Abstract
Palmitic acid (PA) is ubiquitously present in dietary fat guaranteeing an average intake of about 20 g/d. The relative high requirement and relative content in the human body, which accounts for 20–30% of total fatty acids (FAs), is justified by its relevant nutritional role. In particular physiological conditions, such as in the fetal stage or in the developing brain, the respectively inefficient placental and brain blood–barrier transfer of PA strongly induces its endogenous biosynthesis from glucose via de novo lipogenesis (DNL) to secure a tight homeostatic control of PA tissue concentration required to exert its multiple physiological activities. However, pathophysiological conditions (insulin resistance) are characterized by a sustained DNL in the liver and aimed at preventing the excess accumulation of glucose, which result in increased tissue content of PA and disrupted homeostatic control of its tissue concentration. This leads to an overaccumulation of tissue PA, which results in dyslipidemia, increased ectopic fat accumulation, and inflammatory tone via toll-like receptor 4. Any change in dietary saturated FAs (SFAs) usually reflects a complementary change in polyunsaturated FA (PUFA) intake. Since PUFA particularly n-3 highly PUFA, suppress lipogenic gene expression, their reduction in intake rather than excess of dietary SFA may promote endogenous PA production via DNL. Thereby, the increase in tissue PA and its deleterious consequences from dysregulated DNL can be mistakenly attributed to dietary intake of PA.
Keywords: palmitic acid, de novo lipogenesis, fatty acid metabolism, dietary fatty acids, saturated/unsaturated ratio
Introduction
Palmitic acid (PA) is one of the most abundant saturated fatty acids (SFAs) in nature, which is present in animal and human tissues, plants, algae, fungus, yeast, and bacteria. Its distribution varies both within species and among species, and its content can be influenced by several environmental factors as the variation of soil pH, nutrient–ion interaction, age, water, and climate (1, 2).
The average dietary intake of PA is around 20–30 g/d representing about 8–10%en (3–5) and can be found in different vegetable and animal fat sources (Table 1) (6), with levels of 20–30% in animal lipids and 10–45% in vegetable oils. Methods that are used to prepare the food also impact on PA amount; for example, in processed and preserved meats, the content is higher than fresh meat with values up to 7.6/100 g of edible portion in salami and in lard 21/100 g of edible portion. It should be pointed out that due to high within-food variability of PA content, it is very difficult to assess its precise dietary intake. In addition, PA absorption and metabolic fate are strongly influenced by several factors, such as food matrix and pathological or physiological conditions.
Table 1.
Palmitic acid content of oils and fats from vegetable and animal sources (expressed as percentage mass fraction of total FAs) (6).
| Vegetable sources | % Fraction/total FA | Animal sources | % Fraction/total FA |
|---|---|---|---|
| Palm oil | 40.1–47.5 | Lard | 21.07 |
| Cottonseed oil | 21.4–26.4 | Goose | 7.41 |
| Cocoa butter | 25.4 | Whole chicken | 2.19 |
| Olive oil | 7.5–20.0 | Pork loin | 2.06 |
| Oat bran oil | 17.4 | Lamb | 0.58–1.99 |
| Avocado oil | 17.2 | Rabbit | 1.22–1.95 |
| Wheat germ oil | 16.6 | Beef meat | 0.31–1.14 |
| Corn oil | 8.6–16.5 | Horse meat | 1.65 |
| Peanut oil | 8.3–14.0 | Sheep meat | 0.58 |
| Soya bean oil | 8.0–13.3 | Goat meat | 0.40 |
| Grapeseed oil | 5.5–11.0 | Deer meat | 0.12 |
| Sesame oil | 7.9–10.2 | Salami | 5.73–7.55 |
| Coconut oil | 8.2 | Mortadella | 5.70 |
| Walnut oil | 3.9–7.2 | Ham | 3.93–4.93 |
| Linseed oil | 4.0–7.0 | Speck | 3.71 |
| Almond oil | 6.5 | Pancetta | 5.67–5.99 |
| Safflower oil | 4.8–6.2 | Butter | 20.86 |
| Linola oil | 6.0 | Parmesan cheese | 8.04 |
| Cashew nut oil | 4.0–6.0 | Fontina cheese | 7.31 |
| Rapeseed oil | 1.5–6.0 | Cream | 5.72 |
| Sunflower oil | 5.4–5.9 | Ricotta cheese (cow) | 3.49 |
| Hazelnut oil | 5.2 | Ricotta cheese (sheep) | 2.85 |
| Canola oil | 4.0 | Cow's whole milk | 0.92–1.97 |
| Eggs sources | Sheep's whole milk | 1.58 | |
| Hen egg (whole) | 1.90–5.90 | Goat's whole milk | 1.34 |
| Duck egg | 3.00 | Semiskimmed milk | 0.45 |
| Turkey egg | 2.72 | Yogurt | 0.92 |
Importance of the Matrix and PA Distribution on Metabolism
In evaluating the effects of food on health, the overall macronutrient composition and structure need to be considered, i.e. the “food matrix” (7), meaning that food chemical compounds behave differently in isolated form in comparison to part of food structures (8), as well as the resistance of a food to the mastication and the viscosity of aliments, which may affect the bioavailability and digestibility of dietary lipids (9). Dietary fats comprise cholesterol and fatty acids (FAs), which can be free or components of complex lipids, as triacylglycerols (TAGs), and phospholipids (PLs), organized in structures able to modulate FA final metabolic fate. FA esterification on different positions of the TAG glycerol backbone (central sn-2 position, external sn-1 and sn-3 positions) or on a PL may also impact on their digestibility and metabolism (10–16). PA in foods is mainly present esterified in PL and TAG.
Dietary PL represents 1–10% of total daily fat intake (17). PL is mainly catabolized by pancreatic phospholipase A2 (PLA2) that produces free FA (FFA) and lysophosphatidylcholine (lysoPL), which once absorbed by the intestinal epithelium are reacylated or hydrolyzed to form PL or glycerol-3-phosphorylcholine, respectively. FFAs are instead used for TAG synthesis that are subsequently incorporated into chylomicrons (17).
Over 90% of dietary FAs are esterified to TAG preferentially hydrolyzed by digestive lipases (18) on sn-1,3 positions followed by pancreatic lipase to give 2-monoacylglycerol (2-MAG) and FFA (15), which cross the apical membranes of the enterocytes and are reassembled into TAG for secretion to plasma in chylomicrons. SFA released from positions sn-1 and sn-3 may form insoluble soaps with ions as calcium that are not absorbed, a singularity lost if SFA is in the TAG sn-2 position (19), as confirmed by animal and human infant studies which demonstrate that sn-2 esterified FA is efficiently absorbed as 2-MAG (20, 21). The peculiar sn-2 position of PA in human milk results from the activity of the glycerol-3-phosphate (G-3-P) acyltransferase, present in the mammary gland, which acylates an unsaturated FA at the sn-1 position of G-3-P and subsequently a PA at the sn-2 position (22, 23). Human milk, which contains 20–25% of PA with respect to the total FA whose 70% is in sn-2 of TAG, limits PA malabsorption providing the infant with high PA (19, 23–25). Conversely, 45 and 58% of cow and rodent milk fat (25 and 15% of PA on the total FA, respectively) are esterified at the TAG sn-2 position (23, 26). FA composition of the early diet influences intestinal membrane FA, which affects nutrient transport, permeability, and inflammatory pathways that persist into later life (27, 28). Notably, PA also plays an important role in the developing fetus with the term infant reaching 13–15% of body fat of which 45–50% is PA, mostly derived from endogenous synthesis in the fetus (29).
