Abstract
Objective
Instrumentation failure in spine tumor surgery is a common reason for revision operation. Increases in patient survival demand a better understanding of the hardware longevity. The study objective was to investigate risk factors for instrumentation failure requiring revision surgery in patients with spinal tumors.
Methods
A retrospective cohort from a single tertiary care specialty hospital from January 2005 to January 2021, for patients with spinal primary or metastatic tumors who underwent surgical intervention with instrumentation. Demographic and treatment data were collected and analyzed. Kaplan-Meier analysis was performed for overall survival, and separate univariate and multivariate regression analysis was performed.
Results
Three hundred fifty-one patients underwent surgical intervention for spinal tumor, of which 23 experienced instrumentation failure requiring revision surgery (6.6%). Multivariate regression analysis identified pelvic fixation (odds ratio [OR], 10.9), spinal metastasis invasiveness index (OR, 1.11), and survival of greater than 5 years (OR, 3.6) as significant risk factors for hardware failure. One- and 5-year survival rates were 57% and 8%, respectively.
Conclusion
Instrumentation failure after spinal tumor surgery is a common reason for revision surgery. Our study suggests that the use of pelvic fixation, invasiveness of the surgery, and survival greater than 5 years are independent risk factors for instrumentation failure.
Keywords: Spine, Tumor, Hardware, Survival, Surgery
INTRODUCTION
The spine is a frequent site of both primary and metastatic osseous tumors. Metastatic spine tumors account for about 70% of all osseous metastases, while primary spine tumors account for about 5% of all primary bone tumors [1]. Spine metastases often present before their primary source, and account for 10%–30% of new cancer diagnoses each year [2]. Metastatic spine tumors most commonly arise from the primary lung, prostate, kidney, stomach, colon, breast, and thyroid cancers; however, 10% of metastatic spine tumors arise from occult malignancies and have no known primary source at presentation [3.4].
Spinal tumors often present with pain, weakness, neural compression, pathologic fracture, instability, and/or paralysis. The sequela of these tumors are often debilitating—potentially catastrophic—and can lead to a significant impairment of activities of daily living, sphincter control, and ambulation [1,5].
Medical and surgical treatment options are guided by the primary tumor pathology, tumor burden, location, and patient symptoms. Consideration of patient life expectancy, impending paralysis, tumor sensitivity to adjuvant therapy, and probability of systemic control are imperative in determining the appropriate individualized treatment modalities.
When a surgery is indicated, the type of the surgical intervention is guided by the presence or absence of neurologic deficits, spinal instability, and individual patient/tumor factors. The surgery goal is palliation, pain control, maintenance of ambulation, and sphincter control. Quite often, a palliative surgery for metastatic spine lesions will not necessarily affect the survival, but would greatly impact the quality of the remaining life. Surgical options vary, but frequently aim to debulk the tumor and stabilize the spine with instrumentation with or without a fusion [6,7].
Recent data suggest that both the incidence of spinal metastatic disease as well as surgery to address it are increasing rapidly [8]. Despite this trend, there has been a paucity of data regarding the risk factors for surgical and specifically hardware-related complications in the spinal tumor patient population. The ability of the spine to actually fuse in the setting of metastatic disease, possible prior or subsequent radiation, chemotherapy, and generalized catabolic state can be quite compromised. A delayed fusion or a nonunion following the instrumentation in the setting of metastatic disease may result in a hardware loosening or failure. The aim of this study was to investigate the risk factors for instrumentation failure requiring a revision surgery in patients with spinal tumors.
MATERIALS AND METHODS
We have retrospectively reviewed medical records of patients who had undergone a surgery with instrumentation for a spinal tumor at a single tertiary care tumor hospital from January 2005 to January 2021. Inclusion criteria were age ≥ 18 years old, a diagnosis of a spinal primary or metastatic tumor, a presence of clinical symptoms requiring a surgical intervention, and a surgery with instrumentation. The exclusion criteria were an uninstrumented surgery, a lack of an appropriate follow-up, a lack of pre- and/or postsurgical imaging, and incomplete medical records. A total of 2,053 patients who had a spine tumor surgery within the study period were screened. Of those, 351 patients were included in the study.
The study was approved by the Institutional Review Board of Vreden National Medical Research Center of Traumatology and Orthopedics Saint-Petersburg, Russia (003/2021) and complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws.
Indications for surgery included a severe back and/or extremity pain, clinical and/or radiographic evidence of instability, and neurological dysfunction. We performed a standalone posterior column stabilization (without tumor resection or decompression) in cases of radiosensitive or chemotherapy-sensitive tumors with pathological fractures, or signs of spinal instability without spinal cord or nerve roots compression symptoms. Reconstruction of the spinal column with circumferential stabilization of both the anterior and posterior columns was required after tumor resection. Those patients underwent a surgical stabilization with pedicle screw fixation and/or anterior corpectomy with plate fixation. Both conventional iliac screws, as well as S2AI screws, were used for pelvic fixation. Either 5.5-mm (298 patients) or 6.0-mm (53 patients) titanium rods were used. A fusion was done for 1-level instrumentation only. For multilevel cases, only instrumentation was performed without a fusion.
For all patients, we reviewed demographic and treatment data. We used the Charlson Comorbidity Index (CCI), body mass index (BMI), and the Eastern Cooperative Oncology Group (ECOG) scale for assessment of comorbidities and performance status. Tumor type was stratified according to histology for primary spinal neoplasms or primary site for metastatic lesions. The first domain of spinal instability neoplastic score (SINS) defined regions of the spine according to biomechanical considerations. We took into account dissemination of disease into the spine as well as a number of affected vertebrae in the surgical region. Nonsurgical treatment data included local radiotherapy and bone-modifying agent therapy (zoledronic acid, clodronic acid, or denosumab).
Surgical variables included a usage of cement-augmented screws, vertebral body replacement (VBR), pelvic fixation, and number of instrumented levels. Spinal metastasis invasiveness index (SMII) was used for the estimation of surgical invasiveness [9].
