Abstract
Acute pancreatitis is a common inflammatory condition affecting the pancreas, predominantly caused by gallstones, alcohol excess, and hypertriglyceridaemia, with severe disease carrying up to 50% mortality. Despite significant research and preclinical promise, no targeted drug treatments exist for the disease and precision medicine approaches are lacking significantly, when compared to other health conditions. Advances in omics applications will facilitate improved preclinical models and target identification as well as biomarker discovery for refined trial design, focusing on risk stratification, subject selection, and outcome determination. Randomised treatment of Acute Pancreatitis with Infliximab: Double-blind, placebo-controlled, multi-centre trial (RAPID-I) is a pioneering trial, currently under way in acute pancreatitis, which may serve as an innovative model for the implementation of precision medicine strategies for acute pancreatitis in the future.
Keywords: precision medicine, acute pancreatitis, animal models, clinical trials
Introduction
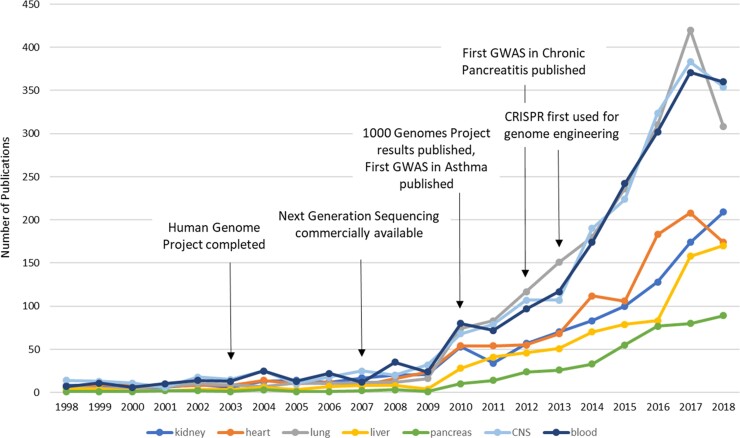
Precision medicine is not a new concept. Hippocrates, the so-called ‘Father of Western Medicine’, wrote over 2500 years ago that ‘different (drugs) to different patients, for the sweet ones do not benefit everyone, nor do the astringent ones, nor are all the patients able to drink the same things.’1 Indeed, the practice of adapting and modifying management strategies depending on the individual being treated has been practiced by excellent physicians and surgeons for centuries. William Osler, the illustrious Canadian physician wrote in 1892, ‘If it were not for the great variability among individuals, medicine might as well be a science and not an art.’2 We now recognise, however, that basing clinical decision making on clinical experience alone is significantly prone to bias and constrained by shortcomings in available scientific knowledge. The means to apply precision medicine is recent and has now become firmly established. Current technological and scientific advances allow us to identify crucial genomic and molecular patterns that facilitate the determination of individual risk, early diagnosis, assessment of severity, disease prognostication, and the determination of optimum management strategies. Nevertheless, the application of these technologies across the panoply of diverse human health conditions has not been equal, with certain organs and disease conditions reaping the benefits faster than others. For instance, the first genome wide association scan (GWAS) for acute respiratory inflammatory conditions (asthma) was published in 2010,3 and although the first GWAS in chronic pancreatitis was published not long after in 2012,4 the first GWAS for acute pancreatitis is still awaited. A comparison of the number of indexed Pubmed publications related to precision medicine based on year and type of organ reveals how far pancreatic disease is behind (Fig. 1). Precision medicine publications focussing on pancreatic diseases have experienced the slowest rise in numbers and were least in total of all organ systems assessed. Although this discrepancy may be explained by differences in disease incidence, lack of targeted treatments, research funding availability, and socio-economic healthcare burdens,5 it is clear that when it comes to precision medicine, pancreatic disease management is currently far from precise.
Figure 1.
Pubmed indexed precision medicine publications by organ system. Number of publications in different disease areas assessed by organ-specific MeSH search and precision medicine within the Pubmed database (10/04/19).
