Abstract
Climate change has devastating effects on plant growth and yield. During ontogenesis, plants are subjected to a variety of abiotic stresses, including drought and salinity, affecting the crop loss (20–50%) and making them vulnerable in terms of survival. These stresses lead to the excessive production of reactive oxygen species (ROS) that damage nucleic acid, proteins, and lipids. Plant growth-promoting bacteria (PGPB) have remarkable capabilities in combating drought and salinity stress and improving plant growth, which enhances the crop productivity and contributes to food security. PGPB inoculation under abiotic stresses promotes plant growth through several modes of actions, such as the production of phytohormones, 1-aminocyclopropane-1-carboxylic acid deaminase, exopolysaccharide, siderophore, hydrogen cyanide, extracellular polymeric substances, volatile organic compounds, modulate antioxidants defense machinery, and abscisic acid, thereby preventing oxidative stress. These bacteria also provide osmotic balance; maintain ion homeostasis; and induce drought and salt-responsive genes, metabolic reprogramming, provide transcriptional changes in ion transporter genes, etc. Therefore, in this review, we summarize the effects of PGPB on drought and salinity stress to mitigate its detrimental effects. Furthermore, we also discuss the mechanistic insights of PGPB towards drought and salinity stress tolerance for sustainable agriculture.
Keywords: antioxidant defense, biostimulants, osmotic stress, plant–microbe interaction, reactive oxygen species, water deficit
1. Introduction
Plants have been increasingly vulnerable to environmental stresses due to climate change during ontogenesis [1], and the rising global mean temperature poses a serious threat to agriculture and socioeconomic development [2]. Climate change globally causes a variety of environmental stresses, which are damaging to agricultural crop production. Environment perturbations that cause metabolic disruption, development, and yield disruption are considered a stress situation during their ontogenesis and cause stress reactions. Stress situations in biological systems are characterized by significant perturbations to the metabolism, development, and yield during their ontogenesis. The natural environment is filled with biotic and abiotic stresses that negatively impact plant growth and productivity [3,4,5]. Among abiotic stresses, salinity and drought account for 50% of productivity losses [6]. The Food and Agricultural Organization (FAO) reported that the annual agricultural productivity losses range between 20 and 40%, totaling US $31 million due to salt stress [7]. At the global level, abiotic stresses such as drought and salinity adversely affect crop output, even though they manifest differently depending on the region [8]. As abiotic stresses are interrelated and often occur together in a natural environment, they have especially severe effects on plants, impacting their cellular, metabolic, and physiological activities and reducing crop yields [1,9]. Abiotic stress may affect the Calvin cycle, photosystems (PS) or photosynthetic enzymatic activity, stomatal function, and even alter electron transport chain (ETC) reactions [10].
The growth, development, and fertility of plants are rigorously influenced by drought stress. During salt and drought conditions, plants respond similarly in physiological and molecular ways, including osmotic imbalance, cell dehydration, and the production of ROS [11,12]. Drought causes water loss and reduces the water potential, which, in turn, reduces cell turgor [13]. Abscisic acid (ABA)-mediated stomata closure is particularly the fastest process induced by drought [14]. Extended drought stress leads to osmotic regulation [15,16], metabolic reprogramming [17], which leads to an accumulation of primary and secondary metabolites [18,19], a decreased root–shoot ratio [20], activation of the antioxidant system [8,21], and cell wall modifications [22,23]. The modifications of these traits can be measured and used to gauge the rigorousness of drought. The soil is classified as saline when its electrical conductivity (EC) is greater than four dS m−1 (approximately 40-mM NaCl) and has an exchangeable sodium content of 15% [24,25]. Soil salinity negatively influences agricultural productivity and soil fertility as well [9]. Additionally, drought stress and salinity affect the photosynthesis, plant transpiration, and functioning of roots [26]. Moreover, because of diverse factors, such as poor irrigation practices, climate alteration, and additional natural processes, croplands are degraded by drought and salinity by about 10% on an annual basis [9,14,20]. Drought stress induces stomatal closure and loses membrane integrity, while salinity stress can cause sodium (Na+) and chloride (Cl−) ion accumulation, thereby reducing plant growth and crop yields [11,12].
Around 1 to 2% of the oxygen (O2) utilized by plants is changed into reactive oxygen species (ROS), particularly singlet oxygen (1O2), hydroxyl radical (OH•), superoxide radical (O2•−), hydrogen peroxide (H2O2), alkoxyl radicals (RO•), peroxy radical (ROO•), and reactive nitrogen species (RNS), as a byproduct of aerobic metabolism in numerous cell organelles, like mitochondria, chloroplasts, nucleolus, and apoplasts [10,15]. Nevertheless, peroxisomes are also recognized to be powerful ROS generators, because photochemical reactions and ETC contribute the most of ROS production [27,28,29,30]. In plant cells, ROS tend to remain at a low level due to the antioxidant systems that regulate them. The rate of ROS production increases exponentially under conditions of drought and salinity stress, exceeding the capacity of antioxidant scavengers and causing an oxidative burst that interrupts the cellular redox homeostasis, affects biomolecules, and modulates the cellular mechanism, resulting in a negative balance between the production and scavenging of these reactive species [31,32,33]. Conversely, controlled ROS production contributes to redox signaling, plant–microbe interactions [28,29], plant growth, and its development under abiotic stress [27,34,35,36]. Occasionally, ROS can damage molecular and cellular components (like nucleic acids, including DNA and RNA and proteins) by oxidizing biomolecules (such as carbohydrates, proteins, lipids, enzymes, and DNA), which can lead to severe plant death [27]. Despite this, the exact mechanisms of ROS-mediated stress alleviation remain unclear. To avoid damage and cell death, the elevated production of ROS and RNS should be suppressed by antioxidant machinery.
Plants employ enzymatic and nonenzymatic antioxidant systems to cope with the damage caused by ROS production. The physiology and metabolism of plants may change either reversibly or irreversibly as a result of abiotic stresses [37]. Several enzymatic antioxidant systems, including catalase (CAT), superoxide dismutase (SOD), peroxidase (POX), ascorbate peroxidase (APX), lipid peroxidase (LPX), glutathione peroxidase (GPX), glutathione reductase (GR), etc., and nonenzymatic antioxidants like vitamins, tocopherols, stilbenes, phenols, ascorbate, glutathione, flavonoids, and carotenoids quench the excess ROS, thereby protecting cells from oxidative stress [20,22,38,39,40,41,42,43]. In fact, all plants possess these mechanisms, which can be referred to as “innate tolerance”. Aside from these forms of responses, certain plants, in comparison to others, have evolved the capacity to thrive under stressful situations. This response is commonly known as the “memory of stress” in plants and can be viewed as an “acquired tolerance”. In recent decades, flavonoids have been extensively debated as potent antioxidants both in plants and animals [40,44,45,46,47,48]. Despite the fact that flavonoids have the ability to mitigate the negative consequences associated with the enormous formation of ROS, authoritative criticism has been raised in a number of circumstances. ROS intermediates affect the PS and induce programmed cell death (PCD) [49] and are accountable for osmotic stress [50,51,52]. Plants have the ability to adapt salt and drought stress in different ways [13,53,54,55,56,57]. In addition, salinity and drought concentrations can decrease the efflux of macro and micronutrients (such as P, N, Mg, Fe, Cu, and Zn), which reduces their solubility and competition with Na+ and Cl− [58,59]. Plant development is thus disturbed in various ways, such as germination, vegetative growth, and reproduction [41,60]. Due to this rising issue, it is appropriate to search for potential methodologies for increasing crop yields under drought and salinity. In contrast, several research studies highlighted the differences between salinity and drought, where both stresses can affect physiology and metabolism differently while, in many pathways, they act similar [61]. Numerous beneficial plant growth-promoting bacteria (PGPB) have been discovered by various researchers that have a positive impact on promoting plant growth by various mechanisms such as phytohormones production, ESP production, regulating the nutrient exchange and internal ionic content, and facilitating the biosynthesis of osmoprotectant compounds (e.g., total soluble sugar (TSS), proline, betaine, or trehalose, etc.) that reduce osmotic stress [18,40,41,62,63,64]. Despite the studies conducted to date on these mechanisms, there are still additional useful impacts of soil microbiota that have not been identified, underscoring the need for further research to optimize PGPB use in agricultural systems. Furthermore, it is imperative to remember that each of these mechanisms is interconnected, but even the same microorganism can have a diverse effect on the same plant. Thus, PGPB might be a useful strategy for boosting plant development and production in drought and salinity-stressed environments. The current review examines the existing literature about the impact of drought and salinity stresses on plant fitness, along with exploring antioxidant-based defense mechanisms that regulate ROS accumulation and reduce oxidative stress. Further, the molecular mechanisms of ROS generation in plants and cost-effective and eco-sustainable PGPB approaches in ameliorating abiotic stress have also been discussed.
2. Abiotic Stress Responses in Plants
While one or more stresses alter the plant’s most favorable environment, the plant employs a special mechanism known as “stress sensing” to detect the change. It is the extremely earliest incidence that occurs upon stressor exposure and initiates the plant stress response(s). Plants use a variety of stress sensing methods, depending on the species, organ, and type of stress [65]. For instance, light and wind stresses influence the aboveground aerial portion of the plants, while drought and salt stress affect the underground portion of the plant, triggering distinctive stress-sensing mechanisms in every case. The receptor substrate and receptor–photon binding models are the most prominent stress transmitting models for radiation and chemical stress [66]. Osmosensors in root cells can detect water availability in the soil, while the sugar production process may be used to detect stressors that alter signaling and growth [67,68].
The Mehler reaction is important in reducing the rate of photosynthetic carbon fixation during drought and salinity, as high levels of O2•− and H2O2 are generated in chlorophyll [69]. In response to drought stress, the concentrations of ABA can rise up to 50-fold [70], which is one of the biggest increases in ABA concentration observed thus far in plants undergoing environmental stimulus. Different stresses stimulate or inhibit stomatal closure, thereby activating anion channels, along with outward K+ channels, and blocking inward-rectifying K+ channels [71,72]. During drought stress, guard cells close their stomata through a complex membrane transport system to conserve water in order to survive and maximize water use, a process that differs from transcriptional regulation for long-term drought adaptation [73]. Salinity causes plants to undergo two types of stress simultaneously, namely osmotic potential change leading to reduced water uptake and ion toxicity due to the accumulation of Na+ and Cl− ions in the soil. Several studies have shown that the large central vacuoles of plants accumulate Na+ through the activity of Na+/H+ exchangers [74]. This mechanism reduces the concentration of Na+ in the cytoplasm by securing it within the central vacuole. In saline conditions, Na+ accumulated in the vacuole serves as an osmolyte and lowers the cellular water potential, thus promoting water uptake. Na+ is accumulated in cells with large vacuoles in roots, such as parenchyma and cortex cells, thereby reducing Na+ entry into root xylems [75]. In the context of cellular redox homeostasis, oxidative stress is an influential and complex phenomenon caused by an exponential enhancement in ROS [76].
Since evolving, plants have learned to cope with the changing environment by producing various enzymatic and nonenzymatic antioxidants that scavenge ROS in numerous cellular organelles in order to neutralize them [52,77,78]. The important ROS scavenging enzymes include SOD, present in almost every cellular compartment, and APX, POX, GPX, LPX, and CAT in peroxisomes [40,41,79]. The widely studied enzyme SOD, dis-mutates O2•− into H2O2, which, subsequently, is detoxified by CAT and a broad set of POXs that further disintegrate H2O2 into water (H2O) and molecular oxygen (O2) [80]. However, these antioxidants do not counteract the uncompensated ROS accumulation during stress conditions, which leads to oxidative damage and oxidative bursts [81].
3. Drought Stress Responses in Plants
Water deficit or drought stress is one of the major abiotic stressors that cause dehydration and decrease crop yields due to a direct impact on all aspects of plant growth and development. The major effects of drought shock are metabolic and osmotic imbalances that result in stomatal closure and turgor loss [82]. This, in turn, prevents carbon dioxide (CO2) from being taken up by the cells, inhibiting cell growth and decreasing photosynthesis [83]. When a plant is stressed by a prolonged water deficit, it reaches a point of permanent wilting and dies. In general, drought stress reduces crop yields, changes chlorophyll components, hampers photosynthetic processes [84], and alters the enzyme activity that is implicated in antioxidant processes and carbon metabolism [85,86]. Under drought stress conditions, plants respond molecularly, physiologically, and biochemically, of which photosynthesis is a major physiological target [87,88]. These changes disrupt the normal homeostasis of plants. During stressful conditions, ROS such as H2O2 can cause cellular damage, toxic effects, and inhibit photosynthesis [89], whereas, in normal conditions, these molecules take part in signal transduction, enabling plant cells to maintain their normal cellular processes [79,90].
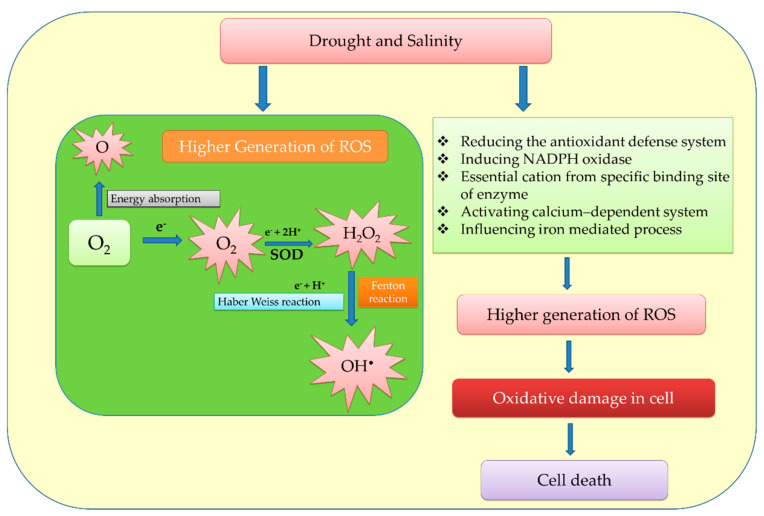
Plants experience oxidative stress when water deficits exist; this leads to the production of various ROS and RNS that adversely affect plant growth, causing cellular processes to slow down [91]. In mitochondria and chloroplasts, high ROS levels cause inequities in electron transport. Photorespiration, which is the primary cause of ROS production under water deficit conditions, produces about 70% of the total H2O2. The presence of drought stress results in stomatal closure, leading to ROS accumulation [92,93]. A variety of enzymatic reactions in plants involving O2•− and H2O2 govern diverse reactions at the cellular level, including the Fenton reaction (an iron-catalyzed reaction) and several enzyme reactions involving POXs, xanthine oxidase, lipoxygenases, and reduced NADPH oxidase (Figure 1). These radicals have the greatest tendency to damage cellular components such as carbohydrates, proteins, lipids (through the peroxidation of unsaturated fatty acids in the membrane), nucleic acid, and enzymes (through denaturation) [94].
Figure 1.
Drought and salinity-induced ROS generation in plants. Drought and salinity stress generates ROS via Fenton and Haber-Weiss reactions. ROS production by abiotic stresses modulates the enzymes (such as inducing NADPH oxidase and decreasing the antioxidant glutathione pool), activating calcium-dependent systems and altering iron-mediated processes. This led to a higher damage of ROS, thereby causing oxidative stress and damaging the cellular organelles.
4. Salinity Stress Responses in Plants
Salinity stress is a significant abiotic factor affecting agricultural systems globally. It affects more than 5% of the globe’s land area, resulting from natural processes [60,95]. It takes a long time for salt to accumulate in arid and semiarid zones [96]. Minerals and nutrients in the soil are important to plants; however, soluble salts present in excessive amounts cause ionic and osmotic stress [97]. Salinity or salt stress results from excessive accumulation of water-soluble salts like sodium nitrate (NaNO3), sodium chloride (NaCl), sodium sulfate (Na2SO4), potassium sulfate (K2SO4), sodium carbonates (NaHCO3 and Na2CO3), calcium sulfate (CaSO4), magnesium chloride (MgCl2), and magnesium sulphate (MgSO4) [97,98]. Almost all of these salts are essential to the plant and contribute to its growth and metabolism. Despite this, they may become toxic if overconsumed or present in excessive concentrations [97]. An optimal concentration of NaCl, for instance, maximizes plant growth, but higher concentrations completely inhibit seed germination and plant growth and development in salt-prone soils [41,99,100].
Various plants and tissues have been reported to undergo oxidative stress when exposed to high salinity [101]. Various plant traits are involved in salt tolerance, from genomic to proteomic and metabolomic levels [102]. A high level of ROS is generated in numerous plant tissues when plants are stressed by salinity due to irregularities in ETCs and the accumulation of photoreductant power. During salinity stress, proteins are altered both during transcription and post-transcriptionally [102]. Therefore, proteomics can contribute to the answers from genomics and transcriptomics. To understand the role of a protein in salinity stress tolerance, the proteomic analysis provides information not only about its down- or upregulation but also about its function, post-transcriptional modifications, and, thus, its interactions with several other proteins and its localization within the cells and tissues [103,104,105]. Different types of proteins begin to change their functional groups when exposed to salinity stress. Among these ion transporters, signal proteins and proteins participate in energy metabolism [106]. Among these proteins, annexin and calmodulin bind calcium and are activated by salinity stress [107]. Their function is to transduce ABA signals. There are several other proteins in Rab’s guanosine triphosphate-binding proteins (GTPase) family involved in transducing salinity stress signaling. Under salinity stress, the OsRPK1 protein kinase regulates the H+-ATPase of the plasma membrane to restore homeostasis to the plasma membrane [108]. The presence of many of these proteins is downregulated in plants that are salt-sensitive, such as potatoes [109]. Protein degradation is another aspect of the changes observed during salinity stress. Similarly, salinity also influences lipid metabolism. The monogalactosyldiacylglycerol synthase enzyme is a component of the galactosylglycerolipids in thylakoids, and chloroplast membranes (digalactosyldiacylglycerol and monogalactosyldiacylglycerol) were reduced during salinity stress, thereby impairing the membrane integrity [107,110].
