Abstract
A healthy brain is critical for living a longer and fuller life. The projected aging of the population, however, raises new challenges in maintaining quality of life. As we age, there is increasing compromise of neuronal activity that affects functions such as cognition, also making the brain vulnerable to disease. Once pathology-induced decline begins, few therapeutic options are available. Prevention is therefore paramount, and primary care can play a critical role. The purpose of this American Heart Association scientific statement is to provide an up-to-date summary for primary care providers in the assessment and modification of risk factors at the individual level that maintain brain health and prevent cognitive impairment. Building on the 2017 American Heart Association/American Stroke Association presidential advisory on defining brain health that included “Life’s Simple 7,” we describe here modifiable risk factors for cognitive decline, including depression, hypertension, physical inactivity, diabetes, obesity, hyperlipidemia, poor diet, smoking, social isolation, excessive alcohol use, sleep disorders, and hearing loss. These risk factors include behaviors, conditions, and lifestyles that can emerge before adulthood and can be routinely identified and managed by primary care clinicians.
Keywords: AHA Scientific Statements, aging, brain, cognitive dysfunction, primary health care, primary prevention, risk factors
The brain, through its capacity for cognition, is the organ that functions to register and record experiences. In youth, sound cognition is expected, but with aging, the increasingly vulnerable brain acquires injuries, and cognitive decline becomes more prevalent. As the average age of the US population grows, this age effect becomes of greater concern as the number of individuals with or at risk for mild cognitive impairment (MCI) and dementia grows.1 As of now, 1 in 5 Americans ≥65 years of age has MCI, and 1 in 7 has dementia2; by 2050, however, the number of Americans with dementia will triple.3 The importance of cognition was affirmed by the Affordable Care Act, which mandated coverage of an assessment of cognitive function as part of the annual wellness benefit for Medicare beneficiaries.4 The question arises, however, about what the health care community can do proactively to mitigate or forestall the onset of decline before it happens. Primary care is well suited to emphasize brain health as part of the goal of many preventive interventions. The purpose of this document is to summarize information that will help primary care providers optimize their effectiveness in maintaining brain health.
For clinicians and scientists, brain health can be approached along many structural, physiological, and epidemiological dimensions. Clinically, optimal brain health is the absence of cognitive impairment/dementia, stroke, and other brain diseases. Pathologically, optimal brain health is the absence of neurodegenerative, cerebrovascular, and comorbid diseases that interfere with everyday physical and cognitive functioning. Pragmatically, it is the preservation of neuronal function to meet the demands of everyday life, operationally defined in terms of the capacity to function adaptively in one’s environment. With aging come increasingly compromised neuronal activity and greater vulnerability to disease.5 The ability to think, solve problems, remember, perceive, and communicate is crucial to successful living; their loss can lead to helplessness and dependency. Once pathologically based decline begins, however, there are few therapeutic options to halt or slow the downward course.
Because of their central role in patient care and management, primary care providers are in a strategic position to identify and manage risk factors for cognitive decline. The goal is to identify and modify risk factors leading to brain injury before it happens. People with dementia experience lower quality of life,6 and caregivers, typically family members, experience high rates of psychological stress and physical ill health.7 Dementia is among the costliest medical conditions, shown to be more expensive in both direct and indirect costs than heart disease or cancer,8 with worldwide costs estimated at $818 billion in 2015.9 Delaying cognitive decline through primary care provider–instigated risk factor modification for even a small percentage of individuals would enable many more people to reach end of life without dementia and would have substantial benefits for patients, caregivers, and society.10
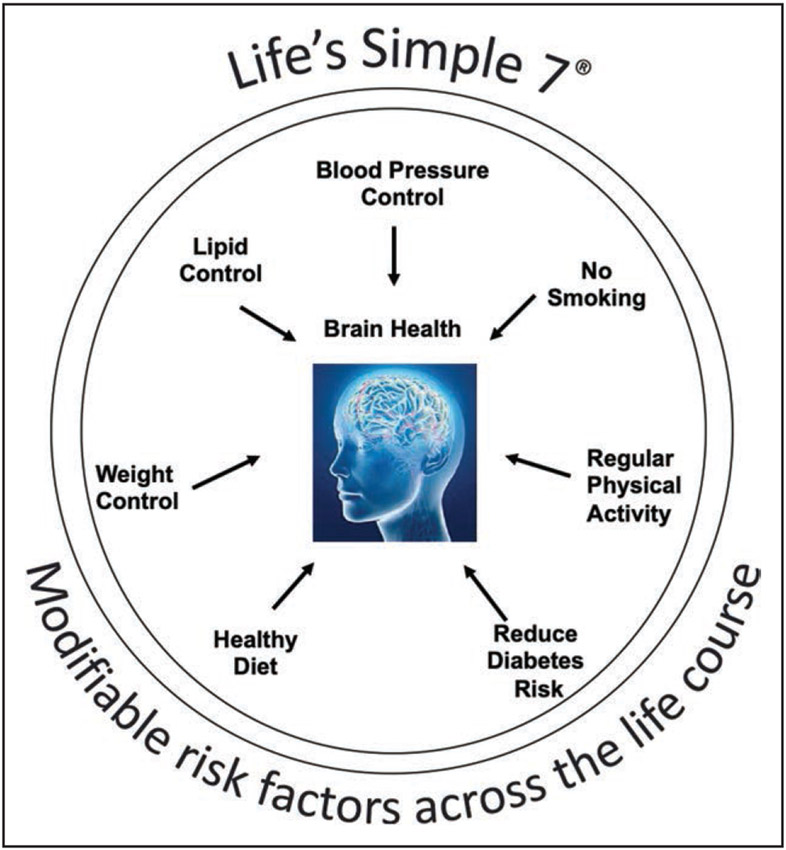
In 2017, the American Heart Association (AHA)/American Stroke Association issued a presidential advisory on defining brain health that included “Life’s Simple 7”11 as a follow-up to an earlier statement on the promotion of cardiovascular health12 (Figure). The goal of that document was to provide a model for operationally defining brain health in highly quantifiable terms and to suggest specific therapeutic targets for preventive intervention that included risk factor management and lifestyle changes. The purpose of the current statement is to update the evidence supporting that strategy and to emphasize the importance of dissemination and surveillance in the primary care setting. We acknowledge that the notion of brain health encompasses other functions such as mobility and emotional status, but they are interdependent with systems and conditions outside the brain. We chose cognition because it is uniquely dependent on the brain.
Figure.
The American Heart Association’s Life’s Simple 7, as described by Lloyd-Jones et al12 and later Gorelick et al.11
METHODS
In April 2019, the AHA Stroke Council convened the Brain Science Subcommittee to address science-based issues related to brain health. The committee recommended a scientific statement on the role of primary care in maintaining brain health. A writing group was assembled and commissioned to focus on 4 topics: definition of brain health, risk factors for cognitive impairment, strategies to safeguard brain health, and integration of best practices into primary care.
The writing group members were chosen for their knowledge in specific areas and experience with guideline development and scientific statements. The participants included representatives from neurology, neuropsychology, internal medicine, family practice, epidemiology, and nursing. The first conference call was held in November 2019. Each of the major sections of the manuscript was assigned a lead author, and the chair and cochair were responsible for drafting the introduction, methods, and concluding sections and editing all subsections of the manuscript. Literature searches were conducted by the lead authors.
The statement was independently reviewed by the AHA Science Advisory and Coordinating Committee. The writing group responded point by point to each comment made by the reviewers. Final approval of the manuscript was provided by the AHA Science Advisory and Coordinating Committee.
COGNITION
Cognition refers to all brain functions that support the perception, acquisition, storage, retrieval, and complex processing of information, including communication, planning, and navigation. It is a rubric, not a single entity, that encompasses many domains.13 A person’s pattern of cognitive impairment depends in part on the location and severity of the brain dysfunction; any combination of impairment in learning, memory, visuospatial skills, language, complex attention, and executive function can be seen. In typical late-onset Alzheimer disease, for example, episodic memory impairment is usually the earliest and most salient feature, consistent with the profound effects of the disease on medial temporal lobe structures.14 We describe below the continuum of cognitive decline that we wish to mitigate.
