Abstract
Chimeric antigen receptor T cell (CAR-T) therapy for the treatment of hematologic tumors has achieved remarkable success, with five CAR-T therapies approved by the United States Food and Drug Administration. However, the efficacy of CAR-T therapy against solid tumors is not satisfactory. There are three existing hurdles in CAR-T cells for solid tumors. First, the lack of a universal CAR to recognize antigens at the site of solid tumors and the compact tumor structure make it difficult for CAR-T cells to locate in solid tumors. Second, soluble inhibitors and suppressive immune cells in the tumor microenvironment can inhibit or even inactivate T cells. Third, low survival and proliferation rates of CAR-T cells in vivo significantly influence the therapeutic effect. As an emerging method, nanotechnology has a great potential to enhance cell proliferation, activate T cells, and restarting the immune response. In this review, we discuss how nanotechnology can modify CAR-T cells through variable methods to improve the therapeutic effect of solid tumors.
Keywords: nanotechnology, CAR-T, solid tumor, immunity, therapeutic effect
Introduction
CAR-T therapy has made remarkable achievements in the research and clinical treatment of cancer, especially in the treatment of B cell malignancies (1–3). Unlike conventional surgery, radiotherapy, chemotherapy, immune checkpoint blocking therapies, targeted drug therapy, and CAR-T cell therapies offer more therapeutic options for patients with previously refractory tumors (4–8). To date, the United States Food and Drug Administration has approved five CAR-T therapies, namely, -Kymriah, Yescarta, Tecartus, Breyanzi and Abecma, -for hematologic malignancies (9). However, CAR-T cell therapy has not achieved satisfactory results in the treatment of solid tumors, such as colon, kidney, and ovarian cancers, for which the best clinical trial outcome is stable disease (10–14).
To improve the efficacy of CAR-T therapy in solid tumors, CAR-T cells must overcome three obstacles. First, the lack of tumor-specific antigens, dense stroma and aberrant vasculature at the tumor site prevent CAR-T cells from efficiently targeting the solid tumor site (15). Second, the tumor immune microenvironment and immunosuppressive mechanisms reduce the antitumor activity of CAR-T cells in solid tumors. Finally, because of the initial differentiation state of selected T cells, the cumbersome production process of CAR-T cells, and the tumor microenvironment (TME) with low oxygen, acidity and nutrition, the survival and proliferation rates of CAR- T cells in vivo were low.
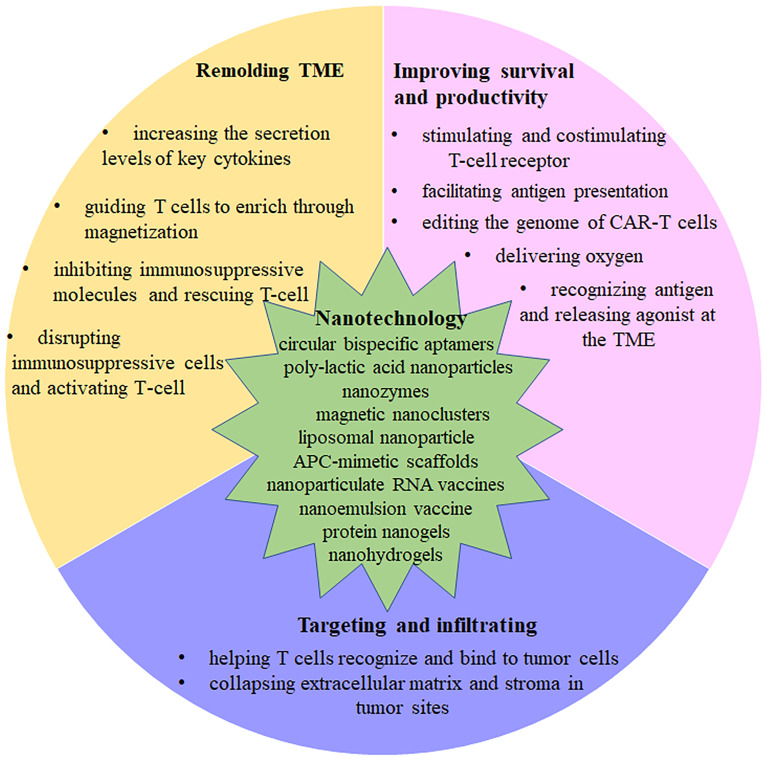
Nanotechnology has multiple features that allow it to address the challenges of CAR T cell therapy in treating solid tumors. With optimal size, high surface area to volume ratio, a variety of shapes and components, as well as surface modification and charge, nanoparticles have a wide range of applications in tumor therapy (16–20). Nanoparticles employed in clinical treatments can be targeted to the site of the lesion with less accumulation in healthy tissue, stronger drug permeability, and retention, and can be rapidly biodegraded and eliminated without pharmacological and toxicological activities (21–23). Therefore, a number of researchers are exploring the use of nanoparticles in combination with CAR-T therapy to improve the efficacy of CAR-T therapy in solid tumors. Herein, we briefly introduce the three major challenges of CAR T cells in solid tumor therapy, and summarize how to combine nanoparticles with CAR T cells from different perspectives to solve the challenges in solid tumor therapy ( Figure 1 ).
Figure 1.
The mechanisms of Nanotechnology affect CAR-T function. Summary of strategies that are discussed in detail in this review.
Current Roadblocks in CAR-T Cell for Solid Tumors
Numerous clinical trials of CAR-T cell therapy for solid tumors have been carried out, and a meta-analysis of the efficacy of CAR-T therapy in solid tumors showed an overall response rate of 9%, although various therapeutic strategies have been implemented (24). There are three major factors that influence CAR-T therapy, as described below.
