Abstract
Objectives
We aimed to compare the success rates and other catheter-related parameters between peripherally inserted central catheters (PICCs) and non-tunnelled ultrasound-guided central venous catheters (USG-CVCs) including femoral, jugular, brachiocephalic and subclavian lines.
Design
This was a retrospective observational study.
Setting
The study was performed in a level III neonatal intensive care unit (NICU) in Qatar, as a single-site study.
Participants
This study included 1333 neonates who required CVC insertion in the NICU from January 2016 to December 2018. Of those, we had 1264 PICCs and 69 non-tunnelled USG-CVCs.
Outcome measures
The success rate and other catheter-related complications in the two groups.
Results
The overall success rate was 88.4% in the USG-CVCs (61/69) compared with 90% in the PICCs (1137/1264) group (p=0.68). However, the first prick success rate was 69.4% in USG-CVCs (43/69) compared with 63.6% in the PICCs (796/1264) group. Leaking and central line-associated blood stream infection (CLABSI) were significantly higher in the USG-CVC group compared with the PICC group (leaking 16.4% vs 2.3%, p=0.0001) (CLABSI 8.2% vs 3.1%, p=0.03). CLABSI rates in the PICC group were 1.75 per 1000 catheter days in 2016 and 3.3 in 2017 compared with 6.91 in 2016 (p=0.0001) and 14.32 in 2017 (p=0.0001) for the USG-CVCs. USG-CVCs had to be removed due to catheter-related complications in 52.5% of the cases compared with 29.9% in PICCs, p=0.001. In 2018, we did not have any non-tunnelled USG-CVCs insertions in our NICU.
Conclusions
The overall complication rate, CLABSI and leaking are significantly higher in non-tunnelled USG-CVCs compared with the PICCs. However, randomised controlled trials with larger sample sizes are desired. Proper central venous device selection and timing, early PICC insertion and early removal approach, dedicated vascular access team development, proper central venous line maintenance, central line simulation workshops and US-guided insertions are crucial elements for patient safety in NICU.
Keywords: NEONATOLOGY, Neonatal intensive & critical care, PERINATOLOGY
Strengths and limitations of this study.
This is an observational study including a large sample of 1333 neonates.
The study provides information on the insertion success rates and complications of peripherally inserted central catheters and non-tunnelled ultrasound-guided central venous catheters in neonates.
It is based on retrospective analyses of collected data.
Introduction
Peripherally inserted central catheters (PICCs) were described for the first time by Shaw in 1973. Since then, they have been used extensively due to their features.1 PICC insertion by direct superficial peripheral vein puncture offers long-term venous access for both term and preterm neonates and is often indicated in neonatal intensive care unit (NICU) for parental nutrition, long-term intravenous medications, antibiotic therapy and vesicant drug administration.2 3
Non-tunnelled ultrasound-guided central venous catheters (USG-CVCs) are inserted in neonates in special circumstances, for example, central venous pressure monitoring, blood withdrawal, haemodialysis and for all other infusions and medications when PICC insertion fails.4 They are inserted in the internal jugular, brachiocephalic, subclavian and femoral veins under ultrasound guidance.1 5–7
There is a limited number of studies comparing PICCs with USG-CVCs in neonates that necessitated further research and comparative analysis. This study aimed to compare the success rates and other catheter-related parameters in PICCs and the non-tunnelled USG-CVCs in NICU between 2016 and 2018.
Methods
This single-centre retrospective study was conducted in the NICU at the Women’s Wellness and Research Centre (WWRC), Hamad Medical Corporation (HMC), Doha, Qatar. WWRC is the main specialist hub for women and newborns health services in Qatar with more than 18 000 deliveries per year. The NICU in WWRC is a level III mainly medical unit with 112 beds and more than 2000 admissions per year with limited congenital cardiac or surgery cases.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Participants
A total of 1333 cases were evaluated in this study. This includes 1264 babies who had PICC insertion and 69 who had non-tunnelled USG-CVC insertion. Related information for all cases between January 2016 and December 2018 was collected from the electronic medical system at the NICU.
A fully dedicated PICC insertion team was launched in January 2017. The PICC team has been expanded over time to include 15 neonatologist physicians, 1 neonatal nurse practitioner as well as seven NICU nurses. The team was trained in central line simulation workshops to insert PICC by the catheter-over-needle technique and the modified Seldinger technique.8 9 The central line simulation workshop is a full-day workshop that was founded by the neonatal simulation team and is accredited by the Department of Healthcare Professions in the Ministry of Public Health with a total of 7 continuous professional development (CPD) hours both category I (1/7) and category III (6/7). The PICC team works in harmony and collaboration with 30 well-trained NICU nurses who are members of the neonatal specialised nursing (NSN) team. The NSN determines the patient’s eligibility, takes care of the central line maintenance using transparent semipermeable dressing, enters the data in the electronic database and gets the blood samples. There is no difference in the central line type of care, frequency or personnel in all types of catheters. In addition to their role in central line insertion and maintenance, the NSN team attends high-risk deliveries and play a pivotal role in neonatal transportation.
