Abstract
BACKGROUND & AIMS:
Campylobacter is the leading cause of bacterial gastroenteritis in the United States. We investigated the prevalence of postinfection irritable bowel syndrome (PI-IBS) in a cohort with culture-confirmed Campylobacter cases; risk factors for PI-IBS based on clinical factors; and shifts in IBS patterns postinfection in patients with pre-existing IBS.
METHODS:
The Minnesota Department of Health collects data on symptoms and exposures upon notification of Campylobacter cases. From 2011 through 2019, we sent surveys (the Rome III and IBS symptom severity surveys) to 3586 patients 6 to 9 months after Campylobacter infection. The prevalence of PI-IBS was estimated and risk factors were assessed using multivariable logistic regression.
RESULTS:
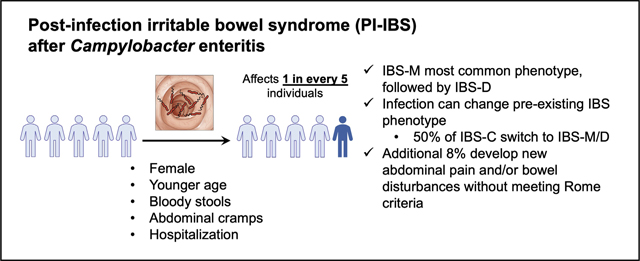
There were 1667 responders to the survey, 249 of whom had pre-existing IBS. Of the 1418 responders without pre-existing IBS, 301 (21%) subsequently developed IBS. Most of these individuals had IBS-mixed (54%), followed by IBS-diarrhea (38%), and IBS-constipation (6%). The mean IBS symptom severity score was 218 (indicating moderate severity). Female sex, younger age, bloody stools, abdominal cramps, and hospitalization during acute enteritis were associated with increased risk, whereas fever was protective for the development of PI-IBS. Antibiotic use and exposure patterns were similar between PI-IBS and control groups. Among patients with IBS-mixed or IBS-diarrhea before infection, 78% retained their subtypes after infection. In contrast, only 50% of patients with IBS-constipation retained that subtype after infection, whereas 40% transitioned to IBS-mixed. Of patients with pre-existing IBS, 38% had increased frequency of abdominal pain after Campylobacter infection.
CONCLUSIONS:
In a cohort of patients with Campylobacter infection in Minnesota, 21% developed PI-IBS; most cases reported mixed IBS or diarrhea of moderate severity. Demographic and clinical factors during acute enterocolitis are associated with PI-IBS development. Campylobacter infection also can result in a switch of a pre-existing IBS phenotype.
Keywords: Gastroenteritis, Functional GI Disorders, Microbiome, Foodborne Illness, Epidemiology
Graphical Abstract
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal (GI) disorders, affecting approximately 12% of the population in North America and 7% to 21% worldwide.1 The pathophysiology of IBS is complex and heterogeneous.2 Acute infectious gastroenteritis is the most commonly identified trigger for development of IBS.3 This entity, described as postinfection IBS (PI-IBS), has been associated with bacterial (Campylobacter, Salmonella, Shigella, and Escherichia coli O157:H7),3,4 protozoal (Giardia),5,6 and viral (Norovirus)7 enteritis. The Rome Foundation working group recently proposed diagnosing PI-IBS as new-onset, Rome criteria–positive IBS, with symptoms developing immediately after resolution of acute gastroenteritis. Acute gastroenteritis can be defined by either a positive stool culture or clinically with the presence of 2 or more symptoms of fever, vomiting, and diarrhea.8 A recent meta-analysis of 45 studies reported a pooled PI-IBS prevalence of 10% within 12 months of an episode of bacterial gastroenteritis, and 4-fold higher odds when compared with uninfected individuals. The risk decreased beyond 12 months of infection but remained increased compared with nonexposed individuals (risk ratio, 2.3), suggesting gradual recovery in a subset of patients.3 A prior study from the United Kingdom showed 13.8% prevalence of PI-IBS after Campylobacter infection.9 A recent claims and encounter database study found an unadjusted risk ratio of 4.3 for IBS risk at 1 year after diagnosis of Campylobacter infection.4 Mathematical modeling has suggested that even with the most conservative estimate, PI-IBS prevalence in the community could be 9%, more than half the overall IBS prevalence in the United States.10
According to the US Centers for Disease Control and Prevention, 1 in 6 adults in the United States suffer from an episode of foodborne illnesses annually. Of these, 9% are estimated to be owing to Campylobacter species, the most commonly diagnosed cause of bacterial gastroenteritis.11,12 Existing large epidemiologic studies are mostly outbreak-associated, often involve more than 1 pathogen, and many are from outside the United States. Some have highlighted that female sex, younger age, antibiotic use, psychological factors, and the severity of the acute illness are associated with PI-IBS development.13,14 Most Campylobacter cases in the United States are sporadic,15 being transmitted as isolated cases. There are limited epidemiologic data on PI-IBS risk associated with community-based sporadic cases of Campylobacter enteritis in the United States. Our aims were as follows: (1) to determine the point prevalence of PI-IBS among a prospective cohort of culture-confirmed Campylobacter cases; (2) to develop a host clinical factor–based risk model for PI-IBS; and (3) to identify shifts in IBS subtype postinfection in individuals with pre-existing IBS.
Methods
As part of statewide surveillance, the Minnesota Department of Health (MDH) requires reporting of all laboratory-confirmed clinical Campylobacter cases in the state. Within 7 to 10 days of notification of Campylobacter cases, the MDH conducts a telephone interview with the affected patient or their guardian to gather information on clinical symptoms of acute infection, exposures, and treatment using a standard surveillance form. To assess PI-IBS development, we sent surveys to patients aged 18 to 80 years from 6 to 9 months after a positive culture for Campylobacter species. Surveys were conducted from November 1, 2011, to September 30, 2019, using postal mailings followed by telephone interviews for nonresponders. Surveyed participants completed the Rome III IBS questionnaire16 and the IBS Symptom Severity Scale (IBS-SSS) questionnaire.17 Questions were asked to exclude a diagnosis of other GI conditions such as celiac disease, Crohn’s disease, ulcerative colitis, and microscopic colitis.
