Abstract
This study aimed to explore whether brachial‐ankle pulse wave velocity (baPWV) and brachial artery flow‐mediated dilation (FMD) or the interaction of both parameters are associated with subclinical target organ damage (STOD) indices in patients with essential hypertension. A total of 4618 patients registered from January 2015 to October 2020 were included. baPWV and FMD were measured to evaluate arterial stiffness and endothelial dysfunction. Whereas left ventricular hypertrophy (LVH), urine albumin‐creatinine ratio (UACR), and carotid intima‐media thickness (CIMT) were obtained as STOD indicators. On multivariable logistic regression analysis with potential confounders, higher quartiles of baPWV and FMD were significantly associated with an increased risk of STOD. In patients <65 years of age, the odds ratio (OR) of LVH, UACR, and CIMT ≥.9 mm for the fourth versus the first quartile of baPWV were 1.765 (1.390–2.240), 2.832 (2.014–3.813), and 3.075 (2.315–4.084), respectively. In interaction analysis, an increase in baPWV shows a progressively higher risk of STOD across the quartiles of FMD. Also, the estimated absolute risks of LVH, UACR, and CIMT ≥.9 mm for the first to fourth quartile of baPWV increased from 1.88 to 2.75, 2.35 to 4.44, and 3.10 to 6.10, respectively, in patients grouped by FMD quartiles. The addition of baPWV to FMD slightly improved risk prediction for STOD. BaPWV and FMD were independently associated with an increased risk of STOD in patients with essential hypertension especially among patients <65 years of age. Patients with elevated baPWV and decreased FMD parameters are at increased risk of STOD.
Keywords: arterial stiffness, brachial artery flow‐mediated dilation, brachial‐ankle pulse wave velocity, endothelial function, subclinical target organ damage
1. INTRODUCTION
Hypertension‐induced subclinical target organ damage (STOD) is the primary risk factor for cardiovascular diseases (CVDs) and mortality. 1 , 2 , 3 Endothelial dysfunction and arterial stiffness, the independent predictors of hypertension, are increasing with age‐related physiological alterations. 4 , 5 , 6 , 7 At the same time, the prevalence of STOD dramatically increases with uncontrolled blood pressure (BP) levels. 8 , 9 Although endothelial dysfunction and arterial stiffness increase with hypertension synchronously, the correlation between endothelial dysfunction and arterial stiffness with STOD was controversial in previous studies. 10 It remains unknown whether endothelial dysfunction and arterial stiffness amplify the risk of STOD in the hypertension population.
Some studies suggested that chronic elevation in BP resulted in arterial stiffness through its unfavorable effects on structural and functional alterations in the walls of central elastic arteries. 11 , 12 , 13 In contrast, other studies showed that arterial stiffness led to higher BP because of the changes in the buffering function of conduit arteries. 14 Scientifically, both theories may justify an increase in BP contributes to arterial stiffness but whether an increase in arterial stiffness predicts the preceding STOD remains unknown. From a hemodynamic perspective, the body maintains normal BP by adjusting peripheral resistance and cardiac output. 15 Also, other studies have suggested that target organ damage characterized by left ventricular hypertrophy (LVH), high levels of albuminuria (UACR), and carotid intima‐media thickness (CIMT) significantly predicts future cardiovascular and renal events in hypertensive patients. 4 , 5 In this context, we hypothesize that vascular resistance resulting from endothelial dysfunction and arterial stiffness may positively associate with STOD indicators.
Both endothelial dysfunction and arterial stiffness contribute to physiological hardening of the arterial system and are surrogate markers for impaired vascular function and structure. One method to assess systemic arterial stiffness is brachial‐ankle pulse wave velocity (baPWV). 16 Whereas, endothelial dysfunction that associate with a decrease in bioavailability of vasodilator factors has been reported to associate with atherogenesis, atherothrombotic complications, and subclinical organ damage8,9 and can be determined using brachial artery flow‐mediated dilation (FMD). 10 , 11 Recent evidence illustrated the relationships among endothelial dysfunction, arterial stiffness, and STOD, based on animal studies 17 and small clinical studies. 10 However, this hypothesis has not been tested in large‐scale epidemiological studies. Elucidating the association between endothelial dysfunction, arterial stiffness and STOD is significant because hypertension‐induced STOD will lead to an increased clinical burden of CVDs and mortality. Thus, we investigated the association between endothelial dysfunction, arterial stiffness, and STOD in a large hospital‐based cohort including 4618 adults.
2. METHODS
2.1. Study participants
We examined patients with hypertension who were hospitalized between January 2015 and October 2020 at the First Affiliated Hospital of Dalian Medical University. Those with diagnosis of secondary hypertension defined based on 2018 ESC/ESH guidelines 18 ) (n = 106), malignancy (n = 21), severe arrhythmia (n = 102), moderate and severe kidney dysfunction (estimated glomerular filtration rate [eGFR] ≤60 ml/min/1.73 m2), proteinuria (>300 mg/24 h) (n = 122), left ventricular systolic dysfunction (LVEF <50%) (n = 58), severe valvular heart disease (n = 26), peripheral vascular diseases (PAD) (n = 62), and myocardial infarction or unstable angina pectoris (n = 117).
Also, electronic health records data comprises substantial missing information that if left unaddressed could decrease the validity of conclusions drawn. 19 Therefore, missing data of the key variables that rule out the aforementioned disease conditions were also excluded to control selection bias and avoid interpretation of our results (n = 2774). Finally, the present study included a total of 4618 patients. Figure 1 describes a brief overview of the selection of study participants. The research was conducted in accordance with the Helsinki declaration guidelines and was approved by the institutional review board of the First Affiliated Hospital of Dalian Medical University. The informed consent provision was waived and all procedures listed here were carried out in compliance with the approved guidelines.
FIGURE 1.
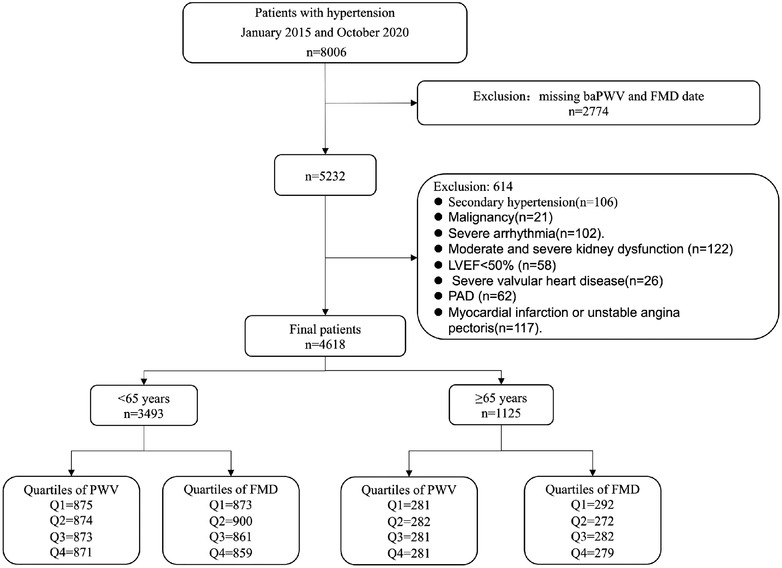
A brief overview of the selection of study participants.