Definitions and Diagnostic Criteria for Subjective Cognitive Decline, MCI, and Dementia
Subjective cognitive decline is a self-experienced, persistent decline in cognitive function from a previous state, lasting >6 months, without (or before) a formal examination by a health care provider or cognitive testing.15,16 MCI is a clinical diagnosis for a symptomatic syndrome on the continuum of cognitive decline between normal cognition and dementia and is characterized by measurable cognitive impairment that does not significantly impair daily, social, or occupational functioning; help is not required to perform basic or instrumental activities of daily living (eg, bathing, dressing, grocery shopping, and taking medicines).17-19 In contrast, dementia is a clinical diagnosis for a syndrome characterized by objective cognitive impairment that occurs over months to years that is severe enough to interfere with daily, social, or occupational functioning; help is needed to perform basic or instrumental activities of daily living.20,21 The fifth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders has different nomenclature, using minor neurocognitive disorder for MCI and major neurocognitive disorder for dementia.22
After determining the severity of the cognitive impairment and its impact on everyday functioning, clinicians can assign a pathogenetic subtype.22 MCI and dementia can be caused by different pathologies, including neurodegenerative disease (Alzheimer disease, Lewy body disease, TAR DNA-binding protein 43, and hippocampal sclerosis) and cerebrovascular disease. Although Alzheimer disease is probably the most common cause, most cases of MCI and dementia are mixed, with contributions by neurodegenerative disease, cerebrovascular disease, and comorbidities.18,20,23
Epidemiology of Subjective Cognitive Decline, MCI, and Dementia
The risk of MCI and dementia increases with aging. Prevalence estimates of subjective cognitive decline, MCI, and dementia differ across studies. Approximately 11.7% of Americans ≥65 years of age report subjective cognitive decline.24 Results from the Aging, Demographics, and Memory Study estimate that the prevalence of MCI is 22.2% and the prevalence of dementia is 13.9% among individuals ≥71 years of age.25,26 Results from the Chicago Health and Aging Project estimate that 10% of adults ≥65 years of age have Alzheimer dementia in particular.3 The numbers of Americans with MCI and dementia are projected to triple by 205016 because of the increases in the population ≥65 years of age and in average life expectancy. It should be pointed out, however, that there is recent evidence that the incidence of Alzheimer disease may be declining, especially in higher-income countries.27-29 There is also considerable evidence that improvements in the management of vascular health may be contributing to this observed decline in dementia incidence across different racial/ethnic groups.30-32
Prognosis is uncertain for individuals with subjective cognitive decline and MCI. Subjective cognitive decline might be an indicator of symptomatic preclinical cognitive decline. However, it does not inevitably progress to MCI, even after 10 to 20 years.33-35 Similarly, MCI does not inevitably progress to dementia,36 even after a decade,37,38 and a small percentage of individuals with MCI have improvements in cognition.39,40 The risk of conversion from MCI to dementia ranges from 3%/y to 15%/y and varies by population, with lower risk in community-based samples and higher risk in samples from Alzheimer disease clinics and research centers.37,38,41 Dementia worsens over time in nearly all individuals.42,43
Risk Factors for Cognitive Impairment
Contributors to progressive intellectual decline include health and lifestyle factors that are potentially modifiable. Although Life’s Simple 7’s ideal cardiovascular health (Table) is defined by the presence of favorable health behaviors, favorable health factors, and the absence of clinical cardiovascular disease, this section shifts to unfavorable or unhealthy factors and behaviors; that is, factors that increase the chance of developing cognitive impairment. Combining findings from a previous systematic literature review, a Delphi consensus study identified the following major, modifiable risk factors for cognitive decline: depression, midlife hypertension, physical inactivity, diabetes, midlife obesity, hyperlipidemia, and smoking.44 Across studies, cardiovascular risk factors, many of which are amenable to change, emerge as highly associated with future cognitive impairment, including dementia.11 A commissioned report on approaches to dementia modeled the life course of modifiable risk factors for dementia and estimated that 35% of dementia cases would be prevented if known risk factors were eliminated, including obesity, hypertension, depression, smoking, physical inactivity, diabetes, hearing impairment, less education, and social isolation.10 New data show that modifiable risk factors, including hypertension, diabetes, and smoking, in midlife not only accelerate cognitive decline in older age but also increase the odds of cognitive decline in middle age.45 A major challenge for providers and public health experts, however, is how to successfully modify these risk factors with interventions that are widely and effectively used. Although some preventive intervention trials have yielded modest or negative results,46,47 recent evidence from multidomain interventions has shown promise for reducing cognitive decline in at-risk older adults,48,49 and the decline in overall incidence rates is felt to be attributable in part to better control of risk factors.29
Table.
Life’s Simple 7: Definition of Ideal Cardiovascular Health
| Goal/metric | Ideal cardiovascular health definition |
|---|---|
| Current smoking | |
| Adults >20 y of age | Never or quit >12 mo ago |
| Children 12–19 y of age | Never tried; never smoked whole cigarette |
| Body mass index | |
| Adults >20 y of age | <25 kg/m2 |
| Children 2–19 y of age | <85th percentile |
| Physical activity | |
| Adults >20 y of age | ≥150 min/wk moderate intensity, ≥75 min/wk vigorous intensity, or combination |
| Children 12–19 y of age | ≥60 min of moderate- or vigorous-intensity activity every day |
| Healthy Diet Score | |
| Adults >20 y of age | 4–5 Components* |
| Children 5–19 y of age | 4–5 Components* |
| Total cholesterol | |
| Adults >20 y of age | <200 mg/dL† |
| Children 6–19 y of age | <170 mg/dL† |
| Blood pressure | |
| Adults >20 y of age | <120/<80 mm Hg† |
| Children 8–19 y of age | <90th percentile† |
| Fasting plasma glucose | |
| Adults >20 y of age | <100 mg/dL† |
| Children 12–19 y of age | <100 mg/dL† |
Low glycemic load, high cereal fiber, high folate, high marine omega-3 fatty acid, a high ratio of polyunsaturated to saturated fat, low trans fat content.
Untreated values.
Adapted from Lloyd-Jones et al.12 Copyright © 2010, American Heart Association, Inc.
ASSOCIATION OF MODIFIABLE RISK FACTORS WITH BRAIN HEALTH
In the past, the evidence guiding principles for preventing cognitive decline stemmed largely from observational studies. More recently, however, SPRINT MIND (Systolic Blood Pressure Intervention Trial–Memory and Cognition in Decreased Hypertension) showed in a randomized controlled trial (RCT) that lowering systolic blood pressure (BP) to <120 mm Hg reduced risk for the combined outcome of MCI and dementia.50 This proof-of-concept research supports the more general thesis that reducing risk for vascular disease may improve brain health. In this section, we summarize risk factors, including hypertension, that can be assessed and modified to maintain brain health and prevent cognitive decline.
Life’s Simple 7
BP Control: Hypertension
Hypertension is a well-established risk factor for stroke and vascular dementia.51,52 A systematic review and meta-analysis of 7 studies found that systolic BP >160 mm Hg was associated with a 25% increase in risk for Alzheimer disease.53 Systolic BP >140 mm Hg was associated with an 18% increased risk. In the Framingham Offspring (n=1440) and the ARIC (Atherosclerosis Risk in Communities; n=4761) prospective cohort studies, midlife hypertension (eg, 40–65 years of age) was associated with increased risk of later-life dementia.54,55 As noted, the most robust data to date on BP management for maintaining brain health come from the SPRINT MIND study.50 Previous observational studies may have been underpowered or had insufficient follow-up time to examine the effect of antihypertensive treatment on rates of cognitive decline. In this RCT of hypertensive adults ≥50 years of age (mean age, 68 years; 28% >75 years) with high cardiovascular disease risk (mean 10-year cardiovascular disease risk, ≈20%), targeting a systolic BP of <120 mm Hg compared with <140 mm Hg reduced the risk for the nonprimary combined outcome of MCI and dementia by 19% (14.6 versus 18.3 cases per 1000 person-years) over a median intervention period of 3.3 years and an overall median follow-up of 5.1 years. Although subsequent meta-analyses provide new support for the management and control of hypertension to reduce risk of MCI and dementia,56,57 more trials are needed to confirm optimal BP targets to maintain cognitive function.
