Abstract
Vitamin E compounds, consisting of α, β, γ, and δ forms of tocopherols and tocotrienols, display different cancer preventive activities in experimental models. Tocotrienols may have higher potential for clinical use due to their lower effective doses in laboratory studies. However, most studies on tocotrienols have been carried out using cancer cell lines. Strong data from animal studies may encourage the use of tocotrienols for human cancer prevention research. To examine the cancer inhibitory activity of different vitamin E forms, we first investigated their inhibitory activities of different vitamin E forms in prostate cancer cell lines. We found that δ-tocotrienol (δT3) was the most effective form in inhibiting cell growth at equivalent doses. Because of this in vitro potency, δT3 was further studied using prostate specific Pten−/− (Ptenp−/−) mice. We found that 0.05% δT3 in diet reduced prostate adenocarcinoma multiplicity by 32.7%, featuring increased apoptosis and reduced cell proliferation. The inhibitory effect of 0.05% δT3 in diet was similar to that of 0.2% δ-tocopherol (δT) in diet reported previously. Our further study on the δT3-induced transcriptome changes indicated that δT3 inhibited genes in blood vessel development in the prostate of Ptenp−/− mice, which was confirmed by immunohistochemistry. Together, our results demonstrate that δT3 effectively inhibits the development of prostate adenocarcinoma in Ptenp−/− mice, which involves inhibition of proliferation and angiogenesis and promotion of apoptosis.
Introduction
Vitamin E compounds, found in vegetable oils, nuts, soybeans, whole grains, and other sources, exist in the α, β, γ, and δ forms of tocopherols and tocotrienols (1–4). This group of compounds is composed of a chromanol ring containing hydroxyl and methyl groups and a 16-carbon side chain. The number and position of methyl groups on the chromanol ring define different forms of tocopherols and tocotrienols: α-tocopherol (αT) and α-tocotrienol (αT3) are trimethylated at the 5-, 7-, and 8-positions; the β-forms, βT and βT3, are 3methylated at the 5- and 8-positions; γT and γT3 are 3methylated at the 7- and 8-positions; while δT and δT3 are methylated at the 8-position. Tocopherols have a saturated phytyl side chain, while tocotrienols have an unsaturated isoprenyl side chain featuring double bonds at the 3`-, 7`-, and 11`-positions. The hydrophobic side chain allows Vitamin E to incorporate into the lipid bilayer of biomembranes. Vitamin E can quench free radicals such as reactive oxygen species (ROS) through a one electron reduction via the 6- hydroxyl group of the chromanol ring, producing a phenoxy radical. The hydroxyl group can be regenerated through reduction by ascorbic acid or glutathione. This regeneration cycle constitutes the most important physiological antioxidant mechanism for protecting cellular membrane integrity. The γ and δ forms also trap reactive nitrogen species (RNS) (4–7).
Vitamin E is absorbed in the small intestine and transported to the liver through the lymphatic pathway (8–10). From the liver, vitamin E is distributed throughout the body to different tissues, incorporating into lipid storage organelles and cellular membranes. Among all forms, αT displays the highest blood and tissue levels, because the hepatic αT transfer protein preferentially transfers αT to blood over other forms, and the highest traditional vitamin E activity; therefore, αT is used in most of the vitamin E studies (10–12).
The cancer preventive activities by different vitamin E forms have recently been reviewed (13). Epidemiological results showed that lower levels of dietary intake or blood levels of αT or γT were often associated with higher risk for cancer (13–16). However, large-scale intervention studies, including the Women’s Health Study (17), the Physicians’ Health Study II Randomized Control Trial (PHSII) (18), and the Selenium and Vitamin E Cancer Prevention Trial (SELECT) (19), found that supplementation with high doses of vitamin E (αT) did not reduce cancer risk. In laboratory studies, αT did not display a robust cancer preventive or inhibiting activity (reviewed in (13,20–22)). In contrast, γT and δT were found to effectively inhibit cancer cell growth and prevent cancer development in a variety of experimental models (13,20–22). In previous studies, we treated prostate cancer cells with different forms of tocopherols and found that δT was the most active tocopherol in inhibiting cell proliferation and inducing apoptosis through attenuating the receptor tyrosine kinase induced Akt activation, while γT was less effective and αT had no effect (23). The inhibition of Akt activation, as well as reduced cell proliferation and increased apoptosis likely resulted from reduced AKT signaling, were further validated in a study using prostate-specific Pten−/− (Ptenp−/−) mice, in which a diet supplemented with 0.2% δT inhibited prostate cancer development (24). In other studies, γT and a γT-rich mixture were found to effectively inhibit cancer development in murine mammary glands by reducing ERα and inducing PPARγ, Pten, and p27 (25–27). Together, these experimental studies demonstrate the cancer preventive activities of δT and γT, suggesting the potential of δT and γT in human cancer prevention (13,21,28,29).
Recent studies suggest that tocotrienols display potent cancer preventive activities at lower concentrations than tocopherols. In a phase II clinical study to treat advanced ovarian cancer patients with δT3 combined with standard bevacizumab therapy, the combination significantly extended median progression-free survival time (30), suggesting potential for tocotrienol use in adjuvant therapy. Various mechanisms, such as inhibition of cell proliferation, metastasis, and angiogenesis as well as induction of apoptosis and autophagy, have been proposed to explain the cancer inhibitory activities of tocotrienols observed in cancer cell line studies (13,31). In order to explore the use of tocotrienols in human cancer prevention, more animal studies are needed.
In the present study, we determined the inhibitory activities of the different forms of vitamin E in prostate cancer cell lines and found that δT3 was the most active form. δT3 (0.05% in diet) was found to effectively inhibit prostate cancer development in Ptenp−/− mice. Our results suggest that the cancer preventive activity of δT3 involves anti-proliferation, pro-apoptosis and anti-angiogenesis.
Materials and Methods
1. Vitamin E compounds and animal diets.
The natural-occurring d-form tocopherols were purified individually from commercial sources to a purity of >99.5% using an automated flash chromatography system as described previously (32). Tocotrienols (≥99.0% purity) were generously provided by Davos Life Science Pte Ltd. (Singapore) and used to treat cultured cells. Large quantity of δT3 (>90.0% purity) were also provided by Davos Life Science and used for preparing diet. Semi-purified rodent diet (AIN93M) and diet supplemented with 0.05% δT3 were prepared by Research Diets, Inc. (New Brunswick, NJ, USA) and stored at 4°C in sealed plastic bags flashed with nitrogen gas.
2. Cell culture, treatments and cell growth assay.
Prostate cancer cell lines, including mouse cell lines, MyC-CaP (ATCC Cat# CRL-3255, RRID:CVCL_J703) and PTEN-Cap8 (ATCC Cat# CRL-3033, RRID:CVCL_0F37), and human cell lines, PC3 (ATCC Cat# CRL-7934, RRID:CVCL_0035), DU145 (ATCC Cat# HTB-81, RRID:CVCL_0105), 22Rv1 (ATCC Cat# CRL-2505, RRID:CVCL_1045), and LNCaP (ATCC Cat# CRL-3313, RRID:CVCL_4783), were obtained from ATCC (Manassas, VA, USA). The human cell lines used were less than 5 passages from the stocks prepared at the 5th passage of the cell lines purchased from ATCC. The stocks were free of mycoplasma by tests using Mycoplasma Universal Mycoplasma Detection Kit (ATCC). In each experiment, we use the cells from our stocks for <2 months and <10 passages. However, the cell lines have not been authenticated in the last 12 months. The mouse cell lines were newly purchased and used under 10 passages within 3 months. Cells were routinely maintained in RPMI medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml of penicillin, and 100 μg/ml of streptomycin at 37°C in 5% CO2.