5. Mechanisms of ROS Regulation in Plant Stress Responses
An imbalance of various ROS species or an accumulation of ROS resulting from high glucose levels degrades active indole-3-acetic acids (IAA), precludes root growth, and impairs root meristem activity through the conserved autophagy/macro-autophagy pathway [111]. Whenever the external environment changes, the metabolic level changes, triggering the formation of ROS in plant cells [112]. Due to diverse cellular metabolic activities in higher plants, ROS production is inevitable [113]. The production of ROS was dramatically increased by different abiotic stress conditions, including metal/metalloid toxicities, drought, and salinity destroying the balance of ROS with antioxidant enzymes [114]. ROS are essential for the regulation of gene expression in an organism under normal conditions, as they regulate numerous processes inside the cell. Further, ROS regulate a wide range of functions, including the cell cycle, plant growth, signaling, abiotic stress response, programmed cell death, pathogen defense, and developmental processes [31]. During the course of photosynthesis, respiration, and other metabolic activities under stress, there is an imbalanced activation or reduction of oxygen, which results in the excessive generation of ROS in various parts of plant cells, such as peroxisomes, plastids, mitochondria, apoplasts, and cytosols that affect proteins, enzymes, chlorophylls, and ETC. The respiratory and photosynthetic ETC, plasma membrane-localized nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, and apoplast POXs are major pathways that are primarily involved in ROS production in plant cells [52]. Chlorophyll is the most important component of the plant cell for ROS production [20,113,115]. ROS are produced either by retrograde signaling or by an oxidative burst in plant cells under unfavorable abiotic conditions. ROS production and detoxification are inequitable, which leads to OS that are harmful to plants. However, ROS can cause further damage unless a mechanism is activated in cellular organelles that limits their production at the beginning. Drought and salinity stress generates ROS via Fenton and Haber–Weiss reactions [116] and decreases the antioxidant glutathione pool, dislodging cations from enzyme-binding sites, altering iron-mediated processes and activating calcium-dependent systems (Figure 1) [114,117,118,119]. Furthermore, directing ROS into signaling pathways reduces oxidative harm and promotes tolerance of a single stressor or, possibly, of a group of stressors.
Oxidative stress causes ROS to be produced that can permanently damage the cellular apparatus. The major components of ROS in plants are typically formed through the excitation of oxygen to form 1O2 or the transfer of one, two, or three electrons to oxygen to create O2•−, H2O2, or OH•. Singlet oxygen is a highly reactive byproduct of oxygenic photosynthesis, and so, it cannot be avoided [120]. In PS II, when O2 reacts with chlorophyll triplet state, singlet oxygen is generated. Further, the synthesis of this ROS in both PS I and PS II is highly effective [121]. Oxidative stress propagates other species through O2•−. As an electron from the photosynthetic electron is placed on the transport chain, it reduces O2 in the Mehler reaction, which produces O2•− in chloroplasts. In chloroplasts, however, the lifetime of O2•− is dependent on CuZnSOD, which consumes O2•− to form H2O2. Hydrogen peroxide, besides having a moderate reactivity, does not possess unpaired electrons, which means that it can move between biological membranes at a high rate, causing damage well away from its point of origin [122]. Hydrogen peroxide plays a role in many physiological processes such as seed germination, regulating stomatal apertures, and even regulating senescence and cell death [123]. OH• is a form of radical with high reactivity, especially in reaction with 3O2. These ROS can damage cells by altering their lipids, proteins, and membrane conformations [124]. OH• is produced at the cytosolic level by ROS-producing cell organelles like chloroplasts (especially at PS-II) or mitochondria [125]. ROS components are produced by a number of organelles, such as the nucleus, chloroplasts, plasma membranes, mitochondria (mainly in ETC), endoplasmic reticulum, and peroxisomes, which are involved especially in the photosynthetic carbon oxidation cycle [32]. The production of ROS by plants in high amounts can cause damages like protein oxidation, fatty acid oxidation or lipid peroxidation, and DNA damage. Additionally, ROS signaling is also characterized by several important components, including receptor proteins, ROS-induced inhibition of phosphatases, and redox-sensitive transcription factors [126].
6. Mechanism of Stress Tolerance in Plants by Modulating the Antioxidants Machinery
Plants sense stress signals through plasma membrane receptors and initiate various pathways of signal transduction from root to shoot, which employ long-distance signaling [127,128]. Initially, plant roots sense osmotic, as well as ionic, stress caused by salinity, and they alter their signaling accordingly. Several secondary messengers are involved in signal transduction, including calcium ions, ROS, inositol phosphate, and phytohormones [129,130]. In response to NaCl stress, the intracellular calcium concentration increases transiently, which activates downstream signal transduction pathways. Calcium-dependent protein kinases and calcium-binding proteins, calcineurin B-like proteins (a SOS family protein: CBL4), sense an increase in cytosolic calcium concentration in the plasma membrane, which activates ion transporters in the plasma membrane [131,132]. Tuteja and Mahajan [133] reported that Na+ ion transporters play a crucial role in maintaining cellular toxicity. To cope with salinity, plants change their physiology, biochemistry, and molecular mechanisms. Plants maintain their water content under osmotic stress by altering some phenotypic characteristics, such as reducing cell division and elongation, inhibiting shoot branching and lateral root formation, and closing their stomata. Furthermore, plant survival under salinity stress is dependent on the ratio of shoots to roots, because heavier roots accumulate more salts and will not allow the salts to bypass the foliage [134]. Different phytohormones regulate these phenotypic changes in plants, including cytokinin, auxin (IAA), gibberellin, ethylene, and ABA [135]. These phytohormones are interdependent and play an essential role in the integration of signaling pathways. Auxins and cytokinins play different roles in cell differentiation, division, and expansion. Through stomatal closure, ABA regulates the water potential inside a cell during osmotic stress and impacts the photosynthetic rates [136]. Abscisic acid also reduces the gibberellic acid content, thus inhibiting shoot growth and leaf expansion. During cellular processes, salt ions are confined within vacuoles, which interfere with osmotic balance. Due to this, cells become dehydrated because water oozes out of the cytoplasm into the extracellular space. In order to maintain this osmotic pressure, plant accumulates a number of low molecular weight organic compounds within their cytoplasm that are compatible with metabolism referred to as compatible solutes. Munns and Tester (2008) pointed out that oxidative stress accumulates solutes such as proline, betaine, glycine, sucrose, trehalose, and mannitol [51]. These compounds are more abundantly accumulated by halophytes (>40 mM) than glycophytes (up to 10 mM) [137]. Similarly, solutes that are compatible with membranes, proteins, and enzymes act as osmoprotectants that help in stabilizing the subcellular structure under dehydration conditions, thereby protecting the plants against oxidative damage by scavenging the free radicals.
Plants are susceptible to ionic stress under prolonged exposure to salinity, causing an accumulation of Na+ that causes cytotoxicity. To prevent Na+ toxicity, plants have developed two mechanisms: increased vacuolar sequestration or intracellular compartmentation and increased Na+ extrusion [138]. Plants are susceptible to salinity tolerance due to the effectiveness of these mechanisms. By excluding salts from roots, glycophytes maintain Na+ toxicity, whereas halophytes use a tonoplast Na+/H+ channel to improve ion compartmentation [139]. As Na+ accumulates in the roots, which is then stored in the xylem, it is transported to the leaves through the transpiration stream. A leaf’s tissues have a greater susceptibility to ionic stress than most other tissues. Additionally, the recirculation of Na+ from shoot to root leaves some amount of Na+ in the shoot [99,140]. Therefore, a regulatory network of different transporters is responsible for the efflux of Na+ from tissues and reabsorption of Na+ from the xylem to allow cells to tolerate ionic stress [141]. Qiu et al. [142] showed that the plasma membrane transporter Na+/H+ antiporter is responsible for the excretion of Na+ ions from cells. Several studies have demonstrated that Salt Overly Sensitive 1 (SOS1) has Na+/H+ antiporter activity in Arabidopsis [143,144,145,146,147]. Additionally, tonoplast antiporters facilitate ion compartmentation within vacuoles. This group includes the Na+/H+ exchangers in Arabidopsis [148]. Additionally, plants also inhibit Na+ uptake by their roots by activating HKTs, which have a high affinity for potassium (K+), making plants more tolerant to salinity [149].
Drought-tolerant plants respond to water scarcity by synthesizing osmolytes, subsequently increasing their osmotic potential [54]. Sometimes, osmolytes are also found in root exudates. Plants can generally be classified into either halophytes or glycophytes based on their potential to grow under conditions of high salinity. In some cases, halophyte plants can tolerate salinities as high as seawater or even higher [97,127]. In order to evade the destructive effects of high salinity, halophytes restrict the absorption of salt and reduce the concentration of salt in their cytoplasm and cell walls [150]. The salt-sensitive glycophytes plants cannot handle the high salinity levels and are incapable of using the approaches that the halophytes use to minimize the effects of high salinity. The cytosol, therefore, accumulates toxic concentrations of salt [97,150]. In addition to affecting plant roots and tubers, salinity also affects seedling viability and seed germination [41,65,127]. In extreme cases, ionic and osmotic stress causes the roots to lose their water uptake, which leads to reduced growth and metabolism [97,98]. A prolonged salinity stress causes stomata to close, which reduces the CO2 uptake. The reduced photosynthetic capacity caused by salt toxicity ultimately results in senescence and death, because the plant is unable to maintain an appropriate growth rate [82,97,98].
Additionally, plant cells also possess mechanisms for reducing ROS-induced toxicity. To combat higher levels of ROS, plant cells produce secondary metabolites such as tocopherols; phenols; flavonoids; carotenoids; polyamines; phenolic compounds; and antioxidative enzymes like SOD, CAT, POX, APX, LPX, and GSH reductase, which help in the deactivation of active oxygen species during multiple redox reactions, making the antioxidant system protective against oxidative stress [40,41,64,151]. These ROS scavengers may improve plants’ tolerance to drought and salinity stress (Figure 2). It is one of the key enzymes in plants’ defense systems against oxidative stress, as it appears ubiquitously in all of the cells of all kinds of plants. These antioxidants combat ROS by converting them into nonreactive forms. To reduce the amount of ROS in the cell, a number of antioxidative enzymes cooperate within different cellular compartments [152]. Under drought and salinity stress conditions, plants show enhanced antioxidant enzyme activities as a mechanism of tolerance to stress [153]. Studies have reported that Arabidopsis overexpresses aldehyde dehydrogenase and is resistant to drought and salinity [154,155,156]. By overexpressing Chlamydomonas GPX in chloroplasts or cytosols of transgenic tobacco plants, stress tolerance can be improved [157].
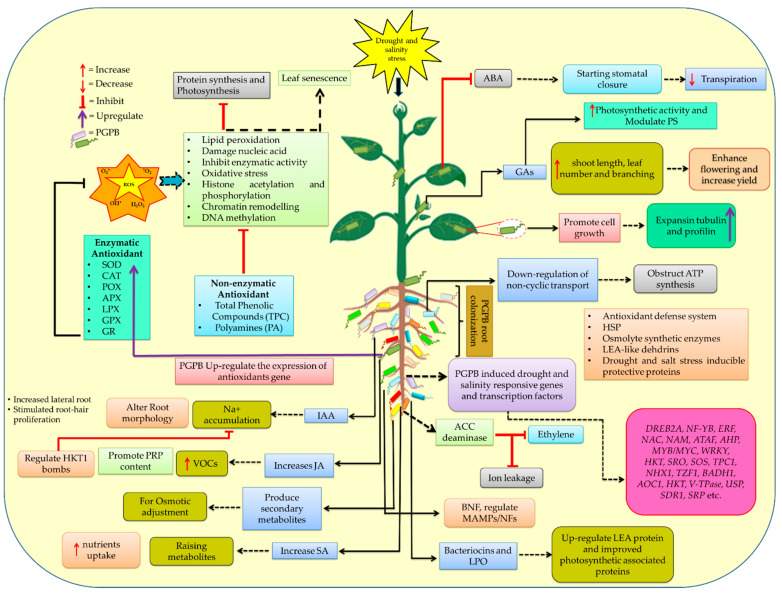
Figure 2.
Schematic representation of plant growth-promoting bacteria (PGPB)—mediated drought and salinity stress tolerance in plants. During drought stress, the plant itself and PGPB are able to detoxify ROS into stable nonreactive compounds. SOD—superoxide dismutase; CAT—catalase; GR—glutathione reductase; GPX—glutathione peroxidase; MAMPs—microbe-associated molecular patterns; NFs—nodulation factors. PGPB modulates the signaling pathways involved in drought and salt response biochemically and molecularly. Drought response is primarily regulated by ABA, which controls other signaling pathways such as SA, IAA, JA, and GA. SA—salicylic acid; ABA—abscisic acid; JAs—jasmonic acid; GAs—gibberellins; IAA—indole-3-acetic acid. PGPR also modulates transcription factors (TFs) that are essential in the drought and salt response and tolerance. NAM, ATAF, NAC, MYB/MYC, and WRKY—transcription factors; NF-Y—nuclear factor-Y; ERF—ethylene-responsive element-binding factor; LCOs—Lipo-chitooligosaccharides; BNF—Biological Nitrogen Fixation; AHP—cytokinin-related genes; AOC1—allene oxide cyclase; HKT—High-affinity K+ transporters; NHX1—vacuolar Na+/H+ antiporter gene; BADH1—Betaine aldehyde dehydrogenase 1; V-ATPase—Vacuolar-H+-pyrophosphatase; USP—Cytosolic universal stress protein; SDR1—salt and drought-responsive gene; LEA—late embryogenesis abundant; TIP1—Tonoplast AQP gene; SRP—Salt-responsive protein-encoding gene; SOS1—Salt overly sensitive gene. Figure created with BioRender.com (https://app.biorender.com/biorender-templates)—accessed on 27 January 2022.
7. PGPB for Plant Growth Promotion and Stress Tolerance
Plants, unlike many other organisms, have evolved different mechanisms to guard themselves against stressful conditions and promote growth and development, as well as to avoid, and protect themselves from, stressful conditions [17,158]. The application of useful bacteria to increase drought and salt tolerance in plants is a substitute that is cheaper and more feasible [50,159,160,161]. Numerous studies have revealed that PGPB can improve both plant growth and nutrition of a variety of crops even when adverse environmental conditions occur, including drought and salinity [18,41,58,63,162,163,164,165,166]. PGPB affects plants directly by producing phytohormones or indirectly by inducing signaling in the host. Most commonly, phytohormones like IAA, gibberellins, cytokinin, ABA, and ethylene; biological nitrogen fixation (BNF); and phosphate solubilization are attributed a direct role [41,63,64]. However, the indirect mechanisms include the production of hydrogen cyanide, antibiotics, volatile organic compounds (VOC), siderophores, and ammonia that suppress phytopathogens. In addition to improving crop water relations and changing the ion balance, these soil microorganisms also modulate abiotic stress regulation via different pathways [50,160]. Over the last several decades, PGPB has been broadly used for sustainable agriculture in several parts of the world in order to reduce chemical pesticides and fertilizers [63,167,168].
7.1. Role of PGPB on Drought Stress Tolerance
Numerous PGPBs synthesize osmolytes and help the plants cope with drought stress (Figure 3). It has been suggested that the production of IAA by PGPB may contribute to the increase in root–shoot biomass under drought stress [169]. Plants are also known to regulate their growth with ethylene, whose production is influenced by conditions such as drought, salinity, and waterlogging [170]. A rhizospheric bacteria producing aminocyclopropane-1-carboxylate deaminase (ACCD) inhibits the ethylene signaling pathway to resist root drying. In tomato and pepper plants, Achromobacter piechaudii exhibits ACCD activity, leading to an improvement in biomass by resisting the water deficit. Similar results were also reported by references [40,41] under salinity stress on pea plants. ACCD-positive isolates reduce the overproduction of ethylene in plants, which enhances injuries caused by water scarcity without affecting the relative water content (RWC) of plants [171]. Plants that have been injected with drought-tolerant bacteria achieve better plant growth and have higher proline contents in their roots and leaves. The effect of PGPB is significant in the presence of water [172]. According to Creus et al. [173], the inoculation of Azospirillum under water scarceness reduced the yield of wheat and increased the ion contents, such as magnesium (Mg2+), potassium (K+), and calcium (Ca2+), in grains. Researchers have found that PGPBs, together with plant growth regulators, provide tolerance to plants under drought stress [174,175,176]. Plant growth and development are significantly influenced by PGPB. In addition to providing micronutrients to the host plants, they can also enhance the availability of growth-promoting chemicals. For instance, they produce exopolysaccharides (ESP), a type of carbohydrate that is released in the rhizospheric region [177]. These ESPs perform a vital function in protecting plants from desiccation [178]. Salicylic acid (SA), a well-known phenolic compound that is secreted by microorganisms, is required for plant growth and development, thereby providing drought tolerance (Table 1 and Figure 3). It works as a signaling molecule under drought stress, triggering genes that function as heat shock proteins (HSP), chaperones, antioxidants, and activate genes that synthesize secondary metabolites [179,180].
