Abstract
The direct (e.g., radiation, microgravity) and indirect (e.g., lifestyle perturbations) effects of spaceflight extend across multiple systems resulting in whole-organism cardiovascular deconditioning. For over 50 years NASA has continually enhanced a countermeasures program designed to characterize and offset the adverse cardiovascular consequences of spaceflight. In this review, we provide a historical overview of research evaluating the effects of spaceflight on cardiovascular health in astronauts and outline mechanisms underpinning spaceflight-related cardiovascular alterations. We also discuss how spaceflight could be leveraged for aging, industry, and model systems such as human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), organoid, and organ-on-a-chip technologies. Finally, we outline the increasing opportunities for scientists and clinicians to engage in cardiovascular research in space and on Earth.
Keywords: Cardiovascular Disease, Exercise, Aging
1. INTRODUCTION
The effects of spaceflight on the cardiovascular system have been extensively characterized for more than 50 years.1 On Earth, approximately 70% of body fluids are below the level of the heart; however, during spaceflight loss of the hydrostatic pressure gradient results in a shift of approximately 2000 mL of fluid towards the head.2 On long duration spaceflight missions, this acute direct insult, coupled with chronic adaptations and indirect effects (e.g., lifestyle perturbations) lead to adverse cardiovascular effects (Figure 1) that may compromise astronaut safety during a mission, as well as increase the risk of long-term cardiovascular events.3 To offset these, and other, adverse physiological consequences, the National Aeronautics and Space Administration (NASA) developed and frequently adapted an advanced exercise countermeasures program used before, during, and after spaceflight.1 As a result of ongoing characterization of adverse effects and exercise countermeasures, astronauts are now able to tolerate spaceflight for over 6 months,4 and most physiological changes recover to baseline levels within 1 month after return to Earth.5 Given the demonstrated success of protecting human health during spaceflight, plans are currently being developed for extended stays on the lunar surface and deep space exploration missions that could last up to three years.
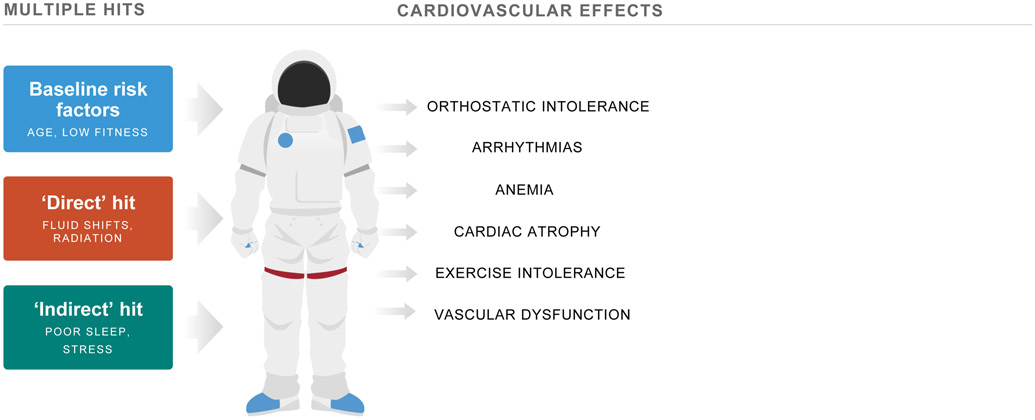
Figure 1. Effects of Spaceflight on the Cardiovascular System.
Multiple hits including baseline risk factors (e.g., older age, low fitness), direct (e.g., fluid shifts, radiation), and indirect (e.g., stress, poor sleep) insults induce adverse cardiovascular effects in astronauts. Adapted from Scott et al.6
A NASA-modeled countermeasures program has potential application to improve clinical cardiovascular research across numerous patient populations.6 Moreover, the physiological changes coupled with the cellular, molecular, and genomic alterations4 experienced by astronauts suggest that spaceflight represents a model of accelerated cardiovascular aging that could be leveraged to study cardiac pathophysiology using model systems such as human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), organoid, and organ-on-a-chip technologies. With the new science and laboratory opportunities created by the expansion of commercial spacecraft entities over the past 10 years, spaceflight represents an innovative platform that could be used to also advance cardiovascular research on Earth.
In this review, we provide a historical overview of 50 years of research evaluating the physiological cardiovascular effects of spaceflight in astronauts. We also outline findings from studies using model systems to evaluate the mechanisms underpinning the adverse cardiovascular effects of spaceflight. Finally, we describe how recent advances in commercial spaceflight platforms could be leveraged to enhance aging, industry, and ex-vivo research, and provide an overview of opportunities for cardiovascular scientists and clinicians to engage in research in space and/or on Earth.
2. Characterization of Spaceflight-Induced Cardiovascular Changes in Astronauts
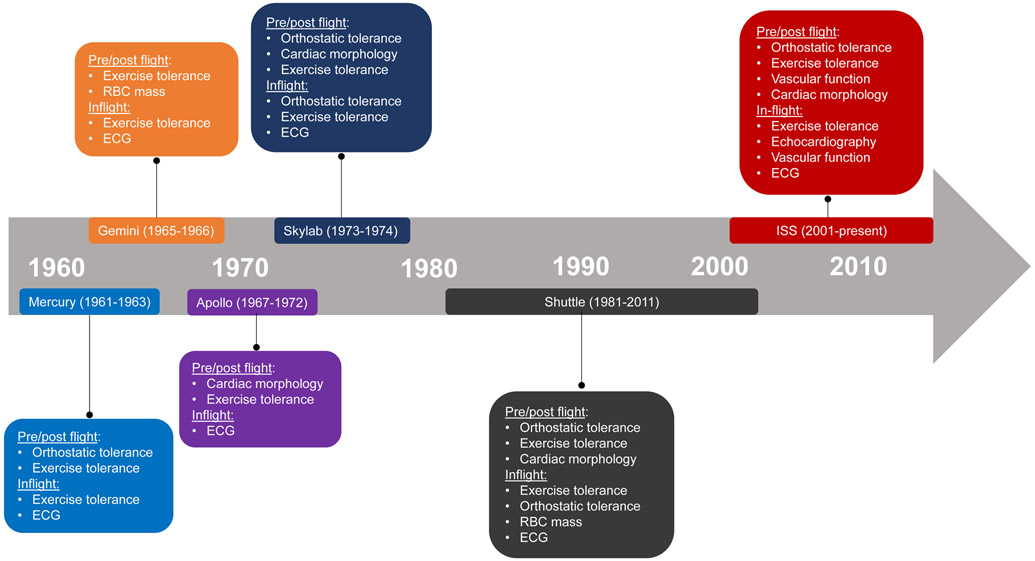
Since Alan Shepard’s historic 15 minute spaceflight on May 5th, 1961, NASA has systematically characterized the effects of spaceflight on cardiovascular health on Mercury (1961-1963; duration range: 15 min-34 h), Gemini (1965-1966; duration range: 4h-13 days), Apollo (1967-1975; duration range: 5-12 days), Skylab (1973-1974; duration range: 28-84 days), Shuttle (1981-2011; duration range: 2-17 days), and International Space Station (ISS; 2000-present; duration range: 70-340 days) missions (Figure 2).1, 7 Here, we provide an overview of key spaceflight-related adverse cardiovascular effects. It is important to note that NASA began implementing exercise training as standard of care in the 1960s with the goal of augmenting reserve pre-flight, mitigating decline in-flight, and accelerating recovery post-flight.6 Thus, cardiovascular changes in astronauts should be considered in the context of adjunct aerobic and resistance exercise training.
Figure 2. Timeline of NASA Spaceflight Missions and Key Cardiovascular Assessments.
The effects of spaceflight on cardiovascular health have been studied on Mercury (1961-1963; duration range: 15 min-34 h), Gemini (1965-1966; duration range: 4h-13 days), Apollo (1967-1975; duration range: 5-12 days), Skylab (1973-1974; duration range: 28-84 days), Shuttle (1981-2011; duration range: 2-17 days), and International Space Station (ISS; 2000-present; duration range: 70-340 days) missions.
2.1. Arrhythmias
A sudden acute cardiovascular event could incapacitate an individual astronaut and put the mission at risk.8 As a result, there has been a continuing effort by NASA to record and categorize inflight rhythm disturbances. The first documented dysrhythmia occurred during the Apollo missions where one astronaut experienced a 22-beat nodal bigeminal rhythm, which was followed by premature atrial beats.9 Twenty-one months later, this astronaut had an acute myocardial infarction.9 During Skylab, all 9 astronauts exhibited some form of rhythm disturbance, although the majority consisted of single premature ventricular contractions and were clinically insignificant.10 However, one astronaut experienced a 5-beat run of ventricular tachycardia during lower body negative pressure (LBNP), and another had periods of “wandering supraventricular pacemaker” during rest and following exercise.10 The Russians also reported an episode of persistent tachydysrhythmia during an extravehicular activity which resulted in the mission duration being shortened from 11 months to 6 months.11 A myocardial infarction in one 49 year old cosmonaut 2 years following a short-duration spaceflight was also recorded.12 Since 2001, 5 out of 100 active astronauts underwent radiofrequency ablation for atrial arrhythmias suggesting astronauts may have an accelerated risk of atrial fibrillation relative to age-matched individuals.8 Using cardiac magnetic resonance (CMR) imaging, Khine et al.8 reported that 6 months of spaceflight caused transient increases in left atrial volume, which when coupled with high heart rate during exercise training, could result in increased risk of atrial fibrillation. Long-term follow-up will be required to determine the clinical importance of acute spaceflight-induced atrial morphology changes.
2.2. Cardiac Atrophy
Cardiac morphology was first assessed using posterior-anterior chest X-rays in Apollo astronauts, where decreased cardiothoracic (C/T) ratios post-spaceflight were observed in 80% of astronauts.9 At the time, radiographic determination of cardiac size was the clinical standard for evaluation of the healthy or failing heart.13 However, NASA researchers noted the limitations of such radiographic techniques including potential variability due to body position and respiration and inability to determine whether alterations in systolic or diastolic function occurred.10 Accordingly, just three years after the first U.S. publication of echocardiographic ultrasound techniques,14 ultrasound was used to evaluate left ventricular (LV) dimensions and mass in Skylab astronauts. On landing day, decreased LV diastolic dimension (−15%), stroke volume (−16%), and mass (−8%) were observed in all astronauts.10 To determine whether declines in stroke volume were related to systolic or diastolic function, images were acquired at comparable pre-flight end-diastolic volumes by using increasing amounts of LBNP.10 LV function curves were created by using volumes at each pressure stage, and, given that no differences in slopes were observed post-flight, researchers concluded that no deterioration in LV systolic function occurred.10 Recognizing the potential for improved accuracy over echocardiography when assessing LV mass, Levine et al.15 used CMR to evaluate LV mass and reported a 12% decrease after 10 days of spaceflight. In contrast, preliminary recent findings using CMR indicate no changes in LV mass, function, or evidence of myocardial fibrosis after 4 to 6 months of spaceflight.16 These findings suggest that in concert with technological advances in evaluation of cardiac morphology and function, improvements in exercise countermeasures may offset the adverse effects of prolonged spaceflight on cardiac morphology and function.
