Abstract
Consciousness is a fascinating field of neuroscience research where questions often outnumber the answers. We advocate an open and optimistic approach where converging mechanisms in neuroscience may eventually provide a satisfactory understanding of consciousness. We first review several characteristics of conscious neural activity, including the involvement of dedicated systems for content and levels of consciousness, the distinction and overlap of mechanisms contributing to conscious states and conscious awareness of transient events, nonlinear transitions and involvement of large-scale networks, and finally the temporal nexus where conscious awareness of discrete events occurs when mechanisms of attention and memory meet. These considerations and recent new experimental findings lead us to propose an inclusive hypothesis involving four phases initiated shortly after an external sensory stimulus: (1) Detect—primary and higher cortical and subcortical circuits detect the stimulus and select it for conscious perception. (2) Pulse—a transient and massive neuromodulatory surge in subcortical-cortical arousal and salience networks amplifies signals enabling conscious perception to proceed. (3) Switch—networks that may interfere with conscious processing are switched off. (4) Wave—sequential processing through hierarchical lower to higher cortical regions produces a fully formed percept, encoded in frontoparietal working memory and medial temporal episodic memory systems for subsequent report of experience. The framework hypothesized here is intended to be nonexclusive and encourages the addition of other mechanisms with further progress. Ultimately, just as many mechanisms in biology together distinguish living from nonliving things, many mechanisms in neuroscience synergistically may separate conscious from nonconscious neural activity.
Keywords: consciousness, thalamus, mechanisms, attention, subcortical, memory
Introduction
Most people agree that it makes a difference whether you are conscious or not. However, there is wide disagreement about what exactly consciousness is and how to explain it. Some argue that truly understanding consciousness depends on internal experiences, which cannot be fully explained through scientific approaches that inherently rely on external observation. Nevertheless, here we will assume that neuroscience can provide valuable insights into the nature of consciousness. Like other challenging topics in science, such as the definition of life, we will approach consciousness by describing its important properties and characteristics rather than by striving for a concise or simple single definition. It is perhaps exactly this richness and beautiful complexity, shared with other important biological phenomena, that creates an intuition that simple or dry explanations of consciousness are unacceptable. Consciousness is not simple but neither are neural circuits or neurons. Hopefully, increased understanding and appreciation of the complex, elegant neural mechanisms that may contribute synergistically will ultimately lead to a satisfactory neuroscientific explanation for consciousness.
Many theories have been offered to explain consciousness, discussed in detail elsewhere and which we will not review here (Dehaene 2014; Koch and others 2016; Lamme 2006; Lau and Rosenthal 2011; Tononi and others 2016). Instead, we will build on previous work to offer a new hypothesis aimed at synthesizing diverse mechanisms in neuroscience that together may produce consciousness. This account is not exclusive; rather, we posit—again like the biological study of life itself—that consciousness is best understood through a synergistic combination of multiple complex mechanisms rather than through a single simple mechanism. We will first discuss several important properties and characteristics of consciousness; then we present the detect, pulse, switch, and wave hypothesis to unify these characteristics in the context of recent experimental findings; before finally turning to future directions and unanswered questions.
Content of Consciousness, Level of Consciousness, and the Consciousness System
Consciousness was described by the neurologists Plum and Posner (1972) as having two components:
Content of consciousness
Level of consciousness
The content of consciousness is the substrate on which consciousness acts and includes hierarchically organized sensory, motor, emotional, and mnemonic systems working at multiple levels. The content of consciousness comprises most systems investigated by neuroscience, including sensory and motor pathways, memory, emotions and drives, language, executive function, visual or other sensory processing, motor planning, and so on (Fig. 1). Each of the individual contents of consciousness can be affected by focal dysfunction that selectively disrupts a specific module while leaving other functions relatively intact (Heilman and Valenstein 2003; Mesulam 2000). For example, if visual pathways are damaged, a person may have impaired visual consciousness but can continue to have normal experiences in other domains. If someone has damage to the language networks, their consciousness of language may be altered while other nonverbal experiences continue unhampered.
Figure 1.
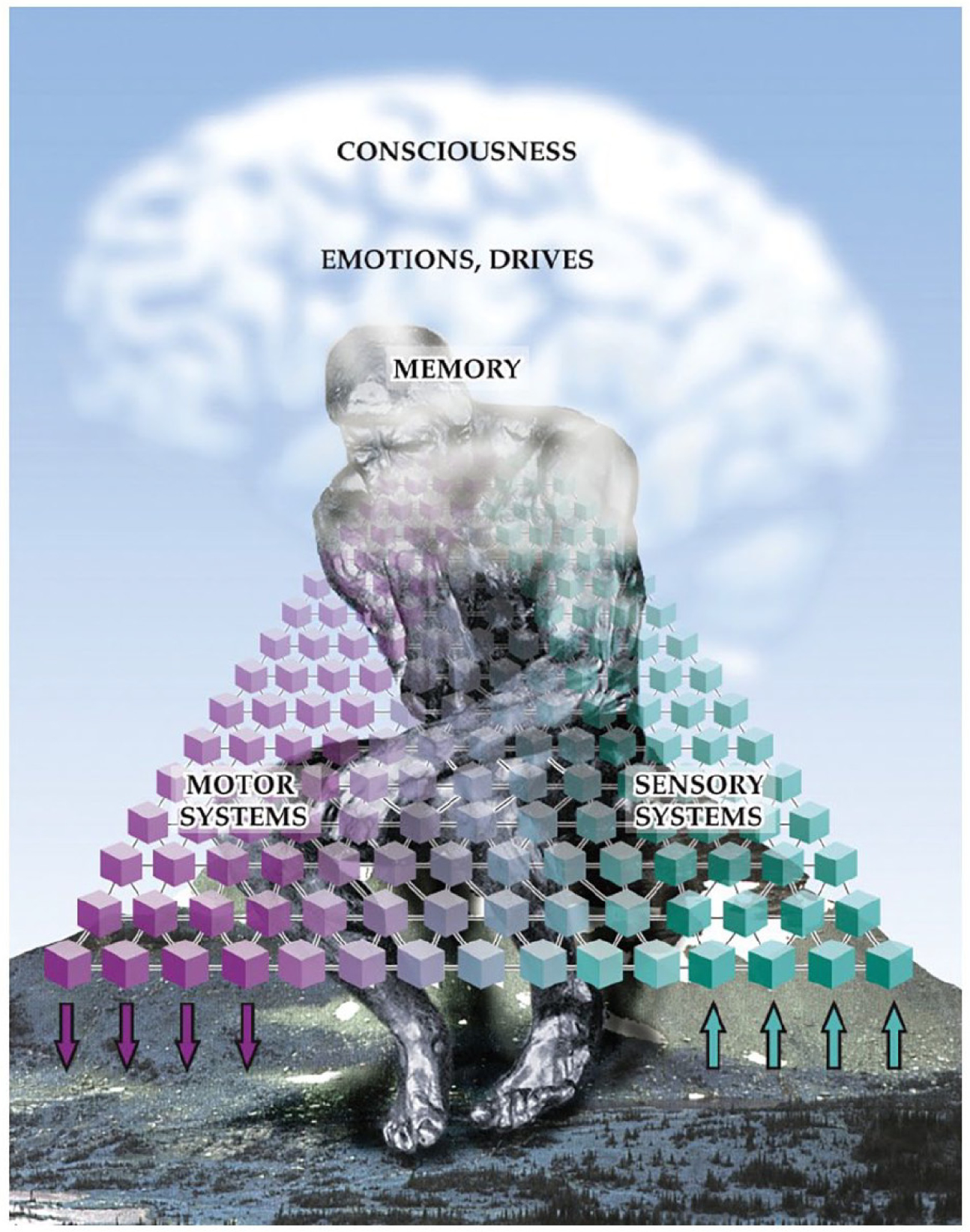
Brain systems for content and level of consciousness. The content or substrate of consciousness is provided by hierarchically organized sensory, motor systems, and cognitive systems, and by brain systems for memory, emotions, and drives. The level of consciousness acts on all of these different modules and is regulated by the consciousness system (see Fig. 2). Reproduced with permission from Blumenfeld (2022).
