Abstract
Objective
To determine whether continuous positive airway pressure (CPAP) adherence reduces health care–related costs or use in patients with obstructive sleep apnea (OSA) and comorbid cardiovascular disease (CVD).
Patients
A total of 23 million patients with CVD were identified in the Medicare fee-for-service database. Of the 65,198 who completed a sleep study between January 2016 and September 2018, 55,125 were diagnosed as having OSA and 1758 were identified in the 5% Medicare durable medical equipment (DME) database.
Methods
Patients with DME claims were categorized as adherent (AD, treatment evidenced ≥91 days after CPAP initiation; n=614) or nonadherent (nAD, n=242) to CPAP therapy. In addition, 9881 individuals with CVD who were not diagnosed as having OSA after sleep testing and without CPAP initiation were included as control patients. Propensity score matching balanced the groups for age, sex, and comorbidities (eg, diabetes mellitus), resulting in 241 participants per cohort. Dependent variables included total episode-of-care, inpatient, outpatient, skilled nursing, home health, and DME costs across 12 months.
Results
Total episode-of-care costs of AD participants ($6825) were lower than those of nAD ($11,312; P<.05) and control ($8102) participants. This difference (Δ) was attributable to fewer outpatient expenses (Δ$2290; P<.05) relative to the nAD group and fewer inpatient expenses (Δ$745) relative to the control group because skilled nursing costs were comparable between groups (P=.73).
Conclusion
Adherence to CPAP treatment reduces annual health care–related expenses by 40% in Medicare patients with CVD and OSA.
Abbreviations and Acronyms: AD, patients adherent to continuous positive airway pressure treatment; CAD, coronary artery disease; CPAP, continuous positive airway pressure; CVD, cardiovascular disease; DME, durable medical equipment; EOC, episode of care; FFS, fee-for-service; HSAT, in-home sleep apnea test; ICD-10, International Statistical Classification of Diseases, Tenth Revision; LOS, length of stay; MA, Medicare Advantage; nAD, patients nonadherent to continuous positive airway pressure treatment; OSA, obstructive sleep apnea; PSG, polysomnography; PSM, propensity score matching
Obstructive sleep apnea (OSA) is among the fastest-growing chronic conditions in the United States,1 with rates increasing consistently for the past 30 years.2 Although approximately 12% of American adults have OSA, it is estimated that 80% with OSA remain undiagnosed.3 The likelihood of developing OSA increases with well-established, nonmodifiable cardiovascular disease (CVD) risk factors, such as age,2 sex (male),4 and body mass,5 in addition to genetic predispositions.6 Moreover, OSA is 3 times more prevalent in patients with CVD7 and is commonly diagnosed concomitant with hypertension,8 type 2 diabetes mellitus,9 heart failure,10 atrial fibrillation,11 and stroke.12 Beyond clinical concerns, untreated OSA is associated with significant costs13 and, overall, the condition places significant financial strain on the health care system.14 More specifically, $150 billion in health care–related expenses have been attributed to OSA, including cost of care, workplace incidents, and automotive accidents, with expenses increasing $30 billion more once CVD-related comorbidities are considered.3 Importantly, it is estimated that $100 billion of this cost could be saved with timely diagnosis and adequate treatment.3
The deleterious effects of OSA are attributed to several mechanisms, namely, hypoxia-normoxia cycling15; as such, airway patency is the principle therapeutic target. Continuous positive airway pressure (CPAP), considered the gold standard treatment for OSA, prevents nocturnal hypoxemia and provides beneficial effects on various cardiometabolic outcomes, including blood pressure,16 insulin sensitivity,17 inflammation,18 oxidative stress,18 and vascular health.18 In addition, we recently reported that older adults who adhere to CPAP treatment have reduced cardiovascular19 and stroke20 risk, which translates into reduced inpatient health care use among Medicare beneficiaries with comorbid OSA and CVD21 and overall positive economic benefits among patients with OSA.22 However, to date, there have been no studies investigating the impact of CPAP adherence on health care costs among patients with high-cost comorbidities (eg, combined OSA and CVD). Tangent to this, numerous reports have found that less than 50% of patients with OSA adhere to CPAP treatment,18,23,24 although this percentage is fluid per the adherence criterion.25 Nevertheless, poor adherence negates the benefits of CPAP18,26 and is associated with a near 5-fold increase in preventable motor vehicle crashes.27 Furthermore, low adherence to CPAP likely adds further economic strain to the health care system, although this is understudied. Therefore, the principle aim of this study was to evaluate potential economic benefits associated with CPAP use in patients with OSA and CVD. The secondary aim was to determine whether health care–related expenses differed in patients adherent to CPAP treatment compared with nonadherent patients during the initial 12 months of treatment.
