Abstract
Background
Navafenterol (AZD8871) belongs to a new class of bronchodilator, the single-molecule muscarinic antagonist and β-agonist, developed for the treatment of COPD. This study aimed to evaluate the efficacy, pharmacokinetics and safety of navafenterol versus placebo and an active comparator treatment for moderate-to-severe COPD.
Methods
This phase 2a, randomised, multicentre (Germany and UK), double-blind, double-dummy, three-way complete crossover study (ClinicalTrials.gov identifier: NCT03645434) compared 2 weeks’ treatment of once-daily navafenterol 600 µg via inhalation with placebo and a fixed-dose combination bronchodilator (umeclidinium/vilanterol (UMEC/VI); 62.5 µg/25 µg) in participants with moderate-to-severe COPD. The primary outcome was change from baseline in trough forced expiratory volume in 1 s (FEV1) on day 15. Secondary end-points included change from baseline in peak FEV1; change from baseline in Breathlessness, Cough and Sputum Scale (BCSS); change from baseline in COPD Assessment Tool (CAT); adverse events; and pharmacokinetics.
Results
73 participants were randomised. After 14 days, trough FEV1 was significantly improved with navafenterol compared with placebo (least-squares (LS) mean difference 0.202 L; p<0.0001). There was no significant difference in FEV1 between navafenterol and UMEC/VI (LS mean difference −0.046 L; p=0.075). COPD symptoms (CAT and BCSS) showed significantly greater improvements with both active treatments versus placebo (all p<0.005). Novel objective monitoring (VitaloJAK) showed that cough was reduced with both active treatments compared with placebo. Safety profiles were similar across the treatment groups and no serious adverse events were reported in the navafenterol treatment period.
Conclusion
Once-daily navafenterol was well tolerated, improved lung function and reduced COPD-related symptoms, similar to an established once-daily fixed-dose combination bronchodilator.
Short abstract
Navafenterol, a novel dual-pharmacology bronchodilator for COPD, improved lung function, reduced COPD symptoms and decreased objective cough counts, to a similar extent to umeclidinium/vilanterol https://bit.ly/3lV886y
Introduction
COPD is a common condition that is a significant cause of morbidity and mortality worldwide [1, 2]. The major clinical symptoms of COPD are chronic and progressive dyspnoea, cough and sputum production [1, 3]. Cough and sputum production are often reported as troubling symptoms, with increased incidence in the morning, which negatively affect health-related quality of life [4, 5].
Regular pharmacological treatment with inhaled long-acting bronchodilators can alleviate and reduce COPD symptoms [1]. Long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs) are mainstays of treatment for COPD, and are preferred over short-acting treatments [1]. LAMA/LABA combination therapy, such as umeclidinium/vilanterol (UMEC/VI), has greater efficacy than monotherapy for improving lung function, symptoms and health-related quality of life in patients with COPD [6, 7]. LAMAs and LABAs induce smooth muscle relaxation and bronchodilation via different mechanisms of action, which leads to additive effects in clinical practice [8].
Dual-pharmacology bronchodilators, a novel class of compounds that combine muscarinic antagonist and β2-adrenoceptor agonist functions in a single molecule, termed muscarinic antagonists and β-agonists (MABAs), may offer advantages over combination therapy that uses two separate drug entities [9]. Because MABAs are single molecules, both pharmacologies are delivered as a fixed ratio, have a single pharmacokinetic profile and have a simplified clinical development programme relative to LAMA/LABA combination therapies [9, 10].
In vitro studies have demonstrated that navafenterol (AZD8871), an inhaled long-acting MABA, has potent M3 antimuscarinic and β2-adrenoceptor agonist activities [11]. In a phase 1, randomised, double-blind crossover study in patients with COPD which compared single doses of navafenterol 400 µg or 1800 µg with placebo, indacaterol and tiotropium, navafenterol delivered sustained bronchodilation over 36 h; both doses of navafenterol were superior to placebo and the higher dose was superior to both indacaterol and tiotropium, with no emerging safety concerns [12]. A phase 2a, randomised, double-blind crossover study of navafenterol 100 μg, navafenterol 600 μg and placebo once daily for 14 days in patients with COPD demonstrated dose-dependent clinically meaningful improvements in bronchodilation over 24 h at day 15, compared with placebo [13]. It is anticipated that navafenterol, as a single-molecule MABA, can provide a novel approach to the treatment of patients with COPD, with greater efficacy than long-acting bronchodilator monotherapy; at least equivalent efficacy to LAMA/LABA dual therapy with a similar safety profile; and potentially provide a platform for future combination with inhaled anti-inflammatory agents.
There are limited data on the effect of COPD treatments on the reduction of cough and little is known about what affects cough frequency in patients with COPD. It is hoped that objective cough monitoring will provide important information on the impact of treatment of cough in COPD [14].
The present study (ClinicalTrials.gov identifier: NCT03645434) was the first to compare navafenterol with a LAMA/LABA combination treatment (UMEC/VI) used in clinical practice. The primary aim of the study was to compare the effects of navafenterol versus UMEC/VI on lung function. Secondary end-points included COPD symptoms and safety assessment, while an objective reduction in cough count was an exploratory end-point.
Methods
Study design
This phase 2a, randomised, double-blind, double-dummy, three-way complete crossover study compared navafenterol 600 µg with placebo and an active comparator LAMA/LABA bronchodilator (UMEC/VI) 62.5 µg/25 µg, administered once daily by dry powder inhaler devices (Genuair/Pressair device (SD3FL) for navafenterol and the Ellipta device for UMEC/VI) in participants with moderate-to-severe COPD. The study was conducted between 10 October 2018 and 7 August 2019 at three sites in Germany and two sites in the UK.
