Abstract
Background
Esophageal and gastric cancer surgery are associated with considerable morbidity, specifically postoperative pulmonary complications (PPCs), potentially accentuated by underlying challenges with malnutrition and cachexia affecting respiratory muscle mass. Physiotherapy regimens aim to increase the respiratory muscle strength and may prevent postoperative morbidity.
Objective
The aim of this study was to assess the impact of physiotherapy regimens in patients treated with esophagectomy or gastrectomy.
Methods
An electronic database search was performed in the MEDLINE, EMBASE, CENTRAL, CINAHL and Pedro databases. A meta-analysis was performed to assess the impact of physiotherapy on the functional capacity, incidence of PPCs and postoperative morbidity, in-hospital mortality rate, length of hospital stay (LOS) and health-related quality of life (HRQoL).
Results
Seven randomized controlled trials (RCTs) and seven cohort studies assessing prehabilitation totaling 960 patients, and five RCTs and five cohort studies assessing peri- or postoperative physiotherapy with 703 total patients, were included. Prehabilitation resulted in a lower incidence of postoperative pneumonia and morbidity (Clavien–Dindo score ≥ II). No difference was observed in functional exercise capacity and in-hospital mortality following prehabilitation. Meanwhile, peri- or postoperative rehabilitation resulted in a lower incidence of pneumonia, shorter LOS, and better HRQoL scores for dyspnea and physical functioning, while no differences were found for the QoL summary score, global health status, fatigue, and pain scores.
Conclusion
This meta-analysis suggests that implementing an exercise intervention may be beneficial in both the preoperative and peri- or postoperative periods. Further investigation is needed to understand the mechanism through which exercise interventions improve clinical outcomes and which patient subgroup will gain the maximal benefit.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-021-11122-7.
Background
Despite improvements in perioperative management, surgery for esophageal and gastric cancer is associated with considerable morbidity, particularly postoperative pulmonary complications (PPCs). PPCs include pneumonia and atelectasis, occur in about 20–40% of patients,1–3 and account for up to 55% of in-hospital deaths.4,5 The risk for PPCs development is multifactorial and includes both patient- and treatment-related factors. Open surgery is associated with significantly higher pain scores, which may interfere with respiratory mechanics and impede adequate mobilization, resulting in atelectasis and shallow breathing.6–10 Other intraoperative procedures that might contribute to PPCs include mechanical ventilation, patient positioning, and administration of sedatives.11–14 Following surgery, patients undergoing esophagogastrectomy often require chest drainage, contributing further to postoperative pain and altered respiratory mechanics.15,16
Preoperative factors also have an impact on the development of PPCs, including preoperative chemoradiotherapy. Although neoadjuvant chemoradiotherapy has led to a significant improvement in survival in esophageal and esophagogastric junction cancer,17 several studies suggest that the associated toxicity affects pulmonary function, causing a decline in diffusion capacity, and total lung and vital capacity.18 In addition to decreased exercise capacity following surgery,19 neoadjuvant chemotherapy is also associated with reduced physical fitness.20 Other predictive factors for severe complications include lower preoperative forced expiratory volume in 1 second (FEV1),21,22 decreased diffusion capacity,23 multiple comorbidities, and smoking.24 Physical activity levels also play an important role in the postoperative period, as patients with higher activity levels appeared to have a significantly lower risk for cancer recurrence, and had higher overall survival rates and better HRQoL scores compared with inactive participants.25 Finally, patients with gastrointestinal cancers often present with malnutrition and cachexia26–28 affecting respiratory muscle mass and strength, and subsequently increasing the risk for development of PPCs29,30 and poor functional capacity.31
Physiotherapy regimens such as early mobilization and breathing exercises aim to decrease the risk for PPCs by reversing atelectasis.32 There is some evidence that breathing exercises, both in the preoperative period32 and during postoperative recovery,33 decrease the incidence of PPCs in upper abdominal surgery. However, due to insufficient strong evidence, routine implementation of respiratory physiotherapy following abdominal surgery has not yet been implemented as a standard of care.32
To date, there has been no published meta-analysis assessing the effect of prehabilitation and peri- or postoperative physiotherapy regimens on postoperative mortality and morbidity in esophageal and gastric cancer surgery. The primary aim of this meta-analysis was to assess the impact of physiotherapy regimens on the incidence of major postoperative morbidity and in-hospital mortality. The secondary aims were to assess whether physiotherapy implementation decreases the length of hospital stay (LOS) and improves the functional exercise capacity and HRQoL.
Methods
Search Strategy
A literature search was performed on the 18 February 2021 to identify relevant studies assessing physiotherapy regimens in patients undergoing esophagectomy or gastrectomy in the MEDLINE (Ovid), EMBASE (Ovid), Cochrane Central Register of Controlled Trials, CINAHL, and Physiotherapy Evidence (Pedro) databases. The search included the following index or free-text words, including synonyms and closely related words: ‘(o)esophagectomy’, ‘gastrectomy’, ‘physiotherapy’, ‘physical therapy’, ‘kinesi(o)therapy’, ‘muscle training’, ‘mobilization’, and ‘breathing techniques’. References of included articles were screened and a hand-search was performed to identify missing articles. The full electronic search strategy is available in electronic supplementary (ES) Table S1.
Two reviewers (KHT and NG) independently assessed the titles and abstracts for inclusion of relevant references. In the case of disagreement for inclusion, a third author (SRM) was consulted. Authors of the included studies were contacted to locate unpublished data.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)33 guidelines were followed (ES Table S2).
Study Selection
Randomized controlled trials (RCTs), quasi-randomized trials, and cohort studies were included, implementing physiotherapy regimens in the pre-, peri- or postoperative periods for patients who have undergone esophagectomy or gastrectomy for malignant disease, either by open surgery or a minimally invasive approach.
Comparative studies were excluded if no outcome data were provided for the control or intervention groups. Studies were excluded if physiotherapy in the form of early mobilization was part of an enhanced recovery pathway and the impact of the pathway was assessed without evaluating the impact of the physiotherapy component.
Outcome Measures
The primary outcomes were the incidence of postoperative morbidity assessed using a Clavien–Dindo classification34 (CDC) of 2 or higher, PPCs, and in-hospital mortality, while the secondary outcomes were assessment of the functional capacity via the 6-minute walking test (6MWT), LOS, and HRQoL.
Quality Assessment of Selected Studies
Two reviewers (KT and NG) assessed the quality of each included study by independently evaluating the risk of bias using the revised Cochrane risk-of-bias tool (RoB2)35 for the assessment of randomized trials and the Newcastle–Ottawa Scale (NOS)36 for the assessment of non-randomized studies. The RoB2 tool categorizes the risk of bias into ‘low’, ‘some concerns’, or ‘high’ risk of bias. For the NOS scores ranging from 0 to 9, we considered a score of 0–3, 4–6, and 7–9 as low, moderate, or high-quality studies, respectively.
Statistical Analysis
Meta-analysis of the data was performed using the Review Manager version 5.3 software (Cochrane Collaboration, Oxford, UK). Both the fixed-effects and random-effects models were considered in the analysis of the data and were the most appropriate models used to pool the results based on the distribution of the data. The standard heterogeneity test, the I2 statistic, was used to assess the consistency of the effect sizes, which indicates the percentage of the variability in effect estimates because of true between-study variance rather than within-study variance. Statistical heterogeneity was graded as low, moderate, or high, with an I2 of above 25%, 50%, and 75%, respectively.37
Results
Literature Search Results
The electronic database searches yielded 11,273 results. The database searches were complemented by a hand search, identifying eight articles through published systematic reviews and protocols. After removal of duplicates, 8048 publications were screened on the abstract. Subsequent screening of the full-text identified 33 relevant records, 17 of which assessed preoperative exercise programs (prehabilitation), while 16 studies assessed peri- or postoperative physiotherapy regimens (Fig. 1).
Fig. 1.
