Abstract
Background
Elevated counts of alveolar macrophages and attenuated phagocytic capacity are associated with chronic obstructive pulmonary disease (COPD). Factors governing macrophage phagocytosis are poorly understood. In this study we aimed to compare the influence of airway epithelial cell secretions from individuals with COPD and without COPD (non-COPD) on macrophage phagocytic activity, and the role of antimicrobial peptides (AMPs).
Methods
Supernatants from non-COPD and COPD small airway epithelial cell (SAEC) cultures exposed to non-typeable Haemophilus influenzae (NTHi) were applied to human monocyte-derived macrophages (MDMs) to assess their influence on phagocytosis. SAECs were analysed for changes in AMP expression by quantitative reverse transcription PCR, and the influence of select AMPs on macrophage phenotype and function was assessed by flow cytometry and metabolic activity assay.
Results
Secretions from the apical and basolateral surface of NTHi-exposed SAECs from non-COPD donors elicited superior phagocytic capacity in MDMs. Moreover, NTHi exposure led to a rapid increase in the expression of a range of AMPs by non-COPD SAECs, but this response was delayed in COPD SAECs. We demonstrate that treatment with AMPs β-defensin 2 and S100 calcium binding protein A8/S100 calcium binding protein A9 (S100A8/A9) improved the phagocytic capacity of MDMs. In-depth analysis of the influence of S100A8/A9 on MDMs revealed a role for this AMP in macrophage phenotype and function. Furthermore, we show that the expression of S100A8 and S100A9 is directly regulated by WNT/β-catenin signalling, a known deregulated pathway in COPD.
Conclusion
In conclusion, for the first time, we demonstrate that airway epithelium from patients with COPD has a reduced capacity to support the phagocytic function of macrophages in response to acute NTHi exposure, and we identify the WNT/β-catenin signalling-modulated and epithelium-derived S100A8/A9 as a potent regulator of macrophage phenotype and function.
Short abstract
COPD airway epithelium has reduced capacity to support the phagocytic function of macrophages in response to acute NTHi exposure, and epithelium-released S100A8/A9 modulates macrophage phenotype and function https://bit.ly/3CVprKM
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide [1], characterised by destruction of the lung parenchyma (emphysema), chronic inflammation [2] and increased lung colonisation with pathogenic bacteria, including non-typeable Haemophilus influenzae (NTHi) [3]. The airway epithelium represents a key barrier to microbes and microbial products through its mucociliary clearance capacity and secretion of antimicrobial peptides (AMPs) [4, 5]. AMPs, also known as host defence peptides, are naturally occurring cationic small proteins that can directly inactivate pathogens [6]. The best-studied groups of human AMPs are the defensins and cathelicidins (LL-37) [7]. Levels of AMPs in sputum and serum are modulated during COPD exacerbations [8]. Furthermore, differences in bacterial killing activity of non-COPD and COPD epithelium secretions have previously been reported and linked to differences in AMP expression (β-defensin 2 (BD2), lipocalin 2 (LCN2) and S100 calcium binding protein A7 (S100A7)) [9]. Pulmonary macrophages are also critical for effective clearance of bacteria in the airways. Elevated macrophage numbers have been reported in COPD; however, their phagocytic capacity is impaired [10, 11]. As critical innate immune mechanisms, disease-related alterations in phagocytosis and AMP production could contribute to increased bacterial colonisation in COPD exacerbations [11]. Interestingly, neutrophil-derived LL-37 and human neutrophil peptide 1–3 AMPs have been reported to exert an immunomodulatory effect on macrophages by improving their phagocytic capacity [12, 13]. However, the mechanisms underlying epithelial secretion-induced changes in macrophage phagocytic capacity and the role of AMPs in this crosstalk are currently unknown.
In this study we sought to understand the differences between the capacity of non-COPD and COPD epithelial secretions to stimulate macrophage phagocytosis and we investigated the involvement of airway epithelium-derived AMPs in this process.
Methods
Isolation of peripheral blood mononuclear cells and monocytes, and generation of macrophages
Leukopak concentrates were obtained from healthy donors at DRK-Blutspendedienst Ulm and all donors provided written consent. Peripheral blood mononuclear cells (PBMCs) were isolated from these leukopak concentrates by standard density gradient centrifugation with Ficoll-Paque.
Monocytes were isolated using the EasyStep Human Monocyte Isolation Kit (#19359, STEMCELL Technologies) in combination with RoboSep (STEMCELL Technologies).
Macrophages were subsequently generated by seeding monocytes in Nunc plates with UpCell surface (ThermoFisher) in XVIVO10 media (with no phenol red and no transferrin, Lonza) with the addition of 100 ng·mL−1 of macrophage colony-stimulating factor (M-CSF) (MILT-130-096-489, Miltenyi Biotec).
Cell culture
Small airway epithelial cells (SAECs) from non-COPD and COPD individuals (characterisation of the participants in table 1) were purchased from Lonza (CC-2547) and cultured in an air–liquid interface (ALI) setting. Cells were grown to 80% confluence in Pneumacult-ex plus media with supplements, 1% hydrocortisone and 1% penicillin-streptomycin (all STEMCELL Technologies). Next, cells were trypsinised and seeded onto transwell inserts (#3470, Corning) coated with bovine collagen (#5005, Medical Industrie GmbH). After cells reached confluence, media from the apical chamber was aspirated so that the cells were exposed to air, and the media in the basolateral chamber was exchanged for the Pneumacult ALI2 media with the addition of maintenance supplements, 2% heparin, 5% hydrocortisone and 1% penicillin-streptomycin (all STEMCELL Technologies). Cells were cultured for 4 weeks, until fully differentiated. This stage was identified by the culture reaching a stable transepithelial electrical resistance (TEER) of 1000 Ω·cm2 (formation of stable barrier), mucus production and cilia beating across the surface of the transwell culture.
TABLE 1.
Subject characteristics
| Non-COPD | COPD | |
| Subjects | 9 | 9 |
| Female/male | 5/4 | 6/3 |
| Age (years) | 53±15 | 58±8 |
| Smoking | ||
| Yes | 3 | 8 |
| No | 6 | 1 |
Data are presented as n or mean±sd.
For NTHi experiments, the basolateral medium was exchanged for medium without antibiotics 24 h before NTHi addition. The apical surface of the SAECs was washed with warm PBS and 20 µL of PBS or NTHi (107 bacteria) was added on top. Cells were incubated for 3 h or 24 h. Afterwards, the basolateral media (basolateral supernatant (SN)) and apical surface washes (apical SN; washed twice with PBS in a total volume of 300 µL) were collected. All SNs were centrifuged at 14 000 g for 10 min to remove cell debris and any bacterial residues.
