Summary
Multiparametric magnetic resonance imaging (MRI)/ultrasound fusion targeted prostate biopsy has been shown to outperform systematic biopsy in the detection of clinically significant prostate cancer. Aside from tumor grade, tumor biomarkers such as phosphatase and tensin homolog (PTEN) and ETS-related gene (ERG) have prognostic significance in prostate cancer and may help direct management of patients with low-grade tumors. Our objective was to compare the detection of PTEN and ERG expression in MRI-targeted versus systematic prostate biopsies. We compared immunohistochemical expression for PTEN and ERG on prostate biopsy cores from patients with Grade Group (GG) 1 or GG2 prostate cancer who had undergone systematic biopsy with concurrent targeted biopsy. Fifty-three cases had both systematic and MRI-targeted prostate tissue available for staining for PTEN; and 52 cases, for ERG. ERG positivity was seen in 37/52 (71.2%) cases, and PTEN loss was seen in 15/53 (28.3%) cases. The detection of ERG expression was not significantly different between MRI-targeted and systematic biopsy (P = .4). Targeted biopsy was superior to systematic biopsy in the detection of PTEN loss (P = .02). MRI-targeted cores detected 14/15 (93.3%) cases of PTEN loss compared to 7/15 (46.7%) cases detected by systematic cores. Most cases with PTEN loss showed heterogeneous expression in both systematic and targeted cores. In 14/15 (93.3%) cases with PTEN loss, GG was the same between targeted and systematic biopsy. Targeted biopsy is superior to systematic biopsy in the detection of PTEN loss in GG1 and GG2 tumors. Inclusion of targeted cores may be helpful for evaluation of certain prognostic biomarkers.
Keywords: Prostate adenocarcinoma, Phosphatase and tensin homolog, ETS-related gene, ERG, Magnetic resonance imaging, Pathology
1. Introduction
In the United States, prostate cancer remains the most common malignancy in men, with the exception of skin cancer [1]. Multiparametric magnetic resonance imaging (MRI)/ultrasound (US) fusion targeted prostate biopsy has been shown in previous studies to outperform systematic biopsy in the detection of clinically significant prostate cancer [2–5]. Targeted biopsy has been shown to detect higher-grade tumors as well as poor prognostic features, such as perineural invasion and possibly extraprostatic extension [4,6–8]. In addition, the implementation of this new technique may affect how urologists manage patients with prostate cancer, with potentially an increased utilization of active surveillance [9,10]. Factors that influence selection of active surveillance include cancer grade, imaging findings, serum prostate specific antigen (PSA) level, and patient preference as part of a shared decision-making model with their physicians.
With the increased utilization of active surveillance and MRI/US fusion targeted biopsy, additional prognostic biomarkers can be useful in helping to determine which patients require definitive therapy. Phosphatase and tensin homolog (PTEN) and ETS-related gene (ERG) expression in prostate cancer has been shown to have prognostic significance and may help direct the management of patients with low- and intermediate-risk tumors [11]. In cases with tumor of the same grade involving multiple cores, tumor heterogeneity might influence the results of any biomarker study.
Although studies have shown that MRI-targeted prostate biopsy better detects higher-grade tumors, whether this technique better samples cancer tissue in terms of detecting prognostic biomarkers is undetermined. To date, there have been limited investigations of prognostic biomarkers in prostate cancer detected by MRI-targeted biopsy compared to systematic biopsy. Our objective was to compare the detection of PTEN and ERG expression in MRI-targeted versus systematic prostate core biopsies to determine if the targeted approach better detects expression of these prognostic biomarkers.