This study was approved by the Institutional Review Boards of MDH and Mayo Clinic. All participants provided either written or oral informed consent for the study.
Statistical Analysis
Continuous variables were summarized as means (SD). In subjects without pre-existing IBS, unadjusted comparisons of demographic data, clinical characteristics of the acute Campylobacter episode, exposures preceding the acute episode (animal and food exposures), travel history, and antibiotic use were performed between participants who developed PI-IBS and those who did not, using the Pearson chi-square and Student t test. A multivariable logistic regression analysis was performed to determine independent factors that predict PI-IBS development based on risk factors selected a priori (age, sex, abdominal pain/cramps, diarrhea duration, hospitalization for infection, fever, vomiting, and bloody diarrhea). The number of candidate variables was limited to avoid overfitting. Age was modeled as a 3 df spline. The duration of diarrhea was modeled as ordered groups (<3 days or unknown, 3–7 days, 8–14 days, and >14 days). Seventy-seven subjects who did not have symptom-related data were excluded from this analysis. In subjects with pre-existing IBS, a logistic regression model was used to test whether the likelihood of a change in type of IBS postinfection was related to pre-infection type. All hypothesis tests were 2-sided with a 0.05 type I error rate. Analyses were conducted using R software (version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Survey Response
The overall survey response was 46.5% (1667 per 3586). Responders had a higher proportion of females (48.0% vs 43.6%; P = .009), Caucasians (92.8% vs 88.1%; P < .001), and a higher mean age (45.4 vs 40.6 y; P < .0001) than nonresponders. The proportion of individuals from urban areas was similar between the 2 groups (76.7% vs 75.2%; P = .29).
Prevalence of Campylobacter Postinfection Irritable Bowel Syndrome
Among the 1667 responders, 249 (14.9%) had IBS before having Campylobacter infection, leaving the remaining 1418 at risk for PI-IBS development (Figure 1). Of these, 301 (21%) met the Rome III criteria for IBS after infection. Criteria for mixed IBS (IBS-M) were met by 54% (n = 159), for diarrhea-predominant IBS (IBS-D) were met by 38% (n = 113), for constipation-predominant IBS (IBS-C) were met by 6% (n = 17), and for unsubtyped IBS were met by 2% (n = 5) (Figure 2A). The mean IBS-symptom severity score was 218 (IBS-SSS range, 0–500). Mild symptom severity (IBS-SSS, 75–175) was reported by 28%, moderate (IBS-SSS, 175–300) was reported by 47%, and severe (IBS-SSS, >300) was reported by 18% (Figure 2B).
Figure 1.
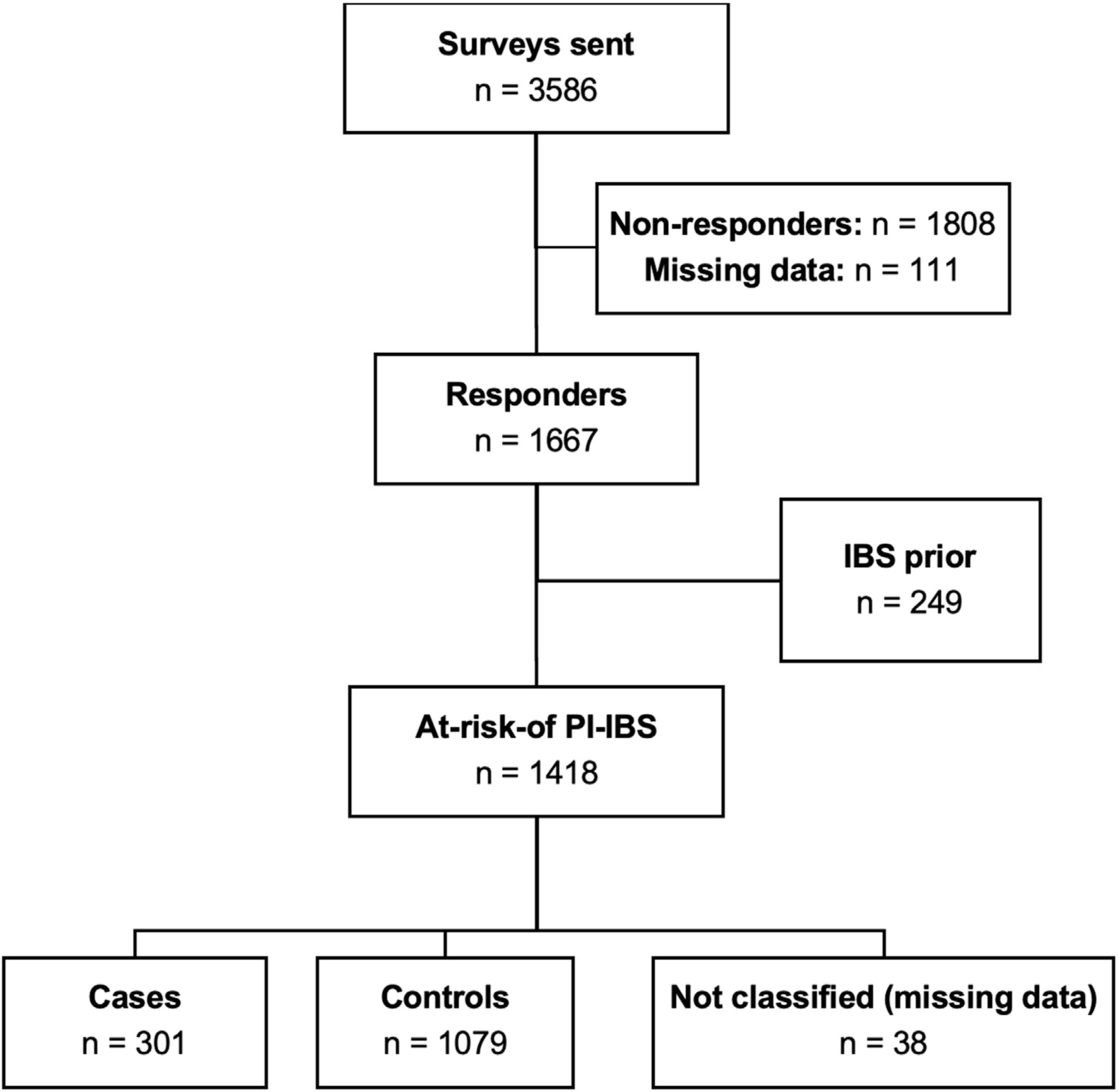
Survey response and identification of Campylobacter postinfection irritable bowel syndrome (PI-IBS) cases and controls.
