Abstract
Total antioxidant activity status (TAS) represents the body's response to oxidative stress, important in the pathogenic assessment of oxidations. Aim: To determine TAS variations in young subjects, with non-lesional cardiac arrhythmias, with/without dyslipidemia and to assess the risk of lipid oxidation. Patients and methods: The research was performed on 120 young subjects (mean age 33 years), with various types of cardiac arrhythmias, on normal heart, without co-existing lesions. Subjects were divided into 3 groups (40 persons). The first 2 groups included subjects with cardiac arrhythmias. Group I also associated dyslipidemia; group II, without dyslipidemia and group III: control. Determination of TAS values was performed using ABTS (2-azino-di-3-ethylbenzthiazoline sulfonate) colorimetic method. Results were statistically processed. Results: TAS values were decreased in all patients with cardiac arrhythmias, representing 52-54% of the values of healthy controls, the data being highly statistically significant. The variation of TAS decrease by types of arrhythmias was thus found in patients with arrhythmias and associated dyslipidemia and, respectively, without dyslipidemia, compared to controls. The deficit of antioxidant activity, between 48%-46% triggers electrochemical processes with implications in arrhythmogenesis and lipid oxidation. Coffee and vegetables-rich diet have antioxidant effect, reducing TAS deficiency. Conclusions: 1. TAS was decreased in all subjects with non-lesional arrhythmias. The study showed decreasing TAS level at 52-54% in patients with arrhythmias, with/without dyslipidemia, compared to controls. 2. TAS deficiency was associated with various types of dysrhythmias, ranging from 62% to 33%. 3. Decreased TAS also triggers lipid oxidation, as risk factor for early atherosclerotic lesions.
Keywords: Total antioxidant activity, oxidative stress, cardiac arrhythmias, dyslipidemia, lipid oxidation
Introduction
Total antioxidant activity status (TAS) is a global parameter that can assess the oxidative activity and status of an organism and also its importance in different pathologies onset, including cardiac ones.
Cardiac arrhythmias are frequently described in young people; their non-lesional pattern, often functional, involves the study of etiopathogenic factors, possibly involved and thus the completion of effective diagnostic methods and therapeutic management [1,2,3].
The excess of oxygen free radicals, which characterizes the oxidative stress, through chemical processes, activates areas and electrophysiopathologic mechanisms responsible for the generation of cardiac arrhythmic disorders, intermittent, persistent or permanent [2,3,4].
Aim and Objectives
The research aims to assess the oxidative stress level through a biomarker-total antioxidant activity status (TAS), related to the existence of some types of non-lesional cardiac arrhythmias, in young people.
The importance of lipid oxidation related to an increased oxidative stress level, thus the development of early atherosclerosis, requires monitoring the lipid profile of the studied subjects [5,6,7].
Patients and Methods
The research represents a prospective study of a biological parameter (biomarker), conducted on a number of 120 young subjects (aged 18-45 years old), during a time period of 3 years follow up, selected and divided into three groups of 40 subjects each.
The following inclusion criteria were used: young age (18-45 years)-regardless of sex and background (rural, urban), Caucasian race and the presence of a cardiac arrhythmia on a normal, non-lesional heart, confirmed clinical and imagistic (electrocardiogram, Holter-ECG device).
Exclusion criteria were represented by: the presence of any acute or chronic, systemic or visceral pathology that may have cardiovascular implications and could alter the oxidative status; previous medication. For the control group, cardiovascular manifestations (including arrhythmias) by clinical examination and biologic investigations were also excluded.
The research was initiated with the written consent of the participating subjects, selected from Emergency Units, Cardiology and Internal Medicine Departments of Emergency Hospital and County Hospital Craiova, as well as healthy volunteers (medical students, doctors, nurses).
The obtained data were processed at University of Medicine and Pharmacy of Craiova, Departments of Biochemistry, Statistics and Informatics. The present study was conducted according to the Principles of Helsinki Declaration and Universitary Code of Ethics, with the approval of the Ethics Comittee of the University of Medicine and Pharmacy of Craiova.
Group I (40 subjects) consisted of subjects with cardiac arrhythmias and dyslipidemia. Group II included patients with cardiac arrhythmias without dyslipidemia and group III, represented the control one, consisting of 40 young subjects without cardiac arrhythmias and without dyslipidemia.