2.3. Anemia
A decline in red blood cell (RBC) mass was a major concern for astronauts, given that impaired oxygen (O2) delivery to skeletal muscle limits exercise tolerance.17 Detailed hematologic investigations were first conducted in Gemini missions using radioisotope-derived plasma volume measurements.18 Using a 51Cr tag, a 20% decrease in RBC mass was observed, accompanied by an abnormally low red cell 51Cr half-life.19 Alfrey and colleagues20-22 began an elegant line of hematological studies in the 1990s to determine the mechanisms of ‘space anemia’ on Shuttle astronauts. Within 24 hours of exposure to spaceflight, there was a ~20% decrease in plasma volume accompanied by a ~10% decline in RBC mass (likened to the removal of ~700 ml of blood).20 Ferrokinetic studies examining plasma iron turnover, erythrocyte iron turnover, and 59Fe disappearance time demonstrated no decline in new RBC production in the bone marrow.20 Next, to evaluate whether there was an increase in RBC destruction, autologous RBCs were labeled with 51Cr and reinjected intravenously before launch.23 Researchers concluded that the decline in RBC mass was due to the selective destruction of circulating RBC that were less than 12 days old.24 Findings from a recent study evaluating space anemia indicate that spaceflight directly induces a persistent 54% increase in hemolysis via mechanisms independent of erythropoietin levels and fluid shifts.25
2.4. Vascular Dysfunction
Relative to research evaluating the effects of spaceflight on previously outlined systems, less is known regarding structural and functional adaptations of the vasculature. Following 5- to 18-day spaceflight missions, total arterial compliance was reduced,26, 27 and Hughson and colleagues28 reported that 6 months of spaceflight induced an increase in carotid artery stiffness similar to more than 10 years of healthy aging. In addition to effects on arterial vasculature, a recent study found that among 11 ISS astronauts, 6 demonstrated stagnant or retrograde flow in the internal jugular vein by early-flight (day 50).29 Importantly, one astronaut developed an occlusive internal jugular vein thrombus during spaceflight that was treated with pharmaceutical interventions,30 and a potential partial internal jugular vein thrombus was identified in another astronaut retrospectively. These findings indicate that additional monitoring is needed to characterize the prevalence of spaceflight-induced altered blood flow and venous thrombi in upper and lower vasculature, and identification of risk factors, underpinning mechanisms, and potential countermeasures are needed.
2.5. Exercise Intolerance
Exercise tests using bungee cords were first conducted before and after spaceflight on Mercury astronauts, who, after 8-34 hours of microgravity demonstrated reduced exercise tolerance compared to preflight.1 Remarkably, the first inflight exercise test was conducted in 1963 using a rudimentary bungee cord where elevated exercise heart rate and slower heart rate recovery were documented.1 To more accurately quantify exercise tolerance in Apollo astronauts, a graded submaximal exercise test with gas exchange was used to evaluate oxygen consumption (VO2), workload, heart rate, and blood pressure.9 Reduced VO2 and workload were documented in 74% of astronauts immediately post-flight.9 Based on these findings, researchers surmised “that man could not be committed to long duration spaceflight until the magnitude and time course of these changes could be established and the underlying physiological mechanisms understood.”9 The 3 Skylab missions were therefore designed to comprehensively characterize physiological changes during spaceflight and a mass spectrometer and cycle ergometer were specifically modified to allow for evaluation of exercise tolerance during spaceflight.10 In-flight, all Skylab astronauts exhibited increased resting heart rate, and decreased VO2 and heart rate recovery, while postflight, a significant decrement in VO2 was noted in all astronauts, primarily due to a 28% reduction in exercise cardiac output.10 Thirty-one days post-flight exercise cardiac output was still 15% lower than pre-flight, which was hypothesized to be due to altered venous return and not impaired myocardial function.10 It is noteworthy that until 1991, all in-flight exercise tests were submaximal due to safety concerns of maximal exercise precipitating a cardiovascular event. In the first study to evaluate peak oxygen consumption (VO2peak) in-flight, Levine et al.31 found that VO2peak was maintained during Shuttle missions, but was reduced by 22% immediately post-flight due to reduced peak cardiac output.31 Between 1993 and 2009, submaximal exercise tests were conducted in-flight due to a lack of metabolic gas analysis hardware. Heart rate data obtained during in-flight tests and submaximal VO2 data obtained during pre-flight tests were used to estimate VO2peak using a linear extrapolation method.32 This method was found to result in errors ranging from 58% over-prediction to 24% under-prediction of VO2peak compared with measured values.32 Accordingly, with new metabolic hardware launched in 2009, Moore et al.33 evaluated VO2peak in ISS astronauts and found that VO2peak was significantly decreased by the 15th day in-flight and remained decreased throughout spaceflight. 10 days post-flight VO2peak was still reduced by 15%, but recovered to pre-flight levels 30 days after return to Earth with post-flight exercise training.
2.6. Orthostatic Intolerance
Post spaceflight orthostatic intolerance was first observed after a Mercury astronaut became hypotensive during an upright 70° tilt test after only 34 hours of spaceflight.1 Thereafter, tilt testing was performed before and after spaceflight throughout Gemini Missions, where post-flight tilt tests consistently yielded increased heart rate, decreased pulse pressure and increased fluid pooling in the lower extremities for up to 50 hours after landing.9 Orthostatic tolerance testing was extended during the Apollo Program; however, because of easier instrumentation, control of different levels of stresses and potential for future inflight use, LBNP was implemented as a test for orthostatic intolerance.9 From these tests, it was concluded that “virtually every astronaut returning from space suffers some degree of orthostatic intolerance”.34 A systematic line of studies was conducted on subsequent Skylab, Shuttle, and ISS missions to understand the etiology of orthostatic intolerance after spaceflight.35,36 To evaluate whether autonomic dysfunction contributed to orthostatic intolerance, vagally mediated carotid baroreceptor-cardiac reflex responses (provoked by neck pressure changes) and change in heart rate and blood pressure from supine to standing was evaluated before and after 4- to 5-day Shuttle missions.34 On landing day, resting heart rate variability, and the slope, range, and position of operational points on the carotid transmural pressure-sinus node response relation were all reduced relative to preflight, suggesting that spaceflight-induced reductions in vagal control of the sinus node or a hypoadrenergic response could contribute to orthostatic intolerance.34 To discern individual susceptibility to orthostatic intolerance after spaceflight, Fritsch-Yelle et al.37 characterized hemodynamic and neuroendocrine responses to orthostatic stress before and after Shuttle missions. Astronauts were classified into presyncopal and nonpresyncopal groups based on the ability to remain standing without assistance for 10 minutes on landing day. Upon standing post-flight, presyncopal astronauts had significantly smaller increases in plasma norepinephrine levels, lower peripheral vascular resistance, and greater decreases in systolic, and diastolic pressures compared with nonpresyncopal astronauts. Together, these studies methodically assessing physiological effects contributing to orthostatic intolerance and characterizing inter-individual differences were critical to the implementation of targeted and effective countermeasures involving in-flight exercise training and volume resuscitation that now prevent orthostatic intolerance in over 95% of astronauts.38, 39
3. Mechanisms of Spaceflight-Induced Cardiovascular Changes
Given the challenges of conducting biological research in space, numerous models have been used to investigate the mechanisms underpinning the cardiovascular effects of microgravity and radiation. The most common in-vivo ground-based analog to simulate microgravity in humans is head-down tilt bed rest.40 Hindlimb unloading (HLU) has been used for over 30 years to simulate microgravity-induced fluid shifts in animal models,41 while more recent ground-based microgravity simulators such as 2D clinostats and rotating wall vessels have been used for in-vitro models. Compared to the approximately 2 millisievert (mSv) of radiation individuals are exposed to on Earth per year, astronauts are exposed to approximately 80 mSv during a 6-month ISS mission, and on exploration missions to the Moon or Mars astronauts may also be exposed to protons and high atomic number and energy particles.42, 43 Accordingly, the NASA Space Radiation Laboratory developed a method to simulate spaceflight radiation in models systems.44 Prior reviews have outlined many mechanisms related to spaceflight-induced cardiovascular changes.45, 46 Here, we focus on evidence from animal and in-vitro models that provide insight into biological pathways potentially contributing to arrythmias, cardiac atrophy, anemia, and vascular dysfunction (Figure 3) that likely contribute to whole-organism impairments such as orthostatic and exercise intolerance. Table 1 summarizes exemplar studies that evaluated the separate and combined effects of microgravity and radiation in model systems.
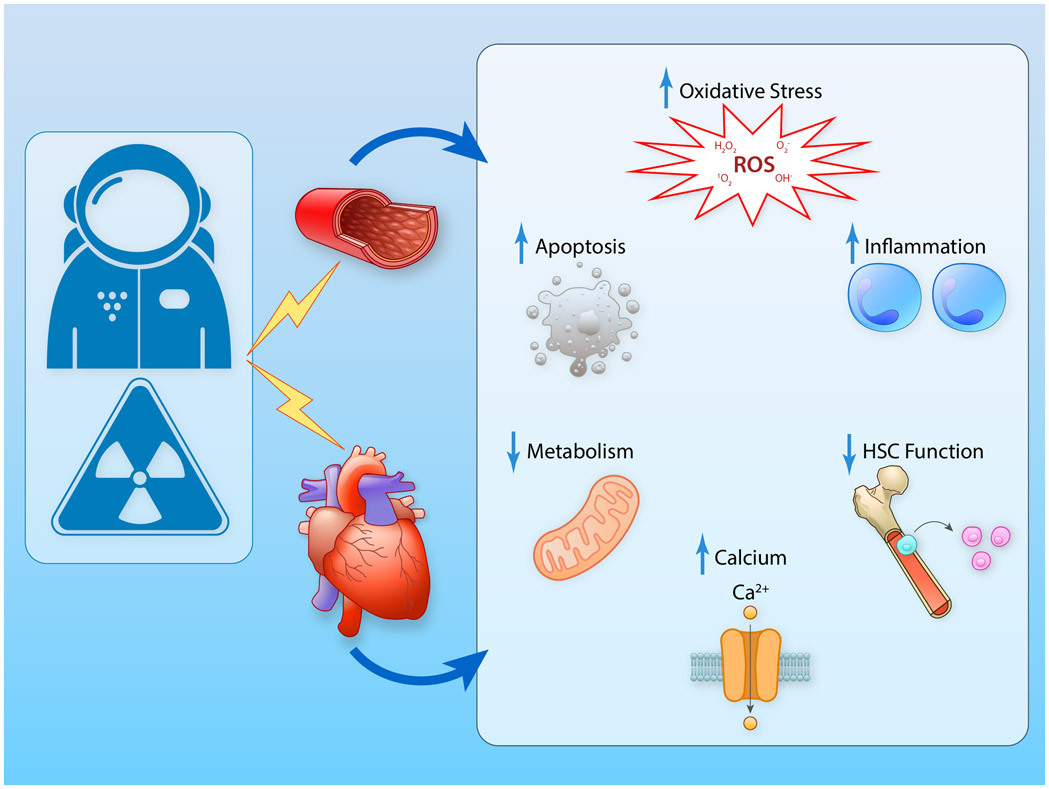
Figure 3. Mechanisms Potentially Underpinning the Adverse Cardiovascular Effects of Spaceflight.
