Abstract
Neuroimmunological diseases and their treatment compromise the immune system, thereby increasing the risk of infections and serious illness. Consequently, vaccinations to protect against infections are an important part of the clinical management of these diseases. However, the wide variety of immunotherapies that are currently used to treat neuroimmunological disease — particularly multiple sclerosis and neuromyelitis optica spectrum disorders — can also impair immunological responses to vaccinations. In this Review, we discuss what is known about the effects of various immunotherapies on immunological responses to vaccines and what these effects mean for the safe and effective use of vaccines in patients with a neuroimmunological disease. The success of vaccination in patients receiving immunotherapy largely depends on the specific mode of action of the immunotherapy. To minimize the risk of infection when using immunotherapy, assessment of immune status and exclusion of underlying chronic infections before initiation of therapy are essential. Selection of the required vaccinations and leaving appropriate time intervals between vaccination and administration of immunotherapy can help to safeguard patients. We also discuss the rapidly evolving knowledge of how immunotherapies affect responses to SARS-CoV-2 vaccines and how these effects should influence the management of patients on these therapies during the COVID-19 pandemic.
Subject terms: Multiple sclerosis, Neuromuscular disease, Inflammatory diseases
In this Review, the authors discuss how various immunotherapies for neuroimmunological diseases interact with vaccination responses, including responses to SARS-CoV-2 vaccinations, and the implications for the safe and effective use of vaccines in patients with these diseases.
Key points
Vaccination against infection is an essential part of the management of neuroimmunological diseases.
All indicated vaccinations should be administered before initiation of immunotherapy whenever possible; appropriate intervals between vaccination and treatment vary with treatment and vaccination.
Inactivated vaccines are considered safe in neuroimmunological diseases but live vaccines are generally contraindicated during immunotherapy.
Vaccination responses during immunotherapy can be diminished or abrogated, depending on the treatment and vaccination; antibody titre testing to monitor responses can be considered where appropriate.
Vaccinations must be avoided during relapses or exacerbations of neuroimmunological diseases.
Vaccination against SARS-CoV-2 is recommended for patients with neuroimmunological disease but some immunotherapies limit the immune response; therefore, timing should be considered carefully.
Introduction
A variety of neuroimmunological diseases can affect the CNS, PNS and the neuromuscular junction, and the pharmacological arsenal for the treatment of these diseases is growing (Table 1). The most common of these diseases is multiple sclerosis (MS), a chronic immune-mediated disease of the CNS that leads to demyelination, axonal damage and reduction of synapses1,2. Pathogenesis of MS involves components of the adaptive immune system, including B cells and T cells, and of the CNS-resident innate immune system, including microglia3. On the basis of these mechanisms, various immunosuppressive therapies have been developed for MS, and more than 15 disease-modifying therapies (DMTs) are currently available.
Table 1.
Neuroimmunological diseases and immunotherapies approved or commonly used for their treatment
| Site of disease | Disease | Pathophysiology | Approved immunotherapies | Other commonly used immunotherapies |
|---|---|---|---|---|
| CNS | Multiple sclerosis | Chronic immune-mediated disease leading to demyelination, axonal damage and reduction of synapses following the loss of immune tolerance against CNS antigens | Alemtuzumab, azathioprine, IFNβ, cladribine, dimethyl fumarate, diroximel fumarate, fingolimod, glatirameroids, mitoxantrone, natalizumab, ocrelizumab, ofatumumab, ozanimod, ponesimod, siponimod, teriflunomide | Cyclophosphamide, glucocorticosteroidsa, high-dose IVIg, plasma exchangea, rituximab, stem cell transplantation |
| NMOSD | Primarily antibody-mediated inflammatory CNS disease directed against neuronal surface molecules that triggers activation of the classical complement cascade to cause granulocyte, eosinophil and lymphocyte infiltration, culminating in injury to astrocytes and then oligodendrocytes, followed by demyelination and neuronal loss | Eculizumab, inebilizumab, sartralizumab | Azathioprine, glucocorticosteroidsa, mycophenolate mofetil, plasma exchangea, rituximab, tocilizumab | |
| MOGAD | Primarily antibody-mediated inflammatory CNS disease directed against neuronal surface molecules, characterized by the coexistence of perivenous and confluent primary demyelination with partial axonal preservation and reactive gliosis in the white and grey matter, with a particular abundance of intracortical demyelinating lesions | None | Azathioprine, glucocorticosteroidsa, plasma exchangea, rituximab | |
| Autoantibody-mediated encephalitides | Auto-antibodies against neuronal surface molecules | None | Cyclophosphamide, glucocorticosteroidsa, high-dose IVIga, plasma exchangea, rituximab | |
| PNS | GBS (and variants) | Acute peripheral neuropathy mediated by molecular mimicry, antiganglioside antibodies, involvement of cellular and humoral immune mechanisms, and probably complement activation | High-dose IVIga, high-dose SCIg, plasma exchangea | |
| CIDP (and variants) | Acquired, immune-mediated chronic inflammatory demyelinating polyneuropathy that affects peripheral nerves and nerve roots, mediated by cellular and humoral mechanisms (T cell activation, immunoglobulin and complement deposition on myelinated nerve fibres) | Glucocorticosteroidsb, high-dose IVIgb, high-dose SCIg, plasma exchangeb | Azathioprine, cyclophosphamide, mycophenolate mofetil, rituximab | |
| Neuromuscular junction | Myasthenia gravis | Auto-antibodies against components of the postsynaptic membrane lead to impairment of neuromuscular transmission by complement-mediated damage | Azathioprine, eculizumab, glucocorticosteroids, high-dose glucocorticosteroidsa | Cyclophosphamide, high-dose IVIga, mycophenolate mofetil, plasma exchangea, rituximab |
| Lambert–Eaton syndrome | Auto-antibodies to the presynaptic voltage-gated calcium channel | Treatment of underlying cancer | Azathioprine, glucocorticosteroids, high-dose IVIgb, plasma exchangeb | |
| Muscle | Polymyositis | T cell-mediated cytotoxic process directed against unidentified muscle antigens | Glucocorticosteroidsb | Azathioprine, cyclophosphamide, high-dose IVIg, mycophenolate mofetil, rituximab |
| Dermatomyositis | Humoral-mediated autoimmune disease in which antigen-specific antibodies are deposited in the microvasculature | Glucocorticosteroidsb | Azathioprine, cyclophosphamide, high-dose IVIg, mycophenolate mofetil, rituximab | |
| Inclusion body myositis | Features of inflammatory and degenerative processes, such as inflammatory infiltrates, but also myonuclear degeneration and protein aggregates | None | High-dose IVIg | |
| Immune-mediated necrotizing myopathies | A group of inflammatory myopathies associated with anti-SRP or anti-HMGCR myositis-specific auto-antibodies | Glucocorticosteroidsb | Azathioprine, cyclophosphamide, high-dose IVIg, mycophenolate mofetil, rituximab |
HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; CIDP, chronic inflammatory demyelinating polyneuropathy; GBS, Guillain–Barré syndrome; IVIg, intravenous immunoglobulins; MOGAD, myelin oligodendrocyte glycoprotein antibody-associated disease; NMOSD, neuromyelitis optica spectrum disorder; SCIg, subcutaneous immunoglobulins; SRP, signal recognition particle. aRelapse treatment. bInduction therapy.
Similarly, three immunosuppressive therapies have now been approved for the treatment of neuromyelitis optica spectrum disorder (NMOSD), which is a primarily antibody-mediated inflammatory CNS disease. In addition, various immunotherapies are used off-label for the treatment of other neuroimmunological diseases for which no therapies are approved such as myelin oligodendrocyte glycoprotein antibody-associated disease, autoantibody-mediated encephalitides, Guillain–Barré syndrome (GBS) and its variants, chronic inflammatory demyelinating polyneuropathy, myasthenic disorders, and inflammatory myopathies (Table 1).
Advances in immunotherapy and the development of targeted therapies on the basis of pathophysiological pathways have greatly improved the management of neuroimmunological diseases but immunotherapies carry the risk of serious infections. Therefore, effective prophylaxis of infections is critical for people undergoing immunotherapy for neuroimmunological diseases4,5, but the effects of these therapies on immune function can also compromise responses to vaccinations. Given that perturbations of the immune system are causative in neuroimmunological diseases and that immunotherapies drastically alter immune function, multiple levels of complexity exist for patients and physicians in relation to the use of vaccinations6,7 (Box 1). A full understanding of the effects of immunotherapies on vaccination responses is needed to ensure maximum protection from infection alongside therapeutic benefits.
In this Review, we first discuss the fundamentals of vaccine immunology before considering in detail how immunotherapies with different modes of action influence responses to vaccines and what these interactions mean for the clinical use and timing of vaccinations in relation to immunotherapies. We focus on MS and NMOSD, as a broad range of licenced DMTs are available for these diseases, yet the same therapies are commonly used in various other neuroimmunological diseases (Table 1). We also consider the current and rapidly evolving knowledge about SARS-CoV-2 vaccines and their use in people with and receiving treatment for neuroimmunological diseases.
Box 1 Interactions between neuroimmunological disease, infections, vaccinations and disease-modifying therapy.
In neuroimmunological diseases, infection, vaccination and disease-modifying therapies (DMTs) all interact with the disease and with each other (Figure), and these interactions need to be managed to minimize the risk of infection and maximize the benefits of vaccination. The course of a neuroimmunological disease influences the risk of infection in the patient (for example, silent aspiration and pneumonia or urinary tract infections) and can also influence the efficacy of vaccination (for example, previous therapies).
Conversely, the disease course can be affected by infections and vaccinations owing to activation of the immune system — for instance, by the Toll-like receptor pathway. For example, myasthenic crisis can occur after infections and multiple sclerosis relapses can follow infection of the upper respiratory tract or pneumonia. Vaccinations that prevent infections have been shown to stabilize the course of neuroimmunological diseases.
DMTs not only alter the course of the disease but, by altering immune function, also increase the risk of infection and affect the efficacy of vaccinations. Vaccinations reduce the risk of infection associated with disease or therapy and can also influence the course of disease (for instance, multiple sclerosis relapses can occur after yellow fever vaccination owing to mild immune activation); these interactions need to be managed carefully. Finally, the effects of infections and vaccinations on immune function could contribute to the aetiology of some neuroimmunological diseases, though such effects remain uncertain (indicated by dashed lines in the figure). For example, increasing evidence indicates that the Epstein–Barr virus can contribute to the pathogenesis of multiple sclerosis227,228 and Campylobacter jejuni infection is thought to trigger Guillain–Barré syndrome as a result of molecular mimicry30.
The effects of vaccination depend on various additional aspects, including the vaccination type (live attenuated, inactivated, conjugate, virus-like particle, recombinant, toxoid or RNA/DNA based), additives (preservatives, stabilizers, adjuvants and manufacturing residuals) and the vaccination history of the patient (whether they are receiving a primary vaccination, revaccination or booster vaccination).
The effects of all of these factors and their interactions can also be influenced by a variety of patient-specific characteristics, including age, sex, comorbidities, immune status, co-medication and drug interactions, use of complementary and alternative medicines, and social issues.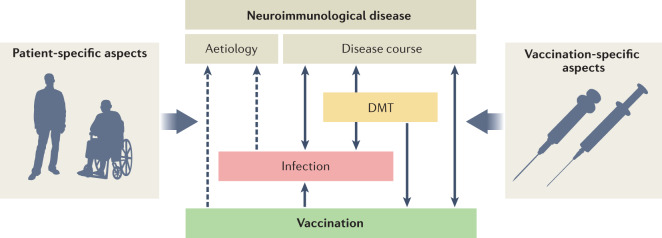
Vaccines and immunological responses
Vaccinations provide active immunization, meaning that an antigen is administered to elicit an antibody and cellular response. This mechanism is in contrast with passive immunization, which is achieved through direct administration of hyperimmune globulins or monoclonal antibodies that provide immediate but short-lasting immunity. Active immunization with vaccines provides longer-lasting protection.
Established forms of vaccines include live attenuated pathogens, inactivated whole pathogens, purified proteins or polysaccharides, and genetically engineered antigens. Various vaccine types are commonly used for a wide variety of diseases and elicit various responses with varying durability (Supplementary Table 1). Novel types of vaccines, such as DNA-based and RNA-based vaccines, are emerging, and this process has been accelerated by the COVID-19 pandemic8–10 — nucleoside-modified mRNA-based vaccines against SARS-CoV-2 (refs11–15) are the first of this type to be approved for use in humans.
Our understanding of how immunity develops after vaccination has evolved over the past two centuries. During the past five decades, research has focused on adaptive immune responses to infectious agents and the capacity of these responses to produce tailored, specific and long-lasting immune effects7,16. Adaptive immune responses involve clonal selection of T cells and antibody production by B cells to respond to antigens. However, the innate immune system is also involved in the response to infection. This response is generally directed against common structures of antigens and does not depend on clonal selection processes and therefore the immune system is immediately available to react. Macrophages, dendritic cells and mast cells bind to pathogens via Toll-like receptors, leading to chemokine and cytokine activation by various intracellular pathways. This innate immune response triggers adaptive immune responses17. In the past 5 years, insight into vaccine immunology has blurred the boundaries between innate and adaptive immune responses and altered the classical view of vaccine-induced immunity.
