Abstract
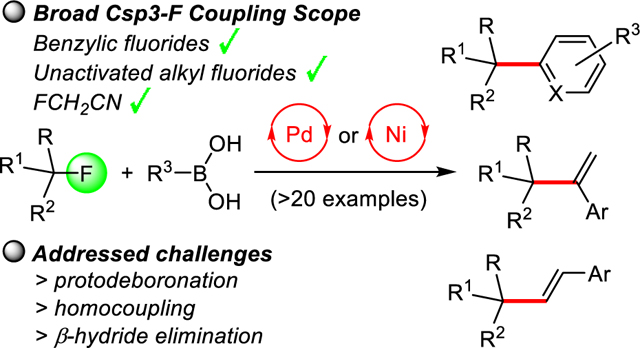
Suzuki cross-coupling of benzylic and unactivated aliphatic fluorides with aryl- and alkenylboronic acids has been achieved via mechanistically distinct Pd and Ni catalyzed pathways that outperform competing protodeboronation, β-hydride elimination and homocoupling processes. The utility is demonstrated with more than twenty examples including heterocyclic structures, 1,1-disubstituted and trans-1,2-disubstituted alkenes, and by the incorporation of acetonitrile into functionalized (hetero)arenes.
Keywords: Suzuki cross-coupling, alkyl fluorides, boronic acids, C-F bond functionalization, carbon-carbon bond formation
Graphical Abstract
The increasing demand for fluorinated compounds has nurtured the introduction of numerous methods that selectively manipulate common functional groups to incorporate fluorine.1 The widespread success of organofluorines entails multifaceted consequences; it provides new synthetic opportunities but also gives rise to environmental concerns. The chemical stability and resistance to metabolic degradation of the C-F bond result in accumulation of potentially toxic pollutants that can ultimately migrate into the food chain. Several groups have started to address this problem with the introduction of hydrodefluorination reactions that convert C-F into C-H bonds.2–5 Alternatively, the steady growth of a large pool of commercially available or easily prepared fluorinated building blocks sets the stage for new synthetic developments that in stark contrast to traditional methods target the C-F bond moiety for selective functionalization.
To date, chemoselective organofluorine functionalization has remained challenging and often requires harsh reaction conditions to overcome the low reactivity and high bond dissociation energy (>100 kcal/mol) of the Csp3-F bond. Significant progress has been reported with substitution, elimination and cross-coupling reactions using aryl or alkenyl fluorides.6 Despite emerging reports on carbon-heteroatom bond formation7–11 and carbon-carbon bond construction12–17 the development of methods that exploit aliphatic substrates still lacks behind Csp2-F bond functionalization capabilities and is often limited to allylic, propargylic or benzylic fluorides.18–23 In particular when highly reactive Lewis acids are required for the carbon-fluorine activation step, the synthetic utility is severely compromised due to low functional group tolerance and competing elimination or rearrangement pathways that result in unsatisfactory yields.
Our laboratory has become increasingly interested in organofluorine chemistry24–36 and we have encountered synthetically useful C-F bond scission pathways over the years.16,37 This encouraged us to investigate if alkyl fluorides can be applied in Suzuki cross-couplings despite the well-known challenges that aliphatic substrate pose. Transition metal catalyzed C-C bond formation with alkyl halides via cross-coupling pathways has proven complicated, in part because of competing β-hydride elimination or other side reactions. Alkyl fluorides have remained particularly difficult substrates. Kumada-Corriu reactions with Grignard reagents have been reported while broadly useful Suzuki coupling protocols remain elusive.38–51 We now demonstrate Csp2-Csp3 bond construction based on catalytic Suzuki cross-coupling of both benzylic and unactivated aliphatic fluorides with arylboronic or vinylboronic acids (Scheme 1). This is accomplished based on mechanistically distinct palladium and nickel catalysis to overcome competing protodeboronation, β-hydride elimination and homocoupling processes. The cross-coupling is generally high-yielding, utilizes commercially available catalysts, inexpensive LiI to generate LiF which serves as a thermodynamic sink to enable C-F bond activation, and exhibits high functional group compatibility.
