Abstract
Diabetic nephropathy (DN) is a major cause of end-stage renal disease, and therapeutic options for preventing its progression are insufficient. The number of patients with DN has been increasing in Asian countries because of westernization of dietary lifestyle, which may be associated with the following changes in gut microbiota. Alterations in the gut microbiota composition can lead to an imbalanced gastrointestinal environment that promotes abnormal production of metabolites and/or inflammatory status. Functional microenvironments of the gut could be changed in the different stages of DN. In particular, altered levels of short chain fatty acids, D-amino acids, and reactive oxygen species biosynthesis in the gut have been shown to be relevant to the pathogenesis of the DN. So far, evidence suggests that the gut microbiota may play a key role in determining networks in the development of DN. Interventions directing the gut microbiota deserve further investigation as a new protective therapy in DN. In this review, we discuss the potential roles of the gut microbiota and future perspectives in the protection and/or treatment of kidneys.
Keywords: Diabetic nephropathy, Short chain fatty acids, Superoxide dismutase, Reactive oxygen species, D-amino acids, Gut microbiota, Diabetes mellitus, Renal disease
Core tip: Evolving evidence suggests that the gut microbiota may play a key role in the development of diabetic nephropathy (DN). Interventions aimed at the gut microbiota deserve further investigation as a novel protective therapy in DN. We review the potential roles of the gut microbiota in the protection of kidneys and in the development of DN.
INTRODUCTION
Diabetic nephropathy (DN) is a chronic disorder occurring in nearly 40% of patients with diabetes[1]. DN is an important cause of end-stage renal disease and a micro-vascular complication of diabetes mellitus (DM)[2,3]. Some dietary factors might be involved in the increase in renal failure in association with DM, showing that the number of patients with DN and/or DM has been increasing in Asian countries because of westernization of dietary lifestyle[2,3]. Pathogenesis of DN may be multifactorial and complex. Early DN has no noticeable clinical symptoms, however, hyperglycemia may be a significant risk factor for DN and/or DM[4]. Sustained elevated blood glucose could lead to changes in the downstream transcription factors and/or gene expression in kidney glomerular cells[5]. Kidney fibrosis and albu-minuria are key pathological processes of the advanced stage of DN[6], but oxidative stress and/or inflammation may also be important mechanisms for the pathogenesis of DN[7]. In general, oxidative stress and inflammatory responses are almost not distinct, because one reaction would intensify the other pathogenesis. Both DM and chronic kidney disease (CKD) may have a common pathophysiological mechanism within a chronic inflammatory state and/or oxidative stresses[8]. Among them, high levels of reactive oxygen species (ROS) could induce inflammatory cytokines in the kidney[9], which might accelerate the development of DN. Inflammation of the kidneys can lead to proteinuria and/or persistent hypertension, which can proceed to renal failure. Hence, successful treatment of the microcirculation in patients with DN has become a superior strategy for the prevention of DN. This reasonable treatment should be discovered immediately. Recently, it has been shown that pathogenesis of DN is associated with certain gut microbiota[10]. The importance of probiotics is widely recognized in various diseases. Besides, studies have shown that crosstalk between host and microbiota might be relevant pathologically in patients with DN[11]. For example, alterations in the gut microbiota are associated with the development of proteinuria[12], and type 2 DM[13]. Changes to the gut microbiota have also been reported in DM and DN[14]. The gut microbiota might well communicate with the kidneys, and the collapse of this relationship might result in the development of renal dysfunction. Accordingly, the gut microbiota could be an important defense against the pathogenesis of kidney disease. Dietary lifestyles have radically changed over the last century in developed countries, and are characterized by reduced dietary fiber and/or increased high-fat consumption[15]. Hence, the changes could be linked to alteration of gut microbiota[16]. Abnormal intestinal metabolites and disruption of the intestinal barrier owing to the gut dysbiosis might facilitate harmful substances produced in the gut entering the circulatory system[17]. These situations allow us to hypothesize that dietary changes could lead to a microbiome that modifies positively the threshold and/or the speed of developing DN and/or DM.
GUT-KIDNEY AXIS IN THE PATHOGENESIS OF DN
Although the significance of the gut microbiota has yet to be completely determined, it is obvious that an intricate symbiotic relationship might exist between host and microbe. In addition, the interaction has recently attracted interest in the study of the pathogenesis of various disorders. The human body holds numerous bacterial and/or microbial cells; the majority of which exist in the gut[18]. The microbiota is a complex community of more than 100 trillion cells in healthy human intestines[19]. The normal gut microbiota could protect the kidney, whereas gut dysbiosis of the microbiota could facilitate kidney disorders[20]. Furthermore, alterations in the microbiota are gradually being linked to the development of various other diseases such as inflammatory bowel disease, cancer, psychiatric disorder, and cardiovascular disease[21]. The gut-kidney axis could additionally affect metabolic and/or immune pathways in addition to the related diseases[22]. The gut-kidney axis is largely mediated by metabolites produced by the gut microbiota, which might regulate physiological function of several organs including the brain, pancreas, adrenal glands, kidneys, etc. (Figure 1). For example, components of the immune system might have a key role with cytokines in communication between the gut and kidneys[23]. Furthermore, crosstalk between the metabolic and immune pathways has a significant role in keeping a good balance in the kidneys[23]. Intestinal responses to inflammation and/or infections are intricate. If microbiota-immune pathways overstimulate tolerance to some inflammation, greater inflammation may accelerate progression of renal disease and/or its complications. Accordingly, gut dysbiosis has frequently been associated with progression of many kidney diseases[24]. In addition, accumulation of uremic toxins, which are derived from dietary metabolism in the gut and/or liver, has distinct effects on the kidneys. For example, the increase in urea increases its influx into the bowel lumen from epithelial cells, where it is hydrolyzed by gut microbiota urease to ammonia[25]. Subsequently, ammonia byproducts may increase the bowel pH, leading to the severe mucosal damage[26]. Accumulation of the uremic toxins in combination with inflammation may also increase the risk of renal disease[27]. Therefore, key factors in kidney disease are function of the gut microbiota and/or the action of gut dysbiosis. Inflammatory bowel disease and DM are indeed multifactorial diseases, and both are chronic diseases associated with increased risk of various diseases including cardiovascular disease, which indicates that the gut is associated with host physiological functions[28]. Interestingly, the prevalence of inflammatory bowel disease in adults with type 1 DM is higher compared to that of nondiabetic controls[29]. It is plausible that the gut-kidney axis might be involved in the pathogenesis of inflammatory bowel disease and DM. Similarly, the gut microbiota may be involved in the damage of other organs, hence targeting the gut microbiota could represent a future therapeutic approach in various diseases. However, the potential impact of gastrointestinal-related disorders on the development and/or progression of DN remains to be elucidated.