For example, evidence has emerged that the innate immune function is altered as a result of Bacille Calmette–Guérin (BCG) immunization against tuberculosis, that BCG vaccination provides protection from reinfection that is independent of T cells and B cells18, and that overall mortality is reduced after BCG vaccination19. Together, these findings suggest a previously unknown process in which haematopoietic stem cells and multipotent progenitor cells are reprogrammed, leading to ‘innate immune memory’ or ‘trained immunity’20. These changes could be driven by epigenetic reprogramming, metabolomic rewiring and/or alterations in gene expression signatures18,21. Micronutrients and nano-biologicals that were originally designed to reduce transplant rejection via modulation of bone marrow cells are currently under investigation for their ability to augment innate immune responses to vaccines18. These insights into the role of innate immune responses to vaccination are important in the context of neuroimmunological disease because some immunotherapies alter components of the innate immune system and could therefore influence the innate immune response to vaccination.
Immune responses to vaccines can be enhanced by the use of adjuvants (such as oil-in-water emulsions, aluminium, Toll-like receptor agonists and virosomes; Supplementary Table 2), which can reduce the dose of antigen needed to achieve protection or can amplify protection in immunocompromised individuals22,23. Concerns have been raised about the possibility that such adjuvants can evoke an autoimmune or inflammatory syndrome24. One well-documented example is the development of narcolepsy in children with a genetic predisposition who received a pandemic influenza vaccine that contained AS03 adjuvant but not in children who received a vaccine that contained MF57 adjuvant24,25. However, all adjuvants seem to generate a comparable inflammatory response in the first few hours after vaccination22, and licenced vaccine adjuvants have generally been proven safe and effective in immunocompromised and non-immunocompromised individuals26.
A common concern with vaccines is the possibility of inducing neuroimmunological disease or worsening of existing neuroimmunological disease. Large cohort studies, meta-analyses and prospective trials have provided convergent evidence that vaccination has no correlation with induction of MS27–29. However, GBS can occur in rare cases after active vaccinations against influenza, yellow fever, rabies, hepatitis A and B, measles, tetanus, and others30–32. The estimated risk of GBS is 1 per million people with the influenza vaccination compared with 17 per million people after influenza infection32.
Immunotherapy and vaccination response
The effects of immunotherapy on the immune system are only partially understood and the effects on vaccine responses also remain unclear. Responses to vaccination vary with different therapies and relate to the drug’s mode of action (Supplementary Table 3). The following sections consider how immunotherapies with various modes of action influence vaccine responses.
Depletion and cytolysis of immune cells
Vaccination responses primarily depend on adaptive immunity. Multiple immunotherapies reduce levels of B cells or T cells and therefore carry the risk of reduced vaccine efficacy. However, responses to vaccination vary between these types of drugs.
Ocrelizumab
Ocrelizumab is an anti-CD20 humanized monoclonal antibody that depletes CD20+ B cells. Ocrelizumab is widely used for the treatment of relapsing–remitting MS and is the first treatment to be licenced for use in primary progressive MS.
The effects of ocrelizumab on humoral responses to selected vaccines were assessed in a phase IIIb controlled prospective multicentre study33. In 68 patients with relapsing–remitting MS who were receiving ocrelizumab treatment, immune responses to vaccination at 12 weeks after initiation of treatment were compared with those in 34 patients receiving IFNβ or no treatment33. At 4 and 8 weeks after the tetanus toxoid booster vaccine, geometric mean levels of IgG and the proportion of patients with a positive response were lower among patients treated with ocrelizumab than among control participants. However, all patients who exhibited a response were seroprotected at 4 and 8 weeks after vaccination. Similarly, at 4 and 8 weeks after vaccination with the pneumococcal polysaccharide vaccine directed against 23 pneumococcal serotypes (23-PPV) and the additional pneumococcal conjugate vaccine directed against 13 pneumococcal serotypes (13-PCV), geometric mean levels of specific antibodies were lower among the ocrelizumab recipients than among control participants. The proportion of individuals who exhibited a positive vaccination response to 23-PPV was lower in the ocrelizumab group than in the control group, and additional vaccination with 13-PCV did not markedly increase the response to the 12 serotypes that it has in common with the 23-PPV vaccine.
At 4 weeks after vaccination against five strains of influenza (seasons 2015–2016 and 2016–2017), 55.6–80% of individuals who received ocrelizumab and 75–97% of control participants developed seroprotective antibody titres33. After vaccination with the keyhole limpet haemocyanin neoantigen (KLH) — an antigen to which previous immune reaction is not suspected — responses were lower in patients receiving ocrelizumab than in control participants. Repeated KLH administration elicited a boosting effect only in the control group33. After all tested vaccines, humoral responses were diminished in patients receiving ocrelizumab compared with patients not receiving B cell depleting treatment at all investigated time points. Nonetheless, patients receiving ocrelizumab were able to mount humoral responses to various vaccines and to neoantigen33.
Though specific studies have not been done to determine the appropriate timing of vaccination relative to ocrelizumab treatment, we recommend that inactivated vaccines should be administered >4 weeks before ocrelizumab treatment to allow adequate time for the immune response to develop. Seasonal influenza vaccination during ongoing treatment is recommended, though the humoral response could be attenuated.
Rituximab
Rituximab is a chimeric anti-CD20 monoclonal antibody that depletes CD20+ B cells. The effects of rituximab on vaccine response have been studied in patients receiving the antibody for NMOSD34. In this study, antibody responses against the H1N1 influenza vaccine were lower among patients receiving rituximab than among people with MS who were receiving treatment with IFNβ or azathioprine. However, antibody titres against hepatitis B virus surface antigen, measles and tetanus were not affected by treatment with rituximab, IFNβ or azathioprine.
Rituximab is also commonly used in rheumatoid arthritis and its effects on vaccine responses have been investigated in this population. Assessment of responses to tetanus toxoid, 23-PPV and KLH and of delayed-type hypersensitivity (DTH) to Candida albicans showed that treatment with rituximab combined with methotrexate produced markedly reduced responses to neoantigens and T cell-independent antigens than those with methotrexate treatment alone. Recall responses to T cell-dependent tetanus toxoid antigen and DTH responses were not affected to the same extent35.
In another small study of patients with rheumatoid arthritis, influenza vaccine and PPVs were administered to 11 patients at 6 months after rituximab treatment (post-rituximab group), to 8 patients at 6 days before rituximab treatment (pre-rituximab group) and to 10 patients who did not receive rituximab (control group). Six days after vaccination, influenza-specific B cell responses were lower in the post-rituximab group than in the other groups and, on day 21, influenza-specific IgG production was undetectable in 55% of individuals in the post-rituximab group. Similarly, 23-PPV-specific IgG production was lower in the post-rituximab group than in the other groups. Thus, rituximab seems to compromise cellular and humoral vaccine responses in patients with rheumatoid arthritis36. On the basis of these studies, primary immunizations should be given before rituximab therapy is initiated where possible to ensure that an adequate immune response can develop.
Ofatumumab
Ofatumumab is a human anti-CD20 monoclonal antibody that was approved for the treatment of MS in 2020 in the USA and in 2021 in the EU and Australia37. The efficacy and safety of protective vaccines in people receiving ofatumumab have not been specifically studied to date. However, given that its mechanism of B cell depletion is the same as that of ocrelizumab and rituximab, the immune response to vaccination is likely to be impaired in a similar way.
Inebilizumab
Inebilizumab is an anti-CD19 monoclonal antibody that depletes CD19+ B cells (a broader population than CD20+ B cells) and is approved for the treatment of NMOSD. In the phase III trial of inebilizumab, antitetanus toxoid IgG levels were measured to assess the effect of inebilizumab on vaccine-generated antibody titres and these levels were not decreased at the end of the study38. However, the safety of immunization with live or live attenuated vaccines after inebilizumab therapy has not been studied, and administration of such vaccines is not recommended during treatment and until B cell repletion is achieved owing to the risk of vaccine-related infection.
Alemtuzumab
Alemtuzumab is an anti-CD52 monoclonal antibody that depletes CD52+ T cells and B cells. In a pilot case–control study that involved 24 patients with MS, the effects of alemtuzumab treatment on the ability to mount antibody responses to diphtheria, tetanus and poliomyelitis vaccine, Haemophilus influenzae type b and meningococcal group C vaccine, and pneumococcal polysaccharide vaccine were assessed39. Results were compared with historical control data. Vaccine responses to T cell-dependent recall antigens (tetanus, diphtheria and poliomyelitis), T cell-dependent novel antigen (meningococcus C) and T cell-independent antigens (pneumococcal) were normal. Follow-up of 5 patients showed that responses to vaccination were not diminished within 6 months of alemtuzumab treatment39. Furthermore, in 20 patients, the persistence of antibodies to mumps, rubella, varicella zoster virus (VZV) and the Epstein–Barr virus were measured before alemtuzumab treatment and at 1 month and 9–11 months after alemtuzumab treatment. Serum antibodies against these common viruses remained detectable after treatment. In conclusion, though vaccine responses seem to be maintained after alemtuzumab therapy, we advocate a 6-month interval between the previous treatment course and vaccination to ensure an adequate immune response.
Cladribine
Cladribine is a synthetic analogue of deoxyadenosine that is incorporated into DNA, leading to apoptosis and depletion of B cells and T cells, including non-proliferating cells40,41. No dedicated studies, other than in the context of SARS-CoV-2 vaccination, have been conducted to examine vaccination responses during oral cladribine treatment42,43. Whether oral cladribine affects immunological memory acquired from previous vaccinations has also not been assessed but patients who are treated with cladribine seem to be capable of mounting an intact cellular response to SARS-CoV-2 mRNA vaccines44,45. Live attenuated vaccines should be administered at least 4–6 weeks before cladribine treatment to avoid the risk of infection. On the basis of the little evidence available, inactivated vaccines seem to be safe during cladribine therapy, but immunization should be completed before therapy whenever possible.
Inhibition of immune cell proliferation
Drug-induced inhibition of immune cell proliferation is expected to reduce the expansion of immune cells that normally follows vaccination, resulting in attenuated vaccine responses. Drugs with this mechanism of action have variable effects on immune cell proliferation, leading to different levels of protection after vaccination.
Teriflunomide
Teriflunomide inhibits pyrimidine synthesis and inhibits the proliferation of rapidly dividing cells such as activated T cells. In one study, the effects of teriflunomide on the immune response to the 2011–2012 seasonal influenza vaccine was investigated46. The study involved patients with MS who were receiving 7 mg or 14 mg teriflunomide or IFNβ-1b. Antibody responses did not differ significantly between the groups except the response to the influenza A (H3N2) antigen — the antibody response was significantly lower in patients receiving 14 mg teriflunomide than in patients receiving the lower dose of teriflunomide or IFNβ-1b46.
In a single-centre, randomized, placebo-controlled study, antibody responses to rabies vaccine were assessed in 46 healthy individuals who received teriflunomide or placebo47. Geometric mean antibody titres were lower in people who received teriflunomide. However, antibody levels after vaccination were above the threshold for seroprotection in all participants. In addition, the proportion of individuals with DTH reactions to various recall antigens (tuberculin, Candida albicans and trichophyton antigens) was similar in both groups and cellular responses to recall antigens did not differ substantially. Overall, teriflunomide therapy does not seem to have a clinically relevant impact on vaccine response48,49.
Azathioprine
Azathioprine inhibits purine synthesis to impair cell proliferation. In a small study of patients receiving azathioprine for neuroimmunological disease, antibody responses to influenza vaccination were not hampered and treatment did not influence pre-existing antibody titres to hepatitis B, measles or tetanus34. On the basis of this evidence, immunization with inactivated vaccines during azathioprine treatment for neuroimmunological diseases seems to induce protection as in patients receiving azathioprine for other underlying diseases.
Cyclophosphamide and mitoxantrone
Cyclophosphamide and mitoxantrone are classical cytostatic drugs that disrupt DNA synthesis and structure. Immune responses after vaccination in patients receiving these drugs for neuroimmunological disease have not been systematically studied, but these drugs impair immune responses and are expected to have a more sustained detrimental impact on vaccination efficacy than other cells that inhibit proliferation. Therefore, vaccines should be administered at least 2 weeks before treatment with these drugs when possible and live and live attenuated vaccines must be avoided for at least 3 months after treatment.
Mycophenolate mofetil
Mycophenolate mofetil depletes guanosine nucleotides in T cells and B cells, thereby preventing their proliferation. Antibody responses with mycophenolate mofetil have been studied in patients with NMOSD34. In this study, antibody responses to influenza vaccination were significantly lower among patients receiving mycophenolate mofetil than among patients receiving IFNβ and among healthy control participants34. Given that treatment with mycophenolate mofetil impairs vaccine immunogenicity, additional vaccine doses are generally advisable (for example, in the case of SARS-CoV-2 vaccination) or should be recommended according to quantifiable antibody responses (for example, in the case of hepatitis A or B vaccination).