To determine the effects of tocopherols and tocotrienols on cell growth, cells were seeded on 96-well plate in 5% FBS medium at the density of 3,000 cells per well and, after overnight, tocopherol or tocotrienol dissolved in DMSO were added into the medium. DMSO was used as the vehicle control. Each concentration was used for a row of 8 wells. The viable cells, at 48 hours after the treatments, were quantified by colorimetric reaction using 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich), the MTT assay, as described previously (23). To determine the induction of apoptosis, cells were seeded at ~50% of confluence in 5% FBS medium on 10 cm plates. After an overnight culture, the cells were treated with tocopherol or tocotrienol for 24 hours and collected in ice-cold NP-40 lysis buffer (25 mM Tris, pH 7.4, 0.5% Nonidet P-40, 150 mM NaCl, 1.5 mM EDTA, 1 mM DTT, 50 mM NaF, 0.5 mM Vanadate, 10% glycerol, and Sigma protease inhibitor cocktail). For the treatment with IGF1 or EGF (R&D systems, Minneapolis, MN, USA), the cells were cultured in 5% FBS medium overnight and then cultured in 0.5% FBS medium for 12 hours in the presence or absence of tocopherol or tocotrienol. At the time of the treatment, the medium was replaced with pre-warmed 0.5% FBS medium without tocopherol and tocotrienol, and 10 ng/ml IGF1 or 20 ng/ml EGF were added into the medium to treat the cells. Cells were collected in NP-40 lysis buffer at 2, 5, 10, 15, 20, and 30 minutes after the treatment.
Protein concentration of samples was determined using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of samples were loaded for SDS-polyacrylamide gel electrophoresis and western blot analysis using antibodies against cleaved-Caspase 3 (C-Casp3) (Abcam Cat# 5000–1, RRID:AB_514418) (Abcam, Cambridge, MA, USA), Akt (Cell Signaling Technology Cat# 9272, RRID:AB_329827), pAkt(S473), Erk1/2 (Cell Signaling Technology Cat# 9102, RRID:AB_330744), and pErk1/2 (Cell Signaling Tech., Danvers, MA, USA). The blots were also probed with antibody against β-actin (Sigma-Aldrich Cat# A5441, RRID:AB_476744) or Gapdh (Cell Signaling Technology Cat# 51332, RRID:AB_2799390) to monitor sample loading. Finally, the blots were probed with the IRdye-labelled secondary antibodies (Li-Cor Biosciences, Lincoln, NE, USA) and quantified by Odyssey Infrared Imaging system (Li-Cor Biosciences).
3. Animal studies.
Male Ptenp−/− mice (Pten-loxP+/+:pB-Cre+; in Fvb background) were produced by breeding male Pten-loxP+/+:pB-Cre+ mice with female Pten-loxP+/+ mice, kindly provided by Dr. Ronald Depinho at MD Anderson Cancer Center (33), as described previously (24). Male Pten-loxP+/+:pB-Cre− mice were used as the wildtype (Wt) controls. Mice were maintained at room temperature with a relative humidity of 50±10% and a 12-hour light/dark cycle housed in the animal facility in the Department of Chemical Biology, Rutgers University. Animal experiments described in this study were conducted in accordance with the protocol approved by the Institutional Animal Care and Use of Rutgers University. Male Ptenp−/− mice born within 3 days were randomly grouped at 4 weeks of age. They were fed either AIN93M or 0.05% δT3 diet starting at 6 weeks of age. At the end point, mice were euthanized by CO2 asphyxiation. For histopathological characterization, the entire prostates were excised and fixed in 10% PBS-buffered formalin (Thermo Fisher Scientific, Waltham, MA, USA) for preparing the paraffin-embedded tissue blocks. For extracting protein or RNA, the prostates were dissected from mice at 20 weeks of age and the anterior and dorsal-lateral-ventral lobes were separated under a dissecting microscope. The dorsal-lateral-ventral lobes were stored at −70°C for protein extraction, or stored in RNAlater solution (Qiagen Co., Germantown, MA, USA) at −70°C for RNA extraction.
4. Histopathological characterization.
The formalin-fixed prostates were dissected and separated into the anterior and dorsal-lateral-ventral lobes, which were then used for preparing two separate paraffin blocks, according to previously described procedures (24). To characterize prostate lesions, histopathological analysis of each prostate was conducted on two hematoxylin and eosin (H&E)-stained sections in the middle of the tissues, approximately 20 sections apart. Prostatic intraepithelial neoplasia (PIN) and adenocarcinoma were identified according to previously characterized histopathological features for Ptenp−/− mice (34,35).
5. Immunohistochemistry staining.
Prostates were also characterized using a standard immunohistochemical (IHC) staining described previously (36). In brief, the IHC staining was carried out using the primary antibody for the target followed by biotinylated secondary antibody and then streptavidin-biotin peroxidase conjugate (Vector Laboratories, Burlingame, CA, USA), and development using 3,3’-diaminobenzidine (DAB) substrate (Vector Laboratories). The slides were then counterstained with hematoxylin for labelling nuclei. Preliminary antibodies were against Ki67 (Abcam Cat# ab6526, RRID:AB_305543), C-Casp3, pErk1/2 and CD31/Pecam1 (Abcam Cat# 2540–1, RRID:AB_1267040). The immuno-stained slides (slides stained at the same time) were analyzed using the Aperio ScanScope GL system (Vista, CA, USA). The slides were scanned using Aperio ScanScope to generate digital images, which were then analyzed by Aperio image analysis software Spectrum using specific algorithms. For example, the Ki67 and C-Casp3 stainings were analyzed by the Nuclear Algorithm, and the results were presented as the percentage of positive stained cells, which were identified by positive IHC staining in nuclei. The total number of cells in the analyzed area was determined by the number of counter stain nuclei identified by the Nuclear Algorithm. The pErk1/2 staining was analyzed by the Positive Pixel Count Algorithm, and the results were presented as the average staining intensity per cell, which was calculated by the total positive staining pixel collected and the total number of cells in the selected area. The analyzed areas included the luminal epithelia or lesioned glands but excluded the basal layer and stroma as well as luminal space that had no cells. The invasion areas were not analyzed, because it was difficult to distinguish between glandular cells from stromal cells. Image analyses usually included ~2,000 cells per mouse in Wt samples and >5000 cells per mouse in Ptenp−/− samples.
The microvessel density were carried out by counting the blood vessel numbers in the highest vascularized areas in the CD31/Pecam-1 stained slides at 200× magnifications according to the established procedure for quantifying the microvessel density in cancer (37). We counted 20 highly vascularized areas inside lesioned glands per mouse, the microvessel density was represented by the average of blood vessel numbers in the top 5 areas. In the stroma, the average of the top 3 areas was used, because we could only count 3–8 areas due to much smaller number of stroma that were large enough to fulfil the imaged area under 200× objective.
6. RNA extraction, RNA-sequencing, and real-time PCR.
The total RNA was extracted from prostate dorsal-lateral-ventral lobes using RNAeasy Mini Kit (Qiagen) as described previously (38). The RNA samples collected from 6 mice on AIN93M and 6 mice on 0.05% δT3 diet were used for the quantification RNA-sequencing performed by BGI US (Cambridge, MA, USA). The expression levels of target genes were also quantified in triplicates using real-time PCR with SuperScript III First-Strand Synthesis SuperMix and Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) on ViiA 7 Real-Time PCR System (Thermo Fisher Scientific). The PCR primer sequences were obtained from PrimerBank (https://pga.mgh.harvard.edu/primerbank/; (39)): β-actin-forward, 5’-GGCTGTATTCCCCTCCATCG-3’; β-actin-reverse, 5’-CCAGTTGGTAACAATGCCATGT-3’; CD31/Pecam1-forward, 5’-CTGCCAGTCCGAAAATGGAAC-3’; CD31/Pecam1-reverse, 5’-CTTCATCCACCGGGGCTATC-3’; Vwf-forward, 5’-CTTCTGTACGCCTCAGCTATG-3’; Vwf-reverse, 5’-GCCGTTGTAATTCCCACACAAG-3’; Vegfd-forward, 5’-TTGAGCGATCATCCCGGTC-3’; and Vegfd-reverse, 5’-GCGTGAGTCCATACTGGCAAG-3’.
