Abstract
The locus coeruleus (LC), or ‘blue spot’, is a small nucleus located deep in the brainstem that provides the far-reaching noradrenergic neurotransmitter system of the brain. This phylogenetically conserved nucleus has proved relatively intractable to full characterization, despite more than 60 years of concerted efforts by investigators. Recently, an array of powerful new neuroscience tools have provided unprecedented access to this elusive nucleus, revealing new levels of organization and function. We are currently at the threshold of major discoveries regarding how this tiny brainstem structure exerts such varied and significant influences over brain function and behaviour. All LC neurons receive inputs related to autonomic arousal, but distinct subpopulations of those neurons can encode specific cognitive processes, presumably through more specific inputs from the forebrain areas. This ability, combined with specific patterns of innervation of target areas and heterogeneity in receptor distributions, suggests that activation of the LC has more specific influences on target networks than had initially been imagined.
Research by a handful of anatomists, behavioural scientists and physiologists over the years has provided limited yet tantalizing clues that the noradrenaline (NA) containing nucleus locus coeruleus (LC) plays a critical role in core behavioural and physiological processes. Evidence of LC-NA involvement in multiple clinical conditions further underscores the need for achieving an advanced understanding of the function of this nucleus.
The position of the LC deep in the brainstem and its small size have made it difficult to record LC neuronal activity and correlate this activity with environmental events and behaviour. The LC, translated from Latin as ‘blue spot, comprises a mere 3,000 brain cells in the rodent and is similarly diminutive in primates. However, those LC-NA neurons display broad and divergent axonal pathways through the CNS. For more than 50 years, the LC was thought to be homogeneous in its structure and function, such that its primary transmitter, noradrenaline (NA), could be released uniformly and act simultaneously on cells and circuits throughout the forebrain, brainstem, cerebellum and spinal cord. Because the LC projects to a large number of CNS areas with widely divergent functions, researchers have tended to see the LC-NA as a system with unified action on neural networks and behavioural outcomes. Under such a unified conceptualization, however, it has been a challenge to develop testable hypotheses regarding a comprehensive and coherent role for the LC in physiology and behaviour. This struggle to find support for such unified theories is compounded by the fact that the input/output relationships of the LC are indeed complex, and for technical reasons have resisted detailed study.
Recent methodological advances have provided powerful new tools that can be applied to unlocking the mysteries of the LC. Studies utilizing optogenetics and chemogenetics, viral tract tracing, RNA sequencing, and neuron- and molecule-specific labelling methods in combination with behavioural measures have begun to dramatically expand our thinking about LC organization and function (see FIG. 1 for an overview). New discoveries indicate that the nucleus and its neurons are more heterogeneous than had previously been appreciated. Technical advances in functional MRI (fMRI) also promise to soon identify behaviour-specific activity in the LC of humans and other primates, opening a new avenue for future investigation. All these advances are enabling new clinical translational studies and treatment innovations (BOXES 1-3), as well as new directions for basic research (FIG. 1).
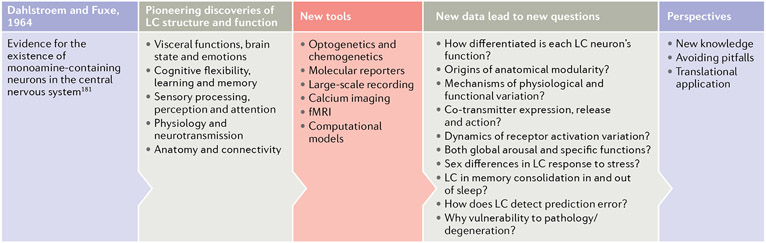
Fig. 1 ∣. The blue spot: past discoveries and future horizons.
The discovery of the existence of monoamine-containing neurons in the CNS by Dahlstroem and Fuxe in 1964 (REF.181) inspired subsequent systematic research on locus coeruleus (LC) structure and function. Use of the available neuroscience methodologies allowed many pioneering discoveries and influential theories of function (second panel). Unprecedented technological advances in recent years have been a catalyst for obtaining new results that confirmed, but also challenged, the existing knowledge about the LC-noradrenaline (NA) system (third panel). Newly revealed complexity of the organization of the LC-NA system has raised many new questions (fourth panel), opening new vistas for future research. fMRI, functional MRI.
Box 1 ∣. LC in ageing and neurodegenerative disorders.
Although it was originally reported that locus coeruleus (LC) degeneration occurs over the lifespan, more recent post-mortem studies have indicated that frank LC neuronal loss does not appear to be a component of normal ageing183. Some LC-sensitive MRI studies have indicated an age-related reduction in LC integrity, but this signal may reflect changes in neuromelanin, neuron shrinkage and/or the loss of proximal dendrites rather than cell body death125,184. LC MRI will be a critical research tool moving forward, as cognitive reserve and memory performance have been linked to higher LC signal intensity and to changes in connectivity in older participants182,185,186. Noradrenergic regulation of plasticity in LC terminals also declines with age125, as does noradrenaline (NA) regulation of hippocampal synaptic plasticity187, perhaps contributing to the increased difficulty with learning flexibility in healthy ageing individuals, as well as to difficulty with reshaping cortical circuits after brain injury in the aged.
Regardless of the controversy concerning LC integrity during normal ageing, it is well established that LC-NA cells are exquisitely sensitive to pathology and death in neurodegenerative diseases of aging; hyperphosphorylated ‘pretangle’ tau appears in the LC prior to any other brain region in Alzheimer disease (AD). LC neurons are also among the first touched by α-synuclein inclusions in Parkinson disease (PD), and LC cell loss becomes catastrophic in both late-stage AD and PD188-190. Because LC neurons can survive for years despite harbouring inclusions of tau or a-synuclein, and because many prodromal symptoms of AD and PD are consistent with LC dysfunction (for example, sleep disturbances, anxiety, depression and so forth)191,192, it is critical to understand the consequences of aberrant tau and α-synuclein for LC physiology. Transgenic animals developed using noradrenergic-selective promoters, LC-specific infection with tau/α-synuclein viral vectors, or intra-LC infusion of preformed fibrils represent fresh approaches to overcome the confounds associated with the ubiquitously expressed protein aggregates in earlier models193-195. Characterizing the molecular signatures and electrophysiological properties of beleaguered LC neurons and assessing LC-relevant behaviours top the list of priorities in this research area. Further development of sensitive and specific MRI methods to assess LC integrity and the use of such integrity as a biomarker for AD and PD have shown promise but are yet to be fully exploited184.
Box 3 ∣. Therapeutics.
Dysfunction of the locus coeruleus-noradrenaline (LC-NA) system has been implicated in many neuropsychiatric and neurological diseases, including depression, anxiety, attention deficit hyperactivity disorder (ADHD), post-traumatic stress disorder (PTSD), Alzheimer disease (AD) and Parkinson disease (PD). Even in cases where the LC is not involved in establishing the disorder itself, it is possible that manipulating LC activity could disrupt the feedback loop that supports the dysfunction, thus re-establishing a healthy physiological response pattern and moving the patient towards normal daily activity.
Given the cornucopia of available pharmacological agents targeting N A synthesis, signalling and metabolism, it is surprising how few are currently in use. The selective NA reuptake inhibitors such as atomoxetine, for ADHD208, and reboxetine, for depression209, the synthetic NA precursor l-3,4-dihydroxyphenylserine (DOPS; droxidopa), for PD210, and the α2-adrenergic receptor agonist lofexidine, for opioid withdrawal211, have all been tried with some success. There are several reasons noradrenergic compounds are not being used more frequently. Although NA dysfunction contributes to many aspects of brain disorders, it is not known to be the specific cause of any symptomatology or known disease process (in contrast to dopamine neuron degeneration, which causes the cardinal motor symptoms of PD and has spawned multiple dopaminergic therapies). Systemic effects of noradrenergic drugs on cardiovascular function prompt caution in their use for treating CNS disorders. Minimally invasive procedures are emerging for manipulating the LC, taking advantage of studies of circuits that regulate LC neurons — for example, the circuit from the suprachiasmatic nucleus (SCN) to the LC via a relay in the dorso-medial hypothalamus23. As specialized retinal ganglion cells strongly innervate the SCN, retinal stimulation (for example, using chemogenetics) may modulate the LC for therapeutic benefit212. Transcutaneous vagus nerve stimulation is another non-invasive procedure that has positive effects on psychiatric and neurological disorders such as depression213, epilepsy214 and cognitive dysfunction215, all of which are mediated, at least in part, through LC activation. As we learn more about the diversity of LC neurons (for example, efferents, afferents, co-transmitters, survival factors and so forth) and their modulatory organization, and as we develop technologies to target subpopulations of these cells, LC-based therapies for neuropsychiatric and neurological diseases will become more credible and effective while minimizing peripheral side effects.
One way to accelerate our understanding of the LC is to map leverage points where new approaches and additional expertise could have maximal impact in advancing the field. This report is the result of an intensive 3-day workshop in which participants were tasked with summarizing and synthesizing the current understanding of LC organization and function, with particular emphasis on recent breakthroughs. Contributors focused on identifying opportunities and priorities for exploitation, including pinpointing critical unanswered questions to guide current and prospective investigators in the field. This report is not a consensus document adopting conventions or formalizing a database, nor is it a detailed review of the state of the field. Rather, it is an attempt (1) to identify major issues of LC organization and physiological function, which were previously intractable or poorly conceived, that can now be realistically addressed with our ever-increasing knowledge base and new methodologies; (2) to suggest new ways to think about how the LC is related to major behavioural and cognitive functions or clinical disorders, as well as describe new ways to use such knowledge translationally; and (3) to present major unsolved questions about LC function whose answers will encourage accelerated research in multiple directions.
Anatomy of the LC
The LC is a cluster of relatively large neurons containing NA that is located bilaterally in the brainstem just under the cerebellum and lateral to the fourth ventricle. What we thought we knew about the widely projecting efferent and convergent afferent anatomy of these neurons, prior to about 10 years ago, was inaccurate. We will here attempt to explain why this decades-long understanding has recently changed and describe the functional and clinical implications of this new understanding. We present several areas of LC functional anatomy in need of further exploration: its efferent and afferent projections patterns, neurotransmitter identities, presynaptic release profiles and reuptake, and the receptor make-up of its terminal fields.
LC efferent anatomy.
Understanding the anatomical organization of the LC is critical to unlocking its function, and this understanding has changed radically over the past 10 years. Nearly a dozen studies from different labs have provided compelling evidence that LC projection patterns may be modular in design, with segregated output channels and the potential for differential release and actions of NA upon its projection fields. Although this work is still ongoing, these new findings have prompted a wholesale shift in our thinking about LC operations and demand a revision of theoretical constructs regarding the impact of the LC-NA system on behavioural outcomes in health and disease. Target-specific projection patterns of individual LC neurons could enable differentiated modulation of diverse behaviours and cognitive functions1-3. New strategies employing transgenic animals and viral vector approaches4-8 are providing a picture of complex, targeted projections from some subsets of LC cells, divergent projections in other subsets, and a mixture of widespread projections with preferred targets in still others.
The distribution of LC-NA axonal fibres is nonuniform across the rat9 and primate10,11 neocortex and is characterized by axon varicosities9. The axon collaterals of distinct LC neurons appear to be distributed in a coordinated fashion to functionally related target circuits, such as those coordinating aversion and/or anxiety in the anterior cingulate and amygdala versus those promoting analgesia in the spinal cord4,6,7,12-15. Thus, clusters of some LC neurons are organized into modules with respect to their efferent targets.
These newly observed organizational features of the LC-NA system are based primarily on studies of a limited set of emotional, cognitive (for example, amygdala versus ventromedial prefrontal cortex circuits for fear learning versus extinction7) and sensory regions of the forebrain and spinal cord12. However, additional evidence for projections from a subgroup of LC neurons to the caudate–putamen, along with increased NA turnover in striatum and the functional connectivity of striatal–motor networks with chemogenetic LC stimulation, is revising previous conceptions that LC-NA projections do not influence striatal–motor function16. Furthermore, the organization of well-known LC projections to other key motor structures (for example, cerebellum, inferior olive, or motor cortex) has largely been ignored. Misconceptions based on gaps in our anatomical understanding still limit theories of LC involvement in basic functions and disorders. However, these initial results point out an enticing area of study and collectively make a strong case for continued investigation of heterogeneity in LC efferent structure. One way forward is to probe the functional consequences of modularity in LC outputs by selective opto- or chemogenetic activation of subsets of terminal-field-specific LC neurons in waking, behaving animals.