Figure 2.
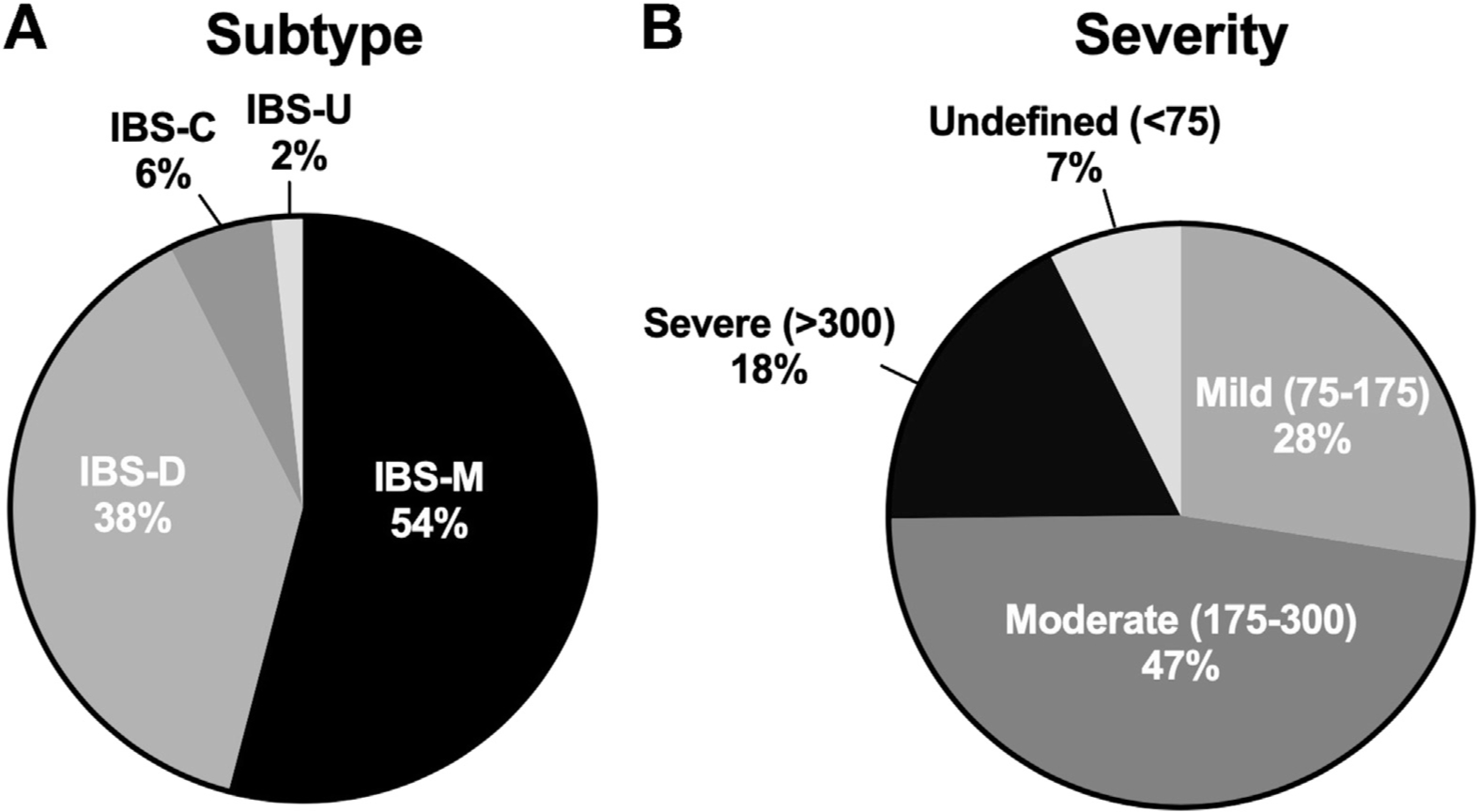
Characteristics of postinfection irritable bowel syndrome. (A) Distribution of IBS subtypes. (B) Distribution of scores on IBS symptom severity scale. IBS-C, constipation-predominant irritable bowel syndrome; IBS-D, diarrhea-predominant irritable bowel syndrome; IBS-M, mixed irritable bowel syndrome; IBS-U, unsubtyped irritable bowel syndrome.