We evaluated ambulatory status at follow-up in 3 grades: nonambulatory, walking with assistive devices, and ambulatory. Local recurrence was defined as a relapse after a complete tumor resection or a local progression after palliative decompression. Overall survival was evaluated from the time of the spinal surgery to the time of death or the time of the last follow-up.
Patients were stratified according to the presence of hardware failure. Hardware failure was defined as a loss of a construct stability due to a rod or screw breakage, mesh cage migration or screw pullout at the upper/lower instrumented vertebra (UIV/LIV) fracture requiring a revision surgery.
Statistical analysis was performed using R ver. 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). A 0.05 level of significance was applied. Descriptive analyses were applied to describe the participants. The results of continuous variables were summarized with medians and interquartile range. Wilcoxon rank-sum test was carried out. For categorical variables, cross-tabulations were generated, and Approximative (Monte Carlo) Pearson chi-square test (number of replicates is 10000) was used. The Kaplan-Meier analysis method estimated postoperative overall survival, 1-year, and 5-year survival rate was calculated. Variables potentially predicting hardware failure following the spinal tumor surgery were selected from the patient characteristic comparisons. Univariate and multivariate logistic regression with analysis of odds ratio estimates was computed for predictor confirmation.
RESULTS
1. Demographics
A total of 351 eligible patients (163 males, 188 females) were included into this study (Fig. 1) with a median age of 57 years (range, 19–85 years). The median CCI was 7 scores (range, 0–12 scores). Patients had a normal BMI (24.4 kg/m2; range, 14.3–43.9 kg/m2). The median follow-up time was 21.4 months (30 days–146 months).
Fig. 1.
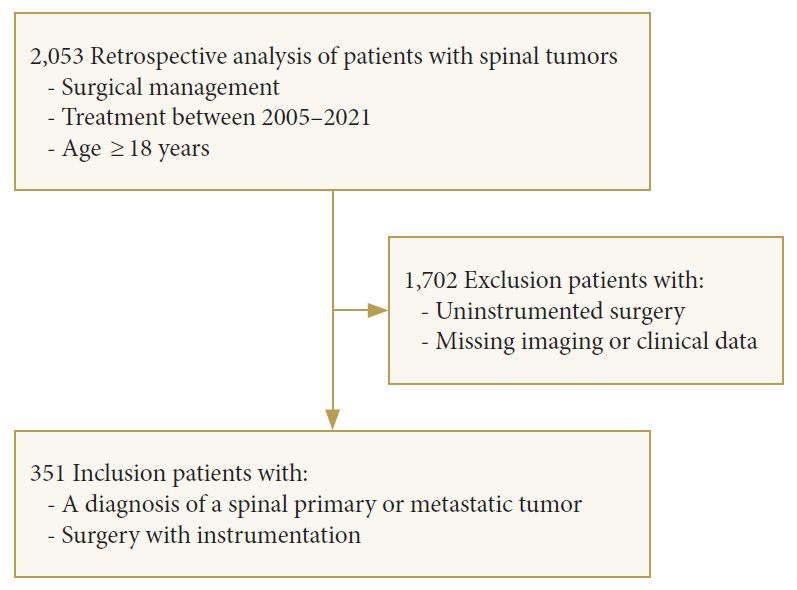
Flow-diagram of patient selection.
2. Tumor Biology
Sevenry-six patients had primary spinal tumors and 275 patients had metastatic tumors. The most common primary tumor type was chordoma (2.6%), followed by plasmacytoma (2.3%), and chondrosarcoma (1.7%). The most prevalent spinal metastases originated from the breast (25%), the kidney (23%), and the lung (7.7%).
The median grade of invasiveness (SMII) was 13 scores (range, 3–27 scores). According to SINS, most tumors involved the junctional region (n=156, 44%), whereby the semirigid region was affected in 92 cases (26%), followed by the mobile spine region (n=90, 26%) and the rigid region (n=13, 3.7%).
We observed 82 events (23%) of local recurrence in our patients. The current dataset includes 7 patients with preoperative embolization only with hypervascular tumors. Our historical results were of questionable significance. We rarely use the embolization today. In the current study, no patients with a hardware failure had embolization.
3. Spine Tumor Burden
Majority of the patients had a 1-level symptomatic lesion with only 1 vertebra being affected with a pathological fracture and/or epidural compression (n = 277, 79%). Those patients may have had other asymptomatic metastases elsewhere in the spinal column. The 2-level (meaning that 2 adjacent vertebrae were affected - Tomita grade 6), 3-level, and 4-level lesions were seen in 41 (12%), 27 (7.7%), and 4 patients (1.1%), respectively. Two of the patients (0.6%) had 5-level lesions.
As far as the total number of spine lesions is concerned, both symptomatic and asymptomatic, 188 patients (54%) had a solitary spinal lesion, whereas 103 patients (29%) presented with several (up to 3) spinal lesions. Sixty patients (17%) had multiple dissemination throughout the spine. Only 21 patients (6.0%) had a preoperative radiotherapy. Preoperative bone-modifying agent therapy was applied in 69 patients (20%) (Table 1).
Table 1.
