Abstract
Background
We investigated whether contrast-enhanced ultrasonography (CEUS) scores can predict lymphocyte-predominant breast cancer (LPBC).
Patients and Methods
We evaluated 75 patients who underwent US and CEUS. LPBC was defined as tissues with ≥50% stromal tumour-infiltrating lymphocytes (TILs) preoperatively. Characteristic US images predicting LPBC were evaluated using TIL-US scores via three ultrasonic tissue characteristics: Shape, internal echo level, and posterior echoes. TIL-CEUS was evaluated based on TIL-US plus CEUS.
Results
TIL-US and TIL-CEUS cut-offs for predicting LPBC were 4 and 6 (area under the curve=0.93 and 0.96, respectively) points based on receiver operating characteristics curves. Sensitivity, specificity, and accuracy values (95% confidence intervaI) were 0.94 (0.77-0.99), 0.75 (0.70-0.77), and 0.80 (0.72-0.82); and 0.94 (0.78-0.99), 0.86 (0.81-0.87), and 0.88 (0.80-0.90) for TIL-US and TIL-CEUS, respectively. TIL-CEUS score was a significant single predictor for LPBC in multivariate logistic regression (p=0.001).
Conclusion
TIL-CEUS can be used for preoperative LPBC prediction and detection.
Keywords: Breast cancer, tumour-infiltrating lymphocytes, lymphocyte-predominant breast cancer, ultrasonography, contrastenhanced ultrasound, ascending slope
Immunological parameters, including tumour-infiltrating lymphocytes (TILs), are prognostic factors for breast cancer (BC) (1-4) and predict response to neoadjuvant chemotherapy (NAC) in BC (5,6). Hence, TILs are important biological markers that predict prognosis and drug treatment effect. The pathological evaluation of TILs is recommended by the International Immuno-Oncology Biomarker Working Group guidelines (7,8). It was reported that LPBC was significantly associated with outcomes in patients with human epidermal growth factor receptor 2 (HER2)-positive BC treated with chemotherapy (2). Moreover, LPBC was an independent prognostic predictor in triple-negative BC (9). Furthermore, for HER2-positive BC, LPBC was a remarkable predictor of pathological complete response after preoperative chemotherapy (5). Although the presence of LPBC is a useful predictor of prognosis and treatment effect in BC, an accurate and convenient evaluation of preoperative and pre-NAC is urgently required.
Ultrasonography (US) is useful for BC detection and diagnosis (10-12). US tissue characterisation is useful for discriminating benign from malignant tumours by evaluating their morphological and internal characteristics (13). Characteristic US images predicting LPBC were scored by the TIL-US scores based on three ultrasonic tissue characteristics: shape, internal echo level and posterior echoes (14). Contrast-enhanced US (CEUS) enables detailed intra-tumoural blood flow visualisation (15,16) and can be superior to b-mode US for differentiating benign tissue from BC (17). CEUS can qualitatively and quantitatively evaluate changes in blood flow, and perfusion parameters assessed quantitatively by CEUS correlate with the microvessel number determined by immunostaining in BC (18). Perfusion parameters on CEUS can provide excellent predictive value for high-grade malignancy and might help determine appropriate therapeutic strategies (19) and predict treatment outcomes for BC after NAC (20). Among CEUS parameters, ascending slope (AS) in particular has been shown to be one of the most important parameters for predicting the efficacy of neoadjuvant chemotherapy (20).
We investigated whether the AS of perfusion parameters assessed from CEUS can predict LPBC and determined whether the TIL-CEUS score can predict LPBC more accurately in combination with CEUS rather than by itself.
Patients and Methods
Participants and study design. We evaluated 75 consecutive patients with clinical BC (T1-4, N0-1, M0) who underwent US before NAC between November 2013 and October 2018. Tumour staging was based on the seventh edition of the American Joint Committee on Cancer staging manual (21). All procedures involving human participants were performed in accordance with the ethical standards of our institutional research committee (Institutional Review Board no. 1166) and the Declaration of Helsinki or comparable ethical standards. Formal consent requirement was waived owing to the study’s retrospective nature.