Fluid shifts and radiation are known to induce adverse cardiac and vascular effects via increased oxidative stress, increased inflammation, increased apoptosis, decreased metabolism, alerted calcium handling, and decreased HSC function. HSC, hematopoietic stem cell function. (Illustration credit: Ben Smith).
Table 1.
Overview of model studies investigating the effects of radiation and/or fluid shifts on the cardiovascular system.
| Author | Model | Exposure | Results |
|---|---|---|---|
| Spaceflight radiation | |||
| Yan et al.93 | Male C57Bl/6NT mice; age: 8-10 mo | Single dose IR: Iron (56Fe 0.15Gy, 1 GeV/n) and proton (1H; 0.5 Gy, 1 GeV); WBE | ↓ systolic and diastolic function 1 mo post-IR ↑ cardiac hypertrophy 3 mo post-IR ↑ cardiac inflammatory marker infiltration 3 mo post-IR ↓ calcium handling |
| Sasi et al.94 | Male C57Bl/6NT mice; age: 8-9 mo | Multiple dose IR: 0.15Gy 56Fe →+ 3 × 56Fe + 1H; WBE | ↑ cardiac hypertrophy 1 mo post-IR ↓ diastolic function 1 mo post-IR ↓ NFATc4 activity 3 mo post-IR |
| Seawright et al.95 | Male C57Bl/6J mice; age: 6 mo | Single dose IR: 16O 0.1-1.0Gy, 600 MeV/n; WBE | ↑ cardiac hypertrophy 2 mo post-IR ↓ systolic function 3 mo post-IR ↑ cleaved caspase-3 activity 3 mo post-IR ↑ cardiac immune markers 3 mo post-IR |
| Koturbash et al. | Male C57BL/6J mice; age: 10 wks | Single dose IR: 56Fe 0.5 Gy ; WBE | DNA hypermethylation 90 days post-IR |
| Grabham et al.96 | HUVEC | 56Fe 1 Gy | ↓ vasculogenesis |
| Fluid shift | |||
| Wang et al.53 | Male Sprague Dawley rats; age: NR | 6 wks HLU | ↓ systolic and diastolic function ↓ cardiac mass ↓ cardiomyocyte contractile function ↓ cardiomyocyte glucose utilization |
| Liang et al.55 | Male C57BL/6 mice; age: 2 mo | 14 or 28 days HLU | ↓ systolic function ↓ cardiac mass ↑ cardiac ROS ↑ calpain |
| Respress et al.47 | C57Bl/6 mice (sex NR), age: NR | 28 days HLU | ↓ systolic function ↑ pacing-induced ventricular arrhythmias ↑ frequency of spontaneous sarcoplasmic reticulum calcium release events ↑ sarcoplasmic reticulum calcium leak |
| Cao et al.58 | Male C57BL/6N and SJL/JOrlIcoCrl mice; age: 3 mo | 28 days HLU | ↓ NK cells, B cells, and erythrocyte precursors in the bone marrow |
| Lui et al.49 | HL-1 cardiomyocytes | 2D clinostat | ↑ cytosolic calcium concentration ↑ cardiomyocyte atrophy |
| Feger et al.54 | Primary rat neonatal cardiomyocytes | Rotating wall vessel | ↓ protein turnover ↔ apoptosis ↑ mitochondrial protein translation |
| Spaceflight radiation + Fluid shift | |||
| Seawright et al.97 | Female C57BL/6J mice; age: 6 mo | 21 days low dose γ-IR (Co-57) WBE + HLU | ↑ cardiac ROS relative to IR or HLU alone ↓ cardiac methylation relative to IR or HLU alone |
| Wnorowski et al.81 | hiPSC-CMs | 5.5 wks on ISS | ↓ decreased calcium recycling rate ↑ upregulation of sarcomere genes ↓ in DNA damage and repair genes |
| Camberos et al.98 | Adult and neonatal cardiac progenitor cells | 30 days on ISS | ↑ induction of transcripts associated with stemness, cell cycle progression, cell differentiation, heart development, oxidative stress and focal adhesion |
Abbreviations: IR, ionizing radiation; Gy, Gray; GeV; gigaelectronvolt; LV, left ventricle; WBE, whole body exposure; HLU, hind limb unloading; NR, not reported; NFATc4, Nuclear Factor Of Activated T Cells 4; HUVEC; Human umbilical vein cells; ROS, reactive oxygen species; hiPSC-CMs, Human induced pluripotent stem cells – derived cardiomyocytes; ISS, International Space Station.
3.1. Arrhythmias
In addition to the combined effect of increased atrial volume and high heart rate during exercise training,8 altered calcium handling may be a key pathway involved in arrythmias.47, 48 Respress and colleagues47 reported that mice exposed to 28 days of HLU were more susceptible to pacing-induced ventricular arrhythmias relative to non-HLU mice, while ventricular myocytes from HLU mice exhibited an increased frequency of spontaneous sarcoplasmic reticulum calcium release events and enhanced sarcoplasmic reticulum calcium leak via cardiac ryanodine receptor. In support of these findings, using a 2D clinostat, Liu et al.49 found that cardiomyocytes exposed to microgravity for 48 h had a significant increase in basal cytosolic calcium, an increase in spontaneous calcium oscillations, as well as a decrease in myosin heavy chain alpha, a marker associated with cardiac remodeling.50 The direct effects of spaceflight radiation alone or combined with microgravity on cardiomyocyte calcium handling is not known. However, spaceflight radiation potentiates reactive oxygen species (ROS) production,51 that in turn, may induce abnormalities in calcium homeostasis and play a pivotal role in the pathogenesis of arrhythmias.52
3.2. Cardiac atrophy
Changes in cardiac morphology have largely been attributed to increased ROS, inflammation, alterations in cardiac energy metabolism,53 and ultrastructural changes to myocytes.54 For instance, HLU in mice reduces cardiomyocyte size, heart weight, and myocardial function via calpain activation and oxidative stress in heart tissues.55 Using a rotary cell culture system, Liang et al.55 simulated microgravity in cultured neonatal mouse cardiomyocytes and demonstrated that calpain facilitates p47 phox phosphorylation via ERK1/2 and p38 pathways. Given that calpains initiate turnover of regulatory and structural myofibrillar proteins,56 these findings suggest that microgravity-induced calpain activation may induce ultrastructural changes to cardiomyocytes. In mice exposed to silicon ions, Tungjai et al.57 demonstrated an increase in cardiac cleaved poly (adenosine diphosphate-ribose) polymerase, a marker of apoptosis, and markers of inflammation, such as increased activated nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), interleukin-1β and interleukin-6, and tumor necrosis factor-α. Intriguingly, unlike in astronauts, HLU- and radiation-induced cardiac atrophy is often coupled with impairments in cardiac systolic and diastolic function. These findings suggest that the commonly used 30° HLU and radiation models may be excessive stressors relative to current spaceflight missions, or that exercise countermeasures in astronauts are effective in offsetting declines in cardiac function but not changes in morphology.
3.3. Anemia
As recently reported in astronauts,25 increased hemolysis likely contributes to anemia; however, altered hematopoietic function may also be a key factor. Cao et al.58 reported that suppression of NK cells, B cells, and erythrocyte precursors in the bone marrow were observed following HLU in mice. These alterations may be due to disrupted cytoskeleton of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and differentially expressed genes (DEG). For instance, the expression levels of hematopoietic-related genes, such as fms-like tyrosine kinase-3 ligand, granulocyte-macrophage colony stimulating factor, interleukin-3, and adipogenic differentiation associated genes, leptin and proliferator-activated receptor γ type 2, were upregulated following HLU in mice.59 Furthermore, using HLU combined with continuous low-dose gamma irradiation, Paul et al.60 reported that the majority of spleen cells displayed DEG involved in signal transduction, metabolism, cell cycle, chromatin organization, and DNA repair, which was coupled with significant reductions in RBC and hemoglobin 7 days post-exposure. These findings collectively suggest that hemolysis coupled with altered proliferation and differentiation of myeloid progenitor cells contribute to spaceflight-related anemia.
3.4. Vascular dysfunction
Endothelial cells are continuously exposed to various hemodynamic forces and are therefore sensitive to changes in fluid dynamics that occur in microgravity and are also impacted by radiation exposure. After 10 days of exposure to microgravity on the ISS, Versari et al.61 reported there were 1023 DEG involved in cell adhesion, oxidative phosphorylation, stress responses, cell cycle, and apoptosis in human umbilical vein endothelial cells. Simulated space radiation also impairs endothelium-dependent vasodilation of the aorta and increases aortic stiffness via increased xanthine oxidase activity and ROS production and decreased nitric oxide production.62, 63 Thus, reduced bioavailability of nitric oxide from increased ROS is a likely pathway for endothelial dysfunction and increased vascular stiffness. In evaluation of the synergistic effects of radiation and microgravity in mice, Ghosh et al.64 reported that simulated space radiation and HLU alone each impaired endothelium-dependent vasodilation, but impairments were potentiated following combined radiation and HLU due to alteration in endothelial nitric oxide synthase signaling pathway. Finally, spaceflight may also contribute to atherosclerosis. Yu et al.65 reported accelerated development of atherosclerotic legions with large necrotic cores in regions of the aorta exposed to simulated spaceflight radiation in apolipoprotein E-deficient mice.
