Abstract
Background: Several perioperative inflammatory markers are postulated to be significant factors for long-term survival after off-pump coronary artery bypass surgery (OPCAB). Hematological parameters, whether single or combined as indices, provide higher predictive values. Methods: The study group comprised 538 consecutive patients (125 (23%) females and 413 (77%) males) with a mean age of 65 ± 9 years, who underwent OPCAB with a mean follow-up time of 4.7 ± 1.7 years. This single-center retrospective analysis included perioperative inflammatory markers such as the neutrophil-to-lymphocyte ratio (NLR), systemic inflammatory response index (SIRI), aggregate index of systemic inflammation (AISI), and systemic inflammatory index (SII). Results: Multivariable analysis identified levels of neutrophils above 4.3 × 109/L (HR 13.44, 95% CI 1.05–3.68, p = 0.037), values of SIRI above 5.4 (HR 0.29, 95% CI 0.09–0.92, p = 0.036) and values of NLR above 3.5 (HR 2.21, 95% CI 1.48–3.32, p < 0.001) as being significant predictors of long-term mortality. The multifactorial models revealed the possibility of strong prediction by combining preoperative factors (COPD, stroke, PAD, and preoperative PLR) and postoperative neutrophil counts (p = 0.0136) or NLR (p = 0.0136) or SIRI (p = 0.0136). Conclusions: Among the postoperative inflammatory indices, the levels of neutrophils, NLR, and SIRI are the most prominent markers for long-term survival after off-pump coronary artery bypass surgery, when combined with preoperative characteristics.
Keywords: OPCAB, NLR, SIRI, AISI, SII
1. Introduction
Coronary atherosclerosis, combined with co-morbidities including obesity, diabetes, and arterial hypertension, as well as gender differences and psychosocial work stress factors, is still a major epidemiological challenge for public health services [1,2,3,4,5]. The origin and progression of atherosclerotic plaques are currently considered to be related to inflammatory process activation [6,7,8].
Complex coronary artery disease can be treated by either coronary percutaneous or surgical revascularization [9,10,11]. The periprocedural inflammatory overreaction is one of the possible factors that indicate a worse long-term prognosis [12,13,14,15,16].
The relationship between surgical intervention and cardiopulmonary bypass has been widely postulated [17,18,19,20]. Despite surgical challenges, the off-pump technique (off-pump coronary artery bypass surgery, OPCAB) can be performed safely by experienced surgeons and may rule out the risk of inflammatory activation that is secondary to CPB application [21]. Despite the elimination of cardiopulmonary application in the OPCAB technique, off-pump surgery still possesses an inflammatory burden that has a detrimental effect on the long-term prognosis [22].
Several inflammatory markers, including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR), have a documented value for predicting worse survival rates following surgical revascularization [12,13].
Numerous novel inflammatory markers have been described that involve the systemic inflammatory response index (SIRI—the quotient of neutrophils and monocytes, divided by lymphocyte count), the aggregate index of systemic inflammation (AISI—the quotient of neutrophils, monocytes, and platelets, divided by lymphocyte count), and the systemic inflammatory index (SII), composed of the quotient of neutrophils and platelets divided by lymphocyte counts [23]. These three indices have been presented as possible mortality prognostic factors in different cardiovascular and non-cardiovascular diseases [24,25,26,27,28]. These novel indices—the aggregate index of systemic inflammation (AISI), systemic inflammatory index (SII), and systemic inflammatory response index (SIRI)—involve the main compounds of previously well-known inflammatory markers, such as neutrophils, monocytes, lymphocytes, and platelets [29,30].
The current study aimed to assess the value of different inflammatory markers, including the more common neutrophil counts and NLR, as well as these novel ones—AISI, SII, and SIRI—for mortality prediction in consecutive patients with chronic coronary syndrome treated with surgical revascularization using the off-pump technique. Furthermore, we also aimed to design a multifactorial model for long-term mortality prediction, to avoid the selectivity of a limitation to a single parameter.
2. Materials and Methods
The study group comprised 538 consecutive patients (125 (23%) females and 413 (77%) males) with a mean age of 65 ± 9 years, who underwent an off-pump coronary artery bypass grafting (OPCAB) procedure between January 2015 and December 2018 in our hospital. The current research presents a single-center study, for which we conducted a retrospective analysis of patients referred for surgical revascularization due to complex chronic coronary syndrome. The patients requiring concomitant valve surgery and those referred for surgery because of acute coronary syndrome were excluded from the study. Additional exclusion criteria included inflammatory, autoimmune, oncological, or hematological proliferative diseases.
The researchers abided by the principles of good clinical practice and the Declaration of Helsinki, and the study was approved by the Local Ethics Committee of the Medical University of Poznan (approval number: 55/20 from 16/01/2020).
The co-morbidities for the study sample included arterial hypertension in 379 patients (71%), diabetes mellitus in 175 patients (33%), hypercholesterolemia in 298 patients (55%), chronic obstructive pulmonary disease (COPD) in 47 patients (9%), and chronic kidney disease in 32 patients (6%)—defined as a glomerular filtration rate (GFR) of ≤60 mL/min/1.63 m2, according to the Cockcroft–Gault equation.
We analyzed the demographic, clinical, and laboratory data; the following indices were calculated to assess the numbers of neutrophils, monocytes, and platelets applied in the NLR, SIRI, SII, and ASIS calculations, utilizing a routine hematology analyzer (Sysmex Europe GmbH, Norderstedt, Germany).
Furthermore, we conducted echocardiography for each patient before the surgery, during hospitalization, and at discharge. Data concerning long-term mortality were collected from the outpatient clinic and the Polish National Health Service database.
Statistics Analysis
We presented the continuous variables as a mean ± standard deviation (SD) or median with an interquartile range; we conducted the analysis using an unpaired Student’s t-test or Mann–Whitney U test since the data did not follow a normal distribution. We presented the categorical variables as frequencies and percentages and analyzed them using a test for proportions. We deployed receiver-operating characteristics (ROC) to determine the cut-off values of the analyzed predictors, to discriminate between individuals enrolled in the study, grouping them into those with and without a mortality endpoint. We also used the log-rank test to check the significance of the survival curves, while using Cox’s proportional hazards model to analyze the long-term mortality predictors. We performed univariate and multivariate analyses (stepwise, backward selection procedure). Furthermore, we transformed the continuous parameters into binary ones (via ROC analysis) to unify the data types. We implemented the hazard ratios (HR) and 95% confidence intervals (95% CI) to interpret and infer from the results.
3. Results
3.1. Clinical Results
During the 4.7 ± 1.7 years of follow-up, fifty-one patients died, irrespective of the cause of death (all-cause mortality of 10%). The survivors and non-survivors did not differ significantly regarding gender (p = 0.589) and age (p = 0.161) (Table 1). In the pre-procedural transthoracic echocardiography (TTE) test, the median left ventricle end-diastolic diameter was not significantly larger (p = 0.056) in the non-survivor group than in survivors, with median values of 50 mm (45–54 mm) and 47 mm (44–52 mm), respectively. There was also a significant difference (p = 0.032) in the preoperative left ventricle ejection fraction (LVEF), with median values of 55% (50–60) vs. 50% (45–60) in survivors and non-survivor groups, respectively.
Table 1.
Patient characteristics (demographical, clinical, and laboratory results).
| Survivors No. = 487 (90%) |
Non-Survivors No. = 51 (10%) |
p | |
|---|---|---|---|
| Demographical data | |||
| Sex (M/F) | 371 (77%)/116 (23%) | 42 (82%)/9 (18%) | 0.589 |
| Age (years) | 64 (60–71) | 67 (62–72) | 0.161 |
| Co-morbidities | |||
| Arterial hypertension (n (%)) | 379 ((71%) | 40 (78%) | 0.109 |
| Diabetes mellitus (n (%)) | 175 (33%) | 16 (31%) | 0.676 |
| Hypercholesterolemia (n (%)) | 298 (55%) | 29 (57%) | 0.173 |
| COPD (n (%)) | 47 (9%) | 12 (24%) | <0.001 * |
| PAD (n (%)) | 80 (15%) | 16 (31%) | <0.001 * |
| Kidney failure (n (%)) | 29 (6%) | 3 (6%) | 0.768 |
| Laboratory tests: | |||
| WBC × 109/L (median (Q1–Q3)) | 7.8 (6.4–9.3) | 7.5 (6.4–8.9) | 0.388 |
| Lymphocytes × 109/L (median (Q1–Q3)) | 1.8 (1.4–2.2) | 1.7 (1.3–2.0) | 0.092 |
| Neutrophils × 109/L (median (Q1–Q3)) | 5 (4–6.3) | 5.1 (4.2–6.1) | 0.886 |
| NLR (median (Q1–Q3)) | 2.8 (2–3.7) | 2.8 (2.1–4.0) | 0.235 |
| Hb × 109/L (median (Q1–Q3)) | 8.7 (8.2–9.2) | 8.6 (7.9–9.3) | 0.658 |
| Platelets × 103/μL (median (Q1–Q3)) | 225 (190–267) | 230 (202–261) | 0.456 |
| Monocytes × 109/L (median (Q1–Q3)) | 0.5 (0.4–0.6) | 0.5 (0.3–0.6) | 0.877 |
| MLR (median (Q1–Q3)) | 0.3 (0.2–0.4) | 0.3 (0.2–0.4) | 0.113 |
| MCHC (mmol/L) (median (Q1–Q3)) | 21.3 (20.8–21.7) | 21 (20.6–21.1) | 0.037 |
| PLR (median (Q1–Q3)) | 125 (98–163) | 140 (114–167) | 0.027 * |
| Troponin I (ng/mL) (median (Q1–Q3)) | 0.01 (0.01–0.02) | 0.02 (0.01–0.03) | 0.13 |
| Creatinine (mg/dL) (median (Q1–Q3)) | 85 (72–102) | 99 (67–132) | 0.044 * |
| SIRI (median (Q1–Q3) | 1.3 (0.8–1.9) | 1.3 (0.9–2.1) | 0.261 |
| SII (median (Q1–Q3)) | 618 (424–903) | 668 (445–982) | 0.174 |
| AISI (median (Q1–Q3)) | 273 (172–440) | 308 (185–489) | 0.199 |
Abbreviations: AISI—aggregate index of systemic inflammation, COPD—chronic obstructive pulmonary disease, Hb—hemoglobin, LV—left ventricle, LVEF—left ventricle ejection fraction, MCHC—mean corpuscular hemoglobin concentration, MLR—monocyte-to-lymphocyte ratio, NLR—neutrophil-to-lymphocyte ratio, PAD—peripheral artery disease, PLR—platelets-to-lymphocyte ratio, SII—systemic inflammatory index, SIRI—systemic inflammatory response index, WBC—white blood cells. * Statistically significant difference. Continuous variables are expressed as the medians with the lower and the upper quartile, whereas categorical variables are expressed as (n) with a percentage (%).
