Abstract
The TWIK-related K+ channels (TREK-1) is a two-pore domain potassium channel that produces background leaky type potassium currents. TREK-1 has a protective role against ischemia-induced neuronal damage. TREK-1 is also expressed in the heart, but its role in myocardial ischemia-reperfusion (IR)-induced injury has not been examined. In the current study, we used a TREK-1 knockout (KO) mouse model to show that TREK-1 has a critical role in the cardiac I/R-induced injury and during remodeling after myocardial infarction (MI). At baseline, TREK-1 KO mice had similar blood pressure and heart rate as the wildtype (WT) mice. However, the lack of TREK-1 was associated with increased susceptibility to ischemic injury and compromised functional recovery following ex-vivo I/R-induced injury. TREK-1 deficiency increased infarct size following permanent coronary artery ligation, resulting in greater systolic dysfunction than the WT counterpart. Electrocardiographic (ECG) analysis revealed QT interval prolongation in TREK-1 KO mice, but normal heart rate (HR). Acutely isolated TREK-1 KO cardiomyocytes exhibited prolonged Ca2+ transients duration associated with action potential duration (APD) prolongation. Our data suggest that TREK-1 has a protective effect against I/R-induced injury and influences the post-MI remodeling processes by regulating membrane potential and maintaining intracellular Ca2+ homeostasis. These data suggest that TREK-1 activation could be an effective strategy to provide cardioprotection against ischemia-induced damage.
Keywords: Potassium channel, ischemia-reperfusion, mice, telemetry, myocardial infarction, TREK-1
Introduction
Two-pore domain potassium channels (K2P channels) are a newly identified class of channels expressed throughout the animal kingdom and in plants (10, 12). K2P channels are encoded by 15 different genes classified in six clades. Assembled as functional dimers, as opposed to other potassium channels, which are tetramers, each subunit has four transmembrane domains (M1-M4) and two-pore domains (36). In contrast to the voltage-dependent channel, K2P channels lack an S4 voltage-sensing domain, and it is now clear that they are functionally important in the thalamus [6], immune cells [12], the heart [10,8], in the adrenal cortex [5] and the lung [28].
Evidence of a functional role for K2P channels in the heart is strongest for TWIK-related acid-sensitive K(+) channel 1 (TASK-1) and TWIK-related potassium channel-1 (TREK-1) channels [14]. The presence of TREK-1 mRNA was detected in cardiac tissue and rat cardiomyocytes (2, 27), functional TREK-1-like channels in ventricular myocytes was demonstrated with patch-clamp technique [34]. In the human heart, TREK-1 channels downregulation has been linked to heart failure and cardiac arrhythmogenesis (25). Although TREK-1 is the most studied among all the K2P members, the importance of TREK-1 in native cells has been difficult to characterize because of the lack of potent or selective inhibitors.
TREK-1 channel was initially regarded as a passive ‘leak channel’ that could influence both the resting membrane potential and the repolarization phase of the action potential along with the voltage-gated and Ca2+-activated outward rectifiers [29]. TREK-1 channels can be activated by a variety of physical stimuli including membrane stretch, pH, temperature, and polyunsaturated fatty acids such as docosahexaenoic acid and arachidonic acid (AA) and by volatile anesthetic [17]. Conversely, TREK-1 channels are inhibited by cAMP and following Gαq and Gαs activation [16,22,26]. Interestingly, cardiac ischemia and ischemia followed by reperfusion are associated with accumulation of fatty acids, including AA and changes in pH that may regulate TREK-1 channels activity with profound effects on the progression of ischemic injury. Nonetheless, the overall contribution of TREK-1 in regulating cardiac excitability is unknown.
Given the potential importance of TREK-1 in cardiac pathophysiology, in this study, we used TREK-1 KO and WT mice to show that loss of TREK-1 exacerbated I/R-induced injury and impaired the recovery of contractile function after an ischemic event. We further demonstrated that absence of TREK-1 increase adverse ventricular remodeling after myocardial infarction. At cellular level, TREK-1 channel is involved in the regulation of Ca2+ transients and cardiac action potential duration (APD). We concluded that TREK-1 channel activity protects the ischemic myocardium and is beneficial during post-MI remodeling by regulating APD and preventing intracellular Ca2+ overload. New pharmacological approaches targeting this channel represents a new class of potential therapeutic agents.
Methods
Mouse experiments.
All mice experiments were performed according to protocols approved by the Institutional Animal Care and Use Committees (IACUC) and comply with USA regulations on animal experimentation. TREK-1 KO and WT mice were bred on the same C57BL/6 genetic background and were a kind gift from Dr. Robert Bryan (Baylor Houston). Mice were housed in a facility maintained at 22°C with an alternating 12-h light/dark cycle and had free access to food and water ad libitum. All mice used in these experiments were between 8-14 weeks of age, weighing between 20-30 gr, and gender-matched.
Telemetric blood pressure and ECG measurements.
Implantable radio transmitters were used to assessECG and blood pressure (BP), the full procedure has been reported previously [4]. Briefly, mice were anesthetized with isoflurane and implanted with radio-transmitters (model HD-X11, Data Sciences International). The catheter was placed near the ascending aorta via the right carotid artery and the emitter inserted subcutaneously in the right dorsal flank. The two ECG leads were implanted subcutaneously, with one lead toward the right upper chest and the other near the left lower chest. Mice were allowed to recover from surgery for 5-7 days before baseline recording started. ECG, heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP) were collected using Dataquest ART data acquisition system (Data Sciences International, Inc.). Upon activation of the transmitter by a magnet, the electrical signals were transmitted wirelessly to a nearby receiver, and amplified signals were sent to a computer system. Data were sampled continuously and stored on a hard disc for offline analysis with LabChart Pro8 (ADInstruments). For analysis purposes, 20-40 second intervals (every 5 minutes) of recording were analyzed and averaged. In another group of animals, ECG activity was monitored during anesthesia as follows: single mice was housed in an anesthesia induction chamber (25 × 10 × 12 cm) containing a mixture of 1.2% isoflurane and room air at a rate of 0.5 l/min (SamnoSuite, Kent Scientific Corporation, Connecticut, USA). The anesthesia was maintained for a 45-min period (anesthetic maintenance state). In preliminary experiments, 3% isoflurane anesthesia often resulted in sick sinus syndrome and these experiments were terminated. All experiments were conducted between 1:00pm and 4:00pm.