In contrast, neural systems that control the level of consciousness often act on many of the contents of consciousness together, producing more global effects on consciousness (Giacino and others 2018; Laureys and others 2015; Posner and others 2019; Schiff 2008). We refer to the specialized cortical and subcortical brain networks that regulate the level of consciousness as the consciousness system, in analogy to other major functional systems in the brain, such as the motor system, somatosensory system, and so on (Blumenfeld 2009; Fig. 2). The consciousness system controls the level of consciousness and therefore regulates access to all of the contents of consciousness. This occurs through three distinct but related processes that maintain (1) alertness, (2) attention, and (3) awareness of self and environment (mnemonic AAA). Consciousness system networks that control these functions include the upper brainstem, thalamic, hypothalamic, and basal fore-brain arousal systems (Li and others 2021; McCormick and others 2015; Motelow and Blumenfeld 2014; Parvizi and Damasio 2001; Redinbaugh and others 2020; Saper and others 2001; Steriade and McCarley 2010), along with the medial and lateral higher-order association cortex (Corbetta and Shulman 2002; Dosenbach and others 2008; Menon and Uddin 2010; Raichle and Snyder 2007; Seeley and others 2007; see Fig. 2). Other structures such as the claustrum, basal ganglia, nucleus accumbens, and cerebellum may also play a role (Crick and Koch 2005; Li and others 2021; Middleton and Strick 2000; Schiff 2010; Schmahmann 2019). The diverse cortical and subcortical networks of the consciousness system overlap in their contributions to alertness, attention, and awareness.
Figure 2.
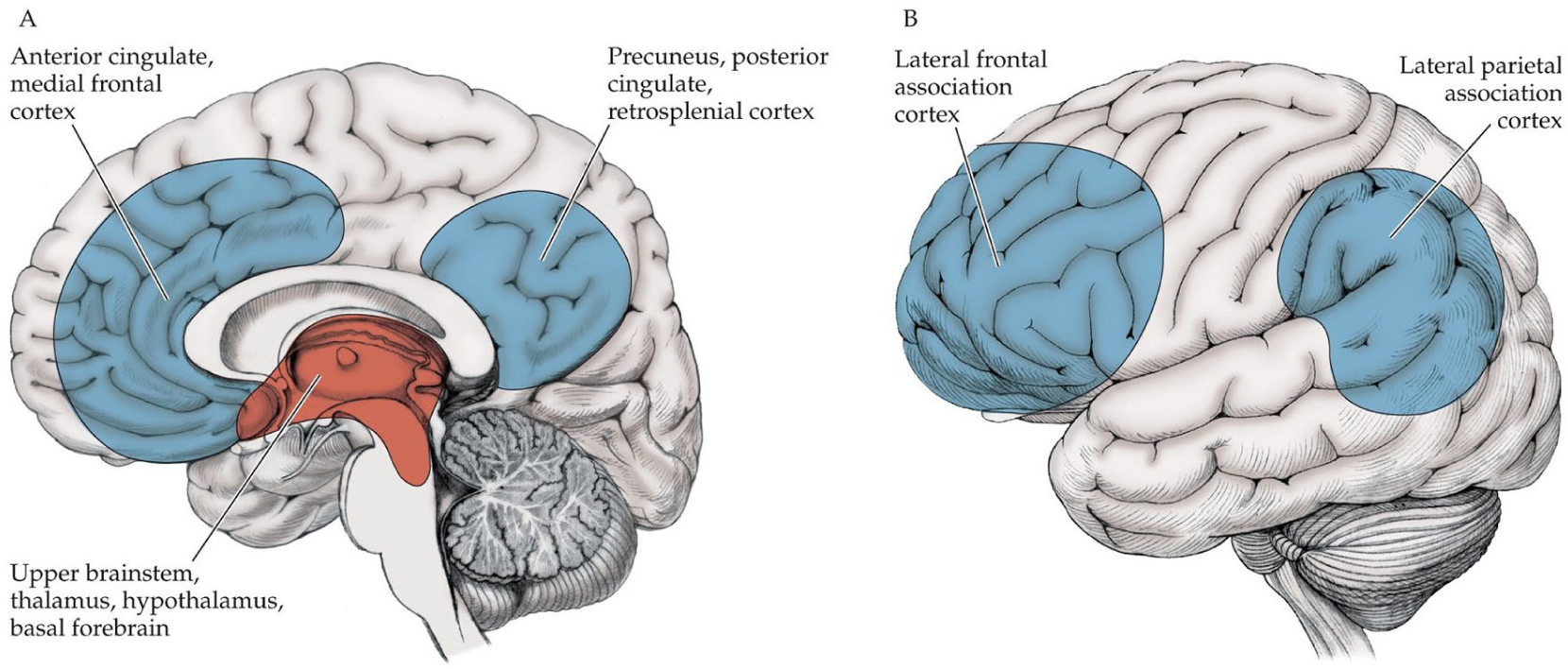
The consciousness system. Anatomical structures involved in regulating the level of alertness, attention, and awareness. (A) Medial view showing cortical (blue) and subcortical (red) components of the consciousness system. (B) Lateral cortical components of the consciousness system. Note that other circuits not pictured here, such as the basal ganglia, nucleus accumbens, claustrum, and cerebellum, may also play a role in attention and other aspects of consciousness. Reproduced with permission from Blumenfeld (2022).