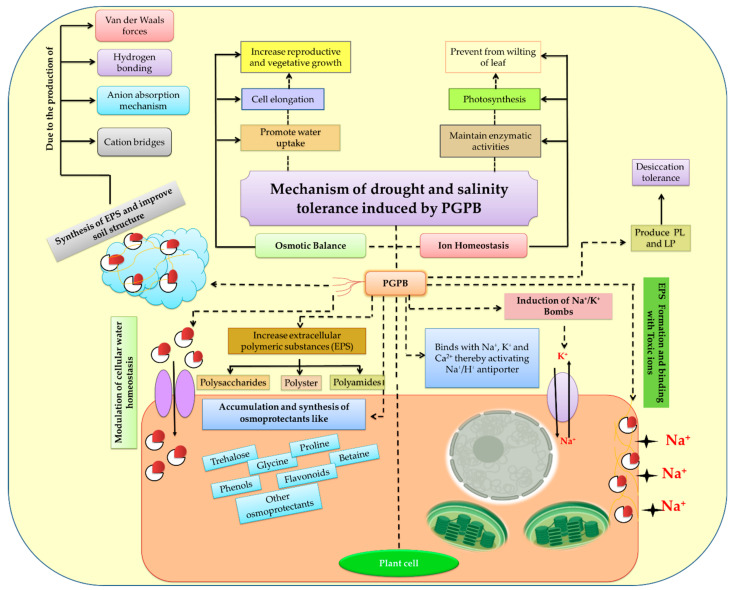
Figure 3.
Mechanisms of plant growth-promoting bacteria (PGPB)-induced tolerance of drought and salinity stress. Plant inoculated with PGPB experienced growth-promoting attributes like EPS and ESP production that modulate cellular water homeostasis. PGPB also induces the accumulation and synthesis of various osmoprotectants like trehalose, proline, glycine, phenols, flavonoids, and so on that help in scavenging ROS and RNS in cells. PGPB are also responsible for maintaining the ion homeostasis (Na+/K+) and removing the toxic ions from the cell. EPS—extracellular polymeric substances; ESP—exopolysaccharide; PL—polysaccharide lipid; LP—lipopolysaccharide protein; Na+—sodium ion; K+—potassium ion. Figure created with BioRender.com (https://app.biorender.com/biorender-templates)—accessed on 21 November 2021.
Table 1.
PGPB-produced mechanisms related to tolerance against drought stress.
| PGPB | Plants | Effects | Mode of Action | References |
|---|---|---|---|---|
| Azospirillum brasilense Sp245 | Triticum aestivum | A higher Mg2+, K+, and Ca2+ content in the grain, as well as higher water content, RWC, and water potential | N2 fixation | [173] |
| ACCD producing rhizobacteria | T. aestivum | Increased root-shoot length, biomass and lateral root number | ACCD production | [184] |
| A. piechaudii | Lycopersicon esculentum | A remarkable mechanism of stress resistance has been found in the production and excretion of glucosyl glycerol. | Use transcriptomic and microscopic approaches to assess osmotic stress tolerance | [185] |
| A. xylosoxidans Cm4, Variovorax paradoxus 5C-2 and Pseudomonas oryzihabitans Ep4 | Solanum tuberosum | Increased plant biomass | Decrease amino acid and ethylene content | [186] |
| Acinetobacter calcoaceticus WP19, Rahnella sp. WP5, Burkholderia sp. WP9, Enterobacter asburiae PDN3, Pseudomonas sp. WW6, Sphingomonas yanoikuyae WW5and Curtobacterium sp. WW7 | Poplar/Populus | Plant growth promotion (increased root-shoot dry weight, total dry weight, total nitrogen); enhanced protection against ROS | Reduced ROS damage, phytohormone production and microbial genes identification for drought tolerance | [187] |
| Azospirillum sp. | T. aestivum | Increased lateral roots formation and root growth, and uptake of nutrients and water content | Production of IAA, high amount of nitrogen, P-solubilization and ACCD activity | [188] |
| B. megaterium CAM12 and P. agglomerans CAH6 | Vigna radiata | Reduced Aluminium uptake in plants; increased plant biomass (Plant growth promotion); higher content of chlorophyll and carotenoids |
Increase IAA production, ACCD activity, EPS and ESP production, siderophore production | [189] |
| B. thuringiensis | Lavandula dentate | Modulate antioxidants enzymes like APX and GR | By controlling shoot proline accumulation and depressing stomatal conductance, IAA increased K+ content | [190] |
| B. cereus AR156, B. subtilis SM21, and Serratia sp. XY21 (BBS) | Cucumis sativus | Proline content of the leaves was increased; enhanced SOD activity in a significant way | BBS treatment downregulate the expression of rbcL, cAPX, and rbcS genes | [191] |
| B. megaterium and Glomus sp. | Trifolium | Increase antioxidant enzymes like GR, SOD, and CAT | IAA and proline production | [192] |
| B. megaterium BOFC15 | Arabidopsis thaliana | Improved root system architecture, enlarged plant biomass, and increased photosynthetic capacity | Elevates cellular polyamine (spermine, spermidine), isoprenoid, ABA, and reduces malonaldehyde content | [193] |
| B. polymyxa | L. esculentum | Physiological and biochemical characteristics of plants were improved by proline accumulation | Phosphate solubilization | [194] |
| Bacillus sp. KB142, KB133, KB129 and KB122 | Sorghum bicolor | Increased plant biomass, RWC, chlorophyll content and soil moisture content | Increase ESP production and Biofilm formation; accumulation of proline and sugars; | [195] |
| B. subtilis GB03 | A. thaliana | Expression of the PEAMT gene in osmotically stressed plants improved leaf RWC and dry DMW as well as the metabolic level of glycine betaine and choline. | Enhances the biosynthesis of Cho and Gly Bet in Arabidopsis; increases ABA synthesis | [196] |
| B. thuringiensis AZP2 | T. aestivum | Increasing photosynthesis and reducing volatile emissions | ACCD production and P-solubilization | [197] |
| B. polymyxa | L. esculentum | Increased RWC, protein, chlorophyll, proline accumulation and yield | Phosphate solubilization | [194] |
| B. phytofirmans | T. aestivum | Improved water-use efficiency, photosynthetic rate, chlorophylls content, nitrogen (N), phosphorus (P), potassium (K), and protein levels in wheat grains | Ameliorating the RWC, improving chlorophyll content and photosynthetic rate | [198] |
| Consortia containing P. synxantha, R81 P.jessenii, R62, and Arthobacter nitroguajacolicus strainYB3, strain YB5 | Oryza sativa | Accumulation of proline improved plant growth and osmotic adjustment | PGPR increases the proline content, CAT, SOD, APX, POX, LPX, and lower level of H2O2, content | [199] |
| Consortia of Bacillus isolate 23-B and Pseudomonas 6- P with Mesorhizobium ciceris | Cicer arietinum | Higher proline concentration, improved seed germination, root-shoot length and fresh weight of the seedlings | ACCD production | [200] |
| E. mori AL, E. asburiae BL and E. ludwigii CL2 | T. aestivum | Increased plant biomass | Higher ACCD production | [201] |
| Gluconacetobacter diazotrophicus PAL5 | Saccharum officinarum | Drought resistance is conferred by the activation of ABA-dependent signaling genes | Activate drought-responsive markers and hormone pathways, such as ABA and Ethylene. | [202] |
| Ochrobactrum pseudogrignonense RJ12, Pseudomonas sp. RJ15, and B. subtilis RJ46 | V. mungo and Pisum sativum | Plant growth promotion (enhanced seed germination, percentage, root-shoot length, and dry weight), enhanced cellular osmolytes and ROS scavenging enzymes, enhanced leaf chlorophyll content | ACCD production | [203] |
| Paenibacilluspolymyxa and Rhizobium tropici | Phaseolus vulgaris | Increased plant growth, N2 content, and nodulation | ACCD production | [204] |
| P. putida H-2–3 | Glycine max | Gibberellins secretion improved plant growth, induced regulation of stress hormones and antioxidants and also increased the crop productivity | Gibberellin production and increased antioxidants enzymes | [205,206] |
| P. polymyxa B2 | A. thaliana | Induction of EARLY RESPONSE TO DEHYDRATION 15 (ERD15) | Produce antibiotic compounds, Hydrogen cyanide and Siderophore | [207] |
| Phyllobacterium brassicacearum STM196 | A. thaliana | Increased plant biomass, lowers transpiration and photosynthesis | Modulate ABA content, delayed reproductive timing | [208] |
| P. brassicacearum strain STM196 | A. thaliana | Reduced leaf transpiration was caused by increased ABA content | Modulate ABA content, delayed reproductive timing | [208] |
| P. brassicacearum | A. thaliana | Increased biomass, ABA content, higher water-use efficiency | Confer stress tolerance by modulating the biochemical parameters | [208] |
| P. chlororaphis O6 | A. thaliana | Transcripts of the jasmonic acid marker genes, pdf-1.2 and VSP1, ethylene-response gene, HEL, PR-1 and SA-regulated gene were up-regulated in colonized plants | Response to ROS, and auxin- and jasmonic acid-responsive genes | [209] |
| P. fluorescens biotype G (ACC5) | P. sativum | Induced longer roots and water uptake | ACCD production | [210] |
| P. putida | C. arietinum | Osmolyte accumulation (proline, betaine, glycine) and ROS scavenging | IAA production and ACCD activity | [211] |
| P. putida P45 | Helianthus annuus | An increase in rhizosphere nutrient and water uptake | ESP production | [212] |
| Pseudomonas sp. | P. sativum | Decreased ethylene production | ACCD production | [204] |
| P. aeruginosa | V. radiata | Increased root and shot length, dry weight and RWC | Production of ROS scavenging enzymes and up-regulation of three drought stress responsive genes (CAT, DREB, and DHN) | [213] |
| P. putida | H. annuus | Increased plant biomass, biofilm formation on roots and soil adhesion | ESP production | [212] |
| R. etli overexpressing trehalose-6-phosphate synthase gene | P. vulgaris | Signaling molecules like trehalose upregulate genes involved in carbon metabolism, nitrogen metabolism, and stress tolerance | Increased activity of nitrogenase gene and overexpression of trehalose-6-phosphate synthase | [214] |
| R. phaseoli (MR-2), R. leguminosarum (LR-30), and M. ciceri (CR-30 and CR-39) | T. aestivum | IAA produced by the consortia improved biomass, growth and drought tolerance index | ESP production and increased catalase activity | [215] |
| V. paradoxus 5C-2 | P. sativum | Growth, yield, nodulation, production and water use efficiency are increased with xylem abscisic acid | Induced ABA and ACCD production | [216] |
| Consortia of B. amylolequefaciens and P. putida | C. arietinum | Growth, production and drought stress tolerance | IAA production, ACCD activity, P solubilization, Siderophore activity | [64] |
RWC—Relative Water Content; N2—Nitrogen; ACCD—1-aminocyclopropane-1-carboxylate deaminase; APX—Ascorbate peroxidase; GR-Glutathione reductase; ROS—Reactive Oxygen Species; Cho—Choline; GlyBet—Glycine Betaine; IAA—Indole Acetic Acid; EPS—Extracellular Polymeric Substances; ESP—Exopolysaccharide; K+—Potassium; SOD—Superoxide Dismutase; CAT—Catalase; POXs—Peroxidases; LPX—Lipid Peroxidase; H2O2—Hydrogen Peroxidase; ABA–Abscisic Acid; SA—Salicylic acid; DREB—Dehydration-Responsive Element-Binding Protein; DHN—Dehydrin; VSP1—Vegetative storage protein; HEL—Ethylene responsive gene; PR-1—SA-regulated gene.
7.1.1. Metabolic Reprogramming
Plants under drought stress may be reprogrammed by numerous microbes by regulating their metabolism and molecular pathways. Some metabolites such as pyruvic acid (PA), succinic acid, thiamine pyrophosphate, uridine diphosphate, and dihydroxyacetone are significantly decreased in wheat under drought conditions, whereas Bacillus velezensis treatment helps to make these metabolites more available to combat drought stress [181]. Azotobacter brasilense, A. chroococcum, and Bacillus sp. increase the accumulation of soluble sugars, proteins, phenols, flavonoids, ABA, and oxygenated monoterpenes in pennyroyal during drought and salinity stress conditions [40,41,182]. Furthermore, a consortium of bacteria, including B. thuringiensis, B. subtilis, and B. megaterium, significantly increased the levels of glycerol, L-asparagine, nicotinamide, riboflavin, total sugar, and 3-hydroxy-3-methylglutarate in chickpea leaves under drought stress [183]. Consequently, plant-associated bacteria may be able to alter the metabolic changes induced by drought in order to reduce the effects.
7.1.2. Biochemical Changes and Molecular Adaptations
Plants respond to drought and salt stress through a variety of processes, including the production of distinct proteins, the secretion of metabolites, and the modulation of genetic expression. Several PGPBs play an essential role in the production of numerous phytohormones (such as IAA, GAs, cytokinin, etc.). These phytohormones play a critical role in regulating plants’ growth and response during salinity stress and water deficit conditions. When the plants are exposed to drought and salinity stress, these phytohormones stimulate various signaling pathways, which result in the greater production of secondary metabolites, antioxidant enzymes, and HSP. Hence, the study and development of numerous phytohormone-related strategies is necessary for increasing drought and salinity tolerance in plants [217].
Apart from biochemical processes, PGPB also modulates molecular mechanisms such as inducing the production of lipochitooligosaccharides (LCOs), late embryogenesis abundant (LEA) proteins, regulating microbe-associated molecular patterns (MAMP), and nodulation factors (NFs), as well as activating several drought and salt-responsive genes (Figure 2). Cellular dehydration tolerance is mediated by LEA proteins [218]. Several microbes produce LCOs, which trigger symbiotic interactions with plants [219]. In response to the flavonoids in root exudates, rhizobacteria secrete Nod factors (NFs), which induce nodule formation [218,220]. Plants have high-affinity K+ transporters (HKT) located on their plasma membranes that mediate Na+ transport, preventing Na+ ions from building up during photosynthesis by excluding them from the shoots (Figure 2) [40,221,222]. Salt stress also ensures cell integrity by increasing the levels of tubulin and profilin, which bind to actin and regulate the cytoskeleton structure [223,224,225,226]. In drought stress, dehydration-responsive element-binding protein 1 (DREB1)/CBF (C-repeat binding factor) and DREB2 regulons function to control the gene expression in an ABA-independent manner [227]. Allene oxide cyclase (AOC1), an enzyme involved in the α-linolenic acid metabolism pathway, was found to increase the salt tolerance in both wheat and Arabidopsis [228]. Here, we outlined and discussed the numerous molecular and biochemical response mechanisms in Figure 2. There are several biocontrol agents and plant growth-promoting substances that are produced by rhizospheric microbes that live around plants [229]. Moreover, they increase the nutrient availability and influence the soil structure, pH, fertility, and oxygen availability [230]. Through numerous processes within the rhizosphere and phyllosphere, these microbes increase the ability of plants to tolerate drought and salinity, thereby promoting plant growth (Figure 2).
7.2. Role of PGPB on Salinity Stress Tolerance
Salinity affects plants in two main ways. High salinity makes the soil hard and dry, which hinders roots from extracting water and causing toxicity to plant cells, thereby affecting plant growth and metabolism. However, toxic concentrations of salt take longer to accumulate in plants [51]. PGPB can alleviate the severity of salinity-related problems. It has been reported that Gram-positive (G+) and Gram-negative (G−) PGPB can colonize the roots of plants and decrease the effects of salinity through direct and indirect mechanisms [62,231,232] (Table 1 and Table 2). These bacteria exhibit chemotaxis and produce ESP, IAA, and ACCD that can withstand salinity stress (Figure 3) [40,41,233]. With PGPB, plants can develop induced systemic tolerance that enables them to cope with the salinity stress [234]. In a study by Yildirim et al. [235], they found that Staphylococcus kloosii and Kocuria erythromyxa can induce salinity tolerance in Raphanus sativus by producing an antioxidants enzyme that scavenges ROS [235,236]. Another study by Nadeem et al. [237] demonstrated that P. fluorescens, P. syringae, and E. aerogenes, possessing ACCD activity, could induce salinity tolerance in maize by regulating the K+/Na+ ratios, chlorophyll levels, and proline levels. According to Hamdia et al. [238], the inoculation of Azospirillum with a high K+/Na+ ratio improved the salt tolerance in maize. According to M’Piga et al. [239], PGPB acts against a variety of phytopathogens by inducing numerous defense enzymes like phenylalanine ammonia-lyase (PAL), POX, chitinase, and β-1,3-glucanase (GLU). It has been demonstrated that IAA acts on the H+ ATPase of the plasma membrane, thereby causing Na+ ions to load into root cells [144,240]. Under salinity stress, another strain of Pseudomonas sp. PDMZnCd2003 can produce high concentrations of IAA. The molecular study reveals that plants suffering from salinity stress had higher levels of ethylene due to higher levels of ACC, which causes changes in various physiological functions. Plants may grow better by any mechanism that reduces the ethylene levels during salinity stress. Under salinity stress conditions, PGPB produced a variety of phytohormones that are capable of enhancing the leaf area, root growth, and a number of root tips, resulting in enhanced nutrient uptake (Table 2) [241]. It has also been demonstrated that P. extremorientalis, P. chlororaphis, P. putida, and P. aurantiaca can produce IAA in a 4% NaCl solution, thereby increasing the plant biomass of Sulla carnosa under salinity stress [241].
Table 2.
