Abstract
Background
Cholecystectomy is the removal of gallbladder and is performed mainly for symptomatic gallstones. Although laparoscopic cholecystectomy is currently preferred over open cholecystectomy for elective cholecystectomy, reports of randomised clinical trials comparing the choice of cholecystectomy (open or laparoscopic) in acute cholecystitis are still being conducted. Drainage in open cholecystectomy is a matter of considerable debate. Surgeons use drains primarily to prevent subhepatic abscess or bile peritonitis from an undrained bile leak. Critics of drain condemn drain use as it increases wound and chest infection.
Objectives
To assess the benefits and harms of routine abdominal drainage in uncomplicated open cholecystectomy.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded until April 2006.
Selection criteria
We included randomised clinical trials comparing 'no drain' versus 'drain' in patients who had undergone uncomplicated open cholecystectomy (irrespective of language, publication status, and the type of drain). Randomised clinical trials comparing one drain with another were also included.
Data collection and analysis
We collected the data on the characteristics and methodological quality of each trial, number of abdominal collections requiring different treatments, bile peritonitis, wound infection, chest complications, and hospital stay from each trial. We analysed the data with both the fixed‐effect and the random‐effects models using RevMan Analysis. For each outcome, we calculated the odds ratio (OR) with 95% confidence intervals (CI) based on intention‐to‐treat analysis.
Main results
Twenty eight trials involving 3659 patients were included. There were 20 comparisons of 'no drain' versus 'drain' and 12 comparisons of one drain with another. There was no statistically significant difference in mortality, bile peritonitis, total abdominal collections, abdominal collections requiring different treatments, or infected abdominal collections. 'No drain' group had statistically significant lower wound infection (OR 0.61, 95% CI 0.43 to 0.87) and statistically significant lower chest infection (OR 0.59, 95% CI 0.42 to 0.84) than drain group. We found no significant differences between different types of drains.
Authors' conclusions
Drains increase the harms to the patient without providing any additional benefit for patients undergoing open cholecystectomy and should be avoided in open cholecystectomy.
Plain language summary
Drains increase the harms to patients undergoing open cholecystectomy
Cholecystectomy is the removal of the gallbladder. It is performed mainly in patients having symptomatic gallstones. Drain usage after open cholecystectomy is controversial. The present review includes 28 trials assessing 20 comparisons of 'no drain' versus 'drain' and 12 comparisons of different drain types. The review reports that drains increase the harms to the patient. Drains do not provide any additional benefit for patients undergoing open cholecystectomy and should be avoided in open cholecystectomy. The review found no significant differences between different drain types.
Background
About 10% to 15% of the adult population in the United States have gallstones (NIH 1992). Only 1% to 4% of them become symptomatic in a year (NIH 1992). Although laparoscopic cholecystectomy is currently preferred over open cholecystectomy for elective cholecystectomy (NIH 1992; Fullarton 1994; Keus 2006a), reports of randomised clinical trials comparing the choice of cholecystectomy (open or laparoscopic) in acute cholecystitis are still being conducted (Johansson 2005; Rai 2005). Furthermore, a number of laparoscopic cholecystectomies have to be converted into open operations (Keus 2006b).
Drainage in open cholecystectomy is a matter of considerable debate. Surgeons use drains primarily to prevent subhepatic abscess or bile peritonitis from an undrained bile leak (Sarr 1987). Other surgeons do not use drainage routinely because of reports of higher incidence of wound infection (Budd 1982; Monson 1991), chest infections (Budd 1982; Monson 1991), and increase in post‐operative temperature (Ronaghan 1986). The possible mechanism of increased chest infections and atelectasis (lung collapse) is decreased ventilation caused by the increased amount of postoperative discomfort related to the use of the drain (Monson 1991). Chest infection and atelectasis may delay hospital discharge. Furthermore, the drains do not perform the function that they are intended to do, like prevent bile peritonitis (Budd 1982) and subhepatic collections (Monson 1991; Irwin 1988), which may require additional interventions such as re‐operation. Other studies have found that there is no significant difference in the incidence of wound infection or chest infection (Playforth 1985; Lewis 1990), or hospital stay (Budd 1982).
Surgical drains may either be open or closed. An open drain is when an artificial conduit is left in the wound to allow drainage of fluids to the exterior (eg, corrugated drain; Penrose drain; Yeates drain). Closed drains may either be suction drains (eg, Redon drain) or passive drains (eg, Robinson drain).
We have not been able to identify any meta‐analyses or systematic reviews comparing routine abdominal drainage versus no abdominal drainage in uncomplicated open cholecystectomy.
Objectives
To assess the benefits and harms of routine abdominal drainage in uncomplicated open cholecystectomy.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised clinical trials (irrespective of language, blinding, or publication status) were considered for this review.
Quasi‐randomised studies (where the method of allocating participants to a treatment are not strictly random (eg, date of birth, hospital record number, alternation) and case control studies were not considered for this review.
Types of participants
Patients who have undergone uncomplicated open cholecystectomy.
Types of interventions
We included trials comparing drainage versus no drainage in uncomplicated open cholecystectomy, irrespective of ‐ the type of the drain; ‐ type of incision; and ‐ site of the drain ‐ main wound or stab incision.
Trials comparing two types of drains (open versus closed) or two types of drainage sites (main wound versus stab incision) were also included in this review.
Co‐interventions were allowed provided they are used equally in the intervention arms.
Types of outcome measures
Primary outcomes
Mortality at maximal follow‐up.
-
Additional procedures for subhepatic collection.
Open procedure.
Radiological drainage requiring insertion of drain.
Radiological drainage requiring percutaneous aspiration.
Bile peritonitis.
Secondary outcomes
Infected wound collections.
Wound infection (as reported by authors).
Respiratory complications (mainly chest infection and atelectasis).
Hospital stay.
Pain (measured using any validated scale).
Search methods for identification of studies
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003). We have given the search strategies with the time span for the searches in Appendix 1 (date of last search April 2006).
We also searched the references of the identified trials to identify further relevant trials.
Data collection and analysis
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2006) and the Cochrane Hepato‐Biliary Group Module (Gluud 2006).
Trial selection and extraction of data
We did not apply any language or publication status restrictions. Both of us, independently of each other, identified the trials for inclusion. Both of us extracted independently the data mentioned above. We assessed the methodological quality of the trials independently, without masking of the study names. We intended to obtain any unclear or missing information by contacting the authors of the individual trials. However, since the last trial was published more than 14 years ago, we did not contact any author. We identified whether the trials shared the same patients (completely or partially) by identifying common authors, centres, inclusion criteria, exclusion criteria, type of drain and other methods of the study such as the policy followed for drain removal. We resolved all differences in opinion through discussion.
Assessment of methodological quality
Due to the risk of overestimation of intervention effects in randomised trials with inadequate methodological quality (Schulz 1995; Moher 1998; Kjaergard 2001), we looked at the influence of methodological quality of the trials on the trial results by evaluating the reported randomisation and follow‐up procedures in each trial. We assessed generation of the allocation sequence, allocation concealment, and follow‐up.
Generation of the allocation sequence
Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice will be considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described.
Inadequate, if a system involving dates, names, or admittance numbers were used for the allocation of patients. These studies are known as quasi‐randomised and will be excluded from the review.
Allocation concealment
Adequate, if the allocation of patients involved a central independent unit, on‐site locked computer, or sealed envelopes.
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described.
Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised (such studies will be excluded).
Blinding
Blinding was not assessed since we expected that there would be no double‐blind trials. However, we recorded whether any of the outcomes were assessed by a blinded observer or assessor blinding.
Follow‐up
Adequate, if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals.
Unclear, if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated.
Inadequate, if the number or reasons for dropouts and withdrawals were not described.
Statistical methods
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2006).
We used the software package RevMan 4.2 (RevMan 2003). For dichotomous variables, we calculated the odds ratio with 95% confidence interval. For the main comparison ('no drain' versus 'drain'), we have reported the random‐effects model (DerSimonian 1986) and the fixed‐effect model (DeMets 1987). Heterogeneity was explored by chi‐squared test with significance set at P value 0.10, and the quantity of heterogeneity was measured by I2 (Higgins 2002). For the other comparisons and for sub‐group analyses, we have reported the results of the fixed‐effect model if the I2 was less than 25% (ie, low heterogeneity) and the random‐effects model if the I2 was equal to or more than 25% (high heterogeneity).
The analysis was performed on an intention‐to‐treat basis (Newell 1992). We did not impute any data and performed the analysis on an available‐case basis (Higgins 2006). We intended to perform a sensitivity analysis with and without empirical continuity correction factors as suggested by Sweeting et al (Sweeting 2004) in case we were to find 'zero‐event' trials in statistically significant outcomes. We have also reported the risk difference.
Subgroup analyses
We performed the following subgroup analyses: ‐ trials with high methodological quality, ie, low risk of bias. Based on the description of quality components, trials with adequate allocation concealment and adequate follow‐up were considered high quality, in spite of very few being with adequate generation of the allocation sequence and none of them blinded.‐ drainage in emergency cholecystectomy. ‐ drainage in elective cholecystectomy. ‐ no drain versus suction drain. ‐ no drain versus passive closed drain. ‐ no drain versus passive open drain. ‐ trials in which routine antibiotic prophylaxis was used. ‐ trials in which the drain was brought out through a separate wound. ‐ trials in which the drain was brought out through the main wound.
Bias exploration
We used the funnel plot to explore bias (Egger 1997; Macaskill 2001). Asymmetry in funnel plot of trial size against treatment effect was used to assess bias. We also assessed the funnel plot asymmetry through the linear regression approach described by Egger et al (Egger 1997) by StatsDirect 2.4.
Results
Description of studies
We identified a total of 540 references through electronic searches of The Cochrane Hepato‐Biliary Group Controlled Trials Register and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 89), MEDLINE (n = 119), EMBASE (n = 239), and Science Citation Index Expanded (n = 93). We excluded 173 duplicates and 318 clearly irrelevant references through reading abstracts. Forty‐nine references were retrieved for further assessment. No references were identified through scanning reference lists of the identified randomised trials. Of the 49 references, we excluded 18 because of the reasons listed in the table 'Characteristics of excluded studies'. In total, 31 reports of 28 randomised trials fulfilled the inclusion criteria. All the 28 trials were completed trials and could provide data for the analyses. Details of the trials are shown in the table 'Characteristics of included studies'. Four trials had three arms providing data for more than one comparison (Budd 1982; McCormack 1983; Loder 1987; Kriplani 1992). In total, there were 20 comparisons of 'no drain' versus 'drain' and 12 comparisons of one type of drain versus another.
In total 3659 patients were involved in the trials included for this review. The average age in 18 trials was 51.7 years. Two trials (Locker 1983; Latif 1989) reported the median age. The mean or median age was not stated in other 8 trials (Trowbridge 1982; Playforth 1985; Loder 1987; Chattopadhyay 1990; Lewis 1990; al‐Arfaj 1992; Kriplani 1992; Saad 1993). Females constituted 85% of the population studied in 21 trials. The number of females was not stated or was not clear in seven trials (Trowbridge 1982; Fraser 1982; Porati 1984; Chattopadhyay 1990; Brewster 1992; Kriplani 1992; Saad 1993).
Separate data for elective and emergency cholecystectomy were available in two trials (Playforth 1985; Monson 1991). Five trials (van der Linden 1980; Trowbridge 1982; Bartolo 1985; Loder 1987; Sarr 1987) clearly mentioned the inclusion of emergency cholecystectomy. However, separate data were not available for elective and emergency cholecystectomy for any of the outcomes reported in this for any of these five trials. It was not clear whether emergency cholecystectomy was included in five trials (Huguier 1980; McCormack 1983; Salam 1984; al‐Arfaj 1992; Saad 1993). In the remaining 16 trials, only elective cholecystectomy was included.
Routine antibiotic prophylaxis was used in six trials (Trowbridge 1982; Playforth 1985; Latif 1989; Chattopadhyay 1990; Monson 1991; Brewster 1992). Routine antibiotic prophylaxis was not used in five trials (van der Linden 1980; Fraser 1982; Porati 1984; Druart 1990; Lewis 1990). In the remaining 17 trials, the prophylactic antibiotic use was not stated or no separate data were available for those who had prophylactic antibiotics and those who did not receive prophylactic antibiotics.
Only one trial reported that the drain was brought out through the main wound (Druart 1990). In three trials (Gordon 1976; van der Linden 1980; Trowbridge 1982), some patients had the drain brought out through main wound and the others had the drain brought out through a separate wound. There are no separate data available for any of the outcomes reported in this review from these three trials. The drain was brought out through a separate wound in 13 trials (Edlund 1979; Huguier 1980; McCormack 1983; Playforth 1985; Sarr 1987; Adloff 1987; Loder 1987; Latif 1989; Monson 1991; al‐Arfaj 1992; Forster 1992; Kriplani 1992; Saad 1993). The reports of the remaining 11 trials did not state whether the drain was brought out through the main wound or through a separate wound.
Risk of bias in included studies
The generation of random sequence was adequate in four (Huguier 1980; Bartolo 1985; Playforth 1985; Forster 1992) of the 26 trials (15.4%) in which this was applicable. In two studies (Locker 1983; Sarr 1987), generation of random sequence was not applicable as the randomisation was performed by drawing a card from a deck of playing cards and by drawing lots respectively. Generation of random sequence was not clear in the remaining 22 trials. Seventeen trials (Gordon 1976; Huguier 1980; van der Linden 1980; Budd 1982; Fraser 1982; Trowbridge 1982; Locker 1983; Salam 1984; Playforth 1985; Adloff 1987; Loder 1987; Sarr 1987; Schaupp 1988; Lewis 1990; Kupczyk‐Joeris 1991; Monson 1991; Kriplani 1992) of the 28 trials (60.7%) had adequate allocation concealment. These 17 trials also had adequate follow‐up and we consider these 17 trials as high‐quality trials, ie, trials with low risk of bias. The allocation concealment is not clear in the remaining 11 trials. None of the trials reported blinding of participants or outcome assessors. The follow‐up was adequate in all trials except one (Porati 1984), ie, 27 of 28 trials (96.4%). None of the trials reported whether they used intention‐to‐treat analysis. Three trials (10.7%) (Lewis 1990; Monson 1991; Forster 1992) reported on sample‐size calculations.
Effects of interventions
No drain versus drain
The results of the meta‐analysis are tabulated in Table 1 and Table 2.
1. Odds ratios and risk difference (95% confidence intervals).
| Outcome | Fixed‐effect | Random‐effects | High method quality | Emergency cholecyst | Elective cholecyst | Suction drain | Passive closed drain | Passive open drain | Risk difference |
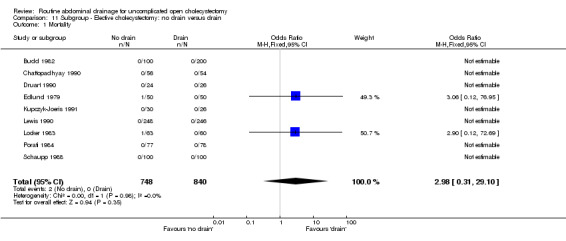
| 01 Mortality | 0.79 [0.21, 2.97] | 0.84 [0.17, 4.08] | 0.54 [0.11, 2.58] | Not applicable. | 2.98 [0.31, 29.10] | 0.55 [0.12, 2.62] | Not estimable. | Not estimable. | 0.00 [‐0.01, 0.01] |
| 02 Abdominal collections requiring re‐operation | Not estimable. | Not estimable. | Not estimable. | Not estimable. | Not estimable. | Not estimable. | Not applicable. | Not estimable. | 0.00 [‐0.01, 0.01] |
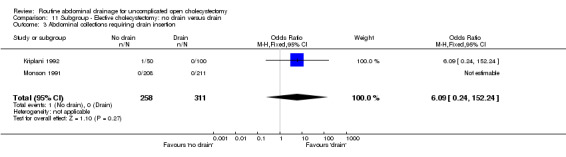
| 03 Abdominal collections requiring drain insertion | 6.09 [0.24, 152.24] | 6.09 [0.24, 152.24] | 6.09 [0.24, 152.24] | Not estimable | 6.09 [0.24, 152.24] | 3.06 [0.12, 76.95] | Not applicable. | 3.06 [0.12, 76.95] | 0.00 [‐0.02, 0.03] |
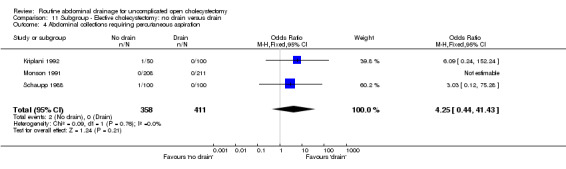
| 04 Abdominal collections requiring percutaneous aspiration | 4.25 [0.44,41.43] | 4.29 [0.44, 41.71] | 4.25 [0.44, 41.43] | Not estimable. | 4.25 [0.44, 41.43] | 3.06 [0.12, 76.95] | Not applicable. | 3.06 [0.12, 76.95] | 0.01 [‐0.01, 0.02] |
| 05 Total abdominal collections | 1.33 [0.93, 1.89] | 1.30 [0.67, 2.53] | 1.00 [0.12, 8.23] | Not applicable. | 1.95 [1.24, 3.08] | 0.82 [0.20, 3.39] | 2.44 [1.14, 5.21] | 1.80 [1.05, 3.07] | 0.07 [‐0.06, 0.20] |
| 06 Infected intra‐abdominal collections | 0.70 [0.14, 3.59] | 0.68 [0.11, 4.37] | 0.70 [0.14, 3.59] | Not applicable. | 0.98 [0.14, 6.96] | 0.33 [0.01, 8.31] | Not estimable. | Not estimable. | 0.00 [‐0.02, 0.01] |
| 07 Bile peritonitis | 1.33 [0.22, 8.01] | 1.47 [0.10, 21.57] | 1.33 [0.22, 8.01] | Not applicable. | 1.47 [0.10, 21.57] | 1.00 [0.14, 7.18] | Not applicable. | 1.00 [0.14, 7.18] | 0.00 [‐0.02, 0.02] |
| 08 Wound infection | 0.61 [0.43, 0.87]* | 0.64 [0.44, 0.93]* | 0.58 [0.38, 0.91]* | 0.56 [0.16, 1.92] | 0.67 [0.43, 1.03] | 0.63 [0.28, 1.44] | 0.68 [0.25, 1.88] | 0.58 [0.29, 1.17] | ‐0.02 [‐0.04, ‐0.01]* |
| 09 Chest infection | 0.59 [0.42, 0.84]* | 0.84 [0.49, 1.44] | 0.63 [0.33, 1.23] | 0.24 [0.05, 1.33] | 0.84 [0.48, 1.50] | 0.51 [0.20, 1.25] | 1.00 [0.48, 2.11] | 1.20 [0.60, 2.39] | ‐0.01 [‐0.04, 0.02] |
| 10 Atelectasis | 0.61 [0.35, 1.08] | 0.62 [0.35, 1.10] | 0.52 [0.23, 1.17] | Not applicable. | 0.61 [0.30, 1.23] | 0.60 [0.29, 1.21] | 0.78 [0.22, 2.79] | 0.53 [0.25, 1.15] | ‐0.04 [‐0.07, 0.00]* |
| 11 Hospital stay | ‐0.39 [‐0.43, ‐0.36]* | ‐0.50 [‐0.90, ‐0.10]* | ‐0.33 [‐0.59, ‐0.07]* | Not applicable. | ‐0.32 [‐0.53, ‐0.11]* | ‐0.46 [‐1.89, 0.98] | ‐0.92 [‐2.01, 0.17] | 0.22 [‐0.28, 0.73] | Not applicable. |