PRISMA flow chart. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Among the studies assessing prehabilitation, seven were RCTs38–44 with an intervention group and a comparative control group, while one RCT45 compared two different types of interventions; the remaining nine publications were cohort studies.2,46–53 For the studies assessing peri- and postoperative physiotherapy, there were six RCTs54–59 comparing an intervention with a control group, and one study60 compared two different types of physiotherapy. Among the cohort studies, eight studies61–68 had an intervention group and a comparative control, and one study69 assessed three groups consisting of two physiotherapy intervention groups and one comparative control group. An overview of interventions for studies included in the meta-analysis is shown in Table 1; a summary of the study characteristics of all studies is shown in ES Table S3, with a comprehensive summary of interventions for all studies provided in shown in ES Table S4. A summary of the statistical method used for each analysis is presented in ES Table S5.
Table 1.
Overview of physiotherapy interventions of the studies included in the meta-analysis
| Authors (year) | Study type | Group (n) | Intervention timing and duration | Type of intervention | Outcomes |
|---|---|---|---|---|---|
| Prehabilitation | |||||
| Dettling et al.52 (2013) | NRS | IG (44) | Preoperative respiratory training (duration: minimum 2 weeks) and postoperative physical therapy (duration: 10 days) |
Preoperative IMT Postoperative respiratory rehabilitation and mobilization |
Incidence pneumonia: 11/44 Incidence other PPCs: 9/44 Incidence in-hospital mortality: 1/44 Median (IQR) LOS: 13.5 (10.0–22.75) |
| CG (39) | – | Postoperative respiratory rehabilitation and mobilization |
Incidence pneumonia: 9/39 Incidence other PPCs: 6/39 Incidence in-hospital mortality: 3/39 Median (IQR) LOS: 12.0 (9.0–14.0) |
||
| Inoue et al.2 (2013) | Cohort study | IG (63) | Preoperatively (duration: minimum 1 week) |
Preoperative IMT, muscle strength training for UL and LL, and abdominal muscles and aerobic exercise Postoperative respiratory rehabilitation and mobilization |
Incidence pneumonia: 4/63 Incidence CDC ≥ II: 4/63 Mean (SD) LOS: 41.2 (32.2) |
| CG (37) | – |
Preoperative rehabilitation insufficiently (or not) received Postoperative respiratory rehabilitation and mobilization |
Incidence pneumonia: 9/37 Incidence CDC ≥ II: 9/37 Mean (SD) LOS: 49.8 (28.9) |
||
| Cho et al.49 (2014) | Cohort study | IG (18) | Preoperatively (duration: 4 weeks) |
Aerobic exercise and stretching before and after exercise Resistance training |
Incidence pneumonia: 0/18 Incidence other PPCs: 3/18 Median (95% CI) LOS: 9.0 (9.0–10.0) |
| CG (54) | – | No preoperative intervention |
Incidence pneumonia: 2/54 Incidence other PPCs: 8/54 Median (95% CI) LOS: 10.0 (9.0–11.0) |
||
| Yamana et al.38 (2015) | RCT | IG (30) | Preoperatively (duration: minimum 1 week) |
Preoperative IMT, muscle strength exercises LL and abdominal muscles, and aerobic exercise Postoperative respiratory rehabilitation and mobilization |
Incidence pneumonia: 10/30 Incidence CDC ≥ II: 8/30 Incidence in-hospital mortality: 0/30 |
| CG (30) | – |
No preoperative intervention Postoperative respiratory rehabilitation and mobilization |
Incidence pneumonia: 17/30 Incidence CDC ≥ II: 18/30 Incidence in-hospital mortality: 0/30 |
||
| Weblin et al.48 (2017) | NRS | IG (13) | Preoperatively (duration: 4 weeks) |
Preoperative respiratory rehabilitation with warm-up, cool down and mobilization ERAS and enhanced postoperative respiratory rehabilitation and mobilization |
Median (IQR) LOS: 13.0 (11.0–20.0) |
| CG (10) | – | ERAS and enhanced postoperative respiratory rehabilitation and mobilization | Median (IQR) LOS: 14.0 (11.5–21.0) | ||
| Valkenet et al.44 (2017) | RCT | IG (120) | Preoperatively (duration: minimum 2 weeks) |
Preoperative IMT Postoperative airway clearance technique and early mobilization |
Incidence pneumonia: 47/120 Incidence other PPCs: 41/118 Incidence in-hospital mortality: 5/120 Mean (SD) LOS: 18.4 (8.0) |
| CG (121) | – |
No preoperative intervention Postoperative airway clearance technique and early mobilization |
Incidence pneumonia: 43/121 Incidence other PPCs: 40/120 Incidence in-hospital mortality: 3/121 Mean (SD) LOS: 20.5 (20.9) |
||
| Christensen et al.46 (2019) | NRS | IG (21) | Preoperatively during neoadjuvant treatment (duration: 9 weeks) | Aerobic and resistance training |
Incidence pneumonia: 4/21 Incidence CDC ≥ II: 11/21 Median (IQR) LOS: 10.0 (9.0–11.0) |
| CG (29 ) | – | Information advice, physiotherapy guidelines |
Incidence pneumonia: 3/29 Incidence CDC ≥ II: 13.29 Median (IQR) LOS: 9.0 (8.0–11.0) |
||
| Minnella et al.40 (2018) | RCT | IG (26) | Preoperatively, duration NS (77% during NACT) |
Aerobic and muscle strength exercises Nutritional advice and support |
Mean (SD) postop 6MWT: 467.5 (65.6) Incidence CDC ≥ II: 12/24 Incidence in-hospital mortality: 0/26 Median (IQR) LOS: 8.0 (5.75–11.75) |
| CG (25) | – | Standardized ERAS with respiratory physiotherapy and early mobilization |
Mean (SD) postop 6MWT: 367.4 (87.0) Incidence CDC ≥ II: 18/25 Incidence in-hospital mortality: 2/25 Median (IQR) LOS: 7.0 (5.5–12.5) |
||
| Guinan et al.42 (2019) | RCT | IG (28) | Subcohort PREPARE trial, intervention as described by Valkenet et al.44 |
Mean (SD) postop 6MWT: 305.6 (116.3) Incidence PPCs: 9/28 Incidence in-hospital mortality: 0/28 Median (IQR) LOS: 17.0 (8.0) |
|
| CG (32) | – |
No preoperative intervention Postoperative airway clearance technique and early mobilization |
Mean (SD) postop 6MWT: 380.2 (47.1) Incidence PPCs: 11/32 Incidence in-hospital mortality: 0/32 Median (IQR) LOS: 18.0 (15.0) |
||
| Lam et al.41 (2018) | RCT, thesis | IG (5) | Preoperatively (duration: 14–16 weeks) |
In-hospital: aerobic and muscle strengthening exercise Home-based IMT |
Incidence PPCs: 4/5 |
| CG (6) | – | Home exercise advice | Incidence PPCs: 4/6 | ||
| Akiyama et al.51 (2021) | Cohort study | IG (23) | Preoperatively (duration: 1 week) |
In-hospital: aerobic exercise and muscle strength training Home-based: 1-month preoperative IMT, mobilization and muscle strength training Postoperative early mobilization, ambulation, IMT and training for chewing and swallowing and aerobic exercise |
Mean (SD) postop 6MWT: 431.5 (80.0) Incidence pneumonia: 1/23 Incidence other PPCs: 1/23 Incidence CDC ≥ II: 2/23 Incidence in-hospital mortality: 0/23 Median (IQR) LOS: 14.0 (12.0–16.0) |
| CG (25) | – |
Historical controls: Home-based: 1-month preoperative IMT, mobilization and muscle strength training Postoperative early mobilization, ambulation, IMT and training for chewing and swallowing and aerobic exercise |
Mean (SD) postop 6MWT: 378.0 (68.7) Incidence pneumonia: 5/25 Incidence other PPCs: 9/25 Incidence CDC ≥ II: 8/25 Incidence in-hospital mortality: 0/25 Median (IQR) LOS: 16.0 (13.5–19.5) |
||
| Halliday et al.47 (2020) | Cohort study | IG (38) | Preoperatively during neoadjuvant treatment, duration NS |
Aerobic and strength exercise training Nutritional support Psychological support ERAS with early mobilization |
