Abstract
Immune cells identify and destroy damaged cells to prevent them from causing cancer or other pathologies by mechanisms that remain poorly understood. Here, we report that the cell cycle inhibitor p21 places cells under immunosurveillance to establish a biological timer mechanism that controls cell fate. p21 activates retinoblastoma protein (Rb)-dependent transcription at select gene promoters to generate a complex bioactive secretome, termed p21-activated secretory phenotype (PASP). The PASP includes the chemokine CXCL14, which promptly attracts macrophages. These macrophages disengage if cells normalize p21 within 4 days, but if p21 induction persists, they polarize towards an M1 phenotype and lymphocytes mount a cytotoxic T cell response to eliminate target cells, including preneoplastic cells. Thus, p21 concurrently induces proliferative arrest and immunosurveillance of cells under duress.
One-sentence summary:
p21 activates Rb to produce a bioactive secretome that places stressed cells under immunosurveillance to set a timer that controls cell fate.
Introduction
Cells in complex multicellular organisms are subject to a myriad of stresses, which can be managed through various cell-autonomous adaptation and repair mechanisms (1). Cells that fail to recuperate, for instance due to severe or prolonged stresses, engage programs that execute regulated cell death or cellular senescence, thereby limiting the risk of neoplastic transformation (2, 3). The cellular senescence program is characterized by permanent cell-cycle withdrawal through the induction of cyclin-dependent kinase inhibitors like p21 and p16, encoded by p21 (CDKN1A) and p16 (CDKN2A), respectively (4–7). In mammals, cellular senescence has also been implicated in biological processes beyond cancer, including development (8, 9), tissue repair (10), aging, and age-related disorders (3, 11–15). Senescent cells presumably exert these functions by virtue of the senescence-associated secretory phenotype (SASP), a diverse collection of secreted factors (SFs) often enriched in immune-modulatory cytokines and chemokines, matrix remodeling enzymes, and growth factors (5, 16–18). Once established, senescent cells can persist in tissues and organs for prolonged periods of time (19), although they can also be recognized and eliminated by lymphocytes through mechanisms that remain ill-defined (4, 16, 20).
We sought to better understand the properties of senescent cells at a molecular mechanistic level by identifying senescence-associated super-enhancer (SASE)-controlled genes. Super-enhancers are large enhancers that bind large amounts of numerous transcription factors (TFs) and co-activators and are highly enriched in certain chromatin modifications (21, 22). Super-enhancers regulate genes with important functions in processes that are cell type-specific or define cell identity (23, 24), which led us to hypothesize that the identification and in-depth characterization of genes that come under the control of super-enhancers with senescence would be particularly informative about the inner workings of this cell fate. In pursuing this idea, we focused our efforts on genes controlled by SASEs that are highly conserved across species, cell types, and senescence-inducing stressors. Our study illuminates that immediate-early cell-autonomous reactions to cellular stress are coordinated with cell non-autonomous responses through p21-mediated changes in the Rb-dependent transcriptional landscape to establish a biological timer mechanism for cell fate decisions.
p21 regulates the SASP through Rb-dependent transcription
In our initial screen, we exposed primary mouse embryonic fibroblasts (MEFs) to three distinct senescence-inducing stressors: γ-irradiation (IR), extensive replication (REP), and oncogene-induced (OI) signaling by overexpression of human KRASG12V (fig. S1). We mapped the common super-enhancer changes as these cells transitioned to a senescent state, and identified the transcriptionally activated genes associated with these super-enhancers (fig. S2, A and B). We uncovered 50 such genes (fig. S2B and tables S1 and S2), three of which were also associated with a SASE in IR-senescent human fetal lung (IMR-90) cells and transcriptionally upregulated in both IR- and OI-senescent IMR-90 cells, including p21 (fig. S2, A to C, and table S3). Importantly, H3K27Ac ChIP-qPCR on OI-senescent cells (SNCs) collected from mouse liver indicated that the SASE identified near the p21 locus was conserved in vivo (fig. S2, D to J).
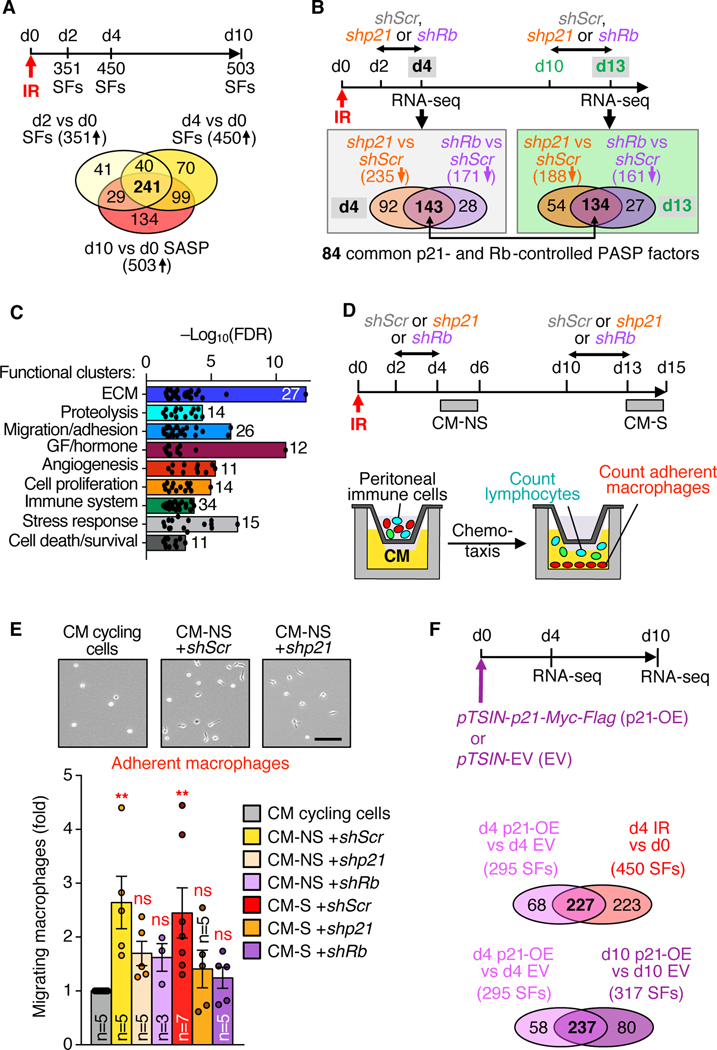
Fully SNCs deficient in p21 incorporated 5-ethynyl-2′-deoxyuridine (EdU) (fig. S3, A to D), indicating that sustaining p21 in the senescent state is important to prevent cell-cycle reentry through continued transcriptional repression of E2F target genes via hypophosphorylation of Rb (25). Intriguingly, knocking down p21 in SNCs also decreased expression of multiple SASP factors as determined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) for a panel of well-established SASP factors (fig. S3E). Comprehensive transcriptomic analysis of IR-senescent MEFs using RNA-sequencing (RNA-seq) revealed that about a third of the SASP (188 of 503 factors) were p21-dependent (Fig. 1A; fig. S4, A to C; and table S4). Similarly, nearly half the SASP (167 of 354 factors) identified in IR-senescent IMR-90 cells were dependent on p21 (Fig. 1A; fig. S4D; and table S4), which prompted us to probe the mechanism(s) underlying these p21-dependent secretory phenotypes, hereafter referred to as the p21-activated secretory phenotype (PASP).
Fig. 1. p21-activated Rb interacts with STAT and SMAD TFs at select gene promoters to establish a bioactive secretome.
(A) Venn diagrams of RNA-seq data depicting downregulated SASP factors with depletion of p21 or Rb in the indicated SNCs. (B) Heatmaps of commonly downregulated SASP factors indicated in (A). FC, fold change. (C) Identification of TFs that transcriptionally activate genes encoding PASP factors using overrepresentation analyses on RNA-seq data from IR-, REP, OI-senescent MEFs, IR-senescent IMR-90 cells, and their non-senescent counterparts. Bolded TFs are significantly activated in SNCs and inhibited upon shp21 and shRb. FDR, false discovery rate. (D) Identification of SASP genes that bind Rb, and TF motif analysis of Rb peaks underlying SFs in OI-senescent IMR-90 cells. (E) Representative Rb occupancy plots at PASP genes. Experiments in the above panels were performed once.
We first focused on Rb and found that its absence in SNCs not only activated E2F target genes (figs. S3, F to I, and S5) but also decreased expression of most of the SASP factors downregulated with p21 deficiency (Fig. 1, A and B; fig. S3J; and table S4), suggesting that p21 confers its effect on the SASP through hypophosphorylation of Rb. To explore how p21-mediated Rb hypophosphorylation might activate SASP genes, we identified TFs that have been linked to the SASP, inflammation, or cytokine production, and used their transcriptional targets in overrepresentation analyses on RNA-seq data from IR-, REP, OI-senescent MEFs, IR-senescent IMR-90 cells, and their non-senescent counterparts. We found that RELA, CEBPβ, SMAD2, SMAD3, STAT1, STAT5A/B, and STAT6 were consistently more transcriptionally active in SNCs than in non-SNCs regardless of senescence-inducing stressor or species (Fig. 1C). RELA, SMAD2, SMAD3, STAT1, and STAT6 lost this status when p21 or Rb were depleted (Fig. 1C). Thus, hypophosphorylated Rb appears to enhance the transcriptional activity of these TFs in SNCs to establish the PASP.
Analysis of publicly available Rb ChIP-seq data from OI-senescent IMR-90 cells (25) revealed that Rb peaks mapped to the promoter regions of 948 SFs and that these peaks were enriched for binding sites of all TFs that we identified as instrumental in establishing the PASP, with the exception of RELA (Fig. 1D and table S5). Rb peaks mapped to promoter regions of 49 of 167 PASP genes identified in IR IMR-90 cells and associated with TFs critical for establishing the PASP (Fig. 1E and table S5). Most of these promoter regions had no such peaks when IMR-90 cells were cycling or quiescent. Furthermore, SMAD2, SMAD3, STAT1, and STAT6 co-immunoprecipitated Rb from IR-senescent MEFs and co-depletion of SMAD2, SMAD3, STAT1, and STAT6 in IR-senescent MEFs reduced transcription of PASP genes where Rb and these TFs colocalize in promoter regions (fig. S6). Thus, a p21-responsive Rb pool interacts with specific SMAD and STAT TFs at PASP gene promoters to enhance their expression.
p21 simultaneously places cells under proliferative arrest and immunosurveillance
To determine whether the PASP is senescence-dependent, we performed RNA-seq on non-senescent MEFs with high p21 collected 2 or 4 days (d2 or d4) post-irradiation (Fig. 2A and fig. S7, A to D). At d2 and d4, IR MEFs upregulated 351 and 450 SFs, respectively, 241 of which were shared with d10 IR MEFs (Fig. 2A and table S6). At d4, IR MEFs depleted for p21 or Rb, lost 235 and 171 of their SFs, respectively, indicating that the PASP is a senescence-independent phenomenon (Fig. 2B and fig. S7, A to D). Eighty-four PASP factors were commonly lost in d4 and d10 IR MEFs when p21 or Rb were depleted, demonstrating that the PASP of non-SNCs becomes an integral part of the SASP as cells advance to a senescent state (Fig. 2B and fig. S7, E and F).
Fig. 2. The p21-activated secretory phenotype places cells under immunosurveillance as they arrest.