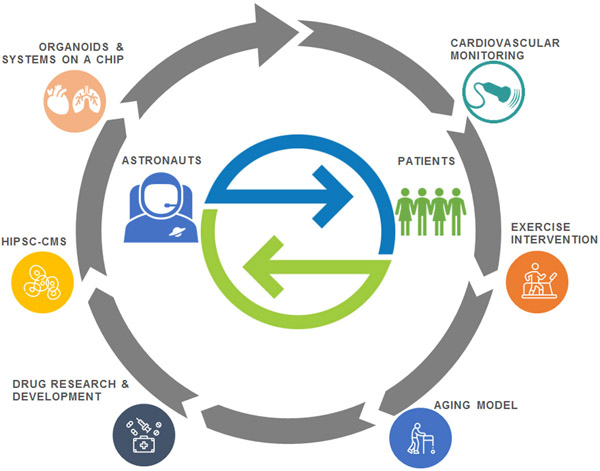
4. Leveraging Spaceflight to Advance Cardiovascular Research on Earth
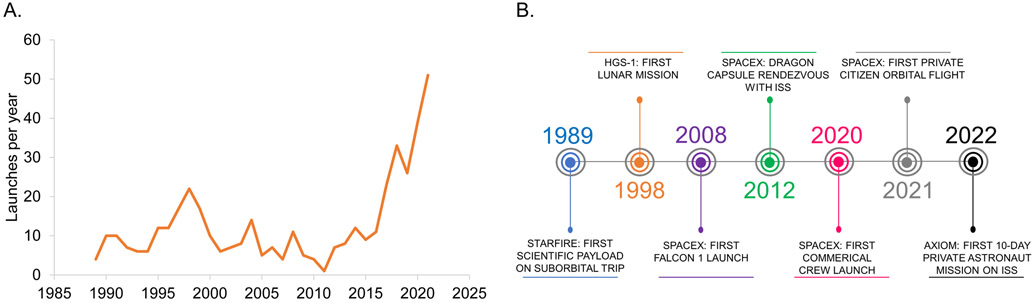
A relative explosion in the promotion and development of commercial spaceflight has occurred in the past decade (Figure 4A).66 With over 85 commercial spaceflight companies and organizations in the United States alone,67 commercial entities have improved access to the unique microgravity and radiation conditions of spaceflight for both clinical and basic research (Figure 4B). As outlined here, we posit that increased access to space coupled with advances in technology may provide unprecedented opportunities to expand cardiovascular research.
Figure 4. Timeline of Commercial Spaceflight.
(A) Number of commercial spaceflight launches per year since inception in 1989. (B) Key commercial spaceflight events over the past 30 years.
4.1. Spaceflight as a Model of Accelerated Aging
The physiological changes coupled with cellular, molecular, and genomic alterations4 suggest that spaceflight may be an exemplar model to study physiological aging sequelae, although many of the underlying biological factors remain to be elucidated (Table 2).68
Table 2.
Spaceflight as a Model of Accelerated Aging.
| ~6 months of Spaceflight + Exercise | ~10 years of Healthy Aging | |
|---|---|---|
| Physiological | ||
| Cardiac Morphology | ↔16 or ↓15 LV mass | ↑99 LV wall thickness ↓99 LV mass |
| Cardiac Function | ↔16 systolic function ↓15 diastolic function |
↔100 systolic function ↓100 diastolic function |
| Cardiac Conduction | ↑8 risk of atrial fibrillation | ↑101 risk of atrial fibrillation |
| Vascular Morphology | ↑102 arterial size above the heart ↑102 intima media thickness |
↑103 arterial size ↑104 intima media thickness |
| Vascular Function | ↑28 arterial stiffness ↓29 jugular venous flow |
↑105 arterial stiffness ↓106 jugular venous flow |
| Orthostatic Tolerance | ↔38, 39 risk of orthostatic intolerance with fluid countermeasures | ↑107 risk |
| Hematological | ↓25 red blood cells | ↓108 red blood cells |
| Exercise Tolerance | ↓5 peak oxygen consumption | ↓109 peak oxygen consumption |
| Spaceflight Model | Aging Model | |
| Biological | ||
| Genomic Stability | ↑110 genomic instability | ↑111 genomic instability |
| Telomere Length | ↑4 telomere length | ↓112 telomere length |
| Epigenetics | ↑113 epigenetic alterations | ↑ 114epigenetic alterations |
| Proteostasis | ↓115 proteostasis (↓ drosophila; ? other models) | ↓116 proteostasis |
| Mitochondrial Function | ↑53 mitochondrial dysfunction | ↑117 mitochondrial dysfunction |
| Cellular Division | ↑118 cellular senescence | ↑68 cellular senescence |
| Stem Cell Function | ↑58 stem cell exhaustion | ↑119 stem cell exhaustion |
| Intracellular Communication | ↑93 altered intercellular communication | ↑120 altered intercellular communication |
4.1.1. Physiological Aging
Similar to other gerontogenic stressors such as cancer therapy,69 prolonged spaceflight exposure not only results in cardiovascular changes that recapitulate ~10 years of aging,5, 28 but also causes other aging-related physiological changes such as muscle atrophy and bone loss.6 Many acute (i.e., during spaceflight) changes appear reversible with postflight rehabilitation, but may ultimately contribute to chronic (i.e., months post-spaceflight) age-related health conditions such as cardiovascular disease and an increased risk of mortality compared to non-astronaut controls.3 Given that these alterations occur on ~6 month ISS missions, assessing aging sequelae using serial monitoring is feasible within a relatively short timeframe unlike other human aging models.69 As previously noted, spaceflight-related aging changes occur despite robust exercise interventions before and during ISS missions; thus, spaceflight represents a platform to evaluate adjunct intervention strategies (e.g., pharmacologic,70 nutrition71) to offset aging phenotypes.
4.1.2. Biological Aging
Several recurrent biological features of spaceflight are similar to molecular and cellular hallmarks of aging72 including oxidative stress, DNA damage, mitochondrial dysregulation, epigenetic/regulatory changes, and shifts in host-microbe interactions.45 Although there is an abundance of studies evaluating the biological effects of spaceflight on the cardiovascular system, several critical questions pertaining to fidelity of model systems and mechanisms underpinning response remain. First, HLU and space radiation studies have primarily been performed in sedentary male mice ranging between 10 weeks and 10 months of age. However, exercise is a mandatory intervention during spaceflight,6 there are well defined differences between males and females in cardiovascular response in astronauts,28 and the average astronaut is ~48 years old.5 Recapitulation of effects in female animal models, older models (e.g., mice aged 10 to 14 months), and including factors like exercise will be needed to improve understanding of a complex biological process and translation to astronauts. Second, evidence to date is largely correlational. There is a need for causal evidence using gain- or loss-of-function in spaceflight and/or spaceflight analogs to elucidate mechanistic links among pathways. Finally, the mechanisms underpinning several cardiovascular effects are not known. For example, given the likely involvement of venous and lymphatic vessels in the development of spaceflight-associated venous thrombosis,30 more research is needed to understand the effects of microgravity and/and space radiation on venous and lymphatic vessels. Addressing these, and other challenges will be critical to facilitate investigation of spaceflight as model of accelerated biological aging.
4.1.3. Integrating Physiological and Biological Models
Recent findings suggest that an integrated approach that combines multiple “omic” data from humans and model systems could be used to define molecular etiologies of adverse cardiovascular effects. For instance, in analyses including various human cell models, tissues, mouse strains (C57BL/6 and BALB/C), and astronaut blood and urine samples, da Silveira and colleagues73 identified that the effects of spaceflight on mitochondrial function at the genetic, protein, and metabolite levels was the key factor impacting innate immunity, lipid metabolism, and gene regulation. The effects of spaceflight were, however, more evident in isolated cells than in whole organs.73 These, and other analyses,74 were feasible because of NASA’s organized platform for deposition, curation, analysis and visualization of complex multi-parametric spaceflight and spaceflight analog data from a host of biological model systems.75 Evaluation of integrated multi-parametric data from several model organisms supports the notion that metadata from various model systems can help catalyze hypothesis-driven investigations.76, 77
4.2. Cardiovascular Drug Research and Development in Space
On the ISS, sedimentation and convection currents are minimized which may help optimize manufacturing and storage of biologics. For instance, in evaluation of Merck’s monoclonal antibody Keytruda® (pembrolizumab) on the ISS, crystalline suspensions produced in microgravity had lower viscosity and were more uniform than ground controls.78 Identification of conditions for producing crystalline suspensions of homogenous particle size and distribution could enable drug delivery via subcutaneous injection versus intravenous dosing. This opportunity has implications for drug purification and storage while improving drug delivery options that align with patient preferences and optimize time in hospital/drug scheduling. While there are past examples of new crystal forms being generated on the ISS that were used for drug targeting and discovery,79 the application of uniform crystals as a therapeutic was not previously recognized as an area of focus or benefit. The continued development of state-of-the-art capabilities for iterative experiments with real time on-orbit analysis, and the increasing access to the microgravity environment in low Earth orbit through the growing number of launches and launch providers creates new research opportunities for the development of novel therapeutics and for manufacturing the growing number of biologics and cell-based therapies.
4.3. Cardiovascular hiPSC-CMs in Space
hiPSC-CMs have emerged as a robust model for studying the molecular and cellular mechanisms of cardiac pathophysiology.80 In a proof-of-concept study, Wnorowski and colleagues81 demonstrated the feasibility of long-term cell culture of hiPSC-CMs on the ISS. Specifically, monolayers of beating hiPSC-CMs from 3 individuals were plated in BioCell, a fully contained cell culture vessel, and launched to the ISS on a SpaceX Dragon spacecraft. BioCells were cultured in a high-nutrient CM maintenance medium that was changed weekly and maintained aboard the ISS in an on-station incubator (Space Automated Bioproduct Laboratory [SABL]) at 37°C and 5% CO2. After sample return from the ISS, cellular phenotypes were evaluated using gene expression, immunofluorescence, calcium imaging, and contractility analyses. One challenge for such approaches, however, is that most iPSC-derived cells are functionally immature and exhibit fetal-like features. Whether spaceflight could accelerate large-scale, high-quality, patient-specific iPSC-CMs for drug development and disease modeling is not known; however, the microgravity environment could provide reduced heterogeneity and protocols for maturation strategies in a single unit to reduce variability. This work represents the first long-duration (30-day) culture of human iPSC’s on the ISS and has generated expanded interest in human iPSC research in microgravity for a variety of cell and tissue types. For instance, Baio and colleagues82 demonstrated that human neonatal cardiovascular progenitor cells (CPCs) exposed to microgravity for 30 days exhibited characteristics of a slightly earlier stage of development compared to adult CPCs exposed to microgravity, as well ground controls. This slight de-differentiation is thought to be associated with enhanced “stemness” (i.e., making the CPCs behave more like stem cells and enhancing potential to develop into different types of cardiovascular cells). In the neonatal CPCs, calcium signaling and Protein kinase B (Akt) signaling were both activated in response to spaceflight.82 Akt is an important molecule in promoting pluripotency and ability of a stem cell to continue to divide, expand and retain its stem-like state.83 The neonatal CPCs grown in microgravity were also found to have enhanced proliferation, while both the adult and neonatal CPCs demonstrated enhanced ability to migrate after exposure to microgravity.82 This work suggests that spaceflight may hold promise to address some of the challenges associated with cardiovascular regenerative medicine therapies.84
4.4. Organoids and Cardiovascular System-on-a-chip in Space
The development of organs-on-a-chip has advanced cell-culture experiments with multi-layered and interconnected tissue architectures that can mimic human physiology and pathophysiology.85 Organoids are 3 dimensional models that incorporate organ-specific cell types derived from stem cells or progenitors to recapitulate organ function and interactions between multiple cell types.86 Using iPSCs in microphysiological systems (MPS or ‘tissue chips’), and organoid models, provides an opportunity to study human disease models for preclinical safety testing, drug development, and testing of therapeutics, and personalized medicine applications.85 Studying these human analog models in microgravity provides an opportunity to understand disease mechanisms at a cellular level in an environment that mimics accelerated aging. Understanding aging sequelae, could in turn, accelerate the development of new therapeutic interventions. Current ISS National Laboratory (CASIS) collaborations with NIH, National Center for Advancing Translational Sciences (NCATS), and the National Science Foundation (NSF) are evaluating a variety of tissue chip organ systems, including blood brain barrier, intestinal, lung, skeletal muscle, kidney, and cardiovascular systems.87, 88 As part of the NSF-CASIS Collaboration on Tissue Engineering, Xu and colleagues89 will be assessing stem cell derived cardiac microtissues in space. The goal of the project is to establish a multipronged approach combining microgravity, tissue engineering, and metabolic regulation to promote maturation of hiPSC-CMs. Building on the 2D cardiomyocyte work done by Wnorowski and colleagues,81 Wu and colleagues90 will be sending 3D hiPSC-CM organoid tissue chip models to the ISS as part of the NIH Tissue Chips in Space program. The team has successfully completed the first phase of the project which involved sending 3D hiPSC-CMs fabricated into a well characterized engineered heart tissue platform to the ISS. Subsequent missions will use 3D hiPSCs containing the induced disease phenotype determined from alterations in cardiac function due to weakened heart muscles noted in samples exposed to microgravity to screen potential drug candidates.
