Abstract
Introduction
The influence of anthropometric characteristics on colorectal neoplasia biology is unclear. We conducted a systematic review and meta-analysis to determine if adult-attained height is independently associated with the risk of colorectal cancer or adenoma.
Methods
We searched MEDLINE, EMBASE, the Cochrane Library, and Web of Science from inception to August 2020 for studies on the association between adult-attained height and colorectal cancer or adenoma. The original data from the Johns Hopkins Colon Biofilm study was also included. The overall hazard ratio/odds ratio of colorectal cancer/adenoma with increased height was estimated using random-effects meta-analysis.
Results
We included 47 observational studies involving 280,644 colorectal cancer and 14,139 colorectal adenoma cases. Thirty-three studies reported data for colorectal cancer incidence per 10-cm increase in height; 19 yielded a hazard ratio (HR) of 1.14 (95% confidence interval [CI], 1.11 to 1.17, P<0.001), and 14 engendered an odds ratio (OR) of 1.09 (95% CI, 1.05 to 1.13, P<0.001). Twenty-six studies compared colorectal cancer incidence between individuals within the highest versus the lowest height percentile; 19 indicated an HR of 1.24 (95% CI, 1.19 to 1.30, P<0.001), and seven resulting in an OR of 1.07 (95% CI, 0.92 to 1.25, P=0.39). Four studies reported data for assessing colorectal adenoma incidence per 10-cm increase in height, showing an overall OR of 1.06 (95% CI, 1.00 to 1.12, P=0.03).
Conclusions
Greater adult attained height is associated with an increased risk of colorectal cancer and adenoma.
Impact:
Height should be considered as a risk factor for colorectal cancer screening.
Keywords: stature, tallness, colorectal carcinoma, colorectal polyps, colorectal neoplasia
Introduction
Globally, colorectal cancer is the third most common cancer in men and the second most in women.(1) Although most colorectal cancers occur in adults ages 50 and older, 12% are diagnosed in people younger than age 50.(2) The overall death rate from colorectal cancers has been declining over several decades. However, deaths from colorectal cancer among individuals younger than age 50 have increased 2% per year from 2007 to 2016(3), emphasizing the importance of identifying and considering additional risk factors for colorectal cancer when evaluating patients for colorectal cancer screening.
There are several well-known risk factors for colorectal cancer. Nonmodifiable factors that increase colorectal cancer risk include a personal or family history of colorectal cancer or adenomas and a personal history of chronic inflammatory bowel disease.(2) In the United States, more than half of all colorectal cancers are attributable to modifiable lifestyle factors, including unhealthy diet, insufficient physical activity, smoking, and high alcohol consumption.(4) A recent review and meta-analyses of prospective observational studies highlighted the role of diet in colorectal cancer incidence, finding an association between lower colorectal cancer risk and high intakes of dietary fiber, dietary calcium, and yogurt and lower intakes of alcohol and red meat.(5) Anthropometric characteristics such as body weight, fat mass, and body mass index are additional important modifiable lifestyle-related risk factors for colorectal cancer(6).
Stature (height) is another attribute that has been actively investigated as a potential non-modifiable risk factor for colorectal cancer. A number of studies have been conducted to determine whether greater attained height is a risk factor for colorectal cancer; some study findings show a positive association between attained height and colorectal cancer while others failed to establish significant results, and still others show a negative association.(7–59)
Clinical gastroenterologists presently focus on genetic and age-related risks for CRC screening, such as a positive family history, high-risk syndromes (Lynch syndrome and polyposis syndromes), age>45 years old. (60) National Comprehensive Cancer Center guidelines also draw attention to increased awareness of modifiable risk factors such as alcohol, smoking, red and processed meat consumption, obesity, and physical activity.(61) However, despite evidence that colorectal cancer risk is increased with increased tallness, height is a non-modifiable risk factor which is currently clinically neglected in the context of screening.(61)
Moreover, only a few studies explored the association between height and risk for colorectal adenoma; two showing no clear association(62, 63), while another found that tallness is associated with an increased risk of colorectal adenoma(64). While three meta-analyses published before 2018 investigated the association between attained height and colorectal cancer(65–67), the height metrics were different, and none have investigated attained height with its neoplastic precursor, colorectal adenoma.
We conducted a systematic review and meta-analysis of observational studies from the peer-reviewed literature to determine if adult-attained height is an independent risk factor for colorectal neoplasia. As a sub-study, we curated, analyzed, and included original data sets collected from the Johns Hopkins Biofilm Colonoscopy Study in the data synthesis to determine if adult-attained height is associated with colonic adenomatosis.
Materials and Methods
This review is reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement(68) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.(69) The review protocol was registered with the international prospective register of systematic reviews (PROSPERO) (CRD42020147732).
Search Strategy and Selection criteria
We searched Medline (PubMed), Embase (Embase.com), Web of Science, and the Cochrane Central Register of Controlled Trials from inception to August 1, 2020, for literature on adult attained height and digestive tract malignancy risk. There was no restriction on publication language or geography. Search strategies were developed in collaboration with an informationist (JN) and are outlined in Supplementary Table 1. We reviewed the reference lists of eligible studies and related reviews for additional eligible studies. Pairs of reviewers (EZ, LW, GM) independently screened the titles and abstracts of identified citations using Covidence systematic review software and assessed the full texts of potentially eligible studies. Discrepancies were resolved by consensus. Studies were included if they were cohort (both prospective and retrospective) or case-control studies that examined the association between the attained height of adults (18 years and above) and risks of colorectal cancer or colorectal adenoma. Studies were required to report the association either as a hazard ratio (HR) or odds ratio (OR) of disease risk per unit change in height or a specific percentile change in height. Review papers or study abstracts were excluded.
