Abstract
Background
Recent studies have found that there are significant associations between body iron status and the development of diabetes. In the present study, we aimed to analyze the association among iron overload (IO), insulin resistance (IR), and diabetes in Chinese adults, and to explore the sex difference.
Methods
Men and women (age >19 years) who participated in the Chinese Health and Nutrition Survey and did not have diabetes at baseline were followed between 2009 and 2015 (n=5,779). Over a mean of 6 years, 75 participants were diagnosed with incident diabetes. Logistic regression was used to assess the risk factors associated with IO. Cox proportional hazard regression was used to estimate the risk of incident diabetes and to determine whether the risk differed among subgroups. Causal mediation analysis (CMA) was used to explore the mechanism linking IO and diabetes.
Results
According to sex-stratified multivariable-adjusted Cox proportional hazards regression, IO increased the risk of incident diabetes. Women with IO had a higher risk of diabetes than men. Subgroup analysis with respect to age showed that the association between IO and diabetes was stronger in older women and younger men (P<0.001). CMA showed that liver injury (alanine transaminase) and lipid metabolism abnormalities (triglyceride, apolipoprotein B) contributed to the association between IO and diabetes.
Conclusion
IO is associated with diabetes and this association is sex-specific. IO may indirectly induce IR via liver injury and lipid metabolism abnormalities, resulting in diabetes.
Keywords: Diabetes mellitus, Hepatic insufficiency, Insulin resistance, Iron overload, Lipid metabolism disorders, Sex characteristics
INTRODUCTION
Iron overload (IO) is usually an excess of iron in the body that cannot be eliminated by physiological mechanisms. IO usually manifests as high ferritin (FET) and transferrin (TRF) saturation. IO can cause serious problems (such as liver fibrosis and heart failure), but it is often overlooked because of a lack of specific symptoms and slow progression of the disease. Recent epidemiologic studies have shown a high incidence of IO in apparently healthy people, especially in the Asia-Pacific region and Africa, which does not have a genetic explanation [1-4]. For most patients diagnosed with IO, the treatment is relatively simple and clear; however, if IO is not treated, fatal organ toxicity will occur. Therefore, screening and diagnosis of IO is necessary in the general population.
Hereditary hemochromatosis was initially discovered to be associated with the development of diabetes [5]. Subsequently, some studies found that systemic iron status is also strongly associated with the development of multiple types of diabetes and their complications in the general population [4,6]. Numerous studies have also revealed that a much milder state of IO, owing to excessive dietary iron intake or a number of other factors, is also a risk factor for the development of type 2 diabetes mellitus and gestational diabetes [5,7,8]. Nevertheless, the complete pathological mechanisms are not fully understood.
Many experimental studies have been conducted to explore the possible effects and mechanisms of IO in diabetes. Potential explanations behind the association between IO and development of diabetes include: (1) excess iron can increase production of reactive oxygen species and liver steatosis; (2) IO can lead to abnormal metabolism of glucose and lipids; and (3) elevated iron levels can trigger β-cell compensation mechanisms and lead to functional failure [8-10]. However, some of these possible mechanisms have not been examined using human data.
Levels of circulating FET vary considerably depending on sex and menopause, suggesting that there may be a sex-dependent relationship between FET and diabetes risk; however, this remains controversial [11-13]. A cross-sectional study of the China Health and Nutrition Survey (CHNS) showed that elevated concentrations of FET were related to a higher risk of diabetes among men but not among women [14]. In another study, higher levels of FET were associated with a higher risk of type 2 diabetes mellitus in both sexes [15]. Menopause may also be an important factor in this association. It is necessary to explore sex differences and the influence of menopause on this association in a Chinese population.
In this study, we aimed to investigate the epidemiology of IO and risk factors for its occurrence in a general Chinese population. We also aimed to identify the association between IO and diabetes according to sex. Mediation analysis was used to explore the potential mediators involved in the association.
METHODS
Study population
The CHNS is an ongoing open-cohort study that was approved by the Ministry of Health and is administered by the University of North Carolina at Chapel Hill, the Chinese Center for Disease Control and Prevention (CCDC), and the China-Japan Friendship Hospital (CJFH). Fifteen provinces and municipal cities in China (Beijing, Chongqing, Guangxi, Guizhou, Heilongjiang, Henan, Hubei, Hunan, Jiangsu, Liaoning, Shanxi, Shandong, Shanghai, Yunnan, and Zhejiang) are participating in the survey, and these vary in their geography, economic development, availability of public resources, and population health status. A multistage, random cluster process was used to draw the samples surveyed in each of the provinces. The survey has been conducted in 10 waves up to now: in 1989, 1991, 1993, 1997, 2000, 2004, 2006, 2009, 2011, and 2015. The participants gave their written informed consent before being included. The survey uses a questionnaire to collect information regarding the community, family, personal, dietary, and other social economic and health factors. The investigators were well trained before the survey commenced and all the procedures strictly observed the protocol written by the CHNS project team from the CJFH, Ministry of Health [16].
In this study, we used the data of participants in three waves of the CHNS conducted between 2009 and 2015; only 2009 blood data were available. In the 2009 wave of the CHNS, 8,607 adult participants were recruited. We defined people of >19 years old as adult, according to the World Health Organization (WHO) age group definition [17]. Participants with a history of myocardial infarction (MI) or stroke were excluded. MI or stroke related to inflammation may have a higher FET lever and the cause is complicated, which may just be a phenomenon accompanying the disease and cannot properly reflect the iron storage in the body. On the other hand, people suffering from MI and stroke tend to change their lifestyle including diet and behavior [18]. We excluded participants with incomplete baseline data (physical examination, dietary assessment, fasting blood glucose, and behavioral status). We also excluded participants who had diabetes prior to the study and who had unknown diabetes statuses before or during the study. Ultimately, data from a total of 5,779 adults were analyzed.
This survey was approved by Institutional Review Boards at the University of North Carolina, Chapel Hill (Chapel Hill, NC), and the National Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention (Beijing, China), and each participant provided written informed consent. Further details of the study population, survey design, sample, data collection, and quality control procedures are available at http://content.digital.nhs.uk/npid and https://www.cpc.unc.edu/projects/china.