2. Materials and methods
A retrospective review was performed of our prospectively maintained, institutional review board-approved, prostate cancer database, searching for patients who underwent MRI/US fusion targeted prostate biopsy and concurrent systematic extended-sextant biopsy from 2014 to 2018. Post–image processing of multiparametric MRI and targeted biopsy of 3D segmented suspicious lesions was performed using the DynaCad and UroNav systems, respectively (Phillips/InVivo, Gainsville, FL). prostate imaging reporting and data system (PIRADS) v2 scoring was assigned by a multidisciplinary consensus conference with fellowship-trained radiologists and urologic oncologists specializing in prostate MRI. Two fellowship-trained urologic oncologists performed all targeted biopsies. For each patient, a minimum of 8 cores was sampled via the standard systematic approach using the conventional extended-sextant template. Each MRI-targeted lesion was sampled by at least 2 needle cores, and all prostate biopsy cores were evaluated for Gleason score and percent tumor involvement, per each MRI-targeted lesion, by the “aggregate of cores methods” as previously described [12].
All histologic evaluation was performed by 2 fellowship-trained genitourinary pathologists (J. B. G. and M. R. P.). Prostate cancer grading was assessed in accordance with the current standard criteria [13,14]. Statistical analyses were done utilizing JMP 13.1.0. Categorical and continuous variables were compared for statistical significance using χ2 test and Student t tests, respectively. The detection rate of ERG positivity and PTEN loss between systematic and MRI-targeted cores was compared using the McNemar test.
Patients were selected that had Grade Group (GG) 2 as the highest grade of prostate cancer detected among all cores sampled during the biopsy session, including both MRI-targeted and systematic cores. The presence of either GG1 or GG2 tumor on either targeted or systematic cores was acceptable for inclusion. No cases with GG3–5 or cases with intraductal carcinoma detected in any cores were included. For inclusion in this data analysis, it was required that prostate cancer was detected on both MRI-targeted cores as well as systematic cores within the case, although the same GG was not required to be present on both biopsy approaches.
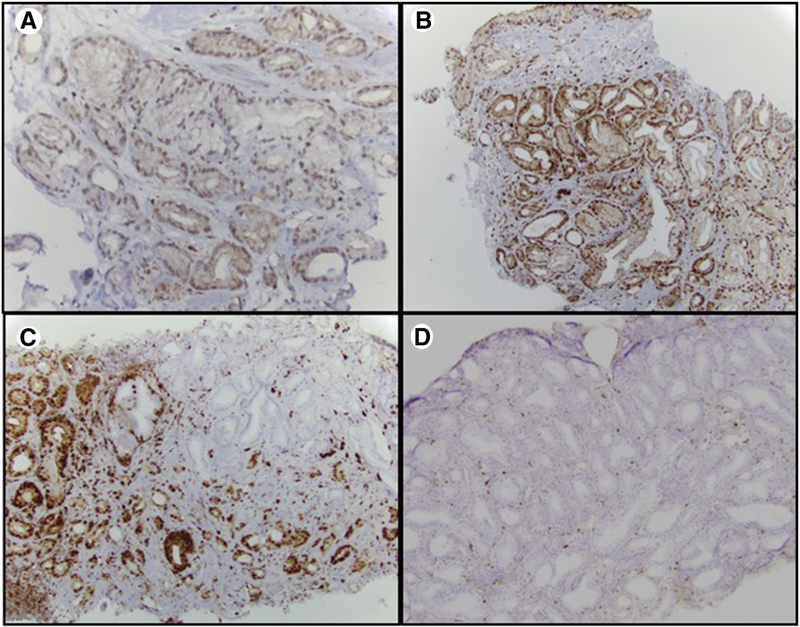
Immunohistochemistry (IHC) for PTEN and ERG was performed on all cores with tumor available in each case. Criteria for PTEN loss were defined as per previously published studies [15] (Figure). ERG positivity was determined by nuclear staining on IHC. For each patient, prostate cancer IHC expression was compared between systematic and MRI-targeted cores.
Figure.
Immunohistochemical stain for PTEN at high-power magnification showing (A) weak but positive PTEN staining in tumor cells, (B) strong positive PTEN staining in tumor cells, (C) heterogeneous loss of PTEN staining in tumor cells, and (D) homogeneous loss of PTEN staining in tumor cells.
