Abstract
With the advent of novel cancer therapeutics and improved screening, more patients are surviving a cancer diagnosis or living longer with advanced disease. Many of these treatments have associated cardiovascular toxicities that can manifest in both an acute and a delayed fashion. Arrhythmias are an increasingly identified complication with unique management challenges in the cancer population. The purpose of this scientific statement is to summarize the current state of knowledge regarding arrhythmia identification and treatment in patients with cancer. Atrial tachyarrhythmias, particularly atrial fibrillation, are most common, but ventricular arrhythmias, including those related to treatment-induced QT prolongation, and bradyarrhythmias can also occur. Despite increased recognition, dedicated prospective studies evaluating true incidence are lacking. Moreover, few studies have addressed appropriate prevention and treatment strategies. As such, this scientific statement serves to mobilize the cardio-oncology, electrophysiology, and oncology communities to develop clinical and scientific collaborations that will improve the care of patients with cancer who have arrhythmias.
Keywords: AHA Scientific Statements; antiarrhythmic agents; anticoagulants; arrhythmias, cardiac; cardiotoxicity; medical oncology
The explosive development of cancer therapeutics has led to improved cancer survival; however, cardiovascular complications can adversely impact overall outcomes.1 As such, the field of cardio-oncology has developed to provide collaborative cardiovascular care to patients with cancer and survivors. The goal of cardio-oncology is the primary prevention and secondary treatment of cancer therapy–related cardiotoxicity to optimize cancer care and cardiovascular health outcomes. There is increasing recognition that some cancer treatments can lead to electrophysiological disease and subsequent tachy- and bradyarrhythmias (Figure 1 ).2 The primary objective of this American Heart Association scientific statement is to present the state-of-the-science on the relationship between cancer therapeutics and cardiac arrhythmias. This statement will highlight the importance of a collaborative multidisciplinary health care team–based approach to prevent adverse cardiovascular events in patients with cancer and to discuss cardioprotective strategies.
Figure 1. Mechanisms of systemic cancer therapy–induced arrhythmias.
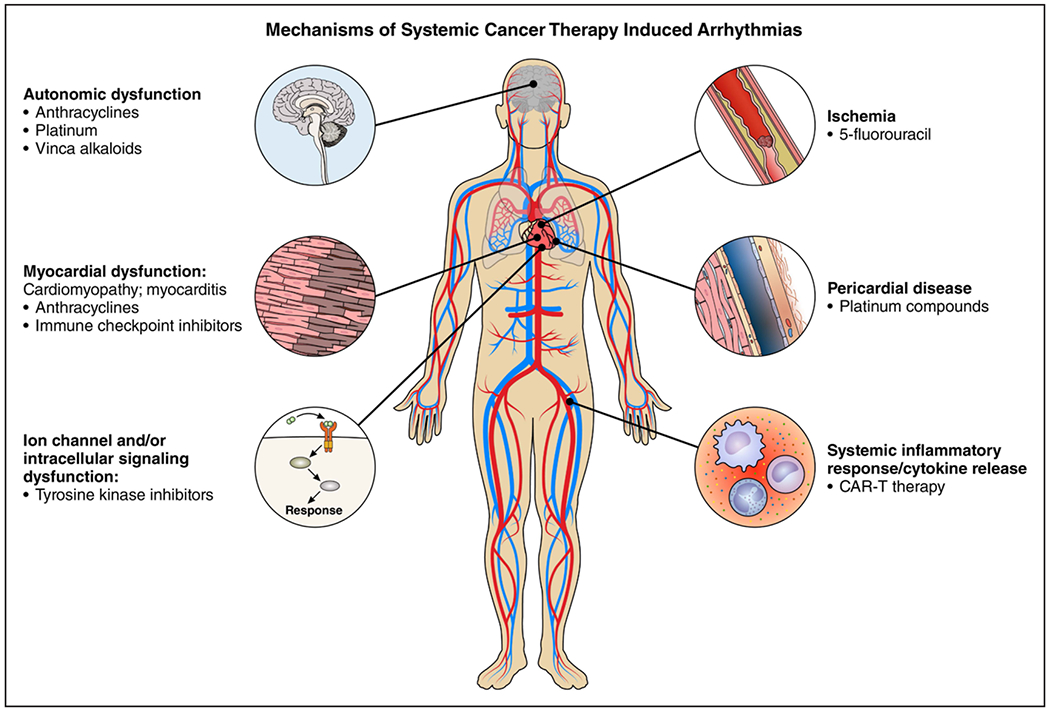
CAR-T indicates chimeric antigen receptor T cell.
CANCER THERAPEUTICS ASSOCIATED WITH ARRHYTHMIAS
Arrhythmic complications can be seen with traditional cytotoxic chemotherapies, targeted agents, and novel immunotherapies including checkpoint inhibitors and chimeric antigen receptor therapy (Table 1).3–10 The most encountered arrhythmia is atrial fibrillation (AF), but ventricular repolarization abnormalities and QT prolongation, ventricular arrhythmias (VAs), and bradycardia and heart block can also occur. The pathophysiology of arrhythmogenesis varies for different cancer therapies and may be attributable to direct cellular effects, electrolyte abnormalities, or the toxicities related to cancer therapy such as heart failure, ischemia, or myocarditis.11 With increasing recognition of the potential for cancer therapies to cause arrhythmias, strategies to mitigate this risk are needed.
Table 1.
Anticancer Agents and Associated Arrhythmias
| Anticancer agents | Cancer use | Type of arrhythmia | Frequency | Additional notes |
|---|---|---|---|---|
| Anthracyclines | ||||
| Doxorubicin | Breast, sarcoma, lung, bladder, gastric, prostate, leukemia, lymphoma | Sinus tachycardia Premature atrial complexes Premature ventricular complexes QTc prolongation Atrial fibrillation |
F | Arrhythmia may be related to cardiotoxicity from anthracyclines rather than direct proarrhythmic drug effects |
| Ventricular tachycardia Sinus bradycardia Supraventricular tachycardia Atrioventricular block Bundle-branch block |
C | |||
| Epirubicin | Breast, esophageal, gastric | Sinus tachycardia Premature atrial complexes Premature ventricular complexes QTc prolongation Atrial fibrillation |
F | |
| Ventricular tachycardia Sinus bradycardia Supraventricular tachycardia Atrioventricular block Bundle-branch block |
C | |||
| Idarubicin | Acute myeloid leukemia | Sinus tachycardia Premature atrial complexes Premature ventricular complexes QTc prolongation Atrial fibrillation |
F | |
| Ventricular tachycardia Sinus bradycardia Supraventricular tachycardia Atrioventricular block Bundle-branch block |
C | |||
| Mitoxantrone | Advanced hormone-refractory prostate cancer, nonlymphocytic leukemia | Sinus tachycardia Premature atrial complexes Premature ventricular complexes QTc prolongation Atrial fibrillation |
F | |
| Ventricular tachycardia Sinus bradycardia Supraventricular tachycardia Atrioventricular block Bundle-branch block |
C | |||
| Antimetabolites | ||||
| Fluorouracil | Colon, pancreatic, breast, head and | Sinus bradycardia Premature ventricular complexes |
F | |
| Atrial fibrillation Ventricular tachycardia |
C | |||
| Capecitabine | Breast, colon, gastric, pancreatic | Sinus tachycardia | F | |
| Atrial fibrillation Sinus bradycardia Premature atrial complexes Premature ventricular complexes QTc prolongation |
C | |||
| Fludarabine | Lymphoma, leukemia, stem cell transplant | Supraventricular tachycardia | C | |
| Gemcitabine | Small cell lung, non–small cell lung, metastatic breast lymphomas | Atrial fibrillation Atrial flutter |
C | Arrhythmia is seen most frequently when gemcitabine is used in combination with vinorelbine |
| Ventricular tachycardia | R | |||
| Alkylating agents | ||||
| Cyclophosphamide | Breast, lymphoma, myeloma, sarcoma, stem cell transplant | Sinus tachycardia | F | Similar to anthracyclines, some of the proarrhythmic effects may be related to associated cardiotoxicity from cyclophosphamide |
| Premature atrial
complexes Supraventricular tachycardia Atrial fibrillation Premature ventricular complexes |
C | |||
| Ventricular tachycardia | R | |||
| Melphalan | Multiple myeloma, ovarian, neuroblastoma, stem cell transplant | Atrial fibrillation | F | |
| Supraventricular tachycardia | C | |||
| Premature ventricular
complexes Ventricular tachycardia |
R | |||
| Platinum-based drugs | ||||
| Cisplatin | Lung, bladder, testicular, breast, esophageal, head and neck | Ventricular
tachycardia Supraventricular tachycardia |
C | Rates of supraventricular tachycardia and atrial fibrillation are significantly higher with infusions into the intrapericardial space or other serous cavities |
| Atrial
fibrillation Bradycardia Atrioventricular block |
R | |||
| Immunomodulatory drugs | ||||
| Lenalidomide | Myelodysplastic syndrome, multiple myeloma, mantle cell lymphoma | Atrial fibrillation | C | Reported rates of atrial fibrillation are from patients with multiple myeloma; risk may be driven by underlying disease with AL amyloid in a proportion of the cohort |
| Ventricular tachycardia | R | |||
| Thalidomide | Multiple myeloma | Atrial fibrillation | R | |
| Premature ventricular
complexes Ventricular tachycardia |
R | |||
| Proteasome Inhibitors | ||||
| Carfilzomib | Multiple myeloma | Atrial fibrillation | R | |
| Small-molecule tyrosine kinase inhibitors | ||||
| Ibrutinib | Chronic lymphocytic leukemia, small lymphocytic lymphoma, mantle cell lymphoma, Waldenstrom macroglobulinemia, chronic graft-versus-host disease | Atrial fibrillation Atrial flutter |
