Abstract
Roquin and Regnase-1 proteins bind and post-transcriptionally regulate proinflammatory target messenger RNAs to maintain immune homeostasis. Either the sanroque mutation in Roquin-1 or loss of Regnase-1 cause systemic lupus erythematosus-like phenotypes. Analyzing mice with T cells that lack expression of Roquin-1, its paralog Roquin-2 and Regnase-1 proteins, we detect overlapping or unique phenotypes by comparing individual and combined inactivation. These comprised spontaneous activation, metabolic reprogramming and persistence of T cells leading to autoimmunity. Here, we define an interaction surface in Roquin-1 for binding to Regnase-1 that included the sanroque residue. Mutations in Roquin-1 impairing this interaction and cooperative regulation of targets induced T follicular helper cells, germinal center B cells and autoantibody formation. These mutations also improved the functionality of tumor-specific T cells by promoting their accumulation in the tumor and reducing expression of exhaustion markers. Our data reveal the physical interaction of Roquin-1 with Regnase-1 as a hub to control self-reactivity and effector functions in immune cell therapies.
Post-transcriptional control of mRNA stability or translation through RNA-binding proteins (RBPs) represents an important level of gene regulation with crucial impact on immune cell fate decisions. This role becomes evident from combined genetic inactivation of alleles encoding for the Roquin-1 and Roquin-2 proteins with redundant functions in mice or in a human patient who developed a severe hyperinflammatory syndrome due to a homozygous nonsense mutation in the RC3H1 gene encoding ROQUIN-1 (refs. 1-3). Moreover, a single amino acid exchange (M199R), called sanroque mutation, in the murine Roquin-1 protein causes lupus-like autoimmunity. Regnase-1-deficient mice exhibit a comparable autoimmune phenotype with activated CD4+ and CD8+ T cells, accumulation of plasma cells, hypergammaglobulinemia and autoantibody production4,5. In response to T cell activation, Roquin-1, its paralog Roquin-2 and Regnase-1 are similarly regulated by MALT1-dependent proteolytic cleavage5,6. All three RBPs share a number of mRNA targets3,5-7, suggesting potential cooperation6,8. Roquin-1/2 proteins repress the expression of Regnase-1 (refs. 6,8). Mapping of binding sites of overexpressed Regnase-1 crosslinked to cellular mRNAs revealed the sequence determinants of Roquin-recognized stem loops of the constitutive decay element (CDE)7,9. Despite an extensive overlap in phenotypes and regulation of these RBPs, they have different functions. Roquin-1 interacts with components of the mRNA deadenylation and decapping machinery9-11, whereas Regnase-1 endonuclease cleaves target mRNAs4,7,12. Because Roquin-1 and Regnase-1 were found enriched in P bodies and at the endoplasmic reticulum (ER), respectively and differed in their requirements for regulation of reporter mRNAs, it was proposed that Roquin-1/2 and Regnase-1 proteins function in a compartmentalized manner independently of each other7,13.
Owing to the prominent humoral autoimmunity occurring in mice with the sanroque mutation or Regnase-1 inactivation4,14 and recapitulation of hallmark phenotypes by T cell-specific deletion of Roquin-1/2 or Regnase-1 (refs.3,5), previous studies mostly focused on CD4+ helper T cells. Nevertheless, the ubiquitous expression of Roquin-1/2 and the prevalence of individual Regnase-1/2/3/4 paralogs suggest an importance of both RBP families in many types of cells and fate decisions15-17. More recently, inactivation of Regnase-1 in tumor-specific CD8+ T cells or chimeric antigen receptor (CAR)-T cells resulted in increased antitumor responses18,19, whereas an involvement of Roquin paralogs in CD8+ effector T cell responses has not yet been studied. In the context of defining tumor antigen-specific T cell antigen receptors (TCRs) or CARs, current efforts also try to modulate tumor-specific T cells. The aim is to bolster activation, prevent regulatory T (Treg) cell-induced suppression, reprogram metabolism or break tumor-imposed exhaustion and make adoptive cell therapies (ACTs) efficient for different blood cancers as well as solid tumors.
Here we explore how the interaction of Roquin-1 with Regnase-1 affects peripheral tolerance and how this program can be used in ACT. We show that both proteins bind to each other in a ternary complex on RNA. This interaction was important for the regulation of shared targets and controlled CD4+ and CD8+ T cell quiescence, metabolic programs, T cell activation, differentiation and effector functions. Weakening the physical interaction of Roquin-1 with Regnase-1 by introducing mutations into the mouse germline caused humoral autoimmunity but led to enhanced responses of cytotoxic T cells directed toward tumor-expressed antigens in the tumor setting.
Results
Roquin-1/2 and Regnase-1 maintain quiescence of T cells.
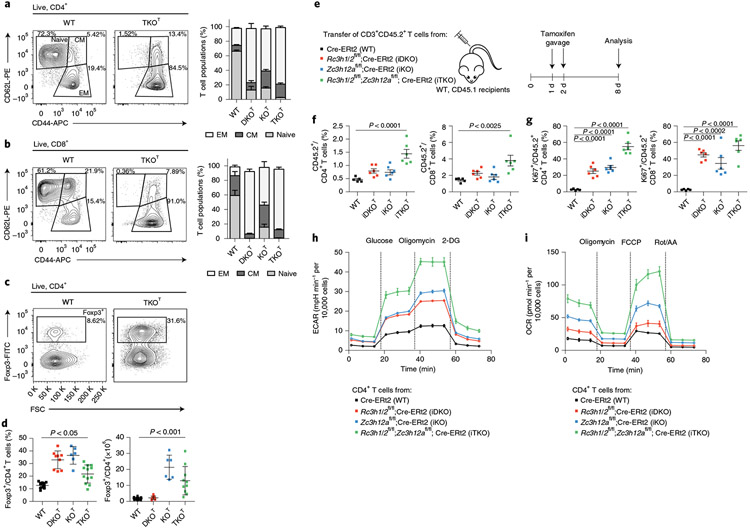
To address the functional relationship of Roquin-1/2 and Regnase-1 proteins we analyzed mice with T cell-specific deletion of the genes encoding Regnase-1 (ref. 20) (Zc3h12afl/fl;Cd4-Cre, termed KOT), Roquin-1 and Roquin-2 (refs. 3,21) (Rc3h1fl/fl;Rc3h2fl/fl;Cd4-Cre, termed DKOT) or a combination of all three genes (Zc3h12afl/fl;Rc3h1fl/fl;Rc3h2fl/fl;Cd4-Cre, termed TKOT). CD4+ and CD8+ T cells from all mutant mice showed a spontaneous reduction of naive T cells (CD62L+CD44lo) and an increase in effector memory T cells (CD62L−CD44hi) (Fig. 1a,b). Accumulation of effector CD4+ and CD8+ T cells was not due to a reduction in Treg cells, which instead increased in frequencies in all knockout mice and also in numbers in KOT and TKOT mice (Fig. 1c,d). Reconstituting lethally irradiated CD45.1/2 congenic mice with mixtures of wild-type (CD45.1) and TKOT (CD45.2) bone marrow (Extended Data Fig. 1a) revealed that the increase in peripheral Treg cells (Fig. 1c,d) was not cell intrinsic, as we found comparable frequencies of wild-type and TKOT Treg cells in mixed-bone-marrow chimeric mice that were increased compared to wild-type/wild-type chimeric mice (Extended Data Fig. 1b). The increased Treg cell frequencies in TKOT but also KOT and DKOT mice presumably occurred secondary to tissue inflammation3,5,6,22, consistent with the observed infiltration of leukocytes into the lung of mutant mice (Extended Data Fig. 1c). We then asked whether the observed activation of conventional CD4+ and CD8+ T cells also occurred in the presence of wild-type Treg cells. We therefore generated mice in which deletion of Roquin-1/2- and Regnase-1-encoding genes, individually or in combination, can be induced by tamoxifen treatment using the Cre-ERt2 transgene23. We adoptively transferred CD3+ T cells (CD45.2) from iKO (Zc3h12afl/fl;Cre-ERt2), iDKO (Rc3h1/2fl/fl;Cre-ERt2) or iTKO (Rc3h1/2fl/fl;Zc3h12afl/fl;Cre-ERt2) mice into congenic (CD45.1) wild-type hosts that were then treated with tamoxifen to acutely delete Roquin-1/2- and Regnase-1-encoding alleles (Fig. 1e). CD45.2+ T cells on day 8 after transfer showed increased frequencies of CD4+ and CD8+ T cells for all knockouts (Fig. 1f). We determined a breakdown of quiescence in knockout CD4+ and CD8+ T cells by increased effector-memory phenotypes (Extended Data Fig. 1d) and enhanced proliferation of these cells indicated by Ki67 staining (Fig. 1g and Extended Data Fig. 1e). These effects were observed in all knockouts but were typically more pronounced upon combined inactivation of Roquin-1/2 and Regnase-1 iTKO T cells. We employed extracellular flux (Seahorse) analyses to determine metabolic reprogramming, a hallmark of T cell activation and functional adaptation, addressing whether inactivation of the different RBPs alters metabolic pathways. During restimulation of CD4+ T cells after in vitro deletion by 4′-OH-tamoxifen treatment we found increased glycolytic activities in all three knockouts compared to wild-type T cells, even under glucose-deprived conditions at baseline. Extracellular acidification rates (ECARs) were even more elevated upon glucose addition and glycolytic capacities were higher in all three knockouts, with the strongest effects in iTKO T cells (Fig. 1h and Extended Data Fig. 2a-c). Mitochondrial respiration was also affected by Roquin-1/2 and Regnase-1 deficiencies and, at baseline, oxygen consumption rates (OCRs) were elevated in all knockouts with a highest increase in iTKO T cells. OCR changes were more pronounced than ECARs suggesting that knockout T cells fuel their energetic demands to a main extent from OXPHOS (Fig. 1h and Extended Data Fig. 2d,e). Consecutively, maximal respiration rates and respiratory spare capacities were increased in all knockout genotypes but were highest in iTKO T cells (Extended Data Fig. 2d,e). With regard to mitochondrial respiration, Regnase-1 deficiency contributed stronger to the deregulation of metabolism compared to Roquin-1/2 deficiency. Similar metabolic reprogramming was also observed for knockout CD8+ T cell cultures; however, iTKOT CD8+ T cells showed variable effects in glycolytic tests, especially in mitochondrial stress tests (Extended Data Fig. 2f,g and Supplementary Table 1). These data show that Roquin-1/2 and Regnase-1 inactivation leads to a general metabolic reprogramming with increased energy generation from mitochondrial respiration and enhanced glycolytic capacity.
Fig. 1 ∣. Roquin-1/2 and Regnase-1 maintain quiescence of T cells.
a–d, Flow cytometry analysis of CD4+ (a), CD8+ (b) subpopulations (WT, n = 9; DKOT and KOT, n = 6; TKOT, n = 5 mice analyzed in at least three independent experiments) and Treg cells (c,d) (WT, n = 15; DKOT, n = 9; KOT, n = 6; TKOT, n = 10 mice analyzed in at least three independent experiments) from spleens of 6–8-week-old WT, DKOT, KOT and TKOT mice. CM, central memory; EM, effector memory; WT, wild type. e, CD45.2+CD3+ T cells from WT, iDKO, iKO and iTKO mice were adoptively transferred into WT CD45.1+ mice. Mice were treated with tamoxifen by oral gavage to induce deletion of floxed alleles. f,g, Frequency (f) and proliferation (g) of CD45.2+, CD4+ and CD45.2+, CD8+ T cells were analyzed by flow cytometry on day 8 after transfer (n = 6 biological replicates). h,i, CD4+ T cells from WT, iDKO, iKO and iTKO mice were treated with 4′-OH-tamoxifen in vitro to induce deletion of floxed alleles. T cells were kept under type 1 helper T cell conditions and expanded with IL-2-containing medium for 2 d. IL-2 was withdrawn overnight followed by restimulation with anti-CD3/28 before glycolytic (h) and mitochondrial stress testing (i) (n = 3 independent experiments). 2-DG, 2-deoxyglucose; Rot, rotenone; AA, antimycin. Data are presented as mean ± s.e.m., analyzed by one-way analysis of variance (ANOVA) with Bonferroni (d) or Dunnett’s (f,g) post hoc test.
Roquin-1/2 and Regnase-1 control humoral autoimmunity.
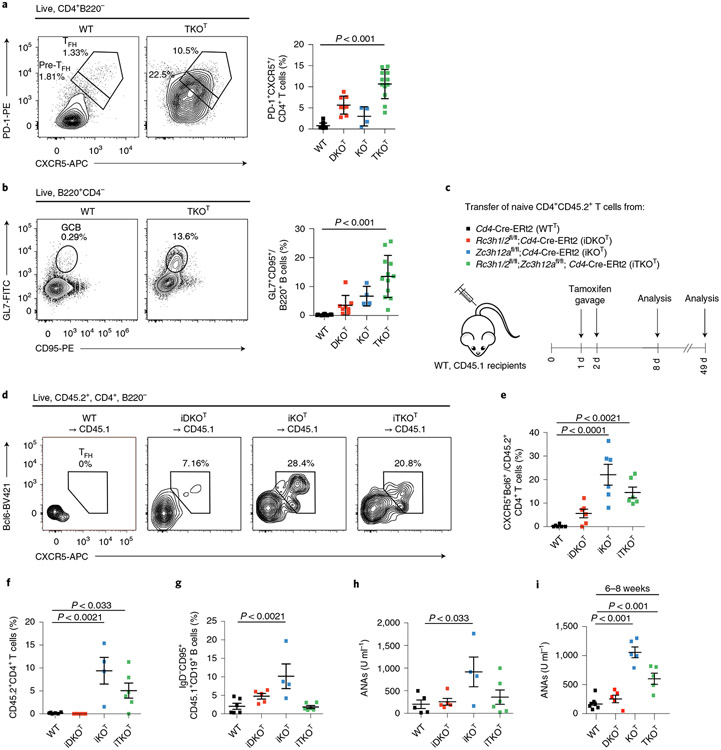
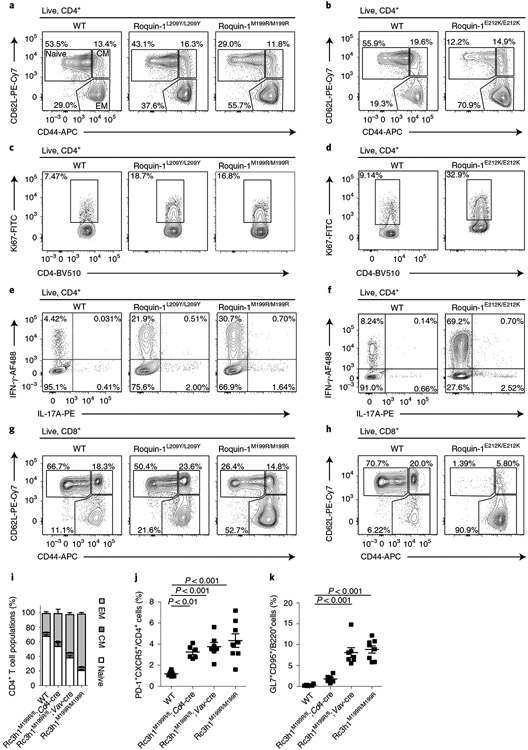
Cd4-Cre mediated deletion of Roquin-1/2 encoding alleles has been associated with follicular helper T (TFH) cell (PD-1hiCxcr5hi, Bcl6+) accumulation3, which we also detected in KOT and even stronger in TKOT mice (Fig. 2a). In addition, all mutant mice showed an accumulation of germinal center (GC) B cells (GL7+CD95+) (Fig. 2b). We combined floxed alleles with an inducible Cd4-Cre-ERt2 knock in allele24,25 and adoptively transferred naive CD4+ T cells (CD45.2+) from wild-type, iKO, iDKO and iTKO mice into congenic (CD45.1+) wild-type mice that were then treated with tamoxifen (Fig. 2c and Extended Data Fig. 2h). Knockout CD4+ T cells spontaneously differentiated into TFH cells in vivo 6 d after acute gene deletion (Fig. 2d,e). Different from non-inducible deletion, the inducible inactivation of Regnase-1 (iKOT) showed strongest TFH cell accumulation compared to iTKO and iDKO, suggesting an advantage of this genotype in the adoptive transfer. Indeed, at a late time point, 7 weeks after transfer, we observed significantly increased numbers of iKO T cells compared to wild-type and iDKO T cells, which also occurred, albeit to a lesser extent, in iTKO T cells (Fig. 2f). Considering intermediate frequencies of dividing iKO CD4+ T cells compared to iDKO and iTKO genotypes early after transfer (Fig. 1f), this finding suggested that Regnase-1 deficiency promotes survival of T cells. Associated with their increased persistence, iKO T cells were capable of inducing autoimmunity in wild-type host mice within 7 weeks (Fig. 2g,h). Transfer of iKO T cells into congenic hosts resulted in accumulation of GC B cells and plasma cells (Fig. 2g and Extended Data Fig. 2i,j) and induced anti-nuclear antibodies (ANAs) in the serum of recipient mice (Fig. 2h). This phenotype was consistent with the appearance of autoantibodies in the serum of 6–8-week-old KOT and TKOT (but not DKOT) mice (Fig. 2i and Supplementary Table 1). Together, these data demonstrate that the autoimmunity associated with the absence of Roquin-1/2 and Regnase-1 genes is caused by deviation of helper T cell functions, because autoimmunity can be transferred with CD4+ T cells, develops in the presence of wild-type Treg cells and originates from a normal T cell receptor repertoire.
