Key Points
Question
What are the proportions of the 4 major subtypes of rosacea among patients with rosacea?
Findings
In this systematic review and meta-analysis of 39 studies including more than 9000 patients, the proportion of erythematotelangiectatic rosacea was 57%, papulopustular rosacea, 43%, phymatous rosacea, 7%, and ocular rosacea, 11%. Subtype distribution occurred equally among men and women except for phymatous rosacea, which was more prevalent in men.
Meaning
These findings suggest that erythematotelangiectatic and papulopustular rosacea are the most common subtypes of rosacea based on global data.
Abstract
Importance
Four distinct rosacea subtypes have traditionally been recognized, but the frequency of these subtypes among patients with rosacea remains unknown.
Objective
To assess the frequency of 4 rosacea subtypes.
Data Sources
This systemic review and meta-analysis included a search of 2 databases, PubMed and Embase, from inception of the databases to November 2, 2021. The search was filtered to include only studies of human participants published in English, French, and German.
Study Selection
Studies were screened independently by 2 of the authors and were included if they were original with a sample size of 25 or more patients and reported absolute numbers or frequency of patients affected by rosacea subtypes. Studies that did not report sufficient data to calculate the proportions of subtypes were excluded.
Data Extraction and Synthesis
Data extraction was performed independently and in duplicate by 2 of the authors, using the search term rosacea, according to the Preferred Reporting items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The search term, objectives, and study protocol methods were defined before the study was initiated. A total of 292 studies were included for full-text assessment. Owing to the heterogeneity of the included studies, a random-effects model was used.
Main Outcome and Measures
The main outcome was the proportion of patients with rosacea in each of the 4 major subtype groups defined by the 2002 National Rosacea Society classification system. Measures were absolute numbers or frequency of patients affected by each of the 4 rosacea subtypes.
Results
A total of 39 studies examining 9190 patients with rosacea were included. The pooled proportion of erythematotelangiectatic rosacea was 56.7% (95% CI, 51.4%-62.0%), of papulopustular rosacea was 43.2% (95% CI, 38.8%-47.6%), of phymatous rosacea was 7.4% (95% CI, 6.1%-8.9%), and of ocular rosacea was 11.1% (95% CI, 6.7%-16.3%). Subtype distribution occurred equally among men and women except for phymatous rosacea, which was more prevalent in men. Studies from Africa showed the lowest proportion of erythematotelangiectatic rosacea. Differences in frequency of subtypes were observed when stratification by publication year was performed.
Conclusion and Relevance
In this systematic review and meta-analysis, differences were found in rosacea subtypes by patient sex and by continent of origin and publication year of included studies. Erythematotelangiectatic and papulopustular rosacea were the most prevalent subtypes, but data should be interpreted with caution. Future studies should use the phenotype-based rosacea approach.
This systematic review and meta-analysis of 39 studies assesses the frequency of the 4 major subtypes in more than 9000 patients with rosacea.
Introduction
Rosacea is a chronic inflammatory skin condition that predominately affects the face.1,2 The global prevalence of rosacea among adults from the general population is estimated at approximately 5.5%.3 Although there is a slight female predominance, rosacea affects both men and women of all ages, and patients usually receive a diagnosis of rosacea during their 30s, 40s, or 50s.4
In 2002, the National Rosacea Society developed a standard classification system.5 Diagnosis of rosacea was based on the presence of at least 1 primary feature (transient or persistent erythema, inflammatory papules or pustules, or telangiectasia) and further classified according to 4 subtypes: erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR), phymatous rosacea (PhR), or ocular rosacea.5,6 This classification system was updated in 2017 and replaced with a phenotype-based approach to diagnosis,7 which was adopted by the National Rosacea Society Expert Committee in the same year.8 This approach should be used in all rosacea research going forward because it allows for much more detailed information to be provided. However, the 4 major subtypes defined in the 2002 classification have been widely used until now, and epidemiologic data based on this classification system may help to better describe the disease and identify the most common clinical subtypes.
To this end, we performed a systematic review and meta-analysis of published literature to examine the frequency of the 4 major subtypes in patients with rosacea.
