Summary
Objective
MicroRNA 140 (miR-140) is a chondrocyte-specific endogenous gene regulator implicated in osteoarthritis (OA). As mechanical injury is a primary aetiological factor in OA, we investigated miR-140-dependent mechanosensitive gene regulation using a novel CRISPR-Cas9 methodology in primary human chondrocytes.
Method
Primary (passage 1/2) human OA chondrocytes were isolated from arthroplasty samples (six donors) and transfected with ribonuclear protein complexes or plasmids using single guide RNAs (sgRNAs) targeting miR-140, in combination with Cas9 endonuclease. Combinations of sgRNAs and single/double transfections were tested. Gene editing was measured by T7 endonuclease 1 (T7E1) assay. miRNA levels were confirmed by qPCR in chondrocytes and in wild type murine femoral head cartilage after acute injury. Predicted close match off-targets were examined. Mechanosensitive miR-140 target validation was assessed in 42 injury-associated genes using TaqMan Microfluidic cards in targeted and donor-matched control chondrocytes. Identified targets were examined in RNAseq data from costal chondrocytes from miR-140−/− mice.
Results
High efficiency gene editing of miR-140 (90–98%) was obtained when two sgRNAs were combined with double RNP-mediated CRISPR-Cas9 transfection. miR-140 levels fell rapidly after femoral cartilage injury. Of the top eight miR-140 gene targets identified (P < 0.01), we validated three previously identified ones (septin 2, bone morphogenetic protein 2 and fibroblast growth factor 2). Novel targets included Agrin, a newly recognised pro-regenerative cartilage agent, and proteins associated with retinoic acid signalling and the primary cilium.
Conclusion
We describe a highly efficient CRISPR-Cas9-mediated strategy for gene editing in primary human chondrocytes and identify several novel mechanosensitive miR-140 targets of disease relevance.
Keywords: Chondrocyte, Human, Osteoarthritis, CRISPR-Cas9, miR-140, Injury
Introduction
Osteoarthritis (OA) is generally accepted as a biologically driven disease where mechanical stresses, combined with other factors, lead to imbalance between catabolic and repair activities within the joint1. Multiple tissues of the joint are affected causing degradation of the articular cartilage, remodelling of subchondral bone and hypertrophy of the synovium2. The chondrocyte is regarded as a critical disease player; being highly mechanosensitive, able to synthesise its own degradative enzymes and having limited renewal, leading to poor tissue repair (reviewed in3). Multiple pathways have been associated with disease modification and many of these have been validated in murine models where the molecule of interest is deleted or suppressed pharmacologically. In recent years, exploitation of some of these pathways has been explored in clinical trials with some promising results, although we are still a way from disease modification in the clinic4.
MicroRNAs (miRs) are important regulatory molecules in all animal and plant cells. They are small endogenous RNAs, typically 20–25 nucleotides in length, that suppress specific mRNAs, by binding and targeting the mRNA for degradation or by suppressing protein translation5. Each miR is formed of a 5 prime (5p) and 3 prime (3p) strand that forms a hairpin loop. miRs undergo a process of maturation using two key enzymes, Drosha and Dicer, deletion of which have profound developmental phenotypes6,7. The mature miR is then loaded onto the Argonaute protein to form the active RNA-induced silencing complex8.
miRs are highly regulated in osteoarthritic cartilage and elsewhere within the joint9,10 and several have been investigated in vitro and in vivo with evidence of disease modification11, 12, 13, 14. One of the best studied of these is MicroRNA 140 (miR-140) which is specifically, and highly expressed in articular cartilage15,16. miR-140 is hosted by the gene WW domain containing protein 2 (WWP2), an E3 ubiquitin ligase. Genetic deletion of either miR-140 or Wwp2 in mice leads to skeletal abnormalities and accelerated OA17. These are thought to occur by affecting catabolic activity of key pathogenic proteinases such as a disintegrin and metalloproteinase with thrombospondin motif-5 (ADAMTS5), although through distinct mechanisms18, 19, 20. miR-140 influences chondrocyte proliferation by reducing Sp1, a transcription factor which controls the cell cycle regulator p15 21 and is modulated by mechanically driven signals22. As mutations affecting miR-140 have also been linked to skeletal abnormalities in humans23, these studies indicate that miR-140 plays an important homeostatic and chondroprotective role in the developing skeleton and adult joint. miRs can be targeted for therapeutic gain and can themselves be used as therapeutic agents, so their biology is of particular interest24.
Both miRs and small interfering RNAs (siRNAs) bind to specific mRNAs to target them for destruction25. siRNAs tend to have single specific targets (designed to recognise foreign mRNAs from invading pathogens) and this has been exploited as an efficient laboratory and in vivo tool. miRs usually have multiple endogenous gene targets through which they modulate cell behaviour. A recent breakthrough molecular approach utilises clustered regularly interspaced short palindromic repeats (CRISPR), an antiviral (anti-bacteriophage) defense system of prokaryotic cells, forming part of their innate immune response26. CRISPR sequences bind to CRISPR-associated proteins (Cas), such as Cas9, an endonuclease, that cuts the DNA after CRISPR recognises complementary DNA sequences in association with a protospacer adjacent motif (PAM). This technology can be adapted to gene editing in eukaryotic cells by designing a CRISPR-Cas9 construct that has a single guide RNA (sgRNA) that recognizes a complementary DNA target region when it is adjacent to a PAM motif (NGG or NRG, commonly found within the mammalian genome)27. After endonuclease action, subsequent repair of the DNA is attempted by non-homologous end joining (NHEJ), which is error prone, and hence usually leads to gene disruption. The advantage of this approach over siRNA-mediated gene suppression, is that it targets genomic DNA and thus gene silencing is permanent and can be transferred to daughter cells.
CRISPR-Cas9 mediated gene editing of primary human chondrocytes is challenging and previous reported transfection efficiencies have varied between 16% and 70%28,29. To explore the effects of gene editing on chondrocyte biology it has been necessary to examine either edited chondrocyte cell lines30, chondrocytes derived from edited induced pluripotent stem cells (iPSCs)31 or using clonally expanded edited chondrocytes that are likely to have lost their chondrocytic phenotype.