Fig. 2 ∣. Roquin-1/2 and Regnase-1 control TFH differentiation and humoral autoimmunity.
a,b, Flow cytometry analysis of TFH (a) and GC B cell (b) subpopulations from spleens of 6–8-week-old WT, DKOT, KOT and TKOT mice (WT, n = 14; DKOT, n = 8; KOT, n = 4; TKOT, n = 12 analyzed mice in at least three independent experiments). c, Naive CD45.2+CD4+ T cells from WT, iDKO, iKO and iTKO mice were adoptively transferred into congenic WT CD45.1 mice. Mice were treated with tamoxifen by oral gavage to induce deletion of floxed alleles. d,e, Adoptively transferred T cells were analyzed by flow cytometry for markers of TFH cell differentiation on day 8 after transfer (n = 6 biological replicates). f–h, Frequencies of adoptively transferred WT, iDKO, iKO and iTKO CD45.2+CD4+ T cells (f), frequencies of recipient GC B cells (g) as well as levels of ANAs in the sera of recipient mice (h) were determined on day 49 after transfer (WT, n = 6; iDKO, n = 5; iKO, n = 4; iTKO, n = 6). i, ANAs in the serum of 6–8-week-old WT, DKOT, KOT and TKOT mice (WT, n = 7; DKOT, KOT and TKOT, n = 5 analyzed mice in three independent experiments). All data are presented as mean ± s.e.m. Statistical significance was calculated by one-way ANOVA with Bonferroni post hoc test (a,b) or Dunnett’s post hoc test (e–i).
Roquin-1/2 and Regnase-1 control CD8+ T cell functions.
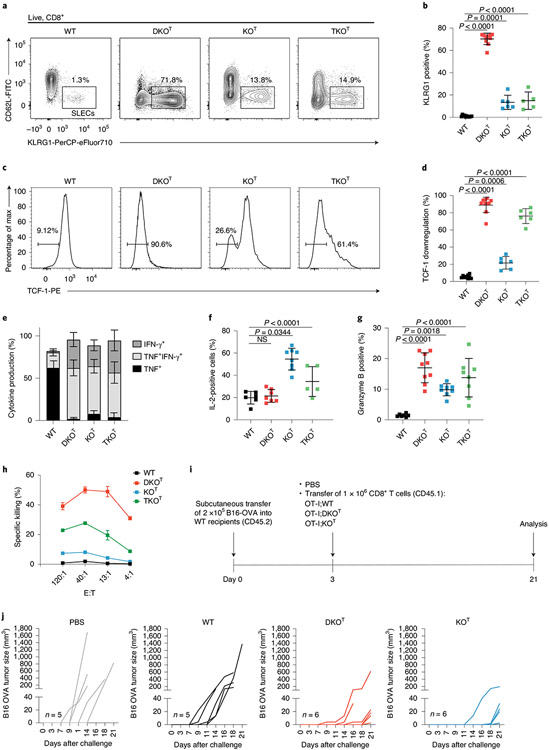
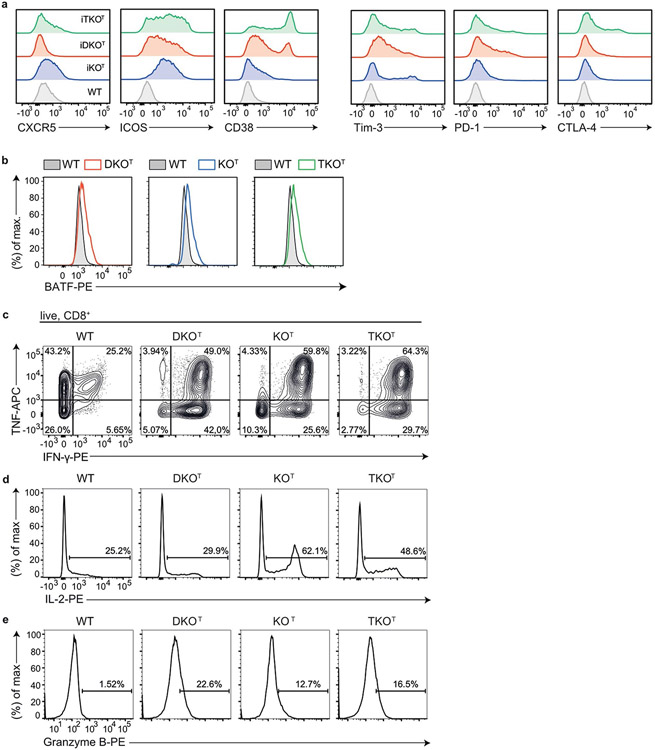
CRISPR–Cas9-mediated inactivation of Regnase-1 yielded in improved antitumor responses of adoptively transferred CD8+ or CAR-T cells18,19. We therefore analyzed CD8+ T cell phenotypes after conditional deletion of the different RBPs by Cd4-Cre-mediated deletion (Fig. 3 and Extended Data Fig. 3). The majority of Roquin-1/2-deficient DKOT CD8+ T cells adopted a short-lived effector cell (SLEC) phenotype with upregulated KLRG1 (Fig. 3a,b) and downregulated TCF-1 expression (Fig. 3c,d). Fewer Regnase-1-deficient (KOT) CD8+ T cells showed increased KLRG1 expression with a majority of cells maintaining high TCF-1 expression (Fig. 3a-d). This finding is consistent with a previous report on retained TCF-1 expression after CRISPR–Cas9-mediated inactivation of Regnase-1 in CAR-T cells19. Notably, the TKOT genotype was similar to either Regnase-1 or Roquin-1/2-deficiencies as it moderately increased KLRG1 and strongly reduced TCF-1 expression (Fig. 3a-d). Analyzing additional markers of activation, stemness and exhaustion26,27 (Extended Data Fig. 3a) we found increased expression of ICOS, CTLA-4 and CD38 in all knockouts. Regnase-1 deficiency increased CXCR5, whereas Roquin-1/2 deficiency induced PD-1 and Tim3 expression (Extended Data Fig. 3a). All knockout T cells showed elevated expression of the transcription factor BATF (Extended Data Fig. 3b), as reported before for inactivation of Regnase-1 (ref. 18). CD8+ T cells from all genotypes showed the capacity to produce tumor necrosis factor (TNF) upon ex vivo stimulation, but only knockout T cells simultaneously produced TNF and interferon (IFN)-γ (Fig. 3e and Extended Data Fig. 3c). Only CD8+ T cells with Regnase-1 deficiency (KOT or TKOT) acquired an enhanced ability to produce interleukin (IL)-2 (Fig. 3f and Extended Data Fig. 3d), whereas all knockout T cells had increased granzyme B expression compared to wild-type counterparts (Fig. 3g and Extended Data Fig. 3e). To quantify cytotoxicity in vitro, we redirected polyclonal CD8+ T cells toward P815 mastocytoma tumor cells in the presence of anti-CD3 (Fig. 3h). All knockout T cells showed enhanced killing in a chromium-release assay, but we observed strongest effects for Roquin-1/2 (DKOT) compared to Regnase-1 deficiencies (KOT) and intermediate effects were seen for TKOT CD8+ T cells. We then addressed whether inactivation of Roquin-1/2 or Regnase-1 leads to increased CD8+ effector responses in a B16-OVA melanoma model by adoptively transferring CD8+ TCR-transgenic OT-I T cells into hosts that had received tumor cells 3 d before (Fig. 3i). Of note, hosts that received PBS or wild-type OT-I T cells showed exponential tumor growth between days 7–21, whereas either Regnase-1 or Roquin-1/2-deficient OT-I T cells suppressed tumor formation resulting in delayed occurrence of measurable tumors and reduced affection rates of recipient mice (Fig. 3j). Together these data show that Roquin-1/2 and Regnase-1 proteins also control shared cellular programs in cytotoxic T cells, but both RBPs have different contributions to the individual phenotypes (Supplementary Table 1).
Fig. 3 ∣. Inactivation of Roquin-1/2 or Regnase-1 in CD8+ T cells enhances cytotoxicity.
a–d, Flow cytometry analysis of splenic WT, DKOT, KOT and TKOT CD8+ T cells for KLRG1 and CD62L expression (a), frequencies of SLEC CD8+ T cells (WT, n = 11; iDKO, n = 10; iKO, n = 6; iTKO, n = 5 individual mice in three independent experiments) (b) and percentage of cells with TCF-1 downregulation (WT, n = 9; iDKO, n = 10; iKO, n = 6; iTKO, n = 6 individual mice in three independent experiments) (c,d). e,f, Quantification of intracellular cytokine staining of IFN-γ, TNF (WT, n = 9; iDKO, n = 9; iKO, n = 11; iTKO, n = 5 individual mice in three independent experiments) (e) and IL-2 (WT, n = 6; iDKO, n = 7; iKO, n = 8; iTKO, n = 5 individual mice in three independent experiments) (f) in CD8+ T cells after PMA/ionomycin stimulation for 4 h. NS, not significant. g, Quantification of granzyme B-positive CD8+ T cells (WT, n = 7; iDKO, n = 11; iKO, n = 8; iTKO, n = 9 individual mice in four independent experiments) in spleens from WT, DKOT, KOT and TKOT mice. h, Chromium-release assay of P815 cells cultivated in effector to target ratios as indicated 4 h after adding splenic CD8+ T cells isolated from WT, DKOT, KOT and TKOT mice (n = 2 individual mice in one experiment). i, Schematic representation of the experimental set-up of the B16-OVA tumor model. j, B16-OVA tumor growth without transfer (PBS control n = 5) or after transfer of either WT (n = 5), DKOT or KOT OT-I T cells (n = 6 individual mice in one experiment). Data are presented as mean ± s.e.m. Statistical significance was calculated by one-way ANOVA with Dunnett’s post hoc test.
Roquin-1 and Regnase-1 exhibit functional interaction.
