Abstract
Background
Autotaxin (ATX) is an ecto-enzyme that catalyses the hydrolysis of lysophospholipids to the lipid mediator lysophosphatidic acid (LPA). LPA/ATX signalling has emerged as a new therapeutic target for pulmonary fibrosis; however, the serum levels and dynamics of ATX during the clinical course of fibrosing interstitial lung disease (ILD) remain unknown. This study sought to examine the serum ATX levels in fibrosing ILD in the chronic phase and in acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF). We aimed to elucidate the association between serum ATX level and clinical characteristics including disease progression and prognosis.
Methods
In total, 119 patients with fibrosing ILD and 38 healthy volunteers as controls were enrolled in the study and their serum ATX activity was analysed. We also included six male patients with AE-IPF in order to analyse the changes in serum ATX at the onset of AE-IPF.
Results
Patients with fibrosing ILD showed significantly higher serum ATX levels compared with healthy controls in both sexes. Per cent change in forced vital capacity after 1 year correlated with serum ATX levels in female patients. High serum ATX levels (>0.721 mg· L−1) were associated with worse outcome in survival curve and multivariate analysis of male patients. Serum ATX activity decreased after the onset of AE-IPF.
Conclusion
Serum ATX levels were significantly higher in patients with fibrosing ILD compared with healthy controls, and this was associated with disease progression and outcome. This suggests the potential of serum ATX as a promising biomarker for the treatment of fibrosing ILD.
Short abstract
Serum ATX levels are higher in fibrosing ILD than in healthy controls; this is associated with disease progression and outcome. Serum ATX levels decrease at the onset of AE-IPF. https://bit.ly/3hLR9jN
Introduction
Fibrosing interstitial lung disease (ILD) is a heterogeneous group of lung disorders characterised by fibrosis in the lung interstitium. Idiopathic pulmonary fibrosis (IPF), a subtype of fibrosing ILD, is a representative phenotype with radiological and/or histopathological usual interstitial pneumonia pattern in which chronic and progressive pulmonary fibrosis of indeterminable cause develops in the lung and finally results in respiratory failure [1]. The clinical course of IPF is usually chronic; however, some patients develop an acute exacerbation of IPF (AE-IPF) with severe worsening of respiratory symptoms and grave prognosis after the onset [2]. Other subtypes of ILD, such as nonspecific interstitial pneumonia (NSIP), connective tissue disease-associated ILD (CTD-ILD), unclassifiable ILD and pleuroparenchymal fibroelastosis also have a progressive fibrotic phenotype [3].
Lysophosphatidic acid (LPA), a bioactive lipid mediator, has been reported to be associated with the development and progression of pulmonary fibrosis through binding to its receptors [4–7]. Autotaxin (ATX) is an extracellular enzyme that catalyses the hydrolytic conversion of lysophospholipids, such as lysophatidylcholine (LPC), to LPA by its phospholipase D activity [8]. ATX is highly expressed in the human fibrotic lung, and pharmacological inhibition of ATX results in attenuation of bleomycin-induced pulmonary fibrosis [9]. The ATX/LPA axis has now emerged as a promising therapeutic target for pulmonary fibrosis, and several clinical studies have been investigating the possible effectiveness of ATX and LPA inhibitors in patients with IPF and progressive fibrosing ILD [10–12]. However, to our knowledge, the association between LPA and ATX levels in blood and the attendant clinical characteristics have not been fully investigated in patients with IPF and other types of fibrosing ILD. Measuring serum ATX levels is more advantageous than LPA measurements in the clinical setting. Primarily, ATX is stable without requiring strict temperature control after sample preparation, while LPA is not stable at room temperature and is only measurable in plasma.
In this study, we aimed to elucidate ATX activity in the chronic phase of fibrosing ILD and after the onset of AE-IPF.
Methods
Patients
We recruited 139 patients with fibrosing ILD in the chronic phase (n=119) and after the onset of AE-IPF (n=6) seen at our institution during the period from November 2017 through October 2020. Since four patients had serum available from both the chronic and acute exacerbation phase, 135 patients were enrolled in the study. In addition, 38 volunteers were recruited to participate as healthy controls.