Incidence pneumonia: 10/38 Incidence other PPCs: 12/38 Incidence CDC ≥ II: 12/38 Median (IQR) LOS: 10.0 (8.0–17.0) |
| CG (38) | – | ERAS with early mobilization |
Incidence pneumonia: 25/38 Incidence other PPCs: 26/38 Incidence CDC ≥ II: 18.38 Median (IQR) LOS: 13.0 (11.0–20.0) |
||
| Swaminathan et al.43 (2020) | RCT | IG (29) | Preoperatively (duration: 1 week) |
IMT with IS ERAS protocol with early mobilization |
Incidence CDC ≥ II: 1/29 Median (IQR) LOS: 11.0 (3.0) |
| CG (29) | – | No preoperative intervention |
Incidence CDC ≥ II: 4/29 Median (IQR) LOS: 13.0 (4.0) |
||
| Zylstra et al.53 (2020) | Cohort | IG (13) | Preoperatively during neoadjuvant treatment, duration NS |
Aerobic and strength training Core strength and stability training Flexibility exercises |
Incidence PPCs: 4/13 Incidence CDC ≥ II: 2/13 Incidence in-hospital mortality: 0/13 Median (IQR) LOS: 10.83 (9.0–13.0) |
| CG (14) | – | No preoperative intervention |
Incidence PPCs: 4/14 Incidence CDC ≥ II: 3/14 Incidence in-hospital mortality: 0/14 Median (IQR) LOS: 9.67 (8.0–12.0) |
||
| Peri- or postoperative rehabilitation | |||||
| Lunardi et al.67 (2011) | Cohort study | IG (40) | Immediate postoperatively, duration NS (until discharge) | IMT, airway clearance maneuvers and early mobilization |
Incidence pneumonia: 1/40 Incidence other PPCs: 1/40 Median (95% CI) LOS: 13.5 (3.6–29.8) |
| CG (30) | – | Historical controls |
Incidence pneumonia: 3/30 Incidence other PPCs: 1/30 Median (95% CI) LOS: 14.0 (8.0–24.2) |
||
| Lococo et al.68 (2012) | Cohort study | IG (8) | Postoperatively (duration: 4 weeks) |
Aerobic exercise, IMT and muscle strength training for UL and LL Educational sessions for nutrition, psychological support and breathing exercises |
Incidence PPCs: 2/8 |
| CG (50) | – |
Historical controls: IMT, to achieve early mobilization General exercise therapy |
Incidence PPCs: 13/50 | ||
| Akiyama et al.61 (2017) | Cohort study | IG (31) | Perioperatively, duration NS | IMT, muscle strength training and early mobilization |
Incidence pneumonia: 4/31 Incidence other PPCs: 14/31 Incidence in-hospital mortality: 0/31 |
| CG (21) | – | Historical controls |
Incidence pneumonia: 5/21 Incidence other PPCs: 8/21 Incidence in-hospital mortality: 0/21 |
||
| Fagevik Olsén et al.55 (2017) | RCT | IG (20) | Postoperatively, at discharge (duration: 3 months) |
IMT with CPAP during ICU; deep breathing exercises with PEP; mobilization; muscle strength training Respiratory rehabilitation: stretching, IMT |
Mean (SD) LOS: 19.7 (10.2) HRQoL: Mean Summary score (SD): 78.37 (7.43) Mean Global Health (SD): 61.6 (20.3) Mean Physical Functioning (SD): 85.3 (15.8) Mean Fatigue score (SD): 31.5 (20.3) Mean Pain score (SD): 18.5 (24.8) Mean Dyspnea score (SD): 33.3 (20.4) |
| CG (23) | – | Advice given to avoid specific interventions during the first 3 postoperative months |
Mean (SD) LOS: 18.3 (6.3) HRQoL: Mean Summary score (SD): 77.52 (11.5) Mean Global Health (SD): 70.1 (22.5) Mean Physical Functioning (SD): 78.8 (16.3) Mean Fatigue score (SD): 38.4 (28.2) Mean Pain score (SD): 20.5 (22.4) Mean Dyspnea score (SD): 42.4 (29.4) |
||
| Chen et al.56 (2017) | RCT | IG (39) | Immediate postoperatively, duration NS (until discharge) |
Early mobilization Nutritional assistance: education, encourage oral intake and feeding assistance if needed |
Median (IQR) LOS: 12.0 (6.0) |
| CG (41) | – | Mobilization encouraged, not enforced | Median (IQR) LOS: 14.0 (9.0) | ||
| O'Neill et al. (2018)58 | RCT | IG (21) | Postoperatively, in long-term survivors (duration: 12 weeks) |
Aerobic exercise Resistance training |
HRQoL: Mean Summary score (SD): 92.64 (11.20) Mean Global Health (SD): 79.17 (29.16) Mean Physical Functioning (SD): 93.33 (20.00) Mean Fatigue score (SD): 22.33 (11.00) Mean Pain score (SD): 0.00 (29.17) Mean Dyspnea score (SD): 0.00 (33.33) |
| CG (22) | – | No postoperative intervention |
HRQoL: Mean Summary score (SD): 96.36 (7.49) Mean Global Health (SD): 75.00 (16.66) Mean Physical Functioning (SD): 83.33 (26.67) Mean Fatigue score (SD): 22.33 (44.67) Mean Pain score (SD): 0.00 (33.33) Mean Dyspnea score (SD): 0.00 (33.33) |
||
| Jianjun et al.62 (2019) | Cohort study | IG (60) | Perioperatively (duration: 1 week) |
Encouraged for preoperative IMT and endurance training Nutritional support if needed |
Incidence pneumonia: 1/60 Mean (SD) LOS: 16.8 (3.5) |
| CG (60) | – | Encouraged ambulation and dietary advice |
Incidence pneumonia: 3/60 Mean (SD) LOS: 18.6 (4.1) |
||
| Wang et al.65 (2020) | Cohort study |
IG (156) PSM (14) |
Perioperatively, duration NS (until discharge) |
Preoperative IMT, expiratory flow rate training; board training Postoperative early mobilization, IMT, airway clearance techniques and hand-assisted sputum excretion Administration of Ambroxol and Doxofylline |
Incidence pneumonia: 7/14 Incidence other PPCs: 7/14 |
|
CG (387) PSM (27) |
– | No perioperative intervention |
Incidence pneumonia: 20/27 Incidence other PPCs: 7/27 |
||
| Jiao et al.54 (2020) | RCT | IG (43) | Perioperatively, duration NS |
Preoperative IMT Postoperative sputum elimination, atomization inhalation if necessary Nutritional support: NG feeding during the first 3 days, gradually receiving high protein, high vitamin, high calorie and digestible food |
Incidence pneumonia: 1/43 Incidence other PPCs: 1/43 |
| CG (43) | – | No perioperative intervention |
Incidence pneumonia: 3/43 Incidence other PPCs: 4/43 |
||
| van Vulpen et al.59 (2021) | RCT | IG (54) | Postoperatively (duration: 12 weeks) |
Aerobic exercise Resistance training Warm-up and cool-down |
HRQoL: Mean Summary score (SD): 86.52 (9.65) Mean Global Health (SD): 76.80 (15.90) Mean Physical Functioning (SD): 89.06 (12.00) Mean Fatigue score (SD): 25.45 (17.96) Mean Pain score (SD): 9.11 (18.35) Mean Dyspnea score (SD): 13.94 (19.66) |
| CG (56) | – | Usual care, no postoperative intervention |
HRQoL: Mean Summary score (SD): 84.15 (13.17) Mean Global Health (SD): 75.06 (16.02) Mean Physical Functioning (SD): 84.72 (12.06) Mean Fatigue score (SD): 26.51 (18.08) Mean Pain score (SD): 13.34 (18.50) Mean Dyspnea score (SD): 22.33 (19.78) |
||
CDC Clavien–Dindo classification, CG control group, CI confidence interval, CPAP continuous positive airway pressure, ERAS enhanced recovery after surgery, HRQoL health-related quality of life, ICU intensive care unit, IG intervention group, IMT inspiratory muscle training, IQR interquartile range, IS incentive spirometer, LL lower limbs, LOS length of hospital stay, NACT neoadjuvant chemotherapy, NRS non-randomized controlled study, NS not specified, PEP positive expiratory pressure, postop postoperative, PPCs postoperative pulmonary complications, PSM propensity score matching, RCT randomized controlled trial, SD standard deviation, UL upper limb, 6MWT 6-min walking test
Finally, six RCTs38,40–44 and eight cohort studies2,46–49,51–53 assessing prehabilitation, and five RCTs54–56,58,59 and five cohort studies61,62,65,67,68 assessing peri- or postoperative physiotherapy, were included in the meta-analysis. Included and excluded studies are presented in ES Table S6 for each analysis.
