Abstract
Background
External rectal prolapse (ERP) is a debilitating condition in which surgery plays an important role. The aim of this study was to evaluate the outcomes of abdominal approaches (AA) and perineal approaches (PA) to ERP.
Methods
This was a PRISMA-compliant systematic review with meta-analysis. Studies published between 1990 and 2021 were retrieved. The primary endpoint was recurrence at the last available follow-up. Secondary endpoints included factors associated with recurrence and function. All studies were assessed for bias using the Newcastle–Ottawa Scale and Cochrane tool.
Results
Fifteen studies involving 1611 patients (AA = 817; PA = 794) treated for ERP were included, three of which were randomized controlled trials (RCTs; 114 patients (AA = 54; PA = 60)). Duration of follow-up ranged from 12 to 82 months. Recurrence in non-randomized studies was 7.7 per cent in AA versus 20.1 per cent in PA (odds ratio (OR) 0.29, 95 per cent confidence interval (c.i.) 0.17 to 0.50; P < 0.001, I2 = 45 per cent). In RCTs, there was no significant difference (9.8 per cent versus 16.3 per cent, AA versus PA (OR 0.82, 95 per cent c.i. 0.29 to 2.37; P = 0.72, I2 = 0.0 per cent)). Age at surgery and duration of follow-up were risk factors for recurrence. Following AA, the recurrence rates were 10.1 per cent and 6.2 per cent in patients aged 65 years and older and less than 65 years of age, respectively (effect size [e.s.] 7.7, 95 per cent c.i. 4.5 to 11.5). Following PA, rates were 27 per cent and 16.3 per cent (e.s. 20.1, 95 per cent c.i. 13 to 28.2). Extending follow-up to at least 40 months increased the likelihood of recurrence. The median duration of hospital stay was 4.9 days after PA versus 7.2 days after AA. Overall, incontinence was less likely after AA (OR 0.32), but constipation occurred more frequently (OR 1.68). Most studies were retrospective, and several outcomes from RCTs were not consistent with those observed in non-RCTs.
Conclusion
The overall risk of recurrence of ERP appears to be higher with PA versus AA. Incontinence is less frequent after AA but at the cost of increased constipation. Age at surgery and duration of follow-up are associated with increased risk of recurrence, which warrants adequate reporting of future studies on this topic.
Recurrence after external rectal prolapse appears to be higher after perineal versus abdominal repair; however, the findings of randomized controlled trials do not confirm such a finding. Duration of follow-up and age at surgery are prognosticators of recurrence. Some functional issues can persist or appear after surgery. Quality of life has been poorly reported and investigated in this population of patients.
Introduction
External rectal prolapse (ERP) is defined as circumferential, full-thickness prolapse of the rectal wall that protrudes outside the anal canal1,2. ERP is also referred to as rectal procidentia or ‘complete’ prolapse, and is often associated with incontinence or defaecation disorders, resulting in a state of significant discomfort and impaired quality of life.
The incidence of ERP has been reported to be six times higher in women, especially those over the age of 60 years3. Initial management is usually conservative or used as a temporizing measure prior to the consideration of surgery. The latter is the only ‘curative’ approach to reduce the ERP if it is irreducible, and it may improve some of the accompanying symptoms. Several surgical techniques have been proposed, which can be divided into perineal approaches (PA) and abdominal approaches (AA). The most common PA procedures are the Delorme procedure and the Altemeier procedure (perineal recto-sigmoidectomy)4. Rectopexy and resection rectopexy (with or without mesh placement) are the most performed AA procedures. Historically, PA has been offered to older patients, owing to the lower morbidity associated with the procedure. However, recent advances in perioperative management, and laparoscopic and robotic surgery allow AA to be performed safely in selected older, fit patients4.
Although both approaches are feasible2, the recurrence rates are presumed to be higher with PA, reported to be as high as 14 to 27 per cent5. However, the superiority of AA over PA has been questioned by recent randomized controlled trials (RCTs)6,7. PA might be associated with fewer complications, justifying its use in frail patients. In addition, the functional outcome of patients after the restoration of anatomy, and whether this can be influenced by surgical approach is unclear.
The aim of this systematic review was to assess the risk of recurrence in patients treated with PA versus AA for ERP, focusing on factors associated with increased risk. Secondary aims included the impact of surgical approach on postoperative function and health-related quality of life (HRQoL).
Methods
The systematic review with meta-analysis was performed following the PRISMA statement8 and Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist9. The trial was registered in PROSPERO (CRD42020176890).