Demographic and Clinical Risk Factors Associated With Campylobacter Postinfection Irritable Bowel Syndrome
Demographic and clinical risk factors were compared between 301 subjects who developed PI-IBS and 1079 who did not (Table 1). Subjects who developed PI-IBS were more likely to be female than those who did not (62% vs 41%; P < .001), and were younger (mean age, 43 vs 46 y; P < .001). A higher proportion of subjects who developed PI-IBS were from a rural area compared with those who did not (30% vs 22%; P = .002). Those who developed PI-IBS had a significantly longer duration of diarrhea, a higher number of stools per day, and a higher prevalence of bloody stools, abdominal cramps, vomiting, and headache during the acute gastroenteritis episode than those who did not develop PI-IBS (Table 1). Fever was less common in those who developed PI-IBS compared with those who did not (67% vs 73%; P = .06). A greater percentage of patients who developed PI-IBS needed hospitalization during the acute infection than those who did not. There was no significant difference in enterocolitis caused by Campylobacter species between the 2 groups, as well as between individuals in different residential settings (Campylobacter jejuni: urban, 90%; large rural, 94%; small rural, 88%; isolated rural, 85%; P = .10). Antibiotic use was not significantly different between those who developed PI-IBS and those who did not (76% vs 78%). The timing of the start of antibiotic treatment after the onset of symptoms also was similar between those who developed PI-IBS and those who did not (mean, 7.7 vs 6.7 d; P = .15). The type of antibiotic used (fluoroquinolone, macrolide, or a combination thereof) also was similar between the 2 groups. International travel was less common in those who developed PI-IBS than in those who did not (23% vs 29%; P = .048). PI-IBS subjects had higher exposure to cats (36% vs 29%; P = .055) and nonpoultry birds (4.1% vs 1.6%; P = .033) than non PI-IBS controls. There was no statistically significant difference in food consumed during the week before illness onset between the 2 groups. When the PI-IBS subjects were compared across disease severity, severely ill patients were older than patients who were mild–moderately ill and less likely to be Caucasian (Supplementary Table 1).
Table 1.
Demographic and Clinical Characteristics of Controls (No PI-IBS) and Cases (PI-IBS)
| No PI-IBS (n = 1079) |
PI-IBS (n = 301) |
P value | |
|---|---|---|---|
|
| |||
| Female, n (%) | 437 (41) | 185 (62) | <.001 |
| Age at time of infection, y, mean (SD) | 46.0 (15) | 42.6 (16) | <.001 |
| Caucasian, n (%) | 971 (90) | 275 (91) | .48 |
| Residence, n (%)a | .006 | ||
| Urban | 847 (79) | 210 (70) | |
| Large rural | 90 (8) | 34 (11) | |
| Small rural | 63 (6) | 19 (6) | |
| Isolated rural | 79 (7) | 38 (13) | |
| Acute gastroenteritis reported symptoms | |||
| Diarrhea, n (%) | 1012 (99.3) | 281 (99.3) | .97 |
| Diarrhea duration, d, means (SD) | 8.0 (6) | 9.1 (6) | .013 |
| Diarrhea duration, n (%) | <.001 | ||
| <3 d or NA | 209 (19) | 89 (30) | |
| 3–7 d | 531 (49) | 116 (39) | |
| 8–14 d | 274 (25) | 67 (22) | |
| >14 d | 65 (6) | 29 (10) | |
| Number of stools/d, means (SD) | 16.9 (14) | 19.4 (17) | .016 |
| Bloody stools, n (%) | 314 (31) | 113 (40) | .005 |
| Abdominal cramps, n (%) | 840 (84) | 256 (92) | <.001 |
| Vomiting, n (%) | 252 (25) | 92 (33) | .007 |
| Fever, n (%) | 733 (73) | 188 (67) | .060 |
| Chills, n (%) | 796 (80) | 217 (79) | .76 |
| Headache, n (%) | 574 (58) | 186 (67) | .005 |
| Muscle aches, n (%) | 562 (57) | 157 (57) | .88 |
| Joint pain, n (%) | 353 (36) | 100 (37) | .76 |
| Campylobacter species, n (%) | .38 | ||
| C jejuni | 939 (87) | 251 (83) | |
| C coli | 101 (9) | 33 (11) | |
| Otherb | 22 (2.0) | 10 (3.3) | |
| Unknown | 19 (1.8) | 7 (2.3) | |
| Quarter of specimen collection, n (%) | .40 | ||
| January–March | 200 (19) | 47 (16) | |
| April–June | 257 (24) | 83 (28) | |
| July–September | 386 (36) | 111 (37) | |
| October–December | 236 (22) | 60 (20) | |
| Hospitalization, n (%) | 113 (11) | 54 (18) | <.001 |
| Antibiotic use, n (%) | 826 (78) | 225 (76) | .45 |
| Days between symptom onset and antibiotic start, means (SD) | 6.7 (9) | 7.7 (7) | .15 |
| Time between symptom onset and antibiotic start, n (%) | .042 | ||
| 0–2 d | 126 (12) | 27 (9) | |
| 3–9 d | 488 (45) | 116 (39) | |
| >9 d | 126 (12) | 48 (16) | |
| Unknown use/onset | 104 (10) | 38 (13) | |
| No antibiotic use | 235 (22) | 72 (24) | |
| Antibiotic type, n (%) | |||
| Fluoroquinolone | 388 (36) | 105 (35) | .73 |
| Macrolide | 403 (37) | 106 (35) | .50 |
| Metronidazole | 75 (7) | 22 (7) | .83 |
| Combination of >1 | 134 (12) | 39 (13) | .80 |
| International travel before onset, n (%) | 303 (29) | 66 (23) | .048 |
| Contact with animals, n (%) | |||
| Dogs | 523 (58) | 159 (62) | .17 |
| Cats | 216 (29) | 80 (36) | .055 |
| Nonpoultry birds | 10 (1.6) | 8 (4.1) | .033 |
| Poultry | 60 (9) | 18 (9) | .97 |
| Pigs | 20 (3.1) | 7 (3.6) | .73 |
| Noncat/dog mammalian pets | 21 (3.3) | 9 (4.6) | .39 |
| Reptiles/amphibians | 9 (1.4) | 4 (2.1) | .53 |
| Ruminants | 67 (10) | 24 (12) | .47 |
| Food consumed the week before, n (%) | |||
| Beef | 561 (80) | 138 (75) | .16 |
| Chicken | 730 (79) | 204 (78) | .75 |
| Turkey | 140 (17) | 39 (17) | .95 |
| Pork | 436 (48) | 116 (46) | .55 |
| Liver pate | 21 (3.0) | 4 (2.1) | .51 |
| Well water | 232 (24) | 68 (25) | .70 |
| Restaurant food | 769 (77) | 217 (78) | .72 |
NOTE. Data were missing for the number of controls and cases, respectively, in the following variables: diarrhea, 60 and 18; diarrhea duration, 190 and 84; stools per day, 80 and 22; bloody stools, 73 and 19; abdominal cramps, 76 and 23; vomiting, 76 and 24; fever, 70 and 20; chills, 81 and 26; headache, 89 and 25; muscle aches, 86 and 26; joint pain, 92 and 29; antibiotics, 18 and 4; time from symptom onset to antibiotic use, 104 and 38; international travel, 30 and 14; contact with dogs, 171 and 46; cats, 330 and 76; nonpoultry birds, 446 and 108; poultry, 426 and 103; other mammalian pets, 439 and 105; reptiles/amphibians, 444 and 107; ruminants, 415 and 99; food consumed: beef, 376 and 117; chicken, 149 and 38; turkey, 248 and 67; pork, 170 and 48; liver pate, 372 and 110; water from a well, 122 and 33; restaurant week prior, 81 and 23. Boldface indicates P < .05.