Functional Exercise Capacity
Due to heterogeneity of outcome measures, a meta-analysis was performed for studies assessing exercise capacity following prehabilitation using the 6MWT. For the final analysis, two RCTs40,42 and one cohort51 were included (ES Fig. S1). Change in functional capacity was reported from baseline to the postoperative period, with random-effects analysis showing no difference in the mean 6MWT between the two groups (pooled mean difference 26.70, 95% confidence interval [CI] − 73.10 to 126.49; p = 0.60).
Incidence of Pneumonia
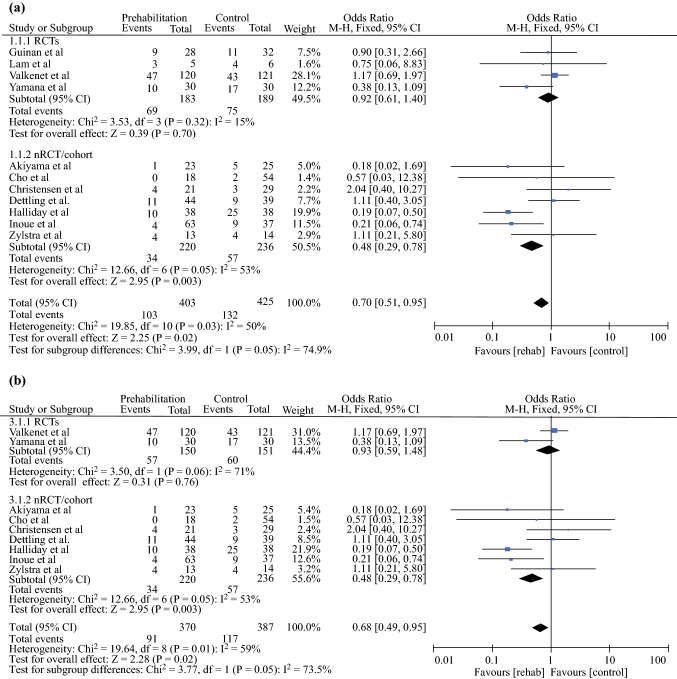
A meta-analysis was performed for all studies assessing the incidence of pneumonia following prehabilitation (Fig. 2a). Fixed-effects analysis demonstrated a significant difference between the two groups, with a lower incidence of pneumonia in patients receiving prehabilitation (pooled odds ratio [OR] 0.70, 95% CI 0.51–0.95; p = 0.02). Further analysis was performed to assess the incidence of pneumonia, with the exclusion of studies providing the combined incidence of pneumonia and other PPCs (Fig. 2b). In agreement with previous findings, fixed-effects analysis showed a significantly lower incidence of pneumonia in patients receiving prehabilitation (pooled OR 0.68, 95% CI 0.49–0.95; p = 0.02).
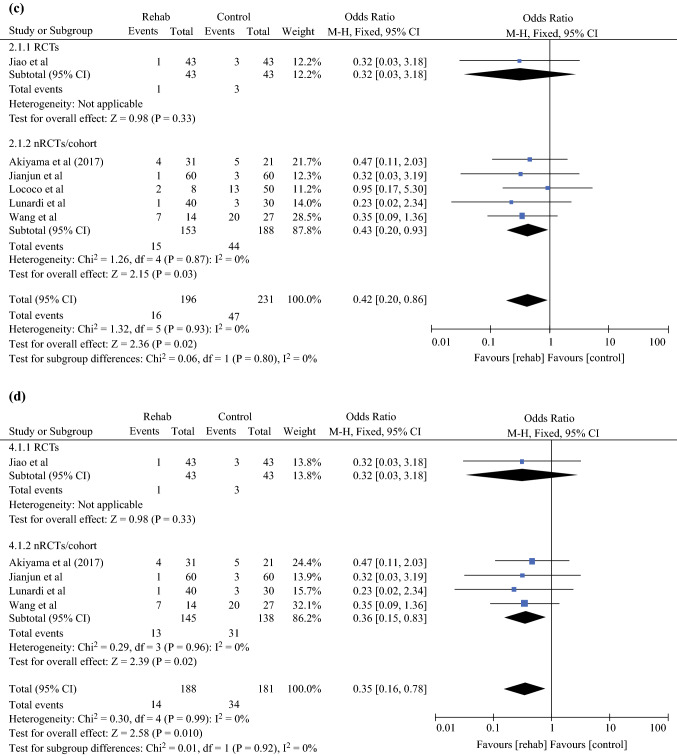
Fig. 2.
a Effect of prehabilitation on the incidence of pneumonia. b Effect of prehabilitation on the incidence of pneumonia, excluding the combined incidence of pneumonia and other PPCs. c Effect of peri- or postoperative rehabilitation on the incidence of pneumonia. d Effect of peri- or postoperative rehabilitation on the incidence of pneumonia, excluding the combined incidence of pneumonia and other PPCs. PPCs postoperative pulmonary complications, M–H Mantel–Haenszel, CI confidence interval, RCTs randomized controlled trials, nRCT non-randomized controlled trials, df degrees of freedom
For peri- or postoperative rehabilitation, a meta-analysis was performed for all studies assessing the incidence of pneumonia (Fig. 2c), with fixed-effects analysis demonstrating a significant difference between the two groups, with a lower incidence of pneumonia in the rehabilitation group (pooled OR 0.42, 95% CI 0.20–0.86; p = 0.02). Further analysis was performed to assess the incidence of pneumonia, excluding the studies providing the combined incidence of pneumonia and other PPCs (Fig. 2d). Similarly, fixed-effects analysis demonstrated a significantly lower incidence of pneumonia in the rehabilitation group (pooled OR 0.35, 95% CI 0.16–0.78; p = 0.01).
Incidence of Other Postoperative Pulmonary Complications (PPCs)
A meta-analysis was performed for all studies assessing the incidence of other PPCs. Fixed-effects analyses showed no significant difference between the intervention and control groups following either prehabilitation (ES Fig. S2a; pooled OR 0.73, 95% CI 0.51–1.05; p = 0.09) or rehabilitation (ES Fig. S2c; pooled OR 1.18, 95% CI 0.60–2.32; p = 0.63).
For further analysis, articles that provided the combined incidence of other PPCs and pneumonia were excluded, thus only including studies that reported the incidence of other PPCs. In agreement with earlier findings, fixed-effects analyses showed no difference between the intervention and control groups for the incidence of other PPCs following prehabilitation (ES Fig. S2b; pooled OR 0.71, 95% CI 0.48–1.05; p = 0.09) or rehabilitation (ES Fig. S2d; pooled OR 1.23, 95% CI 0.59–2.58; p = 0.58).
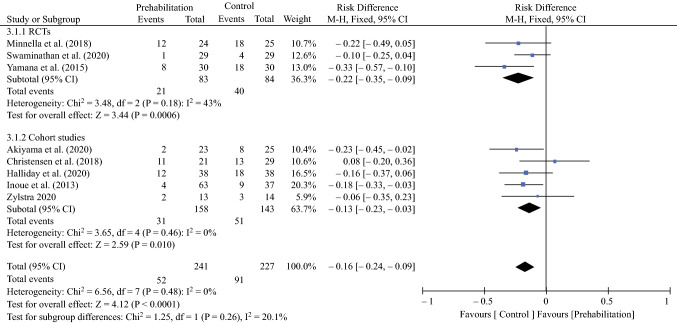
Postoperative Morbidity
Fixed-effects analysis showed fewer complications in the prehabilitation group compared with the control group (risk difference − 0.16, 95% CI − 0.24 to − 0.09; p < 0.0001) (Fig. 3).