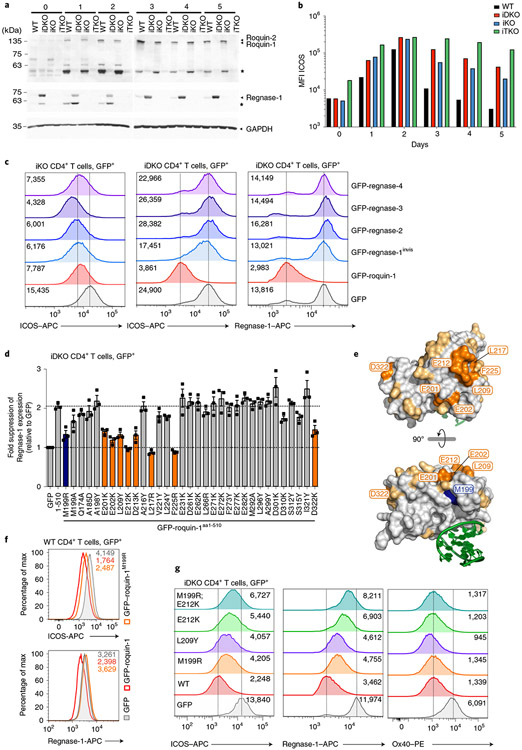
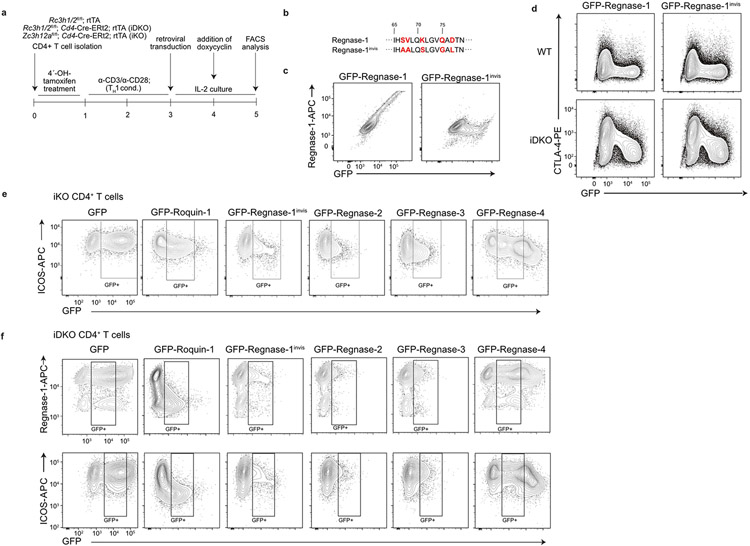
To address post-transcriptional interaction, we tested the contribution of Roquin-1/2 and Regnase-1 to the regulation of ICOS, a well-described target of these RBPs3,5,10,28. We performed tamoxifen gavage of mice to acutely delete floxed alleles by Cd4-Cre-ERt2 in vivo. Isolated naive CD4+ T cells were stimulated in vitro for 2 d with anti-CD3/CD28 under type 1 helper T (TH1) cell conditions, as ICOS expression differs among helper T cell subsets10,29. Appropriate deletion was confirmed in immunoblots (Fig. 4a). In wild-type T cells Roquin-1, the much lower-expressed Roquin-2, as well as Regnase-1 proteins increased during days 1–2 and consistent with TCR-induced MALT1 activity, accumulated as cleavage products. Upon removal of TCR stimulation (days 3–5) the full-length Roquin-1/2 and Regnase-1 proteins increased and the cleavage product of Regnase-1 disappeared. By contrast, cleaved Roquin-1 persisted, suggesting either a longer half-life or constitutive cleavage of this protein (Fig. 4a). Consistent with the Regnase-1-encoding Zc3h12a mRNA being a target of Roquin-1/2 (ref. 6), the Regnase-1 protein became strongly induced in iDKO T cells, whereas Roquin-1/2 expression was unchanged upon Regnase-1 inactivation (Fig. 4a), in contrast to results obtained in a human T cell line22. ICOS protein expression on wild-type TH1 cells increased during stimulation (days 1–2) and returned to basal expression after stimulation (days 3–5). In iTKO compared to wild-type T cells, ICOS protein expression was strongly de-repressed at all time points starting at the naive stage (day 0) (Fig. 4b). Notably, iTKO cells were almost unable to decrease ICOS protein expression after removal of the TCR stimulus (days 3–5) (Fig. 4b). Inactivation of either Roquin-1/2 (iDKO) or Regnase-1 (iKO) increased ICOS protein expression during (day 1–2) and after stimulation (days 3–5) intermediate to wild-type and iTKO TH1 cells. Different from the iTKO genotype, iDKO and iKO TH1 cells were partially able to decrease ICOS protein after stimulation (Fig. 4b). We then tested whether Roquin-1 and Regnase-1 can complement in the absence of each other and whether redundancy existed among Regnase paralogs (Fig. 4c). To address these questions, we reconstituted Regnase-1 (iKO)- or Roquin-1/2 (iDKO)-deficient CD4+ T cells with doxycycline-inducible, green fluorescent protein (GFP)-tagged Roquin-1 or Regnase-1, Regnase-2, Regnase-3 and Regnase-4 proteins (Extended Data Fig. 4a). In these assays we quantified endogenous ICOS and Regnase-1 expression on day 4 of T cell activation. Notably, exogenous GFP–roquin-1 was readily able to decrease Regnase-1 or ICOS expression in iDKO T cells, but was only partially able to downregulate ICOS expression in Regnase-1-deficient (iKO) T cells (Fig. 4c). Endogenous Regnase-1 protein displays strong regulation of Zc3h12a mRNA by Roquin and Regnase-1 (Fig. 4a and refs. 6,30). To distinguish endogenous from overexpressed Regnase-1 protein, we altered the epitope within GFP–regnase-1 that is recognized by the monoclonal antibody used for detection (Extended Data Fig. 4b,c). While becoming invisible to the antibody, GFP–regnase-1invis protein remained fully able to downregulate targets such as CTLA-4 (Extended Data Fig. 4d). The analysis of iKO T cells reconstituted with ectopic Regnase-1invis, Regnase-2, Regnase-3 or Regnase-4 revealed that all four Regnase paralogs were able to downregulate ICOS expression in the absence of endogenous Regnase-1, with only Regnase-4 being slightly less efficient (Fig. 4c and Extended Data Fig. 4e). Ectopic expression of the same Regnase paralogs in Roquin-1/2-deficient T cells showed that neither Regnase-1invis nor Regnase-2, Regnase-3 or Regnase-4 were able to efficiently downregulate endogenous ICOS or Regnase-1 protein expression (Fig. 4c and Extended Data Fig. 4f). Therefore, full regulation of these shared targets required Roquin-1/2 and Regnase-1 proteins.
Fig. 4 ∣. Functional definition of Roquin-1 interaction with Regnase-1.
a,b, WT, iDKO, iKO and iTKO CD4+ T cells were deleted in vivo by tamoxifen gavage, activated in vitro with anti-CD3/28 under TH1 cell conditions (days 0–2) and cultivated in medium containing IL-2 (days 3–5). For each day, expression of Roquin-1/Roquin-2, Regnase-1 and GAPDH (a) or ICOS (b) was analyzed by immunoblot or flow cytometry, respectively. Asterisks mark MALT1 cleavage fragments of Roquin-1 and Regnase-1 (a). MFI, mean fluorescence intensity. c,d CD4+ T cells of indicated genotypes were retrovirally transduced with GFP, GFP–roquin-1, GFP–regnase-1invis, GFP–regnase-2, GFP–regnase-3, GFP–regnase-4 (c) or with GFP, GFP–roquin-1aa1-510 and ROQ mutants introduced into GFP–roquin-1aa1-510 (d). Histograms of ICOS and Regnase-1 expression in GFP+ cells with indication of the respective geometric MFI (gMFI) values (c) or fold suppression of Regnase-1 expression in GFP+ cells transduced with the indicated construct relative to cells transduced with GFP only calculated using gMFI (d). e, Structure of the Roquin-1 ROQ domain with a bound RNA stem loop marked in green, amino acid M199 marked in blue, amino acids essential for Roquin-1 and Regnase-1 functional interaction marked in orange and tested nonessential amino acids in yellow. f,g, WT (f) or iDKO (g) CD4+ T cells were retrovirally transduced with GFP, GFP–roquin-1 or the indicated GFP–roquin-1 mutants. Histograms of ICOS, Regnase-1 and Ox40 expression in GFP+ cells with indication of the respective gMFI. Data are representative of n = 3 independent experiments (a–c,f,g) and are presented as mean ± s.e.m. of n = 3 independent experiments (d).
Molecular determinants of Roquin cooperation with Regnase-1.
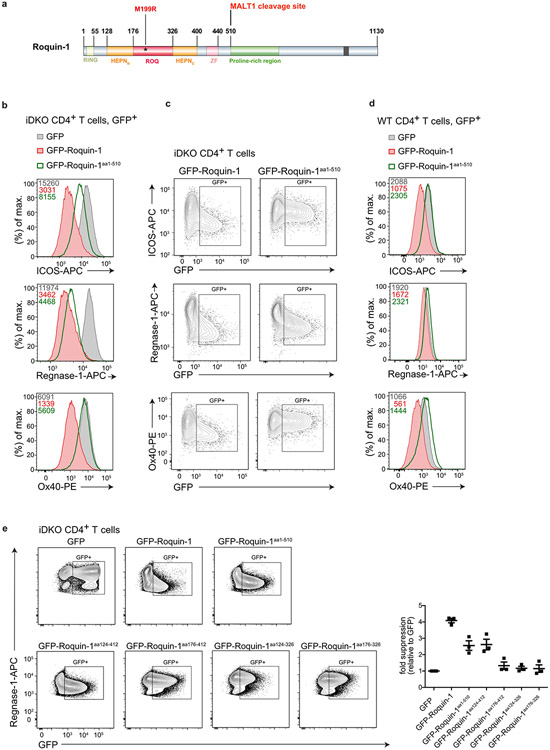
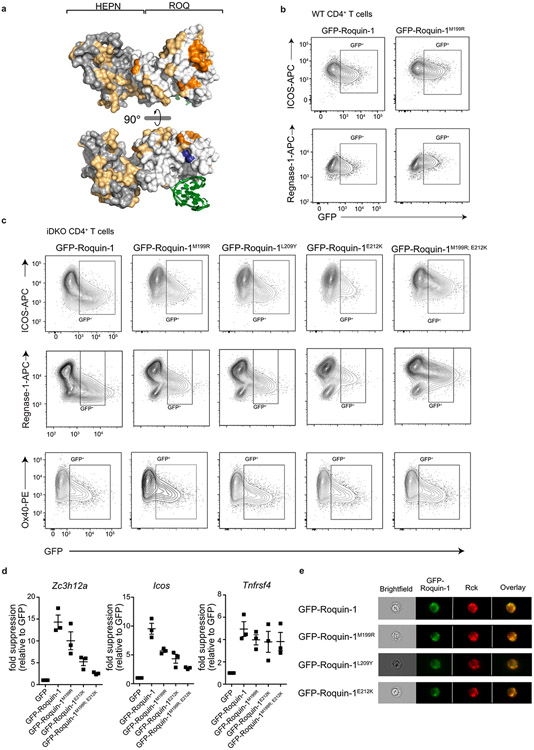
To better understand the functional interaction in the regulation of mRNA targets by Roquin and Regnase-1, we searched for a minimal region in Roquin-1 that was able to regulate shared targets. Notably, in iDKO T cells the amino-terminal MALT1 cleavage product of Roquin-1 (Roquin-1aa1-510) showed partial or almost full activity to downregulate ICOS or Regnase-1 expression, respectively, but was unable to repress Ox40 (Tnfrsf4), another well-known target3,5,31 (Extended Data Fig. 5a-c). This truncated version of Roquin-1 exerted a slight dominant–negative effect on Regnase-1 and Ox40 protein expression when we induced its expression in wild-type T cells (Extended Data Fig. 5d). Deletion analyses indicated that the core RNA-binding domain of Roquin-1 containing HEPNN/ROQ/HEPNC was sufficient to suppress Regnase-1 expression in iDKO T cells (Extended Data Fig. 5e). We then utilized the interdependent regulation of endogenous Regnase-1 as a readout to screen a set of Roquin-1 point mutants. We exchanged residues on the surface of the HEPNN/ROQ/HEPNC domains of Roquin-1 with mutations that alter physical properties but were predicted not to interfere with folding (Extended Data Fig. 6a) and found residues M199, E201, E202, L209, E212, D213, L217, F225, D322 in the ROQ domain essential for Roquin-1aa1-510-mediated repression of Regnase-1 (Fig. 4d). Projecting these residues onto the ROQ domain structure32-34 revealed a site of potential interaction between Roquin-1 and Regnase-1 that is different from the RNA interaction surface (Fig. 4e). Notably, amino acid M199 of Roquin-1, which is mutated in sanroque mice, is part of this patch of residues in the ROQ domain (Fig. 4e) and overexpression of this mutant in wild-type T cells did not exhibit a dominant–negative effect on Regnase-1 or ICOS expression (Fig. 4f and Extended Data Fig. 6b). Instead, M199R, L209Y and E212K Roquin-1 mutants in the full-length protein were impaired to downregulate ICOS and Regnase-1 in iDKO T cells at the protein and mRNA level (Fig. 4g and Extended Data Fig. 6c,d). However, all mutants were fully active to repress Ox40 (Fig. 4g and Extended Data Fig. 6c,d) and, similar to wild-type GFP–roquin-1 protein, localized to P bodies, as identified by the RNA helicase Rck, a marker of P bodies10 (Extended Data Fig. 6e). The sanroque mutation (M199R) as well as the newly identified L209Y and E212K mutants therefore create hypomorphic Roquin-1 variants that have impaired co-regulation activity with Regnase-1 of ICOS and Regnase-1-encoding target mRNAs. These mutations did not affect Ox40 regulation, consistent with regulation of this target mRNA being fully dependent on the carboxy terminus of Roquin-1 and its interaction with the CCR4-NOT complex2. These data demonstrate that shared mRNA targets can be subject to full (Zc3h12a) or partial cooperation (Icos) as well as independent (Tnfrsf4) modes of regulation by Roquin-1 and Regnase-1.
Roquin and Regnase-1 form a ternary complex on RNA.
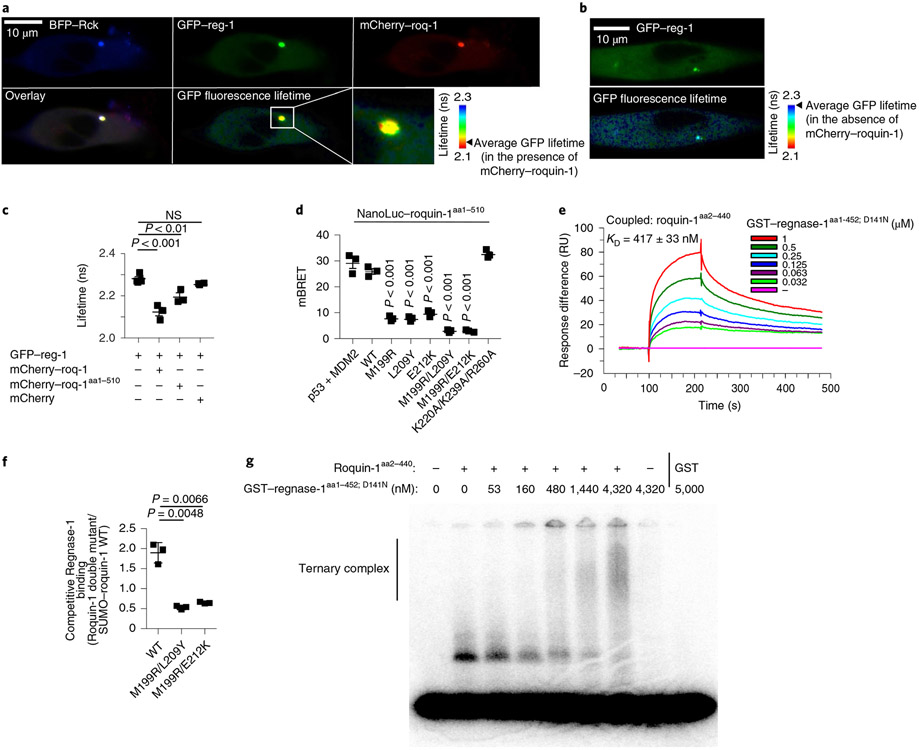
To determine whether post-transcriptional co-regulation can be explained by direct interaction of Roquin-1 with Regnase-1, we performed co-immunoprecipitation and Förster Resonance Energy Transfer (FRET) experiments. Roquin-1 could be co-immunoprecipitated with Regnase-1 from lysates of wild-type but not iKO CD4+ T cells (Extended Data Fig. 7a). To obtain spatial information on this interaction in living cells, we used fluorescence lifetime imaging (FLIM) in HeLa cells co-transfected with GFP–regnase-1 and mCherry–roquin-1. In these experiments the reduction of fluorescence lifetime of GFP is caused upon energy transfer from GFP to mCherry and can be used to quantify donor–acceptor interactions. Both fluorescent proteins localized diffusely in the cytoplasm and colocalized with the P-body marker Rck tagged by blue fluorescent protein (BFP) (Fig. 5a). We found enrichment of GFP–regnase-1 at the ER as reported earlier7, but only in cells that were not co-transfected with Roquin-1 (Extended Data Fig. 7b). Moreover, sucrose gradient centrifugation of T cell extracts showed the majority of endogenous Regnase-1 co-migrating with Roquin-1 in fractions without monosomes or polysomes (Extended Data Fig. 7c). Notably, GFP–regnase-1 interacted with mCherry–roquin-1 as evidenced by the reduced fluorescence lifetime of GFP in the presence of mCherry–roquin-1 (Fig. 5a,c) but not in its absence (Fig. 5b,c). This energy transfer occurred mainly in P bodies but also dispersed in the cytoplasm. Expression of mCherry–roquin-1aa1-510 also reduced the lifetime of GFP–regnase-1 fluorescence, albeit to a lesser extent (Fig. 5c), confirming that the amino terminus of Roquin-1 is sufficient for the interaction. As all four Regnase paralogs were able to downregulate ICOS expression in Regnase-1-deficient iKO T cells (Fig. 4c), we tested the PIN domain of Regnase-1, which is highly conserved among Regnase paralogs35. The truncated GFP–regnase-1aa112-297 protein was localized to the cytoplasm and nucleus and, similar to truncated Roquin-1aa1-510, was no longer enriched in P bodies (Extended Data Fig. 7d,e). The truncated form of GFP–regnase-1aa112-297 also exhibited a reduction in fluorescence lifetime mainly in the cytoplasm where it colocalized with mCherry–roquin-1aa1-510 (Extended Data Fig. 7d,e).