Patient characteristics extracted from the medical records included the following: age, sex, smoking history, laboratory data, pulmonary function test results, final diagnosis of fibrosing ILD, treatment for fibrosing ILD and prognosis. The diagnosis of IPF and other type of fibrosing ILD was determined by using the guidelines of the American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society/Latin American Thoracic Association and the joint statement of the ATS and the ERS [13]. The diagnosis of AE-IPF was based on criteria proposed by the international working group on AE-IPF [2]. The database was locked in October 2020. The institutional review board of Toho University Graduate School of Medicine approved this study (approval number: A20100). All patients and healthy controls provided written informed consent to participate in the study.
Blood collection and autotaxin quantification
Peripheral blood was collected during the stable phase of fibrosing ILD. Blood collection in AE-IPF was performed at the onset before administered corticosteroid treatment. Peripheral blood samples were obtained from the patients in a tube and centrifuged at 3000 xg for 10 min. The resulting supernatant after centrifugation was aliquoted and stored at −80°C. ATX activity was measured by using fluorescence enzyme immunoassay in a commercial clinical laboratory (SRL, Inc., Tokyo, Japan).
Data analysis
Continuous variables comprised the unpaired t-test for both groups. Categorical variables were compared using the χ2 and Fisher's exact tests. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off value of serum ATX for prediction of outcome. Worse outcome was defined as death or inability to continue treatment for fibrosing ILD by transfer to either home healthcare or long-term hospital care. We used the Kaplan–Meier method to determine the prognosis. The log-rank test was used to compare the two groups. Hazard ratios along with 95% confidence intervals were calculated using Cox proportional hazards analysis to determine the independent predictor for onset of AE-IPF. All p-values were two-sided, and p<0.05 was considered to indicate statistical significance. Statistical analysis was performed by using SPSS Statistics for Windows version 27.0 (IBM Corp., Armonk, NY, USA) and GraphPad PRISM version 8.00 (MDF Co., Ltd, San Diego, CA, USA).
Results
Baseline characteristics and sex differences
Baseline characteristics of patients with chronic fibrosing ILD are shown in table 1; 78 IPF patients and 41 non-IPF patients were included in the study. We compared serum ATX levels between male and female patients. Higher ATX levels were seen in females compared with male patients (0.724±0.161 mg ·L−1 versus 0.912±0.224 mg ·L−1; p<0.0001). Thus, for subsequent investigations we divided the patients according to sex. Next, we examined the association of ATX activity and corticosteroid therapy. ATX levels were significantly lower in patients receiving corticosteroid therapy compared with those not receiving corticosteroids in both male and female patients (males: 0.743±0.160 mg· L−1 versus 0.580±0.075 mg· L−1, p=0.002; females: 0.975±0.236 mg ·L−1 versus 0.789±0.137 mg· L−1, p=0.002). We thus excluded those patients receiving corticosteroid therapy for further analysis of chronic fibrosing ILD. No such difference was observed in the presence or absence of treatment with antifibrotic agents including pirfenidone and nintedanib (males: 0.747±0.153 mg· L−1 versus 0.736±0.176 mg· L−1, p=0.681; females: 0.927±0.218 mg ·L−1 versus 0.873±0.243 mg ·L−1, p=0.229).
TABLE 1.
Baseline characteristics of patients with stable fibrosing interstitial lung disease (ILD)
| Patients | Healthy controls | |
| Subjects, n | 119 | 38 |
| Age years | 70±9 | 52±11 |
| Sex (male), n (%) | 69 (58) | 16 (42) |
| Smoking history (yes), n (%) | 73 (61) | 11 (29) |
| Diagnosis of fibrosing ILD, n | ||
| IPF | 78 | |
| NSIP | 11 | |
| Unclassifiable ILD | 10 | |
| PPFE | 8 | |
| CTD-ILD | 12 | |
| Treatment at the time of analysis, n | ||
| Corticosteroids | 25 | |
| Pirfenidone | 19 | |
| Nintedanib | 15 | |
| Laboratory data | ||
| LDH U· L−1 | 252±54 | |
| KL-6 U m·L−1 | 1029±680 | |
| SP-D ng· mL−1 | 215±148 | |
| Pulmonary function | ||
| FVC mL | 2133±743 | |
| %FVC | 75.9±20.4 | |
| DLCO % | 59.8±20.9 | |
Data are presented as mean±sd, unless otherwise stated. IPF: idiopathic pulmonary fibrosis; NSIP: nonspecific interstitial pneumonia; CTD-ILD: connective tissue disease-associated ILD; PPFE: pleuroparenchymal fibroelastosis; LDH: lactate dehydrogenase; SP-D: surfactant protein-D; KL-6: Krebs von den Lungen-6; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide.