2. Odds ratios (continued).
| Outcome | Antibiotic prophylaxis | No prophylaxis | Separate wound | Main wound |
| 01 Mortality | 0.25 [0.03, 2.23] | Not estimable. | 0.55 [0.12, 2.62] | Not estimable. |
| 02 Abdominal collections requiring re‐operation | Not estimable. | Not estimable. | Not estimable | Not applicable. |
| 03 Abdominal collections requiring drain insertion | Not estimable. | Not applicable. | 6.09 [0.24, 152.24] | Not applicable. |
| 04 Abdominal collections requiring percutaneous aspiration | Not estimable. | Not applicable. | 6.09 [0.24, 152.24] | Not applicable. |
| 05 Total abdominal collections | 0.75 [0.15, 3.75] | Not applicable. | 1.22 [0.54, 2.72] | Not applicable. |
| 06 Infected intra‐abdominal collections | 0.33 [0.01, 8.31] | 0.33 [0.01, 8.31] | 0.33 [0.01, 8.31] | Not applicable. |
| 07 Bile peritonitis | Not applicable. | Not applicable. | 6.09 [0.24, 152.24] | Not applicable. |
| 08 Wound infection | 0.70 [0.29, 1.67] | 0.83 [0.35, 1.97] | 0.60 [0.38, 0.94]* | 0.35 [0.01, 8.93] |
| 09 Chest infection | 0.67 [0.22, 2.01] | 0.75 [0.16, 3.47] | 0.78 [0.35, 1.75] | Not applicable |
| 10 Atelectasis | Not applicable | 1.10 [0.24, 4.99] | 0.63 [0.26, 1.58] | 1.10 [0.24, 4.99] |
| 11 Hospital stay | ‐1.20 [‐2.05, ‐0.35]* | ‐0.40 [‐0.75, ‐0.05]* | ‐0.58 [‐1.20, 0.05] | Not applicable |
Mortality at maximal follow‐up
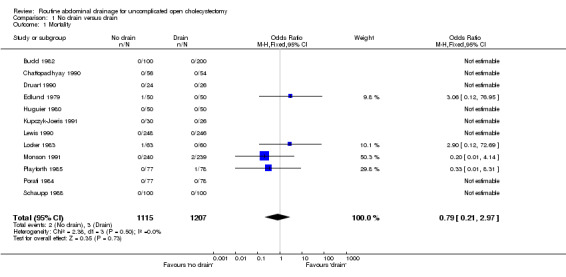
There was no statistically significant difference in mortality (OR 0.79, 95% CI 0.21 to 2.97). There was no change in the results on adopting the random‐effects model, calculating the risk difference, including only trials of high methodological quality or in any of the sub‐group analysis.
Abdominal collections requiring re‐operation
There were no abdominal collections requiring re‐operation in any of the trials that reported on this outcome.
Abdominal collections requiring drain insertion
There was no statistically significant difference in this outcome (OR 6.09, 95% CI 0.24 to 152.24). There was no change in the results on adopting the random‐effects model, calculating the risk difference, including only trials of high methodological quality or in any of the sub‐group analysis.
Abdominal collections requiring percutaneous drainage
There was no statistically significant difference in this outcome (OR 4.25, 95% CI 0.44 to 41.43). There was no change in the results on adopting the random‐effects model, calculating the risk difference, including only trials of high methodological quality or in any of the sub‐group analysis.
Total abdominal collections
There was no statistically significant difference in this outcome (OR 1.33, 95% CI 0.93 to 1.89). There was no change in the results on adopting the random‐effects model, calculating the risk difference, including only trials of high methodological quality or in any of the sub‐group analysis.
Infected abdominal collections
There was no statistically significant difference in this outcome (OR 0.70, 95% CI 0.14 to 3.59). There was no change in the results on adopting the random‐effects model, calculating the risk difference, including only trials of high methodological quality or in any of the sub‐group analysis.
Bile peritonitis
There was no statistically significant difference in this outcome (OR 1.33, 95% CI 0.22 to 8.01). There was no change in the results on adopting the random‐effects model, calculating the risk difference, including only trials of high methodological quality or in any of the sub‐group analysis.
Wound infection
The 'no drain' group had a statistically significant lower wound infection rate (3.4%) compared to that of drain group (5.3%) (OR 0.61, 95% CI 0.43 to 0.87). This continued to remain statistically significant on adopting the random‐effects model, calculating the risk difference and on including only the trials of high methodological quality. In the subgroup analyses, although the odds of developing the wound infection in the 'no drain' group was lower than that of 'drain' group in all subgroups, this difference was statistically significant only in the subgroup analysis of patients in whom the drain was brought out through a separate wound.
Chest infection
The 'no drain' group had a statistically significant lower chest infection rate (5.4%) compared to that of drain group (8.0%) (OR 0.59, 95% CI 0.42 to 0.84). Although it remained lower in the 'no drain' group than 'drain' group on adopting random‐effects model, calculating the risk difference and by including high methodological quality trials only, the difference was not statistically significant. There was also no significant difference in the chest infection rate between the groups in any of the sub‐group analyses performed.
Atelectasis
The 'no drain' group had a lower incidence of atelectasis (6.1%) compared to that of drain group (9.3%). However this difference was not statistically significant in the fixed‐effects model (OR 0.61, 95% CI 0.35 to 1.08), random‐effects model, and by including only high‐quality trials. However, there was a statistically significant risk difference (‐0.04, 95% CI ‐0.07 to 0.00) between the groups favouring the 'no drain' group. The subgroup analyses did not reveal any statistically significant difference in the atelectasis incidence between the groups. However, most of the analyses shows a tendency of lower atelectatic rate in 'no drain' group than in the drain group with some analyses approaching statistical significance.
Hospital stay
The hospital stay was statistically significantly lower in the 'no drain' group compared to the 'drain group' (WMD ‐0.39 days, 95% CI ‐0.43 to ‐0.36). This remained significant on adopting the random‐effects model, calculating the risk difference, and on including only the trials of high methodological quality. In the sub‐group analysis, the hospital stay was statistically significantly lower in the trials that included only elective cholecystectomy and in those in which antibiotic prophylaxis was used routinely and those in which antibiotic prophylaxis was not used routinely. The hospital stay in trials, which reported on this outcome but could not be included in the meta‐analysis because of the non‐availability or standard deviation or because median stay was reported, is tabulated in the Table 3. As seen from the table, the hospital stay is shorter in the 'no drain' group than 'drain' group in most trials, which reported the hospital stay.
3. Hospital stay ('no drain' versus 'drain').
| Study | No drain | Drain | Description | Statistical significance |
| Chattopadhyay 1990 | 6.65 days | 7.63 days | Mean | Significant |
| Al‐Arfaj 1992 | 73/87 | 52/87 drain | Number discharged < 7 days | Significant |
| Budd 1982 | 5.6 days | 5.4 days (suction drain) 6.5 days (passive open drain) | Median | Not stated |
| Edlund 1979 | 7.0 days | 6.8 days | Mean | Not significant. |
| Forster 1992 | 9 days | 9 days | Mean | Not significant. |
| Kupczyk‐Joeris 1991 | 9.2 days | 8.2 days | Mean | Not stated. |
| Latif 1989 | 6 days | 18 days | Mean | Significant |
| Locker 1983 | 53/63 | 35/60 | Number discharged < 6 days | Significant |
| Playforth 1985 | 6 days | 7 days | Median | Not stated. |
| Porati 1984 | 11 days | 13 days | Mean | Not stated. |
| Schaupp 1988 | 9.4 days | 9.9 days | Mean | Not stated. |
Comparison of different types of drains
The results of the meta‐analysis are summarized in the Table 4. The hospital stay in trials, which could not be included for meta‐analysis because of the non‐availability of standard deviation in the report, is tabulated in table 06.
4. Meta‐analysis (one type of drain versus another type of drain).
| Outcome | Suction versus passive closed drain | Column 2 High quality | Column 2 Emergency | Column 2 Elective | Suction versus passive open drain | Column 6 Elective | High pressure versus low pressure suction drain | large bore versus small bore drain | Re‐usable versus disposable drain |
| 01 Mortality | 1.00 [0.06, 16.23] | 1.00 [0.06, 16.23] | 0.32 [0.01, 8.24] | 3.05 [0.12, 76.21] | Not estimable. | Not estimable. | Not applicable. | Not applicable. | Not applicable. |
| 02 Abdominal collections requiring re‐operation | Not applicable. | Not applicable. | Not applicable. | Not applicable. | Not estimable. | Not estimable. | Not applicable. | 0.32 [0.01, 8.25] | Not applicable. |
| 03 Abdominal collections requiring drain insertion | Not applicable | Not applicable | Not applicable | Not applicable | Not estimable | Not estimable | Not applicable. | Not applicable. | Not applicable. |
| 04 Abdominal collections requiring percutaneous aspiration | Not applicable | Not applicable | Not applicable | Not applicable | Not estimable | Not estimable | Not applicable. | Not applicable. | Not applicable. |
| 05 Total abdominal collections | 0.37 [0.06, 2.25] | 0.37 [0.06, 2.25] | Not applicable | 0.37 [0.06, 2.25] | 0.71 [0.28, 1.82] | 0.71 [0.28, 1.82] | Not applicable. | 0.09 [0.00, 1.84] | 0.64 [0.18, 2.20] |
| 06 Infected intra‐abdominal collections | Not applicable. | Not applicable. | Not applicable. | Not applicable. | Not estimable | Not applicable | Not applicable. | Not applicable. | Not applicable. |
| 07 Bile peritonitis | Not applicable. | Not applicable. | Not applicable. | Not applicable. | 1.00 [0.06, 16.21] | 1.00 [0.06, 16.21] | Not applicable | Not applicable. | Not applicable. |
| 08 Wound infection | 0.87 [0.31, 2.41] | 1.14 [0.28, 4.64] | Not applicable. | 2.09 [0.59, 7.34] | 0.77 [0.28, 2.11] | 1.35 [0.29, 6.18] | 0.68 [0.13, 3.55] | Not applicable. | 0.96 [0.06, 16.23] |
| 09 Chest infection | 0.43 [0.10, 1.86] | 0.46 [0.02, 9.23] | Not applicable. | 0.13 [0.03, 0.65]* | 0.81 [0.23, 2.91] | 0.81 [0.23, 2.91] | 1.54 [0.24, 9.75] | 0.32 [0.01, 8.25] | Not applicable. |
| 10 Atelectasis | Not applicable. | Not applicable. | Not applicable. | Not applicable. | 0.71 [0.32, 1.60] | 0.71 [0.32, 1.60] | Not applicable. | Not applicable. | Not applicable. |
| 11 Pain at drain site | 0.16 [0.04, 0.61]* | 0.16 [0.04, 0.61]* | Not applicable. | 0.16 [0.04, 0.61]* | 0.91 [0.12, 6.65] | Not applicable | Not applicable. | Not applicable. | Not applicable. |
| 12 Hospital stay | Not applicable. | Not applicable. | Not applicable. | Not applicable. | 0.39 [0.30, 0.48]* | 0.04 [‐0.44, 0.52] | Not applicable. | Not applicable. | Not applicable. |
Suction drain versus passive closed drain
There is no statistically significant difference in the mortality, total abdominal collections, or wound infection between the two groups (OR 1.00, 95% CI 0.06 to 16.23; OR 0.37, 95% CI 0.06 to 2.25, OR 0.87; 95% CI 0.31 to 2.41). There was no change in results in the subgroup analysis.
The chest infection rate was lower in the suction drain group (2.1%) compared to the passive closed drain group (7.5%). However, this difference is not statistically significant (OR 0.43, 95% CI 0.10 to 1.86). When only elective cholecystectomies were taken into consideration, this difference became statistically significant (OR 0.13, 95% CI 0.03 to 0.65).
The pain was statistically lower at the drain site in the suction group than passive closed drain group (OR 0.16, 95% CI 0.04 to 0.61). The only trial, which reported this outcome (Fraser 1982), included only elective cholecystectomy patients and was of high methodological quality.
As noted in Table 5, there was no statistically significant difference in both the trials that reported on hospital stay.
5. Hospital stay (one type of drain versus another type of drain).
| Study | Comparison | Drain 1 | Drain 2 | Description | Statistical significance |
| Fraser 1982 | Suction vs passive closed drain | 7.1 | 7.1 | Mean | Not significant. |
| McCormack 1983 | Suction vs passive closed drain | 9.6 | 11 | Mean | Not significant. |
| Budd 1982 | Suction vs passive open drain | 5.4 | 6.5 | Median | Not significant. |
| McCormack 1983 | High suction vs low suction | 10.3 | 8.9 | Mean | Not significant. |
Suction drain versus passive open drain
There was no statistically significant difference in any of the outcomes except hospital stay which was 0.39 days (95% CI 0.3 to 0.48) lower in the passive open drain group than the suction group. All the trials comparing suction drain and passive open drain were of high methodological quality. There was no change in the results when only trials in which all cholecystectomies performed electively were included except for hospital stay. The difference between the groups in hospital stay became statistically insignificant when only trials in which all cholecystectomies performed electively were included.
High‐suction versus low‐suction drain
There is no statistically significant difference between the two groups in any of the reported outcomes. The results did not change when only the high methodological quality trial (Loder 1987) was included. In both the trials, no separate data were available with regards to elective and emergency cholecystectomy.
Large bore suction drain versus small bore suction drain
The only trial (Salam 1984), which compared the large bore suction drain and small bore suction drain did not find any significant difference in any of the outcomes reported in this review. This trial was of high methodological quality and it is not clear whether patients who underwent emergency cholecystectomies were included in the trial.
Re‐usable suction drain versus disposable suction drain
The only trial (Bartolo 1985), which compared the re‐usable suction drain and disposable suction drain did not find any significant difference in any of the outcomes reported in this review. This trial was of low methodological quality and no separate data were available with regards to elective and emergency cholecystectomy.
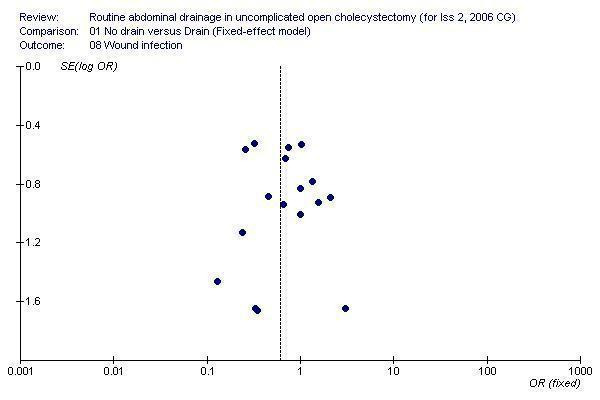
Funnel plot
Visual examination of the funnel plot (Figure 1) does not demonstrate any bias. Statistical examination by Egger's method (Egger 1997) of bias exploration did not arise any cause for concern of bias (P = 0.75).
1.
Discussion
This review has shown that routine drain use after open cholecystectomy does not benefit the patient in any way. However, drain increases the rates of wound infection, chest infection, and atelectasis.
Surgeons use drains primarily to prevent subhepatic abscess or bile peritonitis from an undrained bile leak (Sarr 1987). There was no statistically significant difference in the incidence of bile peritonitis between the 'no drain' group and 'drain' group. The total number of abdominal collections, the infected abdominal collections, and the abdominal collections requiring different interventions were not significantly different between the two groups. All the trials, which reported on the re‐operation for abdominal collections, reported that there was no patient in either group who needed re‐operation. This appears to be a rare event and does not warrant routine drainage.
Critics of the routine drain have used the claim that drain increase wound infection and chest infections (Budd 1982; Monson 1991). In this review, we found that the wound infection rate was statistically significantly higher in the drain group than the 'no drain' group. This was true in spite of adopting various statistical methods like fixed‐effect model, random‐effects model, and risk difference. Many of the sub‐group analyses did not reveal a statistically significant difference. However, the wound infection rate continued to remain lower in the 'no drain' group than 'drain' group. These did not achieve statistical significance, likely because of the smaller number in these sub‐group analyses compared to the main analysis.
The chest infection rate also was higher in the drain group than 'no drain' group. This could be possibly due to the pain induced by the drain. Unfortunately, none of the included trials comparing 'no drain' and 'drain' report on the pain (abdominal or chest pain), and hence this hypothesis could not be tested. Whatever the reason, the fixed‐effects model revealed statistically higher chest infection rate in the drain group. Even in the random‐effects model, trials of high methodological quality, and risk difference, the chest infection rate was higher in the drain group although this was not statistically significant.
The atelectasis rate was also higher in the drain group than the 'no drain' group. The tendency is seen in the fixed‐effect model and random‐effects model, and becomes statistically significant when the risk difference was calculated. The possible reason for this could be the pain induced by the drain as in the case of chest infection.
Hospital stay was statistically significantly lower in the 'no drain' group. But the difference is only 0.39 days that could have arisen spuriously because of the different ways that authors could have calculated the number of days of hospital stay.
None of the causes of mortality reported in the different trials, ie, perforated gastric ulcer (Edlund 1979), ruptured Berry aneurysm (Locker 1983), myocardial infarction (Playforth 1985; Monson 1991), and pulmonary embolism (Monson 1991) seem to have been related to drain use or drain non‐use.
Any systematic review is no better than the trials included in the review. We considered the 61% of the trials as having low risk of bias. However, all trials were conducted without blinded outcome assessment which make the trials prone to bias. We have expected that bias would favour the drain group. In this case, our results may be considered conservative.
To summarize, drains appear to increase the harms to the patient without providing any additional benefit for patients undergoing open cholecystectomy. In this eventuality, we do not attach much importance to the trials comparing different types of drain (which failed to show any significant difference in any of the outcomes measured, except the pain at drain site).
