Summary
Background
There are concerns that the use of non-steroidal anti-inflammatory drugs (NSAIDs) may increase the risk of adverse outcomes among patients with coronavirus COVID-19. This study aimed to synthesize the evidence on associations between the use of NSAIDs and adverse outcomes.
Methods
A systematic search of WHO COVID-19 Database, Medline, the Cochrane Library, Web of Science, Embase, China Biology Medicine disc, China National Knowledge Infrastructure, and Wanfang Database for all articles published from January 1, 2020, to November 7, 2021, as well as a supplementary search of Google Scholar. We included all comparative studies that enrolled patients who took NSAIDs during the COVID-19 pandemic. Data extraction and quality assessment of methodology of included studies were completed by two reviewers independently. We conducted a meta-analysis on the main adverse outcomes, as well as selected subgroup analyses stratified by the type of NSAID and population (both positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or not).
Findings
Forty comparative studies evaluating 4,867,795 adult cases were identified. Twenty-eight (70%) of the included studies enrolled patients positive to SARS-CoV-2 tests. The use of NSAIDs did not reduce mortality outcomes among people with COVID-19 (number of studies [N] = 29, odds ratio [OR] = 0.93, 95% confidence interval [CI]: 0.75 to 1.14, I2 = 89%). Results suggested that the use of NSAIDs was not significantly associated with higher risk of SARS-CoV-2 infection in patients with or without COVID-19 (N = 10, OR = 0.96, 95% CI: 0.86 to 1.07, I2 = 78%; N = 8, aOR = 1.01, 95% CI: 0.94 to 1.09, I2 = 26%), or an increased probability of intensive care unit (ICU) admission (N = 12, OR = 1.28, 95% CI: 0.94 to 1.75, I2 = 82% ; N = 4, aOR = 0.89, 95% CI: 0.65 to 1.22, I2 = 60%), requiring mechanical ventilation (N = 11, OR = 1.11, 95% CI: 0.79 to 1.54, I2 = 63%; N = 5, aOR = 0.80, 95% CI: 0.52 to 1.24, I2 = 66%), or administration of supplemental oxygen (N = 5, OR = 0.80, 95% CI: 0.52 to 1.24, I2 = 63%; N = 2, aOR = 1.00, 95% CI: 0.89 to 1.12, I2 = 0%). The subgroup analysis revealed that, compared with patients not using any NSAIDs, the use of ibuprofen (N = 5, OR = 1.09, 95% CI: 0.50 to 2.39; N = 4, aOR = 0.95, 95% CI: 0.78 to 1.16) and COX-2 inhibitor (N = 4, OR = 0.62, 95% CI: 0.35 to 1.11; N = 2, aOR = 0.73, 95% CI: 0.45 to 1.18) were not associated with an increased risk of death.
Interpretation
Data suggests that NSAIDs such as ibuprofen, aspirin and COX-2 inhibitor, can be used safely among patients positive to SARS-CoV-2. However, for some of the analyses the number of studies were limited and the quality of evidence was overall low, therefore more research is needed to corroborate these findings.
Funding
There was no funding source for this study.
Keywords: NSAIDs, COVID-19, Systematic review, Meta-analysis
Research in context.
Evidence before this study
We searched seven databases and resources from January 1, 2020, through November 7, 2021, with no restriction by language, for any systematic reviews comparing the clinical adverse outcomes between patients receiving NSAIDs. We used database-specific combinations of the following index terms and phrases: COVID-19, SARS-CoV-2, Coronavirus disease-19, 2019-novel coronavirus, 2019-nCoV, Nonsteroidal Anti-Inflammatory, Non-Steroidal Anti-Inflammatory, NSAID*, Antipyretic*, Ibuprofen*, Aspirin, Acetaminophen, and their derivatives. Previous related meta-analyses have only focused on a subset of NSAIDs and included indirect evidence on middle east respiratory syndrome-related coronavirus (MERS) and severe acute respiratory syndrome (SARS) and non-peer-reviewed preprints. Other meta-analyses did not perform subgroup analyses, nor did they grade the quality of evidence for their findings.
Added value of this study
We did a comprehensive systematic review and meta-analysis of forty observational studies across 14 countries and five continents. Our findings suggest that NSAIDs such as ibuprofen, aspirin, and COX-2 inhibitor can be used safely during the COVID-19 pandemic. The use of NSAIDs was not significantly associated with higher risk of SARS-CoV-2 infection, or an increased probability of intensive care unit (ICU) admission, requiring mechanical ventilation, or administration of supplemental oxygen.
Implications of all the available evidence
Our findings suggest that NSAIDs can be used safely in patients to relieve pain, inflammation, and fever during the COVID-19 pandemic. For future studies, there is a lack of high-quality multicenter cohort studies with a large sample size indicating NSAIDs' impact on the quality of life and long-term survival.
Alt-text: Unlabelled box
Introduction
Since December 2019, a new infectious disease, coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2, has swept across the world and brought huge challenges to the public health and medical service systems worldwide.1 Current evidence suggests that fever is one of the main clinical symptoms of COVID-19, with 88.7% of hospitalized adults and 63.3% of hospitalized children with COVID-19 presenting fever. Therefore, symptomatic treatment with non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen is often used in COVID-19 patients.2, 3, 4 However, a study published on March 11, 2020, questioned the safety of ibuprofen in the treatment of COVID-19, suggesting that SARS-CoV-2 could invade human cells by combining with Angiotensin-Converting Enzyme 2 (ACE2), with the goal of infestation. Ibuprofen can strengthen the binding ability of ACE2 and SARS-CoV-2 and enhance the infection process of the virus. The possibility that ibuprofen use can deteriorate the course of COVID-19 should thus not be excluded, and ibuprofen should be used with caution in patients during the pandemic.5
Based on the above research results, Olivier Véran, the Minister of Solidarity and Health of France issued a warning via social media on 14 March 2020, stating that "the use of anti-inflammatory drugs (ibuprofen, cortisone, etc.) may aggravate the course of COVID-19, and paracetamol is recommended if fever symptoms occur".6 On March 17, 2020, the World Health Organization (WHO) also issued a statement that "it is recommended that patients with COVID-19 avoid taking ibuprofen".7 However, at the same time, the European Medicines Agency (EMA) issued a statement that there is no scientific evidence of an association between ibuprofen and COVID-19 deterioration. The EMA further noted that they would closely monitor the situation and review any new information.8 In France, the issuance of the above warnings led to an 80% drop in the prescription rate of ibuprofen in general.9
The evidence on the impact of ibuprofen and other NSAIDs for patients with COVID-19 is still controversial. The first prospective cohort study of ibuprofen and other NSAIDs for treating adults with COVID-19 showed that among 503 adults with confirmed SARS-CoV-2 infection, the use of ibuprofen during the acute phase was not associated with the risk of death (hazard ratio [HR]=0.63, 95% CI: 0.07 to 5.44) or the risk of hospital admission (OR = 1.27, 95% CI: 0.55 to 2.95), compared with no ibuprofen use. Long-term use of NSAIDs was also not found to be associated with a higher risk of death (HR=0.49, 95% CI: 0.18 to 1.36).10 However, another retrospective cohort study from South Korea showed that among 1824 hospitalized adult patients with COVID-19, there was an increased risk of adverse outcomes in patients who used NSAIDs (OR=1.54, 95% CI: 1.13 to 2.11) compared with patients who did not use NSAIDs (n = 87).11 Currently, the research results on whether NSAIDs can be safely used in patients with COVID-19 or not are inconsistent. To answer this question, we included all comparative studies which enrolled patients that took NSAIDs during the COVID-19 pandemic, to conducted this comprehensive systematic review and meta-analysis to explore the association between the use of NSAIDs and adverse outcomes among patients during the COVID-19 pandemic.