ATX activity in chronic fibrosing ILD
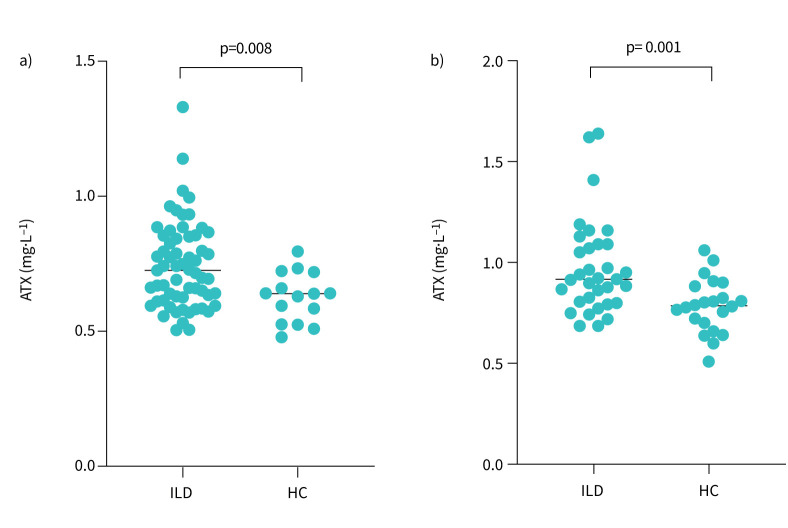
We added a control arm comprising 38 healthy controls to analyse ATX activity in the chronic phase of fibrosing ILD (table 1). Serum ATX levels were compared between patients with fibrosing ILD and healthy controls. Significantly higher ATX levels were seen in both sexes among fibrosing ILD patients compared with healthy controls (figure 1). There were no significant between-group differences in ATX levels among the subtypes of fibrosing ILD (table 2). We then examined the correlation of serum ATX activity with other clinical parameters including laboratory data and pulmonary function (table 3). There was no significant correlation between ATX levels and other clinical parameters at baseline. The per cent predicted change in forced vital capacity (%FVC) after 1 year was correlated with serum ATX levels in female patients.
FIGURE 1.
Autotaxin (ATX) levels in patients with fibrosing interstitial lung disease (ILD) and healthy controls (HC). Fibrosing ILD patients showed significantly increased ATX levels in a) male patients (0.743±0.160 mg ·L−1 versus 0.626±0.092 mg ·L−1; p=0.008) and b) female patients (0.975±0.236 mg· L−1 versus 0.786±0.133 mg· L−1; p=0.001).
TABLE 2.
Autotaxin (ATX) activity by sex in fibrosing interstitial lung disease (ILD) patients without corticosteroid therapy at the time of blood sampling
| Diagnosis of ILD | IPF | NSIP | Unclassifiable ILD | PPFE | CTD-ILD |
| Male | |||||
| Subjects, n | 53 | 3 | 1 | 4 | 0 |
| ATX activity mg· L−1 | 0.746±0.163 | 0.793±0.221 | 0.661 | 0.683±0.988 | |
| Female | |||||
| Subjects, n | 16 | 4 | 3 | 4 | 6 |
| ATX activity mg· L−1 | 1.003±0.258 | 0.933±0.105 | 1.217±0.376 | 0.869±0.195 | 0.876±0.101 |
Data are presented as mean±sd, unless otherwise stated. IPF: idiopathic pulmonary fibrosis; NSIP: nonspecific interstitial pneumonia; PPFE: pleuroparenchymal fibroelastosis; CTD-ILD: connective tissue disease-associated ILD.