Targeting and Infiltrating
CAR T cells are designed to select tumor-associated antigens (TAA) due to the lack of tumor-specific antigens (TSA). In a large number of clinical trials CAR T cell targeting tumor-associated antigens have been found cause damage to normal tissue with low expression of tumor-associated antigens during the process of recognizing and killing tumor cells, which is referred to as the off-target effect (25). Moreover, the reasons behind the success of CAR T cells in the treatment of hematologic tumors is that they can migrate in blood, lymph nodes, and bone marrow to interact with cancer cells (26). By dynamic imaging microscopy on fresh tumor slices from nine patients, Donnadieu et al. (27) investigated T cells with reduced motility in the stroma of human lung tumors, which hinted towards T cells facing difficulties in entering into the tumor due to the presence of obstacles. This makes it easy to understand that there are several other reasons why CAR T cells have difficulty entering solid tumor. Tumor-associated fibroblasts (TAFS) and abnormal vasculature at the tumor site result in compact tumor tissue and a dense extracellular matrix (ECM), which prevent CAR T cell to enter the solid tumor microenvironment (28, 29). The experiments conducted by Peschel et al. (30) confirm the lack of adoptively transferred T cells accumulation in solid tumors, while the infused HER2-specific T cells spread out in the breast cancer patient’s bone marrow. In addition, chemokines can induce T cell migration along the direction of increasing chemokine concentration. However, some solid tumors inhibit chemokine secretion and CAR T cells lack receptors that match chemokines secreted by solid tumors (31, 32), such that chemokine receptors on T cells mismatch with tumor secreted chemokines (33–35). Moreover, the low expression of adhesion molecules including ICAM-1 and 2, VCAM-1 and CD34 in tumor endothelial cells (EC) inhibit the effector T-cell from adhering to the EC and being transported to the tumor (36).
Tumor Immunosuppression
Immunosuppression of the solid tumor microenvironment is another significant challenge for CAR-T therapy. The causes of tumor cells escaping the anti-tumor immune response are complex, including the presence of immunosuppressive cells, the presence of immunosuppressive cytokines and the absence of immune activating factors. The presence of immunosuppressive cells such as dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs), regulatory cells (Tregs), and M2 macrophages in solid tumors sites, which secrete suppressive cytokines-such as transforming the growth factor-β (TGF-β), adenosine, interleukin-10 (IL-10), and vascular endothelial growth factor (VEGF) extracellularly-, suppresses the immune system and reduces the anti-tumor activity of CAR-T (37–40). Moreover, the immune checkpoint molecules PD-1 and CTLA4, when combined with the corresponding ligands, inhibit the killing effect of T cells on the tumor and the activation of T cells (41, 42).
Survival and Proliferation
CAR T cells are targeted to the tumor site by a chimeric receptor mediated expressed on the T cell surface, and eliminate cancer cells through cell killing (43). Studies have shown that the long-term survival and proliferation of CAR T cells capable of maintaining normal function in vivo played a decisive role in the therapeutic effect (44). However, the expansion of the CAR T cells during the treatment of solid tumors is low in vivo. For example, Michael et al. detected a large number of CAR T cells in ovarian cancer patients after 2 days of transfusing in vitro gene-edited T cells back into the body, but the increase only lasted for about 1 month, and quickly declined to be virtually undetectable in the majority of patients (13). Even with large doses of CAR T cells, the presence of CAR T cells in the circulatory system was not detected (45). Moreover, clinical data showed that longer CAR-T cell persistence indicates longer delays, in the development of disease progression (46). The factors that influence the survival of CAR T cells in patients are complex, including the differentiation and functional status of CAR T cells, CAR target affinity, CAR immunogenicity, tedious time-consuming production process, immunosuppressive and hypoxic tumor microenvironment (47–49). Various nanotechnology strategies may improve CAR T cell persistence and expansion in vivo, which would endow CAR-T therapy with superior antitumor activity in the treatment of solid tumors.
Application of Nanotechnology in CAR-T Therapy in Solid Tumors
Nanotechnology to Aid CAR T Cell Target and Accumulate in Solid Tumors
To overcome the off-target effect caused by tumor-associated antigens, one group designed circular bispecific aptamers to help T cells recognize and bind to tumor cells. The aptamer can simultaneously bind naïve T cells and tumor cells, and then specifically activate T cells in the cell-cell junction complex. This strategy helps T cells pinpoint the tumor site and kill cancer cells. Thus, the targeted treatment of all kinds of cancer is possibly realized by the use of specific anticancer aptamers (50).
In an effort to arm CAR T cells to collapse physical barriers caused by angiogenesis, a dense extracellular matrix and stroma in tumor sites, researchers have proposed numerous of NP-based strategies (51, 52). By combing photothermal therapy with the adoptive transfer of CAR T cells, Gu et al. succeeded in promoting the accumulation and enhancing the conventional CAR-T therapy against solid tumors. The indocyanine green (ICG), a near-infrared (NIR) dye, is wrapped in poly(lactic-co-glycolic) acid (PLGA) nanoparticles. Once exposed to NIR light irradiation, ICG is used as the photothermal agent released into solid tumor (53–55). Mild hyperthermia of the tumor disrupts its compact structure, reduces interstitial fluid pressure (IFP), increases blood perfusion, and releases tumor-specific antigens that could significantly stimulate CAR T cells. After about 20 days, tumor growth was significantly inhibited, and no tumor cells were detected in about one-third of the treated mice (56). Other researchers fabricated indocyanine green nanoparticles (INPs) conjugated CAR T cells via the biorthogonal reaction. After mild photothermal intervention, tumor vessels expanded, blood perfusion increased, the ECM ablated and the tumor tissues became loose. Thus, INPs engineered CAR-T biohybrids accumulated and infiltrated extensively in the tumor, remodeled the TME, restarted the immune response, and boosted the efficacy of CAR-T immunotherapy. This microenvironment photothermal-remodeling strategy provides a promising prospect for CAR-T therapy in solid tumors (57).