3. Results
A total of 53 cases with GG1 or GG2 tumors were identified where patients had undergone systematic biopsy with concurrent MRI/US fusion targeted biopsy (Table 1). A total of 66 cancer-positive cores from the systematic approach and a total of 58 cancer-positive cores from the MRI-targeted approach were evaluated with IHC. The average number of total cores sampled was significantly higher for the systematic approach (11.8 ± 0.9) compared to the targeted approach (4.2 ± 2.0), P < .0001. An average of 2.2 ± 1.3 systematic biopsy cores and 2.0 ± 0.9 MRI-targeted biopsy cores per case contained cancer (P = .93). The average age was 66 years (range 44–81), and the average PSA was 6.2 ng/mL (range 1.5–17.2). Races present in our cohort included 41 whites, 10 African Americans, and 2 others. GG1 prostate cancer was the highest GG overall in 28/53 (52.8%) cases, and GG2 prostate cancer was the highest GG overall in 25/53 (47.2%) cases. In our cohort, 30/53 (56.6%) patients went on to choose active surveillance, 8/53 (15.1%) patients were treated with radical prostatectomy, 9/53 (17.0%), patients were treated with radiation therapy, and 6/53 (11.3%) patients were lost to follow-up.
Table 1.
Clinical demographics of patient population that underwent MRI-targeted and systematic prostate needle core biopsy
| Variable | n (%) |
|---|---|
|
| |
| No. of cases | 53 |
| Age (y), mean (range) | 66 (44–81) |
| Race | |
| African American | 10 (18.9%) |
| White | 41 (77.3%) |
| Other | 2 (3.8%) |
| Family history | |
| Yes | 14 (26.4%) |
| No | 38 (71.7%) |
| Unknown | 1 (1.9%) |
| GG | |
| 1 | 28 (52.8%) |
| 2 | 25 (47.2%) |
| PSA, mean ± SD | 6.2 ± 3.4 |
| PIRADS | |
| 3 | 10 (18.9%) |
| 4 | 27 (50.9%) |
| 5 | 16 (30.2%) |
| No. of cores obtained during biopsy, mean ± SD | |
| Systematic | 11.8 ± 0.9 |
| Targeted | 4.2 ± 2.0 |
| No. of cancer-positive cores, mean ± SD | |
| Systematic | 2.2 ± 1.3 |
| Targeted | 2.0 ± 0.9 |
| Management | |
| Active surveillance | 30 (56.6%) |
| Radiation therapy | 9 (17.0%) |
| Radical prostatectomy | 8 (15.1%) |
| Unknown | 6 (11.3%) |
PTEN staining and interpretation were performed on all 53 cases. Staining and interpretation for ERG status were performed on 52 cases; 1 case had tissue depleted after staining with PTEN, and therefore, ERG staining was unable to be performed on both the MRI-targeted and systematic cores.
ERG positivity was seen in 37/52 (71.2%) cases when assessing all biopsy cores available (Table 2). MRI-targeted biopsy detected 29/37 (78.4%) cases of ERG positivity compared to 32/37 (86.5%) cases detected by systematic biopsy. The detection of ERG expression was not significantly different between targeted and systematic biopsy (P = .4).
Table 2.
ERG status detected on systematic and MRI-targeted prostate needle core biopsies
| MRI-targeted biopsy | Systematic biopsy |
||
|---|---|---|---|
| ERG negative | ERG positive | Total | |
|
| |||
| ERG negative | 15 | 8 | 23 |
| ERG positive | 5 | 24 | 29 |
| Total | 20 | 32 | |
NOTE. Total cases n = 52. P = .4.