(A) Timeline and Venn diagrams based on RNA-seq depicting significantly upregulated SFs. (B) Timeline and Venn diagrams comparing significantly downregulated SFs upon p21 or Rb depletion. (C) Functional annotation analyses of 84 PASP factors indicated in (B) displaying overrepresented functional clusters. GF, growth factor. (D) Schematic of CM production and transwell migration assay of peritoneal immune cells in the presence of CM. (E) Representative images and quantitation of adherent macrophages in the bottom transwell chamber. Scale bar: 100 μm. (F) Venn diagrams depicting significantly upregulated PASP factors. Data represent means ± SEM. Experiments in the above panels were performed once except for (E) where data from three experiments were pooled. ns, not significant. **P<0.01. One-way ANOVA with Sidak’s correction (E).
Functional annotation analysis on the 84 shared PASP factors indicated that several traits of SNCs may be p21-Rb-dependent, including features involving cell migration/adhesion and the immune system (Fig. 2C and fig. S7G). This raises the possibility that the PASP plays a role in immunosurveillance. To test this idea, we determined the extent to which the PASP impacts the migratory behavior of mouse peritoneal immune cells in a transwell system (Fig. 2D). Conditioned medium from d4 non-senescent (CM-NS) or d10 senescent (CM-S) IR MEFs promoted transwell migration of macrophages, a property that was lost with CM-NS and CM-S from p21- or Rb-depleted IR MEFs (Fig. 2E). None of the CMs impacted lymphocyte migration in this assay (fig. S8A). In a second migration assay, macrophage numbers selectively increased in the peritoneal lavage 4 days after intraperitoneal injection of CM-NS, but not after injection of CM-NS from p21- or Rb-depleted IR MEFs (fig. S8, B to F). The PASP also stimulated cell movement in a standard scratch assay on cultured MEFs (fig. S8, G and H), indicating that its promigratory properties extend beyond macrophages. NFκB p65 (RELA) appeared to have no role in establishing the PASP or its macrophage-attractant properties (fig. S9 and table S7).
To determine whether the PASP requires an actual senescence-inducing stressor or merely elevated p21 levels, we transduced MEFs with a lentivirus harboring p21-Myc-Flag (fig. S10A). p21-overexpressing (p21-OE) MEFs were subject to growth arrest, initially without elevated p16 and SA-β-Gal activity (d4), and later with these senescence markers (d10) (fig. S10, B to D). At d4, p21-OE MEFs upregulated 295 SFs, 227 of which were also upregulated in d4 IR MEFs, indicating that p21 induction is sufficient to yield a PASP (Fig. 2F and table S8). SMAD2, SMAD3, STAT1, and STAT6 co-immunoprecipitated Rb from the chromatin fraction of d2 p21-OE MEFs (fig. S10E), further supporting the contention that p21-induced, hypophosphorylated Rb interacts with STAT and SMAD TFs at select gene promoters to establish the PASP. PASP factors of d4 p21-OE MEFs were largely preserved in d10 p21-OE MEFs (Fig. 2F, table S8). Thus, the PASP becomes an integral part of the SASP as cells senesce.
p21 enforces immunosurveillance through the chemokine CXCL14
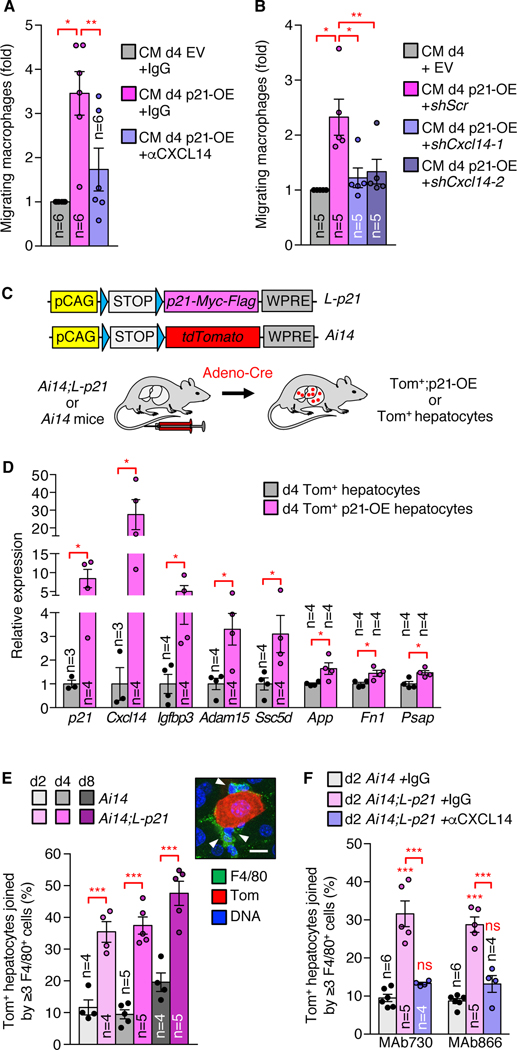
Functional annotation analysis on the PASP of d4 p21-OE MEFs suggested that it has similar biological properties as the PASP of d4 IR-MEFs (fig. S10F). Indeed, CM from d4 p21-OE MEFs stimulated fibroblast migration in our scratch assay and macrophage migration in our transwell assay and increased local macrophage numbers when intraperitoneally injected in mice (fig. S10, G to N). MEF-derived PASPs consistently included Cxcl14 (Fig. 1B and table S8), a member of the CXC chemokine family that acts as a chemoattractant for various immune cell types (26–28). The addition of CXCL14-neutralizing antibodies to CM harvested from d4 p21-OE MEFs ablated stimulation of macrophage migration in our transwell assay, whereas control IgG did not (Fig. 3A and fig. S11A). Moreover, CM from Cxcl14-depleted d4 p21-OE MEFs failed to evoke macrophage migration (Fig. 3B and fig. S11, B and C), further demonstrating that CXCL14 is the key macrophage attractant of the PASP. Complementary experiments in human dermal fibroblasts (HDFs) and human umbilical vein endothelial cells (HUVECs) suggested that the PASP is a common feature of p21 induction and CXCL14 is a signature PASP component (fig. S12).
Fig. 3. p21-induced immunosurveillance requires PASP factor CXCL14.
(A) Transwell migration assay with CM in the presence of CXCL14-neutralizing or IgG antibodies. (B) as in (A) but with CM from shRNA-transduced MEFs. (C) Schematic of the transgenes used for Cre-inducible expression of Myc-Flag-tagged p21 (L-p21 for LoxP/Stop/LoxP-p21) and tdTomato (Ai14) (Top) and the experimental setup for p21-OE induction in hepatocytes via Cre-encoding adenovirus injection (Bottom). (D) RT-qPCR on flow-sorted Tom+ hepatocytes. (E) Representative picture and quantification of Tom+ hepatocytes joined by ≥3 F4/80+ cells. (F) As in (E) but assessing livers from mice treated with CXCL14-neutralizing or IgG control antibodies. Scale bars: 10 μm (E). Data represent means ± SEM. Experiments in the above panels were performed once. ns, not significant. *P<0.05; **P<0.01; ***P<0.001. One-way ANOVA with Sidak’s correction (A, B, E, and F) and unpaired two-tailed t tests (D).
To study the PASP phenomenon at the organismal level, we established a transgenic approach in mice that allows for Cre-inducible coexpression of Myc-Flag-tagged p21 and the fluorescent reporter protein tdTomato (Tom) (Fig. 3C). We induced Myc-Flag-tagged p21 and Tom in ~10% of hepatocytes by tail vein injection of adeno-Cre virus. Hepatocytes with p21 overexpression (p21-OE) were cell-cycle arrested at d4 post-injection and exhibited signs of senescence by d8 post-injection, as evidenced by loss of lamin B1 and nuclear extrusion of HMGB1 (fig. S13, A to C). RNA extracted from FACS-sorted d4 Tom+ hepatocytes with or without p21-OE and used for RT-qPCR analysis of PASP factor gene transcripts indicated that p21-OE induces a PASP in vivo (Fig. 3D and fig. S13D). Cxcl14 was among the upregulated PASP factors, prompting us to test whether p21-OE hepatocytes attract macrophages. Nearly 40% of p21-OE Tom+ hepatocytes were surrounded by three or more macrophages as early as d2 post-adeno-Cre injection versus ~10% of Tom+ hepatocytes without p21-OE (Fig. 3E). Macrophage recruitment to p21-OE hepatocytes was CXCL14-dependent as assessed by injection of anti-CXCL14 antibodies (Fig. 3F). Lymphocytes were also recruited but later, with B and T cells surrounding p21-OE hepatocytes at d4 and d8, respectively (Fig. 4, A and B). By contrast, p21-OE did not prompt recruitment of NK cells (fig. S13E). The number of p21-OE hepatocytes sharply declined by d8, which coincided with a marked increase in dying p21-OE hepatocytes the presence of M1-differentiated macrophages in addition to the presence of both CD4+ and CD8+ T lymphocytes (Fig. 4, C to F, and figs. S13F and S14, A and B). Administration of a CD8α-neutralizing antibody fully prevented the observed decline in d8 p21-OE hepatocytes (fig. S14, C to G), indicating that their elimination is mediated by cytotoxic T cells.
Fig. 4. Hepatocytes under surveillance die upon macrophage differentiation and lymphocyte recruitment.
(A) Representative image and quantification of Tom+ hepatocytes associated with ≥1 B220+ cells. (B) Representative picture and quantification of Tom+ hepatocytes associated with ≥1 CD3ε+ cells. (C) Proportion of Tom+ and healthy (not dying) hepatocytes. (D) Representative picture and quantification of dying Tom+ hepatocytes. (E) Representative picture and quantification of Tom+ hepatocytes associated with ≥1 iNOS+ cells. (F) Quantitation of dying p21-OE Tom+ hepatocytes associated with ≥1 iNOS+ cells. Scale bars: 10 μm (A, B, D, and E). Mice used were on a C57BL/6×129Sv mixed genetic background. Data represent means ± SEM. Experiments in the above panels were performed once. ns, not significant. **P<0.01; ***P<0.001. One-way ANOVA with Sidak’s correction (A to E).
We performed a comparative analysis for overexpression of p16 (p16-OE), a more selective CDK inhibitor, which, unlike p21, only targets G1-CDK activity. At d4, p16-OE MEFs were characterized by growth inhibition, normal p21 levels, and a secretome of 197 factors, 183 of which overlap with the PASP of d4 p21-OE MEFs (fig. S15, A to F, and table S8). Pathway enrichment analyses on the p16-associated secretory phenotype suggested a high degree of similarity in biological properties with the PASP, although the number of immune-system-related annotations was markedly reduced (fig. S15, G and H). CM of d4 p16-OE MEFs failed to promote migration of macrophages in our transwell assay, which correlated with a lack of Cxcl14 induction (fig. S15I). Likewise, using the same transgenic approach as used for p21-OE in mice, we found p16-OE in hepatocytes to trigger cell-cycle arrest but not immunosurveillance, which coincided with a lack of p21 and PASP factor induction, including Cxcl14 (fig. S16). Corresponding analyses of MEFs overexpressing p27, a CDK inhibitor that enables cell-cycle withdrawal during terminal differentiation, revealed that coordinated induction of growth arrest and immunosurveillance is a unique feature of p21 (fig. S17 and table S8).