2.2. Clinical measurements and definition of explanatory variables
Demographic and clinical data including age, gender, current smoking, diabetes mellitus, cardiovascular disease, use of antihypertensive drugs, hypoglycemic drugs and statin were retrieved from the electronic data record of the First Affiliated Hospital of Dalian Medical University. Participants were deemed current smokers if reported they are currently smoking or registered smoking at least 100 cigarettes during their lifetime. 20 , 21 Body mass index (BMI) was calculated as weight in kg per squared height measured in meters. Data on biochemical parameters including total cholesterol (TC), triglycerides (TG), High‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein cholesterol (LDL‐C), hypersensitivity C‐reactive protein (hs‐CRP) were also retrieved from the First Affiliated Hospital of Dalian Medical University and all these biochemical parameters were performed using the standard protocols. eGFR was calculated according to the equation suggested by the Chronic Kidney Disease Epidemiology (CKD‐EPI) Collaboration. 22 Diabetes mellitus (DM) was defined as fasting plasma glucose ≥7.0 mmo/L or 2 h plasma glucose ≥11.1 mmo/L or a self‐reported history of diabetes mellitus or currently receiving antidiabetic treatments. Peripheral artery disease (PAD) was defined as ABI <.9, stent implantation, or according to the past medical records. LVH was defined according to 2018 ESC/ESH guidelines and we strictly adhered in the criteria of echocardiography LVH (LV mass index: men >50 g/m2.7; women >47 g/m2.7 [height in m2.7]; indexation for body surface area [BSA] may be used in normal‐weight patients; LV mass/BSA g/m2 >115 [men] and >95 [women]). Also, data on urinary albumin‐to‐creatinine ratio was produced from the first urine in the morning. Elevate UACR was defined as 30–300 mg/g.
2.3. Echocardiography
All subjects received echocardiography examinations at rest in the left lateral decubitus position using the Vivid E95 ultrasound system. The left ventricular end‐diastolic diameter, left ventricular posterior wall thickness, interventricular septal thickness, and left ventricular ejection fraction were measured. LVMI was calculated by using the Devereux formula 23 via these measurements.
2.4. Ultrasonographic examination of the carotid arteries
All subjects received Bilateral duplex US (Philips iU‐22 Ultrasound System, Philips Medical Systems, Bothell, Washington, USA) examinations in the supine position. Longitudinal images of the common carotid artery, 1 cm proximal to the bifurcation in which the far wall intima‐media interface (M‐line) was clearly defined, were magnified and recorded on videotape for later analysis. The distance between the leading edge of the intima and the media‐adventitia interface was measured with ultrasonic calipers. Three measurements were taken in both the right and left common carotid arteries, and a mean IMT was calculated as previously described. 24 CIMT ≥.9 mm was defined as positive.
2.5. Measurement of FMD
Patients were examined in the supine position after 15 min rest and after at least a 6‐h fasting. An automated sphygmomanometer (Dinamap device) was used to monitor blood pressure and pulse in the left arm at 5‐min intervals throughout the exam. A standard blood pressure cuff was positioned around the right arm, two inches below the antecubital fossa, and the artery was imaged 5–9 cm above the antecubital fossa. A linear‐array multifrequency transducer operating at 9 MHz (GE Logiq 700 Device) was used to acquire images of the right brachial artery. After obtaining baseline images, the cuff was inflated to 50 mm Hg above the participant's systolic blood pressure for 5 min. Images of the right brachial artery were captured continuously for 30 sec before cuff inflation, and for 2 min beginning immediately before cuff deflation to document the vasodilator response. Images of the brachial artery diameters were captured in diastole (gated with electrocardiograph R‐wave). The semi‐automated readings of these digitized images generated the baseline and maximum diameters of the brachial artery from which the rate FMD was computed.
2.6. Measurement of baPWV
BbaPWV measurements were obtained using a volume‐plethysmography device (VP‐1000, OMRON, Dalian, China), which measures both brachial and posterior tibial artery pressure waveforms using an oscillometric method with cuffs placed around both arms and ankles of the patients. baPWV was calculated automatically by time‐phased analysis, and the distance between the upper arm and ankle was estimated based on height. The baPWV values were obtained from the right and left measurements, and the higher reading of the two measurements was used for analysis. 25
2.7. Outcome definition
Hypertension‐related STOD is characterized by significant changes in LVH, CIMT, and UACR. 18 Therefore, we used LVH, CIMT, and UACR as the indices of STOD. We defined STOD if any one of the following exists (i) echocardiographic LVH; (ii) CIMT ≥.9 mm; or (iii) UACR between 30 and 300 mg/g.
2.8. Statistical analysis
All statistical analyses were conducted using SPSS version 26.0. Participants were categorized based on age into two groups, including <65 years of age and ≥65 years of age, and were stratified into quartiles based on the baPWV and FMD values. Kolmogorov–Smirnov test was used to test the normality of distribution. Quantitative variables with a normal distribution were specified as the mean ± standard deviation. Whereas the non‐normal distributed data was present as median (percentile 25, percentile 75). Analysis of variance (ANOVA) was used to compare whether the differences between three or more groups were statistically significant in variables comprising numerical data. Chi‐squared test was computed to analyze categorical data and results were expressed as counts and percentiles. The likelihood of STOD associated with baPWV or FMD was calculated using a logistic regression model. While running logistic regression analysis, the baPWV or FMD quartiles were entered in the models with the first baPWV or FMD‐specific quartile as a reference to assess the odds ratio (OR) and 95% Confidence Interval (95% CI). The logistic regression analysis was carried out in three models. Model 1 was unadjusted, model 2 was adjusted for age and sex, and model 3 was adjusted for age, sex, BMI, eGFR, smoking, SBP, DBP, use of calcium channel blocker, angiotensin‐converting enzyme receptor blocker, β‐Blocker, diuretic, α‐Blocker, use of statins, diabetes, and stable angina. To compute whether elevated baPWV combined with reduced FMD or vice versa influences the risk of STOD, we estimated the OR and 95% CI of STOD using multivariate regression analysis among those participants grouped in different quartiles of baPWV/FMD along with their corresponding FMD/baPWV quartiles. Receiver operator characteristics (ROC) curve analysis was used to evaluate the productive power of baPWV, FMD, and baPWV+FMD for STOD. Also, Youden's index statistics were calculated to capture the performance of the parameters. 26 We further run a sub‐analysis to calculate the OR for STOD associated with quartiles of baPWV grouped by FMD or vice versa to establish the effect of the interaction of the two parameters (baPWV and FMD). All statistical analyses were two‐sided, and a p‐value of less than .05 was considered statistically significant.
3. RESULTS
3.1. Baseline characteristics of the participants
Among the 8006 subjects during the study period, 4618 participants had sufficient data on baPWV and FMD. The prevalence of LVH, UACR, and CIMT were 49.7%, 20.7%, and 84.8%, respectively. The characteristics for patients with age <65 and ≥65 years based on baPWV and FMD quartiles are presented in Tables 1 and 2. Also, characteristics including heart rate, Hs‐CRP and drugs using have been shown in Supplementary Tables S1 and S2, respectively for patients with age <65 and ≥65 years based on baPWV and FMD quartiles. Table 1 showed patients in higher quartiles of baPWV and FMD had higher mean values of SBP, UACR, and LVMI. Also, patients in higher quartiles of baPWV and FMD were more likely to have CIMT ≥.9 mm, LVH, DM, and stable angina. Likewise, they had a higher proportion of older age and decreased rate of LVEF. However, unlike the patients in higher quartiles of baPWV, those patients with higher quartiles of FMD were more likely to be males and smokers. In the group of ≥65 years of age, the proportion of patients with LVH, UACR, and CIMT ≥.9 mm was increasing across the quartiles of baPWV. Conversely, there were no significant differences in age, smoking status, and mean TC and HDL‐C levels across the baPWV and FMD quartiles (Table 2).