For WNT modulation, SAECs were exposed to FH535 (20 µM; #4344/10, Tocris), WNT5A (100 ng·mL−1; #645-WN, R&D Systems) or Chir99021 (2 µM; #4423, Tocris) for 24 h. Afterwards cells were washed once with PBS and stored at −80°C until RNA isolation.
To assess the influence of NTHi on WNT5A expression, M-CSF-generated monocyte-derived macrophages (MDMs) were exposed to NTHi for 24 h, washed with PBS and kept at −80°C until RNA isolation.
Phagocytosis assay
Macrophages were seeded on a 96-well plate (30 000 cells per well) and rested for 2 h. For experiments with SAEC SNs, cells were treated with SN mixed 1:1 with XVIVO10 media and co-exposed to pHrodo Escherichia coli particles (Essen BioScience). For the experiments with recombinant AMPs, cells were pre-treated for 1 h with the following recombinant proteins: BD2 (#167300-49-B, Peprotech), secretory leukocyte peptidase inhibitor (SLPI) (#1274-PI-100, R&D Systems), S100A8 (#9876-S8-050, R&D Systems), S100A9 (#9254-S9-050, R&D Systems) and S100A8/A9 (#8226-S8-050, R&D Systems) (all 5 µg·mL−1, based on the literature [14]) or vehicle as a control. Subsequently, cells were co-treated with pHrodo E. coli or Staphylococcus aureus particles and the particle fluorescence (when localised in the acidic environment of the phagolysosome) was measured using an IncuCyte S3 Live-Cell Analysis System (Essen Bioscience) to monitor phagocytosis progression over time.
Statistical analyses
Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). Data are expressed as mean or mean±sem. Means between two groups were compared using the Mann–Whitney test or t-tests. Comparisons between more than two groups were performed by Kruskal–Wallis test followed by Dunn's post test or one-way ANOVA followed by Sidak multiple comparison test. p-values <0.05 were considered to indicate a statistically significant difference.
Additional methods can be found in the supplementary material.
Results
SNs from NTHi-exposed COPD airway epithelial cells have diminished impact to support the phagocytic capacity of macrophages
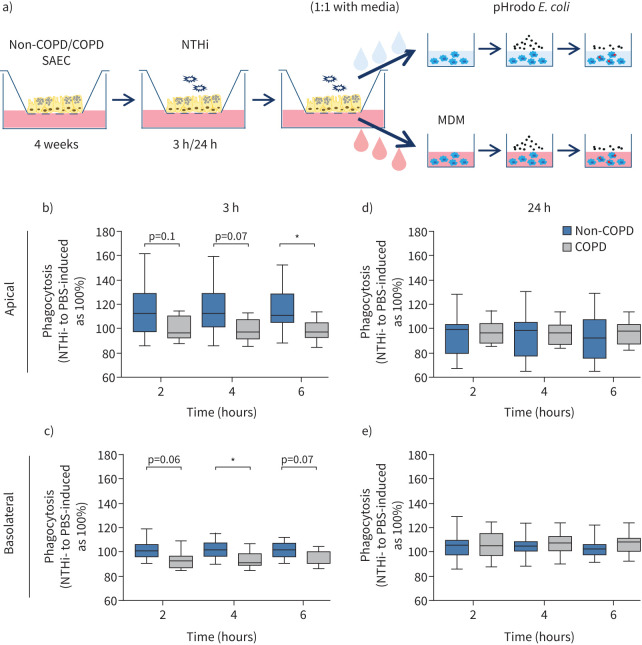
To study the influence of non-COPD and COPD epithelium-derived factors on the function of macrophages, SAECs from non-COPD and COPD individuals (donor information in table 1) were exposed to PBS or NTHi at the apical surface. No differences were apparent between non-COPD and COPD SAECs based on hematoxylin and eosin staining of the SAECs, TEER and expression analysis of epithelial and mesenchymal markers (supplementary figure S1). SN was collected from the apical surface and basolateral media of SAEC cultures and subsequently used to stimulate MDMs (scheme depicted in figure 1a). SN from non-COPD-obtained SAECs exposed to NTH for 3 h, relative to PBS-exposed SAEC SN, supported macrophage phagocytic function significantly better than COPD-obtained SN in the case of both apical and basolateral SN (figure 1b, c; mean±sem for non-COPD versus COPD: 3 h apical: 2 h 114±6.4% versus 99.6±3.1%, 4 h 115±6.1% versus 98.5±2.1%, 6 h 116±5.3% versus 98±2.6%; 3 h basolateral: 2 h 101±2.5% versus 92±2.5%, 4 h 101±2.2% versus 92±2.2%, 6 h 101±2% versus 93±1.8%). However, the differential effect of non-COPD and COPD SNs on macrophage phagocytosis was no longer apparent after longer exposure of epithelium to NTHi over 24 h (figure 1d, e). No obvious differences were observed in the viability of macrophages exposed to non-COPD and COPD SNs (supplementary figure S2a) or in surface marker expression profiles (supplementary figure S2b). Overall, these results suggest a delayed secretory response from COPD epithelium to bacterial exposure.
FIGURE 1.
Supernatants (SNs) from non-typeable Haemophilus influenzae (NTHi)-exposed chronic obstructive pulmonary disease (COPD) small airway epithelial cells (SAECs) have a diminished impact on the phagocytic capacity of macrophages. a) Schematic of the experimental procedure. SAECs obtained from non-COPD and COPD-diagnosed individuals were cultured in an air–liquid-interface set-up to full differentiation. On the apical surface, fully differentiated SAECs were exposed to vehicle (PBS) or heat-inactivated NTHi for 3 h or 24 h. Washings from the apical chamber or media from the basolateral chamber was used for subsequent incubation with human monocyte-derived macrophages (MDMs) to investigate the effects on MDM phagocytosis of the fluorescently labelled pHrodo Escherichia coli particles (based on the fluorescence emission when particles encounter the acidic environment of the phagosome). b–e) MDMs from three healthy donors were exposed to apical washing SN (b, d) or basolateral SN (c, e) obtained from non-COPD (blue) and COPD (grey) SAECs (n=9) exposed to PBS or heat-inactivated NTHi for 3 h (b, c) or 24 h (d, e) (each MDM donor+SAEC donor represents a unique pair). The impact of SN on phagocytosis of pHrodo E. coli particles by MDMs was measured using an IncuCyte S3 Live-Cell Analysis System over time (2 h, 4 h and 6 h time points depicted). Data are presented as a mean relative phagocytosis of MDMs exposed to SN obtained from NTHi-stimulated SAECs to corresponding PBS-exposed SAECs. Means were compared using Kruskal–Wallis test followed by Dunn's post test; *: p<0.05.
COPD airway epithelial cells exert a delayed AMP response to NTHi
While recent experimental evidence has suggested that neutrophil-derived AMPs can modulate the function of macrophages [12, 13], little is known about the potential influence of epithelial-derived AMPs on macrophage function.