Tumor characteristics
| Variable | Overall (n=351) | Hardware failure |
p-value† | ||
|---|---|---|---|---|---|
| No (n=328) | Yes (n=23) | ||||
| Tumor type | 0.829 | ||||
| Cancer of unknown primary site | 6 (1.7) | 6 (1.8) | 0 (0) | ||
| Other (soft tissue sarcoma, germ cell tumor, ovary, adrenal) | 34 (9.7) | 30 (9.1) | 4 (17) | ||
| Stomach | 2 (0.6) | 2 (0.6) | 0 (0) | ||
| Skin (melanoma, basalioma) | 9 (2.6) | 8 (2.4) | 1 (4.3) | ||
| Lung | 27 (7.7) | 26 (7.9) | 1 (4.3) | ||
| Lymphoma | 5 (1.4) | 5 (1.5) | 0 (0) | ||
| Multiple myeloma | 27 (7.7) | 25 (7.6) | 2 (8.7) | ||
| Breast | 86 (25.0) | 80 (24.0) | 6 (26.0) | ||
| Bladder | 2 (0.6) | 2 (0.6) | 0 (0) | ||
| Osteosarcoma | 3 (0.9) | 3 (0.9) | 0 (0) | ||
| Liver | 3 (0.9) | 2 (0.6) | 1 (4.3) | ||
| Plasmacytoma | 8 (2.3) | 8 (2.4) | 0 (0) | ||
| Kidney | 79 (23) | 73 (22.0) | 6 (26.0) | ||
| Prostate | 16 (4.6) | 16 (4.9) | 0 (0) | ||
| Corpus uteri | 8 (2.3) | 8 (2.4) | 0 (0) | ||
| Colon | 9 (2.6) | 9 (2.7) | 0 (0) | ||
| Chondrosarcoma | 6 (1.7) | 5 (1.5) | 1 (4.3) | ||
| Chordoma | 9 (2.6) | 8 (2.4) | 1 (4.3) | ||
| Cervix uteri | 9 (2.6) | 9 (2.7) | 0 (0) | ||
| Thyroid | 3 (0.9) | 3 (0.9) | 0 (0) | ||
| SINS region | 0.587 | ||||
| Junctional | 156 (44.0) | 143 (44.0) | 13 (57.0) | ||
| Mobile spine | 90 (26.0) | 85 (26.0) | 5 (22.0) | ||
| Rigid | 13 (3.7) | 13 (4.0) | 0 (0) | ||
| Semirigid | 92 (26.0) | 87 (27) | 5 (22.0) | ||
| No. of affected vertebrae | 0.493 | ||||
| 1 | 277 (79.0) | 259 (79.0) | 18 (78.0) | ||
| 2 | 41 (12.0) | 38 (12.0) | 3 (13.0) | ||
| 3 | 27 (7.7) | 25 (7.6) | 2 (8.7) | ||
| 4 | 4 (1.1) | 4 (1.2) | 0 (0) | ||
| 5 | 2 (0.6) | 2 (0.6) | 0 (0) | ||
| Spine metastasis number | 0.959 | ||||
| Multiple | 60 (17) | 56 (17) | 4 (17) | ||
| Several (3 or less) | 103 (29) | 97 (30) | 6 (26) | ||
| Solitary | 188 (54) | 175 (53) | 13 (57) | ||
| Preoperative radiotherapy | 21 (6.0) | 20 (6.1) | 1 (4.3) | > 0.999 | |
| Preoperative bone-modifying agent therapy | 69 (20.0) | 67 (20.0) | 2 (8.7) | 0.184 | |
Values are presented as number (%).
SINS, spinal instability neoplastic score.
Approximative (Monte Carlo) Pearson chi-square test.
4. Spine Surgery
The median number of instrumented levels was 5. This is a traditional construct of 2 levels above and below the tumor-involved segment. Screw cement augmentation was used in 48 cases (14%). Corpectomy with VBR was performed in 81 cases (23%). We have used a VBR cage in all spondylectomy cases. The technique of circumferential decompression was similar to a separation surgery and consisted of laminectomy, pediculectomy, and ventral spinal cord/dural sac decompression.
We used pelvic fixation in 10 patients (2.8%). The median grade of invasiveness (SMII) was 13 scores (range, 3–27 scores).
5. Functional Status
Fifty-five patients (68%) presented with severe disability (ECOG 4). Moderate disability ECOG 3 and 2 were in 73 (21%) and 118 patients (34%), respectively. Eighty-nine patients (25%) had mild disability (ECOG 1). Sixteen patients (4.6%) had a normal performance status (ECOG 0) (Table 2).
Table 2.
Patient demographics
| Variable | Overall (n=351) | Hardware failure |
p-value† | |
|---|---|---|---|---|
| No (n=328) | Yes (n=23) | |||
| Sex | 0.518 | |||
| Female | 188 (54) | 174 (53) | 14 (61) | |
| Male | 163 (46) | 154 (47) | 9 (39) | |
| Age (yr) | 57 (19–85) | 57 (19–85) | 59 (42–82) | 0.160 |
| CCI | 7 (0–12) | 7 (0–12) | 7 (0–11) | 0.623 |
| BMI (kg/m2) | 24.3 (14.3–43.9) | 24.3 (14.3–43.9) | 23.1 (18.4–34.6) | 0.329 |
| ECOG | 0.056 | |||
| 0 | 16 (4.6) | 16 (4.9) | 0 (0) | |
| 1 | 89 (25) | 81 (25) | 8 (35) | |
| 2 | 118 (34) | 106 (32) | 12 (52) | |
| 3 | 73 (21) | 70 (21) | 3 (13) | |
| 4 | 55 (16) | 55 (17) | 0 (0) | |
Values are presented as number (%) or median (range).
CCI, Charlson Comorbidity Index; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group.
Approximative (Monte Carlo) Pearson chi-squared test; Wilcoxon rank-sum test.
Most of the patients (n=267, 76%) were ambulatory, 40 patients (11%) needed assistive devices and 44 patients (13%) were nonambulatory at follow-up.
Overall, 199 (57%) and 28 patients (8.0%) survived for more than 1 and 5 years, respectively (Table 3).
Table 3.
Surgical variables and follow-up data
| Variable | Overall (n=351) | Hardware failure |
p-value† | |
|---|---|---|---|---|
| No (n=328) | Yes (n=23) | |||
| Instrumented levels | 5 (3–11) | 5 (3–11) | 5 (3–7) | 0.218 |
| Screw cement augmentation | 48 (14) | 45 (14) | 3 (13) | > 0.999 |
| Vertebral body replacement | 81 (23) | 73 (22) | 8 (35) | 0.196 |
| Pelvic fixation | 10 (2.8) | 5 (1.5) | 5 (22) | < 0.001* |
| SMII | 13 (3–27) | 13 (3–27) | 14 (7–21) | 0.019* |
| Ambulatory status after surgery | 0.162 | |||
| Nonambulatory | 44 (13) | 44 (13) | 0 (0) | |
| Walking with assistive devices | 40 (11) | 37 (11) | 3 (13) | |
| Ambulatory | 267 (76) | 247 (75) | 20 (87) | |
| Local recurrence | 82 (23) | 72 (22) | 10 (43) | 0.025* |
| Survivors of more than 1 year | 199 (57) | 181 (55) | 18 (78) | 0.048* |
| Survivors of more than 5 years | 28 (8.0) | 21 (6.4) | 7 (30) | < 0.001* |
Values are presented as median (range) or number (%).