In addition to studies identified from the literature search, we curated and analyzed original datasets from the Johns Hopkins Biofilm Colonoscopy Study. The study design was described in two previous publications.(70, 71) Briefly, the study recruited adult patients undergoing outpatient colonoscopy between August 2016 and February 2020. The inclusion criteria were individuals aged 40-85 years undergoing outpatient colonoscopy for colorectal cancer screening, had an intact unresected colon, had a complete colonoscopy, and were without a history of known organic colorectal disease. Organic disease in this study population means previously diagnosed colorectal cancer, inflammatory bowel disease, collagenous or autoimmune colitis, ischemic bowel, etc. Since organic diseases such as inflammatory bowel disease are a known risk factor for colorectal neoplasia, and these patients were excluded, this bolsters study validity. Patients who were pregnant, prisoners, on blood thinning medications, or unable to provide informed consent were excluded. Participants were queried during an interviewed by a research assistant prior to the procedure regarding their pertinent diet (i.e.,consumption of red meat, dairy, eggs, yogurt, fish), lifestyle patterns (i.e., tobacco smoking, alcohol consumption, etc.), and other relevant comorbidities (i.e., diabetes, hyperlipidemia, hypertension). The study was approved by the Johns Hopkins Institutional Review Board and was conducted in accordance with recognized ethical guidelines (i.e., Belmont Report, U.S. Common Rule). The precise location, size, diagnosis, and other characteristics of the colorectal polyps were collected from colonoscopy and pathology reports. To standardize polyp diagnosis, histopathology reports of all extracted or biopsied polyps were systematically reviewed by one expert member of the study team (S. Rifkin). Polyp diagnosis was confirmed by a Johns Hopkins pathologist. Colonoscopy was performed by expert gastroenterologists with high adenoma detection rates (>25%), and the cecum was required to be visualized in order to qualify for the study. The association between height and colorectal adenoma was assessed using multivariate logistic regression adjusting for age, race, weight, smoking, diabetes, red meat consumption, hyperlipidemia, and hypertension.
Data Extraction and Risk of Bias Assessment
Pairs of reviewers (EZ, LW, GM) independently assessed the risk of bias of included studies and extracted data items. These included: 1) general study information (author, year, country), 2) study design (case-control, cohort) and population (sample size, age, gender), 3) height measurement, and 4) confounding factors—patient characteristics that the authors adjusted for when they estimated the association between height and cancer risk. We used the Newcastle-Ottawa Scale for assessing the quality of included studies.(72) A study was judged on three perspectives with a maximum rating of nine stars: the selection of the study groups, the comparability of the groups, and the ascertainment of the exposure for case-control studies or outcome for cohort studies.
Data Synthesis and Statistical Methods
We estimated the overall HR or OR and its 95% confidence interval (CI) of colorectal cancer and colorectal adenoma, comparing the highest to the lowest height percentile or with a per 10-cm increase in height. For studies that reported HR or OR of colorectal cancer or colorectal adenoma with per unit change in height other than 10 cm, we derived the HR or OR per 10-cm increase in height based on the log-linear relationship between height and disease risk. We performed a random-effects meta-analysis using DerSimonian and Laird method.(73) We assessed heterogeneity across studies by examining the overlap of CIs for the results of individual studies by forest plots and quantified heterogeneity by the I2 statistic.(74) Sensitivity analysis was performed by removing outlier studies where the point estimate was outside of the 95% CIs of other studies. We used Cochrane Review Manager, version 5.4, for meta-analyses. We investigated the small study effect by visual inspection of funnel plots and the Egger test for comparisons with at least ten studies.(75) We used STATA, version 16, for the Egger test.
Assessing the Certainty of Evidence
We rated the certainty of evidence using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. The certainty of the body of evidence started with a low level (due to the observational nature of included studies). It was rated down based on limitations due to risk of bias, imprecision, inconsistency, indirectness, and publication bias. It was rated up based on the large magnitude of effects (i.e., strong associations), dose-response, and conservative confounding factors (i.e., confounding factors that bias the association toward null).(76)
Results
Characteristics of included studies
Our search identified 3,943 unique citation records that were examined by title and abstract screening. A total of 158 potential eligible records were determined to be eligible for inclusion and underwent full-text screening (Supplementary Figure 1). Forty-six eligible studies (cohort and case-controlled) were included in the quantitative analysis. Of the 46 studies, 36 were cohort studies, whereby participants were followed for incident colorectal cancer or colorectal adenoma after the baseline height measures. Ten were case-control studies, whereby participants with and without colorectal cancer or colorectal adenoma were identified, and height measures were retrospectively collected. Studies were published between 1991-2020, having 280,644 colorectal cancer and 12,680 colorectal adenoma cases from North America, Europe, Asia, and Australia. Aside from data extracted from included studies, we included relevant original data from the Johns Hopkins Biofilm Colonoscopy Study (N=1,459) in the meta-analysis for attained height and colorectal adenoma risk.(70, 71) The characteristics of included studies are described in Supplementary Table 2.
Based on the Newcastle-Ottawa Scale for study quality (maximum score of 9 stars), the 36 cohort studies were allocated an average of 7 stars (range: 5 to 9 stars), and the ten case-control studies were allocated an average of 6 stars (range: 5 to 8 stars). Of the 36 cohort studies, stars were deducted from 16 studies because height was self-reported. Ten of the cohort studies lacked evidence that colorectal cancer or colorectal adenoma was absent at the start of the study. In eight cohort studies, risk factors were not adequately controlled based on study design or analysis. In six studies, the follow-up was insufficient (we required a follow-up duration of 6 years or more to allow cancer development). Of the ten case-control studies, three did not validate cases independently, three recruited controls from settings other than community, four had self-reported height, and nine did not report the completeness of height measurement (i.e., whether there were any missing values in the height measurement). The risk of bias assessment for individual studies is described in Supplementary Table 3.
The Association between Attained Height and Colorectal Cancer
Risk of colorectal cancer per 10-cm increase in height
The estimated overall HR of colorectal cancer with every 10-cm increase in height (N=19 studies) was 1.14 (95% CI, 1.11 to 1.17, P<0.001, I2=79%) (Figure 1). A small study effect was observed in the funnel plot (Supplementary Figure 2) and detected by the Egger test (P=0.008). The estimated overall HR of colon cancer with every 10-cm increase in height (N=7 studies) was 1.15 (95% CI, 1.10 to 1.21, P<0.001, I2=70%) (Supplementary Figure 3). The estimated overall HR of rectum cancer with every 10-cm increase in height (N=9 studies) was 1.09 (95% CI, 1.03 to 1.17, P=0.005, I2=66%) (Supplementary Figure 4)
Figure 1. Hazard ratio of colorectal cancer per 10-cm increase in height.

The forest plot shows the hazard ratio (HR) of colorectal cancer per 10-cm increase in height for each study and for all studies combined. A HR larger than 1 indicates increased colorectal cancer risk with height increase. A HR lower than 1 indicates decreased colorectal cancer risk with height increase. All studies showed increased HR of colorectal cancer with height increase, and most studies reported significant results. The overall HR is therefore highly significant (p<0.00001) and reflects an increased risk.
Abbreviation: CI, confidence interval; SE, standard error; IV, inverse variance.
The estimated overall OR of colorectal cancer with every 10-cm increase in height (N=14 studies) was 1.09 (95% CI, 1.05 to 1.13, P<0.001, I2=21%). (Figure 2). The funnel plot did not detect the small study effect (Supplementary Figure 5) nor by using the Egger test (P=0.65). HR and OR were aligned in directionality; HR reflects instantaneous risk while OR reflects cumulative risk.