Fig. 3.
Effect of prehabilitation on the incidence of postoperative morbidity (Clavien–Dindo grade II or higher). M–H Mantel–Haenszel, CI confidence interval, RCTs randomized controlled trials, df degrees of freedom
Mortality
Fixed-effects analysis was performed and showed no difference in the in-hospital mortality rates between the two groups (pooled OR 0.97, 95% CI 0.31–3.03; p = 0.95) (ES Fig. S3).
Length of Hospital Stay
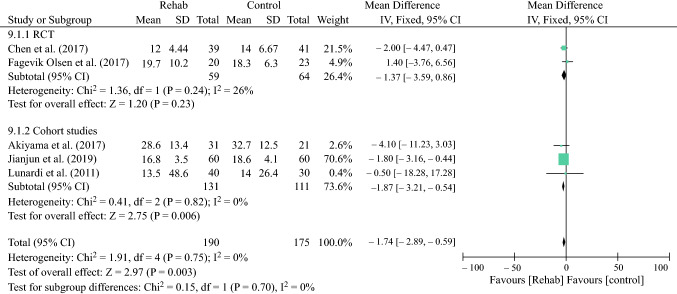
Given the high degree of statistical heterogeneity between studies, random-effects analysis was performed and showed no difference between the two groups (mean difference − 0.44, 95% CI − 1.69 to 0.82; p = 0.50) after prehabilitation (ES Fig. S4). There was no evidence of statistical heterogeneity between studies reporting the LOS after peri- or postoperative rehabilitation, therefore fixed-effects analysis showed shorter hospital stay in the rehabilitation group (mean difference − 1.74, 95% CI − 2.89 to − 0.59; p = 0.003) (Fig. 4).
Fig. 4.
Effect of peri- or postoperative rehabilitation on the LOS. LOS length of hospital stay, SD standard deviation, IV inverse variance, CI confidence interval, RCT randomized controlled trial, df degrees of freedom
Health-Related Quality of Life
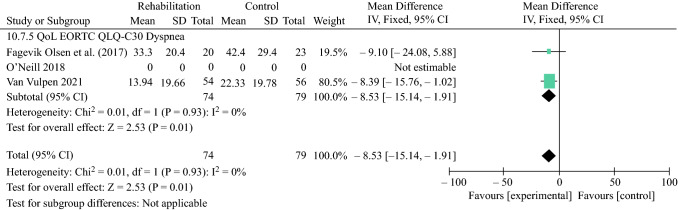
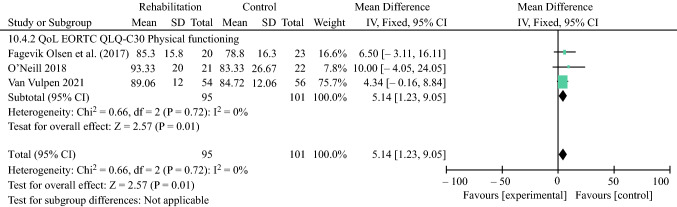
A total of three RCTs55,58,59 were included in the final analysis. The 3-month outcomes were better after rehabilitation for dyspnea (mean difference − 8.53, 95% CI − 15.14 to 1.91) (Fig. 5) and physical functioning (mean difference 5.14, 95% CI 1.23–9.05) (Fig. 6), while no significant difference was observed for the EORTC QLQ-C30 summary score (ES Fig. S5), global health (ES Fig. S6), fatigue (ES Fig. S7) and pain (ES Fig. S8) between the intervention and control groups.
Fig. 5.
Effect of peri- or postoperative rehabilitation on the EORTC QLQ-C30 Dyspnea. EORTC European Organisation for Research and Treatment of Cancer, SD standard deviation, IV inverse variance, CI confidence interval, df degrees of freedom, QoL quality of life
Fig. 6.
Effect of peri- or postoperative rehabilitation on the EORTC QLQ-C30 Physical Functioning. EORTC European Organisation for Research and Treatment of Cancer, SD standard deviation, IV inverse variance, CI confidence interval, df degrees of freedom, QoL quality of life
Methodological Quality of the Included Studies
A summary of the risk of bias of the included RCTs.35 Overall, some concerns were present for the risk of bias assessment of the included RCTs. There were no studies that were considered as high risk of bias. The majority of the included RCTs applied an adequate randomization process with allocation concealment. Half of the studies did not report the method of missing data handling, and, for most studies, there was either inadequate blinding of outcome assessment or no information provided. An evaluation of the cohort studies included in the meta-analysis for the risk of bias is shown in ES Table S7. All but one cohort study received a score of least 6, while seven cohort studies were awarded 7 stars or higher, indicating overall good quality.
Discussion
In this meta-analysis, lower incidence of pneumonia and postoperative morbidity was observed in patients undergoing prehabilitation, however no significant differences were found for other outcomes. Peri- or postoperative rehabilitation resulted in a lower incidence of pneumonia, a shorter LOS and better HRQoL scores for dyspnea and physical functioning, while no effect was observed for the incidence of other PPCs, QoL summary score, global health status, fatigue, and pain scores.
Enhanced recovery after surgery (ERAS) is a multimodal approach aimed at promoting early recovery in patients undergoing major surgery, and commonly includes a physiotherapy component or early mobilization.70,71 These programs have been shown to reduce the risk for complications and decreased the LOS in colorectal surgery.72 Prehabilitation also consists of multiple components, such as preoperative exercise intervention, and nutritional and psychological support. Prehabilitation initially comprised of preoperative exercise training, which was developed to improve functional capacity.73,74 Nutritional support was subsequently implemented to optimize metabolic reserve preoperatively in order to adequately compensate for the catabolic response following surgery.75,76 Similar to findings observed in studies assessing ERAS programs, prehabilitation was shown to improve outcomes in patients undergoing major abdominal or thoracic surgery, with an increase in functional capacity,77,78 reduction in complication rates, and shortening in the LOS.79
Given the clinical importance of pulmonary complications, the incidence of PPCs was also assessed in this meta-analysis. Both prehabilitation and peri- or postoperative rehabilitation have been shown to reduce the risk for postoperative pneumonia, while no differences were observed for other PPC rates between patients receiving intervention and the control group. Interestingly, the magnitude of improvements in pneumonia and LOS were greater with peri- or postoperative rehabilitation than prehabilitation. There are several possible explanations for this, including the proximity in timing of the intervention to the measurement of the outcome, and, second, compliance with the intervention, with peri- and postoperative rehabilitation performed in the hospital setting. However, differences were observed between the RCTs and cohort studies, since RCTs showed no difference in the incidence of pneumonia following prehabilitation and rehabilitation. This could be explained by the low number of RCTs assessing prehabilitation and peri- or postoperative rehabilitation. For the analysis assessing the incidence of pneumonia after prehabilitation, the study by Valkenet et al.44 was given a greater weight than other RCTs, thus determining the outcome. The authors implemented inspiratory muscle training using an inspiratory loading device. The intervention was however home-based and only half of the participants (54.2%) trained at least 80% of the planned sessions and 28% of the participants trained at least 80% of the sessions at the prescribed intensity. The study by Jiao et al.54 was the only RCT included in the analysis that assessed the impact of peri- or postoperative rehabilitation. The physiotherapy intervention consisted of preoperative deep breathing exercises with balloons and abdominal and pursed-lips breathing training followed by postoperative assisted sputum elimination. The exercises were commenced in the preoperative setting and continued in the immediate postoperative period throughout the hospital admission, with no specification of the duration and intensity of the training. Interestingly, both Valkenet et al.44 and Jiao et al.54 reported significantly improved respiratory function in the intervention group compared with the control group, with a higher increase in respiratory muscle strength (maximal inspiratory muscle strength and inspiratory muscle endurance capacity),44 and higher respiratory function indices (forced vital capacity and peak expiratory flow),54 respectively.