TABLE 1.
Characteristics of participants <65 years of age grouped by Quartile of brachial‐ankle pulse wave velocity and flow‐mediated dilation
| Quartile of baPWV, cm/s in patients <65 years of age | Quartile of FMD, % in patients <65 years of age | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |||
| (792.5–1351.5) | (1352–1503.5) | (1504–1682.5) | (1683–3474) | p‐value | (6.8–19.4) | (4.8–6.7) | (2.9–4.7) | (0–2.8) | p‐value | |
| Number of subjects | 875 | 874 | 873 | 871 | 859 | 861 | 900 | 873 | ||
| Age, years | 45.5 ± 11.1 | 47.4 ± 10.8 | 49.3 ± 10.6 | 52.6 ± 10.1 | <.001 | 45.8 ± 11.7 | 47.8 ± 11.1 | 49.5 ± 10.6 | 51.5 ± 9.8 | <.001 |
| Female, N (%) | 331 (37.8) | 346 (39.6) | 360 (41.2) | 399 (45.8) | .005 | 444 (51.7) | 347 (40.3) | 334 (37.1) | 311 (35.6) | <.001 |
| BMI, kg/m2 | 27.2 ± 4.1 | 27.2 ± 3.9 | 27.0 ± 3.9 | 27.0 ± 3.9 | <.001 | 26.6 ± 4.1 | 27.0 ± 4.1 | 27.3 ± 3.8 | 27.1 ± 3.7 | .003 |
| Current smoking, N (%) | 223 (25.5) | 236 (27.0) | 232 (26.6) | 203 (23.3) | .11 | 165 (19.2) | 224 (26.0) | 239 (26.6) | 266 (30.5) | <.001 |
| SBP, mm Hg | 160.2 ± 18.2 | 166.9 ± 19.0 | 172.6 ± 21.7 | 179.0 ± 21.8 | <.001 | 165.3 ± 19.7 | 169.0 ± 20.7 | 171.2 ± 21.8 | 173.1 ± 22.4 | <.001 |
| DBP, mm Hg | 105.7 ± 15.1 | 110.0 ± 14.9 | 111.9 ± 16.5 | 113.5 ± 16.9 | <.001 | 108.2 ± 15.9 | 110.0 ± 15.9 | 111.4 ± 16.3 | 111.5 ± 16.1 | <.001 |
| eGFR, ml/min/1.73 m2 | 110.7 ± 21.4 | 110.6 ± 21.6 | 110.7 ± 23.4 | 108.7 ± 23.9 | .192 | 112.7 ± 22.4 | 111.1 ± 22.9 | 109.4 ± 21.6 | 107.6 ± 23.3 | <.001 |
| TC, mmol/L | 4.8 ± 1.1 | 4.8 ± 1.2 | 4.9 ± 1.0 | 5.0 ± 1.0 | .041 | 4.9 ± 1.0 | 4.8 ± 1.0 | 4.9 ± 1.0 | 4.9 ± 1.4 | .673 |
| TG, mmol/L | 1.5 (1.0–2.1) | 1.5 (1.0–2.2) | 1.5 (1.1–2.2) | 1.6 (1.1–2.2) | .404 | 1.4 (1.0–2.0) | 1.5 (1.1–2.2) | 1.6 (1.1–2.2) | 1.6 (1.1–2.3) | .003 |
| HDL‐C, mmol/L | 1.2 ± .3 | 1.2 ± .3 | 1.2 ± .3 | 1.2 ± .3 | .097 | 1.2 ± .3 | 1.2 ± .3 | 1.2 ± .3 | 1.2 ± .3 | .024 |
| LDL‐C, mmol/L | 2.7 ± .6 | 2.7 ± .7 | 2.7 ± .7 | 2.8 ± .7 | .051 | 2.7 ± .7 | 2.7 ± .7 | 2.7 ± .7 | 2.7 ± .7 | .772 |
| UACR, N (%) | 97 (11.1) | 139 (15.9) | 218 (25.0) | 266 (30.5) | <.001 | 134 (15.6) | 181 (21.0) | 181 (20.1) | 224 (25.7) | <.001 |
| CIMT ≥.9 mm, N (%) | 633 (72.3) | 698 (79.9) | 726 (83.2) | 774 (88.9) | <.001 | 639 (74.4) | 663 (77.0) | 763 (84.8) | 766 (87.7) | <.001 |
| LVEF, % | 59.0 ± 1.6 | 59.1 ± 1.2 | 59.0 ± 1.5 | 58.9 ± 1.5 | .009 | 59.2 ± 1.3 | 59.0 ± 1.3 | 58.9 ± 1.6 | 58.9 ± 1.4 | <.001 |
| LVH, N (%) | 293 (33.5) | 389 (44.5) | 412 (47.2) | 501 (57.5) | <.001 | 366 (42.6) | 372 (43.2) | 403 (44.8) | 454 (52.0) | .008 |
| Diabetes mellitus, N (%) | 100 (11.4) | 125 (14.3) | 174 (19.9) | 227 (26.1) | <.001 | 119 (13.9) | 146 (17.0) | 157 (17.4) | 204 (23.4) | <.001 |
| Stable angina, N (%) | 94 (10.7) | 92 (10.5) | 96 (11.0) | 131 (15.0) | .009 | 69 (8.0) | 98 (11.4) | 103 (11.4) | 143 (16.4) | <.001 |
Abbreviations: BaPWV, brachial‐ankle pulse wave velocity; BMI, body mass index; CIMT, carotid intima‐media thickness; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FMD, flow‐mediated dilation; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; UACR, urine albumin–creatinine ratio.
TABLE 2.