Nanotechnology to Remold Tumor Microenvironment to Stimulate CAR T Cells
To reset immunosuppression of cancer environment and promote the activation of CAR T cells, Zhao and colleagues effectively combined the use of the nanozymes method. They synthesized a tumor-targeting HA@Cu2−xS-PEG (PHCN) nanozyme with photothermal and catalytic properties. After irradiation by a near-infrared laser, the tumor extracellular matrix is damaged by converting light energy into local heat (58–60). Moreover, the reactive oxygen species by nanocatalyzed tumor therapy increased the secretion levels of key cytokines, such as the interferon and tumor necrosis factor as well as tumor-specific antigens, thus activating the corresponding CAR T cells at the tumor site (61).
To surmount the obstacle of hostile microenvironment, researchers tend to combine CAR-T therapy with the use of cytokines and/or antibodies. However, one problem is that CAR T cells and cytokines/antibodies disperse preventing their accumulation in the tumor sites (62, 63). Therefore, Xie et al. used a pH-sensitive benzoic−imine bond and inverse electron demand Diels−Alder cycloaddition to link magnetic nanoclusters (NCs) and the PD-1 antibody (aP) together to form NC-Ap. The constructed NC-aP binds to effector T cells due to their PD-1expression. Magnetic resonance imaging (MRI) guided T cells and aP to enrich in solid tumors through magnetization. Because of the acidic tumor microenvironment, the aP is released after the benzoic−imine bond, and then hydrolyzed. Consequently, the adoptively transferred T cells and aP synergistically inhibit solid tumor growth with a few side effects (64).
One of immunosuppressive molecules that inhibits the immune function of CD4+ and CD8+ T cells is adenosine. On the surface of activated T cells, the A2a adenosine receptor (A2aR) expressed and trigged adenosine to accumulate outside the cell, which suppressed T-cell proliferation and inhibited IFN–γ secretion (65, 66). Thus, using nanotechnology to efficiently transport SCH-58261 (SCH), a small molecule inhibitor of A2aR, to CAR T cells in tumors is a promising method. According to their report, Wang et al. used CAR-T therapy and SCH–loaded cross-linked multilamellar liposomes (cMLV) together, which significantly inhibited the tumor growth and improved the survival of treatment groups, the tumor infiltration rate of T-cells, as well as the expression level of IFN–γ in vivo. Through rescuing tumor-residing T-cell hypofunction, this method augments CAR T-cell efficacy in solid tumors (67).
The presence of immunosuppressive molecules- such as CTLA-4 and PD-L1 is another important cause of tumor immunosuppression. They enable tumor cells to escape surveillance by inhibiting the activation of immune cells, namely the “immune escape” (68, 69). To reset the suppressive solid tumor microenvironment, inhibitors targeting checkpoint molecules (such as CTLA-4, PD-1 and PD-L1) and CAR-T therapy were used in combination (70, 71). The disadvantages of using immune-checkpoint inhibitors (ICIs) include the emergence of a series of new immune-related adverse events and systemic toxicities (72). Stephan et al. designed a liposomal drug-loaded nanoparticle and decorated it with the tumor-targeting peptide iRGD. In addition, PI-3065, a PI3K kinase inhibitor that disrupts the function of immune-suppressive regulatory T cell subsets and myeloid-derived suppressor cell (40), and 7DW8-5, an immunostimulant-invariant natural killer T cell (iNKT) agonist was placed in the liposome (73, 74). They demonstrated that this new target nanoparticle alters the tumor immunosuppression and evidently enhances the anti-tumor activity of CAR T cells (75).
Nanotechnology to Aid CAR T Cells Survive and Proliferate
The number of tumor-infiltrating lymphocytes is positively related with clinical outcomes of CAR-T therapies (36, 76, 77). T cells obtained from patients are limited, such that amplification in vitro may be an effective solution. In the body, the expansion of T cells requires the assistance of antigen-presenting cells (APC), which cannot be achieved in vitro. In light of this problem, Mooney et al. utilized mesoporous silica to create micro-rods and added in the APC-secreting factor interleukin-2, which extends the lifespan of T cells. They also coated the high-aspect ratio mesoporous silica micro-rods (MSRs) with supported lipid bilayers (SLBs) and a variety of antibodies that activate T cells, mimicking APC’s cell membrane. In cell culture, these rods randomly and automatically form a scaffold structure that allows T cells to move around and expand freely. Results showed that APC-mimetic scaffolds generate more CAR T cells and maintain good killing efficacy compared to conventional expansion systems (78).
The lack of proliferation signals in TME results in a low survival rate of CAR T cells. As emerging therapies, nanoparticulate RNA vaccines deliver liposomal antigen-encoding RNA (RNA-LPX) to activate T cells in cancer patients (79). Recently, Sahin et al. combined CAR-T with the nanoparticulate RNA vaccine to achieve the regulated proliferation of CAR-T cell expansion depending on RNA-LPX dose. The mechanism involves that antigen delivery to antigen-presenting cells in the spleen, lymph nodes, and bone marrow by intravenous injection, followed by the initiation of a toll-like receptor-dependent type-I IFN-driven immune-stimulatory program (80). Moreover, Chan et al. used the tailored nanoemulsion (Clec9A-TNE) vaccine to effectively solve the problem of limited antigen presentation, promote the proliferation of CAR T cells in vivo, and augment the efficacy of solid tumor therapy (81).