CEUS and US. Conventional US images were acquired using HIVISION Ascendus (Hitachi, Chiyoda-ku, Tokyo, Japan), and representative images of the index tumour (largest diameter lesion) were acquired. Blood perfusion images, acquired using CEUS with perflubutane microbubbles stabilised with a phosphatidylserine membrane (GE Healthcare Pharma Co., Tokyo, Japan), were assessed qualitatively and quantitatively. CEUS was performed using EUP-L74M linear-array transducer with colour wide-band pulse inversion and coded-phase inversion harmonic US. The CEUS inspection method has been previously described (19,20).
As described previously, CEUS perfusion parameters were created from time–intensity curves based on enhancement intensity and temporal changes to objectively evaluate CEUS findings (18-20). The amount of time required to reach the maximum intensity starting from the moment that the first microbubble entered the lesion was defined as the time to peak (TTP). The peak intensity (PI) of brightness in BC lesions on perflubutane-enhanced US images was calculated as the maximum intensity minus the baseline intensity, and AS was calculated as PI divided by TTP. These perfusion parameters were defined as described previously (18-20). AS has been shown to provide excellent predictive value for high-grade malignancies (19) and can predict the outcome of BC after NAC (20). Therefore, in this study, for CEUS, we assessed the AS of perfusion (18-20).
Scoring of US and CEUS imaging characteristics. As described previously (14), characteristic US image findings that predict LPBC include three ultrasonic tissue characteristics: Shape (more lobulated), internal echo level (weaker) and posterior echoes (stronger). For these ultrasonic tissue characterisations, more characteristic findings were assigned higher scores. The TIL-US scores included the total score, which ranged from 0 to 7 (total points) (14), and these scores showed an excellent performance in predicting LPBC (14). We identified the AS values of CEUS and the best area under the curve (AUC) values for the TIL-US and TIL-CEUS scores via receiver operating characteristic (ROC) analyses. LPBC prediction was performed for all patients.
Pathological diagnosis. Pathological diagnoses were established from specimens obtained from preoperative biopsy before NAC. Primary tumours were evaluated for nuclear grade, estrogen receptor (ER) and HER2 status, and Ki67 proliferation index. Nuclear grade was assigned according to the general rules for clinical and pathological recording of BC, 17th edition (Japanese BC Society, 2012) (22). ER positivity was assessed by immunohistochemical analysis and scored as per the Allred system. HER2 positivity was scored as 3+ by immunohistochemistry alone; 2+ by immunohistochemistry and HER2/chromosome 17 (CEP17) ≥2.0 by fluorescent in situ hybridisation; and 2+ by immunohistochemistry, HER2/CEP17 <2.0, and average HER2 copy number ≥6.0 signals/cell by fluorescent in situ hybridisation. Stromal lymphocytes were evaluated on haematoxylin–eosin-stained sections according to the latest recommendations (International TILs Working Group, 2014) (7) by two experienced pathologists. BC samples with ≥50% stromal TILs and those with <50% stromal TILs were defined as LPBC and non-LPBC, respectively, as a predefined categorical parameter (1,3,7,8).
Statistical analysis. Associations among LPBC, clinicopathological factors, and US tissue characterisation were analysed using chi-squared test. Predictive performance regarding LPBC identification by the TIL-US and TIL-CEUS scores was evaluated by ROC analysis; this was performed using incrementally increasing cutoff values of the TIL-US and TIL-CEUS scores and recalculating the corresponding true-positive and false-negative rates. Univariate and multivariate logistic regression analyses were used for each potential predictor variable for LPBC. Sensitivity, specificity, accuracy, and positive (PPV) and negative (NPV) predictive values were compared for the TIL-US and TIL-CEUS scores and calculated according to standard formulas. Odds ratios (ORs) and 95% confidence intervals (CIs) are reported, and all analyses were performed using JMP version 13.0 (SAS Institute, Cary, NC, USA). Values of p<0.05 were considered statistically significant.
