:1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cochain C, Chaudhari SM, Koch M, Wiendl H, Eckstein HH, Zernecke A. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS One 2014;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee J, Zhuang Y, Wei X, et al. Contributions of PD-1/PD-L1 pathway to interactions of myeloid DCs with T cells in atherosclerosis. J. Mol. Cell. Cardiol 2009;46:169–176. Available at: 10.1016/j.yjmcc.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 71.Li S-H, Chen W-J, Yan M, Shu Y-W, Liao Y-H. Expression of coinhibitory PD-L1 on CD4+CD25+FOXP3+ regulatory T cells is elevated in patients with acute coronary syndrome. Coron. Artery Dis 2015;26. Available at: https://journals.lww.com/coronary-artery/Fulltext/2015/11000/Expression_of_coinhibitory_PD_L1_on.10.aspx. [DOI] [PubMed] [Google Scholar]
- 72.Fernandez DM, Rahman AH, Fernandez N, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med 2019;25:1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michel NA, Zirlik A, Wolf D. CD40L and Its Receptors in Atherothrombosis—An Update. Front. Cardiovasc. Med 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li DK, Wang W. Characteristics and clinical trial results of agonistic anti-CD40 antibodies in the treatment of malignancies (Review). Oncol. Lett 2020;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eliopoulos AG, Young LS. The role of the CD40 pathway in the pathogenesis and treatment of cancer. Curr. Opin. Pharmacol 2004;4:360–367. [DOI] [PubMed] [Google Scholar]
- 76.Richards DM, Sefrin JP, Gieffers C, Hill O, Merz C. Concepts for agonistic targeting of CD40 in immuno-oncology. Hum. Vaccines Immunother 2020;16:377–387. Available at: 10.1080/21645515.2019.1653744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lievens D, Eijgelaar WJ, Biessen EAL, Daemen MJAP, Lutgens E. The multi-functionality of CD40L and its receptor CD40 in atherosclerosis. Thromb. Haemost 2009;102:206–214. [DOI] [PubMed] [Google Scholar]
- 78.Mach F, Schoneck U, Bonnefoy J-Y, Pober JS, Libby P. Activation of Monocyte/Macrophage Functions Related to Acute Atheroma Complication by Ligation of CD40. Circulation 1997;96:396–399. [DOI] [PubMed] [Google Scholar]
- 79.Kotowicz K, Dixon GLJ, Klein NJ, Peters MJ, Callard RE. Biological function of CD40 on human endothelial cells: Costimulation with CD40 ligand and interleukin-4 selectively induces expression of vascular cell adhesion molecule-1 and P-selectin resulting in preferential adhesion of lymphocytes. Immunology 2000;100:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuan M, Fu H, Ren L, Wang H, Guo W. Soluble CD40 ligand promotes macrophage foam cell formation in the etiology of atherosclerosis. Cardiol. 2015;131:1–12. [DOI] [PubMed] [Google Scholar]
- 81.Bruemmer D, Riggers U, Holzmeister J, et al. Expression of CD40 in vascular smooth muscle cells and macrophages is associated with early development of human atherosclerotic lesions. Am. J. Cardiol 2001;87:21–27. [DOI] [PubMed] [Google Scholar]
- 82.Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature 1998;394:200–203. [DOI] [PubMed] [Google Scholar]
- 83.Lutgens E, Cleutjens KBJM, Heeneman S, Koteliansky VE, Burkly LC, Daemen MJAP. Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype. Proc. Natl. Acad. Sci. U. S. A 2000;97:7464–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lutgens E, Gorelik L, Daemen MJAP, et al. Requirement for CD154 in the progression of atherosclerosis. Nat. Med 1999;5:1313–1316. [DOI] [PubMed] [Google Scholar]
- 85.Bavendiek U, Zirlik A, LaClair S, MacFarlane L, Libby P, Schönbeck U. Atherogenesis in mice does not require CD40 ligand from bone marrow-derived cells. Arterioscler. Thromb. Vasc. Biol 2005;25:1244–1249. [DOI] [PubMed] [Google Scholar]
- 86.Robles-Carrillo L, Meyer T, Hatfield M, et al. Anti-CD40L Immune Complexes Potently Activate Platelets In Vitro and Cause Thrombosis in FCGR2A Transgenic Mice. J. Immunol 2010;185:1577–1583. [DOI] [PubMed] [Google Scholar]