As mentioned previously, although laparoscopic cholecystectomy is currently preferred over open cholecystectomy for elective cholecystectomy (NIH 1992; Fullarton 1994), reports of randomised clinical trials comparing the choice of cholecystectomy (open or laparoscopic) in acute cholecystitis are still being conducted (Johansson 2005; Rai 2005). Thus although the overall results of this review may be of limited value in the high‐income countries, the sub‐group analysis of 'no drain' versus 'drain' is very important even in high‐income countries. Only two of the outcomes reported in this review could be assessed separately for emergency cholecystectomies namely wound infection and chest infection. Both these were lower in the 'no drain' group than 'drain' group (ie, they followed the general trend of results). However, this difference was not statistically significant (possibly due to the small number of patients included). The use of drains in open cholecystectomy performed in acute cholecystitis should be restricted to trials.
Authors' conclusions
Implications for practice.
Drains increase the harms to the patient without providing any additional benefit for patients undergoing open cholecystectomy and should be avoided in open cholecystectomy. Our data give no argument for recommending any specific type of drain.
Implications for research.
The use of drains in open cholecystectomy performed in acute cholecystitis should be assessed by adequately powered high‐quality randomised trials. Such trials ought to involve proper randomisation and blinded evaluation of outcome measures and be reported according to the CONSORT statement (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 21 October 2008 | Amended | Converted to new review format. |
Acknowledgements
Martyn Parker, Peterborough District Hospital, Peterborough, author of more than 15 Cochrane reviews, who inspired me to write Cochrane reviews.
The Cochrane Hepato‐Biliary Group for the support that they have provided.
BR Davidson, Royal Free Hospital, London, who has encouraged me to continue writing Cochrane reviews.
Z Yu, who contributed to the background section of the protocol.
Appendices
Appendix 1. Search strategies for identification of studies
| Database | Period | Search strategy used |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | April 2006. | (cholecystecto* or colecystecto*) AND drain* |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 2, 2006. | #1 cholecystecto* or colecystecto* in All Fields in all products #2 MeSH descriptor Cholecystectomy explode all trees in MeSH products #3 drain* in All Fields in all products #4 MeSH descriptor Drainage explode all trees in MeSH products #5 (( #1 OR #2 ) AND ( #3 OR #4 )) |
| MEDLINE (Pubmed) | 1950 to April 2006. | ((cholecystecto* or colecystecto* OR "Cholecystectomy"[MeSH]) AND (drain* OR "Drainage"[MeSH])) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp]) NOT (animals [mh] NOT human [mh])) |
| EMBASE (Dialog datastar) | 1980 to April 2006. | 1 cholecystect$ OR colecystect$ OR CHOLECYSTECTOMY#.W..DE. 2 drain$ OR SURGICAL‐DRAINAGE#.DE. OR DRAIN#.W..DE. 3 1 AND 2 4 RANDOMIZED‐CONTROLLED‐TRIAL#.DE. OR RANDOMIZATION#.W..DE. OR CONTROLLED‐STUDY#.DE. OR MULTICENTER‐STUDY#.DE. OR PHASE‐3‐CLINICAL‐TRIAL#.DE. OR PHASE‐4‐CLINICAL‐TRIAL#.DE. OR DOUBLE‐BLIND‐PROCEDURE#.DE. OR SINGLE‐BLIND‐PROCEDURE#.DE. 5 RANDOM$ OR CROSSOVER$ OR CROSS‐OVER OR CROSS ADJ OVER OR FACTORIAL$ OR PLACEBO$ OR VOLUNTEER$ 6 (SINGLE OR DOUBLE OR TREBLE OR TRIPLE) NEAR (BLIND OR MASK) 7 4 OR 5 OR 6 8 7 AND HUMAN=YES 9 3 AND 8 |
| Science Citation Index Expanded (http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame) | 1970 to April 2006. | #1 TS=((cholecystecto* OR colecystecto*) AND drain*) #2 TS=(random* OR blind* OR placebo* OR meta‐analysis) #3 #2 AND #1 |
Data and analyses
Comparison 1. No drain versus drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 12 | 2322 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.21, 2.97] |
| 2 Abdominal collections requiring re‐operation | 3 | 1123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Abdominal collections requiring drain insertion | 2 | 629 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.09 [0.24, 152.24] |
| 4 Abdominal collections requiring percutaneous aspiration | 3 | 829 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.25 [0.44, 41.43] |
| 5 Total abdominal collections | 5 | 994 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.67, 2.53] |
| 6 Infected intra‐abdominal collections | 8 | 998 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.14, 3.59] |
| 7 Bile peritonitis | 3 | 573 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.10, 21.57] |
| 8 Wound infection | 17 | 3090 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.43, 0.87] |
| 9 Chest infection | 12 | 2128 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.49, 1.44] |
| 10 Atelectasis | 5 | 774 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.35, 1.08] |
| 11 Hospital stay (days) | 7 | 1623 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.90, ‐0.10] |
1.1. Analysis.
Comparison 1 No drain versus drain, Outcome 1 Mortality.
1.2. Analysis.
Comparison 1 No drain versus drain, Outcome 2 Abdominal collections requiring re‐operation.
1.3. Analysis.
Comparison 1 No drain versus drain, Outcome 3 Abdominal collections requiring drain insertion.
1.4. Analysis.
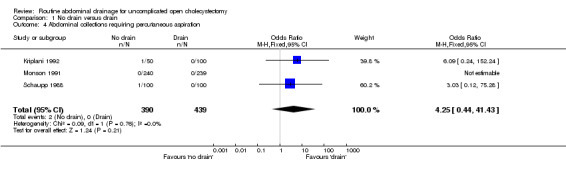
Comparison 1 No drain versus drain, Outcome 4 Abdominal collections requiring percutaneous aspiration.
1.5. Analysis.
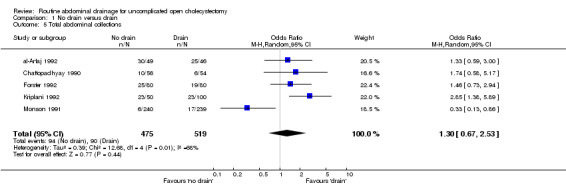
Comparison 1 No drain versus drain, Outcome 5 Total abdominal collections.
1.6. Analysis.
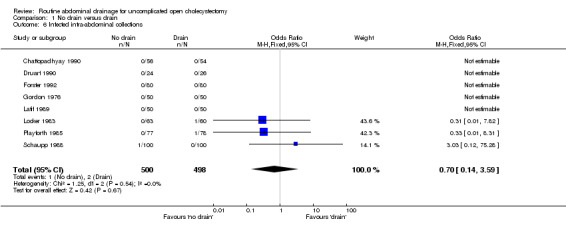
Comparison 1 No drain versus drain, Outcome 6 Infected intra‐abdominal collections.
1.7. Analysis.
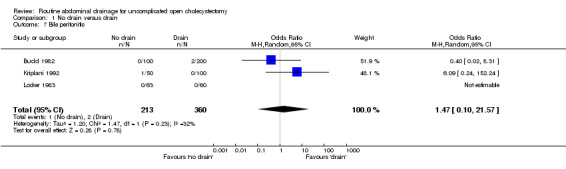
Comparison 1 No drain versus drain, Outcome 7 Bile peritonitis.
1.8. Analysis.
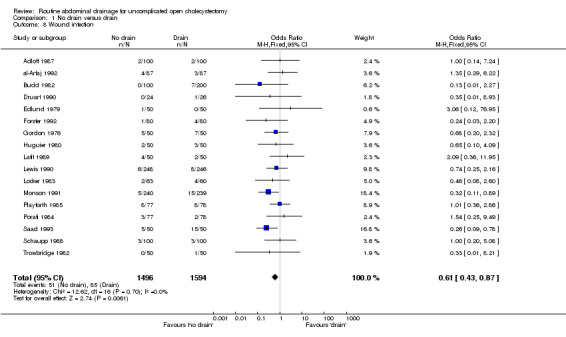
Comparison 1 No drain versus drain, Outcome 8 Wound infection.
1.9. Analysis.
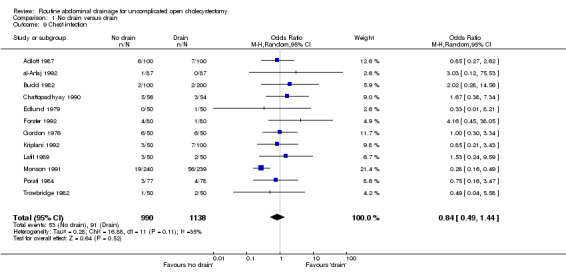
Comparison 1 No drain versus drain, Outcome 9 Chest infection.
1.10. Analysis.
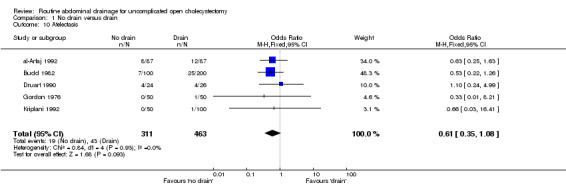
Comparison 1 No drain versus drain, Outcome 10 Atelectasis.
1.11. Analysis.
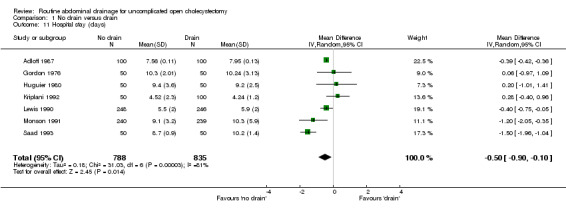
Comparison 1 No drain versus drain, Outcome 11 Hospital stay (days).
Comparison 2. Subgroup ‐ No drain versus suction drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
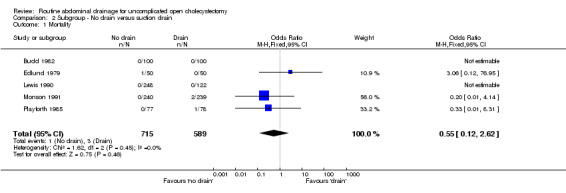
| 1 Mortality | 5 | 1304 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.12, 2.62] |
| 2 Abdominal collections requiring re‐operation | 3 | 949 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Abdominal collections requiring drain insertion | 2 | 579 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.12, 76.95] |
| 4 Abdominal collections requiring percutaneous aspiration | 2 | 579 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.12, 76.95] |
| 5 Total abdominal collections | 3 | 702 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.20, 3.39] |
| 6 Infected intra‐abdominal collections | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.31] |
| 7 Bile peritonitis | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 7.18] |
| 8 Wound infection | 5 | 1073 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.28, 1.44] |
| 9 Chest infection | 5 | 1018 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.20, 1.25] |
| 10 Atelectasis | 3 | 439 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.21] |
| 11 Hospital stay (days) | 2 | 579 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.89, 0.98] |
2.1. Analysis.
Comparison 2 Subgroup ‐ No drain versus suction drain, Outcome 1 Mortality.
2.2. Analysis.
Comparison 2 Subgroup ‐ No drain versus suction drain, Outcome 2 Abdominal collections requiring re‐operation.
2.3. Analysis.
Comparison 2 Subgroup ‐ No drain versus suction drain, Outcome 3 Abdominal collections requiring drain insertion.
2.4. Analysis.
Comparison 2 Subgroup ‐ No drain versus suction drain, Outcome 4 Abdominal collections requiring percutaneous aspiration.
2.5. Analysis.
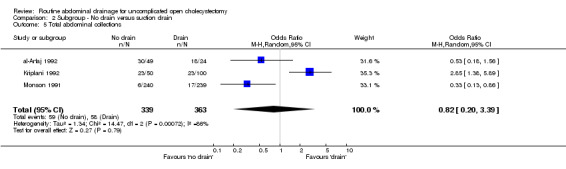
Comparison 2 Subgroup ‐ No drain versus suction drain, Outcome 5 Total abdominal collections.
2.6. Analysis.
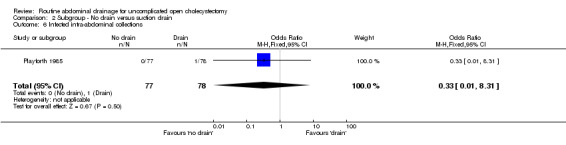
Comparison 2 Subgroup ‐ No drain versus suction drain, Outcome 6 Infected intra‐abdominal collections.
2.7. Analysis.
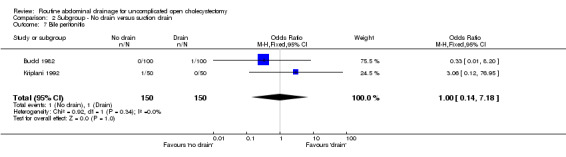
Comparison 2 Subgroup ‐ No drain versus suction drain, Outcome 7 Bile peritonitis.
2.8. Analysis.
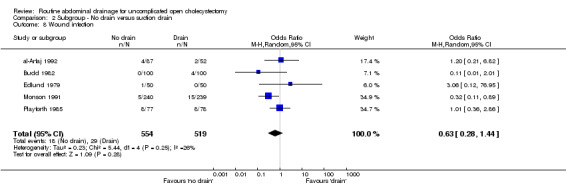
Comparison 2 Subgroup ‐ No drain versus suction drain, Outcome 8 Wound infection.
2.9. Analysis.
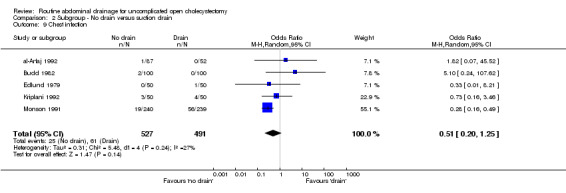
Comparison 2 Subgroup ‐ No drain versus suction drain, Outcome 9 Chest infection.
2.10. Analysis.
Comparison 2 Subgroup ‐ No drain versus suction drain, Outcome 10 Atelectasis.
2.11. Analysis.
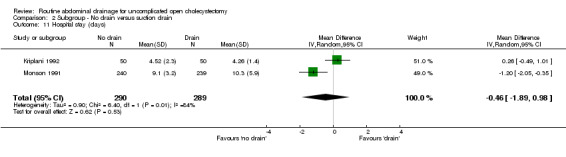
Comparison 2 Subgroup ‐ No drain versus suction drain, Outcome 11 Hospital stay (days).
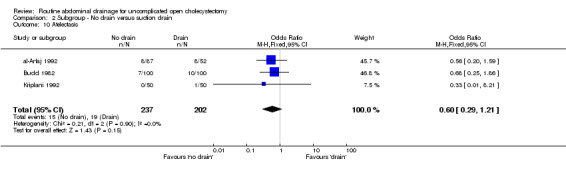
Comparison 3. Subgroup ‐ No drain versus passive closed drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Total abdominal collections | 2 | 181 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.14, 5.21] |
| 3 Infected intra‐abdominal collections | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Wound infection | 4 | 577 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.25, 1.88] |
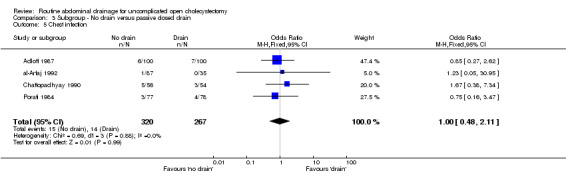
| 5 Chest infection | 4 | 587 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.48, 2.11] |
| 6 Atelectasis | 1 | 122 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.22, 2.79] |
| 7 Hospital stay (days) | 2 | 300 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐2.01, 0.17] |
3.1. Analysis.
Comparison 3 Subgroup ‐ No drain versus passive closed drain, Outcome 1 Mortality.
3.2. Analysis.
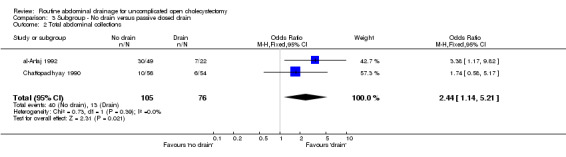
Comparison 3 Subgroup ‐ No drain versus passive closed drain, Outcome 2 Total abdominal collections.
3.3. Analysis.
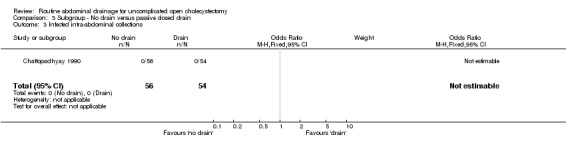
Comparison 3 Subgroup ‐ No drain versus passive closed drain, Outcome 3 Infected intra‐abdominal collections.
3.4. Analysis.
Comparison 3 Subgroup ‐ No drain versus passive closed drain, Outcome 4 Wound infection.
3.5. Analysis.
Comparison 3 Subgroup ‐ No drain versus passive closed drain, Outcome 5 Chest infection.
3.6. Analysis.
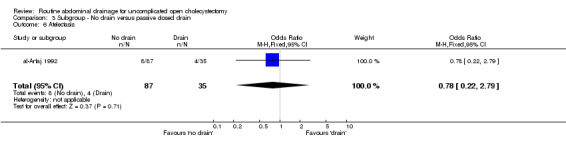
Comparison 3 Subgroup ‐ No drain versus passive closed drain, Outcome 6 Atelectasis.
3.7. Analysis.
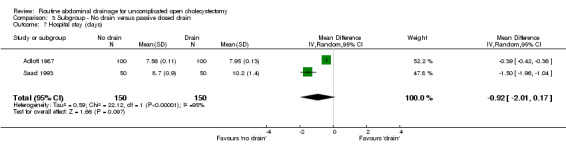
Comparison 3 Subgroup ‐ No drain versus passive closed drain, Outcome 7 Hospital stay (days).
Comparison 4. Subgroup ‐ No drain versus passive open drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 5 | 778 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Abdominal collections requiring re‐operation | 2 | 472 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Abdominal collections requiring drain insertion | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.12, 76.95] |
| 4 Abdominal collections requiring percutaneous aspiration | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.12, 76.95] |
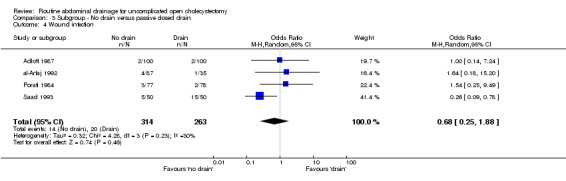
| 5 Total abdominal collections | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.05, 3.07] |
| 6 Infected intra‐abdominal collections | 4 | 410 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Bile peritonitis | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 7.18] |
| 8 Wound infection | 7 | 810 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.29, 1.17] |
| 9 Chest infection | 6 | 760 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.60, 2.39] |
| 10 Atelectasis | 4 | 450 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.15] |
| 11 Hospital stay (days) | 3 | 350 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.28, 0.73] |
4.1. Analysis.
Comparison 4 Subgroup ‐ No drain versus passive open drain, Outcome 1 Mortality.