Impairment of immune cell migration
Several immunotherapies prevent invasion of the CNS by immune cells from the periphery. With these drugs, the response to vaccination would be expected to be maintained. However, the effects on vaccination responses differ between drugs of this type. Those that influence the egress of lymphocytes from the lymphatic tissue seem to impair vaccine responses to a greater extent than those that specifically target the trafficking of immune cells across the blood–brain barrier.
Natalizumab
Natalizumab is a humanized monoclonal antibody that binds to α4β1 integrin on lymphocytes, thereby blocking interactions with cell adhesion molecules that are essential for the lymphocytes to cross the blood–brain barrier. The effects of natalizumab on responses to vaccines have been investigated in several studies.
In a single-centre, randomized, placebo-controlled study, 17 patients who were receiving natalizumab for relapsing–remitting MS and 10 healthy control participants received trivalent influenza A/B vaccine50. Influenza-specific IgG levels were measured up to 12 weeks after vaccination. In both groups, anti-influenza B IgG levels increased substantially after vaccination. Initial increases in levels of anti-influenza A IgG were smaller in both groups; a substantial increase at 4 weeks was seen only in the natalizumab group. IgG titres did not differ significantly between natalizumab-treated individuals and healthy control participants at any time point. Overall, vaccination against influenza in patients receiving natalizumab produced an equivalent humoral immune response to that achieved in healthy individuals.
In a randomized, multicentre, open-label trial51, the effects of natalizumab on responses to tetanus toxoid (a recall antigen) and KLH were investigated in patients with relapsing–remitting MS. Both vaccines were administered to patients who were natalizumab naive receiving no treatment and to patients receiving natalizumab at 6 months after treatment. An adequate response was defined as at least a twofold increase in specific serum IgG levels 28 days after vaccination. The responses to both antigens were adequate and similar in the two groups, indicating that natalizumab does not affect responses to the tested vaccinations in a clinically meaningful way. Consequently, no special measures need to be taken with the use of inactivated vaccines in the context of natalizumab treatment but live attenuated vaccines must be avoided.
Fingolimod
Fingolimod is a non-selective sphingosine 1 phosphate (S1P) receptor modulator that prevents lymphocyte egress from lymph nodes. In a blinded, randomized, multicentre, controlled study, the effects of fingolimod on responses to seasonal influenza and tetanus booster vaccinations were assessed in patients with MS who were receiving fingolimod or placebo52. At 6 weeks after vaccination, responses were lower in patients receiving fingolimod than in those receiving placebo.
In another study, 11 patients with MS received 2 doses of the attenuated live vaccine against VZV before commencing fingolimod treatment. VZV seroconversion was achieved when fingolimod treatment started 26–76 days after the second vaccine dose53. Retesting for VZV IgG after a mean latency of 2.42 years from initiation of therapy revealed a global reduction of antibody titres in all patients and the disappearance of antibodies in 7 patients. After cessation of fingolimod, antibodies reoccurred in 2 of these 7 patients.
Overall, the evidence indicates that fingolimod therapy decreases vaccination-induced humoral immune responses. Therefore, testing for antibody responses, additional booster vaccines or other measures to prevent infections should be considered in the context of fingolimod therapy.
Ozanimod and ponesimod
Ozanimod and ponesimod are second-generation S1P receptor modulators that are more selective than fingolimod — ozanimod is selective for the S1P1 and S1P5 receptors and ponesimod is selective for the S1P1 receptor. The efficacy and safety of vaccines in people receiving these drugs have not been studied to date. However, given the similar mechanism of action to fingolimod, the immune response to vaccination is likely to be compromised in the same way.
Siponimod
Siponimod is a second-generation S1P receptor modulator that is selective for the S1P1 and S1P5 receptors. The effects of siponimod on vaccine responses have been investigated in a double-blind, placebo-controlled, parallel-group study in which 120 healthy adults received T cell-dependent (influenza) and T cell-independent (23-PPV) vaccinations54. Participants were randomly assigned to four groups and underwent either 7 weeks of treatment with 2 mg siponimod daily or placebo. Those who received siponimod were assigned to one of three treatment groups: preceding siponimod, in which siponimod treatment was stopped 7 days before immunization; concomitant siponimod, in which siponimod treatment was continued throughout; and interrupted siponimod, in which treatment with siponimod was stopped 10 days before immunization and restarted 14 days after.
At 4 weeks after vaccination, mean titres of influenza antibodies were similar in the placebo group, the preceding siponimod group and the interrupted siponimod group but were lower in the concomitant siponimod group. However, the proportion of participants in whom seroprotective levels were achieved was similar in all groups for most antigens. For one of the two influenza B virus strains, the seroprotective threshold was not met in the interrupted siponimod and concomitant siponimod groups. 23-PPV vaccination induced a twofold or greater increase in IgG concentrations compared with baseline in 90–100% of participants. Overall, this study provides evidence that siponimod has a limited effect on the efficacy of vaccinations with neoantigens. However, the success of vaccination was not tested in patients with MS. As with other S1P receptor modulators, responses to vaccination might be hampered, and testing for antibody responses, additional booster vaccines or other measures to prevent infections might be necessary.
Pleiotropic therapies
β-Interferons
IFNβ-1a and IFNβ-1b are cytokines with immunomodulatory effects. In a small trial that involved 33 healthy control participants and 26 patients receiving IFNβ-1a or IFNβ-1b for MS, antibody and cellular responses to seasonal influenza vaccine were comparable between the two groups55.
Similar findings were produced by a prospective, non-randomized, open-label study in which 85 patients receiving IFNβ-1a for MS and 77 patients with MS who were receiving no treatment were administered influenza vaccine against various strains56. No significant differences in immune responses were detected between the groups, demonstrating that patients with MS mount an appropriate immune response to influenza vaccine even if they are receiving high-dose, high-frequency IFNβ-1a treatment. Interferons do not seem to attenuate humoral or cellular responses to vaccines and no special measures have to be considered when vaccinations are performed.
Glatiramer acetate
Glatiramer acetate consists of peptides that resemble myelin basic protein and have immunomodulatory effects. Humoral immune responses to protective vaccines in patients treated with glatiramer acetate have mainly been studied in trials with seasonal influenza vaccines. In general, antibody responses were reduced in patients receiving glatiramer acetate compared with those in patients treated with IFNβ57–59. The same was true in a small study of tick-borne encephalitis vaccination60. In general, the evidence suggests that live and inactivated vaccines are safe for patients receiving glatiramer acetate therapy but immune responses are inadequate.
Dimethyl fumarate
Dimethyl fumarate is a prodrug that is metabolized to monomethyl fumarate, which has immunomodulatory effects through the Nrf2 pathway. The drug is approved for the treatment of relapsing–remitting MS and, in some regions, psoriasis. In one study, immune responses to vaccination with tetanus/diphtheria, pneumococcus (polyvalent) and meningococcus (groups A, C, W-135 and Y) antigens were compared in 38 patients receiving dimethyl fumarate and 33 patients receiving non-pegylated interferon61. Response rates were comparable between the two groups, ranging between 53% for meningococcal serogroup C and 95% for pneumococcal serotype 8. Overall, immune responses to vaccination seem to be maintained in patients treated with dimethyl fumarate61,62 so no special measures are needed.
Diroximel fumarate
Diroximel fumarate is a novel oral fumarate. No studies have been conducted to investigate the effects of vaccination during diroximel fumarate treatment to date. However, the response to vaccination is expected to be maintained as with dimethyl fumarate therapy.
Tocilizumab
Tocilizumab is a monoclonal antibody to the IL-6 receptor that is approved for the treatment of NMOSD and has been granted emergency use authorization to prevent severe COVID-19. By binding to soluble and membrane-bound IL-6 receptors, tocilizumab reduces the pro-inflammatory effects of IL-6. The efficacy and safety of vaccines have not been studied in patients receiving tocilizumab for NMOSD or any other neuroimmunological disease. In a study of patients with various other autoimmune inflammatory diseases, antibody responses to pneumococcal polysaccharide vaccination were suppressed in people receiving tocilizumab in comparison with those in people receiving rituximab63. However, a different study showed that responses to hepatitis B vaccination were stronger in people receiving tocilizumab than in people receiving rituximab in autoimmune diseases64. Studies in patients with rheumatoid arthritis have shown that antibody responses to pneumococcal polysaccharide, conjugate and influenza vaccines are not reduced in patients receiving tocilizumab compared with those in untreated patients65,66. Overall, antibody responses to vaccination seem to be maintained in patients treated with tocilizumab therapy so no special measures need to be taken.
Satralizumab
Satralizumab is a humanized monoclonal antibody to the IL-6 receptor that is approved for the treatment of NMOSD in some countries. The efficacy and safety of vaccines with satralizumab treatment have not been studied. Given that this antibody has the same immunological target as tocilizumab, the expected effects on vaccine efficacy are similar. Thus, immunization can be performed in patients who are treated with satralizumab.
Eculizumab
Eculizumab is a C5 complement inhibitor that was originally used for the treatment of haemolytic–uraemic syndrome and paroxysmal nocturnal haemoglobinuria but has been adopted for the treatment of some neuroimmunological diseases. Eculizumab has been approved for the treatment of treatment-refractory acetylcholine receptor antibody-positive myasthenia gravis and of aquaporin 4 antibody-positive NMOSD67,68. Few studies have been conducted to investigate the effects of eculizumab on immune responses to vaccination and none have been conducted in patients with neurological diseases. One methodological difficulty is that commonly used antibody test systems require the presence of complement and therefore rabbit serum is typically used as a complement source to enable the analysis of serum from complement-inhibited patients69.
One retrospective study involving 23 patients with paroxysmal nocturnal haemoglobinuria investigated the effects of eculizumab on serological responses after vaccination with various meningococcal vaccines70. Vaccinations had been administered before treatment initiation for 78% of patients and 43% had been vaccinated more than once owing to chronic eculizumab treatment. No meningococcal infections were observed but overall serological responses differed for the meningococcal subgroups. The protective effects of novel vaccines against meningococcal serogroup B still require assessment. A similar study has been conducted in patients receiving eculizumab for cold agglutinin disease71. In this study, levels of protective antibodies varied for different meningococcal serogroups but declined early or were absent with eculizumab treatment. Based on these findings, we recommend repeated serological analysis for patients receiving chronic eculizumab treatment in addition to revaccination every 3 years and, dependent on serological analysis, early booster vaccinations as recommended for adults with asplenia.
Glucocorticosteroids
Glucocorticosteroids bind to the glucocorticoid receptor and have dose-dependent immunosuppressive effects. Patients with various underlying diseases receiving relatively low doses of glucocorticosteroids (<20 mg prednisolone daily or equivalent in adults) seem to achieve adequate immune responses to vaccination72,73. Among patients receiving glucocorticosteroid therapy for neuromuscular diseases, immune responses to seasonal influenza vaccination were comparable to those among patients who were not receiving glucocorticosteroid therapy74. A meta-analysis showed that antibody responses can be reduced in children receiving low-dose glucocorticosteroids for rheumatoid arthritis but these individuals could still develop seroprotection75 and glucocorticosteroids had no detrimental effect on established antibody responses in this study.
Higher doses of glucocorticosteroids do impair antibody formation although protective effects might be maintained75. In a study of patients with rheumatoid arthritis, antibody responses to pneumococcal polysaccharide or conjugate vaccine were reduced among adults receiving prednisolone at doses above 7.5 mg daily63. Similarly, after influenza vaccination in patients with systemic lupus erythematosus, only 53.9% of patients receiving ≥20 mg prednisolone daily developed protective antibodies compared with 72% of untreated patients and healthy control participants76. By contrast, a study of patients receiving <20 mg prednisolone daily for systemic lupus erythematosus showed no impairment of immune response to the hepatitis B vaccine77. Taken together, the evidence shows that inactivated and live vaccines are safe for patients receiving low-dose systemic glucocorticosteroids; however, for patients receiving higher doses of glucocorticosteroids, live vaccines should be avoided and antibody responses to inactivated vaccines can be impaired.
High-dose intravenous immunoglobulin
High-dose intravenous immunoglobulins (IVIg) are antibodies that are pooled from healthy donors and that have various effects on the immune system, including inhibition of the complement system, effects on macrophages and T cells, and modulation of cytokine networks78,79. Immune responses to vaccination after treatment with IVIg for neuroimmunological diseases have not been studied. In some cases, such as for hepatitis B vaccine, IVIg administered in the days after vaccination could neutralize the vaccine antigen and therefore limit the immune response. Therefore, if IVIg is given within 4 weeks after vaccination, revaccination can be considered at 4 weeks after IVIg therapy to ensure a complete immune response.