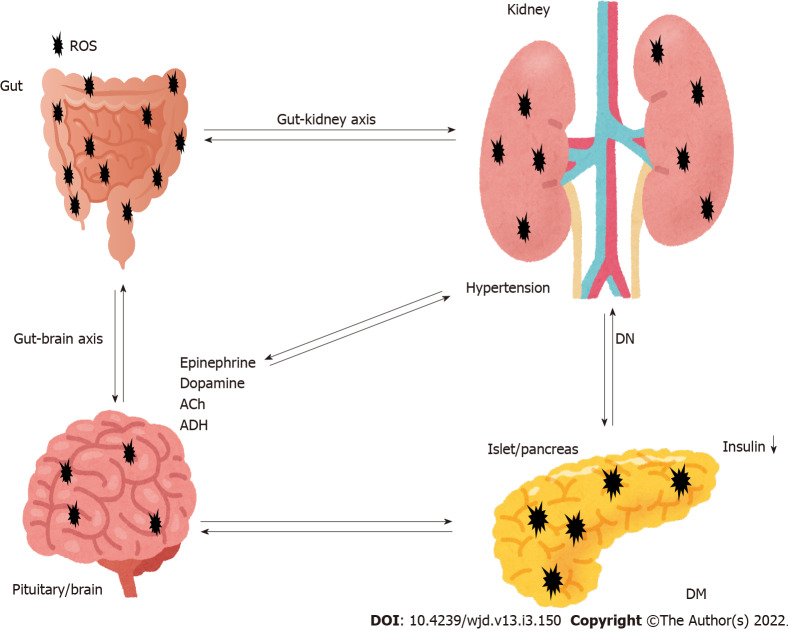
Figure 1.
Representation of the pivotal role of gut-kidney axis crosstalk with the brain and the pancreas in the pathogenesis of diabetic nephropathy. Hypothetical image of the pathogenesis pathway for diabetic nephropathy (DN). Sympathetic activation is a common feature in disorders of the brain as well as gut and kidneys. The brain is responsible for sympathetic outflow contributing to an increase in blood pressure and pathogenesis of the gut and kidneys. Dysbiosis in the gut results in an imbalance of intestinal homeostasis. Pathological events in the brain, pancreas, adrenal glands, gut and kidneys significantly contribute to the development of hypertension and DN. Note that some critical pathways such as inflammation pathway have been omitted for clarity. Ach: Acetylcholine; ADH: Antidiuretic hormone; ROS: Reactive oxygen species; DM: Diabetes mellitus; DN: Diabetic nephropathy.
LEVELS OF SHORT-CHAIN FATTY ACIDS, ROS, AND D-AMINO ACIDS MAY BE INVOLVED IN THE DEVELOPMENT OF DN
Diabetic model mice fed with a high-fiber-diet are less likely to develop DN compared with diabetic control mice fed with a no-fiber diet[30]. High-fiber diet might decrease the expression of genes encoding inflammatory cytokines related to DN[30]. In general, fibers positively improve the dysbiosis of microbiota with promoting the production of short chain fatty acids (SCFAs) (including butyrate, acetate and propionate) in gut microbiota[31], which might also increase the production/release of cytokines and/or chemokines[32]. In addition, SCFAs are able to inhibit intestinal inflammation and/or oxidative stress[33]. Major SCFAs (acetate, propionate and butyrate) are derived through glycolysis of glucose to pyruvate or acetyl-CoA. The SCFAs regularly induce glucagon-like peptide 1 secretion through stimulation of a G-protein-coupled receptor (GPCR)[34]. Gut microbiota in older people may weaken SCFA production[35]. Those SCFAs have various effects on endocrine cells in gut via the GPCRs such as G-protein-coupled receptor (GPR)43 or GPR109A[36]. SCFA-treated diabetic mice have been shown to be protected from nephropathy, suggesting that SCFAs protect renal cells from injury by oxidative stress in DN[37]. It has been shown that butyrate, one of the SCFAs produced by gut microbiota, plays a protective role in DN, which contributes in various physiological processes predominantly by inhibiting histone deacetylases (HDACs)[38]. In addition, providing sodium butyrate has been shown to protect renal cells from oxidative damage and/or apoptosis in type 2 DN mice[39]. Consistently, sodium butyrate has inhibited high-glucose-induced apoptosis of tubular epithelial cells in normal kidneys[40]. Sodium butyrate also lowers plasma glucose and nuclear factor-B expression in the kidneys and attenuates kidney injury[41]. In experimental mice, suppression of HDACs by sodium butyrate may explain the decrease in apoptosis in the kidneys[42]. HDACs can regulate cell proliferation, migration and apoptosis, which are organized by a family of enzymes important for chromatin remodeling, keeping a dynamic balance with histone acetyltransferases in expression of several genes[43]. Valproate, an HDAC inhibitor, has also been shown to decrease renal injury and/or renal fibrosis[44].