The current challenge in understanding LC efferent anatomy is to develop a model that takes into account the limited number of LC neurons and the extensive distribution of LC axons throughout the neuraxis — an arrangement that requires massive arborization of individual axons — while also acknowledging recent findings of focused or modular efferent projections2 (FIG. 2). New imaging methods that target NA receptor occupancy17 hold promise for establishing a detailed mapping of the spatial distribution and timing of NA release and receptors in terminal fields relative to sensory responses and behavioural output. Such information will provide an understanding of the LC axonal organization that is functionally relevant.
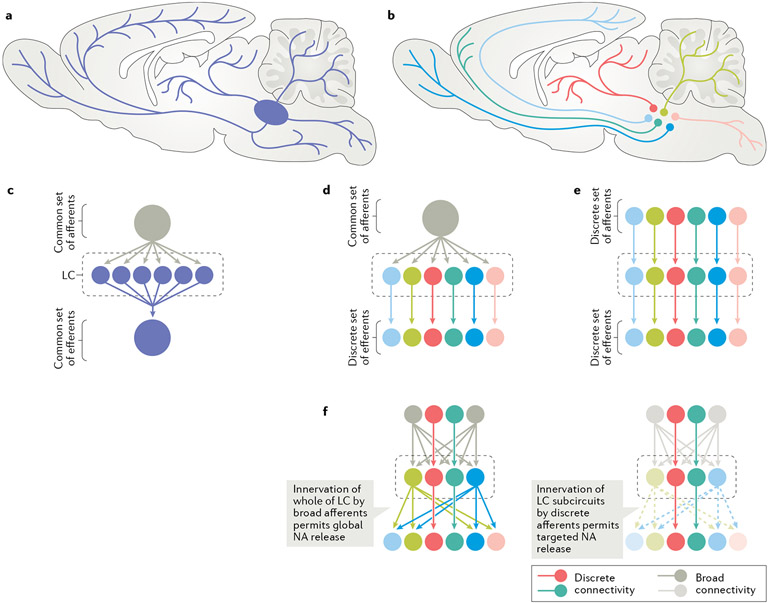
Fig. 2 ∣. Evolving views of the LC synaptic architecture and functional organization.
a ∣ Historically, the locus coeruleus (LC) had been thought to contain functionally homogeneous neurons (indicated here by a uniform blue colour) whose axons extensively collateralize and indiscriminately innervate functionally diverse terminal fields21. b ∣ More recently, several studies4,7,12-14 have shown that the axons of LC neurons are less extensive than had previously been recognized, and instead innervate anatomically and functionally distinct targets. In the schematic, each colour represents a population of LC neurons that projects strongly to a preferred terminal field, including olfactory bulb (medium blue), frontal cortex (medium green), visual cortex (light blue), thalamus and midbrain (red), cerebellum (light green) and spinal cord (pink). Note that this schematic is illustrative only and that there are likely more than six efferent LC pathways. Evidence also suggests that LC neurons also innervate terminal fields beyond their preferred targets, though less densely. c–f ∣ The organization of the presynaptic inputs to LC is less clear. Early studies suggested that all LC neurons were innervated by a limited set of common afferents, which then went on to innervate all regions of the CNS16 (part c). Another possibility is that a common set of afferents equally innervate all LC neurons, which then transmit information in a selective way to specific terminal fields (part d). A more complex arrangement would have unique afferents linked to discrete LC neurons that preferentially innervate functionally distinct terminal fields, such that there is discrete coding of information as it passes through the nucleus (part e). Another possibility is that the LC is organized such that some afferents innervate all of the LC, and so can modulate the activity of the nucleus as a whole, while others are more discrete and allow for point-to-point communication4. The same holds true for LC efferents: some LC neurons have a preferred terminal field that they densely innervate, while others broadly innervate vast expanses of the CNS1. Under this model, the LC is capable of globally broadcasting information to all its terminals simultaneously when its broad afferents are engaged. In some circumstances, however, only discrete afferents may be engaged, to allow for discrete coding of information by LC and transmission only to specific terminals (part f). NA, noradrenaline.
LC afferent anatomy.
Although modularity of the efferent connectivity of LC is an active area of research, the integration of LC efferent modularity and LC afferent patterning has largely been neglected. The LC microcircuitry consists of a dense, cell-rich ‘core’, where NA cell bodies and processes reside, and a pericoeruleus (peri-LC) ‘shell’ into which LC-NA dendrites extend and ramify18. Distinct populations of GABAergic neurons are also found in the peri-LC region and provide local regulation of LC-NA cell activity19,20. Early anatomical-tracing studies identified prominent afferent inputs from medullary nuclei into the LC core21, and subsequent work revealed that LC-NA neurons are innervated by many regions of both the brainstem and forebrain6,20,22. Inputs targeting peri-LC GABAergic cells are partially distinct from the inputs that directly innervate LC-NA neurons20.
Multisynaptic viral-tracing approaches have provided a broader analysis of LC input connectivity23. Inputs from diverse sources can produce differential effects on LC-NA neuronal output directly and indirectly, by differentially targeting the core nucleus versus the pericoerulear regions. One target of opportunity is to investigate how afferent connections and connectivity within the LC and peri-LC regions regulate discrete LC cell populations that themselves have different efferent connectivity. For example, while some LC cells receive common afferent drive, it is unclear whether cell populations with more specific efferent connectivity receive distinct afferent inputs6 (FIG. 2). Another mechanism for afferent-specific regulation would be differential expression of transmitter receptors between LC modules. Elucidating how specific afferents are connected with local LC and peri-LC networks and modules will be important for understanding LC’s diverse functionality.
LC transmitters and co-transmitters.
Another area of LC anatomy that is ripe for investigation is the distribution of neuroactive substances, primarily peptides and dopamine24, that are colocalized in the NA-containing neurons. To date, galanin, neuropeptide Y, brain-derived neurotrophic factor, cocaine- and amphetamine-regulated transcript, dopamine and other neuroactive substances have been identified as co-transmitters in LC-NA cells25-31. Some are expressed in most, but not all, LC-NA neurons, while others are sparsely distributed29. There may be species differences as well32. The functional neuroanatomy of LC co-transmission is an untapped area of inquiry. For example, there is limited information about the efferent targets and discrete versus global inputs to these cells.
We also know little of the extent to which different neuroactive substances are stored in shared or separate vesicles, under what conditions they are released, and the physiological/behavioural effects of LC-mediated neuropeptide and co-transmitter signalling. Recent technological advances in neuroanatomical mapping, opto- and chemogenetic methods of modulating neuronal activity, the detection of transmitter/modulator release, and genetic ablation can now be brought to bear. For example, to map monosynaptic inputs to LC cells that are co-expressing a specific neuropeptide, mouse lines with Cre recombinase knocked into a neuropeptide gene locus can be infused with a Cre-dependent modified rabies virus into the LC33. Similarly, LC-NA/neuropeptide output projection patterns can be identified following intra-LC infusion of neuropeptide gene Cre-recombinase-dependent AAV fluorescent tracers34.
While measuring neuropeptide release in real time has not previously been possible, the suite of new genetically encoded fluorescent neurotransmitter indicators can be expanded beyond those currently available for small-molecule neurotransmitters to include neuropeptides35. Pairing these indicators with optogenetic stimulation or silencing of specific subsets of LC neurons or with calcium-indicated or single-unit electrophysiological recordings of spontaneous activity will reveal, for the first time, the firing-frequency and pattern dependence of LC-NA peptide and dopamine co-transmission. The functional importance of non-noradrenergic LC neurotransmission is illustrated by recent demonstrations that dopamine release from LC terminals in the thalamus and hippocampus has distinct cellular and behavioural effects on stress responses, synaptic plasticity, and learning and memory36-38.
Combinatorial RNAi39 and characterization of conditional-knockout animals that lack a neuropeptide specifically in LC neurons should enable identification of the function of these co-transmitters. This approach was recently deployed to define a role for LC-derived galanin in active coping behaviours40. Utilizing inducible knockouts that remove the neuropeptide in adulthood rather than throughout development will further refine and improve this strategy.
Finally, we have only just begun to identify the mechanisms governing co-transmitter and co-peptide release and reuptake41, the dynamics of which determine transmitter concentration and the duration of exposure to membrane-bound receptors. An important future direction will be to fully characterize the dynamics of LC co-transmitter release and reuptake across LC terminal fields. Microdialysis in combination with high-pressure liquid chromatography is available for examining fluctuations in local extracellular concentrations of NA42,43, and possibly other LC co-transmitters, in anaesthetized and waking animals, but measurements with this technique have poor spatial and temporal resolution. As such, this approach leaves open the question of how rapid transitions in the LC firing rate correspond to transmitter release and to specific physiological outcomes at the cellular, circuit and network levels. Fast-scan voltammetry operates in the range of seconds and has been used extensively in anaesthetized and waking animals for moment-to-moment measurement of extracellular concentrations of dopamine in different brain regions44,45. However, this technique has not been exploited to the same extent for NA. What is needed is an electrochemical or other biosensor methodology whereby rapid measurement of NA levels or of other LC co-transmitters can be made simultaneously in multiple brain sites of waking animals engaged in behavioural tasks46,47. In particular, NA receptor sensors with subsecond fidelity might hold the key to understanding the dynamics of NA release and receptor activation in LC terminal fields on a timescale capable of providing a link between measured release patterns and observable physiologic action17. Such capabilities would permit an evaluation of circuit-specific fluctuations of LC-NA transmitter levels in concert with transitions between behavioural states and concurrent with behavioural and sensory events.
Receptor activation.
The net effect of NA release within a given neural circuit is dependent on the presynaptic and postsynaptic complement of α- and β-adrenergic receptors and their subtypes in that region. The nine known receptor subtypes have long been characterized: α1A, α1B, α1D, α2A, α2B, α2C, β1, β2 and β3 each have unique and sometimes overlapping distribution patterns in the brain, different sensitivities to the NA ligand and different actions upon the cells and the network in which they are embedded. For example, β2-receptors are more abundant in the hippocampus. A genetic variant of this β2-receptor subtype prolongs NA binding and is seen more in those who develop post-traumatic stress disorder48. The literature on the expression, turnover and function of adrenergic receptors across the brain and spinal cord is extensive, describing the NA receptors on neurons, glia, immune cells and even brain vasculature49-55. Any interpretation of the effects of LC output on brain functions and, ultimately, on behavioural outcomes must account for the relative distribution, density, binding properties and physiology of adrenergic receptors on specified cell populations within LC terminal fields. Likewise, information about the distribution of receptors for LC co-transmitters is also critical for a complete understanding of terminal-field responses to LC activation. However, the dynamics of receptor availability and activation are often neglected when considering the net outcome of LC actions on terminal-field operations. Consideration of these factors will provide for a more holistic interpretation of the impact of LC output on CNS function.
Origin and development of the LC
The notable progress in our understanding of the complexity of LC organization3 reveals an urgent need to determine the mechanisms that generate such LC cell heterogeneity. We are only beginning to understand how diversity among LC neurons is obtained and maintained across the lifespan. Molecular and genetic tools appear indispensable for exploring the ontogeny of LC-NA neurons and, in combination with other methods, may provide important insights.
LC embryogenesis.
One possible clue to the origin of distinct LC modules lies in the finding that different populations of NA neurons originate from molecularly distinct progenitors, with most LC neurons emerging exclusively from a single rhombomere, defined by the expression of a factor named engrailed 1 (REF.5). An intersectional genetic fate-mapping method has demonstrated that embryonic origins define features of LC neurons such as their pattern of distribution within the LC complex, their cell morphology and their axonal projection pattern56. The development, maintenance and survival of LC-NA neurons are all regulated by complex molecular signalling cascades involving multiple genes and transcription factors57-61. A recent exciting study showed that by combining only seven transcription factors, astrocytes and fibroblasts could be converted in vitro into NA neurons by direct reprogramming62. A complete set of the genes and transcription factors that are necessary and sufficient for the generation and survival of NA cells in vivo would be helpful for restoring function after neurodegeneration — for example, in Alzheimer disease (where LC pathology begins early) — while the genes and transcription factors that regulate LC cell number and differential circuit formation all need to be catalogued61 in order to ensure replacement of the proper type and number of LC-NA neurons.
LC postnatal development.