Plasma exchange
Plasma exchange is effective in antibody-mediated neurological diseases, such as GBS, and in the treatment of acute exacerbation of neuroimmunological diseases, in which it can yield rapid clinical improvements80–82. The response to vaccines administered during plasma exchange for neuroimmunological diseases has not been studied systematically. In general, responses to vaccinations administered 2–4 weeks before the treatment period and during treatment are likely to be compromised as vaccine antigens are likely to be removed during plasma exchange82,83, and passive vaccination with immunoglobulins is not useful in this period as the treatment removes the antibodies. After completion of plasma exchange, cellular immune response mechanisms are maintained and the production of cytokines and immunoglobulins is expected to normalize within days83. Therefore, vaccination does not necessarily need to be delayed by more than 1–2 weeks, but studies of the immune response to vaccination after plasma exchange are needed to determine the best approach.
Stem cell transplantation
Stem cell transplantation, in particular autologous haematopoietic stem cell transplantation, is a potential treatment option for some patients with MS or NMOSD84. Given the extremity of the procedure, vaccination in this situation is a complex medical issue with various influencing factors. The cellular immune response to previous vaccination might be compromised by stem cell transplantation; therefore, baseline vaccination might need to be repeated after transplantation and reconstitution of the immune system. Vaccinations should be administered according to stem cell transplantation guidelines. Generally, guidelines advise immunization no earlier than 3–6 months after stem cell transplantation, depending on individual immune reconstitution85,86. Basic vaccinations include pneumococcus, tetanus, diphtheria, pertussis, poliomyelitis, Haemophilus influenzae and annual influenza26,84. Live attenuated vaccines, such as measles, mumps, rubella or VZV, are recommended only for patients in whom antibodies are not detectable after complete immune reconstitution84,87.
Comparison of therapies
In several studies, the effects of various immunotherapies on immune responses to seasonal influenza vaccines have been investigated and compared. In one such study58, vaccine-specific antibody responses to the pandemic H1N1 swine flu 2009 vaccine and the seasonal H1N1 and H3N2 influenza 2010 vaccine were analysed in 289 patients with MS who were receiving DMT (IFNβ, glatiramer acetate, natalizumab or mitoxantrone) and 251 healthy control participants. Overall, patients receiving DMT had lower antibody protection rates after H1N1 2009 vaccination compared with control participants (27.4% versus 43.5%). Protection was not influenced by IFNβ but was lowered by all other DMTs included. A similar pattern was seen with the 2010 influenza vaccine.
In a similar study, antibody responses and seroprotection rates after vaccination with the 2012–2013 seasonal influenza vaccine (H1N1, H3N2) in 90 patients receiving various DMTs for MS (fingolimod, glatiramer acetate, IFNβ, natalizumab or no treatment) were compared with those in 62 healthy control participants59. At 3, 6 and 12 months after vaccination, protection rates among patients receiving IFNβ and glatiramer acetate did not differ significantly from those among control participants. By contrast, patients receiving fingolimod had reduced protection at all time points and natalizumab recipients had diminished protection at 3 and 6 months. Among patients with MS who were not receiving DMT, protection rates did not differ from those among control participants.
In a prospective, multicentre, non-randomized observational study of 108 patients with MS, antibody responses and seroprotection to trivalent seasonal influenza vaccination (H1N1, H3N2, B) were analysed. Patients were receiving various DMTs, including IFNβ, glatiramer acetate, natalizumab and fingolimod57. Vaccination elicited robust immune responses in patients receiving IFNβ and glatiramer acetate. Among patients receiving natalizumab and fingolimod, response rates were low, particularly for H3N2. Overall, a longer disease duration was associated with an increased risk of insufficient immune response to vaccination.
Finally, in another prospective, multicentre, non-randomized observational study, the immunogenicity of a tick-borne encephalitis vaccine was analysed in 20 patients with MS who were receiving IFNβ, glatiramer acetate, fingolimod or natalizumab60. Vaccination led to protective antibody titres in 77.8% of participants. Patients who were receiving IFNβ or glatiramer acetate developed sufficient antibody titres, whereas patients receiving fingolimod had low antibody titres.
Practicalities of immunization
Several working groups have published guidelines for the vaccination of patients with neuroimmunological diseases — mainly MS — and of immunocompromised individuals88–91. Implementation of these guidelines in day-to-day clinical practice is vital.
Before initiation of immunotherapy for neuroimmunological diseases, or when switching between therapies, several infection-related aspects need to be considered (Table 2). Regulatory authorities, such as the FDA and EMA, request specific screening to exclude latent infections, such as tuberculosis, and detect the absence of immune protection, including undetectable VZV antibodies, before initiating treatment with some immunotherapies. In addition, some immunotherapies cannot be initiated until after vaccination if immune protection is lacking.
Table 2.
Recommended infection screening and vaccination before immunotherapy
| Drug | FDA and/or EMA requirements | Additional recommendations | |
|---|---|---|---|
| Infection screening | Vaccination | ||
| Alemtuzumab | Annual HPV and Pap smears | Screen for HBV, HCV, HIV, TB, syphilis and VZV; pneumococcal vaccination (regional recommendations); annual influenza vaccination (optional); VZV vaccination if seronegative; avoid attenuated live vaccines (obligatory) | |
| Azathioprine | Live vaccines contraindicated; immune response should be controlled by means of titre determination | Screen for HBV, HCV, HIV, TB and VZV; VZV vaccination if seronegative; pneumococcal vaccination (regional recommendations); annual influenza vaccination (optional); avoid attenuated live vaccines up to 3 months after treatment (obligatory) | |
| Cladribine | Screen for HBV and HCV | VZV if antibody negative | Screen for HBV, HCV, HIV, TB and VZV; VZV vaccination if seronegative; annual influenza vaccination (optional); avoid attenuated live vaccines |
| Cyclophosphamide | Exclude acute infection; screen for HBV, HCV and TB | Live vaccines contraindicated | Screen for HBV, HCV, HIV, TB, syphilis and VZV; consider antimicrobial and antifungal prophylaxis |
| Dimethyl fumarate, diroximel fumarate | Screen for HBV, HCV, HIV and TB (optional); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) | ||
| Eculizumab | Exclude acute infection | Immunizations before initiation according to current guidelines; meningococcal vaccine (serogroups A, C, Y, W-135 and B), consider bridging with antibiotic prophylaxis; Haemophilus influenzae and pneumococcal vaccine (age <18 years) | Screen for HBV, HCV, HIV, TB, syphilis and VZV; pneumococcal (regional recommendations); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) |
| Fingolimod | VZV antibody screening | VZV if antibody negative | Screen for HBV, HCV, HIV and VZV; VZV vaccination if seronegative; HBV and HPV vaccination (optional); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) |
| Glatirameroids | Annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) | ||
| Glucocorticosteroids | Exclude acute infection | Avoid attenuated live vaccines (obligatory) | |
| IFNβ | Annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) | ||
| Inebilizumab | Exclude acute infection; screen for HBV, TB; quantify serum immunoglobulins | Live attenuated or live vaccines not recommended during treatment or until B cell repletion; complete required vaccinations ≥4 weeks before initiation | Check vaccination status before initiation; screen for HBV, HCV, HIV, TB and VZV; seasonal influenza vaccination (optional); avoid attenuated live vaccines (obligatory); test for quantitative serum immunoglobulins |
| Mitoxantrone | Exclude acute infection | Live vaccines contraindicated; delay vaccination for 3 months after treatment | Screen for HBV, HCV, HIV and TB (optional); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) |
| Mycophenolate mofetil | Live vaccines contraindicated | Screen for HBV, HCV, HIV, TB and VZV; VZV vaccination if seronegative; pneumococcal vaccination (regional recommendations); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory); screen for hypogammaglobulinaemia during treatment | |
| Natalizumab | Screen for HBV, HCV, HIV, JCV, TB and VZV (optional); VZV vaccination if seronegative; annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) | ||
| Ocrelizumab | Screen for HBV and HCV | Check vaccination status before initiation; screen for HBV, HCV, HIV, TB and VZV; pneumococcal vaccination (regional recommendations); VZV vaccination if seronegative; HBV vaccination (optional); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) | |
| Ofatumumab | Screen for HBV; quantify serum immunoglobulins | Live attenuated or live vaccines not recommended during treatment or until B cell repletion; complete required vaccinations ≥4 weeks before initiation | Check vaccination status before initiation; screen for HBV, HCV, HIV, TB and VZV; pneumococcal vaccination (regional recommendations); VZV vaccination if seronegative; HBV vaccination (optional); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) |
| Ozanimod | VZV antibody screening | VZV if antibody negative | Screen for HBV, HCV, HIV and VZV; VZV vaccination if seronegative; HBV and HPV vaccination (optional); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) |
| Ponesimod | VZV antibody screening | VZV if antibody negative | Screen for HBV, HCV, HIV and VZV; VZV vaccination if seronegative; HBV and HPV vaccination (optional); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) |
| Rituximab | Screen for HBV, HCV, HIV, TB, syphilis and VZV; VZV vaccination if seronegative; pneumococcal vaccination (regional recommendations); HBV vaccination (optional); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) | ||
| Satralizumab | Exclude acute infection; screen for HBV and TB | Immunizations before initiation according to current guidelines | Screen for HBV, HCV, HIV, TB, syphilis and VZV; pneumococcal (regional recommendations); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) |
| Siponimod | Contraindicated history of PML or cryptococcal meningitis; screen for VZV | VZV if antibody negative; delay treatment initiation until 4 weeks after vaccination | Screen for HBV, HCV, HIV and VZV; VZV vaccination if seronegative; annual influenza vaccination (optional); avoid attenuated live vaccines up to 4 weeks after treatment (obligatory) |
| Teriflunomide | Screen for HBV, HCV, HIV and TB (optional); annual influenza vaccination (optional); avoid attenuated live vaccines | ||
| Tocilizumab | Exclude acute infection; screen for HBV and TB | Immunizations before initiation according to current guidelines | Screen for HBV, HCV, HIV, TB, syphilis and VZV; pneumococcal vaccine vaccination (regional recommendations); annual influenza vaccination (optional); avoid attenuated live vaccines (obligatory) |
When determining the appropriate timing of protective vaccination, the biological half-lives and pharmacodynamics of individual immunotherapies should be considered (Table 3). Special attention is required when live attenuated vaccines are being considered for patients receiving immunotherapies as inappropriate use of these vaccines can lead to severe adverse effects or deterioration of the underlying disease.
Table 3.
Suggested intervals between immunotherapies and vaccinations
| Mechanism of action | Drug | Interval from vaccine to treatment (weeks) | Live vaccine during therapy permitted | Interval from treatment to live vaccine | |
|---|---|---|---|---|---|
| Inactivated vaccine | Live vaccine | ||||
| Direct depletion or cytolysis | Ocrelizumab | >6 | >6 | No | ~18 months + normal B cell count |
| Rituximab | >4 | >4 | No | >12 months + normal B cell count | |
| Ofatumumab | 2–4 | >4 | No | Not studied; after B cell repletion (~40 weeks) | |
| Inebilizumab | >4 | >4 | No | After B cell repletion | |
| Alemtuzumab | 6 | 6 | No | >12 months + normal B cell count | |
| Impairment of cell proliferation | Teriflunomide | 2–4a | 4 | No | >6 months |
| Azathioprine | 2–4a | 4 | No | >3 months | |
| Cladribine | 2–4a | 4–6 | No | 4–6 weeks + normal lymphocyte count | |
| Cyclophosphamide | 2–4a | 4 | No | >3 months | |
| Mitoxantrone | 2–4a | 4 | No | >3 months | |
| Mycophenolate mofetil | 2–4a | >4–6 | No | >2 months | |
| Inhibition of cell migration | Natalizumab | 2–4a | 4 | No | >3 months |
| Fingolimod | 2–4a | 4 | No | >2 months | |
| Ozanimod | 4a | >4 | No | 3 months | |
| Ponesimod | 4a | >4 | No | 2 weeks | |
| Siponimod | 4 | 4 | No | 4 weeks | |
| Pleiotropic effects | IFNβ | 0 | 0 | Individual risk assessment | None |
| Glatiramer acetate | 2–4a | 2–4a | Individual risk assessment (avoid YF vaccine) | None | |
| Dimethyl fumarate | 2–4a | 4 | No (when lymphopenic) | After normalization of lymphocyte count | |
| Tocilizumab | 4a | 4 | No | Not studied | |
| Satralizumab | 2–4a | 4 | No | Not studied | |
| Eculizumab | 2–4 | 4 | Not advised | Not studied | |
| Glucocorticosteroidsb | 0 | 0 | Yes | None | |
| Glucocorticosteroidsb for >2 weeks | 2–4 | 4 | No | >2 months | |
| IVIg | 2–4a | 2–4a | Yes | >3 months (diminished response to measles vaccine up to 1 year) | |
| Plasma exchange | 2–4a | 2–4a | Not advised | None | |
Information based on prescribing information and refs49,73,83,90,222–226. IVIg, intravenous immunoglobulin; YF, yellow fever. aWhere possible, shorter intervals can lead to reduced immune response. If shorter intervals are unavoidable, testing for antibody responses to vaccination and/or additional vaccination might be necessary. bEquivalent to <20 mg prednisolone daily.