Figure 2. Odds ratio of colorectal cancer per 10-cm increase in height.

The forest plot shows the odds ratio (OR) of colorectal cancer per 10-cm increase in height for each study and for all studies combined. An OR larger than 1 indicates increased colorectal cancer risk with height increase. An OR lower than 1 indicates decreased colorectal cancer risk with height increase. Most studies showed increased OR of colorectal cancer with height increase. The two studies with the largest sample sizes (tightest confidence intervals), Boursi et al. 2014, and Demb 2019 et al., showed highly significant results, while studies with smaller sample sizes (wider confidence intervals) reported nonsignificant results. Combining all studies, the overall HR is highly significant (p<0.00001) and reflects an increased risk.
Abbreviation: CI, confidence interval; SE, standard error; IV, inverse variance.
Risk of colorectal cancer comparing the highest versus the lowest height percentile
The estimated overall HR of colorectal cancer comparing the highest versus the lowest height percentile (N=19 studies) was 1.24 (95% CI, 1.19 to 1.30, P<0.001, I2=67%) (Figure 3). The small study effect was not detected by the funnel plot (Supplementary Figure 6) or the Egger test (P=0.20). The estimated overall HR of colon cancer comparing the highest versus the lowest height percentile (N=9 studies) was 1.25 (95% CI, 1.03 to 1.52, P=0.02, I2=91%) (Supplementary Figure 7). The estimated overall HR of rectal cancer (N=5 studies) comparing the highest versus the lowest height percentile was 1.07 (95% CI, 0.98 to 1.17, P=0.11, I2=0%) (Supplementary Figure 8).
Figure 3. Hazard ratio of colorectal cancer comparing the highest versus lowest height percentile.

The forest plot shows the hazard ratio (HR) of colorectal cancer, comparing the highest versus the lowest height percentile for each study and for all studies combined. A HR larger than 1 indicates increased colorectal cancer risk with height increase. A HR lower than 1 indicates decreased colorectal cancer risk with height increase. Most studies showed increased HR of colorectal cancer with height increase, many of which with highly significant results. Combining all studies, the overall HR is highly significant (p<0.00001) and reflects an increased risk.
Abbreviation: CI, confidence interval; SE, standard error; IV, inverse variance.
The estimated overall OR of colorectal cancer comparing the highest versus the lowest height percentile (N=7 studies) was 1.07 (95% CI, 0.92 to 1.25, P=0.39, I2=66%) (Figure 4). A sensitivity analysis excluding the outlier study(9) pulled the result away from null but did not qualitatively change the conclusion, OR 1.10 (0.96 to 1.26, P=0.15, I2=59%).
Figure 4. Odds ratio of colorectal cancer comparing the highest versus the lowest height percentile.
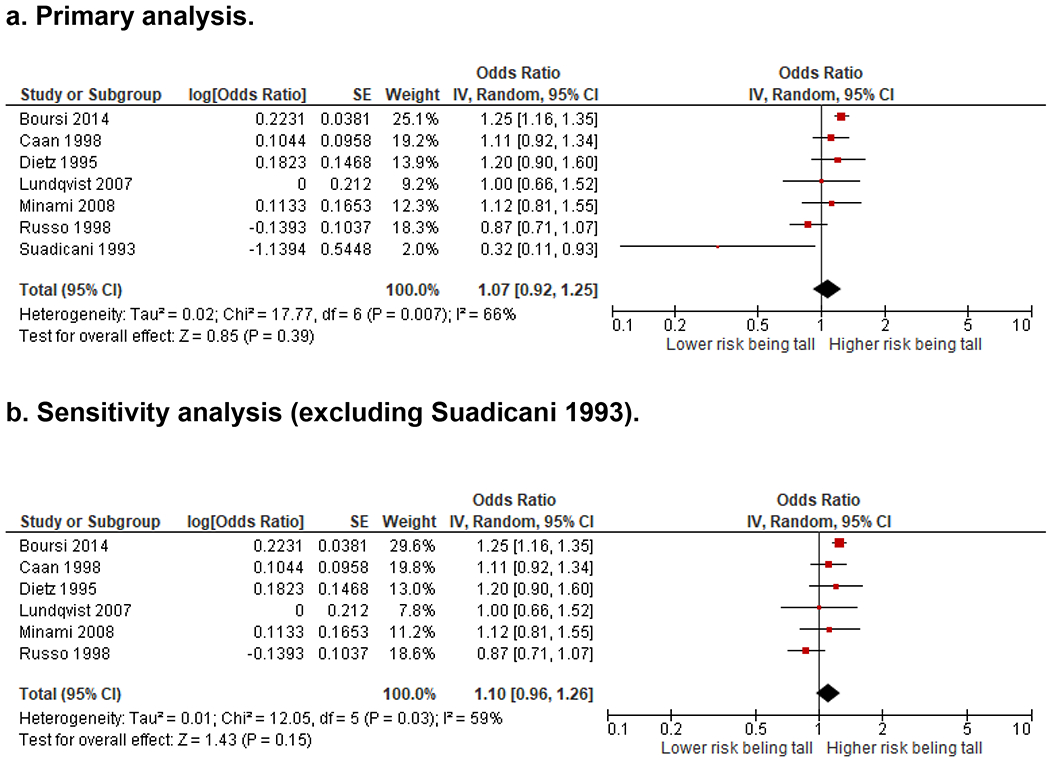
The forest plots show the odds ratio (OR) of colorectal cancer comparing the highest versus the lowest height percentile for each study and for all studies combined. An OR larger than 1 indicates increased colorectal cancer risk with increased height. An OR lower than 1 indicates decreased colorectal cancer risk with increased height. A. Four out of the seven studies showed increased OR of colorectal cancer with an increased height. Combining all studies, the overall OR the estimated overall OR of colorectal cancer comparing the highest versus the lowest height percentile (N=7 studies) was 1.07 (95% CI, 0.92 to 1.25, P=0.39, I2=66%). B. Results of a sensitivity analysis excluding the outlier study by Suadicani et al. pulled the overall results away from null but did not qualitatively change the conclusion [OR 1.10 (0.96 to 1.26, P=0.15, I2=59%)]. Abbreviation: CI, confidence interval; SE, standard error; IV, inverse variance.
The estimated overall OR of colon cancer comparing the highest versus the lowest height percentile (N=4 studies) was 1.13 (95% CI, 0.99 to 1.30, P=0.07, I2=0%) (Supplementary Figure 9). The estimated overall OR of rectal cancer comparing the highest versus the lowest height percentile (N=4 studies) was 0.91 (95% CI, 0.61 to 1.37, P=0.66, I2=43%) (Supplementary Figure 10). HR and OR were aligned in directionality.