Characteristics of participants ≥65 years of age grouped by quartile of brachial‐ankle pulse wave velocity and flow‐mediated dilation
| Quartile of baPWV, cm/s in patients ≥65 years of age | Quartile of FMD, %in patients ≥65Years of age | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |||
| (874–1562) | (1562.5–1770) | (1770.5–2016) | (2016.5–3539) | p‐value | (5.5–16.8) | (3.6–5.4) | (2.2–3.5) | (0–2.1) | p‐value | |
| Number of subjects | 281 | 282 | 281 | 281 | 279 | 282 | 272 | 292 | ||
| Age, years | 69.8 ± 4.8 | 70.7 ± 4.7 | 72.2 ± 5.7 | 74.3 ± 6.1 | <.001 | 71.7 ± 5.7 | 71.4 ± 5.3 | 72.2 ± 5.8 | 71.6 ± 5.7 | .323 |
| Female, N (%) | 163 (58.0) | 166 (58.9) | 183 (65.1) | 189 (67.3) | .057 | 174 (62.4) | 190 (67.4) | 168 (61.8) | 169 (57.9) | .135 |
| BMI, kg/m2 | 26.0 ± 3.2 | 26.0 ± 3.3 | 25.2 ± 3.4 | 25.1 ± 3.5 | <.001 | 25.2 ± 3.4 | 25.80 ± 3.2 | 25.7 ± 3.5 | 25.5 ± 3.5 | .169 |
| Current smoking, N (%) | 37 (13.2) | 35 (12.4) | 28 (10.0) | 25 (8.9) | .477 | 30 (10.8) | 36 (12.8) | 23 (8.5) | 36 (12.3) | .534 |
| SBP, mm Hg | 160.3 ± 18.0 | 166.8 ± 18.3 | 167.9 ± 17.7 | 174.1 ± 19.7 | <.001 | 164.9 ± 18.4 | 167.4 ± 18.3 | 167.6 ± 18.5 | 169.0 ± 20.6 | .077 |
| DBP, mm Hg | 94.1 ± 13.1 | 97.3 ± 13.7 | 97.6 ± 14.0 | 97.7 ± 13.2 | .004 | 96.6 ± 13.0 | 97.6 ± 13.7 | 95.1 ± 13.4 | 97.3 ± 14.1 | .129 |
| eGFR, ml/min/1.73 m2 | 99.7 ± 20.4 | 99.5 ± 21.1 | 102.2 ± 21.9 | 96.7 ± 18.6 | .018 | 99.2 ± 19.4 | 100.5 ± 21.6 | 99.1 ± 21.3 | 99.3 ± 20.1 | .825 |
| TC, mmol/L | 4.8 ± 1.0 | 4.9 ± 1.1 | 4.9 ± 1.1 | 5.0 ± 1.0 | .261 | 4.9 ± 1.1 | 4.9 ± 1.1 | 4.9 ± 1.0 | 4.8 ± 1.0 | .839 |
| TG, mmol/L | 1.2 (.9–1.7) | 1.3 (1.0–1.9) | 1.3 (.9–1.8) | 1.3 (1.0–1.7) | .637 | 1.2 (.9–1.7) | 1.3 (1.0–1.9) | 1.3 (1.0–1.8) | 1.2 (.9–1.7) | .958 |
| HDL‐C, mmol/L | 1.2 ± .3 | 1.2 ± .3 | 1.3 ± .3 | 1.2 ± .3 | .117 | 1.2 ± .3 | 1.2 ± .3 | 1.2 ± .3 | 1.2 ± .3 | .978 |
| LDL‐C, mmol/L | 2.6 ± .7 | 2.7 ± .8 | 2.7 ± .7 | 2.7 ± .7 | .520 | 2.7 ± .7 | 2.7 ± .8 | 2.7 ± .7 | 2.7 ± .7 | .767 |
| UACR, N (%) | 37 (13.2) | 46 (16.3) | 57 (20.3) | 98 (34.9) | <.001 | 60 (21.5) | 58 (20.6) | 53 (19.5) | 67 (22.9) | .778 |
| CIMT ≥.9 mm, N (%) | 261 (92.9) | 274 (97.2) | 273 (97.2) | 276 (98.2) | .004 | 266 (95.3) | 272 (96.5) | 261 (96.0) | 285 (97.6) | .523 |
| LVEF, % | 58.6 ± 1.1 | 58.7 ± 1.4 | 58.6 ± 1.3 | 58.4 ± 2.0 | .083 | 58.6 ± 1.3 | 58.6 ± 1.7 | 58.4 ± 1.7 | 58.6 ± 1.2 | .409 |
| LVH, N (%) | 150 (53.4) | 184 (65.2) | 182 (64.8) | 184 (65.5) | .006 | 174 (62.4) | 181 (64.2) | 165 (60.7) | 180 (61.6) | .852 |
| Diabetes mellitus, N (%) | 68 (24.2) | 79 (28.0) | 86 (30.6) | 109 (38.8) | .002 | 74 (26.5) | 82 (29.1) | 92 (33.8) | 94 (32.2) | .244 |
| Stable angina, N (%) | 68 (24.2) | 83 (29.4) | 73 (26.0) | 79 (28.1) | .514 | 70 (25.1) | 66 (23.4) | 72 (26.5) | 95 (32.5) | .073 |
Abbreviations: BaPWV, brachial‐ankle pulse wave velocity; BMI, body mass index; CIMT, carotid intima‐media thickness; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FMD, flow‐mediated dilation; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; UACR, urine albumin–creatinine ratio.
3.2. Relationship between baPWV/FMD and STOD indices in patients <65 years of age
Table 3 presents the ORs of STOD indices among patients <65 years of age (Table 3). The patients in the highest baPWV/FMD quartile had a significantly increased risk of STOD. This association persisted even after adjusting for potential confounding factors, including age, sex, BMI, eGFR, smoking, SBP, DBP, antihypertensive use including calcium channel blocker, angiotensin‐converting enzyme receptor blocker, β‐Blocker, Diuretic, α‐Blocker, use of statins, diabetes, and stable angina. The OR (95% CI) of LVH and UACR for the fourth versus the first quartile of baPWV were 1.765 (1.390–2.240) and 2.832 (2.014–3.813) and, whereas for the fourth versus the first quartile of CIMT ≥.9 mm was 3.075 (2.315–4.084).
TABLE 3.
ORs (95% CIs) for subclinical target organ damage associated with quartile of brachial‐ankle pulse wave velocity and flow‐mediated dilation in participants <65 years of age
| Quartile of baPWV <65 years of age | Quartile of FMD <65 years of age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P‐for trends | Q1 | Q2 | Q3 | Q4 | P‐for trends | ||
| LVH | Model 1 | 1(Ref) | 1.593 (1.313–1.934) | 1.775 (1.463–2.154) | 2.690 (2.215–3.267) | <.001 | 1(Ref) | 1.025 (.847–1.240) | 1.092 (.905–1.319) | 1.460 (1.208–1.764) | <.001 |
| Model 2 | 1(Ref) | 1.609 (1.312–1.972) | 1.749 (1.425–2.145) | 2.542 (2.063–3.132) | <.001 | 1(Ref) | 1.186 (.967–1.454) | 1.312 (1.070–1.607) | 1.814 (1.474–2.233) | <.001 | |
| Model 3 | 1(Ref) | 1.490 (1.204–1.845) | 1.392 (1.116–1.736) | 1.765 (1.390–2.240) | <.001 | 1(Ref) | 1.055 (.854–1.304) | 1.109 (.898–1.371) | 1.428 (1.151–1.772) | .005 | |
| UACR | Model 1 | 1(Ref) | 1.517 (1.148–2.003) | 2.669 (2.057–3.465) | 3.526 (2.731–4.553) | <.001 | 1(Ref) | 1.440 (1.125–1.843) | 1.362 (1.065–1.742) | 1.867(1.471‐2.371) | <.001 |
| Model 2 | 1(Ref) | 1.679 (1.265–2.229) | 3.300 (2.522–4.319) | 5.169 (3.939–6.782) | <.001 | 1(Ref) | 1.568 (1.220–2.014) | 1.577 (1.226–2.028) | 2.350(1.834‐3.012) | <.001 | |
| Model 3 | 1(Ref) | 1.385 (1.031–1.859) | 2.261 (1.700–3.007) | 2.832 (2.014–3.813) | <.001 | 1(Ref) | 1.293 (.990–1.688) | 1.133 (.865–1.484) | 1.503(1.148‐1.968) | .016 | |
| CIMT≥.9 mm | Model 1 | 1(Ref) | 1.516 (1.214–1.893) | 1.888 (1.499–2.379) | 3.051 (2.357–3.948) | <.001 | 1(Ref) | 1.153 (.925–1.437) | 1.917 (1.512–2.432) | 2.465(1.912‐3.177) | <.001 |
| Model 2 | 1(Ref) | 1.519 (1.217–1.897) | 1.896 (1.504–2.389) | 3.079 (2.378–3.987) | <.001 | 1(Ref) | 1.153 (.925–1.437) | 1.917 (1.512–2.432) | 2.465(1.912‐3.177) | <.001 | |
| Model 3 | 1(Ref) | 1.650 (1.306–2.084) | 1.987(1.551‐2.547) | 3.075 (2.315–4.084) | <.001 | 1(Ref) | 1.146 (.916–1.433) | 1.858 (1.460–2.364) | 2.323 (1.796–3.005) | <.001 | |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; CI, Confidence Interval; FMD, flow‐mediated dilation; CIMT, carotid intima‐media thickness; LVH, left ventricular hypertrophy; OR, odds ratio; UACR, urine albumin–creatinine ratio.