In our NICU, the indications for PICC insertions are the birth weight of <1500 g, the requirement of intravenous fluids for >5 days, the requirement of intravenous medications for >7 days, the requirement of hyperosmolar intravenous fluid therapy >700 mOsmol/L and the requirement of >3 peripheral intravenous catheters (PIVC) insertions in the last 24 hours.10 A successful catheter insertion means a catheter inserted into a proper central venous position that can be used with its tip located either in the superior or inferior vena cava. As per our institutional guideline, two pricks are allowed per operator with a maximum of three in difficult lines. After three unsuccessful pricks, the procedure should be terminated.
Figure 1 shows the three types of PICCs available in our NICU; (NutriLine 2 Fr; Vygon), (PremiCath 1 Fr; Vygon), and (PremiStar 1 Fr; Vygon). PremiStar 1 Fr; Vygon is an antimicrobial impregnated catheter that is used in our unit for babies born less than 28 weeks gestation or when sepsis is suspected. We use NutriLine 2 Fr; Vygon, when a double lumen PICC or a long line is needed in big babies as its length is 30 cm. PremiCath 1 Fr; Vygon is used for the rest of our NICU babies who need PICC insertion. The most common veins used for PICC insertions are the great saphenous vein, small saphenous vein, posterior tibial vein, antecubital vein, cephalic vein, basilic vein and ulnar vein. Figure 2 shows the non-tunnelled CVC available in our unit which is (MultiCath 2; 4.5 Fr; Vygon). The most common veins used for USG-CVC insertions are the internal jugular vein, femoral vein, brachiocephalic vein and subclavian vein.
Figure 1.
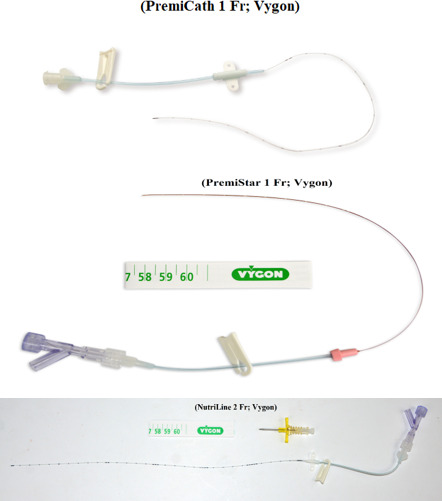
Three types of peripherally inserted central catheters.
Figure 2.

Non-tunnelled ultrasound-guided central venous catheter.
In our practice, non-tunnelled USG-CVC was used only when PICC insertion has failed by two to three operators; two pricks for each. USG-CVCs were inserted either by the paediatric surgeon or the paediatric anaesthetist on-call physician under US guidance. Currently, we use the handheld wireless probe-type ultrasound scanner machine to guide the catheter insertion and for the catheter tip location.
We followed the Centers for Disease Control and Prevention (CDC) definition for central line-associated bloodstream infection (CLABSI) and CLABSI rate. CLABSI is defined as a laboratory-confirmed bloodstream infection (LCBI) where an eligible bloodstream infection (BSI) organism is identified, and an eligible central line is present on the LCBI date of the event or the day before. The infection cannot be related to any other infection the patient might have and must not have been present or incubating when the patient was admitted to the facility. CLABSI rate is the total number of CLABSI divided by the total number of device days 1000.11 12
The differential time to positivity (DTP) is defined as a difference in time to positivity of ≥2 hours between peripheral blood culture and a CVC blood culture (peripheral DTP) or between two CVC blood cultures from different lumens of a multilumen catheter (CVC DTP).13 Due to its limitation reported in the literature, our unit does not prefer to use the DTP for the diagnosis of CLABSI.14
The authors designed an electronic system-based data collection sheet to collect all catheter-related parameters in the two groups.
Statistical analysis
Descriptive statistics were used to summarise and determine the sample characteristics and distribution of participants’ data. The normally distributed data and results were reported with mean and SD; the remaining results were reported with median and IQR. Categorical data were summarised using frequencies and proportions. Associations between two or more qualitative data variables were assessed using χ2 test or Fisher’s exact test as appropriate. Quantitative data between the two independent groups (USG-CVC and PICC) were analysed using unpaired t-test (for normally distributed data) or Mann-Whitney U test (for skewed or non-normally distributed data) as appropriate. Univariate and multivariate logistic regression analysis was applied to determine and assess the potential factors and predictors associated with the catheter insertion success rate adjusted for potential factors and predictors such as catheter types, gestational age, birth weight, the reason for catheter insertion, side of the body, site of insertion and number of pricks. For multivariate logistic regression models, predictor variables were included considering both statistical and clinical significance. The results of logistic regression analysis were presented as ORs with corresponding 95% CIs. Thereafter, we used the receiver operating characteristic curve (ROC) to evaluate the discriminative ability (predictive accuracy of the developed logistic regression model) of potentially significant variables associated with catheter insertion success rate. Box plots were constructed depicting the distribution of gestational age and birth weight across two catheter types. All p values presented were two-tailed, and p values<0.05 were considered statistically significant. All statistical analyses were performed using statistical packages SPSS V.27.0 (IBM Corp) and Epi Info (CDC) software.