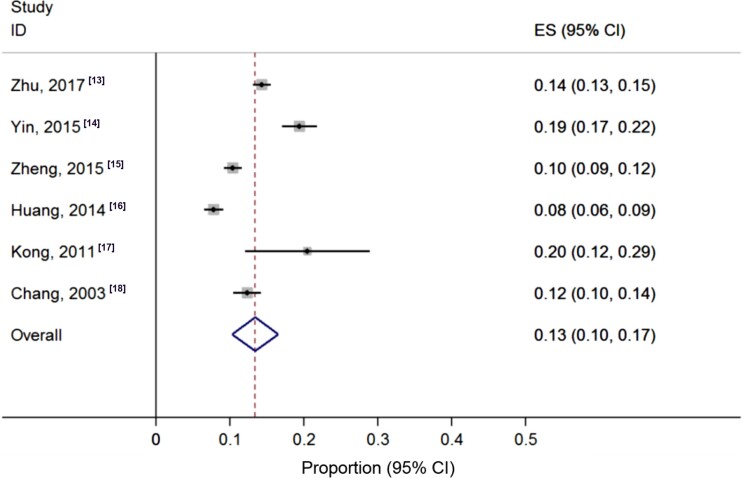
Acute pancreatitis is an inflammatory condition of the pancreas that has increased in global incidence over the last 50 years.6,7 According to the revised Atlanta classification, the condition is categorised as mild in approximately 60% of patients, moderate in 20%–30%, and severe in 5%–10%, with up to 50% mortality risk in severe disease.8–10 Gallstones and alcohol are the two most common aetiologies in adults of Western countries and Japan,11,12 while there is a pronounced incidence of hypertriglyceridaemia-associated acute pancreatitis in China (Fig. 2)13–18 that accounts for approximately 30% of patients in large cohort series.19,20 The risk factors for severe acute pancreatitis include aging, comorbidities, elevated body mass index, and pre-existing diabetes.21,22 Despite a wealth of ongoing international research with significant preclinical promise, currently there are no licensed targeted drug treatments available.23 This fact, together with a major drop in overall research investment, seen in the fall from 25.7% to 10.7% of gastrointestinal inflammatory disorders in the USA over the last 50 years,5 have been significant barriers to the implementation of precision medicine for pancreatitis. Preclinical success has not been translated to trial success for a number of reasons, likely because of inappropriate target selection and insufficient preclinical characterisation, the selection of subsets of patients unlikely to show maximal beneficial effects, speed of treatment delivery, and lack of widely applicable outcome measures.24 Where deficits lie, however, opportunities exist. With a lack of effective drug treatments, precision medicine for acute pancreatitis is at an earlier stage than most other conditions (Fig. 1), with precision medicine efforts primarily directed at prognostication, trial refinement, and optimisation of the treatment of complications. A significant momentum has now gathered in pancreatology, with multiple collaborative national and international networks and initiatives under way,25 poised to exploit technological advancements facilitating personalised approaches. Areas of current application and future opportunity for precision medicine approaches in acute pancreatitis are outlined and discussed.
Figure 2.
Pooled incidence of hypertriglyceridaemia-associated acute pancreatitis from multiple centre studies of China. The pooled incidence was 0.13 (95% CI: 0.1–0.17).
Preclinical models and target identification
The exact mechanisms leading to acute pancreatitis are yet to be fully elucidated, although great progress has been made in recent decades in better understanding the mechanisms of pancreatic acinar injury.26 These have established pancreatic acinar cell calcium overload,27 mitochondrial dysfunction,27,28 premature digestive enzyme activation,29 NF-κB activation,30 disordered autophagy,31 and vacuolisation,32 all critical in acute pancreatitis pathogenesis. Further studies highlight the key role of innate immunity33 and the release of key cellular damage-associated molecular patterns (DAMPs), notably HMGB134 and histones.35,36 Three highly promising pharmacological inhibitory strategies are calcium release activated channel (CRAC) inhibition,37 mitochondrial permeability transition pore (MPTP) inhibition,28 and kynurenine-3-monooxygenase (KMO) inhibition,38 all of which have resulted from extensive animal mechanistic studies. Most of these models use rodent tissues, because of the difficulty of obtaining relevant human tissue. Although studies in pancreatic parenchymal cells have revealed that pathways in rodent cells mirror those in humans,28,39–41 it must be remembered that significant differences remain, particularly as human patients affected by acute pancreatitis remain diverse, displaying genetic and epigenetic heterogeneity as well as different environmental exposures. These all contribute to differences in acute pancreatitis susceptibility, severity, and progression.
The application of omics technologies with a systems medicine approach will facilitate an understanding of the heterogeneity of response in contrasting models, and most importantly within the human condition, allowing appropriate comparisons to be drawn. This will then define the optimal utility of different preclinical models as well as develop more novel clinically relevant models, to aid ongoing translational drug discovery and the identification of further targets. As promising therapeutic strategies progress toward clinical trials, biomarkers will be essential for risk stratification, subject selection, and optimal outcome determination, all applicable to obtain improved outcomes.
Biomarker discovery for improved trial design
Early prediction of severe acute pancreatitis through novel blood and imaging biomarkers is essential for future clinical trial risk stratification. This remains intimately linked to better methods of defining significant biological pathways influencing severity, aiding better patient selection and clearer defined outcomes. Overall, focusing on these areas will allow a more ‘personalised’ approach to therapy.