Over the three 14-day treatment periods, participants received all three treatments in differing sequences, with a 42–49-day washout period (figure 1a). Patients were maintained on daily ipratropium (20 µg×2 puffs four times per day) during washout periods. Participants were randomised using interactive web and interactive voice-response systems. Full methodological details of the study are provided in the supplementary methods.
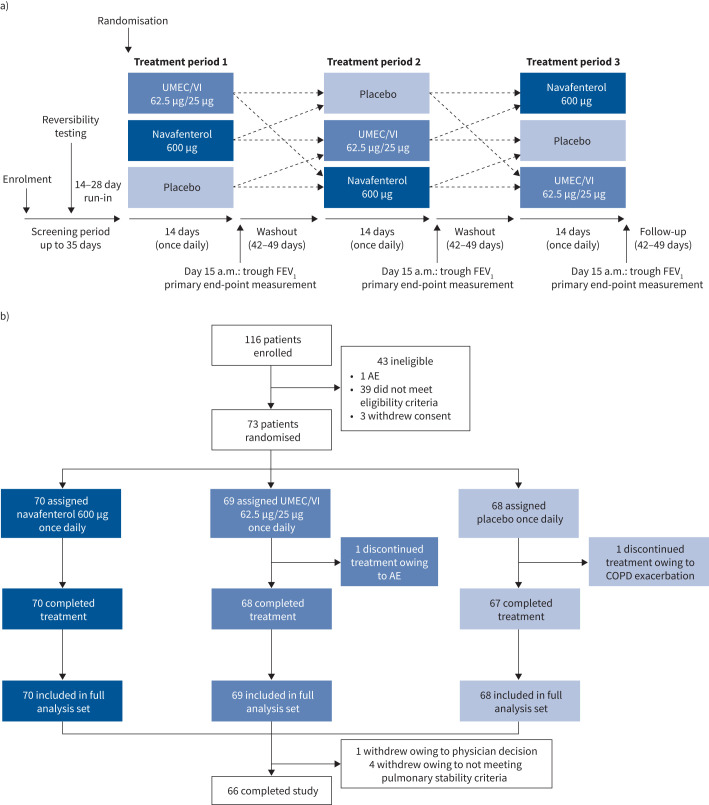
FIGURE 1.
a) Study design; b) patient disposition. UMEC/VI: umeclidinium/vilanterol; FEV1: forced expiratory volume in 1 s; AE: adverse event.
This study was performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with International Council for Harmonisation/Good Clinical Practice applicable regulatory requirements, as well as the AstraZeneca policy on bioethics. The study protocol was approved by independent ethics committees according to local requirements. All patients provided written informed consent. This manuscript has been written in accordance with Consolidated Standards of Reporting Trials guidelines [15].
Patients
Men and women aged 40–85 years with moderate-to-severe COPD were included. Patients were current or former smokers, with a post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity ratio of <70% after inhalation of salbutamol 400 µg and a post-bronchodilator FEV1 ≥40% and ≤80% of the predicted normal value at the second visit.
Patients were excluded if they had significant comorbidities (e.g. significant cardiovascular disease such as myocardial infarction within the 6 months before the screening visit, severe hepatic impairment); α1-antitrypsin deficiency; other active pulmonary disease (predominant asthma, active tuberculosis, lung cancer, bronchiectasis, sarcoidosis, idiopathic interstitial pulmonary fibrosis, primary pulmonary hypertension or uncontrolled sleep apnoea); two or more moderate-to-severe COPD exacerbations in the year before screening; acute worsening of COPD requiring antibiotic or corticosteroid treatment in the 3 months before screening; or had been hospitalised owing to poorly controlled COPD in the 3 months before screening.
Eligible patients were switched from their regular maintenance COPD medication to ipratropium (20 µg×2 puffs four times per day) at enrolment. LABA, LAMA, LABA/LAMA and inhaled corticosteroid/LABA therapies were withdrawn at the start of the study. Patients receiving an inhaled corticosteroid component were allowed to continue taking it as a monotherapy at a stable dose throughout.
A reversibility test was conducted upon washout of prior COPD medication where a reversible status was defined as increased post-bronchodilator FEV1 of ≥12% (percentage reversibility) and ≥200 mL (absolute reversibility) compared with the pre-bronchodilator test. Reversibility status was not part of the eligibility criteria.
Outcomes
The primary objective was to assess the efficacy of navafenterol 600 µg in patients with moderate-to-severe COPD. The primary end-point was the change from baseline in trough FEV1 at day 15.
Secondary end-points included FEV1 area under the curve (AUC); change from baseline in trough FEV1 measured on day 2 and day 8; change from baseline in peak FEV1; change from baseline in total score of the Breathlessness, Cough and Sputum Scale (BCSS) questionnaire [16, 17]; change from baseline in the COPD Assessment Tool (CAT) score [18]; use of rescue medication (salbutamol 100 μg); treatment-emergent adverse events; tolerability; and pharmacokinetics of navafenterol and its primary metabolite, LAS191861 (supplementary figure S1).
Objective cough counts were captured as an exploratory outcome using the VitaloJAK cough monitor on day 1 and day 14 (Vitalograph, Buckingham, UK) [19]. The cough monitor records a patient's cough frequency over a 24-h period via wearable microphones. A condensed recording is produced and analysed to assess the number of coughs per hour [20, 21]. Perceived cough severity was assessed using a visual analogue scale [20].
Statistical analysis
All participants were included in the full analysis set, which was used for the analysis of efficacy variables. The full analysis set was defined as all participants randomised and receiving study treatment, irrespective of their protocol adherence and continued participation in the study. Change from baseline in trough FEV1 and change from baseline in peak FEV1 were analysed using a mixed model with fixed effects for treatment, sequence and period. The participant was fitted as a random effect and the pre-dose FEV1 of each period was included as a covariate. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for the data analysis. The study was powered to demonstrate superiority of navafenterol compared with UMEC/VI for the primary efficacy end-point (supplementary methods). All randomised patients who received at least one dose of study treatment were included in the safety population. Patients who specifically consented were included in the pharmacokinetic analysis subset. Details of the pharmacokinetic analysis are included in the supplementary methods.