The Association between Attained Height and Colorectal Adenoma
Risk of colorectal adenoma per 10-cm increase in height
The estimated overall OR of colorectal adenoma with every 10-cm increase in height (N=4 studies) was 1.06 (95% CI, 1.00 to 1.12, P=0.03, I2=92%). (Supplementary Table 4, Figure 5)
Figure 5. Odds ratio of colorectal adenoma per 10-cm increase in height.

The forest plot shows the odds ratio (OR) of colorectal adenoma per 10-cm increase in height. An OR larger than 1 indicates an increased colorectal cancer risk with increased height. An OR lower than 1 indicates a decreased colorectal cancer risk with increased height. Three out of the four studies showed increased OR of colorectal cancer with increased height. Combining all studies, the overall OR is significant (p=0.03) and reflects increased risk.
Abbreviation: CI, confidence interval; SE, standard error; IV, inverse variance.
The Certainty of Evidence
We graded the overall certainty of the evidence as low to moderate, as shown in the summary of findings table (Table 1). Low grades were initially assigned to the included studies due to their observational nature. These initial grades were further downgraded for risk of bias due to inadequate control of confounding, short follow-up in some studies, and potential publication bias raised by detected small study effects. We upgraded the study when the observed effects were of large magnitude and showed a dose-response gradient.
Table 1. Summary of Findings.
Low grades were initially assigned to the included studies due to their observational nature. These initial grades were further downgraded for risk of bias due to inadequate control of confounding, short follow-up in some studies, and potential publication bias raised by detected small study effects. We upgraded the study when the observed effects were of considerable magnitude and showed a dose-response gradient.
| The association between attained height and colorectal cancer or adenoma risk. | ||||
|---|---|---|---|---|
| Patient population: colorectal cancer or adenoma Settings: community and hospital Risk factor: attained height | ||||
| Outcomes | Relative effect (95% CI) | No. of studies | Quality of the evidence (GRADE) | Comments |
| HR of colorectal cancer per 10-cm increase in height | HR 1.14 (1.11 to 1.17) | 19 | ⊕⊕⊝⊝ low |
Downgraded for: 1. Inadequate control of confounding, short follow-up in some studies 2. Small study effect Upgraded for: 1. Large magnitude of an effect 2. Dose-response gradient |
| OR of colorectal cancer per 10-cm increase in height | RR 1.09 (1.05 to 1.13) | 14 | ⊕⊕⊕⊝ moderate |
Downgraded for: 1. Inadequate control of confounding, short follow-up in some studies Upgraded for: 1. Large magnitude of an effect 2. Dose-response gradient |
| HR of colorectal cancer comparing highest vs. lowest height percentile | HR 1.24 (1.19 to 1.30) | 19 | ⊕⊕⊕⊝ moderate |
Downgraded for: 1. Inadequate control of confounding, short follow-up in some studies Upgraded for: 1. Large magnitude of an effect 2. Dose-response gradient |
| OR of colorectal cancer comparing highest vs. lowest height percentile | OR 1.07 (0.92 to 1.25) | 7 | ⊕⊝⊝⊝ very low |
Downgraded for: 1. Inadequate control of confounding, short follow-up in some studies; 2. Inconsistency of results across studies 3. Imprecision of overall estimate Upgraded for: 1. Dose-response gradient |
| OR of colorectal adenoma per 10-cm increase in height | OR 1.06 (1.00 to 1.12) | 4 | ⊕⊕⊝⊝ low |
Downgraded for: 1. Inadequate control of confounding, short follow-up in some studies Upgraded for: 1. Dose-response gradient |
Discussion
This systematic review and meta-analysis demonstrate an association between taller heights and increased risk for colorectal cancer and colorectal adenoma. In our study, the tallest individuals had a 24% higher risk of developing colorectal cancer than the shortest. Every 10-cm increase in height imposed a 14% higher risk of colorectal cancer after adjusting for demographic, socioeconomic, behavioral, and other known risk factors. The association of increased height with colon cancer appeared to be stronger than with rectal cancer. A higher risk of colorectal cancer or colorectal adenoma was observed with increasing height. Higher adult-attained height appears to be an independent risk factor for both colorectal cancer and colorectal adenoma.
The importance of height as a potential risk for disease relates to the increasing trend of increased tallness in certain populations. A recent British study published by the Institute for the Study of Labor showed that the attained height of adult men in the United Kingdom has increased by 4 inches (10 centimeters) since the turn of the 20th century.(77) Nordic countries have also reported an increase in adult height, alongside a faster growth rate by the earlier timing of puberty, during the last four decades.(78) In the Japanese population, height has been increasing in recent decades.(79) This trend for increases in height has been attributed to nutritional factors, physical activities, improved hygiene, and healthcare delivery services.