Risk stratification
Although major complications related to acute pancreatitis overall remain low, the consequences of persistent organ failure can be life-threatening. As a result, substantial effort has been devoted to early identification of patients at increased risk of complications. Numerous approaches to risk stratification have been developed that include clinical prediction scores, biochemical parameters, and machine learning algorithms.42 A comparison of nine scoring systems in two prospectively collected cohorts of patients hospitalised for acute pancreatitis did not demonstrate clear advantage for any specific approach to identify patients at increased risk for persistent organ failure.42 Recent large cohort studies have suffered from inaccurate prognostic indices to stratify severity.43 This failure of prognostication is of significant detriment to accurate trial stratification and is likely in part to be because of a lack of studies using systems medicine approaches and the application of precision medicine.44 The application of omics strategies to identify novel biomarkers would be of clear benefit, but such studies of acute pancreatitis are lacking.
Outcome determination
In a previous systematic literature review of clinical trials in acute pancreatitis involving human subjects, 61 studies were identified from 1996 to 2014.45 The most common primary outcome was mortality (16%). Other common outcome parameters included organ failure (15%), pancreatic infections (13%), and systemic inflammatory response syndrome (SIRS, 10%). Selection of study end points in acute pancreatitis should be determined based on the context of the proposed intervention. Traditional approaches for the development of novel therapeutics in acute pancreatitis have focused on prevention or reduction of severe forms of illness. These studies incorporated initial risk stratification to identify higher-risk subgroups of patients for amelioration in outcomes such as persistent organ failure or mortality.45 These strategies have not been optimal, particularly in trials with recruitment within 72–96 hours of admission despite the emergency nature of the condition. An alternative approach would be to include all acute pancreatitis patients early after disease onset. In parallel with this, improvement in patient-reported outcomes related to pain, nutritional deficit, and quality of life alongside inclusion of surrogate outcomes of severity, such as C-reactive protein (CRP), albumin, and neutrophils offers the potential for easier trial conduct and greater generalisability of results but requires validation.
RAPID-I: a case study for future precision medicine
Findings from multiple omics platform strategies in acute pancreatitis focused on human genomic, transcriptomic, proteomic, and metabolomic strategies are awaited. Of significant promise and actively applying the points raised in this review is the RAPID-I trial (Randomised treatment of Acute Pancreatitis with Infliximab: Double-blind, placebo-controlled, multi-centre trial). This trial includes transcriptomic biomarker detection and mechanistic evaluation for anti-TNF-α therapy in acute pancreatitis. Adults admitted with a new diagnosis of acute pancreatitis of all severities and pain for less than 24 hours prior to admission are randomised to receive a double-blind infusion of 5 mg/kg or 10 mg/kg infliximab or saline, begun within 12 hours of admission. The primary outcome measure is cumulative CRP (measured at timed intervals over 28 days), with secondary outcome measures of pain, nutritional deficit, SIRS, Sequential Organ Failure Assessment (SOFA) scores, pancreatic injury on computed tomography scan, complications, length of hospital stay, and patient-reported outcomes (i.e. use EQ-5D-5L questionnaire). Transcriptome, cytokine, and leukocyte subset analyses will all be conducted to gain mechanistic insight and test for predictive and prognostic markers of severity and treatment response. RAPID-I is designed to serve as a model for future acute pancreatitis trials to accelerate towards the goal of personalised medicine approaches.
Future personalised medicine trials may require adaptive trial designs for maximal benefit; we would suggest including systems medicine approaches as widely as possible, whether using more traditional or novel designs. Approaches may include combining phases (e.g. phases I and II or phases II and III with appropriate statistical considerations to include go-no go points) with the first priority to obtain a licensed medication that has a major impact on mild, moderate, and/or severe acute pancreatitis. More speculative approaches might include umbrella trials (to study multiple targeted therapies in the context of a single disease), basket trials (to study a single targeted therapy in the context of multiple diseases or disease subtypes) and platform trials (to study multiple targeted therapies in the context of a single disease in a perpetual manner, with therapies allowed to enter or leave the platform on the basis of a decision algorithm).46
Conclusion
Precision medicine for acute pancreatitis remains at an early evolutionary stage, largely hampered by the lack of targeted drug therapy resulting from previous deficient preclinical research strategies and clinical trial design. Great opportunity exists to remedy this position with the results from major omics studies in acute pancreatitis awaited, potentially facilitating target identification and biomarker discovery, both paving the way for improved and potentially more successful future trials. We look forward to the outcomes of the RAPID-I trial and their applications to precision medicine approaches for acute pancreatitis in the future.