Measurement of immune responses to vaccines is not routinely recommended, but correlates of protection can be assessed for, among others, hepatitis A, hepatitis B, measles, tetanus, tick-borne encephalitis, rabies and VZV92–96. Importantly, immune protection can be primarily cell mediated, and therefore antibody titres might not always be the best surrogate marker for vaccine-induced protection.
SARS-CoV-2 in neuroimmunological diseases
Vaccination against SARS-CoV-2 is generally recommended for people with neuroimmunological disease. Indeed, in some countries, this group has been prioritized for vaccination. Overall, the risk of SARS-Cov-2 infection does not seem to be higher among patients with MS than among the general population97,98 but the risk of severe COVID-19 does seem to be increased in patients receiving treatment with specific immunotherapies for neuroimmunological disease99–103. Early data suggest that treatment with monoclonal antibodies that deplete B cells carries an increased risk of serious illness99,100,104–109. Patients with MS or NMOSD who are receiving anti-CD20 therapy have lower antibody responses after SARS-CoV-2 infection than patients receiving other DMTs or no DMT110–113. However, in a Swedish cohort of patients with MS, rituximab treatment did not increase the risk of hospitalization above that with other DMTs and neither the timing of rituximab infusion nor the cumulative lifetime dose influenced COVID-19 severity114. Impaired antibody responses to SARS-CoV-2 infection in patients receiving fingolimod for MS has also been observed113,115. By contrast, treatment with IFNβ for MS does not seem to increase the risk of severe COVID-19 (ref.116).
In this context, vaccination against SARS-CoV-2 is important to reduce the risk of SARS-CoV-2 infection and, more importantly, minimize the severity of COVID-19 in patients with MS and other neuroimmunological diseases117. Data on SARS-CoV-2 vaccination in these patients and the effects of immunotherapies on responses to SARS-CoV-2 vaccinations are accumulating rapidly. A regularly curated literature hub is likely to help all relevant stakeholders stay abreast of new developments118.
SARS-CoV-2 vaccines
Internationally, a large number of SARS-CoV-2 vaccines have been approved and the number is likely to continue increasing13,119–124; the WHO maintains a regularly updated website that provides an overview of the licenced vaccines and candidate vaccines14. Approved vaccines are of various types. Among them are novel nucleoside-modified mRNA vaccines11–15, which consist of delivery vehicles, such as lipid nanoparticles, that encapsulate nucleoside-modified mRNA that encodes the SARS-CoV-2 spike glycoprotein8,125–127. The mRNA-based vaccines produced by BioNTech (BNT162b2) and Moderna (mRNA-1273) do not lead to intracellular virus replication and can therefore be considered non-live vaccines128–130.
Other types of approved SARS-CoV-2 vaccines are recombinant viral vector vaccines (ChAdOx1 COVID-19 vaccine131,132, Ad26.COV2.S133,134, Gam-COVID-Vac/Sputnik V135,136 and Ad5-nCoV137,138), inactivated whole-cell COVID-19 vaccines (CoronaVac/Sinovac COVID-19 vaccine, BIBP COVID-19 vaccine, WIBP-CorV, Covaxin and others135,137,138), and subunit vaccines, in which COVID-19 proteins are used as antigens (EpiVacCorona124, ZF2001, MVC COVID-19 vaccine, Soberana 02 (refs139,140), NVX-CoV2373 (refs141,142) and CoV2 preS dTM143). In general, SARS-CoV-2 vaccines seem to be safe and do not lead to specific adverse events in people with neuroimmunological disease117,135,144–150.
Immunological responses
The immune response to SARS-CoV-2 vaccinations is not yet fully understood, but knowledge is rapidly accumulating. Evidence suggests that a balanced humoral and T helper type 1 cellular immune response are critical for protection against SARS-Cov-2 (ref.15). Quantitative testing for immune responses to SARS-CoV-2 vaccination generally involves measurement of antibodies against the SARS-CoV-2 spike protein. However, the clinical correlates of this antibody response are yet to be defined151–153. Measurement of specific cellular vaccine responses is not routinely available, and whether T cell responses alone will translate into protection against SARS-CoV-2 remains unclear154. BNT162b2 vaccination protects against SARS-CoV-2 (ref.155) and induces a specific T cell response156 that lasts at least 6 months in healthy adults157.
Follow-up studies have shown that antibody responses to SARS-CoV-2 vaccines wane after several months132,158–165, and booster vaccination is required to restore these responses. COVID-19-associated mortality is lower among people who have received two vaccine doses and a booster than among people who have received only two doses of vaccine166. Of note, heterologous booster vaccination using mRNA or Ad26.COV2.S showed similar or higher immunogenicity compared to homologous booster vaccine167.
The extent of protection against severe COVID-19 provided by SARS-CoV-2 vaccines is partly determined by the responsible viral variant. For the delta variant (lineage B.1.617.2), many approved vaccines have proven sufficient to protect against severe disease160,162,168–172 and booster doses prevent hospitalization and death with the delta variant for 97–99% of adults in all age groups for at least 10 weeks after the booster dose166,173. However, a greater antibody response is required for protection against the omicron variant (lineage B.1.1.529) owing to its diminished susceptibility to the vaccine-induced antibody response174–176. Booster vaccination with BNT162 has been shown to elicit a sufficient neutralizing antibody response against the omicron variant177 and could mitigate the reduction in vaccine effectiveness against this variant178–184. Booster vaccination after SARS-CoV2 infection is also recommended for durable protection against reinfection185.
SARS-CoV-2 vaccines and immunotherapy
Studies to date suggest that B cell depletion and other immunosuppressive therapy — particularly S1P1 receptor modulation with fingolimod — reduces antibody responses to SARS-CoV-2 vaccination whereas immunomodulatory therapies and IVIg or plasma exchange do not substantially influence vaccine responses4,6,186–190. One study has also suggested that siponimod treatment in patients with secondary progressive MS reduces the humoral response to SARS-CoV-2 vaccination in comparison with that in healthy control participants191.
In a small study conducted in Israel, the BNT162b2 vaccine was administered to 93 patients with MS who were receiving cladribine, fingolimod, ocrelizumab or no treatment. Antibody responses to the vaccine among patients receiving cladribine were comparable to those among untreated patients188. By contrast, the majority of patients receiving fingolimod did not develop sufficient antibody responses, and protective antibody titres were reached in only 22.7% of patients receiving ocrelizumab188. However, no relevant safety signal was detected; in particular, relapse activity was not increased192.
In a larger study, antibody responses to mRNA SARS-CoV-2 vaccines were lower in patients with MS who were receiving treatment with fingolimod or CD20 antibodies than in patients receiving no treatment193. In this study, vaccination with mRNA-1273 led to higher antibody levels than vaccination with BNT162b2 (ref.193). Moreover, the humoral response was greater with a longer time since the last administration of anti-CD20 treatment and was associated with the number of repopulated B cells at the time of vaccination194. In patients vaccinated with mRNA-1273, the overall seroconversion rates at day 70 were 39.3% among patients receiving ocrelizumab compared with 100% among untreated patients195. The seroconversion rate was 26% among patients who were vaccinated less than 12 weeks after administration of ocrelizumab and 50% among patients who were vaccinated more than 12 weeks after ocrelizumab treatment195. Low B cell counts before vaccination were associated with lower seroconversion rates195,196. The same association has also been observed among patients receiving other anti-CD20 therapies for neuroimmunological disease197. Interestingly, 24.6% of patients with MS who were receiving anti-CD20 therapy developed protective humoral immunity after a booster dose of mRNA SARS-CoV-2 vaccine, whereas only 6.9% of patients who were receiving fingolimod developed an adequate antibody response198.
Additional studies have corroborated the finding that the humoral immune response to mRNA and ChAdOx1 nCoV-19 vaccines is reduced in patients with MS receiving treatment with either a selective S1P1 receptor modulator or CD20 antibody therapy154,199–203, and similar results have been obtained with an inactivated SARS-CoV-2 vaccination (BBIBP-CorV)204,205. However, the overall antibody response to the ChAdOx1 nCoV-19 vaccine seems to be lower than that to the BNT162b2 vaccine200. However, this study also showed that 7 of 16 participants without detectable humoral immunity after vaccination did have SARS-CoV-2-specific T cell responses200.
Robust T cell responses to SARS-CoV-2 vaccination (predominantly mRNA vaccines) have been observed in other cohorts of patients receiving anti-CD20 therapy for MS in whom humoral immune responses to SARS-CoV-2 vaccination are attenuated154,206–208. In a study of 49 patients with MS who were receiving treatment with ocrelizumab, the antibody response to BNT162b2 was diminished but, of 29 patients without a sufficient antibody response, 26 had SARS-CoV-2-specific T cells; this proportion is comparable to that of healthy control participants207. Similarly, in a study of 20 patients with MS receiving treatment with anti-CD20 therapy, 89% developed spike-specific antibodies and only 50% developed receptor-binding domain-specific antibodies after vaccination with mRNA SARS-CoV-2 vaccination, whereas all healthy control participants developed antibodies206. In this study, patients receiving anti-CD20 therapy had lower specific memory B cell responses to mRNA SARS-CoV-2 vaccination than did healthy control participants but their antigen-specific CD4+ T cell and CD8+ T cell responses were comparable to those of healthy control participants206. Overall, evidence suggests that antigen-specific CD4+ and CD8+ T cell responses after vaccination are adequate in patients with MS who are receiving anti-CD20 monoclonal antibodies despite poor spike-specific and receptor-binding domain-specific antibody production62,206,207. Other studies have shown that patients receiving treatment with fingolimod for MS had low humoral and T cell responses to mRNA or vectored SARS-CoV-2 vaccinations62,209.
Studies of patients receiving anti-CD20 therapy for non-neurological disease have demonstrated similar reduced antibody responses to SARS-CoV-2 vaccines210,211. In one comparison of responses in patients receiving anti-CD20 therapy for MS and rheumatoid arthritis, humoral responses to mRNA SARS-CoV-2 vaccination were impaired in both groups compared with those in healthy control participants but T cell responses were adequate212,213.
Long-term vaccination responses in people with MS have been analysed in one study. The study included 414 patients with MS and 89 healthy control participants and showed that the protective response to SARS-CoV-2 vaccination in untreated patients and in patients treated with cladribine, dimethyl fumarate, natalizumab or teriflunomide was similar to that in healthy people214. However, protective antibody titres at the same time point were observed in only 9.5% of patients treated with fingolimod, 22.8% of patients treated with ocrelizumab and 86.4% of patients treated with alemtuzumab214.
Owing to the reduced humoral response to SARS-CoV-2 vaccines in patients receiving anti-CD20 therapy, published recommendations advise vaccination against SARS-CoV-2 at least 3 months after the last anti-CD20 treatment and 4–6 weeks before the next anti-CD20 treatment194,215,216. However, evidence suggests that dosing intervals longer than 6 months can improve the humoral vaccine response194,217. In general, SARS-CoV-2 vaccination should be recommended for all patients receiving immunotherapy. Given the risk of infection during the pandemic, the relative timing of vaccination in relation to dosing of immunotherapy is of minor relevance even if the immune response is reduced144,215,218,219.
Conclusion
Prevention of infectious diseases is an important part of the management of neuroimmunological diseases. The course of these diseases can be negatively affected by infections, and patients with these diseases — particularly those treated with disease-modifying immunotherapy — have a higher risk of acquiring infections and of severe illness as a result of these infections than do healthy individuals; therefore, prophylactic vaccinations are crucial.
Immunotherapies used for neuroimmunological diseases affect the immune system in different ways. Their influence on the individual risk of infection varies and they interact with vaccine immunology (Supplementary Table 3). In general, vaccinations are safe in people with underlying neuroimmunological conditions though live attenuated vaccines should be avoided, especially during concomitant immunotherapy. Both humoral and cellular immune responses to vaccination can be hampered by some immunotherapies though protection from infection can be achieved by vaccination in many cases. Nevertheless, improved knowledge of the interplay between autoimmune dysregulation, immunotherapies and immunization with conventional and novel vaccines is required to improve the care of patients with neuroimmunological diseases. The integration of SARS-CoV-2 vaccinations into treatment paradigms for MS and other neuroimmunological diseases is an ongoing challenge, particularly for patients who are already established on immunotherapy. For these patients, the timing of vaccination is important to ensure maximal safety and protection.
Supplementary information
Glossary
- Delayed-type hypersensitivity
(DHT). A cell-mediated overreaction to foreign antigens that leads to inflammation and tissue damage.
- Recall antigens
Antigens to which healthy people are generally expected to have previously been exposed to such as tetanus, streptococcus, candida or tuberculosis.
Author contributions
A.W. and M.L. wrote and edited the manuscript. M.B. edited the manuscript. A.W., M.L. and H.-P.H. performed the literature search. All authors contributed to conceptualization, data interpretation and review.
Peer review
Peer review information
Nature Reviews Neurology thanks J. Correale, S. Otero and K. Schmierer for their contribution to the peer review of this work.
