Abstract
Hantaviruses can cause two types of infections in humans: hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome. The old world hantaviruses, primarily Hantaan virus (HTNV), responsible for causing HFRS occurs endemically in Asia and Europe. Apodernus agraricus, a striped field mouse, is being considered as main host reservoir for HTNV. Infection in humans is typically accidental and occurs when virus-containing rodent excretions such as urine, feces, or saliva are aerosolized. The major clinical manifestations includes increased vascular permeability causing vascular leakage, acute kidney injury and coagulation abnormalities. The case fatality rate of HFRS varies around 5.0 - 10.0% depending on the causative viral agent. The direct effects of viral infection on endothelial cells, as well as the immunological response to the viral infection, have been suggested to play a key role in the pathogenesis of HFRS. This article summarizes the current knowledge of HFRS epidemiology in Korea and around the globe, etiology, host transmission, clinical presentation, pathogenesis, diagnostic techniques, treatment, and prevention.
Keywords: Bunyavirus, Hemorrhagic fever with renal syndrome, Hantavirus, Pathogenesis, Epidemiology
Introduction
Hemorrhagic fever with renal syndrome (HFRS), a rodent borne viral illness caused by Hantaviruses [1]. The first outbreak of the hantavirus pandemic was discovered during the Korean War (1950 - 53). More than 3,000 United Nations troops have been diagnosed with Korean hemorrhagic fever since the outbreak during the Korean War [2]. Every year, about 100,000 instances of HFRS are reported, the majority of which occur in China, Korea, and Russia [3]. HFRS has become a major epidemic primarily in Asia and Europe. Among all, China is the most severely impacted country and accounting for around more than 90% of all HFRS cases worldwide in the last few decades [4,5].
In Korea, HFRS was originally identified in a United Nations (UN) soldier stationed in the center front, often known as the "Iron Triangle". During the Korean War, it finally spread to Cheorwon, Gimhwa, and Pyeongyang. Because this illness looked to be indigenous to Korea, United States (US) military officials named to it as Korean hemorrhagic fever. A comparable disease, however, was described in a Chinese medical text published around A.D. 960. During the Second World War, HFRS infected 10,000 Japanese military soldiers stationed in Manchuria, and several hundred Russian soldiers in the Far East also contracted the disease. During the Korean War, American researchers founded an epidemic hemorrhagic fever center in Seoul in 1952, but the pathogen remains mysterious [6].
Professor Ho Wang Lee and his fellow researchers detected an antigen in the lung and kidney tissues of Apodemus agrarius, caught in Songnae-dong, Dongducheon-si, and Gyeonggi-do, areas where hemorrhagic fever is common. Using immunofluorescence technique, the antigen was found to react with convalescent phase serum obtained from patients with Korean hemorrhagic fever. In 1978, the team named the antigen as Korean-type antigen and isolated it from blood samples of hemorrhagic fever patients and successfully replicated the virus in A549 cells. This unique virus was termed Hantaan virus (HTNV) after the Hantaan river in 1980 [6]. In 1982, the World Health Organization (WHO) organized a conference with the Working Group on Hemorrhagic Fever with Renal Syndrome in Tokyo, Japan. During this conference, a group of disorders with clinical characteristics comparable to Korean hemorrhagic fever were designated hemorrhagic fever with renal syndrome. In 1984, Hantavirus, a novel genus was identified that includes all pathogens of HFRS [6].
Based on the geographical areas where they found, the Hantaviruses are conventionally divided into two categories: Old world hantaviruses and New world hantaviruses. Amur virus (AMV), Seoul virus (SEOV), HTNV, Dobrava virus (DOBV), Tula virus (TULV), and Puumala virus (PUUV) are pathogenic Old world hantaviruses that cause HFRS in humans [4,7]. HTNV and DOBV tend to produce the most severe form of disease, with mortality rate of around 5.0 - 10.0% [4], whereas Puumala virus is endemic in northern Europe and usually causes a less severe disease, a milder variant of HFRS, also referred as nephropathia epidemica (NE), with a low mortality rate of 0.1 - 0.2% [8]. The first pathogenic of New world hantavirus (Sin Nombre virus) that causes Hantavirus cardiopulmonary syndrome (HCPS), was discovered in the early 1990s in the Four Corners region of the US, resulting in second outbreak of hantavirus [7]. Numerous additional pathogenic New world hantaviruses were identified and characterized [9].
Hantavirus is a virus that primarily infects human vascular endothelial cells (ECs) and causes significant damage to capillaries and small vessels. However, understanding the pathophysiology of HFRS is not sufficient. One of the problems is a scarcity of appropriate animal models. Many scientific and clinical research have recently shed light on the disease's underlying causes and suggested various ways to lessen the severity of the disease. We performed this study to review literature on the pathophysiology of HFRS and predict on future research possibilities. We will start with the basics of Hantaviruses, and then proceed further to rodent reservoirs and human transmission pathways. Afterwards, we will go through the clinical manifestations of HFRS and with its epidemiology and geographical distribution in this region and around the globe. We will further highlight the key pathophysiology causing vascular leakage, coagulation abnormalities, and other symptoms. Finally, we will go through some of the most recent advancements in therapy, with an emphasis on some novel medications that may help to lessen the negative effects of infection on the host.
Etiological Agent and Morphology
Hantaviruses are enveloped RNA viruses with negative-stranded genomes that belong to the Bunyaviridae family and the genus Hantavirus [10]. The viral particle has an oval or spherical shape with a diameter of 80 to 120 nm. The HTNV-RNA genome is divided into three main sections: small [S: between 1,696 and 2,083 nucleotide (nt) in length], medium (M: between 3,613 and 3,707 nt) and large (L: between 6,530 and 6,550 nt). The nucleocapsid (N) protein is encoded by the S segment; the envelope glycoproteins (G1 and G2) are encoded by the M segment; and the RNA dependent polymerase protein is encoded by the L segment [11,12]. The three segments' 3' and 5' termini are preserved and complimentary, permitting the formation of panhandle structures, which are considered to have a function in the viral replication and transcription control [13]. HTNV is quite stable and can remain infectious for two weeks at room temperature and possibly longer at lower temperatures.
Natural Host and Transmission in Humans
In Far East Russia, China, and Korea, surveillance efforts revealed the presence of HTNV and HTNV-like viruses in A. agrarius and A. peninsulae rodents, SEOV and SEOV-like viruses in Rattus norvegicus [14,15]. DOBV, and DOBV-like viruses were found in A. flavicollis, A. agrarius, and A. ponticus in Europe [16,17]. These reservoir animals are asymptomatic following infection. Because the hantaviruses have evolved distinct escape strategies against the innate immune system throughout their long co-evolution within these diverse hosts species [18].
Infection in humans is typically accidental and occurs when virus-containing rodent excretions such as urine, feces, or saliva are aerosolized. Humans are not the natural host of hantavirus and are considered as dead-end host [19]. More than 70.0% of HFRS cases occur in rural regions with poor housing conditions and high rodent population, with local farmers accounting for the majority of infected patients [3]. People who live or work in close proximity to infected rodents (farmers, woodcutter, hikers, etc.) are at a higher risk of infection [4].
HFRS is most common from late autumn until the following spring, with two incidence peaks [20]. The onset of the HFRS epidemic is determined by the kind of pathogenic hantavirus and correlates with increased human outdoor activity in the spring and fall. The size of rodent populations may have an impact on human disease epidemics [21].
General Clinical Characteristics of HFRS
The disease in humans is clinically characterized by acute onset of fever, headache, abdominal discomfort, acute kidney injury (AKI) and hemorrhage. In Korea, fever (94.0%), abdominal discomfort (64.0%) and headache (50.7%) are the most common early clinical symptoms. Although diarrhea as a major presentation is rarely recognized as early indicator, however, a report in Korea suggested that HFRS might present with gastrointestinal symptoms like acute diarrhea [22]. Another recent report in Korea [23], indicated the association of HTNV infection with appendicitis and confirmed the presence of HTNV antigen in the peripheral nerve bundle of appendix tissue via immunohistochemical staining. The diagnosis was further confirmed by analysis of patient’s plasma by immunofluorescence assay and nested reverse transcription-polymerase chain reaction (RT-PCR) [23].