The signaling pathways triggered by hyperglycemia appear to have a pivotal role in diabetic complications due to the production of ROS and/or additional oxidative stress, which finally leads to apoptotic cell death in various tissues[45]. ROS includes superoxide anions, hydroxyl free radicals, and hydrogen peroxide[46]. The mitochon-drial electron transport chain is considered a major endogenous source of ROS[47]. Production of excess ROS leads to increased membrane permeability and serious cellular damage[48]. Such overproduction of ROS links to the pathological condition of altered metabolic pathways in the kidneys and disturbed renal function known as nephropathy[49]. Once ATP synthesis is dysregulated in this hyperglycemic situation, it can result in excess production of ROS, which leads to kidney failure[50]. Furthermore, high glucose exposure with excessive ROS can lead to renal podocyte apoptosis in experimental DN[51]. Antioxidants including ubiquinone (also termed coenzyme Q10), ascorbic acid, and resveratrol have been tested in animal models of kidney diseases with some evidence of therapeutic benefits[52]. Epidemiological studies have also found an association between high levels of ROS and risk of DN[53]. Therefore, downregulation of ROS and/or oxidative stress might have a crucial role in regulating diabetic complications. Besides, ROS have been revealed to function as second messengers in several signal transduction pathways[54,55].
Studies have shown the clinical significance of D-amino acids in several kidney diseases[56]. For example, the combination of blood level and urinary dynamics of D-serine effectively separates CKD from non-CKD[57]. D-amino acids in body fluids are also a promising early detection marker for kidney disease[58]. However, excess D-serine can cause kidney damage in rats[59]. In this case, it has been shown that D-serine administration can initiate extensive necrosis in renal proximal tubules[59]. In contrast, administration of D-alanine does not induce kidney injury[60]. Furthermore, protective effects of low-dose D-serine have likely been shown to suppress renal damage, which may promote the hypoxia-mediated proliferation of tubular epithelial cells[61]. In addition, D-cysteine administration can also protect the kidneys from ischemia-reperfusion injury, which might be useful to treat various renal diseases[62]. D-aspartate plays a role during development and neurogenesis[63]. D-aspartate treatment might produce favorable effects during demyelination and remyelination in the nervous system[64]. Furthermore, the ovary-inducing activity of D-tryptophan is more effective than that of L-tryptophan[65]. These data suggest that D-amino acids have both beneficial and harmful effects on tissue development and/or tissue-protection (Figure 2).
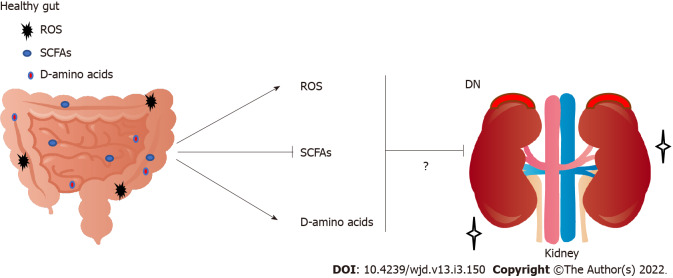
Figure 2.
Implication of increased short-chain fatty acids, decreased reactive oxygen species, and increased D-amino acids derived from gut in the renal protection and/or exacerbation against the progression of diabetic nephropathy. Arrowheads mean stimulation and/or progression, whereas hammerhead represents inhibition. Note that some critical pathways including hormonal regulation have been omitted for clarity. SCFAs: Short-chain fatty acids; ROS: Reactive oxygen species; DN: Diabetic nephropathy.
GUT MICROBIOTA COULD CONTRIBUTE TO HEALTHY KIDNEYS
Carbohydrates are metabolized by gut bacteria into monosaccharides and oligosaccharides, and they could be fermented into SCFAs. As shown above, SCFAs are one of the primary end products of gut fermentation that have considerable effects on host physiology. SCFAs can act as signaling molecules between the gut microbiota and host, and may have a protective effect on the renal function of patients with CKD. In particular, butyrate improves the intestinal barrier and reduces lipopolysaccharide influx into the blood, which could attenuate progression of DN[66]. We provide here a perspective of gut-kidney axis applied in search of renal disease management associated with the gut microbiome, which may theoretically be beneficial for future treatment of DN. Diet is known to be an essential regulator of gut microbiomes[67]. Many studies have confirmed the association between nutrition and the human microbiome in maintaining human health, suggesting significant roles of bacterial metabolites in both health and disease[68]. Trillions of bacteria present in the intestinal and colon lumina constitute the human gut microbiota[69]. Dietary intake could control microbiota whose fermentation may produce various metabolites including SCFAs[70]. The metabolites might additionally regulate the growth of pathogens by competing for of nutrients. For example, parenteral nutrition has been associated with a change in the microbiota, altering SCFA production, and inducing gut mucosal atrophy[71]. The SCFAs made by the healthy gut microbiota have anti-inflammatory properties, including proliferation of regulatory T cells[72,73]. In addition, a significant role for regulatory T cells has been revealed in type 2 diabetes for protection against DN[74]. In addition, SCFAs have favorable effects on β cells, potentiating glucose-stimulated insulin release and/or maintaining β-cell mass through inhibiting apoptosis[75]. Furthermore, propionate, has been shown to prevent adipogenic differentiation of specific stem cells[76].
Many studies have emphasized the relationship between the gut microbiota and oxidative stress[77]. In general, ROS production has a defense mechanism that could elicit cytotoxicity against several pathogens then reduce the burden of infection[78]. Redox signaling is also found in response to microbial signals via the gut epithelial NADPH oxidase 1[79]. Therefore, microbial ROS might rigorously control signaling processes for appropriate immunity and/or the gut barrier[80]. Numerous bacterial species of the microbiota can reduce mitochondrial ROS production[81]. For example, microbial products can upregulate the activity of superoxide dismutase, which results in reduced ROS levels and then decreased cellular apoptosis[82]. In addition, microbial excess ROS might disturb other important pathways of host cells, suggesting that ROS-mediated signaling can regulate various cellular processes in order to keep the host healthy[83]. Epithelial cells may also exhibit increased ROS production in response to several harmful bacteria[84]. In the gut, epithelial appropriate ROS production in response to the gut bacteria may play a signaling role in the host[85]. It is likely that there are many ROS-sensitive important enzymes that could be affected by alterations in the gut redox conditions.