7. Statistical analysis
Statistical analysis was performed using GraphPad Prism 9.1 (GraphPad, San Diego, CA, USA). Student’s t-test was used to determine the difference between two groups. One-way Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison tests was used to determine differences among >2 groups. Statistical significance was indicated by P-value less than 0.05.
Data availability
The RNA sequencing data, including raw and processed files, underlying this article are available in NCBI GEO at https://www.ncbi.nlm.nih.gov/geo/ and can be accessed with accession No. GSE182980.
Results
1. δT3 is the most potent vitamin E form in inhibiting the growth of prostate cancer cells.
To determine the inhibitory activities of different forms of vitamin E on cell growth, we treated a human prostate cancer cell line DU145 and a mouse prostate cancer cell line PTEN-CaP8 with individual forms. We found that tocotrienols effectively inhibited cell growth while δT and γT also inhibited cell growth, and αT had no effect (Figure 1A–B). At the same dose levels, tocopherols and tocotrienols displayed inhibitory activities in the following order: δT3 > γT3 > δT ≈ αT3 > γT. For example, the IC50 values of δT3, γT3, αT3, δT, and γT in DU145 cells were 11.9, 16.6, 25.9, 23.5, and 48.7 μM, respectively. The results of tocopherols were consistent with the data reported previously (23). Similar results were also obtained in the study using other human prostate cancer cell lines, PC-3, LNCaP, and 22Rv1, and a mouse prostate cancer cell line, MyC-CaP (Supplementary Figure 1). These data demonstrated that δT3 is the most potent vitamin E form in inhibiting the growth of prostate cancer cells.
Figure 1. δT3 is the most potent vitamin E form in inhibiting the growth of prostate cancer cells.

(A-B) DU145 and Pten-CaP8 cells were treated with different concentrations of tocopherol or tocotrienol for 48 hours, and the percentage of viable cells were determined by the MTT assay. The error bar represents SD (n=8). (C) DU145 and Pten-CaP8 cells treated with tocopherol or tocotrienol for 24 hours were analysed for apoptosis using the western blot analysis of C-Casp3. The sample loading was monitored using the β-actin levels. The uncropped blot images were provided in Supplementary Figure 2A. (D-E) DU145 cells, treated with DMSO, 24 μM δT or 12 μM δT3 for 12 hours, were challenged with 10 ng/ml IGF1 and then collected for the western blot analyses of pAkt and Akt. Representative results are shown in D. The fold increases of the ratio of pAkt to Akt at 5, 10, 15, and 20 minutes (from the zero time), in three individual experiments, were summarized in E (the uncropped blot images of three experiments were provided in Supplementary Figure 2B). The error bar represents SD. a and b indicate the difference among all groups with statistical significance (ANOVA; P-value < 0.05) at the same time point.
Next, we determined whether tocotrienols induces apoptosis. We performed Western blot analysis for apoptosis marker C-Casp3 using DU145 and PTEN-CaP8 cells treated with tocopherols and tocotrienols at the effective inhibitory concentrations. We found that tocotrienols and δT induced the cleavage/activation of Caspase 3 (Figure 1C) (Supplementary Figure 2), indicating that tocotrienols induced apoptosis. Since the inhibition of δT is mediated through attenuating the receptor tyrosine kinase (RYK)-induced activation/phosphorylation of Akt (pAkt) (23), we assessed the IGF1-induced activation of Akt in DU145 cells pre-treated with δT3 at the IC50 dose. DMSO and δT were used as the controls. We found that δT3 displayed much weaker inhibitory activity on the activation of Akt than δT (Figure 1D–E) (Supplementary Figure 2). Similar result was also obtained in studying the EGF-induced activation of Akt (Supplementary Figure 3). These data implied additional mechanisms for the inhibitory action of δT3.
2. δT3 inhibited the development of prostate adenocarcinoma in Ptenp−/− mice.
Since δT3 is the most potent vitamin E form in inhibiting prostate cancer cell growth, δT3 was further investigated for cancer prevention in prostate cancer mouse model Ptenp−/− mice. Because 0.2% δT in diet effectively inhibited prostate adenocarcinoma development in Ptenp−/− mice (24) and the IC50 of δT3 (9.6 μM) in inhibiting the growth of PTEN-CaP8, a prostate cancer cell line derived from Ptenp−/− mice, was ~4 times lower than that of δT (39.7 μM) (Figure 1B), we supplemented the diet with 0.05% δT3 to treat Ptenp−/− mice. In this study, we randomly separated 28 male Ptenp−/− mice into two groups at 4 weeks of age and fed either AIN93M (n=15) or δT3 diet (n=13) to the mice starting at 6 weeks of age. During the experiment, the body weights of two groups of mice were recorded weekly and showed no difference. Because Ptenp−/− mice developed PIN rapidly (24,35) and we found no effect of δT on the development of PINs (24), we decided to focus on adenocarcinomas and collected the prostates for histopathological characterization of the development of adenocarcinoma at 40 weeks of age. We found that all glands in the dorsal-ventral-lateral lobes of the prostate developed high grade (HG)-PIN, and some progressed to adenocarcinomas with invasiveness features such as rupture or loss of basal membrane and invasion of neoplastic cells into stroma. In contrast, the glands in each anterior lobe fused to form an enlarged fluid cyst. These histological features of prostate lesions were the same as reported previously (24). The adenocarcinoma multiplicities (the percentage of glands in the dorsal-ventral-lateral lobes that developed adenocarcinomas) in Ptenp−/− mice on AIN93M and δT3 diet were 45.3±12.7% and 30.5±7.9%, respectively (Figure 2A), showing a 32.7% reduction in mice on the δT3 diet (P-value = 0.0012). This result is comparable to a 40% reduction by 0.2% δ-T diet reported previously (24), suggesting that δT3 is effective in inhibiting adenocarcinoma development at a lower dose than δT, consistent with their IC50 values on cell growth described above.
Figure 2. Feeding diet supplemented with 0.05% δT3 reduced the multiplicity of adenocarcinoma in the prostate of Ptenp−/− mice.