In this study we optimize a method to use CRISPR-Cas 9 to drive deletion of miR-140 in primary human articular chondrocytes with high efficiency and without affecting WWP2 expression. We examine the regulation of miR-140 upon ex vivo murine cartilage injury and explore the regulation of a number of previously described and novel targets that are relevant to chondrocyte mechanobiology and OA pathogenesis.
Methods
Human tissue: Osteoarthritic human articular chondrocytes were isolated from tissue obtained from individuals undergoing unicompartmental (UKA), or total knee replacement (TKR). There were no exclusion criteria. Samples, Kellgren and Lawrence grades 3–4, were obtained from the Oxford Musculoskeletal Biobank and were collected with informed donor consent in full compliance with national and institutional ethical requirements, the UK Human Tissue Act, and the Declaration of Helsinki (HTA Licence 12,217 and Oxford REC C 09/H0606/11).
sgRNA design: For gene editing, the ALT-R® CRISPR-Cas9 system from Integrated DNA Technologies (IDT, Coralville, IA, USA) was applied. The Cas9 protein, trans-activating CRISPR RNA (tracrRNA) and CRISPR RNA (crRNA) were all acquired from the same company. To form a functional sgRNA duplex, 3 μl of tracrRNA (5 nmol) were mixed with 3 μl of target-specific crRNA (2 nmol) in 94 μl IDT nuclease free duplex buffer and RNA quantified by nanodrop. While the universal tracrRNA forms the backbone, the crRNA is custom designed and target-specific. The required amount was then incubated for 5 min at 95°C and slowly annealed at room temperature for 10 min. Off and on-targets were predicted using a combination of two software packages: IDT (https://eu.idtdna.com/pages) and SANGER - https://www.sanger.ac.uk/htgt/wge/find_off_targets_by_seq.
Transfections: For RNP transfections, sgRNA (400 ng in total) was complexed with Cas9 (1 μg) for 5 min in 50ul reduced serum medium (Opti-MEM, Gibco, NY, USA). Cas9 Plus reagent (2 μl) was added before incubation. Cas9 Plus is part of Lipofectamine CRISPRMAX (Invitrogen, CA, USA). In another tube, Lipofectamine CRISPRMAX (3.5 μl) was added to Opti-MEM (50 μl), and incubated for 5 min. The Opti-MEM media containing the sgRNA/Cas9 complex was carefully added to the Opti-MEM containing Lipofectamine CRISPRMAX and incubated for another 10 min before slowly pipetting the Cas9/sgRNA/Lipofectamine into the cell media. For double transfection, cells were left for 48 h, exchanged into 10%FBS/DMEM for 24 h, then a second round of transfection by addition of freshly prepared Opti-MEM media containing the sgRNA/Cas9 complex/Lipofectamine CRISPRMAX was performed. Cells were incubated in 10%FBS/DMEM for a further 48 h before testing.
T7 Endonuclease 1 (T7E1) assay: DNA was first amplified by quantitative polymerase chain reaction (qPCR) using a high-fidelity DNA Polymerase (Q5® Hot Start High-Fidelity Polymerase; New England Biolabs, Ipswich, MA, USA) and gene-specific primers (Supplementary Table 2). The T7 Endonuclease 1 (New England Biolabs, Ipswich, USA) recognises and cleaves non-perfectly matched DNA such as heteroduplexes and nicked DNA. In a first step, DNA is denatured at 95°C for 5 min, then allowed to reanneal by slowly cooling it down (95 - 85°C: −2°C/s; 85 - 25°C: −0.1°C/s), allowing heteroduplex formation between wild-type DNA and CRISPR-Cas9-mutated DNA. 2 units of T7E1, which recognizes and cleaves mismatched DNA, was added in a final step to digest heteroduplexes for 45 min at 37°C.
Statistical analysis: GraphPad Prism 9.2.1 was used for all statistical analyses. Data are presented as mean ± 95% confidence intervals. Normality of data was tested using Shapiro–Wilk Criteria. Data were determined to be normally distributed unless specified otherwise. For statistical significance between two groups we applied either a t-test, paired (for matched donor samples) or unpaired. One-way analysis of variance (ANOVA) was conducted when comparing more than two groups, followed by Tukey's test (when comparing means with every other mean) or Dunnett's test (when comparing means with control mean) for comparison between the groups. RT-qPCR expression was determined by applying log 2 formula (2−ΔΔCT) using housekeeping genes RNU24 (for microRNA) or RPLP0 (pre-printed on customised microfluidic cards). Where relevant statistical significance was defined by a P value of <0.05.
Results
We first assessed the impact of two different transfection methods on isolated human osteoarthritic chondrocytes (OA hACs). Passage 1 hACs were transfected either with pX330 or using a Ribonucleoprotein (RNP) complex. The pX330 plasmid is an ∼8.5 kb plasmid with the coding sequence for Cas9 and a targeting sgRNA subcloned into it. The RNP complex consisted of the Cas9 protein and targeting sgRNA. RNP transfected and untreated control OA hACs exhibited continuous growth and increased confluency at 24 h and 48 h post transfection. However, plasmid transfected OA hACs appeared to stop proliferating and exhibited high numbers of non-adherent, typan blue positive cells indicating toxicity (Supplementary Fig. 1(A) and (B)). Immunoblots confirmed that RNP transfection of OA hACs with Cas9 had been successful post transfection (Supplementary Fig. 1(C)).
Before exploring the role of miR-140 in hACs, we first confirmed that both arms of miR-140 (140-5p and miR-140-3p), were expressed in cells taken from OA cartilage samples from several different donors (each donor represented by a different colour) (Supplementary Fig. 2). miR-140-5p levels were slightly higher than miR-140-3p (P = 0.0445).