Methods
Our systematic review and meta-analysis were performed in accordance with the Cochrane Handbook.12 We report the results in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.13
Data sources and searches
We performed a systematic literature search of the WHO COVID-19 Database, Medline (via PubMed), The Cochrane Library, Web of Science (WOS), China Biology Medicine disc (CBM), China National Knowledge Infrastructure (CNKI), and Wanfang Database for studies published from January 1, 2020, through November 7, 2021. We used database-specific combinations of the following index terms and phrases: COVID-19, SARS-CoV-2, Coronavirus disease-19, 2019-novel coronavirus, 2019-nCoV, Nonsteroidal Anti-Inflammatory, Non-Steroidal Anti-Inflammatory, NSAID*, Antipyretic*, Ibuprofen*, Aspirin, Acetaminophen, and their derivatives. Supplementary searches were conducted on Google Scholar (https://scholar.google.nl/). Finally, we reviewed the references from the included articles manually to identify any missed potentially relevant records. The inclusion of studies was not restricted by the publication status or language. An information retrieval specialist helped to develop the search strategy. Details of the search strategies are available in eTable 1 in Supplementary.
Inclusion and exclusion criteria
We included all comparative studies in comparing the clinical adverse outcomes during the COVID-19 pandemic (in the time period starting from the end of 2019) between patients receiving and not receiving any NSAIDs. All included studies must be conducted during the COVID-19 pandemic, but the patients could positive or negative to SARS-CoV-2 test. We made no restrictions on age, gender, ethnicity, region, other individual factors, or the COVID-19 status.
We excluded multiple publications on the same population. Studies reporting insufficient details were also excluded unless we were able to retrieve the original data.
Study selection process
Study selection was conducted independently by two investigators. For this purpose, four investigators were divided into two groups (Siya Zhao and Shuai Peng; Zhili Wang and Lidan Gan), and the records were split randomly between these groups. The retrieval consisted of three phases. In phase one, we screened titles and abstracts of search results to exclude literature that obviously did not meet the inclusion and exclusion criteria. In phase two, full-text articles were obtained for articles identified by one or both investigators as potentially relevant. The full texts of eligible articles were reviewed independently by the same two investigators. In phase three, any disagreements in the decision to include or exclude the study were adjudicated through discussion or consultation with a third investigator (Qi Zhou).
Data extraction
Two investigators (Siya Zhao and Lidan Gan) independently extracted data from the included studies using a standardized Microsoft Excel collection form. Disagreements were resolved through discussion or consultation with a third investigator (Qi Zhou) if necessary. The following information was extracted: (1) Basic information: the first author, year of publication, country of origin, sample size, types of study design and complications; (2) Characteristics of the exposure: types of NSAIDs taken; (3) Information on outcomes: mortality, probability of SARS-CoV-2 infection, probability of ICU admission rate, probability of machine ventilation, probability of administration of respiratory support, a composite risk of adverse outcomes (defined as having at least one of the following: requirement for supplemental oxygen, mechanical ventilation, sepsis, ICU admission or death). Mortality was the primary outcome of our study. If sufficient data on characteristics or outcomes were not available, we contacted the authors of studies by email to request them or calculated from other reported data according to methods recommended by the Cochrane Handbook, if available.
Assessment of the methodological quality
Two investigators (Qi Zhou and Siya Zhao) independently assessed the risk of bias in each study included in the systematic review. We used the Newcastle-Ottawa Scale to assess the methodological quality.14 The tool consists of eight items: representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, the demonstration that outcome of interest was not present at the start of study, comparability of cohorts on the basis of the design or analysis, assessment of outcome, sufficient follow-up for outcomes to occur, and adequacy of follow up of cohorts. Each item was rated as either none, one point or two points. We resolved disagreements by discussion or by consultation with another investigator (Yaolong Chen).
Assessment of the certainty of the evidence
We assessed the certainty of the evidence with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach15,16 for all outcomes. We rated the certainty of the evidence for each outcome based on considerations of five factors that may lead to rating down the quality of evidence (risk of bias, inconsistency, imprecision, indirectness, and publication bias), and three factors that may lead to rating up the quality of evidence (dose-response gradient, large magnitude of effect and plausible confounders or biases). The quality of evidence is initially assigned as high for randomized controlled trials (RCTs) without serious defects in methodology, and low for observational studies, after which the quality is gradually up- or downgraded based on the eight factors mentioned before. The quality of the evidence is finally rated as 'high', 'moderate', 'low' or 'very low'. We performed the assessment using the GRADEpro software and generated a summary of findings (SoF) table.17,18
Data analysis
I² statistic was used to quantify the amount of heterogeneity, with I² of greater than 50% representing substantial heterogeneity.19 We did our data analyses with RevMan 5.4 software and STATA15.0 (StataCorp, College Station, Texas). We used a random effects model and calculated odds ratios (OR) with 95% confidence intervals (CI) for dichotomous outcomes, and mean differences (MD) with 95% CI for continuous outcomes.20 We present the pooled results over all studies and performed subgroups analyses by the type of NSAID (ibuprofen vs. aspirin vs. COX-2 inhibitor vs. other drugs ) and population (C+:all patients tested positive for SARS-CoV-2 vs. C±/C-: part or no patients tested positive for SARS-CoV-2). We also performed a meta-analysis of adjusted OR, risk ratio (RR) and HR. If both unadjusted and adjusted ORs were reported from the same study, we extracted both and pooled the unadjusted estimates in the main analysis and the adjusted estimates in the secondary analysis. RR and HR from other studies were regarded as OR. If not available, ORs could be obtained through calculating events and total numbers of patients in two groups. If more than one adjusted ORs were reported, we used the one adjusted for the maximum number of covariables. We also performed a sensitivity analysis to assess the robustness of our findings by excluding one research every analysis. Publication bias was assessed by a funnel plot.21
Role of the funding source
There was no funding source for this study.
Results
Characteristics of the included studies
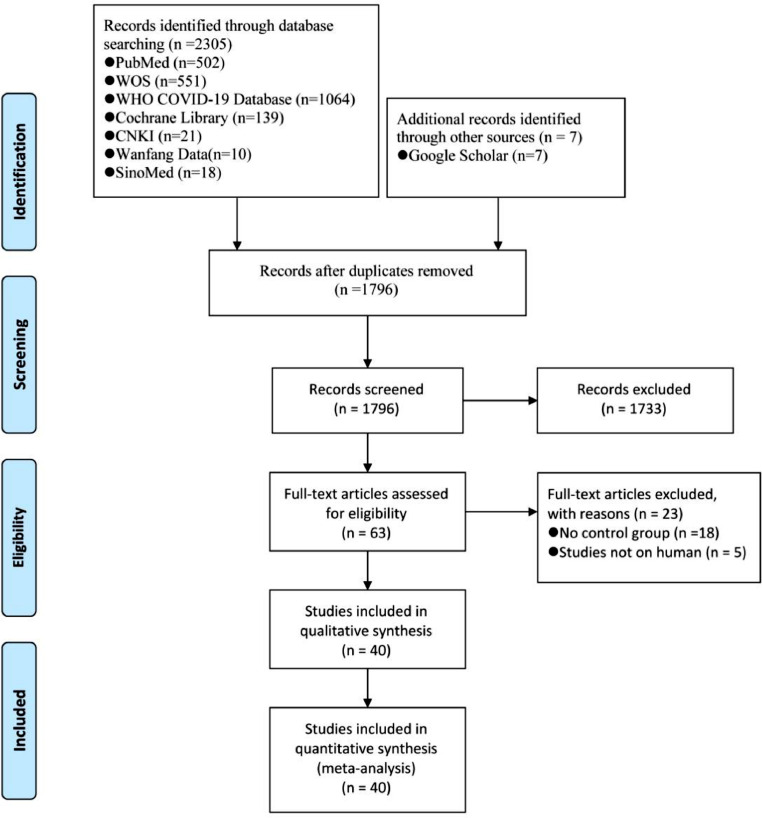
A total of 2312 references were identified in our search. After screening on titles, abstracts and full texts, forty comparative studies involving 4867,795 individuals were included in this review, in which eight are from the United States of America (USA), six are from South Korea, and four are from the United Kingdom (UK) (Figure 1).10,11,22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59 Twenty-eight (70%) of the included studies enrolled patients positive to SARS-CoV-2 tests. All forty studies included only adult cases. Thirty-seven studies were retrospective and the remaining three prospective. Characteristics of the included studies are described in Table 1. Thirty-four (85%) of the included studies did not specify whether the assessment of the outcome was blinded. Moreover, we found a risk of bias in the ascertainment of the exposure in seven studies. Overall, the methodological quality of the included studies was good (eTable 2 in Supplementary).