Search strategy and data sources
A literature search was conducted in MEDLINE (PubMed) and Embase for articles published between 1990 and 2021. Two authors independently performed the literature screening (Gu.S. and G.F.). The following data were independently extracted from the included studies: first author, journal, year of publication, study type, number of patients (AA and PA), mean patient age, and median duration of follow-up. The search terms used were a combination of ‘Delorme’ or ‘Altemeier’ or ‘Rectopexy’ or ‘perineal approach’ or ‘abdominal approach’ AND ‘rectal prolapse’. The full search details are available in Table S1. A cross-reference search was done, searching the reference list of included articles.
Inclusion and exclusion criteria
Studies comparing the outcomes of ERP treated by AA versus PA were evaluated for inclusion. For studies originating from the same centre, only the most recent study with the more complete data was included. Non-comparative studies, studies with calculable endpoints with fewer than 20 patients, studies with a follow-up of less than 12 months, and those published before 1990 were excluded.
Endpoints
The primary endpoint was recurrence at the last available follow-up, defined as the presence of full-thickness prolapse after surgery as diagnosed by rectal examination.
The secondary endpoints were factors associated with the risk of recurrence, including age at surgery and duration of follow-up; duration of hospital stay; postoperative constipation or incontinence; and HRQoL.
Statistical analysis
The meta-analysis was performed according to the Cochrane Collaboration the Quality of Reporting of Meta-analyses (QUORUM)9 guidelines. The estimated effect measures are reported as odds ratios (OR) with 95 per cent confidence intervals (c.i.). The ratio represented the probability of occurrence of an adverse event in the group of patients operated on with AA versus the group of patients operated on with PA. An OR of less than 1 indicated worse outcomes of PA, and the point estimate of OR was considered statistically significant if the 95 per cent c.i. did not include the value ‘1’. For studies that included a zero in a cell for the number of events, the Haldane correction was applied, adding a value of 0.5 in both groups from the respective study10. With regard to follow-up, the cut-off value was chosen using the longest available follow-up allowing for even distribution of the included studies. Odds ratios were combined with the Mantel–Haenszel χ2 method using the random-effect technique11.
Risk-of-bias assessment
RCTs and non-randomized trials were analysed separately. All studies were graded using the Newcastle–Ottawa Scale12. The risk of bias in selected studies was performed using the Cochrane tool risk of bias 2.013 for RCTs and non-RCTs, and the ROBINS-I tool for non-RCTs14.
The publication bias for analysis including at least 10 studies was assessed by means of funnel plot inspection (Fig. S5).
The overall strength of evidence was assessed with the GRADE approach15.
Results
The search yielded 4058 studies, which were analysed by titles and abstracts; 74 duplicates were excluded. All non-comparative studies, reviews, meta-analyses, and case reports were excluded. Twenty-four full-text articles were reviewed, with eight being excluded owing to lack of data needed for the review and one because the duration of follow-up was less than 12 months. Fifteen comparative studies met the inclusion criteria3–7,16–27. These included 12 retrospective studies3,17–20,21–27 and three RCTs6,7,16 (Table 1). The flowchart for study inclusion is reported in Fig. 1. There was 100 per cent agreement among reviewers in the extraction of data, reported in a Microsoft® Excel spreadsheet. In total, 1611 patients who underwent ERP repair between 1990 and 2021 were included in the analysis: 817 patients (50.7 per cent) with AA and 794 patients (49.3 per cent) with PA (Table 1). Duration of follow-up ranged from 12 to 82 months. The types of AA used in the different studies were rectopexy with3,7,16–21,23,27 or without sigmoid resection3,7,17–20,22,24,25,27 (both open and laparoscopic), ventral rectopexy (D’Hoore)6,17,25,26, and rectopexy with mesh (Wells)3,21,23. The PA types used were Delorme3,6,7,17–21,23–25, perineal rectosigmoidectomy (Altemeier)3,7,16,17,20–27, and Thiersh19,25.
Table 1.
Characteristics of included studies
| Author | Year | Study type | No. of patients | Mean age (years) | Mean duration of FU (months) | NOS | |
|---|---|---|---|---|---|---|---|
| AA | PA | ||||||
| Deen et al.16 | 1994 | Randomized | 10 | 10 | 68.5 | 17 | 7 |
| Boccasanta et al.17 | 1999 | Retrospective | 25 | 10 | 60.6 | 35 | 4 |
| Kim et al.18 | 1999 | Retrospective | 176 | 183 | 63.7 | 72 | 5 |
| Aitola et al.19 | 1999 | Retrospective | 104 | 8 | 59 | 62 | 4 |
| Sobrado et al.26 | 2004 | Retrospective | 36 | 15 | 56.7 | 49 | 4 |
| Hammond et al.20 | 2007 | Retrospective | 13 | 62 | 60.8 | 39 | 4 |
| Pescatori and Zbar21 | 2009 | Retrospective | 42 | 75 | 57 | 61 | 5 |
| Riansuwan et al.3 | 2010 | Retrospective | 122 | 55 | 59.9 | 43.8 | 5 |
| Lee et al.22 | 2011 | Retrospective | 8 | 123 | 80.1 | 12.4 | 4 |
| Senapati et al.7 | 2013 | Randomized | 19 | 25 | 63 | 36 | 8 |
| Lee et al.23 | 2014 | Retrospective | 64 | 40 | 57.7 | 24.4 | 5 |
| Mik et al.24 | 2015 | Retrospective | 18 | 68 | 67 | 32 | 6 |
| Emile et al.6 | 2017 | Randomized | 25 | 25 | 39.7 | 18 | 7 |
| Gleditsch et al.25 | 2018 | Retrospective | 73 | 20 | 72 | 82 | 5 |
| Ng et al.27 | 2019 | Retrospective | 82 | 75 | 73.1 | 60 | 5 |
AA, abdominal approach; PA, perineal approach; FU, follow-up; NOS, Newcastle–Ottawa Scale.