Physical examination and behavioral survey
Height and body mass were measured by trained investigators, following a standard protocol that is recommended by the WHO. Height was measured to the nearest 0.1 cm without shoes, and body mass was measured to the nearest 0.1 kg in lightweight clothing [19,20]. Body mass index (BMI) was calculated as body mass in kg divided by height in meters, squared. Systolic and diastolic blood pressures were measured three times and the mean values were analyzed. Smoking status and alcohol intake status were collected using a structured questionnaire and were categorized as yes or no in the analyses.
Dietary assessment
Three consecutive 24-hour records for individuals and a food inventory for each household were made over the same 3-day periods and were combined to determine dietary intake [20,21]. All the participants were interviewed to determine their food consumption both away from home and at home on a 24-hour recall basis. Household food was weighed and the change in the inventory from the start to the end of each day was calculated. The consumption of food according to individual and household data was compared for each individual, and if significant discrepancies were found, the household and individual measurements in question were repeated to resolve these discrepancies. The Chinese Food Composition Table was used for food composition analysis [22].
Fasting blood measurements
Participants were asked to fast for at least 8 hours overnight before venipuncture. Blood samples were collected into three tubes by certified technicians under strict quality control by the CJFH. The sample collected in one 4-mL ethylenediaminetetraacetic acid (EDTA) tube was used for routine analyses on site, a second sample in a 4-mL separation gel tube was used for biochemical tests in a local laboratory, and a third sample collected in the same type of tube was frozen and transported to the central laboratory of the CJFH in Beijing (medical laboratory accreditation certificate ISO 15189:2007) for further biochemical analyses, immunological testing, and storage.
Glycosylated hemoglobin (HbA1c) was measured in provincial laboratories; these met all the requirements for accurate measurement. Glucose concentration was analyzed using a Hitachi 7600 analyzer and glucose oxidase phenol 4-aminoantipyrine peroxidase kits (GOD-PAP, Randox, County Antrim, UK) [14]. The hemoglobin concentration in whole blood was measured using a Beckman Coulter LH750 (Beckman Coulter, Brea, CA, USA). Low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), and total cholesterol (TC) concentrations were measured enzymatically using a Hitachi 7600 automated analyzer (Kyowa, Japan) [19]. Total protein concentration was determined using the biuret method on a Hitachi 7600 automated analyzer (Randox). Apolipoprotein A (Apo-A), apolipoprotein B (Apo-B), and lipoprotein (a) concentrations were measured using the immunoturbidimetric method using a Hitachi 7600 automated analyzer. High-sensitivity C-reactive protein (HSCRP) concentrations was measured using the immunoturbidimetric method on a Hitachi 7600 automated analyzer (Denka Seiken Co. Ltd., Niigata, Japan) [23]. Alanine aminotransferase (ALT) activity and albumin concentration were measured using the International Federation of Clinical Chemistry (IFCC) enzyme and bromocresol green methods, respectively, on a Hitachi 7600 automated analyzer. Insulin (INS) and FET concentrations were analyzed radioimmunologically on a gamma counter (XH-6020, North Institute of Bio-Tech, Beijing, China) and TRF concentration was measured using the nephelometry method (B-type natriuretic peptide assay; Siemens, München, Germany). Only the results of blood tests in 2009 were available and were finally included in our analysis.
Definitions of variables and diseases
Iron deficiency (ID), anemia, and iron deficiency anemia (IDA) were defined in accordance with the WHO thresholds [24]. IDA was defined as the existence of both anemia and ID, and the threshold was adjusted for pregnancy status and altitude. IO was defined as a FET concentration >300 μg/L for adult men and >200 μg/L for adult women [25]. Insulin resistance (IR) was defined as a fasting INS concentration ≥12 mU/L [26,27]. The menopause was defined in this study as women of age equal to or greater than 52years old [28]. Diabetes was identified using the self-reported questionnaire with a tick box response to the question: “Have you ever been diagnosed with diabetes by a doctor?” (yes/no) [19].
Statistical analysis
Continuous variables are expressed as mean±standard deviation or median (interquartile range) and differences between participants with and without IO were identified using the unpaired t-test or the Kruskal-Wallis test. Categorical variables are expressed as percentages and data were compared using Fisher’s exact test.
We used binary logistic regression to explore the underlying variables at baseline that were associated with IO, and the data were adjusted for 13 independent variables including age, physical examination findings, and biological, behavioral, and dietary factors. In order to avoid high correlations between the variables, we have used variance inflation factor to test multicollinearity.
Cox proportional hazards models were used to calculate the hazard ratio (HR) for incident diabetes, comparing participants without (reference standard) to participants with IO. To avoid an effect of sex difference in diabetes prevalence, we decided a priori to stratify the models according to sex [29,30]. The formula ([β0]−[βn])/(β0)×100 was used to describe which groups of covariates were associated with a disparity in incident diabetes between the groups. We used multiple models to explore the relationships between each risk factor group and incident diabetes [29]. In model 1, only age was adjusted for, and in subsequent models, we added each risk factor group to the previous model in order, beginning with the biological factors (model 2) and ending with the behavioral factors (model 5). The final adjusted model (model 5) included all the risk factor groups. In a second modeling approach, we estimated the percentage reduction in the β estimates for each set of covariates from the base model, without adjusting for other sets of covariables. The proportional hazards assumption was tested, and no violations of this assumption were identified.
Causal mediation analysis (CMA) was used to determine the effect of IO on the development of diabetes and identify possible mediators of the relationship. ALT, TG, and TC were ln-transformed because of their skewed distributions. Subgroup analysis was conducted according to age and BMI group. We excluded some variables because of possible collinearity among some of the covariates. A two-tailed P value of <0.05 was considered to represent statistical significance. R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for all the analyses.
RESULTS
We excluded 203 participants who had MI and stroke and 1,403 participants who were unable to provide baseline data for at least one risk factor of interest. We also excluded 199 participants who had diabetes at baseline, 960 participants who were lost to follow-up at baseline, and 63 participants whose diabetes status was undetermined. In total, 5,779 participants were included in the analysis. A flow chart for the participants is shown in Supplementary Fig. 1. A comparison of the baseline characteristics of the included and excluded participants is presented as Supplementary Table 1.