Scheme 1. Scope and challenges of transition metal catalyzed Suzuki cross-coupling chemistry.
At the onset of this study, we examined the palladium catalyzed cross-coupling of 4-nitrobenzyl fluoride, 1, and phenylboronic acid, 2 (Table 1 and SI). We envisaged that the use of a thermodynamic sink to compensate for the endothermic cleavage of the Csp3-F bond might allow transition metal catalyzed Csp2-Csp3 bond formation with aryl- and vinylboronic acids. We selected LiI as the most promising Lewis acid for this task because of the high fluoride affinity of lithium and the expectation that the presence of lithium salts would not interfere with the transition metal catalysis.
Table 1.
Development of palladium catalyzed Suzuki cross-coupling with activated fluorides.

| |||||
|---|---|---|---|---|---|
| Entry | Ligand | Base | Time (h) | Temp. (°C) | Yield (%) |
|
| |||||
| 1 | L1 | K2CO3 | 24 | 25 | 0 |
| 2 | L1 | K2CO3 | 18 | 80 | 33 |
| 3 | L1 | K2CO3 | 2 | 90 | 29a |
| 4 | L1 | K3PO4 | 18 | 80 | 33 |
| 5 | L1 | K3PO4 | 18 | 100 | 40 |
| 6 | L1 | Cs2CO3 | 18 | 70 | 43 |
| 7 | L1 | Cs2CO3 | 18 | 100 | 60b |
| 8 | L1 | Cs2CO3 | 18 | 100 | 54c |
| 9 | L2 | Cs2CO3 | 36 | 100 | 68 |
| 10 | L2 | K2CO3 | 18 | 100 | 28 |
| 11 | L2 | K3PO4 | 18 | 100 | 42 |
| 12 | L3 | Cs2CO3 | 18 | 100 | 54 |
| 13 | L4 | Cs2CO3 | 18 | 100 | 57 |
| 14 | L5 | Cs2CO3 | 18 | 100 | 29 |
| 15 | L6 | Cs2CO3 | 18 | 100 | 51 |
| 16 | L7 | Cs2CO3 | 18 | 100 | 92 |
| 17 | L7 | Cs2CO3 | 18 | 100 | 95d |
| 18 | L7 | Cs2CO3 | 18 | 100 | 91e |
Conditions: 1 (0.2 mmol), 2 (0.6 mmol), LiI (0.3 mmol), Pd(OAc)2 (5 mol%), ligand (10 mol%) in 2.0 mL toluene.
Microwave reaction.
28% of 4 isolated.
m-xylene used as solvent.
0.4 mmol of 2.
0.3 mmol of 2. All reported yields are for isolated product 3.
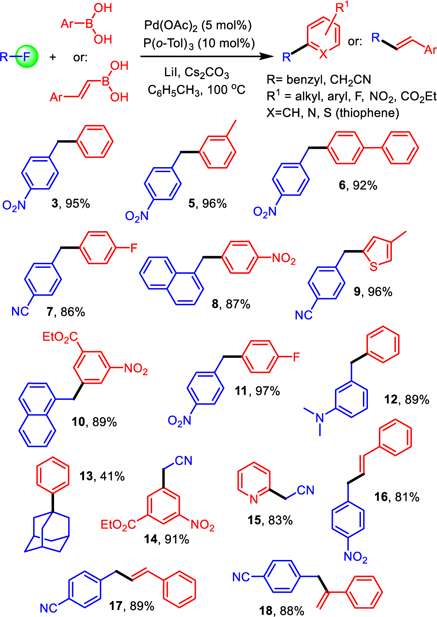
After some initial screening of solvent, base and temperature using palladium acetate and tri-tert-butylphosphine as ligand, we were able to isolate 1-benzyl-4-nitrobenzene, 3, in encouraging 60% yield (entries 1–8 in Table 1). Microwave irradiation proved to accelerate the reaction but did not give superior yields compared to conventional heating (compare entries 2 and 3). Further reaction analysis revealed formation of substantial amounts of biphenyl and 1,2-bis(4-nitrophenyl)ethane, 4, which was unequivocally identified by X-ray analysis. To suppress the homocoupling process, which could result from dynamic exchange between intermediate alkyl(aryl)Pd(II) complexes, we continued with optimizing the catalyst structure using cesium carbonate as base and toluene as solvent at 100 °C. The testing of several mono- and bidentate phosphines under these conditions showed that the desired Csp2-Csp3 cross-coupling product 3 is formed in 95% yield when tri(o-tolyl)phosphine is used as ligand (entries 9–18). It is noteworthy that we obtained 3 in 97% yield when the reaction was conducted at 1.25 mmol under otherwise identical conditions.