PGPB-produced mechanisms related to tolerance against salinity stress.
| PGPB | Plants | Effects | Mode of Action | References |
|---|---|---|---|---|
| P. mendocina | Lactuca sativa L. | Stable soil aggregates in high proportions | ESP production | [259] |
| A. brasilense and Pantoea dispersa | Capsicum annuum L. | Increased dry weight and K+/Na+ ratio | Maintaining of higher stomatal conductance | [276] |
| B. aquimaris | T. aestivum L. | Increased weight, biomass, and leaf nutrients |
Accumulation of osmoprotectants (TSS and proline) | [264] |
| Rhizobium sp. and Pseudomonas sp. | Zea mays L. | Increased plant biomass, development, and nutrient uptake | Accumulation of osmoprotectants (proline, Betaine), water and ion homeostasis | [58] |
| Pseudomonas sp. | S. lycopersicum L. | Higher shoot and root length, total dry weight, and chlorophyll content | ACCD production and osmoprotectants accumulation (trehalose) | [272] |
| B. megaterium | Z. mays L. | Higher root hydraulic conductance | Up-regulation of aquoporin genes (PIPtype) | [274] |
| B. subtilis |
Puccinellia tenuiflora SCRIBN. & MERR. |
Improved shoot and root growth and decreased Na+ ion accumulation | Ion transport genes (HKT type): transcriptional changes | [277] |
| P. simiae | G. max L. | Higher weight, length, and K+/Na+ ratio | Changes in transcriptional regulation of phosphatase activity, proline accumulation, and the production of VOCs | [278] |
| Rhizobium sp. and Pseudomonas sp. | Z. mays L. | Enhanced plant biomass, nutrient uptake and development | Accumulation of proline, water and ion homeostasis | [58] |
| Pseudomonas sp. and Bacillus sp. | G. max L. | Increased water content, plant biomass, and photosynthetic activity | Production of IAA ESP, and ACCD and accumulation of proline | [279] |
| B. aquimaris | T. aestivum L. | Increased weight, biomass, and leaf nutrients |
Accumulation of osmoprotectans (PRP and TSS) | [264] |
| A. lipoferum | T. aestivum L. | Enhanced plant weight and chlorophyll content | N2 fixation and IAA production | [280] |
| Bacillus sp. | P. sativum L. | Enhanced morphological and biochemical parameters | IAA production, P-solubilization, ACCD, and hydrogen cyanide production | [41] |
| Bacillus and Pseudomonas sp. | P. sativum L. | Enhanced morphological and biochemical parameters and modulated antioxidant genes | ACCD production | [40] |
| A. piechaudii | S. lycopersicum L. | Increased dry and fresh weight, and K and P uptake | ACCD production | [171] |
| Burkholdera cepacian, Promicromonospora sp. and A. calcoaceticus | C. sativus L. | Enhanced biomass, photosynthetic pigments, water, and P and K content | Downregulation of ABA genes | [205] |
| Kocuria rhizophila | Z. mays L. | Reduction of Na+ accumulation and increase in productivity parameters | Transcriptional changes in ion transporter genes (NHX and HKT-type) and hormonal changes (ABA and IAA) | [281] |
| B. amyloliquefaciens | Menthax piperita L. | Improved morphological characteristics and higher chlorophyll content | VOCs production and reduction of ABA endogenous levels | [282] |
| Bradyrhizobium japonicum and B. thuringiensis | G. max L. | Germination of seeds and proteome changes | Lipo-chitooligosaccharide and bacteriocin production | [218] |
ESP—Exopolysaccharide; VOCs—Volatile Organic Compounds; ACCD—1-aminocyclopropane-1-carboxylate deaminase; ABA—Abscisic acid; IAA—Indole acetic acid; PRP—Proline-rich protein; TSS—Total soluble sugar; P—Phosphorus; N2—Nitrogen; NHX—vacuolar Na+/H+ antiporter; HKT—Sodium transporter.
7.2.1. Production of Extracellular Polymeric Substances
Many biopolymers were synthesized by microorganisms under natural conditions, including polysaccharides, polyesters, and polyamides (Figure 3). A variety of multifunctional polysaccharides are produced, including intracellular, extracellular, or ESP, structurally [242,243,244]. EPS-producing PGPBs are capable of alleviating salinity stress [245,246], as they bind with cations, such as Na+, and decrease plant accessibility towards these toxic ions. For the classification of stress-tolerant microbes, EPS may be an important criterion, as they help crops thrive under stressful conditions. EPS enhances the water retention capacity of bacteria and regulates the diffusion of organic carbon sources to promote their survival. The desiccation tolerance of bacteria is also attributed to polysaccharide–lipid (PL) and high molecular weight lipopolysaccharide–protein (LP), a carbohydrate complex. Moreover, bacteria also contain polysaccharide–lipid complexes (PLs) and high molecular weight lipopolysaccharide–protein complexes (carbohydrate complexes) that are primarily responsible for desiccation resistance. In addition, EPS can facilitate microbe–plant interactions [244,246,247] by providing microenvironments that enable microbes to survive in stressful conditions. In addition, it helps bacteria colonize the plant by allowing them to attach to root exudates. Under drought and salinity stress conditions, the EPS composition and concentration dramatically change. Microbes secrete EPS in the form of slime material in soil, which is bonded to soil by hydrogen bonds, cation bridges, anion adsorption mechanisms, etc. [248,249]. Thus, slime substance forms around soil aggregates, providing protection against drought and salinity. Plants that are inoculated with EPS-producing microbes display resistance against water deficit and salination conditions (Figure 3). By producing EPS around roots, soil microbes can also increase the water potential and increase the nutrient uptake by plants [246,249]. Under drought and salinity stress, this formation of biofilms is a common mechanism by which various microbes protect themselves from adverse effects. EPS plays an essential role in providing structural stability to biofilms [250,251]. Under saline conditions, EPS may modulate the chemical and physical characteristics of microbes and restrict sodium (Na+) uptake [246].
7.2.2. Osmotic Adjustment
Among the effects caused by salinity, the first is osmotic stress, which disrupts the water balance, resulting in stomatal closure [60,252]. The reduced leaf area and imbalanced gas exchange lead to a decrease in the photosynthesis rates [50,253]. In addition, there is photosynthetic feedback inhibition. During reduced growth, carbohydrates accumulate in storage organs and meristems, which are otherwise used in the expansion and proliferation of new tissues [160,253]. In order to compensate for the effects of drought and salinity, plants must maintain their water balance and preserve their photosynthetic structures. Through various mechanisms, PGPB has demonstrated potential application as a method of enhancing the osmotic balance (Figure 3 and Table 1).
Microbiota (especially, PGPB) are defending themselves against stressful environmental conditions (such as temperature, drought, salinity, and pH) and adhering to biotic and abiotic surfaces with the help of extracellular polysaccharides (EPS) or ESP [159,254,255]. Based on the strain and conditions, the ESP composition and amount can vary [256]. In addition to plant–microbe interactions, ESPs have additional functions. Polysaccharides increase soil particle adhesion and promote macropore generation, which increases the soil porosity and aeration (Figure 3) [159,160,257,258]. In this way, soil particles are bound, and the structure of the soil is improved, thus reducing the effects of the initial osmotic stress [60]. According to one study, when P. mendocina (PGPB) and Glomusintra radices (arbuscular mycorrhizal fungus) were co-inoculated in lettuce, they produced ESP, which produced a high percentage of stable aggregates in soil under field conditions [259]. Furthermore, Qurashi and Sabri (2012) [260] reported that both chickpea growth and soil structure were improved through the inoculation of two bacterial strains, Planococcus rifietoensis RT4 and Halomonas variabilis HT1, in C. arietinum plants that are subjected to soil aggregate formation under salt stress conditions [260].
Salinity reduces the growth of unused photosynthates and is a feedback inhibitor of growth. During the salinity osmotic phase, microorganisms regulate the source–sink relationship of soluble sugars in plants to favor osmotic adjustment and avoid photoinhibition feedback (Figure 3) [50,261]. The roots of plants are a strong source of carbohydrates, and their development can be influenced by the hormonal responses (IAA) associated with the actions of microbes on them. Additionally, microbes can consume a substantial portion of these photosynthates; for instance, Medicago ciliaris lines exhibited salt resistance through the maintenance of nodular symbiotic and sink–source activities [262]. In addition, inoculation with numerous Bacillus strains in wheat and strawberry has increased a variety of physiological parameters, like stomatal conductance or productivity or photosynthesis [263], and nutritional content [264]. Plants can lose intracellular water when subjected to saline stress [265]. To maintain their osmotic state of the cytoplasm and to improve plants’ responses to such stress, vegetative species produce organic osmolytes in the cytoplasm [256]. Apart from that, beneficial bacteria like Azospirillum [266], Burkholderia [222,267], Arthrobacter [268], Bacillus [268,269], Pseudomonas, and Rhizobium [58] also led to the production of certain osmoprotectants, and among them are proline, betaine, trehalose, glycine, phenols, and flavonoids (Figure 3) [160,253,265]. These mechanisms are also used by salt-tolerant bacteria to cope with fluctuating osmotic conditions [256]. Furthermore, osmoprotectants produced in bacteria are biosynthesized more rapidly than in their associated plants [253]. Studies have shown that PGPB inoculation increases plants’ osmolytes levels. The improvement may be due to bacterial solutes being absorbed by roots, or PGPB may enable the de novo synthesis in plants [160,253]. Furthermore, it was demonstrated that the usage of numerous bacterial isolates (B. tequilensis MPP8, B. megaterium MPP7, P. putida MPP18, Alcaligenes faecalis IG27, A. bereziniae IG2, and E. ludwigii IG10) increased the amount of TSS and proline in salt-stressed wheat plants, thereby reducing the electrolyte leakage, reducing the oxidative damage, and enhancing the amount of ROS scavenged [270,271]. Furthermore, mutants of the gene encoding trehalose synthase (treS) in Pseudomonas sp. have been constructed, and their roles in protecting plants against salinity stress have been reported [272]. A high-salt concentration alters the plant’s water potential; therefore, PGPB improves the hydraulic conductivity, thereby regulating the water homeostasis (Figure 3) [160,253,273]. A positive regulation of plasma membrane intrinsic protein (PIP)-type plasma membrane aquaporins was found after exposure to B. megaterium B26 in maize plants under salinity (2.59 dS m−1) [274]. Under the salinity stress conditions (200-mM NaCl), A. brasilense AZ39 inoculation also improved the transcription of a PIP-type aquaporin in barley plants [275].
7.2.3. Ion Homeostasis
Salts cause the accumulation of numerous ions such as Na+, Cl−, Ca2+, Mg2+, SO32−, or CO32−, which leads to ion toxicity. The influx of these ions is greater than the rate of exclusion when exposed to high salt concentrations for a prolonged period of time [51,253]. Initially, plants compartmentalize excess salts in their vacuoles to avoid accumulation in the cytosol and intracellular spaces [50,253], which would limit photosynthesis and respiration. In biochemical reactions, Na+ replaces K+, which results in protein synthesis and conformational changes [60,221]. Researchers have reported that soil microorganisms maintain ion homeostasis, which is necessary for plant development and tolerance during salinity stress [160,283]. Conversely, the majority of these studies have only considered NaCl-induced salinity stress, not the other ions involved in salinity stress. By maintaining high K+/Na+ ions ratios, PGPB can control toxic ion homeostasis, preventing the accumulation of Na+ and Cl− ions in the leaves, increasing ion exclusion by roots, or modulating ion transporter expression [160,253,284]. The high-affinity K+ transporter (HKT) is a plasma membrane protein that mediates Na+ ion transport in plants, preventing the overaccumulation of Na+ ions in shoots by excluding excess Na+ ions in the roots [221,222]. Rhizobacteria have been shown to modulate the expression of these transporters during inoculation. Furthermore, by inoculating Zea mays L. with K. rhizophila Y1, the expression of ion affinity transporters (ZmNHX3, ZMNHX2, ZmNHX1, and ZmHKT1) was upregulated, providing protection from salinity stress [281]. Thus, plant–microbe interactions require the tissue-specific regulation of HKT-type genes to maintain ion homeostasis during salt stress [221,222,277]. In addition, another enzyme is capable of acting as a sodium antiporter, the Salt Overlay Sensitive (SOS) gene, which can help plants cope with salinity stress [270]. The inoculation of wheat plants with three bacterial strains (B. tequilensis MPP8, B. megaterium MPP7, and P. putida MPP18) led to a higher expression of SOS1 and SOS4 genes, both of which were associated with an increase in RWC and photosynthetic pigmentation [270]. A PGPB can regulate the exchange of macro- and micronutrients, in addition to reducing the accumulation of Na+ and Cl− ions. First, a number of microbial processes have been shown to improve plant access to these nutrients, including Pi (inorganic phosphate) solubilization and siderophore production [160,283]. Secondly, PGPB inoculation can increase protein phosphatases (associated with Pi solubilization). Researchers have reported that P. simiae AU treatment increased the presence of VSP (vegetative storage protein) in soybean plants under salinity stress, the enzymatic pathway involved in acid phosphatase activity [278]. This affected the plant’s ability to combat oxidative stress by influencing acid phosphatase activity in the lettuce plants colonized by the P. mendocina, Palleroni strain [285]. In addition, microbes can also reduce the uptake of toxic ions by plants by producing ESP, as these compounds act as a physical barrier around the root system, thereby reducing the impact of ion toxicity [159,283,286]. As ESP bind cations, including Na+, they decrease the ability of toxic ions to be absorbed by plants, thereby reducing salinity stress (Figure 3) [60,159,160,286]. ESP-producing bacteria improved the wheat growth parameters and altered the nutrient uptake by improving the Na+ concentration and boosting K+ and Ca2+ absorption in plants affected by salinity [246,287]. Similarly, ESP-producing rhizobacteria also revealed their importance in alleviating the salt stress conditions in several crops, such as wheat, soybean, pistachio, or alfalfa [264,279,288,289].
8. Drought and Salt-Induced Stress-Responsive Gene Regulation
Plants that exhibit induced systemic tolerance may be influenced by PGPR-mediated stress-responsive genes. When inoculated with P. polymyxa, Arabidopsis expresses certain genes, including BAB18 (encoding LEA proteins) and ERD15 (encoding the early response to dehydration) [290]. The inoculation of cucumber plants with a consortium of bacteria, including B. subtilis, B. cereus, and Serratia sp., has been shown to increase drought resistance by preserving photosynthetic activity through inhibition of the downregulation of APX genes and the RuBisCO large and small subunit genes, rbcL and rbcS. In bean plants treated with R. etli, a macroarray analysis of sequence tags revealed an increase in expression of the trehalose-6-phosphate synthase gene, which regulates nitrogen and carbon metabolism [214]. Under water-scarce conditions, B. licheniformis can cause the overexpression of several stress-responsive proteins in pepper plants such as early nodulin, adenosine kinase, dehydrin-like protein, S-adenosylmethionine synthetase, and vacuolar H+-ATPase. Similar effects have been shown by A. brasilense and B. amyloliquefaciens on wheat leaves, which cause the upregulation of APX1, S-adenosylmethionine synthase, and a heat shock protein gene [291]. Sugarcane has been shown to be responsive to ABA-dependent signaling genes activated by G. diazotrophicus [202], and mungbean plants can be stimulated by P. aeruginosa to enhance the expression of DHN, DREB2A, and CAT1 [213]. In Arabidopsis, under salinity stress conditions, Enterobacter sp. induces the expression of salinity stress-responsive genes (RD29 A, RD29 B, and RAB18); regulons of ABA-responsive elements; DREB2B; and dehydration-responsive elements, thereby causing ABA-independent activation (Figure 2) [292]. In A. thaliana, P. chlororaphis induces the transcription and upregulation of jasmonic acid (JA) marker genes (PDF-1.2 and VSP1), the ethylene-responsive gene (HEL), and the SA-regulated gene (PR-1) while downregulating the drought signaling response genes [209,293].
9. Conclusions and Future Prospects
In plants, abiotic stress, including drought and salinity, causes not only environmental problems but also social and economic ones. The changing environmental conditions are negatively affecting plants, causing lower growth and yields. As abiotic stresses intensify, plant cells produce ROS, which impairs the ecological fitness. The crisis will be exacerbated in the coming decades, threatening plant survival. Antioxidants are recruited by plants to maintain equilibrium between ROS quenching and its generation. The plant’s response to these events is so rapid that it cannot withstand adverse conditions. PGPB promote the plant’s growth by various mechanisms such as phosphate solubilization, nutrient mobilization, production of phytohormones, VOCs, vitamins and modulating the antioxidant machinery, osmotic adjustment, maintaining ion homeostasis, metabolic reprogramming, and modulating biochemical and molecular pathways, regulating drought/salt responsive genes and making the crop more tolerant to drought and salinity stress conditions. Thus, PGPB are a good alternative to conventional fertilizers due to cost-effectiveness, eco-friendliness, and sustainability to increase plant tolerance to multiple stresses, including drought and salinity.
PGPBs that may induce plant resistance to a variety of abiotic stressors, particularly those that resemble field environments, should be investigated for future research projects aimed at promoting sustainable agriculture. For the widespread and efficient use of beneficial microbes, scientists need to embark on field studies and make farmers familiar with the benefits that microbes can have on plant health and soil quality. Furthermore, nanoencapsulation technology has just been invented and can be used in field testing. The approach may be used to protect PGPR against abrupt environmental shocks, increase their distribution, and help regulate microbial release in the rhizosphere/field. Additional research is needed to discover if tripartite “plant–fungal–bacterial” symbioses can generate synergistic effects on plants. These approaches appear promising for drought and saline agriculture in the future. With the above information, it is evident that we are on the right path towards achieving our goal of sustainable food production in a climate that keeps changing. Governments and federal agencies need to promote the utilization of PGPB-formulated biofertilizers as eco-friendly alternatives for crop improvement. More investments should be done by entrepreneurs in biofertilizer companies and allocate financial assistance for start-ups. Additionally, public awareness is needed to show farmers and consumers the benefits of PGPB-based biofertilizers for sustainable agriculture.