Baseline characteristics of all the participants
Baseline characteristics of the 5,779 participants were classified on the basis of their iron status and sex, shown in Table 1. A total of 3,131 (54.18%) women and 2,648 (45.82%) men were included, with mean age 50.94 years. Of these participants, 20.76% (1,200) had abnormal iron status, with 9.22% (533) having ID and 11.54% (667) having IO, the numbers of participants with IO were 215 and 452 in women and men respectively. At the baseline examination, more men (20.58%) than women (7.37%) had IO and the mean age of women with IO was higher than that of men with IO. Although some of these indexes were still within the normal range, participants with IO tended to have higher BMI, HbA1c, HS-CRP, glucose, TG, TC, ALT, and Apo-B than those without IO (P<0.001). In addition, IR was more prevalent in participants with IO. Women with IO have shown the slightly elevated level of mean INS, and men have shown the normal level of mean INS. With respect to dietary factors, there were no differences in the macronutrient or energy intake between participants with and without IO. Behavioral factors did not significantly differ with respect to IO, except that men with IO drank more alcohol.
Table 1.
Participant characteristics at baseline (2009 to 2010), according to sex and the presence or absence of iron overload
| Characteristic | Women (n=3,131) |
Men (n=2,648) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-iron overload (n=2,916) | Iron overload (n=215) | P value | SMD | Non-iron overload (n=2,196) | Iron overload (n=452) | P value | SMD | ||
| Age, yr | 50.14±14.03 | 62.03±10.54 | <0.001 | 0.958 | 51.30±14.83 | 49.01±13.03 | 0.002 | 0.164 | |
| Physical examinations | |||||||||
| Height, cm | 155.52±6.37 | 153.73±6.40 | <0.001 | 0.280 | 166.38±6.68 | 166.78±6.65 | 0.241 | 0.061 | |
| Weight, kg | 56.54±9.54 | 57.81±10.02 | 0.061 | 0.129 | 63.77±11.18 | 68.60±11.13 | <0.001 | 0.433 | |
| BMI, kg/m2 | 23.34±3.46 | 24.37±3.46 | <0.001 | 0.299 | 22.96±3.32 | 24.60±3.31 | <0.001 | 0.493 | |
| Systolic pressure, mm Hg | 122.82±19.51 | 131.97±21.12 | <0.001 | 0.450 | 125.74±17.65 | 125.91±15.86 | 0.850 | 0.010 | |
| Diastolic pressure, mm Hg | 78.81±11.00 | 81.37±11.73 | 0.001 | 0.225 | 81.49±10.96 | 83.40±10.94 | 0.001 | 0.174 | |
| Biological | |||||||||
| Glucose, mmol/L | 5.08 (4.71–5.54) | 5.39 (5.01–6.17) | <0.001 | 0.457 | 5.09 (4.70–5.58) | 5.36 (4.89–5.89) | <0.001 | 0.307 | |
| HbA1c, mmol/L | 5.50 (5.20–5.80) | 5.70 (5.40–6.10) | <0.001 | 0.345 | 5.50 (5.20–5.80) | 5.60 (5.20–6.00) | <0.001 | 0.225 | |
| INS, μIU/mL | 10.45 (7.53–14.86) | 12.44 (8.49–18.73) | <0.001 | 0.102 | 10.05 (6.92–14.48) | 11.44 (7.75–16.65) | <0.001 | 0.056 | |
| IR | |||||||||
| No | 1,768 (60.6) | 106 (49.3) | 0.001 | 0.229 | 1,398 (63.7) | 242 (53.5) | <0.001 | 0.207 | |
| Yes | 1,148 (39.4) | 109 (50.7) | 798 (36.3) | 210 (46.5) | |||||
| Hemoglobin, g/L | 132.00 (122.00–140.00) | 133.00 (125.00–142.00) | 0.095 | 0.059 | 151.00 (141.00–162.00) | 155.00 (146.00–165.00) | <0.001 | 0.242 | |
| HS-CRP, mg/L | 1.00 (0.00–2.00) | 2.00 (1.00–4.00) | <0.001 | 0.174 | 1.00 (1.00–2.00) | 1.00 (1.00–3.00) | <0.001 | 0.115 | |
| FET, ng/mL | 46.38 (22.15–81.95) | 301.87 (235.48–499.30) | <0.001 | 2.217 | 105.24 (66.56–155.58) | 556.36 (416.61–787.13) | <0.001 | 2.628 | |
| TRF, mg/gL | 291.00 (259.00–329.00) | 274.00 (246.00–308.00) | <0.001 | 0.380 | 277.00 (246.00–311.00) | 274.00 (245.00–311.25) | 0.588 | 0.001 | |
| Apo-A, g/L | 1.12 (0.98–1.31) | 1.14 (0.96–1.36) | 0.549 | 0.048 | 1.09 (0.93–1.30) | 1.03 (0.88–1.23) | <0.001 | 0.181 | |
| Apo-B, g/L | 0.88 (0.73–1.08) | 1.06 (0.85–1.25) | <0.001 | 0.564 | 0.88 (0.72–1.05) | 0.96 (0.81–1.15) | <0.001 | 0.334 | |
| LDL-C, mmol/L | 2.94 (2.40–3.60) | 3.26 (2.60–4.14) | <0.001 | 0.302 | 2.88 (2.33–3.45) | 2.94 (2.26–3.65) | 0.300 | 0.053 | |
| HDL-C, mmol/L | 1.45 (1.24–1.70) | 1.39 (1.15–1.61) | 0.004 | 0.178 | 1.36 (1.15–1.62) | 1.23 (1.02–1.50) | <0.001 | 0.288 | |
| LP-A, mg/dL | 83.00 (44.00–179.00) | 93.00 (52.50–200.00) | 0.151 | 0.108 | 75.00 (38.00–161.25) | 64.00 (30.75–134.75) | 0.001 | 0.083 | |
| TG, mmol/L | 1.20 (0.83–1.76) | 1.70 (1.21–2.71) | <0.001 | 0.542 | 1.19 (0.81–1.86) | 1.84 (1.17–2.89) | <0.001 | 0.508 | |
| TC, mmol/L | 4.80 (4.18–5.52) | 5.41 (4.65–6.17) | <0.001 | 0.501 | 4.72 (4.16–5.32) | 4.95 (4.38–5.81) | <0.001 | 0.337 | |
| ALB, g/L | 46.90 (45.00–49.10) | 47.40 (45.55–49.75) | 0.011 | 0.183 | 47.40 (45.20–49.70) | 48.20 (46.00–50.40) | <0.001 | 0.197 | |
| ALT, U/L | 16.00 (12.00–23.00) | 22.00 (15.00–34.50) | <0.001 | 0.460 | 20.00 (15.00–28.00) | 27.00 (18.00–42.00) | <0.001 | 0.448 | |
| TP, g/L | 77.90 (74.70–81.30) | 77.80 (75.20–82.10) | 0.311 | 0.081 | 76.50 (73.10–79.80) | 76.30 (73.20–79.60) | 0.564 | 0.040 | |
| Diet | |||||||||