5. Research and Funding Opportunities to Advance Cardiovascular Research in Space and on Earth
Given the nascent utilization of spaceflight in settings outside of NASA, Table 3 provides an overview of ongoing exemplar studies evaluating cardiovascular model systems in space. Calls for additional projects are released several times per year. For instance, NASA’s Science Mission Directorate’s Biological and Physical Sciences Division recently released a Research Announcement for investigations of Extended Longevity of 3D Tissues and Microphysiological Systems for Modeling of Acute and Chronic Exposure Stressors.91 This is a multi-agency solicitation sponsored by NASA’s Human Exploration and Operations Mission Directorate Human Research Program, NCATS, the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, the Department of Health and Human Services Biomedical Advanced Research and Development Authority, and the Food and Drug Administration, to solicit for research in support of common cross-organizational goals. The research announcement focuses on ground studies aimed at adapting existing 3D tissues and microphysiological systems (tissue chips or organs on chips) to extend the current longevity of these systems to at least 6 months. Advances in Earth-based technologies are also of high interest for NASA. Recently NASA’s Space Health Institute awarded five ground-breaking research grants to mitigate the effects of space radiation on healthy human cell-derived organs-on-chips that include intestinal, vascular, neural, and cardiac models.92 These human analog systems have the opportunity to provide significant utility in preparing for upcoming longer-duration missions both in terms of understanding the long-duration radiation and microgravity human health effects of space travel as well as countermeasure development. Collectively, there is now an unparalleled opportunity for bi-directional translation of knowledge to advance cardiovascular research in space and on Earth (Figure 5).
Table 3.
Ongoing Studies Leveraging Spaceflight for Earth-based research.
| Research Announcement |
Study Title | Institution/Location | Objectives |
|---|---|---|---|
| NIH –NCATS Tissue Chips in Space 1.0 (2017) | Organs-on-Chips as a Platform for Studying Effects of Microgravity on Human Physiology: Blood-Brain-Barrier-Chip in Health and Disease | Emulate, Inc./MA | To validate, optimize and further develop Emulate’s proprietary Organs-On-Chips technology platform for experimentation with human cells in space |
| NIH –NCATS Tissue Chips in Space 1.0 (2017) | Cartilage-Bone-Synovium Microphysiological System: Musculoskeletal Disease Biology in Space | Massachusetts Institute of Technology/MA | To study the effects of space flight on musculoskeletal disease biology, motivated by post-traumatic osteoarthritis and bone loss |
| NIH –NCATS Tissue Chips in Space 1.0 (2017) | Microgravity as Model for Immunological Senescence and its Impact on Tissue Stem Cells and Regeneration | University of California, San Francisco/CA | To investigate the relationship between an individual’s immune aging and healing outcomes, and to investigate the biology of aging during microgravity conditions and also during recovery after returning to Earth’s environment |
| NIH –NCATS Tissue Chips in Space 1.0 (2017) | Effects of Microgravity on the Structure and Function of Proximal and Distal Tubule Microphysiological System | University of Washington/WA | To understand how microgravity and other factors affect kidney function |
| NIH –NCATS Tissue Chips in Space 1.0 (2017) | Lung Host Defense in Microgravity | Children’s Hospital of Philadelphia/PA | To test engineered microphysiological systems, or tissue chips, that model the airway and bone marrow; and to combine the models to emulate and understand the integrated immune responses of the human respiratory system in microgravity |
| NIH –NCATS Tissue Chips in Space 2.0 (2018) | Organ-Chips as a Platform for Studying Effects of Space on Human Enteric Physiology: Interactions of Epithelial Mucosa with Sensory Neurons and Microbiome | Emulate, Inc./MA | To further demonstrate Emulate's proprietary Organs-Chips technology applicability by developing the human innervated Intestine-Chip (hiIC) and the cellular, molecular, and immune responses of the system in the unique environment of space |
| NIH –NCATS Tissue Chips in Space 2.0 (2018) | Electrical Stimulation of Human Myocytes in Microgravity: An In Vitro Model to Evaluate Therapeutics to Counteract Muscle Wasting | University of Florida/FL | To refine a tissue chip to study muscle cells and how they respond to stimulation in regular and low-gravity environments |
| NIH –NCATS Tissue Chips in Space 2.0 (2018) | Effect of Microgravity on Drug Responses Using Engineered Heart Tissues | Stanford University/CA | To develop a mini 3-D model of beating heart tissue and use this model to document the ways low gravity causes changes in the structure and function of heart tissue and find out if returning to a normal-gravity environment reverses these effects |
| NIH –NCATS Tissue Chips in Space 2.0 (2018) | A Human iPSC-Based 3-D Microphysiological System for Modeling Cardiac Dysfunction in Microgravity | University of Washington/WA; Johns Hopkins University/MD | To compare heart tissue generated from iPSCs in regular and low-gravity environments and to improve the heart cells’ contractions |
| NSF-CASIS Tissue Engineering (2018-2019) | ISS: Liver Tissue Engineering in Space | University of California, San Francisco/CA | To create a macroscopic, vascularized liver tissue and to characterize the effect of microgravity and directional angiogenic gradients on 3D intercellular interactions and microvascular organization |
| NSF-CASIS Tissue Engineering (2018-2019) | ISS: Tissue Engineered Muscle in Microgravity as a Novel Platform to Study Sarcopenia | Stanford University/CA | To design and characterize an in vitro engineered skeletal muscle platform in microgravity to model sarcopenia |
| NSF-CASIS Tissue Engineering (2018-2019) | ISS: Microphysiologic Model of Human Cardiovascular Stiffness-Related Diseases in Microgravity | Icahn School of Medicine at Mount Sinai/NY | To characterize a multi-tissue in vitro microfluidic human organoid model of the cardiovascular system, to test micro-CV chips on the ISS, and to identify novel disease biomarkers and pathways post-flight |
| NSF-CASIS Tissue Engineering (2018-2019) | ISS: Cellular Mechanotransduction by Osteoblasts in Microgravity | University of Michigan/MI | To determine if microgravity affects osteoblast mechanosensitivity and apply mechanical compression to osteoblasts to see if they recover their mechanosensitivity post-flight |
| NSF-CASIS Tissue Engineering (2018-2019) | ISS/Collaborative Research: Studying the Effects of Microgravity on 3D Cardiac Organoid Cultures | University of Texas, El Paso/TX; Texas A&M/TX | To compare and contrast the morphology, viability, and altered energy metabolism in 3D bioprinted cardiac organoids under microgravity and Earth's gravity and to study the epigenetic changes in 3D bioprinted cardiac organoids under microgravity and assess how these changes may affect the development of cardiac atrophy when compared to Earth's gravity |
| National Stem Cell Foundation (2019-2020) | The Effects of Microgravity on Microglia 3-Dimensional Models of Parkinson’s Disease and Multiple Sclerosis | The Scripps Research Institute/CA; New York Stem Cell Foundation/NY | To examine how microglial cells grow and move in three-dimensional (3D) cultures as well as any changes in gene expression that occur as a result of microgravity exposure |
| NSF-CASIS Tissue Engineering (2020-2021) | ISS: Collaborative Research: 3D Bone Marrow Analogs to Determine the Contribution of Mechanical Signals to Aging MSC Function | Boise State University/ID; Rensselaer Polytechnic Institute/NY | To develop a 3D printed bone marrow analog system that combines an in vivo environment with the accessibility of an in vitro culture system |
| NSF-CASIS Tissue Engineering (2020-2021) | ISS: Engineering Multiple-Compartment Cartilage Tissue Construct for Space and Terrestrial Applications | University of Connecticut/CT | To create a construct which can automatically supply itself with mechano-responsive microRNA as a therapy to restore cartilage cell chondrogenesis |
| NSF-CASIS Tissue Engineering (2020-2021) | ISS: Unveiling the Mechanical Roles of Gravity and Buoyancy in Embryonic Brain and Heart Torsion | Dartmouth College/NH | To identify the regulative role of physical forces in the early brain and heart development |
| NSF-CASIS Tissue Engineering (2020-2021) | ISS: Mechanisms of Microgravity Accelerated Aging on Human Brain Organoids | University of California, San Diego/CA | To mechanistically investigate aging phenotypes on human brain organoids infused with microglia |
| NSF-CASIS Tissue Engineering (2020-2021) | ISS: Quantifying the Effect of Unloading on Extracellular Matrix Remodeling in the Musculoskeletal System | University of Colorado/CO | To investigate temporal changes in extracellular matrix proteins in response to, and after recovery from, microgravity |
| NSF-CASIS Tissue Engineering (2020-2021) | ISS: Chip-Based in vitro Modeling of Endocortical Microenvironment with Reduced Gravitational Loading | University of Minnesota/MN | To evaluate cellular response to microgravity in monocultures osteoblasts and osteoclasts |
Abbreviations: NIH, National Institute of Health; NCATS, National Center for Advancing Translational Sciences; CAISIS, Center for the Advancement of Science in Space; ISS, International Space Station
Figure 5. Bi-directional Translation of Knowledge to Advanced Cardiovascular Research in Space and on Earth.