Fig. 5 ∣. Molecular determinants of Roquin-1 interactions with Regnase-1.
a–c, HeLa cells were co-transfected with BFP–Rck, GFP–regnase-1 (GFP–reg-1) together with mCherry–roquin-1 (mCherry–roq-1), mCherry–roquin-1aa1-510 or mCherry (a,c) or with GFP–regnase-1 alone (b). Protein localization (a,b), FRET efficiency (GFP–regnase-1 in combination with mCherry 0.67%, mCherry–roq-1 6.6% or mCherry–roq-1aa1-510 3.91%) and lifetime of GFP fluorescence (c) was analyzed via fluorescence lifetime microscopy. d, HEK293T cells were transfected with HaloTag–regnase-1 in combination with the indicated NanoLuc–roquin-1aa1-510 expression plasmids and NanoBret ratio (mBRET) was calculated after measuring NanoLuc and HaloTag signals via NanoBret assay. e, SPR signals after addition of GST–regnase-1aa1-452;D141N to Biacore-chip-immobilized Roquin-1aa2-440. RU, resonance units. f, Competitive in vitro GST-pulldown experiment using GST–regnase-1D141N and wild-type SUMO–roquin-1aa2-440 in combination with the indicated Roquin-1aa2-440 double mutants (untagged). Quantification of eluted Roquin-1aa2-440 mutant relative to SUMO–roquin-1aa2-440 wild-type protein of SDS–PAGE depicted in Extended Data Fig. 7f. g, EMSA using a Zc3h12a 3′-UTR RNA fragment (nt194–212), Roquin-1aa2-440 (320 nM) in combination with increasing levels of GST–regnase-1aa1-452; D141N. Presumably due to inclusion of unlabeled competitor RNA, recognition of the Zc3h12a mRNA stem loop by Regnase-1 could not be detected. Representative data of n = 3 independent experiments (a,b,e,g). Data are presented as mean ± s.e.m. of n = 3 independent experiments (c,d) or mean ± s.d. of n = 3 independent experiments (f). Statistical significance was calculated using one-way ANOVA with Bonferroni post hoc test (c,d) or Student’s t-tests (f).
To finely map the structural details of this interaction, we introduced the identified mutations (Fig. 4d,g) in NanoLuc–roquin-1aa1-510 fusion proteins and coexpressed them with HaloTag–regnase-1 to perform NanoBRET assays (Fig. 5d). Co-transfected cells were analyzed for energy transfer from nano-luciferase to the HaloTag ligand. We observed a similar BRET signal for wild-type Regnase-1 and Roquin-1 proteins as for the positive controls (p53 and MDM2). Of note, single ROQ domain mutants of Roquin-1 (M199R, L209Y and E212K) as well as double mutants (M199R/L209Y or M199R/E212K) effectively reduced the BRET signal. Mutations that interfere with Roquin-1 binding to RNA (K220A/K239A/R260A) (ref. 32) did not reduce the interaction with Regnase-1. To confirm direct protein–protein interaction, we used surface plasmon resonance (SPR) with purified recombinant proteins. By immobilizing Roquin-1aa2-440 on the surface of a Biacore chip and adding Regnase-1aa1-452 protein in solution, we demonstrated formation of a stable binary Roquin-1 and Regnase-1-containing complex at nanomolar affinity (KD = 417 nM) (Fig. 5e). Next, we used a pulldown assay to quantify how the identified mutations in Roquin-1 affect direct binding of recombinant proteins. A larger, SUMO-tagged wild-type Roquin-1 protein was mixed with a shorter (untagged) version of Roquin-1 harboring different mutations. We quantified the ability of both proteins to compete for interaction with immobilized, GST-fused Regnase-1 full-length protein (Fig. 5f). The ratio of Regnase-1 bound untagged Roquin-1 mutants to the SUMO-tagged wild-type Roquin-1 proteins in these pulldown experiments revealed three- to fourfold weaker interactions for M199R/L209Y or M199R/E212K Roquin-1 mutants (Fig. 5f and Extended Data Fig. 7f). Of note, single M199R, L209Y or E212K mutants only resulted in a twofold reduction of binding (Extended Data Fig. 7g, h). We next analyzed the interaction of Roquin-1 and Regnase-1 in RNA-electrophoretic mobility shift assays (EMSAs). We used the RNA-binding sufficient amino terminus of Roquin-1aa2-440 and the RNase-dead version Regnase-1aa1-452; D141N to avoid RNA degradation4 and asked whether these proteins can form a ternary complex with the 3′-UTR stem loop of the Zc3h12a mRNA (nt194–212) (ref. 30) (Fig. 5g). Indeed, the stem loop of the Zc3h12a mRNA was specifically bound by Roquin-1aa2-440 and increasing Regnase-1 concentrations in these binding reactions decreased the Roquin-specific band and induced a supershift (Fig. 5g). Collectively, these data establish Roquin-dependent recognition of the mRNA stem loop and additional interactions of the RNA-bound Roquin-1 protein with Regnase-1.
Roquin-1 interaction with Regnase-1 prevents autoimmunity.
We then addressed the functional consequences of interfering with Roquin-1 and Regnase-1 interaction in vivo. We introduced two different Rc3h1 mutations encoding for Roquin-1 L209Y or E212K, exhibiting weaker or stronger inhibition of interaction and cooperative regulation with Regnase-1, into the mouse germline. Homozygous mice expressing Rc3h1 mutations encoding for E212K, L209Y or M199R increased the frequencies of activated CD44+CD4+ T cells (Fig. 6a,b), increased T cell proliferation (Fig. 6c,d), caused a TH1 cell bias with increased IFN-γ production of ex vivo-stimulated CD4+ T cells (Fig. 6e,f and refs. 22,36) and also induced accumulation of effector memory CD8+ T cells compared to wild-type mice, which was strongest for E212K mutant (Fig. 6g,h). E212K mutant mice showed compromised viability so that only two homozygous animals could be analyzed to date. Together, these data suggest that Roquin-1L209Y/L209Y or Roquin-1E212K/E212K expressing mice phenocopy the sanroque phenotype.
Fig. 6 ∣. Inhibition of Roquin-1 interaction with Regnase-1 breaks T cell quiescence.
a–h, CD4+ and CD8+ T cells from mice with L209Y and M199R mutations (10–12 weeks old) or from mice with E212K mutations (8 weeks old) in Roquin-1 were characterized. CD4+ effector/memory populations (a,b), CD4+ T cell proliferation (c,d), ability of CD4+ T cells to produce IFN-γ and IL-17 after PMA/ionomycin stimulation ex vivo (e,f) or CD8+ effector/memory populations (g,h) were compared. i–k, Frequencies of CD4+ effector/memory T cell populations (i), TFH cells (j) and GC B cells (k) from spleens of 9–14-week-old WT, Rc3h1M199R/fl;Cd4-Cre, Rc3h1M199R/fl;Vav-Cre and Rc3h1M199R/M199R (sanroque) mice were determined by flow cytometry. Contour plots are representative of n = 4 biological replicates analyzed in at least two independent experiments (a,c,e,g) or n = 2 biological replicates in two independent experiments (b,d,e,h). Data are presented as mean ± s.e.m. of WT, Rc3h1M199R/M199R, n = 6; Rc3h1M199R/fl, Cd4-Cre, Rc3h1M199R/fl, Vav-Cre, n = 5 (i) or WT, Rc3h1M199R/M199R, n = 8; Rc3h1M199R/fl, Cd4-Cre, n = 6; Rc3h1M199R/fl, Vav-Cre, n = 7 (j,k) analyzed mice in at least three independent experiments. Statistical significance was calculated by one-way ANOVA with Bonferroni post hoc test (j,k).
We then addressed whether the sanroque phenotype14 develops only due to altered T cell functions. As heterozygosity of the sanroque allele has no phenotype in young mice37, we combined one Rc3h1san and one Rc3h1fl allele with Cd4-Cre or Vav-Cre. This allowed us to compare phenotypes of the sanroque allele originating from T lymphocytes versus hematopoietic cells (Fig. 6i-k and Extended Data Fig. 8a-c). Vav-Cre-mediated deletion of the floxed Rc3h1 allele was much more potent to induce T cell activation (Fig. 6i and Extended Data Fig. 8a) and GC B cell accumulation compared to Cd4-Cre (Fig. 6k and Extended Data Fig. 8c), whereas TFH cell differentiation was similarly increased for both Cre lines (Fig. 6j and Extended Data Fig. 8b). This result indicated T cell-extrinsic contributions of the sanroque allele to the observed activation of T cells and accumulation of GC B cells.
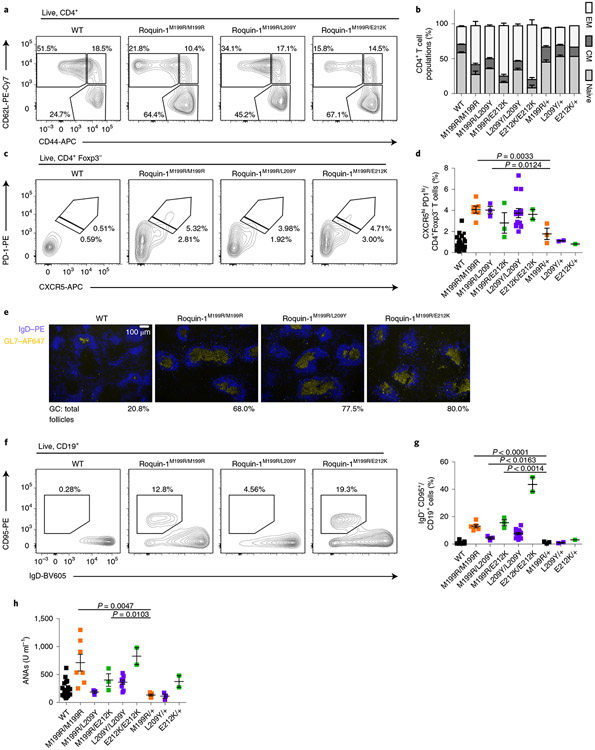
Similar to the sanroque allele, heterozygosity of alleles encoding L209Y/+ or E212K/+ did not induce obvious phenotypes in young mice (Fig. 7). To formally test whether L209Y and E212K induce the same functional impairment in Roquin-1 as the M199R-encoding allele, we generated mice encoding two heterozygous mutations and compared their phenotypes to mice with homozygous M199R-encoding or wild-type alleles. CD4+ T cells from mice carrying mutations encoding Roquin-1M199R/L209Y and Roquin-1M199R/E212K showed a pronounced increase in frequencies of effector memory (Fig. 7a,b) and TFH cells (Fig. 7c,d) compared to wild-type or heterozygous mutant mice with one wild-type allele (Fig. 7a-d). The heterozygous combination with one sanroque allele also caused spontaneous formation of germinal centers in the majority of B cell follicles of the spleen (Fig. 7e) and induced GC B cells in the absence of immunization (Fig. 7f,g). Analyzing autoantibodies in the sera of mice at 8–12 weeks of age (Fig. 7h) we found that homozygous sanroque mice expressing Roquin-1M199R/M199R, different from heterozygous Roquin-1M199R/+ mice, showed a wide range of elevated titers of ANAs, which were partially matched by mice expressing the compound Roquin-1M199/E212K, Roquin-1L209Y/L209Y and Roquin-1E212K/E212K. In fact, mice expressing Roquin-1M199R/E212K showed significantly increased titers of ANAs when compared to mice expressing Roquin-1M199R/+. Collectively, our data demonstrate that the Roquin-1 L209Y and E212K mutants induce phenotypes similar to sanroque mice. Notably, the heterozygous combination of E212K- and M199R-encoding alleles revealed compound effects and autoimmunity equivalent to homozygous sanroque mice expressing the Roquin-1M199R protein.
Fig. 7 ∣. Combined heterozygosity of Roquin-1E212K/M199R shows a synthetic phenotype.
a–d,f–h, T and B cells from 10–12-week-old mice with the M199R mutation in Roquin-1 on one allele and L209Y, E212K or M199R mutations on the other allele were characterized. Frequencies of CD4+ T cell populations (a,b), TFH cells (c,d), immunofluorescence of GL7 (blue) and IgD (yellow) in spleen sections (e), frequencies of GC B cells (f,g) and levels of ANAs in sera determined by ELISA (h). Contour plots or immunofluorescence microscopy images are representative of at least three individual mice analyzed in at least two independent experiments (a,c,e,f). WT, n = 26; M199R/M199R, n = 6; M199R/L209Y, n = 3; M199R/E212K, n = 3; L209Y/L209Y, n = 14; E212K/E212K, n = 2; M199R/+, n = 3; L209Y/+, n = 2; E212K/+, n = 1 (b,d,g,h). Data are shown as mean ± s.e.m. Statistical significance was determined using one-tailed Student’s t-tests. Representative images of n = 3 analyzed mice per genotype (e).
Roquin-1 interaction with Regnase-1 inhibits antitumor immunity.