| Calorie, kcal | 1,994.50±605.06 | 1,935.67±586.26 | 0.168 | 0.099 | 2,366.86±691.93 | 2,357.75±678.95 | 0.798 | 0.013 | |
| Carbohydrate, g | 278.29±94.09 | 267.27±90.88 | 0.097 | 0.119 | 327.23±107.88 | 318.21±107.60 | 0.106 | 0.084 | |
| Fat, g | 70.05±38.54 | 68.72±35.48 | 0.626 | 0.036 | 80.58±39.30 | 81.97±42.67 | 0.500 | 0.034 | |
| Protein, g | 61.77±21.00 | 61.56±21.58 | 0.884 | 0.010 | 72.57±24.80 | 74.10±23.97 | 0.230 | 0.063 | |
| Behavioral | |||||||||
| Smoke | |||||||||
| No | 2,837 (97.3) | 204 (94.9) | 0.054 | 0.124 | 782 (35.6) | 175 (38.7) | 0.217 | 0.064 | |
| Yes | 79 (2.7) | 11 (5.1) | 1,414 (64.4) | 277 (61.3) | |||||
| Alcohol | |||||||||
| No | 2,662 (91.3) | 198 (92.1) | 0.802 | 0.029 | 868 (39.5) | 140 (31.0) | 0.001 | 0.180 | |
| Yes | 254 (8.7) | 17 (7.9) | 1,328 (60.5) | 312 (69.0) | |||||
Values are presented as mean±standard deviation, median (interquartile range), or number (%).
SMD, standard mean difference; HbA1c, glycosylated hemoglobin; INS, insulin; IR, insulin resistance; HS-CRP, high-sensitivity C-reactive protein; FET, ferritin; TRF, transferrin; Apo, apolipoprotein; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; LP-A, lipoprotein (a); TG, triglyceride; TC, total cholesterol; ALB, albumin; ALT, alanine aminotransferase; TP, total protein.
Relationships of biological, dietary, and behavioral factors with IO
We further explored baseline characteristics that were associated with IO using a logistic regression model. In this model, categorical data, including IR, smoking, and alcohol intake and continuous data including age, HS-CRP, TG, Apo-B, ALT, carbohydrate, protein, fat, BMI, and systolic pressure were analyzed at baseline. For women, old age, TG, Apo-B, and ALT were associated with the development of IO, whereas for men, old age, HS-CRP, TG, Apo-B, ALT, BMI, and alcohol intake were associated with the development of IO (Table 2).
Table 2.
Odds ratios and 95% CIs for iron overload, according to baseline risk factors
| Characteristic | Women |
Men |
||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | Z | P value | Odds ratio | 95% CI | Z | P value | |
| Age | 1.07 | 1.06–1.09 | 10.12 | <0.001 | 1.00 | 0.99–1.01 | –0.66 | 0.507 |
| HS-CRP | 1.00 | 0.99–1.01 | 1.24 | 0.214 | 1.02 | 1.00–1.03 | 2.50 | 0.013 |
| TG | 1.26 | 1.15–1.38 | 5.06 | <0.001 | 1.23 | 1.15–1.31 | 6.45 | <0.001 |
| Apo-B | 1.94 | 1.13–3.31 | 2.43 | 0.015 | 2.05 | 1.35–3.11 | 3.35 | 0.001 |
| ALT | 1.02 | 1.01–1.03 | 5.87 | <0.001 | 1.01 | 1.01–1.02 | 4.84 | <0.001 |
| IR | ||||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 1.02 | 0.74–1.39 | 0.12 | 0.908 | 0.99 | 0.79–1.25 | –0.07 | 0.947 |
| Carbohydrate | 1.00 | 1.00–1.00 | –0.73 | 0.468 | 1.00 | 1.00–1.00 | –1.30 | 0.194 |
| Protein | 1.01 | 1.00–1.02 | 2.11 | 0.035 | 1.00 | 1.00–1.01 | 0.56 | 0.574 |
| Fat | 1.00 | 0.99–1.00 | –0.65 | 0.514 | 1.00 | 1.00–1.00 | –0.22 | 0.823 |
| BMI | 1.03 | 0.98–1.07 | 1.17 | 0.243 | 1.08 | 1.04–1.12 | 4.27 | <0.001 |
| Systolic pressure | 1.00 | 0.99–1.01 | –0.64 | 0.520 | 1.00 | 0.99–1.00 | –1.38 | 0.167 |
| Smoke | ||||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 1.05 | 0.50–2.01 | 0.14 | 0.892 | 0.85 | 0.68–1.07 | –1.38 | 0.168 |
| Alcohol | ||||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 0.84 | 0.46–1.43 | –0.61 | 0.545 | 1.39 | 1.10–1.76 | 2.73 | 0.006 |
CI, confidence interval; HS-CRP, high-sensitivity C-reactive protein; TG, triglyceride; Apo, apolipoprotein; ALT, alanine aminotransferase; IR, insulin resistance; BMI, body mass index.
Relationship between IO and incident diabetes
Over the mean 6 years of follow-up (29,428 person-years), 75 participants developed diabetes. Table 3 shows the HRs for diabetes and the percent reductions in β estimates for each model. IO was significantly associated with incident diabetes, but this association was shown to be affected by sex using the models stratified according to sex. In the basic model, women with IO had a much higher risk of developing diabetes than men with IO (HR, 4.74; 95% confidence interval [CI], 2.29 to 9.79 vs. HR, 3.28; 95% CI, 1.66 to 6.46). In model 2, we adjusted for the biological factors (HS-CRP, TG, TC, Apo-B, ALT, IR, LDL-C) and found the disparity in diabetes HR estimates for IO disappeared in men (HR, 2.13; 95% CI, 0.99 to 4.57). After further adjustment for dietary, physical, and behavioral factors, the HR estimates for diabetes in men were further reduced whereas the estimates for women remained significant (P<0.05). Whether in men or women, adjustment for the biological factors was associated with the largest percentage reduction in β estimates (25.64% for women and 36.48% for men). The physical examination findings were associated with the second largest percentage reduction in β estimates in both sexes (Table 3).