PA peculiar tissue distribution results in its better incorporation in several tissues, for example, adipose tissues, with a lower deposition of fat in the visceral depots and higher in the subcutaneous fat (30). Interestingly, several studies demonstrated the protective effect of breastfeeding against obesity in childhood (31, 32) and adulthood (33–36). Also, donkey milk contains high concentrations of PA in sn-2 of TAG and is recognized as the best potential substitute for human milk due to its remarkable nutritional value, good palatability, and reduced allergenicity (37, 38). A recent study in rats showed that oral supplementation with human or donkey milk ameliorated metabolism and reduced inflammation potentially mediated by an improved redox status, mitochondrial uncoupling, and dynamics (39). In addition, it has been demonstrated that PA in sn-2 by modifying endocannabinoids and congeners biosynthesis in different tissues may potentially concur in the physiological regulation of energy metabolism, brain function, and body fat distribution (40). In contrast to milk, in animal tissues that include human adipose tissue and also beef tallow and in soybean oil and cocoa butter, PA is mainly at sn-1,3 position, whereas the sn-2 is occupied by an unsaturated FA (41–45). Lard, having high amounts of PA at TAG sn-2, represents an exception (23), and in animal studies, PA from lard was better absorbed with respect to PA from cocoa butter and palm oil (46, 47). This PA peculiar position led food industries to often use interesterification to produce functional infant formula containing TAG with a high amount of sn-2 PA (48, 49). Amounts of PA in the sn-2 position in breast-fed infants (81%) or in infants fed formula prepared with synthesized TAG (39%), plasma chylomicron TAG containing PA in sn-2 position were higher with respect to those fed with standard infant formula with 6% of PA in sn-2 position (50), and it was shown in infants that PA loss in stools was 8-folds less using infant formula with lard TAG with respect to randomized lard (51). Also, an increase in the proportion of sn-2 PA by interesterification of TAG in coconut oil and palm olein improved PA absorption and metabolism in rats (52, 53). Therefore, the matrix/esterified position plays a crucial role in determining the metabolic fate of dietary PA.
Assessment of Dietary PA Intake in Humans
Most of the studies aimed at evaluating dietary FA intake rely on food frequency questionnaires (FFQs), and food diaries where even repeated measurements do not necessarily provide valid measures of individual intake. Extreme intakes may reflect under- and overreporting rather than true low or high intakes, and subjects most prone to reporting bias may be repeatedly misclassified in quantiles of the distribution (54). In addition, assessing the precise nutrient intake is quite difficult because of the errors made in recalling or the identification of the amounts of foods eaten, especially in processed foods (55).
Measurement of circulating PA is not also a reliable marker of its dietary intake; in fact, the dietary consumption of PA has low impact on plasma levels compared with its endogenous biosynthesis; data from a controlled human feeding trial showed that variations in SFA intake from 11 to 30%en did not change circulating SFA, including PA (56). Accordingly, cohort studies did not show a solid correlation between the PA dietary intake (evaluated by FFQ) and its plasma levels (r = −0.02 to 0.09) (57–60).
Factors other than dietary intake have been suggested to influence FA composition in tissues, first FA metabolism efficiency, genetic variations, and even intrauterine and perinatal program. In fact, considering the relationship between the tissue FA composition and dietary fat, among plasma lipid fractions, only TAGs appear to reflect dietary polyunsaturated FA (PUFA) and SFA, but not monounsaturated FA (MUFA) (61) within the first hours after intake (62). Whereas, FA in serum cholesteryl esters (CEs) and in PL is related to average intake of dietary FA composition during the previous 3–6 weeks, FA of erythrocyte membrane PL and adipose tissue TAG reflect the dietary fat intake of previous months or years, respectively (62).
Noteworthy, it has been demonstrated, by isotope labeling studies in men, that low-fat high-carbohydrate diet stimulates de novo lipogenesis (DNL) with the accumulation of VLDL-TAG PA that led to linoleic acid (LA) reduction probably due to dilution effect, whereas with high-fat (40% fat, 45% carbohydrate) DNL is neglectable (63). This suggests that circulating PA levels are largely driven by endogenous synthesis through DNL rather than direct dietary intake. Therefore, the relative strict regulation of PA tissue concentration, with variable amount of the endogenously produced, leads to a high unreliability of the use of PA plasma levels as a tool to determine its dietary intake.
The potential increase of tissue PA by dietary intake is prevented by the contribution of its conversion to palmitoleic (POA), by the insertion of one double-bond through stearoyl-CoA desaturase-1 (SCD1) (62), which reduces PA availability in tissues, but also via elongation to stearic acid (SA) and further desaturation via SCD1 to form oleic acid (OA). A possible protective capacity of OA to drive PA to be deposited in the neutral form of TAG (64, 65) and POA to improve insulin sensibility has been described (66).
Fate or Metabolism of PA From DNL
When the energetic sources are in excess, the non-fat surplus, mainly carbohydrates, is converted to FA by DNL, a pathway that begins with the conversion of acetyl-CoA into malonyl-CoA by acetyl-CoA carboxylase (ACC). During fed and insulin-stimulated conditions, ACC increases malonyl-CoA levels whereas AMP-activated protein kinase (AMPK) stops the synthesis, probably by inhibiting sterol regulatory element-binding protein (SREBP) (67).
Further evidence indicates that adipose tissue DNL supports metabolic homoeostasis of distant organs, as in liver and muscle, by producing cytokine-like lipids, lipokines, with antidiabetogenic and antiinflammatory activities, such as POA and branched FA esters of hydroxy FA (FAHFA) (66, 68).
In normal conditions, adipose tissue is the major site for DNL, which significantly contributes to body lipid reserves, energy storage, and to the maintenance of serum TAG homeostasis that derived instead from dietary sources (69–75). Furthermore, adipose tissue DNL is considered as an energy-inefficient source of lipids because it yields fewer lipids per calorie consumed, thus being a promising strategy for the treatment of lipotoxicity-related diseases. In fact, adipose tissue DNL is positively correlated with postprandial energy expenditure (76) subsequently to carbohydrate overfeeding, but not fat overfeeding which failed to significantly increase any component of energy expenditure (77, 78).
On the other hand, under specific conditions in the liver, such as insulin resistance, the impaired glycogen biosynthesis and consequent accumulation of glucose induce DNL that may contribute up to 26% to ectopically intrahepatocellular lipids in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). In fact, hepatic DNL is positively correlated with insulin resistance and fatty liver, whereas the correlation with adipose tissue DNL is the opposite (73, 79–81).