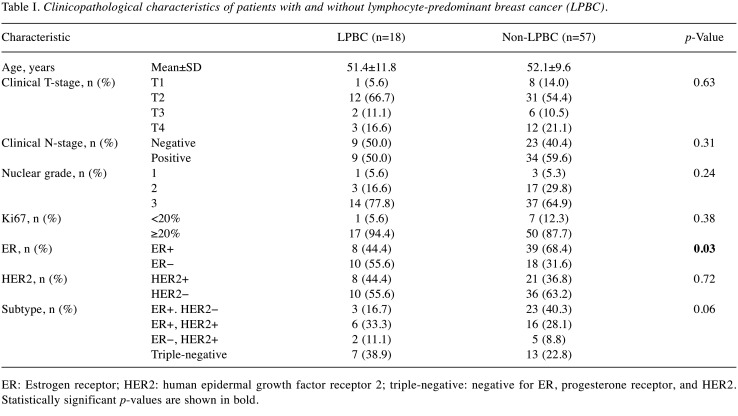
Results
Clinicopathological factors. The clinicopathological characteristics of the 75 study patients (18 in the LPBC group and 57 in the non-LPBC group) as per TIL predominance are summarised in Table I. LPBC using the TIL-US and TIL-CEUS scores was determined in 31 and 26 patients, respectively. TIL was significantly associated with ER− (p=0.03). There was no significant association of TIL with T (p=0.63), clinical N+ (p=0.31), NG (p=0.24), Ki67 proliferation index (p=0.38) and HER2+ disease (p=0.72). Of the patients, 26, 22, 7, and 20 had ER+/HER2−, ER+/HER2+, ER−/HER2+ and triple-negative BC, respectively. TILs were not significantly associated with subtype (p=0.06).
Table I. Clinicopathological characteristics of patients with and without lymphocyte-predominant breast cancer (LPBC).
ER: Estrogen receptor; HER2: human epidermal growth factor receptor 2; triple-negative: negative for ER, progesterone receptor, and HER2. Statistically significant p-values are shown in bold.
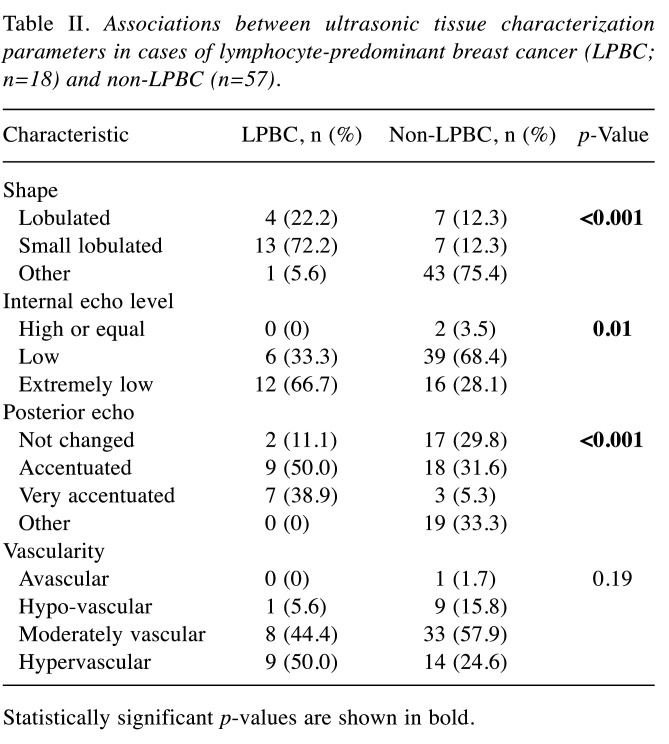
US tissue characterisation. The LPBC group was found to have more lobulated areas (lobulated, 22.2%; small lobulated, 72.2%), whereas the non-LPBC group had only a few lobulated areas (lobulated, 12.3%; small lobulated, 12.3%) (p<0.001; Table II). The small lobulated shape was a characteristic of LPBCs. All LPBC cases had areas with low (33.3%) or extremely low (66.7%) internal echo levels. Posterior echo is a characteristic US finding. Accentuated posterior echoes (no change, 11.1%; accentuated, 50.0%; very accentuated, 38.9%) were found more frequently in cases with LPBC (p<0.001). Vascularity was not significantly different between LPBC and non-LPBC groups (p=0.19).
Table II. Associations between ultrasonic tissue characterization parameters in cases of lymphocyte-predominant breast cancer (LPBC; n=18) and non-LPBC (n=57).
Statistically significant p-values are shown in bold.