Methods
Study Design
We retrospectively analyzed 2 data sets. The first data set included 100% of patients in the Medicare fee-for-service (FFS) program (38 million members) using the Medicare Standard Analytical Files from January 1, 2016, through September 30, 2019. Because CPAP use data are not available from the Medicare FFS program, a second data set representing a random 5% of Medicare patients with durable medical equipment (DME) files was searched from January 1, 2017, through December 31, 2018. Different data retrieval windows were used to exclude patients using CPAP before entering the Medicare database, ensuring that the inclusion and exclusion criteria were met while allowing all patients to be followed longitudinally across a 12-month tracking period.
Participant Identification
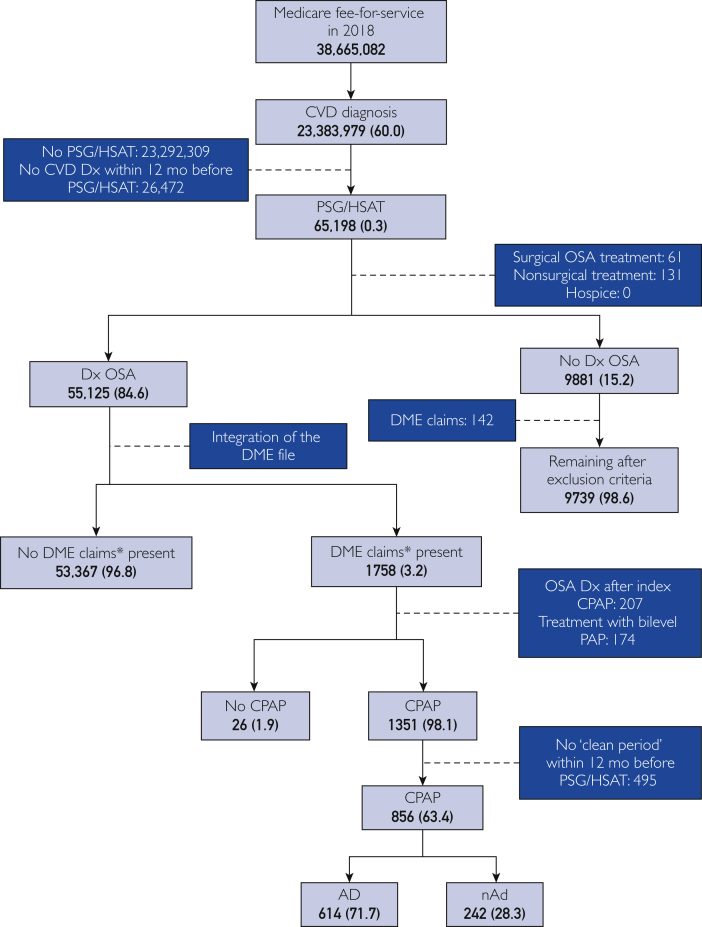
The patient selection process is shown in Figure 1. Because these analyses were conducted retrospectively on administrative claims data, and identifiable health care information was not used, neither institutional review board approval nor informed consent was required per 45 CFR 46.102.28 Potential participants were identified via International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes as having atrial fibrillation, heart failure, stroke, and/or hypertension up to 12 months before completing overnight polysomnography (PSG) or an at-home sleep apnea test (HSAT, identified via Current Procedural Terminology codes) from January 1, 2016, through September 31, 2018. Patients diagnosed as having OSA (per ICD-10 diagnosis codes) in the 12 months after the identified PSG or HSAT were matched to a DME claims file (∼5% of sampled patients), representative of January 1, 2017, through December 31, 2018, to determine presence of CPAP claims (eg, CPAP device, supplies) using the Healthcare Common Procedure Coding System, similar to our previous work.19,20,29 All codes used are reported in the Supplemental Table, available online at http://www.mayoclinicproceedings.org).
Figure 1.
Flow diagram of participant selection. Data are shown as number (% of next highest section). Dashed lines indicate justification for exclusion; solid lines indicate remaining patients. AD = patients adherent to CPAP treatment; CPAP = continuous positive airway pressure; CVD = cardiovascular disease; DME = durable medical equipment; Dx = diagnosis; HSAT = at-home sleep apnea test; nAD = patients nonadherent to CPAP treatment; OSA = obstructive sleep apnea; PSG = polysomnography. ∗Patients identified in the DME file were considered to have DME claims present.
The study population was segmented based on CPAP use. Patients were considered adherent if, per DME data, there was evidence of CPAP treatment or related supply fills on or after day 91 of CPAP initiation, similar to our previous work.13,29 Three cohorts were derived for comparison: those with CVD and OSA who were adherent to CPAP treatment (AD), those with CVD and OSA who were nonadherent (nAD), and those with CVD but not diagnosed as having OSA after PST/HSAT (control). Patients with potentially confounding factors were excluded, as were those without qualifying CVD within the 12 months preceding PSG/HSAT. Additional exclusion criteria included diagnosis of OSA after CPAP initiation and an OSA diagnosis within the 12 months before PSG/HSAT assessment. To focus exclusively on CPAP, patients who received surgical or nonsurgical alternative (eg, bilevel positive airway pressure, mandibular advancement device) treatments for OSA were removed from analysis, as were patients who received hospice care within the study period. After applying all the inclusion and exclusion criteria, 614 AD, 242 nAD, and 9739 control participants were identified.