Results
Patients
In total, 116 patients were enrolled, 73 of whom were randomised into the study. All randomised patients received at least one dose of study treatment, 71 patients completed at least one study period and 66 patients completed all three treatment periods (figure 1b). Two patients discontinued treatment during a study period: one owing to a serious adverse event of acute coronary syndrome while receiving UMEC/VI and one owing to moderate COPD exacerbation while receiving placebo. The mean duration of participation was 186 days. The pharmacokinetic analysis subset included 41 participants.
Patient demographics and baseline data are summarised in table 1. The mean age of participants was 66.0 years and the majority were male (68.5%). The mean post-bronchodilator value for predicted FEV1 at screening was 58.7%; 46 (63.0%) patients had a reversible status at screening. Most patients (76.7%) had not had a COPD exacerbation in the past year and 28 (38.4%) patients were maintained on an inhaled corticosteroid. The mean±sd CAT score at baseline was 15.4±6.1.
TABLE 1.
Patient demographics and baseline characteristics
| Patients | 73 |
| Age, years | 66.0±7.6 |
| Sex | |
| Female | 23 (31.5) |
| Male | 50 (68.5) |
| Race | |
| White | 73 (100.0) |
| Smoking status | |
| Ex-smoker | 45 (61.6) |
| Current smoker | 28 (38.4) |
| Time since COPD diagnosis, years | 12.1±7.4 |
| Number of exacerbations in previous 12 months, mean±sd (min, max) | 0.2±0.4 (0, 1) |
| Number of patients with 0 exacerbations in the previous 12 months | 56 (76.7) |
| Number of patients with 1 exacerbation in the previous 12 months | 17 (23.3) |
| Time since last exacerbation to randomisation, months | 22.6±23.8 |
| Inhaled corticosteroid use# | 28 (38.4) |
| CAT score at baseline | 15.4±6.1 |
| Eosinophil count, 109 cells·L −1 | 0.3±0.2 |
| Lung function at screening (post-bronchodilator) | |
| FEV1, % predicted | 58.7±10.4 |
| FEV1/FVC | 50.1±9.1 |
| Severity of airflow limitation | |
| Moderate (≥50%, <80%) | 56 (76.7) |
| Severe (≥30%, <50%) | 17 (23.3) |
| FEV1 reversibility, % predicted | 20.8±12.8 |
| Reversibility status | |
| Reversible | 46 (63.0) |
| Nonreversible | 27 (37.0) |
| Relevant baseline medical history | |
| Cardiac disorders | 8 (11) |
| Myocardial infarction | 2 (2.7) |
| Myocardial ischaemia | 2 (2.7) |
| Atrioventricular block second degree | 1 (1.4) |
| Cardiac aneurysm | 1 (1.4) |
| Coronary artery disease | 1 (1.4) |
| Extrasystoles | 1 (1.4) |
| Left ventricular dysfunction | 1 (1.4) |
| Palpitations | 1 (1.4) |
| Ventricular hypokinesia | 1 (1.4) |
| Asthma | 4 (5.5) |
| Immune system disorders | 13 (17.8) |
| Seasonal allergy | 5 (6.8) |
| Drug hypersensitivity | 4 (5.5) |
| Allergy to metals | 2 (2.7) |
| Allergy to animal | 1 (1.4) |
| Allergy to arthropod bite | 1 (1.4) |
| Iodine allergy | 1 (1.4) |
Data are presented as n, mean±sd or n (%), unless otherwise stated. CAT: COPD Assessment Tool; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. #: these participants were maintained on stable inhaled corticosteroid treatment throughout the study.
Lung function
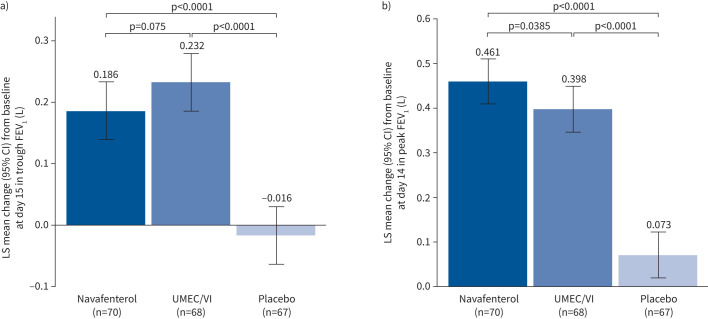
At day 15, trough FEV1 was significantly improved by treatment with either navafenterol or UMEC/VI compared with placebo (navafenterol least-squares (LS) mean difference 0.202 L, 95% CI 0.151–0.253 L; p<0.0001; UMEC/VI LS mean difference 0.248 L, 95% CI 0.197–0.300 L; p<0.0001) (figure 2a). The effect of UMEC/VI was numerically greater compared with navafenterol, but the difference was not statistically significant (LS mean difference −0.046 L, 95% CI −0.097–0.005 L; p=0.075).
FIGURE 2.
a) Least squares (LS) mean change from baseline in trough forced expiratory volume in 1 s (FEV1) at day 15. b) LS mean change from baseline in peak FEV1 at day 14. UMEC/VI: umeclidinium/vilanterol.