Subjects were evaluated clinically and paraclinically (ECG, Holter-ECG, cardiac and general ultrasound, X-rays), biochemical and through specialized investigations.
ECG and Holter-ECG recordings allowed the assessment of heart rhythm disorders, in subjects enrolled in groups I and II, to whom the heart was clinically and paraclinically normal.
So, the arrhythmias occured on a non-lesional background. For group III subjects (controls), no heart rhythm disturbances, nor dyslipidemia were recorded.
In all subjects, in addition to the usual tests (complete blood count-CBC, urea, creatinine, blood glucose, urine summary examination, alanine and aspartate transaminases), lipidogram was determined, in order to assess the associated lipid metabolic status. The presence of dyslipidemia in patients with cardiac arrhythmias included subjects of group I was also assessed through standard colorimetric methods.
In all subjects, total antioxidant activity was determined in blood samples, at the Biochemistry Department of University of Medicine and Pharmacy of Craiova.
TAS measurement was performed using a colorimetric method with ABTS (2-azino-di-3-ethylbenzthiazoline sulfonate), with the kit and control serum from Randox Laboratories limited, GB.
The principle of method is that ABTS, together with oxygen peroxide, by incubation, generates ABTS+ cations, which have a stable greenish blue color, measured at 600nm. The existing antioxidants in the sample reduce the formation of ABTS+ and decrease the intensity of the green color, when read, after 3 minutes.
The presence of risk factors that may influence TAS, such as coffee and type of diet (rich or poor in vegetables-with known antioxidant effect) was also studied. All data obtained were statistically processed, evaluated and discussed, in order to assess the implications of oxidative stress status.
The results of the research (clinical, biochemical) were processed by the Department of Statistics and Informatics, at the University of Medicine and Pharmacy of Craiova, into specific databases, through which correlations and statistical analyzes were performed. For these, programs such as Microsoft Excel (Microsoft Corp., Redmond, WA, USA) were used, which together with XLSTAT 2014 for MS Excel (Addinsoft SARL, Paris, France) and IBM SPSS Statistics 20.0 (IBM Corporation, Armonk, NY , USA), contributed to the creation of parameters database used in statistical processing.
These parameters were analyzed according to certain statistical indicators, through which the most significant were: mean-standard deviation, Student t test, ANOVA and Fisher LSD tests. The graphical representation was achieved in the MS Excel program, using the functions: Functions-Statistical, Pivot Tables, Chart and Data Analysis.
The data of the present research were interpreted and illustrated graphically by means of the following statistical indicators: mean together with standard deviation and correlation coefficient. If the ANOVA test had a statistically significant result, it was analyzed with Fisher LSD test, in order to observe the categories between which statistically significant differences were identified.
Results
Determination of individual values of TAS in all subjects, allowed the assessment of its average values, by groups, in order to compare these values between affected patients and the control ones.
Mean TAS values in all patients with cardiac arrhythmias were lower compared to subjects without cardiac disorders. Compared to the control group, of healthy subjects, in which the average TAS value was 8.81±1.95mmol/L, for the first group of patients with cardiac arrhythmias and dyslipidemia, the average TAS values were 4.61±mmol/L, with a decrease up to 52.34%; for group II-subjects with cardiac arrhythmias but without dyslipidemia, the mean TAS values were slightly higher, but still lower than in controls: 4.77±1.26mmol/L, the decrease being calculated at 54.11%.
Thus, it was concluded that, in groups I and II, there is a deficit of TAS mean values, compared to the control group, of 47.66% for group I and of 45.85% for group II.
In all patients with cardiac arrhythmias, the decrease of TAS was recorded between 52.34% and 54.11%, with deficit between 47.66% and 45.89%, the reduction being at about half of the TAS value in healthy controls (Table 1, Figure 1).
Table 1.
Mean and percentage values of TAS, compared to controls, by groups
|
Group |
No of subjects |
Mean (mmol/L) |
Standard deviation |
Percent |
Difference |
|
Group I |
40 |
4,61 |
1,50 |
52,34% |
47,66% |
|
Group II |
40 |
4,77 |
1,26 |
54,11% |
45,89% |
|
Group III |
40 |
8,81 |
1,95 |
100,00% |
0,00% |
Figure 1.