We then examined the reaction scope and found that high yields are obtained with benzylic fluorides and also with readily available fluoroacetonitrile, providing access to unsymmetrically substituted 1,1’-di(hetero)arylalkanes and practical means to introduce an acetonitrile group into an aromatic ring via Suzuki coupling (Scheme 2). We first screened a variety of arylboronic acids and benzylic fluorides which gave the desired products 3 and 5-12 exhibiting several functional groups that are not tolerated under standard Kumada-Corriu reaction conditions as well as heterocyclic structures in 86–96% yield. The reaction with sterically demanding fluoroadamantane still gave 13 in 41% yield. The same protocol allows coupling of fluoroacetonitrile and we isolated 14 and 15 from the reactions with (3-(ethoxycarbonyl)-5-nitrophenyl)boronic acid and 2-pyridylboronic acid, respectively, in 83–91% yield. Alternatively, vinylboronic acids can be used as well and the trans-1,2- and 1,1-disubstituted alkenes 16-18 were produced in 81–89% yield.
Scheme 2. Reaction scope of the Suzuki cross-coupling with benzylic fluorides and fluoroacetonitrile.
Pd(OAc)2 (5 mol%), P(o-tolyl)3 (10 mol%), fluoroacetonitrile or benzylic fluoride (0.2 mmol), boronic acid (0.4 mmol), LiI (0.3 mmol) and Cs2CO3 (0.4 mmol) in 2 mL of dry toluene under inert atmosphere at 100 °C for 18 hours. All reported yields are for isolated products. See SI for details.
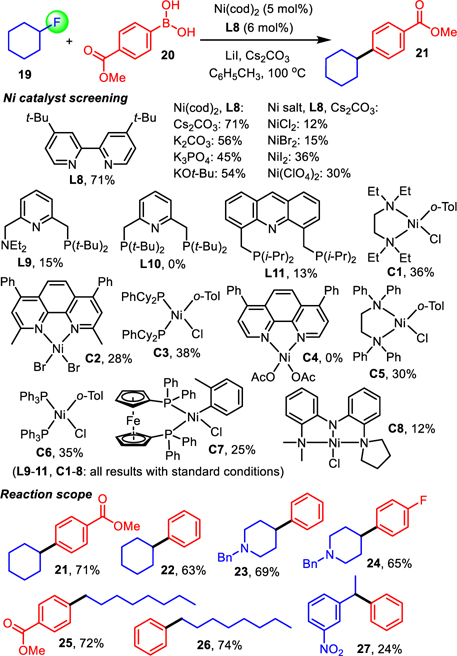
Unfortunately, the reaction with fluorocyclohexane, 19, and 4-methoxycarbonylphenyl boronic acid, 20, was low-yielding which we attributed to competing protodeboronation and uncontrolled HF elimination, a major challenge when electrophiles susceptible to β-hydride elimination are used in late transition metal catalysis. To mitigate these complications and to further expand the realm of Csp2-Csp3 cross-coupling to unactivated alkyl fluorides we resorted to nickel catalysis in order to facilitate the oxidative addition step with these challenging substrates and to reduce the rate of undesirable β-hydride elimination.52 We systematically investigated Ni salts, base additives, solvents, N- and P-donating ligands comprising a wide range of electronic and steric properties, pincer ligands that presumably block a vacant coordination site which is a prerequisite for β-hydride elimination as well as prefabricated complexes (Scheme 3 and SI).
Scheme 3. Development of nickel catalyzed Suzuki cross-coupling with unactivated alkyl fluorides.