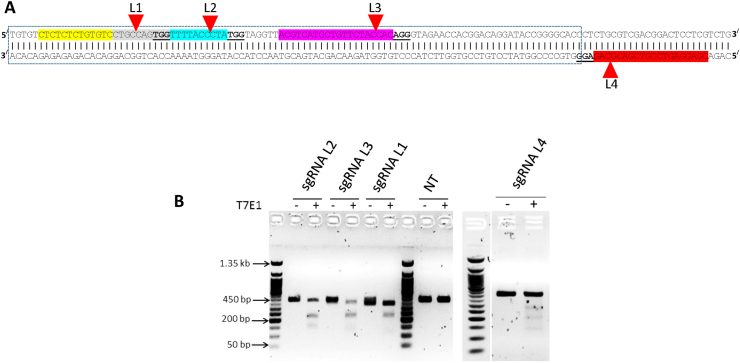
To make the CRISPR-Cas9 RNP, four different single guide RNAs (sgRNAs), with predicted high on-target and low off-target scores, using IDT software (https://eu.idtdna.com/), were designed to target the hairpin structure of miR-140, which sits within an intron of WWP2 [Fig. 1(A)]. Scores for each were as follows: L1 on-target 58, off-target 0; L2 on-target 62, off-target 59; L3 on-target 77, off-target 79; L4 on-target 40, off-target 93. From these results L2 and L3 were predicted to provide the best combination of on and off-targets. Each sgRNA was made up of a universal tracrRNA and a target specific crRNA. miR-140 targeting sgRNAs were complexed with Cas9 as RNPs and transfected using Lipofectamine CRISPRMax. A 439 base pairs (bp) long PCR product was amplified using miR-140 flanking primers (Fig. 1(B), dominant band running at ∼450bp). The T7 Endonuclease 1 (T7E1) assay was used to assess efficiency of CRISPR-Cas9 mediated targeting. The T7E1 assay recognises mismatched regions in double-stranded DNA (dsDNA) and cleaves the DNA at this site to generate smaller products (in this case approximately 180 and 260 bp). By T7E1 assay all four sgRNAs induced DNA fragmentation indicating successful DNA targeting, with sgRNA L4 appearing to be the least efficient [Fig. 2(B)]. This was in keeping with the low IDT scores for L4.
Fig. 1.
CRISPR-Cas9 RNP transfection in primary hACs: Sequence of genomic miR-140 hairpin structure with positions of sgRNAs (L1, L2, L3, L4). Red arrows indicate the predicted cutting sites which cut between the 3rd and 4th bases from the PAM (underlined) (A). The amplified products (around 439bp) were assessed using T7E1 assay. Edited (mismatched) DNA is seen to fragment at around the predicted sizes of 180 and 260bp (B). NT, non-targeted sgRNA control. Representative agarose gel shown. n = 2.
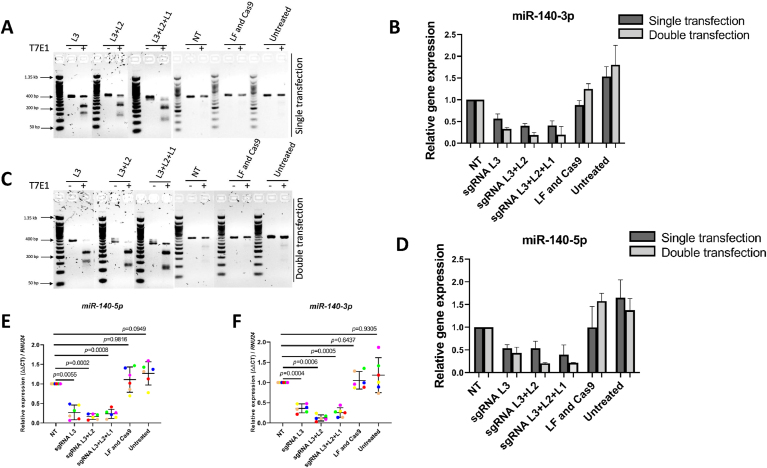
Fig. 2.
Double transfection of CRISPR-Cas9 with RNP enhances gene editing efficiencies in hACs. hACs underwent single or double transfection targeting individual sgRNA (L3) or combination sgRNAs (L3+L2 or L3+L2+1). Lipofectamine + Cas9 + Non-targeting sgRNA (NT), LF and Cas9 treated cells without sgRNA (LF and Cas9), or untreated cells (untreated), served as controls (A–F). T7E1 assay (A, C) and qPCR for miR-140-3p and miR-140-5p from extracted RNA (B, D) from one donor. Error bars are determined by technical (well) replicates (n = 3). Double transfection with single or combination sgRNAs was performed in a further six independent donor cells (each donor colour-coded). qPCR was performed on extracted RNA for miR-140-5p and miR-140-3p. Gene expression, a surrogate marker of editing efficiency, was normalized to NT sgRNA control and expressed relative to RNU24. N = 6 biological replicates (E, F). Statistical testing was performed on the raw delta CT values using the Bonferroni-Dunn method with corrections for multiple comparison.
The impact of single or combination sgRNAs was next examined after single or double transfection. miR-140 was targeted using sgRNA L3 (which had the highest IDT scores) or using the combination of either sgRNAs L2 and L3, or sgRNAs L1, L2 and L3, ensuring that the final concentration of sgRNA remained constant across groups. Three different controls were included: a NT sgRNA control (NT), Lipofectamine (LF) and Cas9 treated cells (no sgRNA) (LF and Cas9), as well as completely untreated cells (untreated). For each donor, each experimental condition was performed either in duplicate or triplicate. DNA cleavage was assessed by the T7E1 assay, after amplifying the 439 bp product described in Fig. 1. Gene editing was confirmed for each set of targeting sgRNAs [Fig. 2(A)] with the suggestion of improved efficiency after double transfection [Fig. 2(C)]. The impact of sgRNA on miR-140 gene expression (a surrogate read out of editing efficiency) was examined in the same samples by qPCR. 50–60% reduction in gene expression of miR-140-3p [Fig. 2(B)] and mir-140-5p [Fig. 2(D)] was apparent after single transfection, and this was enhanced when double transfection was performed. A further six donor cells were then tested with the same combination of sgRNAs after double transfection. Reduction in the expression of both 5p and 3p arms of miR-140 was greatest (>80%) when using the combination of sgRNAs L2+L3 (Fig. 2(E) and (F) and Supplementary Fig. 3). Cell viability, by light microscopy, appeared stable after both double and single transfections, and RNU24 raw CT values were not significantly different between transfected and non-transfected cells in either single or double transfection groups (data not shown).