Ischemia-reperfusion in the isolated heart
Mice were sacrificed, hearts were rapidly excised and retrogradely perfused via the aorta with modified Krebs– Henseleit solution (in mmol/l: NaCl 118.5, CaCl2 1.85, KCl 4.5, glucose 11.1, NaHCO3 25, MgSO4 2.5, NaH2PO4 1.4) gassed with a mixture of 95% O2 and 5% CO2 at 37 °C ± 0.5 °C, pH 7.35 ± 0.05. Perfusion flow through the aorta was constant and perfusion pressure was maintained between 70 and 90 mmHg. Left ventricular developed pressure (LVDP) was measured by a polyethylene tube with a cling film balloon tip filled with degassed water and inserted into the left ventricle through a small incision in the left atrium. The other end of the tube was connected to a pressure transducer. After an equilibration period (15-20 min), hearts were paced electrically paced (6 Hz) and subjected to global no-flow ischemia (20-min), followed by reperfusion (40 min). Data were discarded if one of the following criteria appeared: i) hearts with irreversible arrhythmia during equilibration or after ischemia, and/or ii) absence of recovery after the reperfusion phase.
Assay of myocardial infarct area.
After reperfusion, the heart was arrested in diastole by adding 2,3-butanedione monoxime (10 mM) to the perfusion buffer. Next, the heart was quickly removed and placed at −20°C for a few minutes to harden. The heart was then sliced transversally at a thickness of 1 mm and immersed in neutral 2,3,5-triphenyltetrazolium (TTC) solution for 15 min to 20 min at 37°C, and then placed in 4% formaldehyde solution overnight. The viable myocardium was stained as the water-soluble compound TTC was converted by active mitochondrial dehydrogenases into an insoluble red precipitate. The extent of staining correlates with the number of viable mitochondria and differentiated viable and non-viable tissue [13]. TTC stained area (red staining, ischemic area) and non-TTC stained area (white, infarct area) were analyzed with a digital imaging system by computer, and myocardial infarct area was calculated [36].
Chronic myocardial infarction model.
Two to three-month-old male mice were anesthetized with isoflurane. Mice were intubated and attached to a ventilator. After surgical preparation, a 1 cm vertical incision was performed in the midclavicular line for a lateral thoracotomy. Mice were subjected to myocardial infarction by permanent ligation (8/0 nylon suture) of the left anterior descending coronary artery at about 1 mm below the edge of the left auricle, or to sham operation. The chest was closed, and the pneumothorax reduced. Mice were monitored continuously during recovery until the righting reflex was regained. Post-operative analgesia (buprenorphine, 0.075 mg/kg) was administered subcutaneously twice daily for three days. Non-invasive echocardiographic evaluation was performed to monitor cardiac function. At the end of the fourth week post-MI period, mice were sacrificed, and hearts were collected for further analysis.
Echocardiography.
Mice from both groups were anesthetized with isoflurane and placed on the warming pad of a recording stage of a Vevo 2100 ultrasound machine. The anterior chest was shaved, ultrasound coupling gel was applied, electrodes were connected to each limb, and an electrocardiogram was simultaneously recorded while body temperature was monitored. Two-dimensional (short axis-guided) M-mode measurements (at the level of the papillary muscles) were taken using an 18–32 MHz MS400 transducer as previously described [19,25]. Images were also recorded in the parasternal long-axis. For analysis purposes, three or more beats were averaged; measurements within the same HR interval (450 ± 50 bpm) were used for analysis.
Histological and morphometric analysis.
For morphometric studies, the heart was arrested in diastole by KCl injection and was fixed in 4% paraformaldehyde for 20 minutes under pressure (diastolic arterial pressure). The heart was excised, weighed, and stored for 24-48 hours in 4% paraformaldehyde at 4°C. Picrosirius Red and Masson’s trichrome staining were performed on paraffin-embedded tissue sections from four weeks infarcted hearts, fibrosis and the infarcted area was measured [36].
Cardiomyocytes isolation and intracellular Ca2+ measurements.
Intracellular Ca2+ activity was measured using methods described previously [21,18]. Hearts were Langendorff-perfused with nominally Ca2+-free Tyrode’s solution containing the following (in mM): 137 NaCl, 5.4 KCl, 1 MgCl2, 0.33 NaH2PO4, 10 HEPES, and 10 glucose (pH 7.4) equilibrated with 100% O2 (35 ± 1°C). After the blood was washed out, collagenase type-2 (1 mg/ml; Worthington, Biochemical), protease type XIV (0.02 mg/ml; Sigma), and elastase (0.2 mg/ml; Worthington, Biochemical) were added to the perfusion solution. When the heart became dilated, it was detached from the system, both atria were removed and myocytes from the left ventricle were dispersed in the solution. Ventricular myocytes were subjected to Ca2+ re-adaptation and finally re-suspended in Tyrode’s solution (0.5 mM CaCl2). Cells were used within six hours from the isolation. For intracellular Ca2+ measurements, myocytes were incubated with fura-2 for 30 min at room temperature (22–24°C). Excess fura-2 was washed out and the cells were re-suspended in physiological buffer containing 0.5 mM CaCl2. Cells were placed on a stage of an inverted microscope and continuously perfused with Tyrode’s solution (1 mM CaCl2). Cells were electrically paced at 0.5-Hz to accurately measure resting diastolic intracellular Ca2+ ([Ca2+]i). Fura-2 Ca2+ transients were captured using excitation wavelength set at 340 and 380nm and emission was detected at 510 nm with a dual-excitation fluorescence multiplier system (IonOptics).
Optical membrane potential measurements.
Experiments using FluoVolt-AM ester (ThermoFisher) at 1/1000 dilution was used according to manufacturer’s instructions. FluoVolt loaded cells were allowed to settle at the bottom of a perfusion chamber with a coverslip base, which was mounted on an inverted fluorescence microscope mounting a 40X silicone oil immersion objective. Cells were perfused with a buffer containing 1mM CaCl2, and electrically paced at 1-Hz. Fluorescence emission between 520 and 535 nm was detected by a photomultiplier tube (IonOptics).
Data analysis.
All results are expressed as mean ± S.E.M., and N = sample size (N represents the number of cells, animals or independent experiments as reported in the text). Outlier values were identified as values outside of the mean ± 2 SD interval. Statistical analysis was performed by using a paired t-test or, where appropriate, one-way ANOVA followed by Bonferroni correction, which was calculated with GraphPad Prism, version 7 (GraphPad Software; San Diego, CA, USA). For all statistical comparisons, statistical significance was set at P < 0.05.