Oncogene-induced p21 triggers immunosurveillance
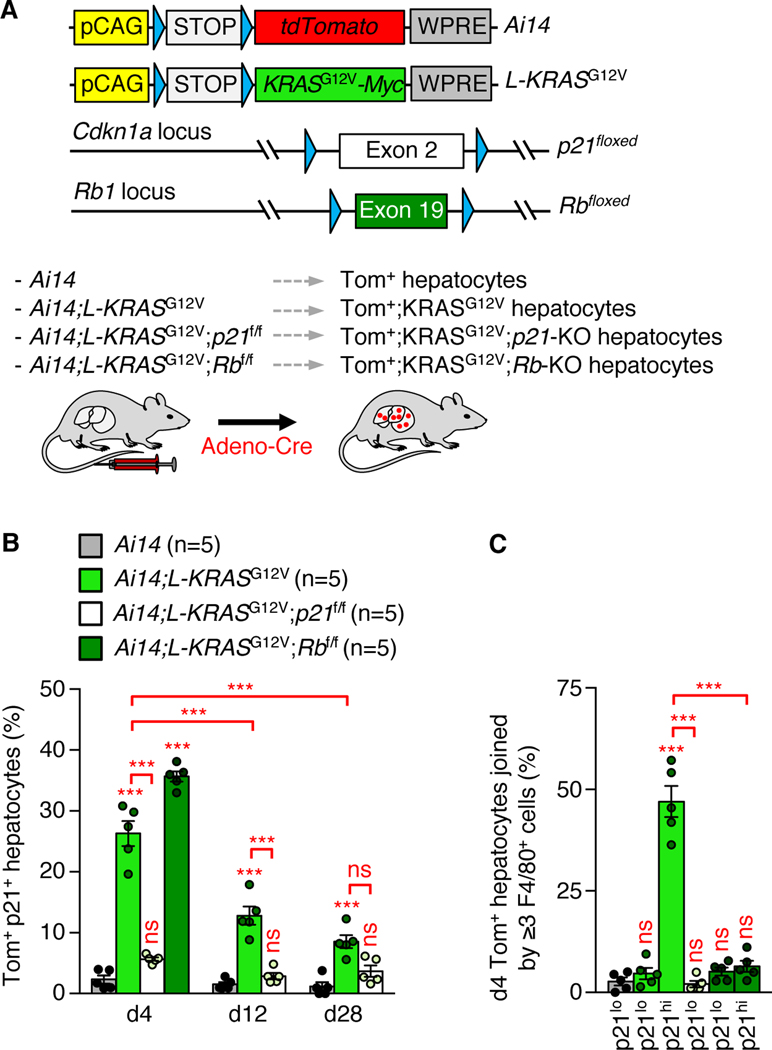
We sought to critically test the physiological relevance of p21-dependent immunosurveillance in a cancer-related context. To this end, we adapted our transgenic approach for co-induction of Tom and p21 in hepatocytes by replacing p21 with KRASG12V (Fig. 5A), an oncoprotein that can induce p21 via mitogenic stress (4). Indeed, approximately 25% of d4 Tom+ KRASG12V hepatocytes had elevated levels of p21 (Fig. 5B and fig. S18A). These hepatocytes attracted macrophages, whereas those that failed to induce p21 did not (Fig. 5C). Use of a newly generated p21-conditional-knockout strain conclusively demonstrated that d4 Tom+ KRASG12V hepatocytes recruit macrophages in a p21-dependent manner (Fig. 5, A to C, and fig. S18B). Furthermore, d4 Tom+ KRASG12V hepatocytes in which Rb was conditionally knocked out retained p21 induction but nevertheless failed to attract macrophages, validating cell culture experiments indicating that p21 places cells under immunosurveillance in an Rb-dependent fashion (Fig. 5, A to C, and fig. S18B).
Fig. 5. p21 induced by oncogenic RAS places cells under immunosurveillance.
(A) (Top) Schematic representation of L-KRASG12V and Ai14 transgenes, and p21- and Rb-conditional knockout alleles. Blue triangles denote LoxP sites. (Bottom) Schematic of the experimental design. (B) Proportion of Tom+ p21+ hepatocytes among Tom+ hepatocytes at indicated days after adeno-Cre injection. (C) Quantification of Tom+ hepatocytes joined by ≥3 F4/80+ macrophages. p21hi, cells with elevated p21 staining; p21lo, cells with baseline or background level p21 staining. Mice used were on a C57BL/6×129Sv mixed genetic background. Data represent means ± SEM. Experiments in the above panels were performed once. ns, not significant. ***P<0.001. Two-way ANOVA with Sidak’s correction (d12 and d28 in B), one-way ANOVA with Sidak’s correction (d4 in B and C).
Hepatocytes from d4 Tom+ KRASG12V mice had a PASP which they lost with conditional inactivation of p21 (Fig. 6A). The PASP included Cxcl14, explaining why d4 Tom+ KRASG12V hepatocytes attract macrophages and their counterparts lacking p21 not. Tom+ hepatocytes numbers remained largely unchanged at d4, d12, and d28 post-induction when KRASG12V was absent (Fig. 6B), but progressively declined due to cell death when KRASG12V was co-expressed (Fig. 6, B and C). However, no such decline occurred when p21 was inactivated upon KRASG12V induction. This was not due to compensatory cell proliferation because p21 inactivation had no impact on the mitotic index of Tom+ KRASG12V hepatocytes (fig. S18, C and D). Consistent with p21-dependent cell elimination, Tom+ KRASG12V hepatocytes with high p21 levels gradually decreased from d4 to d28 (Fig. 5B). At d12, Tom+ KRASG12V hepatocytes were surrounded by M1 macrophages and T lymphocytes, whereas their d4 counterparts were not (Fig. 6, D and E). Thus, p21-mediated macrophage recruitment represents an essential first step towards cell elimination upon KRASG12V induction, which then appears to be executed by subsequently recruited T lymphocytes. This conclusion is supported by the earlier observation that clearance of hepatocytes expressing oncogenic NRAS depends on monocyte/macrophages and CD4 T cells (4).
Fig. 6. p21-dependent immunoclearance protects against oncogenic growth.
(A) RT-qPCR on flow-sorted Tom+ hepatocytes. (B) Proportion of hepatocytes that is Tom+ and appears healthy (not dying). (C) Quantification of dying Tom+ hepatocytes. (D) Quantification of Tom+ hepatocytes joined by ≥1 iNOS+ cells. p21hi, cells with elevated p21 staining; p21lo, cells with baseline or background level p21 staining. (E) As in (D) but for hepatocytes with ≥1 CD3ε+ cells. (F) Representative image and quantitation of Tom+ hepatocyte clusters. (G) Proportion of Tom+ EdU+ hepatocytes in- or outside Tom+ clusters. Scale bar: 20 μm. Mice used were on a C57BL/6×129Sv mixed genetic background. Data represent means ± SEM. Experiments in the above panels were performed once. ns, not significant. *P<0.05; **P<0.01; ***P<0.001. Two-way ANOVA with Sidak’s correction (d12 and d28 in B and C), one-way ANOVA with Sidak’s correction (A and D to F and d4 in B and C) or unpaired two-tailed t test (G).
Regardless of whether p21 was intact or inactivated, Tom+ KRASG12V hepatocytes hardly proliferated and showed signs of cellular senescence from d12 on (fig. S18, C to F). However, small clusters of Tom+ KRASG12V hepatocytes were observed in d28 livers with much higher frequency when p21 was inactivated (Fig. 6F). Hepatocytes within these clusters were cycling at a markedly higher rate than corresponding hepatocytes located in isolation (Fig. 6G). Thus, p21-dependent immunoclearance of cells that experience oncogenic stress constitutes an important first line of defense against neoplastic growth.
p21 engages macrophages to set a biological timer that controls cell fate
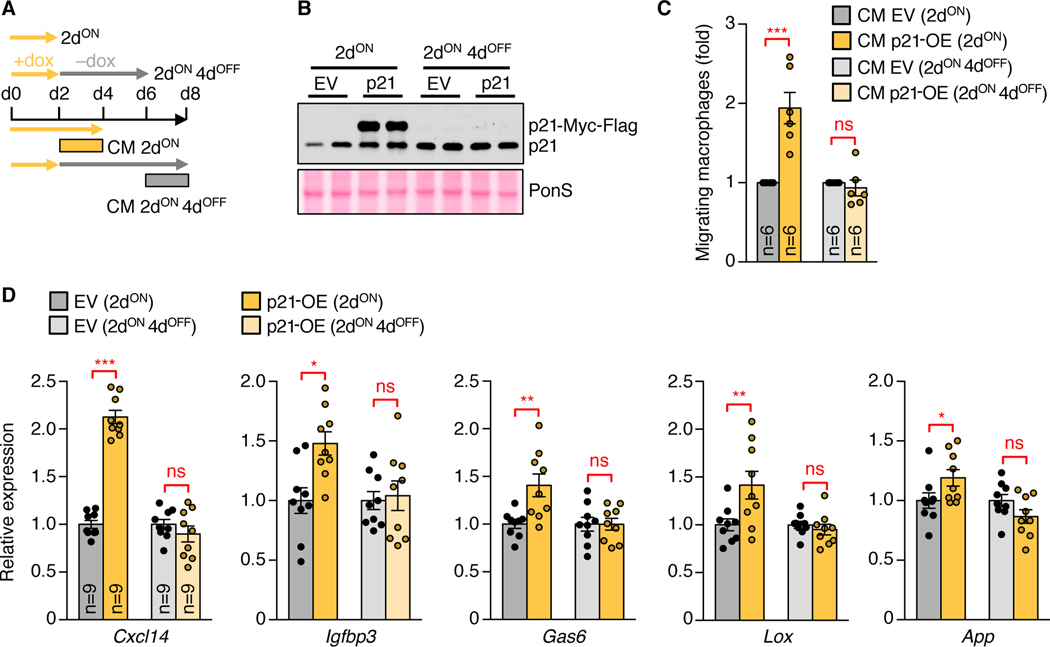
Stress-inducing oncogenic point mutations are irreparable, but many cellular stresses are transient or repairable. To determine whether stressed cells that recuperate and normalize p21 cease to produce a PASP and are released from immunosurveillance, we produced MEFs containing a lentiviral construct that allows for doxycycline (dox)-inducible expression of p21-Myc-Flag. These MEFs stopped proliferating within 2 days after dox administration, but were fully capable of resuming the cell cycle when p21 levels normalized upon dox withdrawal (Fig. 7, A and B, and fig. S19, A to C). CM harvested from d2 p21-OE MEFs stimulated fibroblasts migration in the scratch assay and macrophage migration in the transwell assay with peritoneal immune cells (Fig. 7C and fig. S19, D and E). By contrast, CM prepared from MEFs that had been on dox for 2 days followed by 4 days off dox had no impact on cell migratory properties in these same assays. Cxcl14 expression followed the pro-migratory properties of p21-OE CM, with reduced Cxcl14 expression upon withdrawal corresponding to a more generalized collapse in PASP gene expression (Fig. 7D). Suppression of E2F target genes, proliferative arrest, induction of PASP genes, and chemoattraction of macrophages all occurred within 24 hours after induction of p21 (fig. S19, F to J). Thus, the brake on cell cycle progression and PASP-mediated immune surveillance occur simultaneously and rapidly.
Fig. 7. Cells normalizing p21 cease to produce a PASP and are released from immunosurveillance.
(A) Schematic overview of CM preparations from dox-inducible p21-OE MEFs. (B) Representative immunoblot for p21. PonS served as loading control. (C) Transwell macrophage migration with CM from indicated MEFs. (D) RT-qPCR of the indicated MEFs. Data represent means ± SEM. The blot shown in (B) is representative of 2 independent experiments, and data shown in (C) and (D) were pooled from 2 and 3 experiments, respectively. ns, not significant. *P<0.05; **P<0.01; ***P<0.001. Two-way ANOVA with Sidak’s correction (C and D).