Finally, the gut microbiota have the largest genetic capacity to metabolize D-amino acids that are utilized as nutrients to support bacterial growth to regulate spore germination[86]. Therefore, one possible source of D-amino acids in mammals may be their gut microbiota. In general, many bacterial species encode racemases that convert L-amino acids to D-amino acids[87]. For example, D-alanine production is associated with a relative abundance of bacterial species with racemases such as those of Enterococcus and Lactobacillus in the gut microbiota[88]. Different bacterial species may produce distinct profiles of D-amino acids[89]. Higher D-amino acids levels have been related to the gut microbial mass[90]. Oral intake of a peptide containing specific D-amino acids may reverse the diabetes-associated pathological alterations in the kidneys[91] (Figure 2). Noteworthy differences in the microbiota composition have been discovered in patients with kidney disease compared with healthy controls[92]. Consequently, treatment options for DN should include dietary therapy affecting the gut microbiota. Therapeutic interventions would nevertheless represent a potential target of the microbiota for prevention and/or treatment of DN.
CONCLUSION
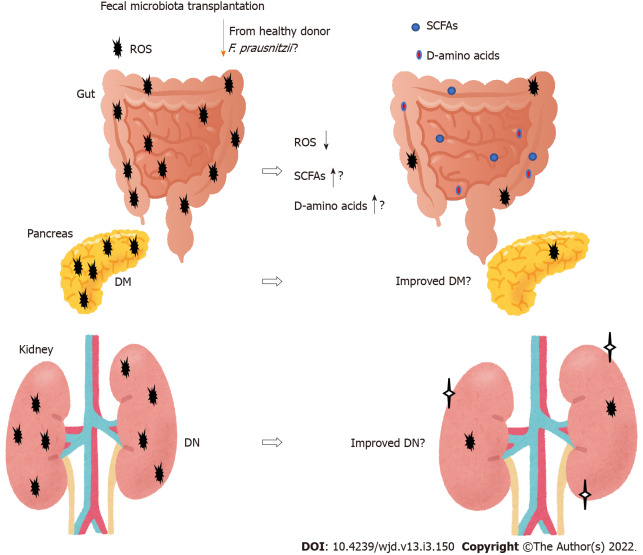
New therapies for DN are emerging. One method that may affect the gut microbiota composition is fecal microbiota transplantation (FMT) (Figure 3). The beneficial effects of the transplantation are dependent on the host responses, however, which may provide a potential treatment strategy for type 2 diabetes[93]. In particular, transplantation of Faecalibacterium prausnitzii (F. prausnitzii) could restore the intestinal structure, which might be used as a potential therapeutic approach against inflammation as well as diabetes[94-96]. Furthermore, F. prausnitzii may serve as a diagnostic and therapeutic biomarker for the use of FMT[97]. The potential role of the gut microbiota has been hypothesized to modulate renal function in experimental DN murine models[98]. Through FMT, the role of the gut microbiota and its SCFA production have been verified in the treatment of DN. Therefore, administration of prebiotics and/or probiotics should individually be tailor-made to prevent and/or cure chronic diseases such as DN. For example, acetate produced by certain gut microbiota reprogramming has been shown to contribute to the tubulointerstitial injury of DN, suggesting that gut microbiota might be a new strategy for DN treatment[99]. Furthermore, FMT from healthy donors considerably attenuates glomerular injury with podocyte improvement in diabetic rats[100].
Figure 3.
The gut microbiota could contribute to the favorable production of short-chain fatty acids, reactive oxygen species and D-amino acids against progression of diabetic nephropathy. Fecal microbiota transplantation consists of fecal microbiota infusion from a healthy donor to a recipient, which has been likely more successful than conventional therapy for diabetic nephropathy. Note that some critical events such as cytokine-induction have been omitted for clarity. SCFAs: Short-chain fatty acids; ROS: Reactive oxygen species; DN: Diabetic nephropathy; DM: Diabetes mellitus.
The above-mentioned topics are only just being explored in preclinical research, suggesting that further studies are required. Owing to a lack of treatments, DN has been a public health concern. Although it is untimely to draw definitive conclusions about the clinical usefulness of microbiota-based treatment strategies for DN, modulation of gut microbiota is an exciting frontier in kidney research. It is clear that intensive evaluation of preclinical studies is necessary to find further insights. In addition, long-term studies are also necessary to clarify the detailed effects of probiotic treatment in the management of DN. A healthy lifestyle with a balanced familiar diet is now one of the main recommendations.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing financial interests.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 5, 2021
First decision: May 3, 2021
Article in press: February 11, 2022
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kobyliak N, Xu PF S-Editor: Wang JJ L-Editor: Kerr C P-Editor: Wang JJ
Contributor Information
Nozomi Nagase, Department of Food Science and Nutrition, Nara Women's University, Nara 630-8506, Japan.
Yuka Ikeda, Department of Food Science and Nutrition, Nara Women's University, Nara 630-8506, Japan.
Ai Tsuji, Department of Food Science and Nutrition, Nara Women's University, Nara 630-8506, Japan.
Yasuko Kitagishi, Department of Food Science and Nutrition, Nara Women's University, Nara 630-8506, Japan.
Satoru Matsuda, Department of Food Science and Nutrition, Nara Women's University, Nara 630-8506, Japan. smatsuda@cc.nara-wu.ac.jp.