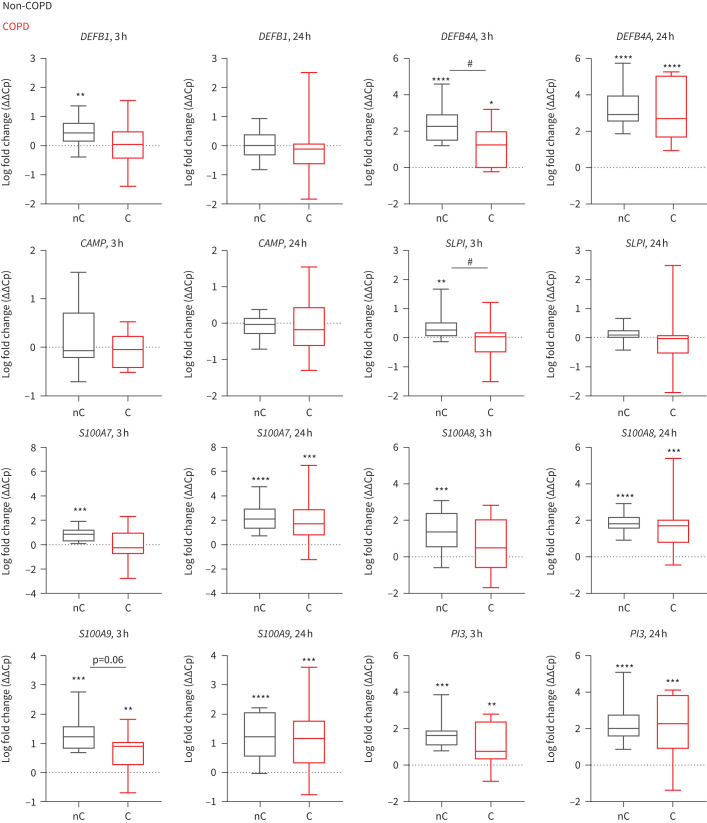
Based on the literature [9, 12] and our unpublished data, we selected a number of known epithelial-derived AMPs to investigate. We assessed potentiation of AMP expression upon NTHi exposure (IL6 mRNA expression used as a positive control for bacterial stimulation shown in supplementary figure S3a; ΔΔCp mean±sem for non-COPD versus COPD: 3 h: 3.2±0.38 versus 2.7±0.28; 24 h: 1.7±0.27 versus 1.34±0.37) in non-COPD and COPD SAECs and identified several differentially regulated AMPs; DEFB4A, SLPI, S100A9 and PI3 were more highly expressed in NTHi-exposed non-COPD than COPD SAECs after 3 h NTHi exposure (ΔΔCp mean±sem for non-COPD versus COPD: DEFB4A 2.44±0.3 versus 1.1±0.38; SLPI 0.38±0.12 versus −0.1±0.1; S100A9 1.3±0.19 versus 0.67±0.19; figure 2) and there was also a trend for higher expression of S100A8 after 3 h NTHi exposure (1.5±0.27 versus 0.66±0.35). However, these differences in epithelial AMP expression were not observed after 24 h of NTHi exposure, consistent with a delayed response to bacterial infection from COPD epithelium.
FIGURE 2.
Chronic obstructive pulmonary disease (COPD) small airway epithelial cells (SAECs) exert a delayed antimicrobial peptide (AMP) response to non-typeable Haemophilus influenzae (NTHi). mRNA expression of AMPs (DEFB1, DEFB4A (gene name for β-defensin 2 protein), CAMP, SLPI, S100A7, S100A8, S100A9, PI3) in non-COPD-derived (nC, grey) and COPD-derived (C, red) SAECs exposed to NTHi for 3 h or 24 h relative to PBS-exposed SAECs. Gene expression comparative crossing point change (ΔΔCp) is presented as a mean (n=11–18). Differences between NTHi and corresponding PBS-exposed SAECs were calculated with paired t-test; *: p<0.05, **: p<0.01, ***: p<0.001, ****: p<0.0001. Differences between means of non-COPD and COPD SAECs were calculated with a Mann–Whitney test; #: p<0.05.
S100A8/A9 heterodimer and BD2 modulate macrophage phagocytosis
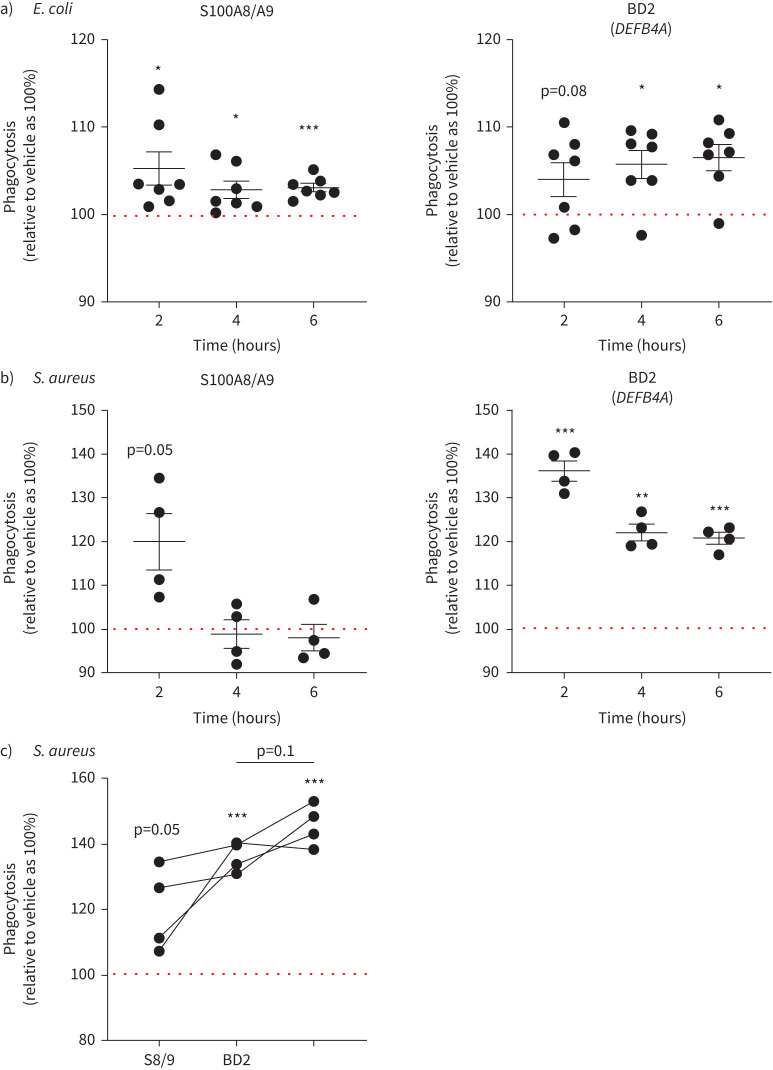
Based on these findings, we next interrogated the ability of selected AMPs (BD2 (encoding gene DEFB4A), SLPI, S100A8, S100A9 and the S100A8/A9 heterodimer) to directly influence macrophage phagocytosis. We used bacterial particles in these assays, rather than live bacteria, to eliminate possible direct bactericidal effects of the AMPs.