Propensity Score Matching
We conducted Pearson χ2 and Fisher exact tests to examine baseline differences in demographic and clinical characteristics. The propensity score match (PSM) was implemented to ensure a more balanced sample among the 3 comparators. The AD were matched to nAD at a ratio of 1:1. Then, the resulting matched AD cohort was matched to patients in the unmatched control cohort at a ratio of 1:1. The following criteria were used in the PSM: age, sex, race, and high-cost comorbidities (coronary artery disease [CAD], chronic obstructive pulmonary disease, diabetes, oncologic disorders, obesity, and smoking). High-cost comorbidities represent the presence of each condition within the 30 days before indexing. After PSM, each cohort consisted of 241 patients balanced across all matching criteria (P=.07 to >.99) (Table 1).
Table 1.
| Characteristic | AD (n=241) | nAD (n=241) | Controlc (n=241) |
P value |
|
|---|---|---|---|---|---|
| AD vs nAD | AD vs control | ||||
| Age group | |||||
| ≤65 y | 41 (17.01) | 41 (17.01) | 40 (16.60) | ||
| 65-69 y | 79 (32.78) | 81 (33.61) | 80 (33.20) | ||
| 70-74 y | 62 (25.73) | 61 (25.31) | 63 (26.14) | .99 | .52 |
| 75-84 y | 50 (20.75) | 49 (20.33) | 55 (22.82) | ||
| ≥85 y | NR | NR | NR | ||
| Sex | |||||
| Male | 124 (51.45) | 130 (53.94) | 124 (51.45) | .65 | >.99 |
| Female | 117 (48.55) | 111 (46.06) | 117 (48.55) | ||
| Race | |||||
| Black | 13 (5.39) | 12 (4.98) | 14 (5.81) | .93 | .99 |
| White | 217 (90.04) | 219 (90.87) | 216 (89.63) | ||
| Otherd | 11 (4.57) | NR | 11 (4.57) | ||
| Comorbidities | |||||
| CAD | 13 (5.39) | 12 (4.98) | 11 (4.56) | >.99 | .84 |
| Diabetes | 15 (6.22) | 16 (6.64) | 14 (5.81) | >.99 | >.99 |
| Obesity | NR | 13 (5.39) | 13 (5.39) | .67 | .67 |
| COPD | NR | NR | NR | >.99 | >.99 |
| Oncologic disorders | 0 | NR | 0 | NR | NR |
| Smoking | NR | NR | NR | >.99 | NR |
| Cardiovascular conditions | |||||
| Atrial fibrillation | 50 (20.74) | 62 (25.72) | 58 (24.06) | .24 | .45 |
| Heart failure | 61 (25.31) | 80 (33.19) | 60 (24.90) | .07 | .07 |
| Hypertension | 230 (95.44) | 235 (97.51) | 228 (94.61) | .32 | .84 |
| Stroke | NR | NR | NR | >.99 | >.99 |
AD = patients with cardiovascular disease (CVD) and obstructive sleep apnea (OSA) who are adherent to continuous positive airway pressure (CPAP) therapy; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; nAD, patients with CVD and OSA who are not adherent to CPAP therapy; NR = not reported (sample size not adequate [n<11] to be reported from the Medicare data set).
Data are shown as number (percentage). Because patients may have had more than one of the cardiovascular conditions, cardiovascular indications do not sum to 100%.
Patients with CVD but without OSA.
Other race includes individuals of Hispanic and unknown descent.
Outcome Variables
We examined the total cost of inpatient, outpatient, skilled nursing, and home health services in the 12 months after either CPAP initiation (AD and nAD) or PSG/HSAT (control), similar to other cohort cost analyses of Medicare populations.30, 31, 32 Annual CPAP costs were estimated based on a typical patient’s CPAP machine and supply needs and were adjusted for inflation. Episode-of-care (EOC) cost was calculated as the sum of all costs associated with the previously mentioned health care–related services. Costs reported herein represent Medicare reimbursement. In addition, the use of each service setting and the length of stay (LOS) of inpatient services were assessed.
Data Analysis
Data are presented as mean cost difference (Δ), percentage use, or percentage use difference (Δ) where appropriate. Demographic data were compared between groups using a Pearson χ2 or Fisher exact test. Kruskal-Wallis 1-way analysis of variance was used to compare cost outcomes, including EOC, LOS, and health care service use across groups. Use outcomes were also analyzed using Pearson χ2 and Fisher exact tests when appropriate. In addition, an outlier analysis (Zk test statistic) was performed to evaluate the impact of aberrant EOC costs on cohort differences.30, 31, 32 All statistical analyses were performed using a statistical software program (SAS Enterprise Guide, Version 7.1; SAS Institute Inc), with significance set a priori at α<0.05.