HRQoL status was assessed in patients undergoing peri- or postoperative rehabilitation at 3 months following surgery. All three RCTs55,58,59 implemented a 12-week rehabilitation program, two58,59 of which implemented a program consisting of aerobic and resistance training, while the third study55 included breathing exercises, strength training, and optimization of thoracic spine mobility. At 2 years after surgery, Fagevik Olsén et al.80 found clinically significant worse QoL scores for dyspnea, fatigue, diarrhea, and appetite loss. Another study presented similar findings, showing persistently worse QoL scores for physical functioning and dyspnea at 3 years after surgery.81 Moreover, pain around chest scars and reduced energy or activity tolerance were associated with long-term poor HRQoL.82 Lastly, the LOS did not differ in the prehabilitation group, while peri- or postoperative exercise intervention resulted into a significantly shorter LOS. Rehabilitation may therefore aid in earlier in-hospital recovery.
There are several limitations present in this meta-analysis. Due to the limited number of RCTs conducted, cohort studies were included in the analysis. For most RCTs, allocation was concealed, there was a low dropout rate following randomization, and the intervention and control groups were similar at baseline for most trials. However, several studies did not report handling of missing data and the reasons for dropout, and the majority of studies reported no blinding of outcome assessment. Several cohort studies used historical controls as a comparison group, consisting of patients who had undergone surgery before physiotherapy implementation. No subgroup analysis could be performed for the type of surgery, including the surgical approach (minimally invasive surgery [MIS] or open surgery), as some studies only provided the number of patients undergoing open surgery or MIS, while the number of patients undergoing either esophagectomy or gastrectomy within these subcohorts was not specified.
The short-term outcomes were assessed by well-defined measures, such as the CDC for surgical complications, and by reporting the mortality rates. The criteria for diagnosis of pneumonia were well-described in four studies,2,38,44,52 which were based on leukocyte count, presence of fever, sputum and chest X-Ray findings. However, the remaining studies reported the incidence of pneumonia or PPCs only. Perioperative rehabilitation was implemented in only a small number of studies, while postoperative rehabilitation was commonly commenced after discharge or in long-term survivors. Although a Clavien–Dindo score of 3 or higher is commonly considered for clinically complications, in this meta-analysis a cut-off at a score of 2 was used to assess the incidence of postoperative morbidity in order to include the incidence of pneumonia, classified as complications requiring pharmacological treatment (Clavien–Dindo score 2).
Patients with esophageal and gastric cancer commonly experience ongoing malnutrition after surgery and often report poor long-term physical functioning and ongoing respiratory symptoms.81,83–85 Physical activity levels were measured by obtaining the step count with an accelerometer. A total of three studies have reported physical activity, two of which assessed this outcome following prehabilitation,41,42 and only one study assessed this after rehabilitation in long-term survivors.58 The respiratory function was evaluated by estimating the respiratory volumes and by measuring the respiratory muscle pressure and endurance. A total of eight studies (four in the preoperative period42,44,45,52 and four in the peri- or postoperative period54,55,60,68) reported the respiratory function, with only one study64 assessing the long-term outcomes. No meta-analysis could be performed due to different timing of measurements and the use of different parameters. This suggests that there is a lack of research assessing functional data in long-term survivorship undergoing rehabilitation. There was no standardized regimen as exercise interventions differed in timing and duration, and were either home-based or in-hospital, with or without supervision. The Borg scale was used to estimate the intensity of the intervention for a large number of studies. However, no standardized measure was available to compare all regimens. To date, there is an evident paucity of research comparing the impact the different components, intensity, and setting of physiotherapy in patients who have undergone esophagectomy or gastrectomy.
Finally, adherence was reported in the majority of studies included in the meta-analysis. For most studies, adherence was monitored by a physiotherapist, while a few studies implemented objective measures such as heart rate monitors, which could possibly improve the objectivity of adherence reporting.
Conclusion
The findings of this meta-analysis showed that implementation of exercise intervention may be beneficial in both the preoperative and peri- or postoperative periods. The next steps of the investigation are to identify which components, or pre-habilitation and peri- and postoperative rehabilitation, have the greatest impact on the clinical outcomes. Furthermore, clearly the Achilles heel to prehabilitation and rehabilitation is patient compliance; more research is needed to understand the human factors and patient barriers around complications to these regimens, to ensure the long-term clinical effectiveness.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Sheraz Markar is funded by the National Institute of Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
Declarations
Conflict of interest
Karina H. Tukanova, Swathikan Chidambaram, Nadia Guidozzi, George B. Hanna, Alison H. McGregor, and Sheraz R. Markar declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg. 2011;91(5):1494–1501. doi: 10.1016/j.athoracsur.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 2.Inoue J, Ono R, Makiura D, et al. Prevention of postoperative pulmonary complications through intensive preoperative respiratory rehabilitation in patients with esophageal cancer. Dis Esophagus. 2013;26(1):68–74. doi: 10.1111/j.1442-2050.2012.01336.x. [DOI] [PubMed] [Google Scholar]
- 3.Atkins BZ, Shah AS, Hutcheson KA, et al. Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg. 2004;78(4):1170–1176. doi: 10.1016/j.athoracsur.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 4.Law S, Wong K-H, Kwok K-F, Chu K-M, Wong J. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg. 2004;240(5):791–800. doi: 10.1097/01.sla.0000143123.24556.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson MK, Durkin AE. Preoperative prediction of the risk of pulmonary complications after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2002;123(4):661–669. doi: 10.1067/mtc.2002.120350. [DOI] [PubMed] [Google Scholar]
- 6.Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin. 2008;26(2):355–367. doi: 10.1016/j.anclin.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers ML, Duffy JP. Surgical aspects of chronic post-thoracotomy pain. Eur J Cardio-Thorac Surg. 2000;18(6):711–716. doi: 10.1016/S1010-7940(00)00569-8. [DOI] [PubMed] [Google Scholar]
- 8.Cook, D, Simons D. Neuromuscular Blockade. Treasure Island, F): StatPearls Publishing. Available at: https://doi.org/https://www.ncbi.nlm.nih.gov/books/NBK538301/. [PubMed]
- 9.Gammon MD, Schoenberg JB, Ahsan H, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89(17):1277–1284. doi: 10.1093/jnci/89.17.1277. [DOI] [PubMed] [Google Scholar]
- 10.Kirmeier E, Eriksson LI, Lewald H, et al. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med. 2019;7(2):129–140. doi: 10.1016/S2213-2600(18)30294-7. [DOI] [PubMed] [Google Scholar]
- 11.Saraswat V. Effects of anaesthesia techniques and drugs on pulmonary function. Indian J Anaesth. 2015;59(9):557–564. doi: 10.4103/0019-5049.165850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med. 2006;32(1):24–33. doi: 10.1007/s00134-005-2817-8. [DOI] [PubMed] [Google Scholar]
- 13.Lumb AB. Why do patients need extra oxygen during a general anaesthetic? BJA Educ. 2019;19(2):37–39. doi: 10.1016/j.bjae.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies OJ, Husain T, Stephens RCM. Postoperative pulmonary complications following non-cardiothoracic surgery. BJA Educ. 2017;17(9):295–300. doi: 10.1093/bjaed/mkx012. [DOI] [Google Scholar]
- 15.Dajczman E, Gordon A, Kreisman H, Wolkove N. Long-term postthoracotomy pain. Chest. 1991;99(2):270–274. doi: 10.1378/chest.99.2.270. [DOI] [PubMed] [Google Scholar]