In addition, a high-carbohydrate diet, particularly rich in simple sugars as fructose (82–84), activates a lipogenic response and increases the synthesis and secretion of VLDL in liver (85) contributing to hypertriglyceridemia (74). DNL contributes to 10–35% of the total VLDL-TAG pool, probably increasing the size (~130 nm), but not the number of VLDL secreted (86), and is in general higher in insulin-resistant states, and in overweight subjects compared to lean individuals (87–91).
Regulation of DNL occurs through the regulation of transcriptional factors as SREBP-1c and carbohydrate-responsive element-binding protein (ChREBP), activated by increased insulin signaling and increased glucose concentrations, respectively, and both induced by feeding (85, 92–95).
In liver, PUFA downregulates DNL via decreased expression of SREBP-1c (96), and leptin reduced adipogenesis through the inhibition of SREBP-1c expression (97). In addition, insulin and SREBP-1c stimulate peroxisome proliferator-activated receptor-γ (PPAR γ) expression (98, 99), which regulates glucose and lipid metabolism thus having adipogenic and lipogenic effects (100) and promotes FA storage in mature adipocytes by the stimulation of lipoprotein lipase (LPL), CD36, and glucose transporter GLUT-4 (101–103).
Therefore, DNL has a dual function, to supply PA in deficiency conditions, such as in the fetus (29) and developing brain (104) to overcome the difficulties of PA to pass, respectively, the placenta and the brain–blood barrier and to prevent the excess accumulation of glucose in the liver. In the latter case, significant increase of tissue PA is detected eluding the homeostatic control of tissue PA concentration and increased endogenous PA production may enhance inflammatory susceptibility through toll-like receptor (TLR4) activation (105) and insulin resistance by ceramide accumulation (106).
Considerations Over High SFA Diets vs. PUFA-Deficient Diets on Metabolism
Dietary guidelines recommend limiting SFA intake to <10% of calories per day. Correlation between dietary SFA intake and cardiovascular disease (CVD) is quite controversial (107). The Cochrane analysis showed an association between reducing SFA intake and a reduction in cardiovascular events and replacing the energy from SFA with PUFA appear to be useful strategies, whereas effects of replacement with MUFA are unclear (108). SFA increases LDL plasma particle concentration but also their size, which is less associated with CVD (109) because more rapidly cleared than small-dense LDL particles from the circulation due to reduced receptor-mediated uptake (110). SFA increases blood total, LDL, and HDL cholesterol concentrations and decreases fasting TAG concentrations not changing the total–HDL cholesterol (TC/HDL) ratio. The capacity of increasing circulating HDL levels decreases with increasing chain length of SFA and for some studies, but not all, myristic acid and PA, and also carbohydrate intake, negatively affect TC/HDL ratio (111).
In vitro cell culture studies showed that PA in the free form in the medium elicits, insulin resistance (112), inflammation via TLR4 (105) and prometastatic activities (113), which implies that increased dietary PA may result in higher PA availability to cell tissues in the free form, while as already mentioned, higher intake of SFA results in a decrease of circulating PA in the free form and increase of its monounsaturated metabolites POA and OA. Therefore, in vitro models, while may elucidate a limited molecular mechanism, are by far not mimicking pathophysiological conditions. The extremely high concentrations typically used in vitro are not achievable in vivo, thus the results obtained do not prove any relevant pathophysiological information.
Any change in dietary SFA reflects a complementary change in MUFA and/or PUFA intake. As mentioned above, PUFA (70), particularly n-3 highly PUFA such as EPA and DHA, suppresses lipogenic gene expression by reducing the nuclear abundance and DNA-binding affinity of transcription factors responsible for imparting insulin and carbohydrate control to lipogenic and glycolytic genes (114); thereby, most of the detrimental effects should be ascribed to the lower PUFA intake rather than high dietary SFA.
From, a meta-analysis of randomized controlled trials emerged that replacing 5% energy from carbohydrate with SFA had no significant effect on fasting glucose but lowered insulin, and replacing SFA with PUFA lowered glucose, HbA1c and HOMA. This suggests that consuming more unsaturated FA in place of either carbohydrates or SFA will help to improve blood glucose control while exchanging dietary carbohydrate with SFA does not appreciably influence markers of blood glucose control, and therefore an approach based only on reducing carbohydrates or SFA intake, without considering the source of energy replacement would not be optimal (115).
Data are often contradictory and may be difficult to interpret into dietary advise: some studies suggested that n-6 PUFA would increase CVD risk (116, 117), and therefore the Institute of Medicine recommends a relatively modest range of 5%–10% energy consumption from PUFA, limiting its plausibility as a meaningful replacement for SFA (118). Increasing dietary PUFA may not be desirable as dietary levels of LA are already higher than recommended (119), particularly the n-6/n-3 PUFA ratio (119). The physiological role played by the SREBP-1c, which is inhibited by n-3 FA (114) and in general by PUFA (18), for glycogen biosynthesis and overall glucose homeostasis (120), stresses the point that balance between different dietary FA is strongly recommended and any unbalance may lead or increase the chance to set into motion a disrupted metabolism. In fact, while replacing SFA with LA has an established cholesterol lowering effect, it has not been shown that this lowering reduces mortality (107).
In addition, recently, it has been shown that lower dietary PUFA/MUFA and n-3/n-6, and not SFA, were associated with disturbances in metabolic syndrome-related indices in postmenopausal women, and that polymorphisms of FA desaturase FADS1 (rs174546) and FADS2 (rs3834458) were associated with unfavorable FA profile in red blood cells (121). It has also been demonstrated that the polymorphism rs1761667 of multifunctional CD36 scavenger receptor that facilitates FA uptake and oxidation, leads to a distinct metabolic pattern in normal weight and in obese subjects (122). Thus, changes of tissue FA profile and associated metabolic changes may also be determined by different genetic polymorphisms, which should be considered in developing personalized therapeutic strategies for ameliorating dyslipidemia and other metabolic disorders.
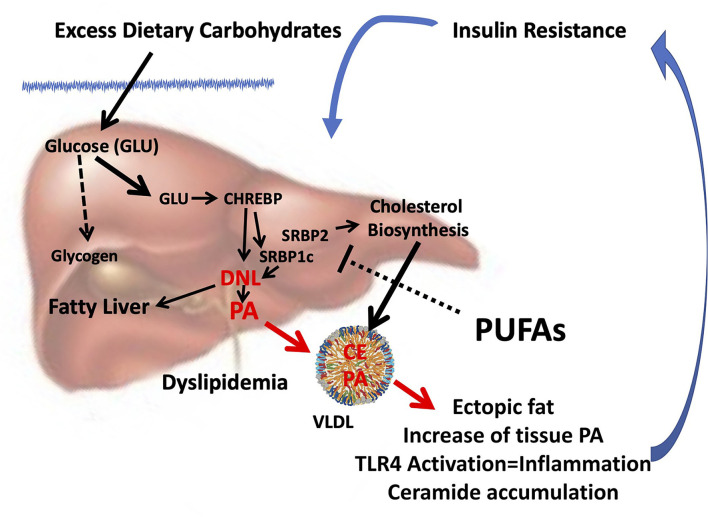
From several studies exploring the molecular mechanism of dietary FA interactions emerged that the reduction of PUFA intake, especially n-3 PUFA, rather than the excess of dietary SFA, may favor insulin resistance (123), promoting endogenous PA production via DNL. Thus, the increase in tissue PA from dysregulated DNL and its deleterious consequences can be mistakenly attributed to dietary intake of PA (Figure 1).