C | Newer-generation Bruton tyrosine kinase inhibitors have shown lower rates for arrhythmias (eg, acalabrutinib) |
| Ventricular tachycardia Premature ventricular complexes |
R | |||
| Vemurafenib | Melanoma | QTc prolongation | F | |
| Atrial fibrillation | C | |||
| Ventricular arrhythmia Supraventricular tachycardia Torsades de pointes Premature ventricular complexes Sinus bradycardia Sinus tachycardia |
R | |||
| Chimeric antigen receptor T-cell therapy | ||||
| Tisagenlecleucel Axicabtagene ciloleucel | B-cell acute lymphoblastic leukemia
(refractory or relapse) Large B-cell lymphoma (refractory or relapse) |
Atrial fibrillation Supraventricular tachycardia |
C | |
| Histone deacetylase inhibitors | ||||
| Romidepsin | Cutaneous T-cell lymphoma | QTc prolongation Supraventricular tachycardia |
C | |
| Atrial fibrillation Torsades de pointes Ventricular tachycardia |
R | |||
| Panobinostat | Multiple myeloma | QTc prolongation | C | |
| Premature ventricular
complexes Ventricular tachycardia |
R | |||
| Vorinostat | Cutaneous T-cell lymphoma | QTc prolongation | C | |
| Premature ventricular complexes | R | |||
| Immune checkpoint inhibitors | ||||
| Nivolumab Ipilimumab Pembrolizumab | Melanoma, lung, kidney, bladder, head and neck, lymphoma | Atrial fibrillation Sinus Bradycardia Atrioventricular block QTc prolongation Ventricular tachycardia Ventricular fibrillation |
R | Risk for arrhythmia may be associated with development of myocarditis |
| Antimicrotubule agents | ||||
| Docetaxel | Breast, lung, prostate, gastric, head and neck | Sinus tachycardia Atrial flutter |
R | |
| Paclitaxel | Breast, ovarian, lung, sarcoma, bladder, cervical, gastric, esophageal, head and neck | Sinus tachycardia Sinus bradycardia |
F | |
| Atrioventricular block Atrial fibrillation Supraventricular tachycardia Atrioventricular block Ventricular tachycardia Premature ventricular complexes |
R | |||
The frequency of toxicity was graded as rare (R) <1% incidence, common (C) 1% to 10% incidence, or frequent (F) >10% incidence in clinical trials or observational studies.
ATRIAL FIBRILLATION AND OTHER ATRIAL ARRHYTHMIAS
Epidemiological studies have demonstrated an association between cancer and AF. This association is bidirectional such that there is a higher incidence of AF in patients with cancer, and an increased incidence of cancer in patients with AF, likely because of shared risk factors.12,13 A study of >15 000 patients found that patients with cancer were more likely to have prevalent AF than those without cancer.12 AF is frequently observed in patients with cancer, driven by comorbidities including advanced age, metabolic disorders, electrolyte fluctuations, or shared mechanistic pathways such as inflammation. Surgical or medical therapies for cancer may have either direct or indirect effects on the development of arrhythmias.12,14 Nevertheless, evidence-based strategies for the prevention and treatment of AF and associated thromboembolic complications in at-risk cancer populations are lacking.
Anthracyclines
Anthracyclines are known to confer a dose-dependent risk for left ventricular (LV) dysfunction and heart failure. However, incident arrhythmias may also occur, both as a primary cardiotoxicity or attributable to anthracycline-induced cardiomyopathy. In a study of 29 patients treated with doxorubicin-containing regimens, supraventricular extrasystoles were most commonly reported, and 10.3% of patients developed AF.15 In patients with anthracycline-associated LV dysfunction, there is a high burden of arrhythmias including AF in up to 56.6% of patients.16,17
Melphalan and Hematopoietic Stem Cell Transplantation
Melphalan, a nitrogen mustard alkylating agent, is commonly used in conditioning chemotherapy regimens before hematopoietic stem cell transplantation (HSCT). In a study of 1221 patients undergoing HSCT for various malignancies, 5.1% of cases were complicated by atrial arrhythmias (supraventricular tachycardia and AF). Melphalan-based regimens had higher rates of supraventricular arrhythmias affecting 11.0% of 438 exposed cases in comparison with any non–melphalan-based regimen.18 Patients with a history of arrhythmia before HSCT are more likely to develop an arrhythmia during transplantation, the majority of which were AF.19 New-onset atrial arrhythmias are correlated with adverse outcomes including all-cause mortality within 1 year after HSCT.19 Strategies to prevent arrhythmias in patients undergoing HSCT should be considered, including risk factor optimization and consideration of prophylactic pharmacotherapy with antiarrhythmic medications in high-risk subgroups such as those with a known history of arrhythmia or underlying cardiovascular disease.
Ibrutinib and Other Bruton Tyrosine Kinase Inhibitors
Ibrutinib is a Bruton tyrosine kinase (BTK) inhibitor frequently used to treat chronic lymphocytic leukemia, mantle cell lymphoma, marginal zone lymphoma, and Waldenstrom macroglobulinemia.20 Although the association between ibrutinib and AF is well established, there is considerable variability in reported rates. A systematic review by Ganatra and colleagues21 found an incidence of AF ranging from 3.5% to 16% across 16 studies, or a rate of 5.8 per 100 person-years. In a recent meta-analysis of 8 randomized clinical trials comprising 2580 patients, the pooled estimate showed that ibrutinib resulted in a relative risk of 4.69 (95% CI, 2.17–7.64, P<0.001) for developing AF.22 There is also evidence to suggest that patients who develop arrhythmias are at increased risk for mortality, possibly from associated ischemic or hemorrhagic cerebrovascular events.23 The mechanism of ibrutinib-associated AF is not well established but may be related to on-target inhibition of cardiac BTK, off-target inhibition of Tec protein kinase, inhibition of phosphoinositide 3-kinase, or enhanced automaticity from altered sarcoplasmic reticulum calcium handling.20,24,25
The newer BTK inhibitors acalabrutinib and zanubrutinib have higher BTK selectivity than ibrutinib, with lower rates of AF/flutter than ibrutinib.26 Although incidence rates have varied by trial, the US Food and Drug Administration fact sheet reports AF/flutter in 4.1% of patients on acalabrutinib14 and 2% of patients treated with zanubrutinib.2
Vascular Endothelial Growth Factor Signaling Pathway Inhibitors
As a class, vascular endothelial growth factor inhibitors have been associated with a relatively low incidence of AF. Sorafenib is a vascular endothelial growth factor inhibitor used to treat unresectable hepatocellular carcinoma, advanced renal cell cancer, and differentiated thyroid cancer. When combined with 5-fluorocuracil, in a small series of 39 patients, 2 patients (5.1%) developed AF.27
Tyrosine Kinase Inhibitors Targeting BCR-ABL
BCR-ABL tyrosine kinase inhibitors target the Philadelphia chromosome translocation identified in chronic myeloid leukemias. In general, AF is not a frequently encountered arrhythmic complication of the first- (imatinib) or second-generation BCR-ABL tyrosine kinase inhibitors (nilotinib, bosutinib, dasatinib).2 In contrast, rates of AF with ponatinib, a third-generation BCR-ABL tyrosine kinase inhibitor, vary significantly with reports as high as 7% in phase 2 studies to only 1.9% in a phase 3 trial.28,29 The exact reason for this disparity is unclear.