Sanger Sequencing was used to confirm the deletion of bp following double transfection with sgRNAs L2+L3. Sequencing results of 15 bacterial clones, that resulted from subcloning of the PCR product, revealed that 13 out of 15 clones showed a deletion of 29 bp between the cleavage sites of sgRNA L2 and sgRNA L3 (Supplementary Fig. 4, clone 1). One clone exhibited a deletion of 31 bp, one base pair upstream of the cutting site of sgRNA L2 (highlighted in yellow) and downstream of sgRNA L3 (highlighted in blue) (clone 2). A third clone showed a deletion of 1 bp upstream of the cleavage site of sgRNA L2 and 6 bp downstream of the cleavage site of sgRNA L3 (clone 3). MiSeq analysis was used to confirm the precision of gene editing following double transfection with sgRNAs L2+L3. MiSeq analysis confirmed a deletion for over 90 % of reads (Supplementary Fig. 4(B)). Deletions of bp, in smaller numbers, were also detected upstream of amplicon position 191 and downstream of amplicon position 220. A very small number of insertions was detected at these positions. A significant majority (>97% of reads) did not exhibit any NHEJ induced bp insertion, the majority of reads (>90 %) exhibited a deletion of 29 bp or larger, with a peak at −29 bp (55 %) (Supplementary Fig. 4(C) and (D)). The absolute number of modified reads amounted to 77,094 (>99.9 %), compared with only 31 (<0.01 %) unmodified reads, suggesting very high gene editing efficiency after RNP double transfection in OA hACs (Supplementary Fig. 4(E)). To check for off-target effects of gene editing we used two different off-target algorithm software packages (see Materials and Methods). No sgRNA L3 and L2 targets with just one sequence mismatch were predicted. Two potential targets were predicted when one considered two sequence mismatches, and three when one considered three sequence mismatches. Specific primers were designed for each predicted off-target site and these were amplified in three different donors which had been double RNP transfected with sgRNA L3+L2. No evidence of DNA cleavage was seen for any of these sequences by T7E1 assay (Supplementary Fig. 5(A)). WWP2, the host of miR-140 was not down-regulated following sgRNA L2+L3 relative to its normalised NT sgRNA control (supplementary Fig. 5(B)).
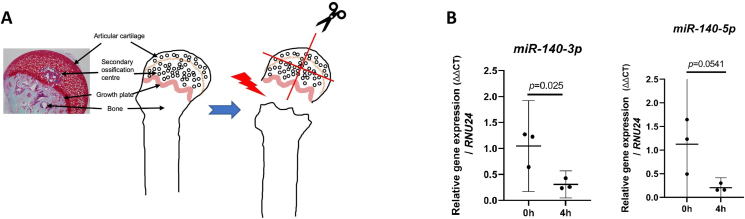
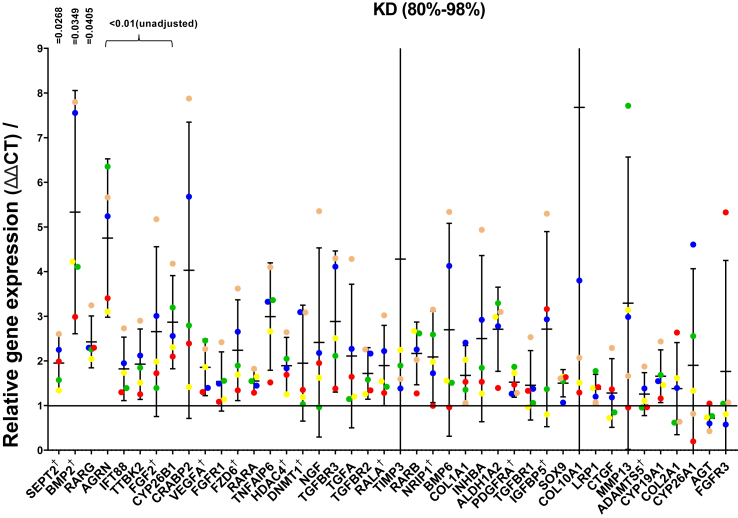
To explore the biology of miR-140 in articular chondrocytes we first established whether miR-140 was regulated by cartilage injury, a critical aetiological factor in OA development and one that drives rapid changes in chondrocyte gene regulation1,32,33. Using a previously validated hip avulsion model in 6 week old wild type mice33,34 [Fig. 3(A)], both miR-140-5p and miR-140-3p were downregulated 4 h after injury (a time at which acute inflammatory genes are regulated optimally), albeit reaching statistical significance only for miR-140-3p (P = 0.0249 (95% CI -1.329, 0.1532); miR-140-5P = 0.0541 (95% CI -1.863, 0.02596) [Fig. 3(B)]. As injury is a powerful regulator of chondrocyte gene regulation and a key etiological factor in OA development, the drop of a highly expressed miR could be directly contributing to the injury response. To test this, we examined a number of genes that were either previously identified miR-140 targets or shown to regulate or to be strongly regulated by cartilage injury (Supplementary Table 1). These included genes involved in cartilage repair pathways, retinoic acid (RA) metabolism, chondrogenesis, cilia biology, and cartilage catabolism. Gene expression was examined in OA human articular chondrocytes (n = 5) after miR-140 deletion and compared with their respective paired NT control. Data are presented in Fig. 4 and ranked according to p value. Each data point represents the ratio of gene expression in the deleted compared with NT control for each patient sample. SEPT2 (1.95 ± 0.51 fold), bone morphogenetic protein 2 (BMP2) (5.33 ± 2.19 fold) and RA receptor gamma (RARG) (2.42 ± 0.47 fold) were upregulated upon miR-140 deletion, displaying statistical significance after stringent correcting for multiple comparisons (Fig. 4). Interestingly, incomplete (50–60%) gene disruption of miR-140 after single transfection, was insufficient to change any of the measured genes (Supplementary Fig. 6) indicating that high editing efficiency is required to demonstrate a biological effect. To validate these potential human chondrocyte miR-140 targets further, we next interrogated RNA sequencing (RNA-seq) data taken from costal cartilage of 7-day old constitutive miR-140 KO mice (available at NCBI GEO datasets with the accession number GSE144360). We considered the top eight genes from our qPCR analysis, whose unadjusted P values were <0.01 (Table I). Of these, Sept2, Agrn, Ift88, Fgf2 and Cyp26b1 showed strong regulation in miR-140 KO costal chondrocytes (all up-regulated apart from Ift88). BMP2, RARG and TTBK2 were not regulated in neonatal mouse costal chondrocytes by deletion of miR-140.