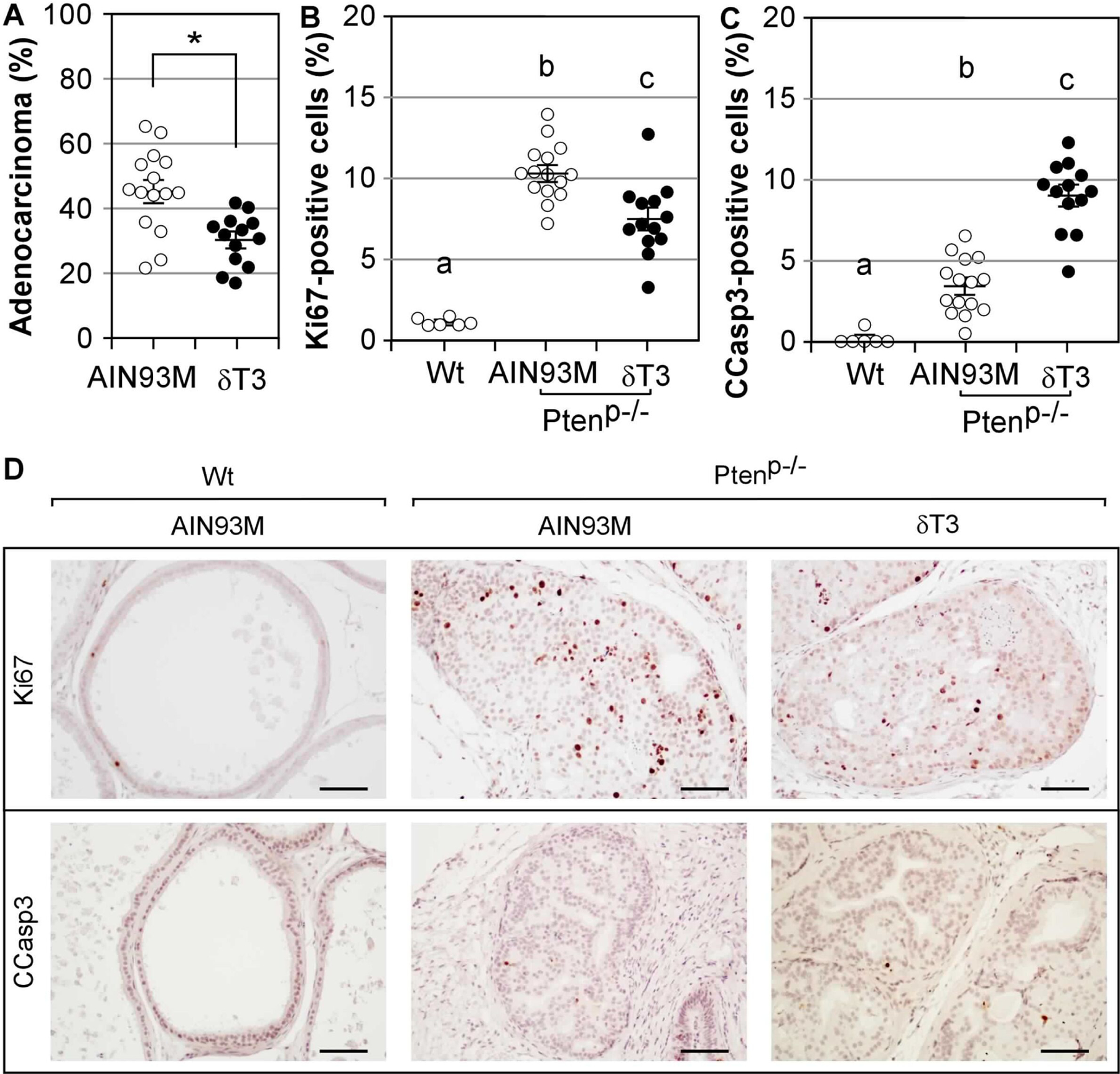
(A) The adenocarcinoma multiplicity in the prostate of Ptenp−/− mice fed either AIN93M (n=15) or 0.05% δT3 diet (n=13) at 40 weeks of age. Data are presented as mean±SD. * indicates the difference with statistical significance (P-value = 0.001). (B-C) The prostates of Ptenp−/− mice on AIN93M or 0.05% δT3 diet were analyzed for cell proliferation and apoptosis by the IHC staining for Ki67 and C-Casp3. The prostates (n=5) of the same aged Wt mice were used as the controls. The percentages of gland luminal epithelial or tumor cells positively stained for Ki67 in the nucleus or C-Casp3 were determined by Aperio’s IHC Nuclear Image Analysis algorithm. a, b and c indicate the difference among all groups with statistical significance (ANOVA; P-value < 0.05). (D) Representative images of the Ki67 and C-Casp3 stained prostate samples of Wt and Ptenp−/− mice. The scale bar represents 50 μm.
3. δT3 reduced cell proliferation and increased apoptosis in the prostate of Ptenp−/− mice.
By IHC staining of Ki67 and C-Casp3, we determined the effects of δT3 on cell proliferation and apoptosis in the prostate of Ptenp−/− mice collected at 40 weeks, with the same aged Wt mice on AIN93M diet as a comparison (Figure 2B–D). The prostates of Wt mice displayed only a few Ki67+ cells (1.15±0.26%) and C-Casp3+ cells (0.02±0.04%). The prostates of Ptenp−/− mice contained significantly more Ki67+ cells. The number of Ki67+ cells was 10.30±1.80% in mice on AIN93M diet and the number was reduced to 7.53±3.25% by δT3 supplementation (P-value < 0.05; ANOVA). The number of C-Casp3+ cells was 0.34±0.17% in the prostates of Ptenp−/− on AIN93M diet and was increased to 0.89±0.13% by δT3 supplementation (P-value < 0.05; ANOVA). These results demonstrated that δT3 reduced cell proliferation and increased apoptosis in the prostate of Ptenp−/− mice.
4. Oxidative stress in prostate of Ptenp−/− mice.
Next, we wondered whether oxidative stress was reduced by δT3 since δT and γT were found to reduce oxidative stress in the prostate and prevent prostate carcinogenesis in mice treated with a carcinogen (40). We assessed oxidative stress by IHC staining of 8-OH-dG and nitrotyrosine, the products of ROS- and RNS-caused damages (41,42). We found that the prostate of Wt mice displayed few cells positively stained for 8-OH-dG in the nuclei, and such a basal level remained unchanged in Ptenp−/− mice on either AIN93M or δT3 diet (Supplementary Figure 4). The nitrotyrosine staining was negative in the prostate of Wt and Ptenp−/− mice (Supplementary Figure 4). These results demonstrated that oxidative stress was not significantly altered in the prostate of Ptenp−/− mice, nor is it affected by δT3, suggesting that oxidative stress is not involved in prostate carcinogenesis in this model and the inhibition of δT3 is independent of its antioxidant activity.
5. The δT3-induced transcriptome alterations in the prostate of Ptenp−/− mice.
To explore the mechanisms of the in vivo action, we studied the transcriptomes of the prostates of Ptenp−/− mice on AIN93M or δT3 diet by quantification RNA-sequencing using the RNA extracted from the dorsal-lateral-ventral lobes collected at 20 weeks of age. The reason for using tissues at this time point was that the actions of δT3 at earlier time point were expected to be critical in inhibiting adenocarcinoma development. The result showed that the expression levels of a large number of genes were altered by δT3 (the raw and processed RNA sequencing data were deposited in NCBI (www.ncbi.nlm.nih.gov/geo/; GEO accession No. GSE182980). These included 85 and 525 genes up- and down-regulated by ≥0.5 log2 fold change in the δT3 group (n=6 in each group; P-value < 0.05) (Supplementary Tables 1 and 2). The numbers of altered genes were increased to 189 up- and 1033 down-regulated genes when ≥0.3 log2 fold change was used as the cut-off threshold. We performed pathway analysis using these 189 up-regulated and 1033 down-regulated genes by Metascape (metascape.org; (43)). Among top 20 enriched pathways (Figure 3A), we found that the δT3-induced transcriptome alterations significantly impacted vasculature development, inflammatory response, and negative regulation of cell proliferation, which could play critical roles in adenocarcinoma development (Figure 2A).
Figure 3. Top 20 pathways and processes enriched by δT3-regulated genes in the prostate of Ptenp−/− mice.