HFRS can show as mild, moderate, or severe illness, depending on the causing virus. DOBV and HTNV cause severe HFRS, SEOV causes moderate HFRS, and Puumala virus causes mild HFRS. The disease has a 1 - 5 week incubation period and starts with a fever and influenza-like symptoms and may progresses to more severe symptoms characterized by hemorrhage, hypotension and acute renal failure [24]. Following the incubation period, the illness progress through a five-phase clinical course: febrile phase (3 - 5 days), hypotensive phase (few hours to few days), oliguric phase (3 - 7 days), and diuretic phase (1 - 2 weeks) leading to convalescent phase (3 - 6 months) [22,25]. Furthermore, in severe cases, some of these stages may overlap, while in moderate cases, one or two phases may be missing. Anemia, leukocytosis, thrombocytopenia, elevated liver enzymes, serum creatinine (renal dysfunction), proteinuria, and hematuria are all common laboratory observations during the acute stage of the illness. The majority of instances recover successfully, however certain severe cases might still result in headaches, restlessness, excessive sweating, hemorrhage, and hyperdiuresis.
Kidney damage is common in HFRS, with acute tubulointerstitial nephritis being the most common clinical manifestation accompanying inflammatory cell infiltration [26]. AKI is a common cause of mortality in individuals with HFRS, especially during the oliguric phase [27]. AKI is characterized clinically by severe proteinuria, haematuria, and a rapid decrease in the glomerular filtration rate (GFR), leading to edema, electrolyte and acid-base balance disturbances [28]. Individuals with old age are more prone to have severe AKI, as well as shock, hematuria, thrombocytopenia, and leukocytosis. Those with severe AKI typically require dialysis or continuous blood purification and spend more time in hospital than patients without AKI [27].
Thrombocytopenia, one of the variables that increases blood vessel permeability, is associated with severe AKI in individuals with acute HTNV infection. Acute thrombocytopenia is a typical HFRS laboratory finding that lasts throughout the Hantavirus infection. As a result, thrombocytopenia is a significant factor in the diagnosis of HFRS [29].
Central nervous system, endocrine and cardiopulmonary and ocular findings are also major clinical manifestations of HFRS. A report from Korea, indicated the occurrence of central diabetes insipidus (DI) and panhypopituitrism caused by HTNV-associated HFRS [30], and suggested the clinicians to distinguish central DI in HFRS patients with a prolonged diuretic phase even if the pituitary MRI results were normal. In the neurological system, Guillain–Barré syndrome, meningoencephalitis, generalized seizures, acute disseminated encephalomyelitis, and urinary bladder paralysis have been described. Pulmonary edema, shock, and perimyocarditis, may develop in cardiopulmonary system. Multiorgan failure, disseminated intravascular coagulopathy, multiple bleedings, pancreatitis, and have also been noticed, all of which can lead to a fatal outcome [31]. A long-term follow up study showed that non-Caucasian patients with HFRS exhibited statistically higher prevalence rates of transient ischemic attack, diseases of musculoskeletal system and, diabetes than non-Caucasian controls [32].
Epidemiology of Old World Hantaviruses causing HFRS with its Geographical Distribution in Korea and around the Globe
Shortly following the identification of HTNV, epidemiological investigations of HFRS in both human and rodent species advanced dramatically (Table 1).
Table 1. Geographical distribution and predominant natural reservoir of pathogenic Old world hantaviruses causing hemorrhagic fever with renal syndrome (HFRS).
| Hantaviruses | Serotype | Disease | Predominant Natural Reservoirs | Geographical Distribution |
|---|---|---|---|---|
| Old World Hantaviruses | Hantaan | HFRS | Apodemus agrarius | Eastern Asia (Korea, Russia and China), Central Europe [14] |
| Seoul | HFRS | Rattus rattus, Rattus norvegicus | Worldwide [15] | |
| Amur | HFRS | Apodemus peninsulae | China, Japan [4] | |
| Puumala | NE | Myodes glareolus | Scandinavia, Russia, Europe [8] | |
| Dobrava | HFRS | Apodemus flavicollis | Balkans, Europe, Syria [16] | |
| Tula | HFRS | Microtus arvalis | Europe [4] | |
| Soocheong | HFRS | Apodemus peninsulae | Korea, Russia, China [34] | |
| Muju | HFRS | Myodes regulus | Korea, China [97] | |
| Imjin | Unknown | Crocidura lasiura | Korea, China [6] | |
| Jeju | Unknown | Corcidura shantungensis | Korea [6] |
NE, nephropathia epidemica.
1. Distribution of hantavirus causing HFRS in host reservoir
The global distribution of HFRS is influenced by the geographic dispersion of their host-reservoirs. The mouse species A. agrarius, a field mouse, is the vector of the prototype, HTNV [14]. In Korea, A. agrarius is the predominate specie of field mouse. In the years 2014 - 2015, in south-west region of Korea, a high prevalence of HTNV (86.7%) was identified in A. agrarius in November (autumn) [33]. A. peninsulae, is the second most prevalent field mouse species in Korea and carries Soocheong virus (SOOV), a genetically unique HFRS-causing hantavirus [34]. The majority of SEOV circulates in the domestic rats and produces urban cases of HFRS in worldwide, Rattus norvegicus is considered as the principal reservoir for SEOV. DOBV is a significant cause of HFRS in the Balkans and in southern Europe. A. flavicollis is the primary reservoir [16]. PUUV causes NE, which is a mild form of HFRS, has been discovered throughout Scandinavia and in areas of Europe. M. glareolus, a bank vole (subfamily Arvicolinae), is the host species. In Korea, the royal vole, M. regulus, is thought to be a possible reservoir of a PUUV and Muju virus (MJUV) [35].
2. Distribution of hantavirus causing HFRS in humans
Infection with HTNV-related HFRS is found primarily in the rural areas of China, Korea and the far east of Russia. Every year, over 10,000 - 20,000 cases of HTNV-related HFRS are reported in China, whereas about 300 - 900 cases are reported in Korea [36]. The incidence peaks in late autumn and early winter. SEOV has a mild disease course with 40,000 to 60,000 cases reported each year, the most of which are from China. The reports are frequently found in other countries as well including, Korea, Japan and Hong Kong [4]. Unlike HTNV, the cases of SEOV infection occurs throughout the year. In Europe, over than 9,000 HFRS are recorded each year, with PUUV infections being the most prevalent. Severe cases of HFRS in Europe are caused by DOBV. In Korea, HTNV, SEOV, SOOV, MJUV, Imjin virus and Jeju virus have been discovered, among them HTNV, SEOV, SOOV and MJUV are the recognized human pathogen [6]. A study performed the phylogenetic analysis of HTNV isolates from diverse regions of Korea (Jeju, Boseong-gun and Gwangju) and revealed that these three clusters were distinct from HTNV isolates previously identified in Korea, China and Russia. This suggests the possible emergence of new HTNV strains in the southwest region of Korea [37].
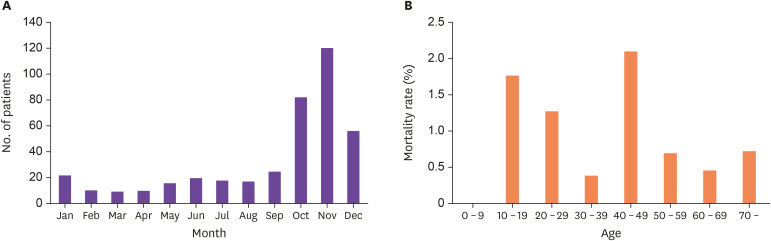
The average number of HFRS cases per month in Korea from 2001 - 2020 is shown in Figure 1. Peak onset occurred in October, November, and December of each year. The largest number of reported cases were observed in November, with an average of 120 cases in each year. From 2011 - 2020, the age-dependent case fatality rate showed the highest rate in the 10 - 19 year age group, followed by the 20 - 29, 40 - 49, and ≥70 year age groups, respectively.
Figure 1. Incidence and death rate of hemorrhagic fever with renal syndrome (HFRS) in Korea (A) Number of patients in each month from 2001 to 2020 (B) Mortality rate by age from 2011 to 2020 (data from Korea Disease Control and Prevention Agency).
Number of patients are presented as average in each month.
Age of the patients are expressed in years.