Ni(cod)2 (5 mol%), L8 (6 mol%), alkyl fluoride (0.2 mmol), boronic acid (0.4 mmol), LiI (0.3 mmol) and Cs2CO3 (0.4 mmol) in 2 mL of dry toluene under inert atmosphere at 100 °C for 48 hours. All reported yields are for isolated products. See SI for more details.
While several combinations gave the desired cross-coupling product 21 from 18 and the arylboronic acid 20 superior results were obtained with 5 mol% of Ni(cod)2 and 4,4’-di-tert-butyl-2,2’-dipyridyl, L8, in the presence of LiI and cesium carbonate in toluene. We were thus able to prepare 21 in 71% yield, and we successfully employed other primary and secondary alkyl fluorides exhibiting β-hydrogen atoms in this protocol, producing 22-26 in 63–74% yield. Under the optimized conditions, formation of by-products due to β-hydride elimination and protodemetalation is generally suppressed to only 10–20% and less than 10%, respectively, while traces of homocoupling products are observed. However, secondary benzylic fluorides remain challenging and 27 was obtained in only 24% yield. A decrease in yields was observed when Ni(cod)2 was replaced by NiCl2, NiBr2, NiI2 or Ni(ClO4)2 and when K2CO3, K3PO4 or KOt-Bu were used as base. The reaction did not occur in dioxane, DMF, IPA and t-BuOH.
Several features of the transition metal catalyzed Suzuki cross-coupling chemistry merit additional discussion. Both benzylic and unactivated alkyl monofluorides containing β-hydrogens can be used which transforms the accessible chemical space of the notoriously inert Csp3-F bond and introduces aliphatic fluorides to synthetic transformations generally associated with chlorides, bromides and iodides. In addition, common functionalities including aryl fluoride bonds, ester, nitro and nitrile groups are tolerated which further extends the application scope and utility. For example, the fluorinated arenes 7, 11 and 24 were produced in 69–97% yield, demonstrating successful Csp3-F bond activation while Csp2-F bonds are left intact. Formation of the desired cross-coupling products was not observed in the absence of a transition metal catalyst or when LiI was replaced by MgI2 under otherwise identical reaction conditions.53 The Pd and Ni catalyzed Suzuki couplings with benzylic and unactivated alkyl fluorides, respectively, appear to occur via mechanistically distinct pathways. The addition of the radical scavenger TEMPO into the standard palladium catalysis protocol did not affect the reaction outcome. In fact, we obtained 3 from phenylboronic acid and 4-nitrobenzyl fluoride in 94–96% yields when we added one or five equivalents of TEMPO which is in agreement with a nonradical two-electron transfer mechanism (Scheme 4).
Scheme 4. Radical trapping experiments with TEMPO and clock reaction analysis with fluoride 28.
See SI for details. n.d. = not detected.
By contrast, the Ni catalyzed Suzuki reaction with fluorocyclohexane did not give the cross-coupling product 22 in the presence of TEMPO which indicates the involvement of a single-electron transfer process and radical intermediates although a reaction shutdown due to catalyst poisoning cannot be excluded. We were able to corroborate the predominance of a radical pathway during Ni catalysis using 6-fluorohex-1-ene, 28, as cyclizable probe in our standard protocol developed for unactivated alkyl fluorides. GC-MS and NMR spectroscopy analysis of the radical clock reaction experiment with 28 revealed that the 5-exo-trig cyclization product 29 was formed in 68% yield while the acyclic cross-coupling product 30 was obtained in only 10% yield. We note, however, that migratory insertion of an organonickel intermediate would provide the same outcome.
In conclusion, we have achieved Suzuki cross-coupling with benzylic and unactivated aliphatic fluorides using arylboronic or vinylboronic acids. The palladium and nickel catalyzed methods utilize commercially available metal complexes, ligands and inexpensive LiI producing LiF which serves as a thermodynamic sink to facilitate C-F bond activation with a variety of monofluoroalkyl compounds under conditions that are compatible with the catalytic carbon-carbon bond formation steps. Mechanistically distinct pathways that effectively control competing protodeboronation, β-hydride elimination and homocoupling processes thus enable generally high-yielding cross-coupling chemistry with high functional group compatibility. The utility is demonstrated with more than twenty examples including heterocyclic structures, 1,1-disubstituted and trans-1,2-disubstituted alkenes, and by the incorporation of the synthetically versatile acetonitrile group into functionalized (hetero)arenes. This study thus significantly extends C-F bond functionalization chemistry to challenging aliphatic substrates that now can be used for the synthesis of multifunctional compounds by late-transition metal catalysis.
