Abstract
Microbiological air contamination in the desert environment is becoming an essential subject for the health of office building occupants and public health. In this study, the concentrations and compositions of airborne microorganisms (bacteria and fungi) were assessed in indoor and outdoor environments using a multistory building complex in Kuwait as a case study. Airborne microorganism samples were collected from 12 sites within the building complex containing nineteen stories over four seasons. Culturable airborne bacteria and fungi were impacted on selected media to determine their concentrations and compositions with a Biolog Omnilog GEN III system and Biolog MicroStation. The indoor mean airborne bacterial count concentrations ranged from 35 to 18,463 CFU/m3, concentrations that are higher than 2,000 CFU/m3, demonstrating high–very high contamination levels in all seasons. Fungal contamination was high in winter and summer, with detected concentrations > 2,000 CFU/m3. Indoor-to-outdoor (I/O) ratios showed that airborne microbial contamination inside building floors originated from indoor air contamination. All the building floors showed bacterial and fungal concentrations ranging from less than 2,000 to more than 2,000 CFU/m3, indicative of a high to very high air contamination level. Statistical analysis showed no correlation between bacterial and fungal concentrations, demonstrating that they originated from unrelated sources. In the indoor building air, the most prevalent bacterial isolate was Bacillus pseudomycoides/cereus, whereas the most dominant fungal isolate was Aspergillus spp. The low count for indoor air bacterial species suggested no particular health risk for the occupants. In contrast, the high count of indoor air fungal species in the winter samples and the presence of potentially allergenic genera detected may suggest possible health risks for the occupants. The results obtained are the basis for the recommendation that the maintenance activities of the HVAC system and the periodical cleaning operation program be revised and preplanned as protective measures.
Keywords: Air-borne microorganisms, Indoor air contamination, Office building, Desert environment
Introduction
Building office air quality has become of great significance for workers’ healthiness and welfare. Previous epidemiological studies of office indoor environments show that more than 30% of office employees complain about their health issues, related to indoor environmental contamination (Gołofit-Szymczak & Górny, 2018). They are exposed to numerous harmful elements, biological and chemical contaminants, mechanical vibration, noise, lighting, electromagnetic fields, static energy, and several microclimates. The building’s offices are at risk of regular exposure to microbial contamination. Once microbiological contaminants are found in low concentrations and no pathogens are discovered, they are widely treated as no threat to human health. The primary problem of microbiological contamination arises when the extent of contamination exceeds a particular limit, which is considered ordinary for a specific environment.
Many studies on the enclosed environment’s microbiological contaminants have been conducted in office buildings, schools, universities, and hospitals (Bonetta et al., 2010; Graudenz et al., 2005; Hospodsky et al., 2012; Huang et al., 2008; Huttunen et al., 2008; Kalogerakis et al., 2005; Kim & Kim, 2007; Law et al., 2001; Łukaszuk et al., 2011; Meadow et al., 2014; Qian et al., 2012; Shoemaker & House, 2006; Stanley et al., 2008; Stetzenbach, 2007; Taubel et al., 2009; Wamedo et al., 2012; Yu et al., 2009). Regarding the assessment of microbial contaminants in office buildings, Awad et al. (2018) studied the microbial indoor air quality in public buildings that included schools, faculties, libraries, hospitals, and child daycare centers and their association with microclimatic conditions, particulate matter (PM), and ventilation type. The results indicated that the fungal and bacterial concentrations varied based on occupancy intensity, ventilation, and human activity. Gołofit-Szymczak and Górny (2018) assessed employees’ exposure to fungal and bacterial aerosols in office buildings with different ventilation structures by evaluating the microbial air quality within the studied premises. The results confirmed that the microbiological air quality in the building offices depended on the type and maintenance condition of the building’s ventilation structure. Brągoszewska et al. (2018) analyzed the microbial air quality of naturally ventilated office rooms and air-conditioned rooms in the Upper Silesia region of Poland. They showed that the mean indoor bacterial aerosol concentrations were less than the Polish suggestions for threshold limit standards in office buildings. Brągoszewska and Biedroń (2018) characterized the quality and quantity of culturable bacterial aerosols in an office building in southern Poland for the duration of the spring season. They found that Staphylococcus xylosus could form biofilms. In another review, Gilbert and Stephens (2018) outlined the history of a building’s environmental microbiology and discussed the current understanding of this microbiome’s ecology and evolution. Grisoli et al. (2019) developed the microbiological contamination global index, the amplification index, and the mesophilic bacterial contamination index to assess the contamination levels in offices, gyms, and libraries. Their outcome confirmed that microbial contamination varied depending on the analyzed environment and highlighted the easy applicability of the proposed indices.
Regarding the assessment of microbial contaminants in schools, Enitan et al. (2017) determined the indoor air bacterial and fungal density in selected private primary schools in Ilishan-Remo of Ogun State at different sampling times of the day using the settle plate method. The results showed that the concentrations of aeroflora above the permissive standard recorded in this study underscore the importance of this microenvironment for children’s high exposure to bioaerosols. Another study by Fsadni et al. (2017) analyzed the association of a school’s indoor air quality contaminants, school characteristics, and respiratory health. They found that bacteria, fungi, dog, and cat allergens directly influence school indoor air quality in the Maltese Islands when compared to European statistics. Brągoszewska et al. (2020) studied students’ exposure to bacterial aerosols in a high school gym in the urban region of southern Poland. The results showed that the bacterial aerosol concentration was 4.20 × 102 ± 49.19 CFU/m3 before gym classes and was more than doubled (8.75 × 102 ± 121.39 CFU/m3) during gym classes. Chegini et al. (2020) investigated the nature of aerosols for culturable fungi and bacteria in indoor and outdoor air in different kindergartens at Rasht, Iran, by over various seasons. The results revealed that 33% and 8% of the indoor and outdoor bacterial and fungal concentrations, respectively, were higher than the recommended value, indicating medium risk. Another study in daycare centers by Harbizadeh et al. (2019) investigated seasonal variations in airborne bacteria in indoor and outdoor air quality in Ahvaz, Iran. The results revealed that the lowest and highest indoor and outdoor concentrations of airborne bacteria were in the daycare centers within the residential and high-traffic regions.
Regarding the assessment of microbial contaminants in universities, Ekhaise and Erhunmwunsee (2013) evaluated the airborne microorganisms associated with the indoor environment and hygienic conditions of the University of Benin, Nigeria, using settle plate methods. Their outcome confirmed the existence of seven airborne bacterial and nine fungal isolates in the residence halls. The quality of indoor air in the Universiti Tun Hussein Onn Malaysia was assessed by Er et al. (2015). The bacterial concentrations in the affected building areas were significantly higher than the maximum exposure limit of 500 CFU/m3. Onet et al. (2018) evaluated the indoor air microbial contamination level for the Polyvalent Sports Hall at the University of Oradea, Romania. The fungal concentration was lower than the bacterial concentration in indoor air and ranged between 0 and 103 CFU/m3. Dang et al. (2020) assessed indoor air microbial contamination in classrooms at the University of Science, Ho Chi Minh City, Vietnam. The results showed fungal and bacterial densities ranged from 106.1–928.9 CFU/m3 and 359.6–2,427.3 CFU/m3, respectively. Other studies in university buildings in Malaysia by Idris et al. (2020) and Mazlan et al. (2020) measured the biological contaminant concentration levels and indoor air pollutants in different laboratories and research facilities. They showed that the bacteria found in laboratories and research facilities were identified as gram-positive bacteria. In addition, they found the occurrence of Bacillus laterosporus, Bacillus cereus, Bacillus sphaericus, Staphylococcus epidermis, Staphylococcus aureus, Micrococcus luteus, Enterobacter cloacae, Pseudomonas stutzeri, Pseudomonas fluorescens and Aeromonas hydrophila bacteria.