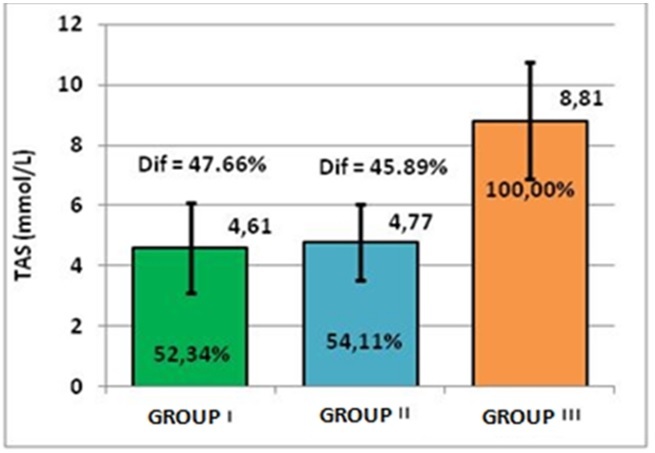
Representation of TAS mean and percentage values, compared to controls, by groups
Performing the ANOVA test, high significant differences between the mean TAS values were shown, the test result being lesser than 0.001 (p<0.001). Because the result of ANOVA test was significant, we used the Fisher LSD post-hoc test to identify the pairs of lots between which the differences are manifested. Thus, we found that there is a significant difference between the group with dyslipidemia and the control group, respectively between the group without dyslipidemia and the control group.
By different cardiac arrhythmia types, TAS values showed a decrease in group I and II compared to controls and also, an antioxidant deficiency, as follows:
-For group I: in atrial flutter, TAS values were 5.87±0.69mmol/L, representing 66.62%, compared to the controls, with a deficit of 33.38%; in paroxysmal supraventricular tachycardia, 5.8±2.61mmol/L, representing 65.78% and a deficit of 34.22%; in extrasystolic ventricular arrhythmia 5.29±1.46mmol/L, representing 60.08% and a deficit of 39.92%; in atrial extrasystolic arrhythmia, 4.98±1.01mmol/L representing 56.2%, with a deficit of 43.48%; in sinus tachycardia, 4.54±0.75mmol/L, representing 51.52% and a deficit of 48.48%; in atrial fibrillation, 3.98±1.3mmol/L, representing 45.13%, with a deficit of 54.87%; in associated cardiac arrhythmias, 3.49±1.4mmol/L, representing 39.57%, with a deficit of 60.43. (Table 2, Figure 2).
Table 2.
TAS mean and percentage values, on arrythmia types, in group I
|
Arrhythmia type in group I |
Mean value (mmol/L) |
Percent |
Standard deviation |
No. of cases |
|
Control TAS (mmol/L) group III |
8,81 |
100% |
1,95 |
40 |
|
Atrial flutter |
5,87 |
66,62% |
0,69 |
2 |
|
Paroxysmal supraventricular tachycardia |
5,80 |
65,78% |
2,61 |
5 |
|
Extrasystolic ventricular arrhythmia |
5,29 |
60,08% |
1,46 |
5 |
|
Atrial extrasystolic arrhythmia |
4,98 |
56,52% |
1,01 |
4 |
|
Sinus tachycardia |
4,54 |
51,52% |
0,75 |
8 |
|
Atrial fibrillation |
3,98 |
45,13% |
1,30 |
10 |
|
Sinus bradycardia |
3,61 |
40,93% |
0,89 |
3 |
|
Associated multiple cardiac disrrhythmias |
3,49 |
39,57% |
1,40 |
3 |
Figure 2.