Fig. 1.
Flowchart of study selection for the current meta-analysis according to PRISMA statement
Postoperative recurrence
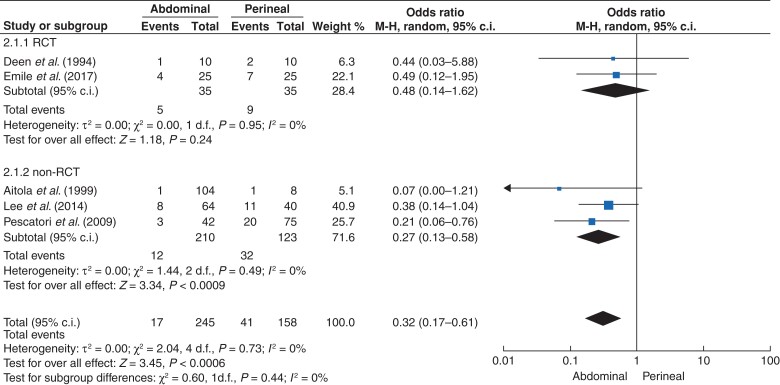
Non-RCT studies reported a lower risk of recurrence with AA than with PA (OR 0.29, 95 per cent c.i. 0.17 to 0.50; P < 0.001, I2 = 45 per cent) (Fig. 2). Overall effect size (ES) in non-RCT trials was 7.7 (95 per cent c.i. 4.5 to 11.5) in AA versus 20.1 (95 per cent c.i. 13 to 28.2) in PA.
Fig. 2.
Recurrence with an abdominal approach (AA) versus perineal approach (PA) to external rectal prolapse
Forest plot with odds ratio of single studies divided into randomized controlled trials (RCTs), non-RCTs, and overall odds ratio. c.i., confidence interval.
In RCTs, there was no difference (P = 0.720) in the risk of recurrence with the AA versus PA (9.8 per cent versus 16.3 per cent (OR 0.82, 95 per cent c.i. 0.29 to 2.37; P = 0.720, I2 = 0 per cent)) (Fig. 2). The heterogeneity was 0.0 per cent. The three studies reported an OR lower than 1, with the exception of that of Senapati et al. (OR 1.43)7. The overall effect size of recurrence across RCTs was 9.8 for AA (95 per cent c.i. 0.1 to 27.5), while for PA it was 16.3 (95 per cent c.i. 7.3 to 27.4).
Funnel plot inspection did not require any further assessment for publication bias.
Factors associated with recurrence
Two groups were established according to patient’s age at surgery: one containing studies in which the average patient age was greater than or equal to 65 years, and another containing studies with patients whose average age was less than 65 years.
In non-RCT, AA had a recurrence rate of 10.1 per cent (95 per cent c.i. 0.7 to 25.9) in studies with a mean patient age of more than 65 years versus 6.2 per cent (95 per cent c.i. 4.2 to 8.4) in studies with a mean patient age of less than 65 years (Fig. S1). The recurrence rate for PA in studies with patients with a mean age of more than 65 years was 27 per cent (95 per cent c.i. 9.2 to 49.7) versus 16.3 (95 per cent c.i. 10.9 to 22.3) in studies with patients with a mean age of less than 65 years (Fig. S2).
In RCTs, the recurrence rates in studies on patients aged more than 65 years were 0 in AA versus 10 per cent in PA, whereas they were 16 per cent in AA and 18 per cent in PA if the mean patient age was less than 65 years.