NA, not applicable; IBS, irritable bowel syndrome; PI-IBS, postinfection irritable bowel syndrome; SD, standard deviation.
Classified using Rural/Urban Commuting Area–ZIP code approximation taxonomy.
Campylobacter species included the following: Campylobacter concisus, Campylobacter curvus, Campylobacter fetus, Campylobacter gracilis, Campylobacter hyointestinalis, Campylobacter lari, and Campylobacter upsaliensis.
Multivariable Analysis for Campylobacter Postinfection Irritable Bowel Syndrome
A logistic regression model was fit to identify independent predictors of PI-IBS development (Table 2). Predictor variables selected a priori were sex, age, diarrhea duration, bloody stools, abdominal cramping, vomiting, fever, and hospitalization. Male sex (odds ratio [OR], 0.47; 95% CI, 0.36–0.62) and fever (OR, 0.62; 95% CI, 0.45–0.83) were associated with lower odds of developing PI-IBS. The prevalence of blood in stools (OR, 1.41; 95% CI, 1.06–1.89), abdominal cramping (OR, 2.05, 95% CI, 1.26–3.33), and hospitalization (OR, 2.04; 95% CI, 1.38–3.02) were associated with PI-IBS development after Campylobacter gastroenteritis.
Table 2.
Multiple Logistic Regression Analysis of Demographic and Clinical Characteristics Related to the Development of Postinfection Irritable Bowel Syndrome
| Estimate | Odds ratio with 95% CI | P value | |
|---|---|---|---|
|
| |||
| Male sex | −0.752 | 0.47 (0.36–0.62) | <.001 |
| Agea | .043 | ||
| Diarrhea durationb | −0.035 | 0.97 (0.81–1.14) | .68 |
| Bloody stools | 0.345 | 1.41 (1.06–1.89) | .020 |
| Abdominal cramps | 0.716 | 2.05 (1.26–3.33) | .004 |
| Vomiting | 0.220 | 1.25 (0.92–1.69) | .15 |
| Fever | −0.485 | 0.62 (0.45–0.83) | .002 |
| Hospitalization | 0.712 | 2.04 (1.38–3.02) | <.001 |
NOTE. Boldface indicates P < .05.
CI, confidence interval.
Age was modeled as a 3 df natural spline, older age was associated with less likelihood of developing irritable bowel syndrome.
Diarrhea duration was categorized into 4 groups of increasing severity and was modeled linearly.
Changes in Phenotypes Among Those With Pre-existing Irritable Bowel Syndrome
Among the participants who had IBS-M (n = 108) or IBS-D (n = 71) before infection, 84 (78%) and 55 (77%), respectively, retained their subtypes after infection. However, among 34 IBS-C patients, only 17 (50%; P < .01 compared with IBS-M and IBS-D) retained the subtype and the rest switched to IBS-M, IBS-D, or unsubtyped IBS after infection (Figure 3). Of the 249 subjects with pre-existing IBS, 94 (38%) had an increase in the frequency of abdominal pain, while 129 (52%) retained the same level of abdominal pain, and 26 (10%) had a decreased frequency of abdominal pain.
Figure 3.