Results
Among the 3 years that this study covered, the usage of USG-CVC has progressively declined to zero in 2018, on which the catheter insertion success rate increased to 97%. Shown in table 1 are the distribution of patients and catheter-related variables associated with the types of catheters. When USG-CVC was compared with PICC about gestational age, the former was significantly higher (33.88±6.34 vs 29.32±4.03, p=0.0001). Birth weight was also significantly higher among USG-CVC compared with PICC (2161.25±1140.26 vs 1234.57±624.90, respectively, p=0.0001). Figure 3 shows the distribution of gestational age (weeks) and birth weight (g) across two catheter types. The duration of catheter insertion was however not significant (USG-CVC 11.69±9.23, PICC 14.57±12.56, p=0.14). Further comparisons between USG-CVC and PICC on several parameters. PICC had a higher success rate (90% vs 88.4%), however, the difference did not reach statistical significance.
Table 1.
Distribution of patients’ profiles and catheter-related parameters and their association with the catheter types
| Variables | Total n=1333 | USG-CVC 69 (5.2%) |
PICC 1264 (94.8%) |
P value |
| Year | ||||
| 2016 2017 2018 |
376 (28.2) 507 (38) 450 (33.8) |
42 (60.9) 27 (39.1) 0 (0) |
334 (26.4) 480 (38) 450 (35.6) |
0.001 |
| Side of the body | ||||
| Left Right |
498 (41.1) 715 (58.9) |
14 (22.6) 48 (77.4) |
484 (42.1) 667 (57.9) |
0.002 |
| Site of insertion | ||||
| Upper extremities Lower extremities |
360 (29.5) 861 (70.5) |
37 (53.6) 32 (46.4) |
323 (28) 829 (72) |
0.001 |
| Number of pricks | ||||
| First prick Second prick Third prick Fourth prick Fifth prick Sixth prick |
839 (63.9) 305 (23.2) 145 (11) 22 (1.7) 1 (0.1) 2 (0.2) |
43 (69.4) 8 (12.9) 6 (9.7) 4 (6.5) 0 (0) 1 (1.6) |
796 (63.6) 297 (23.7) 139 (11.1) 18 (1.4) 1 (0.1) 1 (0.1) |
0.001 |
| Reason for insertion | ||||
| Difficult intravenous insertion Hypoglycaemia Long-term intravenous fluid therapy Long-term intravenous medication therapy |
8 (0.6) 10 (0.8) 1286 (96.6) 27 (2) |
0 (0) 0 (0) 68 (100) 0 (0) |
8 (0.6) 10 (0.8) 1218 (96.4) 27 (2.1) |
0.47 |
| Catheter insertion success rate | ||||
| Successful Not successful |
1198 (89.9) 135 (10.1) |
61 (88.4) 8 (11.6) |
1137 (90) 127 (10) |
0.68 |
| Reason for removal | ||||
| CLABSI Leaking Accidental removal Broken catheter Local redness and swelling Occlusion Malposition Elective Death Phlebitis |
40 (3.4) 36 (3) 8 (0.7) 7 (0.6) 104 (8.7) 42 (3.5) 13 (1.1) 833 (69.9) 39 (3.3) 70 (5.9) |
5 (8.2) 10 (16.4) 1 (1.6) 0 (0) 5 (8.2) 0 (0) 0 (0) 29 (47.5) 5 (8.2) 6 (9.8) |
35 (3.1) 26 (2.3) 7 (0.6) 7 (0.6) 99 (8.8) 42 (3.7) 13 (1.1) 804 (71.1) 34 (3) 64 (5.7) |
0.031 0.001 0.40 0.69 0.88 0.13 0.50 0.001 0.03 0.18 |
| Gestational age (weeks) | ||||
| Mean±SD Median (IQR) |
29.55±4.30 29 (27, 31) |
33.88±6.34 37 (26, 40) |
29.32±4.03 29 (27, 31) |
0.001 |
| Gestational age | ||||
| 22–28 weeks >28–32 weeks >32–36 weeks >36 weeks |
602 (45.2) 490 (36.8) 100 (7.5) 141 (10.6) |
22 (31.9) 4 (5.8) 8 (11.6) 35 (50.7) |
580 (45.9) 486 (38.4) 92 (7.3) 106 (8.4) |
0.001 |
| Birth weight (g) | ||||
| Mean±SD Median (IQR) |
1282.6±692.1 1095 (850, 1400) |
2161.4±1140.3 2530 (970, 3122) |
1234.6±624.9 1080 (840, 1370) |
0.001 |
| Birth weight | ||||
| BW ≤1 kg BW >1–2 kg BW >2 –3 kg BW >3 kg |
561 (42.1) 618 (46.5) 87 (6.5) 67 (5) |
21 (30.4) 10 (14.5) 16 (23.2) 22 (31.9) |
540 (42.7) 608 (48.1) 71 (5.6) 42 (3.6) |
0.001 |
This is a retrospective study design and for some parameters, the data values were incomplete due to the unavailability of the information in the patients’ record files. All percentage (%) was computed using non-missing data values.
CLABSI, central line-associated bloodstream infection; PICCs, peripherally inserted central catheters; USG-CVCs, ultrasound-guided central venous catheters.