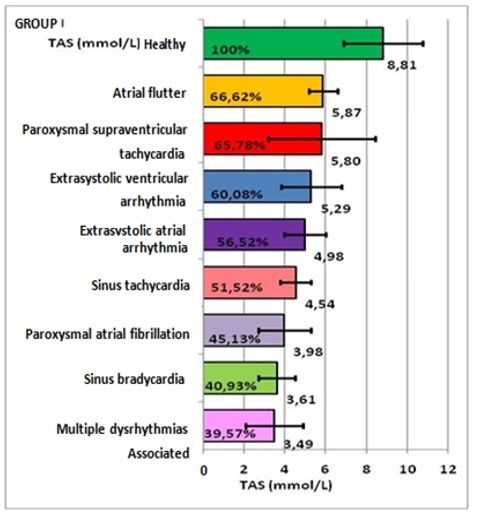
Representation of TAS mean and percentage values, on arrythmia types, in group I
-For group II: in paroxysmal supraventricular tachycardia 5.57±1.73mmol/L, representing 63.19%, compared to the controls, with a deficit of 36.81%; extrasystolic ventricular arrhythmia 5.04±0.83mmol/L, representing 57.22%, with a deficit of 42.78%; atrial extrasystolic arrhythmia 4.89±1.29mmol/L, representing 55.51%, with a deficit of 44.49%; atrial fibrillation 4.46±1.07mmol/L, representing 50.63%, with a deficit of 49.37%; sinus bradycardia 4.42±1.62mmol/L, representing 50.19%, with a deficit of 49.81%; associated arrhythmias 4.42±0.94mmol/L, representing 50.16%, with a deficit of 49.84%; sinus tachycardia 4.3mmol/L, representing 48.8%, with a deficit of 51.2%; atrial flutter 3.39±0.71mmol/L, representing 38.47%, with a deficit of 61.53%. (Table 3, Figures 3,4,5).
Table 3.
TAS mean and percentage values, on arrythmia types, in group II
|
Arrhythmia type in group II |
Mean value (mmol/L) |
Percent |
Standard Deviation |
No. of cases |
|
Control TAS (mmol/L) group III |
8,81 |
100% |
1,95 |
40 |
|
Paroxysmal supraventricular tachycardia |
5,57 |
63,19% |
1,73 |
6 |
|
Extrasystolic ventricular arrhythmia |
5,04 |
57,22% |
0,83 |
6 |
|
Atrial extrasystolic arrhythmia |
4,89 |
55,51% |
1,29 |
11 |
|
Atrial fibrillation |
4,46 |
50,63% |
1,07 |
7 |
|
Sinus bradycardia |
4,42 |
50,19% |
1,62 |
4 |
|
Associated) multiple cardiac disrrhythmias |
4,42 |
50,16% |
0,94 |
3 |
|
Sinus tachycardia |
4,30 |
48,80% |
0,00 |
1 |
|
Atrial flutter |
3,39 |
38,47% |
0,71 |
2 |
Figure 3.
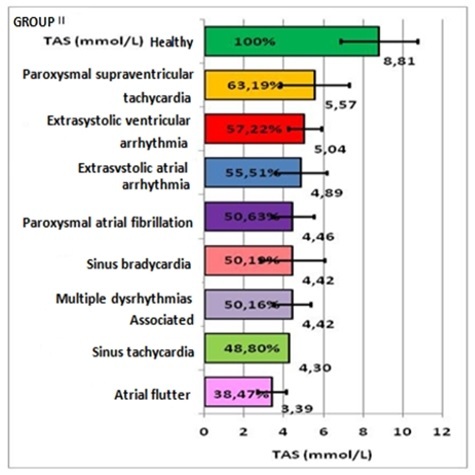
Representation of TAS mean and percentage values, on arrythmia types, in group II
Figure 4.
Extrasystolic atrial arrhythmia in a 33 years old patient with decreased TAS value
Figure 5.
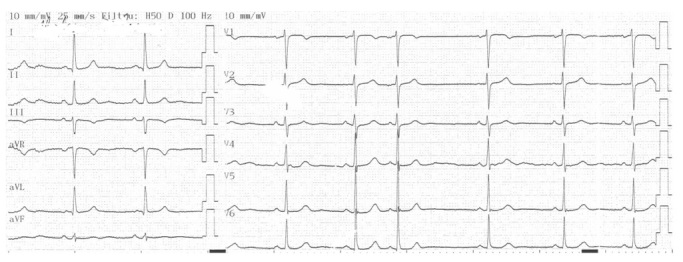
Sinus bradycardia in a 27 years old patient with decreased TAS value
In relation to the exogenous use of antioxidant or prooxidant factors, which may influence the oxidative status, TAS individual parameters were also calculated, depending on the coffee consumption and dietary type.