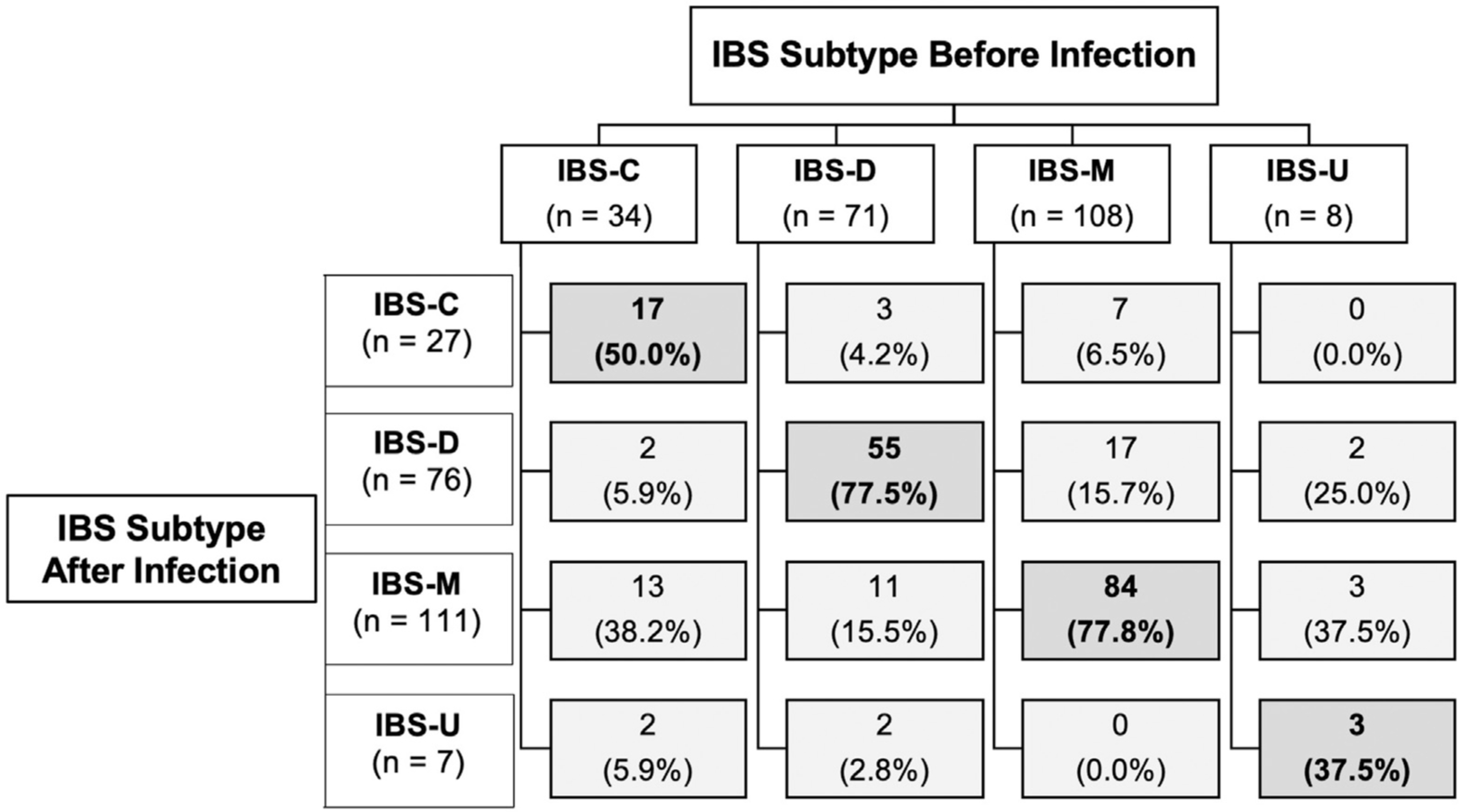
Stability and changes in irritable bowel syndrome phenotypes after Campylobacter enterocolitis in patients who had pre-existing irritable bowel syndrome. IBS-C, constipation-predominant irritable bowel syndrome; IBS-D, diarrhea-predominant irritable bowel syndrome; IBS-M, mixed irritable bowel syndrome; IBS-U, unsubtyped irritable bowel syndrome.
Postinfection Symptoms That Do Not Meet Criteria for Postinfection Irritable Bowel Syndrome
Next, we wanted to determine if certain patients who previously were asymptomatic might develop new abdominal pain and/or bowel disturbances that did not meet the frequency thresholds established in the Rome III criteria for IBS. We found 121 (8.5%) meeting that definition: 47 patients developed new abdominal pain and new bowel disturbances, 73 developed only new bowel disturbances, and 1 developed only new abdominal pain. Thirty-one patients had new onset of loose/mushy/watery stools 25% of the time or more, 20 patients had hard/lumpy stools 25% of the time or more, and 56 patients had both 25% of the time or more.
Timing of Onset of Symptoms After Infection
It is assumed that PI-IBS symptoms start as a continuum after acute symptoms of gastroenteritis subside. However, clinically, a subset of patients had complete resolution of acute symptoms, followed by a transient symptom-free period and then onset of symptoms that subsequently become chronic. One way to capture this was by determining which patients have <6 months of symptom onset at the time of the survey, but fulfill all of the remaining Rome III criteria for IBS. In our data, 112 (7.9%) additional participants were in this category. The most common phenotype was IBS-M (61%) again, followed by IBS-D (29%), IBS-C (7%), and unsubtyped IBS (3%). All of these patients were asymptomatic before their Campylobacter infection.
Discussion
This study shows that 1 in 5 individuals who acquire a sporadic Campylobacter infection in the community develop PI-IBS. Symptoms of PI-IBS usually were mixed diarrhea and constipation or diarrhea alone, but rarely constipation-predominant. These symptoms were moderate in almost half of the patients and severe in one sixth of patients. Those who developed PI-IBS were more likely to be younger, female, and have a greater prevalence of abdominal cramps and bloody stool during infection. Hospitalization also was more common in those who subsequently developed PI-IBS. Interestingly, fever during the gastroenteritis episode was associated inversely with PI-IBS. Antibiotic treatment was approximately 75% in both PI-IBS and non–PI-IBS subjects. International travel was less common among PI-IBS cases; however, domestic exposure to cats and nonpoultry birds was more common. Food consumption patterns, especially eating chicken and eating at a restaurant in the week before the onset of acute symptoms, were similar between cases and controls.
Using the detailed, acute surveillance conducted by the MDH and subsequent surveys to identify new onset of GI symptoms, we are able to determine the prevalence and risk factors for PI-IBS in a large, population-based cohort of laboratory-confirmed Campylobacter patients. A prior model from the Walkerton outbreak cohort study found some similar risk factors to be associated with PI-IBS.14 However, the utility of that model was limited considering that the outbreak resulted from washed livestock fecal contamination of drinking water, which resulted in multipathogen infection (E coli, Campylobacter, Giardia, and so forth). Our risk factors provide a more realistic estimate of PI-IBS risk from sporadic Campylobacter cases derived from communities within the United States. This reduces biases introduced from single-point source outbreaks. In addition, as shown in our univariate analysis, we were able to ascertain associations with a wide range of environmental variables and exposures, including food, travel, and animals.