At birth, LC-NA neurons are functionally active, yet some of their physiological features undergo postnatal developmental alterations, including changes in membrane properties, receptor expression, cellular coupling (from predominantly gap junctions to more synaptic connections) and responsiveness to sensory inputs63. In newborns, the LC’s responsiveness to somatosensory stimuli appears enhanced, whereas later in development, LC neurons become less sensitive to innocuous stimuli and more sensitive to noxious stimuli64. Enhanced response of neonatal LC neurons to sensory stimulation is thought to underlie some unique features of infantile learning, such as attachment65. Indeed, environmental factors in early life can have long-lasting effects on LC function and behaviour into adulthood. For example, high maternal care affects the densities of the benzodiazepine receptor, the α2-adrenoreceptor and the corticotropin-releasing factor receptor in the LC in a way that renders the adult offspring of high-licking mothers more resistant to stress66.
NA has a protective role for other neuronal cell types in the CNS. A recent study reported that early postnatal NA denervation reduces BDNF protein and the activation of TrkB receptors in the ventral midbrain, possibly leading to dopamine neuron degeneration67. The possibility that NA plays a role in preserving neurons in other areas and allowing critical-period plasticity during development has not been adequately addressed.
LC neurons change their electrophysiological characteristics across early development. LC cells in infant rats display slower conduction velocities63 as well as synchronized subthreshold membrane-potential oscillations and synchronized spiking64,68. Interestingly, those LC neurons with oscillatory activity have lower input resistance and a tendency for more electric coupling via dendro-dendritic gap junctions than neurons that do not oscillate. These gap junctions are thought to underlie a high degree of synchrony in the LC of newborns, a synchrony that dissipates by postnatal day 21 (REFS64,68). Interestingly, the expression of NA itself fluctuates among LC cells during postnatal development, with some cells exhibiting only transient noradrenergic characteristics69. There is also a transiently expressed excitatory synaptic coupling among LC neurons in early infancy that is mediated by α1 adrenergic receptors70, whereas α2-mediated inhibitory coupling persists throughout the lifespan68,71. It is unknown how gap junctions and early activity relate to the final phenotype of LC cells. Better understanding of the molecular signalling mechanisms that control the expression and maintenance of the NA phenotype in the LC will likely lead to ground-breaking approaches for the preservation and restoration of LC function in neurodegenerative and neuropsychiatric disorders.
Electrophysiological properties
The electrophysiological properties of LC neurons have been well characterized by means of a number of intracellular and extracellular recording studies72,73. Individual LC neurons in vivo have almost exclusively been measured in adult animals, in which they display a relatively narrow range of discharge rates across varying arousal levels, from almost completely silent in REM sleep to 5–6 Hz in stress74,75. LC neurons ex vivo, recorded almost exclusively from very young brains, display spontaneous firing rates in the range of 0.5–5 Hz72,76. Although synchronous oscillations between LC neurons, mediated by gap junctional coupling of dendrites, have been reported72, ex vivo intracellular brain slice recordings have demonstrated that there remains variability among cells in spontaneous firing rate and resting membrane potential72. Such physiological variation supports the existence of distinct groups of LC neurons, as has been indicated by anatomical studies (FIG. 2).
Several important questions arise from observations of firing-rate variability among LC cells. First, what, if any, are the transcriptional differences between LC cells that could account for variations in their intrinsic physiological properties? A recent publication demonstrated that the LC neurons that innervate medial prefrontal cortex and ventral hippocampus are not only anatomically distinct but also differ in their physiological responses to clonidine, demonstrating phenotypic variability among cells77. The recent development of single-cell sequencing techniques performed in the same cells that have been characterized electrophysiologically offers a powerful window into the molecular determinants of intrinsic physiology. Such electrophysiological and genetic combinations could help reveal why different LC cells behave in unique ways in vivo across behavioural states, both with and without experimental manipulation78. Another important question is whether LC cells with different electrophysiological properties also show distinct patterns of anatomical connectivity or neurochemical profiles in order to differentially affect CNS function.
Novel viral-genetic techniques allow investigation of the electrophysiological attributes of discrete assemblies of LC neurons that can be probed according to their connection patterns and/or their co-transmitter profiles, to reveal unique roles in specific behaviours. Early application of such viral-genetic approaches has started to show the compartmentalization of function within LC cells of different electrophysiological profiles, such that the neurons innervating different terminal fields can show different input resistances, excitability, after-hyperpolarization profiles, action potential widths, excitability, firing rates and plasticity to afferents. For example, LC efferents to the forebrain (that is, basolateral amygdala and infralimbic prefrontal cortex) have higher input sensitivity and firing rates than the slower-firing LC cells that innervate the motor cortex, which themselves are different from those that project to the spinal cord sensory system. These different electrophysiological properties combine with specific projection targets, co-transmitters and receptor arrays to influence discrete behaviours, such as fear conditioning versus fear extinction and anxiety/augmented responses to noxious stimuli versus analgesia7,12.
Similarly, high-density electrode arrays have recently enabled large-scale ensemble recording in the LC, allowing discoveries that could not be inferred from patterns of LC single or multiunit activity. The anaesthetized preparation in these studies permitted the necessary long-duration recording of stable spiking of a large population of LC neurons that has not yet become available for the brainstem of behaving animals. Nevertheless, the LC properties observed under anaesthesia or in vitro require confirmation in awake animals. In anaesthetized rats, spiking among random pairs of LC neurons is predominantly uncorrelated, but on a moment-to-moment basis, clusters of LC neurons may self-assemble to form functional arrays that relay meaningful information79. Individual LC neurons do not respond reliably on a trial-by-trial basis to attention-eliciting stimuli7,79 or to cortical delta waves79, but output from groups of otherwise inconsistently responding cells may produce a high-fidelity ensemble code that represents LC information processing. These and other results suggest that ensemble-based LC coding underlies the spatially and temporally selective neuromodulation of cognitive, sensory, memory and motor functions1. An important open question that is ripe for further investigation is how distinct ensembles arise and are modulated through differences in circuit connectivity and heterogeneous intrinsic physiological properties.
Effects of LC-NA activation
Through its widespread efferent network, the LC-NA system impacts a diverse array of core behavioural processes, including the regulation of waking/arousal and a diversity of state-dependent cognitive and motivational processes. These processes include prefrontal cortex-dependent ‘executive’ functions like attention and working memory80,81, sensory processes, synaptic plasticity and long-term memory82,83, high-arousal/emotional amygdala-dependent memory74,84, cognitive and behavioural responses to stress (BOX 2) and goal-directed motivated behaviour85-87, including motivation for drugs of abuse88. The diverse behavioural effects of this neurotransmitter system reflect the complexity of the effects of NA receptor activation on neuronal signal processing, gene transcription and neuronal plasticity that optimize behavioural outcomes in complex environments.
Box 2 ∣. LC and stress.
The locus coeruleus (LC) has been implicated in neuropsychiatric disorders through its role in the stress response196,197. Stressors engage the LC-noradrenaline (NA) system through corticotropin-releasing factor (CRF), which biases LC neuronal discharge to favour high-tonic activity and diminishes responses to discrete stimuli197,198. These effects could be adaptive in life-threatening environments, where high general arousal and cognitive flexibility would be advantageous. Chemogenetic LC activation in order to mimic acute stress increases brain-wide functional connectivity, particularly in salience networks and amygdala networks, and this is accompanied by decreased exploratory and increased anxiogenic behaviour16. A caveat is that it is not known whether chemogenetic stimulation produces the same rate and pattern changes in LC activity that acute stress does, and these results may differ for different types of stressors.
Enkephalin-containing axon terminals converge on some of the same LC dendrites as CRF-containing axon terminals and have opposing effects on LC discharge199,200 during stress, implicating enkephalin afferents to the LC (acting at μ-opioid receptors) as an important stresscoping and recovery system. Acute stressors engage both CRF and enkephalin afferents to regulate LC activity201,202. Although CRF excitation of LC activity predominates during acute stress, μ-opioid receptor antagonism results in a greater magnitude of LC activation and delays recovery201.
Sex is an important determinant of LC sensitivity to stress, because in rodents the LC neurons of females are more sensitive to CRF and less sensitive to enkephalin than are those of males203-205. This increased female sensitivity to CRF has been linked to synaptic CRF receptor and internalization changes in females compared to males204. This would translate to a greater LC-NA response to stressors and a decreased ability to adapt through the process of receptor internalization. The role of cycling hormones remains to be thoroughly investigated, but oestrogen and progesterone receptors abound on LC cells and likely have mitigating effects on LC activity206. These many sex differences suggest a molecular basis for the higher prevalence of stress-related psychiatric disorders in females207.
LC-NA in sensory processing.
Many studies have shown that local administration of NA or activation of LC can modulate the responses of single cells, local circuits and neural networks to external and internal sensory signals74,89,90. In anaesthetized and unanaesthetized animals, the application of NA or activation of the LC results in a spectrum of changes in sensory neuronal response properties across thalamic and cortical sensory regions, including changes in the magnitude91-96 and timing of stimulus-evoked discharges97,98. Moreover, NA-mediated actions induce alterations in the receptive-field properties of visual cortical neurons99,100, modulate odour detection and discrimination thresholds in the olfactory bulb101, enhance the plasticity of frequency tuning in the auditory cortex93, enhance auditory perception102 and improve performance in a visually guided signal detection task103. Studies in awake animals have underscored the importance of evaluating LC-mediated effects in terminal fields not only at the single-cell level, but also across ensembles of simultaneously recorded neurons, to account for the heterogeneity of effects on target neurons, leading to net changes in neural circuit and neural network output104. Importantly, the experimentally demonstrated modulatory effects of NA and LC activation on cells, circuits and networks follow an inverted-U-shaped function94,104; that is, the facilitating effects of NA and LC activation are initially modest, rise to optimal as NA output elevates, and then gradually diminish with further increases in NA concentrations. In vitro studies have shown that, at moderate concentrations, NA acts at α1 receptors to strengthen peri-threshold sensory neuronal responses, whereas at higher concentrations, NA acts via α2 receptors to degrade signal processing105.
Further experiments in awake, behaving animals will be needed that combine LC manipulations, in vivo recording of neuronal responses to sensory stimulation, and precise psychophysical measures of behavioural responses, to better understand the relationship between differing rates and modes of LC activity, sensory processing, perception and action94,96,104. A recent promising study in that direction with human participants combined the pharmacological manipulation of NA with measurement of visually evoked potentials, fMRI activation in visual cortex and the participants’ performance on various visual perception tasks. Several aspects of visual perception were improved in tandem with increases in the consistency of the evoked potentials and in the fMRI signal when increasing NA; the opposite results were obtained when decreasing NA106.
LC-NA state effects.
Early correlative observations documented a strong positive relationship between LC firing rate and arousal level107,108. Subsequent studies demonstrated that LC neuronal activity and NA receptor activation are sufficient to invoke the alert (conscious) waking state and are necessary for the maintenance of wakefulness74. The arousal-promoting actions of NA, at least in part, involve α1- and β-receptors within a network of subcortical sites75. The relation between NA and arousal is monotonic, with low LC firing rates being associated with sedation or sleep, and the highest rates being associated with high levels of arousal (however, arousal during REM sleep is an exception; see BOX 4). Recent LC optogenetic and chemogenetic studies have confirmed and extended these earlier observations using a variety of arousal measures, such as pupillary diameter, which is highly linked with LC activity and a diversity of behavioural responses. Taken together, these studies indicate a very tight positive linkage between LC activity, a corresponding NA release and arousal, with LC activity playing a sufficient and necessary role in changing the arousal level20,109-113.
Box 4 ∣. LC activity across sleep: relevance to waking functions and disease.
Although the locus coeruleus (LC) firing rate typically slows at sleep onset, within sleep, LC firing rates do not predict either arousal levels or arousal thresholds. In all animals recorded to date, which have included only males, the LC falls nearly silent during the state of rapid eye movement sleep (REMS) and is also silent in the seconds immediately preceding sleep spindles during non-REMS108,216. These sleep states show intense brain activity in limbic circuits, ample synaptic plasticity217,218 and rich cognitive content, in the form of vivid dream reports. LC activity during sleep after learning has been little studied, but the few existing reports reveal an intriguing interplay of activity and silence, both of which could play essential roles in sleep-dependent memory consolidation216,219,220. Specifically, LC activity is timed to deliver noradrenaline at forebrain synapses just in time to strengthen or protect circuits during non-REMS memory replay events — that is, at the peaks of slow oscillations134; however, LC silences precede non-REMS spindles and REMS replay periods, which would uniquely allow a depotentiation of synapses that would be necessary for memory networks to be reshaped124,221,222.