Figure 1.
Literature search and screening process from: Moher D, Liberati A, Tetzlaff J, Altman D G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement BMJ 2009; 339 :b2535 doi:10.1136/bmj.b2535.
Table 1.
Characteristics of the included studies.
| Study ID | Country | Study design | Population | Type of drugs | Dosage (daily) | Sample size |
Age (year) * |
Male (%) |
Diabetes mellitus (%) |
Hypertension (%) |
COPD-Chronic pulmonary disease (%) |
Cardiovascular disease (%) |
Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | C | E | C | E | C | E | C | E | C | E | C | E | C | |||||||
| Abdelwahab et al., 2021 a | Egypt | RS | C+ | Aspirin | 81–162 mg | 31 | 36 | 56.0 ± 16.1 | 44.0 ± 16.5 | 51.6 | 33.3 | 38.7 | 11.1 | 48.4 | 13.9 | NR | NR | 25.8 | 0 | ④ |
| Abdelwahab et al., 2021 b | Egypt | RS | C+ | Aspirin | 81–162 mg | 35 | 123 | 61.0 ± 14.3 | 58.0 ± 14.7 | 48.6 | 46.3 | 48.6 | 43.1 | 62.9 | 45.5 | NR | NR | 22.9 | 3.3 | ④ |
| Abu Esba et al., 2020 | Saudi Arabia | PS | C+ | Unspecified | NR | 146 | 357 | 47.5(33.0–63.0) | 36.0 (27.0–49.0) | 52.1 | 59.4 | 41.1 | 14.8 | 34.9 | 14.6 | NR | NR | 12.3 | 2.5 | ①⑤⑦ |
| Aghajani et al., 2021 | Iran | RS | C+ | Aspirin | NR | 336 | 655 | 65.8 ± 14.3 | 58.5 ± 17.4 | 56.0 | 54.0 | 47.6 | 21.8 | 62.5 | 30.0 | NR | NR | 40.8 | 8.7 | ① ④ |
| Alamdari et al., 2020 | Iran | RS | C+ | Unspecified | NR | 37 | 422 | 61.8 ± 11.9 | 69.7 | NR | NR | NR | NR | NR | NR | NR | NR | ① | ||
| Argenziano et al., 2020 | USA | RS | C+ | Unspecified | NR | 250 | 750 | 63.0 (50.0–75.0) | 59.6 | 37.2 | 60.1 | 6.6 | NR | NR | ③⑦ | |||||
| Blanch-Rubió et al., 2020 | Spain | RS | C± | Unspecified | NR | 318 | 1784 | 66.4 (13.3) | 19.5 | NR | NR | NR | NR | NR | NR | NR | NR | ② | ||
| Bruce et al., 2020 | UK | RS | C+ | Unspecified | NR | 54 | 1168 | NR | NR | 46.3 | 56.9 | 20.4 | 27.3 | 31.5 | 51.1 | NR | NR | NR | NR | ① |
| Chandan et al., 2021 | UK | RS& | Osteoarthritis | Unspecified | NR | 8595 | 8595 | 68.4 ± 10.4 | 68.1 ± 10.5 | 34.8 | 34.2 | 17.0 | 15.9 | NR | NR | 8.1 | 7.1 | 13.7 | 9.7 | ①② |
| Chow et al., 2021 | USA | RS | C+ | Aspirin | 81 mg | 98 | 314 | 61.0 (55.0–72.0) | 52.0 (37.0–65.0) | 62.2 | 58.3 | 55.1 | 29.0 | 78.6 | 52.5 | NR | NR | 34.7 | 5.7 | ①③④ |
| Costantino et al., 2020 | France | RS | Rheumatic diseases | Unspecified | NR | 318 | 337 | 51.0 ± 13.4 | 38.2 | NR | NR | NR | NR | NR | NR | NR | NR | ② | ||
| Drake et al., 2021 | UK | PS& | C+ | Unspecified | NR | 4211 | 67,968 | 70.1 ± 18.7 | 70.2 ± 18.4 | 46.4 | 46.4 | 21.1 | 21.5 | NR | NR | 18.7 | 16.5 | 35.1 | 30.3 | ①③④⑤ |
| Gianfrancesco et al., 2020 | Australia | RS | RA | Unspecified | NR | 111 | 420 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ⑦ |
| Gupta et al., 2020 | USA | PS | C+ | Unspecified | NR | 191 | 2024 | 60.5 (14.5) | 64.8 | NR | NR | NR | NR | NR | NR | NR | NR | ① | ||
| Hong et al., 2020 | China | PS | C+ | COX-2 inhibitor | 200–400 mg | 36 | 7 | 49.3 ± 14.8 | 49.5 ± 18.3 | 47.2 | 57.1 | 8.3 | 14.2 | 8.3 | 14.2 | NR | NR | 5.6 | 14.2 | ① |
| Huh et al., 2021 | South Korea | RS | C± | Unspecified | NR | 7080 | 36,966 | NR | NR | 59.5 | NR | NR | NR | NR | NR | NR | NR | NR | ② | |
| Hwang et al., 2020 | South Korea | RS | C+ | Unspecified | NR | 5 | 98 | 67.6 ± 15.3 | 50.5 | NR | NR | NR | NR | NR | NR | NR | NR | ① | ||
| Imam et al., 2020 | USA | RS | C+ | Unspecified | NR | 466 | 839 | 61.0 ± 16.3 | 53.8 | NR | NR | NR | NR | NR | NR | NR | NR | ① | ||
| Jehi et al., 2020 | USA | RS | C+ | Unspecified | NR | 892 | 3644 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ⑦ |
| Jeong et al., 2020 | South Korea | RS& | C+ | Unspecified | NR | 354 | 1470 | 54.1 ± 17.6 | 47.8 ± 19.1 | 41.5 | 41.0 | 17.0 | 11.0 | 28.0 | 19.0 | 20.0 | 15.0 | NR | NR | ①③④⑥ |
| Karruli et al., 2021 | Italy | RS | C+ | Aspirin | Low dose | 5 | 27 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ① |
| Kim et al., 2021 a | South Korea | RS& | C± | Aspirin | NR | 136 | 136 | NR | NR | 63.2 | 61.8 | 67.6 | 72.1 | 79.4 | 82.4 | 9.6 | 8.1 | 48.5 | 49.3 | ①②③④⑤⑥ |
| Kim et al., 2021 b | South Korea | RS& | C± | Aspirin | NR | 526 | 526 | NR | NR | 58.0 | 56.8 | 50.8 | 49.8 | 75.5 | 76.2 | 9.7 | 7.2 | 36.3 | 35.0 | ①②③④⑤⑥ |
| Kragholm et al., 2020 | Denmark | RS | C+ | Ibuprofen | NR | 264 | 3738 | 58.0(46–68.0) | 57.0(45.0–73.0) | 44.7 | 47.4 | 13.3 | 11.1 | 24.2 | 21.8 | 6.4 | 5.3 | 2.7 | 2.5 | ⑥ |