References
- 1.Du X, Liu J, Xue Y, Kong X, Lv C, Li Z, Huang Y, Wang B. Alteration of gut microbial profile in patients with diabetic nephropathy. Endocrine. 2021;73:71–84. doi: 10.1007/s12020-021-02721-1. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg E, Hospers FA, Navis G, Engberink MF, Brink EJ, Geleijnse JM, van Baak MA, Gans RO, Bakker SJ. Dietary acid load and rapid progression to end-stage renal disease of diabetic nephropathy in Westernized South Asian people. J Nephrol. 2011;24:11–17. doi: 10.5301/jn.2010.5711. [DOI] [PubMed] [Google Scholar]
- 3.Alicic RZ, Johnson EJ, Tuttle KR. Inflammatory Mechanisms as New Biomarkers and Therapeutic Targets for Diabetic Kidney Disease. Adv Chronic Kidney Dis. 2018;25:181–191. doi: 10.1053/j.ackd.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Vergès B. Cardiovascular disease in type 1 diabetes: A review of epidemiological data and underlying mechanisms. Diabetes Metab. 2020;46:442–449. doi: 10.1016/j.diabet.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M, Hansell P, Palm F. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol. 2015;26:328–338. doi: 10.1681/ASN.2013090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christou GA, Kiortsis DN. The role of adiponectin in renal physiology and development of albuminuria. J Endocrinol. 2014;221:R49–R61. doi: 10.1530/JOE-13-0578. [DOI] [PubMed] [Google Scholar]
- 7.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 8.Tanase DM, Gosav EM, Neculae E, Costea CF, Ciocoiu M, Hurjui LL, Tarniceriu CC, Maranduca MA, Lacatusu CM, Floria M, Serban IL. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM) Nutrients. 2020;12 doi: 10.3390/nu12123719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Justin Rucker A, Crowley SD. The role of macrophages in hypertension and its complications. Pflugers Arch. 2017;469:419–430. doi: 10.1007/s00424-017-1950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tesch GH. Diabetic nephropathy - is this an immune disorder? Clin Sci (Lond) 2017;131:2183–2199. doi: 10.1042/CS20160636. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes R, Viana SD, Nunes S, Reis F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1876–1897. doi: 10.1016/j.bbadis.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Yoshifuji A, Wakino S, Irie J, Tajima T, Hasegawa K, Kanda T, Tokuyama H, Hayashi K, Itoh H. Gut Lactobacillus protects against the progression of renal damage by modulating the gut environment in rats. Nephrol Dial Transplant. 2016;31:401–412. doi: 10.1093/ndt/gfv353. [DOI] [PubMed] [Google Scholar]
- 13.Wen L, Duffy A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J Nutr. 2017;147:1468S–1475S. doi: 10.3945/jn.116.240754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Dong H, Wang Y, Jiang Y, Zhang W, Lu Y, Chen Y, Chen L. Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. Int J Biol Macromol. 2020;163:442–456. doi: 10.1016/j.ijbiomac.2020.06.153. [DOI] [PubMed] [Google Scholar]
- 15.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 16.Andoh A, Kuzuoka H, Tsujikawa T, Nakamura S, Hirai F, Suzuki Y, Matsui T, Fujiyama Y, Matsumoto T. Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn's disease. J Gastroenterol. 2012;47:1298–1307. doi: 10.1007/s00535-012-0605-0. [DOI] [PubMed] [Google Scholar]
- 17.Yang G, Wei J, Liu P, Zhang Q, Tian Y, Hou G, Meng L, Xin Y, Jiang X. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. 2021;117:154712. doi: 10.1016/j.metabol.2021.154712. [DOI] [PubMed] [Google Scholar]
- 18.Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Sordi L, Khanna V, Debarbieux L. The Gut Microbiota Facilitates Drifts in the Genetic Diversity and Infectivity of Bacterial Viruses. Cell Host Microbe. 2017;22:801–808.e3. doi: 10.1016/j.chom.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoodpoor F, Rahbar Saadat Y, Barzegari A, Ardalan M, Zununi Vahed S. The impact of gut microbiota on kidney function and pathogenesis. Biomed Pharmacother. 2017;93:412–419. doi: 10.1016/j.biopha.2017.06.066. [DOI] [PubMed] [Google Scholar]
- 21.Li DY, Tang WHW. Contributory Role of Gut Microbiota and Their Metabolites Toward Cardiovascular Complications in Chronic Kidney Disease. Semin Nephrol. 2018;38:193–205. doi: 10.1016/j.semnephrol.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evenepoel P, Poesen R, Meijers B. The gut-kidney axis. Pediatr Nephrol. 2017;32:2005–2014. doi: 10.1007/s00467-016-3527-x. [DOI] [PubMed] [Google Scholar]
- 23.Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14:442–456. doi: 10.1038/s41581-018-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Afsar B, Vaziri ND, Aslan G, Tarim K, Kanbay M. Gut hormones and gut microbiota: implications for kidney function and hypertension. J Am Soc Hypertens. 2016;10:954–961. doi: 10.1016/j.jash.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Vaziri ND, Yuan J, Khazaeli M, Masuda Y, Ichii H, Liu S. Oral activated charcoal adsorbent (AST-120) ameliorates chronic kidney disease-induced intestinal epithelial barrier disruption. Am J Nephrol. 2013;37:518–525. doi: 10.1159/000351171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figura N. Helicobacter pylori factors involved in the development of gastroduodenal mucosal damage and ulceration. J Clin Gastroenterol. 1997;25 Suppl 1:S149–S163. doi: 10.1097/00004836-199700001-00025. [DOI] [PubMed] [Google Scholar]