A cut-off value of 40 months of follow-up allowed for even distribution of the studies into two categories. Studies were divided into two groups, according to duration of follow-up: one with studies with an average duration of follow-up of more than 40 months, and one with studies with an average duration of follow-up of less than 40 months. This analysis was only conducted on non-RCTs because all RCTs had a mean duration of follow-up of less than 40 months. In non-RCTs, recurrence rates increased with increasing duration of follow-up: 8.3 per cent (95 per cent c.i. 4.2 to 13.4) after AA and 24.9 per cent (95 per cent c.i. 12.9 to 39) after PA in studies with more than 40 months of follow-up; and 6.7 per cent (95 per cent c.i. 2.3 to 12.4) after AA and 12.8 per cent (95 per cent c.i. 9 to 17) after PA in those studies with a duration of follow-up of less than 40 months (Figs S3 and S4).
Postoperative function and duration of postoperative stay
Data were available on incontinence and postoperative constipation in five studies6,16,19,21,23. In RCTs, the number of patients who had postoperative faecal incontinence was five for AA and nine for PA (OR 0.48, 95 per cent c.i. 0.14 to 1.62; P = 0.24, I2 = 0) (Fig. 3). Findings were consistent between RCTs and non-RCTs (Fig. 3). Six studies6,16,17,19,21,23 provided results for postoperative constipation. In non-RCTs, the OR for constipation was 2.09 (95 per cent c.i. 1.04 to 4.19; P = 0.04, I2 = 0) in AA versus PA; however, in RCTs, the OR for constipation was 0.81 (95 per cent c.i. 0.23 to 2.88; P = 0.75, I2 = 0) (Fig. 4).
Fig. 3.
Postoperative faecal incontinence with an abdominal approach (AA) versus perineal approach (PA) to external rectal prolapse
Forest plot with odds ratio of studies reporting data on faecal incontinence divided into randomized controlled trials (RCTs), non-RCTs, and overall odds ratio. c.i., confidence interval.
Fig. 4.
Postoperative constipation with an abdominal approach (AA) versus perineal approach (PA) to external rectal prolapse
Forest plot with odds ratio of studies reporting data on constipation divided into randomized controlled trials (RCTs), non-RCTs, and overall odds ratio. c.i., confidence interval.
Eight of 15 studies provided data on duration of postoperative stay3,6,16,18,20,22,23,27. Median duration of postoperative stay was 4.9 days for PA and 7.2 days for AA.
Health-related quality of life
Only three studies3,6,7 reported on HRQoL outcomes following surgery for ERP.
Riansuwan et al.3 assessed HRQoL using the 36-Item Short Form Survey (SF-36) score, reporting lower mean scores in all domains in the PA with a statistically significant difference in both physical and mental components. In contrast, Emile et al.6, using Faecal Incontinence Quality of Life Questionnaire (FIQL) and Gastrointestinal Quality of Life Index (GIQIL) scores, did not find any difference between the two groups.
According to Senapati et al.7, there was no difference between AA and PA using the EuroQol-5 Dimension (EQ-5D) score.
Risk of bias
A summary of the risk of bias is depicted in Fig. 5 and Table 2. The overall bias was high in 65 per cent of the studies and medium among the remaining 35 per cent, due to the higher percentage of selected non-randomized studies, which is reflected in the randomization process.
Fig. 5.
Cochrane risk-of-bias tool outcomes for studies included in the review
Table 2.
ROBIN I tool: risk of bias in non-randomized controlled trials
| Study (year) | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of intervention | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported results | Overall bias |
|---|---|---|---|---|---|---|---|---|
| Ng et al. (2019) | LOW | MODERATE | LOW | LOW | LOW | LOW | MODERATE | LOW |
| Gleditsch et al. (2018) | LOW | MODERATE | LOW | LOW | LOW | LOW | LOW | LOW |
| Mik et al. (2014) | LOW | MODERATE | LOW | LOW | MODERATE | LOW | MODERATE | LOW |
| Pescatori et al. (2009) | LOW | MODERATE | LOW | LOW | LOW | LOW | LOW | LOW |
| Riansuwan et al. (2010) | LOW | MODERATE | LOW | LOW | LOW | LOW | MODERATE | LOW |
| Lee et al. (2011) | LOW | MODERATE | LOW | LOW | LOW | LOW | MODERATE | LOW |
| Lee et al. (2014) | LOW | SERIOUS | LOW | LOW | MODERATE | LOW | MODERATE | MODERATE |
| Boccasanta et al. (1999) | LOW | MODERATE | LOW | LOW | LOW | LOW | LOW | LOW |
| Hammond et al. (2007) | LOW | SERIOUS | LOW | LOW | MODERATE | LOW | MODERATE | MODERATE |
| Kim et al. (1999) | LOW | MODERATE | LOW | LOW | LOW | LOW | LOW | LOW |
| Aitola et al. (1999) | LOW | MODERATE | LOW | LOW | LOW | LOW | MODERATE | LOW |
| Sobrado et al. (2004) | LOW | SERIOUS | LOW | LOW | MODERATE | LOW | MODERATE | MODERATE |
A low risk of bias was observed in the missing outcome data (100 per cent) and in the selection of the reported results (55 per cent). Table 3 summarizes the quality of evidence among the studies.