Results
Participants
Of the 38 million Medicare FFS patients, more than 23 million had a history of atrial fibrillation, heart failure, stroke, and/or hypertension. Of these patients, only 65,198 had a unique PSG or HSAT from January 1, 2016, through September 30, 2018. Patients diagnosed as having OSA after PSG/HSAT (n=55,125) were then matched to Medicare’s 5% DME database to identify CPAP device and supply claims. Of the 1758 patients matched to the DME database, 856 met all the criteria for selection, of which 614 were AD and 242 were nAD (Figure 1). In addition, 9739 of the 65,198 patients who underwent PSG or HSAT met all the criteria for selection in the control group (PSG/HSAT, no OSA diagnosis, and no CPAP initiation). After implementation of PSM, 241 patients remained per cohort (Table 1). Almost one-third of patients were aged 65 to 70 years, and one-fifth were younger than 65 years, with nearly all being White. Approximately 5% of patients per cohort had CAD, with similar prevalence rates of diabetes (6%) and obesity (5%). After PSM, there were no statistically significant differences between groups.
Health Care Costs
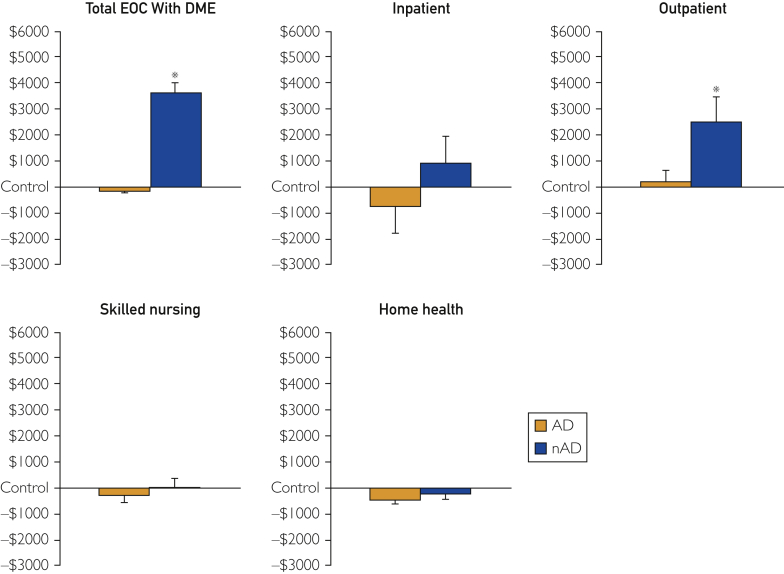
The EOC costs per cohort and place of service are shown in Table 2, with costs relative to control participants illustrated in Figure 2. On average, costs were $4487 lower in the AD cohort relative to the nAD cohort (P=.01), with significantly similar costs as controls (Δ$1277; P=.36). The attenuated costs observed in AD were largely attributable to $2290 less outpatient expenses than nAD (P=.02). Although inpatient costs seemed lower in AD relative to nAD (Δ$1659) and controls (Δ$745), there were no between-group differences (Kruskal-Wallace P=.09). Skilled nursing costs were statistically similar across groups (Kruskal-Wallace P=.73) but tended to be lower in AD relative to nAD (Δ$301) and controls (Δ$277). Home health–related expenses were also lower in AD relative to controls (Δ$461; P=.01), although, statistically, AD and nAD did not differ (Δ$237; P=.16). In addition, the estimated 12-month cost of DME expenses, based on typical costs associated with CPAP use, was $715 greater among AD than nAD ($1115 vs $400, respectively).
Table 2.
| Facility | AD (n=241) | nAD (n=241) | Controlc (n=241) |
|---|---|---|---|
| Total cost | 6825 (4940-8711) | 11,312 (8554-14,067)de | 8102 (6108-10,096) |
| Inpatient | 3441 (2029-4852) | 5100 (3641-6559) | 4185 (2759-5611) |
| Outpatient | 2857 (2131-3582) | 5146 (3309-6984)de | 2651 (2184-3118) |
| Skilled nursing | 328 (86-570) | 629 (142-1116) | 605 (102-1107) |
| Home health | 200 (77-323)e | 437 (128-745) | 661 (399-923) |
| Total cost with DMEdf | 7940 (6055-9826) | 11,712 (8954-14,470) | 8102 (6108-10,096) |
AD = patients with cardiovascular disease (CVD) and obstructive sleep apnea (OSA) who are adherent to continuous positive airway pressure (CPAP) therapy; DME = durable medical equipment; nAD = patients with CVD and OSA who are not adherent to CPAP therapy.
Group data are presented in dollars as mean (95% CI). Because controls were not diagnosed as having OSA and did not receive CPAP treatment they have no DME costs.
Patients with CVD but without OSA.
P<.05 vs AD.
P<.05 vs control.
Includes the cost of DME for CPAP therapy, estimated based on the adjusted annual cost of the CPAP machine and supplies, in addition to the cost of each of the 4 facility settings.