To determine the time allocated to damaged cells to recuperate and avert elimination by immune cells under physiological conditions and to define the underlying timer mechanism, we created transgenic mice in which p21 could be temporally overexpressed in hepatocytes by sequential administration of adeno-Cre injection and dox (Fig. 8A). Macrophages surrounding hepatocytes overexpressing p21 for up to 4 days promptly withdrew upon normalization of p21 (Fig. 8, B and C, and fig. S20, A and B). By contrast, when p21 levels were normalized at day 6, macrophages surrounding hepatocytes failed to disengage. At this stage, hepatocytes were adjoined not only by macrophages that had undergone M1 activation, but also lymphocytes that had been primed for target cell elimination (Fig. 8, D to F). At d6, p21-OE hepatocytes were not yet senescent although some entry into the senescent state occurred during the 2-day off period (fig. S20, C to E). Thus, p21-induction sets a timeframe for stress repair or adaptation that is defined by the time it takes for the immune system to transition from a cell-surveillance to a cell-clearance mode.
Fig. 8. p21 places cells under immunosurveillance to establish a timer mechanism that controls cell fate.
(A) (Top) Schematic representation of the transgenes for dox-inducible repression of Myc-Flag-tagged p21 (iL-p21 for inducible LoxP/Stop/LoxP-p21) and monitoring of ectopic p21 expression status via fluorescent marker proteins (Ai139). Blue triangles denote LoxP sites. (Bottom) Schematic of the experimental design with fluorescent markers for transgenic p21 expression and repression indicated. Rates of p21 overexpression (p21+) among hepatocytes that were positive for Tom and eGFP in the absence of dox (p21-OE “ON”) or only Tom after dox administration (p21-OE “OFF”). (C) Representative image of a p21-OE hepatocyte surrounded by three macrophages, and quantification of fluorescent hepatocytes joined by ≥3 F4/80+ macrophages. (D) Assessment of fluorescent hepatocytes associated with ≥1 iNOS+ cells. (E) As (D) but assessing cells with ≥1 CD3ε+ cells. (F) Representative image of a 6dON+2dOFF dying hepatocyte and quantification of death rates among fluorescent hepatocytes. Scale bars: 10 μm (C and F). Mice used were on a C57BL/6 pure genetic background. Data represent means ± SEM. Experiments in the above panels were performed once. ns, not significant. *P<0.05; **P<0.01; ***P<0.001. Two-way ANOVA with Sidak’s correction (B to F).
Discussion
In response to a wide variety of stress stimuli, p53 induces p21 to halt the cell cycle as one of the hundreds of downstream p53 transcriptional targets. As such, p21 provides time for cells to recuperate through various repair and adaptation mechanisms, or if the damage is too severe, for induction of apoptosis or for cellular senescence. Our studies revealed that p21 also responds to cellular stressors through a cell non-autonomous mechanism by placing cells under immunosurveillance and that p21 does so concomitantly with halting cell cycle progression (fig. S21). These findings extend earlier evidence linking p21 inactivation to immune system deficiencies and predisposition to systemic autoimmunity (29–31). In probing the mechanism, we discovered that the pool of hypophosphorylated Rb that is created in response to p21 induction binds to chromatin to not only establish cell cycle arrest but also to cooperate with select SMAD and STAT TFs to create a bioactive secretome with diverse biological functions, including immunosurveillance.
We found that CXCL14 within this secretome is instrumental in attracting macrophages to cells with elevated p21. By recruiting macrophages, p21 sets a biological timer that allows for a period of repair or adaptation of about 4 days (at least for hepatocytes), which expires when macrophages polarize towards an M1 phenotype and lymphocytes are recruited to mount a cytotoxic T cell response. Therefore, the p21-mediated killing of stressed cells by the leveraging of the immune system represents an important complementary mechanism to p53-mediated apoptosis. Hepatocytes that induce p21 in response to a well-established oncogenic stressor that drives hepatocellular carcinoma are eliminated via this mechanism, underscoring its physiological relevance. Thus, the first line of tumor-protective immunosurveillance appears to already occur at the earliest stages of cellular transformation, complementing established tumor-protective actions of immune cells based on the expression of tumor-specific neoantigens (32). As a transcriptional regulator, p53 has been implicated in diverse aspects of immunity, including tumor-related immune responses (33). The same holds true for Rb, raising the interesting question of the extent to which the roles of these two tumor suppressors are p21-dependent.
Our comparative analysis of p21-OE with p16-OE and p27-OE suggests that coordinated cell cycle arrest and transcriptional activation of select genes is a common feature of cyclin-dependent kinase inhibitors that act through Rb hypophosphorylation. We observed substantial overlap between genes activated by the three inhibitors, but many genes were also uniquely induced. At the surface, this argues against the idea that Rb dephosphorylation is the underlying driver. However, hypophosphorylated Rb appears to be a collection of Rb molecules that are mono-phosphorylated at one of 14 known phosphorylation sites, with each variant having a unique binding-partner profile and a distinctive transcriptional output (34). It is therefore conceivable that p21, p16, and p27 create distinctive collections of mono-phosphorylated Rb molecules, which, through differences in transcription factor binding preferences, activate distinct target genes, thereby yielding secretomes of different complexities and biological properties. Our observation that p16-OE does not induce CXCL14, explains why these cells were not subject to immune clearance. Consistent with this finding, cells undergoing senescence through IFNγ- and TNF-mediated induction of p16 are not subject to elimination (35).
In conclusion, we demonstrate that p21, through Rb hypophosphorylation, induces the macrophage attractant CXCL14 to place cells under immunosurveillance as part of an immediate early response to cellular stress that also includes cell cycle arrest. Our study highlights the role of CXCL14, but the PASP contains several other immune modulatory factors, including IL-7 and IL-34. Both these interleukins induce M1 macrophage differentiation (36, 37), a phenotypic change that we show coincides with the recruitment of cytotoxic T cells to p21-expressing hepatocytes. A greater understanding of IL-7 and IL-34 and other PASP factors in stress-related immunosurveillance could inform innovative therapeutic opportunities for the treatment of cancers, including cancers lacking p53 activity. The observation that low CXCL14 expression correlates with poor clinical outcome in various human cancers, including cervical, colorectal, endometrial, and head and neck cancers (38) underscores the relevance of such future efforts.
Materials and Methods:
Mouse strains
L-KRASG12V mice were generated from KH2 ES (C57BL/6×129Sv) cells according to previously described methods (39) using a modified pBS31 vector. Briefly, the tetracycline-inducible promoter and the SV40 polyA signal in the pBS31 were replaced by a CAG promoter-FRT-loxP-flanked STOP cassette (LoxP-STOP-LoxP, L) and WPRE-bGH-polyA (WPRE-pA) from Ai9 (Addgene, #22799), respectively. The FRT site after the CAG promoter was deleted using site-directed mutagenesis and a multiple cloning site (MCS) was added between L and WPRE-pA. The Myc-tagged human KRASG12V was amplified from pBABE-KRASG12V-puro (Addgene, #9052) and inserted to the MCS. The resultant pBS31-CAG-L-KRASG12V-WPRE-pA plasmid was electroporated into KH2 ES cells and selected clones with Cre-inducible KRASG12V expression were used to generate L-KRASG12V mice according to standard procedures. The same strategy was used to generate L-p21 mice or L-p16 mice using Myc-Flag-tagged cDNAs for mouse p21 or mouse p16 obtained from Origene (#MR227529 or #MR227284, respectively). Obtained founder mice (C57BL/6×129Sv background) were backcrossed to C57BL/6 at least twice before use in breeding for experimentation. To generate iL-p21 transgenic mice on a pure C57BL/6 background, the following targeting construct was cloned: pTRE2-LoxP-STOP-LoxP(LSL)-p21-Myc-Flag-WPRE-pA using the pTRE2 promoter and LSL from the Ai139 transgene (Addgene, #114426) and p21-Myc-Flag from the L-p21 transgene (Origene, #MR227529) as described above. Homology arms spanning 968 bp at the 5′-end and 937 bp at the 3′-end flanked by sgRNA target sites were used to target the construct into the Col1a1 locus of C57BL/6NHsd (Envigo) zygotes using CRISPR-Cas9-mediated gene editing with Cas9 mRNA (Trilink Biotechnologies, #L-7606) and Col1a1-specific sgRNA 5′-GAGGTTCATGAGCCCTCAAA-3′. Obtained founder mice were backcrossed to C57BL/6 once before use for experimentation. To generate p21floxed mice on a pure C57BL/6 background, a targeting vector containing p21 exon 2 flanked by LoxP sites and homology arms spanning 861 bp at the 5′-end and 819 bp at the 3′-end flanked by sgRNA target sites (5′ sgRNA: 5′-TCTTGGTGATTAACTCCATC-3′ and 3′ sgRNA: 5′-CCATAGGCGTGGGACCTCGT-3′) was cloned. The resultant targeting vector was used to target the construct into the p21 locus of C57BL/6NHsd (Envigo) zygotes using CRISPR-Cas9-mediated gene editing with Cas9 mRNA (Trilink Biotechnologies, #L-7606) (40). Obtained founder mice were crossed to C57BL/6 at least once before use for experimentation. Rbfloxed mice (#026563), Ai14 transgenic animals (#007914), and Ai139 transgenic mice (#030219) were purchased from The Jackson Laboratory. The following cohorts were generated for experimentation in this study: Ai14/+ and Ai14/+ L-KRASG12V/+ (C57BL/6×129SV mixed background; fig. S2), Ai14/+ and Ai14/+ L-p21/+ (C57BL/6×129SV mixed background; Figs. 3 and 4, figs. S13 and S14), Ai14/+ and Ai14/+ L-p16/+ (C57BL/6×129SV mixed background; fig. S16), Ai14/+ and Ai14/+ L-KRASG12V/+ and Ai14/+ L-KRASG12V/+ p21floxed/floxed and Ai14/+ L-KRASG12V/+ Rbfloxed/floxed (C57BL/6×129SV mixed background; Figs. 5 and 6 and fig. S18), Ai139/+ and Ai139/+ iL-p21/+ (C57BL/6 pure background; Fig. 8 and fig. S20). Mice were aged until 4 to 6 months of age before use for experimentation unless otherwise noted. Experimental procedures involving laboratory mice were reviewed and approved by the Institutional Animal Care and Use Committee of the Mayo Clinic.
Cell culture
Mouse embryonic fibroblasts (MEFs) were generated and cultured as described previously with each line being derived from a separate C57BL/6 E13.5 embryo containing INK-ATTAC (12, 41). These lines were expanded at 3% oxygen and used for experiments between passage (P)3 and P6. IMR-90 cells were purchased from ATCC (#CCL-186) at P10 and cultured in the same medium as used for MEFs. IMR-90 cells were used for experimentation between P14 and P18. Human dermal fibroblasts (HDFs) were generated from human foreskin of young, healthy donors (2 days to 13 years of age) by the Biochemical Genetics Laboratory at the Mayo Clinic. Each line was derived from a separate donor. HDFs were cultured in the same medium as used for MEFs and used for experimentation between P5 and P8. HUVECs were purchased from ATCC (#PCS-100–013) and were cultured in vascular cell basal medium (ATCC, #PCS-100–030) supplemented with endothelial growth factors (Endothelial Cell Growth Kit-VEGF, ATCC, #PCS-100–041). HUVECs were used for experimentation at P3 to P5 after receival.