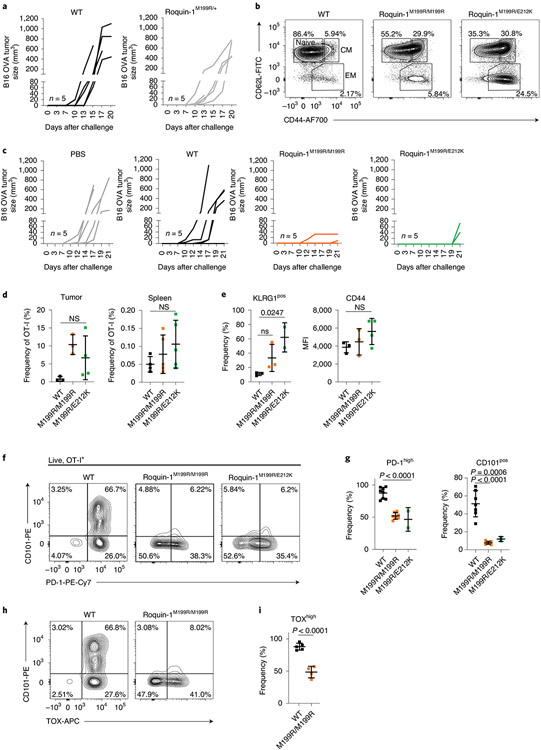
Based on our previous findings demonstrating enhanced cytotoxic activities of Regnase-1 or Roquin-1/2-deficient CD8+ T cells, we asked whether the mixed heterozygous Roquin-1M199R/E212K or sanroque mouse mutants that showed elevated autoantibody titers would also exhibit enhanced antitumor responses. First, we established that OT-I CD8+ T cells from heterozygous sanroque mice (Roquin-1M199R/+) were not effective in conferring protection from tumor growth in the B16-OVA model, as they were comparable to CD8+ T cells from OT-I wild-type mice (Fig. 8a). In the OT-I context we determined only a moderately increased effector memory phenotype in T cells expressing Roquin-1M199R/E212K or Roquin-1M199R/M199R proteins (Fig. 8b). Nevertheless, mice receiving OT-I T cells with either of the two mutations were largely protected from tumor growth as compared to control-treated tumor bearing mice (Fig. 8c). Similar to previous observations on Regnase-1 inactivation18, the frequency of transferred OT-I TCR-transgenic relative to endogenous CD8+ T cells increased more in the tumor than in the spleen for T cells with mutations encoding Roquin-1M199R/E212K or Roquin-1M199R/M199R (Fig. 8d) and these mutant OT-I T cells showed similar upregulation of CD44 but moderately increased KLRG1 expression compared to wild-type OT-I T cells (Fig. 8e). Of note, analyzing PD-1 or TOX as markers of exhaustion38 revealed decreased expression on T cells of mice expressing Roquin-1M199R/E212K or Roquin-1M199R/M199R (Fig. 8f-i). Moreover, CD101, which marks terminally exhausted CD8+ T cells in chronic infections26, was strongly expressed on wild-type OT-I T cells in the tumor, but almost absent from OT-I T cells expressing Roquin-1M199R/E212K or Roquin-1M199R/M199R proteins. Together, these data show that inhibition of Roquin-1 interaction with Regnase-1 promotes the effector function of tumor-specific T cells by increasing their abundance and attenuating their functional inactivation in the tumor.
Fig. 8 ∣. Disrupting Roquin-1 interaction with Regnase-1 improves effector function of tumor-specific T cells.
a,c, Analysis of tumor growth of B16-OVA after transfer of OT-I T cells as described in Fig. 3i and with the indicated genotypes (n = 5 individual mice in one experiment, respectively). b, Flow cytometry analysis of activation markers CD44 and CD62L on OT-I T cells before transfer. d, Frequencies of OT-I T cells relative to all + cells are displayed for tumor and spleen at day 21 after tumor cell transfer. e–i, Flow cytometry analysis of KLRG-1, CD44, CD101, PD-1 and TOX on OT-I T cells in the tumor on day 21 after tumor cell transfer. Data are representative of WT, n = 4; M199R/M199R, n = 5; M199R/E212K, n = 5 individual mice analyzed in one experiment (d). Data are representative of WT, n = 3; M199R/M199R, n = 3; M199R/E212K, n = 2 or 4 each individual mice analyzed in one experiment (e). Data are representative of WT, n = 7; M199R/M199R, n = 7; M199R/E212K, n = 2 individual mice in three independent experiments (g). Data are representative of WT, n = 5 and M199R/M199R, n = 4 individual mice in two independent experiments (h). Data are presented as mean ± s.e.m. Statistical significance was calculated by one-way ANOVA with Bonferroni post hoc test (d,e,g) or unpaired one-tailed Student’s t-test (i).
Discussion
We describe the direct physical interaction of Roquin-1 and Regnase-1 proteins on RNA. Our findings reveal cooperative regulation of Icos and Zc3h12a (Regnase-1) mRNAs within the shared target set7,9,39-41 and explain overlapping phenotypes of Roquin-1/2 and Regnase-1 mutant mice3,5,14. Conditional inactivation of Roquin-1/2 or Regnase-1 in T cells or germline mutations introducing M199R (sanroque), L209Y and E212K substitutions in the ROQ domain of Roquin-1 that similarly interfere with Roquin-1 binding to Regnase-1, caused autoimmunity. This role in peripheral tolerance becomes evident from spontaneous activation of T cells and accumulation of TFH cells and GC B cells in all mouse lines, as well as autoantibody formation in some mouse lines.
Analyzing similar changes induced through acute deletion of RBP-encoding alleles in T cells, we find that the Roquin-1/2 and Regnase-1 proteins are continuously required in naive T cells to maintain quiescence. These proteins silence cell-intrinsic programs of activation and proliferation associated with metabolic reprogramming to increased glycolysis and enhanced oxidative phosphorylation. Although both Roquin-1/2 and Regnase-1 proteins have already been found to be negative regulators of the mTOR pathway, protein biosynthesis and purine metabolism42,43, it is currently unclear which target(s) are driving the observed metabolic changes and trigger spontaneous activation of T cells. Following activation, CD4+ T cells committed to the TFH cell subset and CD8+ T cells acquired polyfunctionality and enhanced cytotoxic activity. In our phenotypic comparisons, contributions from Roquin-1/2 and Regnase-1 were often comparable and combined inactivation typically showed increased effects. The related phenotypes indicate that these RBPs cooperate in the same pathways. Cooperative post-transcriptional regulation can either be explained by binding in a complex to the same mRNA, as established here. It can also be explained by independent binding of the different RBPs to the same mRNA44 or independent binding to different molecules of the same mRNA species as suggested previously7,13,22 or independent binding to different mRNA species that then cooperate in the same pathway. Understanding how cooperation is encoded in the transcripts of Icos and Zc3h12a but not in Tnfrsf4 and thereby allows formation of differential messenger ribonucleoproteins will require extensive structural, biochemical and functional analyses. Of note, our data also revealed selective contributions, especially for CD8+ T cells. Regnase-1 deficiency was associated with increased persistence of CD4+ and CD8+ T cells5,18 and enabled CD8+ T cells to produce copious amounts of IL-2. By contrast, Roquin-1/2 deficiency induced KLRG1 expression in CD8+ T cells, caused downregulation of TCF-1 and strongly increased in vitro cytotoxicity. Nevertheless, inactivation of either Roquin-1/2 or Regnase-1 improved antitumor responses and a comparable improvement resulted from interfering with Roquin-1–Regnase-1 interaction.
Here, we present the interaction of Roquin-1 with Regnase-1 as a molecular mechanism underlying the prevention of autoimmunity and propose that this interaction can become a promising target for improvement of therapeutic approaches with adoptively transferred antigen-specific T cells.
Methods
Mice.
All mice used in this study were on a C57BL/6 background. All animals were housed in a specific-pathogen-free barrier facility under a 12 h/12 h dark/light regime at 20–24 °C and at a humidity of 45–65% in accordance with the Helmholtz Zentrum München and the Ludwig-Maximilians-Universität München institutional, state and federal guidelines. All experimental procedures involving male or female mice were performed in accordance with regulations and were approved by the local government (Regierung von Oberbayern reference nos. 55.2-2532-Vet_02-19-122, 55.2-2532.Vet_02-17-159, 55.2-2532.Vet_02-18-10 and 55.2-2532.Vet_02-19-68). Rc3h1/2fl/fl mice are transgenic for the Roquin-1-encoding gene Rc3h1 (ref. 21) and the Roquin-2-encoding gene Rc3h23. Zc3h12afl/fl mice are transgenic for the Regnase-1-encoding gene Zc3h12a20. Transgenic Rc3h1/2fl/fl mice were crossed to Zc3h12afl/fl mice to reach the final genotype of Rc3h1/2fl/fl;Zc3h12afl/fl, which were crossed with either Cd4-Cre45, Cre-ERt2 (ref. 23) or Cd4-Cre-ERt2 (ref. 24) transgenic mice. For tumor experiments, OVA-specific transgenic TCR was introduced by crossing mice to the OT-I line46. Rc3h1/2fl/fl;Cd4-cre-ERt2;rtTA-M2 or Zc3h12afl/fl;Cd4-cre-ERt2;rtTA-M2 mice were generated by crossing mice with respective loxP sites and Cd4-cre-ERt2 with Gt(ROSA)26Sortm1(rtTA*M2) Jae mice47. The Rc3h1M199R mice (sanroque) (EM:02168) were obtained from the European Mouse Mutant Archive consortium and mice expressing CD45.1 (Ptprca Pepcb/BoyJ), Vav-iCre (Jax no. 00861) as well as Gt(ROSA)26Sortm1(rtTA*M2)Jae mice were obtained from the Jackson Laboratory. Data collection and analysis were not performed blind to the conditions of the experiments. Experimental groups were assigned according to genotype.
Generation of Rc3h1 mutant mouse lines via CRISPR–Cas9-based gene editing.
The Rc3h1_E212K mouse line was generated by Polygene Transgenetics via CRISPR–Cas9 gene editing of the ES cell line and blastocyst injection and the Rc3h1_L209Y mouse line via CRISPR–Cas9-based gene editing by electroporation of one-cell embryos. Specific guide RNAs (Rc3h1_L209Y_gRNA: 5′-CAATGCAGAACCATCTTCTA-3′) were used in form of in vitro transcribed single gRNA (EnGen sgRNA Synthesis kit, NEB, E3322). Before electroporation, the specific sgRNA(200 ng μl−1) and single-strand oligonucleotides (ssODN_Rc3h1_L209Y: 5′-TTGTACCATTTTTCCTAGCGATGCAGGAGGAAGCTCTGAAGCTGGTCTTGTATGCTTTAGAAGATGGTTCTGCATTGTCTCGGAAAGTGTTGGTTCTCTTCGTGGTGCAAAGACTGGAGC-3′; 300 ng μl−1) were diluted in Opti-MEM buffer (Thermo Fisher Scientific) together with recombinant Cas9 protein (200 ng μl−1, IDT) and incubated for 10 min at 20 °C and 10 min at 37 °C to form the active ribonucleoprotein complex. One-cell embryos were obtained by mating C57BL/6N males (Charles River) with C57BL/6N females super-ovulated with 5 U pregnant mare’s serum gonadotropin and 5 U human chorionic gonadotropin and electroporated using an NEPA21 electroporator and a CUY501P1-1.5 electrode (Nepa Gene Co). Zygotes were transferred into pseudopregnant CD1 female mice to obtain live pups. Gene-editing events were analyzed on genomic DNA isolated from ear biopsies of founder mice and F1 progeny using the Wizard Genomic DNA Purification kit (Promega, A1120) following the manufacturer’s instructions.
Isolation, in vitro cultivation and transduction of primary CD4+ T cells.
Isolation, in vitro deletion of floxed alleles, in vitro cultivation and transduction of primary CD4+ T cells was performed as described44. In reconstitution experiments, for induction of pRetro-Xtight-GFP construct expression in rtTA expressing T cells, transduced cells were cultured in the presence of doxycycline (1 μg ml−1) for 16 h before flow cytometry analysis or 6 h before sorting of GFP+ cells for quantitative PCR with reverse transcription (RT–qPCR).
In vivo deletion of floxed alleles.
Male and female Cd4-Cre-ERt2 mice (age 8–16 weeks) with alleles encoding Roquin and Regnase-1 were fed by oral gavage with 5 mg tamoxifen (Sigma) in corn oil per dose on three consecutive days with two doses of tamoxifen on the last day (20 mg total tamoxifen dose per mouse). Mice were killed 3 d after the last gavage and total CD4+ T cells were isolated using the EasySep Mouse T Cell Isolation kit (STEMCELL) according to manufacturers’ instructions.
Generation of mixed-bone-marrow chimeric mice.
Bone marrow cells were isolated from femurs and tibias, frozen in FCS containing 10% dimethylsulfoxide and stored at −80 °C until injected into mice. Male and female CD45.1/2 heterozygous recipient mice (age 8–11 weeks) were lethally irradiated with 2 × 5.5 Gy with a XStrahl CIX2 X-ray device. Then, 4 × 106 bone marrow cells were injected intravenously (i.v.) into CD45.1/2 heterozygous recipient mice. Mice were treated for 2 weeks with water supplemented with antibiotics in drinking water (0.04% Baytril) and 9 weeks after irradiation, reconstituted cells were analyzed by flow cytometry.
Adoptive T cell transfer.
Total CD3+ T cells, naive CD4+ T cells or CD8+ T cells were isolated from donor mice using the EasySep Mouse CD3+ Cell Isolation kit, Mouse Naive CD4+ T Cell Isolation kit or Mouse CD8+ Cell Isolation kit (STEMCELL), respectively, according to manufacturers’ instructions. Then, 1.5 × 106 cells were injected i.v. into 10-week-old male and female CD45.1+ WT recipient mice. On day 1 and 2 after injection all recipient mice were fed 5 mg tamoxifen by oral gavage twice per day (20 mg total tamoxifen per mouse). Mice were killed on day 8 or day 49 after receiving the first tamoxifen dose.
Ex vivo T cell stimulation.
Total splenocytes were stimulated with 20 nM PMA and 1 μM ionomycin for a total of 4 h. After 1 h of stimulation 10 μg ml−1 brefeldin A was added. The reaction was stopped by washing the cells with cold PBS twice before proceeding with antibody staining for flow cytometry.
51Cr-release assay.
Cell-mediated cytotoxicity was determined by a redirected lysis assay. P815 mastocytoma target cells were labeled with 50 μCi 51Cr for 1 h at 37 °C. After washing, 2,000 target cells per 96-well were incubated with effector cells (CD8+ T cells isolated from lymph nodes and spleen) at different effector:target (E:T) ratios in the presence of anti-CD3 (clone (cl.) 145-2C11H, 1 μg ml−1, inhouse production) for 4 h at 37 °C. Subsequently, the amount of radioactivity in the supernatant was measured using a scintillation counter (TopCount NXT).
Melanoma tumor model.
Male and female C57BL/6 wild-type mice were subcutaneously injected with 2 × 105 B16-OVA tumor cells. After 3 d, congenically marked OT-I T cells (1 × 106 i.v.) were adoptively transferred directly after isolation. Tumors were measured manually with a caliper three times per week and tumor size was calculated according to the formula (length × width2) / 2. Mice were humanely killed if the maximal permitted tumor size of 1,400 mm3 was reached. Otherwise spleens and tumors were collected not later than 21 d after tumor engraftment, cut into small pieces and passed through a 100-μm diameter filter.
Flow cytometry and data analysis with FlowJo.