TABLE 3.
Correlation between autotaxin levels and clinical parameters
| r | p-value | |
| Male (n=61) | ||
| KL-6 | −0.117 | 0.370 |
| SP-D | 0.040 | 0.757 |
| %FVC | −0.093 | 0.481 |
| DLCO % | −0.191 | 0.146 |
| Δ1 year %FVC (n=28) | 0.142 | 0.472 |
| Female (n=33) | ||
| KL-6 | −0.009 | 0.957 |
| SP-D | 0.218 | 0.223 |
| %FVC | 0.051 | 0.784 |
| DLCO % | −0.135 | 0.471 |
| Δ1 year %FVC (n=13) | −0.676 | 0.014 |
KL-6: Krebs von den Lungen-6; SP-D: surfactant protein-D; FVC: forced vital capacity; DLCO : diffusing capacity of the lung for carbon monoxide.
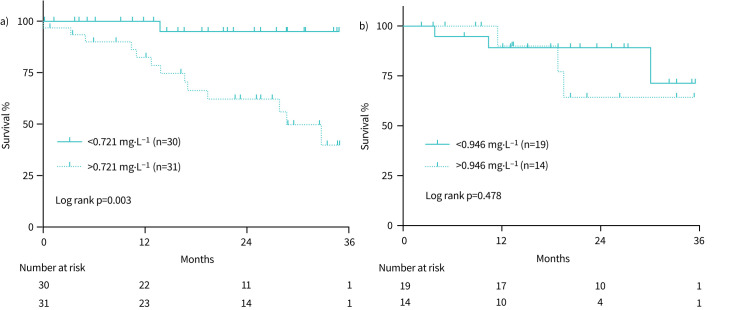
Association between ATX activity and outcome
We identified the optimal cut-off value of ATX level for prediction of outcomes by using ROC curve analysis. The cut-off value was determined as 0.721 mg· L−1 for male (n=61) and as 0.946 mg· L−1 for female (n=33) patients for predicting death or discontinuation of treatment for fibrosing ILD within 3 years (table 4). Survival curve analysis showed significantly worse prognosis in male patients with high serum ATX activity (>0.721 mg·L−1) compared with those with low ATX activity (<0.721 mg· L−1) (figure 2a). In addition, multivariate analysis using Cox proportional hazards model revealed that lower %FVC at baseline and high ATX level were independent predictors of worse outcome (table 5). When limited to IPF patients, high serum ATX activity and %FVC was still a significant predictor of outcome (HR 8.288; 95% CI: 1.021–67.256, p=0.048) (online supplementary table 1). However, there was no significant difference in survival between high and low serum ATX levels among female patients (figure 2b).
TABLE 4.
Receiver operating characteristic (ROC) curve analysis to determine the optimal cut-off value of autotaxin for predicting the worse outcome of fibrosing interstitial lung disease
| Cut-off value mg ·dL−1 | AUC | Sensitivity % | Specificity % | |
| Male (n=61) | 0.721 | 0.766 | 92.9 | 61.7 |
| Female (n=33) | 0.946 | 0.608 | 62.5 | 64.0 |
AUC: area under curve.
FIGURE 2.
Survival curve of fibrosing interstitial lung disease (ILD) patients according to serum autotaxin (ATX) levels. a) Male patients with high ATX levels (>0.721 mg· L−1) showed significantly better outcome than patients with low ATX levels (log rank p=0.003). b) No such difference outcome was observed between high (>0.946 mg ·L−1) and low ATX levels in female patients (log rank p=0.478).
TABLE 5.