| Liu et al., 2021 | China | RS& | C+ | Aspirin | 81 mg | 28 | 204 | 69.5 (61.0–77.0) | 54.0 (42.0–65.0) | 64.3 | 53.4 | 17.9 | 11.3 | 71.4 | 19.6 | 3.6 | 2.5 | NR | NR | ① |
| Lodigiani et al., 2021 | Italy | RS | C+ | Aspirin | NR | 93 | 286 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ①③ |
| Lund et al., 2020 | Denmark | RS& | C+ | Unspecified | NR | 224 | 896 | 54.0(43.0–64.0) | 54.0(41.0–66.0) | 40.2 | 41.9 | NR | NR | NR | NR | 4.0 | 3.9 | 10.3 | 10.2 | ①③④⑦ |
| Mancia et al., 2020 | Italy | RS | C± | Unspecified | NR | 5615 | 31,416 | 68.0 ± 13.0 | 63.0 | NR | NR | NR | NR | NR | NR | NR | NR | ② | ||
| Martínez-Botía et al., 2021 | Spain | RS& | C+ | Unspecified | NR | 366 | 1669 | 66.4 ± 15.8 | 67.3 ± 16.2 | 61.2 | 59.8 | 13.1 | 12.9 | NR | NR | NR | NR | NR | NR | ①③ |
| Meizlish et al., 2021 | USA | RS& | C+ | Aspirin | 81 mg | 964 | 1821 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ① |
| Merzon et al., 2021 | Israel | RS | C± | Aspirin | Low dose | 1621 | 8856 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ①②⑦ |
| Ong et al., 2020 | Singapore | RS | C+ | COX-2 inhibitor | 60–90 mg | 22 | 146 | 56.0 (53.8–61.0) | 62.0 (55.8–68.3) | 50.0 | 56.2 | 18.2 | 31.5 | 31.8 | 50.7 | 0 | 2.7 | 4.5 | 9.6 | ①③④⑤⑥ |
| Osborne et al., 2021 | USA | RS& | C+ | Aspirin | NR | 6300 | 6300 | 67.4 ± 10.8 | 67.3 ± 11.2 | 95.2 | 96.6 | 62.5 | 41.3 | 89.4 | 72.6 | 43.8 | 37.9 | NR | NR | ① |
| Park et al., 2021 | South Korea | RS& | C+ | Unspecified | NR | 397 | 397 | NR | NR | 41.8 | 36.8 | 16.6 | 15.9 | 28.7 | 27.7 | 2.5 | 0.8 | NR | NR | ①④ |
| Reilev et al., 2020 | Denmark | RS | C± | Unspecified | NR | 47,503 | 374,316 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ①②③⑦ |
| Rinott et al., 2020 | Israel | RS | C+ | Ibuprofen | NR | 87 | 316 | 40.0(24.5–64.0) | 46.0(25.0–61.0) | 52.9 | 55.1 | 11.4 | 8.8 | NR | NR | NR | NR | 13.7 | 12.6 | ①③④⑤ |
| Sahai et al., 2021 a | USA | RS& | C+ | Unspecified | NR | 444 | 444 | 58.1 ± 17.0 | 58.2 ± 18.1 | 51.1 | 48.6 | 35.7 | 35.9 | 62.7 | 66.1 | 10.9 | 12.1 | NR | NR | ① |
| Sahai et al., 2021 b | USA | RS& | C+ | Aspirin | 81 mg | 248 | 248 | 68.5 ± 13.6 | 69.5 ± 14.1 | 56.5 | 59.5 | 50.4 | 50 | 84.9 | 85 | 18.7 | 13.6 | NR | NR | ① |
| Son et al., 2021 | South Korea | RS | C± | Aspirin | NR | 844 | 10,631 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ② |
| Vahedian-Azimi et al., 2021 | Iran | RS | C+ | Aspirin | NR | 237 | 350 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ①③ |
| Vila-Corcoles et al., 2020 | Spain | RS | C± | Unspecified | NR | 1650 | 33,286 | NR | NR | 48.1 | 28.1 | NR | NR | NR | NR | NR | NR | ② | ||
| Wong et al., 2021 a | UK | RS | General population | Unspecified | NR | 536,423 | 1,927,284 | 53.0 (42.0–64.0) | 49.0 (36.0–60.0) | 40.8 | 43.3 | 11.0 | 9.0 | 23.9 | 18.4 | 2.9 | 2.2 | NR | NR | ① |
| Wong et al., 2021 b | UK | RS | Rheumatoid arthritis | Unspecified | NR | 175,495 | 1,533,286 | 63.0 (55.0–71.0) | 68.0 (58.0–76.0) | 37.0 | 37.9 | 14.7 | 15.4 | 37.7 | 40.8 | 4.8 | 5.6 | NR | NR | ① |
| Yuan et al., 2021 | China | RS | C+ | Aspirin | 75–150 mg | 52 | 131 | 69.7 ± 1.1 | 71.8 ± 0.9 | 59.6 | 51.9 | 25.0 | 20.6 | 61.5 | 53.4 | 1.9 | 5.3 | NR | NR | |
① Mortality; ② Risk of SARS-CoV-2 infection; ③ ICU admission; ④ Mechanical ventilation; ⑤ Supplemental oxygen; ⑥ Composite adverse outcome; ⑦ Hospital admission; C+: Patients positive to SARS-CoV-2 tests; C±: Partial or none patients positive to SARS-CoV-2 tests; E: Exposure group; C: Control group; NR: Not report; RS: Retrospective study; PS: Prospective study; *: Data are reported as mean ± SD or median (interquartile range); &: Propensity score–matched cohort study.
Mortality
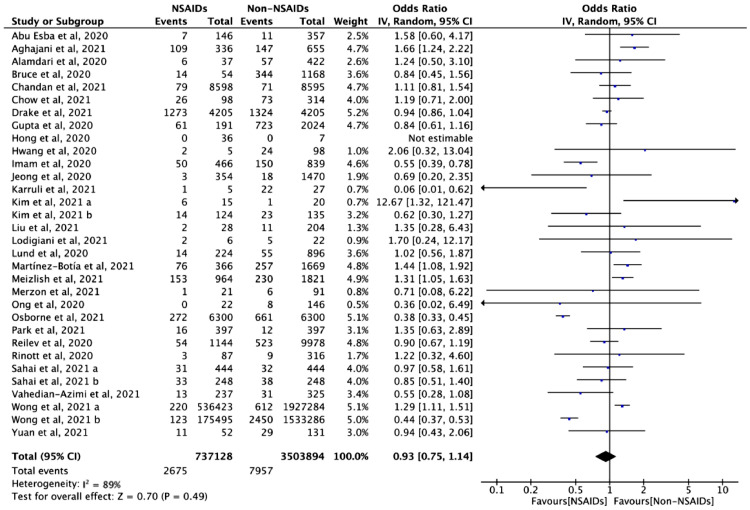
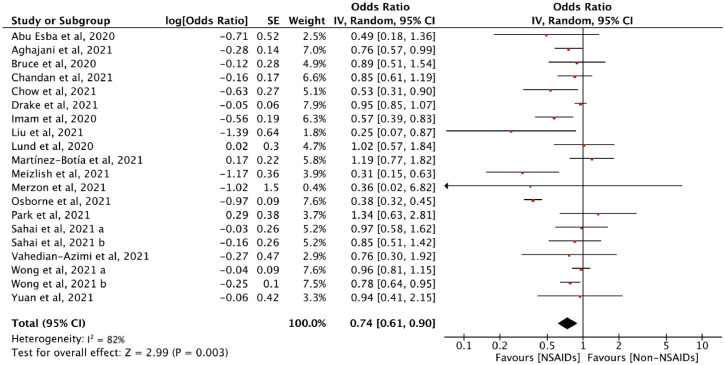
Twenty-nine studies with 4241,022 cases assessed the association between NSAID exposure and mortality in patients who were positive or negative to SARS-CoV-2 tests during the COVID-19 pandemic. The use of NSAIDs did not increase the odds of death (OR = 0.93, 95% CI: 0.75 to 1.14, I2 = 89%) (Figure 2). In the analysis of adjusted estimates, NSAID use was associated with better-adjusted mortality (aOR = 0.74, 95% CI: 0.61 to 0.90, I2 = 82%) (Figure 3).