General Procedure
To a reaction vial containing a Teflon-coated magnetic stirring bar were sequentially added Pd(OAc)2 (15 mg, 0.065 mmol, 5 mol%), P(o-tolyl)3 (39 mg, 0.129 mmol, 10 mol%), 1-(fluoromethyl)-4-nitrobenzene (200 mg, 1.29 mmol), phenylboronic acid (314 mg, 2.58 mmol), LiI (259 mg, 1.94 mmol) and Cs2CO3 (840 mg, 2.58 mmol) followed by 10 mL of toluene under inert atmosphere. The vial was sealed, wrapped with aluminum foil, and stirred at 100 °C in an oil bath for 18 hours. After full conversion was achieved based on TLC analysis, the solvent was removed and the crude product was purified by flash column chromatography on silica gel using 19:1 hexanes/ethyl acetate as the mobile phase. 1-Benzyl-4-nitrobenzene, 3, was obtained as a colorless oil in 97% yield (266 mg, 1.25 mmol). Rf = 0.5 (hexanes/EtOAc, 4:1); 1H NMR (400 MHz, chloroform-d) δ = 8.13 (d, J = 8.4 Hz, 2H), 7.36 – 7.28 (m, 4H), 7.24 (m, 1H), 7.17 (d, J = 6.8 Hz, 2H), 4.07 (s, 2H); 13C NMR (100 MHz, chloroform-d) δ = 149.0, 146.7, 139.3, 129.8, 129.1, 128.9, 126.9, 123.9, 41.9; HRMS (ESI-TOF) m/z: [M+H]+ calcd for C13H12NO2 214.0863, found 214.0866.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge financial support from the US National Institutes of Health (GM106260).
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental details, synthetic procedures, NMR spectra and X-ray crystallographic analysis The Supporting Information is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Zhou Y; Wang J; Gu Z; Wang S; Zhu W; Acena JL; Soloshonok VA; Izawa K; Liu H Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [DOI] [PubMed] [Google Scholar]
- (2).Panisch R; Bolte M; Müller T Hydrogen- and Fluorine-Bridged Disilyl Cations and Their Use in Catalytic C−F Activation. J. Am. Chem. Soc. 2006, 128, 9676–9682. [DOI] [PubMed] [Google Scholar]
- (3).Douvris C; Ozerov OV Hydrodefluorination of Perfluoroalkyl Groups Using Silylium-Carborane Catalysts. Science 2008, 321, 1188–1190. [DOI] [PubMed] [Google Scholar]
- (4).Choi J; Wang DY; Kundu S; Choliy Y; Emge TJ; Krogh-Jespersen K; Goldman AS Net Oxidative Addition of C(sp3)-F Bonds to Iridium via Initial C-H Bond Activation. Science 2011, 332, 1545–1548. [DOI] [PubMed] [Google Scholar]
- (5).Caputo CB; Hounjet LJ; Dobrovetsky R; Stephan DW Lewis Acidity of Organofluorophosphonium Salts: Hydrodefluorination by a Saturated Acceptor. Science 2013, 341, 1374–1377. [DOI] [PubMed] [Google Scholar]
- (6).Amii H; Uneyama K C-F Bond Activation in Organic Synthesis. Chem. Rev. 2009, 109, 2119–2183. [DOI] [PubMed] [Google Scholar]
- (7).Traff AM; Janjetovic M; Hilmersson G C–F Bond Substitution via Aziridinium Ion Intermediates. Chem. Commun. 2015, 51, 13260–13263. [DOI] [PubMed] [Google Scholar]
- (8).Jaiswal AK; Prasad PK; Young RD Nucleophilic Substitution of Aliphatic Fluorides via Pseudohalide Intermediates. Chem. Eur. J. 2019, 25, 6290–6294. [DOI] [PubMed] [Google Scholar]
- (9).Traff AM; Janjetovic M; Ta L; Hilmersson G Selective C-F Bond Activation: Substitution of Unactivated Alkyl Fluorides Using YbI3. Angew. Chem. Int. Ed. 2013, 52, 12073–12076. [DOI] [PubMed] [Google Scholar]