PTEN loss was seen in a total of 15/53 (28.3%) cases. Patients with PTEN loss consisted of 11 whites, 2 African Americans, and 2 others. MRI-targeted biopsy detected 14/15 (93.3%) cases of PTEN loss compared to 7/15 (46.7%) cases detected by systematic biopsy cores. MRI-targeted biopsy was superior to systematic biopsy in the detection of PTEN loss (P = .02) (Table 3). Of the 15 cases that had PTEN loss, 14/15 cases had tumor detected in the same regions on targeted and systematic approaches. Only 1 case had tumor detected in a location that was different between the standard and targeted biopsy. In this particular case, the targeted biopsy was positive in the left mid to left lateral base, whereas the standard biopsy was positive in the right apex and right mid gland. Of the 15 cases with PTEN loss, 12/15 (80%) also had gain of ERG expression. Heterogeneous expression was seen in most cases with PTEN loss. PTEN loss was found to be heterogeneous on systematic biopsy in 5/7 (71.4%) cases and on MRI-targeted biopsy in 11/14 (78.6%) cases.
Table 3.
PTEN status detected on systematic and MRI-targeted prostate needle core biopsies
| MRI-targeted biopsy | Systematic biopsy |
||
|---|---|---|---|
| PTEN Loss | PTEN Retained | Total | |
|
| |||
| PTEN loss | 6 | 8 | 14 |
| PTEN retained | 1 | 38 | 39 |
| Total | 7 | 46 | |
Total cases n = 53. P = .02.
In the 14/15 (93.3%) cases with PTEN loss, GG was the same between the MRI-targeted and systematic biopsy. In 1 case with PTEN loss, the GG was higher in the MRI-targeted cores (GG2) versus the systematic cores (GG1) sampled during the same biopsy session. PTEN loss was seen more often in GG2 tumors in both the systematic and MRI-targeted approach. For the systematic approach, PTEN loss was seen in GG1 tumors in 3/32 (9.4%) cases and in GG2 tumors in 4/21 (19.0%) cases (P = .31). For the MRI-targeted approach, PTEN loss was seen in GG1 tumors in 5/34 (14.7%) cases and in GG2 tumors in 9/19 (47.4%) cases (P = .01) (Table 4). There was no significant difference in race, age, PSA, grade group, or PIRADS score when comparing those patients with and without PTEN loss (Table 5). In the group where PTEN was retained, more patients (65.8%) went on to choose active surveillance versus those with PTEN loss (33.3%). However, this finding was not statistically significant (P = .053) and, importantly, was retrospective in nature, and biomarker information was not used to direct patient management.
Table 4.
PTEN and ERG status on systematic and MRI-targeted biopsy by GG
| GG | Total ERG cases | Total PTEN cases | PTEN loss | ERG gain |
|---|---|---|---|---|
|
| ||||
| Systematic biopsy | ||||
| 1 | 31 | 32 | 3 | 22 |
| 2 | 21 | 21 | 4 | 10 |
| MRI-targeted biopsy | ||||
| 1 | 33 | 34 | 5 | 19 |
| 2 | 19 | 19 | 9 | 10 |
Table 5.
Comparative Clinical and Pathologic Characteristics of Patients with and without PTEN Loss.
| Cases with PTEN loss | Cases with no PTEN loss | P | |
|---|---|---|---|
|
| |||
| No. of cases | 15 | 38 | |
| Detection on biopsy method, n (%) | |||
| Targeted cores | 14 (93%) | ||
| Standard cores | 7 (47%) | ||
| Targeted and standard cores | 6 (40%) | ||
| Age (y), mean ± SD | 66 ± 8 | 65 ± 8 | .66 |
| Race, n (%) | 1.0 | ||
| African American | 2 (15%) | 8 (21%) | |
| White | 11 (85%) | 30 (79%) | .36 |
| GG, n (%) | |||
| 1 | 6 (40%) | 22 (58%) | |
| 2 | 9 (60%) | 16 (42%) | |
| PSA, mean ± SD | 6.3 ± 4.0 | 6.1 ± 3.1 | .79 |
| PIRADS, n (%) | .36 | ||
| 3 | 1 (7%) | 9 (24%) | |
| 4 | 8 (53%) | 19 (50%) | |
| 5 | 6 (40%) | 10 (26%) | .053 |
| Treatment, n (%) | |||
| Active surveillance | 5 (33.3%) | 25 (65.8%) | |
| Radiation therapy | 5 (33.3%) | 4 (10.5%) | |
| Radical prostatectomy | 3 (20.0%) | 5 (13.2%) | |
| Unknown | 2 (13.3%) | 4 (10.5%) | |
Gain of ERG expression was seen more often in GG1 tumors than GG2 tumors in both the MRI-targeted and systematic approaches. For the systematic approach, ERG expression was seen in GG1 tumors in 22/31(71.0%) cases and in GG2 tumors in 10/21 (47.6%) cases (P = .7). For the MRI-targeted approach, ERG expression was seen in GG1 tumors in 19/33 (57.6%) cases and in GG2 tumors in 10/19 (52.6%) cases (P = .09). There was no significant difference in the detection of ERG expression in GG1 versus GG2 tumors.