We did not observe modulation of phagocytosis with SLPI, nor with S100A8 or S100A9 homodimers (supplementary figure S4). However, we found that the S100A8/A9 heterodimer and BD2 could enhance phagocytosis of E. coli (gram-negative) particles (mean±sem for S100A8/A9 relative to vehicle: 2 h 105.3±1.9%, 4 h 102.8±0.9%, 6 h 103±0.45%; for BD2 relative to vehicle: 2 h 104±1.9%, 4 h 105.7±1.6%, 6 h 106.5±1.4%, 12 h 105.2±1.3%; figure 3a) as well as S. aureus (gram-positive) particles (mean±sem for S100A8/A9 relative to vehicle: 2 h 120±6.4%, 4 h 98.8±3.2%, 6 h 98±3%; for BD2 relative to vehicle: 2 h 136±2.2%, 4 h 122±1.8%, 6 h 120.8±1.3%; figure 3b). In the case of S. aureus, the positive effect was most apparent at acute exposure. We therefore investigated the potential additive/synergistic effect at the early time point and found that there was a trend towards an additive effect on MDM phagocytosis when S100A8/A9 and BD2 were applied together (mean±sem for S100A8/A9: 120±6.4%; BD2: 136±2.2%; and S100A8/A9+BD2: 145±3.2%; figure 3c).
FIGURE 3.
Antimicrobial peptides (AMPs) S100 calcium binding protein A8/S100 calcium binding protein A9 (S100A8/A9) and β-defensin 2 (BD2) (gene name DEFB4A) modulate the phagocytic capacity of human monocyte-derived macrophages (MDMs). Cells were pre-treated with indicated proteins or vehicle and the progression of phagocytosis of a) pHrodo Escherichia coli particles (gram-negative) and b) pHrodo Staphylococcus aureus particles (gram-positive) was monitored using the IncuCyte S3 Live-Cell Analysis System. For each time point, data are presented as a % of phagocytosis relative to respective vehicle control (100%, dotted horizontal line) (E. coli n=7, S. aureus n=4). The impact of the recombinant AMP on phagocytosis was calculated using a paired t-test; *: p<0.05; **: p<0.01; ***: p<0.001. c) Comparison of BD2, S100A8/A9 (S8/9) and BD2 co-treatment with S8/9 on phagocytic capacity of MDMs of pHrodo S. aureus particles at the 2 h time point. The impact of the recombinant AMP on phagocytosis was calculated using a paired t-test; ***: p<0.001. Differences between treatments were calculated with one-way ANOVA followed by Sidak multiple comparison test.
S100A8/A9 and BD2 proteins are differentially regulated by NTHi in non-COPD and COPD SAEC secretions
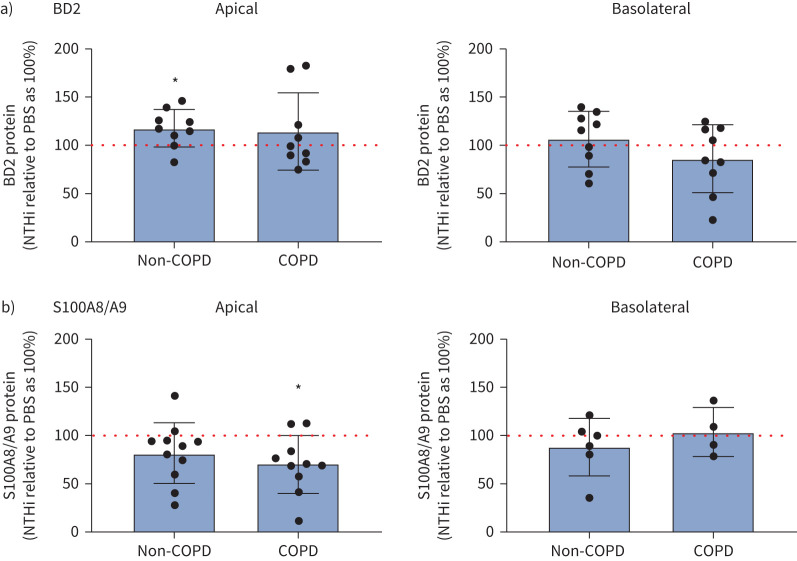
Because we observed that BD2 and S100A8/A9 proteins could improve the phagocytic function of macrophages, we investigated the expression of these proteins in SAEC SN upon NTHi exposure. We found that 3 h of NTHi stimulation significantly increased the expression of BD2 protein in the apical SN of non-COPD SAECs (mean±sem relative to control as 100%: 117±6.4%), but not COPD SAECs (114±13%). In the case of S100A8/A9, NTHi did not increase the detectable levels of this AMP in apical SN of non-COPD SAECs (81±9%), but significantly decreased the expression in SN of COPD SAECs (70±9%). NTHi stimulation did not, however, have an influence on the secretion of BD2 or S100A8/A9 to the basolateral compartment (figure 4 and supplementary figure S5).
FIGURE 4.
S100 calcium binding protein A8/S100 calcium binding protein A9 (S100A8/A9) and β-defensin 2 (BD2) proteins are differentially regulated by non-typeable Haemophilus influenzae (NTHi) in non-chronic obstructive pulmonary disease (non-COPD) and COPD small airway epithelial cell (SAEC) secretions. Expression of a) BD2 and b) S100A8/A9 proteins in supernatants (SNs) obtained from SAEC air–liquid-interface cultures from apical (left) and basolateral (right) compartment measured by ELISA. Data are presented as an expression of the protein in SN obtained from SAECs exposed to NTHi, relative to the expression of protein in SNs obtained from SAEC exposed to PBS for 3 h (PBS-exposed condition as 100%; BD2 n=9; S100A8/A9 apical non-COPD SN n=11, apical COPD SN n=10, basolateral non-COPD SN n=6, basolateral COPD SN n=4). Impact of NTHi on protein expression in non-COPD and COPD SAEC SN was calculated using a one-sample t-test; *: p<0.05.
S100A8/A9 modulates macrophage phenotype and metabolism
Our findings related to BD2 support data published in a previous report showing the role of BD2 in enhancing phagocytosis of bacteria by macrophages [15]. Therefore, we decided to further characterise the as yet unknown role of S100A8/A9 in modulating MDM phenotype and function.