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICIs) are increasingly used for various solid and hematologic malignancies. Although their association with myocarditis is well established, the risk of supraventricular arrhythmia has been less well studied. In a report of 35 patients who presented with ICI-associated myocarditis, 9 (25.7%) patients developed AF/flutter.9 In another study of 30 patients with ICI-related cardiotoxicity, 30% had AF.8 In these cases, underlying myocarditis may be driving or contributing to the risk of arrhythmia.
Chimeric Antigen Receptor T Cells
Chimeric antigen receptor T-cell therapy has significantly improved clinical outcomes in children with acute lymphocytic leukemia and adults with refractory or relapsed large B-cell lymphoma and is being actively investigated as a therapy for other hematologic malignancies. Chimeric antigen receptor T-cell therapy can lead to cytokine release syndrome, which may result in hypotension, demand ischemia, LV dysfunction, pulmonary edema, and cardiogenic shock.7 A retrospective case study of 137 patients reported arrhythmias in 5 (3.6%) patients. Another retrospective case study of 145 patients reported AF in 11 (7.5%) patients and supraventricular tachycardia in 1 patient.30 However, it remains unclear whether this is related to cytokine release syndrome–associated myocardial inflammation or a direct effect of chimeric antigen receptor T-cell therapy.7
MANAGEMENT OF ATRIAL FIBRILLATION: RATE AND RHYTHM CONTROL
Management of AF and other arrhythmias in patients with newly diagnosed cancer should follow algorithms similar to the general population with special consideration taken to avoid unique drug-drug interactions that may exist with certain cancer therapeutics ( Figure 2). Structured screening for potential drug-drug interactions, particularly those impacting cytochrome P450 3A4 and P450 2D6 and P-glycoprotein metabolism, by clinical pharmacologists should be incorporated into care planning.31
Figure 2. Algorithm for the management of atrial fibrillation during cancer treatment.
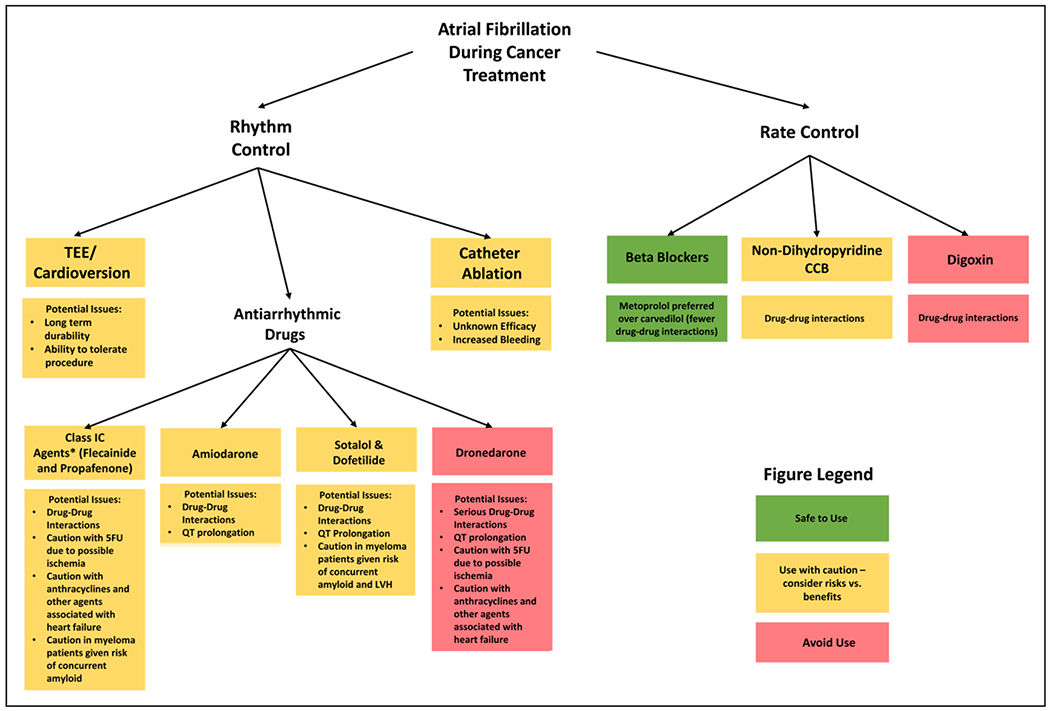
5FU indicates 5-fluorouracil; CCB, calcium channel blocker; LVH, left ventricular hypertrophy; and TEE, transesophageal echocardiography
*Vaughan Williams Classification.
For asymptomatic individuals with AF, a rate control strategy is appropriate, with a goal resting heart rate of <110 beats/min.32 In general, β-blockers are preferred. Nondihydropyridine calcium channel blockers (diltiazem and verapamil) should be used with caution because they inhibit CYP 3A4 metabolism and can lead to increased concentrations of various cancer therapeutics.21 Digoxin should also be used with caution in the cancer population because many cancer treatments, including ibrutinib, inhibit P-glycoprotein which can lead to increased digoxin concentrations and potential toxicity.21 Although tachycardia is more often recognized in patients with cancer because of various factors including deconditioning, pain, dehydration and dysautonomia, bradycardia can occur as a side effect of several treatments including taxanes and anaplastic lymphoma kinase inhibitors.33 As such, concurrent use of atrioventricular nodal–blocking drugs can have an additive heart rate–lowering effect. Moreover, anticancer drugs including imatinib (used in chronic myeloid leukemia) and abiraterone (an antiandrogen for metastatic prostate cancer) impact CYP2D6 metabolism and can increase concentrations of various β-blockers including metoprolol and carvedilol.34 Finally, frequent baseline hypotension in this population may limit implementation of an adequate rate control strategy.
For patients with symptomatic AF, or for those with heart failure felt to be worsened by AF, a rhythm control strategy focusing on the restoration and maintenance of sinus rhythm may be preferable.32 The recently published EAST-AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) suggested a lower rate of adverse events in patients with AF treated with an early rhythm control strategy, even in the absence of symptoms; however, the benefits of this approach in patients with cancer has not been established.35 Although cardioversion is often initially successful, long-term durability is questionable given the inflammatory milieu of cancer along with the continued use of provocative cancer therapeutics.14 Antiarrhythmic medications have not been specifically studied in the cancer population and must be used with caution given the potential for drug-drug interactions leading to QT prolongation. Moreover, many antiarrhythmics interact with the cytochrome P450 CYP 3A4 and 2D6 systems, and with P-glycoprotein, as well, leading to similar drug interactions and toxicities as the nodal-blocking agents.36 Dronedarone should be avoided because of its effects on both the CYP 3A4 and P-glycoprotein systems. Although amiodarone is frequently chosen to treat cancer-associated arrhythmias given its relative ease of use, in particular, in patients with other cardiovascular conditions, it can also increase various cancer drug concentrations via effects on similar metabolic pathways.31 In addition to drug-drug interactions, certain antiarrhythmics may not be an option because of underlying cardiovascular and oncological diseases such as those with underlying coronary disease or LV hypertrophy from amyloidosis. Although catheter ablation is also a potential treatment strategy, the procedural success in the setting of cancer therapy–related arrhythmias is not established. Last, for high-risk populations, the benefits of prophylactic initiation of nodal blocking agents and antiarrhythmic drugs to prevent arrhythmias has not been established.
ANTICOAGULATION AND THROMBOSIS PREVENTION
Both arterial and venous thrombosis are common with active malignancy, and anticoagulation is the mainstay of thromboembolism treatment and prophylaxis. In the setting of AF and atrial flutter, stroke and peripheral embolism can occur because of the stasis of blood in the heart. Recent data suggest that a significant proportion of patients with cancer who have an elevated risk of stroke but lower risk of bleeding are not receiving appropriate anticoagulation for thromboembolism prophylaxis.37 It is recommended to use the CHA2DS2-VASc score to determine the risk of stroke and guide anticoagulation decision making in patients with AF/flutter (Figure 3).33 Anticoagulation is recommended for men with a CHA2DS2-VASc score ≥2 and for women with a score ≥3 without significant contraindications.33 Nevertheless, there is increasing evidence to suggest that this scoring system may not be as accurate for patients who have cancer.38 Moreover, patients who have cancer are more prone to bleeding diatheses for multiple reasons including thrombocytopenia. Although the HAS-BLED score (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly) is frequently used to assess bleeding risk in the general population, it does not take into consideration circumstances unique to patients with cancer including low platelets and intracerebral metastases.39 As such, it may not be reliable in this population. It is essential that cancer-specific risk prediction algorithms are developed to guide decision making regarding anticoagulation in the setting of AF/flutter balanced by the potential risk for bleeding.