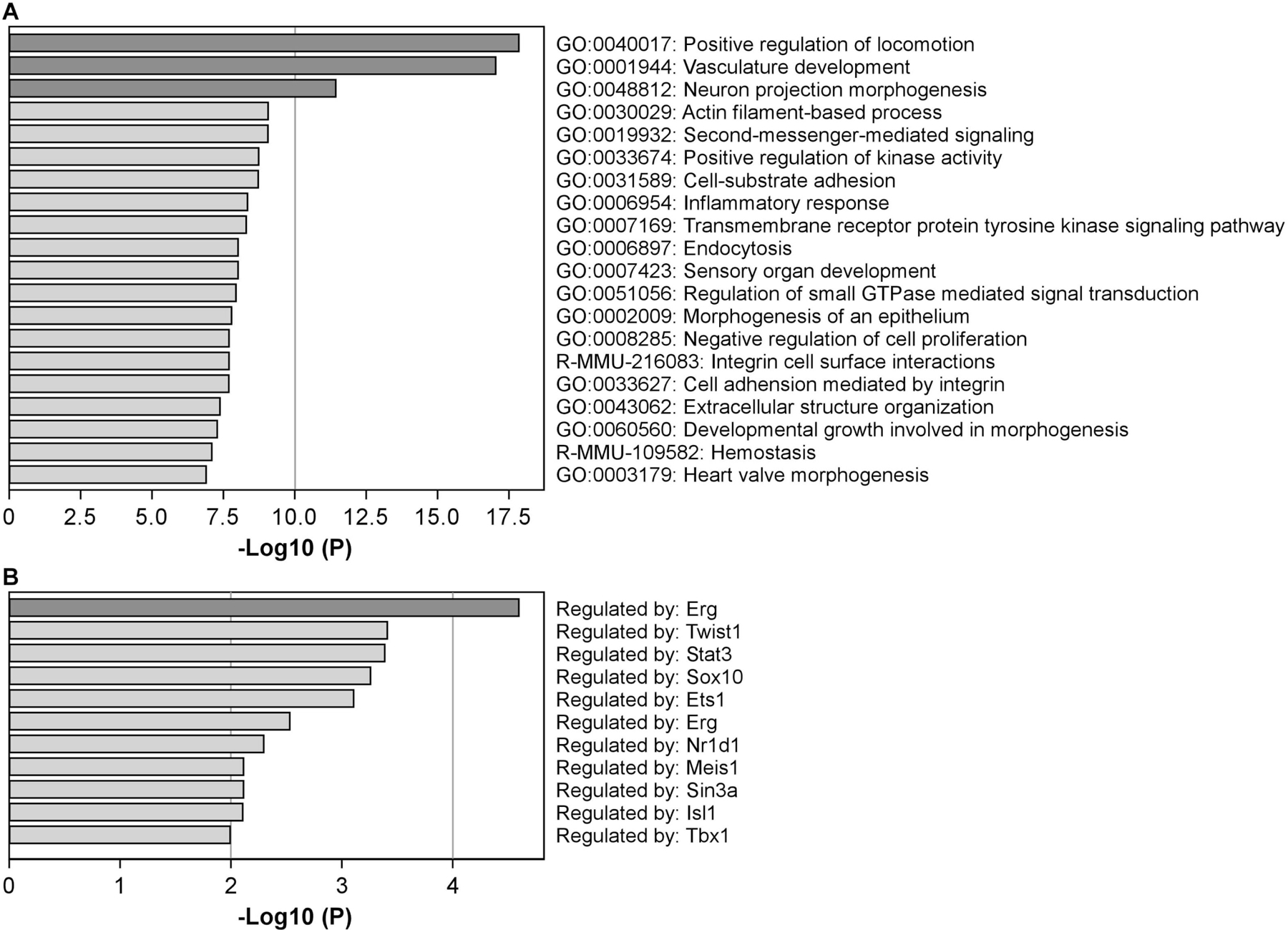
(A) Top 20 pathways and processes that were enriched by 189 up-regulated and 1033 down-regulated genes by δT3 were identified by Metascape. Log10(P) is the P-value in log base 10. (B) The transcription regulatory networks enriched by δT3-regulated genes (P-value ≤ 0.01) were revealed by the TRRUST analysis in Metascape.
Furthermore, transcriptional regulatory networks were analyzed by TRRUST (transcriptional regulatory relationships unraveled by sentence-based text-mining) (44) using Metascape. The result suggests that the δT3-regulated genes enrich the targets of Erg1, Twist1, Stat3, Sox10, Ets1, Nr1d1, Meis1, Sin3a, Isl1, Tbx1 and Irf8 (Figure 3B), indicating that gene regulations by these transcription factors are affected by δT3.
6. δT3 inhibited the angiogenesis in the prostate of Ptenp−/− mice.
Since angiogenesis is one of the hallmarks of cancer (45), the above pathway analysis result indicated that cancer preventive activity of δT3 could involve an anti-angiogenpiesis mechanism. The genes such as endothelial cell specific markers CD31/Pecam1 and Vwf as well as endothelial cell growth factor Vegfd were significantly reduced in the δT3 group, while other major endothelial cell growth factors such as Vegfa, Vegfb, and Vegfc, as well as house-keeping genes Gapdh and β-actin, were not changed (Figure 4A). The reduced expressions of CD31/Pecam1, Vwf, and Vegfd were validated by real-time PCR (Figure 4B – D). These data suggest that δT3 reduces the expression of Vegfd and development of blood vessels.
Figure 4. The expression levels of endothelial cell markers and angiogenesis factors in the prostates of Ptenp−/− mice on AIN93M and δT3 diets at 20 weeks.

(A) The expression levels of β-actin, Gaphd, CD31/Pecam1, Vwf, Vegfa, Vegfb, Vegfc, and Vegfd in the prostates of Ptenp−/− mice on AIN93M and δT3 diets were obtained from the normalized results of the quantification RNA-sequencing. *, **, *** indicate the difference between δT3 and AIN93M groups with statistical significance (n=6 in each group; P-value = 0.0175, 0.0180 and 0.0234, respectively). (B-D) The expression levels of CD31/Pecam1, Vwf and Vegfd were validated by qPCR. The data presented in this figure are normalized by the levels of β-actin. * indicates the difference between δT3 and AIN93M groups with statistical significance (n=6 in each group; P-value < 0.001).
To further characterize the anti-angiogenesis action of δT3, we performed IHC staining of CD31/Pecam1 using prostate samples collected at 40 weeks. In Wt mice, the blood vessels marked by CD31/Pecam1 were present exclusively in the stroma of the prostate (Figure 5A). In Ptenp−/− mice, there were significant numbers of blood vessels developed inside lesioned glands of the dorsal-lateral-ventral lobes (Figure 5B) whereas blood vessels of the anterior lobes remained in the stroma (Supplementary Figure 5). By counting the numbers of blood vessels in the dorsal-lateral-ventral lobes of Ptenp−/− mice using an established method for characterizing the microvessel density in cancerous tissues (37), we found that blood vessel counts were reduced significantly in lesioned glands, but not in the stroma, of the mice on δT3 diet (Figure 5C–G), suggesting that δT3 inhibited tumorigenesis-associated angiogenesis.
Figure 5. The IHC staining of CD31/Pecam1 in the prostates of Ptenp−/− mice on AIN93M and δT3 diets at 40 weeks.

The blood vessels in the prostate of Wt and Ptenp−/− mice at 40 weeks age were identified by the IHC staining of C31/Pecam1. Representative images of the prostate of Wt (A) and Ptenp−/− (B) mice were shown in this figure (S-stromal area; G-lesioned gland area). Representative images of the blood vessel-rich stroma (C and E) and lesioned glands (D and F) in the prostate of Ptenp−/− mice on AIN93M and δT3 diet were also shown in this figure. Microvessel densities (the averages of blood vessel counts) in the stroma and lesioned glands of the mice on AIN93M (n=15) and δT3 (n=13) diet were summarized in G. The scale bar represents 50 μm. * indicates the difference between δT3 and AIN93M groups with statistical significance (P-value < 0.001).
As a comparison, we analyzed the anti-angiogenesis activity of δT by performing IHC staining of CD31/Pecam1 using the prostates of Ptenp−/− mice on AIN93M or 0.2% δT diet at 40 weeks of age collected in a previous study (24). In 5 selected samples whose adenocarcinoma multiplicities were significantly reduced by δT and 5 randomly selected controls, we found that blood vessel counts in the stroma and lesioned glands were not changed by δT (Supplementary Figure 6). Therefore, our data suggest that δT3, but not δT, displays anti-angiogenesis activity.
7. δT3 attenuated the activation of Erk1/2.
To explore the regulation of Vegfd expression by δT3, we examined the promoter of mouse Vegfd gene for the regulatory elements targeted by the transcriptional factors revealed above by TRRUST analysis. Three putative Ets1 binding sites were identified in the promoter, a ~300 bp fragment identified by Encyclopedia of DNA Elements (ENCODE) (Supplementary Figure 7), indicating that Ets1 regulates Vegfd expression, consistent with the well-recognized roles of Ets1 in promoting angiogenesis (46). Because Ets1 acts as an effector of the Ras/MAP kinase signal (47), we wondered whether δT3 affects Ets1 by interfering the activation of MAP kinase. We challenged DU145 cells with IGF1 after the cells had been cultured in the medium with δT3, αT, or DMSO for 12 hours, and determined the levels of the active/phosphorylated MAP kinase Erk1/2 (pErk1/2). We found that δT3 significantly reduced the IGF1-induced activation of Erk1/2 (Figure 6A–B). We further determined the levels of pErk1/2 in the prostates collected from mice at 20 weeks of age and found that the average ratio of pERK1/2 to total Erk1/2 appeared to be lowered in the δT3 group, but statistically not significant due to the very low pErk1/2 in one control sample (Figure 6C–D). By IHC staining of pErk1/2 using the prostates collected at 40 weeks, we found that the average staining intensities of pErk1/2 showed a significant reduction in δT3 group (Figure 6E–F). Together, these data suggest that δT3 attenuated the activation of Erk1/2 and its effector Ets1, resulting in a reduced expression of Vegfd.