Fig. 3.
Cartilage injury suppresses miR-140-3p and miR-140-5p expression. (A) Safranin O/Fast Green-stained section of 6 week old murine femoral head with schematic showing the femoral head before (intact) and after cartilage injury (avulsed and cut). Femoral heads were either snap frozen immediately in liquid nitrogen to provide the negative control (0h) or cut into 4 pieces and cultured in serum-free media for 4 h at 37°C to measure the biological injury response (B) RNA was extracted and miR-140-3p and miR-140-5p expression were quantified by qPCR, normalised to RNU24 and expressed relative to 0h. Data are shown as mean ± SD. Statistical significance by student two-tailed test. n = 3 biological replicates.
Fig. 4.
Human chondrocyte gene editing by CRISPR-Cas9 identifies novel miR-140 gene targets. Gene expression of 46 genes with putative roles in osteoarthritis or previously described miR-140 targets, were analysed by qPCR on pre-printed TaqMan Microfluidic cards. All genes were normalised to RPLP0 and expressed relative to their respective non-targeting (NT) sgRNA control (for each donor). Genes are displayed according to the strength of the statistical significance from left to right. Gene names marked with “†” are previously identified miR-140 targets. Each color represents an individual donor. Statistical significance was determined using the Bonferroni-Dunn method with corrections for multiple comparison. n = 5 biological replicates (donors). Three genes had P < 0.05 after correction. A further 5 genes whose uncorrected P values were <0.01 are also indicated.
Table I.
Genes upregulated in hACs (with P < 0.01) and murine neonatal costal chondrocytes upon deletion of miR-140
| GENE |
miR-140 KD in osteoarthritic, human articular chondrocytes |
P value | P value adjusted | Costal RNA from 7-day-old-miR-140 KO mice |
P value | P value adjusted | Presence of miR-140 seed sequence |
|---|---|---|---|---|---|---|---|
| Mean Fold change normalised to non-targeted control (lower, upper 95% CI) | Mean Fold change normalised to wild type (lower, upper 95% CI) | ||||||
| AGRN | 4.752 (2.978, 6.525) | 0.0019 | 0.0830 | 1.641 (1.434, 1.848) | 4.75E-06 | 9.15E-05 | – |
| BMP2 | 5.333 (2.609, 8.057) | 0.0008 | 0.0349 | 1.170 (0.748, 1.593) | 0.9729 | 0.9880 | 140–5p |
| CYP26B1 | 2.867 (1.824, 3.910) | 0.0089 | 0.3750 | 3.162 (1.985, 4.338) | 1.71E-19 | 4.49E-17 | – |
| FGF2 | 2.657 (0.755, 4.558) | 0.0038 | 0.1632 | 1.523 (1.175, 1.871) | 0.0033 | 0.0219 | 140–5p |
| IFT88 | 1.821 (1.110, 2.531) | 0.0023 | 0.1005 | 0.675 (0.495, 0.856) | 0.0002 | 0.0024 | – |
| RARG | 2.427 (1.845, 3.009) | 0.0009 | 0.0405 | 0.845 (0.629, 1.062) | 0.29772 | 0.5394 | – |
| SEPT2 | 1.95 (1.318, 2.582) | 0.0006 | 0.0268 | 1.836 (1.681, 1.990) | 1.03E-11 | 7.81E-10 | 140–5p |
| TTBK2 | 1.926 (1.138, 2.715) | 0.0029 | 0.1244 | 1.098 (1.004, 1.192) | 0.7826 | 0.8992 | – |
Comparison of human chondrocytes after miR-140 gene editing with genes regulated in costal chondrocytes from 7-day-old miR-140 KO mice compared with wild type animals (determined by RNA-sequencing). Statistical testing of human data was by Student's t-test (two-tailed) (P value) with multiple comparison (n = 47) testing (P value adjusted). For the RNAseq data, we used deseq2 which uses a Wald t-test then a modified Benjamini Hochberg to reduce false positives. Presence of miR-140 seed sequences indicated.
Discussion
In this manuscript we describe a novel approach for efficient genomic editing of miR-140 in primary human chondrocytes by performing transfection of RNP complexes containing Cas9 and sgRNA targeting sequences. This method was superior to that using the pX330 plasmid which exhibited evidence of cell toxicity within 48 h of transfection. Editing efficiency using RNP appeared to be optimal when combining more than one sgRNA, and when double transfection was performed. Efficiency at this level is usually only obtained by clonal expansion of selected targeted clones, which risks losing the chondrocyte phenotype35. MiSeq analysis demonstrated high gene editing efficiencies (>99.9 %) for the populations that were sequenced and qPCR indicated that miR-140 was suppressed by >80% for all donor samples.
To explore the biological significance of miR-140 in human chondrocytes we first assessed whether it was regulated upon cartilage injury, an important and clinically relevant stimulus for the tissue22,36,37. We observed that miR-140 was downregulated 4 h following murine cartilage injury, a time at which many other genes are upregulated. miR-140 might therefore influence the response of the tissue to OA-induced mechanical injury and might explain the enhanced OA phenotype seen in miR-140 knockout mice20. We next investigated the effect of miR-140 deletion on chondrocyte genes that are known to be strongly regulated by injury, in addition to genes that had previously been identified as miR-140 targets by other groups (see Supplementary Table 1and references therein). Several of these genes were upregulated in OA human articular chondrocytes upon miR-140 deletion. None of the genes were regulated when chondrocytes were partially depleted of miR-140 (50–60%) following a single round of transfection indicating that high level depletion is required to uncover biological function.