Figure 3. Algorithm for the management of atrial fibrillation–associated thromboembolism prophylaxis during cancer Treatment.
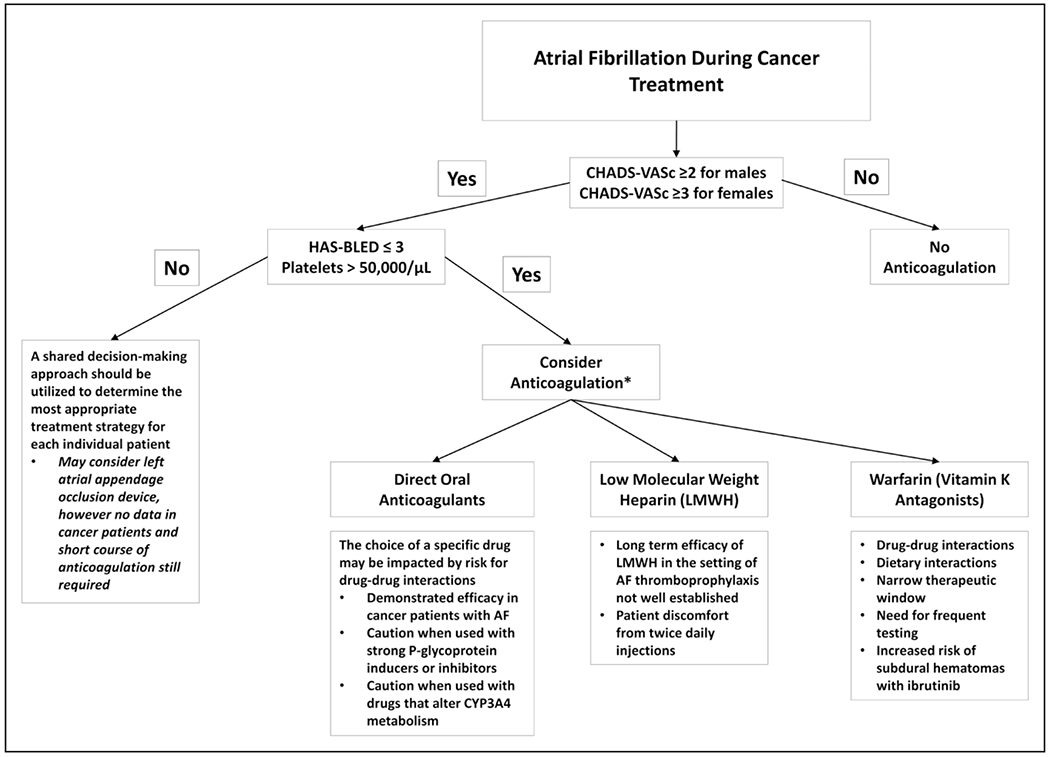
HAS-BLED indicates hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly; and LMWH, low-molecular-weight heparin. *Anticoagulation therapy should be determined based on an individualized shared decision-making approach.
Although low-molecular-weight heparin has been the anticoagulant of choice for patients with cancer based on its superiority over warfarin at preventing deep venous thrombosis, there are no studies evaluating its role to prevent thromboembolism in the setting of AF/flutter.40 There is increasing evidence demonstrating the safety and efficacy of direct oral anticoagulants in patients with cancer who have deep venous thrombosis, and the most recent American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines recommend direct oral anticoagulants over vitamin K antagonists for thromboembolism prophylaxis in nonvalvular AF.32 Although patients with cancer were either excluded from or underrepresented in the original direct oral anticoagulant AF trials, several recent studies have suggested they are likely safe and beneficial for these patients. For example, a study of 1236 patients enrolled in the ARISTOTLE trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) with active or prior cancer confirmed that apixaban was superior to warfarin at preventing stroke and systemic embolism in this population.41 Similar results have been reported with edoxaban.42 Nevertheless, it should be recognized that active cancer therapy can affect the choice of anticoagulant because of potential drug-drug interactions (Supplemental Table I). All direct oral anticoagulants interact with the P-glycoprotein system, but dabigatran is most affected. Rivaroxaban and apixaban are also metabolized by CYP 3A4 and therefore, must be used with caution in conjunction with inducers of this system, including ibrutinib.43 It should be recognized that aspirin to prevent AF-associated thromboembolism is no longer recommended for most patients and may be higher risk in the setting of cancer because of concomitant thrombocytopenia and platelet dysfunction as a direct result of cancer therapeutics such as ibrutinib.44,45 A multidisciplinary approach with input from pharmacists is important to determine appropriate and safe treatment strategies. Moreover, a shared decision-making approach should be considered in all patients with cancer before initiating anticoagulation to develop suitable, individualized treatment.32
Given the complexities associated with anticoagulation in patients who have cancer, including drug interactions and bleeding risk, there may be a role for the early use of left atrial appendage closure devices. However, there are no studies to date specifically evaluating this technology in patients with cancer, and the risk of device-related thrombus could potentially be increased in a hypercoagulable population.46
QT PROLONGATION
The incidence of QT prolongation in patients with cancer is in general up to 22% across a variety of drug classes,47 with the exception of arsenic trioxide (used to treat patients with acute promyelocytic leukemia) that has been associated with QT prolongation in 26% to 93% of patients.48 Although QT prolongation is often related to direct blockade of potassium channels, many antineoplastic agents inhibit intracellular signaling pathways, including phosphoinositide 3-kinase, leading to delays in ventricular repolarization.49 Despite the attention given to QT monitoring, there are no standardized recommendations, and therefore, it is necessary to rely on the specific drug label instructions to guide the frequency of ECG monitoring (Supplemental Table II).47 For novel therapeutics with unknown QT-prolonging effects, ECG monitoring after the first dose and after each change in dose should be pursued.50 Ultimately, standardized QT-monitoring protocols for oncological treatments should be developed.51 Nevertheless, life-threatening arrhythmias are rare, occurring in <1% of patients treated with these drugs,47 and therefore, collaborative conversations between oncology, cardiology, and the patient should occur before deciding to change or withhold optimal cancer treatments.
Accurate and uniform measurement of the QT interval can be challenging. Manual calculation in lead II or V5 is often necessary, averaged over 3 to 5 beats when sinus arrhythmia is present or over 10 beats in the presence of AF, to mitigate the effect of beat-to-beat variability.5,52 The QT interval is longer at slower heart rates and shorter at faster heart rates. Different correction formulae derived from mathematical modeling are commonly used in clinical practice to overcome these limitations and standardize the QT interval over a range of heart rates.5 Although there is substantial familiarity with the Bazett (QTcb) formula (QT/(RR)1/2), the Fridericia (QTcF) formula (QT/(RR)1/3) is preferred in the cancer population because it is often more accurate at heart rate extremes.5,52,53 QT-interval assessment can also be challenging in the setting of interventricular conduction delay from bundle-branch blocks or ventricular pacing. In this situation, calculating the JT interval (difference in the QT interval and the QRS duration) can be considered, but this may be difficult to implement in clinical practice, especially for noncardiologists.