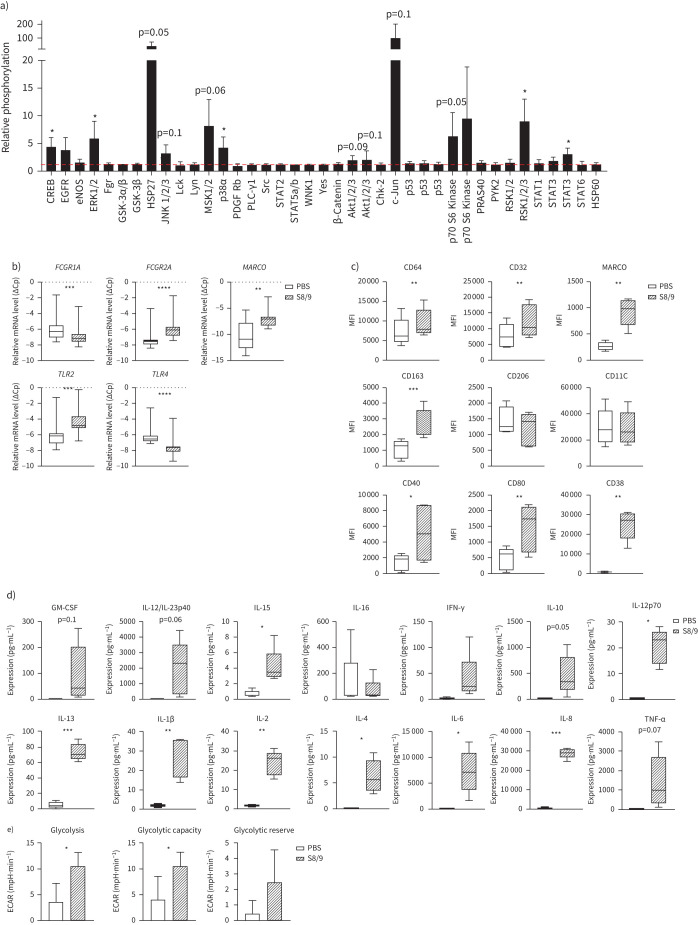
Analysis of the downstream effects of S100A8/A9 on macrophages (evidence for binding of S100A8/A9 to MDM is presented in supplementary figure S6) revealed enhanced phosphorylation of CREB, ERK1/2, HSP27, JNK 1/2/3, MSK 1/2, AKT 1/2/3, c-JUN, p70S6, RSK 1/2/3 and STAT3 (figure 5a and supplementary figure S7). A number of these signalling molecules are known to be associated with phagocytosis [16–18]. Furthermore, we found that S100A8/A9 also significantly altered the expression of receptors known to play a role in phagocytosis, inducing an upregulation of FCGR2A, MARCO and TLR2 mRNA and a downregulation of FCGR1A and TLR4 (figure 5b). We were able to confirm the upregulation of CD32 (FCGR2A) and MARCO protein expression by fluorescence-activated cell sorting (FACS), and despite not changing at the mRNA level, FACS analysis also revealed increased cell surface expression of CD64 (FCGR1A) protein upon S100A8/A9 exposure (figure 5c).
FIGURE 5.
S100 calcium binding protein A8/S100 calcium binding protein A9 (S100A8/A9) modulates macrophage phenotype and metabolism. a) Phosphorylation status of signalling molecules upon 30 min S100A8/A9 (5 µg·mL−1) treatment depicted as fold-difference relative to PBS-induced phosphorylation (n=3). Influence of S100A8/A9 on phosphorylation was calculated using a one-sample t-test. b) mRNA expression of FCGR1A, FCGR2A, MARCO, TLR2 and TLR4 in human monocyte-derived macrophages (MDMs) exposed to PBS or S100A8/A9 (5 µg·mL−1) for 6 h. Relative gene expression, expressed as crossing point change (ΔCp=Cp for GAPDH−Cp for gene of interest) is presented as a mean (n=11). c) Expression of surface markers (CD64, CD332, MARCO, CD163, CD206, CD11C, CD40, CD80 and CD38) presented as geometric mean fluorescence intensity (MFI) on MDMs exposed for 24 h to PBS or S100A8/A9, measured by flow cytometry (n=5). d) Expression of cytokines (pg·mL−1) in supernatants from MDMs exposed to PBS or S100A8/A9 for 24 h. Data are presented as a mean (n=5). e) Glycolysis stress test: glycolysis, glycolytic capacity and glycolytic reserve in MDMs upon PBS or S100A8/A9 exposure for 24 h, presented as a mean (n=3). Impact of S100A8/A9 calculated using paired t-test; *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001.
We investigated the expression of a spectrum of surface markers to characterise changes in macrophage phenotype upon S100A8/A9 and we found that that CD163, CD40, CD80 and CD38 were significantly upregulated, whereas CD206 and CD11C were not affected (figure 5c). Additionally, the secretion profile of macrophages was considerably modulated upon S100A8/A9 exposure, inducing multiple cytokines (figure 5d). These S100A8/A9-induced changes in macrophage phenotype also correlated with enhanced glycolysis (figure 5e and supplementary figure S8). Taken together, these data show that S100A8/A9 is a potent modulator of macrophage phenotype and function.
WNT signalling is influenced by NTHi and can regulate expression of AMPs in airway epithelial cells
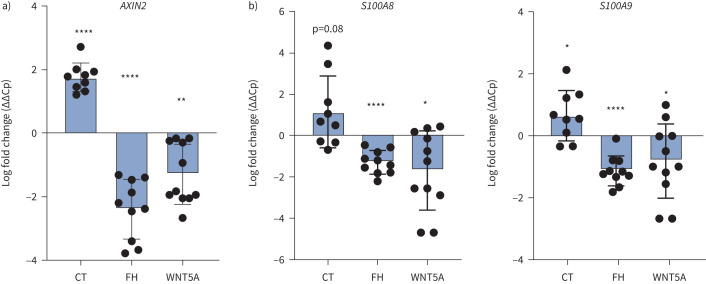
Next, we aimed to understand some of the upstream factors involved in the differential regulation of S100A8/A9 expression in COPD airway epithelium. We performed KEGG pathway analysis on the publicly available datasets from COPD versus non-COPD small airway epithelial cells, which revealed that the WNT signalling pathway is one of the most prominently deregulated pathways in COPD (supplementary figure S9a). Therefore, we focused our attention on whether WNT signalling contributes to the disease-dependent regulation of S100A8/A9. S100A8 and S100A9 promoter analysis revealed several binding sites (TCF/LEF) for β-catenin, a key player of canonical WNT/β-catenin signalling (supplementary figure S9b). Interestingly, we also found that NTHi exposure reduced WNT signalling in COPD-derived SAECs (as shown with AXIN2 target gene expression), while in MDMs we found that it induced the expression of WNT5A (a negative regulator of WNT signalling) (supplementary figure S10). To assess the direct effect of WNT on S100A8 and S100A9 we exposed SAECs to WNT/β-catenin modulators, WNT inducer Chir99021 and WNT inhibitors WNT5A or FH535 (reportedly also an antagonist of PPAR [19]). We confirmed that Chir99021 significantly increased, whereas WNT5A and FH535 significantly reduced, the expression of AXIN2 (WNT/β-catenin target gene) in epithelial cells (figure 6a). Importantly, we found that both S100A8 and S100A9 were regulated along with the WNT signal activity; WNT inducer Chir99021 enhanced their expression whereas WNT inhibitors WNT5A and FH535 attenuated the expression of these AMPs (figure 6b).