Between 2002 and 2016, a research was conducted in Korea that collected HFRS data in Yeoncheon and around the country. The disease's prevalence in Yeoncheon was significantly greater than the national average. Furthermore, when compared to the national average, the seasonal fluctuation pattern was not detected in Yeocheon, indicating that HFRS can occur in endemic regions independent of seasonal variations [38]. In contrast, another research [39] found seasonal variation with most instances recorded between October and December, while some cases identified in Yeoncheon were between January and March. As a result, in order to appropriately analyze the seasonal variation, other endemic areas with high HFRS prevalence will need to be studied.
Pathogenesis of HFRS
HFRS caused by Hantavirus infection causes a nonpathogenic, persistent infection in rodents [40]. On the other hand, clinically productive HFRS in humans, is defined by alterations in EC permeability and vascular edema. Plasma exosmosis or even hemorrhage are caused by increased vascular permeability, which is linked to a variety of clinical symptoms in HFRS, including hemoconcentration, hypotension, shock, and abdominal discomfort [41]. While the processes underlying the pathogenesis of HFRS are not fully recognized, the direct effects of viral infection of ECs and the immune response to hantavirus infection have been considered playing a significant role [42,43].
1. Role of endothelial cells
It was documented that human ECs isolated from adult and fetal veins have been found to be extremely vulnerable to HTNV infection. Increased ECs permeability or direct damage to the vasculature can both produce vascular leakage (Fig. 2) Microscopy has revealed extensive EC swelling, perivascular edema, erythrocyte diapedesis, and mononuclear cell infiltrates in HFRS patients without signs of EC injury. In vitro infection with HTNV, shows no distinct cytopathic effect as shown by phase microscopy and electron microscopy [44]. As a result, hantavirus is classified as a non-cytopathogenic virus that predominantly affects vascular ECs [45,46]. This shows that increased permeability, rather than direct cellular cytotoxicity or vascular damage, causes endothelial barrier function to be lost.
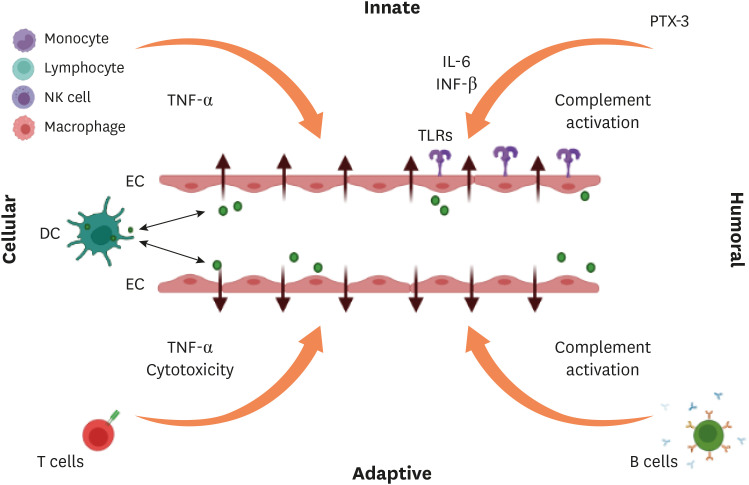
Figure 2. Proposed immune response of hantavirus causing endothelial barrier disruption.
Both humoral and cellular components of innate and adaptive immune systems may cause vascular leakage. In response to hantavirus-infected ECs, neutrophils release inflammatory cytokines like TNF-α which enhance vascular permeability directly or indirectly. The humoral pattern recognition receptor PTX3 and antibodies activate complement system. DCs can transport the virus from lung tissue to ECs of the microvasculature in other organs, or they can get infected after interacting with virus-infected ECs. As hantavirus-infected DCs mature, they move to draining lymph nodes, where they provoke an aggressive CD8+ T cell response. Activated complement components causes cytoskeletal modifications in ECs, which contributes to the ECs barrier’s dysfunction. TLRs detect hantavirus and trigger immune response. B-cells produce antibodies of various subclass.
TNF, tumor necrosis factor; IL, interleukin; INF, interferon; PTX, pentraxin-related protein-3; TLR, toll-like receptor; NK, natural killer; DC, dendritic cell; EC, endothelial cell.
During the acute phase of HFRS, increased levels of soluble EC receptors such as E-Selectin [47], intercellular adhesion molecule [48], and tumor necrosis factor receptor-1 [49] are carried into the circulation. The upregulation of pro-inflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ can all result in activation of endothelium [50,51]. These cytokines cause EC permeability either directly or indirectly through EC activation, resulting in leukocyte recruitment and subsequent EC gap development [52]. Leukocytosis is prominent in hantavirus infections and is most likely related to the inflammatory response to the pathogen. Remarkably, a recent study found that neutrophil activation via extracellular trap formation occurs in the moderate type of HFRS [53]. The pro- or anti-inflammatory responses to hantavirus infection in the rodent host promote viral clearance or tolerance, respectively [54,55,56]. As a result, while the host's pro-inflammatory response is essential for viral clearance, excessive activation appears to result in EC permeability and consequent vascular leakage.
Integrins have also a crucial role in controlling vascular permeability and endothelial cell migration. It has been established that HTNV, SEOV, and PUUV, the HFRS-causing hantaviruses, gain particular αvβ3 or αIIbβ3 integrins for cellular entrance [57,58]. These integrins are heterodimeric receptors made up of α and β subunits that can mediate cell-to-cell adhesion and platelet aggregation [59]. ECs and platelets are important regulators of vascular activities, and integrins play an important role in these cells barrier functions.
2. Role of immune response
HFRS, like many other pathogenic viruses, is predominantly eliminated by the activity of the immune system, both innate and adaptive. As a result, it is widely considered that the etiology of HFRS is mostly immunological, with immune complexes, complement activation, T and B cell responses, and HTNV-induced cytokine release all playing a role [60,61] (Fig. 2).
1) Innate immune response
a. Toll like receptor
The first line of defense against hantavirus infection is the innate immune system, which is characterized by IFN responses and innate immunocyte activation. Different pathways trigger IFN signaling. Following the identification of different pathogen-associated molecular patterns by various pattern-recognition receptors, innate immunity can be initiated. Toll-like receptors (TLRs) are vital in mediating the innate response among the many receptors that engage in the identification of microbial invaders [62]. TLRs are capable of eliciting efficient immunological responses as well as the production of inflammatory cytokines and type I IFN for host defense [63]. A study also observed that TLR4 may be involved in the upregulation of TNF-α, IFN-β, and IL-6 expression in HFRS [64].
b. Inflammatory Cytokines/chemokines
Overexpression of inflammatory cytokines is frequently documented in HFRS patients, giving rise to the theory that a "cytokine storm" may play a key role in the disease's pathophysiology. Cytokines are regarded to be one of the key causes of hantavirus symptoms.
Increased levels of cytokines and chemokines and an imbalance in their production contribute to increasing vascular endothelial permeability and a severe clinical course in patients with HFRS. According to one study [65], the cytokines are generated by many cells, including macrophages, monocytes, and lymphocytes, in response to pro-inflammatory signals and play a role in inflammation control. Cytokines, particularly TNF, IL-1 and IL-6, have been related to fever, septic shock, and the production of acute-phase proteins. A study reported that [66], the concentrations of TNF-α, IL-6, IFN-γ, IL-8, IP-10, and regulated upon activation, normal T cell-expressed and secreted (RANTS) were significantly higher in patients with HFRS compared to controls, and the highest concentrations found typically during the febrile, hypotensive, and oliguric phases, especially in severe and critical-type HFRS cases. Another study suggested that [67], cytokines like TNF- α, IL-6 and IL-1, were mediators causing synthesis of actue phase protein, fever, and septic shock and, while that of TNF-α, IFN-β, and IL-6 were key cytokines for enhanced ECs permeability.
c. Complement system
The complement system, which facilitates opsonization of microorganisms, lysis of gramnegative bacteria, and clearance of immunological complexes, is also thought to be implicated in hantavirus-induced immunopathology. SC5b–9, a soluble form of the terminal complement membrane attack complex, can bind β3 integrin, a possible receptor for the virus, perhaps responsible for increasing ECs permeability in HFRS [68]. Through the production of bradykinin and plateletactivating factor, SC5b–9 may also enhance the permeability of cultured ECs [69]. The complement system activated during the acute phase of PUUV infection, and complement activation levels are associated to disease severity [70,71]. As a result, complement activation might have a role in the development of vascular leakage. The acute phase protein pentraxin-related protein 3 is generated at the site of inflammation and its levels are observed to be elevated during the acute stage of PUUV infection, with high levels being linked to disease severity [72].
d. Nature killer cell
Natural killer (NK) cells, also known as effector and regulatory cells in innate and adaptive immunity are thought to transport into hantavirus-infected tissues to produce cytotoxic chemicals and secrete cytokines and chemokines. In HFRS, NK cells have a role in the pathophysiology and capillary leak syndrome [73], distinctive of hantavirus induced disease. Macrophages and monocytes serve as a link between innate and adaptive immunity. The immune-mediated pathogenesis is aided by high production of activating cytokines in the early stages of HFRS [74].
e. Dendritic cells and Macrophages
During HTNV infection, dendritic cells (DCs) develop and increase the expression and production of class I and class II major histocompatibility complex molecules, along with costimulatory and adhesion molecules [75]. TNF and IFN-γ release are also increased when DCs are activated.