4. Discussion
Prostate cancer is the most common noncutaneous cancer in the United States and the second leading cause of cancer-related deaths in American men. Improvements in MRI now allow for the more precise detection of prostate cancer by imaging [3,5,16,17]. Most notably, the combination of multiparametric MRI and superimposed real-time transrectal US allows for the improved targeting of suspicious lesions [2–4]. Multiple studies have shown the improved sensitivity and specificity of MRI/US fusion-targeted biopsies over the systematic biopsy approach in the detection of clinically significant prostate cancer, while requiring fewer cores [2–5]. In addition to the accurate detection of higher GGs, other clinically significant tumor characteristics such as extraprostatic extension, seminal vesicle invasion, and perineural invasion have also been demonstrated to be more frequently detected on MRI/US fusion biopsy [6–8]. However, despite the diagnostic improvements brought about by MRI targeted biopsy, the question of whether systematic biopsy can be eliminated entirely is still widely debated because of the potential risk of missing clinically significant disease by random sampling [18–20].
In 2014, the University of Alabama at Birmingham implemented the utilization of MRI/US fusion targeted prostate biopsies, with subsequent studies demonstrating optimization of detection of clinically significant prostate cancers [4,9]. In 2018, our group additionally assessed the management choices in patients who undergo MRI-targeted prostate biopsy compared to patients who undergo systematic biopsy [9]. It was found that patients who undergo MRI-targeted biopsy are 3.93 times more likely to choose active surveillance over early definitive treatment compared to men diagnosed on systematic biopsy alone ;, a preference which remained statistically significant after adjusting for age, PSAD, prior biopsy history, provider, tumor grade, and race [9]. As MRI-targeted biopsy becomes increasingly embraced by practitioners globally, it is important to see how prognostic biomarkers perform within this new biopsy technique.
PTEN and ERG mutations are two of the most common mutations found in prostate cancer, occurring in approximately 60% and 40% of prostate cancers, respectively [11]. As the most commonly deleted tumor suppressor gene in prostate cancer, PTEN loss is one of the most promising prognostic and predictive tissue-based biomarkers in prostate cancer [15,21]. A study by Lotan et al found that PTEN loss in biopsy specimens with Gleason score 3 + 3 = 6 prostate cancer is associated with an increased risk of Gleason score upgrading in the final radical prostatectomy specimen. This finding suggests that PTEN mutations may predict which tumors are undergraded on biopsy, thus helping guide clinical decision making [22]. ERG and PTEN mutations tend to occur together, with mouse studies implicating a symbiotic relationship in tumor oncogenesis [23,24]. These findings have led to the hypothesis that there may be a synergistic effect of ERG expression and PTEN loss on prostate cancer progression; several retrospective studies examining PTEN and ERG in human prostate cancer have established that PTEN mutations tend to occur following a preceding ERG mutation, with PTEN deletion occurring more commonly in ERG-rearranged prostate tumors [ 23,24]. Similar to this concept, in our study, we found that 80% of cases with PTEN loss also showed ERG expression on IHC.