4.2. Analysis.
Comparison 4 Subgroup ‐ No drain versus passive open drain, Outcome 2 Abdominal collections requiring re‐operation.
4.3. Analysis.
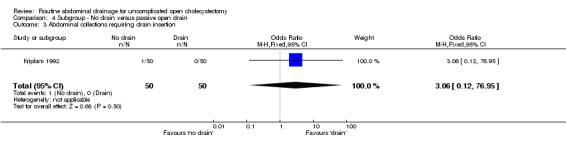
Comparison 4 Subgroup ‐ No drain versus passive open drain, Outcome 3 Abdominal collections requiring drain insertion.
4.4. Analysis.
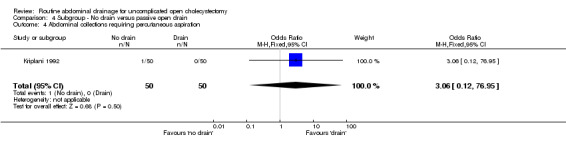
Comparison 4 Subgroup ‐ No drain versus passive open drain, Outcome 4 Abdominal collections requiring percutaneous aspiration.
4.5. Analysis.
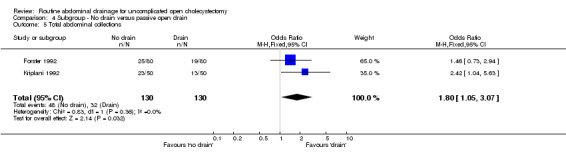
Comparison 4 Subgroup ‐ No drain versus passive open drain, Outcome 5 Total abdominal collections.
4.6. Analysis.
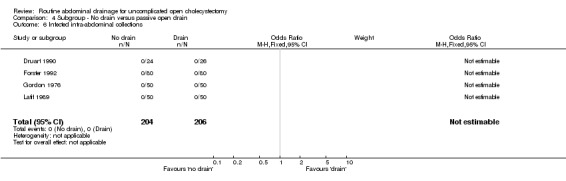
Comparison 4 Subgroup ‐ No drain versus passive open drain, Outcome 6 Infected intra‐abdominal collections.
4.7. Analysis.
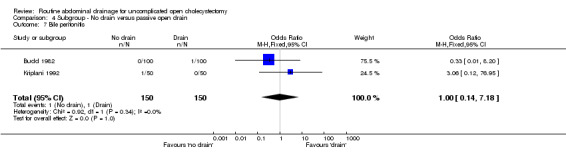
Comparison 4 Subgroup ‐ No drain versus passive open drain, Outcome 7 Bile peritonitis.
4.8. Analysis.
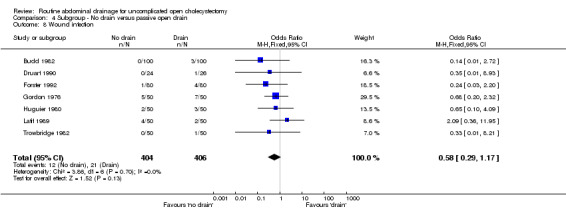
Comparison 4 Subgroup ‐ No drain versus passive open drain, Outcome 8 Wound infection.
4.9. Analysis.
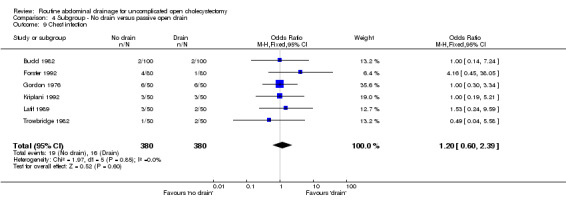
Comparison 4 Subgroup ‐ No drain versus passive open drain, Outcome 9 Chest infection.
4.10. Analysis.
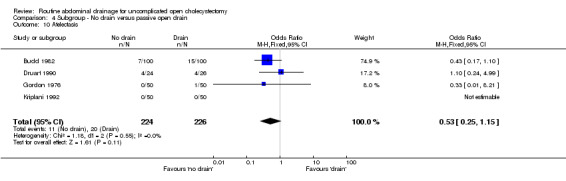
Comparison 4 Subgroup ‐ No drain versus passive open drain, Outcome 10 Atelectasis.
4.11. Analysis.
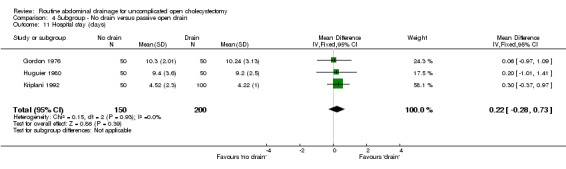
Comparison 4 Subgroup ‐ No drain versus passive open drain, Outcome 11 Hospital stay (days).
Comparison 5. Subgroup ‐ High methodological quality: no drain versus drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 8 | 1907 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.11, 2.58] |
| 2 Abdominal collections requiring re‐operation | 3 | 1123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Abdominal collections requiring drain insertion | 2 | 629 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.09 [0.24, 152.24] |
| 4 Abdominal collections requiring percutaneous aspiration | 3 | 829 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.25 [0.44, 41.43] |
| 5 Total abdominal collections | 2 | 629 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.12, 8.23] |
| 6 Infected intra‐abdominal collections | 4 | 578 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.14, 3.59] |
| 7 Bile peritonitis | 3 | 573 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.22, 8.00] |
| 8 Wound infection | 10 | 2251 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.38, 0.91] |
| 9 Chest infection | 6 | 1329 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.33, 1.23] |
| 10 Atelectasis | 3 | 550 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.23, 1.17] |
| 11 Hospital stay (days) | 6 | 1523 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
5.1. Analysis.
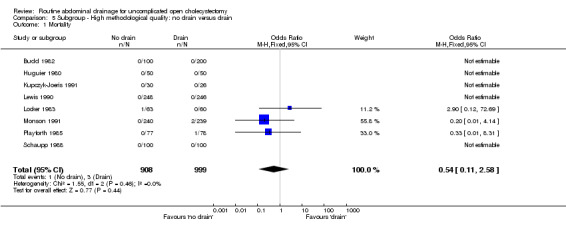
Comparison 5 Subgroup ‐ High methodological quality: no drain versus drain, Outcome 1 Mortality.
5.2. Analysis.
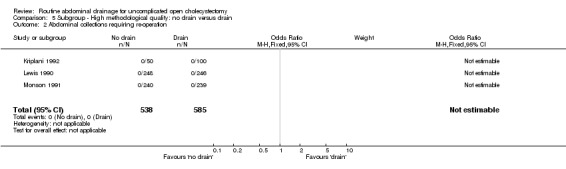
Comparison 5 Subgroup ‐ High methodological quality: no drain versus drain, Outcome 2 Abdominal collections requiring re‐operation.
5.3. Analysis.
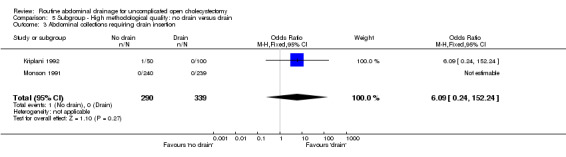
Comparison 5 Subgroup ‐ High methodological quality: no drain versus drain, Outcome 3 Abdominal collections requiring drain insertion.
5.4. Analysis.
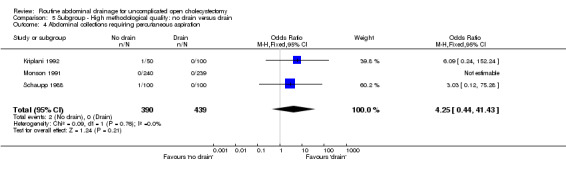
Comparison 5 Subgroup ‐ High methodological quality: no drain versus drain, Outcome 4 Abdominal collections requiring percutaneous aspiration.
5.5. Analysis.
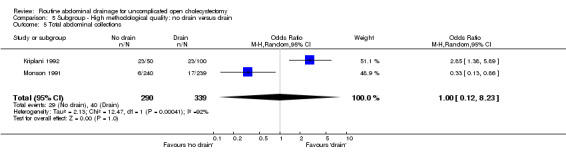
Comparison 5 Subgroup ‐ High methodological quality: no drain versus drain, Outcome 5 Total abdominal collections.
5.6. Analysis.
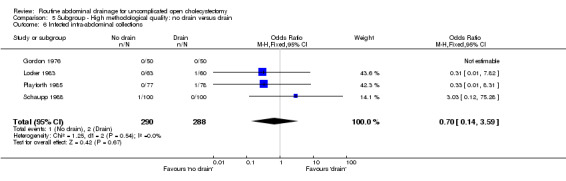
Comparison 5 Subgroup ‐ High methodological quality: no drain versus drain, Outcome 6 Infected intra‐abdominal collections.
5.7. Analysis.
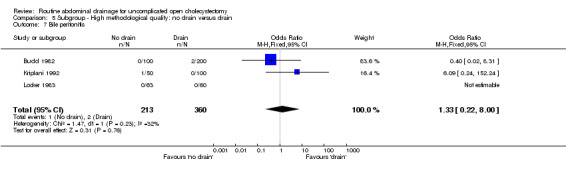
Comparison 5 Subgroup ‐ High methodological quality: no drain versus drain, Outcome 7 Bile peritonitis.
5.8. Analysis.
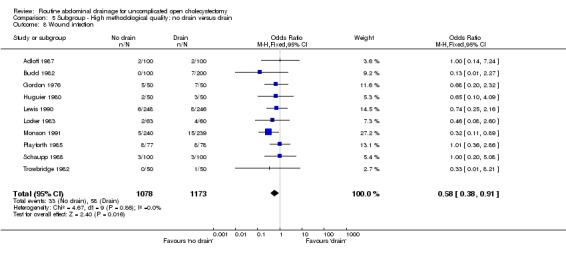
Comparison 5 Subgroup ‐ High methodological quality: no drain versus drain, Outcome 8 Wound infection.
5.9. Analysis.
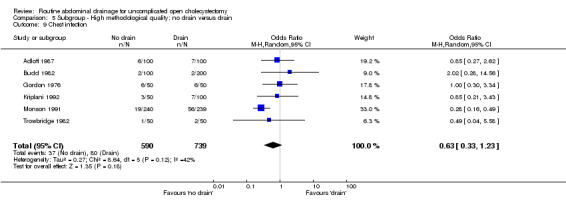
Comparison 5 Subgroup ‐ High methodological quality: no drain versus drain, Outcome 9 Chest infection.
5.10. Analysis.
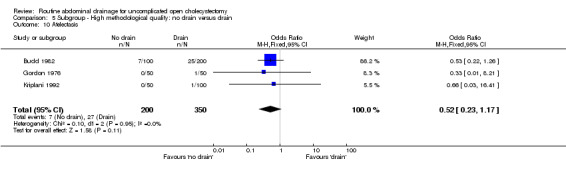
Comparison 5 Subgroup ‐ High methodological quality: no drain versus drain, Outcome 10 Atelectasis.
5.11. Analysis.
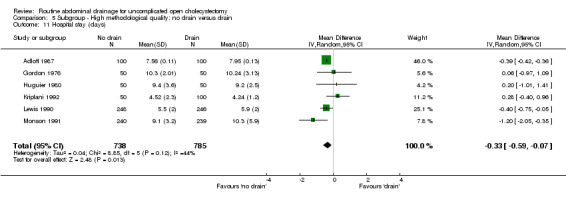
Comparison 5 Subgroup ‐ High methodological quality: no drain versus drain, Outcome 11 Hospital stay (days).
Comparison 6. Subgroup ‐ Routine anitbiotic prophylaxis: no drain versus drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 744 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.23] |
| 2 Abdominal collections requiring re‐operation | 1 | 479 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Abdominal collections requiring drain insertion | 1 | 479 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Abdominal collections requiring percutaneous aspiration | 1 | 479 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Total abdominal collections | 2 | 589 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.15, 3.75] |
| 6 Infected intra‐abdominal collections | 3 | 365 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.31] |
| 7 Wound infection | 4 | 834 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.29, 1.67] |
| 8 Chest infection | 4 | 789 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.22, 2.01] |
| 9 Hospital stay (days) | 1 | 479 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.05, ‐0.35] |
6.1. Analysis.
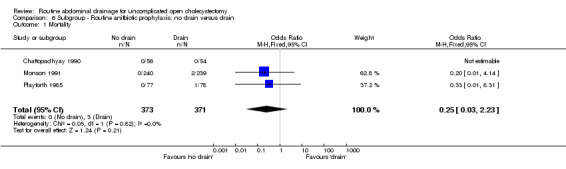
Comparison 6 Subgroup ‐ Routine anitbiotic prophylaxis: no drain versus drain, Outcome 1 Mortality.
6.2. Analysis.
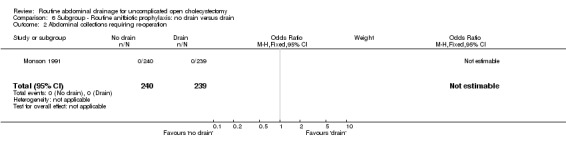
Comparison 6 Subgroup ‐ Routine anitbiotic prophylaxis: no drain versus drain, Outcome 2 Abdominal collections requiring re‐operation.
6.3. Analysis.
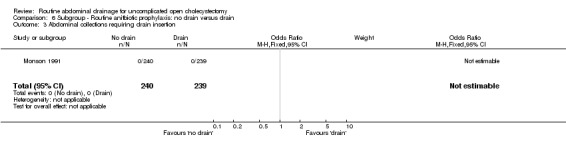
Comparison 6 Subgroup ‐ Routine anitbiotic prophylaxis: no drain versus drain, Outcome 3 Abdominal collections requiring drain insertion.
6.4. Analysis.
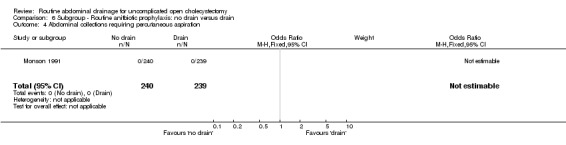
Comparison 6 Subgroup ‐ Routine anitbiotic prophylaxis: no drain versus drain, Outcome 4 Abdominal collections requiring percutaneous aspiration.
6.5. Analysis.
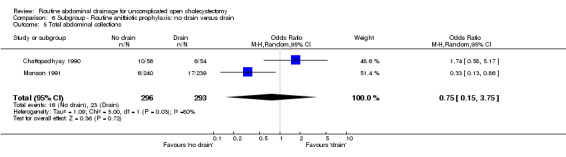
Comparison 6 Subgroup ‐ Routine anitbiotic prophylaxis: no drain versus drain, Outcome 5 Total abdominal collections.
6.6. Analysis.
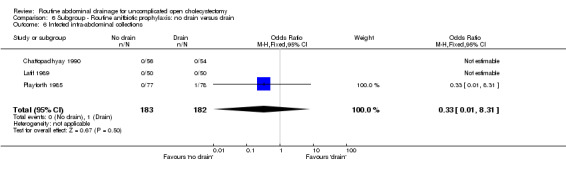
Comparison 6 Subgroup ‐ Routine anitbiotic prophylaxis: no drain versus drain, Outcome 6 Infected intra‐abdominal collections.
6.7. Analysis.
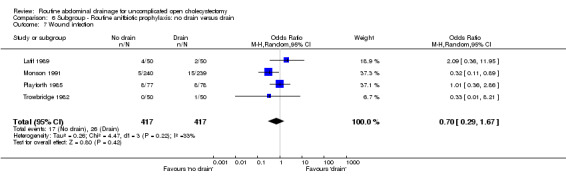
Comparison 6 Subgroup ‐ Routine anitbiotic prophylaxis: no drain versus drain, Outcome 7 Wound infection.
6.8. Analysis.
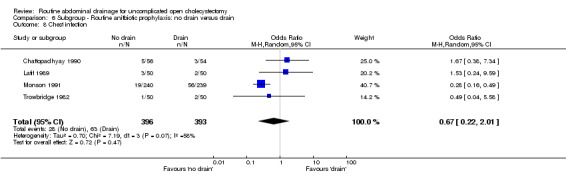
Comparison 6 Subgroup ‐ Routine anitbiotic prophylaxis: no drain versus drain, Outcome 8 Chest infection.
6.9. Analysis.
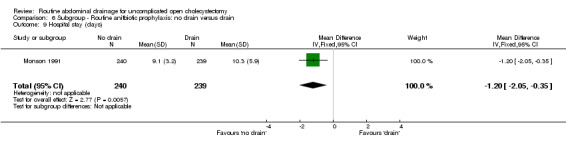
Comparison 6 Subgroup ‐ Routine anitbiotic prophylaxis: no drain versus drain, Outcome 9 Hospital stay (days).
Comparison 7. Subgroup ‐ No routine anitbiotic prophylaxis: no drain versus drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 699 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Abdominal collections requiring re‐operation | 1 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Infected intra‐abdominal collections | 2 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.31] |
| 4 Wound infection | 3 | 699 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.35, 1.97] |
| 5 Chest infection | 1 | 155 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.16, 3.47] |
| 6 Atelectasis | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.24, 4.99] |
| 7 Hospital stay (days) | 1 | 494 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.75, ‐0.05] |
7.1. Analysis.
Comparison 7 Subgroup ‐ No routine anitbiotic prophylaxis: no drain versus drain, Outcome 1 Mortality.
7.2. Analysis.
Comparison 7 Subgroup ‐ No routine anitbiotic prophylaxis: no drain versus drain, Outcome 2 Abdominal collections requiring re‐operation.
7.3. Analysis.
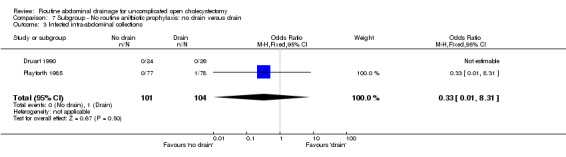
Comparison 7 Subgroup ‐ No routine anitbiotic prophylaxis: no drain versus drain, Outcome 3 Infected intra‐abdominal collections.
7.4. Analysis.
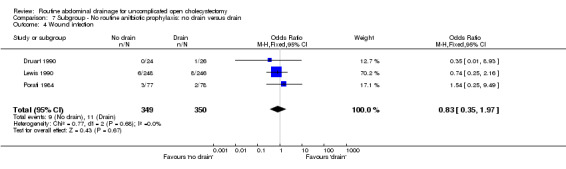
Comparison 7 Subgroup ‐ No routine anitbiotic prophylaxis: no drain versus drain, Outcome 4 Wound infection.