Abbreviations
| ABA | Abscisic acid |
| ACCD | 1-aminocyclopropane-1-carboxylate deaminase |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| EPS | Extracellular polymeric substances |
| ESP | Exopolysaccharide |
| GPX | Glutathione peroxidase |
| GR | Glutathione reductase |
| H2O2 | Hydrogen peroxidase |
| HEL | Ethylene responsive gene |
| HKT | Sodium transporter |
| IAA | Indole-3-acetic acid |
| K+ | Potassium ion |
| LPX | Lipid peroxidase |
| MDA | Malondialdehyde |
| NHX | Vacuolar Na+/H+ antiporter |
| POX | Peroxidases |
| PRP | Proline-rich protein |
| ROS | Reactive oxygen species |
| RWC | Relative water content |
| SOD | Superoxide dismutase |
| TSS | Total soluble sugar |
| VOCs | Volatile organic compounds |
| VSP1 | Vegetative storage protein |
Author Contributions
Conceptualization, A.G. and M.K.; writing—original draft preparation, A.G., M.K. and M.H.; writing—review and editing, A.G., R.M., S.R., A.B., N.P., M.K., M.F. and M.H.; supervision, M.F. and M.H.; and visualization, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bulgari R., Franzoni G., Ferrante A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy. 2019;9:306. doi: 10.3390/agronomy9060306. [DOI] [Google Scholar]
- 2.Nelson G.C., Valin H., Sands R.D., Havlík P., Ahammad H., Deryng D., Elliott J., Fujimori S., Hasegawa T., Heyhoe E., et al. Climate change effects on agriculture: Economic responses to biophysical shocks. Proc. Natl. Acad. Sci. USA. 2014;111:3274–3279. doi: 10.1073/pnas.1222465110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki N., Rivero R.M., Shulaev V., Blumwald E., Mittler R. Abiotic and biotic stress combinations. New Phytol. 2014;203:32–43. doi: 10.1111/nph.12797. [DOI] [PubMed] [Google Scholar]
- 5.Zandalinas S.I., Balfagón D., Arbona V., Gómez-Cadenas A. Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Front. Plant Sci. 2017;8:953. doi: 10.3389/fpls.2017.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saini P., Gani M., Kaur J.J., Godara L.C., Singh C., Chauhan S.S., Francies R.M., Bhardwaj A., Kumar N.B., Ghosh M.K. Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective. Springer; Singapore: 2018. Reactive Oxygen Species (ROS): A way to stress survival in plants; pp. 127–153. [DOI] [Google Scholar]
- 7.Vargas R., Pankova E.I., Balyuk S.A., Krasilnikov P.V., Khasankhanova G.M. Handbook for Saline Soil Management. FAO/LMSU; Moscow, Russia: 2018. p. 132. [DOI] [Google Scholar]
- 8.Ye Q., Yang X., Dai S., Chen G., Li Y., Zhang C. Effects of climate change on suitable rice cropping areas, cropping systems and crop water requirements in southern China. Agric. Water Manag. 2015;159:35–44. doi: 10.1016/j.agwat.2015.05.022. [DOI] [Google Scholar]
- 9.He M., He C.Q., Ding N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018;871:1771. doi: 10.3389/fpls.2018.01771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachdev S., Ansari S.A., Ansari M.I., Fujita M., Hasanuzzaman M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants. 2021;10:277. doi: 10.3390/antiox10020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pushpavalli R., Berger J.D., Turner N.C., Siddique K.H.M., Colmer T.D., Vadez V. Cross-tolerance for drought, heat and salinity stresses in chickpea (Cicer arietinum L.) J. Agron. Crop Sci. 2020;206:405–419. doi: 10.1111/jac.12393. [DOI] [Google Scholar]
- 12.Forni C., Duca D., Glick B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil. 2017;410:335–356. doi: 10.1007/s11104-016-3007-x. [DOI] [Google Scholar]
- 13.Moore J.P., Vicré-Gibouin M., Farrant J.M., Driouich A. Adaptations of higher plant cell walls to water loss: Drought vs. desiccation. Physiol. Plant. 2008;134:237–245. doi: 10.1111/j.1399-3054.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A., Verma J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018;207:41–52. doi: 10.1016/j.micres.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Maurya A.K. Agronomic Crops. Springer; Singapore: 2020. Oxidative stress in crop plants; pp. 349–380. [DOI] [Google Scholar]
- 16.Shah K., Chaturvedi V., Gupta S. Climate change and abiotic stress-induced oxidative burst in rice. In: Hasanuzzaman M., Fujita M., Nahar K., Biswas J.K., editors. Advances in Rice Research for Abiotic Stress Tolerance. Woodhead Publishing; Cambridge, MA, USA: 2019. pp. 505–535. [DOI] [Google Scholar]
- 17.Raza A., Razzaq A., Mehmood S.S., Zou X., Zhang X., Lv Y., Xu J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A Review. Plants. 2019;8:34. doi: 10.3390/plants8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar M., Giri V.P., Pandey S., Gupta A., Patel M.K., Bajpai A.B., Jenkins S., Siddique K.H.M. Plant-Growth-Promoting Rhizobacteria emerging as an effective bioinoculant to improve the growth, production, and stress tolerance of vegetable crops. Int. J. Mol. Sci. 2021;22:12245. doi: 10.3390/ijms222212245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaushal M., Wani S.P. Plant-Growth-Promoting Rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2016;66:35–42. doi: 10.1007/s13213-015-1112-3. [DOI] [Google Scholar]
- 20.Hasanuzzaman M., Bhuyan M.H.M.B., Zulfiqar F., Raza A., Mohsin S.M., Mahmud J.A., Fujita M., Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9:681. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray S.B., Brady S.M. Plant developmental responses to climate change. Dev. Biol. 2016;419:64–77. doi: 10.1016/j.ydbio.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Wongshaya P., Chayjarung P., Tothong C., Pilaisangsuree V., Somboon T., Kongbangkerd A., Limmongkon A. Effect of light and mechanical stress in combination with chemical elicitors on the production of stilbene compounds and defensive responses in peanut hairy root culture. Plant Physiol. Biochem. 2020;157:93–104. doi: 10.1016/j.plaphy.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Florez-Sarasa I., Fernie A.R., Gupta K.J. Does the alternative respiratory pathway offer protection against the adverse effects resulting from climate change? J. Exp. Bot. 2020;71:465–469. doi: 10.1093/jxb/erz428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhuyan M.H.M.B., Hasanuzzaman M., Parvin K., Mohsin S.M., Mahmud J.A., Nahar K., Fujita M. Nitric oxide and hydrogen sulfide: Two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul. 2020;90:409–424. doi: 10.1007/s10725-020-00594-4. [DOI] [Google Scholar]
- 25.Kumar K., Amaresan N., Madhuri K. Alleviation of the adverse effect of salinity stress by inoculation of Plant Growth Promoting Rhizobacteria isolated from hot humid tropical climate. Ecol. Eng. 2017;102:361–366. doi: 10.1016/j.ecoleng.2017.02.023. [DOI] [Google Scholar]
- 26.Khataar M., Mohhamadi M.H., Shabani F. Soil salinity and matric potential interaction on water use, water use efficiency and yield response factor of bean and wheat. Sci. Rep. 2018;8:2679. doi: 10.1038/s41598-018-20968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mhamdi A., Breusegem F.V. Reactive oxygen species in plant development. Development. 2018;145 doi: 10.1242/dev.164376. [DOI] [PubMed] [Google Scholar]
- 28.Maier J., Hecker R., Rockel P., Ninnemann H. Role of nitric oxide synthase in the light-induced development of sporangiophores in Phycomyces blakesleeanus. Plant Physiol. 2001;126:1323–1330. doi: 10.1104/pp.126.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segal L.M., Wilson R.A. Reactive oxygen species metabolism and plant-fungal interactions. Fungal Genet. Biol. 2018;110:1–9. doi: 10.1016/j.fgb.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Pirasteh-Anosheh H., Saed-Moucheshi A., Pakniyat H., Pessarakli M. Water Stress Crop Plants: A Sustainable Approach. John Wiley & Sons; Hoboken, NJ, USA: 2016. Stomatal responses to drought stress; pp. 24–40. [Google Scholar]
- 31.Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Miller G.A.D., Suzuki N., Ciftci-Yilmaz S., Mittler R.O.N. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant. Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 33.Farnese F.S., Menezes-Silva P.E., Gusman G.S., Oliveira J.A. When bad guys become good ones: The key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front. Plant Sci. 2016;7:471. doi: 10.3389/fpls.2016.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corpas F.J., Barroso J.B. Functions of Nitric Oxide (NO) in roots during development and under adverse stress conditions. Plants. 2015;4:240–252. doi: 10.3390/plants4020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanz L., Albertos P., Mateos I., Sánchez-Vicente I., Lechón T., Fernández-Marcos M., Lorenzo O. Nitric oxide (NO) and phytohormones crosstalk during early plant development. J. Exp. Bot. 2015;66:2857–2868. doi: 10.1093/jxb/erv213. [DOI] [PubMed] [Google Scholar]
- 36.Lü P., Kang M., Jiang X., Dai F., Gao J., Zhang C. RhEXPA4, a rose expansin gene, modulates leaf growth and confers drought and salt tolerance to Arabidopsis. Planta. 2013;237:1547–1559. doi: 10.1007/s00425-013-1867-3. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharyya P., Pathak H., Pal S. Impact of climate change on agriculture: Evidence and predictions. Green Energy Technol. 2020:17–32. doi: 10.1007/978-981-15-9132-7_2. [DOI] [Google Scholar]
- 38.Kumar V., Khare T., Sharma M., Wani S.H. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress. Springer; Singapore: 2017. ROS-induced signaling and gene expression in crops under salinity stress; pp. 159–184. [DOI] [Google Scholar]
- 39.Mehla N., Sindhi V., Josula D., Bisht P., Wani S.H. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress. Springer; Singapore: 2017. An introduction to antioxidants and their roles in plant stress tolerance; pp. 1–23. [DOI] [Google Scholar]
- 40.Gupta A., Bano A., Rai S., Kumar M., Ali J., Sharma S. ACC deaminase producing Plant Growth Promoting Rhizobacteria enhance salinity stress tolerance in Pisum sativum. 3 Biotech. 2021;11:1–17. doi: 10.1007/s13205-021-03047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta A., Rai S., Bano A., Khanam A., Sharma S., Pathak N. Comparative evaluation of different salt-tolerant plant growth-promoting bacterial isolates in mitigating the induced adverse effect of salinity in pisum sativum. Biointerface Res. Appl. Chem. 2021;11:13141–13154. doi: 10.33263/briac115.1314113154. [DOI] [Google Scholar]
- 42.Bano A., Gupta A., Rai S., Sharma S., Pathak N. Elucidation of bioactive potential of two commonly grown north indian Psidium guajava viz., lalit and shweta against pathogenic foodborne and mdr bacteria. Biointerface Res. Appl. Chem. 2021;11:14090–14102. doi: 10.33263/briac116.1409014102. [DOI] [Google Scholar]
- 43.Bano A., Gupta A., Rai S., Fatima T. Plant Stress Physiology—Perspectives in Agriculture. IntechOpen; London, UK: 2021. Mechanistic role of reactive oxygen species and its regulation via the antioxidant system under environmental stress; pp. 1–18. [DOI] [Google Scholar]
- 44.Davies K.M., Jibran R., Zhou Y., Albert N.W., Brummell D.A., Jordan B.R., Bowman J.L., Schwinn K.E. The evolution of flavonoid biosynthesis: A bryophyte perspective. Front. Plant Sci. 2020;11:7. doi: 10.3389/fpls.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agati G., Tattini M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010;186:786–793. doi: 10.1111/j.1469-8137.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- 46.Agati G., Azzarello E., Pollastri S., Tattini M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Williams R.J., Spencer J.P.E., Rice-Evans C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Swain T. Plant flavonoids in biology and medicine. Prog. Clin. Biol. Res. 1986;213:1–14. [PubMed] [Google Scholar]
- 49.Storz G., Imlay J.A. Oxidative stress. Curr. Opin. Microbiol. 1999;2:188–194. doi: 10.1016/S1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 50.Ilangumaran G., Smith D.L. Plant Growth Promoting Rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017;8:1768. doi: 10.3389/fpls.2017.01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 52.Apel K., Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 53.Agrawal A.A., Conner J.K., Stinchcombe J.R. Evolution of plant resistance and tolerance to frost damage. Ecol. Lett. 2004;7:1199–1208. doi: 10.1111/j.1461-0248.2004.00680.x. [DOI] [Google Scholar]
- 54.Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S.M.A. Plant drought stress: Effects, mechanisms and management. Sustain. Agric. 2009:153–188. doi: 10.1007/978-90-481-2666-8_12. [DOI] [Google Scholar]
- 55.Ojuederie O.B., Olanrewaju O.S., Babalola O.O. Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy. 2019;9:712. doi: 10.3390/agronomy9110712. [DOI] [Google Scholar]
- 56.Deinlein U., Stephan A.B., Horie T., Luo W., Xu G., Schroeder J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014;19:371–379. doi: 10.1016/j.tplants.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mushtaq Z., Faizan S., Gulzar B. Salt stress, Its impacts on plants and the strategies plants are employing against it: A review. J. Appl. Biol. Biotechnol. 2020;8:81–91. doi: 10.7324/jabb.2020.80315. [DOI] [Google Scholar]
- 58.Bano A., Fatima M. Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils. 2009;45:405–413. doi: 10.1007/s00374-008-0344-9. [DOI] [Google Scholar]
- 59.Talei D., Kadir M.A., Yusop M.K., Valdiani A., Abdullah M.P. Salinity effects on macro and micronutrients uptake in medicinal plant King of Bitters (Andrographis paniculata Nees.) Plant Omics J. 2012;5:271–278. [Google Scholar]
- 60.Shrivastava P., Kumar R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015;22:123–131. doi: 10.1016/j.sjbs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zandalinas S.I., Rivero R.M., Martínez V., Gómez-Cadenas A., Arbona V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 2016;16:105. doi: 10.1186/s12870-016-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta A., Vandana P. Effect of PGPR isolates on plant growth promotion in relation to salinity stress. Bull. Environ. Pharmacol. Life Sci. 2019;8:18–26. [Google Scholar]
- 63.Gupta A., Bano A., Rai S., Dubey P., Khan F., Pathak N., Sharma S. Plant Growth Promoting Rhizobacteria (PGPR): A sustainable agriculture to rescue the vegetation from the effect of biotic stress: A Review. Lett. Appl. NanoBiosci. 2021;10:2459–2465. doi: 10.33263/lianbs103.24592465. [DOI] [Google Scholar]
- 64.Kumar M., Mishra S., Dixit V., Kumar M., Agarwal L., Chauhan P.S., Nautiyal C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.) Plant Signal. Behav. 2016;11:e1071004. doi: 10.1080/15592324.2015.1071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kranner I., Minibayeva F.V., Beckett R.P., Seal C.E. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010;188:655–673. doi: 10.1111/j.1469-8137.2010.03461.x. [DOI] [PubMed] [Google Scholar]
- 66.Verslues P.E., Zhu J.K. Before and beyond ABA: Upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress. Biochem. Soc. Trans. 2005;33:375–379. doi: 10.1042/BST0330375. [DOI] [PubMed] [Google Scholar]
- 67.Urao T., Yakubov B., Satoh R., Yamaguchi-Shinozaki K., Seki M., Hirayama T., Shinozaki K. A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell. 1999;11:1743–1754. doi: 10.1105/tpc.11.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rolland F., Baena-Gonzalez E., Sheen J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi S., Murata N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008;13:178–182. doi: 10.1016/j.tplants.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 70.Zeevaart J.A.D. Changes in the levels of abscisic acid and its metabolites in excised leaf blades of Xanthium strumarium during and after water stress. Plant Physiol. 1980;66:672–678. doi: 10.1104/pp.66.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim T.H., Böhmer M., Hu H., Nishimura N., Schroeder J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi J., Song C.P., Wang B., Zhou J., Kangasjärvi J., Zhu J.K., Gong Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018;60:805–826. doi: 10.1111/jipb.12654. [DOI] [PubMed] [Google Scholar]
- 73.Raghavendra A.S., Gonugunta V.K., Christmann A., Grill E. ABA perception and signalling. Trends Plant Sci. 2010;15:395–401. doi: 10.1016/j.tplants.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 74.Blumwald E., Poole R.J. Na+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol. 1985;78:163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gong Z., Xiong L., Shi H., Yang S., Herrera-Estrella L.R., Xu G., Chao D.Y., Li J., Wang P.Y., Qin F., et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020;63:635–674. doi: 10.1007/s11427-020-1683-x. [DOI] [PubMed] [Google Scholar]
- 76.Roychowdhury R., Khan M.H., Choudhury S. Advances in Rice Research for Abiotic Stress Tolerance. Elsevier Science; Amsterdam, The Netherlands: Woodhead Publishing; Cambridge, MA, USA: 2019. Physiological and molecular responses for metalloid stress in rice—A comprehensive overview; pp. 341–369. [DOI] [Google Scholar]