- (10).Janjetovic M; Ekebergh A; Traff AM; Hilmersson G Catalytic Iodination of the Aliphatic C–F Bond by YbI3(THF)3: Mechanistic Insight and Synthetic Utility. Org. Lett. 2016, 18, 2804–2807. [DOI] [PubMed] [Google Scholar]
- (11).Mandal D; Gupta R; Jaiswal AK; Young RD Frustrated Lewis-Pair-Meditated Selective Single Fluoride Substitution in Trifluoromethyl Groups. J. Am. Chem. Soc. 2020, 142, 2572–2578. [DOI] [PubMed] [Google Scholar]
- (12).Gu W; Haneline MR; Douvris C; Ozerov OV Carbon−Carbon Coupling of C(sp3)−F Bonds Using Alumenium Catalysis. J. Am. Chem. Soc. 2009, 131, 11203–11212. [DOI] [PubMed] [Google Scholar]
- (13).Zhu J; Perez M; Caputo CB; Stephan DW Use of Trifluoromethyl Groups for Catalytic Benzylation and Alkylation with Subsequent Hydrodefluorination. Angew. Chem. Int. Ed. 2016, 55, 1417–1421. [DOI] [PubMed] [Google Scholar]
- (14).Dryzhakov M; Moran J Autocatalytic Friedel–Crafts Reactions of Tertiary Aliphatic Fluorides Initiated by B(C6F5)3·H2O. ACS Catal. 2016, 6, 3670–3673. [Google Scholar]
- (15).Yoshida S; Shimomori K; Kim Y; Hosoya T Single C−F Bond Cleavage of Trifluoromethylarenes with an ortho-Silyl Group. Angew. Chem. Int. Ed. 2016, 55, 10406–10409. [DOI] [PubMed] [Google Scholar]
- (16).Balaraman K; Wolf C Catalytic Enantioselective and Diastereoselective Allylic Alkylation with Fluoroenolates: Efficient Access to C3-Fluorinated and All-Carbon Quaternary Oxindoles. Angew. Chem. Int. Ed. 2017, 56, 1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Willcox DR; Nichol GS; Thomas SP Borane-Catalyzed C(sp3)–F Bond Arylation and Esterification Enabled by Transborylation. ACS Catal. 2021, 11, 3190–3197. [Google Scholar]
- (18).Champagne PA; Benhassine Y; Desroches J; Paquin J -F. Friedel-Crafts Reaction of Benzyl Fluorides: Selective Activation of C-F Bonds as Enabled by Hydrogen Bonding. Angew. Chem. Int. Ed. 2014, 53, 13835–13839. [DOI] [PubMed] [Google Scholar]
- (19).Hamel JD; Paquin J -F. Activation of C–F Bonds α to C–C Multiple Bonds. Chem. Commun. 2018, 54, 10224–10239. [DOI] [PubMed] [Google Scholar]
- (20).Zi Y; Lange M; Schultz C; Vilotijevic I Latent Nucleophiles in Lewis Base Catalyzed Enantioselective N-Allylations of N-Heterocycles. Angew. Chem. Int. Ed. 2019, 58, 10727–10731. [DOI] [PubMed] [Google Scholar]
- (21).Butcher TW; Yang JL; Amberg WM; Watkins NB; Wilkinson ND; Hartwig JF Desymmetrization of Difluoromethylene Groups by C–F Bond Activation. Nature 2020, 583, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Vasilopoulos A; Golden DL; Buss JA; Stahl AS Copper-Catalyzed C–H Fluorination/Functionalization Sequence Enabling Benzylic C–H Cross Coupling with Diverse Nucleophiles. Org. Lett. 2020, 22, 5753–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Nishimine T; Fukushi K; Shibata N; Taira H; Tokunaga E; Yamano A; Shiro M; Shibata N Kinetic Resolution of Allyl Fluorides by Enantioselective Allylic Trifluoromethylation Based on Silicon-Assisted C-F Bond Cleavage. Angew. Chem. Int. Ed. 2014, 53, 517–520. [DOI] [PubMed] [Google Scholar]