Violations either of LC activity at non-REMS slow oscillations or of LC silences before spindles and in REMS could disrupt memory consolidation processes216,223. Indeed, the characteristic sleep aetiology of post-traumatic stress disorder (PTSD), insomnia, and opiate withdrawal (BOX 2) suggests an overactive LC during sleep224, which may underlie the emotional and hippocampal memory consolidation deficits of those disorders225. The overactive LC seen in early Alzheimer disease (AD) (BOX 1) may also cause the disturbed sleep apparent at the earliest diagnoses of that disease, and the catastrophic degradation of the LC in late AD could fail to provide NA at forebrain synapses in support of memory preservation during slow oscillation reactivations, contributing to overall memory erasure. These questions have yet to be addressed.
Given the strong effect of the LC-NA on plasticity, one area for future study is the activity of the LC during sleep in females across the oestrous cycle. Increased LC activity during sleep at some phases of the oestrous/menstrual cycle could underlie increased vulnerability to memory consolidation disturbances, opiate use disorder, AD and anxiety-related disorders such as PTSD226.
LC-NA and plasticity.
A half century ago, Seymour Kety hypothesized that LC release of NA potentiates the activation of small numbers of neurons that, under the influence of NA, strengthen connectivity as a means to store information and adaptively alter sensory and motor circuits69. He suggested that the inhibition of random and spontaneous activity by NA throughout the brain is a characteristic of plasticity-promoting arousal, with smaller, sharply focused and activated circuits mediating learning. Intracellular cAMP-cascade pathways engaged by NA β-adrenoreceptors were proposed to support these learning-associated changes. Developing visual114,115 and olfactory116 networks provided direct evidence of such LC-NA-dependent plasticity. NA-induced long-term potentiation (LTP) of entorhinal throughput in hippocampal dentate gyrus provided mechanistic support for the predicted LC-NA effects117,118.
Since the proposal of Kety’s hypothesis that LC mediates learning through circuitry enhancement, we have learned that LC is involved in multiple distinct and learning-critical forms of hippocampal and cortical plasticity, including habituation119,120, long-term depression (LTD)121-123 and depotentiation124. These contrasting forms of plasticity are related in part to LC effects on specific synaptic pathways121,122 and, in the case of depotentiation, to pauses in LC firing124. LC activity that occurs in conjunction with hippocampal and cortical synaptic transmission triggers input-specific synaptic plasticity122,123, and its temporal parameters can determine whether the direction of change is potentiation or depression122,123.
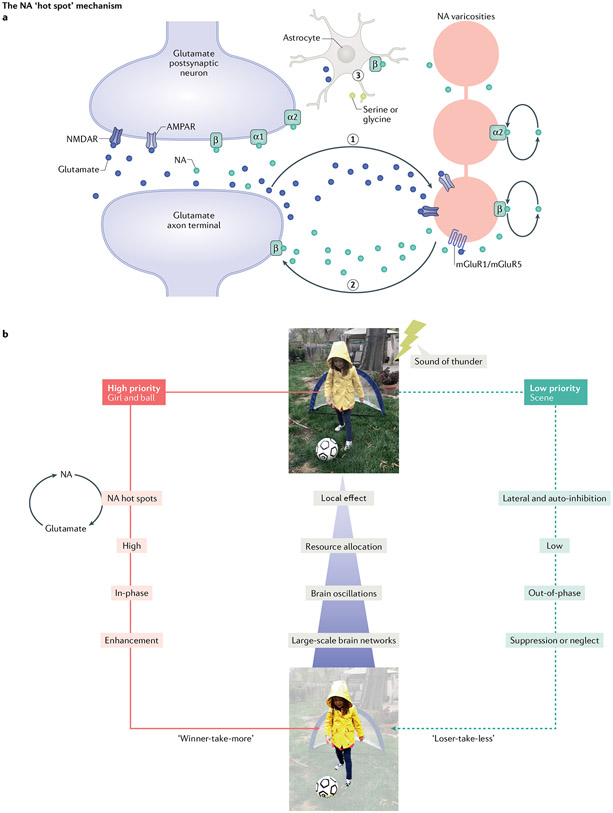
Glutamate amplification of NA (GANE) is a recent proposal to account for the input-specific potentiating and depressing actions of LC-NA on neural circuitry. GANE proposes that NA concentrations linked to LC activity are modulated locally in target structures by glutamate activity125 (FIG. 3). Specifically, GANE hypothesizes that informational glutamate circuits compete for metabolic and molecular plasticity resources, with a winner-take-all outcome. Losers are suppressed. The suppression or enhancement depends on the local NA concentrations. NMDA, AMPA and metabotropic glutamate receptors on NA terminals all increase NA release125. Nearby astrocytes also play a role in supporting GANE-mediated plasticity (FIG. 3a). These competitive interactions underpin the contrasting cognitive effects of LC activation on concurrent inputs, with salient inputs becoming memorable and others being ignored (FIG. 3b).
Fig. 3 ∣. GANE release creates local NA ‘hot spots’ and alters network processing: the network GANE model.
a ∣ A noradrenaline (NA) ‘hot spot’. Local spillover glutamate (blue dots) from active glutamate terminals (step 1) interacts with depolarized NA varicosities. These NA varicosities (pink) are depolarized by locus coeruleus (LC) activation. Spillover glutamate acting on three kinds of glutamate receptors — NMDA receptors (NMDAR), AMPA receptors (AMPAR), and metabotropic glutamate receptors 1/5 (mGluR1/mGluR5) — may increase NA release from these varicosities (green dots; step 2). β-Adrenoceptors on glutamate terminals can promote additional glutamate release, in a positive feedback loop. Spillover glutamate also recruits astrocytes (step 3) to release the NMDA co-agonists serine and glycine (grey dots) and additional, astrocyte-sourced glutamate (blue dots). Finally, on the postsynaptic glutamate neuron, local NA activates higher-affinity α2- and α1-receptors. If NA concentrations are sufficiently high, NA also recruits the lower-affinity β-adrenoreceptors to promote long-term potentiation (LTP) at those glutamate synapses. To generate LTP, a high level of β-adrenoreceptor activation is needed, because lower levels of β-adrenoreceptor activation promote long-term depression, attenuating the input strength. Failure to recruit β-adrenoreceptors will leave the glutamate circuitry unchanged. Increased local NA can also act in an autocrine fashion on α2-and β-adrenoreceptors expressed on the NA varicosities, modulating further NA release. β-Adrenoreceptor activation would also increase NA release in a positive feedback loop. b ∣ The system-wide effects of glutamate amplification of noradrenaline (GANE) interact with local ‘hot spot’ effects. Salience-evaluating structures, such as the anterior cingulate cortex and insula, recruit LC firing in order to enable NA to modulate ongoing processing at multiple levels of brain function. Local glutamate–NA memory-enhancing effects occur in parallel with more broad-scale suppression, as NA recruits lateral and auto-inhibitory processes that suppress weaker glutamate signals in lower-priority processing pathways. These noradrenergic mechanisms lead to ‘winner-take-more’ and ‘loser-take-less’ outcomes in perception and memory under arousal. For example, if the girl in the yellow raincoat has high priority at the moment, either because of her perceptual salience or because of a goal (for example, rooting for her team), memory for her should be enhanced if something else (for example, loud thunder) induces arousal at that moment, whereas the peripheral inputs are more likely to be forgotten. High-priority input interacts with LC-induced arousal and recruits resources and networks to create new memory circuits, whereas lower-priority input is unsupported. Part a adapted with permission from REF.125, Cambridge University Press. Part b image courtesy of David Clewett, UCLA.
Other LC actions are linked to plasticity. LC-NA modulates astrocytes in order to increase cortical perfusion and generate astrocytic calcium waves126,127. There is a growing appreciation of the brain-wide modulation of astrocytic signalling that LC-NA produces127-129, as astrocytes are critically involved in multiple plasticity support functions. The influence of too much or too little NA on the functions of astrocytes for plasticity is open to investigation.
In the hippocampus, optogenetic experiments have revealed a shift in CA1 place cell firing towards a newly rewarded place in an otherwise stable map. Pairing of LC activity with the newly rewarded place is required. The authors suggest that this plasticity is supported by high neuromodulatory tone, changes in local inhibition and astrocytic activation130. These results contrast with results from another experiment showing global remapping in CA1 with phasic glutamatergic LC activation120, strongly supporting a hypothesis of concentration-dependent differences in LC functional effects.
Recent data suggest that long-range glutamate projections play a role in LC plasticity. The pairing of a tone with LC activation both strengthens the representation of the tone in the cortex and leads to the tone now activating the LC through increased synaptic strength of the LC neurons. This tone-evoked LC activation is required to support the enhanced auditory response, which, in turn, improves auditory perception102.
Taken together, both data and theory speak to a complex and multifaceted LC role in neural plasticity and the resultant adaptive behaviour (FIG. 3). The updated view highlights NA suppression as well as enhancement of neural circuitry and suggests that both local and long-distance interactive feedback loops support plasticity. The selective spatial and temporal activations afforded by optogenetics, combined with the ability to monitor LC output through electrophysiological and imaging methods, will allow rapid progress in testing these hypotheses. One example of a hypothesis ripe for further testing is the discovery that LC-released dopamine in the hippocampus, which mediates novelty-enhanced memory consolidation, is dependent on the reversal of NA transporter activity triggered by high levels of local glutamate and activation of NMDA receptors on NA terminals41. Co-release of dopamine in the presence of high local glutamate is a new mechanism consistent with the GANE hypothesis, and its elucidation could increase our understanding of local LC plasticity effects throughout the brain41.
LC-NA and memory formation.
LC neurons fire in bursts in novel environments or when encountering unexpected novelty in a familiar environment119. Consequent NA release and postsynaptic activation of adrenergic receptors at LC targets potentiates evoked responses in the hippocampus131 (see LC-NA and plasticity), changes plasticity-related signal-to-noise ratios122,132,133, facilitates contextual learning38 and enhances everyday memory24. Beyond the hippocampus, NA action at β- and α1-receptors within the amygdala enhances emotional learning2,84. LC activation is critical for offline memory consolidation at discrete time windows after learning in both appetitive and aversive learning134-137. LC input may facilitate cross-regional interactions that underlie offline memory consolidation, particularly during sleep138,139 (BOX 4). NA release impacts various molecular signalling cascades that underlie memory trace stabilization across multiple time windows140,141. Questions that are open to future investigation include the factors triggering experience-dependent delayed activation of the LC-NA system and the function of critical silent periods of LC neurons during REM sleep, which could uniquely allow synaptic depotentiation and the updating of memories (BOX 4).
Executive function.
LC projections to the prefrontal cortex modulate distinct attentional processes, including focused81, flexible142 and spatial attention143. The modulatory actions of NA on attentional processes follow an inverted-U-shaped curve, with optimal function at moderate NA doses, as was reported for working memory144. Interestingly, in contrast to what is seen with working memory, α1 receptor activation promotes both flexible attention and focused attention81. The neural mechanisms for promoting focused attention remain to be elucidated and are an area of opportunity. For instance, can we understand the influence of LC activation on attention solely by looking at the actions of the LC-NA system in the prefrontal cortex, or is LC activation related to a more global influence on networks centred on the prefrontal cortex? Also, what are the specific components of attention that are affected by LC activation?
Behavioural and cognitive flexibility.
A key to understanding the involvement of the LC in cognitive processes is to determine the environmental stimulus pattern or context that elicits changes in LC neuron activity that are associated with the behavioural response. Here the data are sparse, due to the challenges of recording from this small population of neurons located deep in the brainstem. Nevertheless, existing studies in rats and monkeys engaged in formal conditioning protocols have revealed that LC neurons are engaged from the onset of the learning process, responding to both novel stimuli and reward85,86,145,146. Conditioning of LC neurons occurs rapidly, occurring many trials before any behavioural expression of learning or any sign of conditioned responding in cortex73,145. Importantly, once the conditioned behaviour is well-established, LC cells no longer respond reliably to the conditioned stimulus. However, any change in reward contingencies that requires a behavioural adaptation, such as extinction or reversal, elicits a robust response in the LC85,86. This striking sensitivity of LC neurons to changes in a context led to the hypothesis that the LC-NA system is involved in cognitive flexibility and allows rapid behavioural adaptation to changes in environmental contingencies73,85,147. Experiments designed explicitly to test this hypothesis have used pharmacological or genetic manipulation of the NA system and behavioural protocols involving reversal or extra-dimensional shift in rodents142,148-153. The results lend strong support to this idea. Recent refinement of fMRI techniques has allowed functional assessment of areas as small as the LC87, which revealed that this important role of the LC in cognitive flexibility may also operate in humans. These advanced fMRI techniques have opened new vistas for further investigation of the role of the LC-NA system in cognitive and behavioural flexibility16,139,154,155.