Conventional manufacturing of CAR-T cells includes several elaborate procedures such as isolation, modification and expansion, resulting a few effective redirected T cells that can be used. Meanwhile, virus transfection and electroporation are commonly used to help T-cells express targeted chimeric antigen receptors (CARs) or T cell receptors. In turn, these methods have drawbacks as they are time-consuming, have a small application scale (82, 83). Stephan et al. designed a new genetic programming named “hit-and run”, which transports mRNA nanocarriers into cells through simple mixing and transient expression of the target gene. The mRNA nanocarrier has three prominent advantages: (i) lyophilized mRNA NPs can be used for each application that has no effect on its properties and efficacy. (ii) NP uptake and transfection efficiency did not differ whether T cells proliferated or not. (iii) Lymphocyte-targeted mRNA nanocarriers can edit the genome of CAR-T-cells without influencing on their function. The paramount of this method is that it can simply produce CAR T cells at a clinical scale within a short time and without complex handling procedures in vitro (84).
Another novel method was developed to program numerous circulating T cells and effectively remove cancer cells in situ. On the surface of the biodegradable poly (β-aminoester)-based nanoparticles, anti-CD3e f(ab′)2 fragments are coupled with it to target T cells. Inside of the nanoparticles, the poly(beta-aminoester) (PBAE) polymer is assembled with microtubule-associated sequences (MTAS) and nuclear localization signals (NLS), which facilitates the gene transfer in the nucleus of the T cells. To maintain CAR expression in T cells, the CD19 CAR plasmid was flanked by the piggyBac transposase gene through a cut-and-paste mechanism. These stable polymer nanoparticles allow simple manufacture and storage, which provides a practical, economical and widely available pathway for CAR-T therapy (85).
The immunosuppression and hypoxia in the solid tumor microenvironment result in the weaking CAR T cells infiltration and proliferation. One research group constructed an injectable hydrogel-encapsulated porous immune-microchip system (i-G/MC) with oxygen reservoirs to intratumorally deliver CAR T cells. In the injectable i-G/MC system, IL-15-loaded alginate microspheres were made into thin immune-MCs (i-MCs), which were connected with HEMOXCell (Hemo; an oxygen carrier)-loaded alginate, and the alginate forms a gel layer by self-assembly (86). The i-MCs were highly porous and interconnected, which facilitates CAR T cell transport. Hemo, a marine extracellular hemoglobin, has a strong oxygen storage capacity and binds up to 156 oxygen molecules (per Hemo molecule). After the i-G/MC was injected into the solid tumor, the hydrogel (gel) layer degraded quickly, Hemo delivered oxygen to TME, as well as CAR T cells, and decreased the expression level of HIF-1α. Results showed that the immune-niche improves hypoxia TEM and promotes survival and infiltration of CAR T cells in solid tumors.
To avoid the side effects of systemically-administered supporting cytokines like interleukins, protein nanogels (NGs) with interleukin (IL)-15 super-agonist were designed. The NGs recognized the specific cell surface antigen and subsequently released the drug at the sites of antigen encounter, for instance, the tumor microenvironment. Most importantly, the NG delivery enhanced the cell proliferation level 16-fold in tumors and administered eight-fold higher doses of cytokine without toxicity (87).
Conclusion
In preclinical studies, researchers have proposed a number of strategies to improve CAR T cell function through the use of nanotechnology. However, there are still some fundamental issues to be addressed in the clinical application of CAR T therapy. For example, the carcinogenicity, reproductive toxicity and persistence of magnetic nanoclusters are still unknown and therefore it cannot be used in clinical therapy. The use of near infrared laser will cause damage to human skin, short-term use will appear skin swelling phenomenon, long-term may affect human reproductive function and induce cancer. The safety, immunogenicity and toxicity of nano-vaccines have yet to be verified. Will nano-derivative biodegrades induce non-specific immune responses? Due to the specificity of tumor-associated antigens, the preparation cycle of tailored nanoemulsion vaccine is time consuming and involves high cost….
These questions from clinical studies may seem disappointing, but many studies have highlighted the potential of nanotechnology in combination with CAR T therapies for solid cancers, which giving us great hope for CAR T cells. Currently, there are about 40 CAR-T targets in clinical trials in solid tumors, which has significantly outnumbered hematological tumors. Different from CD19, which is often used as a target for CAR-T therapy in hematologic tumors, the main targets of CART development in solid tumors include Mesothelin, GD2, HER2, GPC3, Claudin18.2(CLDN18.2) and so on. Most CAR-T studies in solid tumors have low response rates in the 0-25% range (88). Recently, the EMA granted prime eligibility to CAR T - cell product candidate CT041, which against the claudin18.2 protein (CLDN18.2) for the treatment of gastric/gastroesophageal junction cancer. Results from a phase I clinical trial published in 2019 show a total objective response rate of 33% in a small group of patients with advanced gastric or pancreatic cancers, with no serious side effects (89). This means that CT041 is expected to become the world’s first approved solid tumor CAR T product, thus achieving zero breakthrough in solid tumor treatment.
Author Contributions
JM: Conceptualization; writing-original draft. QY: Writing-review and editing. YM: Conceptualization; writing-review and editing. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
New medicine grant from University of Science and Technology of China, Grant/Award Numbers: WK9110000103.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This work was supported by new medicine grant WK9110000103 from University of Science and Technology of China.