Figure 2.
Health care costs in patients with obstructive sleep apnea and cardiovascular disease who were adherent (AD) and nonadherent (nAD) to continuous positive airway pressure treatment relative to patients with cardiovascular disease but not obstructive sleep apnea (control group). ∗P<.05 vs AD. DME = durable medical equipment; EOC = episode of care.
Use of Health Care Network
Table 3 reports patient use rates for each place of service. No between-group differences were observed for inpatient admissions (Kruskal-Wallace P=.16); however, AD tended to report 27% lower use than nAD (P=.07) and 14% lower use than controls (P=.44). Average LOS tended to be 13% and 32% lower in AD compared with nAD and controls, respectively (Kruskal-Wallace P=.05). Skilled nursing facility use was similar between cohorts (Kruskal-Wallace P=.73). However, outpatient facility use was 4% less by AD and 11% less by nAD relative to controls, with AD using these facilities 7% more than nAD (P<.0001-.01). Whereas use of home health services was similar between AD and nAD (Δ21%, P=.59), controls used these services 53% more often than AD (P=.01) and tended to use them more than nAD (Δ41%; P=.08).
Table 3.
| Facility | AD (n=241) | nAD (n=241) | Controlc (n=241) |
|---|---|---|---|
| Inpatient | 19.9 | 27.4 | 23.2 |
| LOS, mean (95% CI) | 5.42 (3.56-7.27) | 6.26 (4.89-7.63) | 7.98 (5.52-10.44) |
| Outpatient | 95.9 | 89.2de | 100.0d |
| Skilled nursing | NR | 4.6 | 4.6 |
| Home health | 6.2e | 7.9 | 13.3 |
AD = patients with cardiovascular disease (CVD) and obstructive sleep apnea (OSA) who are adherent to continuous positive airway pressure (CPAP) therapy; LOS = length of stay; nAD = patients with CVD and OSA who are not adherent to CPAP therapy; NR = not reported (sample size not adequate [n<11] to be reported from the Medicare data set).
Group data are reported as the percentage of patients using these facilities per cohort except where noted otherwise.
Patients with cardiovascular disease but without obstructive sleep apnea.
P<.05 vs AD.
P<.05 vs control.
Discussion
The present retrospective data suggest that in patients with CVD and OSA, adherence to CPAP treatment reduces annual health care expenses by $4486 (Figure 2). In addition, after estimating annual cost of CPAP machines and supplies, the total EOC cost associated with AD continued to be lower ($3771) than that for nAD. Despite not achieving statistical significance, inpatient costs ($1659) and use (Δ27%) were lower in AD relative to nAD, which likely contributed to the principal findings in Figure 2. This suggests that the increase in routine outpatient management of AD resulted in better health management and fewer experiences of serious health events, including hospital admissions and lower outpatient service costs compared with nAD. Importantly, PSM ensured that the prevalence of high-cost comorbidities (eg, CAD and diabetes) were similar among AD, nAD, and controls, minimizing the influence of aberrant health care expenses. In sum, the present data indicate that patients with OSA and CVD have significantly reduced health care–related expenses when adherent to CPAP treatment.
In addition to establishing the cost-benefit of CPAP adherence, the present study highlights the continuing underdiagnosis of OSA in the United States,33 particularly in patients with CVD. In the present study population, 23 million of the 38 million Medicare patients (∼61%) were diagnosed as having at least 1 of 4 cardiovascular conditions associated with OSA.7 Despite this population being at high risk for OSA, only 65,198 patients with CVD received a PSG/HSAT (0.3%). This finding suggests that patients may not be tested or routinely assessed for OSA or OSA comorbidities at routine care visits or are unable to pay for or access sites to undergo PSG/HSAT or that additional barriers to care exist.3 In addition, of the patients who completed a PSG/HSAT, 85% had an OSA diagnosis. The high rate of OSA among patients with CVD underscores the fact that with or without screening, the prevalence of OSA is high among patients with CVD, indicating the need for OSA screening to become a regular part of cardiovascular care pathways. Furthermore, the cost-savings associated with CPAP adherence observed in the present study echoes the critical need for timely OSA diagnosis and treatment adherence to reduce economic costs and health care use.
Frequently, patients with OSA are diagnosed as having diseases such as hypertension8 and type 2 diabetes mellitus.9 To this point, the Medicare FFS database is uniquely equipped to study OSA because more than 80% of patients in this population have multiple chronic conditions, with hypertension being the most common in both men and women.33 Along these lines, Buttorff et al33 reported that visits to the emergency department as well as inpatient and outpatient settings parallel the number of chronic conditions. Similarly, the amount of prescription medication also follows this trend where patients with 5 or more chronic conditions fill twice as many prescriptions as those with 3 co-diagnoses.33 It is, therefore, evident that annual health care spending increases dramatically as more chronic conditions are diagnosed, underscoring the importance of effectively treating conditions such as OSA, which facilitates the manifestation of other pathologies. Although studies observed that less than 50% of patients prescribed CPAP are adherent,18,23 when adherence is achieved, patients experience a reduction in blood pressure16 and improved insulin sensitivity,34 which co-contribute to the significant cost-savings illustrated in Figure 2. When these results are considered with the approximate $150 billion strain OSA places on the health care network,3 placing greater emphasis on diagnosing patients as having OSA and achieving CPAP adherence would significantly reduce health care–associated financial strain.