Single-cell suspensions were stained with fixable blue viability dye (Thermo Fisher Scientific) for 20 min at 4 °C. For the detection of surface proteins cells were stained with the appropriate antibodies in FACS buffer (2% FCS, 1 mM EDTA in PBS) for 20 min at 4 °C. For intracellular staining of cytokines, Roquin, Regnase-1 or CTLA-4 cells were fixed with 2% formaldehyde at 20 °C for 15 min, washed with saponin permeabilization buffer (0.5% saponin and 1% BSA in PBS) and stained with the appropriate antibodies in saponin buffer for 40 min at 4 °C. For commercially available intracellular antibodies, cells were fixed with Foxp3 Fixation/Perm buffer (eBioscience) according to the manufacturer’s protocol for 30 min at 4 °C, permeabilized with Foxp3 permeabilization buffer (eBioscience) and stained with antibodies diluted in Foxp3 permeabilization buffer for 40 min at 4 °C. Cell populations were acquired on BD LSR Fortessa, BD FACSCanto II or Cytoflex (Beckmann Coulter) flow cytometry devices or sorted using BD FACS Aria III. Data were processed using FlowJo software (v.10.6.0, BD Bioscience). An exemplified gating strategy is shown in Extended Data Fig. 9. The following antibodies were used: anti-CD4 (cl. GK1.5, 1:400 dilution), anti-CD8a (cl. 53-6.7, 1:400 dilution), anti-CD38 (cl. 90, 1:200 dilution), anti-CD44 (cl. IM7, 1:200 dilution), anti-CD62L (cl. MEL-14, 1:200 dilution), anti-CD45.1 (cl. A20, 1:200 dilution), anti-CD45.2 (cl. 104, 1:200 dilution), anti-CD45R (B220; cl. RA3-6B2, 1:200 dilution), anti-CD101 (cl. Moushi101, 1:200 dilution), anti-PD-1 (cl. J43, 1:200 dilution), anti-GL7 (cl. GL7, 1:200 dilution), anti-granzyme B (cl. NGZB, 1:150 dilution), anti-IFN-γ (cl. XMG1.2, 1:200 dilution), anti-ICOS (cl. C398.4A, 1:200 dilution), anti-IL-2 (cl. JES6-5H4, 1:100 dilution), anti-KLRG1 (cl. 2F1, 1:100 dilution), anti-Ox40 (cl. OX-86, 1:200 dilution), anti-CTLA-4 (cl. UC10-4B9, 1:200 dilution), anti-Foxp3 (cl. FJK-16S, 1:100 dilution), anti-Ki67 (cl. SolA15, 1:200 dilution), anti-Tim3 (cl. RMT3-23, 1:200 dilution) and anti-TNF-α (cl. MP6-XT22, 1:100 dilution) all from eBioscience; anti-CD95 (cl. JO2, 1:200 dilution), anti-BATF (cl. S39-1060, 1:40 dilution) and anti-Bcl6 (K112-91, 1:50 dilution) all from BD Bioscience; anti-IL-17A (cl. TC11-18H10.1, 1:100 dilution), anti-CXCR5 (cl. L138D7, 1:50 dilution), anti-CD19 (cl. 6D5, 1:300 dilution), anti-IgD (cl. 11-26c.2a, 1:200 dilution), goat anti-rat antibody (cl. Poly4054, 1:200 dilution) all from BioLegend; and goat anti-rabbit antibody (Invitrogen, 1:200 dilution), anti-CD138 (cl. 281-2, BD Pharmingen, 1:200 dilution), anti-Roquin-1/2 (cl. 3F12, inhouse production, 1:10 dilution), anti-Regnase-1 (cl. 15D11, inhouse production, 1:10 dilution), anti-TCF-1 (cl. S33-966, BD Bioscience, 1:80 dilution), anti-TOX (cl. REA473, Miltenyi Biotech, 1:50 dilution) and anti-Rck (Bethyl).
AMNIS image stream measurements.
Cells were stained as described above and measured using the AMNIS image stream (Millipore).
Immunofluorescence microscopy.
Spleens were frozen in OCT compound (Tissue Tek), cryosections (7 μm) were prepared and fixed in acetone. Slides were stained with anti-IgD-PE (cl. 11-26c.2a, BioLegend) and anti-GL7-Alexa647 (cl. GL7, BioLegend). Images were acquired on an Olympus BX41 fluorescence microscope and processed with Fiji (v.1.0).
Seahorse measurements.
In vitro-deleted and activated CD4+ and CD8+ T cells were starved of IL-2 overnight and restimulated with anti-CD3e and anti-CD28 antibodies (0.5 μg ml−1, BioLegend) for 6–7 h before the seahorse measurement. Cells were washed with PBS, resuspended in seahorse assay medium (XF RPMI, Agilent) containing 2 mM l-glutamine (Thermo Fisher Scientific), with or without 5 mM glucose (Sigma-Aldrich) for glycolytic or mitochondrial stress tests, respectively, and seeded on poly-l-lysine (50 μg ml−1, Sigma-Aldrich) and goat anti-hamster (50 μg ml−1) pre-coated plates at a density of 2–2.2 × 105 cells per well. Cells were degassed using a Cytation1 reader (BioTek) at 37 °C for 1 h. ECARs and OCRs were measured on a 96-well XFe Extracellular Flux Analyzer (Agilent). For each treatment three cycles of 3 min mixing and measurement each were performed. For normalization, nuclei stained with 8 μm Hoechst (Thermo Fisher Scientific) were counted in a Cytation1 reader. To assess basal glycolytic activity during the glycolytic stress test, the ECAR response to an acute 5-mM glucose (Sigma-Aldrich) injection was measured, followed by a 1.5μM oligomycin (Sigma-Aldrich) injection to inhibit mitochondrial respiration and induce maximal glycolytic capacity. Nonglycolytic acidification was assessed after injecting 50 mM 2-DG (Sigma-Aldrich). In the mitochondrial stress test 1.5 μM oligomycin was injected to inhibit mitochondrial proton flux. Maximal mitochondrial respiration was induced by injection of 1 μM FCCP (Sigma-Aldrich) and terminated by 0.5 μM rotenone (Sigma-Aldrich) and antimycin A (Sigma-Aldrich) injections.
Sucrose gradient fractionation.
Sucrose gradient fractionation was performed as described48, with slight modifications. Shortly after, 5 × 107 CD4+ T cells were washed in ice-cold PBS containing cycloheximide (0.1 mg ml−1), resuspended in extraction buffer (20 mM Tris-HCl (pH 7.4), 140 mM KCl, 0.5 mM dithiothreitol (DTT), 5 mM MgCl2, 0.5% Nonidet-P40, 0.1 mg ml−1 cycloheximide and protease inhibitor (PI)), incubated for 15 min on ice and centrifuged for 10 min at 12,000g. Extracts were layered onto a 4.7-ml sucrose gradient (18–50% sucrose (w/v) in 20 mM Tris-HCl, pH 7.4, 140 mM KCl, 0.5 mM DTT, 5 mM MgCl2 and 0.1 mg ml−1 cycloheximide) and centrifuged at 4 °C in a SW55Ti rotor (Beckman) at 35,000 r.p.m. for 90 min. Gradients were fractionated into ten 0.5-ml fractions and absorbance profiles at 254 nm were recorded using the Piston gradient fractionator (Biocomp). For further protein analysis, polysome gradient fractions were subjected to TCA precipitation.
Culture of cell lines.
HeLa, HEK293T, P815 and B16-OVA cells were cultured in DMEM (Invitrogen) supplemented with 10% (v/v) FCS (Gibco), Pen-Strep (100 U ml−1, each, Thermo Fisher Scientific), 10 mM HEPES, pH 7.2–7.5 (Invitrogen) at 37 °C and 10% CO2. HEK293T, HeLa, B16-F10 (no. CRL-6475) and P815 (no. TIB-64) cell lines were purchased from ATCC. The B16-F10 cell line was retrovirally transduced with MigR1-OVA-GFP (provided by D. Zehn, TU Munich).
Calcium phosphate transfection for generation of retroviral particles.
HEK293T cells pre-treated with 25 μM chloroquine, were co-transfected with 5 μg of the packaging vector pCL-Eco (Addgene; 12371) and 50 μg of the respective pRetro-Xtight plasmids using calcium phosphate as a transfection reagent. After 6 h cells were washed and cultured in fresh medium for an additional 48 h. Viral particles were filtered (0.45 μM) and mixed with polybrene (10 μg ml−1) before T cell transduction.
Expression plasmids.
For murine T cell reconstitution experiments, the GFP-coding sequence (CDS), the corresponding murine complete CDS or corresponding shortened versions, as indicated, of Roquin-1, Regnase-1, Regnase-2, Regnase-3 or Regnase-4 C-terminally fused to GFP were inserted into the pRetro-Xtight expression plasmid (Clontech) under the control of a Tet-responsive promotor. For FLIM/FRET experiments, murine Roquin-1 CDS fused C-terminally to the mCherry CDS or the Rck CDS C-terminally fused to eBFP2 was inserted into the pdest12.2 backbone (Invitrogen). For NanoBret assays, the complete CDS of Regnase-1 or Roquin-1aa1-510 was inserted downstream of the HaloTag CDS in the pFN21A HaloTag CMV Vector (Promega) or NanoLuc CDS in the pFN31K Nluc CMV-neo Flexi Vector (Promega), respectively. Mutations in the CDS of Regnase-1 or Roquin-1 were inserted via the QuikChange site-directed-mutagenesis procedure (Stratagene). Primer sequences are available on request.
Cell lysis, SDS–PAGE and immunoblotting.
Cell lysis and SDS–PAGE were performed as described42. For immunoblotting, proteins were transferred to a PVDF membrane and analyzed using primary antibodies followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling). For protein detection, Amersham ECL Prime Western Blotting Detection Reagent and X-ray films were used. The following primary antibodies were used: Roquin-1/2 (cl. 3F12, monoclonal, inhouse production), Regnase-1 (cl. 15D11, inhouse production), Roquin-1 (A300-515A, polyclonal, Bethyl), GAPDH (cl. 6C5, Merck Millipore) and Rpl7a (Abcam, ab70753).
RNA isolation and RT–qPCR.
RNA isolation was performed by column-based RNA isolation utilizing the NucleoSpin RNA isolation kit (Macherey-Nagel) according to the manufacturer’s protocol. RNA was transcribed into complementary DNA using the Quantitect RT kit (Roche) according to the manufacturer’s protocol. To quantify gene expression, the UPL Probe Library System by Roche and the Roche Light Cycler 480 were utilized (Supplementary Table 2).
Co-immunoprecipitation.
To analyze interaction of Roquin and Regnase-1 proteins in T cells, co-immunoprecipitation was performed. T cells were lysed in lysis buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.25% (v/v) Nonidet-P40, 1.5 mM MgCl2, 1 mM DTT supplemented with 1× cOmplete, EDTA-free Protease Inhibitor Cocktail (Roche), 1× Halt Phosphatase Inhibitor Cocktail (Thermo Fisher) and 0.2 U μl−1 RNase inhibitor (RNasin, Promega)) on ice for 15 min and cleared by centrifugation for 15 min at 12.000g and 4 °C. Then, 10 μl Protein-A dynabeads (Invitrogen) were coupled under constant rotation to 10 μg Regnase-1 antibody (R&D systems, no. 604421) in lysis buffer at 4 °C overnight, followed by 1 h at 20 °C. After washing the antibody-coupled beads with phosphate-citrate buffer (24.4 mM citric acid, 65 mM sodium hydrogen phosphate (pH 5), supplemented with 1 mM DTT and PI), washed beads were incubated with 15 mg protein lysate in 900 μl lysis buffer for 4 h at 4 °C while continuously rotating. Then, beads were washed three times with lysis buffer (+ DTT and PI) and resuspended in 40 μl 1× Laemmli buffer. The samples were boiled at 95 °C for 5 min and co-immunoprecipitation was analyzed by SDS–PAGE and immunoblotting with monoclonal antibodies recognizing Regnase-1 (cl. 15D11) and Roquin (cl. 3F12).
NanoBret assay.
NanoBret assays were performed according to the protocol of the NanoBRET Nano-Glo Detection System (Promega). Between 4–6 h after seeding of 1 × 106 HEK293T cells into six-well plates, cells were transfected with 2 μg of HaloTag and 0.02 μg of NanoLuc expression plasmids using FuGENE reagent (Promega) according to the manufacturer’s instructions. After 20 h, cells were trypsinized, resuspended in Opti-MEM I Reduced Serum Medium (no phenol red, with 4% FCS, Thermo Fisher Scientific), re-plated into 96-well plates and incubated with HaloTag NanoBRET 618 Ligand (100 nM final concentration) or dimethylsulfoxide as a no ligand negative control for 22 h. After addition of NanoBRET Nano-Glo Substrate the donor emission (460 nm) and acceptor emission (618 nm) was measured using the GloMax Discover System (Promega). The raw donor and acceptor values were calculated and milliBRET units (mBU) were determined as mBU = acceptor emission (618 nm) / donor emission (460 nm) × 1,000 and afterwards corrected for background signal as mBRET = mean mBU experimental sample – mean mBU no ligand control.
FRET/FLIM experiments.
For confocal microscopy 1 d before analysis HeLa cells were transfected via calcium phosphate precipitation and cells were seeded 6 h before microscopic analysis on eight-well μ-Slides (glass bottom, Ibidi) in Leibovitz’s L-15 medium (no phenol red, Thermo Fisher Scientific). For staining of the ER the cells were washed with HBSS (calcium, magnesium and no phenol red, Thermo Fisher Scientific) and incubated for 25 min at 37 °C in prewarmed staining solution (300 μl HBSS containing 0.8 μM ER-Tracker Red dye, Thermo Fisher Scientific). Confocal and FLIM images were performed with a TCS SP8X FALCON confocal head (Leica Microsystems) mounted on an inverted microscope (DMi8; Leica Microsystems). For confocal imaging, a 405-nm diode and a white-light laser were used as excitations sources (488 nm for GFP and 594 nm for mCherry). Single photons were collected through a 93×/1.3 NA glycerin-immersion objective and detected on Hybrid Detectors (HyD) (Leica Microsystems) with a 414–468 nm, 500–550 nm and 610–722 nm spectral detection window for BFP, GFP and mCherry detection, respectively. The image size was set to 512 × 512 pixels and a 2.5-fold zoom factor was applied, giving a pixel size of 0.098 μm and an image size of 50 × 50 μm. For FLIM, the white-light laser delivered 20 MHz repetition rate at 488 nm. Arrival time of single photons was measured with the included FALCON module and 60 frames were acquired at 1.17 Hz for each time-correlated single-photon counting recording, corresponding to a scanning speed of 600 Hz. FLIM image analyses were performed in the LAS X software. A threshold was applied to the lifetime images before analysis to eliminate background noise. Different regions of interest were fitted with a two-exponential decay model. The mean amplitude-weighted fluorescence lifetime of the area was extracted then reported. The FRET efficiency (EFRET) was calculated according to the following formula:
where τDA is the lifetime of the donor–acceptor sample and τD is the lifetime of the donor alone. Lifetime images shown were produced using the phasor approach49.