The main indication for surgery was three-vessel disease in 188 (35%) patients, followed by left main stem stenosis in 178 (33%) patients, and two-vessel disease in 172 (32%) patients. The mean surgery (skin-to-skin) time was 2.3 ± 0.5 h, and the mean number of performed anastomoses was 2.25 ± 0.2. None of the surgeries were performed as repeat surgery. There were no intra-operative deaths. The 30-day mortality rate was 1.2% (seven patients). Excessive bleeding episodes requiring re-thoracotomy occurred in 18 patients (3%); the median time of intensive care unit (ICU) stay was 27 h (17–35 h) for the presented group. The demographical and clinical characteristics are presented in Table 1.
Perioperative characteristics, including surgical parameters, laboratory test results, and echocardiographic data, were analyzed and revealed significant differences among groups in terms of laboratory inflammatory indexes—SIRI (p = 0.012), SII (p < 0.001), AISI (p < 0.001). All the significant results are presented in Table 2.
Table 2.
Significant differences in postoperative laboratory characteristics between survivors and non-survivors.
| Survivors No. = 487 |
Non-Survivors No. = 51 |
p | |
|---|---|---|---|
| Neutrophils × 109/L (median (Q1–Q3)) | 4.9 (3.7–6.4) | 5.7 (4.7–7.4) | 0.003 |
| NLR (median (Q1–Q3)) | 2.5 (1.8–3.4) | 3.4 (2.3–5.6) | <0.001 |
| Platelets × 103/ μL (median (Q1–Q3)) | 274 (227–338) | 321 (243–409) | 0.009 |
| PLR (median (Q1–Q3)) | 147 (227–338) | 171 (140–237) | <0.001 |
| SIRI (median (Q1–Q3)) | 4.1 (2.6–6.2) | 5.5 (3.6–7.5) | 0.012 |
| SII (median (Q1–Q3)) | 699 (483–1053) | 1074 (565–1590) | <0.001 |
| AISI (median (Q1–Q3)) | 607 (370–1019) | 989 (599–1604) | <0.001 |
Abbreviations: AISI—aggregate inflammatory response index, NLR—neutrophil to lymphocyte ratio, PLR—platelets to lymphocyte ratio, SII—systemic inflammatory index, SIRI—systemic inflammatory response index. Laboratory parameters were performed at the time of admission for surgery. Continuous variables are expressed as the medians with the lower and the upper quartile.
Neither the mean number of performed grafts (2.25 ± 0.3 vs. 2.2 ± 0.2 (p = 0.798)), nor the postoperative maximum values of serum Troponin-I (1.5 (0.8–3.5) ng/mL vs. 1.8 (0.6–5.4) ng/mL (p = 0.578)), nor hospitalization length (11 ± 4 days vs. 10 ± 3 days (p = 0.821)) differed between the survivor and non-survivor groups.
Postoperative laboratory characteristics revealed significant differences between groups in terms of neutrophils (p = 0.003), platelets (p = 0.009), NLR (p < 0.001), PLR (p < 0.001), SIRI (p = 0.012), SII (p < 0.001), and AISI (p < 0.001).
3.2. Receiver Operator Characteristics (ROC) Analysis
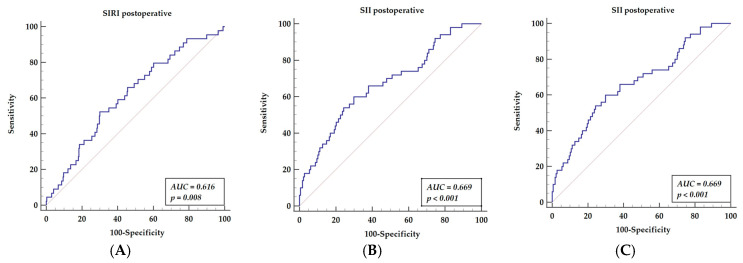
We focused on novel inflammatory markers for long-term mortality prediction after off-pump surgery. ROC analysis revealed significant results for postoperative values of SIRI (AUC = 0.616, p = 0.008), yielding a sensitivity of 52.27% and specificity of 69.92%, with a cut-off value of 5.4; SII (AUC = 0.669, p = 0.001) yielded a sensitivity of 60.00% and specificity of 70.11%, with a cut-off value above 953; AISI (AUC = 0.659, p = 0.0001) yielded a sensitivity of 70% and a specificity of 56.70%, with a cut-off value above 663. The results are presented in Figure 1A–C.
Figure 1.
Receiver operator characteristics curves for postoperative SIRI (A), postoperative SII (B), and postoperative AISI (C). Abbreviations: AISI—aggregate index of systemic inflammation, AUC—area under the curve, SII—systemic inflammatory index, SIRI—systemic inflammatory response index.
3.3. Univariable Analysis
We performed the univariate Cox regression analysis, in which co-morbidities (COPD, stroke, peripheral artery disease (PAD)), preoperative (PLR and serum creatinine), and postoperative parameters, including the inflammatory indexes (SIRI, SII, AISI), followed by the echocardiographic left ventricle ejection fraction were marked as significant risk factors for long-term survival. Co-existing diseases, such as COPD (HR = 2.51, 95% CI 1.29–4.88, p = 0.007), stroke (HR = 4.80, 95% CI 2.53–9.10, p < 0.001), and PAD (HR = 2.96, 95% CI 1.62–5.39, p < 0.001) were statistically significant. Among the preoperative laboratory parameters, PLR (HR = 1.00, 95% CI 1.00–1.01, p = 0.032) and serum creatinine level (HR = 2.59, 95% CI 1.04–6.51, p = 0.042) appeared to be statistically significant. The postoperative parameters are presented in Table 3; among others, a SIRI level above 5.4 (HR = 2.05, 95% CI 1.10–3.83, p = 0.025), an SII level above 953 (HR = 3.26, 95% CI 1.81–5.88, p < 0.001), and an AISI level above 663 (HR = 2.82, 95% CI 1.48–5.39, p = 0.002) were significant for long-term mortality.
Table 3.
Cox regression univariable analysis for long-term survival.
| Parameter | HR | 95% CI | p-Value |
|---|---|---|---|
| Demographical and clinical: | |||
| COPD | 2.51 | 1.29–4.88 | 0.007 |
| Stroke | 4.8 | 2.53–9.10 | <0.001 |
| PAD | 2.96 | 1.62–5.39 | <0.001 |
| Preoperative parameters: | |||
| PLR | 1 | 1.00–1.01 | 0.032 |
| Creatinine | 2.59 | 1.04–6.51 | 0.042 |
| Postoperative parameters: | |||
| Neutrophils | 1.12 | 1.07–1.17 | <0.001 |
| Neutrophils > 4.3 × 109/L | 3.68 | 1.56–8.68 | 0.003 |
| NLR | 1.16 | 1.10–1.22 | <0.001 |
| NLR > 3.5 | 2.74 | 1.54–4.88 | 0.001 |
| Platelets | 1.05 | 1.00–1.01 | 0.002 |
| PLR | 1.01 | 1.00–1.01 | 0.001 |
| SIRI > 5.4 | 2.05 | 1.10–3.83 | 0.025 |
| SII | 1 | 1.00–1.00 | <0.001 |
| SII > 953 | 3.26 | 1.81–5.88 | <0.001 |
| AISI | 1 | 1.00–1.00 | <0.001 |
| AISI > 663 | 2.82 | 1.48–5.39 | 0.002 |
| MLR | 2.2 | 1.08–4.49 | 0.03 |
| Echocardiographic: | |||
| LVEF | 0.928 | 0.90–0.95 | <0.001 |
| LVEF below 45% | 4.41 | 2.43–8.03 | <0.001 |
Abbreviations: AISI—aggregate index of systemic inflammation, COPD—chronic obstructive pulmonary disease, Hb—hemoglobin, LV—left ventricle, LVEF—left ventricle ejection fraction, MCHC—mean corpuscular hemoglobin concentration, MLR—monocyte to lymphocyte ratio, NLR—neutrophil to lymphocyte ratio, PAD—peripheral artery disease, PLR—platelet to lymphocyte ratio, SII—systemic inflammatory index, SIRI—systemic inflammatory response index. Preoperative laboratory parameters were performed at the time of admission for surgery, while postoperative laboratory parameters were performed 24 h after surgery.