Smoking Status
According to a commissioned review, smoking is the third most important modifiable risk factor for dementia, behind hypertension and less education.10 Although the majority of cohort studies found a positive association between smoking and incident cognitive impairment and age-related cognitive decline, some studies may be limited by the differential high mortality of smokers, especially heavy smokers.58,59 Although the exact mechanisms linking smoking to cognitive decline are still unknown, oxidative stress leading to vascular, inflammatory, and neurodegenerative processes may play a central role. A recent meta-analysis involving >900 000 cases showed that whereas smokers clearly had an increased risk of dementia, quitting smoking decreased that risk to close to the level of never smokers.60
Physical Activity
Less physical activity has been associated with a higher risk of cognitive decline in many epidemiological studies.58,61,62 Some were limited by including only older adults, short follow-up, and incomplete data on physical activity after baseline. In the Whitehall II cohort study in participants 35 to 55 years of age at enrollment (n=7424), no association was found between physical activity over time and cognitive decline during 15 years of observation.63 In contrast, a systematic review of studies examining early-life (≤30 years of age) physical activity showed that higher levels of activity were associated with better cognition in later life (≥60 years of age).64 Similar to physical inactivity and stroke risk,65 the relationship may be partially mediated through control of BP, hyperglycemia, body weight, and lipids. Although observational studies suggest that physical activity across the life span will benefit brain health and cognition, clinical trials are not convincing.66 A 2015 Cochrane review of 12 clinical trials (>750 patients) found no evidence that aerobic exercise improves cognitive function in older adults with otherwise normal cognition.67 A more recent randomized, parallel-group, community-based clinical trial of 132 cognitively normal individuals 20 to 67 years of age with below-median aerobic capacity found that compared with stretching/toning, aerobic exercise significantly improved executive function.68 Cortical thickness in a left frontal region increased significantly in the aerobic exercise group, and results did not differ by age. The optimal dose, type, frequency, and duration of physical activity for optimal brain health across the life span remain to be established.
Diabetes
The association between diabetes and increased risk for severe cognitive decline (dementia) is well established,69,70 but the association with milder cognitive impairment has not been thoroughly evaluated. Systematic reviews of prospective cohort studies found that compared with individuals without diabetes, those with diabetes have greater rates of decline in cognitive function and are at 50% increased risk of cognitive decline.71,72 In the ARIC study, participants with midlife diabetes had a 19% significantly greater cognitive decline over 20 years compared with individuals without diabetes.73 In ACCORD-MIND (Action to Control Cardiovascular Risk in Diabetes–Memory in Diabetes), type 1 and 2 diabetes and the metabolic syndrome were associated with increased risk for MCI and progression to dementia.74 In stroke and dementia, these associations may be mediated by endothelial dysfunction or inflammation of the vessel wall, promoting accelerated atherosclerosis and altered metabolism of glucose, insulin, and amyloid.69 Insulin resistance may be a common mechanism linking both diabetes and obesity to increased risk of dementia.75 Although no clinical trials have demonstrated that intensive control of diabetes or treatment of insulin resistance lowers the risk for cognitive impairment,76 the observational data suggest that better control might eventually prove effective.77
Dietary Patterns
Several reviews have summarized the extensive literature related to dietary patterns and cognitive outcomes.78-80 The combined evidence, primarily from observational studies, supports that greater adherence to healthier diets such as the DASH (Dietary Approach to Stop Hypertension), Mediterranean, and MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diets are associated with slower cognitive decline in middle-aged and older adults.79 The Mediterranean diet includes high consumption of fruits, vegetables, and olive oil and moderate alcohol consumption. The MIND diet, a combination of the Mediterranean and DASH diets, shares many food groups with the Mediterranean diet, but the MIND diet differs by special categories for green leafy vegetables and berries. However, comparing 2 derived dietary pattern scores, one characterized by high consumption of meats and fried foods and the other by higher amounts of fruits and vegetables, the ARIC study (n=13 588) found that the 20-year change in global cognitive function and the risk of incident dementia did not differ by dietary pattern.81 Results from an Australian cohort (n=1220) suggest that the MIND diet is more protective against MCI than the Mediterranean diet.82 Among participants enrolled in both the WHI (Women’s Health Initiative) Memory Study and the WHI Dietary Modification trial (n=1606), a low-fat dietary intervention significantly reduced the risk of cognitive impairment defined by the modified Mini-Mental State Examination.83 The few other clinical trials had limitations, including small sample size and short duration of intervention.79,84 More studies, including clinical trials, are needed to better understand mechanisms to guide dietary recommendations for the prevention of MCI.
Body Mass Index: Obesity
Considerable epidemiological evidence supports the association of midlife obesity with increased risk of cognitive impairment,85-87 and with its high prevalence, obesity is identified as one of the most common risk factors for Alzheimer disease and related dementias,80 a collective term for the range of neurodegenerative disease entities that impair cognition. Systematic reviews and meta-analyses related to other cognitive outcomes, however, show inconsistent results.88 Factors contributing to this inconsistency include the assessment of body mass index at 1 point in time, midlife versus late-life assessment of obesity, small sample size, limited exclusion criteria, limited adjustment for potential confounding factors, and reverse causation. In the ARIC study, for example, midlife obesity and other cardiovascular risk factors such as diabetes, hypertension, and total cholesterol were associated with MCI up to 25 years later.89 In a cross-sectional study using data from the Human Connectome Project Healthy Young Adult data set (age, 22–35 years) aimed at identifying a core battery of neurocognitive functions associated with body mass index, body mass index was found to be negatively associated with 7 of 20 neurocognitive measures, including language ability, delay discounting, episodic memory, and cognitive flexibility, but not the executive function measures of inhibition and working memory.88 Whether midlife weight loss or weight loss maintenance benefits cognition remains unknown. Nevertheless, obesity is an important risk factor to consider because it is on the causal pathway between other risk factors and cognitive outcomes, including physical activity, diet, diabetes, and hypertension.
Serum Cholesterol
Relationships between serum cholesterol, cholesterol-lowering treatment, cognitive decline, and dementia are not well understood, in part because of a small number of studies (especially with MCI outcomes), short follow-up times, heterogeneous measurement methods, and differing definitions across studies. A pooled analysis found that high total cholesterol in midlife increased the risk of Alzheimer disease later in life.90 Limited data exist to characterize the relationship between brain health and other fats in the blood such as high-density lipoprotein and triglycerides and trans and saturated fat intake. Evidence from existing clinical trials suggests that statin treatment in late life does not prevent cognitive decline or dementia.91 At the same time, a systematic review and meta-analysis of RCTs showed that statin therapy was not associated with adverse effects on cognition.92 Limited evidence supports relationships between midlife administration of lipid-lowering drugs and reduced risk for dementia or MCI,93 although there was an inverse relationship between duration of statin use after stroke and dementia risk in a population-based cohort in Taiwan.94
Other Factors of Importance
Alcohol Consumption
Light to moderate alcohol consumption has been hypothesized to be protective against MCI, and this observation generally has been found to be true in older ages, with a U-shaped (primarily), J-shaped, or linear association.58 Excessive and prolonged alcohol use can lead to brain injury through neurotoxicity, nutritional deficiency, neuro-inflammation, and changes in neurotransmitter systems.95 The protective effects may be through a reduction in platelet aggregation or modification of serum lipid profile or a direct effect through acetylcholine release in the hippocampus, enhancing memory and learning.58,96
Sleep
Results from numerous observational studies consistently demonstrate that various sleep disorders contribute to cognitive decline.97,98 Sleep disorders such as obstructive sleep apnea and insomnia contribute to vascular dementia via diverse mechanisms. Sleep apnea is associated with hypoxia, systemic inflammation, endothelial dysfunction, hypertension, and atrial fibrillation.99,100 Growing evidence suggests that poor sleep leads to progression of neurodegenerative disease.97 Effective interventions exist to improve sleep such as sleep restriction–sleep compression therapy and multicomponent cognitive-behavioral therapy.101 Studies with longer follow-up and objective sleep measures are needed.