ANDV was found in alveolar macrophages and submandibular glands in human tissue samples from ANDV fatal cases. It has been postulated that replication in human salivary glands and alveolar macrophage secretions may contribute to person-to-person transmission. As a result, it is critical to comprehend the involvement of macrophages in hantavirus infection, not only just as measure of the immune response against the virus, but also in viral transmission [76].
2) Adaptive Immune Response
The absence of cytotoxicity after hantavirus infection of ECs, along with the expression of activated T cells following hantavirus disease pathogenesis, has led to hypothesis that hantavirus disease pathology is mediated, at least to some extent, by hantavirus specific effector T cells.
a. CD8+ T-cell response
T cells play a dual function in viral infections. On the one hand, they are vital aspects of the host's defense against intracellular infections, but on the other, they are frequently at least partially responsible for the organ dysfunction and clinical disease produced by viruses. Although both functions have been proven in mouse models, data from primary human infections is limited and contradictory [77]. During prolonged SEOV infection, regulatory T cells contribute to elevated TGF- β protein levels in the lungs. As a result, regulatory T cells may have a role in the subclinical pathologic findings in rodents following SEOV infection [61].
Immunopathology of HFRS has also been linked to cell mediated responses. T cell activation has been associated to hantavirus pathogenesis, either through an excess of pro-inflammatory cytokines production or by cytotoxic T lymphocytes (CTLs) mediated death of infected cells [78]. CTL activation is considered to be facilitated by hantavirus antigens, which have been discovered in HFRS patients' renal tubular cells [79] and they may attract T cells to damage the renal tubular system. CTL concentration in the kidneys might be linked to epithelial cell leakage and disruption, potentially leading to AKI. Furthermore, NP-specific CD8+ T cells might survive for far more than 15 years following PUUV infection, indicating powerful, lengthy CD8+ T cell responses [80]. With the beginning of symptoms, a robust virus-specific CD8+ T-cell response was detected, with high levels of granzyme B and perforin coupled with increasing cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitory receptor expression [81].
b. CD4+ T-cell response
In research, CD4+ T-cell responses in viral diseases have received less attention than CD8+ T-cell responses. Nonetheless, for HFRS in humans, CD4+ T cells could be involved as a part of complex immune response in the affected organ. Due to the assumption of an exaggerated immune response implicated in hantavirus pathogenesis, regulatory T cells (Tregs) are the most researched subset of CD4+ T cells. It has been proposed that Tregs are required for the establishment of chronic hantavirus infection in rodent hosts and have a role in immunopathology modulation [61,82].
c. B-cell response
IgM antibody. Shortly after hantavirus infection, a significant IgM response arises. IgM antibody is against all the three structural proteins of hantavirus. Patients with HFRS have high levels of hantavirus IgM, which are found concurrently with the beginning of clinical symptoms. IgM serum concentrations will peak 7 - 11 days following the onset of symptoms. During the convalescent phase of HFRS, IgM levels typically decrease while IgG levels increase [21]. As a result, it is frequently employed as an HFRS diagnostic indicator.
IgG antibody. The IgG1 and IgG3 levels frequently rise as the illness progresses. In HFRS patients, however, IgG2 levels often stay stable [83]. IgG3 levels against hantavirus N and G1 proteins generally peak during the convalescent phase of HFRS and then progressively drop over the next few decades. No association has been found between IgG subclass and clinical severity. To further understand the importance of antibody-mediated responses in patient survival, an in-depth study of IgG responses against various hantaviruses is necessary.
IgA antibody. IgA has a key role in mucosal immunity, and aerosol is a major mode of hantavirus transmission. As a result, the existence of neutralizing IgA may play an important role in both acute infection recovery and long-term immunity. The greatest amounts of anti-PUUV IgA may be seen in the serum of acute and early convalescent stage. The levels of IgA will steadily rise as the illness progresses. Therefore, it is considered as one of the marker for early stage of infection [84].
Neutralizing antibodies. During hantavirus infection, G1 and G2 glycoproteins are considered as the potential antigens involved in the production of neutralizing antibodies (nAbs). Increased levels of nAbs were detected in survivors compared to deceased in PUUV-infected patients, suggesting the nAbs production may be directly related to odds of survival [85].
Laboratory Diagnosis
Human HFRS can be diagnosed using clinical and epidemiological data, along with laboratory testing. Patients with unknown fever, thrombocytopenia, renal failure, or respiratory distress who live in hantavirus disease-endemic areas should undergo laboratory testing. The laboratory diagnosis of hantavirus infection is mostly dependent on four types of tests: serology, molecular techniques, viral isolation and immunochemistry [86,87] (Table 2).
Table 2. Laboratory techniques used for the detection of hantaviruses.
| Methods | Benefits | Shortcomings | Instructions | |
|---|---|---|---|---|
| Serological Techniques | ||||
| ELISAs (IgG, IgM) | Sensitive | Cannot utilized for serotyping | Most frequently used | |
| Cross reactivity enables identification of unknown hantaviruses | ||||
| Inexpensive | ||||
| Can be utilized throughout the duration of clinical course | ||||
| ICG | Rapid, sensitive and specific | Cannot utilized for serotyping | Frequently used | |
| Inexpensive | Cost-efficient | |||
| Simple to carry out | ||||
| WB | More sensitive and specific than ELISAs | Costly and time-consuming | Not frequently used | |
| IFA | More specific | Less sensitive and time-consuming | Not frequently used | |
| Neutralization assay | Can be utilized for serotyping | Costly and time-consuming | Not frequently used | |
| Requires BSL-3 | ||||
| Molecular Techniques | ||||
| Real-time RT-PCR | Highly sensitive and specific | Costly than ELISA and time-consuming | Frequently used | |
| Quantitative assay | Could not show viral detection following the viremic phase | Positive earlier than serological assays | ||
| Allow to obtain the sequence of nucleotide | ||||
| NGS | Valuable for genotyping | Costly and complex | Hardly used | |
| Others | ||||
| IHC | Valuable for antigen detection in tissues | Time-consuming | Frequently used for biopsy | |
| Viral culture | Permits virological research | Less sensitive and time-consuming | ||
| Cumbersome and requires BSL-3 | Hardly used | |||
ELISA, enzyme linked immunosorbent assay; ICG, immunocromatography assay; WB, western blot; IFA, immunofluorescence assay; BSL, biosafety level 3 laboratory; RT-PCR, reverse transcription polymerase chain reaction; NGS, next generation sequencing; IHC, immunohistochemistry.
1. Serological assays
The most practical technique is to use ELISAs to identify IgM/IgG antibodies against the three structural hantavirus proteins (Gn, Gc, and N) of hantavirus. Almost all acute HFRS patients exhibit IgM and IgG antibodies against the N protein at the beginning of symptoms. As a result, serological assays that detect IgM and/or IgG antibodies to hantaviral antigens in serum are the most often used techniques for diagnostic testing of HFRS. Unfortunately, ELISA is not currently available in Korea. The indirect immunofluorescence assay, which employed hantavirus-infected cells preserved as an antigen on microscope slides, was one of the first serological tests used to detect HFRS in Europe and Asia. The use of virus-infected cells for serological testing is limited since cell culture infections necessitate BSL-3 facilities.