Figure 2.
The association between NSAID exposure and mortality among patients who were positive or negative to SARS-CoV-2 tests during the COVID-19 pandemic. "Events" is the number of deaths; "Total" is population size; "Weight" is study weight in the analysis. "IV" is inverse variance statistical method of meta-analysis; "Random" is random effects model; "95% CI" is the 95% confidence intervals for the mortality; Each horizontal line in the graphical display represents a study, its width represents 95% CI of the interval estimation of the odds ratio effect of mortality, and the blue midpoint of the line symbolizes the point estimate of the unadjusted odds ratio effect of mortality; "I2″ represents the quantity of heterogeneity (0–100%); "Test for overall effect: Z = 0.70 (P = 0.49)" confirms no statistical difference illustrated by the diamond crossing the line of effect (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Figure 3.
Adjusted odds ratio of the association between NSAID and mortality among patients who were positive or negative to SARS-CoV-2 tests during the COVID-19 pandemic. “Log[Odds Ratio]” is the natural logarithms (In) of odds ratio for each included study; “SE” is the standard error of Log[Odds Ratio]; “Weight” is study weight in the analysis. “IV” is inverse variance statistical method of meta-analysis; “Random” is random effects model; “95% CI” is the 95% confidence intervals for the adjusted mortality; Each horizontal line in the graphical display represents a study, its width represents 95% CI of the interval estimation of the odds ratio effect of adjusted mortality, and the red midpoint of the line symbolizes the point estimate of the adjusted odds ratio effect of mortality; “I2” represents the quantity of heterogeneity (0–100%); “Test for overall effect: Z = 2.99 (P = 0.003)” confirms the statistical difference illustrated by the diamond on the left side of the line of effect (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
The results of subgroup analysis of unadjusted mortality showed that significant associations were not found in the subgroup analyses stratified by the type of NSAID (ibuprofen: OR = 1.09, 95% CI: 0.50 to 2.39; aspirin: OR = 0.93, 95% CI: 0.58 to 1.48; COX-2 inhibitor: OR = 0.62, 95% CI: 0.35 to 1.11) and the population (C+: OR = 0.94, 95% CI: 0.74 to 1.20; C±/C-: OR = 0.91, 95% CI: 0.55 to 1.50). However, subgroup analysis showed that administration of aspirin (aOR = 0.55, 95% CI: 0.40 to 0.78), ibuprofen (aOR = 0.95, 95% CI: 0.78 to 1.16) and COX-2 inhibitor (aOR = 0.73, 95% CI: 0.45 to 1.18) were not statistically different in terms of mortality in patients with COVID-19. The results of the subgroup analysis of the population showed a statistical difference, which means that taking NSAIDs may reduce the mortality among patients positive to SARS-CoV-2 tests (N = 24, OR = 0.94, 95% CI: 0.74 to 1.20, I2=88%; N = 15, aOR=0.72, 95% CI: 0.56 to 0.82, I2=84%). However, the point estimate aORs from different populations (C+, aOR = 0.72, 95% CI: 0.56 to 0.92; C±/C-, aOR=0.87, 95% CI: 0.77 to 0.98) (eFigure 1 in Supplementary) all lied within the 95% CI for the total aOR of the association between NSAID and mortality (aOR = 0.74, 95% CI: 0.61 to 0.90) (Figure 3). Therefore, the adjusted mortality did not differ significantly across patients with or without COVID-19.
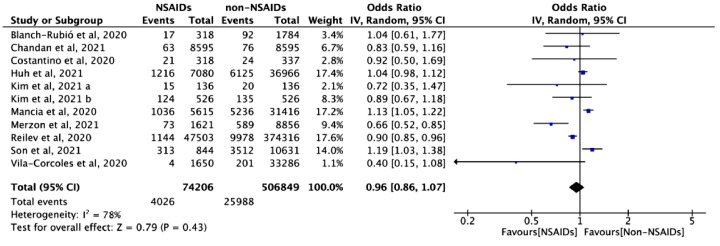
Risk of SARS-CoV-2 infection
Ten studies with 581,055 cases assessed the association between NSAID exposure and the risk of SARS-CoV-2 infection in patients who were negative to SARS-CoV-2 tests during the COVID-19 pandemic. The use of NSAIDs did not increase the odds of SARS-CoV-2 infection (OR = 0.96, 95% CI: 0.86 to 1.07, I2=78%) (Figure 4). Similar results were found in the adjusted analysis (aOR = 1.01, 95% CI: 0.94 to 1.09, I2=26%) (eFigure 2 in Supplementary).
Figure 4.
The association between NSAID exposure and risk of SARS-CoV-2 infection among patients who were negative to SARS-CoV-2 tests during the COVID-19 pandemic. “Events” is the number of SARS-CoV-2 infection; “Total” is population size; “Weight” is study weight in the analysis. “IV” is inverse variance statistical method of meta-analysis; “Random” is random effects model; “95% CI” is the 95% confidence intervals for the risk of SARS-CoV-2 infection; Each horizontal line in the graphical display represents a study, its width represents 95% CI of the interval estimation of the odds ratio effect of risk of SARS-CoV-2 infection, and the blue midpoint of the line symbolizes the point estimate of the unadjusted odds ratio effect of risk of SARS-CoV-2 infection; “I2” represents the quantity of heterogeneity (0–100%); “Test for overall effect: Z = 0.79 (P = 0.43)” confirms no statistical difference illustrated by the diamond crossing the line of effect (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
ICU admission
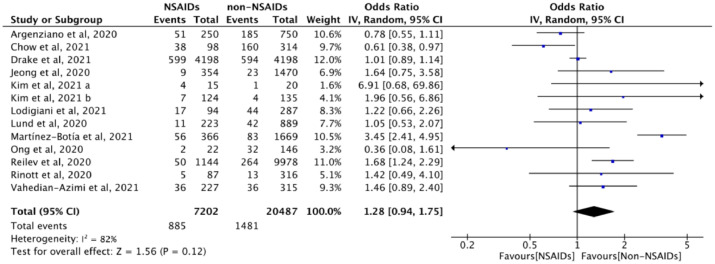
Twelve studies (n = 27,689) evaluated the association between NSAID exposure and ICU admission in patients who were positive or negative to SARS-CoV-2 tests during COVID-19 pandemic. The use of NSAIDs in patients was not associated with the probability of ICU admission (OR = 1.28, 95% CI: 0.94 to 1.75, I2 = 82%) (Figure 5). Similar results were found in the adjusted analysis (aOR = 0.89, 95% CI: 0.65 to 1.22, I2 = 60%) (eFigure 2 in Supplementary).