Although the neuroanatomy of consciousness is relatively well known (Blumenfeld 2015; Fig. 2), the neuro-physiological mechanisms that generate consciousness remain poorly understood. To understand the physiology of consciousness, and how the neuroanatomical components of the consciousness system contribute, it is necessary to consider how consciousness occurs over time.
Conscious States and Conscious Events
Conscious states are situations where a particular level of consciousness lasts for a relatively long period of time. Different conscious states include sleep, wake, drowsiness, heightened vigilance, or disorders of consciousness such as coma, minimally conscious state, or epileptic seizures. Conscious states depend on cortical and subcortical consciousness system networks that control level of consciousness over extended time periods (see Fig. 2). In contrast, in conscious events there is briefer conscious awareness, usually of a specific sensation, action, thought, or combination of these to form an experience. Recent work suggests that the same cortical-subcortical consciousness system networks known to regulate level of consciousness on longer timescales in conscious states also dynamically control level of consciousness on much shorter timescales during brief conscious events (Chica and others 2016; Kronemer and others 2021; Li and others 2021; Sarter and Lustig 2019; Schiff and others 2013; Warren and others 2015).
Consciousness Is Nonlinear and Involves Large-Scale Brain Networks
Two other important properties of consciousness are nonlinearity and the involvement of large-scale neural networks in the brain. Nonlinear transitions between conscious states are common, as we all experience when abruptly returning to a more alert state while drowsing during a lecture, or as occurs in people with epilepsy where the severity of impaired consciousness during seizures tends to be bimodally distributed. There are of course gradations in level of consciousness, and consciousness is not a strictly all-or-none phenomenon; however, transitions in level of consciousness between states tend to occur in a nonlinear manner (Cunningham and others 2014; Del Cul and others 2007; Saper and others 2001). Similarly, nonlinear processes are important for experience of conscious events. Interesting recent work from numerous labs has demonstrated that although neural responses at lower levels tend to be graded more linearly in response to stimulus strength (e.g., signals in primary visual cortex), subsequent higher-order neural responses are highly nonlinear (Del Cul and others 2007; Fisch and others 2009; Herman and others 2019; Noy and others 2015; van Vugt and others 2018). This produces nonlinear signals in higher-order cortical areas that are massively amplified for consciously perceived versus nonperceived events. In addition, both conscious states and conscious events involve large-scale neural networks in the brain. Many converging studies of normal consciousness as well as disorders of consciousness have shown that consciousness is “big” in the brain, meaning that when consciousness occurs it usually involves widely distributed cortical and subcortical networks in both hemispheres (Baars 2005; Dehaene 2014; Koch 2004; Laureys and others 2015; Posner and others 2019; Schiff 2008; Steriade and McCarley 2005; Tononi and others 2016). This broad network involvement of consciousness is in keeping with its crucial functional importance and survival benefits, providing robustness and redundancy that may have evolutionary advantages.
The Timeline of Consciousness
When people use the word “conscious” in common speech, they usually mean either (1) not unconscious (i.e., not in a coma or dreamless sleep) or (2) conscious awareness of something. The mechanisms that prevent unconsciousness and maintain a normal alert state of arousal are less controversial (Posner and others 2019; Steriade and McCarley 2010). Therefore, we will now specifically discuss the more elusive mechanisms of conscious awareness in greater detail. We will focus on conscious awareness of events because these have been most actively studied. Although in reality conscious awareness may occur on more of a continuum (Huk and others 2018), and it is possible to be consciously aware of things that do not occur at a particular moment in time, awareness of discrete events is much easier to study experimentally. Conscious awareness of events may thus serve as the “E. coli of consciousness research,” providing a simple model system, with outcomes extrapolated and interpreted more widely, although all appropriate precautions should be preserved for such a simplified model.
Conscious events can be described as occurring on a timeline of consciousness (Fig. 3). Several investigators have recently distinguished between neural mechanisms contributing to the conscious event itself and other mechanisms that may precede or follow it but are not, strictly speaking, part of consciousness (Aru and others 2012; Pitts and others 2014a; Tsuchiya and others 2015). We will use the example of a brief external stimulus, such as the transient appearance of a face in the visual field (represented by the red arrow at time 0 in Fig. 3), to discuss these concepts. Important precursors or prerequisites of consciousness may contribute to whether or not an event reaches conscious awareness. These precursors of consciousness include level of alertness and arousal, attentional vigilance or anticipation, previous experiences related to an upcoming event, motivational state, and the phase of brain oscillations such as the alpha rhythm (Boly and others 2007; Gonzalez-Garcia and others 2018; Libet and others 1983; Mathewson and others 2009; Palva and others 2005; Sadaghiani and others 2009; Wyart and Tallon-Baudry 2009). Following the conscious event, a number of consequences of consciousness can occur (see Fig. 3). These include so-called postperceptual processing, encoding of information into memory systems, and preparation for subsequent report or description of the experience at a later time (Aru and others 2012; Pitts and others 2014a; Tsuchiya and others 2015). Between the precursors and consequences of consciousness lies the conscious event itself, probably lasting somewhere between 200 and 300 ms (the exact duration is controversial). The conscious event is represented in Figure 3 by a green box instead of the famous black box in the hopeful spirit that it can eventually be explained based on biology.
Figure 3.