3.4. Multivariable Analysis
Parameters that were estimated as significant in the univariable analysis were then verified in a multivariable analysis. Co-morbidities such as COPD (HR = 10.58, 95% CI 2.42–46.36, p = 0.002), stroke (HR = 19.25, 95% CI 5.54–66.94, p < 0.001), and peripheral artery disease (HR = 3.78, 95% CI 1.28–11.15, p = 0.016) were found to represent significant risk factors as presented in Table 4. The preoperative PLR (HR = 0.98, 95% CI 0.96–0.99, p = 0.001) was the only preoperative laboratory parameter influencing long-term survival. The postoperative hemoglobin levels (HR = 3.27, 95% CI 1.09–2.79, p = 0.018), creatinine levels (HR = 1.2, 95% CI 1.01–10.4, p = 0.003), neutrophils at 4.3 × 109/L above the cut-off value (HR = 13.44, 95% CI 1.05–3.68, p = 0.037), SIRI at 5.4 above the cut-off value (HR = 0.29, 95% CI 0.09–0.92, p = 0.036), and NLR at 3.5 above the cut-off value (HR = 2.21, 95% CI 1.48–3.32, p < 0.001) presented significant values for long-term mortality prediction.
Table 4.
Multivariable Cox regression model results.
| Parameter | HR | 95% CI | p-Value |
|---|---|---|---|
| Demographical and clinical: | |||
| COPD | 10.58 | 2.42–46.36 | 0.002 |
| Stroke | 19.25 | 5.54–66.94 | <0.001 |
| PAD | 3.78 | 1.28–11.15 | 0.016 |
| Laboratory parameters: | |||
| preoperative PLR | 0.98 | 0.96–0.99 | 0.001 |
| postoperative Hb | 3.27 | 1.09–2.79 | 0.018 |
| Neutrophils > 4.3 × 109/L | 13.44 | 1.05–3.68 | 0.037 |
| postoperative SIRI > 5.4 | 0.29 | 0.09–0.92 | 0.036 |
| postoperative NLR > 3.5 | 2.21 | 1.48–3.32 | <0.001 |
| postoperative creatinine | 1.02 | 1.01–10.4 | 0.003 |
Abbreviations: COPD—chronic obstructive pulmonary disease, Hb—hemoglobin, NLR—neutrophil to lymphocyte ratio, PAD—peripheral artery disease, PLR—platelet to lymphocyte ratio, SIRI—systemic inflammatory response index. Preoperative laboratory parameters were performed on admission for surgery, postoperative laboratory parameters were performed 24 h after surgery. Statistics were performed using a multivariable proportional hazard Cox regression model.
3.5. Receiver Operator Curve for Postoperative Inflammatory Markers Revealed in the Multivariable Analysis
We compared three postoperative inflammatory indices related to neutrophils, which revealed significant values for long-term mortality prediction in the multivariable analysis.
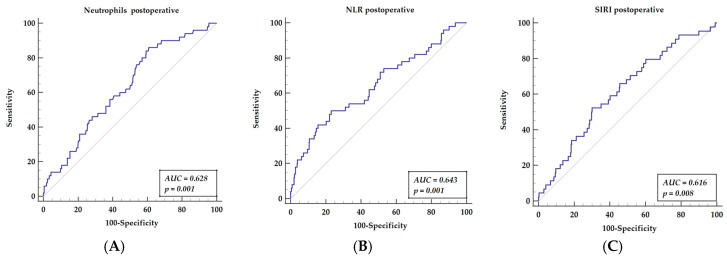
The receiver operator characteristics curve (ROC) was established for neutrophil counts (AUC = 0.628, p = 0.001), yielding a sensitivity of 86% and a specificity of 39.1%, with a cut-off value of 4.3 (Figure 2A); for the neutrophil to lymphocyte ratio (AUC = 0.643, p = 0.001), this yielded a sensitivity of 50% and a specificity of 76.9%, with a cut-off value of above 3.5 (Figure 2B); the systemic index of SIRI (AUC = 0.616, p = 0.008) yielded a sensitivity of 52.27% and a specificity of 69.92%, with a cut-off value of above 5.4 (Figure 2C).
Figure 2.
Receiver operator characteristics curves of three hematologic indices related to neutrophils-neutrophil counts (A), NLR (B), SIRI (C) that are significant for mortality prediction in multivariable analysis. Abbreviations: NLR—neutrophil to lymphocyte ratio, SIRI—systemic inflammatory response index.
3.6. Receiver Operator Curve for Multifactor Models, including Factors Presented in Multivariable Analysis (Preoperative Factors and Postoperative Inflammatory Markers)
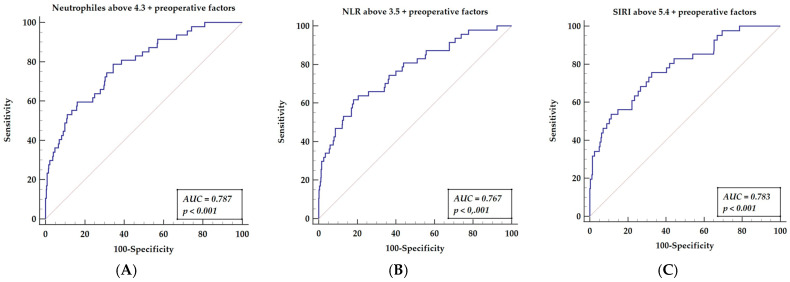
After off-pump surgery, all three inflammatory indices presented a mildly significant predictive value for long-term mortality risk prediction. We performed further analyses, including an analysis of preoperative factors. ROC analysis of long-term mortality prediction, including multifactor score, was performed. We compared the ROC curve as the combination of inflammatory markers (neutrophil counts, NLR, or SIRI) with preoperative factors (stroke, peripheral artery disease, COPD, and PLR). The ROC analysis for neutrophils with a cut-off value of 4.3, combined with preoperative factors, is presented in Figure 3A (AUC = 0.787, p < 0.001), yielding a sensitivity of 78.82% and a specificity of 64.49%. The ROC analysis for NLR with a cut-off value above 3.5. Together with preoperative factors, this is presented in Figure 3B (AUC = 0.767, p < 0.001), yielding a sensitivity of 61.70% and a specificity of 81.86%. The ROC analysis for the systemic index, SIRI, with a cut-off value of above 5.4 and preoperative factors are presented in Figure 3C (AUC = 0.787, p < 0.001), yielding a sensitivity of 75.61% and a specificity of 67.51%.
Figure 3.
Receiver operator characteristics curves of three hematologic indices related to neutrophils (neutrophil counts (A), NLR (B), SIRI (C)), paired with preoperative factors that are significant for mortality prediction in multivariable analysis. Abbreviations: NLR—neutrophil-to-lymphocyte ratio, SIRI—systemic inflammatory response index. Preoperative factors = stroke, peripheral artery disease, COPD, and PLR.
3.7. Multifactorial Models Analysis
The pairwise analysis presented similar results, independent of the inflammatory indices and in addition to the neutrophil counts, as presented in Table 5.
Table 5.
Results of different compositions of multifactorial models affecting long-term survival.
| Variable | AUC | SE | 95% CI | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| 1. Neutrophils > 4.3 + preoperative factors | 0.787 | 0.0355 | 0.748 to 0.822 | 78.72 | 65.49 |
| 2. NLR > 3.5 + preoperative factors | 0.767 | 0.0388 | 0.728 to 0.804 | 61.7 | 81.86 |
| 3. SIRI > 5.4 + preoperative factors | 0.783 | 0.0396 | 0.739 to 0.823 | 75.61 | 67.51 |
Abbreviations: AUC—area under the curve, CI—confidence interval, NLR—neutrophil-to-lymphocyte ratio, SE—standard error, SIRI—systemic inflammatory response index.
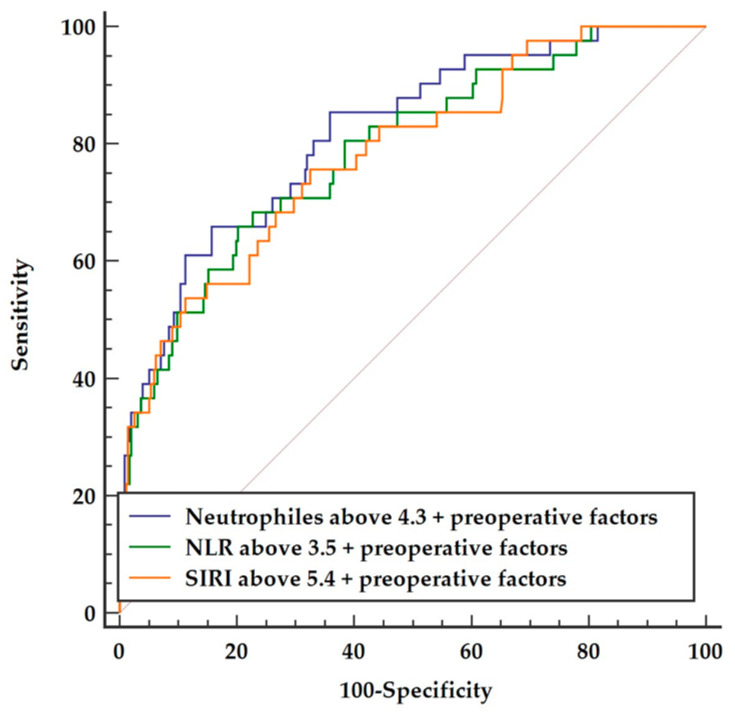
Inclusion of the preoperative factors (COPD, stroke, PAD, and preoperative PLR) to any of the three postoperative inflammatory indices, irrespective of neutrophil counts (AUC = 0.787), NLR (AUC = 0.767), or SIRI (AUC = 0.783), presented a comparable quality of constructed models, as presented in Figure 4.