Social Engagement
Social isolation is defined as having little or no social contact or a diminished social network; loneliness is the subjective feeling related to isolation.102 Growing and consistent evidence shows the effects of social isolation and loneliness as risk factors for cognitive decline and dementia.103-105 Among 1905 participants without dementia in the Betula study in Sweden with an average of 20 years of follow-up, a positive response to the question “Do you often feel lonely?” was associated with a 51% increase in risk for all-cause dementia.102 The underlying mechanism of social isolation and dementia remains to be clarified, but it may be through effects on the cardiovascular system or through its link with depression.106 A review that included 39 studies, of which only 2 were RCTs, examined social networks, social activity, and social support. In the RCTs, a structured social activity improved global cognition but did not affect specific domains.105
Hearing
In a meta-analysis of 36 cross-sectional and longitudinal studies, age-related hearing loss was associated with both cognitive impairment and dementia.107 The significance of these findings lies in the observation that although approximately one-third of older adults experience hearing impairment, it remains largely untreated.108 Potential mechanisms are unclear but may include a theory of sensory deprivation, that perceptual decline may cause cognitive decline. Hearing loss and dementia may be linked because of common factors such as depression and social isolation that affect both at older age.107,109,110 Currently, no RCT evidence supports the benefits of hearing interventions on long-term cognitive outcomes, and the results of a systematic review of observational studies show inconsistent evidence for hearing interventions and cognition.111 Within the Aging and Cognitive Health Evaluation in Elders pilot study, 40 older adults with untreated hearing loss were randomized to a best-practice hearing intervention, including hearing aids, or to a successful aging intervention.112 Results at 6 months showed an improvement in memory in the hearing intervention group, setting the stage for a full-scale RCT of a hearing intervention on cognition.
Mood: Depression
Depression appears to be a risk factor for dementia, yet dementia is also a risk factor for depression in older age.113 Using results from 17 cohort studies, a systematic review and meta-analysis examining risk factors for conversion from MCI to dementia showed an elevated risk for dementia among those with MCI and depression compared with individuals with no depression.114 Results from a large cohort with follow-up over 28 years found that individuals with depressive symptoms in midlife were not at increased risk for dementia, but those with depressive symptoms later in life had a higher risk of dementia, suggesting that perhaps the symptoms are an early sign of dementia.115 Research is needed that examines the role of antidepressants in the depression-dementia relationship among individuals with late-life depression and whether treatment can prevent incident MCI and dementia. The evidence to date is inconsistent because of methodological limitations, including lack of RCTs with sufficient follow-up.
Education
Other risk factors are societal and family life factors, including less education. These factors may also be in the causal pathway of many of the conditions discussed above. In general, studies find higher educational attainment to be protective against cognitive decline in older adults.58,116 High education is also thought to lead to greater cognitive reserve, which enables people to maintain cognitive function despite brain pathology.117 Higher educational levels are also associated with better access to care and control of cardiovascular risk factors.10 A meta-analysis including 13 longitudinal studies found that low educational level (≤8 years) increased risk for dementia or cognitive impairment by 1.8 times.116 Perhaps as much as any risk factor for cognitive decline, modification of education-associated risk starts early in the life span.
INTEGRATION INTO CLINICAL PRACTICE
Public health and primary care share a goal to improve the health of populations. Our summary of modifiable risk factors for cognitive impairment includes behaviors and conditions that emerge as early as childhood and adolescence. Most operate through a lifetime and are theoretically amenable to public health policies that improve education, expand opportunities for college attendance, discourage tobacco use, reduce social isolation, promote physical activity, reduce noise-induced hearing loss, and encourage healthy eating. Leveraging the public-health approach to cognitive health will require help from elected officials. Clinicians can seek this support by reprising their role as activists and advocates for policies that re-engineer our public spaces, mitigate adverse exposures, and expand the opportunities we provide to citizens of all ages to lead healthy lives.
Given that primary care aims to provide patients with rapid access to comprehensive and coordinated care from clinicians who know them over time,118 office-based primary care practice can complement the public health approach to promoting cognitive well-being. Continuity in the clinician-patient relationship underlies the 3 pillars of work in primary care: evaluation and management of acute symptoms, prevention of acute and chronic disease, and management of chronic disease. Promoting overall health, which includes brain health, is deeply embedded in this work, although this fact is rarely acknowledged in discussions with patients or even among professionals.119 It is a hidden purpose and outcome of good primary care.
Standard prevention in primary care addresses every element of the AHA’s Life’s Simple 7.11 Five of the 7 elements are recommended for formal screening by professional guidelines: screening for BP, glucose, cholesterol, smoking status, and weight.120-125 The screening interval varies among these, but screening generally is expected to occur every 1 to 5 years, beginning in very early adulthood. Treatment goals and strategies have been developed and are widely accepted for each of these conditions.126-129 Primary care clinicians can uniquely target those modifiable risk factors that are prevalent at midlife but are known to exist as early as childhood.130 By their education, training, and professional norms, primary care clinicians are well prepared to implement these guidelines, and they are part of standard practice.
For 2 other elements of Life’s Simple 7, healthy diet and physical activity, there are no commonly accepted screening instruments for office practice. Instead, professional guidelines recommend that all patients receive counseling to eat well and exercise a minimum of 150 min/wk.125,131
Three other risk factors for cognitive decline are not among Life’s Simple 7, but they are part of routine primary care practice. The US Preventive Services Task Force has recommended screening for depression since 2016.123 Sleep apnea, although not recommended for routine screening,132 is commonly diagnosed, and affected patients are referred for care. Current guidelines do not recommend testing for hearing loss in asymptomatic adults133 but do recommend testing in patients who report hearing loss or may have hearing loss according to office-based observation. The National Institute for Health and Care Excellence guidelines recommend regular hearing assessment in patients with cognitive impairment.134
Frailty is an emerging concept in geriatrics and primary care that may represent another opportunity to safeguard brain health. Frailty is variably defined as the accumulation of symptoms (eg, weakness, joint pain), signs (eg, weight loss), physical limitations, and comorbid illnesses that increase vulnerability to poor health outcomes. Frailty is independently associated with increased risk for Alzheimer-type dementia.135 Aspects of frailty are amenable to physical therapy, nutritional improvement, and other interventions that theoretically could be effective in preventing cognitive impairment and dementia. Research is ongoing.
However, prevention in primary care should begin at earlier stages in the age spectrum because new data show that modifiable risk factors in younger groups increase the odds of cognitive decline in middle age and older. The CARDIA study (Coronary Artery Risk Development in Young Adults) found that among Black and White middle-aged adults who had been enrolled between 18 and 30 years of age, blood glucose variability below the threshold of diabetes 25 years later was associated with worse processing speed, memory, and language fluency in midlife, independently of fasting glucose levels.136 In the 1946 British birth cohort (Insight 46), higher diastolic BP and change in diastolic BP in the period of 36 to 43 years of age were associated with smaller whole-brain volume later at 70 years of age.137 These data further justify the importance of monitoring risk factors years before the effects on the brain may be apparent.