Figure 6. δT3 attenuated the activation of Erk1/2.
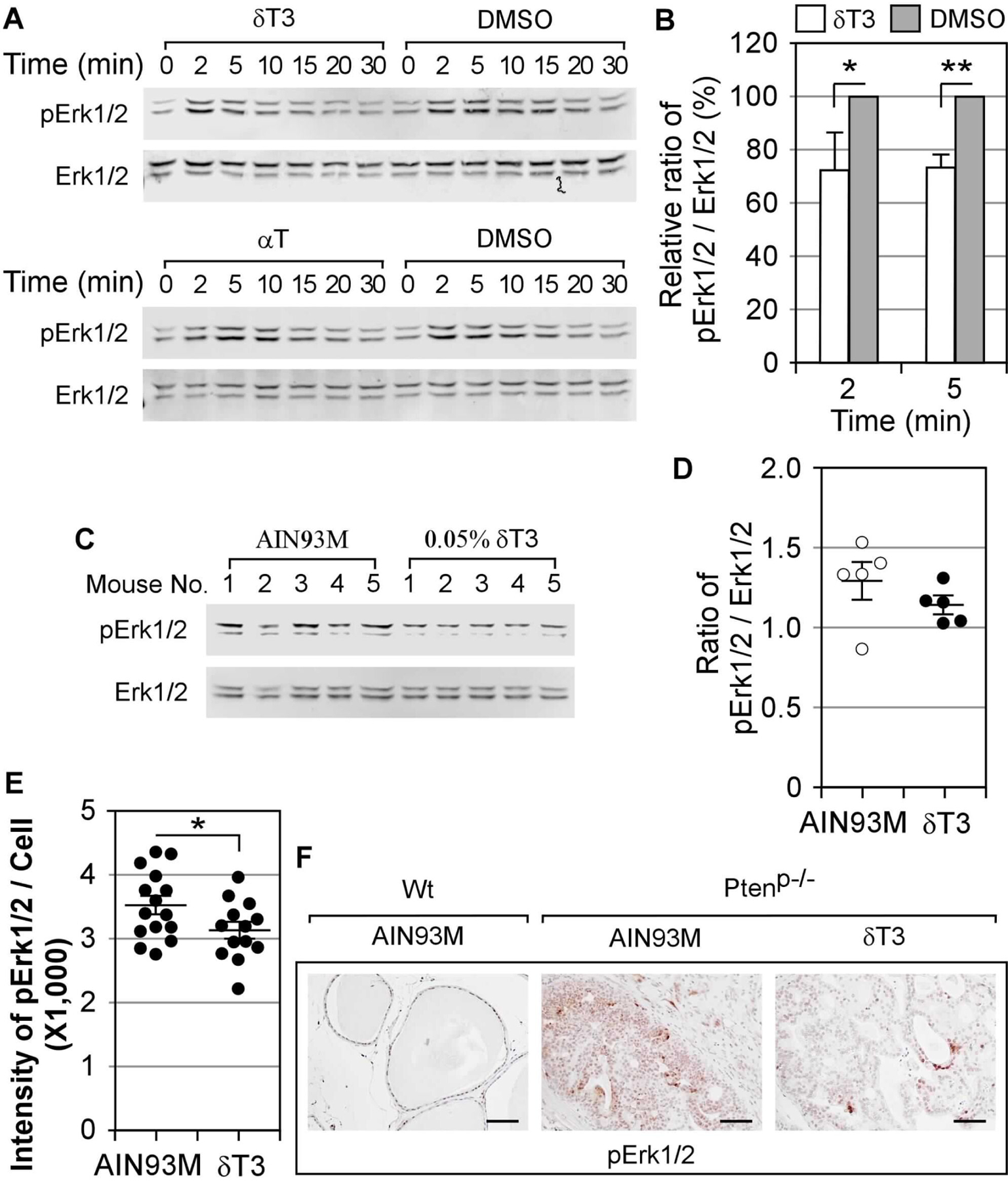
(A-B) DU145 cells were challenged with IGF1 (10 ng/ml) after cultured in the medium with δT3 (5 μM), αT (20 μM), or DMSO for 12 hours. The activation of Erk1/2 was determined by western blot analyses using rabbit antibody against pErk1/2 and mouse antibody against Erk1/2 plus IRDye 680RD-labbeled donkey anti-rabbit and IRDye 800CW-labbeled donkey anti-mouse antibodies. Representative results are shown in A. The fold increases of the ratio of pErk1/2 to Erk1/2 at the peak time points (2 and 5 min), compared to the control (0 min), in three individual experiments (the uncropped blot images were provided in Supplementary Figure 8) using cells treated with δT3 or DMSO was summarized in B (* and ** indicate the differences between two groups with statistical significance; P-value = 0.0311 and 0.0093, respectively). (C-D) The western blot results of pErk1/2 and Erk1/2 in the prostates collected at 20 weeks from Ptenp−/− mice on AIM83M or 0.05% δT3 diet are shown in C. The ratios of pERK1/2 to ERK1/2 were summarized in D. (E-F) The activation of Erk1/2 was determined in the prostates collected at 40 weeks by IHC staining of pErk1/2. The average staining intensities were quantified and are presented in E (n=15 and 13 for AIN93M and δT3 groups, respectively; * indicates the differences between two groups with statistical significance; P-value = 0.0479). Representative images of pErk1/2 stained prostate samples of Wt and Ptenp−/− mice were shown in F. The scale bars in F represent 50 μm.
Discussion
In this study, we demonstrated that δT3 was the most potent vitamin E form in inhibiting the growth of prostate cancer cells, and 0.05% δT3 in diet effectively inhibited prostate cancer development in Ptenp−/− mice. We found that δT3 reduced cell proliferation and increased apoptosis in the prostates of Ptenp−/− mice. Further studies showed that δT3 inhibited tumor-associated angiogenesis, which is expected to contribute to the inhibition of prostate cancer development.
Multiple mechanisms, including antioxidant-dependent and -independent actions, have been proposed for cancer prevention by vitamin E compounds. Protection of cells from oxidative stress-induced damages by δT and γT has been found to be associated with reduced chemical carcinogenesis in the prostate, colon, and mammary gland of rodents (12,13,20,27,40,48,49). In these models, carcinogen-induced oxidative stress probably plays critical roles in carcinogenesis, and reducing oxidative stress by tocopherols is expected to be effective in the prevention. The antioxidant activity may potentially explain the cancer preventive activities of δT and γT but not the ineffectiveness of αT. Cancer prevention by δT and γT have also been attributed to several antioxidant-independent actions which are not found in αT. These actions include inhibition of COX-2 by γT and its metabolites, upregulation of PPARγ by γT, modulation of the ERα activity by γT, and inhibition of the Akt activation by δT (12,13,23,50)). Another possibility for the higher activity of non-αT vitamin E forms than αT is the fact that non-αT vitamin E are not effectively transported out of the liver and are metabolized by side chain cleavage, and the side-chain degradation products may be more active (12,13). Antioxidant-independent mechanisms have also been proposed for tocotrienols involving inhibition of cellular signalings of Wnt, NF-κB, Notch, Akt/mTOR, Stat3, Src, and Her3/Her4, and upregulation of Egr1, miR-34a, p27Kip1, PPARγ, Bax, and other pro-apoptosis genes in cell lines (13,50). Some of these actions could contribute to the cancer preventive activities of tocotrienols, and the relative importance of an action depends on the carcinogenesis mechanisms in the experimental systems.