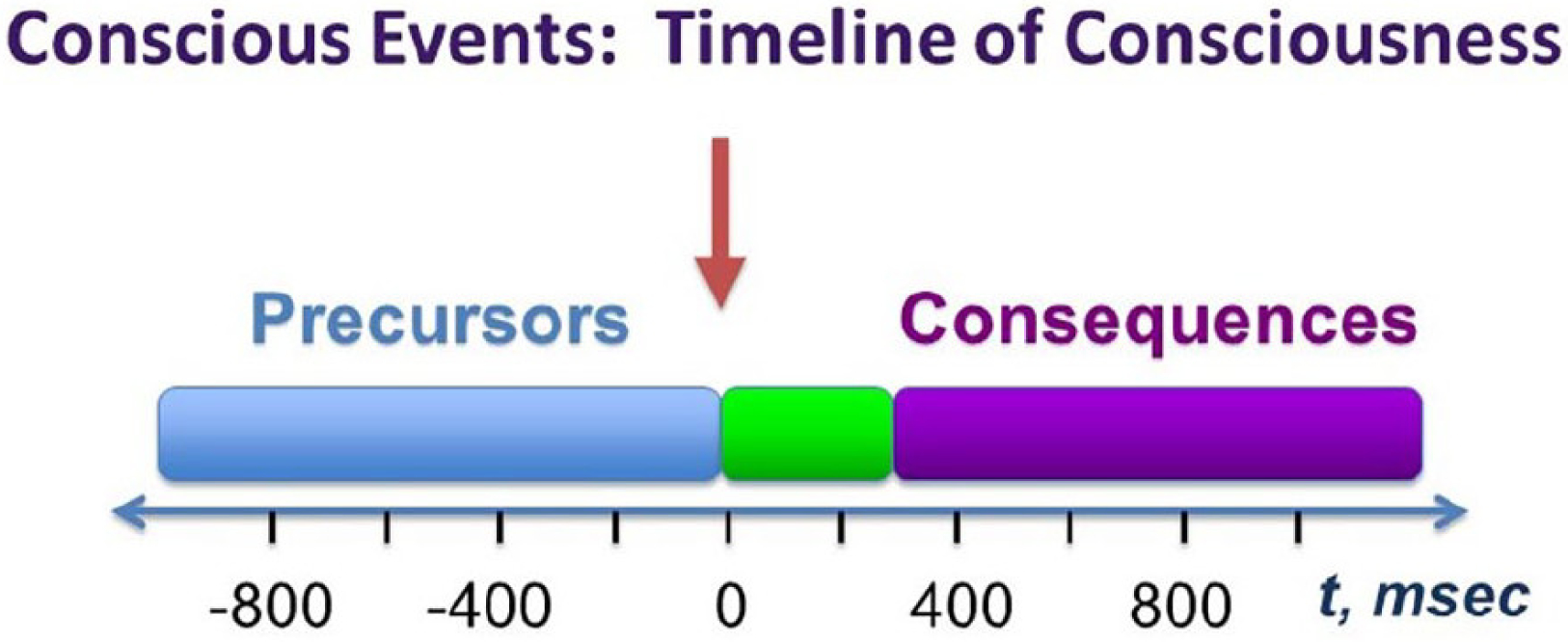
The timeline of consciousness. An event such as a brief external stimulus is indicated by the vertical arrow at time zero. Neural precursors of consciousness are shown in blue, and consequences of consciousness in purple, while the neural mechanisms of the conscious event itself are shown in green. Reproduced with permission from Blumenfeld (2022).
Attention, Memory, and Conscious Awareness
Let us try and open the green box. We will again use the brief appearance of a visual stimulus at time zero as an example of a conscious event. However, the same concepts could be applied to any external sensory (visual, auditory, tactile, etc.) stimulus, as well as to a motor action, or to an internal thought, memory, or feeling, any of which could serve as the contents of consciousness (see Fig. 1). As we have discussed, arousal and attention are likely necessary, but are perhaps not sufficient for conscious awareness. Along the timeline of consciousness (see Fig. 3), attentional engagement is needed at the time of the stimulus for a visual event to reach conscious awareness (Simons and Chabris 1999). In addition to attention, memory provides important clues about the mechanisms of consciousness. For a conscious event to be used for later cognitive operations it must enter dorsal frontoparietal working memory areas (Carver and others 2018; Goldman-Rakic 1996; Persuh and others 2018). Furthermore, to be available for later report as an episodic declarative memory or experience it must reach the medial temporal memory circuits (Hassabis and others 2007; Squire and Wixted 2011; Tulving 2002). Thus, memory systems must be engaged during or toward the end of the conscious event (see Fig. 3). The green box or conscious event is thus at the nexus or temporal transition between attention and memory systems—or, in short, consciousness is where attention meets memory.
However, not all conscious events are in fact remembered. It is possible to be consciously aware of something one moment but to not remember it later either due to amnesia or simply due to normal forgetting. Therefore, consciousness is not the same as memory encoding, but rather may represent the stage just prior to memory encoding, where a potential memory reaches the gateway to memory systems in regions such as the medial temporal lobe. Similarly, it is possible to engage attentional mechanism without forming a conscious experience. For example, some actions involving substantial attention such as driving a car can occur largely automatically, where multiple attended events occur that do not become part of consciousness. Consciousness awareness is, therefore, not identical to attention. Additional neural ingredients are required for an event that reaches attention to also reach conscious awareness.
Detect, Pulse, Switch, and Wave
What happens during the transition from attention to memory? We will now present a new framework for investigating conscious events, called the detect, pulse, switch, and wave hypothesis (Fig. 4). The present hypothesis builds on and incorporates previous theories and findings together with recent work on the neuroscience of consciousness from my laboratory. Returning to the example of a brief visual stimulus at time 0, shortly after the stimulus (<100 ms), primary cortices are activated, specifically visual cortex in this case. To detect the stimulus, signals interact with higher cortical areas including the frontal eye fields and other frontoparietal regions (Bisley and Goldberg 2010; Gregoriou and others 2009; Thompson and Schall 1999) as well as subcortical areas such as the midbrain tectum (Bollimunta and others 2018; Knudsen 2011. see Fig. 4, “Detect”). We recently found support for a rapid early cortical detection network based on human electrocorticography analysis with both visual and auditory stimuli (Christison-Lagay and others 2018; Herman and others 2019; Khalaf and others 2021 [unpublished data]; Kwon and others 2021).
Figure 4.