7.5. Analysis.
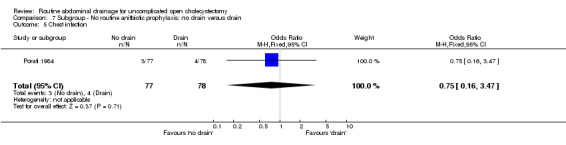
Comparison 7 Subgroup ‐ No routine anitbiotic prophylaxis: no drain versus drain, Outcome 5 Chest infection.
7.6. Analysis.
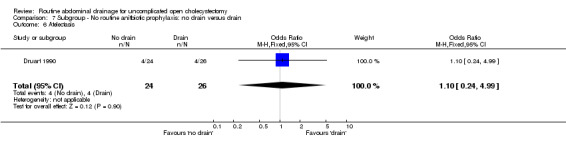
Comparison 7 Subgroup ‐ No routine anitbiotic prophylaxis: no drain versus drain, Outcome 6 Atelectasis.
7.7. Analysis.
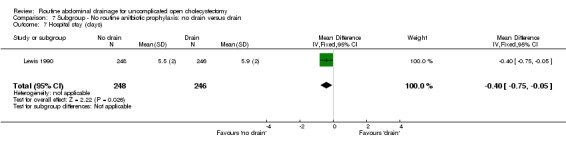
Comparison 7 Subgroup ‐ No routine anitbiotic prophylaxis: no drain versus drain, Outcome 7 Hospital stay (days).
Comparison 8. Subgroup ‐ Brought out through separate wound: no drain versus drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 834 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.12, 2.62] |
| 2 Abdominal collections requiring re‐operation | 2 | 629 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Abdominal collections requiring drain insertion | 2 | 629 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.09 [0.24, 152.24] |
| 4 Abdominal collections requiring percutaneous aspiration | 2 | 629 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.09 [0.24, 152.24] |
| 5 Total abdominal collections | 4 | 884 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.54, 2.72] |
| 6 Infected intra‐abdominal collections | 3 | 415 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.31] |
| 7 Bile peritonitis | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.09 [0.24, 152.24] |
| 8 Wound infection | 9 | 1568 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.38, 0.94] |
| 9 Chest infection | 7 | 1363 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.35, 1.75] |
| 10 Atelectasis | 2 | 324 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.26, 1.58] |
| 11 Hospital stay (days) | 5 | 1029 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.20, 0.05] |
8.1. Analysis.
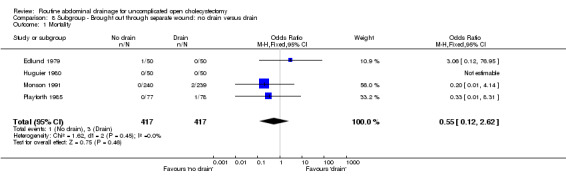
Comparison 8 Subgroup ‐ Brought out through separate wound: no drain versus drain, Outcome 1 Mortality.
8.2. Analysis.
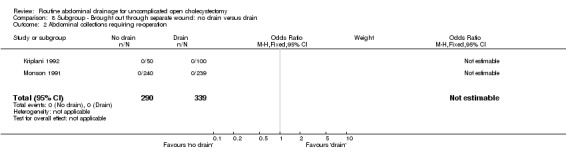
Comparison 8 Subgroup ‐ Brought out through separate wound: no drain versus drain, Outcome 2 Abdominal collections requiring re‐operation.
8.3. Analysis.
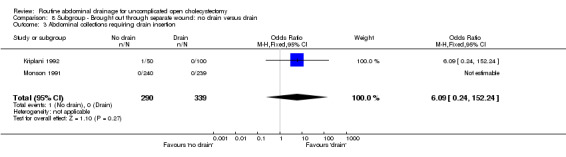
Comparison 8 Subgroup ‐ Brought out through separate wound: no drain versus drain, Outcome 3 Abdominal collections requiring drain insertion.
8.4. Analysis.
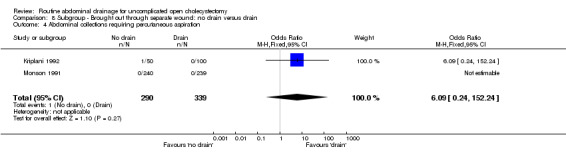
Comparison 8 Subgroup ‐ Brought out through separate wound: no drain versus drain, Outcome 4 Abdominal collections requiring percutaneous aspiration.
8.5. Analysis.
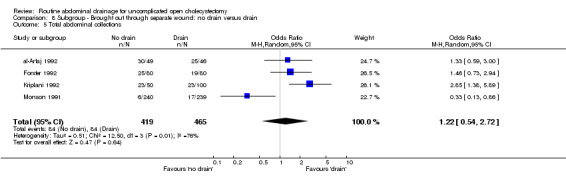
Comparison 8 Subgroup ‐ Brought out through separate wound: no drain versus drain, Outcome 5 Total abdominal collections.
8.6. Analysis.
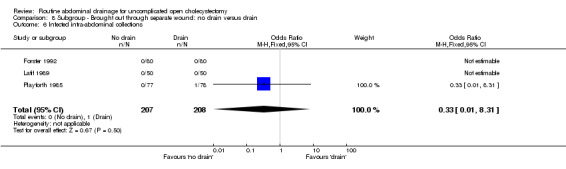
Comparison 8 Subgroup ‐ Brought out through separate wound: no drain versus drain, Outcome 6 Infected intra‐abdominal collections.
8.7. Analysis.
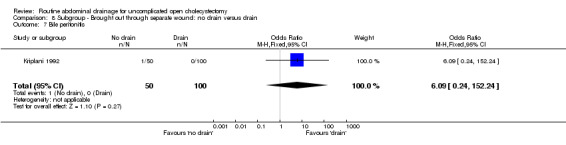
Comparison 8 Subgroup ‐ Brought out through separate wound: no drain versus drain, Outcome 7 Bile peritonitis.
8.8. Analysis.
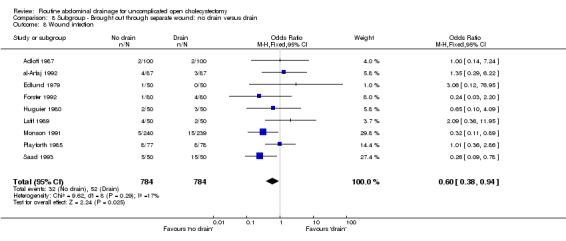
Comparison 8 Subgroup ‐ Brought out through separate wound: no drain versus drain, Outcome 8 Wound infection.
8.9. Analysis.
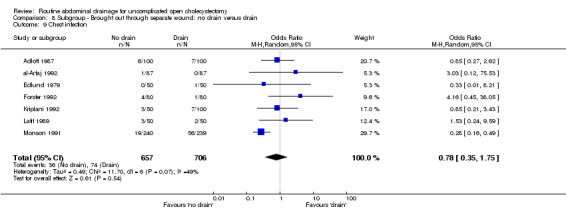
Comparison 8 Subgroup ‐ Brought out through separate wound: no drain versus drain, Outcome 9 Chest infection.
8.10. Analysis.
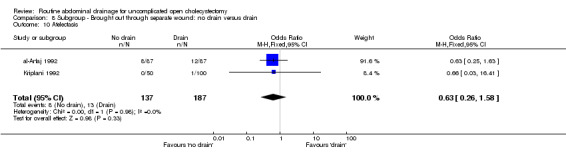
Comparison 8 Subgroup ‐ Brought out through separate wound: no drain versus drain, Outcome 10 Atelectasis.
8.11. Analysis.
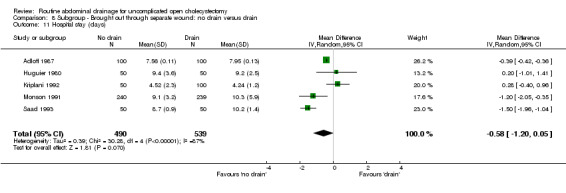
Comparison 8 Subgroup ‐ Brought out through separate wound: no drain versus drain, Outcome 11 Hospital stay (days).
Comparison 9. Subgroup ‐ Brought out through main wound: no drain versus drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Infected intra‐abdominal collections | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Wound infection | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.93] |
| 4 Atelectasis | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.24, 4.99] |
9.1. Analysis.
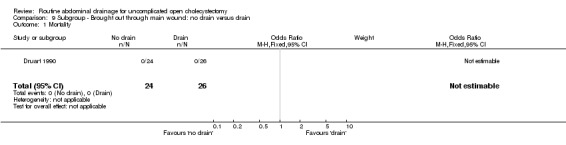
Comparison 9 Subgroup ‐ Brought out through main wound: no drain versus drain, Outcome 1 Mortality.
9.2. Analysis.
Comparison 9 Subgroup ‐ Brought out through main wound: no drain versus drain, Outcome 2 Infected intra‐abdominal collections.
9.3. Analysis.
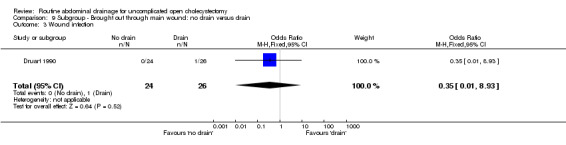
Comparison 9 Subgroup ‐ Brought out through main wound: no drain versus drain, Outcome 3 Wound infection.
9.4. Analysis.
Comparison 9 Subgroup ‐ Brought out through main wound: no drain versus drain, Outcome 4 Atelectasis.
Comparison 10. Subgroup ‐ Emergency cholecystectomy: no drain versus drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal collections requiring re‐operation | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Abdominal collections requiring drain insertion | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Abdominal collections requiring percutaneous aspiration | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
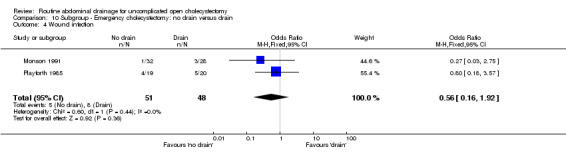
| 4 Wound infection | 2 | 99 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.16, 1.92] |
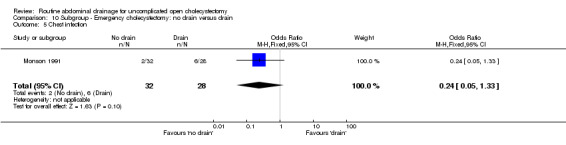
| 5 Chest infection | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.05, 1.33] |
10.1. Analysis.
Comparison 10 Subgroup ‐ Emergency cholecystectomy: no drain versus drain, Outcome 1 Abdominal collections requiring re‐operation.
10.2. Analysis.
Comparison 10 Subgroup ‐ Emergency cholecystectomy: no drain versus drain, Outcome 2 Abdominal collections requiring drain insertion.
10.3. Analysis.
Comparison 10 Subgroup ‐ Emergency cholecystectomy: no drain versus drain, Outcome 3 Abdominal collections requiring percutaneous aspiration.
10.4. Analysis.
Comparison 10 Subgroup ‐ Emergency cholecystectomy: no drain versus drain, Outcome 4 Wound infection.
10.5. Analysis.
Comparison 10 Subgroup ‐ Emergency cholecystectomy: no drain versus drain, Outcome 5 Chest infection.
Comparison 11. Subgroup ‐ Elective cholecystectomy: no drain versus drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 9 | 1588 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.31, 29.10] |
| 2 Abdominal collections requiring re‐operation | 3 | 1063 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Abdominal collections requiring drain insertion | 2 | 569 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.09 [0.24, 152.24] |
| 4 Abdominal collections requiring percutaneous aspiration | 3 | 769 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.25 [0.44, 41.43] |
| 5 Total abdominal collections | 3 | 420 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.24, 3.08] |
| 6 Infected intra‐abdominal collections | 7 | 843 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.96] |
| 7 Bile peritonitis | 3 | 573 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.10, 21.57] |
| 8 Wound infection | 13 | 2517 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.43, 1.03] |
| 9 Chest infection | 10 | 1794 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.48, 1.50] |
| 10 Atelectasis | 4 | 600 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.30, 1.23] |
| 11 Hospital stay (days) | 4 | 944 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.53, ‐0.11] |
11.1. Analysis.
Comparison 11 Subgroup ‐ Elective cholecystectomy: no drain versus drain, Outcome 1 Mortality.
11.2. Analysis.
Comparison 11 Subgroup ‐ Elective cholecystectomy: no drain versus drain, Outcome 2 Abdominal collections requiring re‐operation.
11.3. Analysis.
Comparison 11 Subgroup ‐ Elective cholecystectomy: no drain versus drain, Outcome 3 Abdominal collections requiring drain insertion.
11.4. Analysis.
Comparison 11 Subgroup ‐ Elective cholecystectomy: no drain versus drain, Outcome 4 Abdominal collections requiring percutaneous aspiration.
11.5. Analysis.
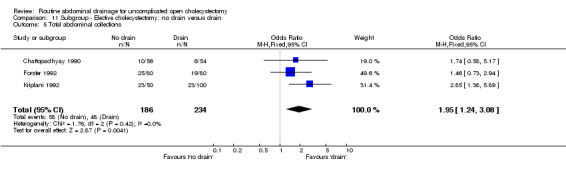
Comparison 11 Subgroup ‐ Elective cholecystectomy: no drain versus drain, Outcome 5 Total abdominal collections.
11.6. Analysis.
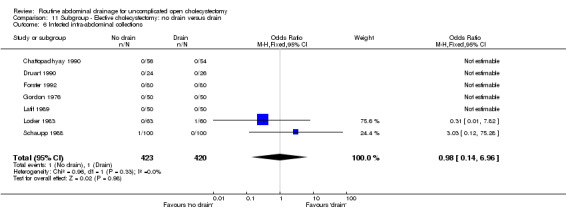
Comparison 11 Subgroup ‐ Elective cholecystectomy: no drain versus drain, Outcome 6 Infected intra‐abdominal collections.
11.7. Analysis.
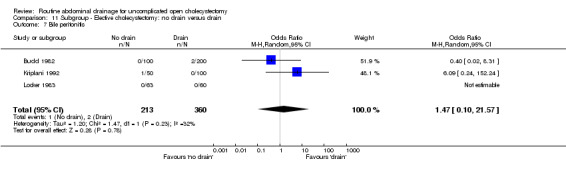
Comparison 11 Subgroup ‐ Elective cholecystectomy: no drain versus drain, Outcome 7 Bile peritonitis.
11.8. Analysis.
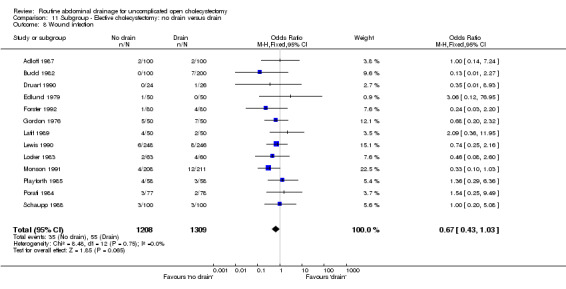
Comparison 11 Subgroup ‐ Elective cholecystectomy: no drain versus drain, Outcome 8 Wound infection.
11.9. Analysis.
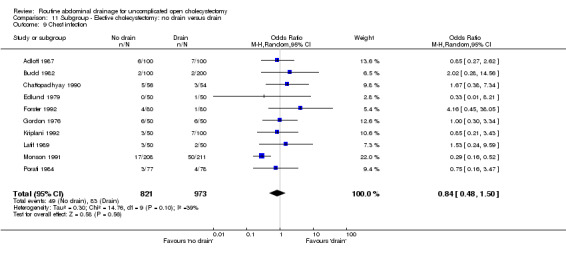
Comparison 11 Subgroup ‐ Elective cholecystectomy: no drain versus drain, Outcome 9 Chest infection.
11.10. Analysis.
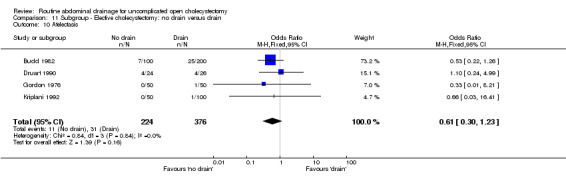
Comparison 11 Subgroup ‐ Elective cholecystectomy: no drain versus drain, Outcome 10 Atelectasis.
11.11. Analysis.
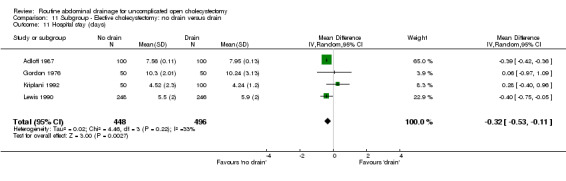
Comparison 11 Subgroup ‐ Elective cholecystectomy: no drain versus drain, Outcome 11 Hospital stay (days).
Comparison 12. No drain versus drain (Risk difference).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 12 | 2322 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 2 Abdominal collections requiring re‐operation | 3 | 1123 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 3 Abdominal collections requiring drain insertion | 2 | 629 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.03] |
| 4 Abdominal collections requiring percutaneous aspiration | 3 | 829 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.01, 0.02] |
| 5 Total abdominal collections | 5 | 994 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.06, 0.20] |
| 6 Infected intra‐abdominal collections | 8 | 998 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 7 Bile peritonitis | 3 | 573 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 8 Wound infection | 17 | 3090 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.04, ‐0.01] |
| 9 Chest infection | 12 | 2128 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 10 Atelectasis | 5 | 774 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.07, 0.00] |
12.1. Analysis.
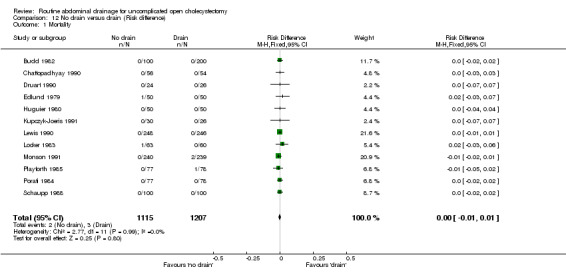
Comparison 12 No drain versus drain (Risk difference), Outcome 1 Mortality.
12.2. Analysis.
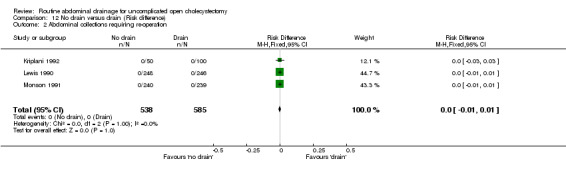
Comparison 12 No drain versus drain (Risk difference), Outcome 2 Abdominal collections requiring re‐operation.