Methods
Literature Search, Data Extraction, Quality Assessment, and Data Analysis
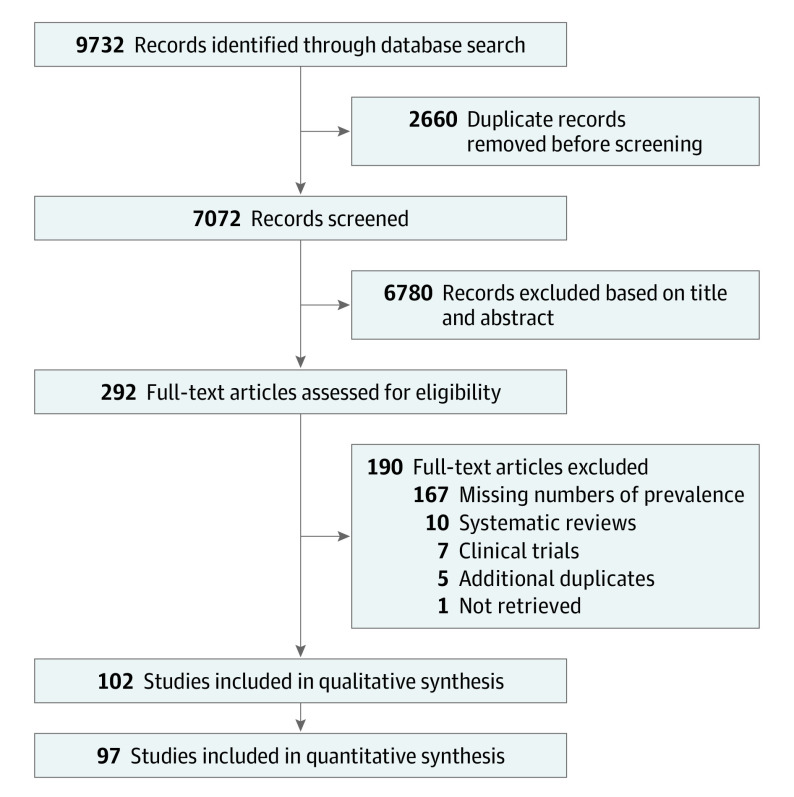
Before the start of the study, a protocol was registered on PROSPERO (CRD42020183288). The systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline,9 and the search and exclusion process is illustrated in Figure 1. Reference lists in articles were screened to identify additional studies, which may have been missed in the initial search. Two authors (Y.A.B. and A.T.M.R.) independently searched PubMed and Embase, using the search term rosacea, and performed data extraction. The search included all articles available from database inception to November 2, 2021. Titles and abstracts were screened by the 2 reviewers, and duplicates were removed. Studies that fulfilled inclusion criteria and any studies with ambiguity of potential eligibility were retrieved as full-text articles and read by the 2 authors. If data were duplicated in more than 1 publication, the publication with the most comprehensive data was included. Any disagreement between the 2 reviewers regarding a potential inclusion of a study was resolved by conferring with a third author (A.S.H., J.P.T., or N.K.F.W.).
Figure 1. PRISMA Flow Diagram.
If studies without primary data presented summary measures for binary data, related CIs, and sample sizes, a reconstruction of primary data was calculated.10 If data were not available, the corresponding author of the publication was contacted with a request for raw data.
The Newcastle-Ottawa scale (NOS) was used to assess the quality of included studies in the quantitative analysis.11 The existing NOS is applicable only to case-control and cohort studies. Therefore, an adapted version of NOS was used to assess the quality of cross-sectional studies. Case-control and cohort studies with an NOS score of 6 or higher were considered to be high quality according to the existing NOS; likewise, cross-sectional studies with a score of 7 or higher were considered to be high quality according to the adapted NOS.12 All studies were included regardless of their NOS score.
Based on data availability from the publications, studies were divided into 3 groups, and the proportion of rosacea subtypes was calculated for each group. The main analysis was done for studies reporting ETR, PPR, PhR, and ocular rosacea; whereas sensitivity analyses were done for studies reporting ETR, PPR, and PhR only and those reporting ETR and PPR only.
Some patients were diagnosed with more than 1 subtype, making the total proportion of subtypes exceed 100%. A study with more than 500 patients was defined as large, and a study with fewer than 500 patients was defined as small. Rosacea subtypes were also stratified by clinical severity of disease when data were available.
Inclusion and Exclusion Criteria
We had no restrictions on study design, but studies had to be original and include absolute numbers or frequency of patients who were affected by rosacea subtypes. Studies that did not report sufficient data to calculate the proportions of subtypes were excluded. Studies with a sample size of 25 or more patients that were published in English, French, or German were eligible for inclusion based on the authors’ linguistic proficiency. After protocol development, all clinical trials were removed from the analysis because of suggestions during the peer review.
Statistical Analysis
The primary end point was the proportion of patients with rosacea in each of the 4 major subtype groups defined by the 2002 National Rosacea Society classification system.5 Sensitivity analyses were done based on sex, study design, study sample size, publication year, study region, severity of rosacea, and quality of the study according to NOS score. A meta-analysis was performed to obtain pooled effect estimates for all end points. Pooled proportions with 95% CIs were calculated using StatsDirect software, version 3 (StatsDirect Ltd). Because of significant between-study heterogeneity, we chose to use the DerSimonian-Laird random-effects model, which describes the percentage of variation throughout studies that is due to heterogeneity rather than pure chance. Heterogeneity was assessed using I2 statistics.
Results
The search resulted in a total of 9732 articles (Embase, 6219; PubMed, 3513). After removal of duplicates, 7072 articles remained. After titles and abstracts were screened, 292 articles were read in full. Of these, 190 were excluded for reasons listed in the PRISMA flow diagram (Figure 1). A total of 102 articles were included in the qualitative analysis, and 97 contained data for the quantitative analysis; 39 studies reporting all 4 subtypes of rosacea were selected for review. Detailed information on included studies is provided in eTable 1 in the Supplement.