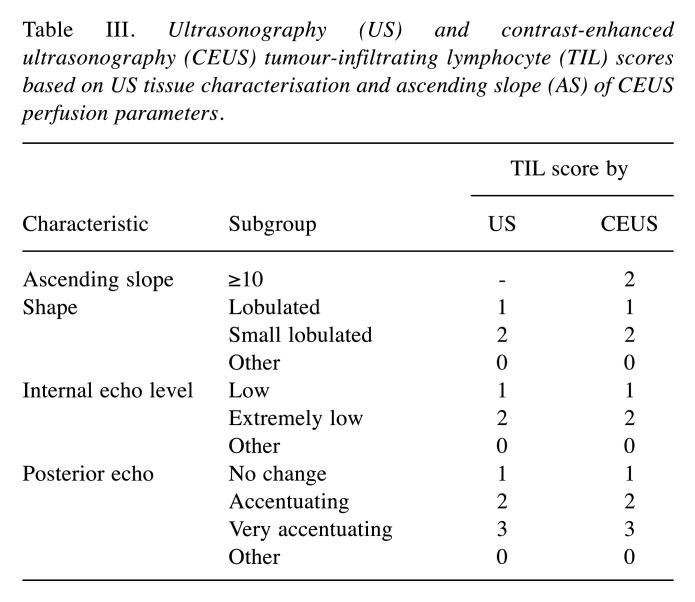
Optimal TIL-US and TIL-CEUS scores. Table III shows US and CEUS TIL scores based on US tissue characterisation and AS using CEUS perfusion parameters. Total US scores range from 0 to 7 (14) while total CEUS scores range from 0 to 9, according to the sub-scores for shape, internal echo level, posterior echoes, and AS (Table III). The mean±standard deviation value for AS in the 75 patients was 10.4±4.2. We plotted ROC curves to establish cutoff values for predicting LPBC based on data derived from AS. The AUC value for AS was 0.85 (95% CI=0.76-0.93; p<0.001; Figure 1). We set the AS cutoff at 10.0 for predicting LPBC based on the ROC curve.
Table III. Ultrasonography (US) and contrast-enhanced ultrasonography (CEUS) tumour-infiltrating lymphocyte (TIL) scores based on US tissue characterisation and ascending slope (AS) of CEUS perfusion parameters.
Figure 1. Receiver operating characteristics (ROC) curve of perfusion parameter ascending slope (AS) to predict lymphocyte-predominant breast cancer (LPBC) in the entire study cohort. ROC curves were used to predict LPBC and establish cutoffs for LPBC based on data derived from AS in contrast-enhanced ultrasonography (CEUS) perfusion parameter for predicting LPBC. The best area under the curve value for AS was 0.85 (95% confidence intervaI=0.76-0.93; p<0.001) using a cutoff (◯) of 10.0 points.
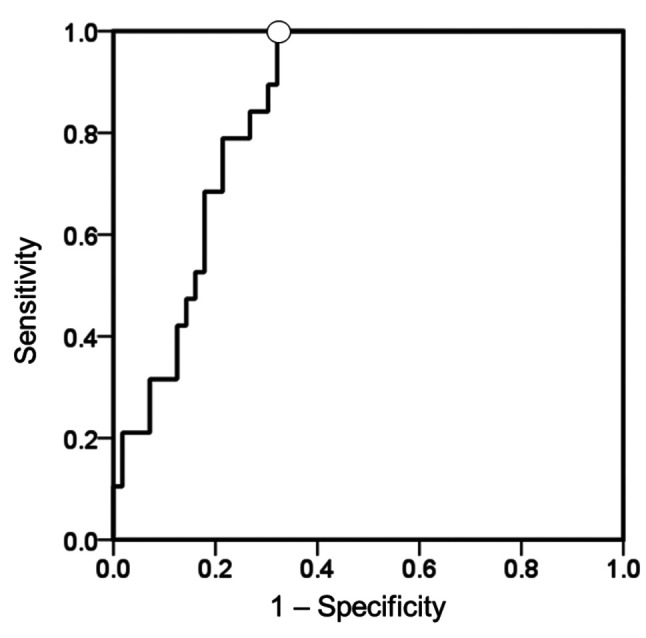
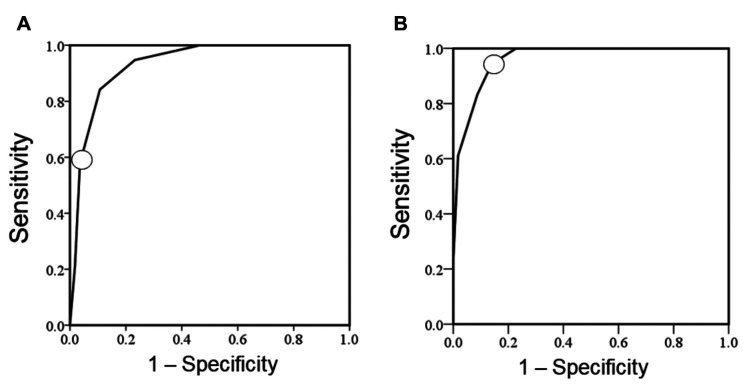
ROC curves were used to establish cutoff values for predicting LPBC based on data derived from the TIL-US and TIL-CEUS scores. The AUC value for TIL-US score was 0.93 (95% CI=0.88-0.99; p<0.001) (Figure 2A), and we set the TIL-US score cutoff for predicting LPBC at 4 points (14). The TIL-CEUS score (total score) ranged from 0 to 9 (total points) and was composed of the TIL-US score (0-7 total points) plus the subscore of AS (0 and 2 points for <10 and ≥10, respectively). The best AUC value for the TIL-CEUS score was 0.96 (95% CI=0.92-1.00; p<0.001) using a cutoff of 6 points (Figure 2B).