12.3. Analysis.
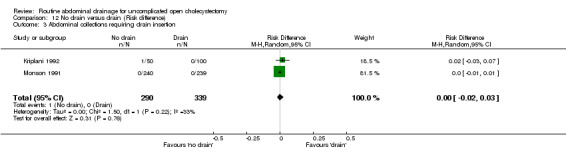
Comparison 12 No drain versus drain (Risk difference), Outcome 3 Abdominal collections requiring drain insertion.
12.4. Analysis.
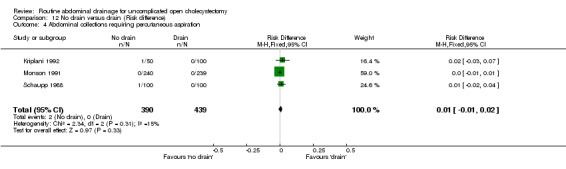
Comparison 12 No drain versus drain (Risk difference), Outcome 4 Abdominal collections requiring percutaneous aspiration.
12.5. Analysis.
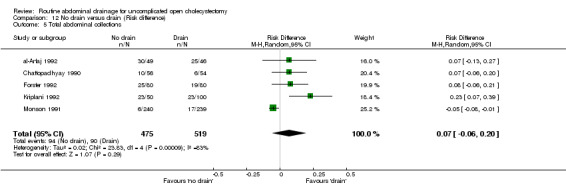
Comparison 12 No drain versus drain (Risk difference), Outcome 5 Total abdominal collections.
12.6. Analysis.
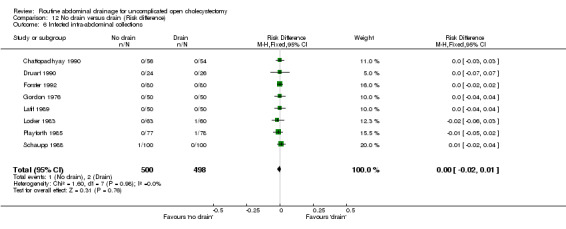
Comparison 12 No drain versus drain (Risk difference), Outcome 6 Infected intra‐abdominal collections.
12.7. Analysis.
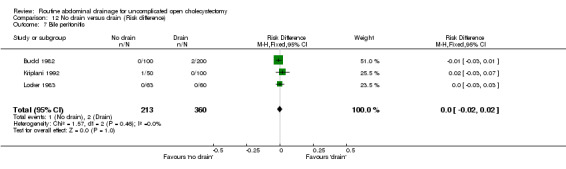
Comparison 12 No drain versus drain (Risk difference), Outcome 7 Bile peritonitis.
12.8. Analysis.
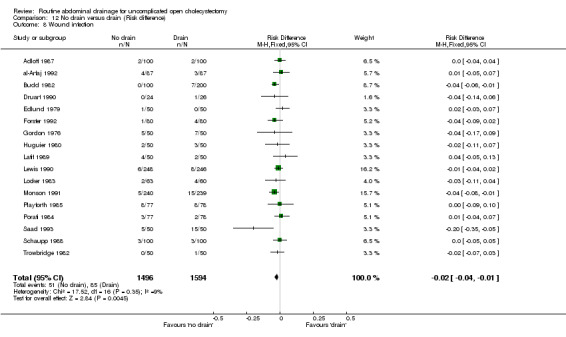
Comparison 12 No drain versus drain (Risk difference), Outcome 8 Wound infection.
12.9. Analysis.
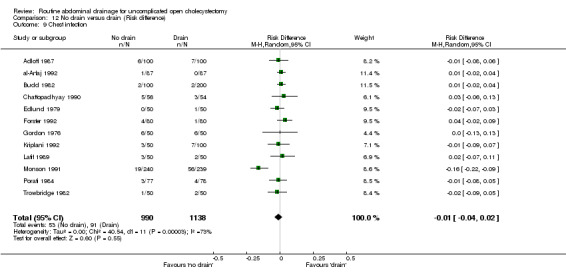
Comparison 12 No drain versus drain (Risk difference), Outcome 9 Chest infection.
12.10. Analysis.
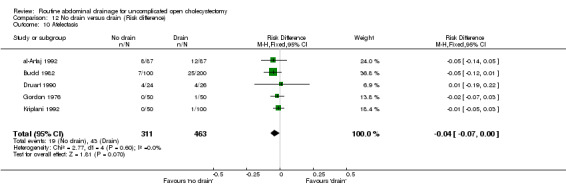
Comparison 12 No drain versus drain (Risk difference), Outcome 10 Atelectasis.
Comparison 13. Suction drain versus passive closed drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.23] |
| 1.1 High methodological quality | 1 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.23] |
| 2 Total abdominal collections | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.06, 2.25] |
| 2.1 Low methodological quality | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.06, 2.25] |
| 3 Wound infection | 3 | 396 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.31, 2.41] |
| 3.1 High methodological quality | 2 | 276 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.28, 4.64] |
| 3.2 Low methodological quality | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.11, 2.00] |
| 4 Chest infection | 3 | 396 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.10, 1.86] |
| 4.1 High methodological quality | 2 | 276 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.02, 9.23] |
| 4.2 Low methodological quality | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 0.6 [0.15, 2.37] |
| 5 Pain at drain site | 1 | 92 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.61] |
| 5.1 High methodological quality | 1 | 92 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.61] |
13.1. Analysis.
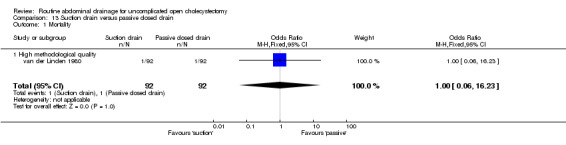
Comparison 13 Suction drain versus passive closed drain, Outcome 1 Mortality.
13.2. Analysis.
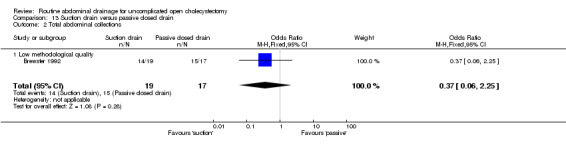
Comparison 13 Suction drain versus passive closed drain, Outcome 2 Total abdominal collections.
13.3. Analysis.
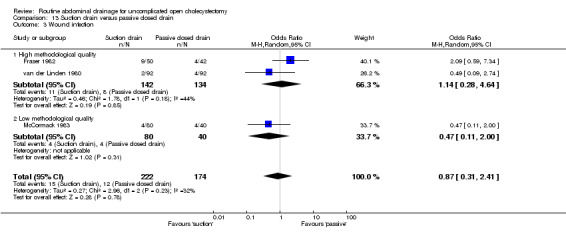
Comparison 13 Suction drain versus passive closed drain, Outcome 3 Wound infection.
13.4. Analysis.
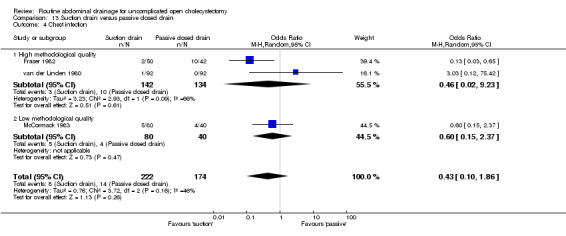
Comparison 13 Suction drain versus passive closed drain, Outcome 4 Chest infection.
13.5. Analysis.
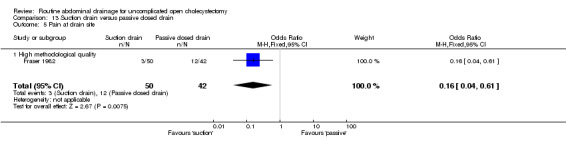
Comparison 13 Suction drain versus passive closed drain, Outcome 5 Pain at drain site.
Comparison 14. Subgroup ‐ Emergency cholecystectomy: suction drain versus passive closed drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.24] |
14.1. Analysis.
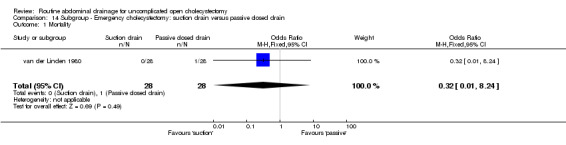
Comparison 14 Subgroup ‐ Emergency cholecystectomy: suction drain versus passive closed drain, Outcome 1 Mortality.
Comparison 15. Subgroup ‐ Elective cholecystectomy: suction drain versus passive closed drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.12, 76.21] |
| 2 Total abdominal collections | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.06, 2.25] |
| 3 Wound infection | 1 | 92 | Odds Ratio (M‐H, Random, 95% CI) | 2.09 [0.59, 7.34] |
| 4 Chest infection | 1 | 92 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.03, 0.65] |
| 5 Pain at drain site | 1 | 92 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.61] |
15.1. Analysis.
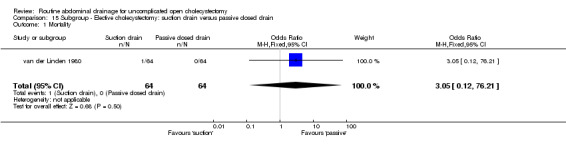
Comparison 15 Subgroup ‐ Elective cholecystectomy: suction drain versus passive closed drain, Outcome 1 Mortality.
15.2. Analysis.
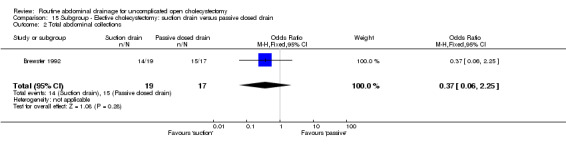
Comparison 15 Subgroup ‐ Elective cholecystectomy: suction drain versus passive closed drain, Outcome 2 Total abdominal collections.
15.3. Analysis.
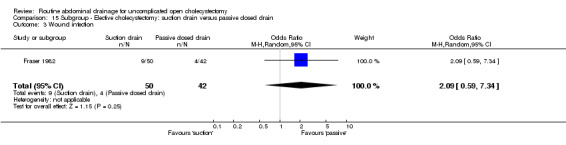
Comparison 15 Subgroup ‐ Elective cholecystectomy: suction drain versus passive closed drain, Outcome 3 Wound infection.
15.4. Analysis.
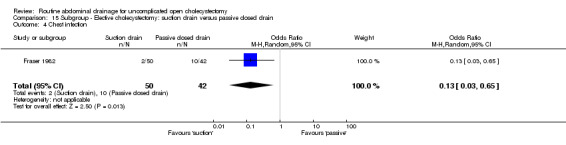
Comparison 15 Subgroup ‐ Elective cholecystectomy: suction drain versus passive closed drain, Outcome 4 Chest infection.
15.5. Analysis.
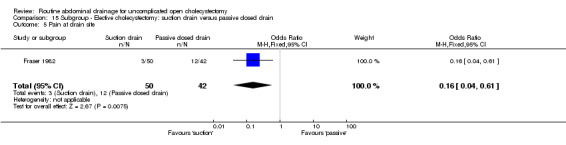
Comparison 15 Subgroup ‐ Elective cholecystectomy: suction drain versus passive closed drain, Outcome 5 Pain at drain site.
Comparison 16. Suction drain versus passive open drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | 328 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Abdominal collections requiring re‐operation | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Abdominal collections requiring drain insertion | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Abdominal collections requiring percutaneous aspiration | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Total abdominal collections | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.28, 1.82] |
| 6 Infected abdominal collections | 1 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Bile peritonitis | 3 | 428 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.21] |
| 8 Wound infection | 3 | 379 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.28, 2.11] |
| 9 Chest infection | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.23, 2.91] |
| 10 Atelectasis | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.32, 1.60] |
| 11 Pain at drain site | 1 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.12, 6.65] |
| 12 Hospital stay (days) | 2 | 228 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [0.30, 0.48] |
16.1. Analysis.
Comparison 16 Suction drain versus passive open drain, Outcome 1 Mortality.
16.2. Analysis.
Comparison 16 Suction drain versus passive open drain, Outcome 2 Abdominal collections requiring re‐operation.
16.3. Analysis.
Comparison 16 Suction drain versus passive open drain, Outcome 3 Abdominal collections requiring drain insertion.
16.4. Analysis.
Comparison 16 Suction drain versus passive open drain, Outcome 4 Abdominal collections requiring percutaneous aspiration.
16.5. Analysis.
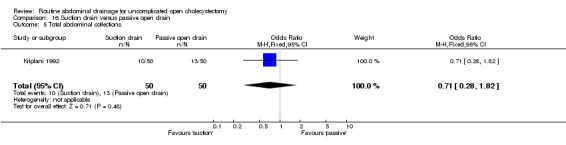
Comparison 16 Suction drain versus passive open drain, Outcome 5 Total abdominal collections.
16.6. Analysis.
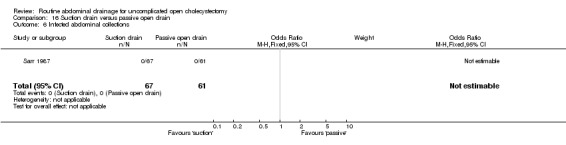
Comparison 16 Suction drain versus passive open drain, Outcome 6 Infected abdominal collections.
16.7. Analysis.
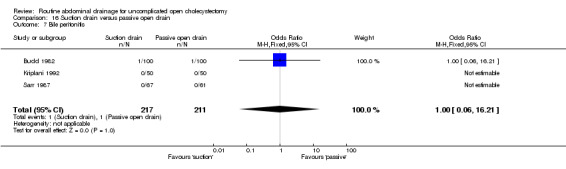
Comparison 16 Suction drain versus passive open drain, Outcome 7 Bile peritonitis.
16.8. Analysis.
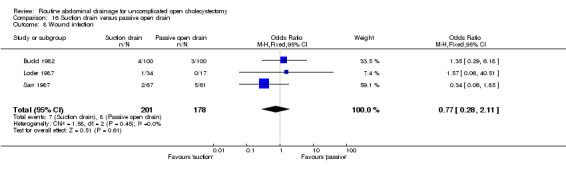
Comparison 16 Suction drain versus passive open drain, Outcome 8 Wound infection.
16.9. Analysis.
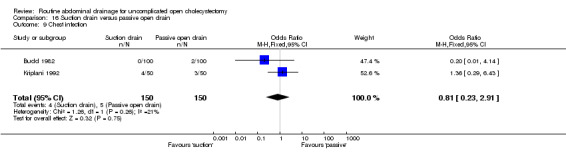
Comparison 16 Suction drain versus passive open drain, Outcome 9 Chest infection.
16.10. Analysis.
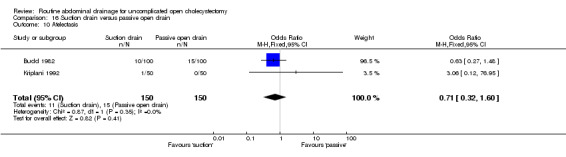
Comparison 16 Suction drain versus passive open drain, Outcome 10 Atelectasis.
16.11. Analysis.
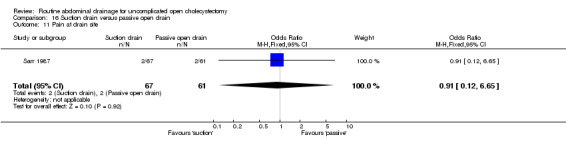
Comparison 16 Suction drain versus passive open drain, Outcome 11 Pain at drain site.
16.12. Analysis.
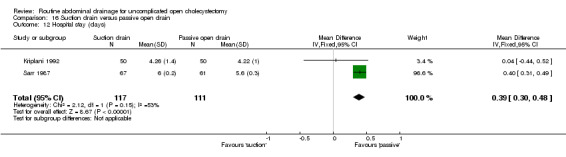
Comparison 16 Suction drain versus passive open drain, Outcome 12 Hospital stay (days).
Comparison 17. Subgroup ‐ Elective cholecystectomy: suction drain versus passive open drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Abdominal collections requiring re‐operation | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Abdominal collections requiring drain insertion | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Abdominal collections requiring percutaneous aspiration | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Total abdominal collections | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.28, 1.82] |
| 6 Bile peritonitis | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.21] |
| 7 Wound infection | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.29, 6.18] |
| 8 Chest infection | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.23, 2.91] |
| 9 Atelectasis | 2 | 300 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.32, 1.60] |
| 10 Hospital stay (days) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.44, 0.52] |
17.1. Analysis.
Comparison 17 Subgroup ‐ Elective cholecystectomy: suction drain versus passive open drain, Outcome 1 Mortality.
17.2. Analysis.
Comparison 17 Subgroup ‐ Elective cholecystectomy: suction drain versus passive open drain, Outcome 2 Abdominal collections requiring re‐operation.
17.3. Analysis.
Comparison 17 Subgroup ‐ Elective cholecystectomy: suction drain versus passive open drain, Outcome 3 Abdominal collections requiring drain insertion.
17.4. Analysis.
Comparison 17 Subgroup ‐ Elective cholecystectomy: suction drain versus passive open drain, Outcome 4 Abdominal collections requiring percutaneous aspiration.
17.5. Analysis.
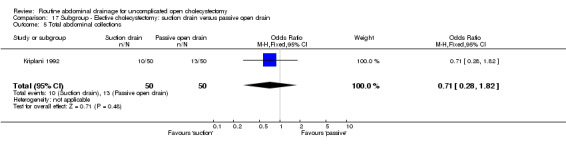
Comparison 17 Subgroup ‐ Elective cholecystectomy: suction drain versus passive open drain, Outcome 5 Total abdominal collections.
17.6. Analysis.
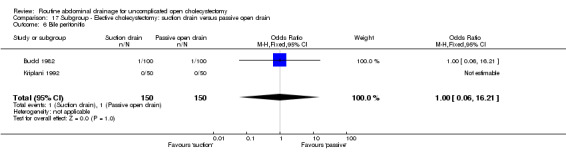
Comparison 17 Subgroup ‐ Elective cholecystectomy: suction drain versus passive open drain, Outcome 6 Bile peritonitis.
17.7. Analysis.
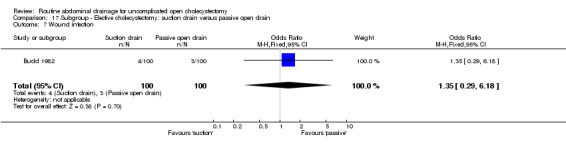
Comparison 17 Subgroup ‐ Elective cholecystectomy: suction drain versus passive open drain, Outcome 7 Wound infection.
17.8. Analysis.
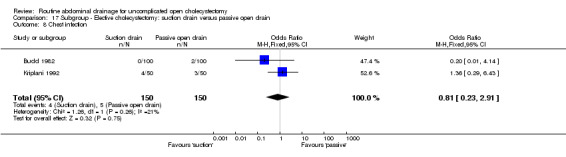
Comparison 17 Subgroup ‐ Elective cholecystectomy: suction drain versus passive open drain, Outcome 8 Chest infection.