Adult-attained stature may also represent a possible marker of the effects of early nutrition on human carcinogenesis and appears to be related to nutritional exposures occurring at the time of human physical maturation.(80) Other factors related to height, such as hormonal and genetic factors, stimulate both cancer development and progression.(30) One proposed reason taller individuals may be at higher risk for cancer is the increased opportunity for a cell mutation, given that taller people have overall more cells. Over the past century, children’s body size has also been increasing and growing to maturity more rapidly.(81) Although a recent systematic review of early-onset colorectal adenoma and cancer has recently reported that smoking and alcohol consumption, obesity, elevated blood glucose, elevated blood pressure, and elevated triglycerides are associated risk factors for younger-onset CRC, height has not been evaluated.(82)
Another proposed mechanism that may link height to cancers is that adult height correlates with body organ size.(83) More active cell proliferation in organs of taller people could increase the possibility of mutations leading to malignant transformation.(37) In addition, multiple interconnected biological pathways have been implicated in the association between height and cancer. Insulin-like growth factor-1 (IGF-1), which correlates with height in children(84), can increase the risk of several cancers(85), including colorectal cancer in adults.(86–88) IGF-1 is known to promote cell proliferation and inhibit apoptosis.(89) Growth hormone also stimulates IGF-1, which is important for determining adult height via regulation of bone growth.(90) Adult-attained height may represent cumulative exposure to IGF-1 throughout important periods of growth and development.(91)
Our findings indicate a dose-response association between height and colorectal neoplasm. By examining the cancer risk by both per 10-cm increase in height and the highest versus the lowest height categories, from all eligible studies published to date, we were able to establish the consistent height-cancer association among studies using different metrics. This is corroborated by previous studies that we excluded from our analysis because they examined height in different metrics and reported the same direction of association (data not shown). (11, 12, 15, 29, 30, 34, 37, 92–95) Khankari et al. published a meta-analysis in 2016, with 24 studies and an unclear number of subjects (66), showing that a 10-cm increase in height was associated with a RR of 1.12 (95% CI 1.10, 1.15) for CRC. Song et al. published a meta-analysis in 2018(67), including 31 studies and 93,818 CRC cases. The pooled RR (95% CI) comparing the highest versus the lowest category of height was 1.25 (1.18-1.32) for CRC. Finally in 2018, Abar et al. published a meta-analysis of 14 studies with 50,936 CRC cases, examining attained height, body fatness, and CRC risk.(65) They found an RR (95% CI) of 1.04 (1.02-1.05) per 5 cm increase in height. Our meta-analysis investigating the association between increased height and colorectal cancer is the most comprehensive to date, with the highest number of studies (n=47) and patients (280,644). Moreover, while three meta-analyses investigated the association between height and colorectal cancer(65–67), to our knowledge, none to date investigated the association between height and colorectal adenoma. In this study, we analyzed primary data collected from the Johns Hopkins Biofilm Colonoscopy Study and examined if adult-attained height is associated with colonic adenomatosis. We found a 6% higher risk for colorectal adenoma with every 10 cm increase in height after adjusting for other risk factors. However, we did not observe a stronger association between attained height and advanced adenoma versus nonadvanced adenoma, consistent with prior studies.(62, 64, 96)
In our study, the effect estimates for colorectal cancer stratified by location (proximal colon, distal colon, and rectum) were too sparse to unify. However, Pust et al. in a large prospective study of 1.3 million UK women reported 18,518 incident colorectal cancers and showed that the relative risk of colon cancer comparing women 165-cm or taller with those below 160-cm was was highest for the proximal tumors 1.28 (95% CI 1.20-1.36), and lowest for rectal cancer 1.21 (95% CI 1.14-1.30) with left-sided colon having intermediate heightened risk 1.24 (95% CI 1.16-1.33).(59)
For comparison to other known risk factors for colorectal cancer, in pooled analyses, processed red meat intake is positively associated with colorectal cancer risk (HR = 1.15, 95% CI: 1.01-1.32; P=0.03) and particularly with distal colon cancer (HR = 1.36; 95% CI: 1.09-1.69; P=0.006).(97) As another comparison, a meta-analysis has shown that the risk of colon cancer is increased among ever smokers (pooled RR, 1.18; 95% CI, 1.11-1.25) compared with never smokers.(98) In our study, attained height demonstrates a strong association with colorectal cancer development (HR of 1.24 (95% CI, 1.19 to 1.30, P<0.001) like these aforementioned well-known risk factors. However, magnitudes of the hazard ratio for height and hazard ratios for these other exposures (i.e., processed red meat intake and smoking) are not directly comparable as they all use different measurement scales (ie., smoking is commonly expressed in pack-years while red meat consumption scales are widely varied).
Due to the observational nature and method limitations of included studies, our findings need to be interpreted with caution. Ideally, a randomized controlled trial is needed to establish the causal association between an exposure and the outcome, as observational studies are subject to confounding. However, in our case, height cannot be randomized. Therefore, the positive association found in observational studies need to be interpreted with caution. And adequate control of confounding is most valuable. The main limitations include self-reported height and inadequate control of confounding detected in multiple studies. Self-reported height may not accurately reflect the true height. For instance, there are reports that as one ages height declines and extraneous factors such as changes in cognition may set in, so reliability on reported height with advanced age may introduce error.(99) Measured adult height is the most accurate means of determining tallness. Overall, aside from the elderly population, the direction of the difference between self-reported height and true height is unknown for all adult populations and therefore, it may introduce an element of inaccuracy with unknown direction.
Risk of bias is a concern as height values in nearly 50% of the included studies were self-reported. The adjustment for other colorectal cancer or colorectal adenoma risk factors was considered inadequate in nearly 20% of the included studies. For example, well-established risk factors such as smoking, alcohol consumption, red meat intake, and obesity were neglected from these studies when attempting to isolate the association between height and colorectal cancer. For instance, Walter 2013 and MacInnis 2004 had only adjusted for a very limited set of confounders. Examing the direction and the magnitude of the association, we did not find systematic differences between these two studies and other studies (i.e., Giovannucci 2004, Hughes 2011, Park 2012) that had controlled for a comprehensive list of confounders. In addition, the follow-up duration was short for some studies, and the completeness of height measures was unclear for other studies. Although some of these limitations may be due to resource limitations, they highlight potential areas of improvement for the design, analysis, and reporting of future research to address mechanisms underlying the association of height and colorectal carcinogenesis. Ideally, future research should use measured height instead of self-reported height, consider adjusting for all well-known risk factors of colorectal neoplasm, and allow sufficient study follow-up for cancer development. In addition, clinicians are interested in knowing more precisely who should undergo earlier screening due to having a greater vertical height. Tallness in a population is defined as having a height greater than or equal to two standard deviations from the mean. According to the 2000 US Census, males 6’3” or women 5’9” or greater would meet this definition.(100) For instance, tall athletes, individuals with inherited tallness, acromegaly (gigantism), Marfan syndrome, and other like disorders could be screened earlier, and its impact could be studied. Future studies should address the risk per the definition of tallness in thier respective populations.
In our study, despite the limitations that we encountered, as iterated above, the large magnitude of the effect of height on colorectal cancer and adenoma risk, and the dose-response gradient observed, warrant attention in clinical practice and future research.
Conclusions
After adjusting for demographic, socioeconomic, behavioral, and other known risk factors, taller adult-attained height is associated with increased colorectal cancer and colorectal adenoma risks in a dose-response manner. Height should be considered as a risk factor in the evaluation of patients for colorectal cancer screening. However, more research is needed to define precise tallness risk parameters to translate this information to clinical care.
Supplementary Material
Acknowledgments
This study was supported by grants R01CA196845 (C.L. Santiago,F. Giardello, C.N.Santiago, J. Drewes, G. Mullin, E. Spence, D. Kafonek, D.M. Cromwell, L. LaLuna), Bloomberg Philanthropies (C.L. Santiago, J.J. Gills), T32DK007632 (S. Rifkin), intramural funds (L.M. Hylind) and the Johns Hopkins Cancer Center Support Grant, NCI P30CA006973 (C.L. Santiago, F. Giardello). All data from R01CA196845 (Johns Hopkins) were stored in Research Electronic Data Capture (REDCap). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or the NIH. The authors thank all members of the Sears laboratory for assistance with the Biofilm Colonoscopy Study.