Generation of senescent and non-senescent MEFs
For H3K27Ac-ChIP-seq experiments, two or three independent MEF lines were generated and induced to senesce via irradiation (IR), serial passaging (REP) or KRASG12V-overexpression (OI). For identification of IR-induced SASEs, we established the following three MEF cultures from each independent MEF line: proliferating P3 MEFs (to derive C1 MEFs); P6 MEFs exposed to 10 Gy γ-radiation (137Caesium source) and cultured for 2 days (to derive C2 MEFs); and P6 MEFs exposed to 10 Gy γ-radiation and cultured for 10 days (to derive IR-senescent MEFs). For identification of REP-induced SASEs, we prepared two MEF cultures from each independent MEF line: proliferating P3 MEFs (to derive C1 MEFs) and P10 MEFs cultured at 20% oxygen between P4 and P10 (to derive REP-senescent MEFs). To identify SASEs in OI-induced senescent MEFs, cells were infected with a KRASG12V-containing lentivirus (prepared using the pLenti-PGK-ER-KRASG12V from Addgene #35635), selected with 250 μg/ml of hygromycin B (EMD Millipore, #400052) and then harvested (to derive C1 MEFs) or treated with 200 nM 4-hydroxytamoxifen (4′-OHT, 1:50,000 from stock in ethanol, Sigma H7904) to induce KRASG12V for 2 days (to derive C2 MEFs) or 10 days (to derive OI-induced senescent MEFs). IR-, REP- and OI-induced senescent MEFs were enriched by sterile FACS as previously described using a BD FACSAria 4-laser digital flow cytometer with FACSDiva v8.0.1 software with 488 nm laser (42). Sorted cells were pelleted, resuspended in fresh culture medium, counted, and used for ChIP-seq and RNA extraction. Small amounts of the sorted cells were reseeded to assess the proportion of cell that was senescent. Samples with ~70% or more SNCs were used for H3K27ac-ChIP-seq experiments. C1 and C2 MEFs cultures were also subjected to FACS but here fractions devoid of SNCs were collected. For all other experiments involving REP-induced SNCs, SNCs were prepared as described above. FACS-enriched SNCs were cultured for at least 24 hours before further use. OI-induced senescent MEFs were also prepared as described above, but instead of the lentiviral KRASG12V expression system we used MEFs derived from L-KRASG12V mice. These MEFs were infected with pTSIN-Cre-PGK-puro2 lentivirus (43) to induce KRASG12V expression. These MEFs were then cultured for 10 days and subject to FACS enrichment of SNCs (the first 2 days in medium containing 2 μg/ml of puromycin).
Generation of IR-senescent and control IMR-90 cells
H3K27Ac-ChIP-seq experiments and matched RNA-sequencing experiments were conducted in triplicate using three technical replicates. IMR-90 cells were expanded at 3% oxygen and used for experiments at P18. For identification of IR-induced SASEs, we established the following three cultures from each of the replicates: proliferating P18 IMR-90 cells (to derive control 1 (C1) cells); P18 IMR-90 cells exposed to 10 Gy γ-radiation (137Caesium source) and cultured for 2 days (to derive control 2 (C2) cells); and P18 IMR-90 cells exposed to 10 Gy γ-radiation and cultured for 10 days (to derive IR-senescent IMR-90 cells). Cells were trypsinized and reseeded to assess the proportion of SNCs Samples with >80% IR-SNCs were used for H3K27Ac ChIP-seq experiments.
ChIP-seq analyses and super-enhancer identification in cultured cells
FACS-enriched MEF or IMR-90 suspensions were pelleted, resuspended in medium, and counted. A total of 2×105 to 1×106 cells were fixed with 1% paraformaldehyde (PFA) for 10 min and then subjected to ChIP-seq as previously described using a rabbit anti-H3K27Ac antibody (Abcam, ab4729, Lot GR150367) (44). Chromatin immunoprecipitation-sequencing (ChIP-seq) libraries were prepared from 1–5 ng of precipitated chromatin or input DNA using the Ovation ultralow DR Multiplex kit (NuGEN) or the ThruPLEX DNA-seq Kit V2 (Rubicon Genomics). ChIP enrichment was validated in library DNAs by performing quantitative PCR in the indicated genomic loci using following primers: mouse mPabpc1-TSS (F): 5′-ATCCCACAGCTTGTGGCGGG-3′; (R): 5′-TCTCGCCATCGGTCGCTCTC-3′; mIntergenic (F): 5′-CCT-GCTGCCTTGTCTCTCTC-3′; (R): 5′-ATGGCCTAGGGATTCCAGCA-3′. The ChIP-seq libraries were sequenced to 51 bp from both ends on an Illumina HiSeq 2000 or HiSeq 4000 instrument in the Mayo Clinic Medical Genomics Core Facility. Fastq files of pair-end reads were mapped with Bowtie 1.1.2 using parameters -k 1 -m 1 -e 70 -l 51 --best to the reference genome as previously described (mm10 for mouse, hg19 for human) (23, 45). We used MACS 1.4.2 to identify peaks for each sample against the background using a P-value cutoff of 10−5 (46). All other parameters were left at default. To identify super-enhancers, neighboring peaks were first stitched together to create a single region capturing these signals as a whole. Peaks occurring within 12.5 kb from each other were combined into stitched enhancers while excluding regions that were within ±2000 bps from any transcription start site (TSS) (23). These stitched enhancers were then ranked by background-subtracted ChIP-seq occupancy ascendingly, and the occupancy was plotted in the unit of reads per million per base pair. From the plot, we identified the point where occupancy started increasing faster by first scaling the x- and y-axes into [0, 1] and then finding the point where a line with a slope of 1 was tangential to the curve. Occupancy increased slowly below but rapidly above this point. The stitched enhancers above this point were defined as super-enhancers. All the above procedures were performed using ROSE (21, 23). In order to determine differential binding for SE between treatment and control samples, we first merged SE regions from all samples into a set of merged regions covering all super-enhancer regions in all samples. Tag counts at each merged region were then extracted and differential analysis on the tag counts were performed using R package DESeq2 1.10.1 using the same settings as described below (see RNA-sequencing) (47). SASEs were defined as super-enhancers with lfcMLE (unshrunk log2 fold change produced by DESeq2) in tag counts ≥0.3 for both senescent vs. proliferating (C1) and senescent vs. induced, non-senescent (C2). Super-enhancers were assigned to genes within ±50 kb of the super-enhancer by calculating the distance between either end of each super-enhancer and TSS of each gene (21). Only super-enhancers ±50 kb from at least one TSS were considered in downstream analyses. For downstream validation, only SASE-controlled genes that were differentially expressed with false discovery rate (FDR)<0.05 in at least two of three senescence mechanisms were considered. BigWig files of H3K27Ac occupancy profiles were generated using deepTools 3.1.0 (48) by first normalizing each ChIP-seq sample and its matching input to cpm (counts per million mapped reads) and then subtracting the input signal from each ChIP sample. H3K27Ac occupancy plots were generated via Integrative Genomics Viewer (IGV) (49). To identify Rb peaks at promoters of SFs, published Rb ChIP-seq data from OI-senescent, quiescent and non-senescent IMR-90 cells were analyzed (GSE19899) (25). Peaks were annotated to genes within 50 kb from either end of any peak. The peak sequences of SASP genes associated to any Rb peak with 2.5-kb padding from each end were used as input to MEME-ChIP (50) to detect enriched motifs using the HOCOMOCO database (51). We used FIMO to locate occurrences of motifs in each input sequence (52).
ChIP on senescent liver cells
FACS-enriched Tom+ cell suspensions from Ai14;L-KRASG12V or Ai14 control livers (see below) were pelleted, resuspended in medium, and counted. A total of 1–4×105 cells were fixed with 1% PFA for 10 min and then subjected to H3K27Ac-ChIP using a rabbit anti-H3K27Ac antibody (Abcam, ab4729, Lot GR150367) or rabbit, IgG (Millipore, #12–370) according to the manufacturers protocol (Active Motif, #53084). Precipitated chromatin or input DNA was subjected to quantitative PCR in the indicated genomic regions in the SASE of the p21 locus using primers indicated in table S9.
RNA isolation and RT-qPCR
MEFs or IMR-90 cells, or flow-sorted liver cells were lysed in RLT buffer supplemented with β-mercaptoethanol according to the RNA extraction protocol. RNA extraction (Qiagen, RNeasy Mini kit, #74104, or RNeasy Micro kit, #74004), cDNA synthesis (Invitrogen, SuperScript III First-Strand Synthesis, #18080051), and quantitative polymerase chain reaction analysis (Applied Biosystem, SYBR Green Real-Time PCR Master Mix, #4309155) were performed according to manufacturer’s instructions. The on-column DNase digestion step was avoided during the RNA extraction procedure unless RNA was used for RNA-sequencing purposes. Primers were optimized via cDNA dilution series. Tbp (TBP in human) was used as a reference gene for RT-qPCR in mouse and human samples. Primer sequences are listed in table S9.
RNA-sequencing
Equal amounts of high-quality RNA (100–200 ng) were subjected to library preparation using the TruSeq RNA Library Prep Kit v2 (Illumina, #RS-122–2001) according to the manufacturer’s instructions. Libraries were sequenced following Illumina’s standard protocol using the Illumina cBot and HiSeq 3000/4000 PE Cluster Kit. Flow cells were sequenced as 100×2 paired-end reads on an Illumina HiSeq 4000 using HiSeq 3000/4000 sequencing kit and HCS 3.3.20 collection software. Base-calling was performed using Illumina’s RTA 2.5.2 software. RNA-sequencing was performed at the Mayo Clinic Center for Individualized Medicine Medical Genomics Facility (Mayo Clinic, Rochester, Minnesota). Fastq files of pair-end RNA-seq reads were aligned with Tophat 2.0.14 to the reference genome (mm10 for mouse, hg19 for human) using Bowtie2 2.2.6 with default parameters (51, 53). Gene level counts were obtained using FeatureCounts 1.4.6 from the SubRead package with gene models from corresponding UCSC annotation packages (54). Differential expression analysis was performed using R package DESeq2 1.10.1 after removing genes with average raw counts less than 10 (47). During the DESeq2 analysis thresholding on Cook’s distance for outliers and independent filtering were turned off so that all genes passed to DESeq2 were assigned P-values for significance of differential expression. Genes with FDR<0.05 were considered significantly differentially expressed. Hierarchical clustering of samples was performed using DESeq2-normalized counts with 1−Pearson correlation as distance and average linkage using R function hclust. Gene Set Enrichment Analysis (GSEA) was performed as previously described against mouse genesets from Enrichment map using gene lists ranked by lfcMLE, which was the unshrunk log2 fold change produced by DESeq2, in descending order (55, 56). Functional annotation analyses were performed via String database v11 focusing on GO BP annotations, KEGG pathways and Reactome pathways with FDR<0.05 (57). Overrepresentation analysis for transcription regulatory targets of individual TFs was performed using the Fisher’s exact test method for selected gene lists against the mouse gene sets from ENCODE and MSigDB collections (56, 58). Mouse TF targets were mapped to human orthologs using MGI’s Vertebrate Homology database and used for overrepresentation analyses in human datasets. Putative SASP factor genes were extracted from Gene Ontology Consortium (Mus musculus MGI and Homo sapiens GO Annotations EBI) and QuickGO database for the annotation GO:0005615 “Extracellular Space” (59–61). Gene lists from both reference databases were merged resulting in the identification of 1845 and 3513 factors for mouse or human, respectively. Heatmaps were generated with Morpheus, Broad Institute (https://software.broadinstitute.org/morpheus). For gene expression heatmaps based on RNA-seq data, lfcMLE values and −log10 of FDR values were used.