Figure 1.
Combined consequences of liver insulin resistance and reduced PUFA intake. Insulin resistance in the liver is characterized by hyperinsulinemia and a reduced ability to store glycogen. In the presence of excess glucose, CHREBP is activated which, in turn, together with hyperinsulinemia, induces SREBP1c, and synergistically induces DNL (124) and thereby the biosynthesis of endogenous PA. Reduced PUFA intake can further promote PA and cholesterol biosynthesis since PUFAs inhibit both SREBP1c (123) and SREBP2 (125). Enhanced DNL can cause fatty liver and formation and release of VLDL enriched with PA and cholesterol esters. As a result, the accumulation of ectopic fat occurs in different tissues, and the increase in tissue PA can sustain insulin resistance by inducing inflammation through the activation of TLR4 (105) and accumulation of ceramides (106), setting in motion a vicious circle. Because reduced PUFA intake is often associated with an unbalanced increase in dietary SFA/PUFA, the rise in tissue PA can be mistakenly attributed to its dietary intake. CHREBP, carbohydrate-responsive element-binding protein; SREBP, sterol regulatory element-binding protein; DNL, de novo lipogenesis; PA, palmitic acid; PUFA, polyunsaturated fatty acid; TLR4, toll-like receptor 4; SFA, saturated fatty acid.
Interestingly, it has been proposed that the claimed adverse effect on cholesterol exerted by high dietary SFA/PUFA ratio may represent a physiological mechanism aimed at fulfilling the needs of tissues for cholesterol (126) and the yield of larger LDL makes this increase not to be related to CVD (7).
Many of the purported harmful effects of dietary PA are based on experimental animal studies, mainly on mice on a high-fat diet, which consists of 45–60%en whereas the optimal fat content in the rodents diets ranges from 9 to 16%en (127). Therefore, high-fat diets contain from 3- to 6-folds of the fat content required, with usually an extremely high percentage of PA and low in PUFA and n-3/n-6 PUFA ratio, which makes difficult to pinpoint the effects of a high-fat content, high concentration of PA, or high n-6/n-3 PUFA ratio. These diets were created to induce obesity as quickly as possible (128) and not to assess the nutritional impact of dietary FA. Therefore, whereas they might be suitable as a model of obesity, they cannot be taken into consideration for translational nutritional studies on the effects of dietary FA (128).
Conclusions
Several pieces of evidence suggest that the nutritional impact of dietary FA is strictly related to the balance among them and with other macronutrients. Most of the studies claiming negative effect of PA rely on in vitro cell culture studies, incubating cells with extremely high concentrations and as a single FA without considering that dietary PA does not modify its tissue concentration, or with animal models of obesity with an extremely high-fat content not achievable by humans (128) and not specifically designed for studying dietary FA and thereby without any translatability to human conditions. More preclinical and clinical studies are needed to better discern the metabolic fate and interaction between dietary and de novo PA particularly in relation to PUFA intake, macronutrient balance, and pathophysiological states.
To blame a single nutrient, such as PA, widely present in our diet from several sources and with several well recognized fundamental physiological properties (129), as detrimental, suggesting that is sufficient to reduce its dietary intake for improving our health and prevent pathological states from CVD to cancer, is rather simplistic but it has a great praise probably because of the human nature to choose less time and energy consuming solutions for complex issues (130).
Thus, guidelines or recommendations to the general population to avoid or increase the intake of single nutrients, without considering the complexity of nutrient-nutrient interactions and the individual-specific nutritional response in relation to age, genetic, environmental, physiological and pathophysiological conditions, do not follow the amount of growing scientific data that suggest we should not be focusing on single nutrients but on increasing diet variability within a personalized nutritional approach.
Author Contributions
EM, CM, GC, and SB: conception and design of the review, organized the literature search, wrote the first draft of the manuscript, and contributed to wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This research was funded by Grants to GC and SB from the University of Cagliari: Fondo Integrativo per la Ricerca (FIR 2020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Clegg AJ. Composition and related nutritional and organoleptic aspects of palm oil. J Am Oil Chem Soc. (1973) 50:321–4. 10.1007/BF02641365 [DOI] [PubMed] [Google Scholar]
- 2.Griffiths RG, Dancer J, O'Neill E, Harwood JL. Lipid composition of Botrytis cinerea and inhibition of its radiolabelling by the fungicide iprodione. New Phytol. (2003) 160:199–207. 10.1046/j.1469-8137.2003.00848.x [DOI] [PubMed] [Google Scholar]
- 3.Sette S, Le Donne C, Piccinelli R, Arcella D, Turrini A, Leclercq C, et al. The third Italian National Food Consumption Survey, INRAN-SCAI 2005-06–part 1: nutrient intakes in Italy. Nutr Metab Cardiovasc Dis. (2011) 21:922–32. 10.1016/j.numecd.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 4.Jensen RG. Lipids in human milk. Lipids. (1999) 34:1243–71. 10.1007/s11745-999-0477-2 [DOI] [PubMed] [Google Scholar]
- 5.Innis SM, Nelson CM. Dietary triacyglycerols rich in sn-2 palmitate alter post-prandial lipoprotein and unesterified fatty acids in term infants. Prostagl Leukot Essent Fatty Acids. (2013) 89:145–51. 10.1016/j.plefa.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 6.Peiretti PG. Palmitic Acid: Effect of Diet Supplementation and Occurrence in Animal Origin Food. Porto LF, editor. New York, NY: Nova Science Publishers Inc. (2014). [Google Scholar]
- 7.Agostoni C, Boccia S, Banni S, Mannucci PM, Astrup A. Sustainable and personalized nutrition: from earth health to public health. Eur J Intern Med. (2021) 86:12–6. 10.1016/j.ejim.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 8.Aguilera JM. The food matrix: implications in processing, nutrition and health. Crit Rev Food Sci Nutr. (2019) 59:3612–29. 10.1080/10408398.2018.1502743 [DOI] [PubMed] [Google Scholar]