Biofilm Study Consortium authors:
Madison McMann (Johns Hopkins University School of Medicine), Courtney Stevens (Johns Hopkins University School of Medicine, Baltimore, MD), Brent Tabisz (Johns Hopkins University School of Medicine), Marshall Bedine (Green Spring Station Endoscopy, Lutherville-Timonium, MD), Eduardo Gonzalez-Velez (Johns Hopkins University School of Medicine, Baltimore, MD), Hazel Marie Galon Veloso (Johns Hopkins University School of Medicine, Baltimore, MD), Pamela Schearer (Reading Hospital, Tower Health, Reading, PA), Stacy Gerhart (Digestive Diseases Associates, Reading, Wyomissing, PA), Amy Schiller (Digestive Disease Associates, Reading, Wyomissing, PA), Karin Donato (Digestive Disease Associates, Reading, Wyomissing, PA), Randi Sweigart (Digestive Disease Associates, Reading, Wyomissing, PA), John Altomare (Digestive Disease Associates, Reading, Wyomissing, PA), Nirav Shah (Digestive Disease Associates, Reading, Wyomissing, PA), Christopher Ibrahim (Digestive Disease Associates, Reading, Wyomissing, PA), Ravi Ghanta (Digestive Disease Associates, Reading, Wyomissing, PA).
Footnotes
Conflicts of interests: The authors declare no potential conflicts of interest.
Institutional review board statement
The Biofilm study was approved by the Johns Hopkins Hospital IRB: IRB00094020 (principal investigators: C.L. Sears, F.M. Giardiello).
References
- 1.Bray FFJ, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2020. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Zhang B, Zhou H, et al. Beneficial influences of dietary Aspergillus awamori fermented soybean meal on oxidative homoeostasis and inflammatory response in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol 2019;93:8–16. [DOI] [PubMed] [Google Scholar]
- 3.Society. AC. American Cancer Society. Key Statistics for Colorectal Cancer. 2020. [Google Scholar]
- 4.Islami FGSA, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018:31–54. [DOI] [PubMed] [Google Scholar]
- 5.Veettil SK, Wong TY, Loo YS, et al. Role of Diet in Colorectal Cancer Incidence: Umbrella Review of Meta-analyses of Prospective Observational Studies. JAMA Netw Open 2021;4:e2037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bou Malhab LJ, Abdel-Rahman WM. Obesity and inflammation: colorectal cancer engines. Curr Mol Pharmacol 2021. [DOI] [PubMed] [Google Scholar]
- 7.Gunnell D, May M, Ben-Shlomo Y, et al. Height, leg length, and cancer: the Caerphilly Study. Nutr Cancer 2003;47:34–9. [DOI] [PubMed] [Google Scholar]
- 8.Chute CG, Willett WC, Colditz GA, et al. A prospective study of body mass, height, and smoking on the risk of colorectal cancer in women. Cancer causes & control : CCC 1991;2:117–24. [DOI] [PubMed] [Google Scholar]
- 9.Suadicani P, Hein HO, Gyntelberg F. Height, weight, and risk of colorectal cancer. An 18-year follow-up in a cohort of 5249 men. Scandinavian journal of gastroenterology 1993;28:285–8. [DOI] [PubMed] [Google Scholar]
- 10.Hebert PR, Ajani U, Cook NR, et al. Adult height and incidence of cancer in male physicians (United States). Cancer causes & control : CCC 1997;8:591–7. [DOI] [PubMed] [Google Scholar]
- 11.Robsahm TE, Tretli S. Height, weight and gastrointestinal cancer: a follow-up study in Norway. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 1999;8:105–13. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu N, Nagata C, Shimizu H, et al. Height, weight, and alcohol consumption in relation to the risk of colorectal cancer in Japan: a prospective study. British journal of cancer 2003;88:1038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oxentenko AS, Bardia A, Vierkant RA, et al. Body size and incident colorectal cancer: a prospective study of older women. Cancer prevention research (Philadelphia, Pa.) 2010;3:1608–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JY, Mitrou PN, Keogh RH, et al. Self-reported and measured anthropometric data and risk of colorectal cancer in the EPIC-Norfolk study. International journal of obesity (2005) 2012;36:107–18. [DOI] [PubMed] [Google Scholar]
- 15.Boursi B, Haynes K, Mamtani R, et al. Height as an independent anthropomorphic risk factor for colorectal cancer. European journal of gastroenterology & hepatology 2014;26:1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietz AT, Newcomb PA, Marcus PM, et al. The association of body size and large bowel cancer risk in Wisconsin (United States) women. Cancer causes & control : CCC 1995;6:30–6. [DOI] [PubMed] [Google Scholar]
- 17.Russo A, Franceschi S, La Vecchia C, et al. Body size and colorectal-cancer risk. International journal of cancer 1998;78:161–5. [DOI] [PubMed] [Google Scholar]
- 18.Otani T, Iwasaki M, Inoue M. Body mass index, body height, and subsequent risk of colorectal cancer in middle-aged and elderly Japanese men and women: Japan public health center-based prospective study. Cancer causes & control : CCC 2005;16:839–50. [DOI] [PubMed] [Google Scholar]
- 19.Bowers K, Albanes D, Limburg P, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. American journal of epidemiology 2006;164:652–64. [DOI] [PubMed] [Google Scholar]
- 20.Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Journal of the National Cancer Institute 2006;98:920–31. [DOI] [PubMed] [Google Scholar]
- 21.Lundqvist E, Kaprio J, Verkasalo PK, et al. Co-twin control and cohort analyses of body mass index and height in relation to breast, prostate, ovarian, corpus uteri, colon and rectal cancer among Swedish and Finnish twins. International journal of cancer 2007;121:810–8. [DOI] [PubMed] [Google Scholar]
- 22.Hughes LA, Simons CC, van den Brandt PA, et al. Body size, physical activity and risk of colorectal cancer with or without the CpG island methylator phenotype (CIMP). PloS one 2011;6:e18571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang RQ, Zheng W, Li HL, et al. Prospective cohort study of body height and cancer incidence among adult men and women in Shanghai. Tumor 2012;32:992–1000. [Google Scholar]
- 24.Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer 2004;108:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacInnis RJ, English DR, Hopper JL, et al. Body size and composition and colon cancer risk in women. Int J Cancer 2006;118:1496–500. [DOI] [PubMed] [Google Scholar]
- 26.Hebert PR, Ajani U, Cook NR, et al. Adult height and incidence of cancer in male physicians (United States). Cancer Causes Control 1997;8:591–7. [DOI] [PubMed] [Google Scholar]
- 27.Shin A, Joo J, Bak J, et al. Site-specific risk factors for colorectal cancer in a Korean population. PLoS One 2011;6:e23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otani T, Iwasaki M, Inoue M, et al. Body mass index, body height, and subsequent risk of colorectal cancer in middle-aged and elderly Japanese men and women: Japan public health center-based prospective study. Cancer Causes Control 2005;16:839–50. [DOI] [PubMed] [Google Scholar]