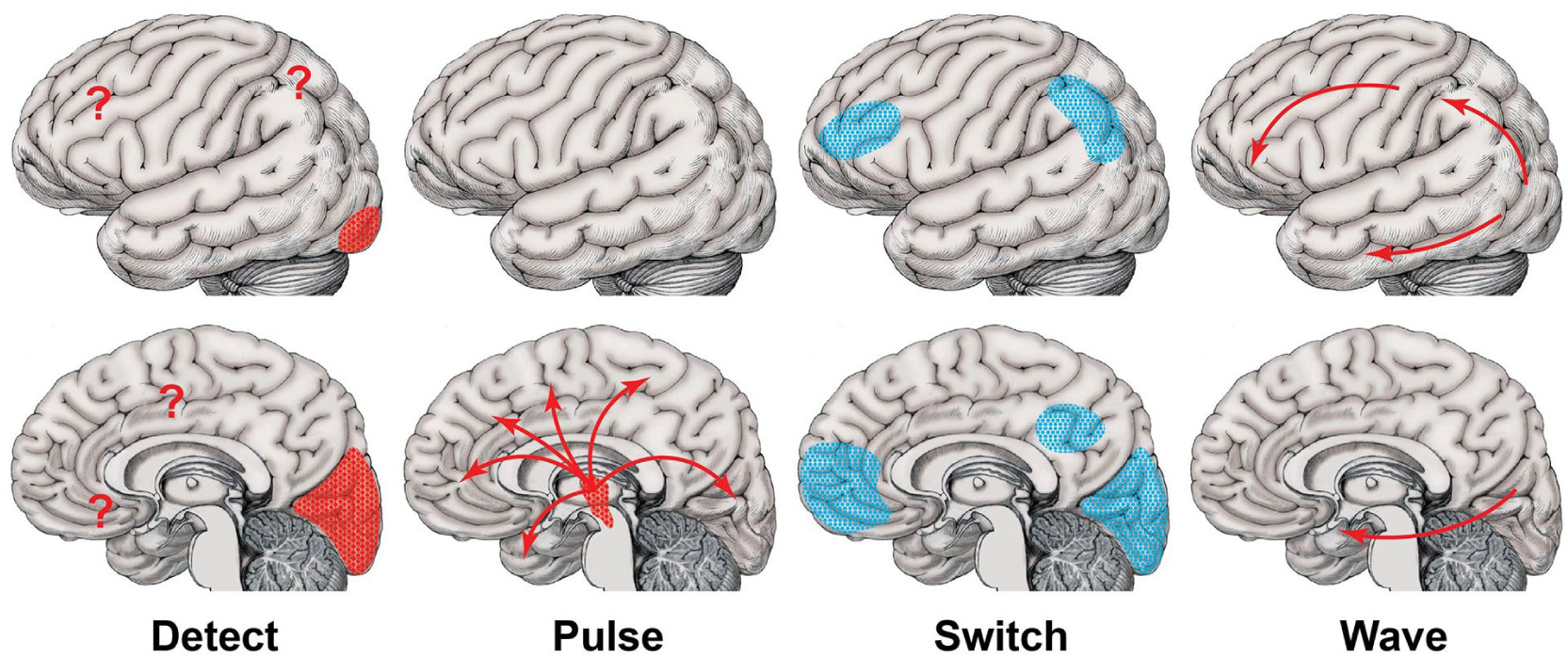
Detect, Pulse, Switch, and Wave hypothesis for consciousness. Sequence of neural mechanisms proposed to produce conscious awareness of events, using an external visual stimulus activating primary visual cortex as an example. Reproduced with permission from Blumenfeld (2022).
Consciously detected stimuli trigger the next three steps within 200 to 300 ms. In the first of these steps, subcortical arousal systems provide a dynamic transient pulse that facilitates subsequent massive nonlinear cortical signals necessary for consciousness (see Fig. 4, “Pulse”). As we have already discussed, other theories endorse nonlinear cortical “ignition” in conscious perception; however, subcortical arousal systems are usually given a slower, permissive role in sustained attention. In contrast, we hypothesize that a transient early subcortical arousal pulse or neuromodulatory surge enhances cortical networks dynamically, facilitating and amplifying rapid and widespread brain involvement in consciousness. We recently found evidence supporting an early transient subcortical-cortical arousal pulse in human visual perception based on both fMRI (functional magnetic resonance imaging) and direct recordings from the human thalamus (Christison-Lagay and others 2018; Kronemer and others 2021; Li and others 2021). Note that the “detect” and “pulse” steps overlap bottom-up subcortical and cortical salience networks previously described for attention (Kinomura and others 1996; Menon and Uddin 2010; Sarter and Lustig 2019; Schiff and others 2013; Seeley and others 2007).
Next, there is a switch off of activity in specific networks to prevent them from interfering with ongoing processing of the conscious stimulus, in this case including primary visual cortex, frontoparietal detection network areas, and the default mode network (see Fig. 4, “Switch”). We hypothesize that such decreases in activity may “chunk” conscious events into discrete moments of time, or serve other functions to selectively control or focus information flow. For example, decreased activity is well known to occur in default mode networks at times when internal attention is switched off and external attention is switched on (Herman and others 2019; Li and others 2019; Miller and others 2009; Ossandón and others 2011; Raichle and Snyder 2007; Singh and Fawcett 2008). In addition to sustained decreases in default mode network activity, we recently found that consciously perceived visual stimuli produce a transient decrease in primary visual cortex as well as in higher order detection networks at about 350 ms after stimulus presentation (Herman and others 2019). The timing of these decreases coincides with the attentional blink or psychological refractory period, and may thus contribute to limiting potentially distracting inputs during conscious processing (Dux and Marois 2009; Hindi Attar and others 2010; Li and others 2013; Sergent and others 2005).
Finally, a broad wave of hierarchical processing sweeps through lower to higher cortical areas (including the dorsal and ventral visual streams in this case) to fully process the event before it is encoded in frontoparietal working memory and medial temporal episodic memory systems (Fig. 4, “Wave”). The earlier portions of the wave may be considered part of the conscious event itself; however, the continued postperceptual processing needed for full memory encoding and preparation for subsequent report blends into the later consequences of consciousness (Fig. 3). We found a wave of increased cortical activity sweeping from back to front through hierarchically organized association cortical networks at a rate of approximately 150 mm/s based on intracranial EEG (electroencephalography) recordings with consciously perceived visual stimuli (Herman and others 2019). A similar forward-sweeping wave of activity was also observed with conscious perception of auditory stimuli (Christison-Lagay and others 2018). In both studies, the wave was not observed with identical visual or auditory stimuli that were not consciously perceived. Interestingly, we found that some of the wave activity in frontoparietal networks was sustained long after the stimulus ended (Herman and others 2019). Recent work with fMRI and EEG using report-independent paradigms for testing conscious perception suggests that the early portions of the wave are related to consciousness, while the later portions of the wave are involved in postperceptual processing (Kronemer and others 2021; Pitts and others 2014b).