Results
TREK-1 KO hearts show increased susceptibility to myocardial I/R-induced injury.
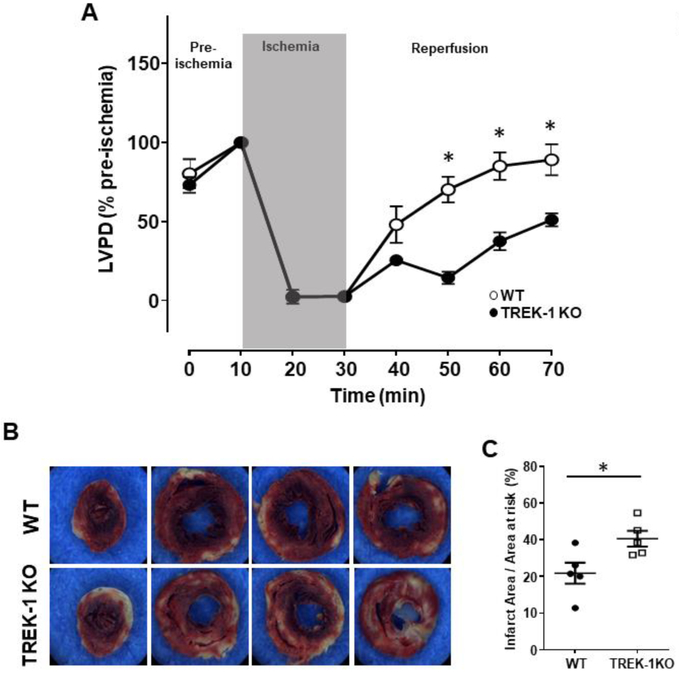
Myocardial ischemia followed by reperfusion has detrimental effects on the heart, often resulting in injured myocardium. TREK-1 channels protect neurons against I/R-induced injury [15]. Therefore, we investigated whether TREK-1 channel activity preserve cardiac function during I/R-induced damage. We studied left ventricular (LV) function in Langendorff-perfused isolated hearts subjected to 20 min of global ischemia followed by 40 min of reperfusion. During equilibration time (pre-ischemia), LVDP was similar between the TREK-1 KO and WT hearts suggesting that at baseline TREK-1 deletion does not alter the mechanical properties of the cardiac muscle (figure 1A). Recovery of LVDP after ischemia was significantly impaired in the TREK-1 KO hearts at each time point examined during the reperfusion period (figure 1A). At the end of the reperfusion phase, TTC staining revealed that the partial LVDP recovery in TREK-1 KO hearts was associated with larger infarct size when compared to the infarct size from the control hearts (figures 1B and C). Thus, disruption of TREK-1 severely compromises recovery of cardiac contractility in TREK-1 KO hearts and results in greater I/R-induced cardiac damage.
Figure 1.
TREK-1 deficiency impairs cardiac recovery after global IR. A) Time course representing changes in LVDP over time. The first LVDP value is obtained from spontaneously beating hearts; thereafter the organ was electrically paced (6Hz). The equilibration phase (10 min) is followed by 20 min of ischemia, where LVDP drops to near zero. Slow, but gradual LVDP recovery can be observed during the reperfusion phase (40 min). Overall, WT hearts showed better functional recovery during reperfusion than TREK-1 KO hearts. LVDP is expressed as % of baseline with data points measured at 10 min intervals. N = 8 hearts each group, *P < 0.05. B) Representative TTC stained ventricular sections from the apex (left picture) to the base (right picture) from WT and TREK-1 KO hearts. Myocardial infarct size was determined by TTC staining, and myocardial infarct size normalized to the area at risk. Unaffected myocardium is stained red; necrotic areas are white. Scale bar = 1mm. C) Quantification of the infarct size, (N = 5, mean ± S.E.), *P < 0.05 vs. WT controls.
Absence of TREK-1 accelerates heart failure post-MI.
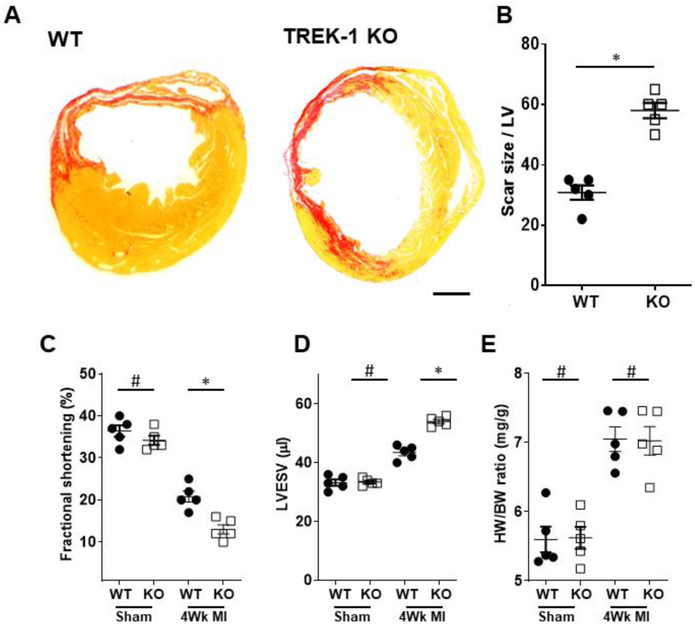
To determine whether TREK-1 is a critical determinant of the cardiac response to in-vivo chronic ischemic injury, we subjected TREK-1 KO and littermate WT mice to permanent coronary artery ligation or sham surgery. Four weeks post-surgery, TREK-1 KO hearts exhibited a much larger infarct size than their WT counterpart as demonstrated by Sirius Red staining (figures 2A and B). Quantitative estimation of LV function by echocardiography showed that cardiac contractility as indicated by fractional shortening (%FS) and left ventricular end-systolic volume (LVESV) were similar between WT and TREK-1 KO sham-operated mice. However, infarcted hearts showed deteriorated cardiac function in both groups as indicated by a drop %FS, which was significantly greater in the TREK-1 KO mice (figure 2C). TREK-1 KO mice post-MI showed a significant increase in LVESV when compared to the WT post-MI group (figure 2D). No difference in heart weight/body weight ratio was observed (figure 2E).
Figure 2.