When comparing the mean values of TAS, for the 3 groups, in subjects who consumed/not consumed coffee, a statistically significant difference was found (p Student=0.022<0.05) in group I (Table 4, Figure 6). The antioxidant effect of coffee may explain a lower decrease of TAS in subjects who consume coffee.
Table 4.
TAS mean values, depending on coffee intake, by groups
|
Diet |
Group I |
Group II |
Group III |
|||
|
TAS |
Coffee intake |
No intake |
Coffee intake |
No intake |
Coffee intake |
No intake |
|
Number |
31 |
9 |
35 |
5 |
28 |
12 |
|
Mean value (mmol/L) |
4,90 |
3,62 |
4,80 |
4,53 |
8,81 |
8,82 |
|
Std. Dev. |
1,52 |
0,92 |
1,32 |
0,85 |
2,19 |
1,29 |
|
Results |
p Student= |
0,022 |
p Student= |
0,653 |
p Student= |
0,991 |
Figure 6.
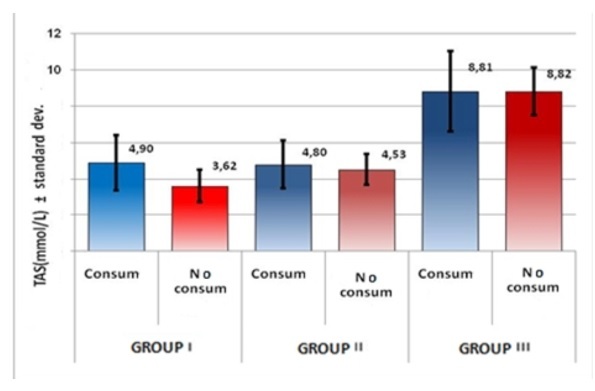
Representation of TAS mean values, on coffee intake, by groups
Depending on the diet type (rich or poor in vegetables), the comparison of TAS values showed statistically significant differences for all 3 groups.
TAS mean value was lower in patients with a vegetable-poor diet, in both groups of patients with arrhythmias (4.12mmol/L and respectively 4.14mmol/L) and slightly higher, in those with a vegetable-rich diet (5,1mmol/L and respectively 5.39mmol/L), compared to the healthy controls (approximately 8mmol/L). (Table 5, Figure 7).
Table 5.
TAS mean values, on diet types, by groups
|
Diet |
Group I |
Group II |
Group III |
|||
|
TAS |
Vegetable poor diet |
Vegetable rich diet |
Vegetable poor diet |
Vegetable rich diet |
Vegetable poor diet |
Vegetable rich diet |
|
Number |
20 |
20 |
20 |
20 |
19 |
21 |
|
Mean value (mmol/L) |
4,12 |
5,10 |
4,14 |
5,39 |
8,06 |
9,49 |
|
Std. Dev. |
1,13 |
1,68 |
0,97 |
1,23 |
1,62 |
2,00 |
|
Result |
p Student= |
0,037 |
p Student= |
0,001 |
p Student= |
0,018 |
Figure 7.
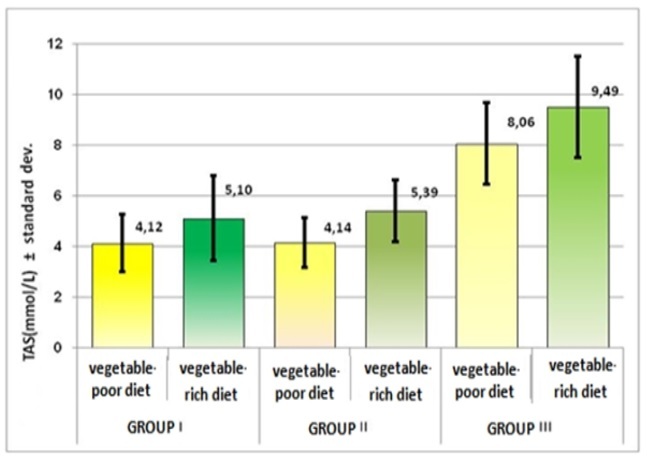
Representation of TAS mean values, on diet types, by groups
Discussions
The total antioxidant activity is a biological marker, which allows a global assessment of antioxidant protection, including all endogenous and exogenous antioxidant forms (enzymatic and non-enzymatic, vitamins) [3,4,7].