Unanswered Questions and Future Work
The detect, pulse, switch and wave hypothesis is intended to summarize several key mechanisms along the timeline of consciousness, filling in the gap between precursors and consequences of consciousness with a plausible sequence of neural events. The hypothesis fulfills the characteristics of consciousness we outlined, including dedicated systems contributing the contents of consciousness for specific modalities such as vision, hearing, action, and so on (Fig. 1); more general systems controlling the level of consciousness via the consciousness system (Fig. 2); dynamic moment-to-moment control of the level of consciousness allowing conscious awareness of discrete events; nonlinear transitions and amplification leading to involvement of large-scale brain networks; and a rapid sequence of neural mechanisms within the “green box” on the timeline of consciousness (Figs. 3 and 4) allowing attention to meet memory.
Although detailed and testable hypotheses of this kind go a long way toward explaining mechanisms of conscious awareness, many important questions remain. For example, the mechanisms of the crucial bifurcation where an event either does or does not enter consciousness require much additional investigation. Interestingly, for a brief time after events occur they may potentially still enter consciousness if attention is refocused on them (Sperling 1960). This creates intriguing questions about when exactly consciousness happens, and what subset of information in the brain at any moment represents consciousness versus nonconscious material, a matter of ongoing debate (Aru and Bachmann 2017; Mack and others 2018).
A related question is whether or not some form of backward reflection or metacognition is needed for consciousness (Fleming and others 2012; Mazor and others 2020). Some posit that consciousness requires the construction of an informational model or attribution of attention to oneself or to others (Graziano 2020; Wilterson and others 2020). In some ways the question is a definitional one, similar to the famous question or whether a tree falling in the forest with no one around to hear it makes a sound. Does conscious awareness require awareness of that awareness, or not? Again timing may be highly relevant to this question. Simple awareness presumably occurs closer to the event, whereas awareness of awareness may occur with some delay.
The role of emotional valence and value, perhaps participating in the modulatory pulse or other steps of the hypothesis described above, also requires extensive additional investigation. For example, recent work has demonstrated involvement of the nucleus accumbens in conscious visual perception (Kronemer and other 2021). Similar to the role of emotion and motivation in attention, limbic contributions may help determine what matters, is important, and therefore enters consciousness (Damasio and Damasio 2021; Romer Thomsen and others 2011).
The “binding problem” is an important unsolved issue in consciousness research, asking how, for example, when you hold an orange, disparate aspects, such as the color, feel, smell, location, and time, are bound together to form a unified experience (Singer 2001). It is possible that aspects of the switch and wave involving higher cortical and medial temporal systems described above may contribute to binding, but the details require much further research. Some clues may come from clinical disorders such as Balint’s syndrome where bilateral dorsolateral parietal damage fractures the normal binding of visual experience into smaller disconnected fragments (Barton 2011).
Another important question is how mechanisms of the kind described here can create a vivid, subjective, and personal (“first-person” perspective) experience of awareness, both of self and of the external world (Blanke and others 2015; Lou and others 2017; Schaefer and Northoff 2017). Again, some clues may come from clinical disorders such as the hemineglect syndrome, where damage—most commonly in the right parietal lobe—may cause individuals to be unaware of the entire left side of the universe, including the left side of their own self (Heilman and others 2000).
Although the timeline of consciousness is a useful heuristic for brief external events, especially in the sensory domain, the timing and mechanisms of consciousness become more complicated when one considers (1) awareness of internal thoughts where the timing of the event is less clear; (2) awareness of motor actions in which some aspects of consciousness may occur both before and after the external event; and (3) more continuous naturalistic experiences where timing is less clear in contrast to the discrete and simple single events often studied experimentally.
Advances in many fields will contribute to answering these questions in the years ahead. Insights will come from exciting progress in the neuroscience of attention, memory, perception, arousal, emotion, motivation, decision making, executive function, mental imagery, and other related topics, including research directly aimed at studying consciousness. Much of neuroscience might be considered “covert consciousness research,” because some investigators prefer to avoid the term consciousness, yet still contribute in important ways to this fascinating field of research. As we have discussed, because consciousness is so fundamentally important, it is likely that a convergence of multiple robust neural mechanisms work together to provide consciousness rather than a single mechanism. By continuing to pursue better understanding of the brain we will hopefully draw closer to explaining the relationship between brain activity and conscious thought, which is one of the great remaining mysteries of modern science.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH/NINDS UG3/UH3 NS112826, R37 NS100901, R01 NS066974, R01 NS096088, R01 NS055829, by the Loughridge Williams Foundation and by the Betsy and Jonathan Blattmachr family.
Footnotes
Portions of the study and figures for this article were reproduced or modified with permission from Blumenfeld (2022).