Considering those genes where the uncorrected P value was <0.01, miR-140-dependent genes included those involved in the RA pathway (RARG, CYP26B1), primary cilia biology (SEPT2, IFT88, and TTBK2), and anabolic factors (BMP2, FGF2 and AGRN). Several, but not all, of these were strongly miR-140-regulated in costal chondrocytes taken from neonatal miR-140 knockout mice, perhaps reflecting differences in species or relating to differences in chondrocyte site and maturity (neonatal murine costal chondrocytes rather than adult human OA articular chondrocytes). SEPT2 was the most robustly miR-140-regulated gene in both human articular and costal chondrocytes and has previously been identified as miR-140-dependent38. It encodes Septin 2, a filamentous GTPase, that directly binds to myosin II, a molecular motor driving muscle contraction. It is found concentrated along the axoneme (central cilium strand) in retinal pigmented epithelial (RPE) cells and deletion inhibits ciliogenesis39. The role of SEPT2 in chondrocytes is unknown but it may be relevant that two other cilia-related genes (IFT88 and TTBK2) were also regulated by miR-140 in human articular chondrocytes. The primary cilium has previously been linked to OA through its established role in modulating hedgehog signalling40. It affects aggrecanase activity in chondrocytes in vitro possibly by controlling the distribution of the scavenger receptor LRP141, and also acts as a modulator of mechanical load in cartilage in vivo42.
Several genes with known pro-regenerative or anabolic roles were also identified as miR-140 targets. FGF2 was strongly miR-140-regulated in human articular and murine costal chondrocytes, as described previously43. FGF2 is released from cartilage upon injury and FGF2 deficient mice develop accelerated OA indicating its chondroprotective role44. Both SEPT2 and FGF2 possess a miR-140-5p seed sequence in their 3p-UTR, and so are predicted to be direct targets of miR-140. Two other anabolic molecules were identified as miR-140-dependent in human articular chondrocytes: BMP2 and AGRN. AGRN is a heparan sulfate proteoglycan, usually associated with the neuromuscular junction45. It has recently been described as a powerful cartilage regenerative agent in damaged articular cartilage in vivo46. AGRN, unlike BMP2, was also strongly miR-140-dependent in neonatal murine costal chondrocytes.
We identified two novel RA regulated genes as miR-140 targets in human articular chondrocytes (RARG and CYP26B1). CYP26B1 was also strongly miR-140-dependent in murine costal chondrocytes. We have recently observed that cartilage injury strongly regulates RA dependent genes, including the CYP26 enzymes, which are the key regulators of cellular RA levels47. Enhancing RA at the time of injury strongly suppresses ‘mechanoflammation’ indicating that RA is a biologically important anti-inflammatory molecule in cartilage48.
Contrary to previous reports16, ADAMTS5 was not regulated in miR-140 knockdown hACs in this study, even though 3′-UTR of ADAMTS5 contains a putative seed sequence for miR-140-3p. Its regulation might have been uncovered had we looked at chondrocytes stimulated with IL1 or equivalent catabolic stimulus. As miR-140 KO mice are characterised by short stature20, it raises the question whether the accelerated OA phenotype seen in these mice is partly due to a mild chondrodysplasia in addition to direct effects on proteases.
We recognise a number of limitations in this study. Firstly, the nature of both the T7E1 and MiSeq assays means that underestimation or overestimation of efficiency, respectively, may occur49. In the case of T7E1, the assay relies on DNA cleavage where there is CRISPR-Cas9 modified DNA attempting to reanneal with native DNA i.e., mismatched. If CRISPR-Cas9 mutated DNA anneals with an identically mutated strand, this will not be recognised as mismatched and therefore could result in an underestimate of gene editing efficiency. In the case of MiSeq, we identified a common polymorphic variant at amplicon position 267 (rs2102066), resulting in homology mismatch between the endogenous sequence and the reference sequence. This single nucleotide polymorphism (SNP) was present in each of the four donors, so this may account for some of the apparent NHEJ scores. This is probably exerting a small overall effect as ∼90 % of the deletions were at the predicted Cas9 target sites for the two sgRNAs for all donors. The qPCR readouts suggest the actual efficiency was between 90% and 98%. We were careful to design sgRNAs that had few predicted off-target effects, and we checked that we were not targeting sequences elsewhere within the genome with up to three mismatches. As the T7E1 assay is not highly sensitive, this result does not exclude there being low numbers of cells where off-targeting has occurred. The ability to detect off-targeting is likely to be affected, in addition, by chromatin accessibility which may be in a less open conformation in assay chondrocytes50. Finally, we selected a restricted number of genes of interest to explore in this study, which were, by nature, biased to those already described in the literature and of specific interest to the group.
To conclude, we have demonstrated that it is possible to get high levels of CRISPR-Cas9 mediated gene deletion in human primary articular chondrocytes. We have validated the functional effect of miR-140 deletion by confirming previously identified targets and identifying new targets. We show, for the first time, that miR-140 is down-regulated upon cartilage injury, and that several injury induced genes are also miR-140 targets. This confirms the important role of miR-140 in cartilage homeostasis, and in the injured joint in the development of OA. As chondroprotective pathways are also regulated by miR-140, it would not seem prudent to regard miR-140 as a target in OA.
Author contributions
-
(1)
The conception and design of the study (TLV, NC, CS, DY), acquisition of data (NC, HM, DD, YH, LZ), analysis and interpretation of data (TLV, NC, DY, CS, DD, YH).
-
(2)
Drafting the article or revising it critically for important intellectual content: all authors
-
(3)
Final approval of the version to be submitted: all authors
Conflict of interest
No conflicts of interest relevant to this work are identified for any of the authors.
Statement of role of funding source
Academic funding sources did not influence the project direction or decision to publish.
Acknowledgements
1. Other contributors – we are grateful to Angus Wann and Clarissa Coveney for histological image.
2. Funding sources: this work was funded through the Centre for OA Pathogenesis Versus Arthritis (grant numbers 20205 and 21621) and the Dunhill Medical Trust (R476/0516). It was also supported by a studentship to Noman Chaudhry from the Kennedy Trust for Rheumatology Research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joca.2022.01.005.