Regarding the assessment of microbial contaminants in hospitals, Fekadu and Getachewu (2015) evaluated the indoor air microorganisms of Jimma University Specialized Hospital wards and determined their health risks. The results indicated that the measured bacterial concentrations were significantly different. Verde et al. (2015) performed the assessment of the fungal load and aerobic mesophilic bacterial counts in the various sites in a Portuguese Hospital (e.g., the emergency service, the operating theater, and the surgical ward). Bacterial concentrations were under the limited criteria in the surgical ward and operating theater. In another study on airborne environmental mesophilic bacteria and fungi at governmental and private Egyptian hospitals, the admission departments, operating theatres, intensive care units, and outdoors were evaluated for comparison by Osman et al. (2017). Bacillus licheniformis was found at the private hospital, and Alloiococcus otitis was found at the governmental hospital. Recently, Busso et al. (2020) applied a QuEChER method to monitor the levels of airborne microorganisms and evaluated possible variables that alter the load of air microorganisms. They showed a straightforward correlation between air exchange rates, airborne particles, and a number of people. Zenaide-Neto and do Nascimento (2020) analyzed airborne fungal density in a hospital in Paraíba, Brazil. The results indicated that 12 of the 23 rooms in the hospital found a fungal density above the acceptable standard limit, with higher occurrence in obstetrics rooms. Recently, another study by Bjelić et al. (2020) examined bacterial and fungal occurrence, relative humidity, and temperature in a clinical hospital in Doboj, the Republic of Srpska. They indicated a high microbiological contamination level in the hospital air. Jankowiak et al. (2020) measured microbiological contamination in dispensing areas in pharmacies in hospital buildings. The results showed that the highest heterotrophic bacteria and staphylococci concentrations recorded were in pharmacies. Finally, the microbiology indoor air quality of COVID-19 patients at the hospital before and during the pandemic was discussed by Rahardhiman et al. (2020). Their results revealed that the number of microorganisms was increased before and during the pandemic, although it was still under the quality standard.
Workers and employees spend more than 90% of their time in an enclosed environment over their lifetime. Although numerous researchers in many countries have carried out several studies assessing microbiological contaminants, airborne bacteria and fungi, investigations of the enclosed environment in desert climates are still lacking. Therefore, the primary goal of this study is to assess the indoor environment of a 19-floor office building equipped with a heating, ventilation, and air conditioning (HVAC) system for bacterial and fungal concentrations and composition. The work comprised measuring and identifying bacterial and fungal concentrations in the air of selected floors. It was executed in response to the complaints of the building occupants.
Materials and methods
Site description
The general characteristic of the weather in Kuwait is that of a typical arid, and hot desert clime (excess of evaporation over precipitation) with a maximum air temperature approaching as high as 50 °C between July and August. Such weather conditions lead the population of Kuwait to spend most of their time in an indoor environment. The multistory complex building used for assessing microbiological contaminants is located on the seaside approximately 2 km west of Kuwait City on the Al-Shuwaikh coast (Fig. 1), away from commercial and recreational activities. Airborne microorganisms were collected from 12 sites in the building. The study was carried out on a nineteen-story 10-year-old building with open-space offices and a central HVAC system. The HVAC system of the building was composed of outdoor air intakes located on the first floor and the rooftop, air removal through ceiling vent ducts, and outdoor air passing duct to the air treatment unit (ATU). As needed, fresh and recycled air streams were mixed and filtered, cooled or heated, and humidified or dehumidified in the ATU. Then, conditioned air was distributed throughout a network duct to the supply vents located in the false ceiling. Cooling and heating devices (fan coil units) to cool or heat recycled indoor air were distributed in open spaces.
Fig. 1.
Satellite image of the multistory building complex with surrounding area
Airborne microorganism sampling
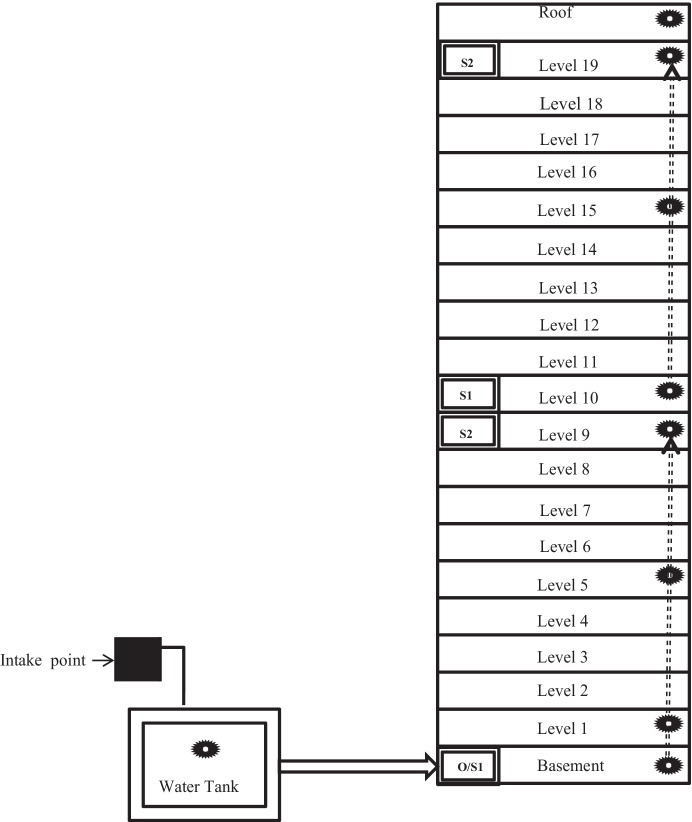
Two sets of sites were designated to follow the duct air distribution from the fresh air intake to the end of the process. The first set of sites went from the basement to the 9th floor, and the second set went from the 10th floor to the rooftop (see Fig. 2 and Table 1). In the center of each office site, the air sample was collected at 1.5 m above the floor twice a day (at 9.00 a.m. and 1.30 p.m.). A total of 120 air samples were collected for bacterial and fungal assessment in each season. A calibrated impactor sampler (a six-stage Anderson air sampler, HRTECH Model: FSC-A6) was used to collect airborne bacteria and fungi at an airflow rate of 0.0283 m3/min for 10 min. At each sampling point, six plates were collected, including one field blank that accompanied the sampler during air monitoring for quality control. The field blanks were processed and analyzed similarly as the rest of the plates. Water samples from the cooling tower water tank were collected for Legionella sp. analysis.
Fig. 2.
Diagram of the sampling points in the office building. O, outdoor air; S1, first ventilation shaft; S2, last ventilation shaft; the black spiky circle, sampling site
Table 1.
Selected site in the building
| Site No | Sample source |
|---|---|
| 1 | Water sample—cooling tower—inlet |
| 2 | Water sample—cooling tower—inside |
| 3 | Air sample—outside-inlet to pump room |
| 4 | Air sample—pump room (basement) |
| 5 | Air sample—main lobby—1st floor |
| 6 | Air sample—meeting room—1st floor |
| 7 | Air sample—office—5th floor |
| 8 | Air sample—office—9th floor |
| 9 | Air sample—office—10th floor |
| 10 | Air sample—office—15th floor |
| 11 | Air sample—gathering room—19th floor |
| 12 | Air sample—roof top—near filter room |
Airborne microorganism’s analyses
Bacterial total counts were examined on trypticase soy agar supplemented with cycloheximide (100 μg/L), and total fungal counts were examined on Rose-Bengal agar supplemented with chloramphenicol (100 μg/L). Petri plates were incubated for 24 h at 37 °C for the bacterial counts and 4–8 days at 24 °C for the fungal counts. The mean colony counts for duplicate samples were estimated and expressed in colony-forming units per cubic meter (CFU/m3). The most dominant bacterial and fungal colonies were selected from each sample and isolated based on their morphology from the indoor and outdoor samples. The water samples from the ATU air humidification tank were analyzed for Legionella spp. according to ISO standard method (ISO, 2004).