Continuity in the patient relationship over time means that primary care clinicians can detect change in function that may signal the onset of MCI or dementia. Neither the US Preventive Services Task Force nor the American Academy of Neurology recommends screening asymptomatic individuals for cognitive impairment, but they do suggest vigilance for change that should warrant case finding.41,138 Specifically, when there is concern for cognitive decline on the basis of patient or informant report or the clinician’s own observations, the clinician should gather pertinent history and consider testing with a validated cognitive assessment instrument. Several brief validated instruments are available, including the Montreal Cognitive Assessment and the Brief Alzheimer Screen.139 What precludes the US Preventive Services Task Force and the American Academy of Neurology from recommending screening of asymptomatic patients is the absence of proof that early detection improves patient-centered or other outcomes. What causes both to leave a door open, however, is the accepted wisdom that early case recognition might identify treatable causes and help patients and families plan for their future. There is also the hope that research may soon identify early interventions that slow pathologically based cognitive decline. Results of FINGER (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) suggest that the optimal intervention may prove to be a broad-based approach involving vascular risk modification and lifestyle improvement,48 a notion supported more recently by a report from the Lancet Commission.130 Such a comprehensive approach must be tailored to sex, race and ethnicity, and socioeconomic status, but we admittedly are still lacking sufficient information for optimal care, and important disparities in access to primary care still exist. In addition, the impact of these risk factors on MCI may differ by sex and race/ethnicity; for example, the association of alcohol consumption on cognition may be stronger in men than women,58 the benefit from aerobic exercise may be greater in men than women,140 and there are studies of diet having differential cognitive effects by racial/ethnic groups.141,142 Risk of cognitive impairment increases with age across all race, education, and socioeconomic status groups; however, higher rates are notable in certain racial/ethnic groups (eg, Black and Latino people in the United States). Unfortunately, these disparities are still poorly understood. However, disproportionate rates of vascular and other factors described here appear to explain at least some of the observed disparities. Thus, these groups would be particularly likely to benefit from the preventive strategies recommended.
Despite broad acceptance of standard operations in primary care practice that can improve brain health, implementation often falls short in what is referred to as a quality gap or evidence-practice gap. This gap is being partially closed by innovations in practice redesign and technology. Successful examples include telehealth,143 which has taken off in the coronavirus disease 2019 (COVID-19) pandemic, self-monitoring for chronic illness,144 and team-based and collaborative care.145 In the area of hypertension control, examples include self-monitoring and self-titration144,146 and collaborative care with pharmacists.147,148
Besides the evidence-practice gap, there are other barriers to success in improving brain health in primary care. The main one is access to primary care services. Even with the Affordable Care Act, an estimated 15% of American adults still lack health insurance.149 For those with insurance, copays for primary care and shortages of caregivers, particularly in rural areas, are serious problems.149 Between 2002 and 2015, the proportion of Americans with a source of primary care decreased from 77% to 75%.150 Downward pressure on wages and profound adverse changes in the primary care workplace have driven physicians-in-training from this field, further exacerbating workforce shortages. Growing numbers of nurse practitioners and physician assistants are helping, but the need for improved primary care services is increasing as the population ages.
Primary care is the right home for practice-based efforts to prevent or postpone cognitive decline among Americans. A wide gap remains, however, between what primary care does in this area and what it can do. Bridging this gap will depend on improving access to high-quality primary care for adults of all ages through reform of health care finance and of primary care practice such that we attract new clinicians. These reforms are tightly interconnected, and there is reason to be hopeful that change will come. Universal health care coverage has moved to the main stage in the national political discussion. Improvements in primary care practice are emerging from dozens of local innovations. The chances that these local innovations will proliferate will be vastly improved by current plans by the Centers for Medicare & Medicaid Services to migrate Medicare toward a value-based system that encourages and reimburses prevention and high-quality patient care. Among the winners will be many millions of precious brains.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
Contributor Information
Ronald M. Lazar, University of Alabama at Birmingham, McKnight Brain Institute
Virginia J. Howard, University of Alabama at Birmingham
Hugo J. Aparicio, Boston University School of Medicine
Lori C. Jordan, Vanderbilt University Medical Center
Walter N. Kernan, Yale School of Medicine
Deborah A. Levine, University of Michigan
David L. Nyenhuis, Hauenstein Neuroscience Center, Saint Mary’s Health
Katherine L. Possin, University of California, San Francisco
Farzaneh A. Sorond, Northwestern University, Feinberg School of Medicine
Anthony J. Viera, Duke University
Carole L. White, University of Texas Health Sciences Center School of Nursing
REFERENCES
- 1.US Census Bureau. 2017 National population projections tables: main series (updated October 8, 2019). 2017. Accessed February 21, 2021. https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html
- 2.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl 1):i162–i171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services, Center for Medicare & Medicaid Services. Rules and regulations. Federal Register (Part II); 752010. 42 CFR Parts 405, 409, 410, et al. Medicare Program; Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2011; Final Rule, Accessed February 21, 2021. 73170 Federal Register / Vol. 75, No. 228 / Monday, November 29, 2010. / Rules and Regulations. [Google Scholar]
- 5.Mattson MP, Arumugam TV. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 2018;27:1176–1199. doi: 10.1016/j.cmet.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen C, Pedersen I, Bergland A, Enders-Slegers MJ, Jøranson N, Calogiuri G, Ihlebæk C. Differences in quality of life in home-dwelling persons and nursing home residents with dementia: a cross-sectional study. BMC Geriatr. 2016;16:137. doi: 10.1186/s12877-016-0312-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilhooly KJ, Gilhooly ML, Sullivan MP, McIntyre A, Wilson L, Harding E, Woodbridge R, Crutch S. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016;16:106. doi: 10.1186/s12877-016-0280-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurd MD, Martorell P, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;369:489–490. doi: 10.1056/NEJMc1305541 [DOI] [PubMed] [Google Scholar]
- 9.Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, Jönsson L, Liu Z, Prince M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13:1–7. doi: 10.1016/j.jalz.2016.07.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, et al. , Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734, doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 11.Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd-Jones DM, Bae HJ, Bauman MA, Dichgans M, Duncan PW, et al. ; on behalf of the American Heart Association/American Stroke Association. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. doi: 10.1161/STR.0000000000000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. ; on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 13.Marshall RS, Lazar RM. Pumps, aqueducts, and drought management: vascular physiology in vascular cognitive impairment. Stroke. 2011;42:221–226. doi: 10.1161/STROKEAHA.110.595645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bondi MW, Edmonds EC, Salmon DP. Alzheimer’s disease: past, present, and future. J Int Neuropsychol Soc. 2017;23:818–831. doi: 10.1017/S135561771700100X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, et al. ; Subjective Cognitive Decline initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15:321–387. [Google Scholar]