Table 3.
Hazard ratios for incident diabetes in participants with and without iron overload and the percentage reductions in the parameter estimatesa
| HRs by model | Women |
Men |
|||||
|---|---|---|---|---|---|---|---|
| IO HR (95% CI) | Reduction in β, %b | P value | IO HR (95% CI) | Reduction in β, %b | P value | ||
| Baseline risk factor adjustment (method 1) | |||||||
| Model 1: age | 4.74 (2.29–9.79) | Reference | <0.001 | 3.28 (1.66–6.46) | Reference | 0.001 | |
| Model 2: model 1+biologicalc | 3.18 (1.56–6.48) | 25.64 | 0.001 | 2.13 (0.99–4.57) | 36.48 | 0.053 | |
| Model 3: model 2+dietd | 3.47 (1.69–7.14) | 20.05 | 0.001 | 2.11 (0.98–4.52) | 37.15 | 0.055 | |
| Model 4: model 3+physical examinationse | 3.34 (1.55–7.2) | 22.56 | 0.002 | 1.85 (0.86–3.97) | 48.19 | 0.114 | |
| Model 5: model 4+behavioralf | 3.35 (1.54–7.26) | 22.37 | 0.002 | 1.91 (0.88–4.13) | 45.49 | 0.100 | |
| Baseline risk factor adjustment (method 2) | |||||||
| Model 1+biologicalc | 3.18 (1.56–6.48) | 25.64 | 0.001 | 2.13 (0.99–4.57) | 36.48 | 0.053 | |
| Model 1+dietd | 4.95 (2.36–10.36) | –2.76 | <0.001 | 3.16 (1.6–6.25) | 3.03 | 0.001 | |
| Model 1+physical examinationse | 4.18 (1.97–8.88) | 8.03 | <0.001 | 2.31 (1.18–4.55) | 29.32 | 0.015 | |
| Model 1+behavioralf | 4.72 (2.28–9.77) | 0.32 | <0.001 | 3.32 (1.69–6.54) | –1.18 | 0.001 | |
HR, hazard ratio; IO, iron overload; CI, confidence interval.
The model was adjusted for baseline risk factors,
Percent reduction in β estimate ([β0–βn]/[β0]×100). β0 indicates an age adjusted reference model. βn indicates the remaining models, ln HR=β,
Biological factors: high-sensitivity C-reactive protein, triglyceride, total cholesterol, apolipoprotein B, alanine aminotransferase, insulin resistance, low-density lipoprotein cholesterol,
Diet factors: carbohydrate intake, fat intake, protein intake,
Physical examinations factors: body mass index, systolic pressure,
Behavioral factors: smoking status, alcohol status.
Risk factors mediating the relationship between IO and IR
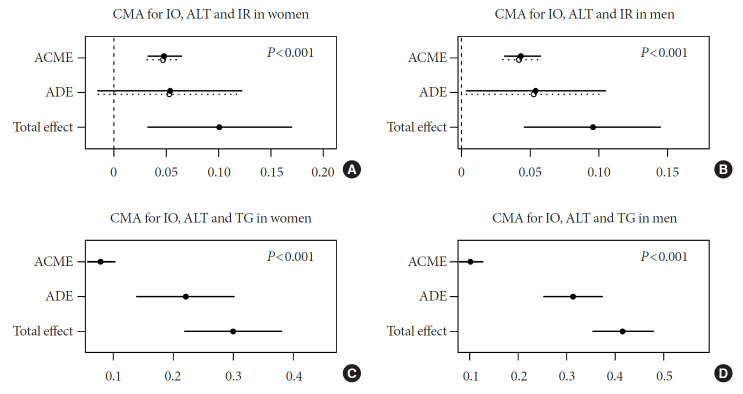
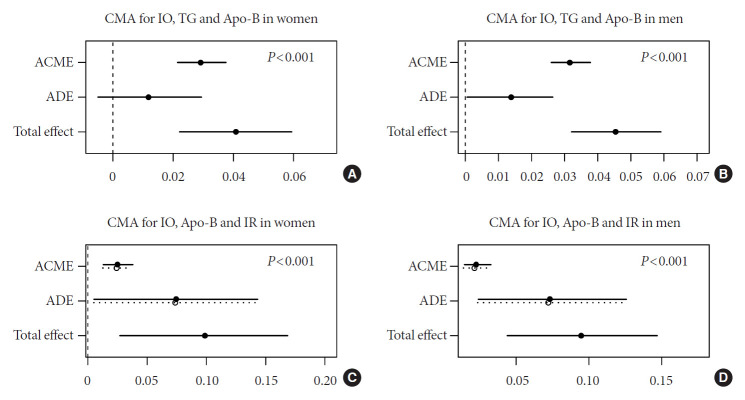
The effects of IO on IR could be accounted for completely by ALT in women (Fig. 1A) and partially in men (Fig. 1B). ALT also acted as a partial mediator for the differences in TG metabolism (Fig. 1C and D). We found the significant completely mediation effect of TG in the association between IO and Apo-B metabolism (P<0.05) (Fig. 2A and B). In addition, Apo-B showed a partial mediation effect on IR (Fig. 2C and D). These results applied to both men and women, although the indirect effects were generally more pronounced in men than women (Supplementary Table 2).
Fig. 1.
Causal mediation analysis (CMA) models for the association of insulin resistance (IR) and triglyceride (TG) metabolism with iron overload (IO). Adjusted for age and high-sensitivity C-reactive protein. (A, B) The circles and solid lines show the point estimates and 95% confidence intervals for the effects of IO on IR and (C, D) TG metabolism. (A, B) The average causal mediation effects (ACMEs) reflect the indirect effects of IO on IR and (C, D) TG metabolism, mediated by alanine aminotransferase. (A, B) The average direct effects (ADEs) reflect the direct effects of IO on IR and (C, D) TG metabolism. The total effects are equal to ACME plus ADE. P indirect represents the P value for ACME.
Fig. 2.