- 77.Foyer C.H., Noctor G. Redox signaling in plants. Antioxid. Redox Signal. 2013;18:2087–2090. doi: 10.1089/ars.2013.5278. [DOI] [PubMed] [Google Scholar]
- 78.Janku M., Luhová L., Petrivalský M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants. 2019;8:105. doi: 10.3390/antiox8040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mittler R. ROS Are Good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 80.Wang F., Liu J., Zhou L., Pan G., Li Z., Cheng F. Senescence-specific change in ROS scavenging enzyme activities and regulation of various SOD isozymes to ROS levels in psf mutant rice leaves. Plant Physiol. Biochem. 2016;109:248–261. doi: 10.1016/j.plaphy.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Hasanuzzaman M., Fujita M., Nahar K., Biswas J.K. Advances in Rice Research for Abiotic Stress Tolerance. Woodhead Publishing; Cambridge, MA, USA: 2019. pp. 1–986. [Google Scholar]
- 82.Zhu J.K. Cell signaling under salt, water and cold stresses. Curr. Opin. Plant Biol. 2001;4:401–406. doi: 10.1016/S1369-5266(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 83.Shinozaki K., Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 2000;3:217–223. doi: 10.1016/S1369-5266(00)80068-0. [DOI] [PubMed] [Google Scholar]
- 84.Muller B., Pantin F., Génard M., Turc O., Freixes S., Piques M., Gibon Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011;62:1715–1729. doi: 10.1093/jxb/erq438. [DOI] [PubMed] [Google Scholar]
- 85.Devi R., Kaur N., Gupta A.K. Potential of antioxidant enzymes in depicting drought tolerance of wheat (Triticum aestivum) Indian J. Biochem. Biophys. 2012;49:257–265. [PubMed] [Google Scholar]
- 86.Kaur K., Gupta A.K., Kaur N. Effect of water deficit on carbohydrate status and enzymes of carbohydrate metabolism in seedlings of wheat cultivars. Indian J. Biochem. Biophys. 2007;44:223–230. [PubMed] [Google Scholar]
- 87.Zinta G., Abdelgawad H., Domagalska M.A., Vergauwen L., Knapen D., Nijs I., Janssens I.A., Beemster G.T.S., Asard H. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob. Chang. Biol. 2014;20:3670–3685. doi: 10.1111/gcb.12626. [DOI] [PubMed] [Google Scholar]
- 88.Chaves M.M., Flexas J., Pinheiro C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choudhury F.K., Rivero R.M., Blumwald E., Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017;90:856–867. doi: 10.1111/tpj.13299. [DOI] [PubMed] [Google Scholar]
- 90.Nath M., Bhatt D., Prasad R., Gill S.S., Anjum N.A., Tuteja N. Reactive oxygen species generation-scavenging and signaling during plant-arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front. Plant Sci. 2016;7:1574. doi: 10.3389/fpls.2016.01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Osmolovskaya N., Shumilina J., Kim A., Didio A., Grishina T., Bilova T., Keltsieva O.A., Zhukov V., Tikhonovich I., Tarakhovskaya E., et al. Methodology of drought stress research: Experimental setup and physiological characterization. Int. J. Mol. Sci. 2018;19:4089. doi: 10.3390/ijms19124089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hasanuzzaman M., Hossain M.A., Silva J.A., Fujita M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In: Venkateswarlu B., Shanker A., Shanker C., Maheswari M., editors. Crop Stressand its Management: Perspectives and Strategies. Springer; Dordrecht, The Netherlands: 2012. pp. 261–315. [DOI] [Google Scholar]
- 93.Raja V., Majeed U., Kang H., Andrabi K.I., John R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017;137:142–157. doi: 10.1016/j.envexpbot.2017.02.010. [DOI] [Google Scholar]
- 94.Mattos L.M., Moretti C.L. Oxidative stress in plants under drought conditions and the role of different enzymes. Enzym. Eng. 2015;5:1–6. doi: 10.4172/2329-6674.1000136. [DOI] [Google Scholar]
- 95.Rath K.M., Rousk J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Biol. Biochem. 2015;81:108–123. doi: 10.1016/j.soilbio.2014.11.001. [DOI] [Google Scholar]
- 96.Bui E.N. Soil salinity: A neglected factor in plant ecology and biogeography. J. Arid Environ. 2013;92:14–25. doi: 10.1016/j.jaridenv.2012.12.014. [DOI] [Google Scholar]
- 97.Nejat N., Mantri N. Plant immune system: Crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Curr. Issues Mol. Biol. 2017;23:1–16. doi: 10.21775/cimb.023.001. [DOI] [PubMed] [Google Scholar]
- 98.Flowers T.J., Troke P.F., Yeo A.R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Physiol. 2003;28:89–121. doi: 10.1146/annurev.pp.28.060177.000513. [DOI] [Google Scholar]
- 99.Tester M., Davenport R. Na+ Tolerance and Na+ transport in higher plants. Ann. Bot. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shabala S., Wu H., Bose J. Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant Sci. 2015;241:109–119. doi: 10.1016/j.plantsci.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 101.Ashraf M., Harris P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–16. doi: 10.1016/j.plantsci.2003.10.024. [DOI] [Google Scholar]
- 102.Robles P., Quesada V. Transcriptional and post-transcriptional regulation of organellar gene expression (OGE) and its roles in plant salt tolerance. Int. J. Mol. Sci. 2019;20:1056. doi: 10.3390/ijms20051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan S., Tang Z., Su W., Sun W. Proteomic analysis of salt stress-responsive proteins in rice root. Proteomics. 2005;5:235–244. doi: 10.1002/pmic.200400853. [DOI] [PubMed] [Google Scholar]
- 104.Ji W., Cong R., Li S., Li R., Qin Z., Li Y., Zhou X., Chen S., Li J. Comparative proteomic analysis of soybean leaves and roots by iTRAQ provides insights into response mechanisms to short-term salt stress. Front. Plant Sci. 2016;7:573. doi: 10.3389/fpls.2016.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li W., Zhao F., Fang W., Xie D., Hou J., Yang X., Zhao Y., Tang Z., Nie L., Lv S. Identification of early salt stress responsive proteins in seedling roots of upland cotton (Gossypium hirsutum L.) employing iTRAQ-based proteomic technique. Front. Plant Sci. 2015;6:732. doi: 10.3389/fpls.2015.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rorat T. Plant dehydrins—Tissue location, structure and function. Cell. Mol. Biol. Lett. 2006;11:536–556. doi: 10.2478/s11658-006-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang H., Zhao X., Sun Q., Yan C., Wang J., Yuan C., Li C., Shan S., Liu F. Comparative transcriptome analysis reveals molecular defensive mechanism of Arachis hypogaea L. in response to salt stress. Int. J. Genom. 2020;2020:6524093. doi: 10.1155/2020/6524093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cheng Y., Qi Y., Zhu Q., Chen X., Wang N., Zhao X., Chen H., Cui X., Xu L., Zhang W. New changes in the plasma-membrane-associated proteome of rice roots under salt stress. Proteomics. 2009;9:3100–3114. doi: 10.1002/pmic.200800340. [DOI] [PubMed] [Google Scholar]
- 109.Aghaei K., Ehsanpour A.A., Komatsu S. Proteome analysis of potato under salt stress. J. Proteome Res. 2008;7:4858–4868. doi: 10.1021/pr800460y. [DOI] [PubMed] [Google Scholar]
- 110.Parihar P., Singh S., Singh R., Singh V.P., Prasad S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015;22:4056–4075. doi: 10.1007/s11356-014-3739-1. [DOI] [PubMed] [Google Scholar]
- 111.Huang H., Ullah F., Zhou D.X., Yi M., Zhao Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019;10:800. doi: 10.3389/fpls.2019.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suzuki N., Miller G., Salazar C., Mondal H.A., Shulaev E., Cortes D.F., Shuman J.L., Luo X., Shah J., Schlauch K., et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell. 2013;25:3553–3569. doi: 10.1105/tpc.113.114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hossain M.A., Piyatida P., Da Silva J.A.T., Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012;2012:872875. doi: 10.1155/2012/872875. [DOI] [Google Scholar]
- 114.Hasanuzzaman M., Fujita M. Heavy metals in the environment: Current status, toxic effects on plants and possible phytoremediation. In: Anjum N.A., Pereira M.E., Ahmad I., Duarte A.C., Umar S., Khan N.A., editors. Phytotechnologies: Remediation of Environmental Contaminants. CRC Press; Boca Raton, FL, USA: 2013. pp. 7–73. [Google Scholar]
- 115.Tripathy B.C., Oelmüller R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012;7:1621–1633. doi: 10.4161/psb.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gossett D.R., Millhollon E.P., Lucas M.C. Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci. 1994;34:706–714. doi: 10.2135/cropsci1994.0011183X003400030020x. [DOI] [Google Scholar]
- 117.Mahmud J.A., Bhuyan M.H.M., Anee T.I., Nahar K., Fujita M., Hasanuzzaman M. Reactive oxygen species metabolism and antioxidant defense in plants under metal/metalloid stress. In: Hasanuzzaman M., Hakeem K., Nahar K., Alharby H., editors. Plant Abiotic Stress Tolerance. Springer; Cham, Switzerland: 2019. pp. 221–257. [DOI] [Google Scholar]
- 118.Panda S.K., Choudhury S., Patra H.K. Abiotic Stress Response Plants. Wiley-VCH Verlag GMBH & Co. KGaA; Weinheim, Germany: 2016. Heavy-metal-induced oxidative stress in plants: Physiological and molecular perspectives; pp. 221–236. [DOI] [Google Scholar]
- 119.Mahmud J.A., Hasanuzzaman M., Nahar K., Bhuyan M.H.M.B., Fujita M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 2018;147:990–1001. doi: 10.1016/j.ecoenv.2017.09.045. [DOI] [PubMed] [Google Scholar]
- 120.Telfer A. Singlet oxygen production by PSII under light stress: Mechanism, detection and the protective role of β-carotene. Plant Cell Physiol. 2014;55:1216–1223. doi: 10.1093/pcp/pcu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nonell S., Flors C. Singlet Oxygen: Application in Biosciences and Nanosciences. Volume 1. Royal Society of Chemistry; London, UK: 2016. Properties of singlet oxygen; pp. 23–46. [Google Scholar]
- 122.Bienert G.P., Møller A.L.B., Kristiansen K.A., Schulz A., Møller I.M., Schjoerring J.K., Jahn T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 123.Gechev T.S., Hille J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005;168:17–20. doi: 10.1083/jcb.200409170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bailly C., El-Maarouf-Bouteau H., Corbineau F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008;331:806–814. doi: 10.1016/j.crvi.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 125.Richards S.L., Wilkins K.A., Swarbreck S.M., Anderson A.A., Habib N., Smith A.G., McAinsh M., Davies J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2015;66:37–46. doi: 10.1093/jxb/eru398. [DOI] [PubMed] [Google Scholar]
- 126.Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. Questions and future challenges. Trends Plant Sci. 2004;10:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 127.Zhu J.K. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/S1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 128.Zhu J.K. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hirayama T., Shinozaki K. Perception and transduction of abscisic acid signals: Keys to the function of the versatile plant hormone ABA. Trends Plant Sci. 2007;12:343–351. doi: 10.1016/j.tplants.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 130.Tuteja N., Mahajan S. Calcium signaling network in plants. Plant Signal. Behav. 2007;2:79–85. doi: 10.4161/psb.2.2.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu J. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA. 2000;97:3730–3734. doi: 10.1073/pnas.97.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y., Lv Y., Jahan N., Chen G., Ren D., Guo L. Sensing of abiotic stress and ionic stress responses in plants. Int. J. Mol. Sci. 2018;19:3298. doi: 10.3390/ijms19113298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tuteja N. Mechanisms of high salinity tolerance in plants. Methods Enzymol. 2007;428:419–438. doi: 10.1016/s0076-6879(07)28024-3. [DOI] [PubMed] [Google Scholar]
- 134.Moya J.L., Primo-Millo E., Talon M. Morphological factors determining salt tolerance in citrus seedlings: The shoot to root ratio modulates passive root uptake of chloride ions and their accumulation in leaves. Plant. Cell Environ. 1999;22:1425–1433. doi: 10.1046/j.1365-3040.1999.00495.x. [DOI] [Google Scholar]
- 135.Waśkiewicz A., Gładysz O., Goliński P. Participation of phytohormones in adaptation to salt stress. Plant Horm. Challenging Environ. Factors. 2016:75–115. doi: 10.1007/978-94-017-7758-2_4. [DOI] [Google Scholar]
- 136.Pliego C., Kamilova F., Lugtenberg B. Plant growth-promoting bacteria: Fundamentals and exploitation. Bact. Agrobiol. Crop Ecosyst. 2011:295–343. doi: 10.1007/978-3-642-18357-7_11. [DOI] [Google Scholar]
- 137.Rhodes D., Nadolska-Orczyk A., Rich P.J. Salinity, osmolytes and compatible solutes. In: Läuchli A., Lüttge U., editors. Salinity—Environment—Plants—Molecules. Springer; Dordrecht, The Netherlands: 2002. pp. 181–204. [DOI] [Google Scholar]
- 138.Sun Y., Cheng Z., Glick B.R. The presence of a 1-aminocyclopropane-1-carboxylate (ACC) deaminase deletion mutation alters the physiology of the endophytic plant growth-promoting bacterium Burkholderia phytofirmans PsJN. FEMS Microbiol. Lett. 2009;296:131–136. doi: 10.1111/j.1574-6968.2009.01625.x. [DOI] [PubMed] [Google Scholar]
- 139.Mahajan S., Tuteja N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 140.Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 141.Davenport R., James R.A., Zakrisson-Plogander A., Tester M., Munns R. Control of sodium transport in durum wheat. Plant Physiol. 2005;137:807–818. doi: 10.1104/pp.104.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qiu Q.-S.S., Guo Y., Dietrich M.A., Schumaker K.S., Zhu J.-K.K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA. 2002;99:8436–8441. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shi H., Ishitani M., Kim C., Zhu J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Silva P., Gerós H. Regulation by salt of vacuolar H+-ATPase and H+-pyrophosphatase activities and Na+/H+ exchange. Plant Signal. Behav. 2009;4:718–726. doi: 10.4161/psb.4.8.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Katiyar-Agarwal S., Zhu J., Kim K., Agarwal M., Fu X., Huang A., Zhu J.K. The plasma membrane Na+/H+ antiporter SOS1 interacts with RCD1 and functions in oxidative stress tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006;103:18816–18821. doi: 10.1073/pnas.0604711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dutta D., Esmaili M., Overduin M., Fliegel L. Expression and detergent free purification and reconstitution of the plant plasma membrane Na+/H+ antiporter SOS1 overexpressed in Pichia pastoris. Biochim. Biophys. Acta Biomembr. 2020;1862:183111. doi: 10.1016/j.bbamem.2019.183111. [DOI] [PubMed] [Google Scholar]
- 147.Chai H., Guo J., Zhong Y., Hsu C.C., Zou C., Wang P., Zhu J.K., Shi H. The plasma-membrane polyamine transporter PUT3 is regulated by the Na+/H+ antiporter SOS1 and protein kinase SOS2. New Phytol. 2020;226:785–797. doi: 10.1111/nph.16407. [DOI] [PubMed] [Google Scholar]
- 148.Pardo J.M., Cubero B., Leidi E.O., Quintero F.J. Alkali cation exchangers: Roles in cellular homeostasis and stress tolerance. J. Exp. Bot. 2006;57:1181–1199. doi: 10.1093/jxb/erj114. [DOI] [PubMed] [Google Scholar]
- 149.Berthomieu P., Conéjéro G., Nublat A., Brackenbury W.J., Lambert C., Savio C., Uozumi N., Oiki S., Yamada K., Cellier F., et al. Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J. 2003;22:2004–2014. doi: 10.1093/emboj/cdg207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Munns R., James R.A., Läuchli A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006;57:1025–1043. doi: 10.1093/jxb/erj100. [DOI] [PubMed] [Google Scholar]
- 151.Khan T.A., Mazid M., Quddusi S. Approaches to Plant Stress and Their Management. Springer; New Delhi, India: 2014. Role of organic and inorganic chemicals in plant-stress mitigation; pp. 39–52. [DOI] [Google Scholar]
- 152.Teotia S., Singh D. Approaches to Plant Stress and Their Management. Springer; New Delhi, India: 2014. Oxidative stress in plants and its management; pp. 227–253. [DOI] [Google Scholar]
- 153.Christou A., Manganaris G.A., Papadopoulos I., Fotopoulos V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013;64:1953–1966. doi: 10.1093/jxb/ert055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang H., Zhang D., Li H., Dong L., Lan H. Ectopic overexpression of the aldehyde dehydrogenase ALDH21 from Syntrichia caninervis in tobacco confers salt and drought stress tolerance. Plant Physiol. Biochem. 2015;95:83–91. doi: 10.1016/j.plaphy.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 155.Kotchoni S.O., Kuhns C., Ditzer A., Kirch H.H., Bartels D. Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant Cell Environ. 2006;29:1033–1048. doi: 10.1111/j.1365-3040.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 156.Xu X., Guo R., Cheng C., Zhang H., Zhang Y., Wang X. Overexpression of ALDH2B8, an aldehyde dehydrogenase gene from grapevine, sustains Arabidopsis growth upon salt stress and protects plants against oxidative stress. Plant Cell. Tissue Organ Cult. 2013;114:187–196. doi: 10.1007/s11240-013-0314-2. [DOI] [Google Scholar]