Airborne microbial identification
Metabolic fingerprint analysis was conducted for bacterial identification using the Biolog Omnilog GEN III system (Biolog, Hayward, CA, USA). The Biolog Omnilog GEN III system is primarily used for soil and water microorganism identification (Avidano et al., 2005). It tests the ability of microorganisms to utilize or oxidize different carbon sources that can be detected by a redox dye (tetrazolium violet). In brief, the isolated bacteria were grown on Biolog Universal Growth agar at 37 °C for 24 h. The pure colonies were suspended in a 0.4% saline solution, and then their inoculum density was adjusted to the specified turbidity range required. Then, the cells were inoculated in a Biolog GP Microplate and incubated at 37 °C. After 24 h, the result was manually read, and the purple well pattern was analyzed by Biolog_Microlog 2 software. Fungal identification was performed by microscopically examining colony characteristics and their micro- and macromorphological characteristics using standard taxonomic keys (Von Arx, 1981) and by using a Biolog MicroStation (Biolog, Hayward, CA, USA).
Statistical analyses
Statistical analysis was performed using SPSS software for Windows, version 19.0. ANOVA variance analysis was performed on bacteria and fungi indoor air concentrations using the season, floor, and morning or afternoon as critical parameters. Pearson’s correlation coefficient was used to analyze the relationship among the selected parameters.
Results and discussion
Microbiological indoor air quality
Tables 2 and 3 summarize the seasonal sampling’s bacterial and fungal indoor contamination levels. The resulting counts were compared with contamination category values designated by the Commission of European Communities (Table 4, CEC, 1993). The range for bacterial concentrations determined at 37 °C was between 35 and 18,463 CFU/m3, with some values higher than the recommended CEC value for indoor contamination (i.e., 2,000 CFU/m3, high-very high contamination levels) in every season (Tables 2 and 5). During the winter, the fungal contamination level was increase in all selected floors with measured concentrations > 2,000 CFU/m3. However, during the summer, only three floors (9, 10, 19) show high contamination with measured concentrations > 2,000 CFU/m3 (Tables 3 and 6). However, during the spring and autumn, the fungal concentrations decreased with the measured concentrations lower than 100 CFU/m3 (very low-low contamination levels).
Table 2.
Indoor air bacterial (37 ◦C) concentrations (CFU/m3) during seasonal samplings
| Sl. No | Location | Bacterial count (CFU/m3)-AM Mean ± SD |
Bacterial count (CFU/m3)-PM Mean ± SD |
Season effect p value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fall | Winter | Spring | Summer | Fall | Winter | Spring | Summer | Am | Pm | ||
| 3 | Outside-inlet to pump room | 21,000 ± 0 | 3185 ± 315 | 438 ± 87 | 6136 ± 13 | 21,000 ± 0 | 123 ± 123 | 735 ± 175 | 0 | 0 | 0 |
| 4 | Basement-pump room | 18,463 ± 1662 | 2625 ± 35 | 683 ± 18 | 6835 ± 42 | 10,885 + 105 | 14,543 ± 2958 | 473 ± 438 | 7525 ± 525 | 0 | 0.011 |
| 5 | 1st floor-lobby | 11,900 ± 525 | 3780 ± 175 | 4901 ± 40 | 893 ± 88 | 13,388 ± 1663 | 1663 ± 53 | 1435 ± 315 | 595 ± 595 | 0 | 0.001 |
| 6 | 1st floor-meeting room | 578 ± 158 | 1733 ± 52 | 11,731 ± 58 | 6305 ± 95 | 10,500 ± 0 | 9083 ± 8418 | 350 ± 350 | 2013 ± 1593 | 0.166 | 0.368 |
| 7 | 5th floor-office | 11,410 ± 315 | 881 ± 8 | 420 ± 420 | 350 ± 350 | 11,900 ± 350 | 2660 ± 525 | 963 ± 18 | 1330 ± 175 | 0 | 0 |
| 8 | 9th floor-office | 3185 ± 140 | 22,239 ± 27 | 473 ± 473 | 15,232 ± 98 | 1645 ± 35 | 1575 ± 315 | 455 ± 245 | 70 ± 70 | 0.093 | 0.012 |
| 9 | 10th floor-office | 1680 ± 70 | 16,975 ± 1225 | 1085 ± 910 | 2363 ± 88 | 945 ± 210 | 53 ± 18 | 508 ± 438 | 578 ± 473 | 0 | 0.425 |
| 10 | 15th floor-office | 1995 ± 245 | 2853 ± 53 | 1231 ± 8 | 1488 ± 263 | 58,106 ± 30 | 1243 ± 508 | 280 ± 210 | 123 ± 88 | 0.002 | 0.002 |
| 11 | 19th floor-gathering room | 1278 ± 53 | 15,752 ± 10 | 3683 ± 33 | 10,332 ± 62 | 10,852 ± 45 | 25,655 ± 7630 | 630 ± 630 | 1190 ± 70 | 0.085 | 0.023 |
| 12 | Roof top | 3063 ± 788 | 35 ± 35 | 560 ± 560 | 1138 ± 88 | 24,502 ± 80 | 10,728 ± 10,693 | 893 ± 157 | 245 ± 35 | 0.041 | 0.545 |
Table 3.
Indoor air fungal concentrations (CFU/m3) during seasonal samplings
| Sl. No | Location | Fungal count (CFU/m3)-AM | Fungal count (CFU/m3)-PM | Season effect p value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fall | Winter | Spring | Summer | Fall | Winter | Spring | Summer | Am | Pm | ||
| 3 | Outside-inlet to pump room | 210 ± 35 | 1470 ± 1295 | 18 ± 18 | 53 ± 18 | 18 ± 18 | 3693 ± 3413 | 123 ± 53 | 0 | 0.43 | 0.433 |
| 4 | Basement-pump room | 158 ± 88 | 3290 ± 1085 | 18 ± 18 | 35 ± 35 | 18 ± 18 | 980 ± 245 | 0 | 0 | 0.031 | 0.011 |
| 5 | 1st floor-lobby | 0 | 5303 ± 18 | 0 | 0 | 35 ± 0 | 8838 ± 1768 | 0 | 0 | 0 | 0.005 |
| 6 | 1st floor-meeting room | 18 ± 18 | 3203 ± 3098 | 0 | 0 | 0 | 12,530 ± 1995 | 35 ± 0 | 18 ± 18 | 0.457 | 0.002 |
| 7 | 5th floor-office | 35 ± 35 | 753 ± 717 | 0 | 0 | 0 | 525 ± 245 | 0 | 0 | 0.457 | 0.087 |
| 8 | 9th floor-office | 18 ± 18 | 16,468 ± 682 | 0 | 3045 ± 2240 | 0 | 1995 ± 455 | 0 | 18 ± 18 | 0.002 | 0.008 |
| 9 | 10th floor-office | 0 | 22,120 ± 1120 | 18 ± 18 | 25,288 ± 10,763 | 70 ± 35 | 0 | 0 | 16,765 ± 4235 | 0.052 | 0.011 |
| 10 | 15th floor-office | 18 ± 18 | 14,753 ± 18 | 53 ± 18 | 333 ± 157 | 53 ± 53 | 1418 ± 52 | 0 | 245 ± 245 | 0 | 0.004 |
| 11 | 19th floor-gathering room | 18 ± 18 | 5548 ± 4988 | 0 | 10,168 ± 1033 | 0 | 10,675 ± 8715 | 0 | 22,225 ± 16,975 | 0.119 | 0.407 |
| 12 | Roof top | 0 | 245 ± 35 | 35 ± 35 | 158 ± 18 | 70 ± 0 | 17,500 ± 3500 | 0 | 18 ± 18 | 0.008 | 0.005 |
Table 4.