Immunoblot and neutralization tests are also used to aid in the serological identification and classification of suspected hantaviral infections. A plaque reduction neutralization test is largely accepted as the most effective approach for hantavirus identification and characterization [88]. However, neutralization tests are time-consuming and need the use of BSL-3 facilities. Nonetheless, these tests remain the gold standard for serologically identifying related hantavirus infections, while early sera may exhibit more wide cross-reactivity than convalescent-phase sera.
2. Molecular assays
Based on the identification of the viral genome, extremely sensitive diagnostic techniques have developed. From the first day of illness, the hantavirus genome may be identified quickly using RT-PCR with clinical samples such as blood, serum, urine or organ segments [89]. The study used urine and saliva specimens to perform RT-nested PCR (RT-nPCR) targeting the L-segment of hantavirus to detect virus in patients during the early phase of infection. Importantly, the virus was detected in urine where as it was not found in serum. The virus was detected in urine and serum specimens up to one month after the initial phase of illness, but not in saliva. Therefore, the urine may serve as an important specimen to detect hantavirus during the early stages of infection and HFRS should not be ruled out based on negative RT-PCR results in serum [89].
It has been reported that viral genomes can be detected in patients before the first day of symptoms [87]. Real-time RT-PCR is a rapid and accurate method for diagnosing HFRS in the laboratory for early stage of infection. Real-time RT-PCR can also be used to determine the viral load in clinical samples. When compared to conventional PCR, RT-nPCR test has a higher degree of sensitivity [89].
3. Viral isolation in cell culture
Because viral isolation from human samples is uncommon, it is not a diagnostic strategy for human hantavirus infection. However, clinical samples inoculated into Vero E-6 cell cultures can be used to isolate hantaviruses for research objectives [90]. Immunofluorescence can be used to identify hantavirus-infected cells one to two weeks after inoculation. The isolation is laborious and time-consuming, poses a significant risk of viral contamination in the laboratory, and necessitates biosecurity precautions to prevent the handler from aerosol transmission.
4. Immunohistochemistry
Immunohistochemistry is useful for detecting viral antigens in tissues, especially in fatal instances where no other types of samples are available.
Treatment and Prevention
The treatment of HFRS is mainly supportive and dependent on the clinical manifestations of the illness and may involve hemodialysis, oxygenation, and shock therapy. There is no specific therapy available. The use of ribavirin (1-beta-D-ribofuranosy 11, 2, 4-triazole- 3-carboxamide), an antiviral drug, has resulted in a decline in morbidity and a decrease in mortality among patients with HFRS. A study performed double-blinded clinical trial of intravenous ribavirin in China, and found that mortality was significantly reduced among 242 patients (seven folds decrease in risk) in ribavirin group. The severity of the disease was associated with the occurrence of oliguric and hemorrhagic phase in the placebo group [91], However, intravenous ribavirin treatment is not yet accessible in Korea. If started in the early course of disease, ribavirin has been found in clinical studies to be effective against hantaviruses that cause HFRS [92]. A meta-analysis was conducted to observe if ribavirin therapy for HPS reduces disease severity [92]. Human and animal studies were included in this analysis. The results found no significant reduction in mortality associated with HPS in humans with ribavirin therapy, however, the drug resulted in an increased survival rate for HPS-like disease in animal group.
Other potential novel approaches includes using a bradykinin receptor antagonist (bradykinin is implicated in vasodilation and increases vascular permeability). Icatibant, a drug that inhibits bradykinin binding, might benefit a significant number of individuals with PUUV and other hantavirus infections [93]. In Korea, icatibant is available but used only for the treatment of hereditary angioedema [94].
Monoclonal antibodies (MAbs) have been developed to combat HTNV. The anti-HTNV MAbs have been shown to exhibit anti-HTNV activity both in vitro and in vivo. As a result, MAbs might be a promising therapy option for HFRS [95]. Passive immune treatment with human plasma, are based mostly on comparable findings in the hamster model, but have not been extensively employed in humans. Several case studies have documented steroid-based anti-inflammatory therapeutic alternatives, notably in individuals with chronic and non-resolving renal failure.
For prevention of infection, it is recommended to avoid areas where high numbers of murid rodents reside in order to prevent getting into contact with virus-laden rat excrement. This includes maintaining dwellings and the surrounding region rodent-free, for example, by removing food sources and crawl spaces to make residences and work locations undesirable to rodents. A significant research attempts have been undertaken in recent years to produce a viable and safe hantavirus vaccine using vaccination techniques ranging from dead virus to recombinant DNA technologies. Hantavax (Korea Green Cross, Seoul, Korea), a formalin-inactivated HTNV vaccine made from rodent brain–derived virus, is commercially accessible in Korea, however, the protective response has been reported to be short-lived [96].
Conclusion and Future Prospective
One of biology's biggest unresolved mysteries is the emergence of zoonotic infections. Hantaviruses have found all over the world and are thought to have co-evolved for a long period of time with certain rodent species that serve as their natural reservoir. The precise contribution of these several host response factors to hantavirus pathology is still unknown, and it is uncertain if one component is the major cause of pathogenesis and the others are secondary contributors. The immunological response is commonly considered to have a key role in hantavirus pathogenesis. The progress of hantavirus pathogenesis investigations, as well as vaccines and therapies, will be aided by the introduction of more animal models. This will aid in elucidating the function of host defense components in protective immunity against hantaviruses following infection or vaccination, as well as proving the principle of Hantavirus-induced immunopathogenesis. The development of medication for HFRS is becoming increasingly important. Intense antiviral treatment has improved the prognosis of many types of viral diseases. Therefore, it is necessary to develop potential antiviral therapy against hantavirus.
In the future, it is anticipated that a greater knowledge of viral biology and pathophysiology would lead to more successful and targeted therapy approaches. A greater knowledge of hantavirus ecology would allow for better management of the infection risk for humans, as well as forecasts of future developments that mankind would face. Further study into the phylogenetic relationships and circulation of viral strains, host specificity, viral persistence mechanisms in animals, and variables affecting virus pathogenicity and distribution is also a major priority in scientific field.
Funding: None.
Conflict of Interest: No conflict of interest.
- Conceptualization: DMK.
- Data curation: MT.
- Formal analysis: MT, DMK.
- Project administration: DMK.
- Supervision: DMK.
- Validation: DMK.
- Visualization: DMK.
- Writing - original draft: MT.
- Writing - review & editing: MT.