A total of 140 458 patients with rosacea were identified in the included articles. The sex of participants was reported for 137 387 cases of rosacea, and 97 788 (71%) were women. The ages of the patients ranged from 8 to 95 years, but it was not possible to provide a mean or median. However, most studies reported a mean age of approximately 50 years. The included studies originated from Africa (n = 4), Asia (n = 52), Europe (n = 36), North America (n = 8), and South America (n = 2); and the publication period was from 1982 to 2021. Of 102 articles included in the qualitative analysis, 56 were cross-sectional studies, 38 were case-control studies, and 8 were cohort studies. The 97 articles included in the quantitative analyses were categorized into 3 different groups, based on available data, as follows: (1) ETR, PPR, PhR, and ocular rosacea (main analysis); (2) ETR, PPR, and PhR; and (3) ETR and PPR. All studies included were given 7 or more stars according to the Newcastle Ottawa Scale, except for 8 studies, which were not considered to be of high quality because they scored less than 7 stars.13,14,15,16,17,18,19,20 All included studies were clinical population studies except for 6 that were conducted in the general population.21,22,23,24,25,26
Studies Reporting All 4 Rosacea Subtypes
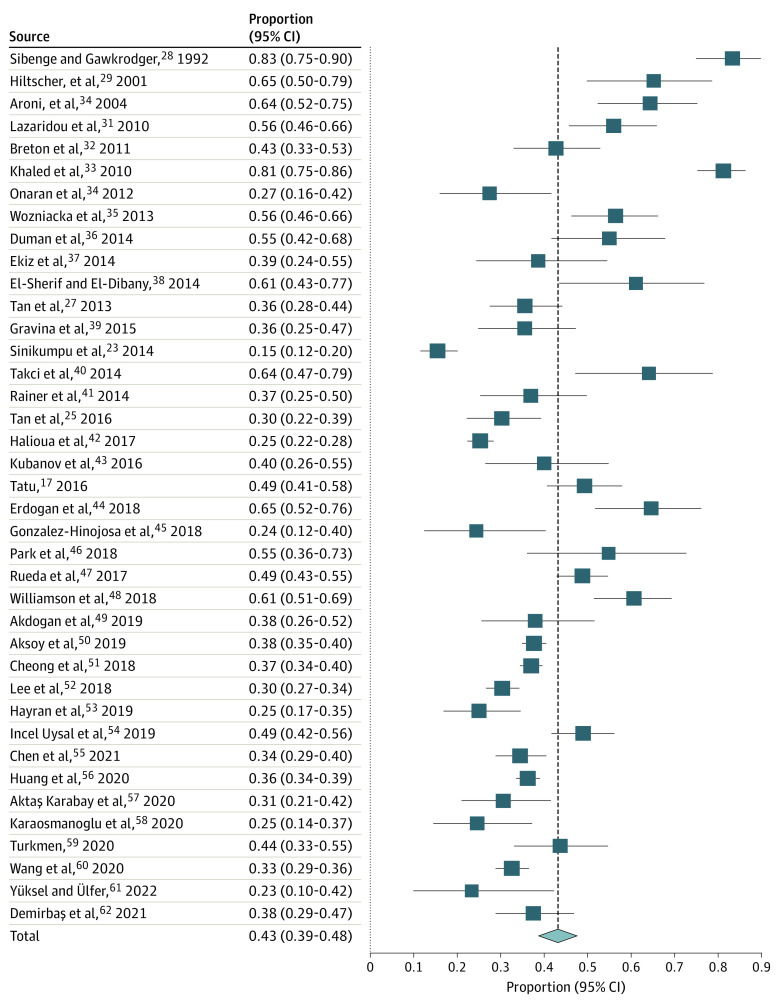
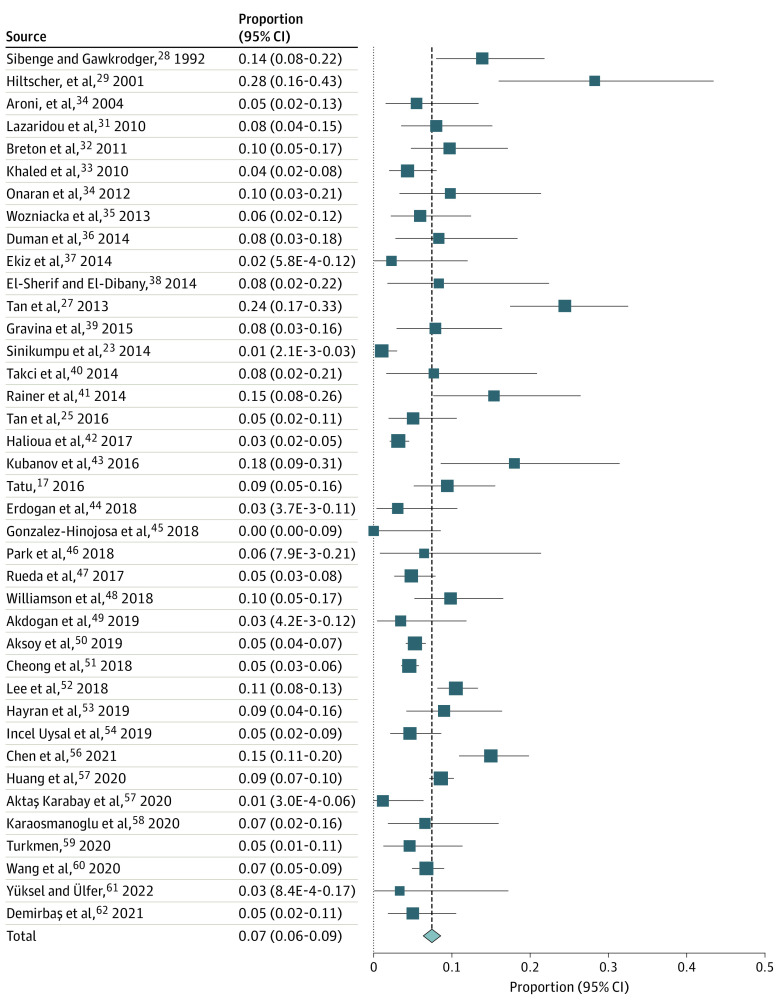
The pooled proportions of ETR, PPR, PhR, and ocular rosacea among 9190 patients with rosacea, based on 39 studies (eTable 2 in the Supplement), were ETR: 56.7% (95% CI, 51.4%-62.0%; I2, 95.9%) (Figure 2),17,23,25,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62 PPR: 43.2% (95% CI, 38.8%-47.6%; I2, 94.0%) (Figure 3),17,23,25,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62 PhR: 7.4% (95% CI, 6.1%-8.9%; I2, 81.4%) (Figure 4),17,23,25,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62 and ocular rosacea: 11.1% (95% CI, 6.7%-16.3%, I2, 98.1%) (Figure 5).17,23,25,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62 The proportions of ETR and PPR appeared to be similar for male and female patients; the proportion of ETR was 58.3% (95% CI, 25.8%-87.2%) for men and 65.5% (95% CI, 41.8%-85.8%) for women based on 3 studies.17,23,27 Papulopustular rosacea was reported for 29.6% (95% CI, 10.5%-53.4%) of men and 34.6% (95% CI, 13.7%-59.3%) of women. Phymatous rosacea was more common among men, affecting 28.6% (95% CI, 10.3%-51.8%) vs 3.6% (95% CI, 0.007%-13.4%) women based on 5 studies.17,23,27,28,29 Ocular rosacea was reported at 36.8% (95% CI, 2.0%-98.4%) for men and 12.6% (95% CI, 0.07%-41.4%) for women based on 3 studies.17,23,27
Figure 2. Forest Plot Showing Pooled Proportion of ETR.