- 87.Lacy M, Bürger C, Shami A, et al. Cell-specific and divergent roles of the CD40L-CD40 axis in atherosclerotic vascular disease. Nat. Commun 2021;12:1–12. Available at: 10.1038/s41467-021-23909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nocentini G, Riccardi C. Therapeutic Targets of the TNF Super Family. 2009. [Google Scholar]
- 89.Mahne AE, Mauze S, Joyce-Shaikh B, et al. Dual roles for regulatory T-cell depletion and costimulatory signaling in agonistic GITR targeting for tumor immunotherapy. Cancer Res. 2017;77:1108–1118. [DOI] [PubMed] [Google Scholar]
- 90.Gerdes N, Zirlik A. Co-stimulatory molecules in and beyond co-stimulation-tipping the balance in atherosclerosis? Thromb. Haemost 2011;106:804–813. [DOI] [PubMed] [Google Scholar]
- 91.Vence L, Bucktrout SL, Curbelo IF, et al. Characterization and comparison of GITR expression in solid tumors. Clin. Cancer Res 2019;25:6501–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zappasodi R, Sirard C, Li Y, et al. Rational design of anti-GITR-based combination immunotherapy. Nat. Med 2019;25:759–766. Available at: 10.1038/s41591-019-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim WJ, Kang YJ, Suk K, Park JE, Kwon BS, Lee WH. Comparative analysis of the expression patterns of various TNFSF/TNFRSF in atherosclerotic plaques. Immunol. Invest 2008;37:359–373. [DOI] [PubMed] [Google Scholar]
- 94.Shami A, Atzler D, Bosmans LA, et al. Glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) drives atherosclerosis in mice and is associated with an unstable plaque phenotype and cerebrovascular events in humans. Eur. Heart J 2020;41:2938–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim WJ, Bae EM, Kang YJ, et al. Glucocorticoid-induced tumour necrosis factor receptor family related protein (GITR) mediates inflammatory activation of macrophages that can destabilize atherosclerotic plaques. Immunology 2006;119:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van De Ven K, Borst J. Targeting the T-cell co-stimulatory CD27/CD70 pathway in cancer immunotherapy: Rationale and potential. Immunotherapy 2015;7:655–667. [DOI] [PubMed] [Google Scholar]
- 97.Ansell SM, Flinn I, Taylor MH, et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, for hematologic malignancies. Blood Adv. 2020;4:1917–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Winkels H, Meiler S, Smeets E, et al. CD70 limits atherosclerosis and promotes macrophage function. Thromb. Haemost 2017;117:164–175. [DOI] [PubMed] [Google Scholar]
- 99.Winkels H, Meiler S, Lievens D, et al. CD27 co-stimulation increases the abundance of regulatory T cells and reduces atherosclerosis in hyperlipidaemic mice. Eur. Heart J 2017;38:3590–3599. [DOI] [PubMed] [Google Scholar]
- 100.Solinas C, Gu-Trantien C, Willard-Gallo K. The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open 2020;5:e000544. Available at: 10.1136/esmoopen-2019-000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Afek A, Harats D, Roth A, Keren G, George J. A functional role for inducible costimulator (ICOS) in atherosclerosis. Atherosclerosis 2005;183:57–63. [DOI] [PubMed] [Google Scholar]
- 102.Gotsman I, Grabie N, Gupta R, et al. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation 2006;114:2047–2055. [DOI] [PubMed] [Google Scholar]
- 103.Kang CW, Dutta A, Chang LY, et al. Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer. Sci. Rep 2015;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chiba S, Baghdadi M, Akiba H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol 2012;13:832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Foks AC, Ran IA, Wasserman L, et al. T-cell immunoglobulin and mucin domain 3 acts as a negative regulator of atherosclerosis. Arterioscler. Thromb. Vasc. Biol 2013;33:2558–2565. [DOI] [PubMed] [Google Scholar]