Adeno-virus injection into mice and isolation of liver cells
To generate in vivo OI-senescent liver cells, we used 4-month-old Ai14;L-KRASG12V or Ai14 control mice injected with 100 μl of 0.9% NaCl containing 109 pfu adeno-Cre-EGFP virus (University of Iowa, Vector Labs, #VVC-U of Iowa-1174) into the tail vein. Eight days post-injection, livers were harvested and the perivenous half of the left lateral lobe was fixed with 4% PFA in PBS for 2 hours and incubated in 30% sucrose overnight. These livers were embedded in OCT (Sakura, #4583) and used for cryosectioning and confocal imaging. To assess proliferation rates in these mice, 50 mg per kg of EdU (5-ethynyl-2′-deoxyuridine, Carbosynth, #NE08701) was IP injected on days 6 and 7 post-adeno-Cre injection for a total of 48 hours before euthanasia of mice. EdU staining was performed on cyrosections with the same kit and protocol used in vitro (see below). To isolate Tom+ liver cells, livers of Ai14;L-KRASG12V or Ai14 control mice 8 days post-injection were perfused with collagenase as previously described (62, 63). Because the parenchymal fraction of Ai14;L-KRASG12V was not viable, the non-parenchymal fraction was subjected to FACS as described above with appropriate lasers and filters. For in vivo p21-OE and p16-OE studies, Ai14;L-p21 or Ai14;L-p16 or Ai14 control mice were injected with 100 μl of 0.9% NaCl containing 108 pfu adeno-Cre-EGFP virus into the tail vein. Livers were harvested and fixed as described above 2, 4, or 8 days post-injection. To assess proliferation rates, these mice were injected intra-peritoneally with 50 mg per kg of EdU on days 2 and 3 after injection of adeno-Cre-EGFP virus and euthanized 48 hours after the first EdU injection. For in vivo KRASG12V-OE studies, Ai14;L-KRASG12V, Ai14;L-KRASG12V p21floxed/floxed, Ai14;L-KRASG12V Rbfloxed/floxed or Ai14 control mice were injected with 100 μl of 0.9% NaCl containing 2.5×107 pfu adeno-Cre-EGFP virus into the tail vein. Livers were harvested and fixed as described above, 4, 12, or 28 days post-injection. To assess proliferation rates in these mice, 50 mg per kg of EdU was injected IP on 2 days and 1 day for a total of 48 hours before euthanasia of mice. To isolate Tom+ hepatocytes for expression analyses, livers were perfused with collagenase and the parenchymal fraction was subjected to FACS as described above. For in vivo inducible p21-OE studies, Ai139;iL-p21 or Ai139 control mice were injected with 100 μl of 0.9% NaCl containing 108 pfu adeno-Cre-EGFP virus into the tail vein. At indicated timepoints (“ON”), livers were harvested and fixed as described above. To suppress p21-OE (“OFF”), mice were treated with doxycycline (Letco, #690902) at 100 mg per kg in water via gavage every 24 hours (for a total of 48 hours) until euthanasia and liver collection.
DNA isolation and PCR for recombined conditional alleles
Livers of A14i;L-KRASG12V, A14i;L-KRASG12V;p21floxed/floxed or A14i;L-KRASG12V;Rbfloxed/floxed mice that received 2.5×107 pfu adeno-Cre virus (containing ~5% Tom+ hepatocytes) or did not receive virus were flash frozen and stored at −80°C. These livers were homogenized via mortar and pestle and DNA was isolated through phenol-chloroform extraction. PCR analysis of p21 exon 2 was performed using the following primers: (F) 5′-GTATCCCAAAGTCCAGGGCACT-3′ and (R) 5′-TGCCAAGGGGAAGGACATCATT-3′ generating 1446 bp, 1549 bp, and 609 bp products for the wild-type, unrecombined-floxed, and recombined-floxed alleles, respectively. PCR analysis of Rb exon 19 was performed as described before (64) using the following primers Rb18 (F) 5′-GGCGTGTGCATCAATG-3′ and Rb212 (R) 5′-GAAAGGAAAGTCAGGGACATTGGG-3′ generating 698 bp, 746 bp, and 260 bp products for the wild-type, unrecombined-floxed, and recombined-floxed alleles, respectively.
Neutralizing antibody experiments in mice
To deplete CD8+ T cells, Ai14;L-p21 and Ai14 mice were IP injected with 500 μg of rat anti-CD8α antibody (clone 53–6.7, BioXcell, #BE0004–1) in 200 μl PBS or 200 μl PBS (as control) each day for 3 consecutive days and again on day 6. On day 7, mice were injected with 100 μl of 0.9% NaCl containing 108 pfu adeno-Cre-EGFP virus into the tail vein. On d12, mice were IP injected once more with anti-CD8α antibody or PBS, mice were euthanized and livers and spleens were collected at d15 (corresponding to d8 post-adeno-Cre injection). Spleens were processed freshly to isolate cells for flow cytometry. Spleens were dissociated between two frosted slides. The cell suspension was then filtered through a 70-μm filter and spun at 400 g for 5 min. Red blood cells were removed via ACK lysis for 8 min on ice. Tubes were filled with PBS, centrifuged, and resuspended. Total cell numbers were then counted. For flow cytometry assessments, 105 cells were used for antibody staining using the following antibodies: hamster FITC-conjugated anti-TCRβ (Tonbo Biosciences, #35–5961, 1:500), PerCP-conjugated rat anti-CD4 (BioLegend, #100538, 1:500), violetFluor 450-conjugated rat anti-CD8α (clone 2.43, Tonbo biosciences, #75–1886, 1:500), and Ghost Dye Red 780 cell viability dye (Tonbo biosciences, #13–0865, 1:1000). Total CD4+ or CD8α+ T cells were calculated using flow cytometry quantifications and the previously noted total cell numbers per spleens. To neutralize CXCL14, Ai14;L-p21 and Ai14 mice were IP injected with the following antibodies in 200 μl of PBS: 500 μg of rat anti-CXCL14 antibody (R&D Systems, #MAb730), 500 μg of mouse anti-CXCL14 antibody (R&D Systems, #MAb866), 500 μg of mouse IgG2a isotype control (BioXcell, #BE0085 as control for Mab730), or 500 μg of rat IgG2b isotype control (BioXcell, #BE0090 as control for MAb866). The following day, mice were again injected with antibody and were also injected with 108 pfu adeno-Cre virus in 100 of μl 0.9% NaCl intravenously as described above. The following day, antibody injection was repeated once more. Mice were euthanized and livers were collected the next day (d3, corresponds to d2 post-adeno-Cre injection).
Cryosectioning and immunofluorescence on liver tissue
OCT-embedded livers were sectioned using a Cryostat (CM 1900, Leica) to generate 20-μm-thick frozen sections. Sections were washed with PBS and permeabilized with 0.5% Triton-X-100 for 20 min. Sections were blocked with 5% BSA/PBS for 1 hour and subsequently incubated overnight with primary antibodies rabbit anti-F4/80 (Cell Signaling, #70076; 1:250), FITC-conjugated rat anti-B220/CD45R (BD BioSciences; #553088; 1:50), FITC-conjugated rat anti-NKp46/CD335 (Biolegend, #580756; 1:50), rabbit anti-CD3ε (Cell Signaling, #99940; 1:50), biotin-conjugated rabbit anti-CD4 (BioLegend, #100508, 1:50; in combination with FITC-conjugated streptavidin, BioLegend, #405201, 1:100), rabbit anti-CD8α (Cell Signaling, #98941, 1:20), rabbit anti-iNOS (Abcam, ab15323, 1:100), rabbit anti-lamin B1 (Abcam, ab16048, 1:500) or rabbit anti-HMGB1 (Abcam, ab18256, 1:500), rabbit anti-p21 (Abcam, ab188224, 1:100 or 1:250), rabbit anti-Myc-tag (Cell Signaling, #2272, 1:100), mouse anti-Myc-tag (Cell Signaling, #2276, 1:100; in combination with Alexa Fluor 647-conjugated goat anti-mouse IgG2a secondary antibody, Invitrogen, #A21241, 1:100), rabbit anti-phospho-Histone H3 (Ser10) (pHH3, Millipore, #06–570, 1:250), or Alexa Fluor 488-conjugated rat anti-F4/80- (Bio-Rad, #MCA497A488T, 1:100; used for co-immunofluorescence in combination with rabbit anti-p21 staining) diluted in 5% BSA/PBS. Secondary antibodies used were Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen, #A11034; 1:250) or Alexa Fluor 647-conjugated goat anti-rabbit IgG (Invitrogen, #A21244; 1:100). These antibodies were incubated for 3 hours. Secondary antibody incubations were avoided if the primary antibody was conjugated to FITC or Alexa Fluor fluorophores. Washes between incubations were performed in PBS (three 5-min washes). Cells were counterstained with Hoechst. A confocal laser-scanning microscope (LSM 880; Zeiss) on an Axio Observer Z1 inverted microscope with spectral detectors (32ch 2PMT GaAsP; Zeiss) and a water-immersion lens (C-Apochromate 40X/1.2 NA Korr. FCS; Zeiss) were used to capture z-stack images with 2-μm step size (F4/80, iNOS, NKp46, CD3ε, CD4, CD8α, and B220 stainings). The percentage of lamin B1+ nuclei was determined as the percentage of Tom+ hepatocytes with lamin B1-staining versus Tom+ hepatocytes without lamin B1 staining. At least 50 hepatocytes or two sections were counted. For HMGB1 staining, the localization of nuclear versus cytoplasmic staining was examined per Tom+ hepatocyte and percentage of Tom+ hepatocytes with nuclear HMGB1 (N>C) was determined compared to Tom+ hepatocytes with loss of nuclear HMGB1 and gain of cytoplasmic staining (N<C). At least 50 hepatocytes or two sections were counted. To determine the proportion of p21-induced hepatocytes, the percentage of Tom+ hepatocytes with nuclear p21-staining versus Tom+ hepatocytes without nuclear p21 were quantified. At least 100 hepatocytes or two sections were counted. Similar analyses were performed to quantify Myc-tag-induced hepatocytes of Ai14;L-p21 mice. To determine the proportion of Myc-tag-induced Ai14;L-KRASG12V hepatocytes, the percentage of Tom+ hepatocytes with Myc-tag-staining at the plasma membrane versus Tom+ hepatocytes without Myc-tag staining were quantified. To count the number of macrophages/Kupffer cells, B cells, T cells, or NK cells associated per Tom+ hepatocyte, the number of F4/80+ cells, B220+, CD3ε+, or NKp46+ cells, respectively, immediately adjacent to Tom+ hepatocytes was counted. At least 100 hepatocytes or two sections were counted. Similar quantifications were performed for the M1 macrophage marker iNOS and T cell subset markers CD4 and CD8α. To assess the proportion of Tom+ hepatocytes actively progressing through the cell cycle, Tom+ hepatocytes with nuclear pHH3 staining versus Tom+ hepatocytes without pHH3 signal were quantified. Cells with pHH3 staining were subdivided into Tom+ pHH3+ before nuclear envelop breakdown as determined via Hoechst signal (considered G2 cells) and after nuclear envelop breakdown (considered mitotic cells). To determine the percentage of Tom+ hepatocytes, at least 400 hepatocytes were scored and the percentage of Tom+ versus Tom− hepatocytes (as determined by nuclear and cellular shape) were determined. To assess the number of dying hepatocytes, at least 100 Tom+ hepatocytes were examined for cellular health and cells with overtly fragmented cytoplasm were considered as dying. Tom+ hepatocyte clusters were defined as three or more Tom+ hepatocytes being immediately adjacent, whereas Tom+ single hepatocytes were assessed when having no other Tom+ hepatocyte immediately adjacent. To quantify Tom+ hepatocyte clusters, large tile images were captured, assessed for the number of Tom+ hepatocyte clusters and normalized to the area of the tile image. Three sections were analyzed and averaged. For all quantifications involving Ai139;iL-p21 or Ai139 mice, similar staining regiments and quantifications were performed, but with the following modifications. In samples without dox (“ON”) Tom+ eGFP+ hepatocytes were selected for quantification, whereas in the presence of dox (“OFF”) Tom+ hepatocytes were selected. At least 50 Tom+ hepatocytes were examined.