Proportion meta-analysis plot (random effect) showing the proportion of ETR. I2 = 95.9%. ETR indicates erythematotelangiectatic rosacea.
Figure 3. Forest Plot Showing Pooled Proportion of PPR.
Proportion meta-analysis plot (random effect) showing the proportion of PPR. I2 = 94.0%. PPR indicates papulopustular rosacea.
Figure 4. Forest Plot Showing Pooled Proportion of PhR.
Proportion meta-analysis plot (random effect) showing the proportion of PhR. I2 = 81.4%. PhR indicates phymatous rosacea.
Figure 5. Forest Plot Showing Pooled Proportion of Ocular Rosacea.
Proportion meta-analysis plot (random effect) showing the proportion of ocular rosacea. I2 = 98.1%.
Among studies reporting ETR, PPR, PhR, and ocular rosacea, there were 20 cross-sectional studies, 16 case-control studies, and 3 cohort studies. Stratification by study design showed the same pooled proportion of rosacea subtypes. Stratification by population type showed significantly lower frequency of PPR in the 2 general population studies (22.3%; 95% CI, 9.7%-38.2%),23,25 compared with clinical populations (44.5%; 95% CI, 40.1%-48.9%) based on 37 studies. Erythematotelangiectatic rosacea was more common in general population studies, 75.8% (95% CI, 58.8%-89.4%) compared with clinical populations, 54.7% (95% CI, 49.3%-60.0%). Stratification by sample size showed a significantly higher proportion of PPR in small studies defined by fewer than 500 patients (45.3%; 95% CI, 38.8%-52.0%) compared with larger studies (32.2%; 95% CI, 29.4%-37.1%). Stratification by publication decade showed a change in the proportion of PPR (34.4%; 95% CI, 31.6%-37.3%) for the period from 2020 to 2021 compared with studies from 2010 to 2019 (42.7%; 95% CI 37.2%-48.3%) and studies from 2000 to 2009 (60.6%; 95% CI, 54.1%-67.0%) (eFigure 1 in the Supplement). The stratification also showed a change in the proportion of ocular rosacea: 2.8% (95% CI, 0.09%-8.9%) from 2020 to 2021 compared with 28.6% (95% CI, 18.0%-40.5%) from 2000 to 2009. Stratification by a publication period of 2 years showed the same changes for PPR and ocular rosacea, but numbers were low. Stratification by continent was based on 36 clinical population studies except for 1 study from North America, which was done in the general population,25 and 1 study23 from Europe, which showed that PPR affected 72.6% (95% CI, 51.4%-90.0%) of the population in Africa, 48.9% (95% CI, 43.5%-54.4%) in South America, 47.6% (95% CI, 34.7%-60.7%) in Europe, 38.4% (95% CI, 22.7%-55.5%) in North America, and 38.1% (95% CI, 35.0%-41.2%) in Asia. Erythematotelangiectatic rosacea affected 80.4% of the population (95% CI, 66.7%-91.1%) in North America, 60.1% (95% CI, 44.5%-74.8%) in Europe, 54.6% (95% CI, 50.6%-58.6%) in Asia, 31.8% (95% CI, 8.8%-61.0%) in South America, and 21.1% (95% CI, 7.9%-38.6%) in Africa (eFigure 2 in the Supplement). Proportion analysis revealed a study heterogeneity (I2) in the range of 81.4% to 98.1%. Heterogeneity (I2) ranged from 0% to 99.5% through all subanalyses.
Sensitivity Analyses on Studies Reporting 2 or 3 Subtypes of Rosacea Only
The pooled proportions of ETR, PPR, and PhR among 6479 patients with rosacea from 25 studies (eTable 3 and eFigures 3, 4, and 5 in the Supplement) and the pooled proportions of ETR and PPR among 2119 patients with rosacea from 33 studies (eTables 4 and 5 and eFigures 6 and 7 in the Supplement) were essentially similar to those in the 39 studies of the main analysis. Stratification by population type showed no significant difference in proportion of subtypes; however, ETR seemed to be more common in the 3 general population studies, 70.7% (95% CI, 43.9%-91.5%),22,24,26 compared with the 22 clinical population studies, 54.4% (95% CI, 45.7%-63.0%), whereas PPR seemed to be less common in general population studies, 20.0% (95% CI, 6.5%-38.4%) compared with clinical population studies, 45.0% (95% CI, 36.0%-54.3%). Stratification by decade of publication showed an increase in proportion of ETR from 2010 to 2019 (57.1%; 95% CI, 40.8%-72.6%) and for 2020 to 2021 (52.0%; 95% CI, 35.5%-68.4%) compared with studies from 1990 to 1999 (28.3%; 95% CI, 21.3%-35.8%). The stratification also showed a decrease in the proportion of PPR for 2020 to 2021 (50.3%; 95% CI, 40.8%-59.9%) compared with studies from 1990 to 1999 (71.7%; 95% CI, 64.2%-78.7%). For detailed sensitivity analyses, see eAppendix in the Supplement.