SMII, spinal metastasis invasiveness index.
p<0.05, statistically significant difference.
Approximative (Monte Carlo) Pearson chi-square test, Wilcoxon rank-sum test.
6. Hardware Failure Rate
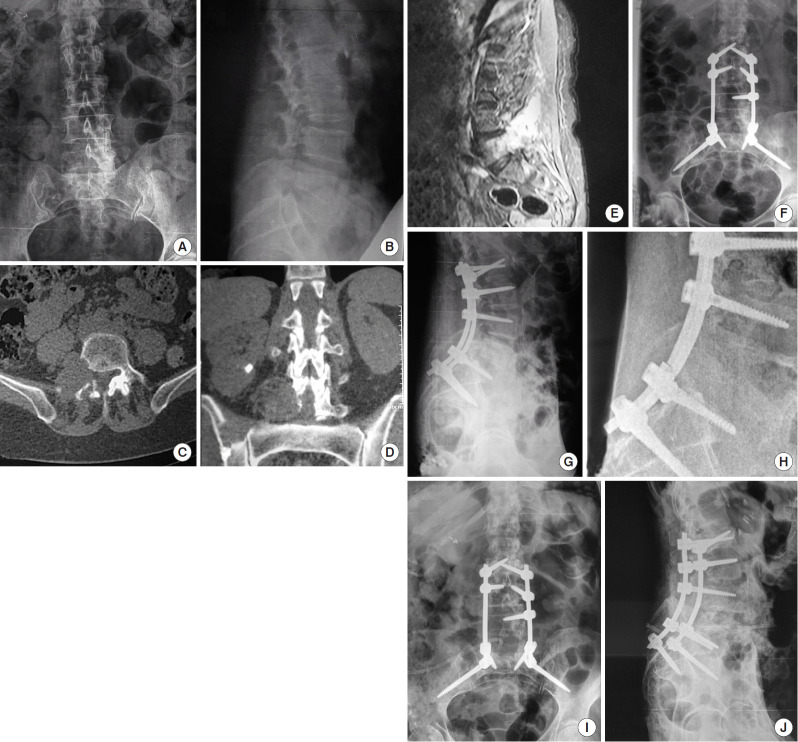
Overall, 23 patients had 31 hardware failures. Bilateral rod breakage was seen in 6 cases (19.4%), whereas unilateral rod breakage was seen in 5 cases (16.1%). An illustrative case of a symptomatic unilateral rod fracture requiring a revision surgery is provided in Fig. 2.
Fig. 2.
(A–E) A 72-year-old female with L4 and L5 metastases of a renal cell carcinoma, presented with a right-sided paresis (⅘ weakness of L4-innervated muscles and ⅗ weakness of L5-innervated muscles according to Medical Research Council muscle grading system) and back pain. (F, G) She was treated with laminectomy, right pediculectomy and L2–S1 fusion with pelvic fixation using S2AI screws. At 9-month follow-up, the patient had complained of a back and leg pain as well as a moderate muscle weakness (⅘ weakness of L4-innervated muscles). (H) A unilateral rod breakage was observed. (I, J) Patient underwent a revision surgery with replacement of the rods. Seven months after the revision surgery, the patient had died due to a progression of the disease.
There were 6 cases (19.4%) of LIV screw pullout whereby 2 patients (6.5%) had additional distal junctional failure (DJF) and 3 cases (9.7%) of UIV screw pullout. One patient (3.2%) had a proximal junctional failure (PJF).
Symptomatic screw breakage was diagnosed in 1 case (3.2%). In respect to the VBR failures, we observed 4 cases (12.9%) of mesh subsidence, 2 cases (6.5%) of anterior mesh migration, and 1 case (3.2%) of pseudoarthrosis (Table 4).
Table 4.
Hardware failures
| Patient No. | Age (yr) | Tumor type | Level | Surgery | Fixation | Screw augmentation | SMII | Hardware failure | Hardware_survival |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | Breast | T8–10 | Circumferential decompression | T6, T7, T11, T12 | No | 19 | Bilateral rod breakage | 19 Days |
| 2 | 51 | Breast | T5 | Spondylectomy | T4, T6, VBR | No | 11 | DJF, LIV screw pullout, mesh subsid- ence | 21 Days |
| 3 | 69 | Breast | L5 | Circumferential decompression | L3, L4, S1, iliac | No | 13 | UIV screw pullout | 1 Month |
| 4 | 66 | Breast | T11 | Circumferential decompression | T7, T8, T10, L1, L2 | Yes | 17 | LIV screw pullout | 2 Months |
| 5 | 75 | Lung | L4 | Laminectomy | L3, L5, S1 | No | 9 | PJF | 3 Months |
| 6 | 69 | Chordoma | T11 | Spondylectomy | T9, T10, T12, L1, VBR | Yes | 17 | DJF, LIV screw pullout, mesh subsid- ence | 4 Months |
| 7 | 44 | Chondrosarcoma | T5–7 | Spondylectomy | T3, T4, T8, T9, VBR | No | 21 | Unilateral rod breakage | 6 Months |
| 8 | 55 | Breast | T1 | Spondylectomy | C6, C7, T2, T3, VBR | No | 15 | Mesh migration | 6 Months |
| 9 | 68 | Liver | L5 | Hemilaminectomy | L4, L5, S1 | No | 10 | Unilateral rod breakage | 6 Months |
| 10 | 71 | Kidney | T10–11 | Spondylectomy | T8, T9, T12, L1, VBR | No | 20 | LIV screw pullout, mesh migration | 6 Months |
| 11 | 59 | Salivary gland | T4 | Circumferential decompression | C7, T1, T2, T6, T7 | No | 15 | LIV screw pullout | 10 Months |
| 12 | 72 | Kidney | L5 | Circumferential decompression | L2, L3, S1, S2AI | No | 14 | Unilateral rod breakage | 10 Months |
| 13 | 42 | Kidney | T8 | Spondylectomy | T4, T5, T6, T7, T10, T11, VBR | No | 20 | Bilateral rod breakage, mesh subsidence | 1 Year |
| 14 | 57 | Kidney | L4 | Spondylectomy | L2, L3, L5, S1, VBR | Yes | 18 | UIV screw pullout | 1 Year 2 months |
| 15 | 59 | Kidney | L1 | Laminectomy | T12, L2 | No | 8 | Unilateral rod breakage | 1 Year 5 months |
| 16 | 52 | Breast | L4–5 | Laminectomy | L2, L3, L4, L5 | No | 14 | Screw breakage | 1 Year 6 months |
| 17 | 52 | Multiple myeloma | L3 | Circumferential decompression | L1, L2, L4, L5 | No | 13 | UIV screw pullout | 2 Years 1 months |
| 18 | 76 | Kidney | L2 | Circumferential decompression | T12, L1, L3, L4 | No | 14 | LIV screw pullout | 1 Year 11 months |
| 19 | 46 | Melanoma | L4–5 | Spondylectomy | L2, L3, S1, iliac, VBR | No | 18 | Unilateral rod breakage, pseudarthrosis | 2 Years 1 months |
| 20 | 65 | Multiple myeloma | S1 | Circumferential decompression | L4, L5, iliac | No | 11 | Bilateral rod breakage | 2 Years 4 months |