The ORs and 95% CI for STOD were further evaluated using the quartile of FMD. Importantly, the patients in the highest FMD quartile had a significantly increased risk of STOD. The OR (95% CI) of LVH, UACR, and CIMT ≥.9 mm for the fourth versus first quartile of FMD were 1.428 (1.151–1.772), 1.503 (1.148–1.968), and 2.323 (1.796–3.005), respectively.
3.3. Relationship between baPWV/FMD and STOD indices in patients ≥65 years of age
The association between the baPWV/FMD and the risk of STOD among patients ≥65 years of age is presented in Table 4. The patients in the highest baPWV quartiles had a significantly increased risk of STOD. Compared with patients in the first quartile of baPWV, the OR (95%CIs) of UACR for the subjects in quartile four was 2.39 (1.53–3.75), P for trends <.001. Likewise, compared with patients of the first quartile of baPWV, the multivariate‐adjusted ORs and 95% CIs of CIMT ≥.9 mm were 2.647 (1.141–6.141), 2.789 (1.192–6.526), 4.374 (1.596–11.986) for the second, third, and fourth quartiles, respectively. However, the risk of STOD, assessed by LVH, was weakly associated with baPWV in patients ≥65 years of age. Also, we observed no significant association between the highest FMD quartiles and STOD indicators.
TABLE 4.
ORs (95% CIs) for subclinical target organ damage associated with quartile of brachial‐ankle pulse wave velocity and flow‐mediated dilation in participants ≥65 years of age
| Quartile of baPWV, cm/s | Quartile of FMD, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P‐for trends | Q1 | Q2 | Q3 | Q4 | P‐for trends | ||
| LVH | Model 1 | 1(Ref) | 1.640 (1.168–2.302) | 1.606 (1.144–2.253) | 1.657 (1.179–2.327) | 0.006 | 1(Ref) | 1.081 (.767–1.525) | .931 (.660–1.312) | .970 (.692–1.360) | .853 |
| Model 2 | 1(Ref) | 1.681 (1.171–2.413) | 1.421 (.987–2.046) | 1.336 (.917–1.946) | .04 | 1(Ref) | 1.017 (.705–1.465) | .912 (.632–1.314) | 1.038 (.724–1.489) | .903 | |
| Model 3 | 1(Ref) | 1.451 (.999–2.107) | 1.315 (.901–1.919) | 1.125 (.751–1.685) | .208 | 1(Ref) | .948 (.655–1.372) | .839 (.579–1.216) | .922 (.639–1.332) | .824 | |
| UACR | Model 1 | 1(Ref) | 1.285 (.805–2.053) | 1.678 (1.068–2.636) | 3.532 (2.3131–5.396) | <.001 | 1(Ref) | .945 (.630–1.419) | .883 (.584–1.337) | 1.087 (.732–1.613) | .779 |
| Model 2 | 1(Ref) | 1.285 (.805–2.053) | 1.678 (1.068–2.636) | 3.532 (2.311–5.396) | <.001 | 1(Ref) | .960 (.638–1.445) | .860 (.567–1.305) | 1.090 (.733–1.622) | .722 | |
| Model 3 | 1(Ref) | 1.022 (.628–1.665) | 1.337 (.833–2.146) | 2.393 (1.527–3.750) | <.001 | 1(Ref) | .883 (.583–1.336) | .793 (.520–1.210) | .962 (.642–1.442) | .71 | |
| CIMT≥ .9 mm | Model 1 | 1(Ref) | 2.625 (1.136–6.063) | 2.615 (1.132–6.041) | 4.230 (1.565–11.435) | .007 | 1(Ref) | 1.990 (.782–5.063) | 1.160 (.510–2.635) | 1.329 (.573–3.084) | .534 |
| Model 2 | 1(Ref) | 2.632 (1.133–6.071) | 2.493 (1.076–5.776) | 3.972 (1.465–10.770) | .01 | 1(Ref) | 2.073 (.812–5.289) | 1.167 (.512–2.659) | 1.276 (.548–2.970) | .493 | |
| Model 3 | 1(Ref) | 2.647 (1.141–6.141) | 2.789 (1.192–6.526) | 4.374 (1.596–11.986) | .006 | 1(Ref) | 2.016 (.789–5.151) | 1.139 (.499–2.602) | 1.224 (.524–2.861) | .524 | |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; CI, Confidence Interval; CIMT, carotid intima‐media thickness; FMD, flow‐mediated dilation; LVH, left ventricular hypertrophy; OR, odds ratio; UACR, urine albumin–creatinine ratio.
3.4. Interaction effect of baPWV and FMD in STOD in patients <65 years of age
To compute the interaction effect of elevated baPWV and reduced FMD among participants <65 years of age, we evaluated the risk of STOD and estimated the OR and 95% CI of STOD among those participants grouped in different quartiles of baPWV along with their corresponding FMD quartiles. An increase in baPWV shows a progressively higher risk of STOD across the quartiles of FMD (Figure 2). Those patients at higher quartiles of baPWV grouped by FMD had a higher risk of LVH, with patients in quartile four accounting for the highest risk of LVH (OR = 2.75). Also, the estimated risks of UACR for the first and fourth quartile of PWV increased from 2.35 to 4.44 in patients grouped by FMD quartiles. Similarly, the estimated risk of CIMT ≥.9 mm surged from 3.10 in quartile 1 to 6.10 in quartile four of baPWV in patients grouped by FMD quartiles.
FIGURE 2.