Discussion
In this systematic review and meta-analysis, the pooled proportion of ETR was 56.7%, of PPR was 43.2%, of PhR was 7.4%, and of ocular rosacea was 11.1% among 9190 patients with rosacea based on 39 studies. In subgroup analyses restricted to articles that presented data reporting fewer than 4 subtypes, the proportions were essentially similar. Proportions appeared to be similar for men and women regarding ETR and PPR, whereas PhR appeared to be more common in men. It was not possible to examine whether ocular rosacea was associated with male or female sex, nor was it possible to examine potential differences with age. Most studies were from Europe and Asia, and these showed similar proportions. Few studies were from Africa where ETR was less common. Important differences in rosacea subtypes by publication year were detected, indicating a shift in these with increasing ETR and lower PPR frequencies. Also, general population studies tended to show higher proportions of ETR compared with PPR.
Data suggested that the proportion of patients with PPR was higher in studies with older publication dates, whereas the proportion of patients with ETR was higher in more recent publications. This may be explained by a change in environmental exposures leading to certain rosacea subtypes, better or earlier interventions to treat PPR, or increased awareness of rosacea; or it may be a random finding. Also, the increase in ETR could reflect overall increased numbers of patients with rosacea due to change in lifestyle, such as increased intake of alcohol and spicy food, increased UV exposure, or perhaps increased access to laser treatments.
We identified differences in proportions of rosacea subtypes when we compared continent of origin of the studies. For example, ETR was less common in studies from Africa, possibly because of a greater number of patients who have darker skin in whom ETR can be more difficult to diagnose63 or because individuals with ETR who have darker skin tend not to seek medical help because the cosmetic burden may be less severe than that for individuals with lighter skin.64 Another explanation could be differences in skin circulation and generalized microvascular endothelial function associated with race.63
We only included 6 general population studies,21,22,23,24,25,26 which revealed results similar to those of clinical population studies except for ETR being more common in general population studies and PPR being more common in clinical population studies, in turn suggesting that PPR leads patients to seek medical help to a higher degree, presumably because PPR is found to have a slightly greater negative impact on quality of life compared with ETR.65 This may in turn depend on access to the health care system and also on availability of lasers to treat ETR and surgery to treat PhR.66 Similarly, behavioral differences between the sexes may have affected the subtypes that were recorded in the studies; for example, it is possible that women with certain subtypes of rosacea seek medical help earlier than men do.3 Findings from general population studies have a high value because they are not affected by access to medical treatments and other potential sources of bias.
Limitations
This study has limitations. Not all of the included studies diagnosed the subtype of rosacea according to the international classification criteria because the standard classification was not published until 2002.5 Also, the many manifestations of ocular rosacea can make this subtype difficult to diagnose, and in only a few studies was ocular rosacea verified by ophthalmologists. Misclassification is therefore a concern for ocular rosacea, and the results should be interpreted with caution. Few studies were conducted outside of Europe and Asia, and few studies reported data stratified by race and ethnicity or skin type. Moreover, very few studies provided subtypes in different severity strata of rosacea. We observed very high study heterogeneity, and the estimates generated from the meta-analysis may therefore be inaccurate.
Conclusions
The findings suggest that ETR and PPR are the most common subtypes of rosacea based on global data, but we emphasize that our data should be interpreted with caution because there was a high degree of heterogeneity. Better and more detailed reporting is warranted in future studies to allow for new and improved insight into subtypes and their possible association with clinical characteristics and comorbidities. We also strongly recommend that future studies use the phenotype-based approach to diagnose and report rosacea.
eTable 1. Detailed Information on All 102 Included Articles
eTable 2. Detailed Information on the 39 Included Articles Reporting All 4 Subtypes
eFigure 1. Pooled Proportion of ETR and PPR from 1980-2020
eFigure 2. Pooled Proportion of Subtypes in Different Study Continents
eTable 3. Detailed Information on the 25 Included Articles Reporting only ETR, PPR and PhR
eFigure 3. Proportion Meta-analysis Plot (Random Effect) Showing the Proportion of ETR.