- 17.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheirner’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen RC. Clinical practice: mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237 [DOI] [PubMed] [Google Scholar]
- 20.Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review.JAMA. 2019;322:1589–1599. doi: 10.1001/jama.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Associaton; 2013. [Google Scholar]
- 23.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 24.Taylor CA, Bouldin ED, McGuire LC. Subjective cognitive decline among adults aged ≥45 years–United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67:753–757. doi: 10.15585/mmwr.mm6727a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008; 148:427–434, doi: 10.7326/0003-4819-148-6-200803180-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, et al. , Prevalence of dementia in the United States: the Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78:1456–1463. doi: 10.1212/WNL.0b013e3182553be6 [DOI] [PubMed] [Google Scholar]
- 28.Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, Brayne C; Medical Research Council Cognitive Function and Ageing Collaboration. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382:1405–1412. doi: 10.1016/S0140-6736(13)61570-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374:523–532. doi: 10.1056/NEJMoa1504327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pase MP, Satizabal CL, Seshadri S. Role of improved vascular health in the declining incidence of dementia. Stroke. 2017;48:2013–2020. doi: 10.1161/STROKEAHA.117.013369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González HM, Tarraf W, Gouskova N, Rodríguez CJ, Rundek T, Grober E, Pirzada A, González P, Lutsey PL, Camacho A, et al. Life’s Simple 7’s cardiovascular health metrics are associated with Hispanic/Latino neurocognitive function: HCHS/SOL results. J Alzheimers Dis. 2016;53:955–965. doi: 10.3233/JAD-151125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González HM, Tarraf W, Harrison K, Windham BG, Tingle J, Alonso A, Griswold M, Heiss G, Knopman D, Mosley TH. Midlife cardiovascular health and 20-year cognitive decline: Atherosclerosis Risk in Communities Study results. Alzheimers Dement. 2018;14:579–589. doi: 10.1016/j.jalz.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaup AR, Nettiksimmons J, LeBlanc ES, Yaffe K. Memory complaints and risk of cognitive impairment after nearly 2 decades among older women. Neurology. 2015;85:1852–1858. doi: 10.1212/WNL.0000000000002153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, Nelson PT, Smith CD, Van Eldik LJ, Wan L, Schmitt FA. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014;83:1359–1365. doi: 10.1212/WNL.0000000000000856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernández-Blázquez MA, Ávila-Villanueva M, Maestú F, Medina M. Specific features of subjective cognitive decline predict faster conversion to mild cognitive impairment. J Alzheimers Dis. 2016;52:271–281. doi: 10.3233/JAD-150956 [DOI] [PubMed] [Google Scholar]
- 36.Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275:214–228. doi: 10.1111/joim.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151–1157. doi: 10.1001/archneurol.2009.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia: meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x [DOI] [PubMed] [Google Scholar]
- 39.Fisk JD, Merry HR, Rockwood K. Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology. 2003;61:1179–1184. doi: 10.1212/01.wnl.0000089238.07771.c7 [DOI] [PubMed] [Google Scholar]
- 40.Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, Barberger-Gateau P, Dartigues JF. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8 [DOI] [PubMed] [Google Scholar]
- 41.Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, Gronseth GS, Marson D, Pringsheim T, Day GS, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:126–135. doi: 10.1212/WNL.0000000000004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knopman DS, Petersen RC. Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc. 2014;89:1452–1459. doi: 10.1016/j.mayocp.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graham JE, Rockwood K, Beattie BL, McDowell I, Eastwood R, Gauthier S. Standardization of the diagnosis of dementia in the Canadian Study of Health and Aging. Neuroepidemiology. 1996;15:246–256. doi: 10.1159/000109914 [DOI] [PubMed] [Google Scholar]
- 44.Deckers K, van Boxtel MP, Schiepers OJ, de Vugt M, Muñoz Sánchez JL, Anstey KJ, Brayne C, Dartigues JF, Engedal K, Kivipelto M, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30:234–246. doi: 10.1002/gps.4245 [DOI] [PubMed] [Google Scholar]
- 45.Yaffe K, Bahorik AL, Hoang TD, Forrester S, Jacobs DR Jr, Lewis CE, Lloyd-Jones DM, Sidney S, Reis JP. Cardiovascular risk factors and accelerated cognitive decline in midlife: the CARDIA Study. Neurology. 2020;95:e839–e846. doi: 10.1212/WNL.0000000000010078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moll van Charante EP, Richard E, Eurelings LS, van Dalen JW, Ligthart SA, van Bussel EF, Hoevenaar-Blom MP, Vermeulen M, van Gool WA. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388:797–805. doi: 10.1016/S0140-6736(16)30950-3 [DOI] [PubMed] [Google Scholar]
- 47.Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S, Bories L, Cufi MN, Dantoine T, Dartigues JF, et al. ; MAPT Study Group. Effect of longterm omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16:377–389. doi: 10.1016/S1474-4422(17)30040-6 [DOI] [PubMed] [Google Scholar]
- 48.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 49.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653–666. doi: 10.1038/s41582-018-0070-3 [DOI] [PubMed] [Google Scholar]
- 50.Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, et al. ; SPRINT MIND Investigators for the SPRINT Research Group. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, et al. ; on behalf of the American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharp SI, Aarsland D, Day S, Sønnesyn H, Ballard C; Alzheimer’s Society Vascular Dementia Systematic Review Group. Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatry. 2011;26:661–669. doi: 10.1002/gps.2572 [DOI] [PubMed] [Google Scholar]
- 53.Lennon MJ, Makkar SR, Crawford JD, Sachdev PS. Midlife hypertension and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2019;71:307–316. doi: 10.3233/JAD-190474 [DOI] [PubMed] [Google Scholar]
- 54.McGrath ER, Beiser AS, DeCarli C, Plourde KL, Vasan RS, Greenberg SM, Seshadri S. Blood pressure from mid- to late life and risk of incident dementia. Neurology. 2017;89:2447–2454. doi: 10.1212/WNL.0000000000004741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker KA, Sharrett AR, Wu A, Schneider ALC, Albert M, Lutsey PL, Bandeen-Roche K, Coresh J, Gross AL, Windham BG, et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA. 2019;322:535–545. doi: 10.1001/jama.2019.10575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding J, Davis-Plourde KL, Sedaghat S, Tully PJ, Wang W, Phillips C, Pase MP, Himali JJ, Gwen Windham B, Griswold M, et al. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19:61–70. doi: 10.1016/S1474-4422(19)30393-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ou YN, Tan CC, Shen XN, Xu W, Hou XH, Dong Q, Tan L, Yu JT. Blood pressure and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 209 prospective studies. Hypertension. 2020;76:217–225. doi: 10.1161/HYPERTENSIONAHA.120.14993 [DOI] [PubMed] [Google Scholar]
- 58.Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campos MW, Serebrisky D, Castaldelli-Maia JM. Smoking and cognition. Curr Drug Abuse Rev. 2016;9:76–79. doi: 10.2174/1874473709666160803101633 [DOI] [PubMed] [Google Scholar]
- 60.Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One. 2015;10:e0118333. doi: 10.1371/journal.pone.0118333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14:510. doi: 10.1186/1471-2458-14-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Souto Barreto P, Demougeot L, Vellas B, Rolland Y. Exercise training for preventing dementia, mild cognitive impairment, and clinically meaningful cognitive decline: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2018;73:1504–1511. doi: 10.1093/gerona/glx234 [DOI] [PubMed] [Google Scholar]
- 63.Sabia S, Dugravot A, Dartigues JF, Abell J, Elbaz A, Kivimäki M, Singh-Manoux A. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ. 2017;357:j2709. doi: 10.1136/bmj.j2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greene C, Lee H, Thuret S. In the long run: physical activity in early life and cognitive aging. Front Neurosci. 2019;13:884. doi: 10.3389/fnins.2019.00884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howard VJ, McDonnell MN. Physical activity in primary stroke prevention: just do it! Stroke. 2015;46:1735–1739. doi: 10.1161/STROKEAHA.115.006317 [DOI] [PubMed] [Google Scholar]
- 66.Brasure M, Desai P, Davila H, Nelson VA, Calvert C, Jutkowitz E, Butler M, Fink HA, Ratner E, Hemmy LS, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:30–38. doi: 10.7326/M17-1528 [DOI] [PubMed] [Google Scholar]