Adverse effect of TREK-1 deletion during post-MI remodeling. A) Transverse ventricular sections from hearts after four weeks of permanent coronary artery ligation stained with Sirius Red. B) The percentage infarct segment in the left ventricle. C) Echocardiographic assessment of cardiac function by fractional shortening (FS) and D) left ventricular end-diastolic volume (LVEDV). E) Heart weight (HW)/body weight (BW) ratio. Data are extracted from WT (N = 5) and TREK-1 KO (N = 5) mice and are expressed as mean ± S.E. #P> 0.5; *P < 0.05 vs. WT controls. Statistical significance was evaluated using one-way ANOVA testing for the difference between multiple groups followed by Bonferroni's post-hoc comparisons tests. Scale bar = 1mm.
Functional alteration is paralleled by structural changes, detailed structural measurements performed on the sham-operated and post-MI mice are included in Table 1. At baseline TREK-1 mice did not ehibit and structural abnormality, however, analysis post-MI mice revealed greater LV diameter in TREK-1 KO hearts associated with thinner posterior wall thickness, which are indicative of accelerated maladaptive cardiac remodeling. These data suggest that TREK-1 deletion does not alter cardiac function and structure at baseline, but accelerates adverse post-MI ventricular remodeling.
Table 1.
Blood pressure values in WT controls and TREK-1 KO mice recorded by telemeters. N = 5 each group. Values are presented as mean ± S.E.
| Sham |
MI |
P<0.05 | |||
|---|---|---|---|---|---|
| Control | TREK-1 | Control | TREK1 | ||
| N | 7 | 7 | 9 | 7 | |
| HR | 450 ± 20 | 425 ± 18 | 435 ± 24 | 415 ± 13 | n.s. |
| LVIDd (mm) | 3.86 ± 0.06 | 3.80 ± 0.05 | 4.56 ± 0.05 | 5.82 ± 0.07* | P<0.05 |
| LVIDs (mm) | 3.08 ± 0.06 | 3.12 ± 0.05 | 3.85 ± 0.04 | 5.23 ± 0.04* | P<0.05 |
| LVPWd (mm) | 0.92 ± 0.06 | 0.96 ± 0.05 | 1.85 ± 0.08 | 1.44 ± 0.04* | P<0.05 |
| LVPWs (mm) | 1.48 ± 0.07 | 1.42 ± 0.04 | 2.34 ± 0.05 | 1.86 ± 0.05* | P<0.05 |
TREK-1 KO mice display prolonged QT-interval
Potassium channels play a vital role in the control of the electrical activity of the heart. Whether TREK-1 is involved in the propagation of electric current through the myocardium during each heartbeat is still undetermined. Non-invasive unrestrained telemetry was used to investigate the cardiac electrical conduction in TREK-1 KO and WT mice.
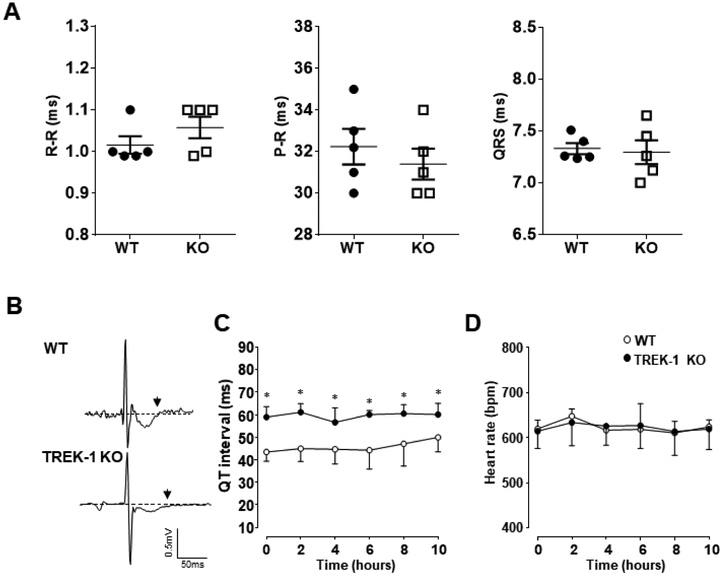
ECG recordings revealed similar R-R, P-R and QRS intervals between TREK-1 KO and WT mice (figure 3A). ECG traces were evaluated for disturbances in rhythm and waveform morphology, and traces from TREK-1 KO mice displayed prolonged QT intervals associated with relatively wide dispersion, averaging 60.1 ± 5.6 ms in TREK-1 KO mice vs. 49.4 ± 7.3 ms in the control group (P<0.05) (figures 3B and C). All mice analyzed maintained a normal sinus rhythm during the recording despite the prolonged QT interval (figure 3D). Previous reports have described the presence of TREK-1 in smooth muscle cells [20,7], therefore, knockout of TREK-1 in smooth muscle could be a significant contribution to the observed mouse cardiac phenotype. Hemodynamic parameters including mean arterial pressure (MAP), systolic and diastolic blood pressure parameters were measured simultaneously to the ECG we found that all hemodynamic parameters measured were within a normal range between the two groups of animals (Table 2).
Figure 3.
Analysis of ECG subintervals recorded using a telemetric system. A) Averages of R-R, P-R, and QRS intervals are provided. All intervals are given in milliseconds (ms). B) Representative ECG traces from 2-lead recordings; waveforms were synchronized on the “R” peak. Top, an example of a recording from a WT mouse presenting a normal QT interval. Bottom, an example of a recording from a TREK-1 KO mouse showing prolonged QT dispersion. The end of the T-wave is indicated by the back arrow. C) Changes in QT intervals over a 10-hour period of continuous recording. D) Heart rate changes over the same period showed in (C). n = 5 per group; *P < 0.05 versus WT mice.
Table 2.
Echocardiographic results from sham-operated WT and TREK-1 KO mice and after four weeks post-myocardial infarction. N: number of animals; HR: heart rate; LVID: left ventricular internal diameter; LVPW: left ventricular diastolic posterior wall thickness; Data are expressed as mean ± S.E. *P<0.05 vs. WT four-week postinfarction group.
| Control | TREK-1 | |
|---|---|---|
| MAP (mmHg) | 96.93 ± 2.63 | 96.64 ± 5.26 |
| Systolic pressure (mmHg) | 113.56 ± 3.73 | 114 ± 4.93 |
| Diastolic pressure (mmHg) | 81.01 ± 6.23 | 79.32 ± 5.89 |
TREK-1 KO exaggerates isoflurane-induced QT prolongation.