Prevention of QT prolongation starts with maintaining familiarity with cardiac, oncological, and other drugs that can prolong the QT interval and minimizing their concomitant use (Supplemental Table II). In general, the arrhythmic risk increases when the QTc is >500 ms or the change from baseline is >60 ms.5,47 If significant QT prolongation is identified, nonessential medications that affect cardiac repolarization should be discontinued and electrolyte (in particular, potassium and magnesium) abnormalities corrected.47 Patients should be counseled about cardiac signs and symptoms including presyncope, syncope, or new rapid palpitations that warrant evaluation.47 In the setting of VAs (premature ventricular contractions, ventricular tachycardia, or torsades de pointes), corrective measures should be promptly delivered including the infusion of magnesium sulfate, and efforts, as well, to maintain a heart rate at >100 beats/min with either isoproterenol or temporary transvenous pacing. In refractory/emergent situations associated with QT-interval prolongation, lidocaine and advanced cardiac life support procedures should be initiated.47,54 Emerging data indicate that mexiletine may help to minimize arsenic-induced QT prolongation, and recurrent torsades de pointes, as well; however, further research is needed to determine overall efficacy and safety with the full range of cancer therapeutics.55,56
VENTRICULAR ARRHYTHMIAS
Chemotherapy-induced VAs, including sustained ventricular tachycardia and fibrillation, are relatively rare. The incidence of VA is higher in patients receiving chemotherapy for advanced metastatic disease but can also be attributable to the cancer itself.57 Prevention of VA in patients with cancer focuses on modifiable risk factors including electrolyte repletion and the avoidance of concomitant medications that may augment the arrhythmic potential. The invoked mechanisms include a direct or indirect effect of the chemotherapeutic agent on cardiomyocytes, prolongation of the QT interval, and concurrent ischemia. Although anthracyclines have been shown to prolong the QT interval, the risk of VA appears to be secondary to LV systolic dysfunction that can be further aggravated by electrolyte abnormalities such as hypokalemia.17,33 The BTK inhibitor ibrutinib is also associated with VA, unrelated to QT prolongation.23,58 In fact, ibrutinib may shorten the QT interval, with enhanced automaticity and triggered activity suggested as mechanisms for arrhythmogenesis.24,25,59,60
The role of implantable cardioverter defibrillator therapy in chemotherapy-induced cardiomyopathy has not been specifically examined. However, current guidelines recommend an implantable cardioverter defibrillator for primary prevention in patients with cardiomyopathy and LV ejection fraction ≤35% despite guideline-directed medical therapy, New York Heart Association class II to III symptoms, and life expectancy >1 year.61 This can, in turn, justify the need for an implantable cardioverter defibrillator in patients with chemotherapy-induced cardiomyopathy who are symptomatic and meet the LV ejection fraction cutoff criteria. This is further supported by several studies showing that the prevalence of VA in anthracycline cardiomyopathy is similar to that in patients with implantable cardioverter defibrillators implanted for non–cancer-related cardiomyopathy.16,17
Chemotherapy-induced cardiomyopathy may also be accompanied by cardiac conduction system disease manifesting as a wide QRS interval (>120 ms). The ensuing abnormal electric activation of the ventricles is associated with mechanical dyssynchrony and adverse ventricular remodeling. Cardiac resynchronization therapy (CRT) can reduce the extent of this dyssynchrony and potentially improve cardiac function and clinical outcomes. Until recently, data supporting CRT in patients with cancer was based on isolated case series.62,63 More recently, the MADIT-CHIC study (Multicenter Automatic Defibrillator Implantation Trial-Chemotherapy Induced Cardiomyopathy) addressed this question prospectively in a single-arm, open-label, multicenter study.64 MADIT-CHIC showed that patients with chemotherapy-induced cardiomyopathy, LV ejection fraction ≤35%, and a wide QRS complex (left bundle-branch block, median QRS duration 152 ms) on optimal guideline-directed medical therapy derive benefit from CRT with significant improvement in LV ejection fraction, reduction in LV volumes and left atrial volume, and improvement in heart failure symptoms at 6 months after CRT implantation.64 Although most CRT implants in the United States are CRT-defibrillators, CRT-pacemakers may be considered for symptom control even in patients with a life expectancy <1 year.3,65
BRADYARRHYTHMIAS AND HEART BLOCK
Assessment of the true incidence of bradycardia and atrioventricular conduction disease related to chemotherapy is difficult because of the variations in surveillance and reporting. Several chemotherapeutic agents in different classes have been associated with benign sinus bradycardia, yet symptomatic bradycardia and advanced atrioventricular block do occur, albeit rarely (Table 1).3 Paclitaxel is associated with reversible asymptomatic sinus bradycardia in up to 30% of patients, but more advanced bradyarrhythmias are atypical.66 In contrast, thalidomide has been reported to cause sinus bradycardia in up to 40% of patients, with some requiring pacemaker implantation for dizziness and syncope.67 5-Fluorocuracil has been associated with symptomatic and asymptomatic bradycardia. In 1 series of 301 subjects, symptomatic sinus bradycardia was observed in nearly 12% of patients, although a systematic review of 5-fluorocuracil- and capecitabine-related cardiotoxicity suggests a lower incidence of clinically significant bradycardia.68,69
Targeted and immunotherapies can also cause bradycardia. In addition to the well-described association with AF, an analysis of 2093 adverse drug reactions attributable to ibrutinib showed a 2.3% occurrence of conduction defects, 42% of which were high-grade or complete atrioventricular block, and 18% of which were fatal.33 Likewise, the anaplastic lymphoma kinase inhibitors, crizotinib and ceritinib, which are used to treat non–small cell lung cancer, are well known to cause bradycardia, although symptomatic episodes requiring intervention are rare.70 ICIs are also increasingly associated with both conduction defects and bradycardia. Presentation with high-degree atrioventricular block can be the first manifestation of ICl-related myocarditis, which is a rare (1%) but potentially fatal (50%) complication of ICI therapy. An analysis of adverse drug event data from the World Health Organization revealed a 0.12% risk of conduction abnormalities reported among 31 321 ICI-related adverse cardiac events.71 In a review of ICI-related cardiotoxicity, conduction disorders were reported in 5 of 30 (1 7%) of patients, including 1 patient who died of a bradycardic arrest.8 In this series, conduction abnormalities were strongly associated with cardiovascular mortality (80% versus 16%, P=0.003).8 Although definitive prevention strategies have not been established, patients taking atrioventricular nodal–blocking drugs may experience augmented bradycardic effects from these cancer therapeutics, and therefore, prophylactic dose reduction before the initiation of the aforementioned cancer treatments should be considered.
AUTONOMIC DYSFUNCTION IN CANCER
Autonomic dysfunction (AD) or dysregulation of the parasympathetic and sympathetic nervous systems is more prevalent in patients with cancer and cancer survivors than in healthy controls.72 Decreased parasympathetic activity or impaired vagal tone is manifested as an increased resting heart rate, decreased heart rate variability, or abnormal heart rate recovery 1 minute after the cessation of exercise. Decreased sympathetic nervous system activity results in abnormal blood pressure responses and orthostasis/syncope. In 1 study, autonomic dysfunction was most frequently identified in patients with hematologic malignancies, with orthostatic hypotension, followed by inappropriate sinus tachycardia and postural orthostatic tachycardia syndrome most often reported.73 Multiple aspects of cancer treatment contribute to AD: cancer therapy, psychological stress, sleep disturbances, weight gain, and loss of cardiometabolic fitness.74 Anthracyclines, taxanes, vinca-alkaloids, platinum-based agents, head and neck irradiation, and HSCT have all been associated with AD.75 The presence of abnormal heart rate recovery was associated with decreased exercise duration and increased mortality in survivors of Hodgkin lymphoma treated with mediastinal radiation.76 Likewise, in survivors of bone marrow transplantation, decreased heart rate variability was associated with fatigue, decreased functional capacity, and increased mortality.77 Patients who have breast cancer with AD also exhibit increased fatigue and decreased exercise capacity.78
It is postulated that AD promotes inflammation and endothelial dysfunction contributing to the development of cardiovascular disease in cancer survivors.79,80 In a rat model of doxorubicin cardiotoxicity, decreased heart rate variability preceded the development of cardiomyopathy.81 Fullerenol and dexrazoxane, both agents that prevent anthracycline-associated cardiotoxicity, have been shown to attenuate AD.81,82 Structured aerobic exercise training, during or after cancer therapy, has also been shown to reverse AD in patients with breast cancer and in survivors of testicular cancer.83,84 In addition to increased salt and fluid intake, leg elevation/compression and avoidance of vasodilatory medications, treatment options for orthostatic hypotension include midodrine, fludrocortisone, pyridostigmine, and droxidopa.85 Although data are lacking, the use of ivabradine may be an attractive option to treat symptomatic inappropriate sinus tachycardia in this population and should be systematically evaluated. In addition, research efforts aimed at attenuating AD in patients with cancer are needed to improve quality of life and potentially reduce long-term cardiovascular toxicity. Moreover, a team-based approach to health care, utilizing exercise therapists, psychologists, and nurses is essential for improving outcomes in this patient population following strategies presented in the AHA scientific statement on cardio-oncology rehabilitation.86
CONCLUSION
Cardio-oncology is an important new subspecialty of medicine that continues to evolve. In this scientific statement, we have summarized the current state of knowledge on the risk of arrhythmias in patients with cancer, including possible therapeutic interventions with antiarrhythmic drugs, and anticoagulation strategies, as well. There are unique challenges when considering these treatments in the cancer population, in particular, given the paucity of evidence and limitations of existing data.