Conceptual model outlining the translation of model systems, clinical technologies, and interventions to accelerate cardiovascular research for human spaceflight and patients on Earth.
Conclusion
The multisystem physiological consequences of spaceflight have been characterized for over 50 years,1 while improvements in spaceflight analogs and increased access to space over the past decade have provoked investigation into the underlying biological effects of spaceflight including oxidative stress, DNA damage, mitochondrial dysregulation, epigenetic/regulatory changes, telomere-length dynamics, and shifts in host-microbe interactions.45 Although future studies are required to further elucidate the mechanisms underlying the effects of microgravity and spaceflight radiation on the cardiovascular system during and after exposure, evidence reviewed here indicates that spaceflight could be leveraged to advance cardiovascular research on Earth. Likewise, tissue chip and human analog models are of high interest to NASA for the potential development of countermeasures for astronauts. Compared to research on model organisms, they could be constructed from an astronaut’s own iPSCs to allow development of personalized countermeasures prior to spaceflight, or to collect cellular level data simultaneously with physiological measurements and routine blood, urine, and saliva samples during a mission. Integration of spaceflight and Earth-based cardiovascular research could help synchronize and potentiate advances to improve both astronaut health on exploration missions and patient health on Earth.
Sources of Funding:
This work is supported by the NASA Human Research Program, the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748), and the National Cancer Institute of the National Institutes of Health under Award Number R37CA248665 (JMS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Nonstandard Abbreviations and Acronyms:
- Akt
protein kinase B
- CPC
cardiovascular progenitor cell
- HLU
hindlimb unloading
- LV
left ventricular
- NASA
National Aeronautics and Space Administration
- NF-κB
nuclear factor kappa-light-chainenhancer of activated B cells RBC red blood cell
Footnotes
Declaration of Interests: JS is an employee of Axiom Space. LD will be an employee of Zymeworks Inc. JMS and MD declare no conflicts of interest.
References
- 1.Kleinknecht K, Bland W. Mercury project summary. 1963 [Google Scholar]
- 2.Hargens AR, Richardson S. Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respiratory physiology & neurobiology. 2009;169 Suppl 1:S30–33 [DOI] [PubMed] [Google Scholar]
- 3.Delp MD, Charvat JM, Limoli CL, Globus RK, Ghosh P. Apollo lunar astronauts show higher cardiovascular disease mortality: Possible deep space radiation effects on the vascular endothelium. Sci Rep. 2016;6:29901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garrett-Bakelman FE, Darshi M, Green SJ, Gur RC, Lin L, Macias BR, McKenna MJ, Meydan C, Mishra T, Nasrini J, Piening BD, Rizzardi LF, Sharma K, Siamwala JH, Taylor L, Vitaterna MH, Afkarian M, Afshinnekoo E, Ahadi S, Ambati A, Arya M, Bezdan D, Callahan CM, Chen S, Choi AMK, Chlipala GE, Contrepois K, Covington M, Crucian BE, De Vivo I, Dinges DF, Ebert DJ, Feinberg JI, Gandara JA, George KA, Goutsias J, Grills GS, Hargens AR, Heer M, Hillary RP, Hoofnagle AN, Hook VYH, Jenkinson G, Jiang P, Keshavarzian A, Laurie SS, Lee-McMullen B, Lumpkins SB, MacKay M, Maienschein-Cline MG, Melnick AM, Moore TM, Nakahira K, Patel HH, Pietrzyk R, Rao V, Saito R, Salins DN, Schilling JM, Sears DD, Sheridan CK, Stenger MB, Tryggvadottir R, Urban AE, Vaisar T, Van Espen B, Zhang J, Ziegler MG, Zwart SR, Charles JB, Kundrot CE, Scott GBI, Bailey SM, Basner M, Feinberg AP, Lee SMC, Mason CE, Mignot E, Rana BK, Smith SM, Snyder MP, Turek FW. The nasa twins study: A multidimensional analysis of a year-long human spaceflight. Science. 2019;364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.English KL, Downs M, Goetchius E, Buxton R, Ryder JW, Ploutz-Snyder R, Guilliams M, Scott JM, Ploutz-Snyder LL. High intensity training during spaceflight: Results from the nasa sprint study. NPJ Microgravity. 2020;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott JM, Dolan LB, Norton L, Charles JB, Jones LW. Multisystem toxicity in cancer: Lessons from nasa's countermeasures program. Cell. 2019;179:1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strughold H, Benson OO Jr. Space medical research. N Engl J Med. 1959;261:494–502 [DOI] [PubMed] [Google Scholar]
- 8.Khine HW, Steding-Ehrenborg K, Hastings JL, Kowal J, Daniels JD, Page RL, Goldberger JJ, Ng J, Adams-Huet B, Bungo MW, Levine BD. Effects of prolonged spaceflight on atrial size, atrial electrophysiology, and risk of atrial fibrillation. Circ Arrhythm Electrophysiol. 2018;11:e005959. [DOI] [PubMed] [Google Scholar]
- 9.Hoffler G, Johnson R. Apollo flight crew cardiovascular evaluations. Biomedical results of apollo. . 1975 [Google Scholar]
- 10.Dietlein LF. The proceedings of the skylab life sciences symposium. 1974 [Google Scholar]
- 11.Fritsch-Yelle JM, Leuenberger UA, D’Aunno DS, Rossum AC, Brown TE, Wood ML, Josephson ME, Goldberger AL. An episode of ventricular tachycardia during long-duration spaceflight. Am J Cardiol. 1998;81:1391–1392 [DOI] [PubMed] [Google Scholar]
- 12.Hamilton DR, Murray JD, Kapoor D, Kirkpatrick AW. Cardiac health for astronauts: Current selection standards and their limitations. Aviation, space, and environmental medicine. 2005;76:615–626 [PubMed] [Google Scholar]
- 13.Bkorck G, Vendsalu A, Johansson S. Studies in functional heart disease. Ii. The roentgenological heart volume. Acta Med Scand. 1957;159:443–452 [DOI] [PubMed] [Google Scholar]
- 14.Popp RL, Harrison DC. Ultrasonic cardiac echography for determining stroke volume and valvular regurgitation. Circulation. 1970;41:493–502 [DOI] [PubMed] [Google Scholar]
- 15.Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol. 2001;91:645–653 [DOI] [PubMed] [Google Scholar]
- 16.Abdullah SM, Hastings JL, Shibata S, Platts SH, Hamilton DR, Thomas JD, Hughes DE, Bungo MW, Levine BD. Abstract 18672: Effects of prolonged space flight on cardiac structure and function. Circulation. 2018;128 [Google Scholar]
- 17.Levine BD. .Vo2max: What do we know, and what do we still need to know? J Physiol. 2008;586:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeitler EO, Rogers TG. The gemini program: Biomedical sciences experiments summary. 1971 [Google Scholar]
- 19.Fischer CL, Johnson PC, Berry CA. Red blood cell mass and plasma volume changes in manned space flight. JAMA. 1967;200:579–583 [PubMed] [Google Scholar]
- 20.Alfrey CP, Udden MM, Leach-Huntoon C, Driscoll T, Pickett MH. Control of red blood cell mass in spaceflight. J Appl Physiol (1985). 1996;81:98–104 [DOI] [PubMed] [Google Scholar]
- 21.Lane HW, Alfrey CP, Driscoll TB, Smith SM, Nyquist LE. Control of red blood cell mass during spaceflight. J Gravit Physiol. 1996;3:87–88 [PubMed] [Google Scholar]
- 22.Rice L, Alfrey CP. Modulation of red cell mass by neocytolysis in space and on earth. Pflugers Arch. 2000;441:R91–94 [DOI] [PubMed] [Google Scholar]
- 23.Alfrey CP, Udden MM, Huntoon CL, Driscoll T. Destruction of newly released red blood cells in space flight. Med Sci Sports Exerc. 1996;28:S42–44 [DOI] [PubMed] [Google Scholar]
- 24.Alfrey CP, Rice L, Udden MM, Driscoll TB. Neocytolysis: Physiological down-regulator of red-cell mass. Lancet. 1997;349:1389–1390 [DOI] [PubMed] [Google Scholar]
- 25.Trudel G, Shahin N, Ramsay T, Laneuville O, Louati H. Hemolysis contributes to anemia during long-duration space flight. Nat Med. 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuday EC, Meck JV, Nyhan D, Shoukas AA, Berkowitz DE. Microgravity-induced changes in aortic stiffness and their role in orthostatic intolerance. J Appl Physiol (1985). 2007;102:853–858 [DOI] [PubMed] [Google Scholar]
- 27.Baevsky RM, Baranov VM, Funtova II, Diedrich A, Pashenko AV, Chernikova AG, Drescher J, Jordan J, Tank J. Autonomic cardiovascular and respiratory control during prolonged spaceflights aboard the international space station. J Appl Physiol (1985). 2007;103:156–161 [DOI] [PubMed] [Google Scholar]
- 28.Hughson RL, Robertson AD, Arbeille P, Shoemaker JK, Rush JW, Fraser KS, Greaves DK. Increased postflight carotid artery stiffness and inflight insulin resistance resulting from 6-mo spaceflight in male and female astronauts. Am J Physiol Heart Circ Physiol. 2016;310:H628–638 [DOI] [PubMed] [Google Scholar]
- 29.Marshall-Goebel K, Laurie SS, Alferova IV, Arbeille P, Aunon-Chancellor SM, Ebert DJ, Lee SMC, Macias BR, Martin DS, Pattarini JM, Ploutz-Snyder R, Ribeiro LC, Tarver WJ, Dulchavsky SA, Hargens AR, Stenger MB. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw Open. 2019;2:e1915011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aunon-Chancellor SM, Pattarini JM, Moll S, Sargsyan A. Venous thrombosis during spaceflight. N Engl J Med. 2020;382:89–90 [DOI] [PubMed] [Google Scholar]