ELISA for detection of anti-nuclear antibodies.
Blood taken from hearts of male and female (age 6–12 weeks) mice was directly centrifuged at 10,000g for 10 min at 4 °C to collect serum. ANA ELISAs were performed using the mouse ANA total IgG ELISA kit (Alpha Diagnostic) according to the manufacturer’s instructions. Optical density at 450 nm was measured on an ELISA reader (Versa Max Microplate reader, Molecular Devices) and concentrations were calculated by using standard serum as a reference.
Prediction of interacting residues on the Roquin surface.
The structures of the ROQ domain bound to RNA (PDB 4QI2) and ROQ-HEPN domain (PDB 4TXA) were superposed using PyMOL and a model of the ROQ-HEPN domain bound to the RNA stem loop was generated. Based on this model, residues on the Roquin surface that are typically involved in protein–protein interactions but not covered by RNA were mutated on the basis of stereochemical and/or electrostatic interference.
Expression and purification of GST–regnase-1D141N.
Full-length GST–regnase-1D141N was expressed from pGEX-6P-3 in Escherichia coli Rosetta 2 (DE3) pLysS. Cells were cultured at 37 °C in 2YT containing 50 μg ml−1 ampicillin, 35 μg ml−1 chloramphenicol and 30 μM ZnCl2. At an optical density (OD600) of 0.8, temperature was reduced to 18 °C and expression was induced by adding isopropyl β-D-1-thiogalactopyranoside (IPTG) to a concentration of 0.7 mM. After 16 h of incubation, cells were collected (5,200g, 4 °C, 30 min). Due to the fast degradation of the protein, the whole purification was conducted at 4 °C in one day. Cells were resuspended in lysis buffer (500 mM NaCl, 50 mM HEPES (pH 7.5), 2 mM MgCl2, 30 μM ZnCl2, 2 mM DTT, 1× cOmplete Protease Inhibitor Cocktail (Roche)) and sonicated on ice. After clarification of the lysate (30,000g, 4 °C, 30 min), supernatant was applied on a pre-equilibrated GSTrap column (GE Healthcare). After washing steps with high-salt buffer (1 M NaCl, 50 mM HEPES (pH 7.5), 2 mM MgCl2, 30 μM ZnCl2, 2 mM DTT) and re-equilibration in lysis buffer, bound proteins were eluted with lysis buffer containing 30 mM reduced glutathione. Eluted fractions were pooled and immediately loaded onto a heparin column (GE Healthcare) equilibrated in 100 mM NaCl, 20 mM HEPES (pH 7.5), 2 mM MgCl2, 30 μM ZnCl2 and 2 mM DTT and eluted using a linear gradient to 60% high-salt buffer in ten column volumes. Pooled fractions were further purified using a Superdex200 column (GE Healthcare) in gel filtration buffer (150 mM NaCl, 20 mM HEPES (pH 7.5), 2 mM MgCl2, 30 μM ZnCl2, 2 mM DTT). Protein-containing fractions were pooled and concentrated.
Expression and purification of GST–regnase-1aa1-452;D141N.
His6–GST–regnase-1aa1-452:D141N with an additional C-terminal His6 tag was expressed from pOPINJ in E. coli Rosetta (DE3) cells. Cells were grown in LB medium with 34 μg ml−1 chloramphenicol and 100 μg ml−1 ampicillin at 37 °C. Expression of the protein was induced at OD600 0.6 by adding 0.5 mM IPTG and overnight growing conditions changed to 18 °C. Next, cells were collected (6,238g, 15 min, 4 °C) and resuspended in lysis buffer (500 mM NaCl, 2 mM DTT, 2 mM MgCl2, 30 μM ZnCl2, PIs and 50 mM HEPES, pH 8) and sonicated on ice. The supernatant of the centrifugation (48,384g, 30 min, 4 °C) was applied to GSTrap column. The column was washed with high-salt buffer (1 M NaCl and 50 mM HEPES, pH 8) and the protein eluted using elution buffer (500 mM NaCl, 30 mM glutathione, 2 mM DTT, 2 mM MgCl2, 30 μM ZnCl2, PIs and 50 mM HEPES, pH 8). After changing the buffer to a low-salt buffer (50 mM NaCl, 35 μM ZnCl2, 2 mM DTT, 2 mM MgCl2 and HEPES, pH 8) the protein was loaded onto a heparin column. The bound protein was eluted with a gradient (20 column volumes 0–100%) using the same buffer including 1 M NaCl. Fractions were pooled, concentrated and further purified using a Superdex 75 10/300 GL column (Amersham Pharmacia Biosciences) in 150 mM NaCl, 35 μM ZnCl2, 2 mM DTT and HEPES, pH 7.5 buffer.
Expression and purification of Roquin-1aa2-440, SUMO–roquin-1aa2-440 and its mutants.
His6–SUMO–roquin-1aa2-440 was expressed from pOPINS3C in E. coli BL21 Star (DE3). Roquin-1aa2-440 and its mutants (Roquin-1aa2-440;M199R, Roquin-1aa2-440;L209Y, Roquin-1aa2-440;E212K, Roquin-1aa2-440;M199R/L209Y and Roquin-1aa2-440;M199R/E212K) were expressed as His6-tagged proteins from pETM11 or pOPINF in E. coli BL21 (DE3) or BL21 Star (DE3). Cells were grown at 37 °C in LB medium with 50 μg ml−1 kanamycin (pETM11) or with 100 μg ml−1 ampicillin (pOPINS3C or pOPINF). At an OD600 of 0.4, cultures were induced by adding 0.5 mM IPTG and overnight growing conditions changed to 20 °C. Cells were collected (6,238g, 15 min, 4 °C), resuspended in lysis buffer (300 mM NaCl, 15 mM imidazole, 1 mg ml−1 lysozyme, 2 mM DTT, PIs and 50 mM Tris, pH 8) and sonicated on ice. After centrifugation (48,384g, 30 min, 4 °C), the supernatant was applied to a HisTrap column (GE Healthcare). Bound protein was eluted, concentrated and further purified using a Superdex 75 10/300 GL column (Amersham Pharmacia Biosciences) in 150 mM NaCl and 50 mM HEPES, pH 7.5 buffer.
Electrophoretic mobility shift assay.
The RNA fragment of Regnase-1 3′-UTR (nt194-212, IBA GmbH) was radioactively labeled using T4 polynucleotide kinase (Thermo Fisher Scientific) and [γ32P] ATP (Hartmann Analytic) at 37 °C for 30 min. The reaction was stopped at 75 °C for 10 min. Sepharose spin columns (NucAway; Invitrogen) were used to separate RNA from free nucleotides. Radioactively labeled RNA (6 nM), proteins (GST–regnase-1aa1-452;D141N, Roquin-1aa2-440) and tRNA competitor (30 μg ml−1) were incubated in HEPES/NaCl/MgCl2 buffer (10 mM HEPES (pH 7.5), 150 mM NaCl and 2 mM MgCl2) and 4% glycerol in a final volume of 20 μl for 30 min at 20 °C. Samples were resolved by native TBE–PAGE (4% polyacrylamide and 1× TBE buffer) or by gradient NativePAGE 4–16% Bis-Tris (Invitrogen) gels. Gels were analyzed using Fuji imaging plates exposed in the FLA-5100, after 10 min incubation in fixing solution (30% (v/v) methanol and 10% (v/v) acetic acid) and vacuum drying.
Competition pulldown assays of GST–regnase-1D141N and Roquin variants.
Competition pulldowns were performed using GSTrap beads (GE Healthcare) pre-equilibrated in binding buffer (150 mM NaCl, 30 mM HEPES (pH 7.5), 2 mM MgCl2, 30 μM ZnCl2 and 2 mM DTT). Then, 0.5 nmol human full-length GST–regnase-1D141N was mixed with 150 μl bead slurry and incubated for 10 min on ice. Subsequently, premixes of 1.2 nmol SUMO–roquin-1aa2-440 wild-type and 1.2 nmol Roquin-1aa2-440 mutant variants were added and incubated for 60 min at 4 °C. After four wash steps (950 μl binding buffer), bound proteins were eluted with 60 μl elution buffer (150 mM NaCl, 30 mM HEPES (pH 7.5), 2 mM MgCl2, 30 μM ZnCl2, 2 mM DTT and 30 mM reduced glutathione). Then, 5% input of individual proteins, wash fractions and elutions were analyzed on 12.5% SDS–PAGE, stained with Coomassie blue and imaged using a ChemiDoc XRS+ (BioRad). Band intensities of Roquin-1aa2-440 mutant variants and SUMO–roquin-1aa2-440 wild-type of three independent experiments were quantified and averaged using Image Lab software and their ratio was plotted.
Surface plasmon resonance.
Binding between Roquin-1aa2-440 and GST–regnase-1aa1-452;D141N was analyzed using BIACORE 3000 instrument (Biacore). Roquin-1aa2-440 was coupled to the CM5 sensor chip (Biacore) at a concentration of 35 μg ml−1 in 10 mM sodium-phosphate buffer (pH 5.7). GST–regnase-1aa1-452;D141N was injected onto the sensor chip using the concentrations 0.032, 0.063, 0.125, 0.25, 0.5 and 1 μM at 30 μl min−1 flow rate in running buffer (150 mM NaCl, 35 μM ZnCl2, 0.05% Tween20, 2 mM DTT and 10 mM HEPES, pH 7.5) at 20 °C. Acquired binding curves were double-referenced against the signal in the buffer run and a ligand-free reference channel. The equilibrium dissociation constant (KD) was calculated from steady-state measurements using the BIAevaluation program (Biacore).
Statistical analysis and experimental design.
Statistical analysis was performed with Prism 5.0b (GraphPad) or Origin. P values were calculated with Student’s t-test or one-way or two-way ANOVA, as indicated. Statistical significance was indicated. Error bars represent mean of all data points ± s.e.m. or mean ± s.d., as indicated. No statistical methods were used to predetermine sample sizes but our sample sizes are similar to those reported in previous publications3,6,18,19. Data distribution was assumed to be normal, but this was not formally tested. Experiments did not involve randomization of animals/samples or conditions or blinding of investigators. No animals or data points were excluded from analyses.
Extended Data
Extended Data Fig. 1 ∣. Roquin-1/2 and Regnase-1 maintain quiescence of T cells.
(a, b) Analysis of mixed bone marrow chimeric mice using either WT (CD45.2) and WT (CD45.1) or TKOT (CD45.2) and WT (CD45.1) bone marrow cells injected into lethally irradiated CD45.1/2 recipient mice. Flow cytometry analysis of CD45.1 and CD45.2 cell populations (a) or Treg cells (b) in splenocytes from recipient mice 9 weeks after reconstitution (WT CD45.1/WT CD45.2 recipients: n = 2, WT CD45.1/TKO CD45.2 recipients: n = 3, analyzed in one experiment). (c) H&E sections of lungs showing alveoli from WT, DKOT, KOT and TKOT mice at the age of 6-8 weeks (Representative data of n ≥3 individual mice). (d, e) Analysis of CD45.2+ CD3+ T cells from Cre-ERt2 (WT), Rc3h1/2fl/fl; Cre-ERt2 (iDKO), Zc3h12afl/fl; Cre-ERt2 (iKO) and Rc3h1/2fl/fl; Zc3h12afl/fl; Cre-ERt2 (iTKO) mice that were adoptively transferred into WT CD45.1+ mice. Recipient mice were treated with tamoxifen by oral gavage to induce deletion of floxed alleles. On day 8 post transfer, T cells were analyzed for their ability to acquire an effector/memory phenotype (d) or to proliferate (e) within the host (n = 6 biological replicates).
Extended Data Fig. 2 ∣. Roquin-1/2 and Regnase-1 control metabolism and humoral autoimmunity.
CD4+ (a–e) or CD8+ (f, g) T cells from Cre-ERt2 (WT), Rc3h1/2fl/fl; Cre-ERt2 (iDKO), Zc3h12afl/fl; Cre-ERt2 (iKO) and Rc3h1/2fl/fl; Zc3h12afl/fl; Cd4-Cre-ERt2 (iTKO) were treated with 4′-OH tamoxifen in vitro to induce deletion of floxed alleles. T cells were activated in vitro and expanded with IL-2 medium for 2d. IL-2 was withdrawn overnight before T cells were restimulated with anti-CD3/28 prior to glycolytic (a, b, f) and mitochondrial stress tests (c–e, g). Shown are calculated ratios for ECAR (mpH/min/10000 cells) (a–c) and OCR (pmol/min/10000 cells) (d–e) relative to WT, respectively. Naïve CD45.2+ CD4+ T cells from Cd4-Cre-ERt2 (WT), Rc3h1/2fl/fl; Cd4-Cre-ERt2 (iDKO), Zc3h12afl/fl; Cd4-Cre-ERt2 (iKO) and Rc3h1/2fl/fl; Zc3h12afl/fl; Cd4-Cre-ERt2 (iTKO) mice were adoptively transferred into CD45.1+ recipient mice. Mice were treated with tamoxifen by oral gavage to induce deletion of floxed alleles. Adoptively transferred cells were identified by congenic markers on day 8 (h) or accumulation of germinal center B (i) or plasma cells (j) was determined on day 49 after induced deletion. (a–e) Data are presented as mean +/− SD of n = 3 biological replicates analyzed over 3 independent experiments, (f, g) representative of 3 independent experiments, (h) n = 6 analyzed mice, or (i, j) WT: n = 6, iDKO: n = 5, iKO: n = 4, iTKO: n = 6 in 2 independent experiments. Statistical significance was calculated by one-way ANOVA with Dunnett’s post-hoc test.
Extended Data Fig. 3 ∣. Describing phenotypes of DKOT, KOT and TKOT CD8+ T cells.
Flow cytometry analysis of markers of activation and exhaustion (a) and BATF transcription factor expression (b) in splenic WT, DKOT, KOT and TKOT CD8+ T cells. Data are representative of at least 5 individual 6-12 week old mice per genotype in at least two independent experiments. Flow cytometry analysis to Fig. 3e, f: intracellular cytokine staining of IFN-γ, TNF (c) and IL-2 (d) after PMA/ionomycin stimulation in CD8+ T cells of splenocytes from WT, DKOT, KOT and TKOT mice for 4 h. Histogram analysis to Fig. 3g: Granzyme B expression in splenic WT, DKOT, KOT and TKOT CD8+ T cells (e).
Extended Data Fig. 4 ∣. Regnase paralogs cannot complement for Roquin loss of function.
(a) Workflow of in vitro reconstitution experiments. (b) Mutations introduced in Regnase-1 coding sequence (marked in red) for the generation of an antibody-invisible GFP-tagged Regnase-1 construct (GFP-Regnase-1invis). (c) Flow cytometry analysis of Regnase-1 expression after retroviral transduction of WT CD4+ T cells with GFP-Regnase-1 or GFP-Regnase-1invis constructs. (d) WT or iDKO CD4+ T cells were retrovirally transduced with GFP–Regnase-1 or GFP-Regnase-1invis. Contour plots of flow cytometry analysis of CTLA-4 expression in dependence of the GFP expression level. (e, f) Flow cytometry analysis of ICOS or Regnase-1 expression after retroviral transduction of iKO or iDKO CD4+ T cells with the indicated GFP-fusion protein. Contour plots of histograms shown in Fig. 4c. (d–f) Data are representative of n = 3 independent experiments.