The time has come for strategic clinical and scientific collaborations between oncologists and electrophysiologists to improve clinical care and develop registries and databases to assess arrhythmia-related outcomes more rigorously in patients with cancer. The CARDIOTOX registry (Cardiovascular Toxicity Induced by Cancer-Related Therapies) can serve as a paradigm for future investigations in this area, even though it does not currently include arrhythmias.87 Accurate assessment of arrhythmia burden can be challenging; however, leveraging mobile and wearable technology may be a useful strategy for patients with cancer and warrants further investigation. In the same vein, more basic and translational research is needed to understand the underlying mechanisms predisposing patients with cancer to arrhythmias, so that we can develop evidence-based strategies for risk mitigation and management for this unique and vulnerable population.
Supplementary Material
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on March 11, 2021, and the American Heart Association Executive Committee on April 22, 2021. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or email Meredith.Edelman@wolterskluwer.com.
Supplemental materials are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIR.0000000000000986
Disclosures
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Brian Olshansky | University of Iowa Hospital & Clinic | None | None | Lundbeck†; Sanofi-Aventis* | None | None | Boehringer Ingelheim*; Lundbeck†; Sanofi Aventis* | None |
| Michael G. Fradley | University of Pennsylvania | Medtronic (investigator-initiated study)* | None | None | None | None | Abbott*; Takeda* | None |
| Theresa M. Beckie | University of South Florida College of Nursing/College of Medicine | None | None | None | None | None | None | None |
| Sherry Ann Brown | Medical College of Wisconsin (MCW)/ Froedtert Hospital in Milwaukee | None | None | None | None | None | None | None |
| Richard K. Cheng | University of Washington Medical Center | None | None | None | None | None | None | None |
| Susan F. Dent | Duke Cancer Institute | Novartis* | None | None | None | None | Novartis* | None |
| Anju Nohria | Brigham and Women’s Hospital | Amgen† | None | None | None | None | Takeda Oncology*; AstraZeneca*; Boehringer Ingelheim* | None |
| Kristen K. Patton | University of Washington | None | None | None | None | None | None | None |
| Jagmeet P. Singh | Massachusetts General Hospital, Harvard Medical School | None | None | None | None | None | Abbott*; Biotronik*; Boston Scientific*; Cardiologs*; EBR†; Impulse Dynamics*; Medtronic*; Microport (unpaid)*; Sanofi*; Toray* | None |
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Joseph Carver | The University of Pennsylvania | None | None | None | None | None | None | None |
| Joerg Herrmann | Mayo Clinic | None | None | None | None | None | Amgen*; Bristol-Myers Squibb*; Takeda Ariad* | None |
| Eric H. Yang | UCLA | None | None | None | None | None | None | None |
REFERENCES
- 1.Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, Herrmann J, Porter C, Lyon AR, Lancellotti P et al. ; ESMO Guidelines Committee. clinicalguidelines@esmo.org. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buza V, Rajagopalan B, Curtis AB. Cancer treatment-induced arrhythmias: focus on chemotherapy and targeted therapies. Circ Arrhythm Electrophysiol. 2017;10:e005443. doi: 10.1161/CIRCEP.117.005443 [DOI] [PubMed] [Google Scholar]
- 3.Herrmann J Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020;17:474–502. doi: 10.1038/s41569-020-0348-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quezado ZM, Wilson WH, Cunnion RE, Parker MM, Reda D, Bryant G, Ognibene FP High-dose ifosfamide is associated with severe, reversible cardiac dysfunction. Ann Intern Med. 1993;118:31–36. doi: 10.7326/0003-4819-118-1-199301010-00006 [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekhar S, Fradley MG. QT Interval prolongation associated with cytotoxic and targeted cancer therapeutics. Curr Treat Options Oncol. 2019;20:55. doi: 10.1007/s11864-019-0657-y [DOI] [PubMed] [Google Scholar]
- 6.Fradley MG, Gliksman M, Emole J, Viganego F, Rhea I, Welter-Frost A, Armanious M, Lee DH, Walko C, Shah B, et al. Rates and risk of atrial arrhythmias in patients treated with ibrutinib compared with cytotoxic chemotherapy. Am J Cardioi. 2019;124:539–544. doi: 10.1016/j.amjcard.2019.05.029 [DOI] [PubMed] [Google Scholar]
- 7.Alvi RM, Frigault MJ, Fradley MG, Jain MD, Mahmood SS, Awadalla M, Lee DH, Zlotoff DA, Zhang L, Drobni ZD, et al. Cardiovascular events among adults treated with chimeric antigen receptor t-cells (CAR-T). J Am Coll Cardioi. 2019;74:3099–3108. doi: 10.1016/j.jacc.2019.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, Monestier S, Grob JJ, Scemama U, Jacquier A, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–2087 doi: 10.1161/CIRCULATIONAHA.117.030571 [DOI] [PubMed] [Google Scholar]
- 9.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitturi KR, Xu J, Araujo-Gutierrez R, Bhimaraj A, Guha A, Hussain I, Kassi M, Bernicker EH, Trachtenberg BH. Immune checkpoint inhibitor-related adverse cardiovascular events in patients with lung cancer. JACC CardioOncology. 2019;1:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alomar M, Fradley MG. Electrophysiology translational considerations in cardio-oncology: QT and beyond. J Cardiovasc Transl Res. 2020;13:390–401. doi: 10.1007/s12265-019-09924-y [DOI] [PubMed] [Google Scholar]
- 12.O’Neal WT, Lakoski SG, Qureshi W, Judd SE, Howard G, Howard VJ, Cushman M, Soliman EZ. Relation between cancer and atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke Study). Am J Cardiol. 2015;115:1090–1094. doi: 10.1016/j.amjcard.2015.01.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinter N, Christesen AMS, Fenger-Grøn M, Tjønneland A, Frost L. Atrial fibrillation and risk of cancer: a Danish population-based cohort study. J Am Heart Assoc. 2018;7:e009543. doi: 10.1161/JAHA.118.009543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63:945–953. doi: 10.1016/j.jacc.2013.11.026 [DOI] [PubMed] [Google Scholar]
- 15.Kilickap S, Barista I, Akgul E, Aytemir K, Aksoy S, Tekuzman G. Early and late arrhythmogenic effects of doxorubicin. South Med J. 2007;100:262–265. doi: 10.1097/01.smj.0000257382.89910.fe [DOI] [PubMed] [Google Scholar]
- 16.Fradley MG, Viganego F, Kip K, Martin A, Patel AA, Ismail-Khan R, Chae S, Herweg B, Labovitz A. Rates and risk of arrhythmias in cancer survivors with chemotherapy-induced cardiomyopathy compared with patients with other cardiomyopathies. Open Heart. 2017;4:e000701. doi: 10.1136/openhrt-2017-000701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazur M, Wang F, Hodge DO, Siontis BL, Beinborn DS, Villarraga HR, Lerman A, Friedman PA, Herrmann J. Burden of cardiac arrhythmias in patients with anthracycline-related cardiomyopathy. JACC Clin Electrophysiol. 2017;3:139–150. doi: 10.1016/j.jacep.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 18.Feliz V, Saiyad S, Ramarao SM, Khan H, Leonelli F, Guglin M. Melphalan-induced supraventricular tachycardia: incidence and risk factors. Clin Cardiol. 2011;34:356–359. doi: 10.1002/clc.20904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonorezos ES, Stillwell EE, Calloway JJ, Glew T, Wessler JD, Rebolledo BJ, Pham A, Steingart RM, Lazarus H, Gale RP et al. Arrhythmias in the setting of hematopoietic cell transplants. Bone Marrow Transplant. 2015;50:1212–1216. doi: 10.1038/bmt.2015.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124:3829–3830. doi: 10.1182/blood-2014-10-604272 [DOI] [PubMed] [Google Scholar]
- 21.Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, Mahmood SS, Barac A, Groarke JD, Hayek SS, et al. Ibrutinib-associated atrial fibrillation. JACC Clin Electrophysiol. 2018;4:1491–1500. doi: 10.1016/j.jacep.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 22.Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PLoS One. 2019;14:e0211228. doi: 10.1371/journal.pone.0211228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salem JE, Manouchehri A, Bretagne M, Lebrun-Vignes B, Groarke JD, Johnson DB, Yang T, Reddy NM, Funck-Brentano C, Brown JR, et al. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol. 2019;74:1667–1678. doi: 10.1016/j.jacc.2019.07.056 [DOI] [PubMed] [Google Scholar]