- 27.Wu PH, Lin YT, Chiu YW, Baldanzi G, Huang JC, Liang SS, Lee SC, Chen SC, Hsu YL, Kuo MC, Hwang SJ. The relationship of indoxyl sulfate and p-cresyl sulfate with target cardiovascular proteins in hemodialysis patients. Sci Rep. 2021;11:3786. doi: 10.1038/s41598-021-83383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torkamani A, Topol EJ, Schork NJ. Pathway analysis of seven common diseases assessed by genome-wide association. Genomics. 2008;92:265–272. doi: 10.1016/j.ygeno.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu S, Gong J, Tan Y, Liu D. Epidemiologic Association between Inflammatory Bowel Diseases and Type 1 Diabetes Mellitus: a Meta-Analysis. J Gastrointestin Liver Dis. 2020;29:407–413. doi: 10.15403/jgld-798. [DOI] [PubMed] [Google Scholar]
- 30.Li YJ, Chen X, Kwan TK, Loh YW, Singer J, Liu Y, Ma J, Tan J, Macia L, Mackay CR, Chadban SJ, Wu H. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J Am Soc Nephrol. 2020;31:1267–1281. doi: 10.1681/ASN.2019101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai Y, Li Y, Marion T, Tong Y, Zaiss MM, Tang Z, Zhang Q, Liu Y, Luo Y. Resistant starch intake alleviates collagen-induced arthritis in mice by modulating gut microbiota and promoting concomitant propionate production. J Autoimmun. 2021;116:102564. doi: 10.1016/j.jaut.2020.102564. [DOI] [PubMed] [Google Scholar]
- 32.Rutting S, Xenaki D, Malouf M, Horvat JC, Wood LG, Hansbro PM, Oliver BG. Short-chain fatty acids increase TNFα-induced inflammation in primary human lung mesenchymal cells through the activation of p38 MAPK. Am J Physiol Lung Cell Mol Physiol. 2019;316:L157–L174. doi: 10.1152/ajplung.00306.2018. [DOI] [PubMed] [Google Scholar]
- 33.Huang W, Guo HL, Deng X, Zhu TT, Xiong JF, Xu YH, Xu Y. Short-Chain Fatty Acids Inhibit Oxidative Stress and Inflammation in Mesangial Cells Induced by High Glucose and Lipopolysaccharide. Exp Clin Endocrinol Diabetes. 2017;125:98–105. doi: 10.1055/s-0042-121493. [DOI] [PubMed] [Google Scholar]
- 34.Zhou D, Chen YW, Zhao ZH, Yang RX, Xin FZ, Liu XL, Pan Q, Zhou H, Fan JG. Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp Mol Med. 2018;50:1–12. doi: 10.1038/s12276-018-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rampelli S, Candela M, Turroni S, Biagi E, Collino S, Franceschi C, O'Toole PW, Brigidi P. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging (Albany NY) 2013;5:902–912. doi: 10.18632/aging.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moniri NH, Farah Q. Short-chain free-fatty acid G protein-coupled receptors in colon cancer. Biochem Pharmacol. 2021;186:114483. doi: 10.1016/j.bcp.2021.114483. [DOI] [PubMed] [Google Scholar]
- 37.Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, Bassi EJ, Moraes-Vieira PM, Hiyane MI, Rodas AC, Peron JP, Aguiar CF, Reis MA, Ribeiro WR, Valduga CJ, Curi R, Vinolo MA, Ferreira CM, Câmara NO. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J Am Soc Nephrol. 2015;26:1877–1888. doi: 10.1681/ASN.2014030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felizardo RJF, de Almeida DC, Pereira RL, Watanabe IKM, Doimo NTS, Ribeiro WR, Cenedeze MA, Hiyane MI, Amano MT, Braga TT, Ferreira CM, Parmigiani RB, Andrade-Oliveira V, Volpini RA, Vinolo MAR, Mariño E, Robert R, Mackay CR, Camara NOS. Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. FASEB J. 2019;33:11894–11908. doi: 10.1096/fj.201901080R. [DOI] [PubMed] [Google Scholar]
- 39.Dong W, Jia Y, Liu X, Zhang H, Li T, Huang W, Chen X, Wang F, Sun W, Wu H. Sodium butyrate activates NRF2 to ameliorate diabetic nephropathy possibly via inhibition of HDAC. J Endocrinol. 2017;232:71–83. doi: 10.1530/JOE-16-0322. [DOI] [PubMed] [Google Scholar]
- 40.Du Y, Tang G, Yuan W. Suppression of HDAC2 by sodium butyrate alleviates apoptosis of kidney cells in db/db mice and HGinduced NRK52E cells. Int J Mol Med. 2020;45:210–222. doi: 10.3892/ijmm.2019.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan S, Jena G. Sodium butyrate, a HDAC inhibitor ameliorates eNOS, iNOS and TGF-β1-induced fibrogenesis, apoptosis and DNA damage in the kidney of juvenile diabetic rats. Food Chem Toxicol. 2014;73:127–139. doi: 10.1016/j.fct.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Kim SW, Hooker JM, Otto N, Win K, Muench L, Shea C, Carter P, King P, Reid AE, Volkow ND, Fowler JS. Whole-body pharmacokinetics of HDAC inhibitor drugs, butyric acid, valproic acid and 4-phenylbutyric acid measured with carbon-11 labeled analogs by PET. Nucl Med Biol. 2013;40:912–918. doi: 10.1016/j.nucmedbio.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 44.Khan S, Jena G, Tikoo K. Sodium valproate ameliorates diabetes-induced fibrosis and renal damage by the inhibition of histone deacetylases in diabetic rat. Exp Mol Pathol. 2015;98:230–239. doi: 10.1016/j.yexmp.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Sávio-Silva C, Soinski-Sousa PE, Simplício-Filho A, Bastos RMC, Beyerstedt S, Rangel ÉB. Therapeutic Potential of Mesenchymal Stem Cells in a Pre-Clinical Model of Diabetic Kidney Disease and Obesity. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22041546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad R, Ahsan H. Singlet oxygen species and systemic lupus erythematosus: a brief review. J Immunoassay Immunochem. 2019;40:343–349. doi: 10.1080/15321819.2019.1616555. [DOI] [PubMed] [Google Scholar]