Figure 2. Receiver operating characteristic (ROC) curve of tumour-infiltrating lymphocyte-ultrasonography (TIL-US) (A) and TIL-contrast-enhanced US (CEUS) (B) scores to predict lymphocyte-predominant breast cancer (LPBC) in the entire study cohort. ROC curves were used to establish cutoffs for predicting LPBC based on data derived from the TIL-US and TIL-CEUS scores. The best area under the curve value for the TIL-US score was 0.93 (95% confidence intervaI=0.88-0.99; p<0.001) using a cutoff of 4 points. The best area under the curve value for TIL-CEUS score was 0.96 (95% confidence intervaI=0.92-1.00; p<0.001) using a cutoff of 6 points. ◯ Optimal cutoff.
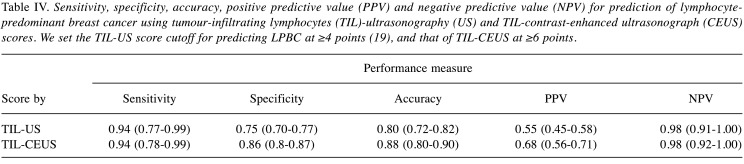
Sensitivity, specificity, accuracy, PPV, and NPV. The sensitivity, specificity, accuracy, PPV and NPV of the TIL-US score for predicting LPBC are given in Table IV. The diagnostic capacity of the TIL-CEUS score for predicting LPBC was as good as or better than that of the TIL-US score. Specificity, accuracy, and PPV were greater with the TIL-CEUS score than with the TIL-US score.
Table IV. Sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) for prediction of lymphocytepredominant breast cancer using tumour-infiltrating lymphocytes (TIL)-ultrasonography (US) and TIL-contrast-enhanced ultrasonograph (CEUS) scores. We set the TIL-US score cutoff for predicting LPBC at ≥4 points (19), and that of TIL-CEUS at ≥6 points.
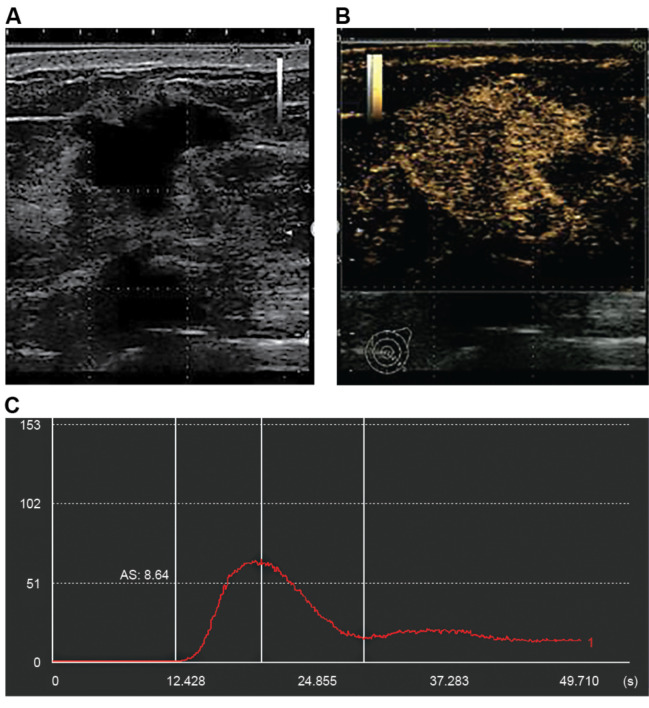
Figure 3 shows a pre-biopsy non-LPBC case. The TIL-US score was 4 points for the irregular shape, extremely low internal echo level, and accentuated posterior echoes (Figure 3A). On the CEUS images (Figure 3B), the AS value calculated from the time–intensity curve (Figure 3C) was 8.64 and the TIL-CEUS score was 4 points.