Figure 3.
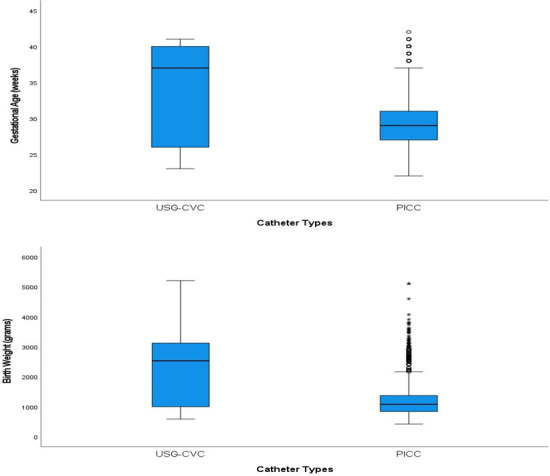
Box plot depicting the distribution of gestational age (weeks) and birth weight (g) across two catheter types. PICC, peripherally inserted central catheters; USG-CVC, ultrasound-guided central venous catheters.
We performed univariate and multivariate logistic regression analysis testing for potential factors and predictors and their possible association with dichotomous outcome variable catheter types (USG-CVC and PICC), it was observed that year of catheter insertion, side of the body, site of insertion, number of pricks ≥3 pricks, reasons for removal (elective vs non-elective), duration of gestation and birth weight were significantly associated with catheter types. The multivariate logistic regression analysis showed that year of catheter insertion, side of the body, site of insertion, number of pricks ≥3 pricks, reasons for removal and birth weight were remained significantly associated with catheter types adjusting other predictors and factors shown in table 2. The discriminative ability of the significant predictors (observed in multivariate analysis) in predictive catheter types were found to be good with an area under the ROC curve value of 0.927 (95% CI 0.90 to 0.96), which indicates that this developed regression model demonstrated an excellent fit (figure 4).
Table 2.
Logistic regression analysis with potential factors and predictors associated with catheter types USG-CVC and PICC
| Variables | Univariate analysis | Multivariate analysis | |||||
| Catheter type PICC, n (%) | Unadjusted OR | 95% CI for OR | P value | Adjusted OR |
95% CI for OR | P value | |
| Year | |||||||
| 2016 2017 2018 |
334 (88.8) 480 (94.7) 450 (100) |
1.0 (reference) 2.24 – |
1.35 to 3.70 – |
0.002 – |
1.0 (reference) 3.43 – |
1.79 to 6.54 – |
0.001 – |
| Side of the body | |||||||
| Left Right |
484 (97.2) 667 (93.3) |
1.0 (reference) 0.40 |
0.22 to 0.74 | 0.003 | 1.0 (reference) 0.34 |
0.17 to 0.70 | 0.003 |
| Site of insertion | |||||||
| Upper extremities Lower extremities |
323 (89.7) 829 (96.3) |
1.0 (reference) 2.97 |
1.82 to 4.85 | 0.001 | 1.0 (reference) 3.39 |
1.81 to 6.33 | 0.001 |
| Number of pricks | |||||||
| 1 Prick 2 pricks ≥3 pricks |
796 (94.9) 297 (97.4) 171 (90.5) |
1.0 (reference) 2.01 0.51 |
0.93 to 4.32 0.29 to 0.91 |
0.075 0.023 |
1.0 (reference) 2.58 0.39 |
0.97 to 6.86 0.16 to 0.95 |
0.058 0.037 |
| Reason for catheter insertion | |||||||
| Long-term intravenous fluid therapy Others* |
1218 (94.7) 45 (100) |
1.0 (reference) – |
– | – | 1.0 (reference) – |
– | – |
| Catheter insertion success rate | |||||||
| Not successful Successful |
127 (94.1) 1137 (94.9) |
1.0 (reference) 1.17 |
0.55 to 2.51 | 0.679 | 1.0 (reference) 1.14 |
0.59 to 2.46 | 0.569 |
| Reasons for removal | |||||||
| Non-elective Elective |
327 (91.1) 804 (96.5) |
1.0 (reference) 2.71 |
1.62 to 4.56 | 0.001 | 1.0 (reference) 2.16 |
1.16 to 4.01 | 0.015 |
| Gestational age (weeks) | |||||||
| PICC vs USG-CVC | 29.3±4.1 vs 33.9±6.3 |
0.82 | 0.78 to 0.86 | 0.001 | 1.03 | 0.90 to 1.17 | 0.715 |
| Birth weight (g) | |||||||
| PICC vs USG-CVC | 1235.6±624.9 vs 2161.4±1140.3 |
0.98 | 0.98 to 0.99 | 0.001 | 0.99 | 0.98 to 0.99 | 0.001 |
Catheter types—USG-CVC was considered as the reference group.
*Others includes: Difficult intravenous insertion, hypoglycaemia and long-term intravenous medication therapy.
PICCs, peripherally inserted central catheters; USG-CVCs, ultrasound-guided central venous catheters.
Figure 4.