Extended Data Fig. 5 ∣. Dissecting protein domains of Roquin-1 sufficient for cooperative target regulation with Regnase-1.
(a) Schematic representation of Roquin-1 domain organization with indication of M199R mutation. iDKO (b, c) or WT (d) CD4+ T cells were retrovirally transduced with GFP, GFP-Roquin-1 or GFP-Roquin-1aa1-510 constructs. Histograms of flow cytometry analysis of ICOS, Regnase-1 or Ox40 expression, as indicated, in GFP+ cells with indication of respective geometric MFI value. (c) Contour plots of histograms depicted in (b). (e) iDKO CD4+ T cells were retrovirally transduced with the constructs encoding GFP, GFP-Roquin-1 or GFP-Roquin-1 mutant proteins. Flow cytometry analysis of Regnase-1 expression and quantification of fold suppression of Regnase-1 expression level in GFP+ cells relative to cells expressing GFP control construct. (b–e) Data are representative of n = 3 independent experiments. (e) Data are presented as mean +/− SEM of n = 3 independent experiments.
Extended Data Fig. 6 ∣. Functional validation of point mutations in Roquin-1 that reduce cooperation with Regnase-1.
(a) Structure of the Roquin-1 HEPNN/ROQ/HEPNC domain with a bound RNA stem-loop marked in green. All amino acids tested are colored, essential amino acids for Roquin-1 and Regnase-1 interaction in the ROQ domain are marked in orange, non-essential ones in yellow and amino acid M199 in blue. WT (b) or iDKO (c) CD4+ T cells were retrovirally transduced with GFP, GFP-Roquin-1 or the indicated GFP-Roquin-1 mutants. Contour plots of flow cytometry measurement of indicated targets in GFP+ cells after 16 h of doxycycline induction of GFP-tagged constructs. (b) Contour plots of histograms shown in Fig. 4f or (c) shown in Fig. 4g. (d) iDKO CD4+ T cells were retrovirally transduced with GFP, GFP-Roquin-1 or the indicated GFP-Roquin-1 mutants and sorted for GFP+ cells 6 h after doxycycline induced expression of the respective constructs. The levels of Zc3h12a, Icos or Tnfrsf4 mRNAs in GFP+ cells were determined via RT-qPCR, normalized to YWHAZ and calculated as fold suppression of the respective construct relative to cells expressing the GFP control construct. Data show mean +/− SEM of n = 3 independent experiments. (e) Representative images of iDKO CD4+ T cells transduced with the indicated GFP-Roquin-1 WT or mutant proteins, stained with anti-Rck (P-body marker) antibody and analyzed via Image Stream. (b, c) Data are representative of n = 3 independent experiments or (e) n = 2 independent experiments.
Extended Data Fig. 7 ∣. Molecular determinants of Roquin-1 interaction with Regnase-1.
(a) Lysates of WT or iKO CD4+ T cells were subjected to immunoprecipitation (IP) with antibodies against Regnase-1. Input lysates before IP and eluates from beads after IP were analyzed in immunoblots with antibodies against Roquin-1 or Regnase-1. (b, d) Fluorescence microscopy images of HeLa cells transfected with BFP-Rck and GFP-Regnase-1 (GFP-Reg-1) and stained with ER staining dye (b), or of cells transfected with GFP-Regnase-1aa112-297 and mCherry-Roquin-1aa1-510 (d). Quantification of GFP fluorescence lifetime in the cytoplasm (cyto) and nucleus (nuc) (e) of transfected cells shown in (d). (c) Cytoplasmic lysates of CD4+ T cells were fractionated after sucrose gradient centrifugation and distribution of Roquin, Regnase-1 and Rpl7a proteins in the individual fractions analyzed via immunoblots, as indicated. Above, representative absorbance profile obtained during fractionation of gradients with indication of localization of 40 S and 60 S ribosomal subunits, 80 S monosomes and polysomes. (f, g) SDS-PAGE of competitive in vitro GST-pulldown experiments using GST-regnase-1D141N and SUMO-roquin-1aa2-440 in combination with the indicated untagged Roquin-1aa2-440 double or single mutants. Purified proteins before pulldown (IN), supernatants of wash steps (W) as well as eluted proteins (E) were loaded. Asterisks mark the migration of degradation products of GST-regnase-1. (h) Quantification of eluted mutant Roquin-1aa2-440 relative to SUMO-roquin-1aa2-440 wild-type protein of in vitro GST-pulldown experiments depicted in (g). (b, d, f, g) Depicted are representative data of n = 3 independent experiments or (a, c) of n = 2 independent experiments. (e) Data are presented as mean +/− SEM or (h) mean +/− SD and (h) statistical significance was calculated using t tests.
Extended Data Fig. 8 ∣. Phenotypes of mice with mutations impairing Roquin-1 interaction with Regnase-1.
Contour plots of flow cytometry analysis of CD4+ T cells (a), TFH cells (b) and GC B cells (c) from spleens of 9-14 weeks old WT, Rc3h1M199R/fl;Cd4-Cre, Rc3h1M199R/fl;Vav-Cre and Rc3h1M199R/M199R mice. Contour plots are representative of WT, Rc3h1M199R/M199R: n = 6, Rc3h1M199R/fl; Cd4-Cre, Rc3h1M199R/fl; Vav-Cre: n = 5 (a), or WT, Rc3h1M199R/M199R: n = 8, Rc3h1M199R/fl; Cd4-Cre: n = 6, Rc3h1M199R/fl; Vav-Cre: n = 7 (b, c) analyzed mice in at least 3 independent experiments.
Extended Data Fig. 9 ∣. Exemplary gating strategy.
Cells were pregated on lymphocytes (FSC-A/SSC-A), single cells (SSC-H/SSC-W and FSC-H/FSC-W) and live cells (Fixable blue -) prior to gating on cell populations of interest.
Supplementary Material
Acknowledgements
We thank D.H. Busch (TU Munich) for critical reading of the manuscript. We thank C. Keplinger (Helmholtz Zentrum München) and L. Esser and J. Klein (Ludwig-Maximilians-Universität, Munich) for excellent technical support. We thank A. Blutke, J. Kranich and R. Schieweck (Ludwig-Maximilians-Universität, Munich) for support with histology and polysome profiles and the BMC Core Facility (Ludwig-Maximilians-Universität, Munich) for support in flow cytometry and the immunoanalytics platform for their support in chromium release assays (E. Noessner, Helmholtz Zentrum München). For the provision of NanoBret constructs we thank K.-P. Knobeloch (University Clinic Freiburg) and D. Zehn (TU Munich) for providing the MigR1-OVA-GFP retroviral plasmid. The work was supported by the German Research Foundation grants SPP-1935 (to D.N. and V.H.), SFB-TRR338 (projects C02 to V.H. and C05 to S.T.), SFB-1054 (project A03) as well as HE3359/7-1 and HE3359/8-1 to V.H. and under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy, ID 390857198 to W.W.) and SFB-870 (project A13 to W.W.) as well as grants from the Wilhelm Sander, Fritz Thyssen, Else Kröner-Fresenius and Krebshilfe foundations to V.H.
Footnotes
Reporting Summary. Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41590-021-01064-3.
Extended data is available for this paper at https://doi.org/10.1038/s41590-021-01064-3.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41590-021-01064-3.
Competing interests
The authors declare no competing interests.
Data availability
All data are provided in the article and its supplementary files or from the corresponding author upon reasonable request. For analysis of the Roquin structure the publicly available structural datasets of Roquin-1 ROQ domain bound to RNA (PDB 4QI2) and Roquin-1 ROQ-HEPN domain (PDB 4TXA) were used. Source data are provided with this paper.
References
- 1.Pratama A et al. Roquin-2 shares functions with its paralog Roquin-1 in the repression of mRNAs controlling T follicular helper cells and systemic inflammation. Immunity 38, 669–680 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Tavernier SJ et al. A human immune dysregulation syndrome characterized by severe hyperinflammation with a homozygous nonsense Roquin-1 mutation. Nat. Commun 10, 4779 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel KU et al. Roquin paralogs 1 and 2 redundantly repress the Icos and Ox40 costimulator mRNAs and control follicular helper T cell differentiation. Immunity 38, 655–668 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Matsushita K et al. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature 458, 1185–1190 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Uehata T et al. Malt1-induced cleavage of regnase-1 in CD4+ helper T cells regulates immune activation. Cell 153, 1036–1049 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Jeltsch KM et al. Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote T(H)17 differentiation. Nat. Immunol 15, 1079–1089 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Mino T et al. Regnase-1 and Roquin regulate a common element in inflammatory mRNAs by spatiotemporally distinct mechanisms. Cell 161, 1058–1073 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Jeltsch KM & Heissmeyer V Regulation of T cell signaling and autoimmunity by RNA-binding proteins. Curr. Opin. Immunol 39, 127–135 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Leppek K et al. Roquin promotes constitutive mRNA decay via a conserved class of stem-loop recognition motifs. Cell 153, 869–881 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Glasmacher E et al. Roquin binds inducible costimulator mRNA and effectors of mRNA decay to induce microRNA-independent post-transcriptional repression. Nat. Immunol 11, 725–733 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Sgromo A et al. A CAF40-binding motif facilitates recruitment of the CCR4-NOT complex to mRNAs targeted by Drosophila Roquin. Nat. Commun 8, 14307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mino T et al. Translation-dependent unwinding of stem-loops by UPF1 licenses Regnase-1 to degrade inflammatory mRNAs. Nucleic Acids Res. 47, 8838–8859 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu M & Blackshear PJ RNA-binding proteins in immune regulation: a focus on CCCH zinc finger proteins. Nat. Rev. Immunol 17, 130–143 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinuesa CG et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Akira S Regnase-1, a ribonuclease involved in the regulation of immune responses. Cold Spring Harb. Symp. Quant. Biol 78, 51–60 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Heissmeyer V & Vogel KU Molecular control of TFH cell differentiation by Roquin family proteins. Immunol. Rev 253, 273–289 (2013). [DOI] [PubMed] [Google Scholar]
- 17.von Gamm M et al. Immune homeostasis and regulation of the interferon pathway require myeloid-derived Regnase-3. J. Exp. Med 216, 1700–1723 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei J et al. Targeting Regnase-1 programs long-lived effector T cells for cancer therapy. Nature 576, 471–476 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng W et al. Regnase-1 suppresses TCF-1+ precursor exhausted T cell formation to limit CAR-T-cell responses against ALL. Blood 138, 122–135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y et al. Central role of myeloid MCPIP1 in protecting against LPS-induced inflammation and lung injury. Signal Transduct. Target Ther 2, 17066 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertossi A et al. Loss of Roquin induces early death and immune deregulation but not autoimmunity. J. Exp. Med 208, 1749–1756 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui X et al. Regnase-1 and Roquin nonredundantly regulate TH1 differentiation causing cardiac inflammation and fibrosis. J. Immunol 199, 4066–4077 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Ventura A et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Sledzinska A TGF-β signalling is required for CD4+ T cell homeostasis but dispensable for regulatory T cell function. PLoS Biol. 11, e1001674 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeitrag J, Alterauge D, Dahlstrom F & Baumjohann D Gene dose matters: considerations for the use of inducible CD4-CreER(T2) mouse lines. Eur. J. Immunol 50, 603–605 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Hudson WH et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity 51, 1043–1058 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLane LM, Abdel-Hakeem MS & Wherry EJ CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev. Immunol 37, 457–495 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Yu D et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature 450, 299–303 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Tan AH, Wong SC & Lam KP Regulation of mouse inducible costimulator (ICOS) expression by Fyn-NFATc2 and ERK signaling in T cells. J. Biol. Chem 281, 28666–28678 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Iwasaki H et al. The IκB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR-IL-1R by controlling degradation of regnase-1. Nat. Immunol 12, 1167–1175 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Janowski R et al. Roquin recognizes a non-canonical hexaloop structure in the 3′-UTR of Ox40. Nat. Commun 7, 11032 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlundt A et al. Structural basis for RNA recognition in roquin-mediated post-transcriptional gene regulation. Nat. Struct. Mol. Biol 21, 671–678 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Srivastava M et al. Roquin binds microRNA-146a and Argonaute2 to regulate microRNA homeostasis. Nat. Commun 6, 6253 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan D, Zhou M, Kiledjian M & Tong L The ROQ domain of Roquin recognizes mRNA constitutive-decay element and double-stranded RNA. Nat. Struct. Mol. Biol 21, 679–685 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki HI et al. MCPIP1 ribonuclease antagonizes dicer and terminates microRNA biogenesis through precursor microRNA degradation. Mol. Cell 44, 424–436 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Lee SK et al. Interferon-γ excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity 37, 880–892 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Ellyard JI et al. Heterozygosity for Roquinsan leads to angioimmunoblastic T-cell lymphoma-like tumors in mice. Blood 120, 812–821 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Blank CU et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol 19, 665–674 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Essig K et al. Roquin targets mRNAs in a 3′-UTR-specific manner by different modes of regulation. Nat. Commun 9, 3810 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murakawa Y et al. RC3H1 post-transcriptionally regulates A20 mRNA and modulates the activity of the IKK/NF-κB pathway. Nat. Commun 6, 7367 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song J et al. Human cytomegalovirus induces and exploits Roquin to counteract the IRF1-mediated antiviral state. Proc. Natl Acad. Sci. USA 116, 18619–18628 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Essig K et al. Roquin suppresses the PI3K-mTOR signaling pathway to inhibit T helper cell differentiation and conversion of Treg to Tfr cells. Immunity 47, 1067–1082 e12 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Nagahama Y et al. Regnase-1 controls colon epithelial regeneration via regulation of mTOR and purine metabolism. Proc. Natl Acad. Sci. USA 115, 11036–11041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoefig KP et al. Defining the RBPome of primary T helper cells to elucidate higher-order Roquin-mediated mRNA regulation. Nat. Commun 12, 5208 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 45.Lee PP et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15, 763–774 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Hogquist KA et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994). [DOI] [PubMed] [Google Scholar]
- 47.Hochedlinger K, Yamada Y, Beard C & Jaenisch R Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121, 465–477 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Schieweck R et al. Pumilio2 and Staufen2 selectively balance the synaptic proteome. Cell Rep. 35, 109279 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Digman MA, Caiolfa VR, Zamai M & Gratton E The phasor approach to fluorescence lifetime imaging analysis. Biophys. J 94, L14–L16 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are provided in the article and its supplementary files or from the corresponding author upon reasonable request. For analysis of the Roquin structure the publicly available structural datasets of Roquin-1 ROQ domain bound to RNA (PDB 4QI2) and Roquin-1 ROQ-HEPN domain (PDB 4TXA) were used. Source data are provided with this paper.