Causal mediation analysis (CMA) models for the association of apolipoprotein B (Apo-B) metabolism and insulin resistance (IR) with iron overload (IO). Adjusted for age and high-sensitivity C-reactive protein. (A, B) The circle and solid line represent the point estimates and 95% confidence intervals for the effects of IO on IR and (C, D) Apo-B metabolism. (A, B) The average causal mediation effects (ACMEs) reflect the indirect effects of IO on IR, mediated by Apo-B; and (C, D) Apo-B metabolism, mediated by triglyceride (TG). (A, B) The average direct effects (ADEs) reflect the direct effects of IO on IR and (C, D) Apo-B metabolism. The total effects are equal to ACME plus ADE. P indirect represents the P value for ACME.
Subgroup analyses of the association between IO and incident diabetes
The incidence of diabetes did not significantly differ between young women or old men with IO and those without. However, in women, age ≥52 years was significantly associated with the risk of incident diabetes; this association was the opposite in men: age <52 years was significantly associated with the risk of incident diabetes. In BMI subgroup analysis, there was a stronger association between IO and diabetes among participants with higher BMI after adjusting for age and HS-CRP. Women with BMI <24 showed a stronger association between IO and diabetes, but this was not the case in men (Table 4).
Table 4.
Hazard ratios for incident diabetes of iron overload in men and women, according to age and BMI category
| Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| Case | Hazard ratio | 95% CI | P value | Case | Hazard ratio | 95% CI | P value | |
| IO by age, yra | ||||||||
| <52 | 1,593 | 5.23 | 0.43–62.95 | 0.093 | 1,326 | 7.13 | 2.35–21.63 | <0.001 |
| ≥52 | 1,538 | 4.47 | 2.13–9.36 | <0.001 | 1,322 | 1.28 | 0.36–4.49 | 0.705 |
| IO by BMI, kg/m2b | ||||||||
| <24 | 1,884 | 4.77 | 1.27–17.92 | 0.021 | 1,604 | 1.02 | 0.13–7.85 | 0.985 |
| 24≤ and <28 | 933 | 4.55 | 0.90–23.11 | 0.068 | 815 | 1.70 | 0.51–5.70 | 0.393 |
| ≥28 | 314 | 4.09 | 1.38–12.15 | 0.011 | 229 | 4.46 | 1.26–15.75 | 0.020 |
BMI, body mass index; CI, confidence interval; IO, iron overload.
The model was adjusted for high-sensitivity C-reactive protein (HS-CRP),
The model was adjusted for age and HS-CRP.
DISCUSSION
In this study, we showed that IO indirectly affects the development of IR, which may cause incident diabetes and be mediated via liver damage and altered lipid metabolism. After adjustment for biological variables, our data suggest that the risk of incident diabetes associated with IO in men and women could be reduced by 25% to 35%. Reducing BMI was the second most effective method, and both biological variables and BMI were much more effective than adjusting smoking or dietary habits.
The prevalence of IO in the general population exceeded our expectations; more than one-tenth of adults in our study had IO. Previously, researchers focused on IO prevention and treatment in hereditary hemochromatosis or red blood cell transfusion. However, attention should also be given to the general population. In comparison with participants without IO at baseline, those with IO displayed more evidence of IR, liver injury, and altered lipid metabolism. Participants with IO had higher ALT activity and TG and Apo-B concentrations, even though the values were within the normal ranges. The logistic regression model also showed these indexes were significantly associated with IO. Valenzuela et al. [9] found that excess hepatic iron storage promotes liver steatosis, increases plasma transaminase activity, and induces oxidative stress. Recent research has shown high FET concentration is associated with higher prevalences of dyslipidemia, metabolic syndrome, and type 2 diabetes mellitus, and that these associations are mediated through this pathway [19,31]. In view of IO’s burden of disease, physicians should be aware of the presence of IO in the general population. We should also pay attention to the small increases in biochemical markers that are related to liver injury and lipid metabolism, even if they are within the normal range, especially in individuals with IO.
Several previous studies have shown that excess iron may cause organ-specific oxidative stress, leading to IR and abnormal lipid metabolism [7,19,31]. However, Clara Podmore et al. [15] suggested the causal role of iron was not clear in development of IR and diabetes, which needed to be clarified. Animal study showed IO may contribute to liver injury, which may result in endoplasmic reticulum stress and IR [9,32]. High INS concentration, which is associated with IR, inhibits fatty acid oxidation and increases TG synthesis by activating insulin receptor substrates 1 and 2 [33]. However, high concentrations of TG can increase Apo-B concentrations, which can induce endoplasmic reticulum stress and hepatic IR [34,35]. Others have also demonstrated that FET directly binds Apo-B via heminmediated binding [36]. In our study, the results of logistic regression did not suggest a direct causal association between IO and IR. However, in further mediation analysis, we found that ALT, TG, and Apo-B may mediate the induction of IR via IO; this process is summarized in Supplementary Fig. 2. To some extent, IO may indirectly promote IR, which is consistent with the results of previous studies. These findings suggest that IO is associated with liver injury and abnormal lipid metabolism. Such liver damage may lead to IR, and abnormal lipid metabolism may further aggravate IR. This may be part of the explanation of why people with IO are prone to develop diabetes.
Another notable finding was the sex differences in the outputs of the Cox proportional hazards models and CMA. In the EPIC-Potsdam study, high FET concentration was still associated with diabetes after adjustment for HS-CRP, γ-glutamyl transferase, ALT, adiponectin, HDL-C, and TG [7]. However, in the present study, the risk of diabetes associated with IO remained in women but disappeared in men after adjustment for biological factors. We speculate sex hormones may explain this difference. Similar to previous studies [15], we found a stronger association between IO and diabetes in leaner participants. However, this association only presents in women whose BMI <18.5 (data not shown). Stratification of the participants according to age illustrated this difference: post-menopausal women (>52 years) showed a significant relationship between IO and the risk of incident diabetes, but the opposite trend was identified in men. Owing to menopause, it is difficult for excess iron to be excreted in older women. Furthermore, there is evidence that estrogen counteracts iron mediated oxidative stress and that androgens stimulate the hematopoietic system and can even be used to treat anemia [37,38]. Several previous studies have also shown that a high FET concentration and marked iron deposition are associated with hypogonadism [39]. Taking these findings together, it is reasonable to believe that sex hormones affect iron metabolism. In addition, our results show old women are more likely to develop diabetes and that protein intake is associated with IO in women but not in men. Therefore, although women are more susceptible to ID, IO is a neglected but potentially significant problem in older women, who should be careful with their dietary protein intake.