Multivariate analysis using Cox proportional hazards model for predicting independent predictors of worse outcome in male patients with fibrosing interstitial lung disease (n=61)
| Hazard ratio | 95% CI | p-value | |
| Age years | 1.005 | 0.943–1.072 | 0.868 |
| Smoking history (yes) | 1.257 | 0.156–10.100 | 0.830 |
| KL-6 | 0.999 | 0.998–1.001 | 0.228 |
| %FVC | 0.964 | 0.930–0.999 | 0.043 |
| ATX >0.721 mg ·L−1 | 8.295 | 1.049–65.558 | 0.045 |
KL-6: Krebs von den Lungen-6; FVC: forced vital capacity; ATX: autotaxin.
ATX activity in acute exacerbation of IPF
Finally, we analysed serum ATX levels in AE-IPF. Clinical characteristics at the onset of AE-IPF and treatment for AE-IPF are shown in table 6. We compared ATX levels between stable male IPF patients without corticosteroid therapy (n=53) and male AE-IPF patients before receiving the first corticosteroid injection (n=6). As shown in figure 3, ATX levels were decreased after AE-IPF onset compared to stable state in male IPF patients (0.746±0.163 mg· L−1 versus 0.564±0.199 mg ·L−1; p=0.006).
TABLE 6.
Baseline characteristics of male patients with acute exacerbation of idiopathic pulmonary fibrosis (n=6)
| Age years | 71±6 |
| PaO2/FIO2 | 315±52 |
| LDH U·L−1 | 332±43 |
| KL-6 U·mL−1 | 1808±990 |
| SP-D ng·mL−1 | 318±137 |
| CRP mg ·dL−1 | 9.3±10.9 |
Data are presented as mean±sd. PaO2: arterial oxygen tension; FIO2: inspiratory oxygen fraction; LDH: lactate dehydrogenase; KL-6: Krebs von den Lungen-6; SP-D: surfactant protein-D; CRP: C-reactive protein.
FIGURE 3.
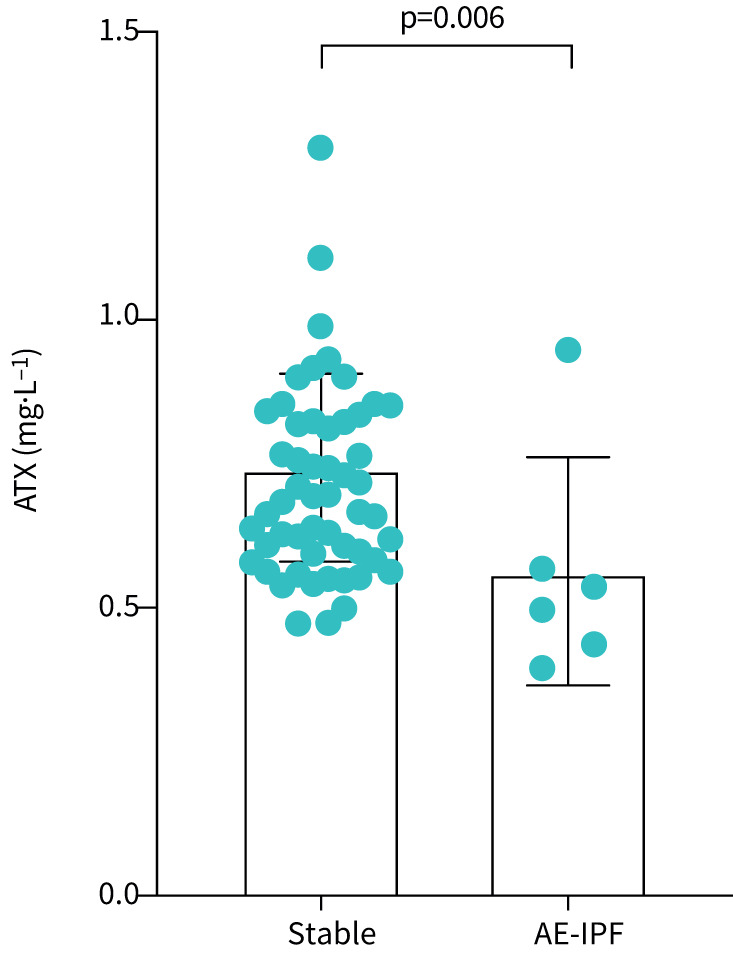
Autotaxin (ATX) levels in male patients with chronic idiopathic pulmonary fibrosis (IPF) and acute exacerbation of IPF (AE-IPF). ATX levels were significantly decreased after the onset of AE-IPF compared with stable IPF (0.746±0.163 mg· L−1 versus 0.564±0.199 mg· L−1; p=0.006).