Declaration of Conflicting Interests
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Aru J, Bachmann T. 2017. Expectation creates something out of nothing: the role of attention in iconic memory reconsidered. Conscious Cogn 53:203–10. [DOI] [PubMed] [Google Scholar]
- Aru J, Bachmann T, Singer W, Melloni L. 2012. Distilling the neural correlates of consciousness. Neurosci Biobehav Rev 36:737–46. [DOI] [PubMed] [Google Scholar]
- Baars BJ. 2005. Global workspace theory of consciousness: toward a cognitive neuroscience of human experience. Prog Brain Res 150:45–53. [DOI] [PubMed] [Google Scholar]
- Barton JJ. 2011. Disorders of higher visual processing. Handb Clin Neurol 102:223–61. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. 2010. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Slater M, Serino A. 2015. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron 88:145–66. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H 2009. Epilepsy and consciousness. In: Laureys S, Tononi G, eds. The neurology of consciousness: cognitive neuroscience and neuropathology . Academic Press. p. 15–30. [Google Scholar]
- Blumenfeld H 2015. Neuroanatomical basis of consciousness. In: Gosseries O, Laureys S, Tononi G, eds. The neurology of consciousness. 2nd ed. Elsevier. [Google Scholar]
- Blumenfeld H 2022. Neuroanatomy through clinical cases. 3rd ed. Sinauer Associates, Oxford University Press. [Google Scholar]
- Bollimunta A, Bogadhi AR, Krauzlis RJ. 2018. Comparing frontal eye field and superior colliculus contributions to covert spatial attention. Nat Commun 9:3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, and others. 2007. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci U S A 104:12187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver FW, Rubinstein DY, Gerlich AH, Fradkin SI, Holroyd T, Coppola R. 2018. Prefrontal high gamma during a magnetoencephalographic working memory task. Hum Brain Mapp 40:1774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica AB, Bayle DJ, Botta F, Bartolomeo P, Paz-Alonso PM. 2016. Interactions between phasic alerting and consciousness in the fronto-striatal network. Sci Rep 6:31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christison-Lagay KL, Micek C, Kronemer SI, Forman S, Aksen M, Abdel-Aty A, and others. 2018. Investigating auditory conscious perception with a threshold task and intracranial EEG. Soc Neurosci Abstracts 2018, Abstract No. 78907. Available from: https://www.abstractsonline.com/pp8/#!/4649/presentation/5352 [Google Scholar]
- Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–15. [DOI] [PubMed] [Google Scholar]
- Crick FC, Koch C. 2005. What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci 360:1271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Chen WC, Shorten A, McClurkin M, Choezom T, Schmidt CP, and others. 2014. Impaired consciousness in partial seizures is bimodally distributed. Neurology 82:1736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Damasio H. 2021. Feeling & knowing: making minds conscious. 1st ed. Pantheon Books. [DOI] [PubMed] [Google Scholar]
- Dehaene S 2014. Consciousness and the brain: deciphering how the brain codes our thoughts. Viking Adult. [Google Scholar]
- Del Cul A, Baillet S, Dehaene S. 2007. Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol 5:e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. 2008. A dual-networks architecture of top-down control. Trends Cogn Sci 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Marois R. 2009. The attentional blink: a review of data and theory. Attention, Perception Psychophysics 71:1683–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch L, Privman E, Ramot M, Harel M, Nir Y, Kipervasser S, and others. 2009. Neural “ignition”: enhanced activation linked to perceptual awareness in human ventral stream visual cortex. Neuron 64:562–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Dolan RJ, Frith CD. 2012. Metacognition: computation, biology and function. Philos Trans R Soc Lond B Biol Sci 367:1280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JT, Katz DI, Schiff ND, Whyte J, Ashman EJ, Ashwal S, and others. 2018. Comprehensive systematic review update summary: disorders of consciousness: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch Phys Med Rehabil 99:1710–9. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. 1996. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A 93:13473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garcia C, Flounders MW, Chang R, Baria AT, He BJ. 2018. Content-specific activity in frontoparietal and default-mode networks during prior-guided visual perception. Elife 7:e36068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MSA. 2020. Consciousness and the attention schema: why it has to be right. Cogn Neuropsychol 37:224–33. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. 2009. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324:1207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. 2007. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci U S A 104:1726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. 2003. Clinical neuropsychology,.4th ed. Oxford University Press. [Google Scholar]
- Heilman KM, Valenstein E, Watson RT. 2000. Neglect and related disorders. Semin Neurol 20:463–70. [DOI] [PubMed] [Google Scholar]
- Herman WX, Smith RE, Kronemer SI, Watsky RE, Chen WC, Gober LM, and others. 2019. A switch and wave of neuronal activity in the cerebral cortex during the first second of conscious perception. Cereb Cortex 29:461–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindi Attar C, Andersen SK, Muller MM. 2010. Time course of affective bias in visual attention: convergent evidence from steady-state visual evoked potentials and behavioral data. Neuroimage 53:1326–33. [DOI] [PubMed] [Google Scholar]
- Huk A, Bonnen K, He BJ. 2018. Beyond trial-based paradigms: continuous behavior, ongoing neural activity, and natural stimuli. J Neurosci 38:7551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas B, Roland PE. 1996. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 271:512–5. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. 2011. Control from below: the role of a midbrain network in spatial attention. Eur J Neurosci 33:1961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C 2004. The quest for consciousness: a neurobiological approach. Roberts and Co. [Google Scholar]
- Koch C, Massimini M, Boly M, Tononi G. 2016. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci 17:307–21. [DOI] [PubMed] [Google Scholar]
- Kronemer SI, Aksen M, Ding J, Ryu JH, Xin Q, Ding Z and others. 2017. Brain networks in human conscious visual perception. BioRxiv https://www.biorxiv.org/. [Google Scholar]
- Kwon H, Kronemer SI, Christison-Lagay KL, Khalaf A, Li J, Ding JZ, and others. 2021. Early Cortical Signals in Visual Stimulus Detection. Neuroimage. 10.1016/j.neuroimage.2021.118608 [DOI] [PubMed] [Google Scholar]
- Lamme VA. 2006. Towards a true neural stance on consciousness. Trends Cogn Sci 10:494–501. [DOI] [PubMed] [Google Scholar]
- Lau H, Rosenthal D. 2011. Empirical support for higher-order theories of conscious awareness. Trends Cogn Sci 15:365–73. [DOI] [PubMed] [Google Scholar]
- Laureys S, Gosseries O, Tononi G. 2015. The neurology of consciousness: cognitive neuroscience and neuropathology. 2nd ed. Academic Press. [Google Scholar]
- Li J, Kronemer SI, Herman WX, Kwon H, Ryu JH, Micek C, and others. 2019. Default mode and visual network activity in an attention task: Direct measurement with intracranial EEG. Neuroimage 201:116003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Ryu JH, Vincent P, Springer M, Kluger D, Levinsohn EA, and others. 2021. The pulse: transient fMRI signal increases in subcortical arousal systems during transitions in attention. Neuroimage 232:117873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lou B, Gao X, Sajda P. 2013. Post-stimulus endogenous and exogenous oscillations are differentially modulated by task difficulty. Front Hum Neurosci 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B, Gleason CA, Wright EW, Pearl DK. 1983. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain 106:623–42. [DOI] [PubMed] [Google Scholar]