Abdominal cramps associated with development of IBS suggesting neuronal sensitization may play a role in the development of chronic visceral hypersensitivity.18 Diarrhea duration was not associated independently with PI-IBS; however, bloody diarrhea was associated with PI-IBS, which suggests that mucosal injury during acute infection may result in greater immune and neuromuscular activation and downstream development of PI-IBS. The lower prevalence of fever in those who did not develop PI-IBS is interesting and, speculatively, may reflect a protective response to acute injury by the host.19 Antibiotic use was high in our cohort and was not associated with PI-IBS development. This is in contrast to some other studies associating antibiotics with PI-IBS development.3 However, in those studies, mostly from outside the United States, overall antibiotic use was much lower (~5%–13%). This might reflect health care anxiety, which has been associated with IBS instead of a true predilection of antibiotic use to be associated with PI-IBS development. Campylobacter species are known to show genomic variability, and strain variations certainly can play a role in acute and chronic host responses.20 The current study only examines host response to infection. Future models combining host and bacterial factors likely will provide stronger prediction models for PI-IBS development.
The PI-IBS phenotype conventionally has been considered to be mostly IBS-D.3 In this study, IBS-M was the most common phenotype. This is important and also makes treatment using motility agents challenging considering that patients can be prone to developing extremes of bowel patterns. However, the Rome IV questionnaire and use of bowel diaries possibly can characterize the IBS-M population better, some of those patients may fit IBS-D or IBS-C classifications.21 In addition, use of the Rome IV criteria may result in a lower overall incidence of PI-IBS, as has been noted with IBS in general.22 Although symptoms in most patients were moderate, 17% were severe. Another important finding from this prospectively recruited cohort was that Campylobacter infection can result in a switch toward IBS-M/D in those with IBS-C before the infection. This suggests that even people with pre-existing IBS can be prone to new bowel disturbances after infection. The causality of infection resulting in a phenotypic switch is plausible considering previous literature showing that IBS phenotypes can switch over long-term follow-up evaluation.23 However, the prior study did not examine if there were enteric infections in the 12-year period between the initial and subsequent surveys. Considering the incidence of foodborne illness, it is possible that several of the subjects in that study had an episode of gastroenteritis in this period. Possible transitions in Campylobacter PI-IBS subtype, especially IBS-M, as well as resolution of IBS symptoms over time, needs to be assessed formally. Clinically, several patients reported bowel irregularities after enteric infections, but not much pain. When we examined whether additional individuals had new GI issues that did not fit Rome criteria for IBS, we realized that 121 patients (8.5%) had either new bowel disturbances or new pain and bowel disturbances that did not meet the thresholds set in the Rome criteria. From these, 73 subjects (5.2%) had long-term bowel changes and no pain. A prior study also found that 5% of patients developed chronically altered bowel habits without pain after Campylobacter enterocolitis. Only 1 patient reported pain but no bowel disturbances, suggesting that isolated new-onset pain is not common after Campylobacter infection. Thus, the burden of postinfection GI issues might be even larger than captured by standardized Rome criterion.
The timing of onset of PI-IBS after infection is unclear. Conventional thinking, as well as the Rome Foundation working group, suggests that PI-IBS originates as a continuum after acute Campylobacter symptoms subside.8 We found that 8% of participants who were asymptomatic before met all of the Rome III criteria for PI-IBS except for onset 6 months ago or more. Considering that the surveys were sent 6 to 9 months after infection, this suggests that the onset was sometime in that period (ie, <6 months after infection). It is possible that if these patients are surveyed further out, many will have persistent symptoms and will meet Rome criteria for PI-IBS, increasing the prevalence in our population close to 30%. This supports the clinical observation that a subset of patients may not have immediate onset of symptoms postinfection because the eliciting and compensatory mechanistic changes may take time to establish before symptoms are experienced.
Limitations of the study were that of a survey-based assessment. Most importantly, response bias can enrich the proportion of patients with PI-IBS. Responder bias could have resulted in a greater proportion of females and the slightly older population in the study. Considering that acute survey data were obtained within 7 to 10 days after reporting of the Campylobacter infection, they would be less likely to be biased by recall issues. Psychological factors such as anxiety, depression, and somatization have been associated with PI-IBS risk,3 however, these were not assessed in the current survey.
In summary, this US population-based study showed a high risk of PI-IBS development among sporadic Campylobacter cases. The model presented can help identify patients at high risk for PI-IBS development. Expansion of the cohort and looking at bacterial and other novel risk factors will allow strengthening of this model, which can be used for preventative interventions for PI-IBS. Another important observation was the changes in pre-existing IBS that acute gastroenteritis appears to precipitate. Additional studies are warranted to study the subsets that show changes in severity and phenotype after infection. This information could provide a mechanistic understanding of IBS in the community in general.
Supplementary Material
What You Need to Know.
Background
Campylobacter is the leading cause of bacterial gastroenteritis in the United States, but little is known about its associated postinfection irritable bowel syndrome (PI-IBS).
Findings
In a cohort of patients with Campylobacter infection in Minnesota, 21% developed PI-IBS; most cases reported mixed IBS or diarrhea of moderate severity. Female sex, younger age, bloody stools, abdominal cramps, and hospitalization during acute enteritis were associated with an increased risk of PI-IBS. Patients with pre-existing IBS with constipation may notice a switch to IBS-mixed or diarrhea, as well as worsening of their abdominal pain.
Implications for patient care
Patients with Campylobacter infection should be monitored for development of IBS.
Acknowledgments
The authors acknowledge Lori Anderson for administrative assistance.
Funding
Supported by National Institutes of Health grants DK 103911 and DK 120745 (M.G.).