References
- 1. June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T Cell Immunotherapy for Human Cancer. Science (2018) 359(6382):1361–5. doi: 10.1126/science.aar6711 [DOI] [PubMed] [Google Scholar]
- 2. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N Engl J Med (2014) 371(16):1507–17. doi: 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD, et al. The Future of Cancer Treatment: Immunomodulation, CARs and Combination Immunotherapy (Vol 13, Pg 273, 2016). Nat Rev Clin Oncol (2016) 13(6):394–4. doi: 10.1038/nrclinonc.2016.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival With Ipilimumab in Patients With Metastatic Melanoma. N Engl J Med (2010) 363(8):711–23. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sznol M, Powderly JD, Smith DC, Brahmer JR, Drake CG, McDermott DF, et al. Safety and Antitumor Activity of Biweekly MDX-1106 (Anti-PD-1, BMS-936558/ONO-4538) in Patients With Advanced Refractory Malignancies. J Clin Oncol (2010) 28(15):2506. doi: 10.1200/jco.2010.28.15_suppl.2506 [DOI] [Google Scholar]
- 6. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science (2017) 357(6349):409–13. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hong M, Clubb JD, Chen YY. Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell (2020) 38(4):473–88. doi: 10.1016/j.ccell.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 8. Bedard PL, Hyman DM, Davids MS, Siu LLL. Small Molecules, Big Impact: 20 Years of Targeted Therapy in Oncology. Lancet (2020) 395(10229):1078–88. doi: 10.1016/S0140-6736(20)30164-1 [DOI] [PubMed] [Google Scholar]
- 9. Nezhad MS, Yazdanifar M, Abdollahpour-Alitappeh M, Sattari A, Seifalian A, Bagheri N. Strengthening the CAR-T Cell Therapeutic Application Using CRISPR/Cas9 Technology. Biotechnol Bioeng (2021) 118(10):3691–705. doi: 10.1002/bit.27882 [DOI] [PubMed] [Google Scholar]
- 10. Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-Specific Chimeric Antigen Receptor mRNA-Engineered T Cells Induce Antitumor Activity in Solid Malignancies. Cancer Immunol Res (2014) 2(2):112–20. doi: 10.1158/2326-6066.CIR-13-0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Correction: Mesothelin-Specific Chimeric Antigen Receptor mRNA-Engineered T Cells Induce Antitumor Activity in Solid Malignancies. Cancer Immunol Res (2015) 3(2):217. doi: 10.1158/2326-6066.CIR-15-0007 [DOI] [PubMed] [Google Scholar]
- 12. Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR, et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-Cell Therapy for CEA(+) Liver Metastases. Clin Cancer Res (2015) 21(14):3149–59. doi: 10.1158/1078-0432.CCR-14-1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A Phase I Study on Adoptive Immunotherapy Using Gene-Modified T Cells for Ovarian Cancer. Clin Cancer Res (2006) 12(20 Pt 1):6106–15. doi: 10.1158/1078-0432.CCR-06-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of Metastatic Renal Cell Carcinoma With Autologous T-Lymphocytes Genetically Retargeted Against Carbonic Anhydrase IX: First Clinical Experience. J Clin Oncol (2006) 24(13):e20–2. doi: 10.1200/JCO.2006.05.9964 [DOI] [PubMed] [Google Scholar]
- 15. Joyce JA, Fearon DT. T Cell Exclusion, Immune Privilege, and the Tumor Microenvironment. Science (2015) 348(6230):74–80. doi: 10.1126/science.aaa6204 [DOI] [PubMed] [Google Scholar]
- 16. Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol Pharm (2008) 5(4):505–15. doi: 10.1021/mp800051m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grimm J, Scheinberg DA. Will Nanotechnology Influence Targeted Cancer Therapy? Semin Radiat Oncol (2011) 21(2):80–7. doi: 10.1016/j.semradonc.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scheinberg DA, Villa CH, Escorcia FE, McDevitt MR. Conscripts of the Infinite Armada: Systemic Cancer Therapy Using Nanomaterials. Nat Rev Clin Oncol (2010) 7(5):266–76. doi: 10.1038/nrclinonc.2010.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, et al. The Effect of Particle Design on Cellular Internalization Pathways. Proc Natl Acad Sci U.S.A. (2008) 105(33):11613–8. doi: 10.1073/pnas.0801763105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaittanis C, Shaffer TM, Thorek DL, Grimm J. Dawn of Advanced Molecular Medicine: Nanotechnological Advancements in Cancer Imaging and Therapy. Crit Rev Oncog (2014) 19(3-4):143–76. doi: 10.1615/CritRevOncog.2014011601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aslan B, Ozpolat B, Sood AK, Lopez-Berestein G. Nanotechnology in Cancer Therapy. J Drug Target (2013) 21(10):904–13. doi: 10.3109/1061186X.2013.837469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duncan R, Gaspar R. Nanomedicine(s) Under the Microscope. Mol Pharmaceut (2011) 8(6):2101–41. doi: 10.1021/mp200394t [DOI] [PubMed] [Google Scholar]