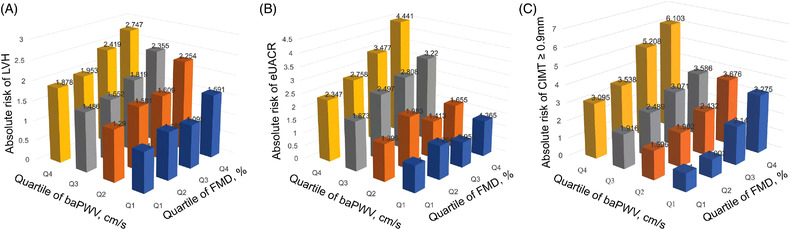
The risk of STOD based on baPWV quartiles in patients grouped by FMD quartiles. (A). The risk of LVH based on baPWV quartiles in patients grouped by FMD quartiles. (B). The risk of UACR based on baPWV quartiles in patients grouped by FMD quartiles. (C). The risk of CIMT ≥.9 mm based on baPWV quartiles in patients grouped by FMD quartiles. STOD, subclinical target organ damage; baPWV, brachial‐ankle pulse wave velocity; FMD, flow‐mediated dilation; LVH, left ventricular hypertrophy; UACR, urine albumin–creatinine ratio; CIMT, carotid intima‐media thickness
3.5. Receiver operating characteristics (ROC) analysis in patients <65 years of age
ROC analysis was used to evaluate the capacity of baPWV and FMD models to predict STOD in patients <65 years of age (Figure 3A). The AUC of baPWV and FMD models to predict LVH were .700 and .683, respectively. The sensitivity and specificity of baPWV were 47.2% and 68.2%, respectively. And Youden's index was .154 with the highest J value of 1576.25 cm/s. Similarly, the sensitivity and specificity of FMD were 74.2% and 32.9%, respectively with a Youden's index of .071. The highest J value was 3.15%. Then we further combined baPWV and FMD in a single model with the aim to evaluate whether the predictive power of the model can increase, and the AUC of the combined model reached .706.
FIGURE 3.
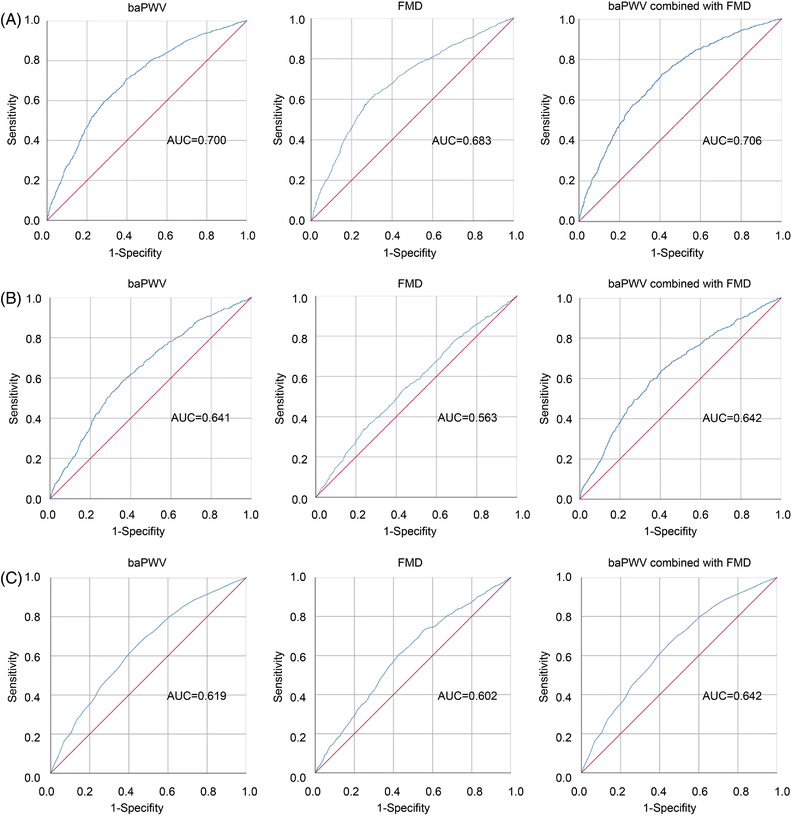
Receiver‐operating characteristics (ROC) curves for prediction of STOD with baPWV, FMD, and combined with baPWV and FMD. (A) ROC curve for prediction of LVH with baPWV, FMD, and combined with baPWV and FMD, respectively. (B) ROC curve for prediction of eUACR with baPWV, FMD, and combined with baPWV and FMD, respectively. (C) ROC curve for prediction of CIMT ≥.9 mm with baPWV, FMD, and combined with baPWV and FMD, respectively. STOD, subclinical target organ damage; baPWV, brachial‐ankle pulse wave velocity; FMD, flow‐mediated dilation; LVH, left ventricular hypertrophy; UACR, urine albumin–creatinine ratio; CIMT, carotid intima‐media thickness
We also performed ROC analysis to determine the diagnostic utility of the baPWV, FMD, and the combined model to identify patients with STOD assessed by UACR. The AUC for baPWV and FMD models were .641 and .563, respectively. The sensitivity and specificity of baPWV were 61.8% and 61.3%, respectively. Youden's index was .231 and the highest J value was 1543.75 cm/s. Likewise, the sensitivity and specificity of FMD were 68.2% and 41.2%, and Youden's index was .094 and the highest J value was 3.45%. We tested then models to improve the identification of STOD by combining both baPWV and FMD parameters. The combination of the baPWV and FMD model reached the highest AUC of .642. The AUC results for the baPWV and FMD models and the combined model are shown in Figure 3B.
Briefly, the AUC of the baPWV, FMD, and the combined models for the risk of STOD assessed by CIMT ≥.9 mm were .619, .602, and .642 (Figure 3C). The sensitivity and specificity of baPWV were 41.2% and 75.2%, respectively, and Youden's index was .173 and the highest J value was 1576.75 cm/s. Whereas the sensitivity and specificity of FMD were 60.9% and 56.8%, respectively, and Youden's index was .177 and the highest J value was 4.95%.
3.6. Sensitivity analysis
We run the sensitivity analysis with the aim to estimate the magnitude of the risk of bias attributed to the missing data. Interestingly. we find that there was no statistically significant difference in the group of patients with missing data and the group of patients without missing data (Supplementary Table S3).
4. DISCUSSION
In this retrospective cross‐sectional study, baPWV and FMD parameters were positively associated with STOD indicators in patients with essential hypertension. This association of the baPWV and FMD with STOD indicators remain consistent even after adjusting for potential confounders. Participants with the highest quartile of baPWV and FMD (baPWV ≥1683 cm/s, FMD ≤2.8% in <65 years of age, respectively) had the highest risk of STOD compared with patients in the lowest quartiles of baPWV and FMD. However, there was no significant relationship between baPWV and FMD measurements and the risk of STOD in patients ≥65 years of age.
In the past, various studies reported that baPWV is associated with cardiovascular morbidity and mortality, as well as with several cardiovascular risk factors. 27 , 28 , 29 , 30 The PWV has also been thoroughly examined, and an elevated baPWV has been identified as a marker of vascular function. 16 Furthermore, arterial stiffness might be involved in a vicious cycle with the development/progression of hypertension, chronic kidney disease, and diabetes mellitus, 31 suggesting high baPWV and low FMD are not only considered as risk factors for hypertension but also consequences of the damage that hypertension causes on the vessels and heart. In this study, the risk of STOD increases across baPWV and FMD quartiles. The findings regarding baPWV and FMD associations with STOD indicators remain consistent after multivariate adjustment for the potential confounding factors, suggesting that a vascular status evaluation is not inferior to the control strategy of hypertension‐related complications. Thus, our results provide insights into the significance of strict measurement of baPWV and FMD parameters to predict STOD as endothelial dysfunction and arterial stiffness processes may lead to an increased socioeconomic burden of hypertension‐associated CVDs and mortality.
baPWV is a marker of arterial stiffness and has been used for the assessment of vascular function. Moreover, baPWV and STOD are known to be independent predictors of future cardiovascular events. 32 According to our study, baPWV was independently associated with an increased risk of LVH, which was consistent with Tomiyama's demonstration. 10 Mechanically, PWV reflects segmental arterial elasticity, and contraction of the left ventricle generates a pulse wave that is propagated throughout the arterial tree. Evidently, an increase in arterial stiffness/baPWV is reported to associate with an increase in the propagation speed of this pulse wave, which could contribute to a greater magnitude of blood flow back from the peripheral artery to the aortic artery during late systole. 33 These changes in the blood circulation could again aggravate the left ventricular afterload and eventually may lead to LVH.