The findings of our study contribute to the existing knowledge regarding the relationship between IO and diabetes. First, we found that IO has a high prevalence in a general Chinese population. Second, we found a significant association between IO and diabetes in this prospective study. Third, we found that the relationship between IO and diabetes is more pronounced in postmenopausal women and young men. We also explored the potential roles of liver injury and abnormal lipid metabolism in the development of diabetes.
There are several limitations in this study. The study participants were all Chinese adults; this limits the generalizability of the study findings. Another drawback is the limited availability of fasting blood samples. Because of this, we were unable to assess the oxidative stress and sex hormone concentrations in the participants. We can’t exclude participants with clinically diagnosed Hereditary hemochromatosis, though according to the extremely low incidence of hereditary hemochromatosis in China, this is unlikely to make a substantial impact on the results. Additionally, diabetes was recorded on the basis of physician-diagnosed self-reporting rather than on oral glucose tolerance testing, which may cause underestimation of morbidity.
In conclusion, this prospective cohort study provided strong evidence to support a positive association between IO and diabetes, which was more pronounced in post-menopausal women and young men. Liver injury and abnormal lipid metabolism partially mediates this association. Further studies are required to clarify the underlying molecular mechanisms.
Acknowledgments
This research uses data from China Health and Nutrition Survey (CHNS). We thank the National Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention, Carolina Population Center, the University of North Carolina at Chapel Hill, the NIH (R01-HD30880, DK056350, and R01-HD38700) and the Fogarty International Center, NIH for financial support for the CHNS data collection and analysis files from 1989 to 2006 and both parties plus the China-Japan Friendship Hospital, Ministry of Health for support for CHNS 2009 and future surveys.
We thank Zidong Chen, and Mark Cleasby PhD from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: M.Y., H.G.
Acquisition, analysis, or interpretation of data: H.G., J.Y., M.Y.
Drafting the work or revising: H.G., J.Y., W.P., M.Y.
Final approval of the manuscript: H.G., J.Y., W.P., M.Y.
FUNDING
This study was funded by Natural Science Foundation of Zhejiang Province (LGF18H260003) and Beijing Municipal Functional Peptide Engineering Research Center Foundation (2018).
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0287.
Comparison of the characteristics of the participants included in the analytic sample and those excluded from the analyses
Results of the causal mediation analysis
Flow chart of participants included in the present study. CHNS, China Health and Nutrition Survey.
Potential mechanism of insulin resis- tance (IR) induced by iron overload (IO). The ↑ indicates the increase of the index, while the remaining arrows indicate the facilitation. ALT, alanine aminotransferase; TG, triglyceride; Apo-B, apolipoprotein B.
REFERENCES
- 1.Eshak ES, Iso H, Maruyama K, Muraki I, Tamakoshi A. Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: a large population-based prospective cohort study. Clin Nutr. 2018;37:667–74. doi: 10.1016/j.clnu.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Ikuta K, Hatayama M, Addo L, Toki Y, Sasaki K, Tatsumi Y, et al. Iron overload patients with unknown etiology from national survey in Japan. Int J Hematol. 2017;105:353–60. doi: 10.1007/s12185-016-2141-9. [DOI] [PubMed] [Google Scholar]
- 3.Wood MJ, Skoien R, Powell LW. The global burden of iron overload. Hepatol Int. 2009;3:434–44. doi: 10.1007/s12072-009-9144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElduff A. Iron: how much is too much? Diabetologia. 2017;60:237–9. doi: 10.1007/s00125-016-4176-0. [DOI] [PubMed] [Google Scholar]
- 5.Simcox JA, McClain DA. Iron and diabetes risk. Cell Metab. 2013;17:329–41. doi: 10.1016/j.cmet.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes Care. 2007;30:1926–33. doi: 10.2337/dc06-2625. [DOI] [PubMed] [Google Scholar]
- 7.Montonen J, Boeing H, Steffen A, Lehmann R, Fritsche A, Joost HG, et al. Body iron stores and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetologia. 2012;55:2613–21. doi: 10.1007/s00125-012-2633-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backe MB, Moen IW, Ellervik C, Hansen JB, MandrupPoulsen T. Iron regulation of pancreatic beta-cell functions and oxidative stress. Annu Rev Nutr. 2016;36:241–73. doi: 10.1146/annurev-nutr-071715-050939. [DOI] [PubMed] [Google Scholar]
- 9.Valenzuela R, Rincon-Cervera MA, Echeverria F, Barrera C, Espinosa A, Hernandez-Rodas MC, et al. Iron-induced prooxidant and pro-lipogenic responses in relation to impaired synthesis and accretion of long-chain polyunsaturated fatty acids in rat hepatic and extrahepatic tissues. Nutrition. 2018;45:49–58. doi: 10.1016/j.nut.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Silva M, Silva ME, de Paula H, Carneiro CM, Pedrosa ML. Iron overload alters glucose homeostasis, causes liver steatosis, and increases serum triacylglycerols in rats. Nutr Res. 2008;28:391–8. doi: 10.1016/j.nutres.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Jiang L, Wang K, Lo K, Zhong Y, Yang A, Fang X, et al. Sex-specific association of circulating ferritin level and risk of type 2 diabetes: a dose-response meta-analysis of prospective studies. J Clin Endocrinol Metab. 2019;104:4539–51. doi: 10.1210/jc.2019-00495. [DOI] [PubMed] [Google Scholar]
- 12.Lee BK, Kim Y, Kim YI. Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean National Health and Nutrition Examination Survey 2008. Metabolism. 2011;60:1416–24. doi: 10.1016/j.metabol.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Huth C, Beuerle S, Zierer A, Heier M, Herder C, Kaiser T, et al. Biomarkers of iron metabolism are independently associated with impaired glucose metabolism and type 2 diabetes: the KORA F4 study. Eur J Endocrinol. 2015;173:643–53. doi: 10.1530/EJE-15-0631. [DOI] [PubMed] [Google Scholar]