Competing interests
A.W. reports personal fees from Alexion, Biogen, Celgene, Merck, Novartis, Sanofi, Roche and Teva that are not directly related to the manuscript. M.L. reports institutional funding from Sanofi Pasteur and personal fees from AbbVie, Bayer, Bristol Myers Squibb, Merck Healthcare and Pfizer that are not directly related to the manuscript. M.B. reports institutional funding from Alexion, Biogen, BMS, Merck, Novartis, and Roche and personal fees from Biogen and Novartis that are not directly related to the manuscript. H.-P.H. reports personal fees from Bayer Healthcare, Biogen, Celgene BMS, GeNeuro, Merck, Novartis, Roche, TG Therapeutics and VielaBio that are not directly related to the manuscript. U.K.Z. reports personal fees from Alexion, Almirall, Bayer, Biogen, Janssen, Merck Serono, Novartis, Octapharm, Roche, Sanofi Genzyme, and Teva as well as grants from the European Union, German Federal Ministry of Education and Research, German Federal Ministry for Economic Affairs and Climate Action, and Deutsche Forschungsgemeinschaft that are not directly related to the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alexander Winkelmann, Micha Loebermann.
These authors jointly supervised this work: Hans-Peter Hartung and Uwe K. Zettl.
Supplementary information
The online version contains supplementary material available at 10.1038/s41582-022-00646-5.
References
- 1.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N. Engl. J. Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front. Immunol. 2018;9:3116. doi: 10.3389/fimmu.2018.03116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97:742–768. doi: 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Loebermann M, et al. Vaccination against infection in patients with multiple sclerosis. Nat. Rev. Neurol. 2012;8:143–151. doi: 10.1038/nrneurol.2012.8. [DOI] [PubMed] [Google Scholar]
- 5.Moiola L, Rommer PS, Zettl UK. Prevention and management of adverse effects of disease modifying treatments in multiple sclerosis. Curr. Opin. Neurol. 2020;33:286–294. doi: 10.1097/WCO.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 6.Zrzavy T, et al. Vaccination in multiple sclerosis: friend or foe? Front. Immunol. 2019;10:1883. doi: 10.3389/fimmu.2019.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegrist, C. A. in Plotkin’s Vaccines (eds Plotkin, S., Orenstein, W., Offit, P., & Edwards, K. M.) 17-36 (Elsevier, 2018).
- 8.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardi N, Hogan MJ, Weissman D. Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 2020;65:14–20. doi: 10.1016/j.coi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Gary EN, Weiner DB. DNA vaccines: prime time is now. Curr. Opin. Immunol. 2020;65:21–27. doi: 10.1016/j.coi.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawat K, Kumari P, Saha L. COVID-19 vaccine: a recent update in pipeline vaccines, their design and development strategies. Eur. J. Pharmacol. 2021;892:173751. doi: 10.1016/j.ejphar.2020.173751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 14.WHO. COVID-19 Vaccine Tracker and Landscapehttps://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (2021). The COVID-19 vaccine tracker assembles up to date information on COVID-19 vaccine candidates currently under development.
- 15.Poland GA, Ovsyannikova IG, Crooke SN, Kennedy RB. SARS-CoV-2 vaccine development: current status. Mayo Clinic Proc. 2020;95:2172–2188. doi: 10.1016/j.mayocp.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plotkin, S. L. & Plotkin, S. A. in Plotkin’s Vaccines (eds Plotkin, S., Orenstein, W., Offit, P., & Edwards, K. M.) (Elsevier, 2018).
- 17.Beutler B. Innate immunity: an overview. Mol. Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Kar UK, Joosten LAB. Training the trainable cells of the immune system and beyond. Nat. Immunol. 2020;21:115–119. doi: 10.1038/s41590-019-0583-y. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netea MG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominguez-Andres J, Netea MG. Long-term reprogramming of the innate immune system. J. Leukoc. Biol. 2019;105:329–338. doi: 10.1002/JLB.MR0318-104R. [DOI] [PubMed] [Google Scholar]
- 22.Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin. Immunol. 2018;39:14–21. doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Villarreal R, Casale TB. Commonly used adjuvant human vaccines: advantages and side effects. J. Allergy Clin. Immunol. Pract. 2020;8:2953–2957. doi: 10.1016/j.jaip.2020.04.045. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrino P, Clementi E, Radice S. On vaccine’s adjuvants and autoimmunity: current evidence and future perspectives. Autoimmun. Rev. 2015;14:880–888. doi: 10.1016/j.autrev.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Stowe J, Andrews N, Miller E. Do vaccines trigger neurological diseases? epidemiological evaluation of vaccination and neurological diseases using examples of multiple sclerosis, Guillain-Barre syndrome and narcolepsy. CNS Drugs. 2020;34:1–8. doi: 10.1007/s40263-019-00670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobermann M, et al. Immunization in the adult immunocompromised host. Autoimmun. Rev. 2012;11:212–218. doi: 10.1016/j.autrev.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Hapfelmeier A, Gasperi C, Donnachie E, Hemmer B. A large case-control study on vaccination as risk factor for multiple sclerosis. Neurology. 2019;93:e908–e916. doi: 10.1212/WNL.0000000000008012. [DOI] [PubMed] [Google Scholar]
- 28.Farez MF, Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J. Neurol. 2011;258:1197–1206. doi: 10.1007/s00415-011-5984-2. [DOI] [PubMed] [Google Scholar]
- 29.Mailand MT, Frederiksen JL. Vaccines and multiple sclerosis: a systematic review. J. Neurol. 2017;264:1035–1050. doi: 10.1007/s00415-016-8263-4. [DOI] [PubMed] [Google Scholar]
- 30.Jasti AK, et al. Guillain-Barre syndrome: causes, immunopathogenic mechanisms and treatment. Expert Rev. Clin. Immunol. 2016;12:1175–1189. doi: 10.1080/1744666X.2016.1193006. [DOI] [PubMed] [Google Scholar]
- 31.Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barre syndrome after exposure to influenza virus. Lancet Infect. Dis. 2010;10:643–651. doi: 10.1016/S1473-3099(10)70140-7. [DOI] [PubMed] [Google Scholar]
- 32.Kwong JC, et al. Risk of Guillain-Barre syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect. Dis. 2013;13:769–776. doi: 10.1016/S1473-3099(13)70104-X. [DOI] [PubMed] [Google Scholar]
- 33.Bar-Or A, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95:e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim W, et al. Reduced antibody formation after influenza vaccination in patients with neuromyelitis optica spectrum disorder treated with rituximab. Eur. J. Neurol. 2013;20:975–980. doi: 10.1111/ene.12132. [DOI] [PubMed] [Google Scholar]
- 35.Bingham CO, 3rd, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62:64–74. doi: 10.1002/art.25034. [DOI] [PubMed] [Google Scholar]
- 36.Rehnberg M, et al. Vaccination response to protein and carbohydrate antigens in patients with rheumatoid arthritis after rituximab treatment. Arthritis Res. Ther. 2010;12:R111. doi: 10.1186/ar3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauser SL, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N. Engl. J. Med. 2020;383:546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 38.Cree BAC, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394:1352–1363. doi: 10.1016/S0140-6736(19)31817-3. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy CL, et al. Immune competence after alemtuzumab treatment of multiple sclerosis. Neurology. 2013;81:872–876. doi: 10.1212/WNL.0b013e3182a35215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moser T, et al. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann. Clin. Transl. Neurol. 2020;7:2199–2212. doi: 10.1002/acn3.51206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin. Neuropharmacol. 2011;34:28–35. doi: 10.1097/WNF.0b013e318204cd90. [DOI] [PubMed] [Google Scholar]
- 42.Brill L, et al. Effect of cladribine on COVID-19 serology responses following two doses of the BNT162b2 mRNA vaccine in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2022;57:103343. doi: 10.1016/j.msard.2021.103343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gold R, et al. Vaccination in multiple sclerosis patients treated with highly effective disease-modifying drugs: an overview with consideration of cladribine tablets. Ther. Adv. Neurol. Disord. 2021;14:17562864211019598. doi: 10.1177/17562864211019598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorensen PS, et al. Expert opinion on the use of cladribine tablets in clinical practice. Ther. Adv. Neurol. Disord. 2020;13:1756286420935019. doi: 10.1177/1756286420935019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bock H, et al. Humoral and cellular immune responses to SARS CoV-2 vaccination in people with multiple sclerosis and NMOSD patients receiving immunomodulatory treatments. Mult. Scler. Relat. Disord. 2022;59:103554. doi: 10.1016/j.msard.2022.103554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bar-Or A, et al. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology. 2013;81:552–558. doi: 10.1212/WNL.0b013e31829e6fbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bar-Or A, et al. Randomized study of teriflunomide effects on immune responses to neoantigen and recall antigens. Neurol. Neuroimmunol. Neuroinflamm. 2015;2:e70. doi: 10.1212/NXI.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bar-Or A, Pachner A, Menguy-Vacheron F, Kaplan J, Wiendl H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs. 2014;74:659–674. doi: 10.1007/s40265-014-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: a review. Mult. Scler. Relat. Disord. 2020;45:102439. doi: 10.1016/j.msard.2020.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vagberg M, Kumlin U, Svenningsson A. Humoral immune response to influenza vaccine in natalizumab-treated MS patients. Neurol. Res. 2012;34:730–733. doi: 10.1179/1743132812Y.0000000059. [DOI] [PubMed] [Google Scholar]
- 51.Kaufman M, et al. Natalizumab treatment shows no clinically meaningful effects on immunization responses in patients with relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2014;341:22–27. doi: 10.1016/j.jns.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 52.Kappos L, et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015;84:872–879. doi: 10.1212/WNL.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 53.Signoriello E, et al. Is antibody titer useful to verify the immunization after VZV vaccine in MS patients treated with fingolimod? A case series. Mult. Scler. Relat. Disord. 2020;40:101963. doi: 10.1016/j.msard.2020.101963. [DOI] [PubMed] [Google Scholar]
- 54.Ufer M, et al. Impact of siponimod on vaccination response in a randomized, placebo-controlled study. Neurol. Neuroimmunol. Neuroinflamm. 2017;4:e398. doi: 10.1212/NXI.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehling M, et al. Preserved antigen-specific immune response in patients with multiple sclerosis responding to IFNbeta-therapy. PLoS One. 2013;8:e78532. doi: 10.1371/journal.pone.0078532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwid SR, Decker MD, Lopez-Bresnahan M, Rebif-Influenza Vaccine Study Investigators. Immune response to influenza vaccine is maintained in patients with multiple sclerosis receiving interferon beta-1a. Neurology. 2005;65:1964–1966. doi: 10.1212/01.wnl.0000188901.12700.e0. [DOI] [PubMed] [Google Scholar]
- 57.Metze C, et al. Immunogenicity and predictors of response to a single dose trivalent seasonal influenza vaccine in multiple sclerosis patients receiving disease-modifying therapies. CNS Neurosci. Ther. 2019;25:245–254. doi: 10.1111/cns.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olberg HK, et al. Immunotherapies influence the influenza vaccination response in multiple sclerosis patients: an explorative study. Mult. Scler. 2014;20:1074–1080. doi: 10.1177/1352458513513970. [DOI] [PubMed] [Google Scholar]
- 59.Olberg HK, et al. Antibody response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. Eur. J. Neurol. 2018;25:527–534. doi: 10.1111/ene.13537. [DOI] [PubMed] [Google Scholar]
- 60.Winkelmann A, et al. Tick-borne encephalitis vaccination in multiple sclerosis: a prospective, multicenter study. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:e664. doi: 10.1212/NXI.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Hehn C, et al. Immune response to vaccines is maintained in patients treated with dimethyl fumarate. Neurol. Neuroimmunol. Neuroinflamm. 2018;5:e409. doi: 10.1212/NXI.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabatino JJ, Jr, et al. Multiple sclerosis therapies differentially affect SARS-CoV-2 vaccine-induced antibody and T cell immunity and function. JCI Insight. 2022;7:e156978. doi: 10.1172/jci.insight.156978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richi P, et al. Impact of biological therapies on the immune response after pneumococcal vaccination in patients with autoimmune inflammatory diseases. Vaccines. 2021;9:203. doi: 10.3390/vaccines9030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richi P, et al. Evaluation of the immune response to hepatitis B vaccine in patients on biological therapy: results of the RIER cohort study. Clin. Rheumatol. 2020;39:2751–2756. doi: 10.1007/s10067-020-05042-2. [DOI] [PubMed] [Google Scholar]
- 65.Mori S, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tocilizumab therapy. Ann. Rheum. Dis. 2013;72:1362–1366. doi: 10.1136/annrheumdis-2012-202658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mori S, et al. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2012;71:2006–2010. doi: 10.1136/annrheumdis-2012-201950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frampton JE. Eculizumab: a review in neuromyelitis optica spectrum disorder. Drugs. 2020;80:719–727. doi: 10.1007/s40265-020-01297-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pittock SJ, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N. Engl. J. Med. 2019;381:614–625. doi: 10.1056/NEJMoa1900866. [DOI] [PubMed] [Google Scholar]
- 69.Lewis LA, Ram S. Meningococcal disease and the complement system. Virulence. 2014;5:98–126. doi: 10.4161/viru.26515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alashkar F, et al. Serologic response to meningococcal vaccination in patients with paroxysmal nocturnal hemoglobinuria (PNH) chronically treated with the terminal complement inhibitor eculizumab. Ann. Hematol. 2017;96:589–596. doi: 10.1007/s00277-017-2924-y. [DOI] [PubMed] [Google Scholar]
- 71.Alashkar F, et al. Serologic response to meningococcal vaccination in patients with cold agglutinin disease (CAD) in the novel era of complement inhibition. Vaccine. 2019;37:6682–6687. doi: 10.1016/j.vaccine.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 72.American Academy of Pediatrics. in Red Book: 2009 Report of the Committee on Infectious Diseases. (eds Pickering, L. K., Baker, C., Kimberlin, D. W. & Long, S.) (American Academy of Pediatrics, 2009).