- 157.Vinocur B., Altman A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005;16:123–132. doi: 10.1016/j.copbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 158.Raza A., Ashraf F., Zou X., Zhang X., Tosif H. Plant adaptation and tolerance to environmental stresses: Mechanisms and perspectives. In: Hasanuzzaman M., editor. Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives. Springer; Singapore: 2020. pp. 117–145. [DOI] [Google Scholar]
- 159.Etesami H., Adl S.M. Can interaction between silicon and non–rhizobial bacteria help in improving nodulation and nitrogen fixation in salinity–stressed legumes? A review. Rhizosphere. 2020;15:100229. doi: 10.1016/j.rhisph.2020.100229. [DOI] [Google Scholar]
- 160.Dodd I.C., Pérez-Alfocea F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012;63:3415–3428. doi: 10.1093/jxb/ers033. [DOI] [PubMed] [Google Scholar]
- 161.Khan N., Bano A., Shahid M.A., Nasim W., Babar M.A. Interaction between PGPR and PGR for water conservation and plant growth attributes under drought condition. Biologia. 2018;73:1083–1098. doi: 10.2478/s11756-018-0127-1. [DOI] [Google Scholar]
- 162.Ayuso-Calles M., García-Estévez I., Jiménez-Gómez A., Flores-Félix J.D., Escribano-Bailón M.T., Rivas R. Rhizobium laguerreae improves productivity and phenolic compound content of lettuce (Lactuca sativa L.) under saline stress conditions. Foods. 2020;9:1166. doi: 10.3390/foods9091166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jiménez-Gómez A., García-Estévez I., García-Fraile P., Escribano-Bailón M.T., Rivas R. Increase in phenolic compounds of Coriandrum sativum L. after the application of a Bacillus halotolerans biofertilizer. J. Sci. Food Agric. 2020;100:2742–2749. doi: 10.1002/jsfa.10306. [DOI] [PubMed] [Google Scholar]
- 164.Goswami D., Dhandhukia P., Patel P., Thakker J.N. Screening of PGPR from saline desert of kutch: Growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol. Res. 2014;169:66–75. doi: 10.1016/j.micres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 165.Armada E., Probanza A., Roldán A., Azcón R. Native plant growth promoting bacteria Bacillus thuringiensis and mixed or individual mycorrhizal species improved drought tolerance and oxidative metabolism in Lavandula dentata plants. J. Plant Physiol. 2016;192:1–12. doi: 10.1016/j.jplph.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 166.Santana S.R.A., Voltolini T.V., Antunes G.D.R., da Silva V.M., Simões W.L., Morgante C.V., de Freitas A.D.S., de Melo Chaves A.R., Aidar S.D.T., Fernandes-Júnior P.I. Inoculation of Plant Growth-Promoting Bacteria attenuates the negative effects of drought on sorghum. Arch. Microbiol. 2020;202:1015–1024. doi: 10.1007/s00203-020-01810-5. [DOI] [PubMed] [Google Scholar]
- 167.Mahanty T., Bhattacharjee S., Goswami M., Bhattacharyya P., Das B., Ghosh A., Tribedi P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2016;24:3315–3335. doi: 10.1007/s11356-016-8104-0. [DOI] [PubMed] [Google Scholar]
- 168.Hirel B., Tétu T., Lea P.J., Dubois F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability. 2011;3:1452–1485. doi: 10.3390/su3091452. [DOI] [Google Scholar]
- 169.Yuwono T., Handayani D., Soedarsono J., Yuwono T., Handayani D., Soedarsono J. The role of osmotolerant rhizobacteria in rice growth under different drought conditions. Aust. J. Agric. Res. 2005;56:715–721. doi: 10.1071/AR04082. [DOI] [Google Scholar]
- 170.Grichko V.P., Glick B.R. Amelioration of flooding stress by ACC deaminase-containing Plant Growth-Promoting Bacteria. Plant Physiol. Biochem. 2001;39:11–17. doi: 10.1016/S0981-9428(00)01212-2. [DOI] [Google Scholar]
- 171.Mayak S., Tirosh T., Glick B.R. Plant Growth-Promoting Bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004;42:565–572. doi: 10.1016/j.plaphy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 172.Casanovas E.M., Barassi C.A., Sueldo R.J. Azospiriflum inoculation mitigates water stress effects in maize seedlings. Cereal Res. Commun. 2002;30:343–350. doi: 10.1007/BF03543428. [DOI] [Google Scholar]
- 173.Creus C.M., Sueldo R.J., Barassi C.A. Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field. Can. J. Bot. 2011;82:273–281. doi: 10.1139/b03-119. [DOI] [Google Scholar]
- 174.Yang Y., Guan H., Batelaan O., McVicar T.R., Long D., Piao S., Liang W., Liu B., Jin Z., Simmons C.T. Contrasting responses of water use efficiency to drought across global terrestrial ecosystems. Sci. Rep. 2016;6:23284. doi: 10.1038/srep23284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Abdelaal K., Alkahtani M., Attia K., Hafez Y., Király L., Künstler A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology. 2021;10:520. doi: 10.3390/biology10060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Khanghahi M.Y., Crecchio C., Verbruggen E. Shifts in the rhizosphere and endosphere colonizing bacterial communities under drought and salinity stress as affected by a biofertilizer consortium. Microb. Ecol. 2021:1–13. doi: 10.1007/s00248-021-01856-y. [DOI] [PubMed] [Google Scholar]
- 177.Vanhaverbeke C., Heyraud A., Mazeau K. Conformational analysis of the exopolysaccharide from Burkholderia caribensis strain MWAP71: Impact on the interaction with soils. Biopolymers. 2003;69:480–497. doi: 10.1002/bip.10432. [DOI] [PubMed] [Google Scholar]
- 178.Pal A., Sharma S. Excess molar volumes and viscosities of binary liquid mixtures of ethylene glycol dimethyl ether + ethylene glycol monomethyl, + diethylene glycol monomethyl, and + triethylene glycol monomethyl ethers at 298.15 and 308.15 K. J. Chem. Eng. Data. 1999;44:212–215. doi: 10.1021/je980215c. [DOI] [Google Scholar]
- 179.Khan M.I.R., Fatma M., Per T.S., Anjum N.A., Khan N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015;6:462. doi: 10.3389/fpls.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Khan A., Khan A.L., Imran M., Asaf S., Kim Y.-H., Bilal S., Numan M., Al-Harrasi A., Al-Rawahi A., Lee I.-J. Silicon-induced thermotolerance in Solanum lycopersicum L. via activation of antioxidant system, heat shock proteins, and endogenous phytohormones. BMC Plant Biol. 2020;20:248. doi: 10.1186/s12870-020-02456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.El-Daim I.A.A., Bejai S., Meijer J. Bacillus velezensis 5113 Induced metabolic and molecular reprogramming during abiotic stress tolerance in wheat. Sci. Rep. 2019;9:16282. doi: 10.1038/s41598-019-52567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Asghari B., Khademian R., Sedaghati B. Plant Growth Promoting Rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. 2020;263:109132. doi: 10.1016/j.scienta.2019.109132. [DOI] [Google Scholar]
- 183.Khan N., Bano A., Rahman M.A., Guo J., Kang Z., Babar M.A. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019;9:2097. doi: 10.1038/s41598-019-38702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shakir M.A., Bano A., Arshad M. Rhizosphere bacteria containing ACC-deaminase conferred drought tolerance in wheat grown under semi-arid climate. Soil Environ. 2012;31:108–112. [Google Scholar]
- 185.Alavi P., Starcher M.R., Zachow C., Müller H., Berg G. Root-microbe systems: The effect and mode of interaction of stress protecting agent (SPA) Stenotrophomonas rhizophila DSM14405T. Front. Plant Sci. 2013;4:141. doi: 10.3389/fpls.2013.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Belimov A.A., Dodd I.C., Safronova V.I., Shaposhnikov A.I., Azarova T.S., Makarova N.M., Davies W.J., Tikhonovich I.A. Rhizobacteria that produce auxins and contain 1-amino-cyclopropane-1-carboxylic acid deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum) Ann. Appl. Biol. 2015;167:11–25. doi: 10.1111/aab.12203. [DOI] [Google Scholar]
- 187.Khan Z., Rho H., Firrincieli A., Hung S.H., Luna V., Masciarelli O., Kim S.H., Doty S.L. Growth enhancement and drought tolerance of hybrid poplar upon inoculation with endophyte consortia. Curr. Plant Biol. 2016;6:38–47. doi: 10.1016/j.cpb.2016.08.001. [DOI] [Google Scholar]
- 188.Arzanesh M.H., Alikhani H.A., Khavazi K., Rahimian H.A., Miransari M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2011;27:197–205. doi: 10.1007/s11274-010-0444-1. [DOI] [Google Scholar]
- 189.Silambarasan S., Logeswari P., Cornejo P., Kannan V.R. Role of plant growth–promoting rhizobacterial consortium in improving the Vigna radiata growth and alleviation of aluminum and drought stresses. Environ. Sci. Pollut. Res. 2019;26:27647–27659. doi: 10.1007/s11356-019-05939-9. [DOI] [PubMed] [Google Scholar]
- 190.Armada E., Roldán A., Azcon R. Differential activity of autochthonous bacteria in controlling drought stress in native lavandula and salvia plants species under drought conditions in natural arid soil. Microb. Ecol. 2014;67:410–420. doi: 10.1007/s00248-013-0326-9. [DOI] [PubMed] [Google Scholar]
- 191.Wang C.J., Yang W., Wang C., Gu C., Niu D.-D., Liu H.-X., Wang Y.-P., Guo J.-H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE. 2012;7:e52565. doi: 10.1371/journal.pone.0052565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Marulanda A., Porcel R., Barea J.M., Azcó R. Microbial ecology drought tolerance and antioxidant activities in lavender plants colonized by native drought-tolerant or drought-sensitive glomus species. Microb. Ecol. 2007;54:543–552. doi: 10.1007/s00248-007-9237-y. [DOI] [PubMed] [Google Scholar]
- 193.Zhou C., Ma Z., Zhu L., Xiao X., Xie Y., Zhu J., Wang J. Rhizobacterial strain Bacillus megaterium BOFC15 induces cellular polyamine changes that improve plant growth and drought resistance. Int. J. Mol. Sci. 2016;17:976. doi: 10.3390/ijms17060976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shintu P.V., Jayaram K.M. Phosphate solubilising bacteria (Bacillus polymyxa)-An effective approach to mitigate drought in tomato (Lycopersicon esculentum Mill.) Trop. Plant Res. 2015;2:17–22. [Google Scholar]
- 195.Grover M., Ali S.Z., Sandhya V., Rasul A., Venkateswarlu B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2010;27:1231–1240. doi: 10.1007/s11274-010-0572-7. [DOI] [Google Scholar]
- 196.Zhang H., Murzello C., Sun Y., Kim M.S., Xie X., Jeter R.M., Zak J.C., Dowd S.E., Paré P.W. Choline and osmotic-stress tolerance induced in Arabidopsis by the soil microbe Bacillus subtilis (GB03) Mol. Plant-Microbe Interact. 2010;23:1097–1104. doi: 10.1094/MPMI-23-8-1097. [DOI] [PubMed] [Google Scholar]
- 197.Timmusk S., El-Daim I.A.A., Copolovici L., Tanilas T., Kännaste A., Behers L., Nevo E., Seisenbaeva G., Stenström E., Niinemets Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE. 2014;9:e96086. doi: 10.1371/journal.pone.0096086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Naveed M., Hussain M.B., Zahir Z.A., Mitter B., Sessitsch A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul. 2014;73:121–131. doi: 10.1007/s10725-013-9874-8. [DOI] [Google Scholar]
- 199.Gusain Y.S., Singh U.S., Sharma A.K. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.) Afr. J. Biotechnol. 2015;14:764–773. doi: 10.4314/ajb.v14i9. [DOI] [Google Scholar]
- 200.Sharma P., Khanna V., Kumari P. Efficacy of aminocyclopropane-1-carboxylic acid (ACC)-deaminase-producing rhizobacteria in ameliorating water stress in chickpea under axenic conditions. Afr. J. Microbiol. Res. 2013;7:5749–5757. doi: 10.5897/ajmr2013.5918. [DOI] [Google Scholar]
- 201.Zhang G., Sun Y., Sheng H., Li H., Liu X. Effects of the inoculations using bacteria producing ACC deaminase on ethylene metabolism and growth of wheat grown under different soil water contents. Plant Physiol. Biochem. 2018;125:178–184. doi: 10.1016/j.plaphy.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 202.Vargas L., Brígida A.B.S., Filho J.P.M., De Carvalho T.G., Rojas C.A., Vaneechoutte D., Van Bel M., Farrinelli L., Ferreira P.C.G., Vandepoele K., et al. Drought tolerance conferred to sugarcane by association with Gluconacetobacter diazotrophicus: A transcriptomic view of hormone pathways. PLoS ONE. 2014;9:e114744. doi: 10.1371/journal.pone.0114744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Saikia J., Sarma R.K., Dhandia R., Yadav A., Bharali R., Gupta V.K., Saikia R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018;8:3560. doi: 10.1038/s41598-018-21921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arshad M., Shaharoona B., Mahmood T. Inoculation with Pseudomonas spp. containing ACC-deaminase partially eliminates the effects of drought stress on growth, yield, and ripening of pea (Pisum sativum L.) Pedosphere. 2008;18:611–620. doi: 10.1016/S1002-0160(08)60055-7. [DOI] [Google Scholar]
- 205.Kang S.M., Khan A.L., Waqas M., You Y.H., Kim J.H.J.G., Kim J.H.J.G., Hamayun M., Lee I.J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014;9:673–682. doi: 10.1080/17429145.2014.894587. [DOI] [Google Scholar]
- 206.Kang S.M., Radhakrishnan R., Khan A.L., Kim M.J., Park J.M., Kim B.R., Shin D.H., Lee I.J. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014;84:115–124. doi: 10.1016/j.plaphy.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 207.Timmusk S., Wagner E.G.H. The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: A possible connection between biotic and abiotic stress responses. Mol. Plant-Microbe Interact. 1999;12:951–959. doi: 10.1094/MPMI.1999.12.11.951. [DOI] [PubMed] [Google Scholar]
- 208.Bresson J., Varoquaux F., Bontpart T., Touraine B., Vile D. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 2013;200:558–569. doi: 10.1111/nph.12383. [DOI] [PubMed] [Google Scholar]
- 209.Cho S.M., Kang B.R., Kim Y.C. Transcriptome analysis of induced systemic drought tolerance elicited by Pseudomonas chlororaphis O6 in Arabidopsis thaliana. Plant Pathol. J. 2013;29:209–220. doi: 10.5423/PPJ.SI.07.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zahir Z.A., Munir A., Asghar H.N., Shaharoona B., Arshad M. Effectiveness of rhizobacteria containing ACC deaminase for growth promotion of peas (Pisum sativum) under drought conditions. J. Microbiol. Biotechnol. 2008;18:958–963. [PubMed] [Google Scholar]
- 211.Tiwari S., Lata C., Chauhan P.S., Nautiyal C.S. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol. Biochem. 2016;99:108–117. doi: 10.1016/j.plaphy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 212.Sandhya V.Z.A.S., SK Z.A., Grover M., Reddy G., Venkateswarlu B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-p45. Biol. Fertil. Soils. 2009;46:17–26. doi: 10.1007/s00374-009-0401-z. [DOI] [Google Scholar]
- 213.Sarma R.K., Saikia R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil. 2014;377:111–126. doi: 10.1007/s11104-013-1981-9. [DOI] [Google Scholar]
- 214.Suárez R., Wong A., Ramírez M., Barraza A., Orozco M.D.C., Cevallos M.A., Lara M., Hernández G., Iturriaga G. Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol. Plant-Microbe Interact. 2008;21:958–966. doi: 10.1094/MPMI-21-7-0958. [DOI] [PubMed] [Google Scholar]
- 215.Hussain M.B., Hussain M.B., Zahir Z.A., Asghar H.N., Asgher M. Can catalase and exopolysaccharides producing rhizobia ameliorate drought stress in wheat? Int. J. Agri. Biol. 2014;16:3–13. [Google Scholar]
- 216.Belimov A.A., Dodd I.C., Hontzeas N., Theobald J.C., Safronova V.I., Davies W.J. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol. 2009;181:413–423. doi: 10.1111/j.1469-8137.2008.02657.x. [DOI] [PubMed] [Google Scholar]
- 217.Ullah A., Mushtaq H., Fahad S., Hakim, Shah A., Chaudhary H.J. Plant growth promoting potential of bacterial endophytes in novel association with Olea ferruginea and Withania coagulans. Microbiology. 2017;86:119–127. doi: 10.1134/S0026261717010155. [DOI] [Google Scholar]
- 218.Subramanian S., Ricci E., Souleimanov A., Smith D.L. A proteomic approach to lipo-chitooligosaccharide and thuricin 17 effects on soybean germination unstressed and salt stress. PLoS ONE. 2016;11:e0160660. doi: 10.1371/journal.pone.0160660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Oldroyd G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013;11:252–263. doi: 10.1038/nrmicro2990. [DOI] [PubMed] [Google Scholar]
- 220.Moretti L.G., Crusciol C.A.C., Bossolani J.W., Momesso L., Garcia A., Kuramae E.E., Hungria M. Bacterial consortium and microbial metabolites increase grain quality and soybean yield. J. Soil Sci. Plant Nutr. 2020;20:1923–1934. doi: 10.1007/s42729-020-00263-5. [DOI] [Google Scholar]
- 221.Zhang H., Kim M.-S.S., Sun Y., Dowd S.E., Shi H., Paré P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant-Microbe Interact. 2008;21:737–744. doi: 10.1094/MPMI-21-6-0737. [DOI] [PubMed] [Google Scholar]
- 222.Pinedo I., Ledger T., Greve M., Poupin M.J. Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front. Plant Sci. 2015;6:1–17. doi: 10.3389/fpls.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wu Y., Sharp R.E., Durachko D.M., Cosgrove D.J. Growth Maintenance of the Maize Primary Root at Low Water Potentials Involves Increases in Cell-Wall Extension Properties, Expansin Activity, and Wall Susceptibility to Expansins. Plant Physiol. 1996;111:765–772. doi: 10.1104/pp.111.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shoji T., Suzuki K., Abe T., Kaneko Y., Shi H., Zhu J.K., Rus A., Hasegawa P.M., Hashimoto T. Salt stress affects cortical microtubule organization and helical growth in Arabidopsis. Plant Cell Physiol. 2006;47:1158–1168. doi: 10.1093/pcp/pcj090. [DOI] [PubMed] [Google Scholar]