This striking sensitivity of LC neurons to environmental imperatives has been observed across species and within different behavioural situations; nevertheless, new evidence suggests that subpopulations of LC neurons respond in specific behavioural/cognitive contexts12,156,157. Two important questions arising from these data are how this type of context-dependent coding is implemented, and whether and how LC neural processing is coordinated within a modular organization (see Anatomy of the LC). Given that small or large numbers of LC neurons can be activated in different contexts, these two questions may be interrelated. Further experiments recording multiple LC single units during behaviour will be needed in order reveal how these subpopulations respond to specific environmental contingencies (see Theories from behavioural correlates for further discussion).
LC-NA in motivation and decision making.
LC neurons are phasically activated by the outcome of task-related decision making145,158. However, the activation of LC neurons is limited to decisions to act and is not involved in the decision to cancel or prevent action159. Recent studies in monkeys have indicated that LC activity correlates with effort production, which is not the case for DA neurons recorded under the same conditions160. In an effort–reward task, decreasing the NA level with clonidine specifically affects effort without affecting reward processing161. While the site of action of NA-dependent modulation of effort is unknown, these effects are compatible with the idea that NA enhances cognitive control and modulates executive functions162,163.
NA has been strongly implicated as a motivational factor in drug-seeking behaviour. Activation of adrenergic receptors in select brain regions reinstates a previously extinguished drug-seeking behaviour, whereas NA receptor blockade prevents reinstatement elicited by various stimuli. In particular, engagement of the LC-NA system is required for drug-primed and cue-induced reinstatement, while other noradrenergic nodes (for example, the A2 group) regulate stress-induced reinstatement164. At least some of these effects involve actions of NA within arousal-related subcortical structures and are associated with increases in affectively neutral arousal165.
Theories from behavioural correlates
Early theoretical models articulated a strong relationship between LC activity and arousal, on the one hand, and the inverted-U relation between arousal and performance in cognitive tasks, on the other. Aston-Jones and Cohen166 developed a model (the adaptive gain theory) centred on modes of LC activity. The phasic mode, characterized by a low baseline firing rate and a strong phasic response to task-relevant stimuli, was seen as enhancing task-related activity, whereas the tonic mode, where LC neurons display sustained activation, would be associated with exploratory behaviour. In early versions of this model, the transient release of NA was proposed to enhance stimulus processing, thereby mediating the role of NA in attention. In later versions, after it was discovered that LC activation was more closely aligned with the behavioural response than with stimulus onset, the NA-induced increase in signal gain in target regions was proposed to facilitate the decision itself — that is, the execution of the action in response to the stimulus. The idea that transient LC activation promotes action execution is compatible with the idea that it enhances effort and is closely related to the notion of cognitive control90,160. Altogether, both the results of experimental studies and the model emphasize the specific temporal relation between transient LC activation and the mobilization of cognitive resources to provide an optimal response.
Arnsten167 provides a neurobiological account of the relation between arousal and cognitive performance through specific sites of NA action. In this framework, optimal performance is achieved at intermediate levels of NA via specific stimulation of high-affinity α2 receptors in the dorso-lateral prefrontal cortex, which supports executive functions. At higher concentrations, NA acts on lower-affinity α1 and β receptors located in posterior, sensory–motor regions, where it promotes fight-or-flight behaviours. Thus, both Aston-Jones and Arnsten provide a noradrenergically based accounting of the inverted-U relation between arousal and performance, with Aston-Jones and Cohen166 focusing on the dynamics of LC activation, and Arnsten80 emphasizing different affinities and the heterogeneous distribution of NA receptors. Further refinements of these models will require a better understanding of the factors underlying LC activation and of the consequence(s) of this activation in LC terminal fields.
Another theory of LC function derived from behavioural correlates of LC activity emphasizes the role of NA in cognitive flexibility. Network reset refers to the reorganization of target networks enabling the rapid switch of the cognitive representation of the world to guide behaviour73,168-173. Bouret and Sara derived this theory from recordings of LC neurons in rats undergoing learning or extinction73,85,145 (see above). More recent studies in humans, using fMRI combined with behavioural protocols designed to evaluate cognitive flexibility, have shown that the LC is indeed engaged when shifts in attention or behavioural strategy are required16,154,155,174. Pupil dilation, as a correlate of LC activation, has been validated in the monkey112 and is now widely used in human research. Here again, engagement of the LC in cognitive flexibility has been corroborated175,176.
A version of this network reset theory of LC function proposed by Dayan and Yu168 suggests that LC neurons are activated in the context of “unexpected uncertainty”, sending a “neural interrupt signal” to prepare the organism for adaptation of behaviour.
These earlier conceptual theories were founded on the idea that LC-NA cells behave homogeneously. However, recent studies have shown that subpopulations of LC neurons can be engaged in a more specific fashion and that their activity patterns, as well as the number and identity of the cells that are activated, can vary depending on the sensory or behavioural context1,145,157. Furthermore, emerging evidence suggests that these different patterns of LC activity are coordinated with distinct cell modules that have unique efferent connectivity (from widely divergent to more specific; see FIG. 2). Together, this suggests a new theory of LC function in which the representational (that is, what information is encoded) and modular features are coordinated. One possibility is that in very critical arousal-eliciting situations, such as exploration of a novel environment or exposure to a stressor, all cell modules are recruited to send a unified, widely broadcast NA signal to many brain regions. In other situations, where information is more subtly scattered in space and time (for example, in response to specific task events or during the initiation of movement), smaller populations of efferent-specific or molecularly identified neurons may respond, sending an NA signal selectively to distinct target sites7. This situationally dependent targeting of responsive LC cells has been termed “context-dependent modular coding”2. Such coding could be implemented through a combination of intrinsic physiological properties that enable LC neurons to switch between coupled and uncoupled modes177 and between partially distinct afferent inputs onto individual cell modules that either engage many or recruit select LC cell populations2 (FIG. 2). Determining whether and how context-dependent modular coding is coordinated with the structural organization of the LC will provide important insights into LC function and potentially link theoretical constructs and LC neuronal representations to anatomical and physiological mechanisms. Exploring the dynamics of activity across these modules as animals experience different sensory and behavioural contexts should provide new insight into the influence of LC-NA on its target regions and its involvement in behavioural adaptation.
Caveats, challenges, conclusions
Until recently, our understanding of the causal relation between LC neuronal activity and behaviour had been limited by an inability to selectively modulate LC-NA neurons in temporally precise and physiologically relevant patterns. This situation has changed dramatically with the advent of optogenetic and chemogenetic approaches, which allow us to activate or inhibit selected subsets of LC projection pathways. However, to provide unambiguous, informative data related to the behavioural consequences of LC activity, it is important that (1) the manipulations accurately reproduce the rate and pattern changes in LC activity that are elicited by physiological stimuli; (2) the efficacy of these manipulations be confirmed through electrophysiological or transmitter-selective sensors under conditions identical to those associated with behavioural testing; and (3) consideration be given to selective (module) versus global (whole-nucleus) activation of LC output channels and their efferent targets.
The establishment of a relation between LC output and behaviour will also require taking timescales into account. LC activity affects behaviour over relatively broad timescales, influencing behavioural states from sleep to arousal, while at the same time, event-related phasic increases in LC activity also influence perceptual processes and promote executive control or single-trial learning at the moment of a decision or at the time of effort production. Many gaps still prevent a full understanding of the local and global dynamics underlying brain-wide NA modulatory effects and the behavioural impact of these actions. We have delineated some of these gaps in each of the sections and subsections of this Perspective, with the aim of orienting current and future investigators.
As we have reviewed, neurophysiological work in rodents and primates indicates that, even if all LC neurons receive inputs related to autonomic arousal, distinct subpopulations of LC neurons can encode specific cognitive processes, presumably through more specific inputs from forebrain areas6,7,156,178. This, combined with specific patterns of innervation of target areas and heterogeneity in receptor distributions16,179, indicates that activation of the LC should have a more specific influence on target networks than had initially been imagined. Future theoretical constructs will need to incorporate not only the fine temporal scale but also the fine anatomical scale of LC function in order to capture the role of the LC in executive function, sensory signal processing, stress responses, motor control and learning, and ultimately in adaptive and maladaptive behaviours. Finally, recent imaging studies in rodents and primates, both human and non-human, have started to unravel the network effect of global LC activation16,155,180. Combining such approaches with local pharmacology and behaviour will be critical to better understanding the complex relation between the LC and behavioural outcomes.
Finally, for neural systems such as the LC-NA, with its multiple brain targets and dynamic patterns of behaviourally related activity, behavioural functions cannot be understood while we know only physiological and anatomical properties. The task of fully integrating such complexity requires computational modelling that can take into account multiple dimensions of action in order to make predictions regarding behavioural outcomes and functions, which in turn can be tested in additional biological experiments; this methodology has proven useful in developing prior LC theories164,166. The findings of higher complexity across LC neurons and projection targets than had previously been realized indicate an increased need for iterative biological–computational approaches to extend theories of the function of the LC system.
The remarkable advances of the past few years, prompting our ‘new look’ at the blue spot, have certainly energized and provided new tactics for the field of LC investigation. Such precise and powerful approaches also provide opportunities for investigators focused on any one of the many neural systems that receive LC input, allowing them to effectively attack questions about how that input participates in the operations of their particular system. However, even with recent methodological advances, the unique anatomy and physiology of the LC continue to pose challenges to its precise characterization and to assigning it functional attributes. More data and innovative hypotheses will be needed to develop a comprehensive picture of LC functions. The present Perspective is meant to attract new investigators to help unravel the mysteries of the ‘blue spot’.
Acknowledgements
Funding for the 3-day workshop that generated this Perspective was provided by a grant from the Albert and Elaine Borchard Foundation Center on International Education to G.R.P. and S.J.S.Research funding to D.M.-V.: German Research Foundation project no.: 316803389, SFB 1280/A04.
Glossary
- Chemogenetics
Viral introduction of chemically engineered neurotransmitter receptors into neuronal membranes. These can be subsequently activated by pharmacological ligands that are specific to the receptor.
- Co-transmitters
Neuromodulators released from a neuron along with a primary neurotransmitter.
- Fast-scan voltammetry
Voltammetry examines fluctuations in current that are driven by variations in voltage/potential. In cyclic voltammetry, after the desired potential is reached, the potential is ramped in the opposite direction to return to the initial potential (time-locked voltage oscillations), causing the substance of interest to be oxidized and reduced in predetermined cycles. The concentration of the substance can be calculated by generating a calibration curve of current against concentration, allowing the relative concentration to be calculated within milliseconds, and thus the real-time detection of neurotransmitter concentration.
- Fear extinction
Learning that a context or cue that was associated with an aversive event no longer predicts that event, and thus the fear response to that context or cue is no longer expressed.
- Frequency tuning
In the auditory cortex, individual neurons exhibit a specific response pattern based on the sound frequency applied. Delivery of a set of different sound frequencies determines the frequency tuning of the neuron.
- Optogenetics
Analysis via the viral introduction of light-sensitive channels or ion pumps into neuronal membranes, which subsequently can be driven by the external application of a specific light wavelength.
- RNAi
RNA interference, which comprises the inhibition of gene expression or translation by silencing the target mRNA.
- Terminal fields
Neural areas targeted by axonal projections.
Footnotes
Competing interest
The authors declare no competing interests
References
- 1.Totah NKB, Logothetis NK & Eschenko O Noradrenergic ensemble-based modulation of cognition over multiple timescales. Brain Res. 1709, 50–66 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Likhtik E & Johansen JP Neuromodulation in circuits of aversive emotional learning. Nat. Neurosci 22, 1586–1597 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Chandler DJ et al. Redefining noradrenergic neuromodulation of behavior: impacts of a modular locus coeruleus architecture. J. Neurosci 39, 8239–8249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kebschull JM et al. High-throughput mapping of single-neuron projections by sequencing of barcoded RNA. Neuron 91, 975–987 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson SD, Plummer NW & Jensen P Uncovering diversity in the development of central noradrenergic neurons and their efferents. Brain Res. 1641, 234–244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz LA et al. Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524, 88–92 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uematsu A et al. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci 20, 1602–1611 (2017). This behavioural study in rats reveals a modular organization of LC with projection and behaviour-specific cell populations.