- 9.Pasquier B, Armand M, Castelain C, Guillon F, Borel P, Lafont H, et al. Emulsification and lipolysis of triacylglycerols are altered by viscous soluble dietary fibres in acidic gastric medium in vitro. Biochem J. (1996) 314:269–75. 10.1042/bj3140269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Astrup A, Bertram HC, Bonjour JP, de Groot LC, de Oliveira Otto MC, Feeney EL, et al. WHO draft guidelines on dietary saturated and trans fatty acids: time for a new approach? BMJ. (2019) 366:l4137. 10.1136/bmj.l4137 [DOI] [PubMed] [Google Scholar]
- 11.Berry SE. Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: an overview and implications for cardiovascular disease. Nutr Res Rev. (2009) 22:3–17. 10.1017/S0954422409369267 [DOI] [PubMed] [Google Scholar]
- 12.Berry SE, Sanders TA. Influence of triacylglycerol structure of stearic acid-rich fats on postprandial lipaemia. Proc Nutr Soc. (2005) 64:205–12. 10.1079/PNS2005422 [DOI] [PubMed] [Google Scholar]
- 13.Bracco U. Effect of triglyceride structure on fat absorption. Am J Clin Nutr. (1994) 60(Suppl. 6):1002S−9S. 10.1093/ajcn/60.6.1002S [DOI] [PubMed] [Google Scholar]
- 14.Hunter JE. Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids. (2001) 36:655–68. 10.1007/s11745-001-0770-0 [DOI] [PubMed] [Google Scholar]
- 15.Michalski MC, Genot C, Gayet C, Lopez C, Fine F, Joffre F, et al. Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Prog Lipid Res. (2013) 52:354–73. 10.1016/j.plipres.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 16.Murru E, Banni S, Carta G. Nutritional properties of dietary omega-3-enriched phospholipids. Biomed Res Int. (2013) 2013:965417. 10.1155/2013/965417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohn JS, Wat E, Kamili A, Tandy S. Dietary phospholipids, hepatic lipid metabolism and cardiovascular disease. Curr Opin Lipidol. (2008) 19:257–62. 10.1097/MOL.0b013e3282ffaf96 [DOI] [PubMed] [Google Scholar]
- 18.Livesey G. The absorption of stearic acid from triacylglycerols: an inquiry and analysis. Nutr Res Rev. (2000) 13:185–214. 10.1079/095442200108729061 [DOI] [PubMed] [Google Scholar]
- 19.Gesteiro E, Guijarro L, Sanchez-Muniz FJ, Vidal-Carou MDC, Troncoso A, Venanci L, et al. Palm Oil on the Edge. Nutrients. (2019) 11:2008. 10.3390/nu11092008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innis SM, Dyer R, Nelson CM. Evidence that palmitic acid is absorbed as sn-2 monoacylglycerol from human milk by breast-fed infants. Lipids. (1994) 29:541–5. 10.1007/BF02536625 [DOI] [PubMed] [Google Scholar]
- 21.Yang LY, Kuksis A. Apparent convergence (at 2-monoacylglycerol level) of phosphatidic acid and 2-monoacylglycerol pathways of synthesis of chylomicron triacylglycerols. J Lipid Res. (1991) 32:1173–86. 10.1016/S0022-2275(20)41980-7 [DOI] [PubMed] [Google Scholar]
- 22.Gimeno RE, Cao J. Thematic review series: glycerolipids. Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity. J Lipid Res. (2008) 49:2079–88. 10.1194/jlr.R800013-JLR200 [DOI] [PubMed] [Google Scholar]
- 23.Innis SM. Dietary triacylglycerol structure and its role in infant nutrition. Adv Nutr. (2011) 2:275–83. 10.3945/an.111.000448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin JC, Bougnoux P, Antoine JM, Lanson M, Couet C. Triacylglycerol structure of human colostrum and mature milk. Lipids. (1993) 28:637–43. 10.1007/BF02536059 [DOI] [PubMed] [Google Scholar]
- 25.Straarup EM, Lauritzen L, Faerk J, Hoy Deceased CE, Michaelsen KF. The stereospecific triacylglycerol structures and Fatty Acid profiles of human milk and infant formulas. J Pediatr Gastroenterol Nutr. (2006) 42:293–9. 10.1097/01.mpg.0000214155.51036.4f [DOI] [PubMed] [Google Scholar]
- 26.Breckenridge WC, Kuksis A. Molecular weight distributions of milk fat triglycerides from seven species. J Lipid Res. (1967) 8:473–8. 10.1016/S0022-2275(20)38904-5 [DOI] [PubMed] [Google Scholar]
- 27.Drozdowski LA, Clandinin T, Thomson AB. Ontogeny, growth and development of the small intestine: understanding pediatric gastroenterology. World J Gastroenterol. (2010) 16:787–99. 10.3748/wjg.v16.i7.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Innis SM Dai C, Wu X, Buchan AM, Jacobson K. Perinatal lipid nutrition alters early intestinal development and programs the response to experimental colitis in young adult rats. Am J Physiol Gastrointest Liver Physiol. (2010) 299:G1376–85. 10.1152/ajpgi.00258.2010 [DOI] [PubMed] [Google Scholar]
- 29.Innis SM. Palmitic acid in early human development. Crit Rev Food Sci Nutr. (2016) 56:1952–9. 10.1080/10408398.2015.1018045 [DOI] [PubMed] [Google Scholar]
- 30.Petrus P, Edholm D, Rosqvist F, Dahlman I, Sundbom M, Arner P, et al. Depot-specific differences in fatty acid composition and distinct associations with lipogenic gene expression in abdominal adipose tissue of obese women. Int J Obes. (2017) 41:1295–8. 10.1038/ijo.2017.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity–a systematic review. Int J Obes Relat Metab Disord. (2004) 28:1247–56. 10.1038/sj.ijo.0802758 [DOI] [PubMed] [Google Scholar]
- 32.Gillman MW, Rifas-Shiman SL, Camargo CA, Jr, Berkey CS, Frazier AL, Rockett HR, et al. Risk of overweight among adolescents who were breastfed as infants. JAMA. (2001) 285:2461–7. 10.1001/jama.285.19.2461 [DOI] [PubMed] [Google Scholar]
- 33.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. (2005) 162:397–403. 10.1093/aje/kwi222 [DOI] [PubMed] [Google Scholar]
- 34.Owen CG, Martin RM, Whincup PH, Davey-Smith G, Gillman MW, Cook DG. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr. (2005) 82:1298–307. 10.1093/ajcn/82.6.1298 [DOI] [PubMed] [Google Scholar]
- 35.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. (2005) 115:1367–77. 10.1542/peds.2004-1176 [DOI] [PubMed] [Google Scholar]
- 36.Quigley MA. Re: Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. (2006) 163:870–2; author reply 2–3. 10.1093/aje/kwj134 [DOI] [PubMed] [Google Scholar]
- 37.Fiocchi A, Brozek J, Schunemann H, Bahna SL, von Berg A, Beyer K, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) guidelines. World Allergy Organ J. (2010) 3:57–161. 10.1097/WOX.0b013e3181defeb9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tafaro A, Magrone T, Jirillo F, Martemucci G, D'Alessandro AG, Amati L, et al. Immunological properties of donkey's milk: its potential use in the prevention of atherosclerosis. Curr Pharm Des. (2007) 13:3711–7. 10.2174/138161207783018590 [DOI] [PubMed] [Google Scholar]