Figure 4.
Comparison of three multifactor models including preoperative parameters and postoperative neutrophils vs. NLR vs. SIRI, respectively. Abbreviations: NLR—neutrophil-to-lymphocyte ratio, SIRI—systemic inflammatory response index.
4. Discussion
To the best of our knowledge, this is the first study presenting the values of different inflammatory indices obtained from peripheral blood count and their comparisons for the purposes of mortality prediction after off-pump coronary bypass surgery. There is a general opinion on the significant value of combined indices, which comprise neutrophil counts from the whole blood analysis and other components, including lymphocytes, platelets, and monocytes. Moreover, demographic and clinical factors seem to present a similar significance.
Neutrophils are defined as short-lived and unrefined phagocytes whose activation is triggered by either bacterial infection or immune activation [31]. Their missions of releasing vast numbers of proteolytic enzymes and reactive oxygen species are responsible for the recruitment and activation of monocytes, macrophages, and dendritic cell subsets [32]. The neutrophil’s life span is dependent on growth factors and cytokine modulation. Neutrophils undergoing apoptosis ameliorate inflammatory processes; conversely, when they become necrotic, they perpetuate inflammation. The latter processes are claimed to stimulate atherosclerotic lesions [33]. Neutrophil granulocyte markers were detected in human carotid arteries in atherosclerotic specimens, supporting their significance in plaque formation [34]. Consistent with previous reports on the impact of activated neutrophils on atherosclerosis plaque formation, our study revealed the relationship between perioperative inflammatory activation and its possible influence on an increased risk regarding long-term mortality.
We evaluated the more commonly used neutrophil counts and NLR, in addition to multifactorial indices. We used univariate modeling to show the significance of neutrophil counts, NLR, SIRI, SII, and AISI. In the next step, we performed a combination of indices and clinical parameters that were shown to be predictive in multivariate analysis. We found that neutrophil counts and their derivative, NLR, have the highest predictive value for estimating mortality, following pairwise analysis. Therefore, we suggest that the neutrophil count and NLR are the most accurate factors and should be predominantly considered for assessing long-term prognoses. Other indices were significant in our univariate analysis. However, we must point out that all the indices mentioned above comprise neutrophil count or NLR; therefore, this observation further confirms the impact of neutrophils.
The main finding of our study encompasses the dominant significance of neutrophil counts on long-term mortality prediction, either alone or in composition, presented as NLR or SIRI. The combination of preoperative factors with postoperative neutrophil counts (above 4.3 × 109/L) or NLR (above 3.5) showed a significant predictive value, with a sensitivity of 78.82% vs. 78.72% and a specificity of 65.49% vs. 64.49%, respectively. Moreover, the pairwise analysis, preceded by multivariable comparison, indicated the significant role of those postoperative inflammatory factors with preoperative ones, to validate a more robust predictive model.
We focused on the perioperative inflammatory response to surgical intervention as a predictive factor due to the reported significance of inflammatory reactions for long-term survival [35,36]. The predictive scores that apply in clinical practice include the EuroSCORE II or STS score; these are dedicated to assessing perioperative mortality and the risk of complications [37,38,39,40,41].
The research on the inflammatory origin of atherosclerosis enables a better understanding of the pathophysiological background of the disease [42,43]. Several reports proved a strict relationship between the progression of coronary disease, inflammatory processes, and mortality [44,45,46]. The recruitment of a particular lineage of immune cells, such as neutrophils, monocytes, and lymphocytes, occurs during revascularization and has a detrimental effect on long-term prognosis [47,48,49,50]. The simple counts of neutrophils and monocyte-to-lymphocyte ratio were proposed as prognostic markers [51,52,53,54,55]. The perioperative inflammatory reactions, presented as the ratios mentioned above, were associated with long-term surgical revascularization results [56,57,58]. Novel markers, such as AISI, SII, and SIRI, were proposed to augment the propensity values of hematological indices in long-term prognosis [59,60,61].
We compared the utility of novel inflammatory systemic indexes in long-term predictions regarding off-pump surgery. Yamamoto et al. showed dynamic changes in oxidative stress components in early reperfusion after surgery, representing an inflammatory response to the procedure [62]. Interestingly, in our analysis, inflammatory markers related to neutrophils appeared to present the highest significance for long-term mortality prediction. The neutrophil counts, neutrophil to lymphocyte ratio, and the systemic inflammatory response were revealed to be significant in multivariable analysis. The comparison of those three indices in our study validates the claim that neutrophil counts and the neutrophil to lymphocyte ratio are the predictive factors.
The systemic inflammatory index (SII) is an inflammatory index that integrates three types of cells that are involved in immune response, including neutrophils, lymphocytes, and platelets. Previously, we described the particular features of all mentioned cells and the significance of their indices (NLR, PLR) to immune response in patients with coronary artery disease [61]. SII is an established prognostic marker of long-term prognosis in coronary revascularization [12], non-cardiac surgery, or neoplasms [62,63]. Platelets, in turn, are also involved in the inflammatory response by facilitating the recruitment of other inflammatory cells and the release of inflammatory mediators [64]. Therefore, SII should better reflect the inflammatory status compared to the three types of cells when assessed separately. Researchers have already described it as a prognostic marker in appendicitis [65], coronary artery disease [66] and neoplasms [67,68]. Patients with a high level of SII had a significantly higher mortality rate than those with a low SII [69].
Interestingly, patients with different dietary patterns showed differences in SII and NLR values in the study by Szymanska et al. (2021) [70]. Those with a lower ratio of omega-6 to omega-3 fatty acids had lower SII and NLR values, which resulted from an anti-inflammatory diet. Adali and collaborators underlined the significance of coronary artery disease pharmacotherapy [71]. Patients treated with ticagrelor had lower SII, NLR, and PLR values than clopidogrel-treated patients. In our study, an SII above 953 was significant for long-term mortality predictions in the univariate analysis. The most plausible explanation is that all components of SII, neutrophils, lymphocytes, and platelets play roles in atherosclerosis initiation, modulation, and aggravation. Thus, higher cell counts may indicate an ongoing disease and augment the phenomenon. Therefore, higher SII values directly show the risk of disease progression and indicate a potentially worse prognosis. SII use, however, appeared to be limited and was not confirmed in our multivariable analysis.
The AISI—aggregate index of systemic inflammation—is similar to the SII, but in addition to neutrophils, lymphocytes, and platelets, it also includes the monocyte count. Monocytes and neutrophils are part of innate immunity and produce proinflammatory cytokines, chemokines, enzymes, and reactive oxidative species. After activation, monocytes may transform into foam cells, destabilizing the atherosclerotic plaque and promoting dysfunctional and atherogenic lipoproteins [56]. Although AISI represents several inflammatory cells as ingredients and should provide more precise prognostic value, it is rarely used or described in the literature. Most recently, Zinellu et al. (2021) underlined its prognostic significance to predict poor outcomes in idiopathic pulmonary fibrosis [72]. Furthermore, Hamad and coworkers (2021) also studied its importance in patients suffering from COVID-19 [23]. Although it was significant in univariate analysis, like SII, it did not convey a sufficient prognostic value in the multivariate analysis.
The systemic inflammatory response index (SIRI) is the immune system’s reaction against infection and invasive pathogens [73]; the name is given to an inflammatory index describing immunological defenses that encompass neutrophils, monocytes, and lymphocytes [74,75,76]. Surgical intervention may serve as an initiative non-immunological trigger [77]. The main issue of the study was the relationship between inflammation activity secondary to the surgery and long-term mortality. Even though inflammatory activation secondary to the off-pump procedure is limited, these reactions still play a crucial role as long-term mortality predictors [78]. We found that SIRI was a significant predictor [79], especially when we integrated data from preoperative factors.
Neutrophils regulate the repair processes in response to injury, inflammatory reactions, and neoplasms. Neutrophils may also contribute to adaptive changes in developing specific adaptive immune responses and may even trigger tissue damage when inappropriate inflammation reactions are activated [80,81]. Both monocytes and neutrophils are attracted to inflammatory sites via cytokines. Mature monocytes are released into the circulation and are recruited for inflammation control and tissue repair [82]. They represent immune cells involved in emerging human inflammatory diseases such as atherosclerosis [83]; their role in the early formation and maturation of atherosclerotic plaques is crucial [84]. The monocytes characterized by the expression of proinflammatory genes and phenotypes were found in patients with coronary artery disease [85]. Lymphocytes are recruited to the sites of inflammation and contribute to chronic inflammatory processes [86]. The lymphocytes’ memory of past stimuli triggers the production of effector cytokines, proliferation, and performance effector functions [87]. The inflammatory markers, including the earlier cells, appear to accurately distinguish the prognostic factors of hematologic response to immune system activation. The proposed SIRI marker is less widely explored than the more commonly used NLR [88,89,90,91,92].