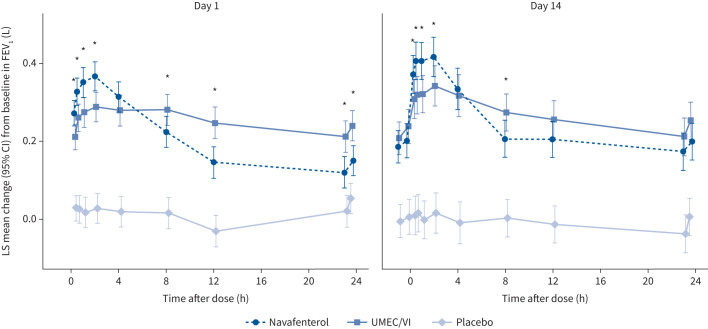
At day 14, there was a significant difference in change from baseline in peak FEV1 for navafenterol and for UMEC/VI compared with placebo (navafenterol LS mean difference 0.388 L, 95% CI 0.329–0.447 L; p<0.0001; UMEC/VI LS mean difference 0.326 L, 95% CI 0.226–0.385 L; p<0.0001; figure 2b). Navafenterol showed a fast onset of action, with the effect of navafenterol on change from baseline in peak FEV1 significantly greater than with UMEC/VI (LS mean difference 0.062 L, 95% CI 0.003–0.121 L; p=0.0385). Additionally, navafenterol showed a greater effect on peak FEV1 than UMEC/VI on day 1 (p<0.05). On day 2, trough FEV1 was higher for UMEC/VI than for navafenterol (p<0.05); however, this difference reduced during the treatment period (figure 3).
FIGURE 3.
Forced expiratory volume in 1 s (FEV1) over 24 h on day 1 and day 14. LS: least-squares; UMEC/VI: umeclidinium/vilanterol. *: p<0.05, significant difference between navafenterol and UMEC/VI.
Navafenterol demonstrated significantly greater improvements in FEV1 AUC than placebo on day 14 at all time points measured (0–4, 0–8, 0–12 and 0–24 h post-dose). Navafenterol showed significantly greater improvements in FEV1 AUC than UMEC/VI from 0 to 4 h post-dose at day 14 (LS mean difference 0.062 L, 95% CI 0.006–0.117 L; p=0.031; table 2 and supplementary table S1).
TABLE 2.
Area under the curve (AUC) forced expiratory volume in 1 s (FEV1) at day 14
| Navafenterol versus placebo | Navafenterol versus UMEC/VI | |||
| FEV1 AUC LS mean difference, L (95% CI) | p-value | FEV1 AUC LS mean difference, L (95% CI) | p-value | |
| 0–4 h | 0.376 (0.320–0.432) | <0.0001 | 0.062 (0.006–0.117) | 0.0308 |
| 0–8 h | 0.328 (0.271–0.385) | <0.0001 | 0.020 (−0.037–0.077) | 0.4948 |
| 0–12 h | 0.296 (0.242–0.349) | <0.0001 | 0.001 (−0.053–0.054) | 0.9827 |
| 0–24 h | 0.244 (0.194–0.294) | <0.0001 | −0.030 (−0.079–0.020) | 0.2426 |
UMEC/VI: umeclidinium/vilanterol; LS: least-squares.
Post hoc analysis of subgroups defined by reversibility status suggested that, for both navafenterol and UMEC/VI, the increase in peak FEV1 from baseline was numerically smaller in patients with a nonreversible status at screening compared with patients who had a reversible status (supplementary figure S2a). The pattern of a greater peak response to navafenterol versus UMEC/VI was observed in both subgroups, although not statistically significant (p=0.12 for both subgroups), while UMEC/VI showed a significant increase in trough FEV1 compared with navafenterol at day 15 in the reversible subgroup only (p=0.008); there was no treatment difference in the nonreversible subgroup (p=0.65) (supplementary figure S2b).
Subgroups defined by eosinophil counts ≥150×106 or <150×106 cells·L−1 at baseline, inhaled corticosteroid use at baseline and current smokers versus former smokers were analysed post hoc. All analyses showed navafenterol and UMEC/VI caused significant improvements in trough and peak FEV1 compared with placebo that were not dependent on eosinophil counts, inhaled corticosteroid use or current smoking status (supplementary figures S3–S5).
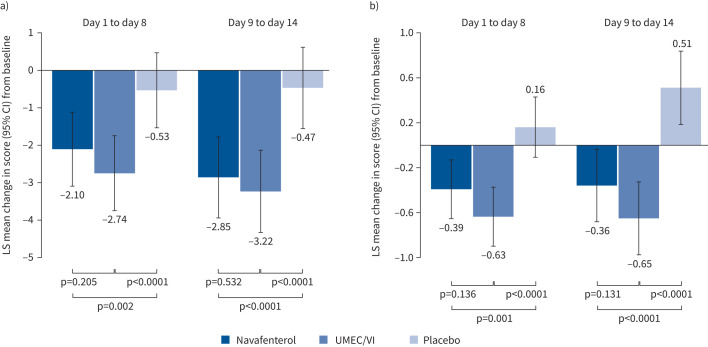
Symptom reduction
The CAT and BCSS questionnaires showed that, compared with baseline assessments, navafenterol and UMEC/VI each significantly improved symptoms of COPD relative to placebo during both the first (days 1–8) and second week (days 9–14) of treatment (all comparisons versus placebo, p<0.005; figure 4). Between the first and second week of treatment, the LS mean changes from baseline in CAT scores were similar (days 1–8: navafenterol −2.10, UMEC/VI −2.74, placebo −0.53; days 9–14: navafenterol −2.85, UMEC/VI −3.22, placebo −0.47). For BCSS, the LS mean changes from baseline score between the first and second weeks were also similar (day 1–8: navafenterol −0.39, UMEC/VI −0.63, placebo 0.16; days 9–14: navafenterol −0.36, UMEC/VI −0.65, placebo 0.51). There were no significant differences between navafenterol and UMEC/VI in CAT or total BCSS (all navafenterol versus UMEC/VI comparisons p>0.05; figure 4). The proportion of CAT responders (defined as a 2.0-point improvement) was higher for both navafenterol and UMEC/VI versus placebo, but there were no differences between UMEC/VI and navafenterol (supplementary table S2). The use of rescue medication was significantly lower with both active treatments than with placebo (all comparisons versus placebo p<0.0001) and was similar between the navafenterol and UMEC/VI treatment groups (all navafenterol versus UMEC/VI comparisons p>0.05; supplementary figure S6).