Although the data in Table 2 and Figure 2 are outside the scope of this study, they could indicate that nonadherence to CPAP therapy increases more costly outpatient services, or that patients adherent to treatment used preventive services to a greater extent. This notion is supported by work from Truong et al35 who found that AD were more than 3.5 times more likely to be readmitted to the hospital within 30 days relative to nAD. Similarly, Hoffman et al36 reported that truck drivers who used their CPAP device more than 4 hours per night had a 33% reduction in health care–related expenses across 2 years compared with a non-OSA control cohort. In addition, AD in their study also missed fewer workdays, augmenting the financial benefits of treatment adherence. Collectively, the financial benefits of CPAP adherence extend beyond reductions in health care–related expenses.
Furthermore, the present study’s findings reaffirm that adherence to CPAP therapy effectively generates cost-savings among Medicare populations with cardiovascular conditions.36 A recent study found that approximately 36% of Medicare beneficiaries were enrolled in commercial insurer Medicare Advantage (MA) plans, nearly 3 times as many as reported in 200537; thus, these findings provide meaningful insight for payers and providers treating the growing MA population. Although there are many similarities between the Medicare (FFS) and MA populations, the latter have lower or comparable rates of chronic conditions, including hypertension, diabetes, and kidney disease, which increase health care costs despite similar average annual costs. With the number of MA enrollees expected to rise, and the similarity of costs and chronic conditions with Medicare FFS, commercial plans should inform their MA coverage decisions with clinical and economic findings of Medicare populations, especially when the plan has a significant MA enrollee population.37,38
We recognize that aspects of this study limit interpretation and generalizability of the data. Principally, CPAP adherence was indirectly assessed by tracking of DME files; although recorded device use would have been more robust, these data were not collected, making retrospective comparisons impossible. However, we confirmed the appropriateness of the 90-day adherence definition with a DME vendor in consultation with the official Centers for Medicare and Medicaid Services guidelines.13,29 Furthermore, the analyzed Medicare FFS DME database represents approximately 5% of the Medicare population, limiting population-scale generalizability; however, studying a sample of this size is commonplace in cost-analysis studies of Medicare enrollees.30, 31, 32,39, 40, 41, 42 Moreover, due to DME data availability, a full 12 months of tracking of post-CPAP initiation DME costs was not available; therefore, a standardized cost estimate of DME, based on previous research, was used to evaluate the total EOC cost inclusive of DME costs. In addition, patients whose PSG/HSAT OSA diagnosis occurred in 2016 who received CPAP treatment were not included in the final study population due to DME data availability being limited to 2017 and 2018. Because this study’s control cohort was defined by the lack of OSA diagnosis after PSG/HSAT, patients with other sleep disorders may have been included during analysis and may have influenced the findings. In addition, 62% of patients included in the present study had essential hypertension as their inclusion CVD diagnosis; thus, the present conclusions may consequently not represent the full breadth of all cardiovascular pathologies. Nearly all patients studied were White (Table 1), which may have introduced bias into the data set because racial minorities have less access to the health care system.43,44 Nearly one-fifth of patients were younger than 65 years (Table 1), which may also have biased the data and limits generalizability of these findings because Medicare patients younger than 65 years have more cognitive/mental impairments and poorer health status, increasing expenses compared with those older than 65 years.45 Last, the present study did not require continuous Medicare coverage; however, Medicare coverage is rarely disrupted. Nevertheless, the present data indicate that patients with OSA and CVD who are AD save more than $4480 relative to nAD largely attributable to reduced outpatient expenses (Figure 2).
Conclusion
The present retrospective cohort analysis examined the impact of CPAP adherence on health care expenses among patients with OSA and CVD and found that AD with OSA and CVD observed significant reductions in annual health care expenses compared with nAD. In addition, AD experienced significantly fewer inpatient admissions with shorter LOS and fewer outpatient treatments than nAD.
Footnotes
Grant Support: This work was supported by grant T32-HL007111 (J.M.B.) from the National Institutes of Health.
Potential Competing Interests: Dr Lerman is a consultant for Itamar Medical. Dr Wickwire has received research funding from the American Academy of Sleep Medicine Foundation, the U.S. Department of Defense, Merck, and ResMed; has served as a scientific consultant for DayZz, Eisai, Merck, and Purdue; and is an equity shareholder in WellTap. Dr Somers is a consultant for Baker Tilly, Respicardia, and Jazz Pharmaceuticals and is on the scientific advisory board for Sleep Number Corp. The other authors report no competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Senaratna C.V., Perret J.L., Lodge C.J., et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Peppard P.E., Young T., Barnet J.H., Palta M., Hagen E.W., Hla K.M. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine Hidden Health Crisis Costing America Billions: Underdiagnosing and Undertreating Obstructive Sleep Apnea Draining Healthcare System. https://aasm.org/resources/pdf/sleep-apnea-economic-crisis.pdf Published 2016.