- 31.Levine BD, Lane LD, Watenpaugh DE, Gaffney FA, Buckey JC, Blomqvist CG. Maximal exercise performance after adaptation to microgravity. J Appl Physiol. 1996;81:686–694 [DOI] [PubMed] [Google Scholar]
- 32.Downs M, Moore AD Jr., Lee SM, Ploutz-Snyder L, Stenger MB, Phillips T, Summers RL, Feeback D, Platts SH. Risk of reduced physical performance capabilities due to reduced aerobic capacity. 2015 [Google Scholar]
- 33.Moore AD Jr. Downs ME, Lee SM, Feiveson AH, Knudsen P, Ploutz-Snyder L. Peak exercise oxygen uptake during and following long-duration spaceflight. J Appl Physiol (1985). 2014;117:231–238 [DOI] [PubMed] [Google Scholar]
- 34.Fritsch JM, Charles JB, Bennett BS, Jones MM, Eckberg DL. Short-duration spaceflight impairs human carotid baroreceptor-cardiac reflex responses. J Appl Physiol (1985). 1992;73:664–671 [DOI] [PubMed] [Google Scholar]
- 35.Buckey JC Jr., Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, Blomqvist CG. Orthostatic intolerance after spaceflight. J Appl Physiol. 1996;81:7–18 [DOI] [PubMed] [Google Scholar]
- 36.Meck JV, Reyes CJ, Perez SA, Goldberger AL, Ziegler MG. Marked exacerbation of orthostatic intolerance after long- vs. Short-duration spaceflight in veteran astronauts. Psychosom Med. 2001;63:865–873 [DOI] [PubMed] [Google Scholar]
- 37.Fritsch-Yelle JM, Whitson PA, Bondar RL, Brown TE. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. J Appl Physiol (1985). 1996;81:2134–2141 [DOI] [PubMed] [Google Scholar]
- 38.Lee SM, Feiveson AH, Stenger MB, Stein SP, Platts SH. Orthostatic hypotension after long-duration space flight: Nasa’s experiences from the international space station. Medicine & Science in Sports & Exercise. 2012;44:S2422157771 [Google Scholar]
- 39.Fu Q, Shibata S, Hastings JL, Platts SH, Hamilton DM, Bungo MW, Stenger MB, Ribeiro C, Adams-Huet B, Levine BD. Impact of prolonged spaceflight on orthostatic tolerance during ambulation and blood pressure profiles in astronauts. Circulation. 2019;140:729–738 [DOI] [PubMed] [Google Scholar]
- 40.Ploutz-Snyder LL, Downs M, Goetchius E, Crowell B, English KL, Ploutz-Snyder R, Ryder JW, Dillon EL, Sheffield-Moore M, Scott JM. Exercise training mitigates multisystem deconditioning during bed rest. Med Sci Sports Exerc. 2018;50:1920–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Globus RK, Bikle DD, Morey-Holton E. The temporal response of bone to unloading. Endocrinology. 1986;118:733–742 [DOI] [PubMed] [Google Scholar]
- 42.Cucinotta FA. Space radiation risks for astronauts on multiple international space station missions. PLoS One. 2014;9:e96099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cucinotta FA, Kim MH, Chappell LJ, Huff JL. How safe is safe enough? Radiation risk for a human mission to mars. PLoS One. 2013;8:e74988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Tessa C, Sivertz M, Chiang IH, Lowenstein D, Rusek A. Overview of the nasa space radiation laboratory. Life Sci Space Res (Amst). 2016;11:18–23 [DOI] [PubMed] [Google Scholar]
- 45.Afshinnekoo E, Scott RT, MacKay MJ, Pariset E, Cekanaviciute E, Barker R, Gilroy S, Hassane D, Smith SM, Zwart SR, Nelman-Gonzalez M, Crucian BE, Ponomarev SA, Orlov OI, Shiba D, Muratani M, Yamamoto M, Richards SE, Vaishampayan PA, Meydan C, Foox J, Myrrhe J, Istasse E, Singh N, Venkateswaran K, Keune JA, Ray HE, Basner M, Miller J, Vitaterna MH, Taylor DM, Wallace D, Rubins K, Bailey SM, Grabham P, Costes SV, Mason CE, Beheshti A. Fundamental biological features of spaceflight: Advancing the field to enable deep-space exploration. Cell. 2020;183:1162–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughson RL, Helm A, Durante M. Heart in space: Effect of the extraterrestrial environment on the cardiovascular system. Nat Rev Cardiol. 2018;15:167–180 [DOI] [PubMed] [Google Scholar]
- 47.Respress JL, Gershovich PM, Wang T, Reynolds JO, Skapura DG, Sutton JP, Miyake CY, Wehrens XH. Long-term simulated microgravity causes cardiac ryr2 phosphorylation and arrhythmias in mice. Int J Cardiol. 2014;176:994–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moffitt JA, Henry MK, Welliver KC, Jepson AJ, Garnett ER. Hindlimb unloading results in increased predisposition to cardiac arrhythmias and alters left ventricular connexin 43 expression. Am J Physiol Regul Integr Comp Physiol. 2013;304:R362–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu C, Zhong G, Zhou Y, Yang Y, Tan Y, Li Y, Gao X, Sun W, Li J, Jin X, Cao D, Yuan X, Liu Z, Liang S, Li Y, Du R, Zhao Y, Xue J, Zhao D, Song J, Ling S, Li Y. Alteration of calcium signalling in cardiomyocyte induced by simulated microgravity and hypergravity. Cell Prolif. 2020;53:e12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carniel E, Taylor MR, Sinagra G, Di Lenarda A, Ku L, Fain PR, Boucek MM, Cavanaugh J, Miocic S, Slavov D, Graw SL, Feiger J, Zhu XZ, Dao D, Ferguson DA, Bristow MR, Mestroni L. Alpha-myosin heavy chain: A sarcomeric gene associated with dilated and hypertrophic phenotypes of cardiomyopathy. Circulation. 2005;112:54–59 [DOI] [PubMed] [Google Scholar]
- 51.Ran F, An L, Fan Y, Hang H, Wang S. Simulated microgravity potentiates generation of reactive oxygen species in cells. Biophys Rep. 2016;2:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ Res. 2017;120:1969–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang XP, Xing CY, Zhang JX, Zhou JH, Li YC, Yang HY, Zhang PF, Zhang W, Huang Y, Long JG, Gao F, Zhang X, Li J. Time-restricted feeding alleviates cardiac dysfunction induced by simulated microgravity via restoring cardiac fgf21 signaling. FASEB J. 2020;34:15180–15196 [DOI] [PubMed] [Google Scholar]
- 54.Feger BJ, Thompson JW, Dubois LG, Kommaddi RP, Foster MW, Mishra R, Shenoy SK, Shibata Y, Kidane YH, Moseley MA, Carnell LS, Bowles DE. Microgravity induces proteomics changes involved in endoplasmic reticulum stress and mitochondrial protection. Sci Rep. 2016;6:34091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang L, Li H, Cao T, Qu L, Zhang L, Fan GC, Greer PA, Li J, Jones DL, Peng T. Calpain activation mediates microgravity-induced myocardial abnormalities in mice via p38 and erk1/2 mapk pathways. J Biol Chem. 2020;295:16840–16851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gustafsson AB, Gottlieb RA. Mechanisms of apoptosis in the heart. J Clin Immunol. 2003;23:447–459 [DOI] [PubMed] [Google Scholar]
- 57.Tungjai M, Whorton EB, Rithidech KN. Persistence of apoptosis and inflammatory responses in the heart and bone marrow of mice following whole-body exposure to (2)(8)silicon ((2)(8)si) ions. Radiat Environ Biophys. 2013;52:339–350 [DOI] [PubMed] [Google Scholar]
- 58.Cao D, Song J, Ling S, Niu S, Lu L, Cui Z, Li Y, Hao S, Zhong G, Qi Z, Sun W, Yuan X, Li H, Zhao D, Jin X, Liu C, Wu X, Kan G, Cao H, Kang Y, Yu S, Li Y. Hematopoietic stem cells and lineage cells undergo dynamic alterations under microgravity and recovery conditions. FASEB J. 2019;33:6904–6918 [DOI] [PubMed] [Google Scholar]
- 59.Dai S, Kong F, Liu C, Xiao F, Dong X, Zhang Y, Wang H. Effect of simulated microgravity conditions of hindlimb unloading on mice hematopoietic and mesenchymal stromal cells. Cell Biol Int. 2020;44:2243–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paul AM, Overbey EG, da Silveira WA, Szewczyk N, Nishiyama NC, Pecaut MJ, Anand S, Galazka JM, Mao XW. Immunological and hematological outcomes following protracted low dose/low dose rate ionizing radiation and simulated microgravity. Sci Rep. 2021;11:11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Versari S, Longinotti G, Barenghi L, Maier JA, Bradamante S. The challenging environment on board the international space station affects endothelial cell function by triggering oxidative stress through thioredoxin interacting protein overexpression: The esa-sphinx experiment. FASEB J. 2013;27:4466–4475 [DOI] [PubMed] [Google Scholar]
- 62.Soucy KG, Lim HK, Kim JH, Oh Y, Attarzadeh DO, Sevinc B, Kuo MM, Shoukas AA, Vazquez ME, Berkowitz DE. Hze (5)(6)fe-ion irradiation induces endothelial dysfunction in rat aorta: Role of xanthine oxidase. Radiat Res. 2011;176:474–485 [DOI] [PubMed] [Google Scholar]
- 63.Soucy KG, Attarzadeh DO, Ramachandran R, Soucy PA, Romer LH, Shoukas AA, Berkowitz DE. Single exposure to radiation produces early anti-angiogenic effects in mouse aorta. Radiat Environ Biophys. 2010;49:397–404 [DOI] [PubMed] [Google Scholar]
- 64.Ghosh P, Behnke BJ, Stabley JN, Kilar CR, Park Y, Narayanan A, Alwood JS, Shirazi-Fard Y, Schreurs AS, Globus RK, Delp MD. Effects of high-let radiation exposure and hindlimb unloading on skeletal muscle resistance artery vasomotor properties and cancellous bone microarchitecture in mice. Radiat Res. 2016;185:257–266 [DOI] [PubMed] [Google Scholar]
- 65.Yu T, Parks BW, Yu S, Srivastava R, Gupta K, Wu X, Khaled S, Chang PY, Kabarowski JH, Kucik DF. Iron-ion radiation accelerates atherosclerosis in apolipoprotein e-deficient mice. Radiat Res. 2011;175:766–773 [DOI] [PubMed] [Google Scholar]
- 66.Administration FA. Commercial space data. 2021;2021 [Google Scholar]
- 67.Commercial spaceflight federation: Member organizations.