The results of our study identify patients who are more prone to SIRI component activation secondary to a surgical trigger. This group is characterized by poorer long-term prognoses. We believe that a single stimulus, such as surgery, may reveal an individual propensity for inflammatory component activation that may lead to atherosclerosis progression. Regarding previous studies [93], neutrophils may initiate plaque formation via multiple roles, but they are also involved in their destabilization [94]. The circulating monocytes in patients with complex coronary artery disease have been postulated to present an increased capacity for cytokine production [94]. Monocytes and macrophages may change their characteristics, secondary to pro-atherogenic stimuli [95], and participate in plaque formation [96]. After endothelial damage, the chemoattractant protein-1 (MCP-1) derived from macrocytes will initiate monocyte migration and secondary foam cell formation, as monocytes are believed to represent one of the initial steps of atherogenesis [97]. The progression of plaques is also related to lymphocyte activation, including B2 and T lymphocytes [98]. Atherosclerotic plaque destabilization, related to activation of neutrophils [99,100], monocytes [101] and lymphocytes [102,103] has also been postulated.
We performed a comparison of these three parameters (neutrophil counts, NLR, and SIRI) to estimate their clinical value for long-term prognosis following off-pump coronary artery bypass grafting. Our multivariate analysis revealed the significance of neutrophil counts, NLR, and SIRI for long-term prognosis. The postoperative inflammatory parameters even possessed a stronger predictive effect when combined with preoperative factors. The analysis performed for this study indicates the two significant components influencing outcomes, represented by co-morbidities and perioperative inflammatory activation. Perioperative inflammatory activation is patient-dependent and possesses a predictive value [104,105,106], although this is only after compilation with preoperative factors.
We want to emphasize that among the preoperative factors, apart from the concomitant diseases (co-morbidities), the platelet-to-lymphocyte ratio (PLR) was also marked as being significant in the multivariable analysis. The PLR reflects an increased level of inflammation and thrombosis, as presented in previous reports [107,108,109]. This preoperative factor may indicate that the individual propensity for inflammatory system activation should be considered before operating.
5. Conclusions
Among the postoperative inflammatory indices, and when combined with preoperative characteristics, the neutrophils, NLR, and SIRI represent the most prominent predictors for long-term survival after off-pump coronary artery bypass surgery. The multivariable analysis established that neutrophils above 4.3, a SIRI above 5.4, and an NLR above 3.5 were significant long-term outcome predictors. The predictive values of the ROC analysis achieved significance as multifactor models were constructed by compiling preoperative co-morbidities (stroke, COPD, PAD) and PLR with postoperative inflammatory markers.
Author Contributions
Conceptualization, T.U., A.O.-W. and M.M.; methodology, T.U. and M.M.; software, M.M.; validation, M.R., A.W. and P.B.; formal analysis, M.M.; investigation, M.R., A.W. and B.P.; resources, M.R., A.W. and B.P.; data curation, T.U. and A.O.-W.; writing—original draft preparation, T.U.; writing—review and editing, T.U., A.O.-W., M.M., A.G., B.P. and M.J.; visualization, T.U.; supervision, M.J.; project administration, T.U.; funding acquisition, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Ethics Committee of Poznan University of Medical Sciences approved the study (protocol code 55/20 and date of approval: 16 January 2020), and the researchers conducted the study according to the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data will be available from the correspondence e-mail address for three years following the publication upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Luo D., Cheng Y., Zhang H., Ba M., Chen P., Li H., Chen K., Sha W., Zhang C., Chen H. Association between high blood pressure and long term cardiovascular events in young adults: Systematic review and meta-analysis. BMJ. 2020;370:m3222. doi: 10.1136/bmj.m3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai X., Zhang Y., Li M., Wu J.H., Mai L., Li J., Yang Y., Hu Y., Huang Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ. 2020;370:m2297. doi: 10.1136/bmj.m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becerra-Tomás N., Blanco Mejía S., Viguiliouk E., Khan T., Kendall C.W.C., Kahleova H., Rahelić D., Sievenpiper J.L., Salas-Salvadó J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019;60:1207–1227. doi: 10.1080/10408398.2019.1565281. [DOI] [PubMed] [Google Scholar]
- 4.Taouk Y., Spittal M.J., LaMontagne A.D., Milner A.J. Psychosocial work stressors and risk of all-cause and coronary heart disease mortality: A systematic review and meta-analysis. Scand. J. Work Environ. Health. 2019;46:19–31. doi: 10.5271/sjweh.3854. [DOI] [PubMed] [Google Scholar]
- 5.Urbanowicz T., Michalak M., Olasińska-Wiśniewska A., Haneya A., Straburzyńska-Migaj E., Bociański M., Jemielity M. Gender differences in coronary artery diameters and survival results after off-pump coronary artery bypass (OPCAB) procedures. J. Thorac. Dis. 2021;13:2867–2873. doi: 10.21037/jtd-20-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedro-Botet J., Climent E., Benaiges D. Atherosclerosis and inflammation. New therapeutic approaches. Med. Clin. 2020;155:256–262. doi: 10.1016/j.medcli.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y.-T., Yuan H.-X., Ou Z.-J., Ou J.-S. Microparticles (Exosomes) and Atherosclerosis. Curr. Atheroscler. Rep. 2020;22:23–29. doi: 10.1007/s11883-020-00841-z. [DOI] [PubMed] [Google Scholar]
- 8.Kuk M., Ward N.C., Dwivedi G. Extrinsic and Intrinsic Responses in the Development and Progression of Atherosclerosis. Heart Lung Circ. 2021;30:807–816. doi: 10.1016/j.hlc.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Thuijs D.J.F.M., Kappetein A.P., Serruys P.W., Mohr F.W., Morice M.C., Mack M.J., Holmes D.R., Curzen N., Davierwala P., Noack T., et al. SYNTAX Extended Survival Investigators. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet. 2019;394:1325–1334. doi: 10.1016/S0140-6736(19)31997-X. [DOI] [PubMed] [Google Scholar]
- 10.Jia S., Liu Y., Yuan J. Evidence in Guidelines for Treatment of Coronary Artery Disease. Adv. Exp. Med. Biol. 2020;1177:37–73. doi: 10.1007/978-981-15-2517-9_2. [DOI] [PubMed] [Google Scholar]
- 11.Stone G.W., Kappetein A.P., Sabik J.F., Pocock S.J., Morice M.C., Puskas J., Kandzari D.E., Karmpaliotis D., Brown W.M., Lembo N.J., et al. EXCEL Trial Investigators. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N. Engl. J. Med. 2019;381:1820–1830. doi: 10.1056/NEJMoa1909406. [DOI] [PubMed] [Google Scholar]
- 12.Urbanowicz T., Michalak M., Gąsecka A., Olasińska-Wiśniewska A., Perek B., Rodzki M., Bociański M., Jemielity M. A Risk Score for Predicting Long-Term Mortality Following Off-Pump Coronary Artery Bypass Grafting. J. Clin. Med. 2021;10:3032. doi: 10.3390/jcm10143032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbanowicz T., Michalak M., Gąsecka A., Perek B., Rodzki M., Bociański M., Straburzyńska-Migaj E., Jemielity M. Postoperative Neutrophil to Lymphocyte Ratio as an Overall Mortality Midterm Prognostic Factor following OPCAB Procedures. Clin. Pract. 2021;11:587–597. doi: 10.3390/clinpract11030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucker B., Vaidya K., Cochran B., Patel S. Inflammation during Percutaneous Coronary Intervention—Prognostic Value, Mechanisms and Therapeutic Targets. Cells. 2021;10:1391. doi: 10.3390/cells10061391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole J., Htun N., Lew R., Freilich M., Quinn S., Layland J. Colchicine to Prevent Periprocedural Myocardial Injury in Percutaneous Coronary Intervention: The COPE-PCI Pilot Trial. Circ. Cardiovasc. Interv. 2021;14:9992. doi: 10.1161/CIRCINTERVENTIONS.120.009992. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L., Li Y., Xu T., Luan Y., Lv Q., Wang Y., Lv X., Fu G., Zhang W. Impact of increased inflammation biomarkers on periprocedural myocardial infarction in patients undergoing elective percutaneous coronary intervention: A cohort study. J. Thorac. Dis. 2020;12:5398–5410. doi: 10.21037/jtd-20-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr B.D., Johnson T.J., Gomez-Rexrode A., Mohammed A., Coughlin M., Toomasian J.M., Rojas-Pena A., Bartlett R.H., Haft J.W. Inflammatory Effects of Blood–Air Interface in a Porcine Cardiopulmonary Bypass Model. ASAIO J. 2020;66:72–78. doi: 10.1097/MAT.0000000000000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston K.A., Westover A.J., Rojas-Pena A., Haft J.W., Toomasian J.M., Johnson T., Buffington D.A., Humes H.D. Novel Leukocyte Modulator Device Reduces the Inflammatory Response to Cardiopulmonary Bypass. ASAIO J. 2019;65:401–407. doi: 10.1097/MAT.0000000000000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giacinto O., Satriano U., Nenna A., Spadaccio C., Lusini M., Mastroianni C., Nappi F., Chello M. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Pat. Inflamm. Allergy Drug Discov. 2019;13:158–173. doi: 10.2174/1872213X13666190724112644. [DOI] [PubMed] [Google Scholar]