| 21 | 82 | Liposarcoma | L5 | Circumferential decompression | L2, L4, S1, iliac | No | 13 | Bilateral rod breakage | 2 Years 7 months |
| 22 | 45 | Aggressive hemangioma | L2 | Laminectomy | L1, L3 | No | 7 | Bilateral rod breakage | 3 Years 7 months |
| 23 | 57 | Hemangioblastoma | T4 | Spondylectomy | T2, T3, T5, T6, T7, T8, VBR | No | 19 | Bilateral rod breakage, mesh subsidence | 3 Years 8 months |
SMII, spinal metastasis invasiveness index; VBR, vertebral body replacement; UIV, upper instrumented vertebra; LIV, lower instrumented vertebra; PJF, proximal junctional failure; DJF, distal junctional failure.
Also, we performed a subgroup analysis of hardware failure over time: less than 1 year and more than 1 year. We observed 70% of junctional failures in the subgroup of patients who developed hardware failure at less than 1 year. On the other hand, 64% of rod breakages were in the subgroup of patients who developed hardware failure at more than 1 year. However, no factors in both subgroups were found to be statistically significant predictors of hardware failure.
When comparing patients with and without hardware failures, we found that they differed in ECOG status, the presence of pelvic fixation, grade of surgery invasiveness, and the frequency of local recurrence. Patients with hardware failures had mild or moderate (ECOG 1–2) disability status (p=0.049). Almost a quarter of hardware failure cases had pelvic fixation (p<0.001). Patients without hardware failures had less invasiveness of surgery and vice versa (p=0.019). Also, we observed a significant difference in the rate of local recurrence, which was higher in patients with hardware failure (p=0.018).
The rate of cases with hardware failures generally increased with an increased overall survival based on the assessment of survivors of more than 1 year (p=0.031) and more than 5 years (p<0.001). The median overall survival of patients with hardware failure was 85 months (95% confidence interval [CI], 44–120). Their 1- and 5-year survival rates were 95.7% and 63.8%, respectively. In contrast, the median overall survival of patients without hardware failure was only 16 months (95% CI, 14–21). Their 1- and 5-year survival rates were 61.3% and 16.5%, respectively.
Logistic regression models to predict the hardware failure were built on the basis of the most relevant parameters independently (Table 4). When considering parameters confirmed by univariate logistic regression altogether, in multivariate analysis we observed that pelvic fixation (OR, 10.9; p<0.001), SMII (OR, 1.11; p=0.042), and survival of more than 5 years (OR, 3.6; p=0.029) were significant predictors of hardware failure (Table 5).
Table 5.
Results of logistic regression analysis
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Male sex | 0.73 | 0.30–1.70 | 0.465 | |||
| Age | 1.04 | 1.00–1.08 | 0.065 | |||
| ECOG | 0.70 | 0.46–1.04 | 0.075 | |||
| SINS junctional region | 1.68 | 0.72–4.05 | 0.230 | |||
| Multiple spine metastasis | 1.14 | 0.49–2.73 | 0.768 | |||
| Preoperative radiotherapy | 0.70 | 0.04–3.62 | 0.721 | |||
| Preoperative bone-modifying agent therapy | 0.37 | 0.06–1.31 | 0.135 | |||
| Multilevel lesion | 1.04 | 0.33–2.72 | 0.937 | |||
| Fixation more than 5 levels | 1.99 | 0.80–4.72 | 0.133 | |||
| Screw cement augmentation | 0.94 | 0.22–2.90 | 0.927 | |||
| Vertebral body replacement | 1.86 | 0.73–4.47 | 0.187 | |||
| Pelvic fixation | 17.9 | 4.62–70.2 | < 0.001* | 10.9 | 2.59, 45.9 | < 0.001* |
| SMII | 1.10 | 1.01–1.20 | 0.025* | 1.11 | 1.01, 1.22 | 0.042* |
| Ambulatory status after surgery | 9.36 | 0.01–16 | 0.987 | |||
| Local recurrence | 2.74 | 1.12–6.48 | 0.027* | 2.06 | 0.72, 5.79 | 0.169 |
| Survival of more than 1 year | 2.92 | 1.14–9.02 | 0.025* | 1.45 | 0.47, 5.02 | 0.528 |
| Survival of more than 5 years | 6.40 | 2.25–16.8 | < 0.001* | 3.60 | 1.09, 11.2 | 0.029* |
OR, odds ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; SINS, spinal instability neoplastic score; SMII, spinal metastasis invasiveness index.
Significant p-value.
DISCUSSION
A patient with a metastatic spine tumor presents multiple challenges to a spinal surgeon. Our study found a 7% rate of hardware failure requiring a revision surgery in this population. This is in line with the hardware complication rates previously reported in the literature (2%–19%) [9-16].