We found a gain of ERG expression in 71.2% of cases in our cohort. This is somewhat higher than the results of previous studies, which report that the ERG gene is rearranged in approximately half of all prostate tumors [21]. This finding is likely the result of multiple factors and may reflect our particular patient demographic. Interestingly, we found no difference in ERG expression between the 2 cohorts, perhaps reflecting its high frequency in our population. ERG expression was seen more frequently in GG1 tumors; however, this was not statistically significant. As gain of ERG expression was found to be a much more prevalent finding, using MRI-targeted tissue for ERG expression may not hold an advantage over tissue acquired by a systematic approach.
As the PTEN gene is almost always lost by deletion in prostate cancer, fluorescence in situ hybridization (FISH) has traditionally been considered the criterion standard assay to detect in situ PTEN loss in tumor tissue [15]. However, the detection of PTEN loss also lends itself well to immunohistochemical assays. A multi-institutional cohort study conducted by Lotan et al investigated the sensitivity and specificity of PTEN immunohistochemistry relative to FISH for detection of PTEN gene deletion in prostate cancer and found that IHC staining had a 97% concordance with homozygous PTEN deletions detected by FISH, showing that automated PTEN immunohistochemistry assay is a sensitive method for the detection of homozygous PTEN gene deletions [15]. In our study, of the 7 systematic cores in which there was PTEN loss, 2 showed homozygous loss (28.6%), with the remaining 5 showing heterozygous loss (71.4%). Of the 14 MRI-targeted cores in which there was PTEN loss, 3 showed homozygous loss (21.4%), with the remaining 11 showing heterozygous loss (78.6%). This finding highlights the issue of tumor heterogeneity and its potential influence on the results of biomarker studies. A study conducted by Lotan et al in 2016 demonstrated that PTEN loss is commonly subclonal and heterogeneous in primary prostate tumors [15,21]. In general, previous PTEN FISH studies have shown that hemizygous deletions are more prevalent than homozygous deletions, a finding illustrated in our cohort, in which most PTEN loss cases were hemizygous [22]. Additional studies by Lotan et al have shown that protein loss is most strongly associated with shorter recurrence-free survival if the loss is homogeneous in all tumor cores sampled and that heterogeneous PTEN loss is a weaker prognostic indicator when compared to homogeneous loss [15,21].
In our study of patients with GG1 and GG2 tumors, we saw PTEN loss in 15/53 (28.3%) cases, which is higher than some other studies but may also reflect the presence of intermediate-risk tumors. Lotan et al showed that PTEN loss by immunohistochemistry is expected to be present in ~11% of Gleason score 6 biopsies overall, making it a rare finding in lower GGs [22]. In addition, they demonstrated that finding PTEN loss in a GG1 tumor may reflect unsampled higher-grade tumor elsewhere [22]. In our study, we also found an association between PTEN loss and higher grade. For the MRI-targeted approach, PTEN loss was seen in GG1 tumors in 14.7% of cases and in GG2 tumors in 47.4% of cases (P = .01). This association was only statistically significant in the MRI-targeted group. In addition, of the 15 cases in which PTEN protein was lost, there was a single case in which the GG differed between the systematic and targeted approaches. In this single case, PTEN loss was found in the target core, which showed GG2 tumor, whereas the systematic core showed GG1 tumor. Interestingly, PTEN loss was recorded as hemizygous in this case. These results can perhaps be explained by the combination of several factors at play. Clinically significant prostate cancer is more likely detected by MRI/US targeted biopsy, and PTEN loss is often associated with clinically significant tumor. In addition, in the targeted approach, more cores are sampled per lesion than the systematic approach, which relies on random sampling. Thereby, our finding of PTEN loss occurring more frequently in targeted cores suggests the ability to identify and target the more significant prostate cancer and sample it more thoroughly to account for the heterogeneity of PTEN expression. Indeed, of the 15 cases that had PTEN loss, 14/15 cases had tumor detected in the same regions on targeted and systematic approaches. Therefore, the difference in PTEN detection between the targeted biopsy and systematic biopsy in most cases was likely explained by tumor heterogeneity and the advantage of better sampling by a targeted approach. Only 1 case had tumor detected in a location that was different between the standard and targeted biopsy, in which the targeted biopsy was positive in the left mid to left lateral base whereas the standard biopsy was positive in the right apex and right mid gland. In this case, the difference in detection of PTEN may reflect multifocal prostate cancer. As MRI-targeted biopsy tissue successfully detected PTEN loss in 93.3% of cases, one could consider adapting a triage method for biomarker testing. One could start with testing of MRI-targeted tissue and then proceed to systematic cores as needed.