- 4.Young T., Shahar E., Nieto F.J., et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 5.Newman A.B., Foster G., Givelber R., Nieto F.J., Redline S., Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165(20):2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 6.Lee W., Nagubadi S., Kryger M.H., Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2(3):349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seet E., Chung F. Management of sleep apnea in adults: functional algorithms for the perioperative period: continuing professional development. Can J Anaesth. 2010;57(9):849–864. doi: 10.1007/s12630-010-9344-y. [DOI] [PubMed] [Google Scholar]
- 8.Sawatari H., Chishaki A., Ando S.I. The epidemiology of sleep disordered breathing and hypertension in various populations. Curr Hypertens Rev. 2016;12(1):12–17. doi: 10.2174/1573402112666160114093307. [DOI] [PubMed] [Google Scholar]
- 9.Fallahi A., Jamil D.I., Karimi E.B., Baghi V., Gheshlagh R.G. Prevalence of obstructive sleep apnea in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Syndr. 2019;13(4):2463–2468. doi: 10.1016/j.dsx.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Lanfranchi P.A., Somers V.K. Sleep-disordered breathing in heart failure: characteristics and implications. Respir Physiol Neurobiol. 2003;136(2-3):153–165. doi: 10.1016/s1569-9048(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 11.Gami A.S., Pressman G., Caples S.M., et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 12.Somers V.K., White D.P., Amin R., et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Wickwire E.M., Tom S.E., Vadlamani A., et al. Older adult US Medicare beneficiaries with untreated obstructive sleep apnea are heavier users of health care than matched control patients. J Clin Sleep Med. 2020;16(1):81–89. doi: 10.5664/jcsm.8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knauert M., Naik S., Gillespie M.B., Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol Head Neck Surg. 2015;1(1):17–27. doi: 10.1016/j.wjorl.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Punjabi N.M. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schein A.S., Kerkhoff A.C., Coronel C.C., Plentz R.D., Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens. 2014;32(9):1762–1773. doi: 10.1097/HJH.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 17.Feng Y., Zhang Z., Dong Z.Z. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15005. doi: 10.1038/npjpcrm.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelic S., Padeletti M., Kawut S.M., et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117(17):2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wickwire E.M., Bailey M.D., Somers V.K., et al. CPAP adherence reduces cardiovascular risk among older adults with obstructive sleep apnea. Sleep Breath. 2021;25(3):1343–1350. doi: 10.1007/s11325-020-02239-2. [DOI] [PubMed] [Google Scholar]
- 20.Wickwire E.M., Bailey M.D., Somers V.K., et al. CPAP adherence is associated with reduced risk for stroke among older adult Medicare beneficiaries with obstructive sleep apnea. J Clin Sleep Med. 2021;17(6):1249–1255. doi: 10.5664/jcsm.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickwire E.M., Bailey M.D., Somers V.K., et al. CPAP adherence is associated with reduced inpatient use among older adult Medicare beneficiaries with pre-existing cardiovascular disease. J Clin Sleep Med. 2022;18(1):39–45. doi: 10.5664/jcsm.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickwire E.M., Albrecht J.S., Towe M.M., et al. The impact of treatments for OSA on monetized health economic outcomes: a systematic review. Chest. 2019;155(5):947–961. doi: 10.1016/j.chest.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Rotenberg B.W., Murariu D., Pang K.P. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45(1):43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickwire E.M., Lettieri C.J., Cairns A.A., Collop N.A. Maximizing positive airway pressure adherence in adults: a common-sense approach. Chest. 2013;144(2):680–693. doi: 10.1378/chest.12-2681. [DOI] [PubMed] [Google Scholar]
- 25.Cistulli P.A., Armitstead J., Pepin J.L., et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med. 2019;59:114–116. doi: 10.1016/j.sleep.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang M.C., Huang Y.C., Lan C.C., Wu Y.K., Huang K.F. Beneficial effects of long-term CPAP treatment on sleep quality and blood pressure in adherent subjects with obstructive sleep apnea. Respir Care. 2015;60(12):1810–1818. doi: 10.4187/respcare.04199. [DOI] [PubMed] [Google Scholar]