Discussion
This study demonstrated that serum ATX levels were elevated in fibrosing ILD patients compared with healthy controls. High ATX levels were associated with disease progression and worse outcome in fibrosing ILD. Low ATX levels were observed in AE-IPF compared with the stable state.
LPA is a bioactive lipid mediator that consists of a glycerol phosphate backbone with a single fatty acid. LPA binds to specific G protein-coupled receptors and mediates various inflammatory and fibrotic responses. LPA1 is the most frequently reported LPA receptor associated with pulmonary fibrosis. LPA/LPA1 signalling has a profibrotic role in epithelial cells, endothelial cells and fibroblasts [4, 6]. Mice with genetic deficiency of LPA1 and pharmacological inhibition of LPA1 both demonstrated protection from fibrosis in a bleomycin mouse model of pulmonary fibrosis [4, 6]. Also, LPA induced fibroblast reorganisation and proliferation thorough LPA2 signalling. 5 LPA/LPA2 signalling activates transforming growth factor-β expression in lung epithelial cells and fibroblasts, leading to myofibroblast differentiation [7].
ATX is a secreted lysophospholipase D that catalyses the hydrolysis of LPC to LPA by phospholipase D activity. It is abundant in most biological fluids, including blood, bronchoalveolar lavage fluid, cerebrospinal fluid and urine [14]. Immunohistochemical studies revealed that ATX is constitutively expressed in bronchial epithelial cells, alveolar epithelial cells and alveolar macrophages in the lung [9]. Furthermore, ATX showed a higher staining intensity in IPF and fibrotic nonspecific interstitial pneumonia (F-NSIP) lung than in control samples of healthy lung, suggesting that ATX is activated in the fibrotic lung [9]. Genetic deletion and pharmacological inhibition of ATX resulted in attenuation of bleomycin-induced pulmonary fibrosis in a mouse model [9]. Taken together, the LPA/ATX axis has now emerged as a potential therapeutic target in pulmonary fibrosis. Several inhibitors targeting LPA1 and ATX have now been entered into clinical trials of IPF and other types of fibrosing ILD [10–12]. The FLORA study, a phase 2a randomised placebo-controlled trial of ATX inhibitors in IPF, reported decreased plasma LPA C18:2 levels in the treatment group compared with the placebo group [10]. However, no study has analysed serum ATX levels in fibrosing ILD to date.
In this study, we showed that serum ATX level is a potential biomarker to predict disease progression and worse outcome in fibrosing ILD. To our knowledge, this is the first report to examine the association of ATX levels with clinical characteristics in fibrosing ILD. Further studies are required to assess the relationship between serum ATX levels and response to LPA/ATX inhibitors if these drugs are proven to be effective.
In terms of utilising blood levels in the clinical setting, serum ATX measurement is preferable to serum LPA. First, ATX is temperature stable after sample preparation [15]. Second, ATX is less affected by metabolic changes such as in diabetes mellitus, chronic kidney disease and food intake [16]. In addition, there were no significant differences between age groups [17]. However, a sex difference in blood ATX levels has been reported [15]. The precise mechanism of this difference is yet to be determined, however; since female subjects and those in pregnancy show high ATX levels in the blood, it might be associated with reproductive biology [15, 18]. In this study, ATX levels were significantly higher in female patients compared with male patients, consistent with previous reports. Therefore, we analysed the association of serum ATX level with clinical characteristics separately in both sexes. Serum ATX level is elevated in pregnancy [18], malignant lymphoma [19] and liver fibrosis [20]. None of the patients in our study were pregnant or had malignant lymphoma or liver fibrosis. Initiation of corticosteroid therapy was reported to decrease ATX levels in a dose-dependent manner [21]. Similarly, in this study, ATX levels were significantly lower in patients receiving corticosteroid therapy compared with those without corticosteroids in both male and female patients. Considering the effect of corticosteroids, patients who were receiving corticosteroid therapy at the time of serum ATX measurement in chronic fibrosing ILD were excluded from further analysis. No such difference was observed with antifibrotic therapy.