Appendix ASupplementary data
The following are the Supplementary data to this article:
References
- 1.Brandt K.D., Dieppe P., Radin E.L. Commentary: is it useful to subset “primary” osteoarthritis? A critique based on evidence regarding the etiopathogenesis of osteoarthritis. Semin Arthritis Rheum. 2009;39:81–95. doi: 10.1016/j.semarthrit.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt L.A., Moreton B.J., Mapp P.I., Wilson D., Hill R., Ferguson E., et al. Histopathological subgroups in knee osteoarthritis. Osteoarthritis Cartilage. 2017;25:14–22. doi: 10.1016/j.joca.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Dell'Accio F., Vincent T.L. Joint surface defects: clinical course and cellular response in spontaneous and experimental lesions. Eur Cell Mater. 2010;20:210–217. doi: 10.22203/ecm.v020a17. [DOI] [PubMed] [Google Scholar]
- 4.Vincent T.L. Of Mice and Men; converging on a common molecular understanding of Osteoarthritis. Lancet Rheumatology. 2020;2:E633–E645. doi: 10.1016/S2665-9913(20)30279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 7.Denli A.M., Tops B.B., Plasterk R.H., Ketting R.F., Hannon G.J. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 8.Gregory R.I., Chendrimada T.P., Cooch N., Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Jones S.W., Watkins G., Le Good N., Roberts S., Murphy C.L., Brockbank S.M.V., et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis and cartilage/OARS. Osteoarthritis Research Society. 2009;17:464–472. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Le L.T.T., Swingler T.E., Crowe N., Vincent T.L., Barter M.J., Donell S.T., et al. The microRNA-29 family in cartilage homeostasis and osteoarthritis. J Mol Med. 2016;94:583–596. doi: 10.1007/s00109-015-1374-z. Berlin, Germany. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Sanchez A., Murphy C.L. miR-1247 functions by targeting cartilage transcription factor SOX9. J Biol Chem. 2013;288:30802–30814. doi: 10.1074/jbc.M113.496729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidl C.I., Martinez-Sanchez A., Murphy C.L. Derepression of MicroRNA-138 contributes to loss of the human articular chondrocyte phenotype. Arthritis Rheumatol. 2016;68:398–409. doi: 10.1002/art.39428. [DOI] [PubMed] [Google Scholar]
- 13.Yan S., Wang M., Zhao J., Zhang H., Zhou C., Jin L., et al. MicroRNA-34a affects chondrocyte apoptosis and proliferation by targeting the SIRT1/p53 signaling pathway during the pathogenesis of osteoarthritis. Int J Mol Med. 2016;38:201–209. doi: 10.3892/ijmm.2016.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coutinho de Almeida R., Ramos Y.F.M., Mahfouz A., den Hollander W., Lakenberg N., Houtman E., et al. RNA sequencing data integration reveals an miRNA interactome of osteoarthritis cartilage. Ann Rheum Dis. 2019;78:270–277. doi: 10.1136/annrheumdis-2018-213882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura Y., Yamamoto K., He X., Otsuki B., Kim Y., Murao H., et al. Wwp2 is essential for palatogenesis mediated by the interaction between Sox9 and mediator subunit 25. Nat Commun. 2011;2:251. doi: 10.1038/ncomms1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyaki S., Nakasa T., Otsuki S., Grogan S.P., Higashiyama R., Inoue A., et al. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60:2723–2730. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shvedova M., Kobayashi T. MicroRNAs in cartilage development and dysplasia. Bone. 2020;140:115564. doi: 10.1016/j.bone.2020.115564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Si H.B., Zeng Y., Liu S.Y., Zhou Z.K., Chen Y.N., Cheng J.Q., et al. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthritis Cartilage. 2017;25:1698–1707. doi: 10.1016/j.joca.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Mokuda S., Nakamichi R., Matsuzaki T., Ito Y., Sato T., Miyata K., et al. Wwp2 maintains cartilage homeostasis through regulation of Adamts5. Nat Commun. 2019;10:2429. doi: 10.1038/s41467-019-10177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyaki S., Sato T., Inoue A., Otsuki S., Ito Y., Yokoyama S., et al. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24:1173–1185. doi: 10.1101/gad.1915510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J., Qin S., Yi C., Ma G., Zhu H., Zhou W., et al. MiR-140 is co-expressed with Wwp2-C transcript and activated by Sox9 to target Sp1 in maintaining the chondrocyte proliferation. FEBS Lett. 2011;585:2992–2997. doi: 10.1016/j.febslet.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 22.De Palma A., Cheleschi S., Pascarelli N.A., Tenti S., Galeazzi M., Fioravanti A. Do MicroRNAs have a key epigenetic role in osteoarthritis and in mechanotransduction? Clin Exp Rheumatol. 2017;35:518–526. [PubMed] [Google Scholar]
- 23.Grigelioniene G., Suzuki H.I., Taylan F., Mirzamohammadi F., Borochowitz Z.U., Ayturk U.M., et al. Gain-of-function mutation of microRNA-140 in human skeletal dysplasia. Nat Med. 2019;25:583–590. doi: 10.1038/s41591-019-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura A., Rampersaud Y.R., Nakamura S., Sharma A., Zeng F., Rossomacha E., et al. microRNA-181a-5p antisense oligonucleotides attenuate osteoarthritis in facet and knee joints. Ann Rheum Dis. 2019;78:111–121. doi: 10.1136/annrheumdis-2018-213629. [DOI] [PubMed] [Google Scholar]
- 25.Dana H., Chalbatani G.M., Mahmoodzadeh H., Karimloo R., Rezaiean O., Moradzadeh A., et al. Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci. 2017;13:48–57. [PMC free article] [PubMed] [Google Scholar]
- 26.Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9:1911. doi: 10.1038/s41467-018-04252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Costa S., Rich M.J., Diekman B.O. Engineered cartilage from human chondrocytes with homozygous knockout of cell cycle inhibitor p21. Tissue Eng. 2020;26:441–449. doi: 10.1089/ten.TEA.2019.0214. [DOI] [PubMed] [Google Scholar]