Figure 5.
The association between NSAID exposure and ICU admission among patients who were positive or negative to SARS-CoV-2 tests during the COVID-19 pandemic. “Events” is the number of ICU admission; “Total” is population size; “Weight” is study weight in the analysis. “IV” is inverse variance statistical method of meta-analysis; “Random” is random effects model; “95% CI” is the 95% confidence intervals for the ICU admission; Each horizontal line in the graphical display represents a study, its width represents 95% CI of the interval estimation of the odds ratio effect of risk of ICU admission, and the blue midpoint of the line symbolizes the point estimate of the unadjusted odds ratio effect of risk of ICU admission; “I2” represents the quantity of heterogeneity (0–100%); “Test for overall effect: Z = 1.56 (P = 0.12)” confirms no statistical difference illustrated by the diamond crossing the line of effect. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
. Mechanical ventilation
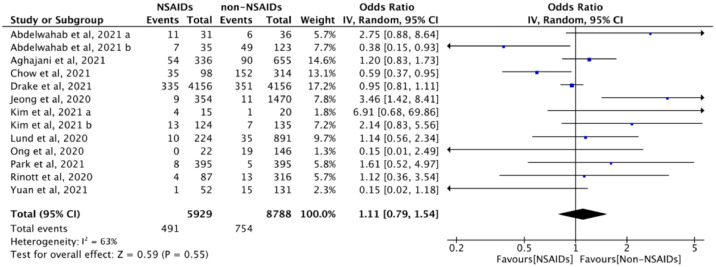
Eleven studies (n = 14,717) evaluated the association between NSAID exposure and mechanical ventilation rate in patients who were positive or negative to SARS-CoV-2 tests. The use of NSAIDs did not increase the probability of requiring mechanical ventilation (OR = 1.11, 95% CI: 0.79 to 1.54, I2 = 63%), compared to those not taking NSAIDs (Figure 6). In the adjusted analysis, there was also no increase in the odds of mechanical ventilation with the use of NSAID (aOR = 0.80, 95% CI: 0.52 to 1.24, I2 = 66%), compared with no NSAID use (eFigure 2 in Supplementary).
Figure 6.
The association between NSAID exposure and mechanical ventilation among patients who were positive or negative to SARS-CoV-2 tests during the COVID-19 pandemic. “Events” is the number of patients mechanical ventilation; “Total” is population size; “Weight” is study weight in the analysis. “IV” is inverse variance statistical method of meta-analysis; “Random” is random effects model; “95% CI” is the 95% confidence intervals for the mechanical ventilation; Each horizontal line in the graphical display represents a study, its width represents 95% CI of the interval estimation of the odds ratio effect of mechanical ventilation, and the blue midpoint of the line symbolizes the point estimate of the unadjusted odds ratio effect of mechanical ventilation; “I2” represents the quantity of heterogeneity (0–100%); “Test for overall effect: Z = 0.59 (P = 0.55)” confirms no statistical difference illustrated by the diamond crossing the line of effect. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Other outcomes
No association between NSAIDs exposure and administration of supplemental oxygen (OR = 0.80, 95% CI: 0.52 to 1.24, I2 = 57%), composite adverse outcome (OR = 1.32, 95% CI: 0.75 to 2.33, I2 = 75%), hospital admission (OR = 1.58, 95% CI: 1.06 to 2.36, I2 = 93%) among patients who were positive or negative to SARS-CoV-2 tests during the COVID-19 pandemic (see eFigure 3 in Supplementary). And the adjusted ORs between NSAIDs exposure and administration of supplemental oxygen (aOR = 1.00, 95% CI: 0.89 to 1.12, I2 = 0%), composite adverse outcome (aOR = 1.07, 95% CI: 0.66 to 1.73, I2 = 72%), hospital admission (aOR = 1.07, 95% CI: 0.74 to 1.55, I2 = 62%) also did not show association(see eFigure 2 in Supplementary).
Sensitivity analysis
We performed a sensitivity analysis on the mortality meta analysis by excluding one study at a time because of the high heterogeneity (I2 = 89%). A major cause of the high heterogeneity was the difference between the very precise estimates in the two sub-studies by Wong et al.37 and Osborne et al.56 (eFigure 4 in Supplementary). The sample size of Wong et al.37 and Osborne et al.56 were observed to be high as compared with other included studies, which might affect the results. Where the population included in the study of Wong et al.37 was non-COVID-19 patients and the drug administrated in the study of Osborne et al.56 was aspirin. Since the results of the sensitivity analysis found that type of NSAID were sources of heterogeneity between studies, so the sources of heterogeneity found in the sensitivity analysis were consistent with sources of heterogeneity found in subgroup analysis.
Quality of evidence and publication bias
Based on the pooled results, we downgraded the quality of evidence for some outcomes by one level due to imprecision (wide confidence interval) or inconsistency. Consequently, the quality of the evidence was classified as “Very low” and “Low” for unadjusted and adjusted mortality, respectively. The GRADE quality summary of findings for all outcomes is shown in eTable 3 in Supplementary. The funnel plot for the studies of mortality as an outcome is provided. It shows a lack of small studies showing lower or no risk with NSAIDs. There was no significant evidence of publication bias (eFigure 5 in Supplementary).
Discussion
This systematic review and meta-analysis included forty studies with a total of 4881,423 adult patients. We found that the use of NSAIDs did not aggravate mortality, ICU admission rate, mechanical ventilation rate, or any other adverse outcome, although the quality of evidence was low or very low. According to our results, there is no need for patients and clinicians to be concerned about the routine use of NSAIDs to relieve pain, inflammation and fever due to COVID-19.
Fever induced by viral infection is usually self-limiting and does not require specific antiviral therapy. Symptomatic and supportive treatments are the main part of the management of patients with COVID-19 at present. The risk of hemodynamic instability caused by fever is high, and NSAIDs (in particular ibuprofen and acetaminophen) are one of the most commonly used antipyretic and analgesic drugs to reduce the body temperature of fever patients and treat acute pain worldwide. Therefore, the current recommendations of some guidelines indicate that the use of antipyretic drugs such as ibuprofen, acetaminophen or any other NSAIDs should not be contraindicated in patients with COVID-19.60,61 The WHO made a rapid systematic review based on 73 studies about SARS, MERS and COVID-19 and found no effect of NSAIDs on the risk of ischemic and hemorrhagic stroke and myocardial infarction in adults with acute respiratory infections. Moderate to high-quality evidence suggests that there is no significant association between ibuprofen or acetaminophen use and all-cause mortality, hospitalization, acute renal failure or acute gastrointestinal bleeding on febrile children. Most included studies found either no adverse events or only mild or moderate adverse events, and there was no evidence indicating that NSAIDs have an impact on the quality of life or long-term survival.62
In our study, both unadjusted and adjusted mortality were analyzed. When the results of the two analyze agreed, we drew conclusions based on the direction of agreement, and when the results of the two analyses did not agree, they were discussed and drew reasonable conclusions primarily based on the adjusted results and quality of the evidence. After the subgroup analysis, we found that ibuprofen, COX-2 inhibitor, and other NSAIDs all could not reduce the risk of death, and the results of analysis of unadjusted and adjusted mortality were consistent. While the adjusted analysis found that aspirin could reduce mortality in patients with COVID-19, however this was not confirmed by the unadjusted analysis. The results of our review were in line with these previous findings.63, 64, 65, 66, 67, 68
This is a comprehensive systematic review and meta-analysis of the correlation between the use of NSAIDs and unfavorable outcomes of COVID-19, and it thus can be expected to reflect the best available evidence on this topic. The study is reported according to the Cochrane guidelines and the PRISMA statement. We performed a meta-analysis of included studies and were thus able to draw quantitative conclusions. Our study has however also some limitations. The forty studies included were all observational studies, some of them had small sample sizes, and all studies presented with a risk of bias in the implementation of blinding methods of outcome evaluators. To get the pooled adjusted OR, HR and RR in the original research were deirectly merged with OR in the meta-analysis, so there are limitations in the results of the adjusted OR. The results of high quality multicenter cohort study with large sample size are therefore still lacking. The clinical heterogeneity among the studies was large and we could thus not draw conclusions or give specific recommendations for different sub-populations (based on e.g. age or severity of disease), or the timing of medication, dosage, or any other aspect, to conduct quantitative analysis. Due to the particularity and urgency of the public health situation, our study was not registered on the international registration platform PROSPERO. The participants of all included studies were all exclusively adults, so the results cannot necessarily be generalized for children.