- (24).Yearick K; Wolf C Catalytic Enantioselective Addition of Diethylzinc to Trifluoromethyl Ketones. Org. Lett. 2008, 10, 3915–3918. [DOI] [PubMed] [Google Scholar]
- (25).Xu H; Wolf C Synthesis of chiral tertiary trifluoromethyl alcohols by asymmetric nitroaldol reaction with a Cu(II)-bisoxazolidine catalyst. Chem. Commun. 2010, 8026–8028. [DOI] [PubMed] [Google Scholar]
- (26).Wolf C; Zhang P Asymmetric Friedel–Crafts Reaction of Indoles with Ethyl Trifluoropyruvate Using a Copper(I)-Bisoxazolidine Catalyst. Adv. Synth. Catal. 2011, 353, 760–766. [Google Scholar]
- (27).Zhang P; Wolf C Synthesis of Pentafluorinated β-Hydroxy Ketones. J. Org. Chem. 2012, 77, 8840–8844. [DOI] [PubMed] [Google Scholar]
- (28).Zhang P; Wolf C Catalytic Enantioselective Difluoroalkylation of Aldehydes. Angew. Chem. Int. Ed. 2013, 52, 7869–7873. [DOI] [PubMed] [Google Scholar]
- (29).Cook AM; Wolf C Efficient Access to Multifunctional Trifluoromethyl Alcohols through Base-Free Catalytic Asymmetric C−C Bond Formation with Terminal Ynamides. Angew. Chem. Int. Ed. 2016, 55, 2929–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ding R; Wolf C Catalytic insertion of aldehydes into dihalonitroacetophenones via sequential bond scission-aldol reaction-acyl transfer. Chem. Commun. 2016, 52, 3576–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Balaraman K; Moskowitz M; Liu Y; Wolf C Detrifluoroacetylative Generation of Halogenated Enolates: Practical Access to Perhalogenated Ketones and Alkenes. Synthesis 2016, 48, 2376–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Balaraman K; Ding R; Wolf C Stereoselective Synthesis of 3,3’-Bisindolines by Organocatalytic Michael Additions of Fluorooxindole Enolates to Isatylidene Malononitriles in Aqueous Solution. Adv. Synth. Catal. 2017, 359, 4165–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Ding R; Wolf C Organocatalytic Asymmetric Synthesis of α-Oxetanyl and α-Azetidinyl Tertiary Alkyl Fluorides and Chlorides. Org. Lett. 2018, 20, 892–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Moskowitz M; Balaraman K; Wolf C Organocatalytic Stereoselective Synthesis of Fluorinated 3,3′-Linked Bisoxindoles. J. Org. Chem. 2018, 83, 1661–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Balaraman K; Moskowitz M; Wolf C Organocatalytic Decarboxylative Cyanomethylation of Difluoromethyl and Trifluoromethyl Ketones. Adv. Synth. Catal. 2018, 360, 4705–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Ding R; De los Santos ZA; Wolf C Catalytic Asymmetric Mannich Reaction of α-Fluoronitriles with Ketimines: Enantioselective and Diastereodivergent Construction of Vicinal Tetrasubstituted Stereocenters. ACS Catal. 2019, 9, 2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Ding R; Bakhshi PR; Wolf C Organocatalytic Insertion of Isatins into Aryl Difluoronitromethyl Ketones. J. Org. Chem. 2016, 82, 1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Terao J; Ikumi A; Kuniyasu H; Kambe N Ni- or Cu-Catalyzed Cross-Coupling Reaction of Alkyl Fluorides with Grignard Reagents. J. Am. Chem. Soc. 2003, 125, 5646–5647. [DOI] [PubMed] [Google Scholar]