Prior studies have found that increasing age, smoking, preoperative radiotherapy, constructs spanning 6, or more levels, and a prior chest wall resection were risk factors for instrumentation failure after spinal surgery for tumor [13-18]. In contrast, in our study those risk factors were increasing SMII, pelvic fixation, and longer survival of more than 5 years.
A comprehensive index of invasiveness (SMII) had a significant impact [19]. The higher the SMII index, the more likely a hardware failure. Even though the SMII was initially developed to predict the operative blood loss and duration, we have used it as a proxy for a surgical invasiveness and magnitude of the surgery [19,20]. The SMII was shown to be a stronger predictor of blood loss and operative time in spinal metastasis patients when compared to the commonly used surgical invasiveness index for spine surgery [19]. In addition, it does not require standing radiographs, which are often difficult to obtain in patients with metastases, due to pain with mobilization. Intuitively, with a greater resection of the involved cancerous tissue, the spine ability to fuse was more compromised. Also, a more invasive resection is bound to destabilize the spine to a greater degree. This would lead to a greater stress on the hardware used to span the resected area. It should be noted that while there was a statistical difference of SMII between the groups, the difference of a single point on the SMII was previously found to translate only to a mean difference of 42 mL and 5 minutes of surgical blood loss and operative time, respectively, which may not be clinically meaningful [19].
Contrary to some of the prior studies, surgical factors such as a construct length and a use of a VBR cage did not significantly affect the incidence of hardware complications [16]. The notable surgical risk factor in our study was pelvic fixation. Of the 23 patients in our study who underwent revision surgery, 22% had pelvic fixation. While there have not been prior studies investigating L5–S1 nonunion risk in the spinal tumor population, L5–S1 pseudarthrosis is a known complication and a risk factor for revision surgery in the degenerative and deformity spine literature [21-23]. Because the region of the lumbosacral junction is exposed to significant loads, bending moments, and stresses, we observed stabilization-related hardware failures. Risk of rods failure after sacropelvic fixation was confirmed by biomechanical and clinical studies [24,25]. In addition, proximal junctional disorders were observed frequently in fusion of the lumbar curve extending to the pelvis [26]. The synergistic effect of the high-stress environment of the lumbosacral spine coupled with a poor bone quality in a compromised patient had likely contributed to our findings [27]. Also, patients who require spinopelvic fixation may have had a more extensive disease requiring more resection of the tumor, resulting in more destabilization. On the other hand, prior studies have found that pelvic fixation can protect against L5–S1 nonunion, S1 screw pullout, and sacral insufficiency fractures [28-32]. Therefore, we recommend that surgeons consider both the potential risks and benefits of added pelvic fixation in this population [24-26].
A recurrence or a local progression of disease had a significant impact only in the univariate analysis. Though not significant in a multivariate analysis, we believe this factor should not be ignored. A local recurrence would possibly lead to an increase in the lytic destruction of the affected vertebra with a potential loss of screw purchase. The stability of a spinal construct is dependent on achieving a timely bony fusion [12]. Local and systemic effects of spine tumors do not favor this outcome [2,14]. In addition, oncological patients often have a poor mobility and nutrition, and frequently undergo radiation and chemotherapy, which would further compromise the fusion bed [12]. As lytic lesions commonly involve the anterior column of the spine, their progression would typically transfer the loads to the posterior column which may result in a rod fracture. This must be taken into account when considering fixation in the patient with a recurrent spinal tumor.
Mortality after surgery for spinal tumors depends on a variety of factors, including patient age, comorbidities, and tumor type [33]. Prior research has shown a longer median survival rate for patients who undergo a revision surgery versus those who do not [14]. Patients in our study with hardware failures had longer overall survival. The mean and median time to hardware failure was 14.4 and 10 months respectively. The rate of cases with hardware failures generally increased with an increased overall survival based on the assessment of survivors of more than 1 year (55% vs. 78%) and more than 5 years (6.4% vs. 30%). These trends imply that some patients did not live long enough to develop a hardware failure.
In contrast to prior studies, our results did not find that preoperative radiotherapy was a risk factor for hardware failure [18]. While osteoradionecrosis is a conceivable mechanism [31] for this this reported association [32], it was derived from a model with 7 covariates but only 3 failure events, creating a high likelihood of a small-sample bias and a model inaccuracy [13]. Moreover, 38% of patients in study of Pedreira et al. [18] had a preoperative radiotherapy compared with only 6% in our patients.
The limitations of this study include its retrospective design, which could introduce a potential bias. Second, the data were collected at a single institution over a decade and a half while significant advances in spinal surgery have taken place. This would limit its generalizability. Third, we have used 2 methods of pelvic fixation (conventional iliac screws and S2AI screws). It is possible that the different pelvic fixation techniques affect the rates of rod fracture differently. Fourth, we have only recently started using triple and quadruple rod constructs, which would potentially mitigate the rod fracture rates at least at a 5-year follow-up. The vast majority of our subjects had a conventional dual rod construct. Fifth, we have only used titanium rods rather than the cobalt-chrome rods due to cost considerations and concerns about higher PJF and DJF rates with stiffer cobalt-chrome rods. Sixth, we have only done fusions for single-level cases. Our philosophy was not to do a fusion for multilevel cases, since, quite frequently, achieving a solid multilevel fusion in a compromised host with metastatic disease is not realistic. Adding a bone graft in those cases will introduce a potential nidus for an infection in an immunocompromised host and would significantly add to the overall costs of treatment. Finally, our database does not contain any information about the smoking status. Although smoking is a well-known risk factor of pseudarthrosis or hardware failure, Longo et al. [17] did not find the influence of smoking history on hardware failure rate. Also, other studies listed in Table 6 did not find smoking status as a risk factor.
Table 6.