The use of NSAIDs (especially, ibuprofen, COX-2 inhibitor and low-dose aspirin) was not found to be associated with higher mortality, ICU admission rate, machine ventilation rate or administration of respiratory support. Despite the concerns that NSAID could enhance the ability of SARS-CoV-2 to invade human cells, there is no evidence to support that NSAID would worsen the prognosis of COVID-19.
Contributions
Qi Zhou and Siya Zhao had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Yaolong Chen, Enmei Liu and Xiaohui Wang.
Acquisition, analysis, or interpretation of data: Qi Zhou, Siya Zhao, Lidan Gan, Zhili Wang, Shuai Peng, Yaolong Chen.
Drafting of the manuscript: Qi Zhou, Siya Zhao, Lidan Gan, Zhili Wang, Shuai Peng.
Critical revision of the manuscript for important intellectual content: Qiyuan Li; Zijun Wang, Xiao Liu; Hui Liu; Qianling Shi; Zhengxiu Luo; Janne Estill; Yaoliong Chen; Xiaohui Wang and Enmei Liu.
Statistical analysis: Qi Zhou and Siya Zhao.
Supervision: Enmei Liu; Yaolong Chen and Xiaohui Wang.
The decision for the manuscript submission and publication: Yaolong Chen.
Declaration of interests
None.
Acknowledgments
Data sharing statement
The data analyzed during the current systematic review and meta-analysis is available from the corresponding author on reasonable request.
Funding
There was no funding source for this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101373.
Contributor Information
Xiaohui Wang, Email: wangxiaohui@lzu.edu.cn.
Enmei Liu, Email: emliu186@126.com.
Yaolong Chen, Email: chenyaolong@lzu.edu.cn.
Appendix. Supplementary materials
References
- 1.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323(8):709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagnoli R., Votto M., Licari A., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents. JAMA Pediatr. 2020;174:882. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 4.Irfan O., Muttalib F., Tang K., et al. Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch Dis Child. 2021;106:440–448. doi: 10.1136/archdischild-2020-321385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . WHO; Geneva: 2020. The Use of Non-Steroidal Anti- Inflammatory Drugs (NSAIDs) in Patients with COVID-19. Available from: https://www.who.int/news-room/commentaries/detail/the-use-of-non-steroidal-anti-inflammatory- drugs-(nsaids)-in-patients-with-covid-19. Accessed 22 April 2020. [Google Scholar]
- 8.Agence nationale de securite du medicament et des produits de sante. Usage des médicaments en ville durant l’épidémie de Covid-19: point de situation après cinq semaines de confinement—Point d’information. https://www.ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Usage-des-medicaments-en-ville-durant-l-epidemie-de-Covid-19-point-de-situation-apres-cinq-semaines-de-confinement-Point-d-information. Accessed 4 May 2020.
- 9.European Medicines Agency . EMA; London: 2020. EMA Gives Advice on the Use of Non-Steroidal Anti-Inflammatories for COVID-19.https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19 [Google Scholar]
- 10.Abu Esba L.C., Alqahtani R.A., Thomas A., et al. Ibuprofen and NSAID use in COVID-19 infected patients is not associated with worse outcomes: a prospective cohort study. Infect Dis Ther. 2020;2:1–16. doi: 10.1007/s40121-020-00363-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong H.E., Lee H., Shin H.J., Choe Y.J., Filion K.B., Shin J.Y. Association between NSAIDs use and adverse clinical outcomes among adults hospitalized with COVID-19 in South Korea: a nationwide study. Clin Infect Dis. 2020:ciaa1056. doi: 10.1093/cid/ciaa1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. 2nd ed. John Wiley & Sons; Chichester (UK): 2019. Cochrane Handbook for Systematic Reviews of Interventions. (editors) [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 15.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norris S.L., Meerpohl J.J., Akl E.A., et al. The skills and experience of GRADE methodologists can be assessed with a simple tool. J Clin Epidemiol. 2016;79:150–158. doi: 10.1016/j.jclinepi.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 17.GRADEpro G.D.T. McMaster University; 2015. GRADEpro Guideline Development Tool[Software] (developed by Evidence Prime, Inc.). Available online: https://gradepro.org/ [Google Scholar]
- 18.Guyatt G., Oxman A.D., Akl E.A., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P., Thompson S.G., Deeks J.J., et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borenstein M., Hedges L.V., Higgins J.P., et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 21.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 22.Lund L.C., Kristensen K.B., Reilev M., et al. Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: a Danish nationwide cohort study. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kragholm K., Gerds T.A., Fosbøl E., et al. Association between prescribed ibuprofen and severe COVID-19 infection: a nationwide register-based cohort study. Clin Transl Sci. 2020;13(6):1103–1107. doi: 10.1111/cts.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong W., Chen Y., You K., et al. Celebrex adjuvant therapy on coronavirus disease 2019: an experimental study. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.561674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rinott E., Kozer E., Shapira Y., et al. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect. 2020;26(9):1259.e5–1259.e7. doi: 10.1016/j.cmi.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce E., Barlow-Pay F., Short R., et al. Prior routine use of non-steroidal anti-inflammatory drugs (NSAIDs) and important outcomes in hospitalised patients with COVID-19. J Clin Med. 2020;9(8):2586. doi: 10.3390/jcm9082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong S.W.X., Tan W.Y.T., Chan Y.H., et al. Safety and potential efficacy of cyclooxygenase-2 inhibitors in coronavirus disease 2019. Clin Transl Immunol. 2020;9(7):e1159. doi: 10.1002/cti2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drake T.M., Fairfield C.J., Pius R., et al. ISARIC4C investigators. Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC clinical characterisation protocol UK cohort: a matched, prospective cohort study. Lancet Rheumatol. 2021;3(7):e498–e506. doi: 10.1016/S2665-9913(21)00104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Botía P., Bernardo Á., Acebes-Huerta A., et al. Clinical management of hypertension, inflammation and thrombosis in hospitalized COVID-19 patients: impact on survival and concerns. J Clin Med. 2021;10(5):1073. doi: 10.3390/jcm10051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S., Hayek S.S., Wang W., et al. STOP-COVID Investigators. factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahai A., Bhandari R., Godwin M., et al. Effect of aspirin on short-term outcomes in hospitalized patients with COVID-19. Vasc Med. 2021 doi: 10.1177/1358863X211012754. × 211012754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alamdari N.M., Afaghi S., Rahimi F.S., et al. Mortality risk factors among hospitalized COVID-19 patients in a major referral center in Iran. Tohoku J Exp Med. 2020;252(1):73–84. doi: 10.1620/tjem.252.73. [DOI] [PubMed] [Google Scholar]