- 106.Qiu MK, Wang SC, Dai YX, Wang SQ, Ou JM, Quan ZW. PD-1 and Tim-3 pathways regulate CD8+ T cells function in atherosclerosis. PLoS One 2015;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Long L, Zhang X, Chen F, et al. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes and Cancer 2018;9:176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andrews LP, Marciscano AE, Drake CG, Vignali DAA. LAG3 (CD223) as a Cancer Immunotherapy Target. Immunol. Rev 2017;276:80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Golden D, Kolmakova A, Sura S, et al. Lymphocyte activation gene 3 and coronary artery disease. JCI Insight 2016;1:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest 1998;101:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rayner KJ. Cell Death in the Vessel Wall: The Good, the Bad, the Ugly. Arterioscler. Thromb. Vasc. Biol 2017;37:e75–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kojima Y, Volkmer JP, McKenna K, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016;536:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Flores AM, Hosseini-Nassab N, Jarr KU, et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol 2020;15:154–161. Available at: 10.1038/s41565-019-0619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y, Nanda V, Direnzo D, et al. Clonally expanding smooth muscle cells promote atherosclerosis by escaping efferocytosis and activating the complement cascade. Proc. Natl. Acad. Sci. U. S. A 2020;117:15818–15826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Advani R, Flinn I, Popplewell L, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med 2018;379:1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jarr K-U, Nakamoto R, Doan BH, et al. Effect of CD47 Blockade on Vascular Inflammation. N. Engl. J. Med 2021;384:382–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Proto JD, Doran AC, Gusarova G, et al. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 2018;49:666–677.e6. Available at: 10.1016/j.immuni.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lauby-Secretan B, Ph D, Scoccianti C, Ph D, Loomis D, Ph D. Body Fatness and Cancer — Viewpoint of the IARC Working Group. N. Engl. J. Med 2016;375:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang B, Song B liang, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat. Metab 2020;2:132–141. Available at: 10.1038/s42255-020-0174-0. [DOI] [PubMed] [Google Scholar]
- 120.Bersanelli M, Cortellini A, Buti S. The interplay between cholesterol (and other metabolic conditions) and immune-checkpoint immunotherapy: shifting the concept from the “inflamed tumor” to the “inflamed patient.” Hum. Vaccines Immunother 2021;00:1–5. Available at: 10.1080/21645515.2020.1852872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Porta C, Marino A, Consonni FM, et al. Metabolic influence on the differentiation of suppressive myeloid cells in cancer. Carcinogenesis 2018;39:1095–1104. [DOI] [PubMed] [Google Scholar]
- 122.Safari E, Ghorghanlu S, Ahmadi-khiavi H, Mehranfar S, Rezaei R, Motallebnezhad M. Myeloid-derived suppressor cells and tumor: Current knowledge and future perspectives. J. Cell. Physiol 2019;234:9966–9981. [DOI] [PubMed] [Google Scholar]
- 123.Ma X, Bi E, Huang C, et al. Cholesterol negatively regulates IL-9-producing CD8+ T cell differentiation and antitumor activity. J. Exp. Med 2018;215:1555–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ma X, Bi E, Lu Y, et al. Cholesterol Induces CD8+ T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019;30:143–156.e5. Available at: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perrone F, Minari R, Bersanelli M, et al. The Prognostic Role of High Blood Cholesterol in Advanced Cancer Patients Treated with Immune Checkpoint Inhibitors. J. Immunother 2020;43:196–203. [DOI] [PubMed] [Google Scholar]