- 29.Kabat GC, Kim MY, Hollenbeck AR, et al. Attained height, sex, and risk of cancer at different anatomic sites in the NIH-AARP diet and health study. Cancer causes & control : CCC 2014;25:1697–706. [DOI] [PubMed] [Google Scholar]
- 30.Wirén S, Häggström C, Ulmer H, et al. Pooled cohort study on height and risk of cancer and cancer death. Cancer Causes and Control 2014;25:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung J, Song YM, Lawlor DA, et al. Height and site-specific cancer risk: A cohort study of a korean adult population. Am J Epidemiol 2009;170:53–64. [DOI] [PubMed] [Google Scholar]
- 32.Boursi B, Haynes K, Mamtani R, et al. Height as an independent anthropomorphic risk factor for colorectal cancer. Eur J Gastroenterol Hepatol 2014;26:1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engeland A, Tretli S, Austad G, et al. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control 2005;16:987–96. [DOI] [PubMed] [Google Scholar]
- 34.Green J, Cairns BJ, Casabonne D, et al. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. The Lancet. Oncology 2011;12:785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega LS, Bradbury KE, Cross AJ, et al. A Prospective Investigation of Body Size, Body Fat Composition and Colorectal Cancer Risk in the UK Biobank. Sci Rep 2017;7:17807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onyeaghala G, Lintelmann AK, Joshu CE, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research cancer prevention guidelines and colorectal cancer incidence among African Americans and whites: The Atherosclerosis Risk in Communities study. Cancer 2020;126:1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi YJ, Lee DH, Han KD, et al. Adult height in relation to risk of cancer in a cohort of 22,809,722 Korean adults. British journal of cancer 2019;120:668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandstedt J, Wangefjord S, Nodin B, et al. Gender, anthropometric factors and risk of colorectal cancer with particular reference to tumour location and TNM stage: a cohort study. Biol Sex Differ 2012;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bostick RM, Potter JD, Kushi LH, et al. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States). Cancer Causes Control 1994;5:38–52. [DOI] [PubMed] [Google Scholar]
- 40.Caan BJ, Coates AO, Slattery ML, et al. Body size and the risk of colon cancer in a large case-control study. Int J Obes Relat Metab Disord 1998;22:178–84. [DOI] [PubMed] [Google Scholar]
- 41.Dietz AT, Newcomb PA, Marcus PM, et al. The association of body size and large bowel cancer risk in Wisconsin (United States) women. Cancer Causes Control 1995;6:30–6. [DOI] [PubMed] [Google Scholar]
- 42.Minami Y, Tochigi T, Kawamura S, et al. Height, urban-born and prostate cancer risk in Japanese men. Japanese journal of clinical oncology 2008;38:205–13. [DOI] [PubMed] [Google Scholar]
- 43.Suadicani P, Hein HO, Gyntelberg F. Height, weight, and risk of colorectal cancer. An 18-year follow-up in a cohort of 5249 men. Scand J Gastroenterol 1993;28:285–8. [DOI] [PubMed] [Google Scholar]
- 44.Si S, Tewara MA, Ji X, et al. Body surface area, height, and body fat percentage as more sensitive risk factors of cancer and cardiovascular disease. Cancer Med 2020;9:4433–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oyeyemi SO, Braaten T, Botteri E, et al. Exploring geographical differences in the incidence of colorectal cancer in the Norwegian Women and Cancer Study: a population-based prospective study. Clin Epidemiol 2019;11:669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demb J, Earles A, Martinez ME, et al. Risk factors for colorectal cancer significantly vary by anatomic site. BMJ Open Gastroenterol 2019;6:e000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cornish AJ, Law PJ, Timofeeva M, et al. Modifiable pathways for colorectal cancer: a mendelian randomisation analysis. Lancet Gastroenterol Hepatol 2020;5:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.La Vecchia C, Negri E, Parazzini F, et al. Height and cancer risk in a network of case-control studies from northern Italy. Int J Cancer 1990;45:275–9. [DOI] [PubMed] [Google Scholar]
- 49.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1995;122:327–34. [DOI] [PubMed] [Google Scholar]
- 50.Ghadirian P, Maisonneuve P, Perret C, et al. Epidemiology of sociodemographic characteristics, lifestyle, medical history, and colon cancer: a case-control study among French Canadians in Montreal. Cancer Detect Prev 1998;22:396–404. [DOI] [PubMed] [Google Scholar]
- 51.Giacosa A, Franceschi S, La Vecchia C, et al. Energy intake, overweight, physical exercise and colorectal cancer risk. Eur J Cancer Prev 1999;8 Suppl 1:S53–60. [PubMed] [Google Scholar]
- 52.Giovannucci E, Rimm EB, Liu Y, et al. Height, predictors of C-peptide and cancer risk in men. Int J Epidemiol 2004;33:217–25. [DOI] [PubMed] [Google Scholar]
- 53.Campbell PT, Cotterchio M, Dicks E, et al. Excess body weight and colorectal cancer risk in Canada: associations in subgroups of clinically defined familial risk of cancer. Cancer Epidemiol Biomarkers Prev 2007;16:1735–44. [DOI] [PubMed] [Google Scholar]
- 54.Shin A, Joo J, Yang HR, et al. Risk prediction model for colorectal cancer: National Health Insurance Corporation study, Korea. PLoS One 2014;9:e88079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simons CC, van den Brandt PA, Stehouwer CD, et al. Body size, physical activity, early-life energy restriction, and associations with methylated insulin-like growth factor-binding protein genes in colorectal cancer. Cancer Epidemiol Biomarkers Prev 2014;23:1852–62. [DOI] [PubMed] [Google Scholar]
- 56.Lin ZW. Analysis of the height dependence of site-specific cancer risk in relation to organ mass. Ann Transl Med 2016;4:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simons CC, Schouten LJ, Godschalk R, et al. Body size, physical activity, genetic variants in the insulin-like growth factor pathway and colorectal cancer risk. Carcinogenesis 2015;36:971–81. [DOI] [PubMed] [Google Scholar]
- 58.Keskin H, Etemadi A, Taylor P. Risk factors of colorectal cancer in Linxian, China: A nutrition intervention trial with 30 years follow-up. Annals of Oncology 2018;29:56-56. [Google Scholar]
- 59.Pust AB, Alison R, Blanks R, et al. Heterogeneity of colorectal cancer risk by tumour characteristics: Large prospective study of UK women. International Journal of Cancer 2017;140:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Force UPST. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:1965–1977. [DOI] [PubMed] [Google Scholar]