Categories of CFU/m3 (mixed population of bacteria and of fungi) for non-industrial indoor environments (CEC, 1993)
| Category | Bacteria | Fungi |
|---|---|---|
| Very low | < 50 | < 25 |
| Low | < 100 | < 100 |
| Intermediate | < 500 | < 500 |
| High | < 2,000 | < 2,000 |
| Very high | > 2,000 | > 2,000 |
Table 5.
Indoor air bacterial (37 °C) categories of contamination during seasonal samplings
| Sl. No | Location | Bacterial colony (CFU/m3)-AM | Bacterial colony (CFU/ m3)-PM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fall | Winter | Spring | Summer | Fall | Winter | Spring | Summer | ||
| 3 | Outside-inlet to pump room | Very high | Very high | Intermediate | High | Very high | Intermediate | High | Very low |
| 4 | Basement-pump room | Very high | Very high | High | High | Very high | Very high | Intermediate | Very high |
| 5 | 1st floor-lobby | Very high | Very high | Intermediate | High | Very high | High | High | High |
| 6 | 1st floor-meeting room | Intermediate | High | High | Intermediate | Very high | Very high | Intermediate | Very high |
| 7 | 5th floor-office | Very high | Low | Intermediate | Intermediate | Very high | Very high | High | High |
| 8 | 9th floor-office | Very high | Very high | Intermediate | High | High | High | Intermediate | Low |
| 9 | 10th floor-office | High | Very high | High | Very high | High | Low | High | High |
| 10 | 15th floor-office | High | Very high | Intermediate | High | High | High | Intermediate | Intermediate |
| 11 | 19th floor-gathering room | High | High | intermediate | high | high | very high | high | high |
| 12 | Roof top | Very high | Very low | high | high | very high | very high | high | intermediate |
Table 6.
Indoor air fungal categories of contamination during seasonal samplings
| Sl. No | Location | Fungal colony (CFU/m3)-AM | Fungal colony (CFU/m3)-PM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fall | Winter | Spring | Summer | Fall | Winter | Spring | Summer | ||
| 3 | Outside-inlet to pump room | Intermediate | High | Very low | Low | Very low | Very high | Intermediate | Very low |
| 4 | Basement-pump room | Intermediate | Very high | Very low | Low | Very low | High | Very low | Very low |
| 5 | 1st floor-lobby | Very low | Very high | Very low | Very low | Low | Very high | Very low | Very low |
| 6 | 1st floor-meeting room | Very low | Very high | Very low | Very low | Very low | Very high | Low | Very low |
| 7 | 5th floor-office | Low | High | Very low | Very low | Very low | High | Very low | Very low |
| 8 | 9th floor-office | Very low | Very high | Very low | Very high | Very low | High | Very low | Very low |
| 9 | 10th floor-office | Very low | Very high | Very low | Very high | Low | Very low | Very low | Very high |
| 10 | 15th floor-office | Very low | Very high | Low | Intermediate | Low | High | Very low | Intermediate |
| 11 | 19th floor-gathering room | Very low | Very high | Very low | Very high | Very low | Very high | Very low | Very high |
| 12 | Roof top | Very low | Intermediate | Low | Intermediate | Low | Very high | Very low | Very low |
The analysis of raw data with ANOVA showed significant differences in indoor bacterial and fungal contaminations are associated with the season (Table 7). The increasing trend for bacterial concentration was from spring to summer, to winter and fall, whereas the increase in fungal concentration was only in winter. Moreover, both the detected daily bacteria concentration measured at 37 °C and indoor fungal contamination showed significant differences. Each showed a tendency of higher contamination levels in the morning than in the afternoon (Table 7). No significant correlation was detected between bacterial concentration at 37 °C and fungal concentration using Pearson’s correlation coefficient.
Table 7.
Variance analysis for bacterial and fungal counts with respect to season and sampling hour
| Variance | Bacteria 37 °C | Fungi 24 °C |
|---|---|---|
| Season | p = 0.001–0.041 (Au > W > Su > Sp) | p = 0–0.031 (W > Su > Au > Sp) |
| Hour | p = 0.001–0.032 (m > a) | p = 0–0.002 (m > a) |
Sp spring, Su summer, W winter, Au autumn, m morning, a afternoon
HVAC system air treatment unit microbial analyses
The effect of the ATU system on IAQ was evaluated by comparing the outdoor air and the indoor air sample microbial concentrations that were collected near air supply diffusers (Tables 8 and 9). The evaluation showed that fungal and bacterial counts from outdoor air increased from summer to winter/autumn. The fungal and bacterial counts were > 2,000 CFU/m3 in both the outdoor air and the indoor air diffusers in the winter samples, indicating that the ATU could not efficiently eliminate the fungal and bacterial material in outdoor air with high contamination. The ratio for indoor/outdoor air samples (I/O; Table 10) was estimated from the microbial counts for indoor and outdoor air samples determined throughout the four seasons. The I/O ratios revealed that the fungal concentrations in autumn, summer, and spring and the bacterial concentrations in autumn were higher outdoors than they were indoors, which is in agreement with other published studies in the area of office building air quality (Bonetta et al., 2010; Tsai et al., 2007). Alternatively, I/O ratios close to 1 or higher were found for fungi in winter, and bacteria in the winter, spring, and summer at 37 °C indicated higher fungal and bacterial air contamination inside than outside the building.
Table 8.
Bacterial and fungal concentration (CFU/m3) in outdoor air and at the ventilation shafts during seasonal samplings (AM)
| Parameter (CFU/m3) | Location | Autumn Mean ± SD |
Winter Mean ± SD |
Spring Mean ± SD |
Summer Mean ± SD |
|---|---|---|---|---|---|
| Bacteria 37 °C | O | 21,000 ± 0 | 3185 ± 315 | 438 ± 87 | 613 ± 613 |
| Fungi 24 °C | O | 210 ± 35 | 1470 ± 1295 | 18 ± 18 | 53 ± 18 |
| Bacteria 37 °C | S1 | 11,900 ± 525 | 3780 ± 175 | 490 ± 140 | 893 ± 88 |
| Fungi 24 °C | S1 | 0 | 5303 ± 18 | 0 | 0 |
| Bacteria 37 °C | S2 | 3185 ± 140 | 2223 ± 927 | 473 ± 473 | 1523 ± 298 |
| Fungi 24 °C | S2 | 18 ± 18 | 16,468 ± 682 | 0 | 3045 ± 2240 |
SD standard deviation, O outdoor air collected at the intake point of the ATU system, S1 indoor air at the level of the first ventilation shaft after the ATU system, S2 indoor air at the level of the last ventilation shaft after the ATU system, a mean value of the two measurements
Table 9.