- 8.Plummer NW et al. An intersectional viral-genetic method for fluorescent tracing of axon collaterals reveals details of noradrenergic locus coeruleus structure. eNeuro 7, ENEURO.0010–20.202 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agster KL, Mejias-Aponte CA, Clark BD & Waterhouse BD Evidence for a regional specificity in the density and distribution of noradrenergic varicosities in rat cortex. J. Comp. Neurol 521, 2195–2207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis DA & Morrison JH Noradrenergic innervation of monkey prefrontal cortex: a dopamine-β-hydroxylase immunohistochemical study. J. Comp. Neurol 282, 317–330 (1989). [DOI] [PubMed] [Google Scholar]
- 11.Morrison JH & Foote SL Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J. Comp. Neurol 243, 117–138 (1986). [DOI] [PubMed] [Google Scholar]
- 12. Hirschberg S, Li Y, Randall A, Kremer EJ & Pickering AE Functional dichotomy in spinal- vs prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats. eLife 6, e29808 (2017). This study reveals the modular organization of LC with projection and behaviour-specific cell populations.
- 13.Waterhouse BD & Chandler DJ Heterogeneous organization and function of the central noradrenergic system. Brain Res. 1641, v–x (2016). [DOI] [PubMed] [Google Scholar]
- 14. Chandler DJ, Gao WJ & Waterhouse BD Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc. Natl Acad. Sci. USA 111, 6816–6821 (2014). This comprehensive study uses anatomical, molecular and electrophysiological approaches to demonstrate the heterogeneity of LC cell populations projecting to prefrontal or motor cortices.
- 15.Chandler DJ, Waterhouse BD & Gao WJ New perspectives on catecholaminergic regulation of executive circuits: evidence for independent modulation of prefrontal functions by midbrain dopaminergic and noradrenergic neurons. Front. Neural Circuits 8, 53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zerbi V et al. Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702–718.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Feng J et al. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 102, 745–761.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shipley MT, Fu L, Ennis M, Liu WL & Aston-Jones G Dendrites of locus coeruleus neurons extend preferentially into two pericoerulear zones. J. Comp. Neurol 365, 56–68 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Aston-Jones G, Zhu Y & Card JP Numerous GABAergic afferents to locus ceruleus in the pericerulear dendritic zone: possible interneuronal pool. J. Neurosci 24, 2313–2321 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breton-Provencher V & Sur M Active control of arousal by a locus coeruleus GABAergic circuit. Nat. Neurosci 22, 218–228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aston-Jones G, Ennis M, Pieribone VA, Nickell WT & Shipley MT The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science 234, 734–737 (1986). [DOI] [PubMed] [Google Scholar]
- 22.Luppi PH, Aston-Jones G, Akaoka H, Chouvet G & Jouvet M Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience 65, 119–160 (1995). [DOI] [PubMed] [Google Scholar]
- 23.Aston-Jones G, Chen S, Zhu Y & Oshinsky ML A neural circuit for circadian regulation of arousal. Nat. Neurosci 4, 732–738 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi T et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castren E, Thoenen H & Lindholm D Brain-derived neurotrophic factor messenger RNA is expressed in the septum, hypothalamus and in adrenergic brain stem nuclei of adult rat brain and is increased by osmotic stimulation in the paraventricular nucleus. Neuroscience 64, 71–80 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Conner JM, Lauterborn JC, Yan Q, Gall CM & Varon S Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J. Neurosci 17, 2295–2313 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koylu EO, Smith Y, Couceyro PR & Kuhar MJ CART peptides colocalize with tyrosine hydroxylase neurons in rat locus coeruleus. Synapse 31, 309–311 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Simpson KL, Waterhouse BD & Lin RC Origin, distribution, and morphology of galaninergic fibers in the rodent trigeminal system. J. Comp. Neurol 411, 524–534 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Xu ZQ, Shi TJ & Hokfelt T Galanin/GMAP- and NPY-like immunoreactivities in locus coeruleus and noradrenergic nerve terminals in the hippocampal formation and cortex with notes on the galanin-R1 and -R2 receptors. J. Comp. Neurol 392, 227–251 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Devoto P, Flore G, Saba P, Fa M & Gessa GL Co-release of noradrenaline and dopamine in the cerebral cortex elicited by single train and repeated train stimulation of the locus coeruleus. BMC Neurosci. 6, 31 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Devoto P, Flore G, Pani L & Gessa GL Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol. Psychiatry 6, 657–664 (2001). This study is an early demonstration that LC axonal terminals can co-release dopamine and noradrenaline.
- 32.Perez SE, Wynick D, Steiner RA & Mufson EJ Distribution of galaninergic immunoreactivity in the brain of the mouse. J. Comp. Neurol 434, 158–185 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A & Uchida N Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Petreanu L, Huber D, Sobczyk A & Svoboda K Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci 10, 663–668 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Jing M & Li Y Lighting up the brain: genetically encoded fluorescent sensors for imaging neurotransmitters and neuromodulators. Curr. Opin. Neurobiol 50, 171–178 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beas BS et al. The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat. Neurosci 21, 963–973 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D & Kandel ER Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl Acad. Sci. USA 113, 14835–14840 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagatsuma A et al. Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc. Natl Acad. Sci. USA 115, E310–E316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pomrenze MB et al. Dissecting the roles of GABA and neuropeptides from rat central amygdala CRF neurons in anxiety and fear learning. Cell Rep. 29, 13–21.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tillage RP et al. Elimination of galanin synthesis in noradrenergic neurons reduces galanin in select brain areas and promotes active coping behaviors. Brain Struct. Funct 225, 785–803 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonneborn A & Greene RW The norepinephrine transporter regulates dopamine-dependent synaptic plasticity in the mouse dorsal hippocampus. Preprint at bioRxiv 10.1101/793265 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berridge CW & Abercrombie ED Relationship between locus coeruleus discharge rates and rates of norepinephrine release within neocortex as assessed by in vivo microdialysis. Neuroscience 93, 1263–1270 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Florin-Lechner SM, Druhan JP, Aston-Jones G & Valentino RJ Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 742, 89–97 (1996). [DOI] [PubMed] [Google Scholar]
- 44.Venton BJ & Cao Q Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 145, 1158–1168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bucher ES & Wightman RM Electrochemical analysis of neurotransmitters. Annu. Rev. Anal. Chem 8, 239–261 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt KT & McElligott ZA Dissecting the catecholamines: how new approaches will facilitate the distinction between noradrenergic and dopaminergic systems. ACS Chem. Neurosci 10, 1872–1874 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts JG & Sombers LA Fast-scan cyclic voltammetry: chemical sensing in the brain and beyond. Anal. Chem 90, 490–504 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liberzon I et al. Interaction of the ADRB2 gene polymorphism with childhood trauma in predicting adult symptoms of posttraumatic stress disorder. JAMA Psychiatry 71, 1174–1182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCune SK & Hill JM Ontogenic expression of two α-1 adrenergic receptor subtypes in the rat brain. J. Mol. Neurosci 6, 51–62 (1995). [DOI] [PubMed] [Google Scholar]
- 50.MacDonald E & Scheinin M Distribution and pharmacology of α2-adrenoceptors in the central nervous system. J. Physiol. Pharmacol 46, 241–258 (1995). [PubMed] [Google Scholar]
- 51.Scheinin M et al. Distribution of α2-adrenergic receptor subtype gene expression in rat brain. Mol. Brain Res 21, 133–149 (1994). [DOI] [PubMed] [Google Scholar]
- 52.Civantos Calzada B & Aleixandre de Artinano A α-Adrenoceptor subtypes. Pharmacol. Res 44, 195–208 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Molinoff PB α- and β-Adrenergic receptor subtypes properties, distribution and regulation. Drugs 28, 1–15 (1984). [DOI] [PubMed] [Google Scholar]
- 54.Hertz L, Chen Y, Gibbs ME, Zang P & Peng L Astrocytic adrenoceptors: a major drug target in neurological and psychiatric disorders? Curr. Drug. Targets CNS Neurol. Disord 3, 239–267 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Nalepa I, Kreiner G, Bielawski A, Rafa-Zablocka K & Roman A α1-Adrenergic receptor subtypes in the central nervous system: insights from genetically engineered mouse models. Pharmacol. Rep 65, 1489–1497 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Plummer NW, Scappini EL, Smith KG, Tucker CJ & Jensen P Two subpopulations of noradrenergic neurons in the locus coeruleus complex distinguished by expression of the dorsal neural tube marker Pax7. Front. Neuroanat 11, 60 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirsch MR, Tiveron MC, Guillemot F, Brunet JF & Goridis C Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development 125, 599–608 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Brunet JF & Pattyn A Phox2 genes — from patterning to connectivity. Curr. Opin. Genet. Dev 12, 435–440 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Holm PC et al. Crucial role of TrkB ligands in the survival and phenotypic differentiation of developing locus coeruleus noradrenergic neurons. Development 130, 3535–3545 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Shi M et al. Notch–Rbpj signaling is required for the development of noradrenergic neurons in the mouse locus coeruleus. J. Cell Sci 125, 4320–4332 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Goridis C & Rohrer H Specification of catecholaminergic and serotonergic neurons. Nat. Rev. Neurosci 3, 531–541 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Li S et al. Conversion of astrocytes and fibroblasts into functional noradrenergic neurons. Cell Rep. 28, 682–697.e687 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Marshall KC, Christie MJ, Finlayson PG & Williams JT Developmental aspects of the locus coeruleus–noradrenaline system. Prog. Brain Res 88, 173–185 (1991). [DOI] [PubMed] [Google Scholar]
- 64.Nakamura S, Kimura F & Sakaguchi T Postnatal development of electrical activity in the locus ceruleus. J. Neurophysiol 58, 510–524 (1987). [DOI] [PubMed] [Google Scholar]
- 65.Debiec J & Sullivan RM The neurobiology of safety and threat learning in infancy. Neurobiol. Learn. Mem 143, 49–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caldji C et al. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl Acad. Sci. USA 95, 5335–5340 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hassani OK et al. The noradrenergic system is necessary for survival of vulnerable midbrain dopaminergic neurons: implications for development and Parkinson’s disease. Neurobiol. Aging 85, 22–37 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Christie MJ Generators of synchronous activity of the locus coeruleus during development. Semin. Cell Dev. Biol 8, 29–34 (1997). [DOI] [PubMed] [Google Scholar]
- 69.Bezin L, Marcel D, Desgeorges S, Pujol JF & Weissmann D Singular subsets of locus coeruleus neurons may recover tyrosine hydroxylase phenotype transiently expressed during development. Mol. Brain Res 76, 275–281 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Williams JT & Marshall KC Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. J. Neurosci 7, 3687–3694 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ennis M & Aston-Jones G Evidence for self- and neighbor-mediated postactivation inhibition of locus coeruleus neurons. Brain Res. 374, 299–305 (1986). [DOI] [PubMed] [Google Scholar]
- 72.Williams JT, North RA, Shefner SA, Nishi S & Egan TM Membrane properties of rat locus coeruleus neurones. Neuroscience 13, 137–156 (1984). [DOI] [PubMed] [Google Scholar]
- 73. Bouret S & Sara SJ Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28, 574–582 (2005). The authors develop the hypothesis that NA released in forebrain structures in response to prediction error promotes resetting of cortical networks and cognitive flexibility.
- 74.Berridge CW & Waterhouse BD The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev 42, 33–84 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Berridge CW, Schmeichel BE & Espana RA Noradrenergic modulation of wakefulness/arousal. Sleep Med. Rev 16, 187–197 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alreja M & Aghajanian GK Use of the whole-cell patch–clamp method in studies on the role of cAMP in regulating the spontaneous firing of locus coeruleus neurons. J. Neurosci. Methods 59, 67–75 (1995). [DOI] [PubMed] [Google Scholar]
- 77.Wagner-Altendorf TA, Fischer B & Roeper J Axonal projection-specific differences in somatodendritic α2 autoreceptor function in locus coeruleus neurons. Eur. J. Neurosci 50, 3772–3785 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Cadwell CR et al. Multimodal profiling of single-cell morphology, electrophysiology, and gene expression using Patch-seq. Nat. Protoc 12, 2531–2553 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Totah NK, Neves RM, Panzeri S, Logothetis NK & Eschenko O The locus coeruleus is a complex and differentiated neuromodulatory system. Neuron 99, 1055–1068.e1056 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Arnsten AF Catecholamine influences on dorsolateral prefrontal cortical networks. Biol. Psychiatry 69, e89–e99 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spencer RC & Berridge CW Receptor and circuit mechanisms underlying differential procognitive actions of psychostimulants. Neuropsychopharmacology 44, 1820–1827 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harley C Noradrenergic and locus coeruleus modulation of the perforant path-evoked potential in rat dentate gyrus supports a role for the locus coeruleus in attentional and memorial processes. Prog. Brain Res 88, 307–321 (1991). [DOI] [PubMed] [Google Scholar]
- 83.Sara SJ, Vankov A & Herve A Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res. Bull 35, 457–465 (1994). [DOI] [PubMed] [Google Scholar]
- 84.McGaugh JL The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci 27, 1–28 (2004). [DOI] [PubMed] [Google Scholar]
- 85. Sara SJ & Segal M Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog. Brain Res 88, 571–585 (1991). The study is one of the first demonstrations in a behaving animal of rapid responses of LC neurons to changes in reinforcement contingencies in a formal learning protocol.