Table 3.
GRADE score: a consensus on rating quality of evidence and strength of recommendations
| Outcome | Pooled effect estimates | Pooled relative effects (95% c.i.) | Number of patients/studies | Heterogeneity I2 | P for overall effect estimate | Quality of evidence (GRADE) | ||
|---|---|---|---|---|---|---|---|---|
| Comparison 1: abdominal approach versus perineal approach (non-RCTs) | ||||||||
| AA | PA | |||||||
| Recurrence | 0.08 | 0.2 | OR 0.29 (0.17–0.50) | 1497/12 | 0.45 | < 0.001 | +++ | |
| Age at surgery (years) | Recurrence rate (%) (95% c.i.) | No. of studies | ||||||
| > 65 | 10.1 (0.7–25.9) | 27 (9.2–49.7) | 4 | + | ||||
| < 65 | 6.2 (4.2–8.4) | 16.3 (10.9–22.3) | 8 | ++ | ||||
| Follow-up duration (months) | ||||||||
| > 40 | 8.3 (4.2–13.4) | 24.9 (12.9–39) | 7 | ++ | ||||
| ≤ 40 | 6.7 (2.3–12.4) | 12.8 (9–17) | 5 | + | ||||
| Postoperative constipation | 0.26 | 0.1 | OR 2.09 (1.04–4.19) | 368/4 | 0 | 0.04 | +++ | |
| Postoperative incontinence | 0.06 | 0.26 | OR 0.27 (0.13–0.58) | 333/3 | 0 | < 0.001 | +++ | |
| Comparison 2: AA versus PA (RCTs) | ||||||||
| AA | PA | |||||||
| Recurrence | 0.13 | 0.17 | OR 0.82 (0.29–2.37) | 114/3 | 0 | 0.72 | ++++ | |
| Age at surgery (years) | Recurrence rate (%) | No. of studies | ||||||
| > 65 | 0 | 10 | 4 | ++ | ||||
| < 65 | 16 | 18 | 8 | +++ | ||||
| Follow-up duration (months) | ||||||||
| > 40 | NA | NA | ||||||
| ≤ 40 | NA | NA | ||||||
| Postoperative constipation | 0.17 | 0.2 | OR 0.81 (0.23–2.88) | 013/2 | 0 | 0.75 | ++++ | |
| Postoperative incontinence | 0.14 | 0.26 | OR 0.48 [0.14–1.62] | 014/2 | 0 | 0.24 | ++++ | |
GRADE score: the quality of evidence for most outcomes varied from very low to high quality. All the outcomes that do not include randomized controlled trials (RCTs) were downgraded. c.i., 95% confidence intervals; AA, abdominal approach; PA, perineal approach; OR, odds ratio; NA, not available.
Discussion
This meta-analysis found that long-term recurrence is higher after PA than after AA for ERP. PA required a shorter postoperative stay. However, this difference was not statistically significant in RCTs. A patient age of less than 65 years at the time of surgery predicted a higher risk of recurrence. The number of recurrences was linked to the duration of FU, being higher for PA in studies with more that 40 months of follow-up, whereas it was higher in studies with shorter follow-up after AA. HRQoL has been poorly investigated, and with different tools. An improvement in overall HRQoL can be expected with both approaches, which is more pronounced in the short term; however, some functional issues might persist or occur after surgery, including a higher likelihood of incontinence after PA versus higher rates of constipation after AA.
Although several studies evaluated the long-term results of surgery for ERP, data from meta-analyses comparing the PA with the AA are lacking. This review found that PA tripled the long-term risk of recurrence versus AA in studies with long follow-up periods. The PROSPER trial7 aimed to demonstrate the best surgical approach for ERP. The authors observed an improvement in Hologram baseline, but no differences were found among the randomized comparisons. Following the PROSPER study, there has been increased use of minimally invasive AA, with a corresponding reduction in PA28.
A study of 50 patients with ERP were randomized into either ventral rectopexy or Delorme procedure, with recurrence rates of 8 per cent and 16 per cent, respectively6. The results of the current meta-analysis are in keeping with the literature, with a recurrence rate of 9.8 per cent for the AA and 16.3 per cent for the PA. These data were consistently found in all included studies, apart from three7,22,26. In the study by Lee et al.22, the small sample size of AA (eight patients) versus PA (123 patients) might explain this finding, in contrast to the low number of PAs included in the study by Sobrado et al.26, which demonstrated a higher risk of recurrence after AA. Interestingly, the current meta-analysis found that recurrence rates for AA were higher in RCTs versus non-RCTs (9.8 per cent versus 7.7 per cent), whereas for PA they were lower in RCTs versus non-RCTs (16.3 per cent versus 20.1 per cent), which resulted in the overall difference not being statistically significant in the meta-analysis of RCTs. Even if this difference might still be clinically relevant, it warrants further prospective, long-term evaluation.