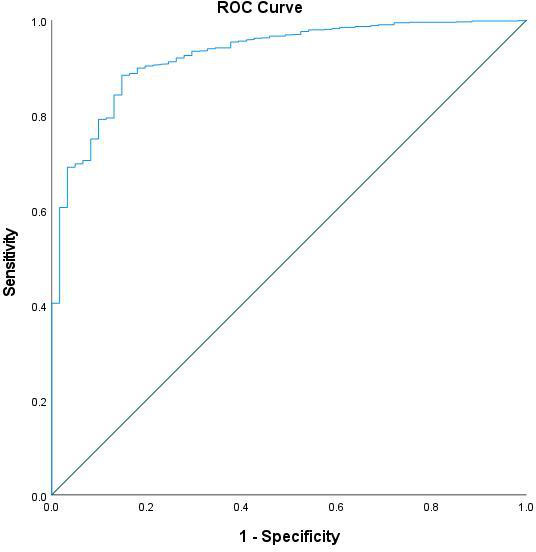
Receiver operating characteristic curve (ROC) to evaluate and assess the predictive accuracy of the developed logistic regression model (using the predicted probabilities) with dichotomous outcome variable catheter types (ultrasound-guided central venous catheters and peripherally inserted central catheter).
CLABSI and leaking were noted to be significantly higher in the USG-CVC group compared with the PICC group. CLABSI rate is defined as the total number of CLABSI divided by the total number of device days 1000.11 12 CLABSI rates in the PICC group were 1.75 in 2016 and 3.3 in 2017 compared with 6.91 in 2016 (p=0.0001) and 14.32 in 2017 (p=0.0001) for the non-tunnelled USG-CVCs. We did not have any USG-CVC inserted in 2018.
In the PICC group, 804 (71.1%) were removed electively after completion of therapy compared with 29 (47.5%) in the USG-CVC group. No significant difference was noted between the two groups regarding the other catheter-related complications. No serious or long-term complications, for example, cardiac arrhythmia, accidental arterial puncture, cardiac tamponade, pericardial or pleural effusion,15 was noted in both groups across the 3 years.
The results of univariate and multivariate logistic regression analysis testing for potential factors and predictors and their possible association with catheter insertion success rates are presented in tables 3 and 4. Univariate results indicated that year of catheter insertion, birth weight and the number of pricks had a significant effect on the likelihood of catheter insertion success rates. In patients who had 2 pricks (unadjusted OR 0.03; 95% CI 0.01 to 0.07, p=0.028) and ≥3 pricks (unadjusted OR 0.01; 95% CI 0.01 to 0.03, p=0.013) were significantly associated with a decreased likelihood of catheter insertion success rates compared with patients who had 1 prick. In addition, it was noted that catheter type PICC was associated with a higher rate of catheter insertion success rates, however, this difference was statistically insignificant (p=0.679).
Table 3.
Univariate logistic regression analyses with potential significant factors and predictors associated with catheter insertion success rates
| Variables | Catheter insertion success rate, n (%) | Unadjusted OR | 95% CI for OR | P value |
| Catheter types | ||||
| USG-CVC PICC |
61 (88.4) 1137 (90) |
1.0 (reference) 1.17 |
0.55 to 2.51 | 0.679 |
| Year | ||||
| 2016 2017 2018 |
309 (81.7) 450 (88.6) 439 (97.6) |
2.0 (reference) 1.73 8.91 |
1.18 to 2.52 4.64 to 17.12 |
0.004 0.001 |
| Gestational age (week) | 29.56±4.20 vs 29.53±5.12 | 1.01 | 0.96 to 1.04 | 0.954 |
| Birth weight (g) | 1270.1±677.5 vs 1394.2±803.3 | 0.98 | 0.98 to 0.99 | 0.045 |
| Reason for catheter insertion | ||||
| Long-term intravenous fluid therapy Others* |
1156 (89.9) 42 (93.3) |
2.0 (reference) 1.57 |
0.48 to 1.51 | 0.453 |
| Side of the body | ||||
| Left Right |
491 (98.6) 706 (98.7) |
2.0 (reference) 1.12 |
0.41 to 3.03 | 0.826 |
| Site of insertion | ||||
| Upper extremities Lower extremities |
353 (98.1) 845 (98.1) |
2.0 (reference) 1.05 |
0.43 to 2.57 | 0.920 |
| Number of pricks | ||||
| 1 prick 2 pricks ≥3 pricks |
833 (99.3) 244 (79.5) 121 (63.7) |
1.0 (reference) 0.03 0.01 |
0.01 to 0.07 0.01 to 0.03 |
0.028 0.013 |
*Others includes: Difficult intravenous insertion, hypoglycaemia and long-term intravenous medication therapy.
PICC, peripherally inserted central catheter; USG-CVC, ultrasound-guided central venous catheter.
Table 4.