FIGURE 6.
WNT signalling regulates expression of antimicrobial peptides (AMPs) in small airway epithelial cells. mRNA expression of a) AXIN2 and b) S100A8 and S100A9 in small airway epithelial cells exposed to WNT inducer Chir99021 (CT) or WNT inhibitors FH535 (FH) or WNT5A for 24 h, relative to vehicle-exposed basal cells. Gene expression comparative ΔΔCp is presented as a mean (n=9–14). The impact of CT, WNT5A and FH was calculated using a paired t-test; *: p<0.05; **: p<0.01; ****: p<0.0001.
Taken together, these results demonstrate that NTHi may attenuate WNT/β-catenin signalling in epithelial cells, both as a direct effect on epithelium and indirectly by inducing expression of the negative WNT pathway regulator WNT5A in macrophages. Moreover, we demonstrate that WNT/β-catenin signalling activity directly impacts expression of S100A8 and S100A9 in lung epithelial cells. Therefore, decreased WNT/β-catenin signalling is likely contributing to impaired expression of S100A8 and S100A9 in lung epithelium.
Discussion
In this study we show for the first time that upon exposure to NTHi, SAECs from COPD patients elicit a delayed AMP response that is associated with impaired macrophage-mediated phagocytosis. We further identify BD2 and S100A8/A9 as airway epithelial-derived AMPs that can enhance phagocytic capacity in human macrophages, and we show that S100A8/A9 is a potent modulator of macrophage phenotype and function. Our data point to aberrant WNT/β-catenin signalling as a potential contributing mechanism of delayed AMP expression by COPD airway epithelial cells upon NTHi exposure. Overall, our study identifies a role for AMPs in regulating the interplay between microbial factors, lung epithelium and macrophage phagocytic activity.
Phagocytosis is the key mechanism used by macrophages to control microbial populations at barrier surfaces. Phagocytic uptake of H. influenzae and S. pneumoniae is impaired in alveolar macrophages, and MDMs in COPD patients [20, 21] and macrophages from some COPD patients exert further impairment in phagocytosis during COPD exacerbation [22]. While multiple factors can be attributed to altered macrophage phenotypes and impaired phagocytic capacity in COPD, aberrant cues from the diseased airway epithelium could play a prominent role given the importance of coordinated macrophage–epithelial crosstalk during bacterial exacerbations. Our data demonstrate that upon exposure to microbial products, secretions from COPD SAECs were inferior in their ability to support MDM phagocytosis. This effect was observed with secretions collected after 3 h, but was diminished after 24 h. These data suggest that healthy airway epithelium can rapidly respond to acute microbial exposure, but this process is delayed in COPD epithelium, resulting in suboptimal support of macrophage phagocytosis. Of note, in our studies we utilised MDMs derived from healthy individuals. Owing to the importance of infiltrating monocytes/macrophages from the periphery for respiratory bacterial clearance [23], and the physical proximity of macrophages to the epithelium, we concluded that MDMs represent a relevant macrophage population for our studies. The possible effects of airway epithelium-derived factors on lung-resident alveolar macrophages would be of interest for future investigation.
Because our primary objective was the comparison of non-COPD and COPD airway epithelium, we intended to work with a primary macrophage population that was most likely to faithfully and reproducibly respond to epithelium-derived factors; therefore, we decided to utilise macrophages derived from healthy individuals. However, it would be an interesting future direction to also assess the influence of the epithelium on macrophages obtained from COPD patients because those patient-derived macrophages may elicit functional properties that are biased by the pre-exposure to stressors like smoke or NTHi.
Our data are consistent with previous mouse studies that indicated a role of mouse colonic epithelial cells and alveolar type II cell-derived SN in macrophage phagocytosis [24, 25] and also are in line with previous reports that expression of the NTHi-stimulated bronchial epithelia AMPs S100A7 and BD2 is reduced in COPD patient cells [9].
Furthermore, we have shown not only that NTHi-induced AMP secretion is delayed in COPD small airway epithelium, but also that S100A8/A9 and BD2 may directly influence the phagocytic capacity of human MDMs. A study conducted with the THP-1 cell line reported that BD2 could enhance engulfment of P. aeruginosa as well as formation of the phagolysosome [16]. Interestingly, levels of BD2 are reduced in the sputum of COPD patients and are inversely correlated with exacerbation frequency [26]. Moreover, BD2 mRNA expression has been shown to be lower in the central airways of COPD patients and positively correlated with forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ratios [8]. These reports, in combination with our data, suggest a role for BD2 in the pathogenesis of COPD exacerbations.
We have shown, for the first time, that the S100A8/A9 heterodimer can enhance phagocytosis in MDMs. A reciprocal relationship between S100A8/A9, microbial exposure and lung epithelium repair has previously been postulated, because deficiency in S100A8/A9 in mice promotes the progression of pneumonia caused by S. aureus infection [27] and treatment with recombinant heterodimer reduces lipopolysaccharide-induced lung injury in vivo [28]. S100A8/A9 was reported to regulate the migration of phagocytes into alveoli in experimental pneumonia [29] and to control polymerisation of tubulin in phagocytes [30]. Even though both S100A8 and S100A9 were shown to be increased in bronchoalveolar lavage fluid of COPD patients, heterodimer S100A8/A9 formation was not assessed [31].
We have further demonstrated that S100A8/A9 has the capacity to alter macrophage phenotype and metabolism. To our knowledge, this is the first in-depth investigation of several aspects of the influence of S100A8/A9 on human macrophages, including protein phosphorylation status, expression of surface markers, phagocytosis-associated receptors, secretion profile and metabolic activity. We have shown that several proteins are phosphorylated upon S100A8/A9 exposure, including JNK [17], c-JUN [18], AKT and p70S6 [19], all signalling pathways previously linked to phagocytosis.