The ability of volatile anesthetics to activate TREK-1 channels is well documented [29]. Clinically relevant concentration of isoflurane is known to prolong the QT interval duration without significant changes in QRS or PR duration in both laboratory animals [30], and in healthy human hearts [33,37,27]. Hence, we examined the effect of isoflurane on TREK-1 KO and WT mice while recording ECG. We reasoned that activation of TREK-1 by isoflurane would result in attenuated isoflurane-induced QT interval prolongation.
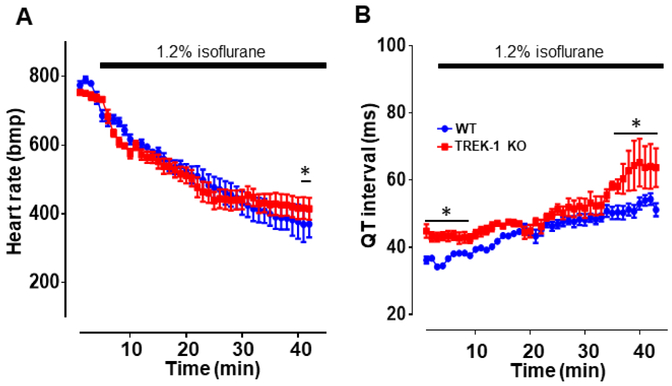
Mice carrying telemetric transmitters were singly housed in an anesthesia induction chamber while ECG recording was capturing the heart electrical behavior. At baseline, in the absence of isoflurane, the heart rate was not significantly different between the two groups (WT, 774 ± 55 vs. TREK-1 KO, 753 ± 68, P<0.05). The heart rate was slightly higher than what is usually observed in this mouse strain. Given that mice that are placed in their cages did not show any difference in heart rate we conclude that the higher heart rate is a result of the mice being placed into the induction chamber. When isoflurane was introduced in the chamber a steady drop of HR was observed in both TREK-1 KO and WT mice. Although HR decrease was similar between the two groups, after prolonged exposure to the anesthetic, WT mice displayed a lower HR than the TREK-1 KO mice (figure 4A). At baseline, QT interval duration was higher in the TREK-1 KO mice as compared to the WT mice. In presence of isoflurane, QT interval was similar in the two groups and only after prolonged exposure to isoflurane TREK-1 KO mice exhibited a greater QT interval duration than the WT counterpart (figure 4B). These results suggest that TREK-1 activation by isoflurane protects the heart against excessive QT interval prolongation.
Figure 4.
Time course of the ECG changes during isoflurane administration in TREK-1 KO and WT mice. A) Heart rate recorded for 30 min in the presence of 1.2% isoflurane. B) QT interval duration extracted from the same traces used in A. Data are presented as mean ± S.E., n=5, *P < 0.05 vs. WT controls.
Loss of TREK-1 delay intracellular Ca2+ clearance and prolongs action potential duration in isolated cardiomyocytes.
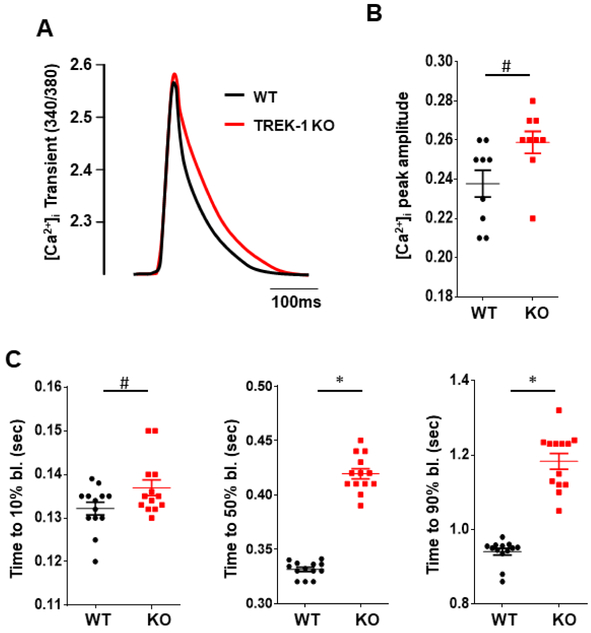
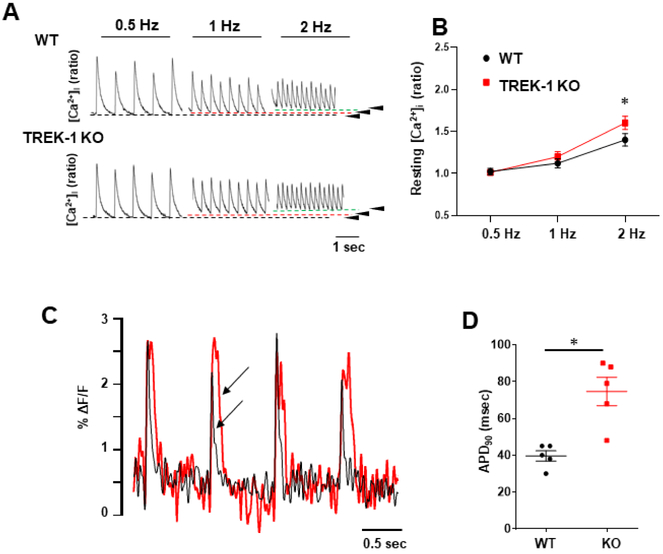
Changes in intracellular Ca2+ dynamics and APD prolongation in cardiomyocytes often precede ischemic damage and maladaptive post-MI remodeling. Intracelluar Ca2+ and membrane potential play critical roles in determining the extent of tissue injury. We measured Ca2+ transients in freshly isolated ventricular cardiomyocytes from TREK-1 KO and WT hearts. Cardiomyocytes isolated from both groups and subjected to electrical field stimulation showed no difference in Ca2+ transient amplitude at steady state (figures 5A and B). Cells exhibited similar decay constant of the Ca2+ transient at 10% decay time (figure 5C). In contrast, time to 50% and 90% Ca2+decay was prolonged in TREK-1 KO cells when compared to WT cells. Interestingly, when cells were paced at higher frequency resting intracellular Ca2+ concentration was higher in TREK-1 KO cells (figures 6A and B). Analysis of optically recorded action potential waveforms from isolated adult ventricular myocytes revealed that action potential duration was significantly prolonged in TREK-1 KO cells as compared to WT cells (figures 6C and D). This is consistent with the prolonged QT interval and the long-lasting Ca2+ decay observed in TREK-1 KO mice. Our results suggest that TREK-1 channels reduce cell repolarization time facilitating intracellular Ca2+ clearance and that TREK-1 deletion can lead to Ca2+ overload and have a harmful effect during I/R and post-MI remodeling.