- 73.Bühler S, et al. Vaccination recommendations for adult patients with autoimmune inflammatory rheumatic diseases. Swiss Med. Wkly. 2015;145:w14159. doi: 10.4414/smw.2015.14159. [DOI] [PubMed] [Google Scholar]
- 74.Golekoh MC, et al. Comparison of the immunogenicity of intramuscular versus subcutaneous administration of trivalent inactivated influenza vaccine in individuals with neuromuscular diseases. J. Child. Neurol. 2013;28:596–601. doi: 10.1177/0883073813480243. [DOI] [PubMed] [Google Scholar]
- 75.Groot N, Heijstek MW, Wulffraat NM. Vaccinations in paediatric rheumatology: an update on current developments. Curr. Rheumatol. Rep. 2015;17:46. doi: 10.1007/s11926-015-0519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borba EF, et al. Influenza A/H1N1 vaccination of patients with SLE: can antimalarial drugs restore diminished response under immunosuppressive therapy? Rheumatology. 2012;51:1061–1069. doi: 10.1093/rheumatology/ker427. [DOI] [PubMed] [Google Scholar]
- 77.Kuruma KA, Borba EF, Lopes MH, de Carvalho JF, Bonfa E. Safety and efficacy of hepatitis B vaccine in systemic lupus erythematosus. Lupus. 2007;16:350–354. doi: 10.1177/0961203307078225. [DOI] [PubMed] [Google Scholar]
- 78.Lunemann JD, Nimmerjahn F, Dalakas MC. Intravenous immunoglobulin in neurology — mode of action and clinical efficacy. Nat. Rev. Neurol. 2015;11:80–89. doi: 10.1038/nrneurol.2014.253. [DOI] [PubMed] [Google Scholar]
- 79.Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int. Immunol. 2017;29:491–498. doi: 10.1093/intimm/dxx039. [DOI] [PubMed] [Google Scholar]
- 80.Blechinger S, et al. Therapeutic plasma exchange in steroid-refractory multiple sclerosis relapses. A retrospective two-center study. Ther. Adv. Neurol. Disord. 2021;14:1756286420975642. doi: 10.1177/1756286420975642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shang P, Feng J, Wu W, Zhang HL. Intensive care and treatment of severe Guillain-Barre syndrome. Front. Pharmacol. 2021;12:608130. doi: 10.3389/fphar.2021.608130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reeves HM, Winters JL. The mechanisms of action of plasma exchange. Br. J. Haematol. 2014;164:342–351. doi: 10.1111/bjh.12629. [DOI] [PubMed] [Google Scholar]
- 83.Guptill JT, et al. Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity. 2016;49:472–479. doi: 10.1080/08916934.2016.1214823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharrack B, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE) Bone Marrow Transpl. 2020;55:283–306. doi: 10.1038/s41409-019-0684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cordonnier C, et al. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7) Lancet Infect. Dis. 2019;19:e200–e212. doi: 10.1016/S1473-3099(18)30600-5. [DOI] [PubMed] [Google Scholar]
- 86.Christopeit M, et al. Prophylaxis, diagnosis and therapy of infections in patients undergoing high-dose chemotherapy and autologous haematopoietic stem cell transplantation. 2020 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO) Ann. Hematol. 2021;100:321–336. doi: 10.1007/s00277-020-04297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rieger CT, et al. Anti-infective vaccination strategies in patients with hematologic malignancies or solid tumors — Guideline of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO) Ann. Oncol. 2018;29:1354–1365. doi: 10.1093/annonc/mdy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Farez MF, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93:584–594. doi: 10.1212/WNL.0000000000008157. [DOI] [PubMed] [Google Scholar]
- 89.Lebrun C, Vukusic S, French Group for Recommendations in Multiple Sclerosis France4MS the Société francophone de la sclérose en plaques SFSEP. Immunization and multiple sclerosis: recommendations from the French Multiple Sclerosis Society. Mult. Scler. Relat. Disord. 2019;31:173–188. doi: 10.1016/j.msard.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 90.Wiedermann U, et al. Guidelines for vaccination of immunocompromised individuals. Wien. Klin. Wochenschr. 2016;128:337–376. doi: 10.1007/s00508-016-1033-6. [DOI] [PubMed] [Google Scholar]
- 91.Wagner N, et al. Impfen bei Immundefizienz. Bundesgesundheitsblatt. 2019;62:494–515. doi: 10.1007/s00103-019-02905-1. [DOI] [PubMed] [Google Scholar]
- 92.Plotkin SA. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Plotkin SA. Updates on immunologic correlates of vaccine-induced protection. Vaccine. 2020;38:2250–2257. doi: 10.1016/j.vaccine.2019.10.046. [DOI] [PubMed] [Google Scholar]
- 94.Loebermann M, et al. A randomized, open-label study of the immunogenicity and reactogenicity of three lots of a combined typhoid fever/hepatitis A vaccine in healthy adults. Clin. Ther. 2004;26:1084–1091. doi: 10.1016/S0149-2918(04)90180-4. [DOI] [PubMed] [Google Scholar]
- 95.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 96.Antia A, et al. Heterogeneity and longevity of antibody memory to viruses and vaccines. PLoS Biol. 2018;16:e2006601. doi: 10.1371/journal.pbio.2006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loonstra FC, Hoitsma E, van Kempen ZL, Killestein J, Mostert JP. COVID-19 in multiple sclerosis: the Dutch experience. Mult. Scler. 2020;26:1256–1260. doi: 10.1177/1352458520942198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Solomon JM, et al. Clinical characteristics and outcomes of multiple sclerosis patients with COVID-19 in Toronto, Canada. Mult. Scler. Relat. Disord. 2022;58:103509. doi: 10.1016/j.msard.2022.103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mohn N, et al. Implications of COVID-19 outbreak on immune therapies in multiple sclerosis patients — lessons learned from SARS and MERS. Front. Immunol. 2020;11:1059. doi: 10.3389/fimmu.2020.01059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hartung HP, Aktas O. COVID-19 and management of neuroimmunological disorders. Nat. Rev. Neurol. 2020;16:347–348. doi: 10.1038/s41582-020-0368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perez CA, et al. COVID-19 severity and outcome in multiple sclerosis: results of a national, registry-based, matched cohort study. Mult. Scler. Relat. Disord. 2021;55:103217. doi: 10.1016/j.msard.2021.103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chaudhry F, Jageka C, Levy PD, Cerghet M, Lisak RP. Review of the COVID-19 risk in multiple sclerosis. J. Cell Immunol. 2021;3:68–77. doi: 10.33696/immunology.3.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Newsome SD, et al. COVID-19 in patients with neuromyelitis optica spectrum disorders and myelin oligodendrocyte glycoprotein antibody disease in North America: from the COViMS Registry. Neurol. Neuroimmunol. Neuroinflamm. 2021;8:e1057. doi: 10.1212/NXI.0000000000001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sormani MP, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89:780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berger JR, Brandstadter R, Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:e761. doi: 10.1212/NXI.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zabalza A, et al. COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur. J. Neurol. 2021;28:3384–3395. doi: 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]
- 107.Simpson-Yap S, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97:e1870–e1885. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng C, et al. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020;34:879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schiavetti I, et al. Severe outcomes of COVID-19 among patients with multiple sclerosis under anti-CD-20 therapies: a systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2022;57:103358. doi: 10.1016/j.msard.2021.103358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bsteh G, et al. Humoral immune response after COVID-19 in multiple sclerosis: a nation-wide Austrian study. Mult. Scler. 2021;27:2209–2218. doi: 10.1177/13524585211049391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Louapre C, et al. Anti-CD20 therapies decrease humoral immune response to SARS-CoV-2 in patients with multiple sclerosis or neuromyelitis optica spectrum disorders. J. Neurol. Neurosurg. Psychiatry. 2022;93:24–31. doi: 10.1136/jnnp-2021-326904. [DOI] [PubMed] [Google Scholar]
- 112.Achtnichts L, et al. Humoral immune response after the third SARS-CoV-2 mRNA vaccination in CD20 depleted people with multiple sclerosis. Vaccines. 2021 doi: 10.3390/vaccines9121470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Conte WL. Attenuation of antibody response to SARS-CoV-2 infection in patients with multiple sclerosis on ocrelizumab: a case-control study. Mult. Scler. Relat. Disord. 2021;52:103014. doi: 10.1016/j.msard.2021.103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McKay KA, et al. Rituximab infusion timing, cumulative dose, and hospitalization for COVID-19 in persons with multiple sclerosis in Sweden. JAMA Netw. Open. 2021;4:e2136697. doi: 10.1001/jamanetworkopen.2021.36697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guerrieri S, et al. Serological response to SARS-CoV-2 vaccination in multiple sclerosis patients treated with fingolimod or ocrelizumab: an initial real-life experience. J. Neurol. 2022;22:39–43. doi: 10.1007/s00415-021-10663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sormani MP, et al. DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann. Clin. Transl. Neurol. 2021;8:1738–1744. doi: 10.1002/acn3.51408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Monschein T, et al. Vaccination and multiple sclerosis in the era of the COVID-19 pandemic. J. Neurol. Neurosurg. Psychiatry. 2021;92:1033–1043. doi: 10.1136/jnnp-2021-326839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen Q, Allot A, Lu Z. LitCovid: an open database of COVID-19 literature. Nucleic Acids Res. 2021;49:D1534–D1540. doi: 10.1093/nar/gkaa952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Le TT, Cramer JP, Chen R, Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:667–668. doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 120.Rodriguez-Coira J, Sokolowska M. SARS-CoV-2 candidate vaccines — composition, mechanisms of action and stages of clinical development. Allergy. 2021;76:1922–1924. doi: 10.1111/all.14714. [DOI] [PubMed] [Google Scholar]
- 121.Sahin U, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 122.Gao Q, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021;21:73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dobrovidova O. Latest Russian vaccine comes with a big dose of mystery. Science. 2021;372:116–117. doi: 10.1126/science.372.6538.116. [DOI] [PubMed] [Google Scholar]
- 125.Jackson LA, et al. An mRNA vaccine against SARS-CoV-2-preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597:318–324. doi: 10.1038/d41586-021-02483-w. [DOI] [PubMed] [Google Scholar]
- 127.Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021;20:817–838. doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thompson MG, et al. Prevention and attenuation of covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N. Engl. J. Med. 2021;385:320–329. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barrett JR, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021;27:279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 133.Sadoff J, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N. Engl. J. Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Corchado-Garcia J, et al. Analysis of the effectiveness of the Ad26.COV2.S adenoviral vector vaccine for preventing COVID-19. JAMA Netw. Open. 2021;4:e2132540. doi: 10.1001/jamanetworkopen.2021.32540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mascellino MT, Di Timoteo F, De Angelis M, Oliva A. Overview of the main anti-SARS-CoV-2 vaccines: mechanism of action, efficacy and safety. Infect. Drug Resist. 2021;14:3459–3476. doi: 10.2147/IDR.S315727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nogrady B. Mounting evidence suggests Sputnik COVID vaccine is safe and effective. Nature. 2021;595:339–340. doi: 10.1038/d41586-021-01813-2. [DOI] [PubMed] [Google Scholar]
- 137.Ndwandwe D, Wiysonge CS. COVID-19 vaccines. Curr. Opin. Immunol. 2021;71:111–116. doi: 10.1016/j.coi.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Soleimanpour S, Yaghoubi A. COVID-19 vaccine: where are we now and where should we go? Expert Rev. Vaccines. 2021;20:23–44. doi: 10.1080/14760584.2021.1875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dai L, et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020;182:722–733.e11. doi: 10.1016/j.cell.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu H, et al. Development of recombinant COVID-19 vaccine based on CHO-produced, prefusion spike trimer and alum/CpG adjuvants. Vaccine. 2021;39:7001–7011. doi: 10.1016/j.vaccine.2021.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heath PT, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N. Engl. J. Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Keech C, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goepfert PA, et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1-2, dose-ranging study. Lancet Infect. Dis. 2021;21:1257–1270. doi: 10.1016/S1473-3099(21)00147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zivkovic SA, Gruener G, Narayanaswami P, Quality A, AANEM Quality and Patient Safety Committee Doctor — should I get the COVID-19 vaccine? Infection and immunization in individuals with neuromuscular disorders. Muscle Nerve. 2021;63:294–303. doi: 10.1002/mus.27179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Otero-Romero S, Ascherio A, Lebrun-Frenay C. Vaccinations in multiple sclerosis patients receiving disease-modifying drugs. Curr. Opin. Neurol. 2021;34:322–328. doi: 10.1097/WCO.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 146.Lotan I, Romanow G, Levy M. Patient-reported safety and tolerability of the COVID-19 vaccines in persons with rare neuroimmunological diseases. Mult. Scler. Relat. Disord. 2021;55:103189. doi: 10.1016/j.msard.2021.103189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kelly H, Sokola B, Abboud H. Safety and efficacy of COVID-19 vaccines in multiple sclerosis patients. J. Neuroimmunol. 2021;356:577599. doi: 10.1016/j.jneuroim.2021.577599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ghasemiyeh P, Mohammadi-Samani S, Firouzabadi N, Dehshahri A, Vazin A. A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. Int. Immunopharmacol. 2021;100:108162. doi: 10.1016/j.intimp.2021.108162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen M, et al. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect. Dis. Poverty. 2021;10:94. doi: 10.1186/s40249-021-00878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Luxi N, et al. COVID-19 vaccination in pregnancy, paediatrics, immunocompromised patients, and persons with history of allergy or prior SARS-CoV-2 infection: overview of current recommendations and pre- and post-marketing evidence for vaccine efficacy and safety. Drug Saf. 2021;44:1247–1269. doi: 10.1007/s40264-021-01131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cromer D, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3:e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gilbert PB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khoury DS, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 154.Gadani SP, et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. 2021;73:103636. doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Haas EJ, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sureshchandra S, et al. Single-cell profiling of T and B cell repertoires following SARS-CoV-2 mRNA vaccine. JCI Insight. 2021;6:e153201. doi: 10.1172/jci.insight.153201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guerrera G, et al. BNT162b2 vaccination induces durable SARS-CoV-2-specific T cells with a stem cell memory phenotype. Sci. Immunol. 2021;6:eabl5344. doi: 10.1126/sciimmunol.abl5344. [DOI] [PubMed] [Google Scholar]