Figure 3. Representative ultrasonography (US) images (A), contrast-enhanced US (CEUS) images (B), and time–intensity curve (C) for a case of non-lymphocyte-predominant breast cancer (LPBC). US tissue characterisation showed an irregular shape, extremely low internal echo level, and accentuated posterior echoes. The ascending slope value calculated from time–intensity curves using CEUS was 8.64. The tumour-infiltrating lymphocyte (TIL)-US and TIL-CEUS scores for this case were 4 points for both; LPBC was predicted from the TIL-US score and non-LPBC from the TIL-CEUS score.
TIL-US score ≥4 (n=31) and TIL-CEUS score ≥6 (n=25) predicted non-LPBC in 14 and eight cases, respectively. Among the non-LPBC cases (n=57), six were predicted as LPBC by the TIL-US score and non-LPBC by the TIL-CEUS score. Likewise, among the non-LPBC cases (n=57), six were predicted as LPBC by the TIL-US score and non-LPBC by the TIL-CEUS score. Hence, the TIL-CEUS score can predict LPBC and non-LPBC more accurately.
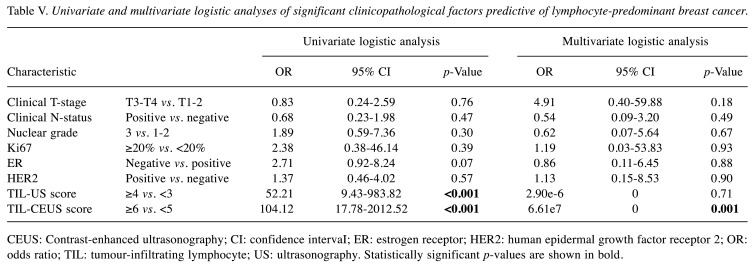
Univariate and multivariate logistic regression analyses. The significant clinicopathological factors predictive of LPBC determined by univariate and multivariate logistic analyses are shown in Table V. On univariate logistic analysis, TIL-US score ≥5 and TIL-CEUS score ≥6 were the significant independent predictors of LPBC. On multivariate logistic analysis, the TIL-CEUS score was significantly associated with effective risk assessment in LPBC. LPBC was demonstrated from preoperative biopsy tissue in 18 patients; both the TIL-US and TIL-CEUS scores predicted LPBC in 17 out of 18 patients. As the bias was extreme, it was not possible to calculate the 95% CIs for the TIL-US and TIL-CEUS scores in multivariate analysis.
Table V. Univariate and multivariate logistic analyses of significant clinicopathological factors predictive of lymphocyte-predominant breast cancer.
CEUS: Contrast-enhanced ultrasonography; CI: confidence intervaI; ER: estrogen receptor; HER2: human epidermal growth factor receptor 2; OR: odds ratio; TIL: tumour-infiltrating lymphocyte; US: ultrasonography. Statistically significant p-values are shown in bold.
Discussion
This study demonstrated that the AS of perfusion parameters assessed from CEUS can predict LPBC. To our knowledge, this is the first report to predict LPBC more accurately in combination with TIL-US. CEUS enables a detailed, real-time evaluation of haemodynamics in BC. We converted brightness on CEUS images into numerical values to evaluate the contrast effects of perflubutane (19,20) and investigated whether perfusion parameters created from CEUS can predict LPBC. We constructed ROC curves for the CEUS perfusion parameter AS to predict LPBC; its AUC value was 0.85, indicating that CEUS is useful for predicting diagnosis. This supports the notion that LPBC is related to haemodynamics.