- 39.Trinchese G, Cavaliere G, De Filippo C, Aceto S, Prisco M, Chun JT, et al. Human milk and donkey milk, compared to cow milk, reduce inflammatory mediators and modulate glucose and lipid metabolism, acting on mitochondrial function and oleylethanolamide levels in rat skeletal muscle. Front Physiol. (2018) 9:32. 10.3389/fphys.2018.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carta G, Murru E, Lisai S, Sirigu A, Piras A, Collu M, et al. Dietary triacylglycerols with palmitic acid in the sn-2 position modulate levels of N-acylethanolamides in rat tissues. PLoS ONE. (2015) 10:e0120424. 10.1371/journal.pone.0120424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bottino NR, Vandenburg GA, Reiser R. Resistance of certain long-chain polyunsaturated fatty acids of marine oils to pancreatic lipase hydrolysis. Lipids. (1967) 2:489–93. 10.1007/BF02533177 [DOI] [PubMed] [Google Scholar]
- 42.Christensen MS, Hoy CE, Redgrave TG. Lymphatic absorption of n - 3 polyunsaturated fatty acids from marine oils with different intramolecular fatty acid distributions. Biochim Biophys Acta. (1994) 1215:198–204. 10.1016/0005-2760(94)90111-2 [DOI] [PubMed] [Google Scholar]
- 43.Lawson LD, Hughes BG. Human absorption of fish oil fatty acids as triacylglycerols, free acids, or ethyl esters. Biochem Biophys Res Commun. (1988) 152:328–35. 10.1016/S0006-291X(88)80718-6 [DOI] [PubMed] [Google Scholar]
- 44.Porsgaard T, Xu X, Gottsche J, Mu H. Differences in the intramolecular structure of structured oils do not affect pancreatic lipase activity in vitro or the absorption by rats of (n-3) fatty acids. J Nutr. (2005) 135:1705–11. 10.1093/jn/135.7.1705 [DOI] [PubMed] [Google Scholar]
- 45.Yang LY, Kuksis A, Myher JJ. Lipolysis of menhaden oil triacylglycerols and the corresponding fatty acid alkyl esters by pancreatic lipase in vitro: a reexamination. J Lipid Res. (1990) 31:137–47. 10.1016/S0022-2275(20)42768-3 [DOI] [PubMed] [Google Scholar]
- 46.Aoe S, Yamamura J, Matsuyama H, Hase M, Shiota M, Miura S. The positional distribution of dioleoyl-palmitoyl glycerol influences lymph chylomicron transport, composition and size in rats. J Nutr. (1997) 127:1269–73. 10.1093/jn/127.7.1269 [DOI] [PubMed] [Google Scholar]
- 47.Porsgaard T, Hoy CE. Lymphatic transport in rats of several dietary fats differing in fatty acid profile and triacylglycerol structure. J Nutr. (2000) 130:1619–24. 10.1093/jn/130.6.1619 [DOI] [PubMed] [Google Scholar]
- 48.Lucas A, Quinlan P, Abrams S, Ryan S, Meah S, Lucas PJ. Randomised controlled trial of a synthetic triglyceride milk formula for preterm infants. Arch Dis Child Fetal Neonatal Ed. (1997) 77:F178–84. 10.1136/fn.77.3.F178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Fouw NJ, Kivits GA, Quinlan PT, van Nielen WG. Absorption of isomeric, palmitic acid-containing triacylglycerols resembling human milk fat in the adult rat. Lipids. (1994) 29:765–70. 10.1007/BF02536698 [DOI] [PubMed] [Google Scholar]
- 50.Nelson CM, Innis SM. Plasma lipoprotein fatty acids are altered by the positional distribution of fatty acids in infant formula triacylglycerols and human milk. Am J Clin Nutr. (1999) 70:62–9. 10.1093/ajcn/70.1.62 [DOI] [PubMed] [Google Scholar]
- 51.Filer LJ, Jr, Mattson FH, Fomon SJ. Triglyceride configuration and fat absorption by the human infant. J Nutr. (1969) 99:293–8. 10.1093/jn/99.3.293 [DOI] [PubMed] [Google Scholar]
- 52.Renaud SC, Ruf JC, Petithory D. The positional distribution of fatty acids in palm oil and lard influences their biologic effects in rats. J Nutr. (1995) 125:229–37. [DOI] [PubMed] [Google Scholar]
- 53.Lien EL, Yuhas RJ, Boyle FG, Tomarelli RM. Corandomization of fats improves absorption in rats. J Nutr. (1993) 123:1859–67. 10.1093/jn/123.11.1859 [DOI] [PubMed] [Google Scholar]
- 54.Black AE, Cole TJ. Biased over- or under-reporting is characteristic of individuals whether over time or by different assessment methods. J Am Diet Assoc. (2001) 101:70–80. 10.1016/S0002-8223(01)00018-9 [DOI] [PubMed] [Google Scholar]
- 55.Ozanne SE, Martensz ND, Petry CJ, Loizou CL, Hales CN. Maternal low protein diet in rats programmes fatty acid desaturase activities in the offspring. Diabetologia. (1998) 41:1337–42. 10.1007/s001250051074 [DOI] [PubMed] [Google Scholar]
- 56.Lee Y, Lai HTM, de Oliveira Otto MC, Lemaitre RN, McKnight B, King IB, et al. Serial biomarkers of de novo lipogenesis fatty acids and incident heart failure in older adults: the cardiovascular health study. J Am Heart Assoc. (2020) 9:e014119. 10.1161/JAHA.119.014119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ, et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr. (2010) 92:1214–22. 10.3945/ajcn.2010.29182 [DOI] [PubMed] [Google Scholar]
- 58.Kroger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Doring F, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. (2011) 93:127–42. 10.3945/ajcn.110.005447 [DOI] [PubMed] [Google Scholar]
- 59.Hodge AM, Simpson JA, Gibson RA, Sinclair AJ, Makrides M, O'Dea K, et al. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr Metab Cardiovasc Dis. (2007) 17:415–26. 10.1016/j.numecd.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 60.Warensjo Lemming E, Nalsen C, Becker W, Ridefelt P, Mattisson I, Lindroos AK. Relative validation of the dietary intake of fatty acids among adults in the Swedish National Dietary Survey using plasma phospholipid fatty acid composition. J Nutr Sci. (2015) 4:e25. 10.1017/jns.2015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikkari T, Luukkainen P, Pietinen P, Puska P. Fatty acid composition of serum lipid fractions in relation to gender and quality of dietary fat. Ann Med. (1995) 27:491–8. 10.3109/07853899709002458 [DOI] [PubMed] [Google Scholar]
- 62.Vessby B, Gustafsson IB, Tengblad S, Boberg M, Andersson A. Desaturation and elongation of Fatty acids and insulin action. Ann N Y Acad Sci. (2002) 967:183–95. 10.1111/j.1749-6632.2002.tb04275.x [DOI] [PubMed] [Google Scholar]
- 63.Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest. (1996) 97:2081–91. 10.1172/JCI118645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. (2001) 50:1771–7. 10.2337/diabetes.50.8.1771 [DOI] [PubMed] [Google Scholar]