The current meta-analysis demonstrated that age is an important factor in predicting recurrence, with both approaches having higher recurrence rates in patients older than 65 years. Lieberth et al.29 reported an overall recurrence rate of 14 per cent in 70 patients operated on using the Delorme technique, but this decreased to 8 per cent when only patients under 50 years of age were considered. Fu et al.30 reported a recurrence rate of 22.1 per cent from a population of 113 patients who underwent laparoscopic ventral rectopexy, reporting age over 70 years as a predictive factor (hazard ratio 2.22). In contrast, a more recent consensus statement published by the Italian Society of Colorectal Surgery2 suggested that age should not be considered as a determining factor in the choice of treatment, as even in older patients the two procedures would be similar.
Patients having a PA with a mean duration of follow-up of more than 40 months had a higher recurrence rate. Conversely patients having an AA with a mean duration of follow-up of less than 40 months had higher recurrence rates. This might suggest that recurrence in the AA may occur earlier than in PA.
Few studies have examined the impact of these procedures on HRQoL and clinical symptoms, but both approaches are effective in achieving improvement in symptoms associated with ERP. A proportion of patients who undergo surgical repair continue to present with symptoms such as obstructed defaecation, constipation, and incontinence. Some studies have shown that the persistence of obstructive defecation, its exacerbation, or even its de novo occurrence is more common with AA than with PA31. Constipation may also occur after abdominal rectopexy, with a reported incidence ranging from 27 to 47 per cent. The reason for this phenomenon is unknown, although it has been hypothesized that it could be due to scar around the rectum and resultant stiffness32.
PA may be associated with an increased frequency of bowel movements, urinary urgency, and faecal incontinence, with the incidence reaching 40 per cent33. This could be explained by the reduced capacity and compliance of the rectal wall. Constipation during perineal surgery is reported in about 10 per cent of cases34. The current study showed that the risk of incontinence was three times higher in PA than in AA; in contrast, the risk of constipation might be higher in AA procedures. However, this was not demonstrated when analysing RCTs separately. The risk of constipation was still higher with PA than with AA in this subgroup of studies (Fig. 4), which merits further investigation.
Given the reported data, the choice of procedure to perform in each patient should be individualized. The relatively high number of recurrences after PA is potentially balanced by the less invasive technique and by the option of redo procedures35.
It is important to consider the complication profile of different approaches, for example damage of hypogastric nerves with an AA when mobilizing the rectum. This can cause subsequent bowel, bladder, and sexual dysfunction. Unfortunately, no consistent objective data that can be compared are available on this topic, which might be relevant to guide the choice of the type of approach.
Duration of stay was addressed in the current meta-analysis because this might be of interest to patients and hospital management. Using techniques such as PA or minimally invasive AA to reduce hospital stay to a minimum can be useful when limited resources and staff are available36,37.
This review has limitations, mainly consisting of the limited number of RCTs available and the low quality of the studies. These considerations warrant careful evaluation and the results need to be interpreted with caution. The heterogeneity of available data limited the possibility of sensitivity and subgroup analyses. There is increased adoption of minimally invasive surgery, which might have an influence on the outcomes.
However, this study has identified areas for further research, including HRQoL and functional outcomes. Several factors relevant when counselling patients on the different treatment approaches to ERP were identified, and decisions should be made within the context of the multidisciplinary team. Follow-up for functional diseases should be long enough to ensure that all recurrences are detected, ideally assessing both anatomical and functional outcomes38. Future studies on ERP are needed, but these do not necessarily need a randomized design. Properly executed non-RCTs might provide useful real-world data and could be more cost-effective. These studies are needed to clarify how surgery impacts on function and HRQoL, using validated tools and incorporating patient-reported outcome measures and patient priorities.
Supplementary Material
Contributor Information
Gianluca Pellino, Colorectal Surgery, Department of Advanced Medical and Surgical Sciences, Università degli Studi della Campania ‘Luigi Vanvitelli’, Naples, Italy; Colorectal Surgery, Vall d’Hebron University Hospital, Barcelona, Spain.
Giacomo Fuschillo, Colorectal Surgery, Department of Advanced Medical and Surgical Sciences, Università degli Studi della Campania ‘Luigi Vanvitelli’, Naples, Italy.
Costantinos Simillis, Cambridge Colorectal Unit, Addenbrookes Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Lucio Selvaggi, Colorectal Surgery, Department of Advanced Medical and Surgical Sciences, Università degli Studi della Campania ‘Luigi Vanvitelli’, Naples, Italy.