- 67.Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2015:CD005381. doi: 10.1002/14651858.CD005381.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stern Y, MacKay-Brandt A, Lee S, McKinley P, McIntyre K, Razlighi Q, Agarunov E, Bartels M, Sloan RP Effect of aerobic exercise on cognition in younger adults: a randomized clinical trial. Neurology. 2019;92:e905–e916. doi: 10.1212/WNL.0000000000007003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2 [DOI] [PubMed] [Google Scholar]
- 70.Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2:246–255. doi: 10.1016/S2213-8587(13)70088-3 [DOI] [PubMed] [Google Scholar]
- 71.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes: systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4 [DOI] [PubMed] [Google Scholar]
- 72.Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144. doi: 10.1371/journal.pone.0004144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, Griswold M, Gottesman RF, Wagenknecht LE, Windham BG, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–793. doi: 10.7326/M14-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, Coker LH, Murray A, Sullivan MD, Marcovina SM, et al. ; Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Investigators. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) trial. Diabetes Care. 2009;32:221–226. doi: 10.2337/dc08-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kellar D, Craft S. Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19:758–766. doi: 10.1016/S1474-4422(20)30231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tuligenga RH. Intensive glycaemic control and cognitive decline in patients with type 2 diabetes: a meta-analysis. Endocr Connect. 2015;4:R16–R24. doi: 10.1530/EC-15-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, et al. ; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X, Maguire B, Brodaty H, O’Leary F. Dietary patterns and cognitive health in older adults: a systematic review. J Alzheimers Dis. 2019;67:583–619. doi: 10.3233/JAD-180468 [DOI] [PubMed] [Google Scholar]
- 79.van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease: a review. Adv Nutr. 2019;10:1040–1065. doi: 10.1093/advances/nmz054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith PJ. Pathways of prevention: a scoping review of dietary and exercise interventions for neurocognition. Brain Plast. 2019;5:3–38. doi: 10.3233/BPL-190083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dearborn-Tomazos JL, Wu A, Steffen LM, Anderson CAM, Hu EA, Knopman D, Mosley TH, Gottesman RF. Association of dietary patterns in midlife and cognitive function in later life in US adults without dementia. JAMA Netw Open. 2019;2:e1916641. doi: 10.1001/jamanetworkopen.2019.16641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. 2019;15:581–589. doi: 10.1016/j.jalz.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 83.Chlebowski RT, Rapp S, Aragaki AK, Pan K, Neuhouser ML, Snetselaar LG, Manson JE, Wactawski-Wende J, Johnson KC, Hayden K, et al. Low-fat dietary pattern and global cognitive function: exploratory analyses of the Women’s Health Initiative (WHI) randomized Dietary Modification trial. EClinicalMedicine. 2020;18:100240. doi: 10.1016/j.eclinm.2019.100240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Radd-Vagenas S, Duffy SL, Naismith SL, Brew BJ, Flood VM, Fiatarone Singh MA. Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am J Clin Nutr. 2018;107:389–404. doi: 10.1093/ajcn/nqx070 [DOI] [PubMed] [Google Scholar]
- 85.Dye L, Boyle NB, Champ C, Lawton C. The relationship between obesity and cognitive health and decline. Proc Nutr Soc. 2017;76:443–454. doi: 10.1017/S0029665117002014 [DOI] [PubMed] [Google Scholar]
- 86.Loef M, Walach H. Midlife obesity and dementia: meta-analysis and adjusted forecast of dementia prevalence in the United States and China. Obesity (Silver Spring). 2013;21:E51–E55. doi: 10.1002/oby.20037 [DOI] [PubMed] [Google Scholar]
- 87.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x [DOI] [PubMed] [Google Scholar]
- 88.Hovens IB, Dalenberg JR, Small DM. A brief neuropsychological battery for measuring cognitive functions associated with obesity. Obesity (Silver Spring). 2019;27:1988–1996. doi: 10.1002/oby.22644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Knopman DS, Gottesman RF, Sharrett AR, Tapia AL, DavisThomas S, Windham BG, Coker L, Schneider ALC, Alonso A, Coresh J, et al. Midlife vascular risk factors and midlife cognitive status in relation to prevalence of mild cognitive impairment and dementia in later life: the Atherosclerosis Risk in Communities Study. Alzheimers Dement. 2018;14:1406–1415. doi: 10.1016/j.jalz.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anstey KJ, Ashby-Mitchell K, Peters R. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J Alzheimers Dis. 2017;56:215–228. doi: 10.3233/JAD-160826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016:CD003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ott BR, Daiello LA, Dahabreh IJ, Springate BA, Bixby K, Murali M, Trikalinos TA. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2015;30:348–358. doi: 10.1007/s11606-014-3115-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Power MC, Rawlings A, Sharrett AR, Bandeen-Roche K, Coresh J, Ballantyne CM, Pokharel Y, Michos ED, Penman A, Alonso A, et al. Association of midlife lipids with 20-year cognitive change: a cohort study. Alzheimers Dement. 2018;14:167–177. doi: 10.1016/j.jalz.2017.07.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pan ML, Hsu CC, Chen YM, Yu HK, Hu GC. Statin use and the risk of dementia in patients with stroke: a nationwide population-based cohort study. J Stroke Cerebrovasc Dis. 2018;27:3001–3007. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.036 [DOI] [PubMed] [Google Scholar]
- 95.Zahr NM, Kaufman KL, Harper CG. Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol. 2011;7:284–294. doi: 10.1038/nrneurol.2011.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Letenneur L, Larrieu S, Barberger-Gateau P. Alcohol and tobacco consumption as risk factors of dementia: a review of epidemiological studies. Biomed Pharmacother. 2004;58:95–99. doi: 10.1016/j.biopha.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 97.Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, Shi J, Vitiello MV, Lu L. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. doi: 10.1016/j.smrv.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 98.Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastião YV, Wen Y, Schwartz S, Borenstein AR, Wu Y, Morgan D, et al. Sleep, cognitive impairment, and Alzheimer’s disease: a systematic review and meta-analysis. Sleep. 2017;40:zsw032. doi: 10.1093/sleep/zsw032 [DOI] [PubMed] [Google Scholar]
- 99.Culebras A, Anwar S. Sleep apnea is a risk factor for stroke and vascular dementia. Curr Neurol Neurosci Rep. 2018;18:53. doi: 10.1007/s11910-018-0855-1 [DOI] [PubMed] [Google Scholar]
- 100.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104 [DOI] [PubMed] [Google Scholar]
- 101.Bloom HG, Ahmed I, Alessi CA, Ancoli-Israel S, Buysse DJ, Kryger MH, Phillips BA, Thorpy MJ, Vitiello MV, Zee PC. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc. 2009;57:761–789. doi: 10.1111/j.1532-5415.2009.02220.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sundström A, Adolfsson AN, Nordin M, Adolfsson R. Loneliness Increases the risk of all-cause dementia and Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci. 2020;75:919–926. doi: 10.1093/geronb/gbz139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuiper JS, Zuidersma M, Oude Voshaar RC, Zuidema SU, van den Heuvel ER, Stolk RP Smidt N. Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2015;22:39–57. doi: 10.1016/j.arr.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 104.Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Tang Y, Bennett DA. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64:234–240. doi: 10.1001/archpsyc.64.2.234 [DOI] [PubMed] [Google Scholar]
- 105.Kelly ME, Duff H, Kelly S, McHugh Power JE, Brennan S, Lawlor BA, Loughrey DG. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: a systematic review. Syst Rev. 2017;6:259. doi: 10.1186/s13643-017-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bhatti AB, Haq AU. The pathophysiology of perceived social isolation: effects on health and mortality. Cureus. 2017;9:e994. doi: 10.7759/cureus.994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018;144:115–126. doi: 10.1001/jamaoto.2017.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Homans NC, Metselaar RM, Dingemanse JG, van der Schroeff MP, Brocaar MP, Wieringa MH, Baatenburg de Jong RJ, Hofman A, Goedegebure A. Prevalence of age-related hearing loss, including sex differences, in older adults in a large cohort study. Laryngoscope. 2017;127:725–730. doi: 10.1002/lary.26150 [DOI] [PubMed] [Google Scholar]