Abbreviations used in this paper:
- GI
gastrointestinal
- IBS
irritable bowel syndrome
- IBS-C
constipation-predominant IBS
- IBS-D
diarrhea-predominant IBS
- IBS-M
mixed IBS
- IBS-SSS
Irritable Bowel Syndrome Symptom Severity Scale
- MDH
Minnesota Department of Health
- OR
odds ratio
- PI-IBS
postinfection irritable bowel syndrome
Footnotes
Conflicts of interest
The authors disclose the following: Robin Patel has received grants from CD Diagnostics, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, ContraFect, TenNor Therapeutics Limited, and Shionogi, is a consultant to Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST, and Qvella (funds are paid to Mayo Clinic), holds a patent on Bordetella pertussis/parapertussis polymerase chain reaction, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an antibiofilm substance issued, receives travel reimbursement from ASM and IDSA, an editor’s stipend from IDSA, and honoraria from the NBME, Up-to-Date, and the Infectious Diseases Board Review Course; and Madhusudan Grover has received grants from Takeda and Dong-A pharmaceuticals. The remaining authors disclose no conflicts.
CRediT Authorship Contributions
Antonio Berumen, MD (Data curation: Supporting; Formal analysis: Supporting; Visualization: Supporting; Writing Supporting; Formal analysis: Supporting;dhusudan@m Supporting);
Ryan Lennon, MS (Data curation: Supporting; Formal analysis: Lead; Writing; dhusuda & editing: Supporting);
Margaret K Breen-Lyles, BA (Writing orting; Formal analysis: Lead;
Jayne Griffith, MPH (Data curation: Supporting; Writing sis: Lead; Writing Supporting);
Robin Patel, MD (Writing curation: Supporting; Writing;
David Boxrud, PhD (Writing – review & editing: Supporting);
Marijke Decuir, MPH (Data curation: Supporting; Software: Supporting; Supervision: Supporting; Writing re: Supporting; Supervision:rvi;
Gianrico Farrugia, MD (Writing Writing Writing ing; Supervisi;
Kirk Smith, PhD (Conceptualization: Supporting; Data curation: Supporting; Methodology: Supporting; Project administration: Supporting; Resources: Supporting; Supervision: Supporting; Writing administration: Supporting; Res;
Madhusudan Grover, MD (Conceptualization: Lead; Data curation: Lead; Funding acquisition: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Resources: Lead; Software: Lead; Supervision: Lead; Visualization: Lead; Writing sources: Lead; Software: Lead; Supervision: Lead; Viting: Lead).
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.07.033.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–721 e4. [DOI] [PubMed] [Google Scholar]
- 2.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology 2017;152:1042–1054 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scallan Walter EJ, Crim SM, Bruce BB, et al. Postinfectious irritable bowel syndrome after Campylobacter infection. Am J Gastroenterol 2019;114:1649–1656. [DOI] [PubMed] [Google Scholar]
- 5.Hanevik K, Dizdar V, Langeland N, et al. Development of functional gastrointestinal disorders after Giardia lamblia infection. BMC Gastroenterol 2009;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wensaas KA, Langeland N, Hanevik K, et al. Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: historic cohort study. Gut 2012;61:214–219. [DOI] [PubMed] [Google Scholar]
- 7.Zanini B, Ricci C, Bandera F, et al. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol 2012;107:891–899. [DOI] [PubMed] [Google Scholar]
- 8.Barbara G, Grover M, Bercik P, et al. Rome Foundation Working Team report on post-infection irritable bowel syndrome. Gastroenterology 2019;156:46–58 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunlop SP, Jenkins D, Neal KR, et al. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 2003;125:1651–1659. [DOI] [PubMed] [Google Scholar]
- 10.Shah ED, Riddle MS, Chang C, et al. Estimating the contribution of acute gastroenteritis to the overall prevalence of irritable bowel syndrome. J Neurogastroenterol Motil 2012;18:200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis 2011;17:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tack DM, Marder EP, Griffin PM, et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food - Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2015–2018. MMWR Morb Mortal Wkly Rep 2019;68:369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall JK, Thabane M, Garg AX, et al. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology 2006; 131:445–450; quiz 660. [DOI] [PubMed] [Google Scholar]
- 14.Thabane M, Simunovic M, Akhtar-Danesh N, et al. Development and validation of a risk score for post-infectious irritable bowel syndrome. Am J Gastroenterol 2009;104:2267–2274. [DOI] [PubMed] [Google Scholar]
- 15.Dewey-Mattia D, Manikonda K, Hall AJ, et al. Surveillance for foodborne disease outbreaks - United States, 2009–2015. MMWR Surveill Summ 2018;67:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 17.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 18.Ibeakanma C, Ochoa-Cortes F, Miranda-Morales M, et al. Brain-gut interactions increase peripheral nociceptive signaling in mice with postinfectious irritable bowel syndrome. Gastroenterology 2011;141:2098–2108 e5. [DOI] [PubMed] [Google Scholar]
- 19.Bettes N, Griffith J, Camilleri M, et al. Su2070 risk and predictors of post-infectious irritable bowel syndrome among community-acquired cases of bacterial enteritis. Gastroenterology 2014; 146:S–538. [Google Scholar]
- 20.Man SM. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 2011;8:669–685. [DOI] [PubMed] [Google Scholar]
- 21.Polster AV, Palsson OS, Tornblom H, et al. Subgroups of IBS patients are characterized by specific, reproducible profiles of GI and non-GI symptoms and report differences in healthcare utilization: a population-based study. Neurogastroenterol Motil 2019;31:e13483. [DOI] [PubMed] [Google Scholar]
- 22.Palsson OS, Whitehead W, Tornblom H, et al. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020;158:1262–1273 e3. [DOI] [PubMed] [Google Scholar]
- 23.Halder SL, Locke GR 3rd, Schleck CD, et al. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology 2007;133:799–807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