- 23. Baetke SC, Lammers T, Kiessling F. Applications of Nanoparticles for Diagnosis and Therapy of Cancer. Br J Radiol (1054) 2015:88. doi: 10.1259/bjr.20150207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hou B, Tang Y, Li WH, Zeng QN, Chang DM. Efficiency of CAR-T Therapy for Treatment of Solid Tumor in Clinical Trials: A Meta-Analysis. Dis Markers (2019) 2019:3425291. doi: 10.1155/2019/3425291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu X, Cai H, Zhao L, Ning L, Lang J. CAR-T Cell Therapy in Ovarian Cancer: From the Bench to the Bedside. Oncotarget (2017) 8(38):64607–21. doi: 10.18632/oncotarget.19929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uribe-Herranz M, Klein-Gonzalez N, Rodriguez-Lobato LG, Juan M, de Larrea CF. Gut Microbiota Influence in Hematological Malignancies: From Genesis to Cure. Int J Mol Sci (2021) 22(3):1026. doi: 10.3390/ijms22031026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, et al. Macrophages Impede CD8 T Cells From Reaching Tumor Cells and Limit the Efficacy of Anti-PD-1 Treatment. Proc Natl Acad Sci USA (2018) 115(17):E4041–50. doi: 10.1073/pnas.1720948115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ager A. High Endothelial Venules and Other Blood Vessels: Critical Regulators of Lymphoid Organ Development and Function. Front Immunol (2017) 8:45. doi: 10.3389/fimmu.2017.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hanahan D, Coussens LM. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell (2012) 21(3):309–22. doi: 10.1016/j.ccr.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 30. Bernhard H, Neudorfer J, Gebhard K, Conrad H, Hermann C, Nahrig J, et al. Adoptive Transfer of Autologous, HER2-Specific, Cytotoxic T Lymphocytes for the Treatment of HER2-Overexpressing Breast Cancer. Cancer Immunol Immunother (2008) 57(2):271–80. doi: 10.1007/s00262-007-0355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Slaney CY, Kershaw MH, Darcy PK. Trafficking of T Cells Into Tumors. Cancer Res (2014) 74(24):7168–74. doi: 10.1158/0008-5472.CAN-14-2458 [DOI] [PubMed] [Google Scholar]
- 32. Dangaj D, Bruand M, Grimm AJ, Ronet C, Barras D, Duttagupta PA, et al. Cooperation Between Constitutive and Inducible Chemokines Enables T Cell Engraftment and Immune Attack in Solid Tumors. Cancer Cell (2019) 35(6):885–+. doi: 10.1016/j.ccell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harlin H, Meng Y, Peterson AC, Zha YY, Tretiakova M, Slingluff C, et al. Chemokine Expression in Melanoma Metastases Associated With CD8(+) T-Cell Recruitment. Cancer Res (2009) 69(7):3077–85. doi: 10.1158/0008-5472.CAN-08-2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mulligan AM, Raitman I, Feeley L, Pinnaduwage D, Nguyen LT, O'Malley FP, et al. Tumoral Lymphocytic Infiltration and Expression of the Chemokine CXCL10 in Breast Cancers From the Ontario Familial Breast Cancer Registry. Clin Cancer Res (2013) 19(2):336–46. doi: 10.1158/1078-0432.CCR-11-3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsumura S, Wang BM, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-Induced CXCL16 Release by Breast Cancer Cells Attracts Effector T Cells. J Immunol (2008) 181(5):3099–107. doi: 10.4049/jimmunol.181.5.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim ST, Jeong H, Woo OH, Seo JH, Kim A, Lee ES, et al. Tumor-Infiltrating Lymphocytes, Tumor Characteristics, and Recurrence in Patients With Early Breast Cancer. Am J Clin Oncol-Cancer Clin Trials (2013) 36(3):224–31. doi: 10.1097/COC.0b013e3182467d90 [DOI] [PubMed] [Google Scholar]
- 37. Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, et al. Coexpression of CD49b and LAG-3 Identifies Human and Mouse T Regulatory Type 1 Cells. Nat Med (2013) 19(6):739–46. doi: 10.1038/nm.3179 [DOI] [PubMed] [Google Scholar]
- 38. Bezie S, Meistermann D, Boucault L, Kilens S, Zoppi J, Autrusseau E, et al. Ex Vivo Expanded Human Non-Cytotoxic CD8(+)CD45RC(low/-) Tregs Efficiently Delay Skin Graft Rejection and GVHD in Humanized Mice. Front Immunol (2017) 8:2014. doi: 10.3389/fimmu.2017.02014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic Intestinal Inflammatory Condition Generates IL-10-Producing Regulatory B Cell Subset Characterized by CD1d Upregulation. Immunity (2002) 16(2):219–30. doi: 10.1016/S1074-7613(02)00274-1 [DOI] [PubMed] [Google Scholar]
- 40. Tian ZG, Gershwin ME, Zhang C. Regulatory NK Cells in Autoimmune Disease. J Autoimmun (2012) 39(3):206–15. doi: 10.1016/j.jaut.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 41. Fedorov VD, Themeli M, Sadelain M. PD-1-and CTLA-4-Based Inhibitory Chimeric Antigen Receptors (iCARs) Divert Off-Target Immunotherapy Responses. Sci Trans Med (2013) 5(215):215ra172. doi: 10.1126/scitranslmed.3006597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE. Tumor Antigen-Specific CD8 T Cells Infiltrating the Tumor Express High Levels of PD-1 and are Functionally Impaired. Blood (2009) 114(8):1537–44. doi: 10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahmad A. CAR-T Cell Therapy. Int J Mol Sci (2020) 21(12). doi: 10.3390/ijms21124303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric Antigen Receptor T Cells Persist and Induce Sustained Remissions in Relapsed Refractory Chronic Lymphocytic Leukemia. Sci Transl Med (2015) 7(303):303ra139. doi: 10.1126/scitranslmed.aac5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive Transfer of Chimeric Antigen Receptor Re-Directed Cytolytic T Lymphocyte Clones in Patients With Neuroblastoma. Mol Ther (2007) 15(4):825–33. doi: 10.1038/sj.mt.6300104 [DOI] [PubMed] [Google Scholar]