Acknowledgements
R.M. holds a University of Liverpool Faculty of Health and Life Sciences Fellowship and is supported by the Royal Liverpool and Broadgreen University Hospitals NHS Trust; R.S. holds a National Institute of Health Research Senior Investigator Award (R.S.) and is supported by the Medical Research Council, National Institute for Health Research, and European Union.
Conflict of interest statement
R.S. has received research funding (paid to the University of Liverpool within the last three years) from AbbVie, CalciMedica, Cypralis, GlaxoSmithKline, Merck/MSD, and Novartis.
References
- 1. Sykiotis GP, Kalliolias GD, Papavassiliou AG. Pharmacogenetic principles in the Hippocratic writings. J Clin Pharmacol 2005;45(11):1218–20. 10.1177/0091270005281091. [DOI] [PubMed] [Google Scholar]
- 2.W., O., The Principles and Practice of Medicine [Book]. 1892.
- 3. Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 2010;363(13):1211–21. 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitcomb DC, LaRusch J, Krasinskas AM, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet 2012;44(12):1349–54. 10.1038/ng.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Szentesi A, Tóth E, Bálint E, et al. Analysis of research activity in gastroenterology: pancreatitis is in real danger. PLoS One 2016;11(10):e0165244. 10.1371/journal.pone.0165244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016;1(1):45–55. 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 7. Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology 2019;156(1):254–272.e11. 10.1053/j.gastro.2018.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mofidi R, Duff MD, Wigmore SJ, et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg 2006;93(6):738–44. 10.1002/bjs.5290. [DOI] [PubMed] [Google Scholar]
- 9. Buter A, Imrie CW, Carter CR, et al. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg 2002;89(3):298–302. 10.1046/j.0007-1323.2001.02025.x. [DOI] [PubMed] [Google Scholar]
- 10. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62(1):102–11. 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 11. Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol 2010;7(3):131–45. 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 12. Wang GJ, Gao CF, Wei D, et al. Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol 2009;15(12):1427–30. 10.3748/wjg.15.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu Y, Pan X, Zeng H, et al.. A study on the etiology, severity, and mortality of 3260 patients with acute pancreatitis according to the revised atlanta classification in Jiangxi, China over an 8-year period. Pancreas 2017;46:504–9. 10.1097/MPA.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 14. Yin G, Cang X, Yu G, et al.. Different clinical presentations of hyperlipidemic acute pancreatitis: a retrospective study. Pancreas 2015;44:1105–10. 10.1097/MPA.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 15. Zheng Y, Zhou Z, Li H, et al.. A multicenter study on etiology of acute pancreatitis in Beijing during 5 years. Pancreas 2015;44:409–14. 10.1097/MPA.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 16. Huang YX, Jia L, Jiang SM, et al.. Incidence and clinical features of hyperlipidemic acute pancreatitis from Guangdong, China: a retrospective multicenter study. Pancreas 2014;43:548–52. 10.1097/MPA.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 17. Kong H, Ding Z, Zhu XC, et al.. d-Dimer change in human acute pancreatitis as determined by serumal triglyceride. Pancreas 2011;40:1103–6. 10.1097/MPA.0b013e3182204ae3. [DOI] [PubMed] [Google Scholar]
- 18. Chang MC, Su CH, Sun MS, et al.. Etiology of acute pancreatitis—a multi-center study in Taiwan. Hepatogastroenterology 2003;50:1655–7. [PubMed] [Google Scholar]
- 19. Shi N, Liu T, De la iglesia-Garcia D, et al. Duration of organ failure impacts mortality in acute pancreatitis. Gut 2019. 10.1136/gutjnl-2019-318241. (https://gut.bmj.com/content/early/2019/02/11/gutjnl-2019-318241). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang R, Deng L, Jin T, et al. Hypertriglyceridaemia-associated acute pancreatitis: diagnosis and impact on severity. HPB (Oxford) 2019. pii: S1365-182X(19)30071-1. 10.1016/j.hpb.2019.01.015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Párniczky A, Kui B, Szentesi A, et al. Prospective, multicentre, nationwide clinical data from 600 cases of acute pancreatitis. PLoS One 2016;11(10):e0165309. 10.1371/journal.pone.0165309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144(6):1252–61. 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moggia E, Koti R, Belgaumkar AP, et al. Pharmacological interventions for acute pancreatitis. Cochrane Database Syst Rev 2017;4:CD011384. 10.1002/14651858.CD011384.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberts SE, Akbari A, Thorne K, et al. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Aliment Pharmacol Ther 2013;38(5):539–48. 10.1111/apt.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abu-El-Haija M, Gukovskaya AS, Andersen DK, et al. Accelerating the drug delivery pipeline for acute and chronic pancreatitis: summary of the Working Group on Drug Development and Trials in Acute Pancreatitis at the National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Pancreas 2018;47(10):1185–92. 