- 16.McAnena OJ, Rogers J, Williams NS. Right thoracoscopically assisted oesophagectomy for cancer. Br J Surg. 1994;81(2):236–238. doi: 10.1002/bjs.1800810225. [DOI] [PubMed] [Google Scholar]
- 17.Biere SS, Maas KW, Bonavina L, et al. Traditional invasive versus minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial) BMC Surg. 2011;11:2. doi: 10.1186/1471-2482-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biere SSAY, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379(9829):1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 19.Inoue T, Ito S, Ando M, et al. Changes in exercise capacity, muscle strength, and health-related quality of life in esophageal cancer patients undergoing esophagectomy. BMC Sport Sci Med Rehabil. 2016;8(1):34. doi: 10.1186/s13102-016-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Restrepo RD, Braverman J. Current challenges in the recognition, prevention and treatment of perioperative pulmonary atelectasis. Expert Rev Respir Med. 2015;9(1):97–107. doi: 10.1586/17476348.2015.996134. [DOI] [PubMed] [Google Scholar]
- 21.Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56(6):1285–1289. doi: 10.1016/0003-4975(93)90667-7. [DOI] [PubMed] [Google Scholar]
- 22.Markar SR, Ni M, Gisbertz SS, et al. Implementation of minimally invasive esophagectomy from a randomized controlled trial setting to national practice. J Clin Oncol. 2020;38(19):2130–2139. doi: 10.1200/JCO.19.02483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goense L, Meziani J, Bülbül M, Braithwaite SA, van Hillegersberg R, Ruurda JP. Pulmonary diffusion capacity predicts major complications after esophagectomy for patients with esophageal cancer. Dis esophagus. 2019 doi: 10.1093/dote/doy082. [DOI] [PubMed] [Google Scholar]
- 24.Akaishi T, Kaneda I, Higuchi N, et al. Thoracoscopic en bloc total esophagectomy with radical mediastinal lymphadenectomy. J Thorac Cardiovasc Surg. 1996;112(6):1533–1540. doi: 10.1016/S0022-5223(96)70012-0. [DOI] [PubMed] [Google Scholar]
- 25.Klevebro F, Elliott JA, Slaman A, et al. Cardiorespiratory comorbidity and postoperative complications following esophagectomy: a European multicenter cohort study. Ann Surg Oncol. 2019;26(9):2864–2873. doi: 10.1245/s10434-019-07478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersson J, Glenny RW. Gas exchange and ventilation–perfusion relationships in the lung. Eur Respir J. 2014;44(4):1023–1041. doi: 10.1183/09031936.00037014. [DOI] [PubMed] [Google Scholar]
- 27.Kneyber MCJ, Zhang H, Slutsky AS. Ventilator-induced lung injury. Similarity and differences between children and adults. Am J Respir Crit Care Med. 2014;190(3):258–265. doi: 10.1164/rccm.201401-0168CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelb AW, Southorn P, Rehder K. Effect of general anaesthesia on respiratory function. Lung. 1981;159(1):187–198. doi: 10.1007/BF02713915. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki N, Meyer MJ, Eikermann M. Postoperative respiratory muscle dysfunction: pathophysiology and preventive strategies. Anesthesiology. 2013;118(4):961–978. doi: 10.1097/ALN.0b013e318288834f. [DOI] [PubMed] [Google Scholar]
- 30.Drummond GB. The abdominal muscles in anaesthesia and after surgery. BJA Br J Anaesth. 2003;91(1):73–80. doi: 10.1093/bja/aeg145. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Cruz E, Camacho-Limas CP. Association of nutritional status and functional capacity in gastrointestinal cancer patients. Gac Med Mex. 2017;153(5):575–580. doi: 10.24875/GMM.17002776. [DOI] [PubMed] [Google Scholar]
- 32.Pasquina P, Tramér MR, Granier J-M, Walder B. Respiratory physiotherapy to prevent pulmonary complications after abdominal surgery: a systematic review. Chest. 2006;130(6):1887–1899. doi: 10.1378/chest.130.6.1887. [DOI] [PubMed] [Google Scholar]
- 33.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 35.Sterne JAC, Savović J, Page MJ, et al. RoB2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 36.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2008. Available at: https://doi.org/http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 8 Apr 2021.
- 37.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamana I, Takeno S, Hashimoto T, Maki K, Shibata R, Shiwaku H, et al. Randomized controlled study to evaluate the efficacy of a preoperative respiratory rehabilitation program to prevent postoperative pulmonary complications after esophagectomy. Dig Surg. 2015;32(5):331–337. doi: 10.1159/000434758. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y-J, Cheng JC-H, Lee J-M, Huang P-M, Huang G-H, Chen CC-H. A walk-and-eat intervention improves outcomes for patients with esophageal cancer undergoing neoadjuvant chemoradiotherapy. Oncologist. 2015;20(10):1216–1222. doi: 10.1634/theoncologist.2015-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minnella EM, Awasthi R, Loiselle S-E, Agnihotram RV, Ferri LE, Carli F. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg. 2018;153(12):1081–1089. doi: 10.1001/jamasurg.2018.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam S. Physical activity in the aetiology and preoperative management of oesophageal adenocarcinoma. PhD thesis, University of East Anglia, Norwich. 2018.
- 42.Guinan EM, Forde C, O’Neill L, et al. Effect of preoperative inspiratory muscle training on physical functioning following esophagectomy. Dis Esophagus. 2019;32(2):doy091. doi: 10.1093/dote/doy091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swaminathan N, Kundra P, Ravi R, Kate V. ERAS protocol with respiratory prehabilitation versus conventional perioperative protocol in elective gastrectomy—a randomized controlled trial. Int J Surg. 2020;81:149–157. doi: 10.1016/j.ijsu.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 44.Valkenet K, Trappenburg JCA, Ruurda JP, et al. Multicentre randomized clinical trial of inspiratory muscle training versus usual care before surgery for oesophageal cancer. Br J Surg. 2018;105(5):502–511. doi: 10.1002/bjs.10803. [DOI] [PubMed] [Google Scholar]
- 45.van Adrichem EJ, Meulenbroek RL, Plukker JT, Groen H, van Weert E. Comparison of two preoperative inspiratory muscle training programs to prevent pulmonary complications in patients undergoing esophagectomy: a randomized controlled pilot study. Ann Surg Oncol. 2014;21(7):2353–2360. doi: 10.1245/s10434-014-3612-y. [DOI] [PubMed] [Google Scholar]
- 46.Christensen JF, Simonsen C, Banck-Petersen A, et al. Safety and feasibility of preoperative exercise training during neoadjuvant treatment before surgery for adenocarcinoma of the gastro-oesophageal junction. BJS Open. 2019;3(1):74–84. doi: 10.1002/bjs5.50110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halliday LJ, Doganay E, Wynter-Blyth VA, Hanna GB, Moorthy K. The impact of prehabilitation on post-operative outcomes in oesophageal cancer surgery: a propensity score matched comparison. J Gastrointest Surg. 2020 doi: 10.1007/s11605-020-04881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weblin J, Mcwilliams D, Tucker O. Feasibility of implementing prehabilitation in patients undergoing major oesophagogastric cancer resection: a single centre experience. ACPRC J. 2017;49:82–94. [Google Scholar]
- 49.Cho H, Yoshikawa T, Oba MS, et al. Matched pair analysis to examine the effects of a planned preoperative exercise program in early gastric cancer patients with metabolic syndrome to reduce operative risk: the Adjuvant Exercise for General Elective Surgery (AEGES) study group. Ann Surg Oncol. 2014;21(6):2044–2050. doi: 10.1245/s10434-013-3394-7. [DOI] [PubMed] [Google Scholar]
- 50.Herrstedt H, Bay ML, Simonsen C, et al. Exercise-mediated improvement of depression in patients with gastro-esophageal junction cancer is linked to kynurenine metabolism. Acta Oncol (Madr) 2019;58(5):579–587. doi: 10.1080/0284186X.2018.1558371. [DOI] [PubMed] [Google Scholar]
- 51.Akiyama Y, Sasaki A, Fujii Y, et al. Efficacy of enhanced prehabilitation for patients with esophageal cancer undergoing esophagectomy. Esophagus. 2021;18(1):56–64. doi: 10.1007/s10388-020-00757-2. [DOI] [PubMed] [Google Scholar]
- 52.Dettling DS, van der Schaaf M, Blom RLGM, et al. Feasibility and effectiveness of pre-operative inspiratory muscle training in patients undergoing oesophagectomy: a pilot study. Physiother Res Int. 2013;18(1):16–26. doi: 10.1002/pri.1524. [DOI] [PubMed] [Google Scholar]
- 53.Zylstra JL. Structured exercise during neo-adjuvant chemotherapy in patients with operable adenocarcinoma of the oesophagus and gastro-oesophageal junction. PhD thesis, John Moore’s University, Liverpool. 2020.