Bacterial and fungal concentration (CFU/m3) in outdoor air and at the ventilation shafts during seasonal samplings (PM)
| Parameter (CFU/m3) | Location | Autumn Meana ± SD |
Winter Meana ± SD |
Spring Meana ± SD |
Summer Meana ± SD |
|---|---|---|---|---|---|
| Bacteria 37 °C | O | 21,000 ± 0 | 3185 ± 315 | 438 ± 87 | 613 ± 613 |
| Fungi 24 °C | O | 210 ± 35 | 1470 ± 1295 | 18 ± 18 | 53 ± 18 |
| Bacteria 37 °C | S1 | 1680 ± 70 | 16,975 ± 1225 | 1085 ± 910 | 2363 ± 88 |
| Fungi 24 °C | S1 | 0 | 22,120 ± 1120 | 18 ± 18 | 25,288 ± 10,763 |
| Bacteria 37 °C | S2 | 1278 ± 53 | 1575 ± 210 | 368 ± 333 | 1033 ± 262 |
| Fungi 24 °C | S2 | 18 ± 18 | 5548 ± 4988 | 0 | 10,168 ± 1033 |
SD standard deviation, O outdoor air collected at the intake point of the ATU system, S1 indoor air at the level of the first ventilation shaft after the ATU system, S2 indoor air at the level of the last ventilation shaft after the ATU system, a mean value of the two measurements
Table 10.
Indoor/outdoor ratios calculated for bacterial and fungal loads in seasonal samplings
| Season | Ia/Ob | |
|---|---|---|
| Bacteria 37 °C | Fungi 24 °C | |
| Autumn | 0.57 | 0 |
| Winter | 1.2 | 3.61 |
| Spring | 1.12 | 0 |
| Summer | 1.46 | 0 |
aMeans of microbiological counts obtained at the level of open-spaces (level1, lobby)
bMicrobiological counts obtained at the intake point of the ATU system (outside-inlet)
HVAC system humidification water tank microbial analyses
Contamination of the indoor environment by Legionella spp. is among the most common causes of severe pneumonia in society and is the cause of approximately 1–40% of hospital-acquired pneumonia cases (Diederen, 2008). Legionella spp. was isolated from the water inside the cooling tower tank in the spring and summer with a trend of increasing concentration throughout the warmer seasons (spring to summer) (Table 11). The Legionella concentration was > 1000 CFU/L, which means that the water tank needs to be disinfected and cleaned within 7 days, and the cleaning and disinfecting program should be reviewed (AWT, 2003).
Table 11.
Legionella spp. contamination of humidification water tank
| Microorganism | Autumn | Winter | Spring | Summer |
|---|---|---|---|---|
| Legionella spp. (CFU/L) | a < 100 CFU/L | a < 100 CFU/L | 2 × 106 | 107 |
a < 100 CFU/L: detection limit for Legionella spp. analysis
Identification of airborne bacteria
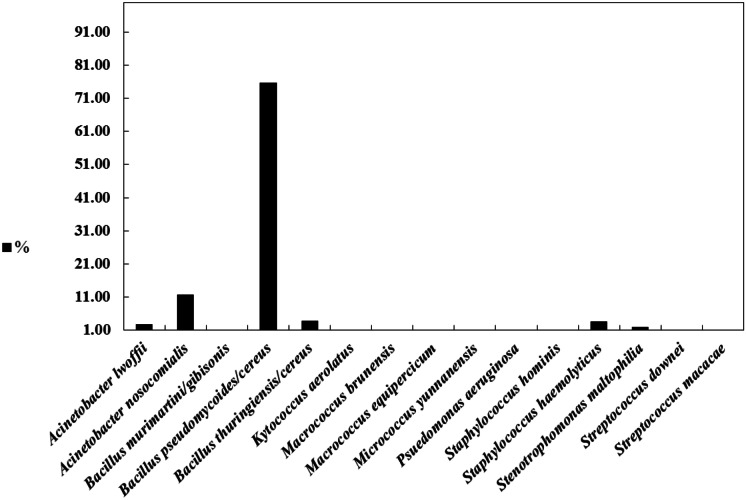
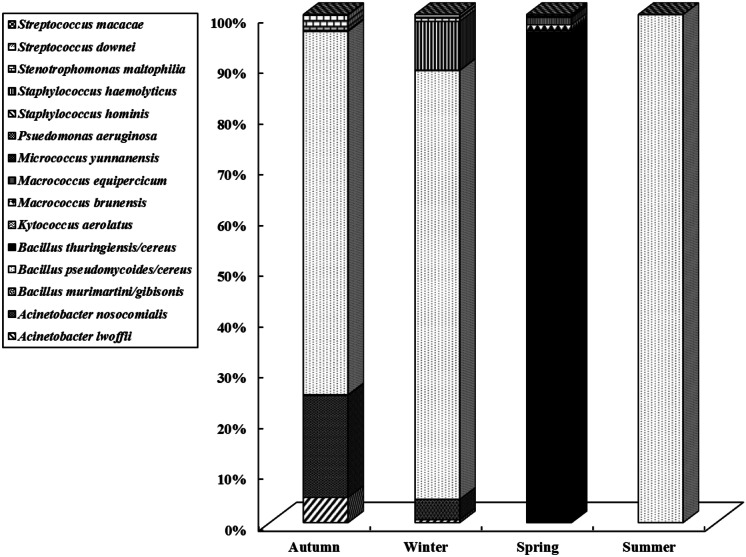
The most prevalent bacterial species identified in the indoor air of the building were Bacillus pseudomycoides/cereus (75.7%), Acinetobacter nosocomialis (11.7%), Bacillus thuringiensis/cereus (3.9%), Staphylococcus hemolyticus (3.6%), Acinetobacter lwoffii (2.7%), and Stenotrophomonas maltophilia (1.9%) (Fig. 3). Figure 4 shows the seasonal distribution of bacterial species in the indoor air. Bacillus pseudomycoides/cereus was identified in the autumn indoor air samples (71.5%) in winter (84.4%), and in summer (100%). Nevertheless, it disappeared in spring, and Bacillus thuringiensis/cereus (96.6%) was identified in all spring indoor air samples. Species normally occurring on human skin (Acinetobacter lwoffii, Staphylococcus hominis, Staphylococcus hemolyticus, Micrococcus yunnanensis, Streptococcus macacae) were also identified. Some of the identified bacteria (Acinetobacter lwoffii, Acinetobacter nosocomialis, Bacillus thuringiensis/cereus, Pseudomonas aeruginosa, Staphylococcus hemolyticus, Staphylococcus hominis, Stenotrophomonas maltophilia, and Streptococcus downei) can be considered opportunistic pathogens, while others (Bacillus murimartini/gibsonii, Bacillus pseudomycoides/cereus, Macrococcus brunensis, Micrococcus yunnanensis, Streptococcus macacae) are typically found in environmental samples. According to other studies, the most dominant bacteria in indoor environments were Bacillus spp. (Andualem et al., 2019; Idris et al., 2020). In the outdoor air, environmental species such as Acinetobacter lwoffii, Acinetobacter nosocomialis, Bacillus pseudomycoides/cereus, Bacillus thuringiensis/cereus, Stenotrophomonas maltophilia, and Micrococcus yunnanensis were mainly encountered. Acinetobacter lwoffii and Micrococcus yunnanensis were only encountered in the outdoor air.
Fig. 3.
Percentage of the total isolated bacterial species in the building in all seasons
Fig. 4.