eFigure 4. Proportion Meta-analysis Plot (Random Effect) Showing the Proportion of PPR
eFigure 5. Proportion Meta-analysis Plot (Random Effect) Showing the Proportion of PhR
eTable 4. Detailed Information on the 33 Included Articles Reporting Only ETR and PPR
eTable 5. Pooled Proportion of Subtypes Throughout the Three Groups
eFigure 6. Proportion Meta-analysis Plot (Random Effect) Showing the Proportion of ETR
eFigure 7. Proportion Meta-analysis Plot (Random Effect) Showing the Proportion of PPR
eAppendix. Sensitivity Analyses on Studies Reporting Two or Three Subtypes of Rosacea Only
eReferences
References
- 1.van Zuuren EJ, Fedorowicz Z, Carter B, van der Linden MM, Charland L. Interventions for rosacea. Cochrane Database Syst Rev. 2015;2015(4):CD003262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oge’ LK, Muncie HL, Phillips-Savoy AR. Rosacea: diagnosis and treatment. Am Fam Physician. 2015;92(3):187-196. [PubMed] [Google Scholar]
- 3.Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282-289. doi: 10.1111/bjd.16481 [DOI] [PubMed] [Google Scholar]
- 4.Elewski BE, Draelos Z, Dréno B, Jansen T, Layton A, Picardo M. Rosacea - global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25(2):188-200. doi: 10.1111/j.1468-3083.2010.03751.x [DOI] [PubMed] [Google Scholar]
- 5.Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46(4):584-587. doi: 10.1067/mjd.2002.120625 [DOI] [PubMed] [Google Scholar]
- 6.Wilkin J, Dahl M, Detmar M, et al. ; National Rosacea Society Expert Committee . Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50(6):907-912. doi: 10.1016/j.jaad.2004.01.048 [DOI] [PubMed] [Google Scholar]
- 7.Tan J, Almeida LM, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176(2):431-438. doi: 10.1111/bjd.15122 [DOI] [PubMed] [Google Scholar]
- 8.Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148-155. doi: 10.1016/j.jaad.2017.08.037 [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Pietrantonj C. Four-fold table cell frequencies imputation in meta analysis. Stat Med. 2006;25(13):2299-2322. doi: 10.1002/sim.2287 [DOI] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O’Connell DO, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed December 1, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 13.Monpoint S, Languedoc-Roussillon ADVL. Etude epidemiologique sur la rosacee. Nouvelles Dermatologiques. 2001;20(4):262-263. [Google Scholar]
- 14.Michel JL, Cabibel F. Frequency, severity and treatment of ocular rosacea during cutaneous rosacea. Article in French. Ann Dermatol Venereol. 2003;130(1 Pt 1):20-24. [PubMed] [Google Scholar]
- 15.Thurkkaram I. Clinical forms and epidermiological features of rosacea. Eur J Med Res. 2010;15(suppl 1):39. [Google Scholar]
- 16.Perrigouard C, Peltre B, Cribier B. [A histological and immunohistological study of vascular and inflammatory changes in rosacea]. Ann Dermatol Venereol. 2013;140(1):21-29. doi: 10.1016/j.annder.2012.10.592 [DOI] [PubMed] [Google Scholar]
- 17.Tatu AL. Clinicodermoscopic correlations observed in a rosacea group of patients. J Am Acad Dermatol. 2016;74(5)(suppl 1):AB104. doi: 10.1016/j.jaad.2016.02.409 [DOI] [Google Scholar]
- 18.Pindado-Ortega C, Saceda-Corralo D, Buendía-Castaño D, et al. Frontal fibrosing alopecia and cutaneous comorbidities: a potential relationship with rosacea. J Am Acad Dermatol. 2018;78(3):596-597.e1. doi: 10.1016/j.jaad.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 19.Molina DAC, Zuniga DMM, Romero YL, et al. Skin phototype and positive antinuclear antibodies in rosacea patients in a dermatologic center in Bogota, Colombia. J Am Acad Dermatol. 2019;81(4)(suppl 1):AB277. doi: 10.1016/j.jaad.2019.06.1204 [DOI] [Google Scholar]
- 20.Lee SH, Lee SB, Heo JH, et al. Sebaceous glands participate in the inflammation of rosacea. J Eur Acad Dermatol Venereol. 2020;34(3):e144-e146. doi: 10.1111/jdv.16055 [DOI] [PubMed] [Google Scholar]
- 21.Berg M, Lidén S. An epidemiological study of rosacea. Acta Derm Venereol. 1989;69(5):419-423. [PubMed] [Google Scholar]
- 22.Abram K, Silm H, Oona M. Prevalence of rosacea in an Estonian working population using a standard classification. Acta Derm Venereol. 2010;90(3):269-273. doi: 10.2340/00015555-0856 [DOI] [PubMed] [Google Scholar]
- 23.Sinikumpu SP, Huilaja L, Jokelainen J, et al. High prevalence of skin diseases and need for treatment in a middle-aged population: a Northern Finland Birth Cohort 1966 study. PLoS One. 2014;9(6):e99533. doi: 10.1371/journal.pone.0099533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moustafa F, Hopkinson D, Huang KE, Feldman S. Prevalence of rosacea in community settings. J Cutan Med Surg. 2015;19(2):149-152. doi: 10.2310/7750.2014.14087 [DOI] [PubMed] [Google Scholar]
- 25.Tan J, Schöfer H, Araviiskaia E, Audibert F, Kerrouche N, Berg M; RISE study group . Prevalence of rosacea in the general population of Germany and Russia - The RISE study. J Eur Acad Dermatol Venereol. 2016;30(3):428-434. doi: 10.1111/jdv.13556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Wang B, Deng Y, et al. Epidemiological features of rosacea in Changsha, China: a population-based, cross-sectional study. J Dermatol. 2020;47(5):497-502. doi: 10.1111/1346-8138.15301 [DOI] [PubMed] [Google Scholar]