- 20.Naase H., Harling L., Kidher E., Sepehripour A., Nguyen B., Kapelouzou A., Cokkinos D., Stavridis G., Angelini G., Evans P.C., et al. Toll-like receptor 9 and the inflammatory response to surgical trauma and cardiopulmonary bypass. J. Cardiothorac. Surg. 2020;15:137. doi: 10.1186/s13019-020-01179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirhafez S.R., Khadem S.H., Sahebkar A., Movahedi A., Rahsepar A.A., Mirzaie A., Jamialahmadi T., Ferns G.A., Ghayour-Mobarhan M. Comparative effects of on-pump versus off-pump coronary artery bypass grafting surgery on serum cytokine and chemokine levels. IUBMB Life. 2021;73:1423–1431. doi: 10.1002/iub.2566. [DOI] [PubMed] [Google Scholar]
- 22.Nooh H.A., Abdellateif M.S., Refaat L., Kandeel E.Z., Bayoumi A., Samra M., Khafagy M. The role of inflammatory indices in the outcome of COVID-19 cancer patients. Med. Oncol. 2021;39:6. doi: 10.1007/s12032-021-01605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamad D.A., Aly M.M., Abdelhameid M.A., Ahmed S.A., Shaltout A.S., Abdel-Moniem A.E., Ragheb A.M.R., Attia M.N., Meshref T.S. Combined Blood Indexes of Systemic Inflammation as a Mirror to Admission to Intensive Care Unit in COVID-19 Patients: A Multicentric Study. J. Epidemiol. Glob. Health. 2021;12:64–73. doi: 10.1007/s44197-021-00021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fois A.G., Paliogiannis P., Scano V., Cau S., Babudieri S., Perra R., Ruzzittu G., Zinellu E., Pirina P., Carru C., et al. The Systemic Inflammation Index on Admission Predicts In-Hospital Mortality in COVID-19 Patients. Molecules. 2020;25:5725. doi: 10.3390/molecules25235725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paliogiannis P., Ginesu G.C., Tanda C., Feo C.F., Fancellu A., Fois A.G., Mangoni A.A., Sotgia S., Carru C., Porcu A., et al. Inflammatory cell indexes as preoperative predictors of hospital stay in open elective thoracic surgery. ANZ J. Surg. 2018;88:616–620. doi: 10.1111/ans.14557. [DOI] [PubMed] [Google Scholar]
- 26.Eissa M., Shaarawy S., Abdellateif M.S. The Role of Different Inflammatory Indices in the Diagnosis of COVID-19. Int. J. Gen. Med. 2021;14:7843–7853. doi: 10.2147/IJGM.S337488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng Y., Huang W., Shi Z., Chen Y., Ma J. Positive association between systemic immune-inflammatory index and mortality of cardiogenic shock. Clin. Chim. Acta. 2020;511:97–103. doi: 10.1016/j.cca.2020.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Yatabe S., Eto K., Haruki K., Shiba H., Kosuge M., Ohkuma M., Ito D., Takeda Y., Sugano H., Sasaki S., et al. Signification of Systemic Immune-Inflammation Index for prediction of prognosis after resecting in patients with colorectal cancer. Int. J. Colorectal Dis. 2020;35:1549–1555. doi: 10.1007/s00384-020-03615-w. [DOI] [PubMed] [Google Scholar]
- 29.Luo H., He L., Zhang G., Yu J., Chen Y., Yin H., Goyal H., Zhang G.-M., Xiao Y., Gu C., et al. Normal Reference Intervals of Neutrophil-To-Lymphocyte Ratio, Platelet-To-Lymphocyte Ratio, Lymphocyte-To-Monocyte Ratio, and Systemic Immune Inflammation Index in Healthy Adults: A Large Multi-Center Study from Western China. Clin. Lab. 2019;65:65–74. doi: 10.7754/Clin.Lab.2018.180715. [DOI] [PubMed] [Google Scholar]
- 30.Akboga S.A., Gokce A., Hatipoglu M., Beyoglu M.A., Inan K., Sezen A.I., Dal H.C., Akkas Y., Turan S., Kocer B. The relationship between mortality and inflammatory markers and the systemic immune inflammatory index in patients in the intensive care unit with a pneumothorax as a complication of COVID-19 disease. Ir. J. Med Sci. 2021;2021:1–6. doi: 10.1007/s11845-021-02740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 32.Soehnlein O., Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 33.Döring Y., Drechsler M., Soehnlein O., Weber C. Neutrophils in atherosclerosis: From mice to man. Arterioscler. Thromb. Vasc. Biol. 2015;35:288–295. doi: 10.1161/ATVBAHA.114.303564. [DOI] [PubMed] [Google Scholar]
- 34.Ionita M.G., Borne P.V.D., Catanzariti L.M., Moll F.L., De Vries J.-P.P., Pasterkamp G., Vink A., De Kleijn D.P., De Vries J.-P.P.M. High Neutrophil Numbers in Human Carotid Atherosclerotic Plaques Are Associated With Characteristics of Rupture-Prone Lesions. Arterioscler. Thromb. Vasc. Biol. 2010;30:1842–1848. doi: 10.1161/ATVBAHA.110.209296. [DOI] [PubMed] [Google Scholar]
- 35.Sardu C., Massetti M., Testa N., Di Martino L., Castellano G., Turriziani F., Sasso F.C., Torella M., De Feo M., Santulli G., et al. Effects of Sodium-Glucose Transporter 2 Inhibitors (SGLT2-I) in Patients With Ischemic Heart Disease (IHD) Treated by Coronary Artery Bypass Grafting via MiECC: Inflammatory Burden, and Clinical Outcomes at 5 Years of Follow-Up. Front. Pharmacol. 2021;12:777083. doi: 10.3389/fphar.2021.777083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plicner D., Stoliński J., Wąsowicz M., Gawęda B., Hymczak H., Kapelak B., Drwiła R., Undas A. Preoperative values of inflammatory markers predict clinical outcomes in patients after CABG, regardless of the use of cardiopulmonary bypass. Indian Heart J. 2016;68((Suppl. 3)):S10–S15. doi: 10.1016/j.ihj.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan P.G., Wallach J.D., Ioannidis J.P. Meta-Analysis Comparing Established Risk Prediction Models (EuroSCORE II, STS Score, and ACEF Score) for Perioperative Mortality During Cardiac Surgery. Am. J. Cardiol. 2016;118:1574–1582. doi: 10.1016/j.amjcard.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z., He R., Wu C., Xia Y. Transfemoral versus Transapical Aortic Implantation for Aortic Stenosis Based on No Significant Difference in Logistic EuroSCORE: A Meta-Analysis. Thorac. Cardiovasc. Surg. 2015;64:374–381. doi: 10.1055/s-0035-1555606. [DOI] [PubMed] [Google Scholar]
- 39.Guida P., Mastro F., Scrascia G., Whitlock R., Paparella D. Performance of the European System for Cardiac Operative Risk Evaluation II: A meta-analysis of 22 studies involving 145,592 cardiac surgery procedures. J. Thorac. Cardiovasc. Surg. 2014;148:3049–3057.e1.. doi: 10.1016/j.jtcvs.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 40.Parolari A., Pesce L.L., Trezzi M., Loardi C., Kassem S., Brambillasca C., Miguel B., Tremoli E., Biglioli P., Alamanni F. Performance of EuroSCORE in CABG and off-pump coronary artery bypass grafting: Single institution experience and meta-analysis. Eur. Heart J. 2008;30:297–304. doi: 10.1093/eurheartj/ehn581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urbanowicz T., Staburzyńska-Migaj E., Pawłowska M., Żabicki B., Michalak M., Filipiak M., Grajek S., Jemielity M. EuroSCORE is a Predictor of Postoperative Pericardial Effusion following Heart Transplantation. Ann. Transplant. 2015;20:193–197. doi: 10.12659/AOT.892582. [DOI] [PubMed] [Google Scholar]
- 42.Libby P. Inflammation in Atherosclerosis—No Longer a Theory. Clin. Chem. 2021;67:131–142. doi: 10.1093/clinchem/hvaa275. [DOI] [PubMed] [Google Scholar]
- 43.Chan Y.-H., Ramji D.P. A perspective on targeting inflammation and cytokine actions in atherosclerosis. Future Med. Chem. 2020;12:613–626. doi: 10.4155/fmc-2019-0301. [DOI] [PubMed] [Google Scholar]
- 44.Mauricio D., Castelblanco E., Alonso N. Cholesterol and Inflammation in Atherosclerosis: An Immune-Metabolic Hypothesis. Nutrients. 2020;12:2444. doi: 10.3390/nu12082444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kocyigit D., Gurses K.M., Tokgozoglu L. Anti-inflammatory therapy in atherosclerosis. Front. Biosci. 2020;25:242–269. doi: 10.2741/4805. [DOI] [PubMed] [Google Scholar]
- 46.Ministrini S., Carbone F., Montecucco F. Updating concepts on atherosclerotic inflammation: From pathophysiology to treatment. Eur. J. Clin. Investig. 2020;51:e13467. doi: 10.1111/eci.13467. [DOI] [PubMed] [Google Scholar]