References
- 1.Schmaljohn C, Hjelle B. Hantaviruses: a global disease problem. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paul JR, McClure WW. Epidemic hemorrhagic fever attack rates among United Nations troops during the Korean war. Am J Hyg. 1958;68:126–139. doi: 10.1093/oxfordjournals.aje.a119957. [DOI] [PubMed] [Google Scholar]
- 3.Zhang YZ, Zou Y, Fu ZF, Plyusnin A. Hantavirus infections in humans and animals, China. Emerg Infect Dis. 2010;16:1195–1203. doi: 10.3201/eid1608.090470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonsson CB, Figueiredo LT, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev. 2010;23:412–441. doi: 10.1128/CMR.00062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou LX, Chen MJ, Sun L. Haemorrhagic fever with renal syndrome: literature review and distribution analysis in China. Int J Infect Dis. 2016;43:95–100. doi: 10.1016/j.ijid.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Noh JY, Jung J, Song JW. Hemorrhagic fever with renal syndrome. Infect Chemother. 2019;51:405–413. doi: 10.3947/ic.2019.51.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hjelle B, Jenison S, Torrez-Martinez N, Yamada T, Nolte K, Zumwalt R, MacInnes K, Myers G. A novel hantavirus associated with an outbreak of fatal respiratory disease in the southwestern United States: evolutionary relationships to known hantaviruses. J Virol. 1994;68:592–596. doi: 10.1128/jvi.68.2.592-596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Settergren B. Clinical aspects of nephropathia epidemica (Puumala virus infection) in Europe: a review. Scand J Infect Dis. 2000;32:125–132. doi: 10.1080/003655400750045204. [DOI] [PubMed] [Google Scholar]
- 9.Mittler E, Dieterle ME, Kleinfelter LM, Slough MM, Chandran K, Jangra RK. Hantavirus entry: Perspectives and recent advances. Adv Virus Res. 2019;104:185–224. doi: 10.1016/bs.aivir.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmaljohn CS, Nichol ST. In: Fields virology. 5th ed. Fields BN, Knipe DM, Howley PM, Griffin DE, editors. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. Bunyaviridae; pp. 1741–1778. [Google Scholar]
- 11.Elliott RM, Schmaljohn CS, Collett MS. Bunyaviridae genome structure and gene expression. Curr Top Microbiol Immunol. 1991;169:91–141. doi: 10.1007/978-3-642-76018-1_4. [DOI] [PubMed] [Google Scholar]
- 12.Antic D, Kang CY, Spik K, Schmaljohn C, Vapalahti O, Vaheri A. Comparison of the deduced gene products of the L, M and S genome segments of hantaviruses. Virus Res. 1992;24:35–46. doi: 10.1016/0168-1702(92)90029-9. [DOI] [PubMed] [Google Scholar]
- 13.Schmaljohn CS. In: The Bunyaviridae. Elliot RM, editor. New York: Plenum Press; 1996. Molecular biology of hantaviruses; pp. 63–90. [Google Scholar]
- 14.Jiang JF, Zhang WY, Yao K, Wu XM, Zuo SQ, Zhan L, Zhang PH, Cao WC. A new Hantaan-like virus in rodents (Apodemus peninsulae) from Northeastern China. Virus Res. 2007;130:292–295. doi: 10.1016/j.virusres.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Wang S, Yin W, Liang M, Li J, Zhang Q, Feng Z, Li D. Epidemic characteristics of hemorrhagic fever with renal syndrome in China, 2006-2012. BMC Infect Dis. 2014;14:384. doi: 10.1186/1471-2334-14-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avsic-Zupanc T, Toney A, Anderson K, Chu YK, Schmaljohn C. Genetic and antigenic properties of Dobrava virus: a unique member of the Hantavirus genus, family Bunyaviridae. J Gen Virol. 1995;76:2801–2808. doi: 10.1099/0022-1317-76-11-2801. [DOI] [PubMed] [Google Scholar]
- 17.Klempa B, Stanko M, Labuda M, Ulrich R, Meisel H, Krüger DH. Central European Dobrava Hantavirus isolate from a striped field mouse (Apodemus agrarius) J Clin Microbiol. 2005;43:2756–2763. doi: 10.1128/JCM.43.6.2756-2763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rang A. Modulation of innate immune responses by hantaviruses. Crit Rev Immunol. 2010;30:515–527. doi: 10.1615/critrevimmunol.v30.i6.20. [DOI] [PubMed] [Google Scholar]
- 19.Krüger DH, Schönrich G, Klempa B. Human pathogenic hantaviruses and prevention of infection. Hum Vaccin. 2011;7:685–693. doi: 10.4161/hv.7.6.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean Hemorrhagic fever. J Infect Dis. 1978;137:298–308. doi: 10.1093/infdis/137.3.298. [DOI] [PubMed] [Google Scholar]
- 21.Khaiboullina SF, Morzunov SP, St Jeor SC. Hantaviruses: molecular biology, evolution and pathogenesis. Curr Mol Med. 2005;5:773–790. doi: 10.2174/156652405774962317. [DOI] [PubMed] [Google Scholar]
- 22.Kim HK, Chung JH, Kim DM, Yun NR, Kim CM, Jalal S. Hemorrhagic fever with renal syndrome as a cause of acute diarrhea. Am J Trop Med Hyg. 2019;100:1236–1239. doi: 10.4269/ajtmh.18-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim SC, Lee YM, Kim CM, Yun NR, Kim DM. Acute appendicitis associated with Hantaan virus infection. Am J Trop Med Hyg. 2021;105:801–806. doi: 10.4269/ajtmh.20-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krautkrämer E, Zeier M, Plyusnin A. Hantavirus infection: an emerging infectious disease causing acute renal failure. Kidney Int. 2013;83:23–27. doi: 10.1038/ki.2012.360. [DOI] [PubMed] [Google Scholar]
- 25.McCAUGHEY C, Hart CA. Hantaviruses. J Med Microbiol. 2000;49:587–599. doi: 10.1099/0022-1317-49-7-587. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Sung SH, An HR, Jun YH, Yu M, Ryu DR, Kim SJ, Kang DH, Choi KB. A case report of crescentic glomerulonephritis associated with Hantaan virus infection. Nephrol Dial Transplant. 2010;25:2790–2792. doi: 10.1093/ndt/gfq253. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Wang J, Wang T, Li J, Hui L, Ha X. Thrombocytopenia as a predictor of severe acute kidney injury in patients with Hantaan virus infections. PLoS One. 2013;8:e53236. doi: 10.1371/journal.pone.0053236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferluga D, Vizjak A. Hantavirus nephropathy. J Am Soc Nephrol. 2008;19:1653–1658. doi: 10.1681/ASN.2007091022. [DOI] [PubMed] [Google Scholar]
- 29.Denecke B, Bigalke B, Haap M, Overkamp D, Lehnert H, Haas CS. Hantavirus infection: a neglected diagnosis in thrombocytopenia and fever? Mayo Clin Proc. 2010;85:1016–1020. doi: 10.4065/mcp.2009.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn HJ, Chung JH, Kim DM, Yoon NR, Kim CM. Hemorrhagic fever with renal syndrome accompanied by panhypopituitarism and central diabetes insipidus: a case report. J Neurovirol. 2018;24:382–387. doi: 10.1007/s13365-018-0624-6. [DOI] [PubMed] [Google Scholar]
- 31.Vaheri A, Henttonen H, Voutilainen L, Mustonen J, Sironen T, Vapalahti O. Hantavirus infections in Europe and their impact on public health. Rev Med Virol. 2013;23:35–49. doi: 10.1002/rmv.1722. [DOI] [PubMed] [Google Scholar]
- 32.Mathes RW, Page WF, Crawford HM, McBean AM, Miller RN. Long-term sequelae of hemorrhagic fever with renal syndrome attributable to hantaan virus in Korean War veterans. Mil Med. 2005;170:315–319. doi: 10.7205/milmed.170.4.315. [DOI] [PubMed] [Google Scholar]
- 33.Jalal S, Jha B, Kim CM, Kim DM, Yun NR, Kim YS, Park JW, Chung JK. Molecular detection of viruses causing hemorrhagic fevers in rodents in the south-west of Korea. J Neurovirol. 2019;25:239–247. doi: 10.1007/s13365-018-0708-3. [DOI] [PubMed] [Google Scholar]
- 34.Baek LJ, Kariwa H, Lokugamage K, Yoshimatsu K, Arikawa J, Takashima I, Kang JI, Moon SS, Chung SY, Kim EJ, Kang HJ, Song KJ, Klein TA, Yanagihara R, Song JW. Soochong virus: an antigenically and genetically distinct hantavirus isolated from Apodemus peninsulae in Korea. J Med Virol. 2006;78:290–297. doi: 10.1002/jmv.20538. [DOI] [PubMed] [Google Scholar]