FIGURE 4.
Effect of treatment on the symptoms of COPD, as measured by a) the COPD Assessment Tool (CAT) and b) the Breathlessness, Cough and Sputum Scale (BCSS). LS: least-squares; UMEC/VI: umeclidinium/vilanterol.
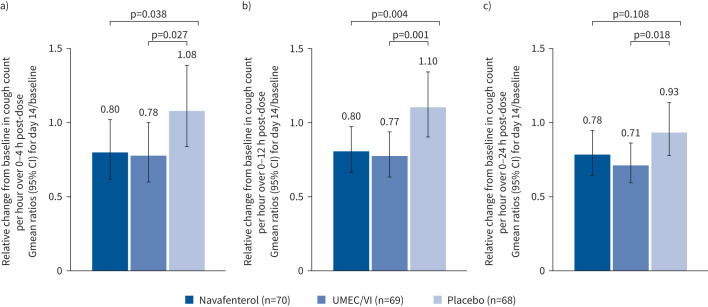
In an exploratory analysis, VitaloJAK objective cough monitoring measured cough frequency over 24 h at baseline and on day 14. Current smokers reported a higher frequency of cough at baseline than ex-smokers (supplementary table S3). At day 14, cough frequency was numerically lower over the 0–24-h period with navafenterol and UMEC/VI treatment compared with placebo (navafenterol versus placebo p=0.108; UMEC/VI versus placebo p=0.018; figure 5). Additionally, over the time period corresponding to maximum peak effect from spirometry (0–4 h), navafenterol and UMEC/VI showed significantly greater improvements in cough frequency compared with placebo (p=0.038 and p=0.027, respectively). This effect was sustained throughout the daytime period (0–12 h; p=0.004 and p=0.001, respectively). At day 14, improvements in cough with navafenterol and UMEC/VI compared with placebo were seen using a visual analogue scale (supplementary table S4).
FIGURE 5.
Relative change from baseline (geometric mean (Gmean) ratios) in objective cough count for day 14 over a) 0–4 h post-dose, b) 0–12 h post-dose and c) 0–24 h post-dose. UMEC/VI: umeclidinium/vilanterol.
Safety
61 (83.6%) participants experienced an adverse event during the study period. The proportions of patients who reported treatment-emergent adverse events were similar among the treatment groups: 39 (55.7%) participants during the navafenterol treatment period, 38 (55.1%) participants during the UMEC/VI treatment period and 35 (51.5%) participants during the placebo treatment period (table 3 and described in detail in the supplementary results).
TABLE 3.
Summary of treatment-emergent adverse events (AEs)
| Navafenterol | UMEC/VI | Placebo | All patients | |
| Patients | 70 | 69 | 68 | 73 |
| Any AE | 39 (55.7) | 38 (55.1) | 35 (51.5) | 61 (83.6) |
| Any SAE# | 0 (0.0) | 2 (2.9) | 2 (2.9) | 4 (5.5) |
| Any AE leading to discontinuation | 0 (0.0) | 1 (1.4) | 0 (0.0) | 1 (1.4) |
| Most frequently reported AEs¶ | ||||
| Headache | 14 (20.0) | 13 (18.8) | 14 (20.6) | 23 (31.5) |
| Nasopharyngitis | 6 (8.6) | 8 (11.6) | 3 (4.4) | 16 (21.9) |
| Rhinitis | 2 (2.9) | 3 (4.3) | 3 (4.4) | 8 (11.0) |
| Cough | 3 (4.3) | 3 (4.3) | 0 (0.0) | 6 (8.2) |
Data are presented as n or n (%). UMEC/VI: umeclidinium/vilanterol; SAE: serious adverse event. #: participants with multiple SAEs were counted once for each system organ class/preferred term. The summary includes SAEs starting on or after the first administration of study drug, up to and including 42 days after the final dose of study treatment. Washout period was considered part of the prior treatment; ¶: AEs reported in more than five patients are included using the Medical Dictionary for Regulatory Activities (version 21.0) preferred term.
No serious adverse events were reported during the navafenterol treatment period. Four participants reported serious adverse events: vestibular neuronitis (n=1, 1.5%) and humerus fracture (n=1, 1.5%) were reported during the placebo treatment period, and tooth abscess (n=1, 1.4%) and acute coronary syndrome (n=1, 1.4%) were reported during the UMEC/VI treatment period. None of the serious adverse events were considered by the investigators as related to the study treatment. The participant who reported acute coronary syndrome discontinued UMEC/VI and was withdrawn from the study. No clinically relevant differences in vital signs, laboratory findings or echocardiogram results were identified among the treatment groups. No deaths were reported during the study.
Pharmacokinetics
Navafenterol was rapidly absorbed after single (day 1) and multiple (day 14) doses (supplementary table S5, supplementary figure S7). The range for median time to maximum plasma concentration (tmax) was 0.45–2.05 h after dose administration. Evidence of accumulation was seen after repeated dosing, with accumulation ratios of 1.72 for maximum plasma concentration and 2.41 for AUC. The metabolite LAS191861 was rapidly formed (median tmax was ∼2 h after dosing) with AUC approximately two-fold lower than for navafenterol. Additional pharmacokinetic parameters are shown in supplementary table S5. In 15 out of 25 samples, navafenterol at concentrations of 3.63–15.85 pg·mL−1 was detected in pre-dose samples for the treatment period immediately following the navafenterol treatment period. LAS191861 was also detected in 20 out of 25 samples at concentrations of 2.02–17.71 pg·mL−1. This is probably due to the long terminal half-life of both navafenterol and LAS191861. Sensitivity analysis of change in trough FEV1 from baseline to day 15 suggests that the influence of the carryover on the estimated treatment effect observed for navafenterol or the active comparator was likely to be small (data not shown).