Recently, several studies recommended UACR 18 , 34 as predictors of STOD in patients with hypertension. However, there was a lack of robust knowledge on whether the combination of the endothelial dysfunction and arterial stiffness indicators is sufficient to predict the STOD assessed by the UACR. To the extent of our knowledge, this was the first study to evaluate the association between baPWV and FMD and STOD, and we found that elevated baPWV and FMD were independently linked with STOD indicators including UACR in middle‐aged hypertension patients. Also, the interaction analyses between baPWV and FMD parameters show that patients in higher baPWV quartiles grouped by the FMD quartiles reflect prominent UACR, indicating that high/low values baPWV/FMD are associated with an increased risk of STOD.
Earlier evidence established that elevated arterial stiffness linked with early stages of chronic kidney disease, 35 and vascular calcification related to advanced chronic kidney disease may amplify this increase in arterial stiffness. 36 Various reasons could explain the observed relationship between endothelial dysfunction and arterial stiffness indicators and UACR. Hypertension is a component of metabolic syndrome, and endothelial dysfunction has been reported as an early pathogenic event in metabolic syndrome. 37 What's more, Tomiyama and colleagues 38 found that persistent metabolic syndrome status aggravates the progression of arterial stiffness. The other possible linkage is through the renal resistive index. Previous studies have reported that microalbuminuria and arterial stiffness are related to resistive index. 39 Also, chronic inflammation‐induced endothelial dysfunction increases the progression to atherosclerosis and aggravates permeability of the capillary basement membrane of the glomerulus, which could further lead to albumin leak from the basement membrane and may cause the appearance of UACR. 40 , 41
Arterial stiffness and atherosclerosis often coexist in the same vascular territories and share similar risk factors. And the presence of atherosclerosis decreases the compliance of blood vessels, leading to stiffening of the arteries. 28 In addition, atherosclerosis may also increase arteriosclerosis in its advanced stage. 29 In the present study, we found that baPWV and FMD were positively associated with CIMT ≥.9 mm. This could be explained by the fact that arterial stiffness could be related to atherosclerosis through endothelial dysfunction, mechanical force on the inner wall of blood vessels, extracellular matrix disorder, elevated endothelial permeability, and vascular aging. 26 , 27
The high values of baPWV were significantly associated with the risk of STOD in participants younger than 65 years of age, whereas this association was weakened to a nonsignificant level when we analyzed data in the age group ≥65 years. PWV is known to be positively associated with age. 42 Of the many risk factors associated with endothelial dysfunction and arterial stiffness, age and hypertension are by far the most important factors influencing vascular function. 43 , 44 Aging per se is a promoter of arterial stiffness, especially in the older population. 45 Consequently, this may mask the associations between baPWV for STOD in the age category ≥65, but not in patients <65 years of age. Therefore, further longitudinal studies are needed to validate the potential benefit of implementing baPWV and FMD screening in order to detect STOD in the middle‐aged population.
The present study demonstrated that baPWV and FMD biomarkers own a good prognostic performance for STOD independently. Compared with the baPWV model alone, the unified model that combined the baPWV and FMD offered a slight increase in the AUC in STOD detection. Therefore, simultaneous application of both baPWV and FMD parameters facilitates the prediction of the risk of STOD more accurately among patients with essential hypertension.
5. LIMITATIONS
The current study has certain advantages and disadvantages. To the best of our knowledge, no study has looked into the interaction between baPWV and FMD to predict STOD in hypertension. patients using hospital data. This study, however, should be interpreted with some limitations. First, the single‐centered retrospective design has limited the cause‐and‐effect relationship between baPWV/FMD and the risk of STOD in hypertension. Also, our data cannot provide data on the long‐term visit‐to‐visit variabilities of baPWV and FMD and their association with STOD prognosis. Third, the prevalence of STOD increases dramatically in cases of uncontrolled blood pressure, and in many cases, BP was not controlled. Consequently, it is unclear whether the STOD risk is related to the presence of essential hypertension or to the effectiveness or otherwise of therapy as every medication is known to have a positive or negative effect on cardiovascular risk. Thus, our study would have benefited from dividing patients according to blood pressure values at the time of evaluation of baPWV and FMD parameters. However, we considered the adjustment of the individual antihypertensive drugs including calcium channel blocker, angiotensin‐converting enzyme receptor blocker, β‐Blocker, Diuretic, and α‐Blocker in the regression model to minimize the risk of bias inherited to the possible positive and negative effects of the different types of antihypertensive use.
6. CONCLUSION
We found strong independent associations between baPWV and FMD parameters and the risk of STOD, assessed by LVH, UACR, CIMT ≥.9 mm, in hypertension patients. Also, our study identified that elevated baPWV and decreased FMD were independent prognostic markers for STOD in middle‐aged hypertension patients. The combination of baPWV and FMD significantly improved the prediction of STOD in hypertension patients, indicating that the simultaneous application of this unified model can slightly improve discrimination for STOD in hypertension patients.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
PATIENT CONSENT STATEMENT
Informed consent was obtained from all individual participants involved in the study.
AUTHOR CONTRIBUTIONS
Yancui Sun contributed to study concept and design, acquisition of data, and interpretation of data. Fei Liu contributed to acquisition of data and interpretation of data. Ying Zhang, Yan Lu, Zhuolin Su and Haizhe Ji contributed to study design and statistical analysis. Yunpeng Cheng, Wei Song and Tesfaldet H. Hidru contributed to acquisition of data. Xiaolei Yang contributed to study concept and design, interpretation of data and study supervision. Yinong Jiang contributed to study concept and design, interpretation of data and critical revision of manuscript for intellectual content.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
This research was funded by the National Natural Science Foundation of China (grant number 81900439 and 82070427), the Science Foundation of Doctors of Liaoning Province (grant number 2020‐BS‐197), the Liaoning Revitalization Talents Program (grant number XLYC2002096), and Liaoning province science and technology project (2021JH1/10400050).
Sun Y, Liu F, Zhang Y, et al. The relationship of endothelial function and arterial stiffness with subclinical target organ damage in essential hypertension. J Clin Hypertens. 2022;24:418–429. 10.1111/jch.14447
Yancui Sun and Fei Liu contributed equally to this article and are considered co‐first authors
Contributor Information
Xiaolei Yang, Email: yangxl1012@yeah.net.
Yinong Jiang, Email: yinongjiang@126.com.