Figure 5.
Intracellular Ca2+ transients analysis in single cardiomyocytes isolated from TREK-1 KO and WT hearts. A) Representative traces of Ca2+ transient from TREK-1 KO and WT cells, traces are normalized and superimposed to facilitate comparison. B) Quantitative analysis showed no difference in the amplitude of Ca2+ transients between the two groups. C) Time to 10%, 50% and 90% decay of Ca2+ transient signal. N = 9 to 14cells/5 hearts. Values are presented as mean ± S.E. *P < 0.05 vs. WT.
Figure 6.
Intracellular Ca2+ transients in response to increase in frequency and membrane potential measurements. Stimulation from 0.5 to 4 Hz. A) representative traces of intracellular Ca2+ in myocytes from adult Wt (n = 35) and TREK-1 KO (n = 27) myocytes (isolated from 4 mice each group). The numbers on the traces indicate the frequency of stimulation and transients evoked at 0.5, 1, and 2 Hz. B) Intracellular Ca2+ at resting level while stimulated at the correspondent frequency, values measured at the resting level indicated by the arrow-heads in panel A. C) Optical measurements of action potentials duration (AP) by using a voltage-sensitive dye (FluoVolt). Comparison of action potential measured using FluoVolt loaded cardiomyocyte isolated from TREK-1 KO and WT hearts. Cells were paced electrically at 1-Hz and AP waveform was recorded as a FluoVolt fluorescence signal. The figure shows a representative trace from each group containing five peaks superimposed to emphasize action potential prolongation in TREK-1 KO cardiomyocytes (arrow-heads, red trace). B) Peaks were evaluated for time to baseline (APD90%), five peaks from each cell were averaged, each point represents APD averaged from each heart 15 cells/5 hearts. Values are presented as mean ± S.E.*P < 0.05 compared to the control group.
Discussion.
The presence of TREK-1 channels in the cardiomyocytes and the likelihood that these channels regulate cardiac function has been reported [1,3,8]. However, it is not clear whether TREK-1 activity has any role during pathological conditions such as ischemia or during the progression of post-MI injury. Pharmacological approaches that attempt to manipulate TREK-1 function are difficult to interpret because TREK-1 is insensitive to most of the classical K+ channel blockers. Using a TREK-1 KO mouse model, we showed that loss of TREK-1 exacerbated myocardial IR-injury resulting in increased myocardial damage and worsening functional recovery during reperfusion. We also provided evidence that TREK-1 could be protective against post-MI maladaptive remodeling. We further demonstrated that TREK-1 actively regulated cardiac repolarization and intracellular Ca2+ clearance by shortening the APD. Overall, our data show a critical role of TREK-1 in the healthy heart and during pathological conditions.
TREK-1 contribute to the cardiac electrical signal. Telemetry recordings from awake unanesthetized mice revealed a significant increase of QT intervals at baseline condition. Our data are supported by similar results reported earlier using a cardiac-specific TREK-1 KO mouse model and a mouse model lacking TASK-1 channels, another member of the K2P channel [31,11]. Furthermore, exposure to clinically relevant concentrations of isoflurane is known to prolong the QT-interval in the rodents and the human heart mainly by suppression of Ito current. Indeed, isoflurane significantly increased QT interval duration in TREK-1 KO mice, suggesting that the absence of TREK-1 current delays ventricular repolarization. Although TREK-1 KO mice showed a normal cardiac rhythm at rest, the HR in TREK-1 KO mice exposed to isoflurane was higher than the HR in WT mice; this suggest a functional role of TREK-1 in the sino-atrial nodal cell. These findings are supported by earlier reports which identified functional TREK-1 currents during sinoatrial node excitation [31]. The potential role of TREK-1 on these cells need to be further explored.
The most striking finding was that TREK-1 KO hearts were more susceptible to I/R-induced cardiac damage than the control counterpart. This is somewhat different from a previous finding showing that TREK-1 knockdown in neonatal rat cardiomyocytes is protective against ischemic damage in vitro [35]. Nevertheless, neonatal rat cardiomyocytes are not fully differentiated characterized by an hypometabolic status and are more tolerant to ischemia than the adult mouse hearts [2,23]. Our results are more in line to those studies that utilize in vivo models, for instance, it has been shown that TREK-1 KO adult mice are susceptible to brain ischemia [15]. Mechanistically, TREK-1 channel may be activated by intracellular acidification and AA accumulation known to occur during ischemia [32,34,9]. TREK-1 activation could facilitate membrane repolarization and shorten the APD protecting the cells from Ca2+ overload, hence, preventing cell death and tissue damage.
Studies on computational models have shown that subtraction of TREK-1 currents from rodent action potential results in about 12 % increase in APD [31]. Here we have employed cell-permeable fluorophores for a rapid and convenient measure of the cardiomyocyte membrane potential and intracellular Ca2+ changes. These experiments demonstrate that TREK-1 is active during membrane repolarization and its absence results in a significantly prolonged APD in TREK-1 KO cardiomyocytes, which is associated with a delay in Ca2+ transient decay. Although these fluorophores are not suitable for assessing resting membrane potential, previous studies have shown that TREK-1 contributes to the setting of resting membrane potential [38]. Prolongation of APD, coupled with prolonged Ca2+ clearance time, suggests that TREK-1 KO cells are prone to Ca2+ overload during ischemic conditions. Disturbance of the Ca2+ clearance and, therefore, increasing intracellular Ca2+ concentration became more evident when cells were stimulated at higher frequency, again suggesting that these cells are prone to Ca2+ overload and explains TREK-1 KO cells vulnerability to ischemic injury. Because these cells are mechanically unoaded they cannot reach the same frequency of the mouse heart rate. Further investigation into TREK-1 role in the regulation of intracellular Ca2+ and ADP during ischemic conditions may provide new important regulatory mechanisms involving TREK-1 in the protection against IR -induced damage and post-MI cardiac remodeling. Prolongation of both action potential and Ca2+ transients can have inotropic effects due to an increase in sarcoplasmic reticulum Ca2+ uptake. We did not observe an increase in contractility in TREK-1 KO hearts, probably due to the permanent deletion of TREK-1 and potential remodeling of the excitation contraction-coupling key components to compensate for the loss of TREK-1 channel.