In this study, cancer development in Ptenp−/− mice is driven by the loss of tumor suppressor Pten, and our data did not show the involvement of oxidative stress. We found that δT3 attenuated the activation of MAP kinase Erk1/2 in prostate cancer cells. Since Ras/MAP kinase pathway regulates various cellular processes, including cell proliferation and apoptosis (51), the inhibition on Erk1/2 by δT3 could be critical in reducing proliferation and promoting apoptosis. Moreover, this finding is in line with the δT3-induced transcriptome changes suggesting that the gene regulation by Ets1, a Ras/MAP kinase effector, is interfered by δT3. Since Ets1 plays critical roles in promoting angiogenesis (46) and there are three putative Ets1 binding sites in Vegfd promoter, it is highly likely that the transcription of Vegfd is regulated by Ets1; therefore, δT3 could reduce Vegfd expression by interfering Ets1 through attenuating the activation of Erk1/2. Although the regulation of Vegfd expression remains to be defined, this possibility provides a working model for further investigating anti-angiogenesis mechanism of δT3.
How δT3 attenuates the activation of Erk1/2 is unclear. This action may be due to its incorporation into lipid membrane, which changes the membrane properties. Cell signalings initiated on the cell surface, including the activation of receptor tyrosine kinases that trigger Akt and Ras/MAP kinase, are believed to be integrated in cholesterol/sphingolipid-rich membrane, termed lipid raft, which functions as docking and trafficking domain for cell surface receptors (52). It has been reported that γT3 disrupted lipid raft, resulting in the inhibition of the activation of HER2 and growth of HER2+ breast cancer (53,54). We found that the ligand-induced internalization of receptor tyrosine kinase was inhibited by δT presumably by interfering lipid raft-mediated trafficking (23). The higher activities of tocotrienols than tocopherols may be attributed to the higher fluidity of the unsaturated isoprenyl side chain of tocotrienols in the membrane (13).
Our finding of that δT3 reduced the Vegfd expression and inhibited angiogenesis in lesioned glands is consistent with the anti-angiogenesis activity of δT3 or γT3 observed in xenograft tumors of pancreatic cancer cell line (55), gastric cancer cell line (56), colon cancer cell line (57), and liver cancer cell line in immunodeficient mice (58), as well as pancreatic tumors developed in transgenic mice (59). Our finding is in agreement with the reports that δT3 or γT reduced expression of angiogenesis factors such as Vegfa (56,59) and Angpt1 (60) in cancer cells treated with δT3 or γT3. However, direct inhibition on endothelial cells by γT3 through blocking the activation of Vegfr2 in endothelial cells has also been reported by others (58,61).
Prostate cancer is generally believed to develop from earlier non-invasive PINs to adenocarcinoma and further progress to metastatic cancer (reviewed in (62)). Ptenp−/− mice develop PIN rapidly and have a long latency before progressing to adenocarcinoma, which is rarely metastatic (35). Such a long latency provides an opportunity to study the prevention of the development of adenocarcinoma by δT3. However, the Ptenp−/− mouse model is not a suitable model to study the prevention of PIN and metastasis (24).
In summary, we demonstrated that δT3 inhibited the development of prostate adenocarcinoma from PIN in Ptenp−/− mice, and this is associated with the increased apoptosis and inhibited proliferation and angiogenesis in lesioned prostate glands. Since genetic alterations of PTEN are the most common drivers of human prostate cancer (63,64), the results of our study using a relevant animal model suggest the potential of the use of δT3 in prostate cancer prevention in humans.
Supplementary Material
Prevention Relevance Statement.
We demonstrated that δ-tocotrienol is the most active vitamin E form in inhibiting the growth of several prostate cancer cell lines. In transgenic Ptenp−/− mice, δ-tocotrienol inhibited the formation of prostate cancer. This result would encourage and help design clinical studies for the application of δ-tocotrienol for prostate cancer prevention.
Acknowledgements
We thank Dr. Ronald DePinho for kindly providing Fvb background prostate-specific Pten knockout mice. We thank Davos Life Science Pte for generously providing tocotrienols. We thank Margareta Zhang for technical assistance. This study was supported by the John L. Colaizzi Chair Endowment Fund (to C.S. Yang).
Footnotes
Conflicts of interest:
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Eitenmiller RR, Lee J. Vitamin E: food chemistry, composition, and analysis. CRC Press; 2005. [Google Scholar]
- 2.Shahidi F, de Camargo AC. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. International journal of molecular sciences 2016;17(10):1745–73 doi 10.3390/ijms17101745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Quinn PJ. Vitamin E and its function in membranes. Progress in lipid research 1999;38(4):309–36 doi 10.1016/s0163-7827(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 4.Patel A, Liebner F, Netscher T, Mereiter K, Rosenau T. Vitamin E chemistry. Nitration of non-alpha-tocopherols: products and mechanistic considerations. J Org Chem 2007;72(17):6504–12. [DOI] [PubMed] [Google Scholar]
- 5.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci U S A 1997;94(7):3217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci U S A 1993;90(5):1771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med 2002;33(11):1534–42. [DOI] [PubMed] [Google Scholar]
- 8.Bjørneboe A, Bjørneboe GE, Drevon CA. Absorption, transport and distribution of vitamin E. J Nutr 1990;120(3):233–42 doi 10.1093/jn/120.3.233. [DOI] [PubMed] [Google Scholar]
- 9.Schneider C Chemistry and biology of vitamin E. Mol Nutr Food Res 2005;49(1):7–30 doi 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- 10.Rigotti A. Absorption, transport, and tissue delivery of vitamin E. Mol Aspects Med 2007;28(5–6):423–36 doi 10.1016/j.mam.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Qian J, Morley S, Wilson K, Nava P, Atkinson J, Manor D. Intracellular trafficking of vitamin E in hepatocytes: the role of tocopherol transfer protein. J Lipid Res 2005;46(10):2072–82. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Q Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med 2014;72:76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang CS, Luo P, Zeng Z, Wang H, Malafa M, Suh N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol Carcinog 2020;59(4):365–89 doi 10.1002/mc.23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein SJ, Peters U, Ahn J, Friesen MD, Riboli E, Hayes RB, et al. Serum alpha-tocopherol and gamma-tocopherol concentrations and prostate cancer risk in the PLCO Screening Trial: a nested case-control study. PLoS One 2012;7(7):e40204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang HY, Alberg AJ, Norkus EP, Hoffman SC, Comstock GW, Helzlsouer KJ. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol 2003;157(4):335–44 doi 10.1093/aje/kwf210. [DOI] [PubMed] [Google Scholar]
- 16.Key TJ, Appleby PN, Travis RC, Albanes D, Alberg AJ, Barricarte A, et al. Carotenoids, retinol, tocopherols, and prostate cancer risk: pooled analysis of 15 studies. Am J Clin Nutr 2015;102(5):1142–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. Jama 2005;294(1):56–65. [DOI] [PubMed] [Google Scholar]
- 18.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. Jama 2009;301(1):52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama 2009;301(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 2010;31(4):533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang CS, Suh N. Cancer prevention by different forms of tocopherols. Top Curr Chem 2013;329:21–33. [DOI] [PubMed] [Google Scholar]