- 158.Collier AY, et al. Differential kinetics of immune responses elicited by Covid-19 vaccines. N. Engl. J. Med. 2021;385:2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bar-On YM, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Andrews N, et al. Duration of protection against mild and severe disease by covid-19 vaccines. N. Engl. J. Med. 2022;386:340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gilboa M, et al. Early Immunogenicity and safety of the third dose of BNT162b2 mRNA Covid-19 vaccine among adults older than 60 years; real world experience. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab584. [DOI] [PubMed] [Google Scholar]
- 162.Goldberg Y, et al. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl. J. Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gupta RK, Topol EJ. COVID-19 vaccine breakthrough infections. Science. 2021;374:1561–1562. doi: 10.1126/science.abl8487. [DOI] [PubMed] [Google Scholar]
- 164.Munro APS, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Levin EG, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Arbel R, et al. BNT162b2 vaccine booster and mortality due to covid-19. N. Engl. J. Med. 2021;385:2413–2420. doi: 10.1056/NEJMoa2115624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Atmar RL, et al. Homologous and heterologous Covid-19 booster vaccinations. N. Engl. J. Med. 2022 doi: 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chadeau-Hyam M, et al. SARS-CoV-2 infection and vaccine effectiveness in England (REACT-1): a series of cross-sectional random community surveys. Lancet Respir. Med. 2022 doi: 10.1016/S2213-2600(21)00542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chemaitelly H, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N. Engl. J. Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dickerman BA, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. Veterans. N. Engl. J. Med. 2022;386:105–115. doi: 10.1056/NEJMoa2115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lin DY, et al. Effectiveness of Covid-19 vaccines over a 9-month period in North Carolina. N. Engl. J. Med. 2022 doi: 10.1056/NEJMoa2117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lopez Bernal J, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Andrews N, et al. Effectiveness of COVID-19 booster vaccines against covid-19 related symptoms, hospitalisation and death in England. Nat. Med. 2022 doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bates TA, et al. Antibody response and variant cross-neutralization after SARS-CoV-2 breakthrough infection. JAMA. 2022;327:179–181. doi: 10.1001/jama.2021.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hoffmann M, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022;185:447–456.e11. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sievers BL, et al. Antibodies elicited by SARS-CoV-2 infection or mRNA vaccines have reduced neutralizing activity against Beta and Omicron pseudoviruses. Sci. Transl. Med. 2022 doi: 10.1126/scitranslmed.abn7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cele S, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N. Engl. J. Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Garcia-Beltran WF, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Muik A, et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022;375:678–680. doi: 10.1126/science.abn7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nemet I, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. N. Engl. J. Med. 2022;386:492–494. doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu M, et al. Three-dose vaccination elicits neutralising antibodies against omicron. Lancet. 2022;399:715–717. doi: 10.1016/S0140-6736(22)00092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Planas D, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 184.Zuo F, et al. Heterologous immunization with inactivated vaccine followed by mRNA booster elicits strong humoral and cellular immune responses against the SARS-CoV-2 Omicron variant. medRxiv. 2022 doi: 10.1101/2022.01.04.22268755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Angyal A, et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe. 2022;3:e21–e31. doi: 10.1016/S2666-5247(21)00275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pawlitzki M, et al. Merits and culprits of immunotherapies for neurological diseases in times of COVID-19. EBioMedicine. 2020;56:102822. doi: 10.1016/j.ebiom.2020.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Baker D, et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin. Exp. Immunol. 2020;202:149–161. doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Achiron A, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14:1–8. doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Giossi R, et al. Anti-Spike IgG in multiple sclerosis patients after BNT162b2 vaccine: an exploratory case-control study in Italy. Mult. Scler. Relat. Disord. 2022 doi: 10.1016/j.msard.2021.103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Etemadifar M, et al. SARS-CoV-2 serology among people with multiple sclerosis on disease-modifying therapies after BBIBP-CorV (Sinopharm) inactivated virus vaccination: same story, different vaccine. Mult. Scler. Relat. Disord. 2021 doi: 10.1016/j.msard.2021.103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Krbot Skoric M, Rogic D, Lapic I, Segulja D, Habek M. Humoral immune response to COVID-19 vaccines in people with secondary progressive multiple sclerosis treated with siponimod. Mult. Scler. Relat. Disord. 2022;57:103435. doi: 10.1016/j.msard.2021.103435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Achiron A, et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. 2021;27:864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sormani MP, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72:103581. doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Disanto G, et al. Association of disease-modifying treatment and anti-CD20 infusion timing with humoral response to 2 SARS-CoV-2 vaccines in patients with multiple sclerosis. JAMA Neurol. 2021 doi: 10.1001/jamaneurol.2021.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.van Kempen ZLE, et al. Longitudinal humoral response after SARS-CoV-2 vaccination in ocrelizumab treated MS patients: to wait and repopulate? Mult. Scler. Relat. Disord. 2022;57:103416. doi: 10.1016/j.msard.2021.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Boekel L, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3:e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kornek B, et al. B cell depletion and SARS-CoV-2 vaccine responses in neuroimmunologic patients. Ann. Neurol. 2022;91:342–352. doi: 10.1002/ana.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.König M, et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response After COVID-19 vaccination. JAMA Neurol. 2022 doi: 10.1001/jamaneurol.2021.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bigaut K, et al. Impact of disease-modifying treatments on humoral response after COVID-19 vaccination: a mirror of the response after SARS-CoV-2 infection. Rev. Neurol. 2021;177:1237–1240. doi: 10.1016/j.neurol.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tallantyre EC, et al. COVID-19 vaccine response in people with multiple sclerosis. Ann. Neurol. 2022;91:89–100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Konig M, et al. Humoral immunity to SARS-CoV-2 mRNA vaccination in multiple sclerosis: the relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J. Neurol. Neurosurg. Psychiatry. 2021 doi: 10.1136/jnnp-2021-327612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Payne RP, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184:5699–5714.e11. doi: 10.1016/j.cell.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pitzalis M, et al. Effect of different disease-modifying therapies on humoral response to BNT162b2 vaccine in Sardinian multiple sclerosis patients. Front. Immunol. 2021;12:781843. doi: 10.3389/fimmu.2021.781843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Etemadifar M, et al. SARS-CoV-2 serology among people with multiple sclerosis on disease-modifying therapies after BBIBP-CorV (Sinopharm) inactivated virus vaccination: same story, different vaccine. Mult. Scler. Relat. Disord. 2022;57:103417. doi: 10.1016/j.msard.2021.103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ozakbas S, et al. Comparison of SARS-CoV-2 antibody response after two doses of mRNA and inactivated vaccines in multiple sclerosis patients treated with disease-modifying therapies. Mult. Scler. Relat. Disord. 2022;58:103486. doi: 10.1016/j.msard.2022.103486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Apostolidis SA, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Brill L, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021;78:1510–1514. doi: 10.1001/jamaneurol.2021.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rauber S, et al. Immune response to SARS-CoV-2 vaccination in relation to peripheral immune cell profiles among patients with multiple sclerosis receiving ocrelizumab. J. Neurol. Neurosurg. Psychiatry. 2022 doi: 10.1136/jnnp-2021-328197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tortorella C, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98:e541–e554. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Moor MB, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cai H, Zhou R, Jiang F, Zeng Q, Yang H. Vaccination in neuromyelitis optica spectrum disorders: friend or enemy? Mult. Scler. Relat. Disord. 2022;58:103394. doi: 10.1016/j.msard.2021.103394. [DOI] [PubMed] [Google Scholar]
- 212.Jyssum I, et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol. 2022;4:e177–e187. doi: 10.1016/S2665-9913(21)00394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Madelon N, et al. Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Achiron A, et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: Up to 6 months cross-sectional study. J. Neuroimmunol. 2021;361:577746. doi: 10.1016/j.jneuroim.2021.577746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Centonze D, et al. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert consensus. J. Neurol. 2021;268:3961–3968. doi: 10.1007/s00415-021-10545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cabreira V, Abreu P, Soares-Dos-Reis R, Guimaraes J, Sa MJ. Multiple sclerosis, disease-modifying therapies and COVID-19: a systematic review on immune response and vaccination recommendations. Vaccines. 2021;9:773. doi: 10.3390/vaccines9070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Baker D, et al. CD19 B cell repopulation after ocrelizumab, alemtuzumab and cladribine: implications for SARS-CoV-2 vaccinations in multiple sclerosis. Mult. Scler. Relat. Disord. 2022;57:103448. doi: 10.1016/j.msard.2021.103448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Doneddu PE, et al. Acute and chronic inflammatory neuropathies and COVID-19 vaccines: practical recommendations from the task force of the Italian Peripheral Nervous System Association (ASNP) J. Peripher. Nerv. Syst. 2021;26:148–154. doi: 10.1111/jns.12435. [DOI] [PubMed] [Google Scholar]
- 219.Toscano S, Chisari CG, Patti F. Multiple sclerosis, COVID-19 and vaccines: making the point. Neurol. Ther. 2021;10:627–649. doi: 10.1007/s40120-021-00288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Agarwal N, Ollington K, Kaneshiro M, Frenck R, Melmed GY. Are immunosuppressive medications associated with decreased responses to routine immunizations? A systematic review. Vaccine. 2012;30:1413–1424. doi: 10.1016/j.vaccine.2011.11.109. [DOI] [PubMed] [Google Scholar]
- 221.Winthrop KL, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors) Clin. Microbiol. Infect. 2018;24:S21–S40. doi: 10.1016/j.cmi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 222.Andrade P, Santos-Antunes J, Rodrigues S, Lopes S, Macedo G. Treatment with infliximab or azathioprine negatively impact the efficacy of hepatitis B vaccine in inflammatory bowel disease patients. J. Gastroenterol. Hepatol. 2015;30:1591–1595. doi: 10.1111/jgh.13001. [DOI] [PubMed] [Google Scholar]
- 223.Furer V, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020;79:39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 224.Garcia Garrido HM, et al. Hepatitis A vaccine immunogenicity in patients using immunosuppressive drugs: a systematic review and meta-analysis. Travel. Med. Infect. Dis. 2019 doi: 10.1016/j.tmaid.2019.101479. [DOI] [PubMed] [Google Scholar]
- 225.Huber F, et al. Safety of live vaccines on immunosuppressive or immunomodulatory therapy — a retrospective study in three Swiss travel clinics. J. Travel. Med. 2018 doi: 10.1093/jtm/tax082. [DOI] [PubMed] [Google Scholar]
- 226.McMahan ZH, Bingham CO., 3rd Effects of biological and non-biological immunomodulatory therapies on the immunogenicity of vaccines in patients with rheumatic diseases. Arthritis Res. Ther. 2014;16:506. doi: 10.1186/s13075-014-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Levin LI, et al. Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA. 2005;293:2496–2500. doi: 10.1001/jama.293.20.2496. [DOI] [PubMed] [Google Scholar]
- 228.Bjornevik K, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375:296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