- 46. Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor Activity and Long-Term Fate of Chimeric Antigen Receptor-Positive T Cells in Patients With Neuroblastoma. Blood (2011) 118(23):6050–6. doi: 10.1182/blood-2011-05-354449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beatty GL, O’Hara M. Chimeric Antigen Receptor-Modified T Cells for the Treatment of Solid Tumors: Defining the Challenges and Next Steps. Pharmacol Ther (2016) 166:30–9. doi: 10.1016/j.pharmthera.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat Med (2018) 24(5):541–50. doi: 10.1038/s41591-018-0014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zou WP. Immunosuppressive Networks in the Tumour Environment and Their Therapeutic Relevance. Nat Rev Cancer (2005) 5(4):263–74. doi: 10.1038/nrc1586 [DOI] [PubMed] [Google Scholar]
- 50. Yang Y, Sun X, Xu J, Cui C, Safari Yazd H, Pan X, et al. Circular Bispecific Aptamer-Mediated Artificial Intercellular Recognition for Targeted T Cell Immunotherapy. ACS Nano (2020) 14(8):9562–71. doi: 10.1021/acsnano.9b09884 [DOI] [PubMed] [Google Scholar]
- 51. Jain RK, Stylianopoulos T. Delivering Nanomedicine to Solid Tumors. Nat Rev Clin Oncol (2010) 7(11):653–64. doi: 10.1038/nrclinonc.2010.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, et al. Heparanase Promotes Tumor Infiltration and Antitumor Activity of CAR-Redirected T Lymphocytes. Nat Med (2015) 21(5):524–9. doi: 10.1038/nm.3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen Q, Liang C, Wang C, Liu Z. An Imagable and Photothermal “Abraxane-Like” Nanodrug for Combination Cancer Therapy to Treat Subcutaneous and Metastatic Breast Tumors. Adv Mater (2015) 27(5):903–10. doi: 10.1002/adma.201404308 [DOI] [PubMed] [Google Scholar]
- 54. Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal Therapy With Immune-Adjuvant Nanoparticles Together With Checkpoint Blockade for Effective Cancer Immunotherapy. Nat Commun (2016) 7:13193. doi: 10.1038/ncomms13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Makadia HK, Siegel SJ. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) (2011) 3(3):1377–97. doi: 10.3390/polym3031377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen Q, Hu Q, Dukhovlinova E, Chen G, Ahn S, Wang C, et al. Photothermal Therapy Promotes Tumor Infiltration and Antitumor Activity of CAR T Cells. Adv Mater (2019) 31(23):e1900192. doi: 10.1002/adma.201900192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen Z, Pan H, Luo Y, Yin T, Zhang B, Liao J, et al. Nanoengineered CAR-T Biohybrids for Solid Tumor Immunotherapy With Microenvironment Photothermal-Remodeling Strategy. Small (2021) 17(14):e2007494. doi: 10.1002/smll.202007494 [DOI] [PubMed] [Google Scholar]
- 58. Gawande MB, Goswami A, Felpin FX, Asefa T, Huang XX, Silva R, et al. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Review Catalysis. Chem Rev (2016) 116(6):3722–811. doi: 10.1021/acs.chemrev.5b00482 [DOI] [PubMed] [Google Scholar]
- 59. Chen PY, Ma YC, Zheng Z, Wu CF, Wang YC, Liang GL. Facile Syntheses of Conjugated Polymers for Photothermal Tumour Therapy. Nat Commun (2019) 10:1192. doi: 10.1038/s41467-019-09226-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhao Z, Chen C, Wu WT, Wang FF, Du LL, Zhang XY, et al. Highly Efficient Photothermal Nanoagent Achieved by Harvesting Energy via Excited-State Intramolecular Motion Within Nanoparticles. Nat Commun (2019) 10:768. doi: 10.1038/s41467-019-08722-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhu LP, Liu J, Zhou GY, Liu TM, Dai YL, Nie GJ, et al. Remodeling of Tumor Microenvironment by Tumor-Targeting Nanozymes Enhances Immune Activation of CAR T Cells for Combination Therapy. Small (2021). doi: 10.1002/smll.202102624 [DOI] [PubMed] [Google Scholar]
- 62. Beckman RA, Weiner LM, Davis HM. Antibody Constructs in Cancer Therapy - Protein Engineering Strategies to Improve Exposure in Solid Tumors. Cancer (2007) 109(2):170–9. doi: 10.1002/cncr.22402 [DOI] [PubMed] [Google Scholar]
- 63. Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic Antibodies: Successes, Limitations and Hopes for the Future. Br J Pharmacol (2009) 157(2):220–33. doi: 10.1111/j.1476-5381.2009.00190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nie W, Wei W, Zuo L, Lv C, Zhang F, Lu GH, et al. Magnetic Nanoclusters Armed With Responsive PD-1 Antibody Synergistically Improved Adoptive T-Cell Therapy for Solid Tumors. ACS Nano (2019) 13(2):1469–78. doi: 10.1021/acsnano.8b07141 [DOI] [PubMed] [Google Scholar]
- 65. Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, et al. CD73 on Tumor Cells Impairs Antitumor T-Cell Responses: A Novel Mechanism of Tumor-Induced Immune Suppression. Cancer Res (2010) 70(6):2245–55. doi: 10.1158/0008-5472.CAN-09-3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lappas CM, Rieger JM, Linden J. A2A Adenosine Receptor Induction Inhibits IFN-Gamma Production in Murine CD4+ T Cells. J Immunol (2005) 174(2):1073–80. doi: 10.4049/jimmunol.174.2.1073 [DOI] [PubMed] [Google Scholar]
- 67. Siriwon N, Kim YJ, Siegler E, Chen X, Rohrs JA, Liu Y, et al. CAR-T Cells Surface-Engineered With Drug-Encapsulated Nanoparticles Can Ameliorate Intratumoral T-Cell Hypofunction. Cancer Immunol Res (2018) 6(7):812–24. doi: 10.1158/2326-6066.CIR-17-0502 [DOI] [PubMed] [Google Scholar]
- 68. Littman DR. Releasing the Brakes on Cancer Immunotherapy. Cell (2015) 162(6):1186–90. doi: 10.1016/j.cell.2015.08.038 [DOI] [PubMed] [Google Scholar]