10.1097/MPA.0000000000001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gukovskaya AS, Pandol SJ, Gukovsky I. New insights into the pathways initiating and driving pancreatitis. Curr Opin Gastroenterol 2016;32(5):429–35. 10.1097/MOG.0000000000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang W, Cane MC, Mukherjee R, et al. Caffeine protects against experimental acute pancreatitis by inhibition of inositol 1,4,5-trisphosphate receptor-mediated Ca2+ release. Gut 2017;66(2):301–13. 10.1136/gutjnl-2015-309363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukherjee R, Mareninova OA, Odinokova IV, et al. Mechanism of mitochondrial permeability transition pore induction and damage in the pancreas: inhibition prevents acute pancreatitis by protecting production of ATP. Gut 2016;65(8):1333–46. 10.1136/gutjnl-2014-308553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Logsdon CD, Ji B. The role of protein synthesis and digestive enzymes in acinar cell injury. Nat Rev Gastroenterol Hepatol 2013;10(6):362–70. 10.1038/nrgastro.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang H, Liu Y, Daniluk J, et al. Activation of nuclear factor-κB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology 2013;144(1):202–10. 10.1053/j.gastro.2012.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biczo G, Vegh ET, Shalbueva N, et al. Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology 2018;154(3):689–703. 10.1053/j.gastro.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Voronina S, Collier D, Chvanov M, et al. The role of Ca2+ influx in endocytic vacuole formation in pancreatic acinar cells. Biochem J 2015;465(3):405–12. 10.1042/BJ20140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao Q, Wei Y, Pandol SJ, et al. STING signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology 2018;154(6):1822–1835.e2. 10.1053/j.gastro.2018.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang R, Zhang Q, Hou W, et al. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology 2014;146(4):1097–107. 10.1053/j.gastro.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu T, Huang W, Szatmary P, et al. Accuracy of circulating histones in predicting persistent organ failure and mortality in patients with acute pancreatitis. Br J Surg 2017;104(9):1215–25. 10.1002/bjs.10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Merza M, Hartman H, Rahman M, et al. Neutrophil extracellular traps induce trypsin activation, inflammation, and tissue damage in mice with severe acute pancreatitis. Gastroenterology 2015;149(7):1920–1931.e8. 10.1053/j.gastro.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 37. Wen L, Voronina S, Javed MA, et al. Inhibitors of ORAI1 prevent cytosolic calcium-associated injury of human pancreatic acinar cells and acute pancreatitis in 3 mouse models. Gastroenterology 2015;149(2):481–492.e7. 10.1053/j.gastro.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mole DJ, Webster SP, Uings I, et al. Kynurenine-3-monooxygenase inhibition prevents multiple organ failure in rodent models of acute pancreatitis. Nat Med 2016;22(2):202–9. 10.1038/nm.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wan MH, Huang W, Latawiec D, et al. Review of experimental animal models of biliary acute pancreatitis and recent advances in basic research. HPB (Oxford) 2012;14(2):73–81. 10.1111/j.1477-2574.2011.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murphy JA, Criddle DN, Sherwood M, et al. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology 2008;135(2):632–41. 10.1053/j.gastro.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 41. Criddle DN, Booth DM, Mukherjee R, et al. Cholecystokinin-58 and cholecystokinin-8 exhibit similar actions on calcium signaling, zymogen secretion, and cell fate in murine pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 2009;297(6):G1085–92. 10.1152/ajpgi.00119.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology 2012;142(7):1476–82. quiz e15-6 10.1053/j.gastro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 43. Sellers ZM, MacIsaac D, Yu H, et al. Nationwide trends in acute and chronic pancreatitis among privately insured children and non-elderly adults in the United States, 2007–2014. Gastroenterology 2018;155(2):469–478.e1. 10.1053/j.gastro.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uc A, Andersen DK, Borowitz D, et al. Accelerating the drug delivery pipeline for acute and chronic pancreatitis—knowledge gaps and research opportunities: overview summary of a National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Pancreas 2018;47(10):1180–4. 10.1097/MPA.0000000000001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Afghani E, Pandol SJ, Shimosegawa T, et al. Acute pancreatitis-progress and challenges: a report on an international symposium. Pancreas 2015;44(8):1195–210. 10.1097/MPA.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med 2017;377(1):62–70. 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]