ROC curves were used to establish cutoffs for LPBC based on data derived from the TIL-US and TIL-CEUS scores combined with AS, with AUC values of 0.93 and 0.96, respectively. The diagnostic capacity of the TIL-CEUS score for predicting LPBC was equal to or better than that of the TIL-US score; the TIL-CEUS score had higher specificity, accuracy, and PPV than the TIL-US score and may be an applicable index for the preoperative evaluation of LPBC. In the non LPBC case shown in Figure 3, even though the TIL-US score was predictive of LPBC, there were low haemodynamics in CEUS, and non-LPBC was predicted using the TIL-CEUS score. In this case, the TILs-US score may have been a more accurate assessment of TILs. The TIL-CEUS score was also a significant independent preoperative predictor of LPBC. Our findings indicate that the TIL-CEUS score can predict LPBC and may be used for a more accurate evaluation of LPBC preoperatively.
The limitations of our study must be acknowledged. Firstly, this was a retrospective study involving a single institute. Secondly, we analysed whether preoperative biopsy tissues obtained before NAC are useful for LPBC evaluation. To evaluate LPBC accurately, determinants useful for LPBC prediction are required based on pathological specimens obtained after surgery in patients not undergoing NAC and clinicopathological factors, including TIL-CEUS scores. Thirdly, the cutoff used in the study classified the patients into two groups (<50% and ≥50%) and did not group them using 10% intervals. Classifying the patients using cutoffs of small specific ranges may make the TIL-CEUS score more useful. Our data showed an excellent LPBC predictive performance, but the underlying mechanism for this remains unclear. Future studies are needed to comparatively analyse the US and pathological images to clarify the underlying mechanisms. In the future, the TIL-CEUS score might be useful for predicting the therapeutic effect of NAC.
Conflicts of Interest
The Authors declare that they have no conflicts of interest.
Authors’ Contributions
Kayo Fukui and Norio Masumoto were involved in study design and data interpretation. Kayo Fukui, Norio Masumoto, Erika Yokoyama, and Akiko Kanou were involved in data analysis. All authors critically revised the article, commented on drafts of the manuscript, and approved the final article.
Acknowledgements
The Authors thank Noriyuki Shiroma at the Department of Aki-health Center for preparing and managing the patients’ data.
References
- 1.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, Wolff AC, Wood WC, Davidson NE, Sledge GW, Sparano JA, Badve SS. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, Baehner FL. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016;2(1):56–64. doi: 10.1001/jamaoncol.2015.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, Piccart MJ, Loibl S, Denkert C, Smyth MJ, Joensuu H, Sotiriou C. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, Schem C, Fisch K, Darb-Esfahani S, Mehta K, Sotiriou C, Wienert S, Klare P, André F, Klauschen F, Blohmer JU, Krappmann K, Schmidt M, Tesch H, Kümmel S, Sinn P, Jackisch C, Dietel M, Reimer T, Untch M, Loibl S. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 6.Denkert C, Von minckwitz G, Darb-esfahani S, Lederer B, Heppner B, Weber K, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt W, Blohmer J, Karn T, Pfitzner B, Kümmel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching P, Jackisch C, Van mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. The Lancet Oncology. 2020;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 7.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S, International TILs Working Group 2014 The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denkert C, Wienert S, Poterie A, Loibl S, Budczies J, Badve S, Bago-Horvath Z, Bane A, Bedri S, Brock J, Chmielik E, Christgen M, Colpaert C, Demaria S, Van den Eynden G, Floris G, Fox SB, Gao D, Ingold Heppner B, Kim SR, Kos Z, Kreipe HH, Lakhani SR, Penault-Llorca F, Pruneri G, Radosevic-Robin N, Rimm DL, Schnitt SJ, Sinn BV, Sinn P, Sirtaine N, O’Toole SA, Viale G, Van de Vijver K, de Wind R, von Minckwitz G, Klauschen F, Untch M, Fasching PA, Reimer T, Willard-Gallo K, Michiels S, Loi S, Salgado R. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29(10):1155–1164. doi: 10.1038/modpathol.2016.109. [DOI] [PubMed] [Google Scholar]