The frequency of cardiac arrhythmias is high, the causes of which appear, apart from some cardiac or general lesions, sometimes remain difficult to elucidate.
Heart rhythm disorders, as a heterogeneous group of diseases, on non-lesion heart, both worldwide and in Europe, affect 0.3-1% of the total general population, being 2 times more common in females than males.
The prevalence among general population is 1.15/1000, with an incidence of 23/100000) [1,3].
In young people with cardiac arrhythmias (group I and II), TAS showed decreasing variations, up to about half of the value of healthy controls, regardless of the dyslipidemia status (present/absent).
Thus, the TAS deficit was 48% (mean value: 4.6mmol/L), by mean values decrease up to 52%, in subjects of group I (with arrhythmias and dyslipidemia) and also TAS deficit of 46%, by decreasing mean TAS values to 54% (4.77mmol/L) in subjects of group II, compared to controls.
TAS deficit, by types of arrhythmias, was found in those with associated dyslipidemia (group I), as follows: in atrial flutter-62%, sinus tachycardia-51%, associated arrhythmias-50%, sinus bradycardia-50%, atrial fibrillation-49%, atrial extrasystolic arrhythmia-44%, ventricular extrasystolic arrhythmia-43%, paroxysmal supraventricular tachycardia-37% and those without dyslipidemia (group II), respectively: in associated arrhythmias, an antioxidant deficiency TAS of 60%, sinus bradycardia-59%, atrial fibrillation-55%, sinus tachycardia-48%, atrial extrasystolic arrhythmia-43%, ventricular extrasystolic arrhythmia-40%, paroxysmal supraventricular tachycardia-34%, atrial flutter-33%.
The comparison of TAS decreased values with the control ones, in the two groups of subjects with arrhythmic pathology, registered highly statistically significant differences (p<0.001).
In relation to the profile of the observed arrhythmias, the decrease of TAS was found in all types of studied arrhythmias, with values of up to 66% in atrial flutter and up to 39% in associated cardiac arrhythmias with values of up to 66% in atrial flutter and up to 39% in associated cardiac arrhythmias.
Re-entry tachyarrhythmia such as paroxysmal supraventricular tachycardia was associated with the lowest decrease of TAS, due to the pathophysiological mechanisms of fascicular reentry, but biochemical changes may influence and trigger arrhythmic maintenance mechanisms.
Studies by Niki et al. [26] showed that arrhythmias and oxidative stress levels are correlated.
Either reduction or excess of Na+channels (encoded by the SCN5A gene), Ca2+, K+ as well as voltage-dependent ion channel alterations, along with other mitochondrial and DNA dysfunctions are the main pathways of initiation and aberrant nerve impulse conduction, participating in arrhythmogenesis.
Reactive oxygen species can lead to ectopic cardiac activity.
Experimental studies [26,27] have shown that they participate in prolonging the duration of the action potential, leading to early but also delayed post-depolarization and triggering aberrant fascicles activity.
In all subjects with cardiac arrhythmias, TAS had a lower value, regardless of coffee consumption, with a slightly lower decrease in coffee consumers than non-consumers, which is also explained by the antioxidant effect of coffee associated with other mechanisms of caffeine involved in arrhythmogenesis.
The antioxidant deficit was of 44% for dyslipidemic consumers and 56% for non-consumers [8,9,10].
In relation to diet, although TAS was decreased in all patients with arrhythmias (group I and II), the decrease being lesser in those with a diet rich in vegetables, due to the intake of exogenous antioxidants and higher in those with a diet poor in vegetables [11,12,13,14,15,16,17,18,19,20,21,22,23,24].
Monitoring the lipid profile is important because, in the presence of a low antioxidant capacity, the existence of oxidative stress produces lipid oxidation, leading to the onset of an immune reaction, by the formation of anti-LDL-oxidized antibodies and endothelial lesions with subintimal deposition of degraded lipids and early atherosclerotic processes [25,26,27,28].
Conclusions
1. As measurable biomarker, total antioxidant activity (TAS) allows the assessment of oxidative/antioxidative imbalance, with implications in arrhythmogenic electrochemical processes.