- 61.Provenzale D, Ness RM, Llor X, et al. NCCN Guidelines Insights: Colorectal Cancer Screening, Version 2.2020. J Natl Compr Canc Netw 2020;18:1312–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pyo JH, Hong SN, Min BH, et al. Is height a risk factor for colorectal adenoma? Korean J Intern Med 2016;31:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boutron-Ruault MC, Senesse P, Meance S, et al. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutrition and cancer 2001;39:50–7. [DOI] [PubMed] [Google Scholar]
- 64.Nimptsch K, Giovannucci E, Willett WC, et al. Body fatness during childhood and adolescence, adult height, and risk of colorectal adenoma in women. Cancer Prev Res (Phila) 2011;4:1710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abar L, Vieira AR, Aune D, et al. Height and body fatness and colorectal cancer risk: an update of the WCRF-AICR systematic review of published prospective studies. European journal of nutrition 2018;57:1701–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khankari NK, Shu XO, Wen W, et al. Association between Adult Height and Risk of Colorectal, Lung, and Prostate Cancer: Results from Meta-analyses of Prospective Studies and Mendelian Randomization Analyses. PLoS medicine 2016;13:e1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song X, Gong X, Zhang T, et al. Height and risk of colorectal cancer: a meta-analysis. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 2018;27:521–529. [DOI] [PubMed] [Google Scholar]
- 68.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 70.Rifkin SB, Giardiello FM, Zhu X, et al. Yogurt consumption and colorectal polyps. Br J Nutr 2020:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santiago CN, Rifkin S, Drewes J, et al. Self-reported Metabolic Risk Factor Associations with Adenomatous, Sessile Serrated, and Synchronous Adenomatous and Sessile Serrated Polyps. Cancer PreScit 2021;14:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.GA Wells BS, D O’Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 2020. August 26]; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 73.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 74.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 75.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bailey RHT, Inwood K. . Health, Height and the Household at the Turn of the 20th Century. Institute for the Study of Labor (IZA) April 2014; Discussion Paper No. 8128. [Google Scholar]
- 78.Holmgren A, Niklasson A, Aronson AS, et al. Nordic populations are still getting taller - secular changes in height from the 20th to 21st century. Acta Paediatr 2019;108:1311–1320. [DOI] [PubMed] [Google Scholar]
- 79.N Y. Taii Kijunchi (in Japanese). Rinsho Eiyo 1999:267–270. [Google Scholar]
- 80.Albanes D, Jones DY, Schatzkin A, et al. Adult stature and risk of cancer. Cancer research 1988;48:1658–62. [PubMed] [Google Scholar]
- 81.Chen ST. Secular trend of growth in Malaysian children. J Singapore Paediatr Soc 1990;32:102–7. [PubMed] [Google Scholar]
- 82.Breau G, Ellis U. Risk Factors Associated With Young-Onset Colorectal Adenomas and Cancer: A Systematic Review and Meta-Analysis of Observational Research. Cancer Control 2020;27:1073274820976670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Albanes DW M Are cell number and cell proliferation risk factors for cancer? J. Natl. Cancer Inst. 1988:772–775. [DOI] [PubMed] [Google Scholar]
- 84.Aea Juul. Serum insulin-like growth factor-I in 1030 healthy children, ado- lescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J. Clin. Endocrinol. Metab. 1994:744–752. [DOI] [PubMed] [Google Scholar]
- 85.AsghariHanjani N, Vafa M. The role of IGF-1 in obesity, cardiovascular disease, and cancer. Med J Islam Repub Iran 2019;33:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Renehan AGea. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004:1346–1353 [DOI] [PubMed] [Google Scholar]
- 87.Simons CCJM, Schouten LJ, Godschalk R, et al. Body size, physical activity, genetic variants in the insulin-like growth factor pathway and colorectal cancer risk. Carcinogenesis 2015;36:971–981. [DOI] [PubMed] [Google Scholar]
- 88.Giovannucci E, Rimm EB, Liu Y, et al. Height, predictors of C-peptide and cancer risk in men. International journal of epidemiology 2004;33:217–25. [DOI] [PubMed] [Google Scholar]
- 89.Pollak MN SE, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer 2004:505–518. [DOI] [PubMed] [Google Scholar]
- 90.Yakar S RC, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu J-L, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 2002;110:771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.RG R Insulin-like growth factors and the basis of growth. N Engl J Med 2003;349:2184–2186. [DOI] [PubMed] [Google Scholar]
- 92.Kabat GC, Anderson ML, Heo M, et al. Adult stature and risk of cancer at different anatomic sites in a cohort of postmenopausal women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2013;22:1353–63. [DOI] [PubMed] [Google Scholar]
- 93.Kabat GC, Heo M, Kamensky V, et al. Adult height in relation to risk of cancer in a cohort of Canadian women. International journal of cancer 2013;132:1125–32. [DOI] [PubMed] [Google Scholar]
- 94.La Vecchia C, Negri E, Parazzini F, et al. Height and cancer risk in a network of case-control studies from northern Italy. International journal of cancer 1990;45:275–9. [DOI] [PubMed] [Google Scholar]
- 95.Walter RB, Brasky TM, Buckley SA, et al. Height as an explanatory factor for sex differences in human cancer. Journal of the National Cancer Institute 2013;105:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He X, Wu K, Ogino S, et al. Association Between Risk Factors for Colorectal Cancer and Risk of Serrated Polyps and Conventional Adenomas. Gastroenterology 2018;155:355–373 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bernstein AM, Song M, Zhang X, et al. Processed and Unprocessed Red Meat and Risk of Colorectal Cancer: Analysis by Tumor Location and Modification by Time. PLoS One 2015;10:e0135959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765–78. [DOI] [PubMed] [Google Scholar]
- 99.Sahyoun NR, Maynard LM, Zhang XL, et al. Factors associated with errors in self-reported height and weight in older adults. J Nutr Health Aging 2008;12:108–15. [DOI] [PubMed] [Google Scholar]
- 100.Fryar CD, Kruszon-Moran D, Gu Q, et al. Mean Body Weight, Height, Waist Circumference, and Body Mass Index Among Adults: United States, 1999-2000 Through 2015-2016. Natl Health Stat Report 2018:1–16. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