Immunostaining and confocal microscopy
For p21 or 53BP1 immunostaining, flow-sorted MEFs were seeded on 10-well chambered slides (HTC supercured, Thermo Fisher Scientific, #30966S Black) at 2000 cells per well. The following day, cells were fixed in PBS/4% PFA for 15 min, permeabilized in PBS/0.2% Triton X-100 for 15 min and blocked in PBS/5% BSA for 1 hour. Primary antibodies mouse anti-p21 (Santa Cruz, sc-53870; 1:200) or rabbit anti-53BP1 (Novus Biological, #NB100–305; 1:200) were diluted in PBS/5% BSA and subsequently incubated with primary antibodies overnight and secondary antibodies (goat anti-rabbit AlexaFluor488, Invitrogen, #A11034; 1:250) for 3 hours. Washes between incubations were performed in PBS (three 5-min washes). Cells were counterstained with Hoechst and the percentage of p21+ nuclei was determined. For 53BP1 staining, the number of clearly visible 53BP1 foci per cell was counted and percentage of 53BP1+ cells with more than one focus was determined. At least 100 cells or 50 cells per sample were counted for p21 or 53BP1 staining, respectively. A confocal laser-scanning microscope (LSM 880; Zeiss) on an Axio Observer Z1 inverted microscope with spectral detectors (32ch 2PMT GaAsP; Zeiss) and a water-immersion lens (C-Apochromate 40X/1.2 NA Korr. FCS; Zeiss) were used to capture images.
Plasmid constructs
shRNA oligo sequences were obtained from the RNAi Consortium (TRC, Broad Institute) and cloned into pLKO.1 vector (Addgene, #10878) according to a corresponding Addgene protocol (65). Four to five shRNAs per gene were tested for their knockdown potential and the two most efficient shRNAs were used in experiments. The non-targeting TRC2 shRNA (referred to as scrambled shRNA shScr, Sigma-Aldrich, #SCH202) was used as a negative control. For shRNA sequences see table S9. The Myc-Flag-tagged cDNA for mouse p21 was obtained from Origene (#MR227529) and subcloned into the lentiviral pTSIN-PGK-puro2 backbone (43, 66) or dox-inducible pTRIPZ-PKG-puro backbone (modified from GE Dharmacon) (67). Similarly, the Myc-Flag-tagged cDNAs for mouse p16 (Origene, #MR227284) and mouse p27 (Origene, #MR201957) were also subcloned into the lentiviral pTSIN-PGK-puro2 backbone.
Lentivirus production and cell transduction
Lentiviral particles were produced in HEK-293T cells using Lipofectamine 2000 (Invitrogen, #11668) and appropriate helper plasmids: pLP1, pLP2, VSV-G (pLKO.1 vectors and pLenti vectors), VSV-G and pHR-CMV8.9 (for pTSIN vectors) or trans-lentiviral packaging mix (GE Dharmacon, #TLP4606) (for pTRIPZ vectors). After 48 hours, virus supernatant was harvested by filtration of HEK-293T supernatant through a 0.45-μm syringe filter. Virus was frozen at −80°C in small aliquots and freshly thawed for each infection cycle.
SA-β-Gal staining
MEFs and IMR-90 cells were seeded on 10-well chambered slides (HTC supercured, Thermo Fisher Scientific, #30966S Black) at 2000 cells per well. Flow-sorted cells were fixed the next day and stained. To assess senescence induction kinetics after irradiation or gene overexpression or gene knockdown, cells were irradiated with 10 Gy or infected twice with appropriate virus supernatants. At indicated times, cells were fixed and stained for SA-β-Gal activity according to manufacturer’s protocol (Cell Signaling, #9860S). MEFs were stained for 24 hours, whereas human cells were stained for 12 hours. Cells were counterstained with Hoechst and the percentage of SA-β-Gal+ cells was then determined. At least 100 cells per sample were counted. To determine the proportion of SA-β-Gal+ hepatocytes in adeno-Cre induced livers, 8-μm-thick cryosections were cut and stained similarly to described previously (68). Briefly, sections were fixed for 10 min according to manufacturer’s protocol (Cell Signaling, #9860S) and staining was performed for 14 hours. Sections were counterstained with Hoechst. At least 200 hepatocytes (as determined by cell and nuclear shape) were examined for SA-β-Gal+ staining.
Growth curves
Growth curves were generated using senescent MEFs as well as their respective proliferating controls (P5 non-irradiated for IR, P3 for REP, pLenti-PGK-ER- KRASG12V-infected, ethanol-treated cells for OI). At d0, flow-sorted cells were plated in a 12-well plate at a density of 25,000 cells per well in duplicates. At day 4, sub-confluent cultures were trypsinized, counted and re-seeded at 25,000 cells per well. Counting was repeated at day 7. Duplicate measures were averaged and cumulative cell number was calculated according to the following formula (69) Tx = Tx−1 * Nx / N0, where T is the cumulative cell number, x the passage number, Nx the counted cell number at passage x, and N0 the initially seeded cell number. For growth curves of p21-OE or p16-OE MEFs, P3 cells were infected with pTSIN empty, pTSIN-p21-Myc-Flag or pTSIN-p16-Myc-Flag on two consecutive days. The next day (day 3) cells were trypsinized, counted and reseeded in six-well plates at 100,000 cells per well (three wells per condition). Cells were counted every 24 hours until day 6. In parallel, cells were selected with puromycin and reseeded at day 7, when counting was continued.
EdU incorporation assay
Sorted senescent and non-SNCs were seeded on 10-well chambered slides at 2000 cells per well. The next day medium was replaced with medium containing 1 μM EdU (5-ethynyl-2′-deoxyuridine, 1:10,000 dilution, stock in DMSO) and cells were allowed to incorporate EdU for 48 hours. Cells were then fixed and subjected to EdU staining according to the manufacturer’s instructions (Thermo Scientific, Click-iT Plus EdU Alexa Fluor 555 Imaging Kit, #C10637). To assess DNA reduplication after knockdown of SASE-controlled genes, senescent MEFs were seeded on 10-well chambered slides at 2000 cells per well and infected with shRNA-containing virus on the 2 following consecutive days. Forty-eight hours after the first infection, medium was replaced with medium containing 1 μM EdU for 48 hours. Four days after the first infection, cells were fixed and subjected to EdU staining. To assess proliferation of irradiated, non-senescent, P3 MEFs were seeded at 2000 cells per well. The next day, cells were irradiated with 10 Gy. Two days post-IR, EdU was added for 24 hours, or cells were infected with shRNA-virus on two consecutive days. On day 4 post-IR, EdU was added for 24 hours. To assess proliferation of p21-OE human cells or p27-OE MEFs, cycling cells were infected with appropriate virus supernatants for 2 consecutive days as described above, selected for the next 48 hours with 2 μg/ml of puromycin. At day 4, cells were reseeded at 2000 cells per well and EdU was allowed to be incorporated for 24 hours. For inducible p21-overexpression, stably virus-infected cells were reseeded at 2000 cells per well and 4 μg/ml of dox was added the next day. At indicated days, EdU was added for 24 hours, except for short p21-OE induction experiments represented in fig. S19G where EdU was allowed to be incorporated for 12 hours. To quantify the EdU+ cell fraction, cells were counterstained with Hoechst and percentage of EdU+ cells was determined. At least 100 cells were counted.
Immunoblot analysis and co-immunoprecipitation
Co-immunoprecipitations and immunoblot analyses were performed as previously described (70). Subcellular fractionation for co-immunoprecipitations on chromatin fractions was performed using the Subcellular Protein Fractionation Kit (Thermo Scientific, #78840) according to the manufacturer’s instructions. The primary antibodies used were as follows: mouse anti-p21 (Santa Cruz, sc-53870; 1:8000 used for both mouse and human samples), rabbit anti-Myc-tag (Cell Signaling, #2272; 1:1000); rabbit anti-Rb (Abcam, ab181616; 1:2000), rabbit anti-STAT1 (Abcam, ab92506; 1:1000), rabbit anti-STAT6 (Cell Signaling, #5397; 1:1000), rabbit anti-SMAD2 (Cell Signaling, #5339, 1:1000), rabbit anti-SMAD3 (Cell Signaling, #9513; 1:1000), and mouse anti-p27 (BD Biosciences, #610242, 1:1000). All antibodies were detected with secondary HRP-conjugated goat anti-mouse or anti-rabbit antibodies (Jackson Immunoresearch; 1:10,000). PonS staining (0.2% w/v in 5% glacial acetic acid, Sigma-Aldrich, #P3504) served as a loading control. Immunoblots are representative of at least two independent experiments.
Conditioned medium
To generate CM from IR-induced cells, MEFs were seeded in T75 flasks at low density. The following day, cells were exposed to 10 Gy IR. Two days post-IR, cells were infected with shRNA virus as described above. At day 4 post-IR, these cells as well as cycling control cells of similar density were washed twice and 5 ml of culture medium as added. After 48 hours of conditioning, CM was harvested, filtered through a 0.2-μm syringe filter, and stored in small aliquots at −80°C. To generate CM from IR-SNCs, cells 10 days after IR were used and treated the same way. To produce CM from gene overexpressing MEFs, cells were seeded in T75 flasks, infected with appropriate virus supernatants on the next two consecutive days. Cells were selected with puromycin until d4 or d10 post-infection. Cells were again washed twice before of 5 ml of culture medium was added. CM was harvested as described above. For inducible pTRIPZ-p21-Flag-Myc overexpression, 4 μg/ml of dox was added to cells for 48 hours, then cells were washed and subjected to conditioning in the presence of dox, or cells were washed twice immediately and regular culture medium was added. These cells were washed twice a day to remove any residual dox and conditioning of medium was started 4 days after removal of dox. For short-term p21-OE overexpression experiments shown in fig. S19, medium was allowed to be conditioned for 12 hours. For CM from shCxcl14 knockdown cells, cycling cells were first infected with p21-OE virus for 2 days, followed by infection with shCxcl14 virus for the next 2 consecutive days after which, on day 4, conditioning was started.
Scratch assays
Cycling P3 MEFs were seeded in 24-well plates and grown to confluence for ~3 days. Medium was removed and CM was added. Immediately afterwards, using a P20 pipette tip a linear vertical scratch was made from the top well center to the bottom well center. Cells were promptly imaged to document the initial scratch width (0 hours). Cells were grown in regular 3% O2 incubators until 2, 12, 24, and 48 hours post-scratch when cells were imaged again. To count cells emigrating from the cell dense area into the scratch space, three to six fields of a 10X objective were quantified and invading cell number was normalized to scratch length which these cells occupied. The average scratch width was measured from two microscopy fields of a 4X objective. Per field we collected at least 10 horizontal measurements (spaced 200 μm apart) from scratch edge to scratch edge.