2. The decrease of TAS was 52-54% (approximately half) in patients with cardiac arrhythmias, statistically significant compared to healthy subjects.
3. Decreased TAS values were recorded in both dyslipidemic and non-dyslipidemic subjects, more significant in dyslipidemic ones.
4. Diet rich in vegetables and coffee consumption, as associated factors, decreased the existing deficit of TAS in heart rhythm disorders.
5. TAS deficiencies imply the need for antioxidants use, for correcting and improving arrhythmias and also decreasing early cardiovascular risk.
Conflict of interests
None to declare.
References
- 1.Martínez EE, Segura C. Systemic Oxidative Stress: A key Point in Neurodegeneration-A Review. J Nutr Health Aging. 2019;23(8):694–699. doi: 10.1007/s12603-019-1240-8. [DOI] [PubMed] [Google Scholar]
- 2.Apak R. Current Issues in Antioxidant Measurement. J Agric Food Chem. 2019;67(33):9187–9202. doi: 10.1021/acs.jafc.9b03657. [DOI] [PubMed] [Google Scholar]
- 3.Beznă MC, Cârstea D, Beznă M, Deliu IC, Alexandru DO, Ciurea P. Clinical study regarding arrhythmogenic risk factors and oxidative stress inductibility in young people. Current Health Journal. 2015;41(3):251–257. doi: 10.12865/CHSJ.41.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beznă MC, Cârstea D, Beznă M, Pisoschi C, Istrătoaie O, Alexandru DO, Efrem C, Melinte PR. Estimation of oxidative stress involvment by superoxide dismutase variation in cardiac arrhythmias. Current Helath Journal. 2017;43(2):119–126. doi: 10.12865/CHSJ.43.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winterbourn CC, Kettle AJ. Radical-radical reactions of superoxide: a potential route to toxicity. Biochem Biophys Res Commun. 2003;305(3):729–736. doi: 10.1016/s0006-291x(03)00810-6. [DOI] [PubMed] [Google Scholar]
- 6.Chainy GBN, Sahoo DK. Hormones and oxidative stress: an overview. Free Radic Res. 2020;54(1):1–26. doi: 10.1080/10715762.2019.1702656. [DOI] [PubMed] [Google Scholar]
- 7.Chiarugi P. Reactive oxygen species as mediators of cell adhesion. Ital J Biochem. 2003;52(1):28–32. [PubMed] [Google Scholar]
- 8.Ha K, Kim K, Sakaki JR, Chun OK. Relative Validity of Dietary Total Antioxidant Capacity for Predicting All-Cause Mortality in Comparison to Diet Quality Indexes in US Adults. Nutrients. 2020;12(5):1210–1210. doi: 10.3390/nu12051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acidri R, Sawai Y, Sugimoto Y, Handa T, Sasagawa D, Masunaga T, Yamamoto S, Nishihara E. Phytochemical Profile and Antioxidant Capacity of Coffee Plant Organs Compared to Green and Roasted Coffee Beans. Antioxidants (Basel) 2020;9(2):93–93. doi: 10.3390/antiox9020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgado SR, Arbelaez AFA, Rojano B. Antioxidant capacity, bioactive compounds in coffee pulp and implementation in the production of infusions. Acta Sci Pol Technol Aliment. 2019;18(3):235–248. doi: 10.17306/J.AFS.0663. [DOI] [PubMed] [Google Scholar]
- 11.Nascimento-Souza MA, Paiva PG, Silva AD, Duarte MSL, Ribeiro AQ. Coffee and Tea Group Contribute the Most to the Dietary Total Antioxidant Capacity of Older Adults: A Population Study in a Medium-Sized Brazilian City. J Am Coll Nutr. 2020;3:1–11. doi: 10.1080/07315724.2020.1823281. [DOI] [PubMed] [Google Scholar]
- 12.Phan MT, Paterson J, Bucknall M, Arcot J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit Rev Food Sci Nutr. 2018;58(8):1310–1329. doi: 10.1080/10408398.2016.1254595. [DOI] [PubMed] [Google Scholar]
- 13.Dragoi GS, Dinca I, Melinte PR, Silca GA, Radu L, Diaconu C. Forensic and anatomic signification of phenotype transformations inside fetus membranes. Part One: Amnion and lamina chorionica. Rom J Leg Med. 2009;4:257–264. [Google Scholar]