Distribution of bacteria species in indoor air during different seasons
Identification of airborne fungi
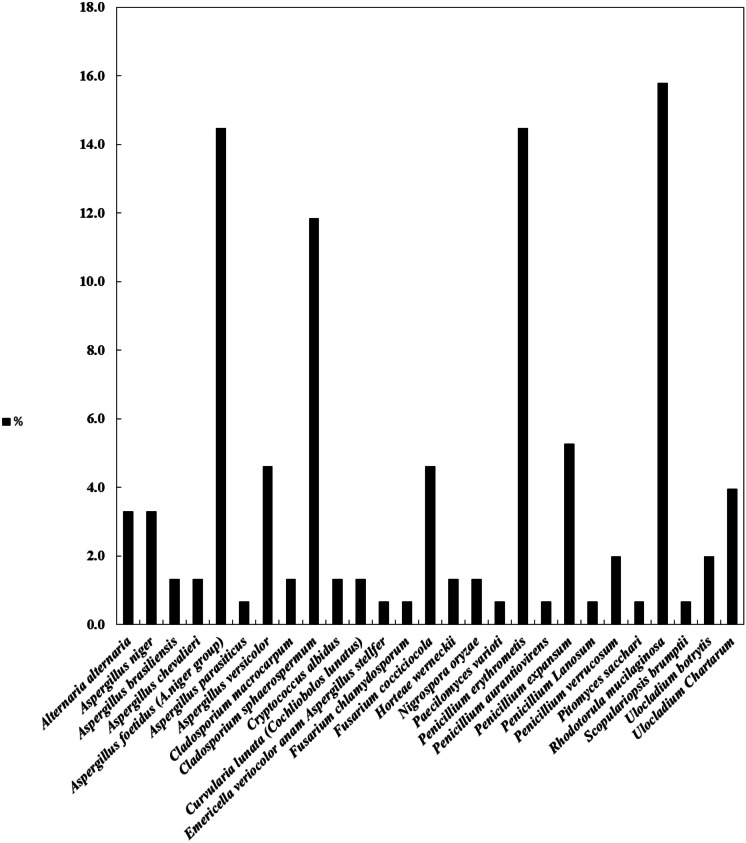
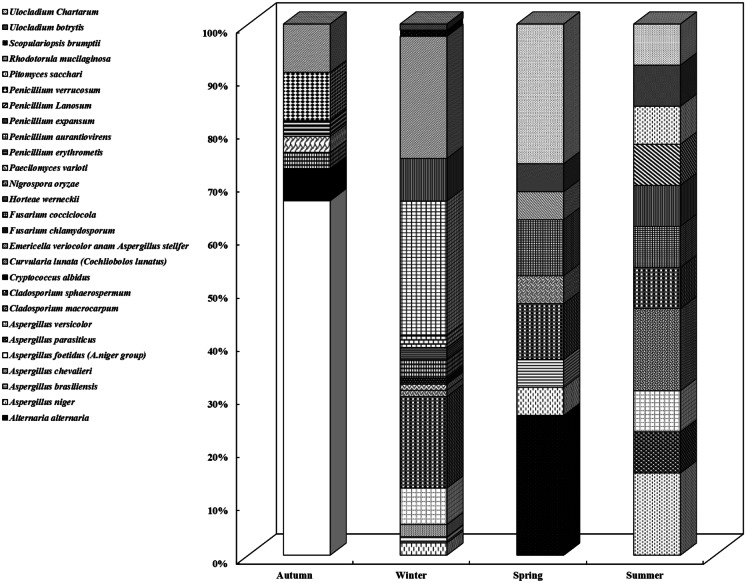
The most widespread fungal genera identified in the indoor air of the building were Aspergillus spp. (25.7%), Penicillium spp. (23%), Rhodotorula spp. (15.8%), Cladosporium spp. (13.2%), Ulocladium spp. (5.9%), and Fusarium spp. (5.3%) (Fig. 5). Figure 6 shows the seasonal distribution of fungal species in the indoor air. Aspergillus foetidus (66.7%) was identified in all autumn indoor air samples, Penicillium aurantiovirens (25.3%) and Rhodotorula mucilaginosa (23%) were found in winter, Alternaria alternata (26.3%) and Ulocladium chartarum (26.3%) were found in spring, and Aspergillus niger (15.4%) and Cladosporium macrocarpum (15.4%) were found in summer. Other studies identified Aspergillus spp., Penicillium spp., and C. sphaerospermum as the most prevalent fungal genera in the environment of building indoor air (Nevalainen & Seuri, 2005; Rejc et al., 2020). Penicillium spp., Rhodotorula mucilaginosa, and Cladosporium spp. were predominant in winter indoor air (33.3%), (23%), and (17.2%), respectively. In contrast, in autumn, Aspergillus spp. was the most widespread genus (66.7%). The identified fungal genera (Aspergillus, Penicillium, and Cladosporium) in the indoor air were previously documented as potential causes of respiratory allergies (Schwab & Straus, 2004; Tasic & Tasic, 2007), which also associated Penicillium species with sick building syndrome.
Fig. 5.
Percentage of total isolated fungal species in the building in all seasons
Fig. 6.
Distribution of fungal species in indoor air during different seasons
Conclusion
This study assessed the airborne microbial concentrations and compositions in the indoor and outdoor environments of a multistory building complex equipped with an HVAC system. It was executed in response to the complaints of the building occupants. Our data showed that airborne bacterial and fungal concentrations in all floors ranged from less than 2,000 to more than 2,000 CFU/m3, demonstrating a high–very high contamination level according to the Commission of European Communities (CEC, 1993). In indoor air, higher concentrations of airborne bacteria compared to that detected outdoors may be associated with several internal sources, including human activity. During the winter, airborne fungal concentrations on all floors ranged from less than 2,000 to more than 2,000 CFU/m3, demonstrating a high–very high contamination level according to the Commission of European Communities. Statistical analysis showed no correlation between the bacterial and fungal concentrations, demonstrating that they originated from unrelated sources. Opportunistic pathogen bacterial species were isolated from indoor air (Acinetobacter lwoffii, Acinetobacter nosocomialis, Bacillus thuringiensis/cereus, Pseudomonas aeruginosa, Staphylococcus hemolyticus, Staphylococcus hominis, Stenotrophomonas maltophilia, and Streptococcus downei); however, the low counts recorded and the heterogeneity of the bacterial species do not indicate any potential risk for the health of building occupants. In contrast, during the summer and winter sampling, the isolation of potentially allergenic genera with high concentrations (Aspergillus, Cladosporium, Penicillium, and Rhodotorula) indicated a potential health risk for the building occupants. The results obtained are the basis for the recommendation that the maintenance activities of the HVAC system and the periodical cleaning operation program be revised and preplanned as protective measures. Moreover, the water tank within the ATU system should be cleaned with extreme care during the operation.
Acknowledgements
The authors gratefully acknowledge the financial support of this research study of the indoor/outdoor air quality at the Oil Sector Complex Building (EM073C) from Kuwait Petroleum Corporation (KPC). The continuous support and cooperation of Mr. Abdulraheem Al-Rashidi, Environment Manager, are also gratefully acknowledged and highly appreciated.
Nomenclatures and acronyms
- ANOVA
Analysis of variance
- ATU
Air treatment unit
- HVAC system
Heating, ventilation, and air conditioning system
- I/O
Indoor-to-outdoor ratios
- IAQ
Indoor air quality
- ISO
International organization for standardization
- CEC value
Commission of European Communities value
- CFU/m3
Colony-forming units per cubic meter of air
- CFU/L
Colony-forming units per liter of water
- COVID-19
Coronavirus disease 2019
- PM
Particulate matter
- QuEChER method
Quick, easy, cheap, effective, rugged, and safe method
Author contribution
QA supervised the microbiological work, analyzed and interpreted the data, and wrote the manuscript. MA is the research leader who participated in reviewing and writing the manuscript. MY is the research team member who participated in reviewing and writing the manuscript. VJ performed and analyzed the microbiological work. All authors read and approved the final manuscript.