Discussion
This randomised controlled trial showed that navafenterol was superior to placebo in improving lung function outcomes in patients with moderate-to-severe COPD. The primary end-point analysis on day 15 showed no statistically significant difference between navafenterol and UMEC/VI on trough FEV1. For both treatments, there were similar improvements in COPD symptoms as measured by CAT, BCSS and objective cough monitoring.
The lung function profiles of navafenterol and UMEC/VI showed different patterns, when assessed over 24 h on day 1 and day 15. On day 1, navafenterol showed a greater peak effect than UMEC/VI. However, navafenterol exhibited a numerically lower trough FEV1 than UMEC/VI on day 2. After 14 days of treatment, navafenterol retained a greater peak FEV1 than UMEC/VI, but with a more gradual decline in FEV1 after the peak, and therefore both treatments had similar values from 12 to 24 h post-dose. It is possible that the greater peak effect of navafenterol could result in a lower burden of symptoms in the mornings, although this was not studied here and needs to be evaluated further. The effect of navafenterol on trough FEV1 increased from day 1 to day 15; this effect was less evident in patients treated with UMEC/VI. Pharmacokinetic analysis showed that the absorption of navafenterol and appearance of its metabolite LAS191861 was rapid. Importantly, there was substantial accumulation after repeated dosing. This accumulation of navafenterol from day 1 to day 14 was consistent with the pattern of change in FEV1 over the same time period.
The minimal clinically important difference (MCID) has been estimated at 1–3 points for CAT and >0.3 points for BCSS [17, 22]. For BCSS, a 1-point change represents substantial symptomatic improvement, a 0.6-point change is considered moderate and a 0.3-point change is considered small [22]. In this study, improvements greater than MCIDs were reported for navafenterol versus placebo for both CAT and BCSS at the end of the 14-day treatment period. Objective cough monitoring and the visual analogue scale were evaluated as exploratory end-points. The cough visual analogue scale is a practical tool used to evaluate cough severity, but is a subjective assessment that reflects the patient's perception of their own symptoms [20, 23]. The VitaloJAK is an objective method of measuring cough frequency over time and may be more sensitive than the subjective visual analogue scale [24]. Several clinical studies in patients with COPD and asthma have used the VitaloJAK for the assessment of cough and its relationship with other disease parameters [21, 25–27]. Although the MCID for reduction in cough counts has not been determined for COPD, data from refractory chronic cough suggests that a 20% reduction would be considered clinically meaningful [28]. The present study provides the first evidence that long-acting bronchodilators can decrease cough frequency in patients with COPD, with significant and sustained reductions during the daytime period compared with placebo. Because cough can reduce the quality of life of patients, sensitive objective cough monitoring techniques may have considerable value in COPD clinical trials [4].
In this study, we found that a higher proportion of patients met bronchodilator reversibility criteria than was observed in phase 3 trials of long-acting bronchodilators and a previous real-world study of patients with COPD [29–32]. In published studies, 11–34% of patients were classified as having a reversible status, whereas in the present study 63% of patients were classified as having a short-acting bronchodilator reversible status.
A number of other MABAs have been in development for the treatment of COPD including batefenterol, AZD8999, AZD2115, CHF6366 and THRX200495 [33]. The majority of these are no longer in active clinical development. Batefenterol completed phase 2b clinical trials, but has not progressed to phase 3. In terms of β2-adrenoceptor agonist/muscarinic antagonist activity ratios, batefenterol has a stronger β2-adrenoceptor agonist function whereas navafenterol has a stronger M3 muscarinic antagonist activity [33]. In a phase 2 trial, batefenterol 300 μg in combination with fluticasone furoate 100 μg showed improvements in change from baseline in FEV1 compared with placebo over 42 days of treatment [34]. Differences in trial design make it challenging to compare across studies. Although synergy between LABA and LAMA therapies has been demonstrated in vitro, this remains unproven in clinical practice. A potential benefit of MABAs is the future possibility of co-formulation with an anti-inflammatory compound(s), which could offer an opportunity for novel triple (or quadruple) pharmacology fixed-dose combination products that would be technically less demanding to develop than a product containing LAMA and LABA as separate molecular entities.
This study had a crossover design that used double-dummy and double-blinding. The crossover design minimised interparticipant variability and optimised sample size. To reduce the possibility of carryover effects as a consequence of the long terminal half-life of navafenterol and LAS191861, a long washout period was implemented. Although there were measurable concentrations of navafenterol after washout, sensitivity analyses including carryover variables were performed and did not indicate the presence of significant carryover in the FEV1 efficacy results. A limitation of the study is that the therapeutic dose of navafenterol is unknown, because the full dose response has not yet been explored. Although this may limit comparisons with known therapeutic doses of established bronchodilators, efficacy comparisons with placebo are unaffected. Given that navafenterol is well tolerated so far, higher doses of navafenterol may be explored to examine the potential positive effects on efficacy. An additional limitation of the study was that there was no robust control of type I errors for any of the secondary end-points, although this is standard for phase 2 studies. This study may not be fully representative of the broader COPD patient population owing to the high proportion of patients with a short-acting bronchodilator reversible status.