- 24.Chang PC, Wo HT, Lee HL, Lin SF, Wen MS, Chu Y, Yeh SJ, Chou CC. Role of sarcoplasmic reticulum calcium in development of secondary calcium rise and early afterdepolarizations in long QT syndrome rabbit model. PLoS One. 2015;10:e0123868. doi: 10.1371/journal.pone.0123868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang L, Li L, Ruan Y, Zuo S, Wu X, Zhao Q, Xing Y, Zhao X, Xia S, Bai R, et al. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;16:1374–1382. doi: 10.1016/j.hrthm.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 26.Owen C, Berinstein NL, Christofides A, Sehn LH. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol. 2019;26:e233–e240. doi: 10.3747/co.26.4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrini I, Lencioni M, Ricasoli M, lannopollo M, Orlandini C, Oliveri F, Bartolozzi C, Ricci S. Phase II trial of sorafenib in combination with 5-fluorouracil infusion in advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2012;69:773–780. doi: 10.1007/s00280-011-1753-2 [DOI] [PubMed] [Google Scholar]
- 28.Henry ML, Niu J, Zhang N, Giordano SH, Chavez-MacGregor M. Cardiotoxicity and cardiac monitoring among chemotherapy-treated breast cancer patients. JACC Cardiovasc Imaging. 2018;11:1084–1093. doi: 10.1016/j.jcmg.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Assouline S, Etienne G, Nicolini FE, le Coutre P, Clark RE, et al. ; EPIC investigators. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:612–621. doi: 10.1016/S1470-2045(16)00080-2 [DOI] [PubMed] [Google Scholar]
- 30.Lefebvre B, Kang Y, Smith AM, Frey NV, Carver JR, Scherrer-Crosbie M. Cardiovascular effects of CAR T cell therapy: a retrospective study. JACC CardioOncol. 2020;2:193–203. doi: 10.1016/j.jaccao.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Leeuwen RWF, Jansman FGA, van den Bemt PMLA, de Man F, Piran F, Vincenten I, Jager A, Rijneveld AW, Brugma JD, Mathijssen RHJ, et al. Drug-drug interactions in patients treated for cancer: a prospective study on clinical interventions. Ann Oncol. 2015;26:992–997. doi: 10.1093/annonc/mdv029 [DOI] [PubMed] [Google Scholar]
- 32.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 33.Rhea I, Burgos PH, Fradley MG. Arrhythmogenic anticancer drugs in cardiooncology. Cardiol Clin. 2019;37:459–468. doi: 10.1016/j.ccl.2019.07.011 [DOI] [PubMed] [Google Scholar]
- 34.Jamani R, Lee EK, Berry SR, Saluja R, DeAngelis C, Giotis A, Emmenegger U. High prevalence of potential drug-drug interactions in patients with castration-resistant prostate cancer treated with abiraterone acetate. Eur J Clin Pharmacol. 2016;72:1391–1399. doi: 10.1007/s00228-016-2120-3 [DOI] [PubMed] [Google Scholar]
- 35.Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, et al. ; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 36.Yamreudeewong W, DeBisschop M, Martin LG, Lower DL. Potentially significant drug interactions of class III antiarrhythmic drugs. Drug Saf. 2003;26:421–438. doi: 10.2165/00002018-200326060-00004 [DOI] [PubMed] [Google Scholar]
- 37.Fradley MG, Ellenberg K, Alomar M, Swanson J, Kharod A, Nguyen ATH, Khodor S, Mishra S, Duong LM, Shah N, et al. Patterns of anticoagulation use in patients with cancer with atrial fibrillation and/or atrial flutter. JACC CardioOncology. 2020;2:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Souza M, Carlson N, Fosbøl E, Lamberts M, Smedegaard L, Nielsen D, Torp-Pedersen C, Gislason G, Schou M. CHA2DS2-VASc score and risk of thromboembolism and bleeding in patients with atrial fibrillation and recent cancer. Eur J Prev Cardiol. 2018;25:651–658. doi: 10.1177/2047487318759858 [DOI] [PubMed] [Google Scholar]
- 39.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–180. doi: 10.1016/j.jacc.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 40.Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, et al. ; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313 [DOI] [PubMed] [Google Scholar]
- 41.Melloni C, Dunning A, Granger CB, Thomas L, Khouri MG, Garcia DA, Hylek EM, Hanna M, Wallentin L, Gersh BJ, et al. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and a history of cancer: insights from the ARISTOTLE trial. Am J Med. 2017;130:1440–1448.e1. doi: 10.1016/j.amjmed.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 42.Fanola CL, Ruff CT, Murphy SA, Jin J, Duggal A, Babilonia NA, Sritara P, Mercuri MF, Kamphuisen PW, Antman EM, et al. Efficacy and safety of edoxaban in patients with active malignancy and atrial fibrillation: analysis of the ENGAGE AF-TIMI 48 trial. J Am Heart Assoc. 2018;7:e008987. doi: 10.1161/JAHA.118.008987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fitzgerald JL, Howes LG. Drug interactions of direct-acting oral anticoagulants. Drug Saf. 2016;39:841–845. doi: 10.1007/s40264-016-0443-8 [DOI] [PubMed] [Google Scholar]
- 44.Alkhouli M, Noseworthy PA, Rihal CS, Holmes DR Jr. Stroke prevention in nonvalvular atrial fibrillation: a stakeholder perspective. J Am Coll Cardiol. 2018;71:2790–2801. doi: 10.1016/j.jacc.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 45.Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1:772–778. doi: 10.1182/bloodadvances.2016001883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sequeira AR, Bhandari A, Kilpatrick B, Fradley MG, Mohanty BD. Managing thromboembolic risk from atrial fibrillation in patients with cancer: a role for nonpharmacologic approaches. Future Cardiol. 2020;16:687–693. doi: 10.2217/fca-2020-0005 [DOI] [PubMed] [Google Scholar]
- 47.Porta-Sánchez A, Gilbert C, Spears D, Amir E, Chan J, Nanthakumar K, Thavendiranathan P Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: a systematic review. J Am Heart Assoc. 2017;6:e007724. doi: 10.1161/JAHA.117.007724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–3860. doi: 10.1200/JCO.2001.19.18.3852 [DOI] [PubMed] [Google Scholar]
- 49.Ballou LM, Lin RZ, Cohen IS. Control of cardiac repolarization by phosphoinositide 3-kinase signaling to ion channels. Circ Res. 2015;116:127–137. doi: 10.1161/CIRCRESAHA.116.303975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W; on behalf of the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121:1047–1060. doi: 10.1161/CIRCULATIONAHA.109.192704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abu Rmilah AA, Lin G, Begna KH, Friedman PA, Herrmann J. Risk of QTc prolongation among cancer patients treated with tyrosine kinase inhibitors. Int J Cancer. 2020;147:3160–3167 doi: 10.1002/ijc.33119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, et al. ; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Circulation. 2009;119:e241–e250. doi: 10.1161/CIRCULATIONAHA.108.191096 [DOI] [PubMed] [Google Scholar]
- 53.Fradley MG, Moslehi J. QT prolongation and oncology drug development. Card Electrophysiol Clin. 2015;7:341–355. doi: 10.1016/j.ccep.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 54.Keren A, Tzivoni D, Gavish D, Levi J, Gottlieb S, Benhorin J, Stern S. Etiology, warning signs and therapy of torsade de pointes. A study of 10 patients. Circulation. 1981;64:1167–1174. doi: 10.1161/01.cir.64.6.1167 [DOI] [PubMed] [Google Scholar]
- 55.Badri M, Patel A, Patel C, Liu G, Goldstein M, Robinson VM, Xue X, Yang L, Kowey PR, Yan GX. Mexiletine prevents recurrent torsades de pointes in acquired long QT syndrome refractory to conventional measures. JACC Clin Electrophysiol. 2015;1:315–322. doi: 10.1016/j.jacep.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 56.Shakir M, Reiss J, Buckley M, Surkis W, Yan GX. Mexiletine: a treatment for all cause acquired QT interval prolongation. J Am Coll Cardiol. 2020;75(11 suppl 1):397. [Google Scholar]