- Lou HC, Changeux JP, Rosenstand A. 2017. Towards a cognitive neuroscience of self-awareness. Neurosci Biobehav Rev 83:765–73. [DOI] [PubMed] [Google Scholar]
- Mack A, Clarke J, Erol M. 2018. Attention, expectation and iconic memory: a reply to Aru and Bachmann (2017). Conscious Cogn 59:60–3. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. 2009. To see or not to see: prestimulus alpha phase predicts visual awareness. J Neurosci 29:2725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor M, Friston KJ, Fleming SM. 2020. Distinct neural contributions to metacognition for detecting, but not discriminating visual stimuli. Elife 9:e53900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, McGinley MJ, Salkoff DB. 2015. Brain state dependent activity in the cortex and thalamus. Curr Opin Neurobiol 31:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. 2000. Principles of behavioral and cognitive neurology. 2nd ed. Oxford University Press. [Google Scholar]
- Middleton FA, Strick PL. 2000. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn 42:183–200. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Weaver KE, Ojemann JG. 2009. Direct electrophysiological measurement of human default network areas. Proc Natl Acad Sci U S A 106:12174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motelow J, Blumenfeld H. 2014. Consciousness and subcortical arousal systems. In: Faingold CL, Blumenfeld H, eds. Neuronal networks in brain function, CNS disorders, and therapeutics. Elsevier. p. 277–98. [Google Scholar]
- Noy N, Bickel S, Zion-Golumbic E, Harel M, Golan T, Davidesco I, and others. 2015. Ignition’s glow: ultra-fast spread of global cortical activity accompanying local “ignitions” in visual cortex during conscious visual perception. Conscious Cogn 35:206–24. [DOI] [PubMed] [Google Scholar]
- Ossandón T, Jerbi K, Vidal JR, Bayle DJ, Henaff MA, Jung J, and others. 2011. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. J Neurosci 31:14521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Linkenkaer-Hansen K, Naatanen R, Palva JM. 2005. Early neural correlates of conscious somatosensory perception. J Neurosci 25:5248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Damasio A. 2001. Consciousness and the brainstem. Cognition 79:135–60. [DOI] [PubMed] [Google Scholar]
- Persuh M, LaRock E, Berger J. 2018. Working memory and consciousness: the current state of play. Front Hum Neurosci 12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts MA, Metzler S, Hillyard SA. 2014a. Isolating neural correlates of conscious perception from neural correlates of reporting one’s perception. Front Psychol 5:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts MA, Padwal J, Fennelly D, Martinez A, Hillyard SA. 2014b. Gamma band activity and the P3 reflect postperceptual processes, not visual awareness. Neuroimage 101:337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum F, Posner JB. 1972. The diagnosis of stupor and coma. Contemp Neurol Ser 10:1–286. [PubMed] [Google Scholar]
- Posner JB, Saper CB, Schiff ND, Claassen J. 2019. Plum and Posner’s diagnosis and treatment of stupor and coma (Contemporary Neurology Series; 5th ed.). Oxford University Press. [Google Scholar]
- Raichle ME, Snyder AZ. 2007. A default mode of brain function: a brief history of an evolving idea. Neuroimage 37:1083–90. [DOI] [PubMed] [Google Scholar]
- Redinbaugh MJ, Phillips JM, Kambi NA, Mohanta S, Andryk S, Dooley GL, and others. 2020. Thalamus modulates consciousness via layer-specific control of cortex. Neuron 106:66–75.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer Thomsen K, Lou HC, Joensson M, Hyam JA, Holland P, Parsons CE, and others. 2011. Impact of emotion on consciousness: positive stimuli enhance conscious reportability. PLoS One 6:e18686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A. 2009. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci 29:13410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. 2001. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 24:726–31. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C. 2019. Cholinergic double duty: cue detection and attentional control. Curr Opin Psychol 29:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Northoff G. 2017. Who am I: the conscious and the unconscious self. Front Hum Neurosci 11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND. 2008. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci 1129:105–18. [DOI] [PubMed] [Google Scholar]
- Schiff ND. 2010. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci 33:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND, Shah SA, Hudson AE, Nauvel T, Kalik SF, Purpura KP. 2013. Gating of attentional effort through the central thalamus. J Neurophysiol 109:1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. 2019. The cerebellum and cognition. Neurosci Lett 688:62–75. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, and others. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C, Baillet S, Dehaene S. 2005. Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci 8:1391–400. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Chabris CF. 1999. Gorillas in our midst: sustained inattentional blindness for dynamic events. Perception 28:1059–74. [DOI] [PubMed] [Google Scholar]
- Singer W 2001. Consciousness and the binding problem. Ann N Y Acad Sci 929:123–46. [DOI] [PubMed] [Google Scholar]
- Singh K, Fawcett I. 2008. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage 41:100–12. [DOI] [PubMed] [Google Scholar]
- Sperling G 1960. The information available in brief visual presentations. Psychol Monogr 74:1–29. [Google Scholar]
- Squire LR, Wixted JT. 2011. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci 34: 259–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. 2005. Brain control of wakefulness and sleep. 2nd ed. Kluwer Academic/Plenum. [Google Scholar]
- Steriade MM, McCarley RW. 2010. Brain control of wakefulness and sleep. 2nd ed. Springer. [Google Scholar]
- Thompson KG, Schall JD. 1999. The detection of visual signals by macaque frontal eye field during masking. Nat Neurosci 2:283–8. [DOI] [PubMed] [Google Scholar]
- Tononi G, Boly M, Massimini M, Koch C. 2016. Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci 17:450–61. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Wilke M, Frassle S, Lamme VA. 2015. No-report paradigms: extracting the true neural correlates of consciousness. Trends Cogn Sci 19:757–70. [DOI] [PubMed] [Google Scholar]
- Tulving E 2002. Episodic memory: from mind to brain. Annu Rev Psychol 53:1–25. [DOI] [PubMed] [Google Scholar]
- van Vugt B, Dagnino B, Vartak D, Safaai H, Panzeri S, Dehaene S, and others. 2018. The threshold for conscious report: signal loss and response bias in visual and frontal cortex. Science 360:537–42. [DOI] [PubMed] [Google Scholar]
- Warren CM, Nieuwenhuis S, Donner TH. 2015. Perceptual choice boosts network stability: effect of neuromodulation? Trends Cogn Sci 19:362–4. [DOI] [PubMed] [Google Scholar]
- Wilterson AI, Kemper CM, Kim N, Webb TW, Reblando AMW, Graziano MSA. 2020. Attention control and the attention schema theory of consciousness. Progr Neurobiol 195:101844. [DOI] [PubMed] [Google Scholar]
- Wyart V, Tallon-Baudry C. 2009. How ongoing fluctuations in human visual cortex predict perceptual awareness: baseline shift versus decision bias. J Neurosci 29:8715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]