- 69. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on Tumor Cells in the Escape From Host Immune System and Tumor Immunotherapy by PD-L1 Blockade. Proc Natl Acad Sci USA (2002) 99(19):12293–7. doi: 10.1073/pnas.192461099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 Antibody Therapy Potently Enhances the Eradication of Established Tumors by Gene-Modified T Cells. Clin Cancer Res (2013) 19(20):5636–46. doi: 10.1158/1078-0432.CCR-13-0458 [DOI] [PubMed] [Google Scholar]
- 71. Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, et al. Multifactorial T-Cell Hypofunction That Is Reversible Can Limit the Efficacy of Chimeric Antigen Receptor-Transduced Human T Cells in Solid Tumors. Clin Cancer Res (2014) 20(16):4262–73. doi: 10.1158/1078-0432.CCR-13-2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ajina R, Zahavi DJ, Zhang YW, Weiner LM. Overcoming Malignant Cell-Based Mechanisms of Resistance to Immune Checkpoint Blockade Antibodies. Semin Cancer Biol (2020) 65:28–37. doi: 10.1016/j.semcancer.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W, Lim EL, et al. Inactivation of PI(3)K P110delta Breaks Regulatory T-Cell-Mediated Immune Tolerance to Cancer. Nature (2014) 510(7505):407–11. doi: 10.1038/nature13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li XM, Fujio M, Imamura M, Wu D, Vasan S, Wong CH, et al. Design of a Potent CD1d-Binding NKT Cell Ligand as a Vaccine Adjuvant. Proc Natl Acad Sci USA (2010) 107(29):13010–5. doi: 10.1073/pnas.1006662107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang F, Stephan SB, Ene CI, Smith TT, Holland EC, Stephan MT. Nanoparticles That Reshape the Tumor Milieu Create a Therapeutic Window for Effective T-Cell Therapy in Solid Malignancies. Cancer Res (2018) 78(13):3718–30. doi: 10.1158/0008-5472.CAN-18-0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science (2006) 313(5795):1960–4. doi: 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 77. Kmiecik J, Poli A, Brons NHC, Waha A, Eide GE, Enger PO, et al. Elevated CD3(+) and CD8(+) Tumor-Infiltrating Immune Cells Correlate With Prolonged Survival in Glioblastoma Patients Despite Integrated Immunosuppressive Mechanisms in the Tumor Microenvironment and at the Systemic Level. J Neuroimmunol (2013) 264(1-2):71–83. doi: 10.1016/j.jneuroim.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 78. Cheung AS, Zhang DKY, Koshy ST, Mooney DJ. Scaffolds That Mimic Antigen-Presenting Cells Enable Ex Vivo Expansion of Primary T Cells. Nat Biotechnol (2018) 36(2):160–9. doi: 10.1038/nbt.4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. Systemic RNA Delivery to Dendritic Cells Exploits Antiviral Defence for Cancer Immunotherapy. Nature (2016) 534(7607):396–401. doi: 10.1038/nature18300 [DOI] [PubMed] [Google Scholar]
- 80. Reinhard K, Rengstl B, Oehm P, Michel K, Billmeier A, Hayduk N, et al. An RNA Vaccine Drives Expansion and Efficacy of Claudin-CAR-T Cells Against Solid Tumors. Science (2020) 367(6476):446–53. doi: 10.1126/science.aay5967 [DOI] [PubMed] [Google Scholar]
- 81. Chan JD, von Scheidt B, Zeng B, Oliver AJ, Davey AS, Ali AI, et al. Enhancing Chimeric Antigen Receptor T-Cell Immunotherapy Against Cancer Using a Nanoemulsion-Based Vaccine Targeting Cross-Presenting Dendritic Cells. Clin Transl Immunol (2020) 9(7):e1157. doi: 10.1002/cti2.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nightingale SJ, Hollis RP, Pepper KA, Petersen D, Yu XJ, Yang C, et al. Transient Gene Expression by Nonintegrating Lentiviral Vectors. Mol Ther (2006) 13(6):1121–32. doi: 10.1016/j.ymthe.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 83. Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A Guide to Manufacturing CAR T Cell Therapies. Curr Opin Biotechnol (2018) 53:164–81. doi: 10.1016/j.copbio.2018.01.025 [DOI] [PubMed] [Google Scholar]
- 84. Moffett HF, Coon ME, Radtke S, Stephan SB, McKnight L, Lambert A, et al. Hit-And-Run Programming of Therapeutic Cytoreagents Using mRNA Nanocarriers. Nat Commun (2017) 8:389. doi: 10.1038/s41467-017-00505-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, et al. In Situ Programming of Leukaemia-Specific T Cells Using Synthetic DNA Nanocarriers. Nat Nanotechnol (2017) 12(8):813–20. doi: 10.1038/nnano.2017.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rodriguez-Brotons A, Bietiger W, Peronet C, Langlois A, Magisson J, Mura C, et al. Comparison of Perfluorodecalin and HEMOXCell as Oxygen Carriers for Islet Oxygenation in an In Vitro Model of Encapsulation. Tissue Eng Part A (2016) 22 p(23-24):1327–36. doi: 10.1089/ten.tea.2016.0064 [DOI] [PubMed] [Google Scholar]
- 87. Tang L, Zheng Y, Melo MB, Mabardi L, Castano AP, Xie YQ, et al. Enhancing T Cell Therapy Through TCR-Signaling-Responsive Nanoparticle Drug Delivery. Nat Biotechnol (2018) 36(8):707–16. doi: 10.1038/nbt.4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Moreno V, Hernandez T, de Miguel M, Doger B, Calvo E. Adoptive Cell Therapy for Solid Tumors: Chimeric Antigen Receptor T Cells and Beyond. Curr Opin Pharmacol (2021) 59:70–84. doi: 10.1016/j.coph.2021.05.004 [DOI] [PubMed] [Google Scholar]
- 89. CARsgen Announces CAR T-Cell Product Candidate CT041 Granted PRIME Eligibility by the EMA. Available at: https://www.prnewswire.com/news-releases/carsgen-announces-car-t-cell-product-candidate-ct041-granted-prime-eligibility-by-the-ema-301424092.html (Accessed 15 November, 2021).