17.9. Analysis.
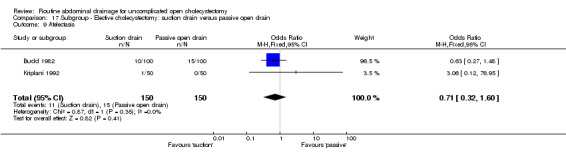
Comparison 17 Subgroup ‐ Elective cholecystectomy: suction drain versus passive open drain, Outcome 9 Atelectasis.
17.10. Analysis.
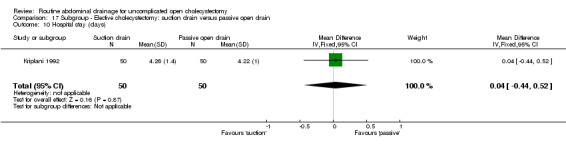
Comparison 17 Subgroup ‐ Elective cholecystectomy: suction drain versus passive open drain, Outcome 10 Hospital stay (days).
Comparison 18. High suction drain versus low suction drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound infection | 2 | 114 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.13, 3.55] |
| 1.1 High methodological quality | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 7.36] |
| 1.2 Low methodological quality | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.47] |
| 2 Chest infection | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.24, 9.75] |
| 2.1 Low methodological quality | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.24, 9.75] |
18.1. Analysis.
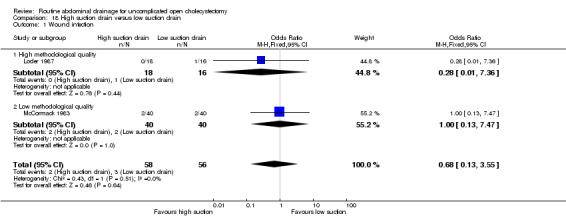
Comparison 18 High suction drain versus low suction drain, Outcome 1 Wound infection.
18.2. Analysis.
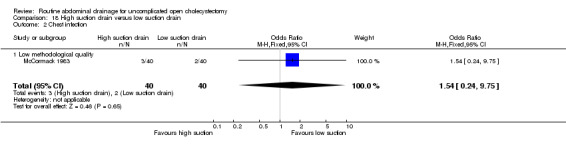
Comparison 18 High suction drain versus low suction drain, Outcome 2 Chest infection.
Comparison 19. Large bore suction drain versus small bore suction drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal collections requiring re‐operation | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.25] |
| 2 Total abdominal collections | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.84] |
| 3 Chest infection | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.25] |
19.1. Analysis.
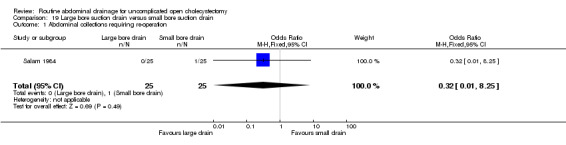
Comparison 19 Large bore suction drain versus small bore suction drain, Outcome 1 Abdominal collections requiring re‐operation.
19.2. Analysis.
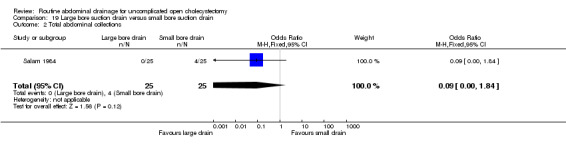
Comparison 19 Large bore suction drain versus small bore suction drain, Outcome 2 Total abdominal collections.
19.3. Analysis.
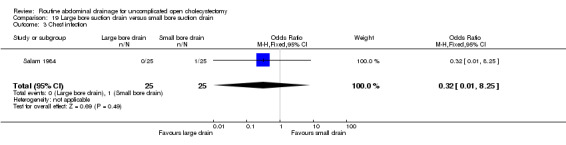
Comparison 19 Large bore suction drain versus small bore suction drain, Outcome 3 Chest infection.
Comparison 20. Disposable suction drain verus re‐usable suction drain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total abdominal collections | 1 | 51 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.18, 2.20] |
| 2 Wound infection | 1 | 51 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.06, 16.23] |
20.1. Analysis.
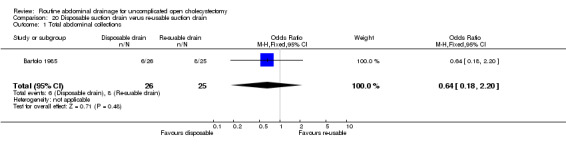
Comparison 20 Disposable suction drain verus re‐usable suction drain, Outcome 1 Total abdominal collections.
20.2. Analysis.
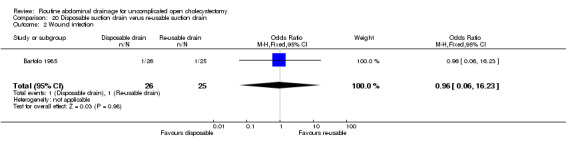
Comparison 20 Disposable suction drain verus re‐usable suction drain, Outcome 2 Wound infection.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adloff 1987.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate. Sealed box. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: France.
Number randomised: 200.
Mean age: 49.9 years.
Females: 164. Inclusion criteria: Elective cholecystectomy. Exclusion criteria: CBD exploration. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: closed passive drain (n = 100). Group 2: no drain (n = 100). Co‐interventions: Drain brought through: separate wound. Antibiotic use: not stated. Duration of drain: not stated. |
|
| Outcomes | The main outcome measures were wound infection, chest infection, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
al‐Arfaj 1992.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: unclear. Follow‐up: adequate. Blinding: none (inadequate). Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: Saudi Arabia.
Number randomised: 174.
Mean age: not stated.
Females: 135. Inclusion criteria: Gallbladder disease requiring cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: drain (some suction, some passive at surgeon's discretion) (n = 87). Group 2: no drain (n = 87). Co‐interventions: Drain brought through: separate wound. Antibiotic use: surgeon's preference. Duration of drain: 48 to 72 hours. |
|
| Outcomes | The main outcome measures were abdominal collections, wound infection, chest infection, atelectasis, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bartolo 1985.
| Methods | Randomised clinical trial Generation of the allocation sequence: adequate. Computer generated . Allocation concealment: unclear. Follow‐up: adequate. Blinding: none (inadequate). Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: United Kingdom.
Number randomised: 51.
Mean age: 60 years.
Females: 40. Inclusion criteria: Cholecystectomy (includes CBD exploration, emergency cholecystectomy). |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: re‐usable suction drain (n = 25). Group 2: disposable suction drain (n = 26). Co‐interventions: Drain brought through: not stated. Antibiotic use: not stated. Duration of drain: not stated. |
|
| Outcomes | The main outcome measures were abdominal collections and wound infection. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Brewster 1992.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: unclear. Follow‐up: adequate. Blinding: none (inadequate). Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: United Kingdom.
Number randomised: 36.
Mean age: 58 years.
Females: Not stated. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: suction drain (n = 19). Group 2: closed passive drain (n = 17). Co‐interventions: Drain brought through: Not stated. Antibiotic use: Yes. Duration of drain: till < 50 ml and not bile stained. |
|
| Outcomes | The main outcome measure was abdominal collection. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Budd 1982.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate. Sealed envelope. Follow‐up: adequate. Blinding: none (inadequate). Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: United States of America.
Number randomised: 300.
Mean age: 43.5 years.
Females: 209. Inclusion criteria: Cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to three groups. Group 1: suction drain (n = 100). Group 2: passive open drain (n = 100). Group 3: no drain (n = 100). Co‐interventions: Drain brought through: not stated. Antibiotic use: not stated. Duration of drain: suction drain: 3rd POD (unless drainage in previous 24 hours > 50 ml). Penrose drain: twisted on 1st POD, advanced 2nd POD and removed on 3rd POD. |
|
| Outcomes | The main outcome measures were mortality, bile peritonitis, wound infection, chest infection, atelectasis and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Chattopadhyay 1990.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: unclear. Follow‐up: adequate. Blinding: none (inadequate). Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: India.
Number randomised: 110.
Mean age: not stated.
Females: not stated. Inclusion criteria: Elective cholecystectomy Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: passive closed drain (n = 54). Group 2: no drain (n = 56). Co‐interventions: Drain brought through: not stated. Antibiotic use: yes. Duration of drain: when drainage was insignificant (exact quantity not stated). |
|
| Outcomes | The main outcome measures were mortality, abdominal collection, wound complications, chest infection, atelectasis, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Druart 1990.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: unclear. Follow‐up: adequate. Intention‐to‐treat analysis: no. Blinding: none (inadequate). Sample size calculation: no. |
|
| Participants | Country: Belgium.
Number randomised: 50.
Mean age: 58 years.
Females: 40. Inclusion criteria: Elective cholecystectomy. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: passive open drain (n = 26). Group 2: no drain (n = 24). Co‐interventions: Drain brought through: main wound. Antibiotic use: not used routinely. Duration of drain: < 50 ml usually 4 or 5th POD. |
|
| Outcomes | The main outcome measures were mortality, abdominal collection, wound infection and chest complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Edlund 1979.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate. Sealed envelope. Follow‐up: adequate. Blinding: none (inadequate). Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: Sweden.
Number randomised: 100.
Mean age: 52.8 years.
Females: 64. Inclusion criteria: Elective cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: suction drain (n = 50). Group 2: no drain (n = 50). Co‐interventions: Drain brought through: separate wound. Antibiotic use: not stated. Duration of drain: < 50 ml usually 4 or 5th POD. |
|
| Outcomes | The main outcome measures were mortality, wound infection, chest infection and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Forster 1992.
| Methods | Randomised clinical trial Generation of the allocation sequence: adequate. Computer generated. Allocation concealment: unclear. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: yes. |
|
| Participants | Country: Germany.
Number randomised: 160.
Mean age: 49 years.
Females: 121. Inclusion criteria: Elective uncomplicated cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: passive open drain (n = 80). Group 2: no drain (n = 80). Co‐interventions: Drain brought through: separate wound. Antibiotic use: not stated. Duration of drain: not stated. |
|
| Outcomes | The main outcome measures were abdominal collections, wound infection, chest infection and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fraser 1982.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate. Sealed envelope technique. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: United Kingdom.
Number randomised: 92.
Mean age: 48.6 years.
Females: not clear. Inclusion criteria: Elective cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: suction (n = 50). Group 2: passive closed drain (n = 42). Co‐interventions: Drain brought through: not stated. Antibiotic use: no. Duration of drain: not stated. |
|
| Outcomes | The main outcome measures were wound infection, chest infection, hospital stay and pain at drain site. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gordon 1976.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate (sealed envelope technique). Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: United Kingdom.
Number randomised: 100.
Mean age: 48 years.
Females: 67. Inclusion criteria: Elective cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: passive open drain (n = 50). Group 2: no drain (n = 50). Co‐interventions: Drain brought through: separate wound (right paramedian incision) and main wound (right subcostal incision). Antibiotic use: not stated. Duration of drain: after 48 hours unless continued significant discharge. |
|
| Outcomes | The main outcome measures were infected abdominal collections, wound infection, chest infection, atelectasis, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Huguier 1980.
| Methods | Randomised clinical trial Generation of the allocation sequence: adequate. Random number table. Allocation concealment: adequate. Sealed envelope technique. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: France.
Number randomised: 100.
Mean age: 51.2 years.
Females: 70. Inclusion criteria: Simple cholecystectomies where the operative field at end of the operation was clear of bile and bleeding. Exclusion criteria: CBD exploration, empyema gall bladder, other malignancies. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: passive open drain (n = 50). Group 2: no drain (n = 50). Co‐interventions: Drain brought through: separate wound. Antibiotic use: not stated. Duration of drain: not stated. |
|
| Outcomes | The main outcome measures were mortality, wound infection, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kriplani 1992.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate. Sealed envelope technique. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: India.
Number randomised: 150.
Mean age: not stated.
Females: not stated. Inclusion criteria: Elective cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to three groups. Group 1: suction drain (n = 50). Group 2: passive open drain (n = 50). Group 3: no drain (n = 50). Co‐interventions: Drain brought through: separate wound. Antibiotic use: In high risk cases such as airway disease, diabetes mellitus, old age. Duration of drain: drain removed in 24 hours unless > 30 ml or more than 2 pads soaked. |
|
| Outcomes | The main outcome measures were bile peritonitis, abdominal collections, chest infection, atelectasis, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kupczyk‐Joeris 1991.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate (sealed envelope technique). Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: Germany.
Number randomised: 56.
Mean age: 52 years.
Females: 38. Inclusion criteria: Elective cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: passive open drain (n = 26). Group 2: no drain (n = 30). Co‐interventions: Drain brought through: not stated. Antibiotic use: not stated. Duration of drain: not stated. |
|
| Outcomes | The main outcome measures were mortality, wound complications and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Latif 1989.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: unclear. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: Saudi Arabia.
Number randomised: 100.
Median age: 36 years.
Females: 82. Inclusion criteria: Elective cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: Passive open drain (n = 50). Group 2: No drain (n = 50). Co‐interventions: Drain brought through: separate wound. Antibiotic use: yes. Duration of drain: within 48 hours. |
|
| Outcomes | The main outcome measures were abdominal collections, wound infection, chest infection and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lewis 1990.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate (sealed envelope technique). Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: yes. |
|
| Participants | Country: Canada.
Number randomised: 494.
Mean age: not stated.
Females: 400. Inclusion criteria: Simple elective cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to three groups. Group 1: suction drain (n = 122). Group 2: passive open drain (n = 124). Group 3: no drain (n = 248). Co‐interventions: Drain brought through: not stated. Antibiotic use: not used routinely ( except for heart valve prophylaxis). Duration of drain: shortened on 1st POD and removed on 2nd POD (unless > 20 ml/eight hours). |
|
| Outcomes | The main outcome measures were mortality, abdominal collections requiring re‐operation, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Locker 1983.
| Methods | Randomised clinical trial Generation of the allocation sequence: Not applicable. Allocation concealment: adequate. Drawing a card from a deck of playing cards at the end of cholecystectomy. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: United States of America.
Number randomised: 123.
Median age: 38.
Females: 96. Inclusion criteria: Elective cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: drain (n = 60). Group 2: no drain (n = 63). Co‐interventions: Drain brought through: not stated. Antibiotic use: not stated. Duration of drain: not stated. |
|
| Outcomes | The main outcome measures were mortality, biliary peritonitis, abdominal collections, wound infection, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Loder 1987.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate. Sealed envelope technique. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: Australia.
Number randomised: 51.
Mean age: not stated.
Females: 38. Inclusion criteria: Cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to three groups. Group 1: high suction drain (n = 18). Group 2: low suction drain (n = 16). Group 3: passive open drain (n = 17). Co‐interventions: Drain brought through: separate wound. Antibiotic use: not stated. Duration of drain: removed on 1st POD. Other co‐interventions: gentamycin 80 mg in 100 ml warmed isotonic saline was used to determine the completeness of drainage. |
|
| Outcomes | The main outcome measures were wound infection and pain. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
McCormack 1983.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: unclear. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: United Kingdom.
Number randomised: 120.
Mean age: 61 years.
Females: 86. Inclusion criteria: Cholecystectomy. |
|
| Interventions | Participants were randomly assigned to three groups. Group 1: high suction drain (n = 40). Group 2: low suction drain (n = 40). Group 3: passive closed drain (n = 40). Co‐interventions: Drain brought through: Separate wound. Antibiotic use: Used in 59 patients, but criteria not stated. Duration of drain: Removed on 3rd POD. |
|
| Outcomes | The main outcome measures were wound infection, chest infection and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Monson 1991.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate. Sealed envelope. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: yes. |
|
| Participants | Country: United Kingdom.
Number randomised: 479.
Mean age: 50.7 years.
Females: 369. Inclusion criteria: Elective and emergency cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: suction drain (n = 239). Group 2: no drain (n = 240). Co‐interventions: Drain brought through: separate wound. Antibiotic use: yes. Duration of drain: not stated. |
|
| Outcomes | The main outcome measures were mortality, abdominal collections, wound infection, chest infection, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Playforth 1985.
| Methods | Randomised clinical trial Generation of the allocation sequence: adequate. Random number table. Allocation concealment: adequate. Held by a third party. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: United Kingdom.
Number randomised: 155.
Mean age: not stated.
Females: 97. Inclusion criteria: Elective and emergency cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: suction drain (n = 78). Group 2: no drain (n = 77). Co‐interventions: Drain brought through: separate wound. Antibiotic use: yes. Duration of drain: 3 to 5 days (criteria not stated). |
|
| Outcomes | The main outcome measures were mortality, re‐operation, wound infection, chest complications, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Porati 1984.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: unclear. Blinding: none (inadequate). Follow‐up: unclear. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: Italy.
Number randomised: 155.
Mean age: 40.7 years.
Females: not stated. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: passive closed drain (n = 78). Group 2: no drain (n = 77). Co‐interventions: Drain brought through: not stated. Antibiotic use: no. Duration of drain: 6 days. |
|
| Outcomes | The main outcome measures were mortality, wound infection, chest infection, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Saad 1993.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: unclear. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: Sudan.
Number randomised: 100.
Mean age: not stated.
Females: not stated. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: passive closed drain (n = 50). Group 2: no drain (n = 50). Co‐interventions: Drain brought through: separate wound. Antibiotic use: not stated. Duration of drain: < 30 ml usually 48 to 72 hours. |
|
| Outcomes | The main outcome measures were wound infection, septicaemia, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Salam 1984.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate. Sealed envelope technique. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: Ireland.
Number randomised: 50.
Mean age: 52 years.
Females: 36. Inclusion criteria: Simple cholecystectomy. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: large bore suction drain (n = 25). Group 2: small bore suction drain (n = 25). Co‐interventions: Drain brought through: not stated. Antibiotic use: not stated. Duration of drain: when the drains stopped draining. |
|
| Outcomes | The main outcome measures were abdominal collections and chest infection. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sarr 1987.
| Methods | Randomised clinical trial Generation of the allocation sequence: not applicable. Allocation concealment: adequate (lots). Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: United States of America.
Number randomised: 128.
Mean age: 48.6 years.
Females: 89. Inclusion criteria: Cholecystectomy for acute or chronic cholecystitis. Exclusion criteria: Undergoing CBD exploration. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: suction drain (n = 67). Group 2: passive open drain (n = 61). Co‐interventions: Drain brought through: separate wound. Antibiotic use: according to discretion of surgeon. Duration of drain: not stated. |
|
| Outcomes | The main outcome measures were mortality, infected abdominal collection, wound infection, bile peritonitis, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schaupp 1988.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate. Sealed envelope technique. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: Germany.
Number randomised: 200.