Funding
This study was funded by the Kuwait Petroleum Corporation (KPC) and Kuwait Institute for Scientific Research (KISR).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andualem Z, Gizaw Z, Bogale L, Dagne H. Indoor bacterial load and its correlation to physical indoor air quality parameters in public primary schools. Multidisciplinary Respiratory Medicine. 2019;14:1–7. doi: 10.1186/s40248-018-0167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidano L, Gamalero E, Cossa GP, Carraro E. Characterization of soil health in an Italian polluted site by using microorganisms as bioindicators. Applied Soil Ecology. 2005;30:21–33. doi: 10.1016/j.apsoil.2005.01.003. [DOI] [Google Scholar]
- Awad AH, Saeed Y, Hassan Y, Fawzy Y, Osman M. Air microbial quality in certain public buildings, Egypt: A comparative study. Atmospheric Pollution Research. 2018;9:617–626. doi: 10.1016/j.apr.2017.12.014. [DOI] [Google Scholar]
- AWT. (2003). Legionella 2003: An update and statement by the Association of Water Technologies (AWT). Association of Water Technologies. Rockville, M.D. Available at http: www.legionella.com/images/awtlegionella2003.pdf; Accessed on June 2003.
- Bjelić, L. S., Ilić, P., & Farooqi Z. U. R. (2020). Indoor microbiological air pollution in the hospital. Quality of Life (Banja Luka) – APEIRON. 11, 5–10. 10.7251/QOL2001005S.
- Bonetta SA, Bonetta SI, Mosso S, Sampò S, Carraro E. Assessment of microbiological indoor air quality in an Italian office building equipped with an HVAC system. Environmental Monitoring and Assessment. 2010;161:473–483. doi: 10.1007/s10661-009-0761-8. [DOI] [PubMed] [Google Scholar]
- Brągoszewska E, Biedroń I. Indoor air quality and potential health risk impacts of exposure to antibiotic resistant bacteria in office rooms in Southern Poland. International Journal of Environmental Research and Public Health. 2018;15:2604. doi: 10.3390/ijerph15112604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brągoszewska E, Biedroń I, Kozielska B, Pastuszka JS. Microbiological indoor air quality in an office building in Gliwice, Poland: Analysis of the case study. Air Quality, Atmosphere, and Health. 2018;11:729–740. doi: 10.1007/s11869-018-0579-z. [DOI] [Google Scholar]
- Brągoszewska E, Biedroń I, Mainka A. Microbiological air quality in a high school gym located in an urban area of Southern Poland—Preliminary research. Atmosphere. 2020;11:797. doi: 10.3390/atmos11080797. [DOI] [Google Scholar]
- Busso IT, Herrera F, Tames MF, Gasquez IG, Camisassa LN, Carreras HA. QuEChER method for air microbiological monitoring in hospital environments. Journal of Infection in Developing Countries. 2020;14:66–73. doi: 10.3855/jidc.11563. [DOI] [PubMed] [Google Scholar]
- CEC. (1993). Indoor air quality & its impact on man. Biological particles in indoor environments. Commission of European Communities. Report 12. Cost Project 613. EUR. 14988 EN.
- Chegini FM, Baghani AN, Hassanvand MS, Sorooshian A, Golbaz S, Bakhtiari R, Ashouri A, Joubani MN, Alimohammadi M. Indoor and outdoor airborne bacterial and fungal air quality in kindergartens: Seasonal distribution, genera, levels, and factors influencing their concentration. Building and Environment. 2020;175:106690. doi: 10.1016/j.buildenv.2020.106690. [DOI] [Google Scholar]
- Diederen BMW. Legionella spp. and Legionnaires’ disease. Journal of Infection. 2008;56:1–12. doi: 10.1016/j.jinf.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Dang, D. Y. N., Vuong, H. N., Nguyen, T. T., & Phan, T. T. T. (2020). Microbiological contamination of indoor air in university classrooms (Case study: University of Science-Vietnam National University, Ho Chi Minh city). Vietnam Journal of Science, Technology and Engineering. 62, 30–35. https://vietnamscience.vjst.vn/index.php/VJSTE/article/view/369
- Ekhaise FO, Erhunmwunsee MI. Microbiological indoor air quality of male student hostels in University of Benin, (Ugbowo Campus), Benin City, Nigeria. NISEB Journal. 2013;13:91–106. [Google Scholar]
- Enitan SS, Ihongbe JC, Ochei JO, Effedua HI, Adeyemi O, Phillips T. Microbiological assessment of indoor air quality of some selected private primary schools in Ilishan-Remo, Ogun state, Nigeria. International Journal of Medical and Health Research. 2017;3:8–19. [Google Scholar]
- Er CM, Sunar NM, Leman AM, Othman N, Emparan Q, Parjo UK, Gani P, Jamal NA, Ideris NA. The evaluation of indoor microbial air quality in two new commissioning higher educational buildings in Johor, Malaysia. Applied Mechanics and Materials. 2015;773–774:1068–1072. doi: 10.4028/www.scientific.net/AMM.773-774.1068. [DOI] [Google Scholar]
- Fekadu S, Getachewu B. Microbiological assessment of indoor air of Teaching hospital wards: A case of Jimma University specialized hospital. Ethiopian Journal of Health Sciences. 2015;25:117–122. doi: 10.4314/ejhs.v25i2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fsadni P, Frank B, Fsadni C, Montefort S. The impact of microbiological pollutants on school indoor air quality. Journal of Geosciences and Environment Protection. 2017;5:54–65. doi: 10.4236/gep.2017.55004. [DOI] [Google Scholar]
- Gilbert JA, Stephens B. Microbiology of the built environment. Nature Reviews Microbiology. 2018;16:661–670. doi: 10.1038/s41579-018-0065-5. [DOI] [PubMed] [Google Scholar]
- Gołofit-Szymczak M, Górny RL. Microbiological air quality in office buildings equipped with dventilation systems. Indoor Air. 2018;28:792–805. doi: 10.1111/ina.12495. [DOI] [PubMed] [Google Scholar]
- Graudenz GS, Oliveira CH, Tribess A, Jr, Mendes C, Latorre MR, Kalil J. Association of air conditioning with respiratory symptoms in office workers in tropical climate. Indoor Air. 2005;15:62–66. doi: 10.1111/j.1600-0668.2004.00324.x. [DOI] [PubMed] [Google Scholar]
- Grisoli P, Albertoni M, Rodolfi M. Application of airborne microorganism indexes in offices, gyms, and libraries. Applied Sciences. 2019;9:1101. doi: 10.3390/app9061101. [DOI] [Google Scholar]
- Harbizadeh A, Mirzaee SA, Khosravi AD, Shoushtari FS, Goodarzi H, Alavi N, Ankali KA, Rad HD, Maleki H, Goudarzi G. Indoor and outdoor airborne bacterial air quality in day care centers (DCCs) in greater Ahvaz, Iran. Atmospheric Environment. 2019;216:116927. doi: 10.1016/j.atmosenv.2019.116927. [DOI] [Google Scholar]
- Hospodsky D, Qian J, Nazaroff WW, Yamamoto N, Bibby K, Rismani-Yazdi H, Peccia J. Human occupancy as a source of indoor airborne bacteria. PLoS ONE. 2012;7:e34867. doi: 10.1371/journal.pone.0034867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Agranovski I, Pyankov O, Grinshpun S. Removal of viable bioaerosol particles with a low-efficiency HVAC filter enhanced by continuous emission of unipolar air ions. Indoor Air. 2008;18:106–112. doi: 10.1111/j.1600-0668.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- Huttunen K, Rintala H, Hirvonen MR, Vepsäläinen A, Hyvärinen A, Meklin T, et al. Indoor air particles and bioaerosols before and after renovation of moisture-damaged buildings: The effect on biological activity and microbial flora. Environmental Research. 2008;107:291–298. doi: 10.1016/j.envres.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Idris SAA, Hanafiah MM, Ismail M, Abdullah S, Khan MF. Laboratory air quality and microbiological contamination in a university building. Arabian Journal of Geosciences. 2020;13:580. doi: 10.1007/s12517-020-05564-8. [DOI] [Google Scholar]
- ISO. (2004). ISO 11731, 2004, Water Quality—Detection and Enumeration of Legionella—Part 2: Direct Membrane Filtration Method for Waters with Low Bacterial Counts; ISO: Geneva, Switzerland.