- 54.Jiao J, Fan X, Dang H, Yin J, Wang Q, Zhang G, Fu J. Effects of comprehensive nursing care on recovery of respiratory function after thoracic surgery. Int J Clin Exp Med. 2020;13(7):5328–5334. [Google Scholar]
- 55.Fagevik-Olsén M, Kjellby Wendt G, Hammerlid E, et al. Effects of a training intervention for enhancing recovery after Ivor-Lewis esophagus surgery: a randomized controlled trial. Scand J Surg. 2017;106(2):116–125. doi: 10.1177/1457496916655499. [DOI] [PubMed] [Google Scholar]
- 56.Chen CCH, Li HS, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg. 2017;152(9):827–834. doi: 10.1001/jamasurg.2017.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang Y-L, Tsai Y-F, Hsu C-L, et al. The effectiveness of a nurse-led exercise and health education informatics program on exercise capacity and quality of life among cancer survivors after esophagectomy: a randomized controlled trial. Int J Nurs Stud. 2020;101:103418. doi: 10.1016/j.ijnurstu.2019.103418. [DOI] [PubMed] [Google Scholar]
- 58.O’Neill LM, Guinan E, Doyle SL, et al. The RESTORE randomized controlled trial: impact of a multidisciplinary rehabilitative program on cardiorespiratory fitness in esophagogastric cancer survivorship. Ann Surg. 2018;268(5):747–755. doi: 10.1097/SLA.0000000000002895. [DOI] [PubMed] [Google Scholar]
- 59.van Vulpen JK, Hiensch AE, van Hillegersberg R, et al. Supervised exercise after oesophageal cancer surgery: the PERFECT multicentre randomized clinical trial. Br J Surg. 2021;108(7):786–796. doi: 10.1093/bjs/znab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fagevik Olsén M, Wennberg E, Johnsson E, et al. Randomized clinical study of the prevention of pulmonary complications after thoracoabdominal resection by two different breathing techniques. Br J Surg. 2002;89(10):1228–1234. doi: 10.1046/j.1365-2168.2002.02207.x. [DOI] [PubMed] [Google Scholar]
- 61.Akiyama Y, Iwaya T, Endo F, et al. Effectiveness of intervention with a perioperative multidisciplinary support team for radical esophagectomy. Support Care Cancer. 2017;25(12):3733–3739. doi: 10.1007/s00520-017-3801-x. [DOI] [PubMed] [Google Scholar]
- 62.Jianjun W, Xing W, Guozhong Y, Chuming Z, Jiang Y. Application of exercised-based pre-rehabilitation in perioperative period of patients with gastric cancer. Open Med (Warsaw, Poland) 2019;14:875–882. doi: 10.1515/med-2019-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura M, Iwahashi M, Nakamori M, et al. An analysis of the factors contributing to a reduction in the incidence of pulmonary complications following an esophagectomy for esophageal cancer. Langenbeck’s Arch Surg. 2008;393(2):127–133. doi: 10.1007/s00423-007-0253-7. [DOI] [PubMed] [Google Scholar]
- 64.van Egmond MA, Engelbert RHH, Klinkenbijl JHG, van Berge Henegouwen MI, van der Schaaf M. Physiotherapy with telerehabilitation in patients with complicated postoperative recovery after esophageal cancer surgery: feasibility study. J Med Internet Res. 2020;22(6):e16056. doi: 10.2196/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W, Liu Q, Yu Y, et al. Hand-assisted sputum excretion can effectively reduce postoperative pulmonary complications of esophageal cancer. Ann Palliat Med. 2020;9(6):3721–3730. doi: 10.21037/apm-20-1267. [DOI] [PubMed] [Google Scholar]
- 66.Simonsen C, Thorsen-Streit S, Sundberg A, et al. Effects of high-intensity exercise training on physical fitness, quality of life and treatment outcomes after oesophagectomy for cancer of the gastro-oesophageal junction: PRESET pilot study. BJS Open. 2020;4(5):855–864. doi: 10.1002/bjs5.50337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lunardi AC, Cecconello I, Celso F, et al. Postoperative chest physical therapy prevents respiratory complications in patients undergoing esophagectomy. Rev Bras Fisioter. 2011;15(2):160–165. doi: 10.1590/S1413-35552011000200012. [DOI] [PubMed] [Google Scholar]
- 68.Lococo F, Cesario A, Sterzi S, et al. Rationale and clinical benefits of an intensive long-term pulmonary rehabilitation program after oesophagectomy: preliminary report. Multidiscip Respir Med. 2012;7(2):21. doi: 10.1186/2049-6958-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vats N. Effect of deep breathing exercises and incentive spirometry in the prevention of post operative pulmonary complications in the patients of cancer esophagus undergoing esophagectomy. Indian J Physiother Occup Ther. 2009;3(3):60–67. [Google Scholar]
- 70.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292–298. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 71.Melnyk M, Casey RG, Black P, Koupparis AJ. Enhanced recovery after surgery (ERAS) protocols: time to change practice? Can Urol Assoc J. 2011;5(5):342–348. doi: 10.5489/cuaj.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg. 2014;101(3):172–188. doi: 10.1002/bjs.9394. [DOI] [PubMed] [Google Scholar]
- 73.Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care. 2005;8(1):23–32. doi: 10.1097/00075197-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg. 2017;39:156–162. doi: 10.1016/j.ijsu.2017.01.111. [DOI] [PubMed] [Google Scholar]
- 75.West MA, Wischmeyer PE, Grocott MPW. Prehabilitation and nutritional support to improve perioperative outcomes. Curr Anesthesiol Rep. 2017;7(4):340–349. doi: 10.1007/s40140-017-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Durrand J, Singh SJ, Danjoux G. Prehabilitation. Clin Med. 2019;19(6):458–464. doi: 10.7861/clinmed.2019-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27(4):1072–1082. doi: 10.1007/s00464-012-2560-5. [DOI] [PubMed] [Google Scholar]
- 78.Pouwels S, Fiddelaers J, Teijink JAW, Ter Woorst JF, Siebenga J, Smeenk FWJM. Preoperative exercise therapy in lung surgery patients: a systematic review. Respir Med. 2015;109(12):1495–1504. doi: 10.1016/j.rmed.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 79.Valkenet K, van de Port IGL, Dronkers JJ, de Vries WR, Lindeman E, Backx FJG. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clin Rehabil. 2011;25(2):99–111. doi: 10.1177/0269215510380830. [DOI] [PubMed] [Google Scholar]
- 80.Fagevik Olsén M, Larsson M, Hammerlid E, Lundell L. Physical function and quality of life after thoracoabdominal oesophageal resection. Results of a follow-up study. Dig Surg. 2005;22(1–2):63–68. doi: 10.1159/000085348. [DOI] [PubMed] [Google Scholar]
- 81.Lagergren P, Avery KNL, Hughes R, et al. Health-related quality of life among patients cured by surgery for esophageal cancer. Cancer. 2007;110(3):686–693. doi: 10.1002/cncr.22833. [DOI] [PubMed] [Google Scholar]
- 82.Markar SR, Zaninotto G, Castoro C, et al. Lasting symptoms after esophageal resection (LASER): European multicenter cross-sectional study. Ann Surg. Epub 17 Nov 2020. 10.1097/SLA.0000000000003917 [DOI] [PubMed]
- 83.Djärv T, Lagergren J, Blazeby JM, Lagergren P. Long-term health-related quality of life following surgery for oesophageal cancer. Br J Surg. 2008;95(9):1121–1126. doi: 10.1002/bjs.6293. [DOI] [PubMed] [Google Scholar]
- 84.Avery KNL, Metcalfe C, Barham CP, Alderson D, Falk SJ, Blazeby JM. Quality of life during potentially curative treatment for locally advanced oesophageal cancer. Br J Surg. 2007;94(11):1369–1376. doi: 10.1002/bjs.5888. [DOI] [PubMed] [Google Scholar]
- 85.Yu W, Park KB, Chung HY, Kwon OK, Lee SS. Chronological changes of quality of life in long-term survivors after gastrectomy for gastric cancer. Cancer Res Treat. 2016;48(3):1030–1036. doi: 10.4143/crt.2015.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.