- 225.Huang G.-T., Ma S.-L., Bai L.-P., Zhang L., Ma H., Jia P., Liu J., Zhong M., Guo Z.-F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2011;39:969–987. doi: 10.1007/s11033-011-0823-1. [DOI] [PubMed] [Google Scholar]
- 226.Singh R.P., Runthala A., Khan S., Jha P.N. Quantitative proteomics analysis reveals the tolerance of wheat to salt stress in response to Enterobacter cloacae SBP-8. PLoS ONE. 2017;12:e0183513. doi: 10.1371/journal.pone.0183513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nakashima K., Ito Y., Yamaguchi-Shinozaki K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 2009;149:88–95. doi: 10.1104/pp.108.129791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhao Y., Dong W., Zhang N., Ai X., Wang M., Huang Z., Xiao L., Xia G. A wheat allene oxide cyclase gene enhances salinity tolerance via jasmonate signaling. Plant Physiol. 2014;164:1068–1076. doi: 10.1104/pp.113.227595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ullah A., Manghwar H., Shaban M., Khan A.H., Akbar A., Ali U., Ali E., Fahad S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018;25:33103–33118. doi: 10.1007/s11356-018-3364-5. [DOI] [PubMed] [Google Scholar]
- 230.Finkel O.M., Castrillo G., Paredes S.H., González I.S., Dangl J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017;38:155–163. doi: 10.1016/j.pbi.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Di Benedetto N.A., Corbo M.R., Campaniello D., Cataldi M.P., Bevilacqua A., Sinigaglia M., Flagella Z. The role of Plant Growth Promoting Bacteria in improving nitrogen use efficiency for sustainable crop production: A focus on wheat. AIMS Microbiol. 2017;3:413–434. doi: 10.3934/microbiol.2017.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chauhan H., Bagyaraj D.J., Selvakumar G., Sundaram S.P. Novel Plant Growth Promoting Rhizobacteria—Prospects and potential. Appl. Soil Ecol. 2015;95:38–53. doi: 10.1016/j.apsoil.2015.05.011. [DOI] [Google Scholar]
- 233.Glick B.R., Karaturovic D.M., Newell P.C. A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can. J. Microbiol. 1995;41:533–536. doi: 10.1139/m95-070. [DOI] [Google Scholar]
- 234.Yang J., Kloepper J.W., Ryu C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14:1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 235.Yildirim E., Turan M., Guvenc I. Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. J. Plant Nutr. 2008;31:593–612. doi: 10.1080/01904160801895118. [DOI] [Google Scholar]
- 236.Figueiredo M.V.B., Burity H.A., Martínez C.R., Chanway C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008;40:182–188. doi: 10.1016/j.apsoil.2008.04.005. [DOI] [Google Scholar]
- 237.Nadeem S.M., Zahir Z.A., Naveed M., Arshad M. Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can. J. Microbiol. 2007;53:1141–1149. doi: 10.1139/W07-081. [DOI] [PubMed] [Google Scholar]
- 238.Abd M., Hamdia E.-S., Shaddad M.A.K., Doaa M.M. Mechanisms of salt tolerance and interactive effects of Azospirillum brasilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul. 2004;44:165–174. doi: 10.1023/b:grow.0000049414.03099.9b. [DOI] [Google Scholar]
- 239.M’piga P., Bélanger R.R., Paulitz T.C., Benhamou N. Increased resistance to Fusarium oxysporum f. sp. radicis-lycopersiciin tomato plants treated with the endophytic bacterium Pseudomonas fluorescens strain 63-28. Physiol. Mol. Plant Pathol. 1997;50:301–320. doi: 10.1006/pmpp.1997.0088. [DOI] [Google Scholar]
- 240.Cho H.T., Hong Y.N. Effect of IAA on synthesis and activity of the plasma membrane H+-ATPase of sunflower hypocotyls, in relation to IAA-induced cell elongation and H+ Excretion. J. Plant Physiol. 1995;145:717–725. doi: 10.1016/S0176-1617(11)81286-1. [DOI] [Google Scholar]
- 241.Egamberdieva D., Kucharova Z. Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol. Fertil. Soils. 2009;45:563–571. doi: 10.1007/s00374-009-0366-y. [DOI] [Google Scholar]
- 242.Verma J.P., Jaiswal D.K., Meena V.S., Kumar A., Meena R.S. Issues and challenges about sustainable agriculture production for management of natural resources to sustain soil fertility and health. J. Clean. Prod. 2015:793–794. doi: 10.1016/j.jclepro.2015.04.130. [DOI] [Google Scholar]
- 243.Gupta J., Rathour R., Singh R., Thakur I.S. Production and characterization of extracellular polymeric substances (EPS) generated by a carbofuran degrading strain Cupriavidus sp. ISTL7. Bioresour. Technol. 2019;282:417–424. doi: 10.1016/j.biortech.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 244.Vurukonda S.S.K.P., Vardharajula S., Shrivastava M., SkZ A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 245.Upadhyay S.K., Singh J.S., Singh D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere. 2011;21:214–222. doi: 10.1016/S1002-0160(11)60120-3. [DOI] [Google Scholar]
- 246.Ashraf M., Hasnain S., Berge O., Mahmood T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils. 2004;40:157–162. doi: 10.1007/s00374-004-0766-y. [DOI] [Google Scholar]
- 247.Nishanth S., Bharti A., Gupta H., Gupta K., Gulia U., Prasanna R. Cyanobacterial extracellular polymeric substances (EPS): Biosynthesis and their potential applications. Microb. Nat. Macromol. 2021:349–369. doi: 10.1016/b978-0-12-820084-1.00015-6. [DOI] [Google Scholar]
- 248.Ansari F.A., Ahmad I. Plant growth promoting attributes and alleviation of salinity stress to wheat by biofilm forming Brevibacterium sp. FAB3 isolated from rhizospheric soil. Saudi J. Biol. Sci. 2018 doi: 10.1016/j.sjbs.2018.08.003. [DOI] [Google Scholar]
- 249.Naseem H., Bano A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014;9:689–701. doi: 10.1080/17429145.2014.902125. [DOI] [Google Scholar]
- 250.Zhang Z.J., Chen S.H., Wang S.M., Luo H.Y. Characterization of extracellular polymeric substances from biofilm in the process of starting-up a partial nitrification process under salt stress. Appl. Microbiol. Biotechnol. 2011;89:1563–1571. doi: 10.1007/s00253-010-2947-y. [DOI] [PubMed] [Google Scholar]
- 251.Zheng D., Chang Q., Li Z., Gao M., She Z., Wang X., Guo L., Zhao Y., Jin C., Gao F. Performance and microbial community of a sequencing batch biofilm reactor treating synthetic mariculture wastewater under long-term exposure to norfloxacin. Bioresour. Technol. 2016;222:139–147. doi: 10.1016/j.biortech.2016.09.114. [DOI] [PubMed] [Google Scholar]
- 252.Mukhopadhyay R., Sarkar B., Jat H.S., Sharma P.C., Bolan N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021;280:111736. doi: 10.1016/j.jenvman.2020.111736. [DOI] [PubMed] [Google Scholar]
- 253.Zulfiqar F., Akram N.A., Ashraf M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta. 2020;251:1–17. doi: 10.1007/s00425-019-03293-1. [DOI] [PubMed] [Google Scholar]
- 254.Hooshdar P., Kermanshahi R.K., Ghadam P., Khosravi-Darani K. A Review on production of exopolysaccharide and biofilm in probiotics like lactobacilli and methods of analysis. Biointerface Res. Appl. Chem. 2020;10:6058–6075. doi: 10.33263/BRIAC105.60586075. [DOI] [Google Scholar]
- 255.Gupta P., Diwan B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017;13:58–71. doi: 10.1016/j.btre.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Egamberdieva D., Wirth S., Bellingrath-Kimura S.D., Mishra J., Arora N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019;10:2791. doi: 10.3389/fmicb.2019.02791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Martens D.A., Frankenberger W.T. Decomposition of bacterial polymers in soil and their influence on soil structure. Biol. Fertil. Soils. 1992;13:65–73. doi: 10.1007/BF00337337. [DOI] [Google Scholar]
- 258.Rashid M.I., Mujawar L.H., Shahzad T., Almeelbi T., Ismail I.M.I., Oves M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016;183:26–41. doi: 10.1016/j.micres.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 259.Kohler J., Caravaca F., Carrasco L., Roldán A. Contribution of Pseudomonas mendocina and Glomus intraradices to aggregate stabilization and promotion of biological fertility in rhizosphere soil of lettuce plants under field conditions. Soil Use Manag. 2006;22:298–304. doi: 10.1111/j.1475-2743.2006.00041.x. [DOI] [Google Scholar]
- 260.Qurashi A.W., Sabri A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012;43:1183–1191. doi: 10.1590/S1517-83822012000300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dodd I.C., Zinovkina N.Y., Safronova V.I., Belimov A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010;157:361–379. doi: 10.1111/j.1744-7348.2010.00439.x. [DOI] [Google Scholar]
- 262.Ben Salah I., Albacete A., Messedi D., Gandour M., Andújar C.M., Zribi K., Martinez V., Abdelly C., Pérez-Alfocea F. Hormonal responses of nodulated Medicago ciliaris lines differing in salt tolerance. Environ. Exp. Bot. 2013;86:35–43. doi: 10.1016/j.envexpbot.2011.04.013. [DOI] [Google Scholar]
- 263.Mahantappa S., Singh N.B., Sanjeev T., Saresh N.V., Archana V. Application of RAPD and ISSR markers for fingerprinting of promising myrobalan accessions (Terminalia Chebula Retz.): An indigenous minor agroforestry tree species. Indian J. Ecol. 2017;44:13–20. [Google Scholar]
- 264.Upadhyay S.K., Singh D.P. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015;17:288–293. doi: 10.1111/plb.12173. [DOI] [PubMed] [Google Scholar]
- 265.Porcel R., Aroca R., Ruiz-Lozano J.M. Salinity stress alleviation using Arbuscular Mycorrhizal Fungi. A review. Agron. Sustain. Dev. 2012;32:181–200. doi: 10.1007/s13593-011-0029-x. [DOI] [Google Scholar]
- 266.Rodríguez-Salazar J., Suárez R., Caballero-Mellado J., Iturriaga G. Trehalose accumulation in Azospirillum brasilense improves drought tolerance and biomass in maize plants. FEMS Microbiol. Lett. 2009;296:52–59. doi: 10.1111/j.1574-6968.2009.01614.x. [DOI] [PubMed] [Google Scholar]
- 267.Barka E.A., Nowak J., Clément C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl. Environ. Microbiol. 2006;72:7246–7252. doi: 10.1128/AEM.01047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sziderics A.H., Rasche F., Trognitz F., Sessitsch A., Wilhelm E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.) Can. J. Microbiol. 2007;53:1195–1202. doi: 10.1139/W07-082. [DOI] [PubMed] [Google Scholar]
- 269.Vardharajula S., Ali S.Z., Grover M., Reddy G., Bandi V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011;6:1–14. doi: 10.1080/17429145.2010.535178. [DOI] [Google Scholar]
- 270.Haroon U., Khizar M., Liaquat F., Ali M., Akbar M., Tahir K., Batool S.S., Kamal A., Chaudhary H.J., Munis M.F.H. Halotolerant plant growth-promoting rhizobacteria induce salinity tolerance in wheat by enhancing the expression of SOS genes. J. Plant Growth Regul. 2021:1–14. doi: 10.1007/s00344-021-10457-5. [DOI] [Google Scholar]
- 271.Sapre S., Gontia-Mishra I., Tiwari S. Plant growth-promoting rhizobacteria ameliorates salinity stress in pea (Pisum sativum) J. Plant Growth Regul. 2021;41:647–656. doi: 10.1007/s00344-021-10329-y. [DOI] [Google Scholar]
- 272.Orozco-Mosqueda M.D.C., Duan J., DiBernardo M., Zetter E., Campos-García J., Glick B.R., Santoyo G. The production of ACC deaminase and trehalose by the plant growth promoting bacterium Pseudomonas sp. UW4 synergistically protect tomato plants against salt stress. Front. Microbiol. 2019;10:1392. doi: 10.3389/fmicb.2019.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Groppa M.D., Benavides M.P., Zawoznik M.S. Root hydraulic conductance, aquaporins and plant growth promoting microorganisms: A revision. Appl. Soil Ecol. 2012;61:247–254. doi: 10.1016/j.apsoil.2011.11.013. [DOI] [Google Scholar]
- 274.Marulanda A., Azcón R., Chaumont F., Ruiz-Lozano J.M., Aroca R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta. 2010;232:533–543. doi: 10.1007/s00425-010-1196-8. [DOI] [PubMed] [Google Scholar]
- 275.Zawoznik M.S., Ameneiros M., Benavides M.P., Vázquez S., Groppa M.D. Response to saline stress and aquaporin expression in Azospirillum- inoculated barley seedlings. Appl. Microbiol. Biotechnol. 2011;90:1389–1397. doi: 10.1007/s00253-011-3162-1. [DOI] [PubMed] [Google Scholar]
- 276.Del Amor F.M., Cuadra-Crespo P., del Amor F.M., Cuadra-Crespo P. Plant Growth-Promoting Bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2011;39:82–90. doi: 10.1071/FP11173. [DOI] [PubMed] [Google Scholar]
- 277.Niu S.Q., Li H.R., Paré P.W., Aziz M., Wang S.M., Shi H., Li J., Han Q.Q., Guo S.Q., Li J., et al. Induced growth promotion and higher salt tolerance in the halophyte grass Puccinellia tenuiflora by beneficial rhizobacteria. Plant Soil. 2016;407:217–230. doi: 10.1007/s11104-015-2767-z. [DOI] [Google Scholar]
- 278.Vaishnav A., Kumari S., Jain S., Varma A., Choudhary D.K. Putative bacterial volatile-mediated growth in soybean (Glycine max L. Merrill) and expression of induced proteins under salt stress. J. Appl. Microbiol. 2015;119:539–551. doi: 10.1111/jam.12866. [DOI] [PubMed] [Google Scholar]
- 279.Kumari S., Vaishnav A., Jain S., Varma A., Choudhary D.K. Bacterial-mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in soybean (Glycine max L. Merrill) J. Plant Growth Regul. 2015;34:558–573. doi: 10.1007/s00344-015-9490-0. [DOI] [Google Scholar]
- 280.El-Akhdar I., El-Sheekh M., Allam N.G., Kamal F., Abou-Shanab R., Staehelin C. Evaluation of salt-tolerant Azospirillum lipoferum and its role in improvement of Wheat growth parameters. Environ. Biodivers. Soil Secur. 2019;3:163–178. doi: 10.21608/jenvbs.2019.16428.1069. [DOI] [Google Scholar]
- 281.Li X., Sun P., Zhang Y., Jin C., Guan C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020;174:104023. doi: 10.1016/j.envexpbot.2020.104023. [DOI] [Google Scholar]
- 282.Cappellari L.D.R., Banchio E. Microbial volatile organic compounds produced by Bacillus amyloliquefaciens GB03 ameliorate the effects of salt stress in Mentha piperita principally through acetoin emission. J. Plant Growth Regul. 2020;39:764–775. doi: 10.1007/s00344-019-10020-3. [DOI] [Google Scholar]
- 283.Vaishnav A., Varma A., Tuteja N., Choudhary D.K. PGPR-mediated amelioration of crops under salt stress. In: Choudhary D., Varma A., Tuteja N., editors. Plant-microbe Interaction: An Approach to Sustainable Agriculture. Springer; Singapore: 2016. pp. 205–226. [DOI] [Google Scholar]
- 284.Ha-Tran D.M., Nguyen T.T.M., Hung S.H., Huang E., Huang C.C. Roles of plant growth-promoting rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A review. Int. J. Mol. Sci. 2021;22:3154. doi: 10.3390/ijms22063154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kohler J., Hernández J.A., Caravaca F., Roldán A. Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct. Plant Biol. 2008;35:141. doi: 10.1071/FP07218. [DOI] [PubMed] [Google Scholar]
- 286.Arora N.K., Tewari S., Singh S., Lal N., Maheshwari D.K. PGPR for protection of plant health under saline conditions. Bact. Agrobiol. Stress Manag. 2012:239–258. doi: 10.1007/978-3-642-23465-1_12. [DOI] [Google Scholar]
- 287.Atouei M.T., Pourbabaee A.A., Shorafa M. Alleviation of salinity stress on some growth parameters of wheat by Exopolysaccharide-producing bacteria. Iran J. Sci. Technol. Trans A Sci. 2019;43:2725–2733. doi: 10.1007/s40995-019-00753-x. [DOI] [Google Scholar]
- 288.Martínez R., Espejo A., Sierra M., Ortiz-Bernad I., Correa D., Bedmar E., López-Jurado M., Porres J.M. Co-inoculation of Halomonas maura and Ensifer meliloti to improve alfalfa yield in saline soils. Appl. Soil Ecol. 2015;87:81–86. doi: 10.1016/j.apsoil.2014.11.013. [DOI] [Google Scholar]
- 289.Khalilpour M., Mozafari V., Abbaszadeh-Dahaji P. Tolerance to salinity and drought stresses in pistachio (Pistacia vera L.) seedlings inoculated with indigenous stress-tolerant PGPR isolates. Sci. Hortic. 2021;289:110440. doi: 10.1016/j.scienta.2021.110440. [DOI] [Google Scholar]
- 290.Sukweenadhi J., Kim Y.J., Choi E.S., Koh S.C., Lee S.W., Kim Y.J., Yang D.C. Paenibacillus yonginensis DCY84T induces changes in Arabidopsis thaliana gene expression against aluminum, drought, and salt stress. Microbiol. Res. 2015;172:7–15. doi: 10.1016/j.micres.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 291.Tiwari S., Prasad V., Chauhan P.S., Lata C. Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in Rice. Front. Plant Sci. 2017;8:1510. doi: 10.3389/fpls.2017.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kim K., Jang Y.J., Lee S.M., Oh B.T., Chae J.C., Lee K.J. Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol. Cells. 2014;37:109. doi: 10.14348/molcells.2014.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Notununu I., Moleleki L., Roopnarain A., Adeleke R. Effects of plant growth-promoting rhizobacteria on the molecular responses of maize under drought and heat stresses: A review. Pedosphere. 2022;32:90–106. doi: 10.1016/S1002-0160(21)60051-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the article.