- 9.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein SP, Localio AR, Conant EF, Rosen M, Thomas KM, Schnall MD. Multimodality screening of high-risk women: a prospective cohort study. J Clin Oncol. 2009;27(36):6124–6128. doi: 10.1200/JCO.2009.24.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, Pisano ED, Jong RA, Evans WP, Morton MJ, Mahoney MC, Larsen LH, Barr RG, Farria DM, Marques HS, Boparai K, ACRIN 6666 Investigators Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299(18):2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohuchi N, Suzuki A, Sobue T, Kawai M, Yamamoto S, Zheng Y, Shiono Y, Saito H, Kuriyama S, Tohno E, Endo T, Fukao A, Tsuji I, Yamaguchi T, Ohashi Y, Fukuda M, Ishida T. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. The Lancet. 2018;387(10016):341–348. doi: 10.1016/S0140-6736(15)00774-6. [DOI] [PubMed] [Google Scholar]
- 13.Rahbar G, Sie AC, Hansen GC, Prince JS, Melany ML, Reynolds HE, Jackson VP, Sayre JW, Bassett LW. Benign versus malignant solid breast masses: US differentiation. Radiology. 1999;213(3):889–894. doi: 10.1148/radiology.213.3.r99dc20889. [DOI] [PubMed] [Google Scholar]
- 14.Fukui K, Masumoto N, Shiroma N, Kanou A, Sasada S, Emi A, Kadoya T, Yokozaki M, Arihiro K, Okada M. Novel tumor-infiltrating lymphocytes ultrasonography score based on ultrasonic tissue findings predicts tumor-infiltrating lymphocytes in breast cancer. Breast Cancer. 2019;26(5):573–580. doi: 10.1007/s12282-019-00958-3. [DOI] [PubMed] [Google Scholar]
- 15.Sasajima J, Mizukami Y, Sugiyama Y, Nakamura K, Kawamoto T, Koizumi K, Fujii R, Motomura W, Sato K, Suzuki Y, Tanno S, Fujiya M, Sasaki K, Shimizu N, Karasaki H, Kono T, Kawabe J, Ii M, Yoshiara H, Kamiyama N, Ashida T, Bardeesy N, Chung DC, Kohgo Y. Transplanting normal vascular proangiogenic cells to tumor-bearing mice triggers vascular remodeling and reduces hypoxia in tumors. Cancer Res. 2010;70(15):6283–6292. doi: 10.1158/0008-5472.CAN-10-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriyasu F, Itoh K. Efficacy of perflubutane microbubble-enhanced ultrasound in the characterization and detection of focal liver lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol. 2009;193(1):86–95. doi: 10.2214/AJR.08.1618. [DOI] [PubMed] [Google Scholar]
- 17.Kapetas P, Clauser P, Woitek R, Wengert GJ, Lazar M, Pinker K, Helbich TH, Baltzer PAT. Quantitative multiparametric breast ultrasound: application of contrast-enhanced ultrasound and elastography leads to an improved differentiation of benign and malignant lesions. Invest Radiol. 2019;54(5):257–264. doi: 10.1097/RLI.0000000000000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan CF, Du J, Fang H, Li FH, Zhu JS, Liu Q. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology. 2012;262(2):450–459. doi: 10.1148/radiol.11110789. [DOI] [PubMed] [Google Scholar]
- 19.Masumoto N, Kadoya T, Amioka A, Kajitani K, Shigematsu H, Emi A, Matsuura K, Arihiro K, Okada M. Evaluation of malignancy grade of breast cancer using perflubutane-enhanced ultrasonography. Ultrasound Med Biol. 2016;42(5):1049–1057. doi: 10.1016/j.ultrasmedbio.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Amioka A, Masumoto N, Gouda N, Kajitani K, Shigematsu H, Emi A, Kadoya T, Okada M. Ability of contrast-enhanced ultrasonography to determine clinical responses of breast cancer to neoadjuvant chemotherapy. Jpn J Clin Oncol. 2016;46(4):303–309. doi: 10.1093/jjco/hyv215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 22.Japanese Breast Cancer Society . Tokyo, Kanehara Shuppan. 2012. General Rules for Clinical and Pathological Recording of Breast Cancer. 17th Ed. [Google Scholar]