- (39).Matsubara K; Ishibashi T; Koga Y C-F Bond Cleavage Reactions of Fluoroalkanes with Magnesium Reagents and without Metal Catalysts. Org. Lett. 2009, 11, 1765–1768. [DOI] [PubMed] [Google Scholar]
- (40).Blessley G; Holden P; Walker M; Brown JM; Gouverneur V Palladium-Catalyzed Substitution and Cross-Coupling of Benzylic Fluorides. Org. Lett. 2012, 14, 2754–2757. [DOI] [PubMed] [Google Scholar]
- (41).Erickson LW; Lucas EL; Tollefson EJ; Jarvo ER Nickel-Catalyzed Cross-Electrophile Coupling of Alkyl Fluorides: Stereospecific Synthesis of Vinylcyclopropanes. J. Am. Chem. Soc. 2016, 138, 14006–15011. [DOI] [PubMed] [Google Scholar]
- (42).Iwasaki T; Yamashita K; Kuniyasu H; Kambe N Co-Catalyzed Cross-Coupling Reaction of Alkyl Fluorides with Alkyl Grignard Reagents. Org. Lett. 2017, 19, 3691–3694. [DOI] [PubMed] [Google Scholar]
- (43).Wolfe MMW; Shanahan JP; Kampf JW; Szymczak NK Defluorinative Functionalization of Pd(II) Fluoroalkyl Complexes. J. Am. Chem. Soc. 2020, 142, 18698–18705. [DOI] [PubMed] [Google Scholar]
- (44).Hatakeyama T; Ito S; Nakamura M; Nakamura E Alkylation of Magnesium Enamide with Alkyl Chlorides and Fluorides. J. Am. Chem. Soc. 2005, 127, 14192–14193. [DOI] [PubMed] [Google Scholar]
- (45).Sai M; Someya H; Yorimitsu H; Oshima K Copper-Catalyzed Reaction of Alkyl Halides with Cyclopentadienylmagnesium Reagent. Org. Lett. 2008, 10, 2545–2547. [DOI] [PubMed] [Google Scholar]
- (46).Mo Z; Zhang Q; Deng L Dinuclear Iron Complex-Catalyzed Cross-Coupling of Primary Alkyl Fluorides with Aryl Grignard Reagents. Organometallics 2012, 31, 6518–6521. [Google Scholar]
- (47).Iwasaki T; Shimizu R; Imanishi R; Kuniyasu H; Kambe N Cross-coupling Reaction of Alkyl Halides with Alkyl Grignard Reagents Catalyzed by Cp-Iron Complexes in the Presence of 1,3-Butadiene. Chem. Lett. 2018, 47, 763–766. [Google Scholar]
- (48).Terao J; Watabe H; Kambe N Ni-Catalyzed Alkylative Dimerization of Vinyl Grignard Reagents Using Alkyl Fluorides. J. Am. Chem. Soc. 2005, 127, 3656–3657. [DOI] [PubMed] [Google Scholar]
- (49).Iwasaki T; Shimizu R; Imanishi R; Kuniyasu H; Kambe N Copper-Catalyzed Regioselective Hydroalkylation of 1,3-Dienes with Alkyl Fluorides and Grignard Reagents. Angew. Chem. Int. Ed. 2015, 54, 9347–9350. [DOI] [PubMed] [Google Scholar]
- (50).Iwasaki T; Min X; Fukuoka A; Kuniyasu H; Kambe N Nickel-Catalyzed Dimerization and Alkylarylation of 1,3-Dienes with Alkyl Fluorides and Aryl Grignard Reagents. Angew. Chem. Int. Ed. 2016, 55, 5550–5554. [DOI] [PubMed] [Google Scholar]
- (51).Iwasaki T; Fukuoka A; Yokoyama W; Min X; Hisaki I; Yang T; Ehara M; Kuniyasu H; Kambe N Nickel-catalyzed Coupling Reaction of Alkyl Halides with Aryl Grignard Reagents in the Presence of 1,3-Butadiene: Mechanistic Studies of Four-Component Coupling and Competing Cross-coupling Reactions. Chem. Sci. 2018, 9, 2195–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Tasker SZ; Standley EA; Jamison TF Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).GC-MS analysis of the reactions showed formation of alkyl iodide intermediates.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.