Isolation and characterization of peritoneal immune cells
Two-to-four-month-old wildtype mice were used to collect the peritoneal lavage (71) using 10 ml of ice-cold PBS applied via a 20G needle. The lavage was centrifuged at 500g for 10 min at 4 °C. Cells were counted and subjected to transwell migration assays or used for flow cytometry. Peritoneal immune cells from wildtype control mice or wildtype mice injected with CM were resuspended in 300 μl of DMEM. A 100-μl cell suspension was used for antibody staining using eFluor 450-conjugated anti-CD11b (eBioscience, #48–0112; 1:100), FITC-conjugated anti-B220/CD45R (BD BioSciences; #553088; 1:100), and APC-conjugated anti-TCRβ (BD BioSciences; #553174; 1:100). Cells were stained 20 min on ice in the dark, after which 200 μl of DMEM was added and cells were analyzed via a FACSCanto X (BD BioSciences). Cell counts within 60 s were noted.
Transwell migration assay
To perform transwell migration assays using peritoneal immune cells, 500 μl of CM was added to a 24-well plate. A transwell inset (3-μm pore size, Costar, #3415 or #3472) was loaded with ~2×105 peritoneal immune cells in 100 μl of medium (matching the medium used for CM production). Cells were allowed to migrate for 12 hours. Then, the transwell was carefully removed and the medium containing suspension cells was collected. Attached cells on the well bottom were washed twice with PBS, trypsinized and scraped. Suspension cells and attached cells were spun at 500g for 10 min, resuspended and counted. Cell counts were normalized to cell numbers of control condition (CM cycling cells or CM EV) for each mouse separately. For CXCL14 neutralization experiments, CM from EV- or p21-OE cells was added to a 24-well plate together with 20 μg/ml of goat anti-CXCL14 (R&D Systems, #AF866) or 20 μg/ml of goat anti-IgG (R&D Systems, #AB-108-C) (72). Transwell migration assays were performed as described above.
Injection of CM in wildtype mice
To determine the immune cell-eliciting potential of CM, CM was generated as described above except that culture medium with 0.5% FBS was used. One milliliter of CM was aspirated with a 25G needle and 3-ml syringe. The needle was switched to 27G and CM was slowly injected into the peritoneum of 8–10-week-old C57BL/6 wildtype mice. Four days post-injection, the peritoneal lavage was harvested and subjected to antibody staining and flow cytometry as described above.
Statistical analysis
Prism software (GraphPad Software) was used for statistical analyses. Unless otherwise stated, Student’s two-tailed paired t tests (in MEFs and HDFs) or Student’s two-tailed unpaired t tests (in IMR-90 cells and HUVECs) were used for pairwise significance involving two groups. For all experiments involving three or more groups, one-way analysis of variance (ANOVA) with Sidak’s correction or two-way ANOVA with Sidak’s or Bonferroni correction for multiple comparisons were performed. In these comparisons, the following denotes significance in all figures: *P<0.05, **P<0.01 and ***P<0.001.
Supplementary Material
Acknowledgements:
We thank B.G. Childs, J.F. Limzerwala, C.J. Sieben, R. Bram, J. Elisseeff, and B. van de Sluis for helpful and stimulating discussions and/or careful evaluation of the manuscript. We also thank R. Velasco Fierro for valuable technical assistance. We are grateful to C. Ross for his work on the identification of super-enhancers and the genes they control, M.H. Hofker for conceptual discussions, and R. Thaler for assistance in generating heatmaps. Flow cytometry was performed by the Mayo Clinic Microscopy and Cell Analysis Core, sequencing by Mayo Clinic Medical Genomics Facility Sequencing Core, and ChIP in the Epigenomics Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology.
Funding: This work was supported by grants from Mayo Clinic’s Center for Biomedical Discovery, the Paul F. Glenn Foundation for Medical Research, the Keck Foundation, the US National Institutes of Health (grants AG057493, P30 DK084567 and R01 AG056318), the Mayo Clinic Cancer Center, and the David F. and Margaret T. Grohne Cancer Immunology and Immunotherapy Program.
Competing interests: J.M.v.D. is a cofounder of Unity Biotechnology. J.M.v.D., D.J.B. and R.M.L. are co-inventors on patents licensed to or filed by Unity Biotechnology. J.M.v.D., I.S. and H.L. are co-inventors on a planned patent related to this work. J.M.v.D., H.L. R.M.L and D.J.B. are current Unity Biotechnology shareholders. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic conflict of interest policies.
Footnotes
Data availability: ChIP-seq and RNA-seq data sets have been deposited in the Gene Expression Omnibus: the following secure token has been created to allow review of record GSE117278 while it remains in private status: sbwvqaqinlyjtsz.
References and notes
- 1.Galluzzi L, Yamazaki T, Kroemer G, Linking cellular stress responses to systemic homeostasis. Nat Rev Mol Cell Biol 19, 731–745 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Koren E, Fuchs Y, Modes of Regulated Cell Death in Cancer. Cancer discovery 11, 245–265 (2021). [DOI] [PubMed] [Google Scholar]
- 3.van Deursen JM, The role of senescent cells in ageing. Nature 509, 439–446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang TW et al. , Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Eggert T et al. , Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell 30, 533–547 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuilman T et al. , Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Tasdemir N et al. , BRD4 Connects Enhancer Remodeling to Senescence Immune Surveillance. Cancer discovery 6, 612–629 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munoz-Espin D et al. , Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Storer M et al. , Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Chiche A et al. , Injury-Induced Senescence Enables In Vivo Reprogramming in Skeletal Muscle. Cell Stem Cell 20, 407–414 e404 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Baker DJ et al. , Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker DJ et al. , Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Childs BG et al. , Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeon OH et al. , Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 23, 775–781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bussian TJ et al. , Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krizhanovsky V et al. , Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppe JP et al. , Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6, 2853–2868 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgilis A et al. , PTBP1-Mediated Alternative Splicing Regulates the Inflammatory Secretome and the Pro-tumorigenic Effects of Senescent Cells. Cancer Cell 34, 85–102 e109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omori S et al. , Generation of a p16 Reporter Mouse and Its Use to Characterize and Target p16(high) Cells In Vivo. Cell Metab 32, 814–828 e816 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Hoare M et al. , NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol 18, 979–992 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loven J et al. , Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hnisz D et al. , Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whyte WA et al. , Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowen JM et al. , Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell 159, 374–387 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chicas A et al. , Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell 17, 376–387 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nara N et al. , Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J Biol Chem 282, 30794–30803 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Hara T, Tanegashima K, Pleiotropic functions of the CXC-type chemokine CXCL14 in mammals. J Biochem 151, 469–476 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Kurth I et al. , Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. The Journal of experimental medicine 194, 855–861 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santiago-Raber ML et al. , Role of cyclin kinase inhibitor p21 in systemic autoimmunity. J Immunol 167, 4067–4074 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Lawson BR et al. , Deficiency of the cyclin kinase inhibitor p21(WAF-1/CIP-1) promotes apoptosis of activated/memory T cells and inhibits spontaneous systemic autoimmunity. The Journal of experimental medicine 199, 547–557 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balomenos D et al. , The cell cycle inhibitor p21 controls T-cell proliferation and sex-linked lupus development. Nat Med 6, 171–176 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Zitvogel L, Tesniere A, Kroemer G, Cancer despite immunosurveillance: immunoselection and immunosubversion. Nature reviews. Immunology 6, 715–727 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Munoz-Fontela C, Mandinova A, Aaronson SA, Lee SW, Emerging roles of p53 and other tumour-suppressor genes in immune regulation. Nature reviews. Immunology 16, 741–750 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanidas I et al. , A Code of Mono-phosphorylation Modulates the Function of RB. Mol Cell 73, 985–1000 e1006 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braumuller H et al. , T-helper-1-cell cytokines drive cancer into senescence. Nature 494, 361–365 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Kim SJ et al. , Macrophages are the primary effector cells in IL-7-induced arthritis. Cell Mol Immunol 17, 728–740 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulakirba S et al. , IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential. Scientific reports 8, 256 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westrich JA, Vermeer DW, Colbert PL, Spanos WC, Pyeon D, The multifarious roles of the chemokine CXCL14 in cancer progression and immune responses. Mol Carcinog 59, 794–806 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nam HJ, van Deursen JM, Cyclin B2 and p53 control proper timing of centrosome separation. Nat Cell Biol 16, 538–549 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao X et al. , Homology-mediated end joining-based targeted integration using CRISPR/Cas9. Cell Res 27, 801–814 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babu JR et al. , Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J Cell Biol 160, 341–353 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flor AC, Doshi AP, Kron SJ, Modulation of therapy-induced senescence by reactive lipid aldehydes. Cell Death Discov 2, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamada M et al. , Ran-dependent docking of importin-beta to RanBP2/Nup358 filaments is essential for protein import and cell viability. J Cell Biol 194, 597–612 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong J et al. , Purification of nanogram-range immunoprecipitated DNA in ChIP-seq application. BMC Genomics 18, 985 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langmead B, Trapnell C, Pop M, Salzberg SL, Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10, R25 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y et al. , Model-based analysis of ChIP-Seq (MACS). Genome Biol 9, R137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Love MI, Huber W, Anders S, Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramirez F et al. , deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res 44, W160–165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson JT et al. , Integrative genomics viewer. Nat Biotechnol 29, 24–26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bailey TL et al. , MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37, W202–208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langmead B, Salzberg SL, Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grant CE, Bailey TL, Noble WS, FIMO: scanning for occurrences of a given motif. Bioinformatics 27, 1017–1018 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim D et al. , TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14, R36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao Y, Smyth GK, Shi W, The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Research 41, e108-e108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subramanian A et al. , Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merico D, Isserlin R, Stueker O, Emili A, Bader GD, Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PloS one 5, e13984 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szklarczyk D et al. , STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47, D607-D613 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liberzon A et al. , Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashburner M et al. , Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25, 25–29 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.The Gene Ontology C, Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res 45, D331-D338 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Binns D et al. , QuickGO: a web-based tool for Gene Ontology searching. Bioinformatics 25, 3045–3046 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kahraman A et al. , TRAIL mediates liver injury by the innate immune system in the bile duct-ligated mouse. Hepatology 47, 1317–1330 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aziz K et al. , Ccne1 Overexpression Causes Chromosome Instability in Liver Cells and Liver Tumor Development in Mice. Gastroenterology 157, 210–226 e212 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayhew CN et al. , Liver-specific pRB loss results in ectopic cell cycle entry and aberrant ploidy. Cancer Res 65, 4568–4577 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Moffat J et al. , A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Miest T, Saenz D, Meehan A, Llano M, Poeschla EM, Intensive RNAi with lentiviral vectors in mammalian cells. Methods 47, 298–303 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weaver RL et al. , BubR1 alterations that reinforce mitotic surveillance act against aneuploidy and cancer. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang MJ et al. , Reversal of hepatocyte senescence after continuous in vivo cell proliferation. Hepatology 60, 349–361 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Chen X et al. , Endogenous expression of Hras(G12V) induces developmental defects and neoplasms with copy number imbalances of the oncogene. Proceedings of the National Academy of Sciences of the United States of America 106, 7979–7984 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kasper LH et al. , CREB binding protein interacts with nucleoporin-specific FG repeats that activate transcription and mediate NUP98-HOXA9 oncogenicity. Mol Cell Biol 19, 764–776 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ray A, Dittel BN, Isolation of mouse peritoneal cavity cells. J Vis Exp, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu J et al. , IRX1 hypomethylation promotes osteosarcoma metastasis via induction of CXCL14/NF-kappaB signaling. The Journal of clinical investigation 125, 1839–1856 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.