- 47.Shah B., Pillinger M., Zhong H., Cronstein B., Xia Y., Lorin J.D., Smilowitz N.R., Feit F., Ratnapala N., Keller N.M., et al. Effects of Acute Colchicine Administration Prior to Percutaneous Coronary Intervention: COLCHICINE-PCI Randomized Trial. Circ. Cardiovasc. Interv. 2020;13:e008717. doi: 10.1161/CIRCINTERVENTIONS.119.008717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King A.H., Kwan S., Schmaier A.H., Kumins N.H., Harth K.C., Colvard B.D., Wong V.L., Kashyap V.S., Cho J.S. Elevated neutrophil to lymphocyte ratio is associated with decreased amputation-free survival after femoropopliteal percutaneous revascularization. Int. Angiol. 2021;40:442–449. doi: 10.23736/S0392-9590.21.04699-X. [DOI] [PubMed] [Google Scholar]
- 49.Ceyhun G., Engin M. Çağatay The Monocyte/High Density Lipoprotein Cholesterol Ratio (MHR) as an Indicator of the Need for Amputation in Patients With Peripheral Artery Disease Developing Critical Limb Ischemia. Angiology. 2021;72:268–273. doi: 10.1177/0003319720965808. [DOI] [PubMed] [Google Scholar]
- 50.Guclu K., Celik M. Prognostic Value of Inflammation Parameters in Patients With Non-ST Elevation Acute Coronary Syndromes. Angiology. 2020;71:825–830. doi: 10.1177/0003319720936500. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z., Wang J., Cao D., Han L. Correlation of neutrophil-to-lymphocyte ratio with the prognosis of non-ST-segment elevation in patients with acute coronary syndrome undergoing selective percutaneous coronary intervention. J. Int. Med. Res. 2020;48:300060520959510. doi: 10.1177/0300060520959510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butt K., D’Souza J., Yuan C., Jayakumaran J., Nguyen M., Butt H.I., Abusaada K. Correlation of the Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) with Contrast-Induced Nephropathy in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Interventions. Cureus. 2020;12:11879. doi: 10.7759/cureus.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zengin A., Karaca M., Aruğaslan E., Yıldırım E., Karataş M.B., Çanga Y., Emre A., Tayyareci G. Performance of neutrophil to lymphocyte ratio for the prediction of long-term morbidity and mortality in coronary slow flow phenomenon patients presented with non-ST segment elevation acute coronary syndrome. J. Cardiovasc. Thorac. Res. 2021;13:125–130. doi: 10.34172/jcvtr.2021.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seaoud E., Mohamed A.A.H.A., Elkot M.A. The Role of the Platelet/Lymphocyte Ratio and Neutrophil/Lymphocyte Ratio in Predicting High-Risk Heart Score in Patients Admitted with Non-ST Elevation Acute Coronary Syndrome. Pulse. 2020;8:66–74. doi: 10.1159/000508592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang T.Y., Zhao Q., Liu Z.S., Zhang C.Y., Yang J., Meng K. Relationship between monocyte/lymphocyte ratio and non-culprit plaque vulnerability in patients with acute coronary syndrome: An optical coherence tomography study. Medicine. 2020;99:e21562–e21668. doi: 10.1097/MD.0000000000021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perros A.J., Esguerra-Lallen A., Rooks K., Chong F., Engkilde-Pedersen S., Faddy H.M., Hewlett E., Naidoo R., Tung J.P., Fraser J.F., et al. Coronary artery bypass grafting is associated with immunoparalysis of monocytes and dendritic cells. J. Cell. Mol. Med. 2020;24:4791–4803. doi: 10.1111/jcmm.15154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dagher O., Mury P., Noly P.-E., Fortier A., Lettre G., Thorin E., Carrier M. Design of a Randomized Placebo-Controlled Trial to Evaluate the Anti-inflammatory and Senolytic Effects of Quercetin in Patients Undergoing Coronary Artery Bypass Graft Surgery. Front. Cardiovasc. Med. 2021;8:741542. doi: 10.3389/fcvm.2021.741542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gürbüz O., Kumtepe G., Ozkan H., Karal I.H., Velioğlu Y., Ercan A., Yüksel A., Ener S. Predictive Value of Neutrophil-Lymphocyte Ratio for Long-Term Cardiovascular Event Following Coronary Artery Bypass Grafting. Braz. J. Cardiovasc. Surg. 2020;35:274–284. doi: 10.21470/1678-9741-2018-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji Y., Wang H. Prognostic prediction of systemic immune-inflammation index for patients with gynecological and breast cancers: A meta-analysis. World J. Surg. Oncol. 2020;18:197–208. doi: 10.1186/s12957-020-01974-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Y.-L., Wu C.-H., Hsu P.-F., Chen S.-C., Huang S.-S., Chan W.L., Lin S.-J., Chou C.-Y., Chen J.-W., Pan J.-P., et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur. J. Clin. Investig. 2020;50:e13230. doi: 10.1111/eci.13230. [DOI] [PubMed] [Google Scholar]
- 61.Wang L., Zhou Y., Xia S., Lu L., Dai T., Li A., Chen Y., Gao E. Prognostic value of the systemic inflammation response index (SIRI) before and after surgery in operable breast cancer patients. Cancer Biomark. 2020;28:537–547. doi: 10.3233/CBM-201682. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto M., Nishimori H., Fukutomi T., Yamaguchi T., Orihashi K. Dynamics of Oxidative Stress Evoked by Myocardial Ischemia Reperfusion After Off-Pump Coronary Artery Bypass Grafting Elucidated by Bilirubin Oxidation. Circ. J. 2017;81:1678–1685. doi: 10.1253/circj.CJ-16-1116. [DOI] [PubMed] [Google Scholar]
- 63.Urbanowicz T., Olasińska-Wiśniewska A., Michalak M., Rodzki M., Witkowska A., Straburzyńska-Migaj E., Perek B., Jemielity M. The Prognostic Significance of Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR) and Platelet to Lymphocyte Ratio (PLR) on Long-Term Survival in Off-Pump Coronary Artery Bypass Grafting (OPCAB) Procedures. Biology. 2021;11:34. doi: 10.3390/biology11010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diem S., Schmid S., Krapf M., Flatz L., Born D., Jochum W., Templeton A.J., Früh M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 65.Hajibandeh S., Hajibandeh S., Hobbs N., Mansour M. Neutrophil-to-lymphocyte ratio predicts acute appendicitis and distinguishes between complicated and uncomplicated appendicitis: A systematic review and meta-analysis. Am. J. Surg. 2020;219:154–163. doi: 10.1016/j.amjsurg.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 66.Aydın C., Engin M. The Value of Inflammation Indexes in Predicting Patency of Saphenous Vein Grafts in Patients With Coronary Artery Bypass Graft Surgery. Cureus. 2021;13:e16646–e16655. doi: 10.7759/cureus.16646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li W., Ma G., Deng Y., Chen W., Liu Z., Chen F., Wu Q. Systemic Immune-Inflammation Index Is a Prognostic Factor for Breast Cancer Patients After Curative Resection. Front. Oncol. 2021;11:570208–570218. doi: 10.3389/fonc.2021.570208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chien T.-M., Li C.-C., Lu Y.-M., Chou Y.-H., Chang H.-W., Wu W.-J. The Predictive Value of Systemic Immune-Inflammation Index on Bladder Recurrence on Upper Tract Urothelial Carcinoma Outcomes after Radical Nephroureterectomy. J. Clin. Med. 2021;10:5273. doi: 10.3390/jcm10225273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang Y., Zeng X., Feng Y., Chen Q., Liu Z., Luo H., Zha L., Yu Z. Association of Systemic Immune-Inflammation Index With Short-Term Mortality of Congestive Heart Failure: A Retrospective Cohort Study. Front. Cardiovasc. Med. 2021;8:753133–8753148. doi: 10.3389/fcvm.2021.753133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szymanska P., Rozalski M., Wilczynski M., Golanski J. Systemic immune-inflammation index (SII) and neutrophil to lymphocyte ratio (NLR) are useful markers for assessing effects of anti-inflammatory diet in patients before coronary artery bypass grafting. Rocz. Panstw. Zakl. Hig. 2021;72:327–335. doi: 10.32394/rpzh.2021.0170. [DOI] [PubMed] [Google Scholar]
- 71.Adali M.K., Buber I., Kilic O., Turkoz A., Yilmaz S. Ticagrelor improves systemic immune-inflammation index in acute coronary syndrome patients. Acta Cardiol. 2021;2021:15385. doi: 10.1080/00015385.2021.1973770. [DOI] [PubMed] [Google Scholar]
- 72.Zinellu A., Collu C., Nasser M., Paliogiannis P., Mellino S., Zinellu E., Traclet J., Ahmad K., Mangoni A.A., Carru C., et al. The Aggregate Index of Systemic Inflammation (AISI): A Novel Prognostic Biomarker in Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2021;10:4134. doi: 10.3390/jcm10184134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balk R.A. Systemic inflammatory response syndrome (SIRS): Where did it come from and is it still relevant today? Virulence. 2014;5:20–26. doi: 10.4161/viru.27135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaukonen K.-M., Bailey M., Pilcher D., Cooper D.J., Bellomo R. Systemic Inflammatory Response Syndrome Criteria in Defining Severe Sepsis. N. Engl. J. Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 75.Koirala U., Thapa P.B., Joshi M.R., Singh D.R., Sharma S.K. Systemic Inflammatory Response Syndrome following Gastrointestinal Surgery. J. Nepal Med. Assoc. 2017;56:221–225. doi: 10.31729/jnma.3144. [DOI] [PubMed] [Google Scholar]