Mean age: 53.2 years
Females: 131. Inclusion criteria: Cholecystectomy. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: drain (type not stated) (n = 100). Group 2: no drain (n = 100). Co‐interventions: Drain brought through: not stated. Antibiotic use: not stated. Duration of drain: not stated. |
|
| Outcomes | The main outcome measures were mortality, abdominal collections, wound infection, and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Trowbridge 1982.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate. Sealed envelope technique. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: United States of America.
Number randomised: 100.
Mean age: not stated.
Females: not stated. Inclusion criteria:
Exclusion criteria: Bile spillage. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: passive open drain (n = 50). Group 2: no drain (n = 5 0). Co‐interventions: Drain brought through: separate wound (half) and main wound (half). Antibiotic use: yes. Duration of drain: not stated. |
|
| Outcomes | The main outcome measures were wound infection and chest infection. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
van der Linden 1980.
| Methods | Randomised clinical trial Generation of the allocation sequence: unclear. Allocation concealment: adequate. Sealed envelope technique. Blinding: none (inadequate). Follow‐up: adequate. Intention‐to‐treat analysis: no. Sample size calculation: no. |
|
| Participants | Country: Sweden.
Number randomised: 184.
Mean age: 53.3 years.
Females: 125. Inclusion criteria: Patients undergoing cholecystectomy (elective or emergency; with or without CBD exploration). |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: suction drain (n = 92). Group 2: passive closed drain (n = 92). Co‐interventions: Drain brought through: separate wound (half) and main wound (half). Antibiotic use: no. Duration of drain: < 25 ml. |
|
| Outcomes | The main outcome measures were mortality, re‐operation, wound infection and chest infection. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
No attempts were made to contact any author as all the studies were published 14 years ago. CBD = common bile duct. deg F = degrees Fahrenheit.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Diez 1990 | Allocation concealment ‐ inadequate. Randomisation by clinical records. |
| Farha 1981 | Allocation concealment ‐ inadequate. Allocated by rotation. |
| Gupta 1978 | Not a randomised clinical trial. |
| Hanna 1970 | Not performed in humans. |
| Irwin 1988 | No sample size for the different groups. |
| Jha 1986 | No mention of randomisation. |
| Kapoor 1993 | No separate data available for only those who were randomised. |
| Maull 1978 | Allocation concealment inadequate. Drawing cards arranged in random order. No mention of concealing the randomisation. |
| Maull 1981 | Same as above. |
| Peer 1993 | Allocation concealment not stated. The sentence "non‐drainage group included patients with simple cholecystectomy without CBD exploration, no evidence of pericholecystic abscess, empyema of gallbladder, dry liver bed" suggests that this was not a randomised trial. |
| Ragoonanan 1983 | Allocation concealment ‐ inadequate. Randomisation by date of birth. |
| Rivas 1980 | Not a randomised clinical trial. |
| Salam 1994 | Letter to editor with details of an included trial (Salam 1984). |
| Salles 1992 | Not a randomised clinical trial. |
| Shirazi 1982 | Allocation concealment inadequate. Drawing cards arranged in random order. No mention of concealing the randomisation. |
| Stone 1978 | Allocation concealment inadequate. Randomisation by hospital number. |
| Truedson 1983 | Allocation concealment ‐ inadequate. Randomisation by date of birth. |
| van der Linden 1981 | Does not contain any of the outcomes stated in the protocol. |
Contributions of authors
K Gurusamy is the lead author of the review and identified the trials for inclusion, extracted the data, performed statistical analysis, and wrote the review. K Samraj identified the trials and extracted the data independently. K Samraj also critically commented on the discussion.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Adloff 1987 {published data only}
- Adloff M, Arnaud JP, Ollier JC. Cholecystectomy with or without prophylactic drainage. Randomised prospective study of 200 cases. Medecine & Chirurgie Digestives 1987;16(1):9‐12. [Google Scholar]
al‐Arfaj 1992 {published data only}
- al‐Arfaj AL, Shahab K, al‐Ghassab G, al‐Breiki H, al‐Shawan S, Azab A. Drainage after cholecystectomy. A prospective randomized clinical trial. International Surgery 1992;77(4):274‐6. [PubMed] [Google Scholar]
Bartolo 1985 {published data only}
- Bartolo DC, Andrews H, Virjee J, Leaper DJ. The new ReliaVac drain after cholecystectomy. A comparative clinical and ultrasonic trial. Journal of the Royal College of Surgeons of Edinburgh 1985;30(6):358‐9. [PubMed] [Google Scholar]
Brewster 1992 {published data only}
- Brewster NT, King PM, Cunningham C, Adam RD, Griffiths JM. Passive tube and suction drainage after elective cholecystectomy ‐ a comparison using ultrasonography. Journal of the Royal College of Surgeons of Edinburgh 1992;37(5):325‐7. [PubMed] [Google Scholar]
Budd 1982 {published data only}
- Budd DC, Cochran RC, Fouty WJ Jr. Cholecystectomy with and without drainage. A randomized, prospective study of 300 patients. American Journal of Surgery 1982;143(3):307‐9. [DOI] [PubMed] [Google Scholar]
Chattopadhyay 1990 {published data only}
- Chattopadhyay TK, Kapoor VK, Gupta S. Cholecystectomy with and without drainage. Indian Journal of Gastroenterology 1990;9(4):322‐3. [PubMed] [Google Scholar]
Druart 1990 {published data only}
- Druart ML, Fetelian D, Engelman E, Limbosch JM. Elective cholecystectomy without drainage and without prophylactic antibiotics. A prospective randomized trial with clinical and bacteriological aspects. Acta Chirurgica Belgica 1990;90(3):79‐85. [PubMed] [Google Scholar]
Edlund 1979 {published data only}
- Edlund G, Gedda S, Linden W. Intraperitoneal drains and nasogastric tubes in elective cholecystectomy. A controlled clinical trial. American Journal of Surgery 1979;137(6):775‐9. [DOI] [PubMed] [Google Scholar]
Forster 1992 {published data only}
- Forster R, Schnabel M, Krahl M, Lindlar R, Rothmund M. Routine drainage following uncomplicated, elective cholecystectomy? A prospective, randomized study. Der Chirurg 1992;63(7):558‐62. [PubMed] [Google Scholar]
Fraser 1982 {published data only}
- Fraser I, Everson NW, Nash JR. A randomised prospective trial of two drainage methods after cholecystectomy. Annals of the Royal College of Surgeons of England 1982;64(3):183‐5. [PMC free article] [PubMed] [Google Scholar]
Gordon 1976 {published data only}
- Gordon AB, Bates T, Fiddian RV. A controlled trial of drainage after cholecystectomy. The British Journal of Surgery 1976;63(4):278‐82. [DOI] [PubMed] [Google Scholar]
Huguier 1980 {published data only}
- Huguier M. Drainage after cholecystectomy. A controlled trial. Annales de Chirurgie 1980;34(1):48‐50. [PubMed] [Google Scholar]
Kriplani 1992 {published data only}
- Kriplani AK, Sawhney S, Kumar S, Kapur BM. Influence of intraperitoneal drainage after cholecystectomy; a prospective ultrasonographic study. Tropical Gastroenterology 1992;13(4):146‐51. [PubMed] [Google Scholar]
Kupczyk‐Joeris 1991 {published data only}
- Kupczyk‐Joeris D, Schumpelick V, Toens C, Truong S, Goltz I. Elective cholecystectomy with and without subhepatic drainage. A controlled, prospective study. Zentralblatt fur Chirurgie 1991;116(20):1187‐93. [PubMed] [Google Scholar]
Latif 1989 {published data only}
- Latif AA, Hussain SA, Choudhary A, Al‐Saigh A. Cholecystectomy without drainage: a controlled clinical trial. Annals of Saudi Medicine 1989;9(1):36‐8. [Google Scholar]
Lewis 1990 {published data only}
- Lewis RT, Allan CM, Goodall RG, Marien B, Park M, Lloyd‐Smith W, et al. The conduct of cholecystectomy: incision, drainage, bacteriology and postoperative complications. Canadian Journal of Surgery 1982;25(3):304‐7. [PubMed] [Google Scholar]
- Lewis RT, Goodall RG, Marien B, Park M, Lloyd‐Smith W, Wiegand FM. Simple elective cholecystectomy: to drain or not. American Journal of Surgery 1990;159(2):241‐5. [DOI] [PubMed] [Google Scholar]
Locker 1983 {published data only}
- Locker D, Norwood SH, Torma MJ, Fontenelle LJ. A prospective randomized study of drained and undrained cholecystectomies. The American Surgeon 1983;49(10):528‐30. [PubMed] [Google Scholar]
Loder 1987 {published data only}
- Loder PB, Smith GH, Morris S, Bambach CP, Smith RC. A randomised comparison of three drainage systems following cholecystectomy. The Australian and New Zealand Journal of Surgery 1987;57(8):531‐5. [DOI] [PubMed] [Google Scholar]
McCormack 1983 {published data only}
- McCormack TT, Abel PD, Collins CD. Abdominal drainage following cholecystectomy: high, low, or no suction?. Annals of the Royal College of Surgeons of England 1983;65(5):326‐8. [PMC free article] [PubMed] [Google Scholar]
Monson 1991 {published data only}
- Monson JR, Guillou PJ, Keane FB, Tanner WA, Brennan TG. Cholecystectomy is safer without drainage: the results of a prospective, randomized clinical trial. Surgery 1991;109(6):740‐6. [PubMed] [Google Scholar]
- Monson JR, MacFie J, Irving H, Keane FB, Brennan TG, Tanner WA. Influence of intraperitoneal drains on subhepatic collections following cholecystectomy: a prospective clinical trial. The British Journal of Surgery 1986;73(12):993‐4. [DOI] [PubMed] [Google Scholar]
- Monson JRT, Irving H, Tanner AW, Macfie J, Brennan TG. Does drainage increase the risk of subhepatic collection following cholecystectomy? ‐ a prospective study. Gut 1985;26(10):A1158. [Google Scholar]
Playforth 1985 {published data only}
- Playforth MJ, Sauven P, Evans M, Pollock AV. Suction drainage of the gallbladder bed does not prevent complications after cholecystectomy: a random control clinical trial. The British Journal of Surgery 1985;72(4):269‐71. [DOI] [PubMed] [Google Scholar]
Porati 1984 {published data only}
- Porati M, Sina G, Monguzzi A, Ballabio R, Colombi I. [Cholecystectomy without drainage. Randomized clinical study]. Minerva Chirurgica 1984;39(15‐16):1081‐6. [PubMed] [Google Scholar]
Saad 1993 {published data only}
- Saad AM, Hassan AM. Cholecystectomy with and without drainage: a prospective randomised study. East African Medical Journal 1993;70(8):499‐501. [PubMed] [Google Scholar]
Salam 1984 {published data only}
- Salam IM, McMullin JP, O'Higgins NJ. A comparison of two types of vacuum drainage after cholecystectomy. Annals of the Royal College of Surgeons of England 1984;66(3):190‐1. [PMC free article] [PubMed] [Google Scholar]
Sarr 1987 {published data only}
- Sarr MG, Parikh KJ, Minken SL, Zuidema GD, Cameron JL. Closed‐suction versus Penrose drainage after cholecystectomy. A prospective, randomized evaluation. The American Journal of Surgery 1987;153(4):394‐8. [DOI] [PubMed] [Google Scholar]
Schaupp 1988 {published data only}
- Schaupp W, Menges HW, Schworm HD. The "ideal" cholecystectomy. A prospective, randomized study. Der Chirurg 1988;59(10):661‐4. [PubMed] [Google Scholar]
Trowbridge 1982 {published data only}
- Trowbridge PE. A randomized study of cholecystectomy with and without drainage. Surgery, Gynecology & Obstetrics 1982;155(2):171‐6. [PubMed] [Google Scholar]
van der Linden 1980 {published data only}
- Linden W, Gedda S, Edlund G. Randomized trial of drainage after cholecystectomy. Suction versus static drainage through a main wound versus a stab incision. American Journal of Surgery 1980;141(2):289‐94. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Diez 1990 {published data only}
- Diez JA, Pujato MR, Ferreres AR. The need of drainage after cholecystectomy. HPB Surgery: a World Journal of Hepatic, Pancreatic and Biliary Surgery 1990;3(1):5‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farha 1981 {published data only}
- Farha GJ, Chang FC, Matthews EH. Drainage in elective cholecystectomy. American Journal of Surgery 1981;142(6):678‐80. [DOI] [PubMed] [Google Scholar]
Gupta 1978 {published data only}
- Gupta S, Rauscher G, Stillman R, Fitzerald J, Powers J. The rational use of drains after cholecystectomy. Surgery, Gynecology & Obstetrics 1978;146(2):191‐2. [PubMed] [Google Scholar]
Hanna 1970 {published data only}
- Hanna E. Efficiency of peritoneal drainage. Surgery, Gynecology & Obstetrics 1970;131(5):983‐5. [PubMed] [Google Scholar]
Irwin 1988 {published data only}
- Irwin ST, Moorehead RJ, Parks TG. Effect of drainage on subhepatic collections and respiratory function after elective cholecystectomy. The British Journal of Surgery 1988;75(5):476. [PubMed] [Google Scholar]
Jha 1986 {published data only}
- Jha IN, Singh HB, Prasad N. Clinical trial of drainage following cholecystectomy: suction versus static drainage. Journal of the Indian Medical Association 1986;84(11):341‐2. [PubMed] [Google Scholar]
Kapoor 1993 {published data only}
- Kapoor VK, Ibrarullah M, Baijal SS, Kulshreshtha A, Mittal BR, Saxena R, et al. Cholecystectomy and drainage: ultrasonographic and radioisotopic evaluation. World Journal of Surgery 1993;17(1):101‐4. [DOI] [PubMed] [Google Scholar]
Maull 1978 {published data only}
- Maull KI, Daugherty ME, Shearer GR, Sachatello CR, Ernst CB, Meeker WR, et al. Cholecystectomy: to drain or not to drain. A randomized prospective study of 200 patients. The Journal of Surgical Research 1978;24(4):259‐63. [DOI] [PubMed] [Google Scholar]
Maull 1981 {published data only}
- Maull KI, Shirazi KK, Whitley RE, Halloran LG, Gayle WE, Haynes BW. The effect of prophylactic drainage on sub‐hepatic fluid collections after elective cholecystectomy ‐ a prospective randomized ultrasonographic study. American Surgeon 1981;47(2):85‐8. [PubMed] [Google Scholar]
Peer 1993 {published data only}
- Peer GQ, Wani NA. Role of intraperitoneal drains on subhepatic collection following routine uncomplicated cholecystectomy. Journal of the Indian Medical Association 1993;91(7):175‐6. [PubMed] [Google Scholar]
Ragoonanan 1983 {published data only}
- Ragoonanan C, Crosby DL, Morgan WP, Rees BI. Peritoneal drainage following cholecystectomy: a controlled trial. Annals of the Royal College of Surgeons of England 1983;65(6):403. [PMC free article] [PubMed] [Google Scholar]
Rivas 1980 {published data only}
- Rivas A, Holliday H, Wright J. Cholecystectomy with closed suction drainage. Southern Medical Journal 1980;73(2):161‐2. [DOI] [PubMed] [Google Scholar]
Salam 1994 {published data only}
- Salam IM. Passive tube and suction drainage after elective cholecystectomy: a comparison using ultrasonography. Journal of the Royal College of Surgeons of Edinburgh 1994;39(2):134‐5. [PubMed] [Google Scholar]
Salles 1992 {published data only}
- Salles RA, Santos Jr AP, Freitas RG, Silva ML, Coelho CA. Cholecystectomy with or without drainage. Revista do Colegio Brasileiro de Cirurgioes 1992;19(6):269‐71. [Google Scholar]
Shirazi 1982 {published data only}
- Shirazi KK, Maull KI. Subhepatic sonography following cholecystectomy. Journal of Ultrasound in Medicine 1982;1(7):271‐3. [DOI] [PubMed] [Google Scholar]
Stone 1978 {published data only}
- Stone HH, Hooper CA, Millikan WJ Jr. Abdominal drainage following appendectomy and cholecystectomy. Annals of Surgery 1978;187(6):606‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Truedson 1983 {published data only}
- Truedson H. Cholecystectomy with and without intraperitoneal drain. Acta Chirurgica Scandinavica 1983;149(4):393‐9. [PubMed] [Google Scholar]
van der Linden 1981 {published data only}
- Linden W, Gedda S, Edlund G. Sump drainage versus static drainage after cholecystectomy. Surgery, Gynecology & Obstetrics 1981;152(6):829‐30. [PubMed] [Google Scholar]
Additional references
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fullarton 1994
- Fullarton GM, Bell G. Prospective audit of the introduction of laparoscopic cholecystectomy in the west of Scotland. West of Scotland Laparoscopic Cholecystectomy Audit Group. Gut 1994;35(8):1121‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2006
- Gluud C, Als‐Nielsen B, D'Amico G, Fingerhut A, Gluud LL, Khan S, et al. Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2006, Issue 3. Art. No.: LIVER.
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. The Cochrane Library, Issue 4, 2006. Chichester, UK: John Wiley & sons, Ltd. [Google Scholar]
Johansson 2005
- Johansson M, Thune A, Nelvin L, Stiernstam M, Westman B, Lundell L. Randomized clinical trial of open versus laparoscopic cholecystectomy in the treatment of acute cholecystitis. The British Journal of Surgery 2005;92(1):44‐9. [DOI] [PubMed] [Google Scholar]
Keus 2006a
Keus 2006b
- Keus F, Jong JAF, Gooszen HG, Laarhoven CJHM. Laparoscopic versus small‐incision cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No.: CD006229. DOI: 10.1002/14651858.CD006229. [DOI] [PMC free article] [PubMed]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
NIH 1992
- NIH consensus statement on gallstones and laparoscopic cholecystectomy. National Institutes of Health Consensus Development Conference Statement September 14‐16, 1992. http://consensus.nih.gov/1992/1992GallstonesLaparoscopy090html.htm (accessed 15 November 2006).
Rai 2005
- Rai R, Sinha A, Rai S. Randomized clinical trial of open versus laparoscopic cholecystectomy in the treatment of acute cholecystitis (Br J Surg 2005; 92: 44‐49). The British Journal of Surgery 2005;92(4):494. [DOI] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Ronaghan 1986
- Ronaghan JE, Miller SF, Finley RK Jr, Jones LM, Elliott DW. A statistical analysis of drainage versus nondrainage of elective cholecystectomy. Surgery, Gynecology & Obstetrics 1986;162(3):253‐5. [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
StatsDirect 2.4 [Computer program]
- StatsDirect Ltd. StatsDirect Statistical software Version 2.4.5. StatsDirect Ltd, 2005.
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [DOI] [PubMed] [Google Scholar]