Summary of studies reporting on the risk factors for instrumentation failure
| Study | Follow-up period (min–max, mean/median) | Patient total | Increasing age | Smoking | Preoperative radiotherapy | Construct length >6 levels | Prior chest wall reconstruction | Vertebral body replacement | Pelvic fixation | Survival time |
|---|---|---|---|---|---|---|---|---|---|---|
| Yee et al. [13] 2021 | 6 Months–2 years; not reported | 164 | + | N/A | N/A | - | N/A | N/A | - | Median survival of 11 months |
| Longo et al. [17] 2019 | Not reported; median, 8.1 months | 58 | - | - | - | N/A | N/A | - | N/A | Median follow-up time 7.4 months in no hardware failure group and 17.7 months in hardware failure group |
| Alamanda et al. [14] 2018 | 6 Months–5 years; not reported | 55 | - | - | - | N/A | N/A | N/A | N/A | Revision group survival = 3.0 years; nonrevision group survival = 1.5 years (p = 1.05) |
| Pedreira et al. [18] 2017 | 3 Months–not reported; not reported | 159 | - | N/А | + | N/A | N/A | N/A | N/A | Mean survival in the patients without hardware failure was 16.7 ± 22.6 months, while in the cohort with hardware failure, mean survival was found to be 33 ± 30 months |
| Amankulor et al. [15] 2014 | 9 Days–7 years; median, 399 days | 318 | N/A | N/A | N/A | + | + | N/A | - | 75% risk of death at 2 years without hardware failure |
| De Ruiter et al. [16] 2014 | 0 Month–6 years; mean, 10 months | 60 | N/A | N/A | N/A | N/A | N/A | 56% complication rate | N/A | 53% 1-year survival rate |
| Current study | 30 Days–146 months; median, 21.4 months | 351 | - | N/A | - | - | N/A | - | + | Hardware failure associated with survival >5 years |
Follow-up notes, patient total, and patient survival notes are summarized.
+, identified risk factor for instrumentation failure; -, not a significant risk factor for instrumentation failure; N/A, not analyzed in statistical analysis.
Our study has some significant strengths. The first strength of our study is in a significant number of patients treated and a robust follow-up, though limited by a high mortality in cancer patients.The current study is the largest series looking at the hardware failure in tumor patients. The second strength is that we have analyzed a variety of tumors with various regimens of chemo and radiotherapy, which would make our results very applicable in a real-world scenario. The third strength is that the risk factors that we have identified are relatively novel and differ from those previously reported. This might be due to the improvement in patient survival due to ever evolving cancer treatments over the last 2 decades, improvement in surgical strategy and instrumentation, and improvement of surgical skills due to the higher volume of cancer patients. Those differences in risk factors might also be due to the increasing popularity of pelvic fixation in the past decade and introduction of S2AI screws several years ago.
Based on the results of our study, we are proposing several changes to the surgical and postoperative protocols. First, a triple and quadruple rod construct should be considered in patients with a longer expected survival. Alternatively, those patients might also require cobalt-chrome rods to mitigate the risk of a rod fracture. Second, the postoperative follow-up should be at least yearly for life to provide an early recognition of a hardware failure and a loss of construct stability.
CONCLUSION
Our study highlights several novel risk factors for instrumentation failure requiring a revision surgery in patients with spinal tumors, including increasing SMII, pelvic fixation, and a survival longer than 5 years. It also contributes to the survival data for patients who undergo surgery for various tumors of the spine. Future studies are needed to determine the risks of instrumentation failure at greater follow-up. Future studies will also be needed to determine if a fusion is always necessary when spine instrumentation is performed in a cancer patient.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: NZ, AS, DP, DM, SM, OS, DK; Data curation: NZ, AS, DP, DM, SM, OS, DK; Formal analysis: NZ, AS, DP, DM, SM, OS, JS, DK; Methodology: NZ, AS, DP, DM, SM, OS, DK; Project administration: NZ, AS, DP, DM, SM, OS, DK; Visualization: NZ, AS, DP, DM, SM, OS, DK; Writing - original draft: NZ, AS, DP, DM, SM, OS, JS, DK; Writing - review & editing: NZ, AS, DP, DM, SM, OS, JS, DK.
REFERENCES
- 1.Wewel JT, O'Toole JE. Epidemiology of spinal cord and column tumors. Neurooncol Pract. 2020;7(Suppl 1):i5–9. doi: 10.1093/nop/npaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White AP, Kwon BK, Lindskog DM, et al. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14:587–98. doi: 10.5435/00124635-200610000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Constans JP, de Divitiis E, Donzelli R, et al. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59:111–8. doi: 10.3171/jns.1983.59.1.0111. [DOI] [PubMed] [Google Scholar]
- 4.Gasbarrini A, Cappuccio M, Mirabile L, et al. Spinal metastases: treatment evaluation algorithm. Eur Rev Med Pharmacol Sci. 2004;8:265–74. [PubMed] [Google Scholar]
- 5.Meyer SA, Singh H, Jenkins AL. Surgical treatment of metastatic spinal tumors. Mt Sinai J Med. 2010;77:124–9. doi: 10.1002/msj.20162. [DOI] [PubMed] [Google Scholar]
- 6.Williams R, Foote M, Deverall H. Strategy in the surgical treatment of primary spinal tumors. Global Spine J. 2012;2:249–66. doi: 10.1055/s-0032-1329886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunning EC, Butler JS, Morris S. Complications in the management of metastatic spinal disease. World J Orthop. 2012;3:114–21. doi: 10.5312/wjo.v3.i8.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsiue PP, Kelley BV, Chen CJ, et al. Surgical treatment of metastatic spine disease: an update on national trends and clinical outcomes from 2010 to 2014. Spine J. 2020;20:915–24. doi: 10.1016/j.spinee.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Placantonakis DG, Laufer I, Wang JC, et al. Posterior stabilization strategies following resection of cervicothoracic junction tumors: review of 90 consecutive cases. J Neurosurg Spine. 2008;9:111–9. doi: 10.3171/SPI/2008/9/8/111. [DOI] [PubMed] [Google Scholar]