TREK-1 global KO show worsening of post-MI remodeling when compared to the WT group, however, it is difficult to pinpoint which cell population is contributing to the MI injury. Careful interpretation of the results of these experiments is critically important. For instance, it could be argued that since TREK-1 has been reported in smooth muscle, changes in coronary artery function may be responsible for worsening of the cardiac function after injury. Earlier reports have clearly shown that absence of TREK-1 does not affect the vascular system [24]. This accords with our findings where basic hemodynamic and blood pressure is not changed in the TREK-1 KO mice when compared to data from the WT mice. Moreover, during the IR experiments, perfusion pressure and flow rate of the coronary were not different between WT and TREK-1 KO hearts. We did not attempt a detailed analysis of cardiac remodeling post-MI because of the involvement of immnune-response and cardiac fibroblast during this process which can also be affected by TREK-1 deletion [1]. Our studies in acutely isolated cardiomyocytes suggest a functional role of TREK-1 in the myocardium. Further studies in this direction could benefit from tissue-specific and inducible TREK-1 KO models. Overall, the data presented here suggest the involvement of TREK-1 channels in regulating cardiac excitability. The possibility that TREK-1 channel openers can be cardio-protective without causing adverse effects on the vasculature deserves further investigation.
Acknowledgments.
We would like to thank Mr. Jesse Gammons for his help in finalizing this manuscript. This work was supported by intramural department funds and by National Heart, Lung and Blood Institute (grant number HL114869, to Dr. S.M. and HL131526 and HL123540 to CMW).
Footnotes
Disclosures.
There are no competing interests.
Compliance with Ethical Standards:
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committees and comply with USA regulations on animal experimentation.
Informed consent:
Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Bibliography.
- 1.Abraham DM, Lee TE, Watson LJ, Mao L, Chandok G, Wang HG, Frangakis S, Pitt GS, Shah SH, Wolf MJ, Rockman HA (2018) The two-pore domain potassium channel TREK-1 mediates cardiac fibrosis and diastolic dysfunction. The Journal of clinical investigation 128:4843–4855. doi: 10.1172/JCI95945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adolph EF (1948) Tolerance to cold and anoxia in infant rats. The American journal of physiology 155:366–377. doi: 10.1152/ajplegacy.1948.155.3.366 [DOI] [PubMed] [Google Scholar]
- 3.Aimond F, Rauzier JM, Bony C, Vassort G (2000) Simultaneous activation of p38 MAPK and p42/44 MAPK by ATP stimulates the K+ current ITREK in cardiomyocytes. The Journal of biological chemistry 275:39110–39116. doi: 10.1074/jbc.M008192200 [DOI] [PubMed] [Google Scholar]
- 4.Alam MA, Parks C, Mancarella S (2016) Long-term Blood Pressure Measurement in Freely Moving Mice Using Telemetry. Journal of visualized experiments : JoVE. doi: 10.3791/53991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandulik S, Tauber P, Lalli E, Barhanin J, Warth R (2015) Two-pore domain potassium channels in the adrenal cortex. Pflugers Archiv : European journal of physiology 467:1027–1042. doi: 10.1007/s00424-014-1628-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bista P, Cerina M, Ehling P, Leist M, Pape HC, Meuth SG, Budde T (2015) The role of two-pore-domain background K(+) (K(2)p) channels in the thalamus. Pflugers Archiv : European journal of physiology 467:895–905. doi: 10.1007/s00424-014-1632-x [DOI] [PubMed] [Google Scholar]
- 7.Blondeau N, Petrault O, Manta S, Giordanengo V, Gounon P, Bordet R, Lazdunski M, Heurteaux C (2007) Polyunsaturated fatty acids are cerebral vasodilators via the TREK-1 potassium channel. Circulation research 101:176–184. doi: 10.1161/CIRCRESAHA.107.154443 [DOI] [PubMed] [Google Scholar]
- 8.Bodnar M, Schlichthorl G, Daut J (2015) The potassium current carried by TREK-1 channels in rat cardiac ventricular muscle. Pflugers Archiv : European journal of physiology 467:1069–1079. doi: 10.1007/s00424-014-1678-9 [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet E (1999) Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev 79:917–1017 [DOI] [PubMed] [Google Scholar]
- 10.Decher N, Kiper AK, Rolfes C, Schulze-Bahr E, Rinne S (2015) The role of acid-sensitive two-pore domain potassium channels in cardiac electrophysiology: focus on arrhythmias. Pflugers Archiv : European journal of physiology 467:1055–1067. doi: 10.1007/s00424-014-1637-5 [DOI] [PubMed] [Google Scholar]
- 11.Decher N, Wemhoner K, Rinne S, Netter MF, Zuzarte M, Aller MI, Kaufmann SG, Li XT, Meuth SG, Daut J, Sachse FB, Maier SK (2011) Knock-out of the potassium channel TASK-1 leads to a prolonged QT interval and a disturbed QRS complex. Cell Physiol Biochem 28:77–86. doi: 10.1159/000331715 [DOI] [PubMed] [Google Scholar]
- 12.Ehling P, Cerina M, Budde T, Meuth SG, Bittner S (2015) The CNS under pathophysiologic attack--examining the role of K(2)p channels. Pflugers Archiv : European journal of physiology 467:959–972. doi: 10.1007/s00424-014-1664-2 [DOI] [PubMed] [Google Scholar]
- 13.Ferrera R, Larese A, Berthod F, Guidollet J, Rodriguez C, Dureau G, Dittmar A (1993) Quantitative reduction of MTT by hearts biopsies in vitro is an index of viability. J Mol Cell Cardiol 25:1091–1099. doi: 10.1006/jmcc.1993.1121 [DOI] [PubMed] [Google Scholar]
- 14.Gurney A, Manoury B (2009) Two-pore potassium channels in the cardiovascular system. Eur Biophys J 38:305–318. doi: 10.1007/s00249-008-0326-8 [DOI] [PubMed] [Google Scholar]
- 15.Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M (2004) TREK-1, a K+ channel involved in neuroprotection and general anesthesia. The EMBO journal 23:2684–2695. doi: 10.1038/sj.emboj.7600234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang D, Han J, Kim D (2006) Mechanism of inhibition of TREK-2 (K2P10.1) by the Gq-coupled M3 muscarinic receptor. American journal of physiology Cell physiology 1:C649–656. doi: 10.1152/ajpcell.00047.2006 [DOI] [PubMed] [Google Scholar]