Multivariate logistic regression analyses with potential significant factors and predictors associated with catheter insertion success rates
| Variables | Catheter insertion success rate N (%) | Adjusted OR | 95% CI for OR | P value |
| Gestational age (week) | 29.56±4.20 vs 29.53±5.12 | 1.23 | 1.03 to 1.44 | 0.015 |
| Number of pricks | ||||
| 1 prick 2 pricks ≥3 pricks |
833 (99.3) 244 (79.5) 121 (63.7) |
1.0 (reference) 0.07 0.02 |
0.01 to 0.57 0.01 to 0.13 |
0.014 0.001 |
The multivariate logistic regression analysis showed that duration of gestation (weeks) and the number of pricks were remained significantly (p<0.05) associated with the catheter insertion success rate after controlling and adjusting potential factors and predictors as shown in table 4. The higher catheter insertion success rates were associated with increasing gestational age (adjusted OR 1.23; 95% CI 1.03 to 1.44, p=0.015). Whereas, in patients who had 2 pricks (adjusted OR 0.07; 95% CI 0.0 to, 0.57, p=0.014) and ≥3 pricks (adjusted OR 0.02; 95% CI 0.01 to 0.13, p=0.001) were significantly associated with a reduction in the likelihood of catheter insertion success rates when compared with patients who had 1 prick. Thereafter, we computed a prediction model to evaluate the discriminative ability of potentially significant predictors (observed in the developed multivariate logistic regression model) associated with catheter insertion success rates using ROC curve analysis. The value of area under the curve observed was found to be 0.841 (95% CI 0.81 to 0.87), which is indicating that this developed regression model demonstrated an excellent fit, figure 5.
Figure 5.
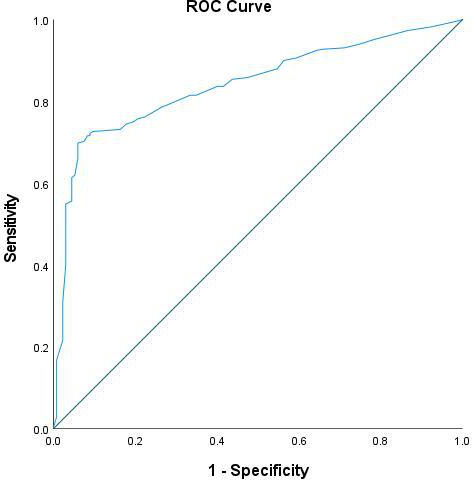
Receiver operating characteristic curve (ROC) to evaluate and assess the predictive accuracy of the developed logistic regression model (using the predicted probabilities) with dichotomous outcome variable catheter insertion success rate (successful/not successful).
Discussion
Both PICCs and non-tunnelled USG-CVCs have risks associated with their usage. Immediate risks include injury to local structures, accidental arterial puncture, phlebitis at the insertion site, air embolism, haematoma, arrhythmia and catheter damage and malposition. Late complications include infection, occlusion, thrombosis, infiltration, extravasation and catheter migration.16–18 Infection, thrombosis, embolisation, hydrocephalus, are complications reported in premature babies receiving central venous lines.3
The current study compared PICC to USG-CVC in a sample of cases from Qatar. The results also showed a progressive reduction in the usage of USG-CVC across the 3 years until reached 0% in 2018. This is due to the implementation of a PICC insertion team in early 2017 with a progressive build-up of the team skills.19 Since then, overall success and first prick rates have significantly increased. Reports of an overall success rate of 94% were indicated elsewhere.20 A systematic review highlighted the importance and necessity of a vascular access team in the NICU, as it reflects positively on the rate of BSI.21 This was also confirmed in another study where the rate of infections was reduced by 50% after the establishment of a PICC team in the NICU.22
Only 29 (47.5%) of our USG-CVC were electively removed after completion of therapy while the rest were removed due to death, phlebitis, CLABSI or other catheter-related complications. In the PICC group, elective removal was noted to be significantly higher 804 (71.1%) than USG-CVC (p=0.0001). The higher rate of CLABSI in USG-CVCs compared with PICCs is mainly related to the vulnerable insertion sites being close to infection or joint areas.23 Also, the higher rate of catheter leaking in USG-CVCs might be due to occlusions resulting from mechanical or postural factors, catheter malpositioning or undesirable catheter-tip location. CLABSI and thrombosis might also lead to catheter leaking.24 Approximately one-third of PICCs were associated with complications in another study which is close to our PICC data.25
Ragavan et al described the advantages of using PICCs inserted in the cubital veins as to have a reduced complication incidence rate, as well as maintenance rates in comparison to USG-CVCs inserted in the internal jugular vein. The authors concluded by recommending the usage of PICCs routinely when dealing with neonatal surgical patients.26 On the other hand, a recent study reported a 100% success rate of 30 preterm babies who underwent an USG brachiocephalic CVC insertion. No case of accidental arterial or pleural puncture was noted by the researchers.27 In another study involving neonates with femoral central venous catheterisation,28 the overall success rate was 100% of neonates (n=82/82), first attempt 63/74 (85%), second attempt 8/74 (11%) and third attempt 3/74 (4%). Another two studies reported no statistical difference in the complication rate or efficacy between those who had PICC and those who had USG-CVC.4 29
The limitation of this study is being retrospective with potential risks of bias and confounding factors especially when single-centre studies. The imbalance in numbers between the two groups suggest that the inferences may not be robust. Another limitation of the study is that the PICC team was properly trained to insert PICCs while the USG-CVC were placed by operators not belonging to the team (surgeons or anaesthetists). Potential bias by indication might be an issue as percutaneous CVCs were considered if some attempts for a PICC insertion failed. As reported by other researchers,28 USG-CVCs sometimes needed multiple pricks to get the catheter successfully inserted as reported in our study. This might be related to the level of experience, the number of exposures and lack of training as this task is not the main task daily performed by the operators (surgeons and anaesthetists). Besides, being inserted as rescue mode, not for selected patients is a stressor that might be a factor in increasing the number of pricks.