- Jankowiak E, Kubera Ł, Małecka-Adamowicz M, Dembowska E. Microbiological air quality in pharmacies and an antibiotic resistance profile of staphylococci species. Aerobiologia. 2020;36:551–563. doi: 10.1007/s10453-020-09651-x. [DOI] [Google Scholar]
- Kalogerakis N, Paschali D, Lekaditis V, Pantidou A, Eleftheriadis K, Lazaridis M. Indoor air quality-bioaerosol measurements in domestic and office premises. Journal of Aerosol Science. 2005;36:751–761. doi: 10.1016/j.jaerosci.2005.02.004. [DOI] [Google Scholar]
- Kim KY, Kim CN. Airborne microbiological characteristics in public buildings of Korea. Builting and Environment. 2007;42:2188–2196. doi: 10.1016/j.buildenv.2006.04.013. [DOI] [Google Scholar]
- Law AKY, Chau CK, Chan GYS. Characteristics of bioaerosol profile in office buildings in Hong Kong. Building and Environment. 2001;36:527–541. doi: 10.1016/S0360-1323(00)00020-2. [DOI] [Google Scholar]
- Łukaszuk CR, Krajewska-Kułak E, Kułak W. Effects of fungal air pollution on human health. Progress in Health Sciences. 2011;1:156–164. [Google Scholar]
- Mazlan, S. M., Hamzah, A., Noor, W. S., & Abas, A. (2020). Assessment of Indoor Microbiological Air Contamination in Research Facility at University of Malaysia. 10.21203/rs.3.rs-42886/v1
- Meadow JF, Altrichter AE, Kembel SW, Kline J, Mhuireach G, Moriyama M, &, , et al. Indoor airborne bacterial communities are influenced by ventilation, occupancy, and outdoor air sources. Indoor Air. 2014;24:41–48. doi: 10.1111/ina.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen A, Seuri M. Of microbes and men. Indoor Air. 2005;15:58–64. doi: 10.1111/j.1600-0668.2005.00344.x. [DOI] [PubMed] [Google Scholar]
- Onet A, Ilies DC, Buhas S, Rahota D, Ilies A, Balas S, Marcu F, Herman GV. Microbial air contamination in indoor environment of university sports hall. Journal of Environmental Protection and Ecology. 2018;19:694–703. [Google Scholar]
- Osman ME, Ibrahim HY, Yousef FA, Elnasr AA, Saeed Y, Hameed AA. A study on microbiological contamination on air quality in hospitals in Egypt. Indoor and Built Environment. 2017;27:953–968. doi: 10.1177/1420326X17698193. [DOI] [Google Scholar]
- Qian J, Hospodsky D, Yamamoto N, Nazaroff WW, Peccia J. Size-resolved emission rates of airborne bacteria and fungi in an occupied classroom. Indoor Air. 2012;22:339–351. doi: 10.1111/j.1600-0668.2012.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahardhiman A, Yudhastuti R, Azizah R. Microbiology indoor air quality at hospital during the covid19 pandemic. Jurnal Kesehatan Lingkungan. 2020;12:89–92. doi: 10.20473/jkl.v12i1si.2020.89-92. [DOI] [Google Scholar]
- Rejc T, Kukec A, Bizjak M, GodičTorkar K. Microbiological and chemical quality of indoor air in kindergartens in Slovenia. International Journal of Environmental Health Research. 2020;30:49–62. doi: 10.1080/09603123.2019.1572870. [DOI] [PubMed] [Google Scholar]
- Schwab CJ, Straus DC. The roles of Penicillium and Aspergillus in sick building syndrome. Advances in Applied Microbiology. 2004;55:215–238. doi: 10.1016/S0065-2164(04)55008-6. [DOI] [PubMed] [Google Scholar]
- Shoemaker RC, House DE. Sick building syndrome (SBS) and exposure to water-damaged buildings: Time series study, clinical trial and mechanisms. Neurotoxicology and Teratology. 2006;28:573–588. doi: 10.1016/j.ntt.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Stanley NJ, Kuehn TH, Kim SW, Raynor PC, Anantharaman S, Ramakrishnan MA, &, , et al. Background culturable bacteria aerosol in two large public buildings using HVAC filters as long-term, passive, high-volume air samplers. Journal of Environmental Monitoring. 2008;10:474–481. doi: 10.1039/b719316e. [DOI] [PubMed] [Google Scholar]
- Stetzenbach, L. D. (2007). Introduction to Aerobiology. In C. Hurst, R. Crawford, J. Garland, D. Lipson, A. Mills, & L. Stetzenbach (Eds.), Manual of Environmental Microbiology (3rd ed., pp 925–938). ASM Press, Washington, DC. 10.1128/9781555815882.ch73
- Tasic S, Tasic MN. Cladosporium spp.-cause of opportunistic mycoses. Acta Facultatis Medicae Naissensis. 2007;24:15–19. [Google Scholar]
- Taubel M, Rintala H, Pitkaranta M, Paulin L, Laitinen S, Pekkanen J, et al. The occupant as a source of house dust bacteria. Journal of Allergy and Clinical Immunology. 2009;124:834–840. doi: 10.1016/j.jaci.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Tsai F, Macher J, Hung Y. Biodiversity and concentrations of airborne fungi in large US office buildings from the BASE study. Atmospheric Environment. 2007;41:5181–5191. doi: 10.1016/j.atmosenv.2006.06.069. [DOI] [Google Scholar]
- Verde SC, Almeida SM, Matos J, Guerreiro D, Meneses M, Faria T, Botelho D, Santos M, Viegas C. Microbiological assessment of indoor air quality at different hospital sites. Microbiological Research. 2015;166:557–563. doi: 10.1016/j.resmic.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Von Arx JA. The genera of fungi sporulating in pure culture. Lubrecht & Cramer Ltd.; 1981. [Google Scholar]
- Wamedo SA, Ede PN, Chuku A. Interaction between building design and indoor airborne microbial load in Nigeria. Asian Journal of Biological Sciences. 2012;5:183–191. doi: 10.3923/ajbs.2012.183.191. [DOI] [Google Scholar]
- Yu BF, Hu ZB, Liu M, Yang HL, Kong QX, Liu YH. Review of research on air-conditioning systems and indoor air quality control for human health. International Journal of Refrigeration. 2009;32:3–20. doi: 10.1016/j.ijrefrig.2008.05.004. [DOI] [Google Scholar]
- Zenaide-Neto, H., & Nascimento, J. S. do. (2020). Air quality and microbiological control in a hospital in Paraíba, Brazil. International journal of advanced research in science. 7, 99–108. doi:10.22161/ijaers.79.13 Available at: http://journal-repository.com/index.php/ijaers/article/view/2474 (Accessed: 6April2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.