Notable limitations of this study are the relatively small sample size and retrospective nature. In the group where PTEN was retained, more patients (65.8%) went on to choose active surveillance versus those with PTEN loss (33.3%). However, this finding was not statistically significant (P = .053). This is expected because the study was retrospective in nature and clinicians did not have biomarker information to direct patient management. Future studies will need to further investigate if hemizygous PTEN deletions detected via IHC on biopsy accurately predict upgrading to allow for recommendations in terms of active surveillance. Other confounding factors include racial and ethnic backgrounds of the patient populations studied. Similar to other centers, at our institution, a lower number of African American men have undergone an MRI-targeted biopsy sessions compared to white men. Larger numbers would be required to evaluate the impact of prognostic biomarker expression in a race-stratified manner. Another potential factor may be access to health care by means of the referral patterns to our tertiary care center and distances associated with the regional area that our institution serves.
5. Conclusions
MRI/US fusion targeted biopsy is superior to systematic biopsy sampling in the detection of PTEN loss by IHC in GG1 and GG2 prostate cancer. Detection of ERG expression by IHC was equivalent between the MRI-targeted and systematic approach. These findings suggest that inclusion of tissue from MRI-targeted cores may be helpful for the assessment of some prognostic biomarkers.
Funding/Support:
This work was funded in part by a Junior Faculty Development Grant (ACS-IRG 001-53), by a pilot grant from the Young Supporters Board of the UAB Comprehensive Cancer Center, and by developmental funds from the UAB Comprehensive Cancer Center Support Grant (NCI P30 CA 013148) to Soroush Rais-Bahrami.
Footnotes
Competing interests: Jeffrey W. Nix and Soroush Rais-Bahrami serve as consultants for Philips/InVivo Corp. Soroush Rais-Bahrami also serves as a consultant to Blue Earth Diagnostics and Genomic Health Inc. All other authors report no conflicts of interest or financial disclosures that were pertinent to the following study.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- [2].Yarlagadda VK, Lai WS, Gordetsky JB, et al. MRI/US fusion-guided prostate biopsy allows for equivalent cancer detection with significantly fewer needle cores in biopsy-naive men. Diagn Interv Radiol 2018;24(3):115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313(4):390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gordetsky JB, Thomas JV, Nix JW, Rais-Bahrami S. Higher prostate cancer grade groups are detected in patients undergoing multiparametric MRI-targeted biopsy compared with standard biopsy. Am J Surg Pathol 2017;41(1):101–5. [DOI] [PubMed] [Google Scholar]
- [5].Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378(19):1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Truong M, Rais-Bahrami S, Nix JW, Messing EM, Miyamoto H, Gordetsky JB. Perineural invasion by prostate cancer on MR/US fusion targeted biopsy is associated with extraprostatic extension and early biochemical recurrence after radical prostatectomy. HUM PATHOL 2017;66:206–11. [DOI] [PubMed] [Google Scholar]
- [7].Gordetsky JB, Nix JW, Rais-Bahrami S. Perineural invasion in prostate cancer is more frequently detected by multiparametric MRI targeted biopsy compared with standard biopsy. Am J Surg Pathol 2016;40(4):490–4. [DOI] [PubMed] [Google Scholar]