No USG-CVC was inserted in our unit for the last 2 years, however, it might be needed in the future in certain indications. Randomised controlled trials (RCTs) to study the feasibility of intracavitary ECG in catheter insertion and tip location in neonates are strongly recommended. Also, the use of US guidance during PIVC insertion and the frequency of its use in tip location monitoring of correctly positioned central lines to confirm the tip positions and diagnose catheter migration are both rich areas for future prospective studies.
Conclusion
The overall complication rate, CLABSI and leaking are significantly higher in non-tunnelled USG-CVCs compared with the PICCs. However, RCTs with larger sample sizes are desired. Proper central venous device selection and timing, early PICC insertion and early removal approach, dedicated vascular access team development, proper central venous line maintenance, central line simulation workshops and US-guided insertions are crucial elements for patient safety in NICU.
Supplementary Material
Acknowledgments
This research was funded and supported by the Medical Research Center, Hamad Medical Corporation, Doha, Qatar. Special thanks to the entire peripherally inserted central catheter and neonatal specialised nursing teams in Women’s Wellness and Research Centre who provide high-quality care to our newborns.
Footnotes
Contributors: MAAB is the principal author responsible for the overall content as guarantor. He accepts full responsibility for the finished work, the conduct of the study, had access to the data, and controlled the decision to publish. MAAB and DS conceptualised and designed the study. RvR and MAAB collected, cleaned and anonymised the data. PC designed and performed the data analysis. MAAB, PC and SH drafted the initial manuscript. MAAB and PC designed the figures. EEE, MAAB, AG and PC intellectually revised the manuscript. All authors reviewed and revised the manuscript, and approved the final submitted manuscript.
Funding: This work was supported by the Medical Research Center (MRC), Hamad Medical Corporation, Doha, Qatar (Protocol number MRC-01-18-151).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request from the corresponding author, Dr Mohammad A A Bayoumi (moh.abdelwahab@hotmail.com). Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Institutional Review Board at Hamad Medical Corporation before the study procedures commenced (MRC-01-18-151).
References
- 1.Barone G, Pittiruti M. Epicutaneo-caval catheters in neonates: new insights and new suggestions from the recent literature. J Vasc Access 2020;21:805–9. 10.1177/1129729819891546 [DOI] [PubMed] [Google Scholar]
- 2.McCay AS, Elliott EC, Walden M. Videos in clinical medicine. PICC placement in the neonate. N Engl J Med 2014;370:e17. 10.1056/NEJMvcm1101914 [DOI] [PubMed] [Google Scholar]
- 3.Yu X, Yue S, Wang M, et al. Risk factors related to peripherally inserted central venous catheter nonselective removal in neonates. Biomed Res Int 2018;2018:1–6. 10.1155/2018/3769376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foo R, Fujii A, Harris JA, et al. Complications in tunneled CVL versus PICC lines in very low birth weight infants. J Perinatol 2001;21:525–30. 10.1038/sj.jp.7210562 [DOI] [PubMed] [Google Scholar]
- 5.Araujo CC, Lima MC, Falbo GH. Percutaneous subclavian central venous catheterization in children and adolescents: success, complications and related factors. J Pediatr 2007;83:64–70. 10.2223/JPED.1583 [DOI] [PubMed] [Google Scholar]
- 6.Lausten-Thomsen U, Merchaoui Z, Dubois C. Eleni DIT Trolli S, Le Sache N, Mokhtari M, et al. ultrasound-guided subclavian vein cannulation in low birth weight neonates. Pediatr Crit Care Med 2017;18:172–5. [DOI] [PubMed] [Google Scholar]
- 7.Tan Y, Tu Z, Ye P, et al. Ultrasound guidance for internal jugular vein cannulation in neonates: modified dynamic needle tip positioning short-axis out-of-plane technique versus long-axis in-plane technique, a randomized controlled trial. J Vasc Access 2021;11297298211015043:112972982110150. 10.1177/11297298211015043 [DOI] [PubMed] [Google Scholar]
- 8.Uygun I, Okur MH, Otcu S, et al. Peripherally inserted central catheters in the neonatal period. Acta Cir Bras 2011;26:404–11. 10.1590/S0102-86502011000500014 [DOI] [PubMed] [Google Scholar]
- 9.Qin KR, Nataraja RM, Pacilli M. Long peripheral catheters: is it time to address the confusion? J Vasc Access 2019;20:457–60. 10.1177/1129729818819730 [DOI] [PubMed] [Google Scholar]
- 10.Gorski LA, Hadaway L, Hagle ME, et al. Infusion therapy standards of practice, 8th edition. J Infus Nurs 2021;44:S1–224. 10.1097/NAN.0000000000000396 [DOI] [PubMed] [Google Scholar]