- 27.Tan J, Blume-Peytavi U, Ortonne JP, et al. An observational cross-sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169(3):555-562. doi: 10.1111/bjd.12385 [DOI] [PubMed] [Google Scholar]
- 28.Sibenge S, Gawkrodger DJ. Rosacea: a study of clinical patterns, blood flow, and the role of Demodex folliculorum. J Am Acad Dermatol. 1992;26(4):590-593. doi: 10.1016/0190-9622(92)70086-U [DOI] [PubMed] [Google Scholar]
- 29.Hiltscher D, Boslet WT, Fuchslocher M, Sinkgraven R, Rzany B. Lebensqualitat bei Patienten mit Rosacea und Rhinophym. Aktuelle Derm. 2001;27(12):391-394. doi: 10.1055/s-2001-19630 [DOI] [Google Scholar]
- 30.Aroni K, Tsagroni E, Lazaris AC, Patsouris E, Agapitos E. Rosacea: a clinicopathological approach. Dermatology. 2004;209(3):177-182. doi: 10.1159/000079886 [DOI] [PubMed] [Google Scholar]
- 31.Lazaridou E, Apalla Z, Sotiraki S, Ziakas NG, Fotiadou C, Ioannides D. Clinical and laboratory study of rosacea in northern Greece. J Eur Acad Dermatol Venereol. 2010;24(4):410-414. doi: 10.1111/j.1468-3083.2009.03424.x [DOI] [PubMed] [Google Scholar]
- 32.Breton AL, Truchetet F, Véran Y, et al. Prevalence analysis of smoking in rosacea. J Eur Acad Dermatol Venereol. 2011;25(9):1112-1113. doi: 10.1111/j.1468-3083.2010.03802.x [DOI] [PubMed] [Google Scholar]
- 33.Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88(8):597-601. [PubMed] [Google Scholar]
- 34.Onaran Z, Karabulut AA, Usta G, Örnek K. Central corneal thickness in patients with mild to moderate rosacea. Can J Ophthalmol. 2012;47(6):504-508. doi: 10.1016/j.jcjo.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 35.Woźniacka A, Salamon M, McCauliffe D, Sysa-Jędrzejowska A. Antinuclear antibodies in rosacea patients. Postepy Dermatol Alergol. 2013;30(1):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duman N, Ersoy Evans S, Atakan N. Rosacea and cardiovascular risk factors: a case control study. J Eur Acad Dermatol Venereol. 2014;28(9):1165-1169. doi: 10.1111/jdv.12234 [DOI] [PubMed] [Google Scholar]
- 37.Ekiz O, Balta I, Sen BB, Dikilitaş MC, Ozuğuz P, Rifaioğlu EN. Vitamin D status in patients with rosacea. Cutan Ocul Toxicol. 2014;33(1):60-62. doi: 10.3109/15569527.2013.797907 [DOI] [PubMed] [Google Scholar]
- 38.El-Sherif NA, El-Dibany SA. Clinical evaluation of Libyan patients with rosacea and its correlation with seropositivity to Helicobacter pylori. J Dermatol Surg.2014;18(1-2):13-16. doi: 10.1016/j.jssdds.2013.12.005 [DOI] [Google Scholar]
- 39.Gravina A, Federico A, Ruocco E, et al. Helicobacter pylori infection but not small intestinal bacterial overgrowth may play a pathogenic role in rosacea. United European Gastroenterol J. 2015;3(1):17-24. doi: 10.1177/2050640614559262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takci Z, Bilgili SG, Karadag AS, Kucukoglu ME, Selek S, Aslan M. Decreased serum paraoxonase and arylesterase activities in patients with rosacea. J Eur Acad Dermatol Venereol. 2015;29(2):367-370. doi: 10.1111/jdv.12556 [DOI] [PubMed] [Google Scholar]
- 41.Rainer BM, Fischer AH, Luz Felipe da Silva D, Kang S, Chien AL. Rosacea is associated with chronic systemic diseases in a skin severity-dependent manner: results of a case-control study. J Am Acad Dermatol. 2015;73(4):604-608. doi: 10.1016/j.jaad.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 42.Halioua B, Cribier B, Frey M, Tan J. Feelings of stigmatization in patients with rosacea. J Eur Acad Dermatol Venereol. 2017;31(1):163-168. doi: 10.1111/jdv.13748 [DOI] [PubMed] [Google Scholar]
- 43.Kubanov AA, Gallyamova YA, Grevceva AS. Influence of mites genus demodex on clinical picture of the disease at patients with acne and rosacea. Int J Pharm Technol. 2016;8(2):13694-13705. [Google Scholar]
- 44.Erdogan HK, Bulur I, Saracoglu ZN, Bilgin M. The evaluation of contact sensitivity with standard and cosmetic patch test series in rosacea patients. Ann Dermatol. 2018;30(3):290-295. doi: 10.5021/ad.2018.30.3.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Hinojosa D, Jaime-Villalonga A, Aguilar-Montes G, Lammoglia-Ordiales L. Demodex and rosacea: is there a relationship? Indian J Ophthalmol. 2018;66(1):36-38. doi: 10.4103/ijo.IJO_514_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park BW, Ha JM, Cho EB, et al. A study on vitamin d and cathelicidin status in patients with rosacea: serum level and tissue expression. Ann Dermatol. 2018;30(2):136-142. doi: 10.5021/ad.2018.30.2.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rueda LJ, Motta A, Pabón JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56(5):510-513. doi: 10.1111/ijd.13491 [DOI] [PubMed] [Google Scholar]
- 48.Williamson T, Cheng WY, McCormick N, Vekeman F. Patient preferences and therapeutic satisfaction with topical agents for rosacea: a survey-based study. Am Health Drug Benefits. 2018;11(2):97-106. [PMC free article] [PubMed] [Google Scholar]