- 22.Yang CS, Chen JX, Wang H, Lim J. Lessons learned from cancer prevention studies with nutrients and non-nutritive dietary constituents. Mol Nutr Food Res 2016;60(6):1239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Hong J, Yang CS. δ-Tocopherol inhibits receptor tyrosine kinase-induced AKT activation in prostate cancer cells. Mol Carcinog 2016;55(11):1728–38 doi 10.1002/mc.22422. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Yang X, Liu A, Wang G, Bosland MC, Yang CS. δ-Tocopherol inhibits the development of prostate adenocarcinoma in prostate specific Pten−/− mice. Carcinogenesis 2018;39(2):158–69 doi 10.1093/carcin/bgx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das Gupta S, Sae-tan S, Wahler J, So JY, Bak MJ, Cheng LC, et al. Dietary gamma-Tocopherol-Rich Mixture Inhibits Estrogen-Induced Mammary Tumorigenesis by Modulating Estrogen Metabolism, Antioxidant Response, and PPARgamma. Cancer Prev Res (Phila) 2015;8(9):807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smolarek AK, So JY, Thomas PE, Lee HJ, Paul S, Dombrowski A, et al. Dietary tocopherols inhibit cell proliferation, regulate expression of ERalpha, PPARgamma, and Nrf2, and decrease serum inflammatory markers during the development of mammary hyperplasia. Mol Carcinog 2013;52(7):514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smolarek AK, So JY, Burgess B, Kong AN, Reuhl K, Lin Y, et al. Dietary administration of delta- and gamma-tocopherol inhibits tumorigenesis in the animal model of estrogen receptor-positive, but not HER-2 breast cancer. Cancer Prev Res (Phila) 2012;5(11):1310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang CS, Li G, Yang Z, Guan F, Chen A, Ju J. Cancer prevention by tocopherols and tea polyphenols. Cancer Lett 2013;334(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang CS, Suh N, Kong AN. Does vitamin E prevent or promote cancer? Cancer Prev Res (Phila) 2012;5(5):701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomsen CB, Andersen RF, Steffensen KD, Adimi P, Jakobsen A. Delta tocotrienol in recurrent ovarian cancer. A phase II trial. Pharmacol Res 2019;141:392–6 doi 10.1016/j.phrs.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Constantinou C, Charalambous C, Kanakis D. Vitamin E and cancer: an update on the emerging role of gamma and delta tocotrienols. Eur J Nutr 2020;59(3):845–57 doi 10.1007/s00394-019-01962-1. [DOI] [PubMed] [Google Scholar]
- 32.Guan F, Li G, Liu AB, Lee MJ, Yang Z, Chen YK, et al. delta- and gamma-tocopherols, but not alpha-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev Res (Phila) 2012;5(4):644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature 2011;470(7333):269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ittmann M, Huang J, Radaelli E, Martin P, Signoretti S, Sullivan R, et al. Animal models of human prostate cancer: the consensus report of the New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res 2013;73(9):2718–36 doi 10.1158/0008-5472.Can-12-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svensson RU, Haverkamp JM, Thedens DR, Cohen MB, Ratliff TL, Henry MD. Slow disease progression in a C57BL/6 pten-deficient mouse model of prostate cancer. Am J Pathol 2011;179(1):502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li G, Wang H, Liu AB, Cheung C, Reuhl KR, Bosland MC, et al. Dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced prostate carcinogenesis in CYP1A-humanized mice. Cancer Prev Res 2012;5(7):963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med 1991;324(1):1–8 doi 10.1056/nejm199101033240101. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Wang DH, Yang X, Sun Y, Yang CS. Colitis-induced IL11 promotes colon carcinogenesis. Carcinogenesis 2021;42(4):557–69 doi 10.1093/carcin/bgaa122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Spandidos A, Wang H, Seed B. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic acids research 2012;40(Database issue):D1144–9 doi 10.1093/nar/gkr1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JX, Li G, Wang H, Liu A, Lee MJ, Reuhl K, et al. Dietary tocopherols inhibit PhIP-induced prostate carcinogenesis in CYP1A-humanized mice. Cancer Lett 2016;371(1):71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009;27(2):120–39. [DOI] [PubMed] [Google Scholar]
- 42.Ibitoye R, Kemp K, Rice C, Hares K, Scolding N, Wilkins A. Oxidative stress-related biomarkers in multiple sclerosis: a review. Biomark Med 2016;10(8):889–902 doi 10.2217/bmm-2016-0097. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nature communications 2019;10(1):1523 doi 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han H, Shim H, Shin D, Shim JE, Ko Y, Shin J, et al. TRRUST: a reference database of human transcriptional regulatory interactions. Sci Rep 2015;5:11432 doi 10.1038/srep11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–74 doi 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Dittmer J The biology of the Ets1 proto-oncogene. Mol Cancer 2003;2:29 doi 10.1186/1476-4598-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci 1998;23(6):213–6 doi 10.1016/s0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- 48.Chen JX, Liu A, Lee MJ, Wang H, Yu S, Chi E, et al. delta- and gamma-tocopherols inhibit phIP/DSS-induced colon carcinogenesis by protection against early cellular and DNA damages. Mol Carcinog 2016;13(10):172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das Gupta S, So JY, Wall B, Wahler J, Smolarek AK, Sae-Tan S, et al. Tocopherols inhibit oxidative and nitrosative stress in estrogen-induced early mammary hyperplasia in ACI rats. Mol Carcinog 2015;54(9):916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Constantinou C, Charalambous C, Kanakis D. Vitamin E and cancer: an update on the emerging role of γ and δ tocotrienols. European journal of nutrition 2020;59(3):845–57 doi 10.1007/s00394-019-01962-1. [DOI] [PubMed] [Google Scholar]
- 51.Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med 2020;19(3):1997–2007 doi 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mollinedo F, Gajate C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: implications in tumor progression and therapy: Thematic Review Series: Biology of Lipid Rafts. J Lipid Res 2020;61(5):611–35 doi 10.1194/jlr.TR119000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alawin OA, Ahmed RA, Dronamraju V, Briski KP, Sylvester PW. gamma-Tocotrienol-induced disruption of lipid rafts in human breast cancer cells is associated with a reduction in exosome heregulin content. J Nutr Biochem 2017;48:83–93 doi 10.1016/j.jnutbio.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 54.Alawin OA, Ahmed RA, Ibrahim BA, Briski KP, Sylvester PW. Antiproliferative effects of γ-tocotrienol are associated with lipid raft disruption in HER2-positive human breast cancer cells. The Journal of nutritional biochemistry 2016;27:266–77. [DOI] [PubMed] [Google Scholar]
- 55.Husain K, Centeno BA, Coppola D, Trevino J, Sebti SM, Malafa MP. δ-Tocotrienol, a natural form of vitamin E, inhibits pancreatic cancer stem-like cells and prevents pancreatic cancer metastasis. Oncotarget 2017;8(19):31554–67 doi 10.18632/oncotarget.15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manu KA, Shanmugam MK, Ramachandran L, Li F, Fong CW, Kumar AP, et al. First evidence that γ-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-κB pathway. Clin Cancer Res 2012;18(8):2220–9 doi 10.1158/1078-0432.Ccr-11-2470. [DOI] [PubMed] [Google Scholar]
- 57.Shibata A, Nakagawa K, Tsuduki T, Miyazawa T. δ-Tocotrienol treatment is more effective against hypoxic tumor cells than normoxic cells: potential implications for cancer therapy. J Nutr Biochem 2015;26(8):832–40 doi 10.1016/j.jnutbio.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Siveen KS, Ahn KS, Ong TH, Shanmugam MK, Li F, Yap WN, et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014;5(7):1897–911 doi 10.18632/oncotarget.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Husain K, Centeno BA, Chen DT, Hingorani SR, Sebti SM, Malafa MP. Vitamin E δ-tocotrienol prolongs survival in the LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) transgenic mouse model of pancreatic cancer. Cancer Prev Res (Phila) 2013;6(10):1074–83 doi 10.1158/1940-6207.Capr-13-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang KD, Liu J, Russell PJ, Clements JA, Ling MT. Gamma-Tocotrienol Induces Apoptosis in Prostate Cancer Cells by Targeting the Ang-1/Tie-2 Signalling Pathway. International journal of molecular sciences 2019;20(5) doi 10.3390/ijms20051164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Sun WG, Liu HK, Qi GY, Wang Q, Sun XR, et al. γ-Tocotrienol inhibits angiogenesis of human umbilical vein endothelial cell induced by cancer cell. J Nutr Biochem 2011;22(12):1127–36 doi 10.1016/j.jnutbio.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 62.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev 2010;24(18):1967–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010;18(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161(5):1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA sequencing data, including raw and processed files, underlying this article are available in NCBI GEO at https://www.ncbi.nlm.nih.gov/geo/ and can be accessed with accession No. GSE182980.