- 14.Han LL, Wang YX, Li J, Zhang XL, Bian C, Wang H, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189–95. doi: 10.1002/mnfr.201400088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podmore C, Meidtner K, Schulze MB, Scott RA, Ramond A, Butterworth AS, et al. Association of multiple biomarkers of iron metabolism and type 2 diabetes: the EPIC-InterAct study. Diabetes Care. 2016;39:572–81. doi: 10.2337/dc15-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Zhai FY, Du SF, Popkin BM. The China Health and Nutrition Survey, 1989-2011. Obes Rev. 2014;15 Suppl 1:2–7. doi: 10.1111/obr.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV Infection: recommendations for a public health approach. 2nd ed. Geneva: World Health Organization; 2013. Chapter, Definition of key terms; p13-6 [cited 2021 Aug 31]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK374295. [PubMed] [Google Scholar]
- 18.He J, Fang A, Yu S, Shen X, Li K. Dietary nonheme, heme, and total iron intake and the risk of diabetes in adults: results from the China Health and Nutrition Survey. Diabetes Care. 2020;43:776–84. doi: 10.2337/dc19-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu L, Yan J, Zhang Q, Lin H, Zhu L, Liu Q, et al. Association between serum ferritin and blood lipids: influence of diabetes and hs-CRP levels. J Diabetes Res. 2020;2020:4138696. doi: 10.1155/2020/4138696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X, Byles JE, Shi Z, Hall JJ. Evaluation of older Chinese people’s macronutrient intake status: results from the China Health and Nutrition Survey. Br J Nutr. 2015;113:159–71. doi: 10.1017/S0007114514003444. [DOI] [PubMed] [Google Scholar]
- 21.Batis C, Sotres-Alvarez D, Gordon-Larsen P, Mendez MA, Adair L, Popkin B. Longitudinal analysis of dietary patterns in Chinese adults from 1991 to 2009. Br J Nutr. 2014;111:1441–51. doi: 10.1017/S0007114513003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan Y, Chen R, Zheng W, Guo C, Lu L, Ji X, et al. Association between serum magnesium and anemia: China health and nutrition survey. Biol Trace Elem Res. 2014;159:39–45. doi: 10.1007/s12011-014-9967-x. [DOI] [PubMed] [Google Scholar]
- 23.Li X, He T, Yu K, Lu Q, Alkasir R, Guo G, et al. Markers of iron status are associated with risk of hyperuricemia among Chinese adults: nationwide population-based study. Nutrients. 2018;10:191. doi: 10.3390/nu10020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO/UNICEF/UNU . Iron deficiency anaemia assessment, prevention and control. A guide for programme managers. Geneva: World Health Organization; 2001. p. 33. [Google Scholar]
- 25.StatPearls . StatPearls Publishing; 2020. Treasure Island. Chapter, Iron overload [cited 2021 Aug 31]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526131. [Google Scholar]
- 26.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320–5. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 27.McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, et al. Diagnosing insulin resistance in the general population. Diabetes Care. 2001;24:460–4. doi: 10.2337/diacare.24.3.460. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi TA, Johnson KM. Menopause. Med Clin North Am. 2015;99:521–34. doi: 10.1016/j.mcna.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Bancks MP, Kershaw K, Carson AP, Gordon-Larsen P, Schreiner PJ, Carnethon MR. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA. 2017;318:2457–65. doi: 10.1001/jama.2017.19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan NA, Wang H, Anand S, Jin Y, Campbell NR, Pilote L, et al. Ethnicity and sex affect diabetes incidence and outcomes. Diabetes Care. 2011;34:96–101. doi: 10.2337/dc10-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitchika A, Schipf S, Nauck M, Dorr M, Lerch MM, Felix SB, et al. Associations of iron markers with type 2 diabetes mellitus and metabolic syndrome: results from the prospective SHIP study. Diabetes Res Clin Pract. 2020;163:108149. doi: 10.1016/j.diabres.2020.108149. [DOI] [PubMed] [Google Scholar]
- 32.Jahng JW, Alsaadi RM, Palanivel R, Song E, Hipolito VE, Sung HK, et al. Iron overload inhibits late stage autophagic flux leading to insulin resistance. EMBO Rep. 2019;20:e47911. doi: 10.15252/embr.201947911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weickert MO, Pfeiffer AF. Signalling mechanisms linking hepatic glucose and lipid metabolism. Diabetologia. 2006;49:1732–41. doi: 10.1007/s00125-006-0295-3. [DOI] [PubMed] [Google Scholar]
- 34.Hevi S, Chuck SL. Ferritins can regulate the secretion of apolipoprotein B. J Biol Chem. 2003;278:31924–9. doi: 10.1074/jbc.M303081200. [DOI] [PubMed] [Google Scholar]
- 35.Su Q, Tsai J, Xu E, Qiu W, Bereczki E, Santha M, et al. Apolipoprotein B100 acts as a molecular link between lipid-induced endoplasmic reticulum stress and hepatic insulin resistance. Hepatology. 2009;50:77–84. doi: 10.1002/hep.22960. [DOI] [PubMed] [Google Scholar]
- 36.Seki T, Kunichika T, Watanabe K, Orino K. Apolipoprotein B binds ferritin by hemin-mediated binding: evidence of direct binding of apolipoprotein B and ferritin to hemin. Biometals. 2008;21:61–9. doi: 10.1007/s10534-007-9093-8. [DOI] [PubMed] [Google Scholar]
- 37.Das SK, Patel VB, Basu R, Wang W, DesAulniers J, Kassiri Z, et al. Females are protected from iron-overload cardiomyopathy independent of iron metabolism: key role of oxidative stress. J Am Heart Assoc. 2017;6:e003456. doi: 10.1161/JAHA.116.003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Invest. 2009;32:704–16. doi: 10.1007/BF03345745. [DOI] [PubMed] [Google Scholar]
- 39.Yang JH, Chou CH, Yang WS, Ho HN, Yang YS, Chen MJ. Iron stores and obesity are negatively associated with ovarian volume and anti-Mullerian hormone levels in women with polycystic ovary syndrome. Taiwan J Obstet Gynecol. 2015;54:686–92. doi: 10.1016/j.tjog.2014.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the characteristics of the participants included in the analytic sample and those excluded from the analyses
Results of the causal mediation analysis
Flow chart of participants included in the present study. CHNS, China Health and Nutrition Survey.
Potential mechanism of insulin resis- tance (IR) induced by iron overload (IO). The ↑ indicates the increase of the index, while the remaining arrows indicate the facilitation. ALT, alanine aminotransferase; TG, triglyceride; Apo-B, apolipoprotein B.