- 49.Akdogan N, Alli N, Incel Uysal P, Candar T. Role of serum 25-hydroxyvitamin D levels and vitamin D receptor gene polymorphisms in patients with rosacea: a case-control study. Clin Exp Dermatol. 2019;44(4):397-403. doi: 10.1111/ced.13769 [DOI] [PubMed] [Google Scholar]
- 50.Aksoy B, Ekiz Ö, Unal E, et al. Systemic comorbidities associated with rosacea: a multicentric retrospective observational study. Int J Dermatol. 2019;58(6):722-728. doi: 10.1111/ijd.14353 [DOI] [PubMed] [Google Scholar]
- 51.Cheong KW, Yew YW, Lai YC, Chan R. Clinical characteristics and management of patients with rosacea in a tertiary dermatology center in Singapore from 2009 to 2013. Int J Dermatol. 2018;57(5):541-546. doi: 10.1111/ijd.13954 [DOI] [PubMed] [Google Scholar]
- 52.Lee JB, Moon J, Moon KR, et al. Epidemiological and clinical features of rosacea in Korea: A multicenter cross-sectional study. J Dermatol. 2018;45(5):546-553. doi: 10.1111/1346-8138.14281 [DOI] [PubMed] [Google Scholar]
- 53.Hayran Y, Lay I, Mocan MC, Bozduman T, Ersoy-Evans S. Vascular endothelial growth factor gene polymorphisms in patients with rosacea: A case-control study. J Am Acad Dermatol. 2019;81(2):348-354. doi: 10.1016/j.jaad.2019.03.055 [DOI] [PubMed] [Google Scholar]
- 54.Incel Uysal P, Akdogan N, Hayran Y, Oktem A, Yalcin B. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94(6):704-709. doi: 10.1016/j.abd.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen B, Yu F, Chen W, et al. Contact sensitization to cosmetic series of allergens in female patients with rosacea: A prospective controlled study in China. J Cosmet Dermatol. 2021;20(8):2627-2634. doi: 10.1111/jocd.13902 [DOI] [PubMed] [Google Scholar]
- 56.Huang YX, Li J, Zhao ZX, et al. Effects of skin care habits on the development of rosacea: A multi-center retrospective case-control survey in Chinese population. PLoS One. 2020;15(4):e0231078. doi: 10.1371/journal.pone.0231078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aktaş Karabay E, Karşıyakalı N, Karabay E. Evaluation of sexual functions in female rosacea patients: a prospective, case-control study. Int J Impot Res. 2020;32(6):628-634. doi: 10.1038/s41443-020-0275-z [DOI] [PubMed] [Google Scholar]
- 58.Karaosmanoglu N, Karaaslan E, Ozdemir Cetinkaya P. Evaluation of serum uric acid levels in patients with rosacea. Arch Dermatol Res. 2020;312(6):447-451. doi: 10.1007/s00403-020-02033-w [DOI] [PubMed] [Google Scholar]
- 59.Turkmen D. Serum bilirubin and uric acid antioxidant levels in rosacea patients. J Cosmet Dermatol. 2020;19(10):2717-2720. doi: 10.1111/jocd.13395 [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Xie H, Gong Y, et al. Relationship between rosacea and sleep. J Dermatol. 2020;47(6):592-600. doi: 10.1111/1346-8138.15339 [DOI] [PubMed] [Google Scholar]
- 61.Yüksel M, Ülfer G. Measurement of the serum zonulin levels in patients with acne rosacea. J Dermatolog Treat. 2022;33(1):389-392. doi: 10.1080/09546634.2020.1757015 [DOI] [PubMed] [Google Scholar]
- 62.Demirbaş A, Yümer Y, Elmas ÖF, et al. Relationship between rosacea and chronic obstructive pulmonary disease: Rosacea and comorbidities. J Cosmet Dermatol. 2021. Published online August 19, 2021. doi: 10.1111/jocd.14389 [DOI] [PubMed] [Google Scholar]
- 63.Alexis AF, Callender VD, Baldwin HE, Desai SR, Rendon MI, Taylor SC. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80(6):1722-1729.e7. doi: 10.1016/j.jaad.2018.08.049 [DOI] [PubMed] [Google Scholar]
- 64.Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17(1):70-73. doi: 10.1016/S0190-9622(87)70173-X [DOI] [PubMed] [Google Scholar]
- 65.Zeichner JA, Eichenfield LF, Feldman SR, Kasteler JS, Ferrusi IL. Quality of life in individuals with erythematotelangiectatic and papulopustular rosacea: findings from a web-based survey. J Clin Aesthet Dermatol. 2018;11(2):47-52. [PMC free article] [PubMed] [Google Scholar]
- 66.McGinley M, Alinia H, Kuo S, Huang KE, Feldman SR. Patient perspectives on low level light therapy and laser therapies for rosacea-associated persistent facial redness. Dermatol Online J. 2014;21(2):13030/qt2q39x891. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Detailed Information on All 102 Included Articles
eTable 2. Detailed Information on the 39 Included Articles Reporting All 4 Subtypes
eFigure 1. Pooled Proportion of ETR and PPR from 1980-2020
eFigure 2. Pooled Proportion of Subtypes in Different Study Continents
eTable 3. Detailed Information on the 25 Included Articles Reporting only ETR, PPR and PhR
eFigure 3. Proportion Meta-analysis Plot (Random Effect) Showing the Proportion of ETR.
eFigure 4. Proportion Meta-analysis Plot (Random Effect) Showing the Proportion of PPR
eFigure 5. Proportion Meta-analysis Plot (Random Effect) Showing the Proportion of PhR
eTable 4. Detailed Information on the 33 Included Articles Reporting Only ETR and PPR
eTable 5. Pooled Proportion of Subtypes Throughout the Three Groups
eFigure 6. Proportion Meta-analysis Plot (Random Effect) Showing the Proportion of ETR
eFigure 7. Proportion Meta-analysis Plot (Random Effect) Showing the Proportion of PPR
eAppendix. Sensitivity Analyses on Studies Reporting Two or Three Subtypes of Rosacea Only
eReferences