- 65.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA. (2003) 100:3077–82. 10.1073/pnas.0630588100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. (2008) 134:933–44. 10.1016/j.cell.2008.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. (2011) 13:376–88. 10.1016/j.cmet.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. (2014) 159:318–32. 10.1016/j.cell.2014.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aarsland A, Chinkes D, Wolfe RR. Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding. Am J Clin Nutr. (1997) 65:1774–82. 10.1093/ajcn/65.6.1774 [DOI] [PubMed] [Google Scholar]
- 70.Ameer F, Scandiuzzi L, Hasnain S, Kalbacher H, Zaidi N. De novo lipogenesis in health and disease. Metabolism. (2014) 63:895–902. 10.1016/j.metabol.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 71.Bjorntorp P, Sjostrom L. Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism. (1978) 27(Suppl. 2):1853–65. 10.1016/S0026-0495(78)80004-3 [DOI] [PubMed] [Google Scholar]
- 72.Letexier D, Pinteur C, Large V, Frering V, Beylot M. Comparison of the expression and activity of the lipogenic pathway in human and rat adipose tissue. J Lipid Res. (2003) 44:2127–34. 10.1194/jlr.M300235-JLR200 [DOI] [PubMed] [Google Scholar]
- 73.Minehira K, Vega N, Vidal H, Acheson K, Tappy L. Effect of carbohydrate overfeeding on whole body macronutrient metabolism and expression of lipogenic enzymes in adipose tissue of lean and overweight humans. Int J Obes Relat Metab Disord. (2004) 28:1291–8. 10.1038/sj.ijo.0802760 [DOI] [PubMed] [Google Scholar]
- 74.Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. (2003) 77:43–50. 10.1093/ajcn/77.1.43 [DOI] [PubMed] [Google Scholar]
- 75.Strawford A, Antelo F, Christiansen M, Hellerstein MK. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol Endocrinol Metab. (2004) 286:E577–88. 10.1152/ajpendo.00093.2003 [DOI] [PubMed] [Google Scholar]
- 76.Marques-Lopes I, Ansorena D, Astiasaran I, Forga L, Martinez JA. Postprandial de novo lipogenesis and metabolic changes induced by a high-carbohydrate, low-fat meal in lean and overweight men. Am J Clin Nutr. (2001) 73:253–61. 10.1093/ajcn/73.2.253 [DOI] [PubMed] [Google Scholar]
- 77.Dirlewanger M, di Vetta V, Guenat E, Battilana P, Seematter G, Schneiter P, et al. Effects of short-term carbohydrate or fat overfeeding on energy expenditure and plasma leptin concentrations in healthy female subjects. Int J Obes Relat Metab Disord. (2000) 24:1413–8. 10.1038/sj.ijo.0801395 [DOI] [PubMed] [Google Scholar]
- 78.Minehira K, Bettschart V, Vidal H, Vega N, Di Vetta V, Rey V, et al. Effect of carbohydrate overfeeding on whole body and adipose tissue metabolism in humans. Obes Res. (2003) 11:1096–103. 10.1038/oby.2003.150 [DOI] [PubMed] [Google Scholar]
- 79.Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab. (2002) 282:E46–51. 10.1152/ajpendo.2002.282.1.E46 [DOI] [PubMed] [Google Scholar]
- 80.Eissing L, Scherer T, Todter K, Knippschild U, Greve JW, Buurman WA, et al. De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat Commun. (2013) 4:1528. 10.1038/ncomms2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salans LB, Knittle JL, Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. (1968) 47:153–65. 10.1172/JCI105705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hudgins LC, Seidman CE, Diakun J, Hirsch J. Human fatty acid synthesis is reduced after the substitution of dietary starch for sugar. Am J Clin Nutr. (1998) 67:631–9. 10.1093/ajcn/67.4.631 [DOI] [PubMed] [Google Scholar]
- 83.Neese RA, Benowitz NL, Hoh R, Faix D, LaBua A, Pun K, et al. Metabolic interactions between surplus dietary energy intake and cigarette smoking or its cessation. Am J Physiol. (1994) 267:E1023–34. 10.1152/ajpendo.1994.267.6.E1023 [DOI] [PubMed] [Google Scholar]
- 84.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest. (1999) 104:1087–96. 10.1172/JCI6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. (2010) 45:199–214. 10.3109/10409231003667500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Timlin MT, Parks EJ. Temporal pattern of de novo lipogenesis in the postprandial state in healthy men. Am J Clin Nutr. (2005) 81:35–42. 10.1093/ajcn/81.1.35 [DOI] [PubMed] [Google Scholar]
- 87.Cai D, Yuan M, Jia Y, Liu H, Hu Y, Zhao R. Maternal gestational betaine supplementation-mediated suppression of hepatic cyclin D2 and presenilin1 gene in newborn piglets is associated with epigenetic regulation of the STAT3-dependent pathway. J Nutr Biochem. (2015) 26:1622–31. 10.1016/j.jnutbio.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 88.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab. (2011) 22:353–63. 10.1016/j.tem.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. (2005) 115:1343–51. 10.1172/JCI23621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. (2002) 277:34182–90. 10.1074/jbc.M204887200 [DOI] [PubMed] [Google Scholar]
- 91.Ma W, Wu JH, Wang Q, Lemaitre RN, Mukamal KJ, Djousse L, et al. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: the Cardiovascular Health Study. Am J Clin Nutr. (2015) 101:153–63. 10.3945/ajcn.114.092601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herman MA, Peroni OD, Villoria J, Schon MR, Abumrad NA, Bluher M, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. (2012) 484:333–8. 10.1038/nature10986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. (2001) 2:282–6. 10.1093/embo-reports/kve071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oosterveer MH, Schoonjans K. Hepatic glucose sensing and integrative pathways in the liver. Cell Mol Life Sci. (2014) 71:1453–67. 10.1007/s00018-013-1505-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shao W, Espenshade PJ. Expanding roles for SREBP in metabolism. Cell Metab. (2012) 16:414–9. 10.1016/j.cmet.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jump DB, Clarke SD, Thelen A, Liimatta M. Coordinate regulation of glycolytic and lipogenic gene expression by polyunsaturated fatty acids. J Lipid Res. (1994) 35:1076–84. 10.1016/S0022-2275(20)40103-8 [DOI] [PubMed] [Google Scholar]
- 97.Swierczynski J. Leptin and age-related down-regulation of lipogenic enzymes genes expression in rat white adipose tissue. J Physiol Pharmacol. (2006) 57(Suppl. 6):85–102. Available online at: https://www.jpp.krakow.pl/journal/archive/11_06_s6/pdf/85_11_06_s6_article.pdf [PubMed] [Google Scholar]