- [8].Baumgartner EM, Porter KK, Nix JW, Rais-Bahrami S, Gordetsky JB. Detection of extraprostatic disease and seminal vesicle invasion in patients undergoing magnetic resonance imaging-targeted prostate biopsies. Trans Androl Urol 2018;7(Suppl. 4):S392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gordetsky JB, Saylor B, Bae S, Nix JW, Rais-Bahrami S. Prostate cancer management choices in patients undergoing multiparametric magnetic resonance imaging/ultrasound fusion biopsy compared to systematic biopsy. Urol Oncol 2018;36(5):241.e7–241.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lai WS, Gordetsky JB, Thomas JV, Nix JW. Rais-Bahrami S. Factors predicting prostate cancer upgrading on magnetic resonance imaging-targeted biopsy in an active surveillance population. Cancer 2017;123(11):1941–8. [DOI] [PubMed] [Google Scholar]
- [11].Ullman D, Dorn D, Rais-Bahrami S, Gordetsky J. Clinical utility and biologic implications of phosphatase and tensin homolog (PTEN) and ETS-related gene (ERG) in prostate cancer. Urology 2018;113:59–70. [DOI] [PubMed] [Google Scholar]
- [12].Gordetsky JB, Schultz L, Porter KK, Nix JW, Thomas JV. Del Carmen Rodriguez Pena M, et al. Defining the optimal method for reporting prostate cancer grade and tumor extent on magnetic resonance/ultrasound fusion-targeted biopsies. HUM PATHOL 2018;76:68–75. [DOI] [PubMed] [Google Scholar]
- [13].Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 2016;40(2):244–52. [DOI] [PubMed] [Google Scholar]
- [14].Gordetsky J, Epstein J. Grading of prostatic adenocarcinoma: current state and prognostic implications. Diagn Pathol 2016;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lotan TL, Wei W, Ludkovski O, et al. Analytic validation of a clinical-grade PTEN immunohistochemistry assay in prostate cancer by comparison with PTEN FISH. Mod Pathol 2016;29(8):904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gordetsky JB, Ullman D, Schultz L, et al. Histologic findings associated with false-positive multiparametric magnetic resonance imaging performed for prostate cancer detection Hum Pathol. 2019;83:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Engelhard K, Labanaris AP, Bogner K, Lübke L, Dworak O, Kühn R. How good is post-biopsy multiparametric magnetic resonance imaging in detecting and characterising the index lesion of localised prostate cancer? Scand J Urol 2014;48(6):499–505. [DOI] [PubMed] [Google Scholar]
- [18].Ma TM, Tosoian JJ, Schaeffer EM, et al. The role of multiparametric magnetic resonance imaging/ultrasound fusion biopsy in active surveillance. Eur Urol 2017. Feb;71(2):174–80. [DOI] [PubMed] [Google Scholar]
- [19].Hansen NL, Kesch C, Barrett T, et al. Multicentre evaluation of targeted and systematic biopsies using magnetic resonance and ultrasound image-fusion guided transperineal prostate biopsy in patients with a previous negative biopsy. BJU Int 2017. Nov;120(5):631–8. [DOI] [PubMed] [Google Scholar]
- [20].Borkowetz A, Platzek I, Toma M, et al. Direct comparison of multiparametric magnetic resonance imaging (MRI) results with final histopathology in patients with proven prostate cancer in MRI/ultrasonography-fusion biopsy. BJU Int 2016. Aug;118(2):213–20. [DOI] [PubMed] [Google Scholar]
- [21].Lotan TL, Wei W, Morais CL, et al. PTEN loss as determined by clinical-grade immunohistochemistry assay is associated with worse recurrence-free survival in prostate cancer. Eur Urol Focus 2016;2(2):180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lotan TL, Carvalho FL, Peskoe SB, et al. PTEN loss is associated with upgrading of prostate cancer from biopsy to radical prostatectomy. Mod Pathol 2015;28(1):128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carver BS, Tran J, Gopalan A, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet 2009;41:619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].King JC, Xu J, Wongvipat J, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet 2009;41:524–6. [DOI] [PMC free article] [PubMed] [Google Scholar]