Multiple groups of receptors are engaged in phagocytosis, including opsonic receptors Fc (e.g. CD32 and CD64) and non-opsonic receptors, (e.g. scavenger receptor MARCO and TLR receptors) [32]. Here, we have shown that S100A8/A9 directly upregulates the expression of CD32, CD64, MARCO and TLR2 receptors in MDMs. Interestingly, it has been shown that MARCO is downregulated in COPD, and that its restoration upregulates phagocytosis in COPD macrophages [33]. Similarly, TLR2, which binds lipopeptides from H. influenzae and S. pneumoniae, is decreased in COPD macrophages [34]. We have also shown that S100A8/A9 alters the expression of surface markers, consistent with changed MDM polarisation. In addition, we found that the secretion profile of S100A8/A9-exposed macrophages also changed, including a number of cytokines previously associated with phagocytosis, e.g. interleukin (IL)-1β, interferon-γ, tumour necrosis factor-α [35], IL-6 and IL-10 [36]. The secretions from S100A8/A9-treated MDMs could be attributed to both M1 and M2 polarisation and thus exert both pro- and anti-inflammatory properties. We found that S100A8/A9 also enhanced the glycolytic activity in macrophages, consistent with a metabolism preference that would be expected in the context of M1-biased polarisation [37].
Analysis of upstream pathways potentially regulating S100A8/A9 expression revealed WNT signalling as one of the most deregulated pathways in COPD epithelial cells, confirming previous reports [38]. Furthermore, a link between WNT/β-catenin activity and AMP expression has been established in other cell types [39]. Therefore, we explored the hypothesis that the WNT/β-catenin pathway may regulate the expression of AMPs in airway epithelial cells. We found several binding sites for β-catenin in the promoter for S100A8 and S100A9. We have also shown for the first time that WNT/β-catenin signal activity directly influences AMP expression in primary human small airway epithelium. While literature evidence highlights the contribution of several signalling pathways and external factors, including cigarette smoke, to the regulation of AMP expression [9], there are a number of studies linking WNT signal activity to phagocytosis [40] and AMP production [39, 40]. Thus, it is tempting to speculate that therapeutic modulation of WNT/β-catenin signalling could be explored as an approach for pharmacological calibration of macrophage phagocytic activity.
In conclusion, we have shown that COPD small airway epithelium exhibits a delayed AMP induction response to NTHi compared to non-COPD epithelium and this corresponds to a significantly lower impact of COPD epithelial secretions on macrophage phagocytosis. We have identified epithelial AMPs, BD2 and S100A8/A9 as having a positive impact on MDM phagocytic capacity. Moreover, our comprehensive analysis of the influence of the S100A8/A9 heterodimer has revealed its role in regulating macrophage polarisation and metabolism, as well its own regulation by WNT/β-catenin signalling, a prominent deregulated pathway in COPD airway epithelium. Further studies addressing the potential utility of S100A8/A9 to improve the function of macrophages in COPD as well as affecting other pathological features of the disease could therefore provide new therapeutic avenues for COPD.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figures ERJ-02732-2020_Supplementary_figures (1.1MB, pdf)
Supplementary methods ERJ-02732-2020_Supplementary_materials (208.8KB, pdf)
Shareable PDF
Acknowledgement
The authors are grateful to Cornelia Tilp and Jochen Blender (Immunology and Respiratory Disease Research, Boehringer Ingelheim) for their help with the generation of heat-inactivated NTHi as well as Bernd Guiliard for his help with ALI cultures and Jennifer Schuett (Immunology and Respiratory Disease Research, Boehringer Ingelheim) for performing MSD measurements. The authors thank Birgit Stierstorfer and Nadine Rehm (Drug Discovery Sciences, Boehringer Ingelheim) for providing immunohistochemistry stainings.
Footnotes
This article has supplementary material available from erj.ersjournals.com
Author contributions: W. Skronska-Wasek and S. Pflanz conceived the project. W. Skronska-Wasek, K. Kitt, J.P. Garnett and S. Pflanz were involved in the experimental design. W. Skronska-Wasek, S. Durlanik, H.Q. Le and V. Schroeder performed the experiments. W. Skronska-Wasek analysed the data. W. Skronska-Wasek and S. Pflanz drafted the manuscript. All authors participated in writing or editing the paper.
Conflict of interest: W. Skronska-Wasek has nothing to disclose.
Conflict of interest: S. Durlanik has nothing to disclose.
Conflict of interest: H.Q. Le has nothing to disclose.
Conflict of interest: V. Schroeder has nothing to disclose.
Conflict of interest: K. Kitt has nothing to disclose.
Conflict of interest: J.P. Garnett has nothing to disclose.
Conflict of interest: S. Pflanz has nothing to disclose.
References
- 1.Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology 2016; 21: 14–23. doi: 10.1111/resp.12660 [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2016; 138: 16–27. doi: 10.1016/j.jaci.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 3.Patel IS, Seemungal TA, Wilks M, et al. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002; 57: 759–764. doi: 10.1136/thorax.57.9.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J 2004; 23: 327–333. doi: 10.1183/09031936.03.00098803 [DOI] [PubMed] [Google Scholar]
- 5.Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest 2002; 109: 693–697. doi: 10.1172/JCI0215218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi KY, Chow LN, Mookherjee N. Cationic host defence peptides: multifaceted role in immune modulation and inflammation. J Innate Immun 2012; 4: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals (Basel) 2013; 6: 1543–1575. doi: 10.3390/ph6121543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pace E, Ferraro M, Minervini MI, et al. β-defensin 2 is reduced in central but not in distal airways of smoker COPD patients. PLoS One 2012; 7: e33601. doi: 10.1371/journal.pone.0033601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amatngalim GD, Schrumpf JA, Henic A, et al. Antibacterial defense of human airway epithelial cells from chronic obstructive pulmonary disease patients induced by acute exposure to nontypeable haemophilus influenzae: modulation by cigarette smoke. J Innate Immun 2017; 9: 359–374. doi: 10.1159/000455193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rytila P, Plataki M, Bucchieri F, et al. Airway neutrophilia in COPD is not associated with increased neutrophil survival. Eur Respir J 2006; 28: 1163–1169. doi: 10.1183/09031936.00149005 [DOI] [PubMed] [Google Scholar]
- 11.Berenson CS, Kruzel RL, Eberhardt E, et al. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis 2013; 208: 2036–2045. doi: 10.1093/infdis/jit400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan M, van der Does AM, Tang X, et al. Antimicrobial peptide LL-37 promotes bacterial phagocytosis by human macrophages. J Leukoc Biol 2014; 95: 971–981. doi: 10.1189/jlb.0513304 [DOI] [PubMed] [Google Scholar]