- 11.O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011;52:e162–93. 10.1093/cid/cir257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain ASS, Ali SR, Ariff S, et al. A protocol for quality improvement programme to reduce central line-associated bloodstream infections in NICU of low and middle income country. BMJ Paediatr Open 2017;1:e000008. 10.1136/bmjpo-2017-000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Juaid A, Walkty A, Embil J, et al. Differential time to positivity: vascular catheter drawn cultures for the determination of catheter-related bloodstream infection. Scand J Infect Dis 2012;44:721–5. 10.3109/00365548.2012.678883 [DOI] [PubMed] [Google Scholar]
- 14.Orihuela-Martín J, Rodríguez-Núñez O, Morata L, et al. Performance of differential time to positivity as a routine diagnostic test for catheter-related bloodstream infections: a single-centre experience. Clin Microbiol Infect 2020;26:383.e1–383.e7. 10.1016/j.cmi.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Wang X, Fan L, et al. Iatrogenic pleural effusion due to extravasation of parenteral nutrition via an Epicutaneo cava catheter in neonates: a prospective cohort study. Front Pediatr 2020;8:570978. 10.3389/fped.2020.570978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornbau C, Lee KC, Hughes GD, et al. Central line complications. Int J Crit Illn Inj Sci 2015;5:170–8. 10.4103/2229-5151.164940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wrightson DD. Peripherally inserted central catheter complications in neonates with upper versus lower extremity insertion sites. Adv Neonatal Care 2013;13:198–204. 10.1097/ANC.0b013e31827e1d01 [DOI] [PubMed] [Google Scholar]
- 18.Santos FKY, Flumignan RLG, Areias LL, et al. Peripherally inserted central catheter versus central venous catheter for intravenous access: a protocol for systematic review and meta-analysis. Medicine 2020;99:e20352. 10.1097/MD.0000000000020352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayoumi MAA, Van Rens MFP, Chandra P, et al. Effect of implementing an Epicutaneo-Caval catheter team in neonatal intensive care unit. J Vasc Access 2021;22:243–53. 10.1177/1129729820928182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linck DA, Donze A, Hamvas A. Neonatal peripherally inserted central catheter team. evolution and outcomes of a bedside-nurse-designed program. Adv Neonatal Care 2007;7:22–9. 10.1097/00149525-200702000-00009 [DOI] [PubMed] [Google Scholar]
- 21.Legemaat MM, Jongerden IP, van Rens RMFPT, et al. Effect of a vascular access team on central line-associated bloodstream infections in infants admitted to a neonatal intensive care unit: a systematic review. Int J Nurs Stud 2015;52:1003–10. 10.1016/j.ijnurstu.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 22.Taylor T, Massaro A, Williams L, et al. Effect of a dedicated percutaneously inserted central catheter team on neonatal catheter-related bloodstream infection. Adv Neonatal Care 2011;11:122–8. 10.1097/ANC.0b013e318210d059 [DOI] [PubMed] [Google Scholar]
- 23.Haddadin Y, Annamaraju P, Regunath H. Central line associated blood stream infections. Treasure Island: StatPearls, 2021. [PubMed] [Google Scholar]
- 24.Bonizzoli M, Batacchi S, Cianchi G, et al. Peripherally inserted central venous catheters and central venous catheters related thrombosis in post-critical patients. Intensive Care Med 2011;37:284–9. 10.1007/s00134-010-2043-x [DOI] [PubMed] [Google Scholar]
- 25.Pet GC, Eickhoff JC, McNevin KE, et al. Risk factors for peripherally inserted central catheter complications in neonates. J Perinatol 2020;40:581–8. 10.1038/s41372-019-0575-7 [DOI] [PubMed] [Google Scholar]
- 26.Ragavan M, Gazula S, Yadav DK, et al. Peripherally inserted central venous lines versus central lines in surgical newborns--a comparison. Indian J Pediatr 2010;77:171–4. 10.1007/s12098-009-0291-y [DOI] [PubMed] [Google Scholar]
- 27.Barone G, Pittiruti M, Ancora G, et al. Centrally inserted central catheters in preterm neonates with weight below 1500 G by ultrasound-guided access to the brachio-cephalic vein. J Vasc Access 2020;1129729820940174. [DOI] [PubMed] [Google Scholar]
- 28.Ostroff M, Zauk A, Chowdhury S, et al. A retrospective analysis of the clinical effectiveness of subcutaneously tunneled femoral vein cannulations at the bedside: a low risk central venous access approach in the neonatal intensive care unit. J Vasc Access 2021;22:926–34. 10.1177/1129729820969291 [DOI] [PubMed] [Google Scholar]
- 29.Hosseinpour M, Mashadi MR, Behdad S, et al. Central venous catheterization in neonates: comparison of complications with percutaneous and open surgical methods. J Indian Assoc Pediatr Surg 2011;16:99–101. 10.4103/0971-9261.83487 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request from the corresponding author, Dr Mohammad A A Bayoumi (moh.abdelwahab@hotmail.com). Not applicable.