- 10.Wang JC, Boland P, Mitra N, et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:287–98. doi: 10.3171/spi.2004.1.3.0287. [DOI] [PubMed] [Google Scholar]
- 11.Akeyson EW, McCutcheon IE. Single-stage posterior vertebrectomy and replacement combined with posterior instrumentation for spinal metastasis. J Neurosurg. 1996;85:211–20. doi: 10.3171/jns.1996.85.2.0211. [DOI] [PubMed] [Google Scholar]
- 12.Kumar N, Patel R, Wadhwa AC, et al. Basic concepts in metal work failure after metastatic spine tumour surgery. Eur Spine J. 2018;27:806–14. doi: 10.1007/s00586-017-5405-z. [DOI] [PubMed] [Google Scholar]
- 13.Yee TJ, Saadeh YS, Strong MJ, et al. Survival, fusion, and hardware failure after surgery for spinal metastatic disease. J Neurosurg Spine. 2021;34:665–72. doi: 10.3171/2020.8.SPINE201166. [DOI] [PubMed] [Google Scholar]
- 14.Alamanda VK, Robinson MM, Kneisl JS, et al. Survival outcomes and factors associated with revision surgery for metastatic disease of the spine. J Oncol. 2018;2018:6140381. doi: 10.1155/2018/6140381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amankulor NM, Xu R, Iorgulescu JB, et al. The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J. 2014;14:1850–9. doi: 10.1016/j.spinee.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 16.De Ruiter GC, Lobatto DJ, Wolfs JF, et al. Reconstruction with expandable cages after single- and multilevel corpectomies for spinal metastases: a prospective case series of 60 patients. Spine J. 2014;14:2085–93. doi: 10.1016/j.spinee.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Longo M, De la Garza Ramos R, Gelfand Y, et al. Incidence and predictors of hardware failure after instrumentation for spine metastasis: a single-institutional series. World Neurosurg. 2019;125:e1170–5. doi: 10.1016/j.wneu.2019.01.272. [DOI] [PubMed] [Google Scholar]
- 18.Pedreira R, Abu-Bonsrah N, Karim Ahmed A, et al. Hardware failure in patients with metastatic cancer to the spine. J Clin Neurosci. 2017;45:166–71. doi: 10.1016/j.jocn.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 19.Kumar N, Ramos MRD, Patel R, et al. The “spinal metastasis invasiveness index”: a novel scoring system to assess surgical invasiveness. Spine (Phila Pa 1976) 2021;46:478–85. doi: 10.1097/BRS.0000000000003823. [DOI] [PubMed] [Google Scholar]
- 20.Mirza SK, Deyo RA, Heagerty PJ, et al. Development of an index to characterize the “invasiveness” of spine surgery: validation by comparison to blood loss and operative time. Spine (Phila Pa 1976) 2008;33:2651–61. doi: 10.1097/BRS.0b013e31818dad07. [DOI] [PubMed] [Google Scholar]
- 21.Kebaish KM. Sacropelvic fixation: techniques and complications. Spine (Phila Pa 1976) 2010;35:2245–51. doi: 10.1097/BRS.0b013e3181f5cfae. [DOI] [PubMed] [Google Scholar]
- 22.El Dafrawy MH, Raad M, Okafor L, et al. Sacropelvic fixation: a comprehensive review. Spine Deform. 2019;7:509–16. doi: 10.1016/j.jspd.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Kim YJ, Bridwell KH, Lenke LG, et al. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine (Phila Pa 1976) 2006;31:2329–36. doi: 10.1097/01.brs.0000238968.82799.d9. [DOI] [PubMed] [Google Scholar]
- 24.Barton C, Noshchenko A, Patel V, et al. Risk factors for rod fracture after posterior correction of adult spinal deformity with osteotomy: a retrospective case-series. Scoliosis. 2015;10:30. doi: 10.1186/s13013-015-0056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mushlin HM, Shea P, Brooks DM, et al. The effect of sacroiliac fusion and pelvic fixation on rod strain in thoracolumbar fusion constructs: a biomechanical investigation. Spine (Phila Pa 1976) 2021;46:E769–75. doi: 10.1097/BRS.0000000000003911. [DOI] [PubMed] [Google Scholar]
- 26.Zaborovskii N, Ptashnikov D, Mikhaylov D, et al. Spinal deformity in elderly patients: comparison of two distal termination sites of lumbar curve fusion. Eur J Orthop Surg Traumatol. 2017;27:73–8. doi: 10.1007/s00590-016-1858-8. [DOI] [PubMed] [Google Scholar]
- 27.Nanda A, Manghwani J, Kluger PJ. Sacropelvic fixation techniques - current update. J Clin Orthop Trauma. 2020;11:853–62. doi: 10.1016/j.jcot.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuman BJ, Ailon T, Scheer JK, et al. Development and validation of a novel adult spinal deformity surgical invasiveness score: analysis of 464 patients. Neurosurgery. 2018;82:847–53. doi: 10.1093/neuros/nyx303. [DOI] [PubMed] [Google Scholar]
- 29.Passias PG, Horn SR, Soroceanu A, et al. Development of a novel cervical deformity surgical invasiveness index. Spine (Phila Pa 1976) 2020;45:116–23. doi: 10.1097/BRS.0000000000003175. [DOI] [PubMed] [Google Scholar]
- 30.Nuttall GA, Horlocker TT, Santrach PJ, et al. Predictors of blood transfusions in spinal instrumentation and fusion surgery. Spine (Phila Pa 1976) 2000;25:596–601. doi: 10.1097/00007632-200003010-00010. [DOI] [PubMed] [Google Scholar]
- 31.Donovan DJ, Huynh TV, Purdom EB, et al. Osteoradionecrosis of the cervical spine resulting from radiotherapy for primary head and neck malignancies: operative and nonoperative management. Case report. J Neurosurg Spine. 2005;3:159–64. doi: 10.3171/spi.2005.3.2.0159. [DOI] [PubMed] [Google Scholar]
- 32.Mesfin A, Sciubba DM, Dea N, et al. Changing the adverse event profile in metastatic spine surgery: an evidence-based approach to target wound complications and instrumentation failure. Spine (Phila Pa 1976) 2016;41 Suppl 20:S262–70. doi: 10.1097/BRS.0000000000001817. [DOI] [PubMed] [Google Scholar]
- 33.Patil CG, Patil TS, Lad SP, et al. Complications and outcomes after spinal cord tumor resection in the United States from 1993 to 2002. Spinal Cord. 2008;46:375–9. doi: 10.1038/sj.sc.3102155. [DOI] [PubMed] [Google Scholar]