ATX levels were decreased after the onset of AE-IPF. Since corticosteroids are used in all patients for the treatment of AE-IPF in our facility, it is difficult to define how to interpret the usefulness of ATX concentration as a biomarker, which is affected by corticosteroid use. We excluded the patients who had received the first dose of corticosteroids at the time of blood collection. The pathogenesis of AE-IPF characteristically is by increased type II alveolar epithelial cell injury and/or proliferation, coagulation disorders and fibrotic deposition [2, 22]. ATX is mainly expressed in bronchial epithelial cells and alveolar macrophage in the lung [9]. Therefore, a possible explanation for decreased ATX levels in AE-IPF is that severe epithelial damage in AE-IPF resulted in lower expression and production of ATX in bronchial epithelial cells.
This study has several limitations. First, this is a single-centre analysis, and we could not observe longitudinal changes in serum ATX levels in patients with chronic fibrosing ILD. Changes in serum ATX activity would be useful to predict disease progression. Future study with a validation cohort is needed to confirm our findings, since we include a heterogeneous fibrotic ILD phenotype in this study. Second, the healthy controls were younger than the patients and there were less subjects with smoking history. This could have added some potential bias. Sex differences have been reported in several studies, but a previous study showed similar levels among different ages groups [17]. There was no significant difference between patients with or without smoking history in both sexes (data not shown). Third, because of the small number of female patients who were not receiving corticosteroid therapy, we did not perform a multivariate analysis in female patients with fibrosing ILD. Similarly, we could not analyse ATX levels in AE-IPF among female patients.
Conclusions
In this study, elevated serum ATX levels in fibrosing ILD patients compared with healthy controls and high ATX levels were associated with disease progression and worse outcome. Our findings indicated that serum ATX is a potential blood biomarker for diagnosing and predicting outcome in fibrosing ILD. Inhibitors targeting LPA/ATX signalling have emerged as new treatment candidates for fibrosing ILD. The association of serum ATX with response to LPA and ATX inhibitors should be examined in the future if their utility is to be proven in clinical trials.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00683-2021.SUPPLEMENT (19.5KB, pdf)
Acknowledgements
We thank Florence Ene for medical English editing.
Provenance: Submitted article, peer reviewed.
Author contributions: T. Isshiki had access to the data and takes responsibility for data accuracy. S. Sakamoto, S. Homma and K. Kishi contributed to the design of the study. T. Isshiki, H. Shimizu, A. Yamasaki, Y. Nakamura and S. Miyoshi contributed to data collection. All authors were involved in drafting and revising the manuscript, and gave their final approval of the version to be published.
Conflicts of interest: This study received no specific funding from any company. S. Homma received research grants from Nippon Boehringer Ingelheim Co., Ltd, Shionogi & Co., Ltd, and Chugai Pharmaceutical Co., Ltd. T. Isshiki, S. Sakamoto, A. Yamasaki, H. Shimizu, S. Miyoshi, Y. Nakamura and K. Kishi have no conflicts of interest to declare.