- 57.Enriquez A, Biagi J, Redfearn D, Boles U, Kamel D, Ali FS, Hopman WM, Michael KA, Simpson C, Abdollah H, et al. Increased incidence of ventricular arrhythmias in patients with advanced cancer and implantable cardioverter-defibrillators. JACC Clin Electrophysiol. 2017;3:50–56. doi: 10.1016/j.jacep.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 58.Guha A, Derbala MH, Zhao Q, Wiczer TE, Woyach JA, Byrd JC, Awan FT, Addison D. Ventricular arrhythmias following ibrutinib initiation for lymphoid malignancies. J Am Coll Cardiol. 2018;72:697–698. doi: 10.1016/j.jacc.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fradley MG, Welter-Frost A, Gliksman M, Emole J, Viganego F, Lee DH, Shah B, Chavez JC, Pinilla-Ibarz J, Schabath MB. Electrocardiographic changes associated with ibrutinib exposure. Cancer Control. 2020;27:1073274820931808. doi: 10.1177/1073274820931808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Jong J, Hellemans P, Jiao JJ, Huang Y, Mesens S, Sukbuntherng J, Ouellet D. Ibrutinib does not prolong the corrected QT interval in healthy subjects: results from a thorough QT study Cancer Chemother Pharmacol. 2017;80:1227–1237 doi: 10.1007/s00280-017-3471-x [DOI] [PubMed] [Google Scholar]
- 61.Epstein AE, DiMarco JP Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, et al. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742 [DOI] [PubMed] [Google Scholar]
- 62.Ajijola OA, Nandigam KV, Chabner BA, Orencole M, Dec GW, Ruskin JN, Singh JP Usefulness of cardiac resynchronization therapy in the management of doxorubicin-induced cardiomyopathy Am J Cardiol. 2008;101:1371–1372. doi: 10.1016/j.amjcard.2007.12.037 [DOI] [PubMed] [Google Scholar]
- 63.Rickard J, Kumbhani DJ, Baranowski B, Martin DO, Tang WH, Wilkoff BL. Usefulness of cardiac resynchronization therapy in patients with adriamycin-induced cardiomyopathy Am J Cardiol. 2010;105:522–526. doi: 10.1016/j.amjcard.2009.10.024 [DOI] [PubMed] [Google Scholar]
- 64.Singh JP, Solomon SD, Fradley MG, Barac A, Kremer KA, Beck CA, Brown MW, McNitt S, Schleede S, Zareba W, et al. ; MADIT-CHIC Investigators. Association of cardiac resynchronization therapy with change in left ventricular ejection fraction in patients with chemotherapy-induced cardiomyopathy. JAMA. 2019;322:1799–1805. doi: 10.1001/jama.2019.16658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, et al. ; ESC Committee for Practice Guidelines (CPG); Document Reviewers. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on Cardiac Pacing and Resynchronization Therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281–2329. doi: 10.1093/eurheartj/eht150 [DOI] [PubMed] [Google Scholar]
- 66.Arbuck SG, Strauss H, Rowinsky E, Christian M, Suffness M, Adams J, Oakes M, McGuire W, Reed E, Gibbs H, et al. A reassessment of cardiac toxicity associated with Taxol. J Natl Cancer Inst Monogr. 1993:117–130. [PubMed] [Google Scholar]
- 67.Minoia C, Giannoccaro M, Iacobazzi A, Santini D, Silvestris N, Fioretti A, Oliva S, Guarini A. Antineoplastic drug-induced bradyarrhythmias. Expert Opin Drug Saf. 2012;11:739–751. doi: 10.1517/14740338.2012.705826 [DOI] [PubMed] [Google Scholar]
- 68.Khan MA, Masood N, Husain N, Ahmad B, Aziz T, Naeem A. A retrospective study of cardiotoxicities induced by 5-fluouracil (5-FU) and 5-FU based chemotherapy regimens in Pakistani adult cancer patients at Shaukat Khanum Memorial Cancer Hospital & Research Center. J Pak Med Assoc. 2012;62:430–434. [PubMed] [Google Scholar]
- 69.Polk A, Vaage-Nilsen M, Vistisen K, Nielsen DL. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev. 2013;39:974–984. doi: 10.1016/j.ctrv.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 70.Ou SH, Tang Y Polli A, Wilner KD, Schnell P Factors associated with sinus bradycardia during crizotinib treatment: a retrospective analysis of two large-scale multinational trials (PROFILE 1005 and 1007). Cancer Med. 2016;5:617–622. doi: 10.1002/cam4.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP Balko JM, Bonaca MP et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin R, Delgado JM, Moltò JM, Vicent JM, Manzanares R, Insa R, Matias-Guiu J. Cardiovascular reflexes in patients with malignant disease. Ital J Neurol Sci. 1992;13:125–129. doi: 10.1007/BF02226960 [DOI] [PubMed] [Google Scholar]
- 73.Noor B, Akhavan S, Leuchter M, Yang EH, Ajijola OA. Quantitative assessment of cardiovascular autonomic impairment in cancer survivors: a single center case series. Cardiooncology. 2020;6:11. doi: 10.1186/s40959-020-00065-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lakoski SG, Jones LW, Krone RJ, Stein PK, Scott JM. Autonomic dysfunction in early breast cancer: incidence, clinical importance, and underlying mechanisms. Am Heart J. 2015;170:231–241. doi: 10.1016/j.ahj.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coumbe BGT, Groarke JD. Cardiovascular autonomic dysfunction in patients with cancer. Curr Cardiol Rep. 2018;20:69. doi: 10.1007/s11886-018-1010-y [DOI] [PubMed] [Google Scholar]
- 76.Groarke JD, Tanguturi VK, Hainer J, Klein J, Moslehi JJ, Ng A, Forman DE, Di Carli MF, Nohria A. Abnormal exercise response in long-term survivors of Hodgkin lymphoma treated with thoracic irradiation: evidence of cardiac autonomic dysfunction and impact on outcomes. J Am Coll Cardiol. 2015;65:573–583. doi: 10.1016/j.jacc.2014.11.035 [DOI] [PubMed] [Google Scholar]
- 77.Deuring G, Kiss A, Halter JP, Passweg JR, Grossman P Cardiac autonomic functioning is impaired among allogeneic hematopoietic stem cell transplantation survivors: a controlled study. Bone Marrow Transplant. 2017;52:66–72. doi: 10.1038/bmt.2016.176 [DOI] [PubMed] [Google Scholar]
- 78.Vigo C, Gatzemeier W, Sala R, Malacarne M, Santoro A, Pagani M, Lucini D. Evidence of altered autonomic cardiac regulation in breast cancer survivors. J Cancer Surviv. 2015;9:699–706. doi: 10.1007/s11764-015-0445-z [DOI] [PubMed] [Google Scholar]
- 79.Mühlfeld C, Das SK, Heinzel FR, Schmidt A, Post H, Schauer S, Papadakis T, Kummer W, Hoefler G. Cancer induces cardiomyocyte remodeling and hypoinnervation in the left ventricle of the mouse heart. PLoS One. 2011;6:e20424. doi: 10.1371/journal.pone.0020424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013 [DOI] [PubMed] [Google Scholar]
- 81.Potočnik N, Perše M, Cerar A, Injac R, Finderle Ž. Cardiac autonomic modulation induced by doxorubicin in a rodent model of colorectal cancer and the influence of fullerenol pretreatment. PLoS One. 2017;12:e0181632. doi: 10.1371/journal.pone.0181632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun F, Shi J, Geng C. Dexrazoxane improves cardiac autonomic function in epirubicin-treated breast cancer patients with type 2 diabetes. Medicine (Baltimore). 2016;95:e5228. doi: 10.1097/MD.0000000000005228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adams SC, DeLorey DS, Davenport MH, Stickland MK, Fairey AS, North S, Szczotka A, Courneya KS. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: a phase 2 randomized controlled trial. Cancer. 2017;123:4057–4065. doi: 10.1002/cncr.30859 [DOI] [PubMed] [Google Scholar]
- 84.Kirkham AA, Lloyd MG, Claydon VE, Gelmon KA, McKenzie DC, Campbell KL. A longitudinal study of the association of clinical indices of cardiovascular autonomic function with breast cancer treatment and exercise training. Oncologist. 2019;24:273–284. doi: 10.1634/theoncologist.2018-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ricci F, De Caterina R, Fedorowski A. Orthostatic hypotension: epidemiology, prognosis, and treatment. J Am Coll Cardiol. 2015;66:848–860. doi: 10.1016/j.jacc.2015.06.1084 [DOI] [PubMed] [Google Scholar]
- 86.Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, La Gerche A, Ligibel JA, Lopez G, Madan K, et al. ; on behalf of the American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Peripheral Vascular Disease. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:e997–e1012. doi: 10.1161/CIR.0000000000000679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.López-Sendón J, Álvarez-Ortega C, Zamora Auñon P, Buño Soto A, Lyon AR, Farmakis D, Cardinale D, Canales Albendea M, Feliu Batlle J, Rodríguez Rodríguez I, et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J. 2020;41:1720–1729. doi: 10.1093/eurheartj/ehaa006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