- 98.Fajas L, Schoonjans K, Gelman L, Kim JB, Najib J, Martin G, et al. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol. (1999) 19:5495–503. 10.1128/MCB.19.8.5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vidal-Puig AJ, Considine RV, Jimenez-Linan M, Werman A, Pories WJ, Caro JF, et al. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest. (1997) 99:2416–22. 10.1172/JCI119424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res. (2006) 45:120–59. 10.1016/j.plipres.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 101.Wu Z, Xie Y, Morrison RF, Bucher NL, Farmer SR. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J Clin Invest. (1998) 101:22–32. 10.1172/JCI1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. (2001) 276:37731–4. 10.1074/jbc.R100034200 [DOI] [PubMed] [Google Scholar]
- 103.Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem. (1998) 273:16710–4. 10.1074/jbc.273.27.16710 [DOI] [PubMed] [Google Scholar]
- 104.Edmond J, Higa TA, Korsak RA, Bergner EA, Lee WN. Fatty acid transport and utilization for the developing brain. J Neurochem. (1998) 70:1227–34. 10.1046/j.1471-4159.1998.70031227.x [DOI] [PubMed] [Google Scholar]
- 105.Li B, Leung JCK, Chan LYY, Yiu WH, Tang SCW. A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog Lipid Res. (2020) 77:101020. 10.1016/j.plipres.2019.101020 [DOI] [PubMed] [Google Scholar]
- 106.Turpin-Nolan SM, Bruning JC. The role of ceramides in metabolic disorders: when size and localization matters. Nat Rev Endocrinol. (2020) 16:224–33. 10.1038/s41574-020-0320-5 [DOI] [PubMed] [Google Scholar]
- 107.Calder PC. Lipids: a hole in the diet-heart hypothesis? Nat Rev Cardiol. (2016) 13:385–6. 10.1038/nrcardio.2016.78 [DOI] [PubMed] [Google Scholar]
- 108.Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. (2020) 5:CD011737. 10.1002/14651858.CD011737.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krauss RM. All low-density lipoprotein particles are not created equal. Arterioscler Thromb Vasc Biol. (2014) 34:959–61. 10.1161/ATVBAHA.114.303458 [DOI] [PubMed] [Google Scholar]
- 110.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. (2010) 122:876–83. 10.1161/CIRCULATIONAHA.109.915165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. (2003) 77:1146–55. 10.1093/ajcn/77.5.1146 [DOI] [PubMed] [Google Scholar]
- 112.Xu L, Wang W, Zhang X, Ke H, Qin Y, You L, et al. Palmitic acid causes insulin resistance in granulosa cells via activation of JNK. J Mol Endocrinol. (2019) 62:197–206. 10.1530/JME-18-0214 [DOI] [PubMed] [Google Scholar]
- 113.Pascual G, Dominguez D, Elosua-Bayes M, Beckedorff F, Laudanna C, Bigas C, et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature. (2021) 599:485–90. 10.1038/s41586-021-04075-0 [DOI] [PubMed] [Google Scholar]
- 114.Nakatani T, Kim HJ, Kaburagi Y, Yasuda K, Ezaki O. A low fish oil inhibits SREBP-1 proteolytic cascade, while a high-fish-oil feeding decreases SREBP-1 mRNA in mice liver: relationship to anti-obesity. J Lipid Res. (2003) 44:369–79. 10.1194/jlr.M200289-JLR200 [DOI] [PubMed] [Google Scholar]
- 115.Imamura F, Micha R, Wu JH, de Oliveira Otto MC, Otite FO, Abioye AI, et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. (2016) 13:e1002087. 10.1371/journal.pmed.1002087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hamazaki T, Okuyama H. The Japan Society for Lipid Nutrition recommends to reduce the intake of linoleic acid. A review and critique of the scientific evidence. World Rev Nutr Diet. (2003) 92:109–32. 10.1159/000073796 [DOI] [PubMed] [Google Scholar]
- 117.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. (2008) 233:674–88. 10.3181/0711-MR-311 [DOI] [PubMed] [Google Scholar]
- 118.Trumbo P, Schlicker S, Yates AA, Poos M. Food, Nutrition Board of the Institute of Medicine TNA. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. (2002) 102:1621–30. 10.1016/S0002-8223(02)90346-9 [DOI] [PubMed] [Google Scholar]
- 119.Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri JM, Guesnet P. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res. (2006) 45:203–36. 10.1016/j.plipres.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 120.Ruiz R, Jideonwo V, Ahn M, Surendran S, Tagliabracci VS, Hou Y, et al. Sterol regulatory element-binding protein-1 (SREBP-1) is required to regulate glycogen synthesis and gluconeogenic gene expression in mouse liver. J Biol Chem. (2014) 289:5510–7. 10.1074/jbc.M113.541110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Muzsik A, Jelen HH, Chmurzynska A. Metabolic syndrome in postmenopausal women is associated with lower erythrocyte PUFA/MUFA and n-3/n-6 ratio: a case-control study. Prostaglandins Leukot Essent Fatty Acids. (2020) 159:102155. 10.1016/j.plefa.2020.102155 [DOI] [PubMed] [Google Scholar]
- 122.Melis M, Carta G, Pintus S, Pintus P, Piras CA, Murru E, et al. Polymorphism rs1761667 in the CD36 gene is associated to changes in fatty acid metabolism and circulating endocannabinoid levels distinctively in normal weight and obese subjects. Front Physiol. (2017) 8:1006. 10.3389/fphys.2017.01006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr. (2001) 131:1129–32. 10.1093/jn/131.4.1129 [DOI] [PubMed] [Google Scholar]
- 124.Linden AG, Li S, Choi HY, Fang F, Fukasawa M, Uyeda K, et al. Interplay between ChREBP and SREBP-1c coordinates postprandial glycolysis and lipogenesis in livers of mice. J Lipid Res. (2018) 59:475–87. 10.1194/jlr.M081836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu J, Cho H, O'Malley S, Park JH, Clarke SD. Dietary polyunsaturated fats regulate rat liver sterol regulatory element binding proteins-1 and−2 in three distinct stages and by different mechanisms. J Nutr. (2002) 132:3333–9. 10.1093/jn/132.11.3333 [DOI] [PubMed] [Google Scholar]
- 126.Zinocker MK, Svendsen K, Dankel SN. The homeoviscous adaptation to dietary lipids (HADL) model explains controversies over saturated fat, cholesterol, and cardiovascular disease risk. Am J Clin Nutr. (2021) 113:277–89. 10.1093/ajcn/nqaa322 [DOI] [PubMed] [Google Scholar]
- 127.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. (1997) 127(Suppl. 5):838S−41S. 10.1093/jn/127.5.838S [DOI] [PubMed] [Google Scholar]
- 128.Speakman JR. Use of high-fat diets to study rodent obesity as a model of human obesity. Int J Obes. (2019) 43:1491–2. 10.1038/s41366-019-0363-7 [DOI] [PubMed] [Google Scholar]
- 129.Carta G, Murru E, Banni S, Manca C. Palmitic acid: physiological role, metabolism and nutritional implications. Front Physiol. (2017) 8:902. 10.3389/fphys.2017.00902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hagura N, Haggard P, Diedrichsen J. Perceptual decisions are biased by the cost to act. Elife. (2017) 6:e18422. 10.7554/eLife.18422 [DOI] [PMC free article] [PubMed] [Google Scholar]