- 27.Burks S.V., Anderson J.E., Bombyk M., et al. Nonadherence with employer-mandated sleep apnea treatment and increased risk of serious truck crashes. Sleep. 2016;39(5):967–975. doi: 10.5665/sleep.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn P.A., Worsham C.M., Berland G. Characterization of prescription patterns and estimated costs for use of oxygen concentrators for home oxygen therapy in the US. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.29967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wickwire E.M., Jobe S.L., Oldstone L.M., Scharf S.M., Johnson A.M., Albrecht J.S. Lower socioeconomic status and co-morbid conditions are associated with reduced continuous positive airway pressure adherence among older adult medicare beneficiaries with obstructive sleep apnea. Sleep. 2020;43(12):zsaa122. doi: 10.1093/sleep/zsaa122. [DOI] [PubMed] [Google Scholar]
- 30.Glantz N.M., Duncan I., Ahmed T., et al. Racial and ethnic disparities in the burden and cost of diabetes for US Medicare beneficiaries. Health Equity. 2019;3(1):211–218. doi: 10.1089/heq.2019.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lokhandwala T., Bittoni M.A., Dann R.A., et al. Costs of diagnostic assessment for lung cancer: a Medicare claims analysis. Clin Lung Cancer. 2017;18(1):e27–e34. doi: 10.1016/j.cllc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Clark M.A., Arnold S.V., Duhay F.G., et al. Five-year clinical and economic outcomes among patients with medically managed severe aortic stenosis: results from a Medicare claims analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):697–704. doi: 10.1161/CIRCOUTCOMES.112.966002. [DOI] [PubMed] [Google Scholar]
- 33.Buttorff C., Ruder T., Bauman M. Rand Corp; 2017. Multiple Chronic Conditions in the United States. [Google Scholar]
- 34.Abud R., Salgueiro M., Drake L., Reyes T., Jorquera J., Labarca G. Efficacy of continuous positive airway pressure (CPAP) preventing type 2 diabetes mellitus in patients with obstructive sleep apnea hypopnea syndrome (OSAHS) and insulin resistance: a systematic review and meta-analysis. Sleep Med. 2019;62:14–21. doi: 10.1016/j.sleep.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Truong K.K., De Jardin R., Massoudi N., Hashemzadeh M., Jafari B. Nonadherence to CPAP associated with increased 30-day hospital readmissions. J Clin Sleep Med. 2018;14(2):183–189. doi: 10.5664/jcsm.6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffman B., Wingenbach D.D., Kagey A.N., Schaneman J.L., Kasper D. The long-term health plan and disability cost benefit of obstructive sleep apnea treatment in a commercial motor vehicle driver population. J Occup Environ Med. 2010;52(5):473–477. doi: 10.1097/JOM.0b013e3181dbc8ab. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser Family Foundation . Kaiser Family Foundation; April 22, 2020. A Dozen Facts About Medicare Advantage in 2020. [Google Scholar]
- 38.Creighton S. Medicare Advantage achieves cost-effective care and better outcomes for beneficiaries with chronic conditions relative to fee-for-service Medicare. https://www.risehealth.org/insights-articles/medicare-advantage-achieves-cost-effective-care-and-better-outcomes-for-beneficiaries-with-chronic-conditions-relative-to-fee-for-service-medicare/ Published March 25, 2019.
- 39.Chhatre S., Chang Y.H.A., Gooneratne N.S., Kuna S., Strollo P., Jayadevappa R. Association between adherence to continuous positive airway pressure treatment and cost among medicare enrollees. Sleep. 2020;4343(1):zsz188. doi: 10.1093/sleep/zsz188. [DOI] [PubMed] [Google Scholar]
- 40.Delea T.E., Vera-Llonch M., Edelsberg J.S., et al. The incidence and cost of hospitalization for 5-FU toxicity among Medicare beneficiaries with metastatic colorectal cancer. Value Health. 2002;5(1):35–43. doi: 10.1046/j.1524-4733.2002.51083.x. [DOI] [PubMed] [Google Scholar]
- 41.Sivakanthan S., Van Gompel J.J., Alikhani P., van Loveren H., Chen R., Agazzi S. Surgical management of trigeminal neuralgia: use and cost-effectiveness from an analysis of the Medicare Claims Database. Neurosurgery. 2014;75(3):220–226. doi: 10.1227/NEU.0000000000000430. [discussion 225-226] [DOI] [PubMed] [Google Scholar]
- 42.Adeyemi A., Delhougne G. Incidence and economic burden of intertrochanteric fracture: a Medicare claims database analysis. JB JS Open Access. 2019;4(1) doi: 10.2106/JBJS.OA.18.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stepanikova I., Oates G.R. Perceived discrimination and privilege in health care: the role of socioeconomic status and race. Am J Prev Med. 2017;52(1S1):S86–S94. doi: 10.1016/j.amepre.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheeler S.M., Bryant A.S. Racial and ethnic disparities in health and health care. Obstet Gynecol Clin North Am. 2017;44(1):1–11. doi: 10.1016/j.ogc.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Cubanski J., Neuman T., Damico A. Medicare’s role for people under age 65 with disabilities. The Henry J. Kaiser Family Foundation. https://files.kff.org/attachment/issue-brief-Medicares-Role-for-People-Under-Age-65-with-Disabilities Published August 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.