- 13.Soehnlein O, Kai-Larsen Y, Frithiof R, et al. Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest 2008; 118: 3491–3502. doi: 10.1172/JCI35740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogl T, Stratis A, Wixler V, et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J Clin Invest 2018; 128: 1852–1866. doi: 10.1172/JCI89867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Li D, Wang Y, et al. β-defensin 2 and 3 promote bacterial clearance of Pseudomonas aeruginosa by inhibiting macrophage autophagy through downregulation of early growth response gene-1 and c-FOS. Front Immunol 2018; 9: 211. doi: 10.3389/fimmu.2018.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang L, Wu HM, Ding PS, et al. TLR2 mediates phagocytosis and autophagy through JNK signaling pathway in Staphylococcus aureus-stimulated RAW264.7 cells. Cell Signal 2014; 26: 806–814. doi: 10.1016/j.cellsig.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 17.Macdonald JM, Doherty J, Hackett R, et al. The c-Jun kinase signaling cascade promotes glial engulfment activity through activation of draper and phagocytic function. Cell Death Differ 2013; 20: 1140–1148. doi: 10.1038/cdd.2013.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganesan LP, Wei G, Pengal RA, et al. The serine/threonine kinase Akt Promotes Fcγ receptor-mediated phagocytosis in murine macrophages through the activation of p70S6 kinase. J Biol Chem 2004; 279: 54416–54425. doi: 10.1074/jbc.M408188200 [DOI] [PubMed] [Google Scholar]
- 19.Berenson CS, Garlipp MA, Grove LJ, et al. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis 2006; 194: 1375–1384. doi: 10.1086/508428 [DOI] [PubMed] [Google Scholar]
- 20.Handeli S, Simon JA. A small-molecule inhibitor of Tcf/β-catenin signaling down-regulates PPARγ and PPARλ activities. Mol Cancer Ther 2008; 7: 521–529. doi: 10.1158/1535-7163.MCT-07-2063 [DOI] [PubMed] [Google Scholar]
- 21.Taylor AE, Finney-Hayward TK, Quint JK, et al. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J 2010; 35: 1039–1047. doi: 10.1183/09031936.00036709 [DOI] [PubMed] [Google Scholar]
- 22.Singh R, Such K, Kowlessar B, et al. Macrophage phagocytosis in COPD patients at exacerbation compared to stable state. Thorax 2013; 68: A159. [Google Scholar]
- 23.Mould KJ, Barthel L, Mohning MP, et al. Cell origin dictates programming of resident versus recruited macrophages during acute lung injury. Am J Respir Cell Mol Biol 2017; 57: 294–306. doi: 10.1165/rcmb.2017-0061OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristek M, Collins LE, DeCourcey J, et al. Soluble factors from colonic epithelial cells contribute to gut homeostasis by modulating macrophage phenotype. Innate Immun 2015; 21: 358–369. doi: 10.1177/1753425914538294 [DOI] [PubMed] [Google Scholar]
- 25.Chuquimia OD, Petursdottir DH, Periolo N, et al. Alveolar epithelial cells are critical in protection of the respiratory tract by secretion of factors able to modulate the activity of pulmonary macrophages and directly control bacterial growth. Infect Immun 2013; 81: 381–389. doi: 10.1128/IAI.00950-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCrae CZ, Qian J, Zheng J, et al. Human β-defensin 2 levels are reduced in the sputum of chronic obstructive pulmonary disease (COPD) patients and are associated with the occurrence of exacerbations. Am J Respir Crit Care Med 2016; 193: A6334. [Google Scholar]
- 27.Achouiti A, Vogl T, Van der Meer AJ, et al. Myeloid-related protein-14 deficiency promotes inflammation in staphylococcal pneumonia. Eur Respir J 2015; 46: 464–473. doi: 10.1183/09031936.00183814 [DOI] [PubMed] [Google Scholar]
- 28.Hiroshima Y, Hsu K, Tedla N, et al. S100A8/A9 and S100A9 reduce acute lung injury. Immunol Cell Biol 2017; 95: 461–472. doi: 10.1038/icb.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raquil MA, Anceriz N, Rouleau P, et al. Blockade of antimicrobial proteins S100A8 and S100A9 inhibits phagocyte migration to the alveoli in streptococcal pneumonia. J Immunol 2008; 180: 3366–3374. doi: 10.4049/jimmunol.180.5.3366 [DOI] [PubMed] [Google Scholar]
- 30.Vogl T, Ludwig S, Goebeler M, et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood 2004; 104: 4260–4268. doi: 10.1182/blood-2004-02-0446 [DOI] [PubMed] [Google Scholar]
- 31.Merkel D, Rist W, Seither P, et al. Proteomic study of human bronchoalveolar lavage fluids from smokers with chronic obstructive pulmonary disease by combining surface-enhanced laser desorption/ionization-mass spectrometry profiling with mass spectrometric protein identification. Proteomics 2005; 5: 2972–2980. doi: 10.1002/pmic.200401180 [DOI] [PubMed] [Google Scholar]
- 32.Freeman SA, Grinstein S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev 2014; 262: 193–215. doi: 10.1111/imr.12212 [DOI] [PubMed] [Google Scholar]
- 33.Harvey CJ, Thimmulappa RK, Sethi S, et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med 2011; 3: 78ra32. doi: 10.1126/scitranslmed.3002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Droemann D, Goldmann T, Tiedje T, et al. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res 2005; 6: 68. doi: 10.1186/1465-9921-6-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pechkovsky DV, Potapnev MP, Zalutskaya OM. Different patterns of cytokine regulation of phagocytosis and bacterial killing by human neutrophils. Int J Antimicrob Agents 1996; 7: 33–40. doi: 10.1016/0924-8579(96)00007-6 [DOI] [PubMed] [Google Scholar]
- 36.Kumakura S, Yamaguchi Y, Murakawa Y, et al. Effect of cytokines and hyperthermia on phagocytosis and phosphatidylserine externalization: implication for the pathophysiology of hemophagocytic syndrome. Ann Clin Lab Sci 2018; 48: 314–322. [PubMed] [Google Scholar]
- 37.Van den Bossche J, Baardman J, de Winther MP. Metabolic characterization of polarized M1 and M2 bone marrow-derived macrophages using real-time extracellular flux analysis. J Vis Exp 2015; 105: 53424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang R, Ahmed J, Wang G, et al. Down-regulation of the canonical Wnt β-catenin pathway in the airway epithelium of healthy smokers and smokers with COPD. PLoS One 2011; 6: e14793. doi: 10.1371/journal.pone.0014793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benakanakere MR, Zhao J, Galicia JC, et al. Sphingosine kinase-1 is required for toll mediated β-defensin 2 induction in human oral keratinocytes. PLoS One 2010; 5: e11512. doi: 10.1371/journal.pone.0011512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen K, Fu Q, Li D, et al. Wnt3a suppresses Pseudomonas aeruginosa-induced inflammation and promotes bacterial killing in macrophages. Mol Med Rep 2016; 13: 2439–2446. doi: 10.3892/mmr.2016.4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figures ERJ-02732-2020_Supplementary_figures (1.1MB, pdf)
Supplementary methods ERJ-02732-2020_Supplementary_materials (208.8KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02732-2020.Shareable (284.7KB, pdf)