- 86.Aston-Jones G, Rajkowski J & Kubiak P Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience 80, 697–715 (1997). [DOI] [PubMed] [Google Scholar]
- 87.Jahn CI et al. Dual contributions of noradrenaline to behavioural flexibility and motivation. Psychopharmacology 235, 2687–2702 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weinshenker D & Schroeder JP There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32, 1433–1451 (2007). [DOI] [PubMed] [Google Scholar]
- 89.Waterhouse BD & Navarra RL The locus coeruleus–norepinephrine system and sensory signal processing: A historical review and current perspectives. Brain Res. 1709, 1–15 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Sara SJ & Bouret S Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76, 130–141 (2012). [DOI] [PubMed] [Google Scholar]
- 91. Foote SL, Freedman R & Oliver AP Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 86, 229–242 (1975). This is the first demonstration in a behaving animal (in the awake monkey) that NA modulates signal to noise ratios in a sensory cortex.
- 92.Rogawski MA & Aghajanian GK Modulation of lateral geniculate neurone excitability by noradrenaline microiontophoresis or locus coeruleus stimulation. Nature 287, 731–734 (1980). [DOI] [PubMed] [Google Scholar]
- 93.Manunta Y & Edeline JM Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J. Neurophysiol 92, 1445–1463 (2004). [DOI] [PubMed] [Google Scholar]
- 94.Devilbiss DM, Page ME & Waterhouse BD Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J. Neurosci 26, 9860–9872 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCormick DA Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 12, 215–221 (1989). [DOI] [PubMed] [Google Scholar]
- 96.Vazey EM, Moorman DE & Aston-Jones G Phasic locus coeruleus activity regulates cortical encoding of salience information. Proc. Natl Acad. Sci. USA 115, E9439–E9448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lecas JC Locus coeruleus activation shortens synaptic drive while decreasing spike latency and jitter in sensorimotor cortex. Implications for neuronal integration. Eur. J. Neurosci 19, 2519–2530 (2004). [DOI] [PubMed] [Google Scholar]
- 98.Bouret S & Sara SJ Locus coeruleus activation modulates firing rate and temporal organization of odour-induced single-cell responses in rat piriform cortex. Eur. J. Neurosci 16, 2371–2382 (2002). [DOI] [PubMed] [Google Scholar]
- 99.McLean J & Waterhouse BD Noradrenergic modulation of cat area 17 neuronal responses to moving visual stimuli. Brain Res. 667, 83–97 (1994). [DOI] [PubMed] [Google Scholar]
- 100.Waterhouse BD, Azizi SA, Burne RA & Woodward DJ Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res. 514, 276–292 (1990). [DOI] [PubMed] [Google Scholar]
- 101.Escanilla O, Arrellanos A, Karnow A, Ennis M & Linster C Noradrenergic modulation of behavioral odor detection and discrimination thresholds in the olfactory bulb. Eur. J. Neurosci 32, 458–468 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Martins AR & Froemke RC Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat. Neurosci 18, 1483–1492 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Navarra RL, Clark BD, Gargiulo AT & Waterhouse BD Methylphenidate enhances early-stage sensory processing and rodent performance of a visual signal detection task. Neuropsychopharmacology 42, 1326–1337 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Devilbiss DM & Waterhouse BD The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J. Neurosci 24, 10773–10785 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Devilbiss DM & Waterhouse BD Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse 37, 273–282 (2000). [DOI] [PubMed] [Google Scholar]
- 106.Gelbard-Sagiv H, Magidov E, Sharon H, Hendler T & Nir Y Noradrenaline modulates visual perception and late visually evoked activity. Curr. Biol 28, 2239–2249.e2236 (2018). [DOI] [PubMed] [Google Scholar]
- 107.McCarley RW & Hobson JA Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science 189, 58–60 (1975). [DOI] [PubMed] [Google Scholar]
- 108.Aston-Jones G & Bloom FE Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci 1, 876–886 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carter ME et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci 13, 1526–1533 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lovett-Barron M et al. Ancestral circuits for the coordinated modulation of brain state. Cell 171, 1411–1423.e1417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hayat H et al. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv 6, eaaz4232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Joshi S, Li Y, Kalwani RM & Gold JI Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89, 221–234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reimer J et al. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun 7, 13289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pettigrew JD Pharmacologic control of cortical plasticity. Retina 2, 360–372 (1982). [PubMed] [Google Scholar]
- 115.Pettigrew JD & Kasamatsu T Local perfusion of noradrenaline maintains visual cortical plasticity. Nature 271, 761–763 (1978). [DOI] [PubMed] [Google Scholar]
- 116.Sullivan RM, Wilson DA & Leon M Norepinephrine and learning-induced plasticity in infant rat olfactory system. J. Neurosci 9, 3998–4006 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Neuman RS & Harley CW Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Res. 273, 162–165 (1983). [DOI] [PubMed] [Google Scholar]
- 118.Stanton PK & Sarvey JM Blockade of norepinephrine-induced long-lasting potentiation in the hippocampal dentate gyrus by an inhibitor of protein synthesis. Brain Res. 361, 276–283 (1985). [DOI] [PubMed] [Google Scholar]
- 119.Vankov A, Herve-Minvielle A & Sara SJ Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur. J. Neurosci 7, 1180–1187 (1995). [DOI] [PubMed] [Google Scholar]
- 120.Grella SL et al. Locus coeruleus phasic, but not tonic, activation initiates global remapping in a familiar environment. J. Neurosci 39, 445–455 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hagena H, Hansen N & Manahan-Vaughan D β-Adrenergic control of hippocampal function: subserving the choreography of synaptic information storage and memory. Cereb. Cortex 26, 1349–1364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lemon N, Aydin-Abidin S, Funke K & Manahan-Vaughan D Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on β-adrenergic receptor activation. Cereb. Cortex 19, 2827–2837 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Salgado H, Kohr G & Trevino M Noradrenergic ‘tone’ determines dichotomous control of cortical spike-timing-dependent plasticity. Sci. Rep 2, 417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Poe GR, Walsh CM & Bjorness TE Both duration and timing of sleep are important to memory consolidation. Sleep 33, 1277–1278 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mather M, Clewett D, Sakaki M & Harley CW Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav. Brain Sci 39, e200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Toussay X, Basu K, Lacoste B & Hamel E Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J. Neurosci 33, 3390–3401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.O’Donnell J, Ding F & Nedergaard M Distinct functional states of astrocytes during sleep and wakefulness: is norepinephrine the master regulator? Curr. Sleep Med. Rep 1, 1–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oe Y et al. Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nat. Commun 11, 471 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Porter-Stransky KA et al. Noradrenergic transmission at α 1-adrenergic receptors in the ventral periaqueductal gray modulates arousal. Biol. Psychiatry 85, 237–247 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaufman AM, Geiller T & Losonczy A A role for the locus coeruleus in hippocampal CA1 place cell reorganization during spatial reward learning. Neuron 105, 1018–1026.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kitchigina V, Vankov A, Harley C & Sara SJ Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur. J. Neurosci 9, 41–47 (1997). [DOI] [PubMed] [Google Scholar]
- 132.Hansen N & Manahan-Vaughan D Hippocampal long-term potentiation that is elicited by perforant path stimulation or that occurs in conjunction with spatial learning is tightly controlled by β-adrenoreceptors and the locus coeruleus. Hippocampus 25, 1285–1298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hansen N & Manahan-Vaughan D Locus coeruleus stimulation facilitates long-term depression in the dentate gyrus that requires activation of β-adrenergic receptors. Cereb. Cortex 25, 1889–1896 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sara SJ Reactivation, retrieval, replay and reconsolidation in and out of sleep: connecting the dots. Front. Behav. Neurosci 4, 185 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ferry B, Roozendaal B & McGaugh JL Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between β- and α1-adrenoceptors. J. Neurosci 19, 5119–5123 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clayton EC & Williams CL Posttraining inactivation of excitatory afferent input to the locus coeruleus impairs retention in an inhibitory avoidance learning task. Neurobiol. Learn. Mem 73, 127–140 (2000). [DOI] [PubMed] [Google Scholar]
- 137.Cahill L Neurobiological mechanisms of emotionally influenced, long-term memory. Prog. Brain Res 126, 29–37 (2000). [DOI] [PubMed] [Google Scholar]
- 138.Eschenko O, Magri C, Panzeri S & Sara SJ Noradrenergic neurons of the locus coeruleus are phase locked to cortical up–down states during sleep. Cereb. Cortex 22, 426–435 (2012). [DOI] [PubMed] [Google Scholar]
- 139.Sara SJ Locus coeruleus in time with the making of memories. Curr. Opin. Neurobiol 35, 87–94 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Bernabeu R et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl Acad. Sci. USA 94, 7041–7046 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O’Dell TJ, Connor SA, Guglietta R & Nguyen PA β-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn. Mem 22, 461–471 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McGaughy J, Ross RS & Eichenbaum H Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience 153, 63–71 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reynaud AJ et al. Atomoxetine improves attentional orienting in a predictive context. Neuropharmacology 150, 59–69 (2019). [DOI] [PubMed] [Google Scholar]
- 144.Berridge CW & Spencer RC Differential cognitive actions of norepinephrine a2 and a1 receptor signaling in the prefrontal cortex. Brain Res. 1641, 189–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bouret S & Sara SJ Reward expectation, orientation of attention and locus coeruleus–medial frontal cortex interplay during learning. Eur. J. Neurosci 20, 791–802 (2004). [DOI] [PubMed] [Google Scholar]
- 146.Xiang L et al. Behavioral correlates of activity of optogenetically identified locus coeruleus noradrenergic neurons in rats performing T-maze tasks. Sci. Rep 9, 1361 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aston-Jones G, Rajkowski J & Cohen J Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 46, 1309–1320 (1999). [DOI] [PubMed] [Google Scholar]
- 148.Devauges V & Sara SJ Activation of the noradrenergic system facilitates an attentional shift in the rat. Behav. Brain Res 39, 19–28 (1990). [DOI] [PubMed] [Google Scholar]
- 149.Tait DS et al. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur. J. Neurosci 25, 3719–3724 (2007). [DOI] [PubMed] [Google Scholar]
- 150.Snyder K, Wang WW, Han R, McFadden K & Valentino RJ Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology 37, 520–530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cope ZA, Vazey EM, Floresco SB & Aston Jones GS DREADD-mediated modulation of locus coeruleus inputs to mPFC improves strategy set-shifting. Neurobiol. Learn. Mem 161, 1–11 (2019). [DOI] [PubMed] [Google Scholar]
- 152.Tervo DGR et al. Behavioral variability through stochastic choice and its gating by anterior cingulate cortex. Cell 159, 21–32 (2014). [DOI] [PubMed] [Google Scholar]
- 153.Janitzky K et al. Optogenetic silencing of locus coeruleus activity in mice impairs cognitive flexibility in an attentional set-shifting task. Front. Behav. Neurosci 9, 286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.von der Gablentz J, Tempelmann C, Munte TF & Heldmann M Performance monitoring and behavioral adaptation during task switching: an fMRI study. Neuroscience 285, 227–235 (2015). [DOI] [PubMed] [Google Scholar]
- 155.Hermans EJ et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334, 1151–1153 (2011). [DOI] [PubMed] [Google Scholar]
- 156.Bouret S & Richmond BJ Sensitivity of locus ceruleus neurons to reward value for goal-directed actions. J. Neurosci 35, 4005–4014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Uematsu A, Tan BZ & Johansen JP Projection specificity in heterogeneous locus coeruleus cell populations: implications for learning and memory. Learn. Mem 22, 444–451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rajkowski J, Majczynski H, Clayton E & Aston-Jones G Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J. Neurophysiol 92, 361–371 (2004). [DOI] [PubMed] [Google Scholar]
- 159.Kalwani RM, Joshi S & Gold JI Phasic activation of individual neurons in the locus ceruleus/subceruleus complex of monkeys reflects rewarded decisions to go but not stop. J. Neurosci 34, 13656–13669 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Varazzani C, San-Galli A, Gilardeau S & Bouret S Noradrenaline and dopamine neurons in the reward/effort trade-off: a direct electrophysiological comparison in behaving monkeys. J. Neurosci 35, 7866–7877 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Borderies N, Mattioni J, Bornert P, Gilardeau S & Bouret S Pharmacological evidence for the implication of noradrenaline in effort. Preprint at bioRxiv 10.1101/714923 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shenhav A et al. Toward a rational and mechanistic account of mental effort. Annu. Rev. Neurosci 40, 99–124 (2017). [DOI] [PubMed] [Google Scholar]
- 163.Berridge CW & Arnsten AF Psychostimulants and motivated behavior: arousal and cognition. Neurosci. Biobehav. Rev 37, 1976–1984 (2013). [DOI] [PubMed] [Google Scholar]
- 164.Schmidt KT & Weinshenker D Adrenaline rush: the role of adrenergic receptors in stimulant-induced behaviors. Mol. Pharmacol 85, 640–650 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Espana RA, Schmeichel BE & Berridge CW Norepinephrine at the nexus of arousal, motivation and relapse. Brain Res. 1641, 207–216 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Aston-Jones G & Cohen JD An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci 28, 403–450 (2005). The authors present a computational approach to modelling the relation between mode of firing of LC neurons and adaptive behavioural performance.