Support statement: This study was supported by Project Research Grant number 20–6 from Toho University School of Medicine with partial support by a grant from the Ministry of Health, Labour and Welfare of Japan, awarded to the Study Group on Diffuse Pulmonary Disorders. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An Official ATS/ERS/JRS/ALAT Clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 2.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis an international working group report. Am J Respir Crit Care Med 2016; 194: 265–275. doi: 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. doi: 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tager AM, LaCamera P, Shea BS, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 2008; 14: 45–54. doi: 10.1038/nm1685 [DOI] [PubMed] [Google Scholar]
- 5.Huang LS, Fu P, Patel P, et al. Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice. Am J Respir Cell Mol Biol 2013; 49: 912–922. doi: 10.1165/rcmb.2013-0070OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funke M, Zhao Z, Xu Y, et al. The lysophosphatidic acid receptor LPA 1 promotes epithelial cell apoptosis after lung injury. Am J Respir Cell Mol Biol 2012; 46: 355–364. doi: 10.1165/rcmb.2010-0155OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu MY, Porte J, Knox AJ, et al. Lysophosphatidic acid induces αvβ36 integrin-mediated TGF-β activation via the LPA2 receptor and the small G protein Gαq. Am J Pathol 2009; 174: 1264–1279. doi: 10.2353/ajpath.2009.080160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tokumura A, Majima E, Kariya Y, et al. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 2002; 277: 39436–39442. doi: 10.1074/jbc.M205623200 [DOI] [PubMed] [Google Scholar]
- 9.Oikonomou N, Mouratis MA, Tzouvelekis A, et al. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol 2012; 47: 566–574. doi: 10.1165/rcmb.2012-0004OC [DOI] [PubMed] [Google Scholar]
- 10.Maher TM, van der Aar EM, van de Steen O, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat idiopathic pulmonary fibrosis (FLORA): a phase 2a randomised placebo-controlled trial. Lancet Respir Med 2018; 6: 627–635. doi: 10.1016/S2213-2600(18)30181-4 [DOI] [PubMed] [Google Scholar]
- 11.Palmer SM, Snyder L, Todd JL, et al. Randomized, double-blind, placebo-controlled, phase 2 trial of BMS-986020, a lysophosphatidic acid receptor antagonist for the treatment of idiopathic pulmonary fibrosis. Chest 2018; 154: 1061–1069. doi: 10.1016/j.chest.2018.08.1058 [DOI] [PubMed] [Google Scholar]
- 12.Lee G, Kang SU, Ryou J-H, et al. Late Breaking Abstract – BBT-877, a potent autotaxin inhibitor in clinical development to treat idiopathic pulmonary fibrosis. Eur Respir J 2019; 54: PA1293. [Google Scholar]
- 13.Raghu G, Collard HR, Egan JJ, et al. An Official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbayianni E, Kaffe E, Aidinis V, et al. Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer. Prog Lipid Res 2015; 58: 76–96. doi: 10.1016/j.plipres.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Ohkawa R, Okubo S, et al. Measurement of lysophospholipase D/autotaxin activity in human serum samples. Clin Biochem 2007; 40: 274–277. doi: 10.1016/j.clinbiochem.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 16.Ikeda H, Kobayashi M, Kumada H, et al. Performance of autotaxin as a serum marker for liver fibrosis. Ann Clin Biochem 2018; 55: 469–477. doi: 10.1177/0004563217741509 [DOI] [PubMed] [Google Scholar]
- 17.Usami Y, Ishimine N, Shiba A, et al. Performance evaluation of a new liver fibrosis marker autotaxin immunoassay. Igakukensa 2019; 68: 99–104. [Google Scholar]
- 18.Iwasawa Y, Fujii T, Nagamatsu T, et al. Expression of autotaxin, an ectoenzyme that produces lysophosphatidic acid, in human placenta. Am J Reprod Immunol 2009; 62: 90–95. doi: 10.1111/j.1600-0897.2009.00715.x [DOI] [PubMed] [Google Scholar]
- 19.Masuda A, Nakamura K, Izutsu K, et al. Serum autotaxin measurement in haematological malignancies: a promising marker for follicular lymphoma. Br J Haematol 2008; 143: 60–70. doi: 10.1111/j.1365-2141.2008.07325.x [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa H, Ikeda H, Nakamura K, et al. Autotaxin as a novel serum marker of liver fibrosis. Clin Chim Acta 2011; 412: 1201–1206. doi: 10.1016/j.cca.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 21.Sumida H, Nakamura K, Yanagida K, et al. Decrease in circulating autotaxin by oral administration of prednisolone. Clin Chim Acta 2013; 415: 74–80. doi: 10.1016/j.cca.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 22.Collard HR, Calfee CS, Wolters PJ, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2010; 299: L3–L7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00683-2021.SUPPLEMENT (19.5KB, pdf)