- 35.Song KJ, Baek LJ, Moon S, Ha SJ, Kim SH, Park KS, Klein TA, Sames W, Kim HC, Lee JS, Yanagihara R, Song JW. Muju virus, a novel hantavirus harboured by the arvicolid rodent Myodes regulus in Korea. J Gen Virol. 2007;88:3121–3129. doi: 10.1099/vir.0.83139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim WK, Kim JA, Song DH, Lee D, Kim YC, Lee SY, Lee SH, No JS, Kim JH, Kho JH, Gu SH, Jeong ST, Wiley M, Kim HC, Klein TA, Palacios G, Song JW. Phylogeographic analysis of hemorrhagic fever with renal syndrome patients using multiplex PCR-based next generation sequencing. Sci Rep. 2016;6:26017. doi: 10.1038/srep26017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jalal S, Kim CM, Kim DM, Song HJ, Lee JC, Shin MY, Lim HC. Geographical clustering of Hantavirus isolates from Apodemus agrarius identified in the Republic of Korea indicate the emergence of a new Hantavirus genotype. J Clin Virol. 2022;146:105030. doi: 10.1016/j.jcv.2021.105030. [DOI] [PubMed] [Google Scholar]
- 38.Park YH. Absence of a seasonal variation of hemorrhagic fever with renal syndrome in Yeoncheon compared to nationwide Korea. Infect Chemother. 2018;50:120–127. doi: 10.3947/ic.2018.50.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YR. There is still seasonal variation of hemorrhagic fever with renal syndrome in Gyeonggi province. Infect Chemother. 2018;50:280–282. doi: 10.3947/ic.2018.50.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer BJ, Schmaljohn CS. Persistent hantavirus infections: characteristics and mechanisms. Trends Microbiol. 2000;8:61–67. doi: 10.1016/s0966-842x(99)01658-3. [DOI] [PubMed] [Google Scholar]
- 41.Hayasaka D, Maeda K, Ennis FA, Terajima M. Increased permeability of human endothelial cell line EA.hy926 induced by hantavirus-specific cytotoxic T lymphocytes. Virus Res. 2007;123:120–127. doi: 10.1016/j.virusres.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Hepojoki J, Vaheri A, Strandin T. The fundamental role of endothelial cells in hantavirus pathogenesis. Front Microbiol. 2014;5:727. doi: 10.3389/fmicb.2014.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schönrich G, Krüger DH, Raftery MJ. Hantavirus-induced disruption of the endothelial barrier: neutrophils are on the payroll. Front Microbiol. 2015;6:222. doi: 10.3389/fmicb.2015.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pensiero MN, Sharefkin JB, Dieffenbach CW, Hay J. Hantaan virus infection of human endothelial cells. J Virol. 1992;66:5929–5936. doi: 10.1128/jvi.66.10.5929-5936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guhl S, Franke R, Schielke A, Johne R, Krüger DH, Babina M, Rang A. Infection of in vivo differentiated human mast cells with hantaviruses. J Gen Virol. 2010;91:1256–1261. doi: 10.1099/vir.0.019505-0. [DOI] [PubMed] [Google Scholar]
- 46.Mackow ER, Gavrilovskaya IN. Hantavirus regulation of endothelial cell functions. Thromb Haemost. 2009;102:1030–1041. doi: 10.1160/TH09-09-0640. [DOI] [PubMed] [Google Scholar]
- 47.Takala A, Lähdevirta J, Jansson SE, Vapalahti O, Orpana A, Karonen SL, Repo H. Systemic inflammation in hemorrhagic fever with renal syndrome correlates with hypotension and thrombocytopenia but not with renal injury. J Infect Dis. 2000;181:1964–1970. doi: 10.1086/315522. [DOI] [PubMed] [Google Scholar]
- 48.Han Q, Zhang L, Liu Z, Kang W, Lou S, Qiu J, Li Z, Zhang G, Wang Y, Li M, Li N. Elevated sICAM-1 levels in patients with hemorrhagic fever with renal syndrome caused by Hantaan virus. Eur J Clin Microbiol Infect Dis. 2010;29:1507–1511. doi: 10.1007/s10096-010-1032-x. [DOI] [PubMed] [Google Scholar]
- 49.Kyriakidis I, Papa A. Serum TNF-α, sTNFR1, IL-6, IL-8 and IL-10 levels in hemorrhagic fever with renal syndrome. Virus Res. 2013;175:91–94. doi: 10.1016/j.virusres.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 50.Sadeghi M, Eckerle I, Daniel V, Burkhardt U, Opelz G, Schnitzler P. Cytokine expression during early and late phase of acute Puumala hantavirus infection. BMC Immunol. 2011;12:65. doi: 10.1186/1471-2172-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saksida A, Wraber B, Avšič-Županc T. Serum levels of inflammatory and regulatory cytokines in patients with hemorrhagic fever with renal syndrome. BMC Infect Dis. 2011;11:142. doi: 10.1186/1471-2334-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marcos-Ramiro B, García-Weber D, Millán J. TNF-induced endothelial barrier disruption: beyond actin and Rho. Thromb Haemost. 2014;112:1088–1102. doi: 10.1160/TH14-04-0299. [DOI] [PubMed] [Google Scholar]
- 53.Raftery MJ, Lalwani P, Krautkrämer E, Peters T, Scharffetter-Kochanek K, Krüger R, Hofmann J, Seeger K, Krüger DH, Schönrich G. β2 integrin mediates hantavirus-induced release of neutrophil extracellular traps. J Exp Med. 2014;211:1485–1497. doi: 10.1084/jem.20131092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Easterbrook JD, Klein SL. Immunological mechanisms mediating hantavirus persistence in rodent reservoirs. PLoS Pathog. 2008;4:e1000172. doi: 10.1371/journal.ppat.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guivier E, Galan M, Salvador AR, Xuéreb A, Chaval Y, Olsson GE, Essbauer S, Henttonen H, Voutilainen L, Cosson JF, Charbonnel N. Tnf-α expression and promoter sequences reflect the balance of tolerance/resistance to Puumala hantavirus infection in European bank vole populations. Infect Genet Evol. 2010;10:1208–1217. doi: 10.1016/j.meegid.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 56.Li W, Klein SL. Seoul virus-infected rat lung endothelial cells and alveolar macrophages differ in their ability to support virus replication and induce regulatory T cell phenotypes. J Virol. 2012;86:11845–11855. doi: 10.1128/JVI.01233-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER. beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc Natl Acad Sci U S A. 1998;95:7074–7079. doi: 10.1073/pnas.95.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mou DL, Wang YP, Huang CX, Li GY, Pan L, Yang WS, Bai XF. Cellular entry of Hantaan virus A9 strain: specific interactions with beta3 integrins and a novel 70kDa protein. Biochem Biophys Res Commun. 2006;339:611–617. doi: 10.1016/j.bbrc.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 59.Peters CJ, Khan AS. Hantavirus pulmonary syndrome: the new American hemorrhagic fever. Clin Infect Dis. 2002;34:1224–1231. doi: 10.1086/339864. [DOI] [PubMed] [Google Scholar]
- 60.Khaiboullina SF, Netski DM, Krumpe P, St Jeor SC. Effects of tumor necrosis factor alpha on sin nombre virus infection in vitro . J Virol. 2000;74:11966–11971. doi: 10.1128/jvi.74.24.11966-11971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Easterbrook JD, Zink MC, Klein SL. Regulatory T cells enhance persistence of the zoonotic pathogen Seoul virus in its reservoir host. Proc Natl Acad Sci U S A. 2007;104:15502–15507. doi: 10.1073/pnas.0707453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 63.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang H, Wang PZ, Zhang Y, Xu Z, Sun L, Wang LM, Huang CX, Lian JQ, Jia ZS, Li ZD, Bai XF. Hantaan virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon, interleukin-6 and tumor necrosis factor-alpha. Virology. 2008;380:52–59. doi: 10.1016/j.virol.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Vaheri A, Strandin T, Hepojoki J, Sironen T, Henttonen H, Mäkelä S, Mustonen J. Uncovering the mysteries of hantavirus infections. Nat Rev Microbiol. 2013;11:539–550. doi: 10.1038/nrmicro3066. [DOI] [PubMed] [Google Scholar]
- 66.Wang PZ, Li ZD, Yu HT, Zhang Y, Wang W, Jiang W, Bai XF. Elevated serum concentrations of inflammatory cytokines and chemokines in patients with haemorrhagic fever with renal syndrome. J Int Med Res. 2012;40:648–656. doi: 10.1177/147323001204000227. [DOI] [PubMed] [Google Scholar]
- 67.Lohoff M, Mak TW. Roles of interferon-regulatory factors in T-helper-cell differentiation. Nat Rev Immunol. 2005;5:125–135. doi: 10.1038/nri1552. [DOI] [PubMed] [Google Scholar]
- 68.Tsukada H, Ying X, Fu C, Ishikawa S, McKeown-Longo P, Albelda S, Bhattacharya S, Bray BA, Bhattacharya J. Ligation of endothelial alpha v beta 3 integrin increases capillary hydraulic conductivity of rat lung. Circ Res. 1995;77:651–659. doi: 10.1161/01.res.77.4.651. [DOI] [PubMed] [Google Scholar]