- 109.Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am J Psychiatry. 2018;175:215–224. doi: 10.1176/appi.ajp.2017.17040423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maharani A, Pendleton N, Leroi I. Hearing impairment, loneliness, social isolation, and cognitive function: longitudinal analysis using English Longitudinal Study on Ageing. Am J Geriatr Psychiatry. 2019;27:1348–1356. doi: 10.1016/j.jagp.2019.07.010 [DOI] [PubMed] [Google Scholar]
- 111.Dawes P. Hearing interventions to prevent dementia. HNO. 2019;67:165–171. doi: 10.1007/s00106-019-0617-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deal JA, Albert MS, Arnold M, Bangdiwala SI, Chisolm T, Davis S, Eddins A, Glynn NW, Goman AM, Minotti M, et al. A randomized feasibility pilot trial of hearing treatment for reducing cognitive decline: results from the Aging and Cognitive Health Evaluation in Elders Pilot Study. Alzheimers Dement (NY). 2017;3:410–415. doi: 10.1016/j.trci.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rubin R Exploring the relationship between depression and dementia. JAMA. 2018;320:961–962. doi: 10.1001/jama.2018.11154 [DOI] [PubMed] [Google Scholar]
- 114.Li JQ, Tan L, Wang HF, Tan MS, Tan L, Xu W, Zhao QF, Wang J, Jiang T, Yu JT. Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: a systematic review and meta-analysis of cohort studies. J Neurol Neurosurg Psychiatry. 2016;87:476–484. doi: 10.1136/jnnp-2014-310095 [DOI] [PubMed] [Google Scholar]
- 115.Singh-Manoux A, Dugravot A, Fournier A, Abell J, Ebmeier K, Kivimäki M, Sabia S. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry. 2017;74:712–718. doi: 10.1001/jamapsychiatry.2017.0660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang XJ, Xu W, Li JQ, Cao XP, Tan L, Yu JT. Early-life risk factors for dementia and cognitive impairment in later life: a systematic review and meta-analysis. J Alzheimers Dis. 2019;67:221–229. doi: 10.3233/JAD-180856 [DOI] [PubMed] [Google Scholar]
- 117.Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12:11–22. doi: 10.3233/jad-2007-12103 [DOI] [PubMed] [Google Scholar]
- 118.Starfield B Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992. [Google Scholar]
- 119.Maust DT, Solway E, Langa KM, Kullgren JT, Kirch M, Singer DC, Malani P. Perception of dementia risk and preventive actions among US adults aged 50 to 64 years. JAMA Neurol. 2020;77:259–262. doi: 10.1001/jamaneurol.2019.3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.US Preventive Services Task Force. Screening for high blood pressure: recommendations and rationale. Am J Prev Med. 2003;25:159–164.12880885 [Google Scholar]
- 121.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.US Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med.. 2003;139:930–932. [DOI] [PubMed] [Google Scholar]
- 123.US Preventive Services Task Force. Screening for depression in adults: US Preventive Services Task Force Recommendation. JAMA. 2016;315:380–387. doi: 10.1001/jama.2015.18392 [DOI] [PubMed] [Google Scholar]
- 124.Siu AL US Preventive Services Task Force. Screening for abnormal blood glucose and type 2 diabetes mellitus: U. S. Preventive Services Task Force Recommendaiton Statement. Ann Intern Med.. 2016;163:861–868. doi: 10.7326/M15-2345 [DOI] [PubMed] [Google Scholar]
- 125.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published corrections appear in Circulation. 2019;140:e649–e650e60, Circulation. 2020;141:e60, and Circulation. 2020;141:e774]. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Circulation. 2019;139:e1182–e1186]. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Hypertension. 2018; 71:e140–e144]. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 128.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D’Alessio DA, Davies MJ. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:487–493. doi: 10.2337/dci19-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.US Preventive Services Task Force. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force Recommendation Statement. JAMA 2018;320:1163–1171. [DOI] [PubMed] [Google Scholar]
- 130.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.US Preventive Services Task Force. Screening for obstructive sleep apnea in adults. US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:407–414. [DOI] [PubMed] [Google Scholar]
- 133.Moyer VA; U.S. Preventive Services Task Force. Screening for hearing loss in older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:655–661. doi: 10.7326/0003-4819-157-9-201211060-00526 [DOI] [PubMed] [Google Scholar]
- 134.Ftouh S, Harrop-Griffiths K, Harker M, Munro KJ, Leverton T; Guideline Committee. Hearing loss in adults, assessment and management: summary of NICE guidance. BMJ. 2018;361:k2219. doi: 10.1136/bmj.k2219 [DOI] [PubMed] [Google Scholar]
- 135.Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019;18:177–184. doi: 10.1016/S1474-4422(18)30371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bancks MP, Carnethon MR, Jacobs DR Jr, Launer LJ, Reis JP, Schreiner PJ, Shah RV, Sidney S, Yaffe K, Yano Y, et al. Fasting glucose variability in young adulthood and cognitive function in middle age: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes Care. 2018;41:2579–2585. doi: 10.2337/dc18-1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lane CA, Barnes J, Nicholas JM, Sudre CH, Cash DM, Parker TD, Malone IB, Lu K, James SN, Keshavan A, et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): an epidemiological study. Lancet Neurol. 2019;18:942–952. doi: 10.1016/S1474-4422(19)30228-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moyer VA; U.S. Preventive Services Task Force. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:791–797. doi: 10.7326/M14-0496 [DOI] [PubMed] [Google Scholar]
- 139.Hemmy LS, Linskens EJ, Silverman PC, Miller MA, Talley KMC, Taylor BC, Ouellette JM, Greer NL, Wilt TJ, Butler M, et al. Brief cognitive tests for distinguishing clinical Alzheimer-type dementia from mild cognitive impairment or normal cognition in older adults with suspected cognitive impairment. Ann Intern Med. 2020;172:678–687. doi: 10.7326/M19-3889 [DOI] [PubMed] [Google Scholar]
- 140.Stern Y, Lee S, Predovan D, Sloap RP. Sex moderates the effect of aerobic exercise on some aspects of cognition in cognitively intact younger and middle-age adults. J Clin Med. 2019;8:886. doi: 10.3390/jcm8060886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Beydoun MA, Fanelli-Kuczmarski MT, Kitner-Triolo MH, Beydoun HA, Kaufman JS, Mason MA, Evans MK, Zonderman AB. Dietary anti-oxidant intake and its association with cognitive function in an ethnically diverse sample of US adults. Psychosom Med. 2015;77:68–82. doi: 10.1097/PSY.0000000000000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koyama A, Houston DK, Simonsick EM, Lee JS, Ayonayon HN, Shahar DR, Rosano C, Satterfield S, Yaffe K. Association between the Mediterranean diet and cognitive decline in a biracial population. J Gerontol A Biol Sci Med Sci. 2015;70:354–359. doi: 10.1093/gerona/glu097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tuckson RV, Edmunds M, Hodgkins ML. Telehealth. N Engl J Med. 2017;377:1585–1592. doi: 10.1056/NEJMsr1503323 [DOI] [PubMed] [Google Scholar]
- 144.McManus RJ, Mant J, Franssen M, Nickless A, Schwartz C, Hodgkinson J, Bradburn P, Farmer A, Grant S, Greenfield SM, et al. ; TASMINH4 investigators. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949–959. doi: 10.1016/S0140-6736(18)30309-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Unützer J, Katon W, Callahan CM, Williams JW Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noël PH, Lin EH, et al. ; IMPACT Investigators. Improving Mood-Promoting Access to Collaborative Treatment. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836 [DOI] [PubMed] [Google Scholar]
- 146.McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, Jones MI, Jowett S, Little P, Penaloza C, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014;312:799–808. doi: 10.1001/jama.2014.10057 [DOI] [PubMed] [Google Scholar]
- 147.McAlister FA, Majumdar SR, Padwal RS, Fradette M, Thompson A, Buck B, Dean N, Bakal JA, Tsuyuki R, Grover S, et al. Case management for blood pressure and lipid level control after minor stroke: PREVENTION randomized controlled trial. CMAJ. 2014;186:577–584. doi: 10.1503/cmaj.140053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, Carrell D, Tyll L, Larson EB, Thompson RS. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008;299:2857–2867. doi: 10.1001/jama.299.24.2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hawks L, Himmelstein DU, Woolhandler S, Bor DH, Gaffney A, McCormick D. Trends in unmet need for physician and preventive service in the United States 1998–2017. JAMA Intern Med. 2020;180:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180:463–466. doi: 10.1001/jamainternmed.2019.6282 [DOI] [PMC free article] [PubMed] [Google Scholar]