- 17.Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M (2000) Polyunsaturated fatty acids are potent neuroprotectors. The EMBO journal 19:1784–1793. doi: 10.1093/emboj/19.8.1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, Mancarella S, Coromilas J, Lederer WJ, Kass RS, Marks AR (2006) Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proceedings of the National Academy of Sciences of the United States of America 103:7906–7910. doi: 10.1073/pnas.0602133103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Matta SM, Sullivan RD, Bahouth SW (2014) Carvedilol reverses cardiac insufficiency in AKAP5 knockout mice by normalizing the activities of calcineurin and CaMKII. Cardiovascular research 104:270–279. doi: 10.1093/cvr/cvu209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma R, Seifi M, Papanikolaou M, Brown JF, Swinny JD, Lewis A (2018) TREK-1 Channel Expression in Smooth Muscle as a Target for Regulating Murine Intestinal Contractility: Therapeutic Implications for Motility Disorders. Front Physiol 9:157. doi: 10.3389/fphys.2018.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancarella S, Yue Y, Karnabi E, Qu Y, El-Sherif N, Boutjdir M (2008) Impaired Ca2+ homeostasis is associated with atrial fibrillation in the alpha1D L-type Ca2+ channel KO mouse. American journal of physiology Heart and circulatory physiology 295:H2017–2024. doi: 10.1152/ajpheart.00537.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathie A (2007) Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. The Journal of physiology 578:377–385. doi: 10.1113/jphysiol.2006.121582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortola JP (1999) How newborn mammals cope with hypoxia. Respir Physiol 116:95–103 [DOI] [PubMed] [Google Scholar]
- 24.Namiranian K, Lloyd EE, Crossland RF, Marrelli SP, Taffet GE, Reddy AK, Hartley CJ, Bryan RM Jr. (2010) Cerebrovascular responses in mice deficient in the potassium channel, TREK-1. Am J Physiol Regul Integr Comp Physiol 299:R461–469. doi: 10.1152/ajpregu.00057.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parks C, Alam MA, Sullivan R, Mancarella S (2016) STIM1-dependent Ca(2+) microdomains are required for myofilament remodeling and signaling in the heart. Sci Rep 6:25372. doi: 10.1038/srep25372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M (1998) A mammalian two pore domain mechano-gated S-like K+ channel. The EMBO journal 17:4283–4290. doi: 10.1093/emboj/17.15.4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmeling WT, Warltier DC, McDonald DJ, Madsen KE, Atlee JL, Kampine JP (1991) Prolongation of the QT interval by enflurane, isoflurane, and halothane in humans. Anesthesia and analgesia 72:137–144 [DOI] [PubMed] [Google Scholar]
- 28.Schwingshackl A, Teng B, Makena P, Ghosh M, Sinclair SE, Luellen C, Balasz L, Rovnaghi C, Bryan RM, Lloyd EE, Fitzpatrick E, Saravia JS, Cormier SA, Waters CM (2014) Deficiency of the two-pore-domain potassium channel TREK-1 promotes hyperoxia-induced lung injury. Critical care medicine 42:e692–701. doi: 10.1097/CCM.0000000000000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terrenoire C, Lauritzen I, Lesage F, Romey G, Lazdunski M (2001) A TREK-1-like potassium channel in atrial cells inhibited by beta-adrenergic stimulation and activated by volatile anesthetics. Circulation research 89:336–342 [DOI] [PubMed] [Google Scholar]
- 30.Tong L, Cai M, Huang Y, Zhang H, Su B, Li Z, Dong H (2014) Activation of K(2)P channel-TREK1 mediates the neuroprotection induced by sevoflurane preconditioning. British journal of anaesthesia 113:157–167. doi: 10.1093/bja/aet338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unudurthi SD, Wu X, Qian L, Amari F, Onal B, Li N, Makara MA, Smith SA, Snyder J, Fedorov VV, Coppola V, Anderson ME, Mohler PJ, Hund TJ (2016) Two-Pore K+ Channel TREK-1 Regulates Sinoatrial Node Membrane Excitability. J Am Heart Assoc 5:e002865. doi: 10.1161/JAHA.115.002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Vusse GJ, Reneman RS, van Bilsen M (1997) Accumulation of arachidonic acid in ischemic/reperfused cardiac tissue: possible causes and consequences. Prostaglandins Leukot Essent Fatty Acids 57:85–93 [DOI] [PubMed] [Google Scholar]
- 33.Whyte SD, Booker PD, Buckley DG (2005) The effects of propofol and sevoflurane on the QT interval and transmural dispersion of repolarization in children. Anesthesia and analgesia 100:71–77. doi: 10.1213/01.ANE.0000140781.18391.41 [DOI] [PubMed] [Google Scholar]
- 34.Xian Tao L, Dyachenko V, Zuzarte M, Putzke C, Preisig-Muller R, Isenberg G, Daut J (2006) The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovascular research 69:86–97. doi: 10.1016/j.cardiores.2005.08.018 [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Guo P, Li J, Wang W, Xu S, Wang L, Wang X (2014) Functional study of TREK-1 potassium channels during rat heart development and cardiac ischemia using RNAi techniques. Journal of cardiovascular pharmacology 64:142–150. doi: 10.1097/FJC.0000000000000099 [DOI] [PubMed] [Google Scholar]
- 36.Yeap XY, Dehn S, Adelman J, Lipsitz J, Thorp EB (2013) Quantitation of acute necrosis after experimental myocardial infarction. Methods in molecular biology 1004:115–133. doi: 10.1007/978-1-62703-383-1_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yildirim H, Adanir T, Atay A, Katircioglu K, Savaci S (2004) The effects of sevoflurane, isoflurane and desflurane on QT interval of the ECG. Eur J Anaesthesiol 21:566–570 [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Shepherd N, Creazzo TL (2008) Temperature-sensitive TREK currents contribute to setting the resting membrane potential in embryonic atrial myocytes. The Journal of physiology 586:3645–3656. doi: 10.1113/jphysiol.2008.153395 [DOI] [PMC free article] [PubMed] [Google Scholar]