- 69.Bossi F, Fischetti F, Pellis V, Bulla R, Ferrero E, Mollnes TE, Regoli D, Tedesco F. Platelet-activating factor and kinin-dependent vascular leakage as a novel functional activity of the soluble terminal complement complex. J Immunol. 2004;173:6921–6927. doi: 10.4049/jimmunol.173.11.6921. [DOI] [PubMed] [Google Scholar]
- 70.Paakkala A, Mustonen J, Viander M, Huhtala H, Pasternack A. Complement activation in nephropathia epidemica caused by Puumala hantavirus. Clin Nephrol. 2000;53:424–431. [PubMed] [Google Scholar]
- 71.Sane J, Laine O, Mäkelä S, Paakkala A, Jarva H, Mustonen J, Vapalahti O, Meri S, Vaheri A. Complement activation in Puumala hantavirus infection correlates with disease severity. Ann Med. 2012;44:468–475. doi: 10.3109/07853890.2011.573500. [DOI] [PubMed] [Google Scholar]
- 72.Outinen TK, Mäkelä S, Huhtala H, Hurme M, Meri S, Pörsti I, Sane J, Vaheri A, Syrjänen J, Mustonen J. High pentraxin-3 plasma levels associate with thrombocytopenia in acute Puumala hantavirus-induced nephropathia epidemica. Eur J Clin Microbiol Infect Dis. 2012;31:957–963. doi: 10.1007/s10096-011-1392-x. [DOI] [PubMed] [Google Scholar]
- 73.Björkström NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaëlsson J, Malmberg KJ, Klingström J, Ahlm C, Ljunggren HG. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mustonen J, Mäkelä S, Outinen T, Laine O, Jylhävä J, Arstila PT, Hurme M, Vaheri A. The pathogenesis of nephropathia epidemica: new knowledge and unanswered questions. Antiviral Res. 2013;100:589–604. doi: 10.1016/j.antiviral.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Raftery MJ, Kraus AA, Ulrich R, Krüger DH, Schönrich G. Hantavirus infection of dendritic cells. J Virol. 2002;76:10724–10733. doi: 10.1128/JVI.76.21.10724-10733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pizarro E, Navarrete M, Mendez C, Zaror L, Mansilla C, Tapia M, Carrasco C, Salazar P, Murua R, Padula P, Otth C, Rodríguez EM. Immunocytochemical and ultrastructural evidence supporting that andes Hantavirus (ANDV) is transmitted person-to-person through the respiratory and/or salivary pathways. Front Microbiol. 2020;10:2992. doi: 10.3389/fmicb.2019.02992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Terajima M, Ennis FA. T cells and pathogenesis of hantavirus cardiopulmonary syndrome and hemorrhagic fever with renal syndrome. Viruses. 2011;3:1059–1073. doi: 10.3390/v3071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Groen J, Bruijn JA, Gerding MN, Jordans JG, Moll van Charante AW, Osterhaus AD. Hantavirus antigen detection in kidney biopsies from patients with nephropathia epidemica. Clin Nephrol. 1996;46:379–383. [PubMed] [Google Scholar]
- 80.Van Epps HL, Terajima M, Mustonen J, Arstila TP, Corey EA, Vaheri A, Ennis FA. Long-lived memory T lymphocyte responses after hantavirus infection. J Exp Med. 2002;196:579–588. doi: 10.1084/jem.20011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lindgren T, Ahlm C, Mohamed N, Evander M, Ljunggren HG, Björkström NK. Longitudinal analysis of the human T cell response during acute hantavirus infection. J Virol. 2011;85:10252–10260. doi: 10.1128/JVI.05548-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schountz T, Prescott J, Cogswell AC, Oko L, Mirowsky-Garcia K, Galvez AP, Hjelle B. Regulatory T cell-like responses in deer mice persistently infected with Sin Nombre virus. Proc Natl Acad Sci U S A. 2007;104:15496–15501. doi: 10.1073/pnas.0707454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lundkvist A, Hörling J, Niklasson B. The humoral response to Puumala virus infection (nephropathia epidemica) investigated by viral protein specific immunoassays. Arch Virol. 1993;130:121–130. doi: 10.1007/BF01319001. [DOI] [PubMed] [Google Scholar]
- 84.de Carvalho Nicacio C, Björling E, Lundkvist A. Immunoglobulin A responses to Puumala hantavirus. J Gen Virol. 2000;81:1453–1461. doi: 10.1099/0022-1317-81-6-1453. [DOI] [PubMed] [Google Scholar]
- 85.Pettersson L, Thunberg T, Rocklöv J, Klingström J, Evander M, Ahlm C. Viral load and humoral immune response in association with disease severity in Puumala hantavirus-infected patients--implications for treatment. Clin Microbiol Infect. 2014;20:235–241. doi: 10.1111/1469-0691.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaheri A, Vapalahti O, Plyusnin A. How to diagnose hantavirus infections and detect them in rodents and insectivores. Rev Med Virol. 2008;18:277–288. doi: 10.1002/rmv.581. [DOI] [PubMed] [Google Scholar]
- 87.Machado AM, de Figueiredo GG, Sabino dos Santos G, Jr, Figueiredo LTM. Laboratory diagnosis of human hantavirus infection: novel insights and future potential. Future Virol. 2009;4:383–389. [Google Scholar]
- 88.Chu YK, Rossi C, Leduc JW, Lee HW, Schmaljohn CS, Dalrymple JM. Serological relationships among viruses in the Hantavirus genus, family Bunyaviridae. Virology. 1994;198:196–204. doi: 10.1006/viro.1994.1022. [DOI] [PubMed] [Google Scholar]
- 89.Seo JW, Kim DY, Kim CM, Yun NR, Lee YM, Lawrence Panchali MJ, Kim DM. Utility of nested reverse-transcriptase polymerase chain reaction of clinical specimens for early diagnosis of hemorrhagic fever with renal syndrome. Am J Trop Med Hyg. 2021;105:1285–1289. doi: 10.4269/ajtmh.21-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Binder F, Reiche S, Roman-Sosa G, Saathoff M, Ryll R, Trimpert J, Kunec D, Höper D, Ulrich RG. Isolation and characterization of new Puumala orthohantavirus strains from Germany. Virus Genes. 2020;56:448–460. doi: 10.1007/s11262-020-01755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huggins JW, Hsiang CM, Cosgriff TM, Guang MY, Smith JI, Wu ZO, LeDuc JW, Zheng ZM, Meegan JM, Wang QN, et al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis. 1991;164:1119–1127. doi: 10.1093/infdis/164.6.1119. [DOI] [PubMed] [Google Scholar]
- 92.Moreli ML, Marques-Silva AC, Pimentel VA, da Costa VG. Effectiveness of the ribavirin in treatment of hantavirus infections in the Americas and Eurasia: a meta-analysis. Virusdisease. 2014;25:385–389. doi: 10.1007/s13337-014-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vaheri A, Strandin T, Jääskeläinen AJ, Vapalahti O, Jarva H, Lokki ML, Antonen J, Leppänen I, Mäkelä S, Meri S, Mustonen J. Pathophysiology of a severe case of Puumala hantavirus infection successfully treated with bradykinin receptor antagonist icatibant. Antiviral Res. 2014;111:23–25. doi: 10.1016/j.antiviral.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 94.Jung JW, Suh DI, Park HJ, Kim S, Kwon HS, Yang MS, Park CS, Kim JH, Kim SH, Lee YW, Hur GY, Ye YM, Kwon YE, Park HK, Kim CW, Koh YI, Park JW, Lee JM, Min KU, Wickner P, Kang HR. Clinical features of hereditary angioedema in Korean patients: A nationwide multicenter study. Int Arch Allergy Immunol. 2018;176:272–279. doi: 10.1159/000488350. [DOI] [PubMed] [Google Scholar]
- 95.Xu R, Yang XY, Yang DF, Zou CY, Gong PL, Zeng FD. Phase I evaluation of the safety and pharmacokinetics of a single-dose intravenous injection of a murine monoclonal antibody against Hantaan virus in healthy volunteers. Antimicrob Agents Chemother. 2009;53:5055–5059. doi: 10.1128/AAC.00728-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cho HW, Howard CR, Lee HW. Review of an inactivated vaccine against hantaviruses. Intervirology. 2002;45:328–333. doi: 10.1159/000067925. [DOI] [PubMed] [Google Scholar]
- 97.Park K, Kim WK, Lee SH, Kim J, Lee J, Cho S, Lee GY, No JS, Lee KH, Song JW. A novel genotype of Hantaan orthohantavirus harbored by Apodemus agrarius chejuensis as a potential etiologic agent of hemorrhagic fever with renal syndrome in Republic of Korea. PLoS Negl Trop Dis. 2021;15:e0009400. doi: 10.1371/journal.pntd.0009400. [DOI] [PMC free article] [PubMed] [Google Scholar]