- 47.Auger C, Vinaik R, Appanna VD, Jeschke MG. Beyond mitochondria: Alternative energy-producing pathways from all strata of life. Metabolism. 2021;118:154733. doi: 10.1016/j.metabol.2021.154733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonora M, Patergnani S, Ramaccini D, Morciano G, Pedriali G, Kahsay AE, Bouhamida E, Giorgi C, Wieckowski MR, Pinton P. Physiopathology of the Permeability Transition Pore: Molecular Mechanisms in Human Pathology. Biomolecules. 2020;10 doi: 10.3390/biom10070998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jha JC, Banal C, Chow BS, Cooper ME, Jandeleit-Dahm K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid Redox Signal. 2016;25:657–684. doi: 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badal SS, Danesh FR. New insights into molecular mechanisms of diabetic kidney disease. Am J Kidney Dis. 2014;63:S63–S83. doi: 10.1053/j.ajkd.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 52.Huang SS, Ding DF, Chen S, Dong CL, Ye XL, Yuan YG, Feng YM, You N, Xu JR, Miao H, You Q, Lu X, Lu YB. Resveratrol protects podocytes against apoptosis via stimulation of autophagy in a mouse model of diabetic nephropathy. Sci Rep. 2017;7:45692. doi: 10.1038/srep45692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res. 2012;40:95–112. doi: 10.1007/s00240-011-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiarugi P, Buricchi F. Protein tyrosine phosphorylation and reversible oxidation: two cross-talking posttranslation modifications. Antioxid Redox Signal. 2007;9:1–24. doi: 10.1089/ars.2007.9.1. [DOI] [PubMed] [Google Scholar]
- 55.Linnane AW, Kios M, Vitetta L. Healthy aging: regulation of the metabolome by cellular redox modulation and prooxidant signaling systems: the essential roles of superoxide anion and hydrogen peroxide. Biogerontology. 2007;8:445–467. doi: 10.1007/s10522-007-9096-4. [DOI] [PubMed] [Google Scholar]
- 56.Kaimori JY, Maehara K, Hayashi-Takanaka Y, Harada A, Fukuda M, Yamamoto S, Ichimaru N, Umehara T, Yokoyama S, Matsuda R, Ikura T, Nagao K, Obuse C, Nozaki N, Takahara S, Takao T, Ohkawa Y, Kimura H, Isaka Y. Histone H4 lysine 20 acetylation is associated with gene repression in human cells. Sci Rep. 2016;6:24318. doi: 10.1038/srep24318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hesaka A, Sakai S, Hamase K, Ikeda T, Matsui R, Mita M, Horio M, Isaka Y, Kimura T. D-Serine reflects kidney function and diseases. Sci Rep. 2019;9:5104. doi: 10.1038/s41598-019-41608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimura T, Hamase K, Miyoshi Y, Yamamoto R, Yasuda K, Mita M, Rakugi H, Hayashi T, Isaka Y. Chiral amino acid metabolomics for novel biomarker screening in the prognosis of chronic kidney disease. Sci Rep. 2016;6:26137. doi: 10.1038/srep26137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ganote CE, Peterson DR, Carone FA. The nature of D-serine--induced nephrotoxicity. Am J Pathol. 1974;77:269–282. [PMC free article] [PubMed] [Google Scholar]
- 60.Maekawa M, Okamura T, Kasai N, Hori Y, Summer KH, Konno R. D-amino-acid oxidase is involved in D-serine-induced nephrotoxicity. Chem Res Toxicol. 2005;18:1678–1682. doi: 10.1021/tx0500326. [DOI] [PubMed] [Google Scholar]
- 61.Nakade Y, Iwata Y, Furuichi K, Mita M, Hamase K, Konno R, Miyake T, Sakai N, Kitajima S, Toyama T, Shinozaki Y, Sagara A, Miyagawa T, Hara A, Shimizu M, Kamikawa Y, Sato K, Oshima M, Yoneda-Nakagawa S, Yamamura Y, Kaneko S, Miyamoto T, Katane M, Homma H, Morita H, Suda W, Hattori M, Wada T. Gut microbiota-derived D-serine protects against acute kidney injury. JCI Insight. 2018;3 doi: 10.1172/jci.insight.97957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide. 2014;41:4–10. doi: 10.1016/j.niox.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 63.van den Pol AN, Obrietan K, Cao V, Trombley PQ. Embryonic hypothalamic expression of functional glutamate receptors. Neuroscience. 1995;67:419–439. doi: 10.1016/0306-4522(95)96912-w. [DOI] [PubMed] [Google Scholar]
- 64.de Rosa V, Secondo A, Pannaccione A, Ciccone R, Formisano L, Guida N, Crispino R, Fico A, Polishchuk R, D'Aniello A, Annunziato L, Boscia F. D-Aspartate treatment attenuates myelin damage and stimulates myelin repair. EMBO Mol Med. 2019;11 doi: 10.15252/emmm.201809278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobayashi K, Maezawa T, Tanaka H, Onuki H, Horiguchi Y, Hirota H, Ishida T, Horiike K, Agata Y, Aoki M, Hoshi M, Matsumoto M. The identification of ᴅ-tryptophan as a bioactive substance for postembryonic ovarian development in the planarian Dugesia ryukyuensis. Sci Rep. 2017;7:45175. doi: 10.1038/srep45175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabatino A, Regolisti G, Cosola C, Gesualdo L, Fiaccadori E. Intestinal Microbiota in Type 2 Diabetes and Chronic Kidney Disease. Curr Diab Rep. 2017;17:16. doi: 10.1007/s11892-017-0841-z. [DOI] [PubMed] [Google Scholar]
- 67.Chen PB, Black AS, Sobel AL, Zhao Y, Mukherjee P, Molparia B, Moore NE, Aleman Muench GR, Wu J, Chen W, Pinto AFM, Maryanoff BE, Saghatelian A, Soroosh P, Torkamani A, Leman LJ, Ghadiri MR. Directed remodeling of the mouse gut microbiome inhibits the development of atherosclerosis. Nat Biotechnol. 2020;38:1288–1297. doi: 10.1038/s41587-020-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P ANR MicroObes consortium, Doré J, Zucker JD, Clément K, Ehrlich SD. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 69.Guo Y, Kitamoto S, Kamada N. Microbial adaptation to the healthy and inflamed gut environments. Gut Microbes. 2020;12:1857505. doi: 10.1080/19490976.2020.1857505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shanahan F, van Sinderen D, O'Toole PW, Stanton C. Feeding the microbiota: transducer of nutrient signals for the host. Gut. 2017;66:1709–1717. doi: 10.1136/gutjnl-2017-313872. [DOI] [PubMed] [Google Scholar]
- 71.Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papatriantafyllou M. T cells: maintaining T cell homeostasis. Nat Rev Immunol. 2013;13:546–547. doi: 10.1038/nri3504. [DOI] [PubMed] [Google Scholar]
- 73.Papatriantafyllou M. Regulatory T cells: distilling regulatory T cell inducers. Nat Rev Immunol. 2013;13:546. doi: 10.1038/nri3506. [DOI] [PubMed] [Google Scholar]
- 74.Abouzeid S, Sherif N. Role of alteration in Treg/Th17 cells' balance in nephropathic patients with Type 2 diabetes mellitus. Electron Physician. 2015;7:1613–1618. doi: 10.19082/1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pingitore A, Chambers ES, Hill T, Maldonado IR, Liu B, Bewick G, Morrison DJ, Preston T, Wallis GA, Tedford C, Castañera González R, Huang GC, Choudhary P, Frost G, Persaud SJ. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes Metab. 2017;19:257–265. doi: 10.1111/dom.12811. [DOI] [PubMed] [Google Scholar]