Conclusions
Treatment with navafenterol 600 µg once daily was well tolerated and provided improvements in overall lung function and COPD symptoms reduction, to a similar extent to UMEC/VI, an established LAMA/LABA combination bronchodilator. The results from this study support further investigation of navafenterol in larger and longer clinical trials in patients with COPD.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods and results ERJ-00972-2021.Supplement (142.6KB, pdf)
Supplementary tables and figures ERJ-00972-2021.Tables (276.6KB, pdf)
Shareable PDF
Acknowledgements
We thank the patients and their carers as well as the site staff and investigators who participated in this study. We thank Laura Drought (PharmaGenesis London, London, UK), who provided medical writing support, which was funded by AstraZeneca. We thank Evelina Björnsson, Henrik Forsman and Victor Balaguer (AstraZeneca, Gothenburg, Sweden) for support in the execution and conduct of the study.
Footnotes
This article has supplementary material available from erj.ersjournals.com
This study is registered at clinicaltrials.gov with identifier NCT03645434. Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
Author contributions: D. Singh, J. Beier, C. Astbury, M.G. Belvisi, C.A. Da Silva, A. Jauhiainen, E. Jimenez, A. Lei, S. Necander, J.A. Smith, U. Wählby Hamrén and I. Psallidas contributed substantially to the study design and concept. D. Singh and J. Beier were involved in data acquisition. A. Jauhiainen, E. Jimenez, A. Lei, S. Necander, U. Wählby Hamrén, W. Xin and I. Psallidas conducted the data analyses. D. Singh, J. Beier, C. Astbury, M.G. Belvisi, C.A. Da Silva, A. Jauhiainen, E. Jimenez, A. Lei, S. Necander, J.A. Smith, U. Wählby Hamrén, W. Xin and I. Psallidas assisted with interpretation of the data, were involved in drafting of the manuscript, provided critical revisions for important intellectual content, approved the final version submitted for publication, and agreed to be accountable for all aspects of the work.
Conflict of interest: D. Singh has received grants and personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Epiendo, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Mundipharma, Novartis, Pfizer, Pulmatrix, Sanofi, Teva, Theravance and Verona, and personal fees from Cipla, Genentech and Peptinnovate. J. Beier has received consultation fees from AstraZeneca, Berlin Chemie/Menarini, Chiesi and Pohl-Boskamp, and participated in scientific advisory boards that were funded by AstraZeneca and Chiesi. C. Astbury, M.G. Belvisi, C.A. Da Silva, A. Jauhiainen, E. Jimenez, A. Lei, S. Necander, U. Wählby Hamrén, W. Xin and I. Psallidas are employees of AstraZeneca and may hold stock or stock options. The VitaloJAK algorithm has been licensed by Manchester University Foundation Trust (MFT) and the University of Manchester to Vitalograph Ltd and Vitalograph Ireland (Ltd); MFT receives royalties which may be shared with the clinical division in which J.A. Smith works, and J.A. Smith has received personal fees from AstraZeneca.
Support statement: AstraZeneca funded this study, and participated in the study design, data collection, data analysis, data interpretation and writing of the study report. AstraZeneca reviewed the publication, without influencing the opinions of the authors, to ensure medical and scientific accuracy, and the protection of intellectual property. The corresponding author had access to all data in the study, and had the final responsibility for the decision to submit the manuscript for publication. M.G. Belvisi and J.A. Smith receive funding from the Wellcome Trust (207504/B/17/Z). J.A. Smith is also funded by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre and is an NIHR Senior Investigator. D. Singh is supported by the NIHR Manchester Biomedical Research Centre.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf Date last updated: 2020. Date last accessed: 10 July 2021.
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3: e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rennard SI, Drummond MB. Early chronic obstructive pulmonary disease: definition, assessment, and prevention. Lancet 2015; 385: 1778. doi: 10.1016/S0140-6736(15)60647-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res 2017; 18: 67. doi: 10.1186/s12931-017-0548-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook N, Gey J, Oezel B, et al. Impact of cough and mucus on COPD patients: primary insights from an exploratory study with an online patient community. Int J Chron Obstruct Pulmon Dis 2019; 14: 1365–1376. doi: 10.2147/COPD.S202580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray R, Tombs L, Naya I, et al. Efficacy and safety of the dual bronchodilator combination umeclidinium/vilanterol in COPD by age and airflow limitation severity: a pooled post hoc analysis of seven clinical trials. Pulm Pharmacol Ther 2019; 57: 101802. doi: 10.1016/j.pupt.2019.101802 [DOI] [PubMed] [Google Scholar]
- 7.Maltais F, Bjermer L, Kerwin EM, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res 2019; 20: 238. doi: 10.1186/s12931-019-1193-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calzetta L, Matera MG, Rogliani P, et al. Dual LABA/LAMA bronchodilators in chronic obstructive pulmonary disease: why, when, and how. Expert Rev Respir Med 2018; 12: 261–264. doi: 10.1080/17476348.2018.1442216 [DOI] [PubMed] [Google Scholar]
- 9.Cazzola M, Lopez-Campos JL, Puente-Maestu L. The MABA approach: a new option to improve bronchodilator therapy. Eur Respir J 2013; 42: 885–887. doi: 10.1183/09031936.00067013 [DOI] [PubMed] [Google Scholar]
- 10.Matera MG, Page CP, Calzetta L, et al. Pharmacology and therapeutics of bronchodilators revisited. Pharmacol Rev 2020; 72: 218–252. doi: 10.1124/pr.119.018150 [DOI] [PubMed] [Google Scholar]
- 11.Aparici M, Carcasona C, Ramos I, et al. Pharmacological profile of AZD8871 (LAS191351), a novel inhaled dual M3 receptor antagonist/β2-adrenoceptor agonist molecule with long-lasting effects and favorable safety profile. J Pharmacol Exp Ther 2019; 370: 127–136. doi: 10.1124/jpet.118.255620 [DOI] [PubMed] [Google Scholar]
- 12.Singh D, Balaguer V, Astbury C, et al. Navafenterol (AZD8871) in patients with COPD: a randomized, double-blind, phase I study evaluating safety and pharmacodynamics of single doses of this novel, inhaled, long-acting, dual-pharmacology bronchodilator. Respir Res 2020; 21: 102. doi: 10.1186/s12931-020-01347-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh D, Fuhr R, Jimenez L, et al. A randomized trial of dual-acting bronchodilator AZD8871 for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2019; 199: 1282–1284. doi: 10.1164/rccm.201812-2345LE [DOI] [PubMed] [Google Scholar]
- 14.Smith J, Woodcock A. Cough and its importance in COPD. Int J Chron Obstruct Pulmon Dis 2006; 1: 305–314. doi: 10.2147/copd.2006.1.3.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CONSORT . CONSORT 2010 Checklist of Information to Include When Reporting a Randomised Trial. Available from: www.consort-statement.org/ Date last updated: 2010. Date last accessed: 2 June 2021.