- 68.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J Clin Invest. 2013;123:966–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guida JL, Agurs-Collins T, Ahles TA, Campisi J, Dale W, Demark-Wahnefried W, Dietrich J, Fuldner R, Gallicchio L, Green PA, Hurria A, Janelsins MC, Jhappan C, Kirkland JL, Kohanski R, Longo V, Meydani S, Mohile S, Niedernhofer LJ, Nelson C, Perna F, Schadler K, Scott JM, Schrack JA, Tracy RP, van Deursen J, Ness KK. Strategies to prevent or remediate cancer and treatment-related aging. Journal of the National Cancer Institute. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scott JM, Martin D, Ploutz-Snyder R, Downs M, Dillon EL, Sheffield-Moore M, Urban RJ, Ploutz-Snyder LL. Efficacy of exercise and testosterone to mitigate atrophic cardiovascular remodeling. Med Sci Sports Exerc. 2018;50:1940–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hackney KJ, English KL. Protein and essential amino acids to protect musculoskeletal health during spaceflight: Evidence of a paradox? Life (Basel). 2014;4:295–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.da Silveira WA, Fazelinia H, Rosenthal SB, Laiakis EC, Kim MS, Meydan C, Kidane Y, Rathi KS, Smith SM, Stear B, Ying Y, Zhang Y, Foox J, Zanello S, Crucian B, Wang D, Nugent A, Costa HA, Zwart SR, Schrepfer S, Elworth RAL, Sapoval N, Treangen T, MacKay M, Gokhale NS, Horner SM, Singh LN, Wallace DC, Willey JS, Schisler JC, Meller R, McDonald JT, Fisch KM, Hardiman G, Taylor D, Mason CE, Costes SV, Beheshti A. Comprehensive multi-omics analysis reveals mitochondrial stress as a central biological hub for spaceflight impact. Cell. 2020;183:1185–1201 e1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beheshti A, Cekanaviciute E, Smith DJ, Costes SV. Global transcriptomic analysis suggests carbon dioxide as an environmental stressor in spaceflight: A systems biology genelab case study. Sci Rep. 2018;8:4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ray S, Gebre S, Fogle H, Berrios DC, Tran PB, Galazka JM, Costes SV. Genelab: Omics database for spaceflight experiments. Bioinformatics. 2019;35:1753–1759 [DOI] [PubMed] [Google Scholar]
- 76.Sanford JA, Nogiec CD, Lindholm ME, Adkins JN, Amar D, Dasari S, Drugan JK, Fernandez FM, Radom-Aizik S, Schenk S, Snyder MP, Tracy RP, Vanderboom P, Trappe S, Walsh MJ, Molecular Transducers of Physical Activity C. Molecular transducers of physical activity consortium (motrpac): Mapping the dynamic responses to exercise. Cell. 2020;181:1464–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation. 2018;137:1176–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reichert P, Prosise W, Fischmann TO, Scapin G, Narasimhan C, Spinale A, Polniak R, Yang X, Walsh E, Patel D, Benjamin W, Welch J, Simmons D, Strickland C. Pembrolizumab microgravity crystallization experimentation. NPJ Microgravity. 2019;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ng JD, Baird JK, Coates L, Garcia-Ruiz JM, Hodge TA, Huang S. Large-volume protein crystal growth for neutron macromolecular crystallography. Acta Crystallogr F Struct Biol Commun. 2015;71:358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas D, Cunningham NJ, Shenoy S, Wu JC. Human ipscs in cardiovascular research: Current approaches in cardiac differentiation, maturation strategies, and scalable production. Cardiovasc Res. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wnorowski A, Sharma A, Chen H, Wu H, Shao NY, Sayed N, Liu C, Countryman S, Stodieck LS, Rubins KH, Wu SM, Lee PHU, Wu JC. Effects of spaceflight on human induced pluripotent stem cell-derived cardiomyocyte structure and function. Stem Cell Reports. 2019;13:960–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baio J, Martinez AF, Bailey L, Hasaniya N, Pecaut MJ, Kearns-Jonker M. Spaceflight activates protein kinase c alpha signaling and modifies the developmental stage of human neonatal cardiovascular progenitor cells. Stem Cells Dev. 2018;27:805–818 [DOI] [PubMed] [Google Scholar]
- 83.Romorini L, Garate X, Neiman G, Luzzani C, Furmento VA, Guberman AS, Sevlever GE, Scassa ME, Miriuka SG. Akt/gsk3beta signaling pathway is critically involved in human pluripotent stem cell survival. Sci Rep. 2016;6:35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Braunwald E Cell-based therapy in cardiac regeneration: An overview. Circ Res. 2018;123:132–137 [DOI] [PubMed] [Google Scholar]
- 85.Paloschi V, Sabater-Lleal M, Middelkamp H, Vivas A, Johansson S, van der Meer A, Tenje M, Maegdefessel L. Organ-on-a-chip technology: A novel approach to investigate cardiovascular diseases. Cardiovasc Res. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim J, Koo BK, Knoblich JA. Human organoids: Model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Low LA, Giulianotti MA. Tissue chips in space: Modeling human diseases in microgravity. Pharm Res. 2019;37:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Three tissue engineering projects awarded from joint national science foundation and casis solicitation to leverage the space station. The ISS National Lab Blog https://www.issnationallab.org/iss360/nsf-casis-award-tissue-engineering-projects/. 2020
- 89.Xu C Iss: Engineering stem cell-derived cardiac microtissues with metabolic regulators in space to promote cardiomyocyte maturation. 2019 [Google Scholar]
- 90.Wu JC, Pruitt BL. Effect of microgravity on drug responses using engineered heart tissues. 2018 [Google Scholar]
- 91.Extended longevity of 3d tissues and microphysiological systems for modeling of acute and chronic exposures to stressors. https://nspires.nasaprs.com/external/index.do. 2021
- 92.Space health institute awards 5 ground-breaking research grants to mitigate the effects of space radiation on healthy human cell-derived organs-on-a-chip. https://www.nasa.gov/feature/5-research-grants-awarded-on-human-cell-derived-organs-on-a-chip. 2020
- 93.Yan X, Sasi SP, Gee H, Lee J, Yang Y, Mehrzad R, Onufrak J, Song J, Enderling H, Agarwal A, Rahimi L, Morgan J, Wilson PF, Carrozza J, Walsh K, Kishore R, Goukassian DA. Cardiovascular risks associated with low dose ionizing particle radiation. PLoS One. 2014;9:e110269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sasi SP, Yan X, Zuriaga-Herrero M, Gee H, Lee J, Mehrzad R, Song J, Onufrak J, Morgan J, Enderling H, Walsh K, Kishore R, Goukassian DA. Different sequences of fractionated low-dose proton and single iron-radiation-induced divergent biological responses in the heart. Radiat Res. 2017;188:191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seawright JW, Sridharan V, Landes RD, Cao M, Singh P, Koturbash I, Mao XW, Miousse IR, Singh SP, Nelson GA, Hauer-Jensen M, Boerma M. Effects of low-dose oxygen ions and protons on cardiac function and structure in male c57bl/6j mice. Life Sci Space Res (Amst). 2019;20:72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grabham P, Sharma P, Bigelow A, Geard C. Two distinct types of the inhibition of vasculogenesis by different species of charged particles. Vasc Cell. 2013;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seawright JW, Samman Y, Sridharan V, Mao XW, Cao M, Singh P, Melnyk S, Koturbash I, Nelson GA, Hauer-Jensen M, Boerma M. Effects of low-dose rate gamma-irradiation combined with simulated microgravity on markers of oxidative stress, DNA methylation potential, and remodeling in the mouse heart. PLoS One. 2017;12:e0180594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Camberos V, Baio J, Mandujano A, Martinez AF, Bailey L, Hasaniya N, Kearns-Jonker M. The impact of spaceflight and microgravity on the human islet-1+ cardiovascular progenitor cell transcriptome. Int J Mol Sci. 2021;22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hees PS, Fleg JL, Lakatta EG, Shapiro EP. Left ventricular remodeling with age in normal men versus women: Novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol. 2002;90:1231–1236 [DOI] [PubMed] [Google Scholar]
- 100.Maceira AM, Prasad SK, Khan M, Pennell DJ. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur Heart J. 2006;27:2879–2888 [DOI] [PubMed] [Google Scholar]
- 101.Manolio TA, Furberg CD, Rautaharju PM, Siscovick D, Newman AB, Borhani NO, Gardin JM, Tabatznik B. Cardiac arrhythmias on 24-h ambulatory electrocardiography in older women and men: The cardiovascular health study. J Am Coll Cardiol. 1994;23:916–925 [DOI] [PubMed] [Google Scholar]
- 102.Arbeille P, Provost R, Zuj K. Carotid and femoral artery intima-media thickness during 6 months of spaceflight. Aerosp Med Hum Perform. 2016;87:449–453 [DOI] [PubMed] [Google Scholar]
- 103.Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am. 2009;93:583–604, Table of Contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagai Y, Metter EJ, Earley CJ, Kemper MK, Becker LC, Lakatta EG, Fleg JL. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation. 1998;98:1504–1509 [DOI] [PubMed] [Google Scholar]
- 105.Gepner AD, Korcarz CE, Colangelo LA, Hom EK, Tattersall MC, Astor BC, Kaufman JD, Liu K, Stein JH. Longitudinal effects of a decade of aging on carotid artery stiffness: The multiethnic study of atherosclerosis. Stroke. 2014;45:48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chung CP, Lin YJ, Chao AC, Lin SJ, Chen YY, Wang YJ, Hu HH. Jugular venous hemodynamic changes with aging. Ultrasound Med Biol. 2010;36:1776–1782 [DOI] [PubMed] [Google Scholar]
- 107.Goswami N, Blaber AP, Hinghofer-Szalkay H, Montani JP. Orthostatic intolerance in older persons: Etiology and countermeasures. Front Physiol. 2017;8:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Patel KV. Epidemiology of anemia in older adults. Semin Hematol. 2008;45:210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682 [DOI] [PubMed] [Google Scholar]
- 110.Furukawa S, Nagamatsu A, Nenoi M, Fujimori A, Kakinuma S, Katsube T, Wang B, Tsuruoka C, Shirai T, Nakamura AJ, Sakaue-Sawano A, Miyawaki A, Harada H, Kobayashi M, Kobayashi J, Kunieda T, Funayama T, Suzuki M, Miyamoto T, Hidema J, Yoshida Y, Takahashi A. Space radiation biology for "living in space". Biomed Res Int. 2020;2020:4703286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, Fraifeld VE. The role of DNA damage and repair in aging through the prism of koch-like criteria. Ageing Res Rev. 2013;12:661–684 [DOI] [PubMed] [Google Scholar]
- 112.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579 [DOI] [PubMed] [Google Scholar]
- 113.Singh KP, Kumari R, Dumond JW. Simulated microgravity-induced epigenetic changes in human lymphocytes. J Cell Biochem. 2010;111:123–129 [DOI] [PubMed] [Google Scholar]
- 114.Gonzalo S Epigenetic alterations in aging. J Appl Physiol (1985). 2010;109:586–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Walls S, Diop S, Birse R, Elmen L, Gan Z, Kalvakuri S, Pineda S, Reddy C, Taylor E, Trinh B, Vogler G, Zarndt R, McCulloch A, Lee P, Bhattacharya S, Bodmer R, Ocorr K. Prolonged exposure to microgravity reduces cardiac contractility and initiates remodeling in drosophila. Cell Rep. 2020;33:108445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res Rev. 2011;10:205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blaber EA, Pecaut MJ, Jonscher KR. Spaceflight activates autophagy programs and the proteasome in mouse liver. Int J Mol Sci. 2017;18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729 [DOI] [PubMed] [Google Scholar]
- 120.Fafian-Labora JA, O’Loghlen A. Classical and nonclassical intercellular communication in senescence and ageing. Trends Cell Biol. 2020;30:628–639 [DOI] [PubMed] [Google Scholar]