Giuseppe Signoriello, Section of Statistic, Department of Experimental Medicine, Università degli Studi della Campania ‘Luigi Vanvitelli’, Naples, Italy.
Danilo Vinci, Colorectal Surgery, Department of Advanced Medical and Surgical Sciences, Università degli Studi della Campania ‘Luigi Vanvitelli’, Naples, Italy.
Christos Kontovounisios, Department of Colorectal Surgery, Royal Marsden Hospital, London, UK; Department of Colorectal Surgery, Chelsea and Westminster Hospital, London, UK; Department of Surgery and Cancer, Imperial College London, London, UK.
Francesco Selvaggi, Colorectal Surgery, Department of Advanced Medical and Surgical Sciences, Università degli Studi della Campania ‘Luigi Vanvitelli’, Naples, Italy.
Guido Sciaudone, Colorectal Surgery, Department of Advanced Medical and Surgical Sciences, Università degli Studi della Campania ‘Luigi Vanvitelli’, Naples, Italy.
Funding
None declared.
Disclosure. The authors declare no conflict of interest
Supplementary material
Supplementary material is available at BJS Open online
Data availability
Additional data can be found as supplementary material and are available from the corresponding author on reasonable request.
References
- 1. Lowry AC, Simmang CL, Boulos P, Farmer CK, Finan PJ, Hyman Net al. Consensus statement of definitions for anorectal physiology and rectal cancer, Washington DC May 1, 1999. Dis Colon Rectum 2001;44:915–919 [DOI] [PubMed] [Google Scholar]
- 2. Gallo G, Martellucci J, Pellino G, Ghiselli R, Infantino A, Pucciani Fet al. Consensus Statement of the Italian Society of Colorectal Surgery (SICCR): management and treatment of complete rectal prolapse. Tech Coloproctol 2018;22:919–931 [DOI] [PubMed] [Google Scholar]
- 3. Riansuwan W, Hull TL, Bast J, Hammel JP, Church JM. Comparison of perineal operations with abdominal operations for full-thickness rectal prolapse. World J Surg 2010;34:1116–1122 [DOI] [PubMed] [Google Scholar]
- 4. Mustain WC, Davenport DL, Parcells JP, Vargas HD, Houriganm JS. Abdominal versus perineal approach for treatment of rectal prolapse: comparable safety in a propensity-matched cohort. Am Surg 2013;79:686–692 [PubMed] [Google Scholar]
- 5. Bordeianou L, Paquette I, Johnson E, Holubar SD, Gaertner W, Feingold DLet al. Clinical practice guidelines for the treatment of rectal prolapse. Dis Colon Rectum 2017;60:1121–1131 [DOI] [PubMed] [Google Scholar]
- 6. Emile SH, Elbanna H, Youssef M, Thabet W, Omar W, Elshobaky Aet al. Laparoscopic ventral mesh rectopexy vs Delorme's operation in management of complete rectal prolapse: a prospective randomized study. Colorectal Dis 2017;19:50–57 [DOI] [PubMed] [Google Scholar]
- 7. Senapati A, Gray RG, Middleton LJ, Harding J, Hills RK, Armitage NCet al. PROSPER: a randomised comparison of surgical treatments for rectal prolapse. Colorectal Dis 2013;15:858–868 [DOI] [PubMed] [Google Scholar]
- 8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CDet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol 2021;134:178–189 [DOI] [PubMed] [Google Scholar]
- 9. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie Det al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 10. Haldane JB. The estimation and significance of the logarithm of a ratio of frequencies. Ann Hum Genet 1956;20:309–311 [DOI] [PubMed] [Google Scholar]
- 11. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–748 [PubMed] [Google Scholar]
- 12. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos Met al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 21 February 2021)
- 13. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron Iet al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 14. Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan Met al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello Pet al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deen KI, Grant E, Billingham C, Keighley MRB. Abdominal resection rectopexy with pelvic floor repair versus perineal rectosigmoidectomy and pelvic floor repair for full-thickness rectal prolapse. Br J Surg 1994;81:302–304 [DOI] [PubMed] [Google Scholar]
- 17. Boccasanta P, Rosati R, Venturi M, Cioffi UGO, Simone MD, Montorsi M, et al. Surgical treatment of complete rectal prolapse: results of abdominal and perineal approaches. J Laparoendosc Adv Surg Tech 1999;9:235–238 [DOI] [PubMed] [Google Scholar]