- 76.Hagen M., Sembill J.A., Sprügel M.I., Gerner S.T., Madžar D., Lücking H., Hölter P., Schwab S., Huttner H.B., Kuramatsu J.B. Systemic inflammatory response syndrome and long-term outcome after intracerebral hemorrhage. Neurol. Neuroimmunol. Neuroinflamm. 2019;6:e588. doi: 10.1212/NXI.0000000000000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaikittisilpa N., Krishnamoorthy V., Lele A.V., Qiu Q., Vavilala M.S. Characterizing the relationship between systemic inflammatory response syndrome and early cardiac dysfunction in traumatic brain injury. J. Neurosci. Res. 2018;96:661–670. doi: 10.1002/jnr.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin Z., Hao D., Song Y., Zhuang L., Wang Q., Yu X. Systemic inflammatory response index as an independent risk factor for ischemic stroke in patients with rheumatoid arthritis: A retrospective study based on propensity score matching. Clin. Rheumatol. 2021;40:3919–3927. doi: 10.1007/s10067-021-05762-z. [DOI] [PubMed] [Google Scholar]
- 79.Kobayashi H., Okuma T., Okajima K., Ishibashi Y., Zhang L., Hirai T., Ohki T., Ikegami M., Sawada R., Shinoda Y., et al. Systemic inflammation response index (SIRI) as a predictive factor for overall survival in advanced soft tissue sarcoma treated with eribulin. J. Orthop. Sci. 2020;20:30346–30358. doi: 10.1016/j.jos.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 80.Zhang P., Li Y., Zhang H., Wang X., Dong L., Yan Z., She L., Wang X., Wei M., Tang C. Prognostic value of the systemic inflammation response index in patients with aneurismal subarachnoid hemorrhage and a Nomogram model construction. Br. J. Neurosurg. 2020;2020:1–7. doi: 10.1080/02688697.2020.1831438. [DOI] [PubMed] [Google Scholar]
- 81.Margraf A., Ludwig N., Zarbock A., Rossaint J. Systemic Inflammatory Response Syndrome After Surgery: Mechanisms and Protection. Anesth. Analg. 2020;131:1693–1707. doi: 10.1213/ANE.0000000000005175. [DOI] [PubMed] [Google Scholar]
- 82.Lehmann S., Dieterlen M.T., Flister A., Klaeske K., Jawad K., Garbade J., Borger M.A., Kostelka M. Differences of early immunological responses in on-pump versus off-pump cardiac surgery. Perfusion. 2019;34:399–407. doi: 10.1177/0267659118823137. [DOI] [PubMed] [Google Scholar]
- 83.Liew P.X., Kubes P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019;99:1223–1248. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 84.Castanheira F.V.S., Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133:2178–2185. doi: 10.1182/blood-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- 85.Chan M.W.Y., Viswanathan S. Recent progress on developing exogenous monocyte/macrophage-based therapies for inflammatory and degenerative diseases. Cytotherapy. 2019;21:393–415. doi: 10.1016/j.jcyt.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 86.Kapellos T.S., Bonaguro L., Gemünd I., Reusch N., Saglam A., Hinkley E.R., Schultze J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019;10:2035. doi: 10.3389/fimmu.2019.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weber C., Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 88.Bekkering S., Quintin J., Joosten L.A., van der Meer J.W., Netea M.G., Riksen N.P. Oxidized Low-Density Lipoprotein Induces Long-Term Proinflammatory Cytokine Production and Foam Cell Formation via Epigenetic Reprogramming of Monocytes. Arterioscler. Thromb. Vasc. Biol. 2014;34:1731–1738. doi: 10.1161/ATVBAHA.114.303887. [DOI] [PubMed] [Google Scholar]
- 89.Noack M., Miossec P. Importance of lymphocyte-stromal cell interactions in autoimmune and inflammatory rheumatic diseases. Nat. Rev. Rheumatol. 2021;17:550–564. doi: 10.1038/s41584-021-00665-4. [DOI] [PubMed] [Google Scholar]
- 90.Cronkite D.A., Strutt T.M. The Regulation of Inflammation by Innate and Adaptive Lymphocytes. J. Immunol. Res. 2018;2018:1467538. doi: 10.1155/2018/1467538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murad L.D., Silva T.Q., Schilithz A.O.C., Monteiro M.C., Murad L.B., Fialho E. Body Mass Index Alters the Predictive Value of the Neutrophil-to-Lymphocyte Ratio and Systemic Inflammation Response Index in Laryngeal Squamous Cell Carcinoma Patients. Nutr. Cancer. 2021;7:1–9. doi: 10.1080/01635581.2021.1952447. [DOI] [PubMed] [Google Scholar]
- 92.Liu X., Ge H., Feng X., Hang J., Zhang F., Jin X., Bao H., Zhou M., Han F., Li S., et al. The Combination of Hemogram Indexes to Predict Exacerbation in Stable Chronic Obstructive Pulmonary Disease. Front. Med. 2020;7:572435. doi: 10.3389/fmed.2020.572435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soehnlein O. Multiple Roles for Neutrophils in Atherosclerosis. Circ. Res. 2012;110:875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 94.van der Valk F.M., Bekkering S., Kroon J., Yeang C., Van den Bossche J., van Buul J.D., Ravandi A., Nederveen A.J., Verberne H.J., Scipione C., et al. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation. 2016;134:611–624. doi: 10.1161/CIRCULATIONAHA.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Netea M.G., Quintin J., Van Der Meer J.W. Trained Immunity: A Memory for Innate Host Defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 96.Munjal A., Khandia R. Atherosclerosis: Orchestrating cells and biomolecules involved in its activation and inhibition. Adv. Protein Chem. Struct. Biol. 2020;120:85–122. doi: 10.1016/bs.apcsb.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 97.Mehu M., Narasimhulu C.A., Singla D.K. Inflammatory Cells in Atherosclerosis. Antioxidants. 2022;11:233. doi: 10.3390/antiox11020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ammirati E., Moroni F., Magnoni M., Camici P.G. The role of T and B cells in human atherosclerosis and atherothrombosis. Clin. Exp. Immunol. 2015;179:173–187. doi: 10.1111/cei.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Silvestre-Roig C., Braster Q., Ortega-Gomez A., Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020;17:327–340. doi: 10.1038/s41569-019-0326-7. [DOI] [PubMed] [Google Scholar]
- 100.Mawhin M.-A., Tilly P., Zirka G., Charles A.-L., Slimani F., Vonesch J.-L., Michel J.-B., Bäck M., Norel X., Fabre J.-E. Neutrophils recruited by leukotriene B4 induce features of plaque destabilization during endotoxaemia. Cardiovasc. Res. 2018;114:1656–1666. doi: 10.1093/cvr/cvy130. [DOI] [PubMed] [Google Scholar]
- 101.Fracassi F., Niccoli G., Cosentino N., Eligini S., Fiorelli S., Fabbiocchi F., Vetrugno V., Refaat H., Montone R.A., Marenzi G., et al. Human monocyte-derived macrophages: Pathogenetic role in plaque rupture associated to systemic inflammation. Int. J. Cardiol. 2021;325:1–8. doi: 10.1016/j.ijcard.2020.09.071. [DOI] [PubMed] [Google Scholar]
- 102.Profumo E., Buttari B., Tosti M.E., Tagliani A., Capoano R., D’Amati G., Businaro R., Salvati B., Riganò R. Plaque-infiltrating T lymphocytes in patients with carotid atherosclerosis: An insight into the cellular mechanisms associated to plaque destabilization. J. Cardiovasc. Surg. 2012;54:349–357. [PubMed] [Google Scholar]
- 103.Komissarov A., Potashnikova D., Freeman M.L., Gontarenko V., Maytesyan D., Lederman M.M., Vasilieva E., Margolis L. Driving T cells to human atherosclerotic plaques: CCL3/CCR5 and CX3CL1/CX3CR1 migration axes. Eur. J. Immunol. 2021;51:1857–1859. doi: 10.1002/eji.202049004. [DOI] [PubMed] [Google Scholar]
- 104.Roig C.S., Daemen M., Lutgens E., Soehnlein O., Hartwig H. Neutrophils in atherosclerosis. Hämostaseologie. 2015;35:121–127. doi: 10.5482/HAMO-14-09-0040. [DOI] [PubMed] [Google Scholar]
- 105.Zeng X., Liu G., Pan Y., Li Y. Development and validation of immune inflammation–based index for predicting the clinical outcome in patients with nasopharyngeal carcinoma. J. Cell. Mol. Med. 2020;24:8326–8349. doi: 10.1111/jcmm.15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takenaka Y., Oya R., Kitamiura T., Ashida N., Shimizu K., Takemura K., Yamamoto Y., Uno A. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: A meta-analysis. Head Neck. 2018;40:647–655. doi: 10.1002/hed.24986. [DOI] [PubMed] [Google Scholar]
- 107.Zhang S., Diao J., Qi C., Jin J., Li L., Gao X., Gong L., Wu W. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: A meta-analysis. BMC Cardiovasc. Disord. 2018;18:75–82. doi: 10.1186/s12872-018-0812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lowry S.F. The Evolution of an Inflammatory Response. Surg. Infect. 2009;10:419–425. doi: 10.1089/sur.2009.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jakab L. A szervezeti önvédelem módja: A gyulladás The way of self-defence of the organism: Inflammation. Orv. Hetil. 2013;154:1247–1255. doi: 10.1556/OH.2013.29670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be available from the correspondence e-mail address for three years following the publication upon reasonable request.