- 76.Iván J, Major E, Sipos A, Kovács K, Horváth D, Tamás I, Bay P, Dombrádi V, Lontay B. The Short-Chain Fatty Acid Propionate Inhibits Adipogenic Differentiation of Human Chorion-Derived Mesenchymal Stem Cells Through the Free Fatty Acid Receptor 2. Stem Cells Dev. 2017;26:1724–1733. doi: 10.1089/scd.2017.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kong Y, Olejar KJ, On SLW, Chelikani V. The Potential of Lactobacillus spp. for Modulating Oxidative Stress in the Gastrointestinal Tract. Antioxidants (Basel) 2020;9 doi: 10.3390/antiox9070610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghosh S, Dai C, Brown K, Rajendiran E, Makarenko S, Baker J, Ma C, Halder S, Montero M, Ionescu VA, Klegeris A, Vallance BA, Gibson DL. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am J Physiol Gastrointest Liver Physiol. 2011;301:G39–G49. doi: 10.1152/ajpgi.00509.2010. [DOI] [PubMed] [Google Scholar]
- 79.Neish AS. Redox signaling mediated by the gut microbiota. Free Radic Res. 2013;47:950–957. doi: 10.3109/10715762.2013.833331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2012;180:626–635. doi: 10.1016/j.ajpath.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lobet E, Letesson JJ, Arnould T. Mitochondria: a target for bacteria. Biochem Pharmacol. 2015;94:173–185. doi: 10.1016/j.bcp.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 82.Liu TF, Vachharajani VT, Yoza BK, McCall CE. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem. 2012;287:25758–25769. doi: 10.1074/jbc.M112.362343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Belizário JE, Faintuch J, Garay-Malpartida M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediators Inflamm. 2018;2018:2037838. doi: 10.1155/2018/2037838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 85.Neish AS, Jones RM. Redox signaling mediates symbiosis between the gut microbiota and the intestine. Gut Microbes. 2014;5:250–253. doi: 10.4161/gmic.27917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cava F, Lam H, de Pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011;68:817–831. doi: 10.1007/s00018-010-0571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Radkov AD, Moe LA. Bacterial synthesis of D-amino acids. Appl Microbiol Biotechnol. 2014;98:5363–5374. doi: 10.1007/s00253-014-5726-3. [DOI] [PubMed] [Google Scholar]
- 88.Gilmore MS, Skaugen M, Nes I. Enterococcus faecalis cytolysin and lactocin S of Lactobacillus sake. Antonie Van Leeuwenhoek. 1996;69:129–138. doi: 10.1007/BF00399418. [DOI] [PubMed] [Google Scholar]
- 89.Lam H, Oh DC, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science. 2009;325:1552–1555. doi: 10.1126/science.1178123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ketting D, Wadman SK, Spaapen LJ, Van der Meer SB, Duran M. Gas chromatography method for the separation of amino acids enantiomers in plasma and urine. Application in a case of short bowel syndrome. Clin Chim Acta. 1991;204:79–86. doi: 10.1016/0009-8981(91)90219-3. [DOI] [PubMed] [Google Scholar]
- 91.Chai Z, Wu T, Dai A, Huynh P, Koentgen F, Krippner G, Ren S, Cooper ME. Targeting the CDA1/CDA1BP1 Axis Retards Renal Fibrosis in Experimental Diabetic Nephropathy. Diabetes. 2019;68:395–408. doi: 10.2337/db18-0712. [DOI] [PubMed] [Google Scholar]
- 92.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 93.Wang H, Lu Y, Yan Y, Tian S, Zheng D, Leng D, Wang C, Jiao J, Wang Z, Bai Y. Promising Treatment for Type 2 Diabetes: Fecal Microbiota Transplantation Reverses Insulin Resistance and Impaired Islets. Front Cell Infect Microbiol. 2019;9:455. doi: 10.3389/fcimb.2019.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ganesan K, Chung SK, Vanamala J, Xu B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19123720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu J, Liang R, Zhang W, Tian K, Li J, Chen X, Yu T, Chen Q. Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression. J Diabetes. 2020;12:224–236. doi: 10.1111/1753-0407.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Björkqvist O, Rangel I, Serrander L, Magnusson C, Halfvarson J, Norén T, Bergman-Jungeström M. Faecalibacterium prausnitzii increases following fecal microbiota transplantation in recurrent Clostridioides difficile infection. PLoS One. 2021;16:e0249861. doi: 10.1371/journal.pone.0249861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen HT, Huang HL, Xu HM, Luo QL, He J, Li YQ, Zhou YL, Nie YQ, Zhou YJ. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp Ther Med. 2020;19:2650–2660. doi: 10.3892/etm.2020.8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Su X, Gao Y, Lv C, Gao Z, Liu Y, Wang Y, Li S, Wang Z. The potential role of the gut microbiota in modulating renal function in experimental diabetic nephropathy murine models established in same environment. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165764. doi: 10.1016/j.bbadis.2020.165764. [DOI] [PubMed] [Google Scholar]
- 99.Hu ZB, Lu J, Chen PP, Lu CC, Zhang JX, Li XQ, Yuan BY, Huang SJ, Ruan XZ, Liu BC, Ma KL. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. Theranostics. 2020;10:2803–2816. doi: 10.7150/thno.40571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu J, Chen PP, Zhang JX, Li XQ, Wang GH, Yuan BY, Huang SJ, Liu XQ, Jiang TT, Wang MY, Liu WT, Ruan XZ, Liu BC, Ma KL. GPR43 deficiency protects against podocyte insulin resistance in diabetic nephropathy through the restoration of AMPKα activity. Theranostics. 2021;11:4728–4742. doi: 10.7150/thno.56598. [DOI] [PMC free article] [PubMed] [Google Scholar]