- 29.Seidl C.I., Fulga T.A., Murphy C.L. CRISPR-Cas9 targeting of MMP13 in human chondrocytes leads to significantly reduced levels of the metalloproteinase and enhanced type II collagen accumulation. Osteoarthritis Cartilage. 2019;27:140–147. doi: 10.1016/j.joca.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Horita M., Nishida K., Hasei J., Furumatsu T., Sakurai M., Onodera Y., et al. Involvement of ADAM12 in chondrocyte differentiation by regulation of TGF-beta1-induced IGF-1 and RUNX-2 expressions. Calcif Tissue Int. 2019;105:97–106. doi: 10.1007/s00223-019-00549-6. [DOI] [PubMed] [Google Scholar]
- 31.Adkar S.S., Wu C.L., Willard V.P., Dicks A., Ettyreddy A., Steward N., et al. Step-wise chondrogenesis of human induced pluripotent stem cells and purification via a reporter allele generated by CRISPR-cas9 genome editing. Stem Cell. 2019;37:65–76. doi: 10.1002/stem.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent T.L. Mechanoflammation in osteoarthritis pathogenesis | elsevier enhanced reader. Semin Arthritis Rheum. 2019;49:S36–S38. doi: 10.1016/j.semarthrit.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Chong K.-W., Chanalaris A., Burleigh A., Jin H., Watt F.E., Saklatvala J., et al. Fibroblast growth factor 2 drives changes in gene expression following injury to murine cartilage in vitro and in vivo. Arthritis Rheum. 2013;65:2346–2355. doi: 10.1002/art.38039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ismail H.M., Miotla-Zarebska J., Troeberg L., Tang X., Stott B., Yamamoto K., et al. Brief report: JNK-2 controls aggrecan degradation in murine articular cartilage and the development of experimental osteoarthritis. Arthritis Rheumatol. 2016;68:1165–1171. doi: 10.1002/art.39547. [DOI] [PubMed] [Google Scholar]
- 35.Brodkin K.R., Garcia A.J., Levenston M.E. Chondrocyte phenotypes on different extracellular matrix monolayers. Biomaterials. 2004;25:5929–5938. doi: 10.1016/j.biomaterials.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.H., Lee G., Won Y., Lee M., Kwak J.S., Chun C.H., et al. Matrix cross-linking-mediated mechanotransduction promotes posttraumatic osteoarthritis. Proc Natl Acad Sci U S A. 2015;112:9424–9429. doi: 10.1073/pnas.1505700112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watt F.E., Paterson E., Freidin A., Kenny M., Judge A., Saklatvala J., et al. Acute molecular changes in synovial fluid following human knee injury: association with early clinical outcomes. Arthritis Rheumatol. 2016;68:2129–2140. doi: 10.1002/art.39677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu J., Zhang W., Tang H., Qian H., Yang J., Zhu Z., et al. Septin 2 accelerates the progression of biliary tract cancer and is negatively regulated by mir-140-5p. Gene. 2016;589:20–26. doi: 10.1016/j.gene.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Ghossoub R., Hu Q., Failler M., Rouyez M.C., Spitzbarth B., Mostowy S., et al. Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J Cell Sci. 2013;126:2583–2594. doi: 10.1242/jcs.111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin A.C., Seeto B.L., Bartoszko J.M., Khoury M.A., Whetstone H., Ho L., et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15:1421–1425. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 41.Coveney C.R., Collins I., Mc Fie M., Chanalaris A., Yamamoto K., Wann A.K.T. Cilia protein IFT88 regulates extracellular protease activity by optimizing LRP-1-mediated endocytosis. Faseb J. 2018 doi: 10.1096/fj.201800334. official publication of the Federation of American Societies for Experimental Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coveney C.R., Zhu L., Miotla-Zarebska J., Stott B., Parisi I., Batchelor V., et al. The ciliary protein IFT88 controls post-natal cartilage thickness and influences development of osteoarthritis. Arthritis Rheumatol. 2021;74:49–59. doi: 10.1002/art.41894. [DOI] [PubMed] [Google Scholar]
- 43.Ornitz D.M., Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chia S.-L., Sawaji Y., Burleigh A., McLean C., Inglis J., Saklatvala J., et al. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009;60:2019–2027. doi: 10.1002/art.24654. [DOI] [PubMed] [Google Scholar]
- 45.Glass D.J., Bowen D.C., Stitt T.N., Radziejewski C., Bruno J., Ryan T.E., et al. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- 46.Eldridge S.E., Barawi A., Wang H., Roelofs A.J., Kaneva M., Guan Z., et al. Agrin induces long-term osteochondral regeneration by supporting repair morphogenesis. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aax9086. [DOI] [PubMed] [Google Scholar]
- 47.Zhu L., Chanalaris A., Groves K., Furniss D., Watt F., Gardiner M., et al. Polymorphic variants in ALDH1A2 determine the expression level of ALDH1A2 and CYP19A1 in the cartilage of patients undergoing trapeziectomy for severe thumb osteoarthritis. Osteoarthritis Cartilage. 2018;26 [Google Scholar]
- 48.Zhu L., Kamalathevan P., Koneva L., Miotla Zarebska J., Chanalaris A., Ismail H.M., et al. Variants in ALDH1A2 reveal an anti-inflammatory role for retinoic acid and a new class of disease-modifying drugs in osteoarthritis. BioRxiv preprint. 2021 doi: 10.1101/2021.09.10.457848. [DOI] [PubMed] [Google Scholar]
- 49.Sentmanat M.F., Peters S.T., Florian C.P., Connelly J.P., Pruett-Miller S.M. A survey of validation strategies for CRISPR-cas9 editing. Sci Rep. 2018;8:888. doi: 10.1038/s41598-018-19441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh R., Kuscu C., Quinlan A., Qi Y., Adli M. Cas9-chromatin binding information enables more accurate CRISPR off-target prediction. Nucleic Acids Res. 2015;43:e118. doi: 10.1093/nar/gkv575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.