- 14.Dragoi GS, Dinca I, Melinte PR, Silca GA, Radu L, Diaconu C. Forensic and anatomic signification of phenotype transformations inside fetus membranes. Part Two: Trophoblastic membrane. Rom J Leg Med. 2009;4:257–264. [Google Scholar]
- 15.Mozaffari H, Daneshzad E, Surkan PJ, Azadbakht L. Dietary Total Antioxidant Capacity and Cardiovascular Disease Risk Factors: A Systematic Review of Observational Studies. J Am Coll Nutr. 2018;37(6):533–545. doi: 10.1080/07315724.2018.1441079. [DOI] [PubMed] [Google Scholar]
- 16.Jun S, Chun OK, Joung H. Estimation of dietary total antioxidant capacity of Korean adults. Eur J Nutr. 2018;57(4):1615–1625. doi: 10.1007/s00394-017-1447-6. [DOI] [PubMed] [Google Scholar]
- 17.Duthie SJ, Duthie GG, Russell WR, Kyle JAM, Macdiarmid JI, Rungapamestry V, Stephen S, Megias-Baeza C, Kaniewska JJ, Shaw L, Milne L, Bremner D, Ross K, Morrice P, Pirie LP, Horgan G, Bestwick CS. Effect of increasing fruit and vegetable intake by dietary intervention on nutritional biomarkers and attitudes to dietary change: a randomised trial. Eur J Nutr. 2018;57(5):1855–1872. doi: 10.1007/s00394-017-1469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarker U, Hossain MM, Oba S. Nutritional and antioxidant components and antioxidant capacity in green morph Amaranthus leafy vegetable. Sci Rep. 2020;10(1):1336–1336. doi: 10.1038/s41598-020-57687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valoppi F, Haman N, Ferrentino G, Scampicchio M. Inhibition of lipid autoxidation by vegetable waxes. Food Funct. 2020;22;11(7):6215–6225. doi: 10.1039/d0fo01022g. [DOI] [PubMed] [Google Scholar]
- 20.Bacchetti T, Turco I, Urbano A, Morresi C, Ferretti G. Relationship of fruit and vegetable intake to dietary antioxidant capacity and markers of oxidative stress: A sex-related study. Nutrition. 2019;61:164–172. doi: 10.1016/j.nut.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 21.Daneshzad E, Tehrani H, Bellissimo N, Azadbakht L. Dietary Total Antioxidant Capacity and Gestational Diabetes Mellitus: A Case-Control Study. Oxid Med Cell Longev. 2020;8:5471316–5471316. doi: 10.1155/2020/5471316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan BL, Norhaizan ME, Liew WP. Nutrients and Oxidative Stress: Friend or Foe. Oxid Med Cell Longev. 2018;2018:9719584–9719584. doi: 10.1155/2018/9719584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellegrini N, Vitaglione P, Granato D, Fogliano V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: merits and limitations. J Sci Food Agric. 2020;100(14):5064–5078. doi: 10.1002/jsfa.9550. [DOI] [PubMed] [Google Scholar]
- 24.Van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019;21(4):425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhary P, Pandey A, Azad CS, Tia N, Singh M, Gambhir IS. Association of oxidative stress and endothelial dysfunction in hypertension. Anal Biochem. 2020;590:113535–113535. doi: 10.1016/j.ab.2019.113535. [DOI] [PubMed] [Google Scholar]
- 26.Niki E. Oxidant-specific biomarkers of oxidative stress. Association with atherosclerosis and implication for antioxidant effects. Free Radic Biol Med. 2018;120:425–440. doi: 10.1016/j.freeradbiomed.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Orzechowski A, Cywińska A, Rostagno AA, Rizzi FM. Oxidative Stress, Chronic Inflammation, and Amyloidoses. Oxid Med Cell Longev. 2019;2019:6024975–6024975. doi: 10.1155/2019/6024975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassalle C, Maltinti M, Sabatino L. Targeting Oxidative Stress for Disease Prevention and Therapy: Where Do We Stand, and Where Do We Go from Here. Molecules. 2020;25(11):2653–2653. doi: 10.3390/molecules25112653. [DOI] [PMC free article] [PubMed] [Google Scholar]