REFERENCES
- 1. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pontremoli R, Leoncini G, Viazzi F, et al. Evaluation of subclinical organ damage for risk assessment and treatment in the hypertensive patient: role of microalbuminuria. J Am Soc Nephrol. 2006;17(4 Suppl 2):S112–114. [DOI] [PubMed] [Google Scholar]
- 3. Agabiti‐Rosei E, Muiesan ML, Salvetti M. Evaluation of subclinical target organ damage for risk assessment and treatment in the hypertensive patients: left ventricular hypertrophy. J Am Soc Nephrol. 2006;17(4 Suppl 2):S104–108. [DOI] [PubMed] [Google Scholar]
- 4. Fantin F, Mattocks A, Bulpitt CJ, Banya W, Rajkumar C. Is augmentation index a good measure of vascular stiffness in the elderly? Age Ageing. 2007;36(1):43–48. [DOI] [PubMed] [Google Scholar]
- 5. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr. , Lerman A. Long‐term follow‐up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. [DOI] [PubMed] [Google Scholar]
- 6. Shirai K, Saiki A, Nagayama D, Tatsuno I, Shimizu K, Takahashi M. The role of monitoring arterial stiffness with cardio‐ankle vascular index in the control of lifestyle‐related diseases. Pulse (Basel). 2015;3(2):118–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yildiz O. Vascular smooth muscle and endothelial functions in aging. Ann N Y Acad Sci. 2007;1100:353–360. [DOI] [PubMed] [Google Scholar]
- 8. Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116(6):1007–1021. [DOI] [PubMed] [Google Scholar]
- 9. Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin. 2006;24(1):135–146. [DOI] [PubMed] [Google Scholar]
- 10. Tomiyama H, Ishizu T, Kohro T, et al. Longitudinal association among endothelial function, arterial stiffness and subclinical organ damage in hypertension. Int J Cardiol. 2018;253:161–166. [DOI] [PubMed] [Google Scholar]
- 11. Tomiyama H, Yamashina A. Non‐invasive vascular function tests: their pathophysiological background and clinical application. Circ J. 2010;74(1):24–33. [DOI] [PubMed] [Google Scholar]
- 12. Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73(3):411–418. [DOI] [PubMed] [Google Scholar]
- 13. Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow‐mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109(5):613–619. [DOI] [PubMed] [Google Scholar]
- 14. Takase H, Dohi Y, Toriyama T, et al. Brachial‐ankle pulse wave velocity predicts increase in blood pressure and onset of hypertension. Am J Hypertens. 2011;24(6):667–673. [DOI] [PubMed] [Google Scholar]
- 15. Hall JE. Guyton and Hall textbook of medical physiology, 13th ed. Guyton and Hall textbook of medical physiology, 13th ed. [Google Scholar]
- 16. Tanaka A, Tomiyama H, Maruhashi T, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72(5):1060–1071. [DOI] [PubMed] [Google Scholar]
- 17. Neves MF, Kasal DA, Cunha AR, Medeiros F. Vascular dysfunction as target organ damage in animal models of hypertension. Int J Hypertens. 2012;2012:187526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. [DOI] [PubMed] [Google Scholar]
- 19. Li R, Chen Y, Moore JH. Integration of genetic and clinical information to improve imputation of data missing from electronic health records. J Am Med Inform Assoc. 2019;26(10):1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bild DE, Bluemke DA, Burke GL, et al. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 21. Ford ES, Giles WH, Mokdad AH. The distribution of 10‐year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol. 2004;43(10):1791–1796. [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Devereux RB, Dahlof B, Gerdts E, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110(11):1456–1462. [DOI] [PubMed] [Google Scholar]
- 24. O'Leary DH, Bots ML. Imaging of atherosclerosis: carotid intima‐media thickness. Eur Heart J. 2010;31(14):1682–1689. [DOI] [PubMed] [Google Scholar]
- 25. Lee JY, Ryu S, Lee SH, et al. Association between brachial‐ankle pulse wave velocity and progression of coronary artery calcium: a prospective cohort study. Cardiovasc Diabetol. 2015;14:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Santulli G, Pascale V, Finelli R, et al. We are what we eat: impact of food from short supply chain on metabolic syndrome. J Clin Med. 2019;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. [DOI] [PubMed] [Google Scholar]
- 29. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. [DOI] [PubMed] [Google Scholar]
- 30. Tomiyama H, Matsumoto C, Shiina K, Yamashina A. Brachial‐ankle PWV: current status and future directions as a useful marker in the management of cardiovascular disease and/or cardiovascular risk factors. J Atheroscler Thromb. 2016;23(2):128–146. [DOI] [PubMed] [Google Scholar]
- 31. Tomiyama H, Shiina K. State of the art review: brachial‐ankle PWV. J Atheroscler Thromb. 2020;27(7):621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Asselbergs FW, de Boer RA, Diercks GF, et al. Vascular endothelial growth factor: the link between cardiovascular risk factors and microalbuminuria? Int J Cardiol. 2004;93(2‐3):211–215. [DOI] [PubMed] [Google Scholar]
- 34. Haffner SM, Stern MP, Gruber MK, Hazuda HP, Mitchell BD, Patterson JK. Microalbuminuria. Potential marker for increased cardiovascular risk factors in nondiabetic subjects? Arteriosclerosis. 1990;10(5):727–731. [DOI] [PubMed] [Google Scholar]
- 35. Yoshida M, Tomiyama H, Yamada J, et al. Relationships among renal function loss within the normal to mildly impaired range, arterial stiffness, inflammation, and oxidative stress. Clin J Am Soc Nephrol. 2007;2(6):1118–1124. [DOI] [PubMed] [Google Scholar]
- 36. Lioufas NM, Pedagogos E, Hawley CM, et al. Aortic calcification and arterial stiffness burden in a chronic kidney disease cohort with high cardiovascular risk: baseline characteristics of the impact of phosphate reduction on vascular end‐points in chronic kidney disease trial. Am J Nephrol. 2020;51(3):201–215. [DOI] [PubMed] [Google Scholar]
- 37. Deedwania PC. Mechanisms of endothelial dysfunction in the metabolic syndrome. Curr Diab Rep. 2003;3(4):289–292. [DOI] [PubMed] [Google Scholar]
- 38. Tomiyama H, Hirayama Y, Hashimoto H, et al. The effects of changes in the metabolic syndrome detection status on arterial stiffening: a prospective study. Hypertens Res. 2006;29(9):673–678. [DOI] [PubMed] [Google Scholar]
- 39. Sengstock DM, Vaitkevicius PV, Supiano MA. Arterial stiffness is related to insulin resistance in nondiabetic hypertensive older adults. J Clin Endocrinol Metab. 2005;90(5):2823–2827. [DOI] [PubMed] [Google Scholar]
- 40. Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB, Sr. , Wilson PW. C‐reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110(4):380–385. [DOI] [PubMed] [Google Scholar]
- 41. Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol. 2013;9(8):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Avolio AP, Kuznetsova T, Heyndrickx GR, Kerkhof PLM, Li JK. Arterial flow, pulse pressure and pulse wave velocity in men and women at various ages. Adv Exp Med Biol. 2018;1065:153–168. [DOI] [PubMed] [Google Scholar]
- 43. Taddei S, Virdis A, Mattei P, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91(7):1981–1987. [DOI] [PubMed] [Google Scholar]
- 44. Bruno RM, Duranti E, Ippolito C, et al. Different impact of essential hypertension on structural and functional age‐related vascular changes. Hypertension. 2017;69(1):71–78. [DOI] [PubMed] [Google Scholar]
- 45. AlGhatrif M, Wang M, Fedorova OV, Bagrov AY, Lakatta EG. The pressure of aging. Med Clin North Am. 2017;101(1):81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