- 126.Young AC, Quach HT, Song H, et al. Impact of body composition on outcomes from anti-PD1 +/−anti-CTLA-4 treatment in melanoma. J. Immunother. Cancer 2020;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leon C, Hill JS, Wasan KM. Potential role of Acyl-coenzyme A:cholesterol transferase (ACAT) inhibitors as hypolipidemic and antiatherosclerosis drugs. Pharm. Res 2005;22:1578–1588. [DOI] [PubMed] [Google Scholar]
- 129.Antalis CJ, Uchida A, Buhman KK, Siddiqui RA. Migration of MDA-MB-231 breast cancer cells depends on the availability of exogenous lipids and cholesterol esterification. Clin. Exp. Metastasis 2011;28:733–741. [DOI] [PubMed] [Google Scholar]
- 130.Geng F, Cheng X, Wu X, et al. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-mediated lipogenesis. Clin. Cancer Res 2016;22:5337–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tabas I, Marathe S, Keesler GA, Beatini N, Shiratori Y. Evidence that the initial upregulation of phosphatidylcholine biosynthesis in free cholesterol-loaded macrophages is an adaptive response that prevents cholesterol-induced cellular necrosis. Proposed role of an eventual failure of this response in foam c. J. Biol. Chem 1996;271:22773–22781. Available at: 10.1074/jbc.271.37.22773. [DOI] [PubMed] [Google Scholar]
- 132.Tardif JC, Grégoire J, L’Allier PL, et al. Effects of the acyl coenzyme A:cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation 2004;110:3372–3377. [DOI] [PubMed] [Google Scholar]
- 133.Bocan TMA, Krause BR, Rosebury WS, et al. The combined effect of inhibiting both ACAT and HMG-CoA reductase may directly induce atherosclerotic lesion regression. Atherosclerosis 2001;157:97–105. [DOI] [PubMed] [Google Scholar]
- 134.InsullJr W, Koren MJ, Davignon J, et al. Efficacy and short-term safety of a new ACAT inhibitor, avasimibe, on lipids, lipoproteins and apolipoproteins, in patientswith combined hyperlipidemia. Atherosclerosis 2001;157:137–144. [DOI] [PubMed] [Google Scholar]
- 135.Yang W, Bai Y, Xiong Y, et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 2016;531:651–655. Available at: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gelsomino F, Fiorentino M, Zompatori M, et al. Programmed death-1 inhibition and atherosclerosis: Can nivolumab vanish complicated atheromatous plaques? Ann. Oncol 2018;29:284–285. Available at: 10.1093/annonc/mdx718. [DOI] [PubMed] [Google Scholar]
- 137.Cautela J, Rouby F, Salem J-E, et al. Acute Coronary Syndrome With Immune Checkpoint Inhibitors: A Proof-of-Concept Case and Pharmacovigilance Analysis of a Life-Threatening Adverse Event. Can. J. Cardiol 2020;36:476–481. Available at: 10.1016/j.cjca.2019.11.035. [DOI] [PubMed] [Google Scholar]
- 138.Kwan JM, Cheng R, Feldman LE. Hepatotoxicity and Recurrent NSTEMI While on Pembrolizumab for Metastatic Giant Cell Bone Tumor. Am. J. Med. Sci 2019;357:343–347. Available at: http://www.sciencedirect.com/science/article/pii/S0002962918304476. [DOI] [PubMed] [Google Scholar]
- 139.Hu Y-B, Zhang Q, Li H-J, et al. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a meta-analysis. Transl. Lung Cancer Res 2017;6:S8–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oren O, Yang EH, Molina JR, Bailey KR, Blumenthal RS, Kopecky SL. Cardiovascular Health and Outcomes in Cancer Patients Receiving Immune Checkpoint Inhibitors. Am. J. Cardiol 2020;125:1920–1926. Available at: 10.1016/j.amjcard.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 141.Calabretta R, Hoeller C, Pichler V, et al. Immune Checkpoint Inhibitor Therapy Induces Inflammatory Activity in Large Arteries. Circulation 2020:1–3. [DOI] [PubMed] [Google Scholar]
- 142.Poels K, van Leent MMT, Boutros C, et al. Immune Checkpoint Inhibitor Therapy Aggravates T Cell–Driven Plaque Inflammation in Atherosclerosis. JACC CardioOncology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Newman JL, Stone JR. Immune checkpoint inhibition alters the inflammatory cell composition of human coronary artery atherosclerosis. Cardiovasc. Pathol 2019;43:107148. Available at: http://www.sciencedirect.com/science/article/pii/S1054880719302339. [DOI] [PubMed] [Google Scholar]