- 18. Kim DS, Tsang CB, Wong WD, Lowry AC, Goldberg SM, Madoff RD. Complete rectal prolapse: evolution of management and results. Dis Colon Rectum 1999;42:460–466 [DOI] [PubMed] [Google Scholar]
- 19. Aitola PT, Hiltunen KM, Matikainen MJ. Functional results of operative treatment of rectal prolapse over an 11-year period: emphasis on transabdominal approach. Dis Colon Rectum 1999;42:655–660 [DOI] [PubMed] [Google Scholar]
- 20. Hammond K, Beck DE, Margolin DA, Whitlow CB, Timmcke AE, Hicks TC. Rectal prolapse: a 10-year experience. Ochsner J 2007;7:24–32 [PMC free article] [PubMed] [Google Scholar]
- 21. Pescatori M, Zbar AP. Tailored surgery for internal and external rectal prolapse: functional results of 268 patients operated upon by a single surgeon over a 21-year period. Colorectal Dis 2009;11:410–419 [DOI] [PubMed] [Google Scholar]
- 22. Lee SH, Lakhtaria P, Canedo J, Lee Y-S, Wexner SD. Outcome of laparoscopic rectopexy versus perineal rectosigmoidectomy for full-thickness rectal prolapse in elderly patients. Surg Endosc 2011;25:2699–2702 [DOI] [PubMed] [Google Scholar]
- 23. Lee JL, Yang SS, Park IJ, Yu CS, Kim JC. Comparison of abdominal and perineal procedures for complete rectal prolapse: an analysis of 104 patients. Ann Surg Treat Res 2014;86:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mik M, Trzcinski R, Kujawski R, Dziki L, Tchorzewski M, Dziki A. Rectal prolapse in women-outcomes of perineal and abdominal approaches. Indian J Surg 2015;77:1121–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gleditsch D, Wexels WA, Nesbakken A. Surgical options and trends in treating rectal prolapse: long-term results in a 19-year follow-up study. Langenbecks Arch Surg 2018;403:991–998 [DOI] [PubMed] [Google Scholar]
- 26. Sobrado CW, Kiss DR, Nahas SC, Araújo SE, Seid VE, Cotti Get al. Surgical treatment of rectal prolapse: experience and late results with 51 patients. Rev Hosp Clin Fac Med Sao Paulo 2004;59:168–171 [DOI] [PubMed] [Google Scholar]
- 27. Ng ZQ, Levitt M, Tan P, Makin G, Platell C. Long-term outcomes of surgical management of rectal prolapse. ANZ J Surg 2019;89:E231–E235 [DOI] [PubMed] [Google Scholar]
- 28. Gunner CK, Senapati A, Northover JM, Brown SR. Life after PROSPER. What do people do for external rectal prolapse? Colorectal Dis 2016;18:811–814 [DOI] [PubMed] [Google Scholar]
- 29. Lieberth M, Kondylis LA, Reilly JC, Kondylis PD. The Delorme repair for full-thickness rectal prolapse: a retrospective review. Am J Surg 2009;197:418–423 [DOI] [PubMed] [Google Scholar]
- 30. Fu CW, Stevenson AR. Risk factors for recurrence after laparoscopic ventral rectopexy. Dis Colon Rectum 2017;60:178–186 [DOI] [PubMed] [Google Scholar]
- 31. Marzouk D, Ramdass MJ, Haji A, Akhtar M. Digital assessment of lower rectum fixity in rectal prolapse (DALR): a simple clinical anatomical test to determine the most suitable approach (abdominal versus perineal) for repair. Surg Radiol Anat 2005;27:414–419 [DOI] [PubMed] [Google Scholar]
- 32. Senapati A, Nicholls RJ, Thomson JP, Phillips RK. Results of Delorme’s procedure for rectal prolapse. Dis Colon Rectum 1994;37:456–460 [DOI] [PubMed] [Google Scholar]
- 33. Madiba TE, Baig MK, Wexner SD. Surgical treatment of rectal prolapse. Arch Surg 2005;140:63–73 [DOI] [PubMed] [Google Scholar]
- 34. Xynos E. Functional results after surgery for overt rectal prolapse. Acta Chir Iugosl 2012;59:21–24 [DOI] [PubMed] [Google Scholar]
- 35. Trompetto M, Tutino R, Realis Luc A, Novelli E, Gallo G, Clerico G. Altemeier's procedure for complete rectal prolapse: outcome and function in 43 consecutive female patients. BMC Surg 2019;19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. COVIDSurg Collaborative . Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg 2020;107:1440–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spinelli A, Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg 2020;107:785–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gallo G, Trompetto M, Realis Luc A, Novelli E, De Paola G, Clerico Get al. Anatomo-functional outcomes of the laparoscopic Frykman-Goldberg procedure for rectal prolapse in a tertiary referral centre. Updates Surg 2021;73:1819–1828 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data can be found as supplementary material and are available from the corresponding author on reasonable request.