- 16.DeVries R, Kriebel D, Sama S. Validation of the breathlessness, cough and sputum scale to predict COPD exacerbation. NPJ Prim Care Respir Med 2016; 26: 16083. doi: 10.1038/npjpcrm.2016.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leidy NK, Rennard SI, Schmier J, et al. The breathlessness, cough, and sputum scale: the development of empirically based guidelines for interpretation. Chest 2003; 124: 2182–2191. doi: 10.1378/chest.124.6.2182 [DOI] [PubMed] [Google Scholar]
- 18.Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34: 648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 19.Smith JA, Holt K, Dockry R, et al. Performance of a digital signal processing algorithm for the accurate quantification of cough frequency. Eur Respir J 2021; 58: 2004271. 10.1183/13993003.04271-2020 [DOI] [PubMed] [Google Scholar]
- 20.Spinou A, Birring SS. An update on measurement and monitoring of cough: what are the important study endpoints? J Thorac Dis 2014; 6: S728–S734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodhi S, Smith JA, Satia I, et al. Cough rhythms in asthma: potential implication for management. J Allergy Clin Immunol Pract 2019; 7: 2024–2027. doi: 10.1016/j.jaip.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cazzola M, Hanania NA, MacNee W, et al. A review of the most common patient-reported outcomes in COPD – revisiting current knowledge and estimating future challenges. Int J Chron Obstruct Pulmon Dis 2015; 10: 725–738. doi: 10.2147/COPD.S77368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith J, Owen E, Earis J, et al. Effect of codeine on objective measurement of cough in chronic obstructive pulmonary disease. J Allergy Clin Immunol 2006; 117: 831–835. doi: 10.1016/j.jaci.2005.09.055 [DOI] [PubMed] [Google Scholar]
- 24.Smith J, Owen E, Earis J, et al. Cough in COPD: correlation of objective monitoring with cough challenge and subjective assessments. Chest 2006; 130: 379–385. doi: 10.1016/S0012-3692(15)51851-5 [DOI] [PubMed] [Google Scholar]
- 25.Marsden PA, Satia I, Ibrahim B, et al. Objective cough frequency, airway inflammation, and disease control in asthma. Chest 2016; 149: 1460–1466. doi: 10.1016/j.chest.2016.02.676 [DOI] [PubMed] [Google Scholar]
- 26.Satia I, Watson R, Scime T, et al. Allergen challenge increases capsaicin-evoked cough responses in patients with allergic asthma. J Allergy Clin Immunol 2019; 144: 788–795. doi: 10.1016/j.jaci.2018.11.050 [DOI] [PubMed] [Google Scholar]
- 27.Sumner H, Woodcock A, Kolsum U, et al. Predictors of objective cough frequency in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 943–949. doi: 10.1164/rccm.201211-2000OC [DOI] [PubMed] [Google Scholar]
- 28.Nguyen AM, Muccino D, Birring S, et al. Defining minimal clinically important differences (MCID) in chronic cough: analyses of objective cough counts from a phase 2 randomized controlled trial. J Allergy Clin Immunol 2019; 143: Suppl., AB169. doi: 10.1016/j.jaci.2018.10.047 [DOI] [Google Scholar]
- 29.Müller V, Gálffy G, Orosz M, et al. Characteristics of reversible and nonreversible COPD and asthma and COPD overlap syndrome patients: an analysis of salbutamol Easyhaler data. Int J Chron Obstruct Pulmon Dis 2016; 11: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maleki-Yazdi MR, Kaelin T, Richard N, et al. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trial. Respir Med 2014; 108: 1752–1760. doi: 10.1016/j.rmed.2014.10.002 [DOI] [Google Scholar]
- 31.Donohue JF, Niewoehner D, Brooks J, et al. Safety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52-week, randomized, double-blind, placebo-controlled study. Respir Res 2014; 15: 78. doi: 10.1186/1465-9921-15-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. Eur Respir J 2016; 48: 1030–1039. doi: 10.1183/13993003.00216-2016 [DOI] [PubMed] [Google Scholar]
- 33.Ora J, Coppola A, Cazzola M, et al. Long-acting muscarinic antagonists under investigational to treat chronic obstructive pulmonary disease. J Exp Pharmacol 2020; 12: 559–574. doi: 10.2147/JEP.S259330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crim C, Gotfried M, Spangenthal S, et al. A randomized, controlled, repeat-dose study of batefenterol/fluticasone furoate compared with placebo in the treatment of COPD. BMC Pulm Med 2020; 20: 119. doi: 10.1186/s12890-020-1153-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods and results ERJ-00972-2021.Supplement (142.6KB, pdf)
Supplementary tables and figures ERJ-00972-2021.Tables (276.6KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00972-2021.Shareable (291.2KB, pdf)