- 144.Weitz-Schmidt G, Welzenbach K, Brinkmann V, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat. Med 2001;7:687–692. [DOI] [PubMed] [Google Scholar]
- 145.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 146.Bird L Statins as adjuvants. Nat. Rev. Immunol 2018;18:669. [DOI] [PubMed] [Google Scholar]
- 147.Omori M, Okuma Y, Hakozaki T, Hosomi Y. Statins improve survival in patients previously treated with nivolumab for advanced non-small cell lung cancer: An observational study. Mol. Clin. Oncol 2018:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cantini L, Pecci F, Hurkmans DP, et al. High-intensity statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced non-small cell lung cancer patients. Eur. J. Cancer 2021;144:41–48. Available at: 10.1016/j.ejca.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 149.Liu X, Bao X, Hu M, et al. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature 2020;588:693–698. Available at: 10.1038/s41586-020-2911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Drobni ZD, Murphy SP, Alvi RM, et al. Association between incidental statin use and skeletal myopathies in patients treated with immune checkpoint inhibitors. Immunother. Adv 2021;1:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol 2013;31:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Coward J, Lemech C, Meniawy T, et al. Phase I/II study of CTLA-4 inhibitor AGEN1884 + PD-1 Inhibitor AGEN2034 in patients with advanced/refractory solid tumors, with expansion into 2L cervical cancer and solid tumors. Ann. Oncol 2018;29:viii417. [Google Scholar]
- 153.Migden MR, Rischin D, Schmults CD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med 2018:341–351. [DOI] [PubMed] [Google Scholar]
- 154.Guo L, Wei R, Lin Y, Kwok HF. Clinical and Recent Patents Applications of PD-1/PD-L1 Targeting Immunotherapy in Cancer Treatment—Current Progress, Strategy, and Future Perspective. Front. Immunol 2020;11:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med 2020;383:1328–1339. [DOI] [PubMed] [Google Scholar]
- 156.Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med 2020;383:1218–1230. [DOI] [PubMed] [Google Scholar]
- 157.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med 2018;379:2342–2350. [DOI] [PubMed] [Google Scholar]
- 158.Acharya N, Acharya N, Sabatos-Peyton C, Anderson AC, Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J. Immunother. Cancer 2020;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lecocq Q, Keyaerts M, Devoogdt N, Breckpot K. The next-generation immune checkpoint lag-3 and its therapeutic potential in oncology: Third time’s a charm. Int. J. Mol. Sci 2021;22:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Buzzatti G, Dellepiane C, Del Mastro L. New emerging targets in cancer immunotherapy: The role of GITR. ESMO Open 2020;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Byrne KT, Betts CB, Mick R, et al. Neoadjuvant Selicrelumab, an Agonist CD40 Antibody, Induces Changes in the Tumor Microenvironment in Patients with Resectable Pancreatic Cancer. Clin. Cancer Res 2021;27:4574–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marin-Acevedo JA, Kimbrough EMO, Manochakian R, Zhao Y, Lou Y. Immunotherapies targeting stimulatory pathways and beyond. J. Hematol. Oncol 2021;14:1–31. Available at: 10.1186/s13045-021-01085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Starzer AM, Berghoff AS. New emerging targets in cancer immunotherapy: CD27 (TNFRSF7). ESMO Open 2020;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang W, Huang Q, Xiao W, et al. Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis. Front. Immunol 2020;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]