- 167. Arnsten AF Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast. 7, 133–146 (2000). This paper reviews experiments mainly in primates supporting the notion that optimal concentration of NA plays an important role in the cognitive function of prefrontal cortex.
- 168. Yu AJ & Dayan P Uncertainty, neuromodulation, and attention. Neuron 46, 681–692 (2005). The authors present a computational approach to support the notion that the LC-NA system responds to unexpected uncertainty in the environment.
- 169.Nassar MR et al. Rational regulation of learning dynamics by pupil-linked arousal systems. Nat. Neurosci 15, 1040–1046 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Preuschoff K, T Hart BM & Einhauser W Pupil dilation signals surprise: evidence for noradrenaline’s role in decision making. Front. Neurosci 5, 115 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jepma M & Nieuwenhuis S Pupil diameter predicts changes in the exploration–exploitation trade-off: evidence for the adaptive gain theory. J. Cogn. Neurosci 23, 1587–1596 (2011). [DOI] [PubMed] [Google Scholar]
- 172.Muller TH, Mars RB, Behrens TE & O’Reilly JX Control of entropy in neural models of environmental state. eLife 8, e39404 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sales AC, Friston KJ, Jones MW, Pickering AE & Moran RJ Locus Coeruleus tracking of prediction errors optimises cognitive flexibility: An Active Inference model. PLoS Comput. Biol 15, e1006267 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Raizada RD & Poldrack RA Challenge-driven attention: interacting frontal and brainstem systems. Front. Hum. Neurosci 1, 3 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Giller F, Muckschel M, Ziemssen T & Beste C A possible role of the norepinephrine system during sequential cognitive flexibility — evidence from EEG and pupil diameter data. Cortex 128, 22–34 (2020). [DOI] [PubMed] [Google Scholar]
- 176.Wolff N, Muckschel M, Ziemssen T & Beste C The role of phasic norepinephrine modulations during task switching: evidence for specific effects in parietal areas. Brain Struct. Funct 223, 925–940 (2018). [DOI] [PubMed] [Google Scholar]
- 177.Alvarez VA, Chow CC, Van Bockstaele EJ & Williams JT Frequency-dependent synchrony in locus ceruleus: role of electrotonic coupling. Proc. Natl Acad. Sci. USA 99, 4032–4036 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ennis M, Shipley MT, Aston-Jones G & Williams JT Afferent control of nucleus locus ceruleus: differential regulation by ‘shell’ and ‘core’ inputs. Adv. Pharmacol 42, 767–771 (1998). [DOI] [PubMed] [Google Scholar]
- 179.Cerpa JC, Marchand AR & Coutureau E Distinct regional patterns in noradrenergic innervation of the rat prefrontal cortex. J. Chem. Neuroanat 96, 102–109 (2019). [DOI] [PubMed] [Google Scholar]
- 180.Guedj C et al. Boosting norepinephrine transmission triggers flexible reconfiguration of brain networks at rest. Cereb. Cortex 27, 4691–4700 (2017). [DOI] [PubMed] [Google Scholar]
- 181. Dahlstroem A & Fuxe K Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand Suppl. 62 (Suppl. 232), 1–55 (1964). This seminal paper reports the discovery of nuclei of noradrenergic neurons in the brain.
- 182.Dahl MJ et al. Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nat. Hum. Behav 3, 1203–1214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Theofilas P et al. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: a stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 13, 236–246 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mann DM & Yates P O. Lipoprotein pigments — their relationship to ageing in the human nervous system. II. The melanin content of pigmented nerve cells. Brain 97, 489–498 (1974). [DOI] [PubMed] [Google Scholar]
- 185.Betts MJ et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 142, 2558–2571 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu KY et al. Noradrenergic-dependent functions are associated with age-related locus coeruleus signal intensity differences. Nat. Commun 11, 1712 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Twarkowski H & Manahan-Vaughan D Loss of catecholaminergic neuromodulation of persistent forms of hippocampal synaptic plasticity with increasing age. Front. Synaptic Neurosci 8, 30 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Weinshenker D Long road to ruin: noradrenergic dysfunction in neurodegenerative disease. Trends Neurosci. 41, 211–223 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Braak H & Del Tredici K Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 121, 589–595 (2011). [DOI] [PubMed] [Google Scholar]
- 190.Braak H et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003). [DOI] [PubMed] [Google Scholar]
- 191.Ehrenberg AJ et al. Neuropathologic correlates of psychiatric symptoms in Alzheimer’s disease. J. Alzheimers Dis 66, 115–126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vermeiren Y & De Deyn PP Targeting the norepinephrinergic system in Parkinson’s disease and related disorders: The locus coeruleus story. Neurochem. Int 102, 22–32 (2017). [DOI] [PubMed] [Google Scholar]
- 193.Butkovich LM, Houser MC & Tansey MG α-Synuclein and noradrenergic modulation of immune cells in Parkinson’s disease pathogenesis. Front. Neurosci 12, 626 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ghosh A et al. An experimental model of Braak’s pretangle proposal for the origin of Alzheimer’s disease: the role of locus coeruleus in early symptom development. Alzheimers Res. Ther 11, 59 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Henrich MT et al. A53T-α-synuclein overexpression in murine locus coeruleus induces Parkinson’s disease-like pathology in neurons and glia. Acta Neuropathol. Commun 6, 39 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Koob GF Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psychiatry 46, 1167–1180 (1999). [DOI] [PubMed] [Google Scholar]
- 197.Valentino RJ & Van Bockstaele E Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol 583, 194–203 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McCall JG et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87, 605–620 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tjoumakaris SI, Rudoy C, Peoples J, Valentino RJ & Van Bockstaele EJ Cellular interactions between axon terminals containing endogenous opioid peptides or corticotropin-releasing factor in the rat locus coeruleus and surrounding dorsal pontine tegmentum. J. Comp. Neurol 466, 445–456 (2003). [DOI] [PubMed] [Google Scholar]
- 200.Valentino RJ & Wehby RG Morphine effects on locus ceruleus neurons are dependent on the state of arousal and availability of external stimuli: studies in anesthetized and unanesthetized rats. J. Pharmacol. Exp. Ther 244, 1178–1186 (1988). [PubMed] [Google Scholar]
- 201.Curtis AL, Leiser SC, Snyder K & Valentino RJ Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology 62, 1737–1745 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Reyes BA, Zitnik G, Foster C, Van Bockstaele EJ & Valentino RJ Social stress engages neurochemically-distinct afferents to the rat locus coeruleus depending on coping strategy. eNeuro 2, ENEURO.0042–15.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Curtis AL, Bethea T & Valentino RJ Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31, 544–554 (2006). This study is an important example of how gender impacts the function of LC-NA system.
- 204.Bangasser DA et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry 15, 896–904 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Guajardo HM, Snyder K, Ho A & Valentino RJ Sex differences in μ-opioid receptor regulation of the rat locus coeruleus and their cognitive consequences. Neuropsychopharmacology 42, 1295–1304 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Helena C et al. Effects of estrogen receptor α and β gene deletion on estrogenic induction of progesterone receptors in the locus coeruleus in female mice. Endocrine 36, 169–177 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Brady KT & Randall CL Gender differences in substance use disorders. Psychiatr. Clin. North. Am 22, 241–252 (1999). [DOI] [PubMed] [Google Scholar]
- 208.Clemow DB & Bushe CJ Atomoxetine in patients with ADHD: A clinical and pharmacological review of the onset, trajectory, duration of response and implications for patients. J. Psychopharmacol 29, 1221–1230 (2015). [DOI] [PubMed] [Google Scholar]
- 209.Sepede G, Corbo M, Fiori F & Martinotti G Reboxetine in clinical practice: a review. Clin. Ter 163, e255–e262 (2012). [PubMed] [Google Scholar]
- 210.Fukada K et al. l-threo-3,4-dihydroxyphenylserine (L-DOPS) co-administered with entacapone improves freezing of gait in Parkinson’s disease. Med. Hypotheses 80, 209–212 (2013). [DOI] [PubMed] [Google Scholar]
- 211.Doughty B, Morgenson D & Brooks T Lofexidine: a newly FDA-approved, nonopioid treatment for opioid withdrawal. Ann. Pharmacother 53, 746–753 (2019). [DOI] [PubMed] [Google Scholar]
- 212.Bowrey HE, James MH & Aston-Jones G New directions for the treatment of depression: targeting the photic regulation of arousal and mood (PRAM) pathway. Depress. Anxiety 34, 588–595 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Conway CR & Xiong W The mechanism of action of vagus nerve stimulation in treatment-resistant depression: current conceptualizations. Psychiatr. Clin. North. Am 41, 395–407 (2018). [DOI] [PubMed] [Google Scholar]
- 214.Oliveira T, Francisco AN, Demartini ZJ & Stebel SL The role of vagus nerve stimulation in refractory epilepsy. Arq. Neuropsiquiatr 75, 657–666 (2017). [DOI] [PubMed] [Google Scholar]
- 215.Vonck K et al. Vagus nerve stimulation 25 years later! What do we know about the effects on cognition? Neurosci. Biobehav. Rev 45, 63–71 (2014). [DOI] [PubMed] [Google Scholar]
- 216.Swift KM et al. Abnormal locus coeruleus sleep activity alters sleep signatures of memory consolidation and impairs place cell stability and spatial memory. Curr. Biol 28, 3599–3609.e3594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ribeiro S et al. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J. Neurosci 22, 10914–10923 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ribeiro S, Goyal V, Mello CV & Pavlides C Brain gene expression during REM sleep depends on prior waking experience. Learn. Mem 6, 500–508 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sara SJ Sleep to remember. J. Neurosci 37, 457–463 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Poe GR Sleep is for forgetting. J. Neurosci 37, 464–473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Booth V & Poe GR Input source and strength influences overall firing phase of model hippocampal CA1 pyramidal cells during theta: relevance to REM sleep reactivation and memory consolidation. Hippocampus 16, 161–173 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Poe GR, Nitz DA, McNaughton BL & Barnes CA Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 855, 176–180 (2000). [DOI] [PubMed] [Google Scholar]
- 223.Novitskaya Y, Sara SJ, Logothetis NK & Eschenko O Ripple-triggered stimulation of the locus coeruleus during post-learning sleep disrupts ripple/spindle coupling and impairs memory consolidation. Learn. Mem 23, 238–248 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vanderheyden WM, Poe GR & Liberzon I Trauma exposure and sleep: using a rodent model to understand sleep function in PTSD. Exp. Brain Res 232, 1575–1584 (2014). [DOI] [PubMed] [Google Scholar]
- 225.Wassing R et al. Restless REM sleep impedes overnight amygdala adaptation. Curr. Biol 29, 2351–2358.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 226.Cabrera Y, Holloway J & Poe GR Sleep changes across the female hormonal cycle affecting memory: implications for resilient adaptation to traumatic experiences. J. Womens Health 29, 446–451 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]