- 33.Hwang J.M., Kim J.H., Park J.S., et al. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurol Sci. 2020;41(9):2317–2324. doi: 10.1007/s10072-020-04541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imam Z., Odish F., Gill I., et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandan J.S., Zemedikun D.T., Thayakaran R., et al. Nonsteroidal antiinflammatory drugs and susceptibility to COVID-19. Arthritis Rheumatol. 2021;73(5):731–739. doi: 10.1002/art.41593. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J., Lee S.H., You S.C., et al. Non-steroidal anti-inflammatory agent use may not be associated with mortality of coronavirus disease 19. Sci Rep. 2021;11(1):5087. doi: 10.1038/s41598-021-84539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong A.Y., MacKenna B., Morton C.E., et al. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts. Ann Rheum Dis. 2021;80(7):943–951. doi: 10.1136/annrheumdis-2020-219517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gianfrancesco M., Hyrich K.L., Al-Adely S., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costantino F., Bahier L., Tarancón L.C., et al. COVID-19 in French patients with chronic inflammatory rheumatic diseases: clinical features, risk factors and treatment adherence. Jt Bone Spine. 2021;88(1) doi: 10.1016/j.jbspin.2020.105095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huh K., Ji W., Kang M., et al. Association of prescribed medications with the risk of COVID-19 infection and severity among adults in South Korea. Int J Infect Dis. 2021;104:7–14. doi: 10.1016/j.ijid.2020.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanch-Rubió J., Soldevila-Domenech N., Tío L., et al. Influence of anti-osteoporosis treatments on the incidence of COVID-19 in patients with non-inflammatory rheumatic conditions. Aging. 2020;12(20):19923–19937. doi: 10.18632/aging.104117. (Albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vila-Corcoles A., Satue-Gracia E., Ochoa-Gondar O., et al. Use of distinct anti-hypertensive drugs and risk for COVID-19 among hypertensive people: a population-based cohort study in Southern Catalonia, Spain. J Clin Hypertens. 2020;22(8):1379–1388. doi: 10.1111/jch.13948. (Greenwich) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancia G., Rea F., Ludergnani M., et al. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jehi L., Ji X., Milinovich A., et al. Development and validation of a model for individualized prediction of hospitalization risk in 4,536 patients with COVID-19. PLoS ONE. 2020;15(8) doi: 10.1371/journal.pone.0237419. Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reilev M., Kristensen K.B., Pottegård A., et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49(5):1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Argenziano M.G., Bruce S.L., Slater C.L., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdelwahab H.W., Shaltout S.W., Sayed Ahmed H.A., et al. Acetylsalicylic acid compared with enoxaparin for the prevention of thrombosis and mechanical ventilation in COVID-19 patients: a retrospective cohort study. Clin Drug Investig. 2021;41(8):723–732. doi: 10.1007/s40261-021-01061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haji Aghajani M., Moradi O., Amini H., et al. Decreased in-hospital mortality associated with aspirin administration in hospitalized patients due to severe COVID-19. J Med Virol. 2021;93(9):5390–5395. doi: 10.1002/jmv.27053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chow J.H., Khanna A.K., Kethireddy S., et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth Analg. 2021;132(4):930–941. doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 50.Karruli A., Boccia F., Gagliardi M., et al. Multidrug-resistant infections and outcome of critically ill patients with coronavirus disease 2019: a single center experience. Microb Drug Resist. 2021;27(9):1167–1175. doi: 10.1089/mdr.2020.0489. [DOI] [PubMed] [Google Scholar]
- 51.Kim I., Yoon S., Kim M., et al. Aspirin is related to worse clinical outcomes of COVID-19. Medicina. 2021;57(9):931. doi: 10.3390/medicina57090931. (Kaunas)Published 2021 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Q., Huang N., Li A., et al. Effect of low-dose aspirin on mortality and viral duration of the hospitalized adults with COVID-19. Medicine. 2021;100(6):e24544. doi: 10.1097/MD.0000000000024544. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meizlish M.L., Goshua G., Liu Y., et al. Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis. Am J Hematol. 2021;96(4):471–479. doi: 10.1002/ajh.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merzon E., Green I., Vinker S., et al. The use of aspirin for primary prevention of cardiovascular disease is associated with a lower likelihood of COVID-19 infection. FEBS J. 2021;288(17):5179–5189. doi: 10.1111/febs.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osborne T.F., Veigulis Z.P., Arreola D.M., Mahajan S.M., Röösli E., Curtin C.M. Association of mortality and aspirin prescription for COVID-19 patients at the veterans health administration. PLoS ONE. 2021;16(2) doi: 10.1371/journal.pone.0246825. Published 2021 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Son M., Noh M.G., Lee J.H., Seo J., Park H., Yang S. Effect of aspirin on coronavirus disease 2019: a nationwide case-control study in South Korea. Medicine. 2021;100(30):e26670. doi: 10.1097/MD.0000000000026670. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vahedian-Azimi A., Rahimibashar F., Najafi A., et al. Associastion of in-hospital use of statins, aspirin, and renin-angiotensin-aldosterone inhibitors with mortality and ICU admission due to COVID-19. Adv Exp Med Biol. 2021;1327:205–214. doi: 10.1007/978-3-030-71697-4_17. [DOI] [PubMed] [Google Scholar]
- 59.Yuan S., Chen P., Li H., Chen C., Wang F., Wang D.W. Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease. J Cell Mol Med. 2021;25(2):1263–1273. doi: 10.1111/jcmm.16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alhazzani W., Møller M.H., Arabi Y.M., et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.COVID-19 Treatment Guidelines Panel . National Institutes of Health; 2021. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed May 6. [PubMed] [Google Scholar]
- 62.World Health Organization . World Health Organization; Geneva: 2020. The Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Patients With COVID-19.https://www.who.int/news-room/commentaries/detail/the-use-of-non-steroidal-anti- inflammatory-drugs-(nsaids)-in-patients-with-covid-19 [Google Scholar]
- 63.Yousefifard M., Zali A., Zarghi A., et al. Non-steroidal anti-inflammatory drugs in management of COVID-19; a systematic review on current evidence. Int J Clin Pract. 2020;74(9):e13557. doi: 10.1111/ijcp.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore N., Bosco-Levy P., Thurin N., et al. NSAIDs and COVID-19: a systematic review and meta-analysis. Drug Saf. 2021:1–10. doi: 10.1007/s40264-021-01089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kow C.S., Hasan S.S. The risk of mortality in patients with COVID-19 with pre-diagnosis use of NSAIDs: a meta-analysis. Inflammopharmacology. 2021;29(3):641–644. doi: 10.1007/s10787-021-00810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wijaya I., Andhika R., Huang I., et al. The effects of aspirin on the outcome of COVID-19: a systematic review and meta-analysis. Clin Epidemiol Glob Health. 2021;12 doi: 10.1016/j.cegh.2021.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martha J.W., Pranata R., Lim M.A., et al. Active prescription of low-dose aspirin during or prior to hospitalization and mortality in COVID-19: a systematic review and meta-analysis of adjusted effect estimates. Int J Infect Dis. 2021;108:6–12. doi: 10.1016/j.ijid.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kow C.S., Hasan S.S. Use of antiplatelet drugs and the risk of mortality in patients with COVID-19: a meta-analysis. J Thromb Thrombolysis. 2021;52(1):124–129. doi: 10.1007/s11239-021-02436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.